Abstract
In the last few years, the muscular system has gained attention due to the discovery of the muscle-secretome and its high potency for retaining or regaining health. These cytokines, described as myokines, released by the working muscle, are involved in anti-inflammatory, metabolic and immunological processes. These are able to influence human health in a positive way and are a target of research in metabolic diseases, cancer, neurological diseases, and other non-communicable diseases. Therefore, different types of exercise training were investigated in the last few years to find associations between exercise, myokines and their effects on human health. Particularly, resistance training turned out to be a powerful stimulus to enhance myokine release. As there are different types of resistance training, different myokines are stimulated, depending on the mode of training. This narrative review gives an overview about resistance training and how it can be utilized to stimulate myokine production in order to gain a certain health effect. Finally, the question of why resistance training is an important key regulator in human health will be discussed.
Keywords: myokine, resistance training, Irisin, IL-6, myostatin, PGC-1 alpha, BDNF
1. Introduction
Resistance training is known to be effective for maintaining and gaining muscle mass and strength [1]. Concerning mental-and physical health, this type of training stood in the shadow of endurance exercise, known for its positive effects on cardio-vascular health and functional capacity. This rethinking started to change with the discovery of myokines, a group of cytokines, small peptides, and proteoglycan peptides, secreted by contracting skeletal muscle cells [2]. Paracrine, autocrine and endocrine effects of these myokines were detected [2,3]. This led to a new understanding for the importance of muscular activity for human health. As discussed in this review, myokines have positive effects on metabolic, cardiovascular, mental, and immunological processes [4,5,6,7,8]. It is already well established that the human body possesses a complex muscle–body–crosstalk [3,8]. By stimulating the skeletal muscle in a certain way, we can make use of this cross talk and improve health [4,5,6,7,8].
Resistance training offers a variety of training methods to stimulate skeletal muscles that are more or less popular. For instance, resistance training with a goal of promoting muscle hypertrophy is triggering muscular growth by a stimulus to enhance muscle fibre growth leading to building muscles, so we can even enhance the health effects of our working muscle just by moving it in an appropriate way [9]. This narrative review will focus on the health-benefits of resistance training in general by explaining the “myokinological” background and enlighten the importance of resistance training in the therapy of the most common non-communicable diseases [5,10,11]. In Section 3, we will describe the investigated types of resistance training.
2. The Healthy Muscle Cell and Its Reaction to Resistance Training
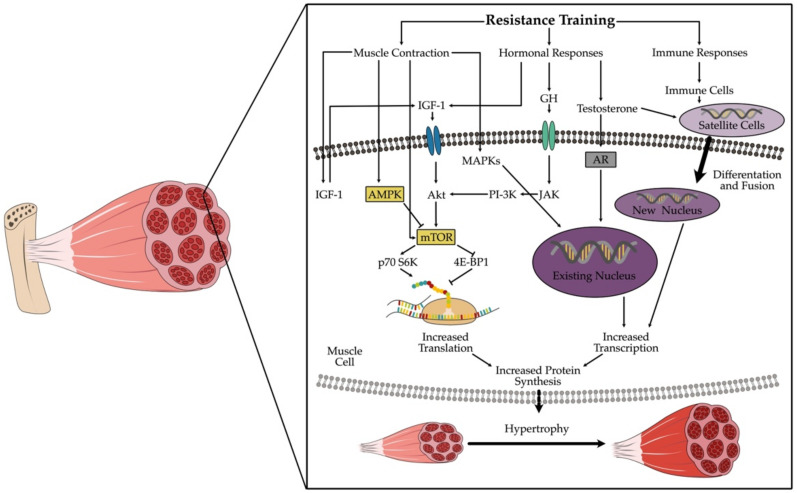
Regular repetitive training leads to adjustment processes on neuronal and morphological levels. The neuronal adaptations lead to an increase of maximal strength and quick force in the early training phase. Following this first phase, morphological adaptations occur when regular training continues. These adaptations are mainly induced by three mechanisms: 1. mechanical tension, 2. muscle damage and 3. metabolic stress [12]. These mechanisms can lead to increased protein biosynthesis, enhanced endocrinological activity and activation of satellite cells (Figure 1). This in turn stimulates hypertrophy of myofibrils, changes in the muscle fibre spectrum and hypertrophy of sarcoplasm and connective tissue structures [12].
Figure 1.
Intracellular adaptions following resistance training and leading to increased protein synthesis. Only the most important steps are pictured, the processes in vivo are much more complex. Abbreviations: Akt = protein kinase B; AMPK = adenosine monophosphate-activated protein kinase; AR = androgen receptor; GH = growth hormone; IGF-1 = insulin-like growth factor 1; JAK = janus kinase; MAPKs = mitogen-activated protein kinase; mTOR = mammalian target of rapamycin; PI-3K = phosphatidylinositol-3 kinase; p70 S6K = 70 kDa ribosomal protein S6 kinase; 4E-BP1 = eukaryotic initiation factor 4 E.
2.1. Mechanical Tension
Contraction and stretching generates mechanical tension in skeletal muscle, which seems to be the most important trigger leading to fibre hypertrophy by inducing molecular cascades that lead to protein synthesis; this process is called mechanotransduction [13]. The signaling involves increased levels of phosphatidic acid (PA) via diaglycerol kinase zeta (DKGz) [14]. PA in turn induces the mammalian target of rapamycin (mTOR), which is well known as the key regulator in protein synthesis [15]. Mainly responsible for this effect are tension dependent Ca2+ ion channels and membrane-bound mechanosensors like Integrine [16], and they react to the amount and duration of a mechanical stress [12].
2.2. Muscle Damage
Exercise induced microtrauma (muscle damage) is another cause for hypertrophy [12]. The extent of the damage depends on the intensity and duration of exercise, as well as the method of training and the training level of the athlete [12]. Microtrauma leads to an inflammatory reaction, which causes infiltration with granulocytes and macrophages. Cytokines and growth factors are released. This in turn activates the satellite cells [17]. Satellite cells belong to the pool muscle stem cells and are localized in the extracellular matrix, between sarcolemma and basal membrane [18]. After activation, these cells start to divide and migrate to the place of damage. Proliferating satellite cells are called myogenic precursor cells (MPC) [19]. Depending on the further steps of cell division, new myotubes or whole muscle fibres are created, either as single MPC, or as multiple melted MPCs [20]. While proliferating, these MPCs also create new satellite cells to refill the pool of muscle stem cells [19,20,21]. Under optimal conditions, the new muscle tissue is innervated, vascularized, and cannot be distinguished from uninjured muscle tissue, neither morphologically nor functionally [21].
2.3. Metabolic Stress
Examining the diverse metabolic changes in the muscle cell during resistance training shows that two further players are involved: the mTOR complex, also called Master Growth Regulator [15], and the AMPK- PGC-1 alpha axis—the energy regulators [22].
Once a contraction stimulus is received by muscle cells, ATP and Ca2+ are required for muscular action [23]. The available ATP lasts only for a few contractions. In the next step, the cell uses creatine phosphate to resynthesize ATP. This short-lived energy source is consumed within seconds. Then, the anaerobic glycolysis follows, producing not only ATP, but also lactate and NADH/H+ and phosphate (Pi) [24]. When these anaerobic metabolites accumulate, the pH level of the cell decreases. This change is relayed to the hypothalamus via chemosensitive afferent pathways where the secretion of growth hormones (GH), testosterone, as well as certain anabolic myokines, specifically IL-6, is induced. The described mechanisms are responsible for the induction of mTOR, the regulator of the protein synthesis [15]. Physical changes related to cell volume and hydrostatic pressure also occur, which after being recognized by certain mechanosensors, lead to the induction of mTOR [16].
If the contraction of the cell continues, the available energy resources quickly decrease, resulting in the rise of AMP concentration. AMP then activates AMP-kinase, which leads to enhanced energy uptake into the cell, activating lipid oxidation. AMP-kinase activates PGC-1 alpha by phosphorylation, therefore stimulating mitochondrial biogenesis [25]. In short, PGC-1 alpha plays a central role in the regulation of energy and mitochondrial biogenesis, as well as in oxidative stress defense mechanisms. A high activity of AMP-kinase inhibits the mTOR- pathway [12]. This means that when energy levels decline due to high energy demanding processes, the protein synthesis is stopped. Both pathways are in competition with each other [20] (Figure 1).
3. Different Types of Resistance Training
Concerning myokine production, there are mainly two investigated types of resistance training so far: hypertrophy training and strength endurance training. Further literature concerning other types of resistance training like maximal strength training is missing to date. The reason for the specific interest in hypertrophy and strength endurance training is the cellular signal cascade following the training stimulus, as mentioned above (Figure 1). The AMP-kinase pathway and the mTOR–pathway seem to be the key point in the production of certain important myokines [12,15,20,26].
In the following, we will give a short characterization of these two types of training.For a consistent definition of the investigated types of resistance training, we used the ACSM guidelines [27]. The specifications of repetitions and percent of the one repetition maximum are only examples. Due to the focus of this review on myokines, we renounce further training recommendations.
3.1. Hypertrophy Training
The current state of evidence suggests that the total volume of training is important to maximize muscle growth [28]; however, generalized loads cannot be prescribed due to the individual responses to resistance training [28]. This means that there are several recommendations concerning the definition of hypertrophy training [29]. The comparability of hypertrophy training study protocols is therefore impeded [29,30]. The definition for hypertrophy training and strength endurance training used in this review are taken from the American College of Sports Medicine position statement [27]: “An extensive utilization of energy-rich phosphates along with the consecutive accumulation of metabolites like H+, P (i), ADP, AMP and lactate is recommended. For example: 6–12 repetitions with 70–85% of the one repetition maximum (1 RM), in 1–3 series, with 1–3 min rest intervals between the series” [31] is recommended to attain this target. The rest period is required for the recovery of creatine phosphate, but not for a full metabolic recovery. The goal of hypertrophy training is to enhance the muscle fibre cross-sectional area (fCSA) in the slow Type-I, and mainly in the fast Type-IIA fibres, and to promote creatine phosphate and glycogen storage [1]. Furthermore, there is an improvement of the alactic as well as lactic metabolism via increased enzymatic activity [26].
3.2. Strength Endurance Training
In contrast to hypertrophy training, strength endurance training involves the use of lower weights, usually 50–70% of 1 RM, performing more repetitions [31]. For example, 1–3 sets with 10–15 repetitions for beginners, or multiple sets with 10–25 repetitions, or even more, for advanced athletes, rest intervals should last <90 s [27]. Extreme settings of strength endurance training are reported to work with 150 repetitions in less than 5 min per set [32].
The main goal of strength endurance training is not an increase of the fCSA, but an increase of time to exhaustion and maximal aerobic power [20]. These effects are unaffected by changes in VO2 max and seem to be related to increases in lactate threshold and lower limb strength, as well as improving running or cycling economy [33]. Just like hypertrophy training, strength endurance training has the potential to cause conversions from Type IIB to Type IIA muscle fibres [34].
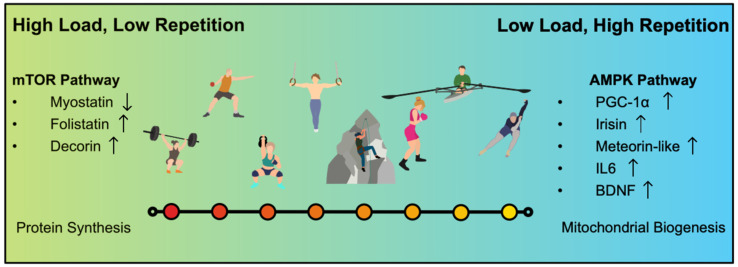
The transitions from resistance training to strength endurance to muscle endurance is flowing [27]. There is no hard threshold; it seems more likely to be a continuum, a “strength-endurance continuum”, with high load/low repetitions on the one side and low load/high repetitions on the other side [32,33] (Figure 2).
Figure 2.
The Strength–Endurance Continuum shows exemplarily different types of sports, associated with strength training. In general, there is not only weightlifting, but also other kinds of strength training that lead to the health promoting effects.
4. Myokines
Myokines, which are secreted by muscle contractions, are able to restore metabolic balance in the human body and are able to regain health. Several hundred myokines have been identified to date, and presumably many hundred more are waiting to be discovered [35]. For simplicity, the review focuses on the most prominent myokines for potentially playing a role in the treatment of common non-communicable diseases such as the metabolic syndrome, cancer and neuronal degenerative diseases.
4.1. IL-6
Interleukin-6 (IL-6) was the first myokine that was discovered and is one of the most popular myokines discussed in several original data and reviews [6,7,11,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Years before the discovery of myokines, IL-6 was mentioned as an interferon (interferon beta2) [52]. In 1989, the molecule received its new name “Interleukin-6” [39]. In 2003, Pederson et al. presented this molecule as a potential “exercise factor” [7,41].
Although we know that IL-6 is a pro-inflammatory cytokine, it also has anti-inflammatory capacities when it is released by the working muscle [39]. The measurable plasma levels can increase up to 100-fold of the pre-exercise baseline and decrease back to baseline in a short period of time [53]. The anti-inflammatory effects seem to depend on the muscle as the secreting organ and a rapid onset peak effect. Furthermore, IL-6 seems to induce the production of other anti-inflammatory cytokines and to inhibit the production of TNF alpha and IL-1 beta [39,54]. Another important target for IL-6 is the metabolic system where it plays an important regulatory role by enhancing insulin-sensitivity and the accumulation of GLUT-4 [42]. IL-6 exerts its effect both locally within the muscle through activation of AMPK, and, when released into circulation, peripherally in a hormonelike fashion [39].
IL-6 not only has anti-inflammatory effects but is also involved in stimulating the multiplication of satellite cells in the muscular system, leading to hypertrophy of the muscle [55]. There are also hints that repetitive eccentric contraction leads to an increase in the number of stem cells and fusion of muscle fibres when compared against concentric training [56]. Via activation of the mTOR- signal cascade, IL-6 increases the protein synthesis in the myotubes [57].
Although IL-6 plays a very important role in exercise-metabolism, putting it in a pill seems not to be recommendable. High circulating levels of IL-6 are associated with inflammation. The structural differences between muscle cell IL-6 and other forms of IL-6 are unknown. Furthermore, there are different pathways following the activation of IL-6 receptor (IL-6R) that are responsible for either proinflammatory or anti-inflammatory effects of IL-6 [58,59]. There might be more cofactors involved as well, which could be responsible for the variant effects of IL-6.
4.2. Myostatin Group
4.2.1. Myostatin
Myostatin is the only myokine that is reduced by exercise. It was identified in 1997 as a negative regulator of skeletal muscle growth [60]. The molecule belongs to the transforming growth factor beta superfamily (TGF-ß) [61]. The function of Myostatin is to limit muscle growth during embryogenetic development, but it is also expressed in adulthood [62]. The activated Myostatin has a high affinity to the Activin type 2 receptor (ACTRIIB) [63]. This activation triggers a catabolic cascade via SMAD1/SMAD2 and activates the forkhead box transcription factors family (FOXO1,2,3) [64]. Furthermore, mTOR and AMPK pathways are inactivated [64].
High levels of Myostatin inhibit the satellite cell proliferation and differentiation and block the action of muscle fiber protein accretion [65]. High levels are significantly associated with muscle wasting diseases, myopathy and sarcopenia [66]. Myostatin can also be expressed in cardiomyocytes, thereby associated with the development of heart failure [67]. A positive correlation between obesity, insulin-resistance and high levels of Myostatin has also been observed [65,68]. One of the most prescribed drugs, glucocorticoids, stimulate the expression of Myostatin that leads to iatrogenic muscle loss [69,70].
Myostatin plays a central role in the regulation of muscle growth and therefore also in the regulation of metabolism. In the past years, there have also been several pharmacological developments concerning suppression of Myostatin as a target for curing muscular dystrophies [69,71].
This is only one example of pharmacological development concerning the use of the Myostatin-system for therapeutic purposes in muscle dystrophies or secondary cachexia. After 20 years of Myostatin research, a successful transition from mouse-model to human-model is still missing. Too many cofactors that need to be considered are still prohibiting the implementation of myostatin-inhibition in therapy [71]. This makes physical activity and exercise induced Myostatin-inhibition even more important for therapeutic use.
4.2.2. Decorin and Follistatin
The Myostatin–Antagonist Decorin was discovered in 1991 and later classified as a myokine [72]. It suppresses the Myostatin-effect by binding this myokine [73]. Another study confirmed that a Decorin-overexpression could enhance the proliferation of myoblasts and lead to the growth of myotubes [74]. Within muscle-body-crosstalk, Decorin inhibits angiogenesis and tumorgenesis [75].
Follistatin is another important antagonist of Myostatin, which cannot only bind Myostatin, but also blocks Myostatin receptors [76]. It was first discovered in follicular fluid and was found to be an FSH-suppressing protein [77]. It was classified as an antagonist of the TGF-beta- superfamily. Besides Myostatin, it can also bind other members of the TGF-beta family [76,78]. Concerning the muscular system, Follistatin can induce the proliferation of satellite cells, leading to protein synthesis [79,80]. Follistatin was also able to accelerate the healing of muscle injuries in mice, and reduced muscle fibrosis [81].
4.3. BDNF
The brain-derived neurotropic factor (BDNF) is produced by a wide spectrum of cells, including skeletal muscle cells, cardiac myocytes, smooth muscle cells, and cells in the liver and brain [82]. BDNF was first discovered in 1982 in the brain of pigs, long before it was identified as a myokine [83]. As a neurotrophine, BDNF is involved in the differentiation of neurons, synaptic plasticity and neurogenesis in the amygdala, prefrontal cortex and hippocampus [84,85,86]. It is furthermore a positive regulator of axonal and dendritic growth in the central nerval system (CNS) [87,88]. These functions promise potential for BDNF- associated therapies in the treatment of neuronal diseases like Alzheimer’s disease, Parkinson’s disease or Huntington’s Chorea. BDNF also has important influence on other tissues. It is engaged in the endogenous reparation of myocardial and skeletal muscle cells [89,90]. BDNF activity is also negatively correlated with insulin resistance, obesity and blood glucose levels [91,92]. Via the hypothalamic melatonin pathway, BDNF can regulate metabolism and may protect against obesity [93].
In the muscle itself, BDNF can be released by satellite cells in the case of injury and is involved in the regeneration of the damaged muscle [94,95,96,97]. Via the AMPK-pathway, BDNF enhances the lipid oxidation in the muscle cell [98], and improves glucose utilization [99]. Several studies show that circulating BDNF levels are elevated after resistance training, independent of the kind of resistance training [100]. Furthermore, the BDNF levels increased after training with regular repetition over several weeks [101]. Therefore, resistance training performed regularly can increase the positive effects of BDNF in the human body.
In summary, BDNF is a multifunctional protein with organ protective capabilities and therefore a very interesting molecule for therapeutic potential regarding the treatment of non-communicable diseases.
The myokine BDNF has only auto- and paracrine functions, as far as is currently known [98]. This means that BDNF produced by the muscle cell cannot be responsible for the elevated circulating levels seen after resistance training. The enhanced levels observed after training must be produced by other tissues, such as liver or adipose tissue [4]. In general, resistance training stimulates not only the muscle cell, but also different other organs to produce BDNF.
It is important to know that BDNF cannot pass the blood–brain barrier. This is one reason why it is difficult to develop BDNF-associated therapeutics for the treatment of neuronal disease [102]. In 2010, Linker et al. were successful in channeling BDNF through the blood–brain barrier by using T-cells as a transporter [103]. In the following years, different studies were performed to investigate the effect of exogenous BDNF in patients’ brains [104,105,106]. Although there were positive results in vitro as well as in mice-models concerning Multiple Sclerosis, the positive results in human patients with Parkinson’s disease failed to appear [102]. In conclusion, the positive effects of BDNF in the brain that appear by performing resistance training did not appear when BDNF was applied in the form of a medication—neither directly nor via genetic modifications [102].
4.4. The PGC-1 Alpha Group
4.4.1. Irisin
Irisin is one of the most recent myokines that is described to date [107]. Biochemically, it is a PGC-1 alpha dependent molecule, cleaved of the transmembrane protein FNDC5. Irisin is proposed to be an exercise induced myokine, which is able to induce the browning of white adipose tissue [107]. The process of browning leads to increased thermogenesis and enhanced energy metabolism [108,109]. Lively discussions regarding this molecule ensued, due to controversial opinions regarding the expression of Irisin. It was labelled as a mythos rather than a myokine [110]. This debate could be solved by using a different detection method. The formerly used commercial ELISA-kits seem to be rather unspecific: They detected not only Irisin, but also different cross-reacting proteins. Through implementation of tandem mass spectrometry, the results started to become clearer [111]. Another point that leads to confusion was the time of measurement. Since Irisin is a short-lived molecule, different times of measuring after training lead to different conclusions about the exercise induced expression of Irisin [112,113,114]. For later studies, this was considered. In the following years, numerous studies concerning Irisin were performed, underpinning Irisin’s existence (Table 1).
Table 1.
Summary of human studies investigating myokine secretion via resistance training protocols.
| Myokine | Study | n | Study Cohort | Training Protocol | Trial | Results |
|---|---|---|---|---|---|---|
| IL-6 | Buford et al. [145] | 24 | Physically active postmenopausal women | 3 × (10 × 80% 1 RM) | 1 session | Muscle biopsies: IL-6-mRNA ↑ |
| Della Gatta et al. [147] | 16 | Young men n = 8; elderly men n = 8 |
2 × (8–12 × 50–80% 1 RM), progressive increase | 12 weeks | Muscle biopsies: IL-6 ↑; not significant different between the groups |
|
| Mendham et al. [44] | 12 | Sedentary men | 3 × (10 × 60% 1 RM) vs. 3 × (10 × 80% 1 RM) vs. Low intensity aerobic exercise (40 min) vs. Moderate intensity aerobic exercise (40 min) |
4 sessions, randomized cross-over | Serum- IL-6 ↑ in the moderate intensity groups (RE and AE) | |
| Phillips et al. [146] | 14 | Healthy men | 3 × (12 × 65% 1 RM) vs. 3 × (8 × 85% 1 RM) |
2 sessions, controlled | Serum-IL-6 ↑, with association to total volume load | |
| Quiles et al. [148] | 15 | RE-experienced men | 4–5 × (8–12 × 60–70% 1 RM) vs. 8–10 × (2–6 × 75–85% 1 RM) |
3 weekly, 6 weeks, randomized | Plasma-IL-6 ↑ in both groups; no significant effect on BDNF |
|
| Tomeleri et al. [149] | 38 | Obese older women | 3 × (10–15 maximum repetitions) | 3 × weekly, 8 weeks, randomized controlled | Baseline-Serum-IL-6 ↓ | |
| Myostatin | Kazemi et al. [150] | 24 | Healthy men | 3 × (15 × 55% 1 RM) | 1 session | Plasma-Myostatin ↓ |
| Raue et al. [151] | 14 | Young women n = 8 Old women n = 6 |
3 × (10 × 70% 1 RM) | 1 session | Muscle biopsies: Myostatin mRNA ↓ | |
| Decorin | Bugera et al. [152] | 10 | Physically active young men | 4 × (7 × 80% 1 RM) vs. 4 × (15–30 × 30% 1 RM) vs. BFR 3 × (15–30 × 30% 1 RM) | 1 session | Plasma-Decorin ↑ |
| Kanzleiter et al. [153] | 10 | Young men | 3 × (8 × max weight) | Human study I, single bout | Plasma-Decorin ↑ | |
| Follistatin | Bagheri et al. [154] | 40 | Middle aged men | 3–4 × (15 > 12 > 10 > 8 × 50–80% 1 RM) Upper body vs. lower body vs. both vs. control |
3 × weekly, 8 weeks, 10% increase every 2 weeks, randomized controlled | Serum-Follistatin ↑, serum-Myostatin ↓, depending on the volume of activated muscle mass |
| Hofmann et al. [157] | 91 | Elderly women | 1–2 × 15, elastic bands vs. Training + nutritional supplements vs. Cognitive training |
Progressive increase of resistance; 6 months; randomized | Serum-Follistatin ↑ only in the training group | |
| Negaresh et al. [155] | 31 | Elderly men n = 15 Young men n = 16 |
4 × (10 × 50%−85% 1 RM) | 3 × weekly, 8 weeks, 5% increase per week | Plasma-Follistatin ↑; plasma-Myostatin ↓ in both groups | |
| Irisin | Blizzard Leblanc et al. [9] | 11 | Obese youth | 4 × (12–15 × 60–65% 1 RM) | 3 × weekly, 6 weeks | Plasma-Irisin → after a single bout of RE, but ↑ after a single bout of AE; with greater ↑↑ after 6 weeks of RE |
| Ellefsen et al. [158] | 18 | Untrained women | Progressive full body heavy strength | 3 × weekly, 12 weeks | Serum-Irisin → | |
| Huh et al. [117] | 20 | Sedentary healthy men n = 14 Men with metabolic syndrome n = 6 |
3 × (8–12 × 75–80% 1 RM) vs. HIIT (4 ×4 min 90% Vo2 max) vs. Continuous moderate exercise (36 min 65% Vo2 max) |
1 session, randomized cross-over | Serum-Irisin ↑ immediately and ↓ after 1 h back to baseline; no difference between the groups | |
| Kim et al. [159] | 28 | Obese adults | 3 × (10–12 × 65–80% 1 RM) vs. Aerobic exercise (50 min 65–80% HR max) |
5 × weekly, 8 weeks, randomized controlled | Plasma-Irisin ↑ only in the RE group | |
| Norheim et al. [160] | 26 | Healthy physically inactive men n = 13 Pre-diabetic men n = 13 |
Combined strength and endurance training, 2 × weekly 60 min ergometer and 2 × weekly 60 min full body strength workout | 4 × weekly, 12 weeks | Plasma-Irisin higher in the prediabetic group; acute ↑ after exercise; baseline ↓ after 12 weeks | |
| Nygaard et al. [161] | 9 | Moderately trained, healthy adults | 3 × (10–12 × max weight) vs. 6 × 5 min high intensity treadmill, Borg > 18 |
1 session, randomized cross-over | Plasma-Irisin ↑ in both groups, but remained higher in the RE group | |
| Pekkala et al. [162] | 56 | Untrained healthy men | Aerobic exercise (60 min 50% Vo2 max) vs. 5 × 10 repetitions until failure (leg press only) vs. Long term endurance exercise vs. Long term RE and endurance exercise |
Single bout vs. 2 × weekly, 21 weeks |
Serum-Irisin → | |
| Tibana et al. [163] | 49 | Inactive women: Obese n = 26 Non obese n = 23 |
3 × (6–12 × repetitions maximum) | 2 × weekly, 16 weeks | Plasma-Irisin ↓ in the non-obese group after intervention; no change in the obese group | |
| Tsuchiya et al. [116] | 10 | Healthy men | 3–4 × (12 ×65% 1 RM) vs. Aerobic exercise (60 min 65% Vo2 max) vs. 30 min RE + 30 min AE |
3 single bouts of exercise; randomized cross-over | Plasma-Irisin ↑, significant higher in the RE group | |
| Zhao et al. [164] | 17 | Older male adults | Class of leg muscle strength and core strength training | 2 × weekly, 12 weeks, randomized controlled | Serum-Irisin ↑ | |
| BDNF | Church et al. [101] | 20 | RE-experienced young men | 4 × (10–12 × 70% 1 RM) vs. 4 × (3–5 × 90% 1 RM) |
4 × weekly, 7 weeks | Plasma-BDNF ↑ |
| Domínguez-Sanchéz et al. [166] | 51 | Physically inactive, obese men | (12–15 × 50–70% 1 RM) vs. HIIT vs. HIIT + RE |
1 session, randomized controlled | Plasma-BDNF ↑, highest ↑↑ in the combined group | |
| Figueiredo et al. [89] | 21 | Physically active men | 1 min 100%VO2 max + 8 exercises 8–12 RM | 8 weeks, control group | Plasma-BDNF ↑ | |
| Forti et al. [167] | 65 | Healthy elderly | 2 × (10–15 × 80% 1 RM) vs. 1 × (80–100 × 20% 1 RM) vs. 1 × (60 × 20% 1 RM) + 1 × (10–20 × 40% 1 RM) |
3 × weekly, 12 weeks, randomized | Serum-BDNF ↑ in male participants, only in the 3rd group | |
| Lodo et al. [168] | 20 | Young healthy adolescents | 4 × (5 × 70% 1 RM) vs. 4 × (10 × 35% 1 RM) |
2 bouts of exercise with equated total load lifted | Serum-BDNF → | |
| Jørgensen et al. [169] | 30 | Persons with multiple sclerosis | Progressive high intensity | 2 × weekly, 24 weeks, randomized controlled | Plasma-BDNF → | |
| Marston et al. [171] | 45 | Healthy adults | 5 × (5 × 85% 1 RM) vs. 3 × (10 × 70% 1 RM) vs. |
2 × weekly, 12 weeks, randomized controlled | Serum-BDNF (↑) only in the high load group | |
| Marston et al. [170] | 16 | Untrained men n = 11 Untrained women n = 5 |
5 × 5 repetitions to-fatigue vs. 3 × 10 repetitions to fatigue |
2 bouts of exercise; cross-over | Greater serum-BDNF ↑ in the second group | |
| McKay et al. [90] | 29 | Male adolescents | 300 × maximal eccentric contractions | Single bout | Muscle-biopsy: BDNF ↑ | |
| Roh et al. [172] | 26 | Elderly, obese women | Elastic bands, intensity:10–14 RPE | 3 × weekly, 12 weeks, randomized controlled | Serum-BDNF ↑ | |
| Rojas Vega et al. [174] | 11 | Healthy adults | 3 repetitions of maximal effort isokinetic work (knee extension): 40% 1 RM vs. 110% 1 RM |
2 single bouts | Serum-BDNF (↑), but no significance | |
| Urzi et al. [173] | 20 | Elderly women | Elastic band resistance training | 12 weeks, randomized controlled | Plasma-BDNF ↑ | |
| Walsh et al. [175] Alberga et al. [177,178] |
202 | Postpubertal adolescent with obesity | HEARTY-Trial: 2–3 × (6–15 × maximum reps) vs. 20–40 min 70–85% HRmax vs. combination |
4 × weekly, 22 weeks, randomized controlled | Baseline plasma-BDNF ↑ | |
| Walsh et al. [179] | 10 | Older adults | 4 × (8–10 × 60–80% 1 RM) | 3 × weekly, 8 weeks | Serum -BDNF ↑ | |
| Wens et al. [180] | 41 | Persons with multiple sclerosis n = 22 Healthy persons n = 19 |
1 × (10 × 12–14 RPE) increased to 4 × (15 x12–14 RPE) |
5 sessions per 2 weeks, 24 weeks, randomized controlled | Baseline serum-BDNF were lower in persons with MS; Serum-BDNF ↑ after exercise in both groups |
|
| Yarrow et al. [100] | 20 | Healthy young adults | 4 × (6 × 52,5% 1 RM) trad. vs. 4 × (6 × 40% 1 RM) concentric vs. 3 × (6 × 100% 1 RM) eccentric |
5 weeks | Serum BDNF ↑, with higher response at the end of intervention. No baseline change |
Abbrevations in order of appearance: RM: repetition maximum; RE: resistance exercise; AE: aerobic exercise; HIIT: high intensity interval training; RPE: rate of perceived exertion; MS: multiple sclerosis.
Irisin can be produced in muscle, adipose tissue and in the myocardium [115]. A powerful stimulator for muscle cells to produce Irisin is physical exercise. Among different exercise studies, resistance training crystallized to be most effective; however, this has not been confirmed until today [113,116,117].
The most interesting effect of Irisin is the browning of white adipose tissue by increasing the expression of mitochondrial uncoupling protein 1 (UCP 1) [107]. This leads to an activation of non-shivering thermogenesis. Furthermore, Irisin improves glucose homeostasis and lipid metabolism, and reduces insulin-resistance as well as adipose tissue inflammation [108,109,118]. An important benefit for the myocardium is the reduction of endothelial function abnormalities. Irisin protects the myocardium against ischemia and reperfusion injuries through the AMPK-Akt-eNOS-NO-pathway [119,120].
Additionally, Irisin seems to enhance bone mass with positive effects on cortical mineral density and bone geometry via alphaV/beta5-integrin [121,122].
Concerning neurological diseases, Irisin has positive effects on Alzheimer’s disease by reducing insulin-resistance and improving glucose metabolism. Irisin is also involved in the process of neurogenesis in the CNS [123].
In the case of cancer, there are varying results, but most results confirm a positive effect on oncologic diseases [124]. Studies show that Irisin induces cancer cell apoptosis and reduces the migration of breast cancer cells [125]. Irisin is also able to enhance the sensibility of cancer cells for Doxorubicin, a well-known chemotherapy agent. This led to the hypothesis that, in combination with Irisin, lower Doxorubicin doses could be sufficient [125]. Breast cancer patients also seem to have a lower base level of Irisin compared to a healthy population [126]. Similar positive effects could be found by treating colorectal-, prostate, lung-, bone-, and pancreas cancer cells [127,128,129,130]. Different results were found by investigating Irisin and hepatocellular cancer. An overexpression of Irisin mRNA was observed in liver cells [131], along with reduced sensibility for Doxorubicin [132]. Concerning cancer and Irisin, a general recommendation cannot be given, as multiple factors are involved—the primary factor is the type of cancer being studied. More studies are needed.
Though Irisin was first discovered in 2012, there are already several well-known drugs that influence PGC-1 alpha and Irisin. Metformin, a drug for treating diabetes type 2, enhances the expression of FNDC5 mRNA and circulating Irisin levels in mice [133]. These results have not yet been confirmed in humans. Other drugs for treating cardiac diseases (for example, Losartan, Catecholamine, Bezafibrate, Fenofibrate and Pioglitazone) are shown to stimulate PGC-1 alpha expression and activate a certain pathway [134,135,136,137,138]. Further studies are needed to investigate a potential correlation with Irisin.
Another point to consider is the dependence of Irisin on PGC-1 alpha. It is a central player involved with the regulation of mitochondrial biogenesis and cell metabolism and can be induced by various stimuli. This in mind could help identify heretofore unexplored ways of Irisin induction. Some of these have already been observed, like inducing Irisin through cold-exposure, or other external stimuli, leading to an increased ATP demand [139].
4.4.2. Meteorin-Like
Meteorin-like (Metrnl) is another recently discovered adipomyokine, dependent on PGC-1 alpha. Metrnl was discovered in 2014 by using the splice variant PGC-1 alpha4 [140]. It was first found to be preferentially induced in muscle cells by resistance training and linked to muscle hypertrophy [141]. Later, another study showed that Metrnl is generally inducible by exercise. However, this study could not specify whether resistance training or endurance training is more likely to induce this myokine [37]. More studies are required.
Like Irisin, Metrnl is able to induce the browning of white adipose tissue, not by direct action on adipocytes but rather via recruitment of IL-4 and IL-13 expressing eosinophils in adipose tissue [140]. This leads to enhanced glucose tolerance and improved insulin sensitivity in mice. Even a loss of 25% body fat in the Metrnl treated mice could be observed [140]. These are reasons why Metrnl could be a potential target fighting metabolic diseases and obesity. Studies with humans were more complicated, as various cofactors (like patients’ medications) had to be considered [142]. This leads to different outcomes concerning Metrnl and diabetes type 2 patients [142]. Further studies need to be performed. Recently, Metrnl has been reported as a cytokine expressed by macrophages and barrier tissues like skin and respiratory tract epithelium [142,143]. It seems closely involved in innate and acquired immune responses and inflammatory regulation [144].
5. Myokines and Resistance Exercise
5.1. IL-6
Concerning resistance training, there are controversial results in different studies regarding measured post exercise IL-6 levels [45,145]. There might be a necessary minimum total volume of training [146], just like a threshold or a sweet spot, required to attain this peak effect of IL-6. The studies with the highest IL-6 output involved a full body resistance work-out including large muscle groups [44,146]. This hypothesis matches the described reaction of a muscle cell to an adequate stimulus, described in the first part of this review.
By taking a closer look on the studies, the biggest differences that occur are concerning the method of measurement of IL-6. Buford et al. used muscle biopsies for the purpose of determining IL-6-mRNA levels [145]. Della Gatta et al. also performed muscle biopsies, but for determining IL-6 itself and not IL-6-mRNA levels [147]. Mendham et al. and Phillips et al. measured serum-IL-6 levels after exercise [44,146]. Quiles et al. determined plasma-IL-6 levels after exercise [148].
All studies concerning the acute response after exercise could present an increase in their measured IL-6 levels, independent from the type of measurement (Table 1).
Tomeleri et al. used the baseline serum-IL-6 level as a main target [149]. This was the only human study which could be found, concerning resistance training and the baseline serum-IL-6 level. The decrease could be interpreted as an improvement of the inflammatory level.
5.2. Myostatin
As has already been mentioned, Myostatin operates as an inhibitor of muscle growth [60]. Therefore, lowering the Myostatin-level via training is the worthwhile goal for muscle growth [65]. Kazemi et al. were able to show that even a single session of exercise could reduce the plasma-Myostatin level [150]. Raue et al. found similar results: after one session of resistance exercise, the Myostatin-mRNA levels in the collected muscle biopsies were significantly reduced [151]. This means, for practical implementation, every session of resistance training counts.
5.3. Decorin
Decorin is an important antagonist of Myostatin [72]. This means that Decorin and Myostatin levels correlate inversely. The fact that resistance exercise is an appropriate method to enhance Plasma-Decorin levels, which could be demonstrated by Bugera et al. and Kanzleiter et al. [152,153]. Both studies performed their measurements after a single bout of exercise.
5.4. Follistatin
Different studies present the same results concerning the increase of plasma or serum-Follistatin levels after resistance training [154,155,156,157]. Bagheri et al. performed a randomized controlled trial over eight weeks, with the result of elevated serum-Follistatin, depending on the volume of activated muscle groups [154]. Increased serum-Follistatin was also the result in the study of Hofmann et al. in the Vienna Active Ageing Study [157]. Negaresh et al. could show an increase in plasma-Follistatin levels as well, independent from the age of the investigated volunteers [155].
5.5. Irisin
As described above, Irisin is a frequently discussed myokine. One of the reasons is the fact that we still do not exactly know how this molecule is stimulated in the most effective way. In Table 1, you can see the current number of studies performed to find a correlation between a certain type of exercise and Irisin production, with very different results.
Blizzard Leblanc et al. first compared a single bout of aerobic exercise with a single bout of resistance exercise, followed then by six weeks of resistance exercise (RE) [9]. The plasma-Irisin level did not change after a single session of RE, but after a single session of aerobic exercise (AE). After six weeks of RE though, there was a measurable increase in the plasma-Irisin level [9]. In contrast, Ellefsen et al. did not find any changes in the serum-Irisin level after 12 weeks of full body heavy strength training [158].
Huh et al. compared RE and AE. They found an elevated serum-Irisin in both groups, immediately after exercise, which returned to baseline 1 h after the end of training [117]. Another study which compared RE and AE was performed by Kim et al. in a randomized controlled trial [159]. In this study, only the RE group could present an elevated plasma-Irisin level. Norheim et al. performed a mixed form of training for the duration of 12 weeks and differed between inactive and prediabetic volunteers [160]. They also observed elevated plasma-Irisin levels with a stronger increase for the prediabetic group. Interestingly, the baseline level of Irisin was reduced after 12 weeks of training. Nygaard et al. compared AE and RE in a single session randomized cross-over design [161]. Once again, plasma-Irisin levels increased after both exercise interventions, but remained higher after RE. Pekkala et al. compared four different types of exercise protocols for a duration of 21 weeks, but they could not measure any change in the serum-Irisin levels [162].
Tibana et al. is one of the rare studies which did not show an increase but a decrease in plasma-Irisin levels of non-obese volunteers after 16 weeks of RE [163].
Tsuchiya et al. performed another randomized cross-over study with healthy men [116]. Plasma-Irisin levels increased in all three intervention groups, with a significantly higher increase in the RE group. Zhao et al. performed their study with core and leg strength training, and they also could find increased Serum–Irisin levels [164].
Although there are contradictory results now and then, the vast number of studies predicts a positive effect of RE concerning Irisin. There also seems to be an advantage over AE [116,161,165].
5.6. BDNF
For this review, 16 studies concerning BDNF and RE could be found, which fits the inclusion criteria mentioned above. Compared with the other myokines of this review, this is the largest number of studies. The strong interest in BDNF can be explained by its positive effects on neuronal diseases as already explained in the first part of this review using a resistance training protocol targeting strength (e.g., 5 × 85% 1 RM).
Church et al., Domínguez-Sanchéz et al. and Figueiredo et al. could all find an increase in plasma-BDNF levels after RE [89,101,166]. The study of Domínguez-Sanchéz et al. was performed as a randomized controlled trial with RE, high intensity interval training (HIIT) and a combined group [166]. The combined group showed the highest increase in serum-BDNF levels. Forte et al. also compared three groups of different types of RE [167]. Only in one group could an increase of serum-BDNF be detected.
Lodo et al. and Jørgensen et al. are two studies that could not find any change in serum- or plasma-BDNF levels after RE [168,169].
Marston et al. published two studies with different intervention protocols [170,171]. Both studies could show an increase in serum-BDNF, but only for the high load group in the study from 2019 [171], and a higher increase for the 3 × 10 repetition group in the study from 2017 [170]. This underlines the hypothesis of a minimum total load of exercise needed to gain a measurable effect.
McKay et al. performed muscle biopsies after a single bout of 300 × maximal eccentric contractions [90]. After this muscle damaging exercise, BDNF was elevated in the biopsies, and a correlation between BDNF and the proliferation as well as differentiation of satellite cells could be demonstrated for the first time [90].
Roh et al. and Urzi et al. both performed studies with elastic band resistance training in elderly women [172,173]. Both studies could detect an increase of the serum- or plasma-BDNF levels.
The study of Rojas Vega et al. could only find a non-significant increase of serum-BDNF after isokinetic knee extension [174]. A potential explanation for this result could be the upper mentioned minimum load hypothesis.
A huge study with several publications was the HEARTY-TRIAL [175,176,177,178]. Concerning BDNF and RE, Walsh et al. and Alberga et al. could demonstrate that the baseline serum BDNF level of obese adolescents was elevated after 22 weeks of training, either RE or AE, or combined [175,178]. In another study with older adults, Walsh et al. found elevated serum-BDNF levels after eight weeks of RE as well [179].
Wens et al. compared healthy persons with multiple sclerosis (MS) patients [180]. A lower baseline serum-BDNF level was noticed in the MS group. In both groups, serum-BDNF increased after RE.
Yarrow et al. found a higher response of serum-BDNF to RE at the end of a 5-week RE intervention, but no baseline change [100].
The wide range of studies performed with resistance training in order to elevate BDNF levels is impressive. Most of these studies could present positive results. This underlines the importance of resistance training in therapy and prevention of neuronal diseases.
6. Discussion—Myokines and Resistance Training
This review shows that resistance training can induce the production of different myokines, which are meaningful to retain or even regain health. The anti-inflammatory effects (IL-6) [39], the enhancement of insulin sensitivity (Mtrnl) [140] or the browning of adipose tissue (Irisin, Mtrnl) [107,140] with subsequent optimizing of the status of metabolic diseases, and many other positive effects, as highlighted in this review, underline the importance of giving resistance training the status of a medical treatment for certain diseases [181]. The myokines IL-6 and Irisin seem to develop their positive effects directly by inducing certain pathways [54,107], whereas Mtrnl first recruits eosinophils in adipose tissue [140]. Follistatin and Decorin develop their effects by being an antagonist of Myostatin and binding this myokine [73,76]. BDNF seems to be produced by a variety of tissues, such as muscle, liver, adipose tissue and brain [82].
Depending on the therapeutical goal, you can activate either the mTOR- pathway, or the AMPK-pathway for myokine production (Figure 1 and Figure 2). It seems obvious that metabolic diseases like Type 2 Diabetes or obesity and cardiovascular diseases could benefit from the activation of the AMPK-pathway. Most studies considered in this review just focused on myokine production itself, if myokines can be stimulated via resistance training at all. This is a first step and is important to know, but now we have to look further. We have to consider body composition, gender, age, and probably even hormonal status as well for future studies.
We could also show that there are various types of exercise protocols, investigated to their beneficial effect on myokine production. The studies included in this review are very heterogeneous. Population, study designs and even resistance training criteria are not comparable to each other. However, most studies have one thing in common: they induce myokine production.
Some of the Myokines, like Irisin, seem to be more stimulated via resistance training compared to endurance training. Another point of interest seems to be the intensity of exercise. Therefore, different types of resistance training were investigated in the considered studies for this review. Hypertrophy training stimulated different types of myokines compared to strength endurance training, although the transition between both is flowing (Figure 2). Most protocols used the method of “1 Repetition maximum” (1RM), or other variants of RM, to find the required training zones. This tool is an important factor for the comparability of the studies. Investigators therefore have the opportunity to use a certain protocol for different groups of participants, compare the results and adapt the training to the possibilities of each individual participant. We recommend for further investigations to use the ASCM-guidelines [27] to give a clear definition of the investigated types of training, to make further studies more comparable.
However, as already considered above, the myokine stimulation seems to depend on more factors than only on the intensity and duration of training including cofactors like nutrition, lifestyle, medication, circadian rhythmic and several more influences, presumably the myokine production [182]. (Figure 3) Therefore, we should consider a multidimensional context in the exercise induced cell metabolism for further studies. Particularly, we should pay attention to the nutritional influence on cell metabolism. We already know, for example, that amino acids can induce mTOR and lead to protein biosynthesis [183]. Some studies already investigate the influence of nutritional facts on myokine production [182].
Figure 3.
Potential factors that influence the myokine production. Not only sports, but also body weight management, stress reduction, nutrition, daily movement, and other lifestyle factors could enhance the myokine production. Vice versa, an unhealthy lifestyle could lead to loss of muscle mass and an impaired myokine production.
7. Conclusions
The difficulty of interpreting the results of these studies is the wide variety of exercise protocols and participants. A “one fits all” recommendation concerning the right training protocol for a certain person, regarding health and training status, for the stimulation of a certain myokine, cannot be given at present. This fact is in turn not only the result of this wide range, but it is also the reason why so many different study and exercise protocols exist. However, we can see a direction, and we now know that resistance training is a crucial part of maintaining health [184,185]. More studies are needed to investigate individual cofactors like nutrition, illness, and lifestyle, to specify individual training recommendations.
We already know a lot about the healthy muscle metabolism [186], but we need to know more about the different types of disruption of muscle metabolism, with the intention of creating a healing individual training protocol for every type of patient.
Acknowledgments
We acknowledge the contributions of Eric Jagar, (USA) in reviewing and editing this manuscript. The present work was performed in fulfillment of the requirements for obtaining the medical doctoral degree.
Author Contributions
Conceptualization, B.E.M.Z., N.B.W., M.L.E., C.S. and O.M.; Imaging, S.H. and B.E.M.Z.; Writing—original draft preparation: B.E.M.Z., N.B.W., M.L.E. and O.M.; Writing—review and editing: B.E.M.Z., N.B.W., M.L.E., L.S., J.R.S., S.H., C.S., L.R. and O.M. Supervision: O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mero A.A., Hulmi J.J., Salmijärvi H., Katajavuori M., Haverinen M., Holviala J., Ridanpää T., Häkkinen K., Kovanen V., Ahtiainen J.P., et al. Resistance Training Induced Increase in Muscle Fiber Size in Young and Older Men. Eur. J. Appl. Physiol. 2013;113:641–650. doi: 10.1007/s00421-012-2466-x. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen B.K., Åkerström T.C.A., Nielsen A.R., Fischer C.P. Role of Myokines in Exercise and Metabolism. J. Appl. Physiol. 2007;103:1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 3.Delezie J., Handschin C. Endocrine Crosstalk between Skeletal Muscle and the Brain. Front. Neurol. 2018;9:698. doi: 10.3389/fneur.2018.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen B.K. Physical Activity and Muscle-Brain Crosstalk. Nat. Rev. Endocrinol. 2019;15:383–392. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen B.K. Anti-Inflammatory Effects of Exercise: Role in Diabetes and Cardiovascular Disease. Eur. J. Clin. Investig. 2017;47:600–611. doi: 10.1111/eci.12781. [DOI] [PubMed] [Google Scholar]
- 6.Karstoft K., Pedersen B.K. Exercise and Type 2 Diabetes: Focus on Metabolism and Inflammation. Immunol. Cell Biol. 2016;94:146–150. doi: 10.1038/icb.2015.101. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen B.K., Febbraio M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 8.Bay M.L., Pedersen B.K. Muscle-Organ Crosstalk: Focus on Immunometabolism. Front. Physiol. 2020;11:567881. doi: 10.3389/fphys.2020.567881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leblanc D.R.B., Rioux B.V., Pelech C., Moffatt T.L., Kimber D.E., Duhamel T.A., Dolinsky V.W., McGavock J.M., Senechal M. Exercise-Induced Irisin Release as a Determinant of the Metabolic Response to Exercise Training in Obese Youth: The Exit Trial. Physiol. Rep. 2017;5:e13539. doi: 10.14814/phy2.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knudsen S.H., Pedersen B.K. Targeting Inflammation Through a Physical Active Lifestyle and Pharmaceuticals for the Treatment of Type 2 Diabetes. Curr. Diabetes Rep. 2015;15:82. doi: 10.1007/s11892-015-0642-1. [DOI] [PubMed] [Google Scholar]
- 11.Severinsen M.C.K., Pedersen B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020;41:594–609. doi: 10.1210/endrev/bnaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenfeld B.J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength Cond. Res. 2010;24:2857–2872. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez A.M., Hoffman J.R., Stout J.R., Fukuda D.H., Willoughby D.S. Intramuscular Anabolic Signaling and Endocrine Response Following Resistance Exercise: Implications for Muscle Hypertrophy. Sports Med. 2016;2016 46:671–685. doi: 10.1007/s40279-015-0450-4. [DOI] [PubMed] [Google Scholar]
- 14.Bamman M.M., Roberts B.M., Adams G.R. Molecular Regulation of Exercise-Induced Muscle Fiber Hypertrophy. Cold Spring Harb. Perspect. Med. 2018;8:a029751. doi: 10.1101/cshperspect.a029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wackerhage H., Ratkevicius A. Signal Transduction Pathways That Regulate Muscle Growth. Essays Biochem. 2008;44:99–108. doi: 10.1042/BSE0440099. [DOI] [PubMed] [Google Scholar]
- 16.Hornberger T.A. Mechanotransduction and the Regulation of MTORC1 Signaling in Skeletal Muscle. Int. J. Biochem. Cell Biol. 2011;43:1267–1276. doi: 10.1016/j.biocel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toigo M. Trainingsrelevante Determinanten Der Molekularen Und Zellul??Ren Skelettmuskeladaptation—Teil 2: Adaptation von Querschnitt Und Fasertypusmodulen. Schweiz. Z. Sportmed. Sporttraumatol. 2006;54:121–132. [Google Scholar]
- 18.Paulsen G., Mikkelsen U.R., Raastad T., Peake J.M. Leucocytes, Cytokines and Satellite Cells: What Role Do They Play in Muscle Damage and Regeneration Following Eccentric Exercise? Exerc. Immunol. Rev. 2012;18:42–97. [PubMed] [Google Scholar]
- 19.Dumont N.A., Wang Y.X., Rudnicki M.A. Intrinsic and Extrinsic Mechanisms Regulating Satellite Cell Function. Development. 2015;142:1572–1581. doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damas F., Libardi C.A., Ugrinowitsch C. The Development of Skeletal Muscle Hypertrophy through Resistance Training: The Role of Muscle Damage and Muscle Protein Synthesis. Eur. J. Appl. Physiol. 2018;118:485–500. doi: 10.1007/s00421-017-3792-9. [DOI] [PubMed] [Google Scholar]
- 21.Toigo M. MuskelRevolution. Springer; Berlin/Heidelberg, Germany: 2019. [Google Scholar]
- 22.Di W., Lv J., Jiang S., Lu C., Yang Z., Ma Z., Hu W., Yang Y., Xu B. PGC-1: The Energetic Regulator in Cardiac Metabolism. Curr. Issues Mol. Biol. 2018;28:29–46. doi: 10.21775/cimb.028.029. [DOI] [PubMed] [Google Scholar]
- 23.Hargreaves M., Spriet L.L. Exercise Metabolism: Fuels for the Fire. Cold Spring Harb. Perspect. Med. 2018;8:a029744. doi: 10.1101/cshperspect.a029744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rigoulet M., Bouchez C.L., Paumard P., Ransac S., Cuvellier S., Duvezin-Caubet S., Mazat J.P., Devin A. Cell Energy Metabolism: An Update. Biochim. Biophys. Acta. Bioenerg. 2020;1861:148276. doi: 10.1016/j.bbabio.2020.148276. [DOI] [PubMed] [Google Scholar]
- 25.Hoppeler H., Baum O., Mueller M., Lurman G. Molekulare Mechanismen Der Anpassungsfähigkeit Der Skelettmuskulatur. Schweiz. Z. Sportmed. Sporttraumatol. 2011;59:6–13. [Google Scholar]
- 26.Kraemer W.J., Deschenes M.R., Fleck S.J. Physiological Adaptations to Resistance Exercise. Implications for Athletic Conditioning. Sports Med. 1988;6:246–256. doi: 10.2165/00007256-198806040-00006. [DOI] [PubMed] [Google Scholar]
- 27.American College of Sports Medicine American College of Sports Medicine Position Stand. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 28.Howe L.P., Read P., Waldron M. Muscle Hypertrophy: A Narrative Review on Training Principles for Increasing Muscle Mass. Strength Cond. J. 2017;39:72–81. doi: 10.1519/SSC.0000000000000330. [DOI] [Google Scholar]
- 29.Fisher J. A Critical Commentary on the Practical Application of Resistance Training Studies. J. Trainol. 2013;2:10–12. doi: 10.17338/trainology.2.2_10. [DOI] [Google Scholar]
- 30.Carpinelli R.N. Critical Commentary on the Stimulus for Muscle Hypertrophy in Experienced Trainees. Med. Sport. Pract. 2020;21:1–37. [Google Scholar]
- 31.Kraemer W.J., Adams K., Cafarelli E., Dudley G.A., Dooly C., Feigenbaum M.S., Fleck S.J., Franklin B., Fry A.C., Hoffman J.R., et al. American College of Sports Medicine Position Stand. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2002;34:364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 32.Anderson T., Kearney J.T. Effects of Three Resistance Training Programs on Muscular Strength And Absolute and Relative Endurance. Res. Q. Exerc. Sport. 1982;53:1–7. doi: 10.1080/02701367.1982.10605218. [DOI] [PubMed] [Google Scholar]
- 33.Campos G., Luecke T., Wendeln H., Toma K., Hagerman F., Murray T., Ragg K., Ratamess N., Kraemer W., Staron R. Muscular Adaptations in Response to Three Different Resistance-Training Regimens: Specificity of Repetition Maximum Training Zones. Eur. J. Appl. Physiol. 2002;88:50–60. doi: 10.1007/s00421-002-0681-6. [DOI] [PubMed] [Google Scholar]
- 34.Kraemer W.J., Patton J.F., Gordon S.E., Harman E.A., Deschenes M.R., Reynolds K., Newton R.U., Triplett N.T., Dziados J.E. Compatibility of High-Intensity Strength and Endurance Training on Hormonal and Skeletal Muscle Adaptations. J. Appl. Physiol. 1995;78:976–989. doi: 10.1152/jappl.1995.78.3.976. [DOI] [PubMed] [Google Scholar]
- 35.Khan S.U., Ghafoor S. Myokines: Discovery Challenges and Therapeutic Impediments. J. Pak. Med. Assoc. 2019;69:1014–1017. [PubMed] [Google Scholar]
- 36.Agarwal M., Singh S., Narayan J., Pandey S., Tiwari S., Sharma P. Cardiovascular Response and Serum Interleukin-6 Level in Concentric vs. Eccentric Exercise. J. Clin. Diagn. Res. 2017;11:CC04–CC08. doi: 10.7860/JCDR/2017/25281.9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eaton M., Granata C., Barry J., Safdar A., Bishop D., Little J.P. Impact of a Single Bout of High-Intensity Interval Exercise and Short-Term Interval Training on Interleukin-6, FNDC5, and METRNL MRNA Expression in Human Skeletal Muscle. J. Sport Health Sci. 2018;7:191–196. doi: 10.1016/j.jshs.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen B.K. The Anti-Inflammatory Effect of Exercise: Its Role in Diabetes and Cardiovascular Disease Control. Essays Biochem. 2006;42:105–117. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen B.K., Febbraio M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 40.Petersen A.M.W., Pedersen B.K. The Role of IL-6 in Mediating the Anti-Inflammatory Effects of Exercise. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2006;57((Suppl. 1)):43–51. [PubMed] [Google Scholar]
- 41.Pedersen B.K., Steensberg A., Fischer C., Keller C., Keller P., Plomgaard P., Febbraio M., Saltin B. Searching for the Exercise Factor: Is IL-6 a Candidate? J. Muscle Res. Cell Motil. 2003;24:113–119. doi: 10.1023/A:1026070911202. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda S.-I., Tamura Y., Kakehi S., Sanada H., Kawamori R., Watada H. Exercise-Induced Increase in IL-6 Level Enhances GLUT4 Expression and Insulin Sensitivity in Mouse Skeletal Muscle. Biochem. Biophys. Res. Commun. 2016;473:947–952. doi: 10.1016/j.bbrc.2016.03.159. [DOI] [PubMed] [Google Scholar]
- 43.Cornish S.M., Chase J.E., Bugera E.M., Giesbrecht G.G. Systemic IL-6 and Myoglobin Response to Three Different Resistance Exercise Intensities in Older Men. J. Aging Phys. Act. 2018;26:451–456. doi: 10.1123/japa.2017-0167. [DOI] [PubMed] [Google Scholar]
- 44.Mendham A.E., Donges C.E., Liberts E.A., Duffield R. Effects of Mode and Intensity on the Acute Exercise-Induced IL-6 and CRP Responses in a Sedentary, Overweight Population. Eur. J. Appl. Physiol. 2011;111:1035–1045. doi: 10.1007/s00421-010-1724-z. [DOI] [PubMed] [Google Scholar]
- 45.Cullen T., Thomas A.W., Webb R., Hughes M.G. The Relationship between Interleukin-6 in Saliva, Venous and Capillary Plasma, at Rest and in Response to Exercise. Cytokine. 2015;71:397–400. doi: 10.1016/j.cyto.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Krause M.d.S., de Bittencourt P.I.H.J. Type 1 Diabetes: Can Exercise Impair the Autoimmune Event? The L-Arginine/Glutamine Coupling Hypothesis. Cell Biochem. Funct. 2008;26:406–433. doi: 10.1002/cbf.1470. [DOI] [PubMed] [Google Scholar]
- 47.Gmiat A., Micielska K., Kozłowska M., Flis D.J., Smaruj M., Kujach S., Jaworska J., Lipińska P., Ziemann E. The Impact of a Single Bout of High Intensity Circuit Training on Myokines’ Concentrations and Cognitive Functions in Women of Different Age. Physiol. Behav. 2017;179:290–297. doi: 10.1016/j.physbeh.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Gil A.M., Elizondo-Montemayor L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients. 2020;12:1899. doi: 10.3390/nu12061899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahmoud N., Mohammadreza H.A., Abdolhosein T.K., Mehdi N., Arent S.M. Serum Myokine Levels after Linear and Flexible Non-Linear Periodized Resistance Training in Overweight Sedentary Women. Eur. J. Sport Sci. 2021:1–11. doi: 10.1080/17461391.2021.1895893. [DOI] [PubMed] [Google Scholar]
- 50.Furuichi Y., Manabe Y., Takagi M., Aoki M., Fujii N.L. Evidence for Acute Contraction-Induced Myokine Secretion by C2C12 Myotubes. PLoS ONE. 2018;13:e0206146. doi: 10.1371/journal.pone.0206146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mageriu V., Manole E., Bastian A.E., Staniceanu F. Role of Myokines in Myositis Pathogenesis and Their Potential to Be New Therapeutic Targets in Idiopathic Inflammatory Myopathies. J. Immunol. Res. 2020;2020:9079083. doi: 10.1155/2020/9079083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weissenbach J., Chernajovsky Y., Zeevi M., Shulman L., Soreq H., Nir U., Wallach D., Perricaudet M., Tiollais P., Revel M. Two Interferon MRNAs in Human Fibroblasts: In Vitro Translation and Escherichia Coli Cloning Studies. Proc. Natl. Acad. Sci. USA. 1980;77:7152–7156. doi: 10.1073/pnas.77.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raschke S., Eckel J. Adipo-Myokines: Two Sides of the Same Coin—Mediators of Inflammation and Mediators of Exercise. Mediat. Inflamm. 2013;2013:320724. doi: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinbacher P., Eckl P. Impact of Oxidative Stress on Exercising Skeletal Muscle. Biomolecules. 2015;5:356–377. doi: 10.3390/biom5020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cornish S.M., Bugera E.M., Duhamel T.A., Peeler J.D., Anderson J.E. A Focused Review of Myokines as a Potential Contributor to Muscle Hypertrophy from Resistance-Based Exercise. Eur. J. Appl. Physiol. 2020;120:941–959. doi: 10.1007/s00421-020-04337-1. [DOI] [PubMed] [Google Scholar]
- 56.Proske U., Morgan D.L. Muscle Damage from Eccentric Exercise: Mechanism, Mechanical Signs, Adaptation and Clinical Applications. J. Physiol. 2001;537:333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao S., Durstine J.L., Koh H.J., Carver W.E., Frizzell N., Carson J.A. Acute Myotube Protein Synthesis Regulation by IL-6-Related Cytokines. Am. J. Physiol. Cell Physiol. 2017;313:C487–C500. doi: 10.1152/ajpcell.00112.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rose-John S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uciechowski P., Dempke W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology. 2020;98:131–137. doi: 10.1159/000505099. [DOI] [PubMed] [Google Scholar]
- 60.McPherron A.C., Lawler A.M., Lee S.J. Regulation of Skeletal Muscle Mass in Mice by a New TGF-Beta Superfamily Member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 61.Iizuka K., Machida T., Hirafuji M. Skeletal Muscle Is an Endocrine Organ. J. Pharmacol. Sci. 2014;125:125–131. doi: 10.1254/jphs.14R02CP. [DOI] [PubMed] [Google Scholar]
- 62.Argilés J.M., Orpí M., Busquets S., López-Soriano F.J. Myostatin: More than Just a Regulator of Muscle Mass. Drug Discov. Today. 2012;17:702–709. doi: 10.1016/j.drudis.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 63.De Caestecker M. The Transforming Growth Factor-β Superfamily of Receptors. Cytokine Growth Factor Rev. 2004;15:1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Braun T., Gautel M. Transcriptional Mechanisms Regulating Skeletal Muscle Differentiation, Growth and Homeostasis. Nat. Rev. Mol. Cell Biol. 2011;12:349–361. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- 65.Allen D.L., Hittel D.S., McPherron A.C. Expression and Function of Myostatin in Obesity, Diabetes, and Exercise Adaptation. Med. Sci. Sports Exerc. 2011;43:1828–1835. doi: 10.1249/MSS.0b013e3182178bb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez-Cadavid N.F., Taylor W.E., Yarasheski K., Sinha-Hikim I., Ma K., Ezzat S., Shen R., Lalani R., Asa S., Mamita M., et al. Organization of the Human Myostatin Gene and Expression in Healthy Men and HIV-Infected Men with Muscle Wasting. Proc. Natl. Acad. Sci. USA. 1998;95:14938–14943. doi: 10.1073/pnas.95.25.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berezin A.E., Berezin A.A., Lichtenauer M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers. 2021;2021:6644631. doi: 10.1155/2021/6644631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hittel D.S., Berggren J.R., Shearer J., Boyle K., Houmard J.A. Increased Secretion and Expression of Myostatin in Skeletal Muscle from Extremely Obese Women. Diabetes. 2009;58:30–38. doi: 10.2337/db08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gilson H., Schakman O., Combaret L., Lause P., Grobet L., Attaix D., Ketelslegers J.M., Thissen J.P. Myostatin Gene Deletion Prevents Glucocorticoid-Induced Muscle Atrophy. Endocrinology. 2007;148:452–460. doi: 10.1210/en.2006-0539. [DOI] [PubMed] [Google Scholar]
- 70.Ma K., Mallidis C., Bhasin S., Mahabadi V., Artaza J., Gonzalez-Cadavid N., Arias J., Salehian B. Glucocorticoid-Induced Skeletal Muscle Atrophy Is Associated with Upregulation of Myostatin Gene Expression. Am. J. Physiol. Endocrinol. Metab. 2003;285:E363–E371. doi: 10.1152/ajpendo.00487.2002. [DOI] [PubMed] [Google Scholar]
- 71.Nielsen T.L., Vissing J., Krag T.O. Antimyostatin Treatment in Health and Disease: The Story of Great Expectations and Limited Success. Cells. 2021;10:533. doi: 10.3390/cells10030533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brandan E., Fuentes M.E., Andrade W. The Proteoglycan Decorin Is Synthesized and Secreted by Differentiated Myotubes. Eur. J. Cell Biol. 1991;55:209–216. [PubMed] [Google Scholar]
- 73.Miura T., Kishioka Y., Wakamatsu J., Hattori A., Hennebry A., Berry C.J., Sharma M., Kambadur R., Nishimura T. Decorin Binds Myostatin and Modulates Its Activity to Muscle Cells. Biochem. Biophys. Res. Commun. 2006;340:675–680. doi: 10.1016/j.bbrc.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 74.Kishioka Y., Thomas M., Wakamatsu J.-I., Hattori A., Sharma M., Kambadur R., Nishimura T. Decorin Enhances the Proliferation and Differentiation of Myogenic Cells through Suppressing Myostatin Activity. J. Cell. Physiol. 2008;215:856–867. doi: 10.1002/jcp.21371. [DOI] [PubMed] [Google Scholar]
- 75.Baghy K., Reszegi A., Tátrai P., Kovalszky I. Decorin in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020;1272:17–38. doi: 10.1007/978-3-030-48457-6_2. [DOI] [PubMed] [Google Scholar]
- 76.Tortoriello D.V., Sidis Y., Holtzman D.A., Holmes W.E., Schneyer A.L. Human Follistatin-Related Protein: A Structural Homologue of Follistatin with Nuclear Localization. Endocrinology. 2001;142:3426–3434. doi: 10.1210/endo.142.8.8319. [DOI] [PubMed] [Google Scholar]
- 77.Hemmati-Brivanlou A., Kelly O.G., Melton D.A. Follistatin, an Antagonist of Activin, Is Expressed in the Spemann Organizer and Displays Direct Neuralizing Activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 78.Hill J.J., Qiu Y., Hewick R.M., Wolfman N.M. Regulation of Myostatin in Vivo by Growth and Differentiation Factor-Associated Serum Protein-1: A Novel Protein with Protease Inhibitor and Follistatin Domains. Mol. Endocrinol. 2003;17:1144–1154. doi: 10.1210/me.2002-0366. [DOI] [PubMed] [Google Scholar]
- 79.Gilson H., Schakman O., Kalista S., Lause P., Tsuchida K., Thissen J.-P. Follistatin Induces Muscle Hypertrophy through Satellite Cell Proliferation and Inhibition of Both Myostatin and Activin. Am. J. Physiol. Endocrinol. Metab. 2009;297:E157–E164. doi: 10.1152/ajpendo.00193.2009. [DOI] [PubMed] [Google Scholar]
- 80.Suryawan A., Frank J.W., Nguyen H.V., Davis T.A. Expression of the TGF-Beta Family of Ligands Is Developmentally Regulated in Skeletal Muscle of Neonatal Rats. Pediatric Res. 2006;59:175–179. doi: 10.1203/01.pdr.0000196718.47935.6e. [DOI] [PubMed] [Google Scholar]
- 81.Zhu J., Li Y., Lu A., Gharaibeh B., Ma J., Kobayashi T., Quintero A.J., Huard J. Follistatin Improves Skeletal Muscle Healing after Injury and Disease through an Interaction with Muscle Regeneration, Angiogenesis, and Fibrosis. Am. J. Pathol. 2011;179:915–930. doi: 10.1016/j.ajpath.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukumoto M., Takeuchi T., Koubayashi E., Harada S., Ota K., Kojima Y., Higuchi K. Induction of Brain-derived Neurotrophic Factor in Enteric Glial Cells Stimulated by Interleukin-1β via a C-Jun N-terminal Kinase Pathway. J. Clin. Biochem. Nutr. 2020;66:103–109. doi: 10.3164/jcbn.19-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barde Y.A., Edgar D., Thoenen H. Purification of a New Neurotrophic Factor from Mammalian Brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camandola S., Mattson M.P. Brain Metabolism in Health, Aging, and Neurodegeneration. EMBO J. 2017;36:1474–1492. doi: 10.15252/embj.201695810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vilar M., Mira H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front. Neurosci. 2016;10:26. doi: 10.3389/fnins.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee J., Duan W., Mattson M.P. Evidence That Brain-Derived Neurotrophic Factor Is Required for Basal Neurogenesis and Mediates, in Part, the Enhancement of Neurogenesis By Dietary Restriction in the Hippocampus of Adult Mice. J. Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 87.Chao M.V. Neurotrophins and Their Receptors: A Convergence Point for Many Signalling Pathways. Nat. Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 88.Diniz B.S., Teixeira A.L. Brain-Derived Neurotrophic Factor and Alzheimer’s Disease: Physiopathology and Beyond. Neuromol. Med. 2011;13:217–222. doi: 10.1007/s12017-011-8154-x. [DOI] [PubMed] [Google Scholar]
- 89.Figueiredo C., Antunes B.M., Giacon T.R., Vanderlei L.C.M., Campos E.Z., Peres F.P., Clark N.W., Panissa V.L.G., de Lira F.S. Influence of Acute and Chronic High-Intensity Intermittent Aerobic plus Strength Exercise on BDNF, Lipid and Autonomic Parameters. J. Sports Sci. Med. 2019;18:359–368. [PMC free article] [PubMed] [Google Scholar]
- 90.McKay B.R., Nederveen J.P., Fortino S.A., Snijders T., Joanisse S., Kumbhare D.A., Parise G. Brain-Derived Neurotrophic Factor Is Associated with Human Muscle Satellite Cell Differentiation in Response to Muscle-Damaging Exercise. Appl. Physiol. Nutr. Metab. 2020;45:581–590. doi: 10.1139/apnm-2019-0501. [DOI] [PubMed] [Google Scholar]
- 91.Krabbe K.S., Nielsen A.R., Krogh-Madsen R., Plomgaard P., Rasmussen P., Erikstrup C., Fischer C.P., Lindegaard B., Petersen A.M.W., Taudorf S., et al. Brain-Derived Neurotrophic Factor (BDNF) and Type 2 Diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 92.Jamshed H., Beyl R.A., Manna D.L.D., Yang E.S., Ravussin E., Peterson C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients. 2019;11:1234. doi: 10.3390/nu11061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu B., Goulding E.H., Zang K., Cepoi D., Cone R.D., Jones K.R., Tecott L.H., Reichardt L.F. Brain-Derived Neurotrophic Factor Regulates Energy Balance Downstream of Melanocortin-4 Receptor. Nat. Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mousavi K., Parry D.J., Jasmin B.J. BDNF Rescues Myosin Heavy Chain IIB Muscle Fibers after Neonatal Nerve Injury. Am. J. Physiol. Cell Physiol. 2004;287:C22–C29. doi: 10.1152/ajpcell.00583.2003. [DOI] [PubMed] [Google Scholar]
- 95.Omura T., Sano M., Omura K., Hasegawa T., Doi M., Sawada T., Nagano A. Different Expressions of BDNF, NT3, and NT4 in Muscle and Nerve after Various Types of Peripheral Nerve Injuries. J. Peripher. Nerv. Syst. JPNS. 2005;10:293–300. doi: 10.1111/j.1085-9489.2005.10307.x. [DOI] [PubMed] [Google Scholar]
- 96.Clow C., Jasmin B.J. Brain-Derived Neurotrophic Factor Regulates Satellite Cell Differentiation and Skeltal Muscle Regeneration. Mol. Biol. Cell. 2010;21:2182–2190. doi: 10.1091/mbc.e10-02-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu T., Chang Y., Gao X.L., Li H., Zhao P. Dynamic Expression and the Role of BDNF in Exercise-Induced Skeletal Muscle Regeneration. Int. J. Sports Med. 2017;38:959–966. doi: 10.1055/s-0043-118343. [DOI] [PubMed] [Google Scholar]
- 98.Matthews V.B., Aström M.-B., Chan M.H.S., Bruce C.R., Krabbe K.S., Prelovsek O., Akerström T., Yfanti C., Broholm C., Mortensen O.H., et al. Brain-Derived Neurotrophic Factor Is Produced by Skeletal Muscle Cells in Response to Contraction and Enhances Fat Oxidation via Activation of AMP-Activated Protein Kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 99.Yamanaka M., Tsuchida A., Nakagawa T., Nonomura T., Ono-Kishino M., Sugaru E., Noguchi H., Taiji M. Brain-Derived Neurotrophic Factor Enhances Glucose Utilization in Peripheral Tissues of Diabetic Mice. Diabetes Obes. Metab. 2007;9:59–64. doi: 10.1111/j.1463-1326.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- 100.Yarrow J.F., White L.J., McCoy S.C., Borst S.E. Training Augments Resistance Exercise Induced Elevation of Circulating Brain Derived Neurotrophic Factor (BDNF) Neurosci. Lett. 2010;479:161–165. doi: 10.1016/j.neulet.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 101.Church D.D., Hoffman J.R., Mangine G.T., Jajtner A.R., Townsend J.R., Beyer K.S., Wang R., la Monica M.B., Fukuda D.H., Stout J.R. Comparison of High-Intensity vs. High-Volume Resistance Training on the BDNF Response to Exercise. J. Appl. Physiol. 2016;121:123–128. doi: 10.1152/japplphysiol.00233.2016. [DOI] [PubMed] [Google Scholar]
- 102.Palasz E., Wysocka A., Gasiorowska A., Chalimoniuk M., Niewiadomski W., Niewiadomska G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020;21:1170. doi: 10.3390/ijms21031170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Linker R.A., Lee D.-H., Demir S., Wiese S., Kruse N., Siglienti I., Gerhardt E., Neumann H., Sendtner M., Lühder F., et al. Functional Role of Brain-Derived Neurotrophic Factor in Neuroprotective Autoimmunity: Therapeutic Implications in a Model of Multiple Sclerosis. Brain. 2010;133:2248–2263. doi: 10.1093/brain/awq179. [DOI] [PubMed] [Google Scholar]
- 104.Whone A., Luz M., Boca M., Woolley M., Mooney L., Dharia S., Broadfoot J., Cronin D., Schroers C., Barua N.U., et al. Randomized Trial of Intermittent Intraputamenal Glial Cell Line-Derived Neurotrophic Factor in Parkinson’s Disease. Brain. 2019;142:512–525. doi: 10.1093/brain/awz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Denyer R., Douglas M.R. Gene Therapy for Parkinson’s Disease. Parkinson’s Dis. 2012;2012:757305. doi: 10.1155/2012/757305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Géral C., Angelova A., Lesieur S. From Molecular to Nanotechnology Strategies for Delivery of Neurotrophins: Emphasis on Brain-Derived Neurotrophic Factor (BDNF) Pharmaceutics. 2013;5:127–167. doi: 10.3390/pharmaceutics5010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boström P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C., Rasbach K.A., Boström E.A., Choi J.H., Long J.Z., et al. A PGC1α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Polyzos S.A., Anastasilakis A.D., Efstathiadou Z.A., Makras P., Perakakis N., Kountouras J., Mantzoros C.S. Irisin in Metabolic Diseases. Endocrine. 2018;59:260–274. doi: 10.1007/s12020-017-1476-1. [DOI] [PubMed] [Google Scholar]
- 109.Perakakis N., Triantafyllou G.A., Fernández-Real J.M., Huh J.Y., Park K.H., Seufert J., Mantzoros C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017;13:324–337. doi: 10.1038/nrendo.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Albrecht E., Norheim F., Thiede B., Holen T., Ohashi T., Schering L., Lee S., Brenmoehl J., Thomas S., Drevon C.A., et al. Irisin—A Myth Rather than an Exercise-Inducible Myokine. Sci. Rep. 2015;5:8889. doi: 10.1038/srep08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jedrychowski M.P., Wrann C.D., Paulo J.A., Gerber K.K., Szpyt J., Robinson M.M., Nair K.S., Gygi S.P., Spiegelman B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015;22:734–740. doi: 10.1016/j.cmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hecksteden A., Wegmann M., Steffen A., Kraushaar J., Morsch A., Ruppenthal S., Kaestner L., Meyer T. Irisin and Exercise Training in Humans—Results from a Randomized Controlled Training Trial. BMC Med. 2013;11:235. doi: 10.1186/1741-7015-11-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fox J., Rioux B.V., Goulet E.D.B., Johanssen N.M., Swift D.L., Bouchard D.R., Loewen H., Sénéchal M. Effect of an Acute Exercise Bout on Immediate Post-Exercise Irisin Concentration in Adults: A Meta-Analysis. Scand. J. Med. Sci. Sports. 2018;28:16–28. doi: 10.1111/sms.12904. [DOI] [PubMed] [Google Scholar]
- 114.Qiu S., Cai X., Sun Z., Schumann U., Zügel M., Steinacker J.M. Chronic Exercise Training and Circulating Irisin in Adults: A Meta-Analysis. Sports Med. 2015;45:1577–1588. doi: 10.1007/s40279-014-0293-4. [DOI] [PubMed] [Google Scholar]
- 115.Colaianni G., Cinti S., Colucci S., Grano M. Irisin and Musculoskeletal Health. Ann. N. Y. Acad. Sci. 2017;1402:5–9. doi: 10.1111/nyas.13345. [DOI] [PubMed] [Google Scholar]
- 116.Tsuchiya Y., Ando D., Takamatsu K., Goto K. Resistance Exercise Induces a Greater Irisin Response than Endurance Exercise. Metabolism. 2015;64:1042–1050. doi: 10.1016/j.metabol.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 117.Huh J.Y., Siopi A., Mougios V., Park K.H., Mantzoros C.S. Irisin in Response to Exercise in Humans with and without Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2015;100:E453–E457. doi: 10.1210/jc.2014-2416. [DOI] [PubMed] [Google Scholar]
- 118.Mahgoub M.O., D’Souza C., al Darmaki R.S.M.H., Baniyas M.M.Y.H., Adeghate E. An Update on the Role of Irisin in the Regulation of Endocrine and Metabolic Functions. Peptides. 2018;104:15–23. doi: 10.1016/j.peptides.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 119.Wang H., Zhao Y.T., Zhang S., Dubielecka P.M., Du J., Yano N., Chin Y.E., Zhuang S., Qin G., Zhao T.C. Irisin Plays a Pivotal Role to Protect the Heart against Ischemia and Reperfusion Injury. J. Cell. Physiol. 2017;232:3775–3785. doi: 10.1002/jcp.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Y., Song H., Zhang Y., Wu F., Mu Q., Jiang M., Wang F., Zhang W., Li L., Shao L., et al. Irisin Inhibits Atherosclerosis by Promoting Endothelial Proliferation Through MicroRNA126-5p. J. Am. Heart Assoc. 2016;5:e004031. doi: 10.1161/JAHA.116.004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim H., Wrann C.D., Jedrychowski M., Vidoni S., Kitase Y., Nagano K., Zhou C., Chou J., Parkman V.-J.A., Novick S.J., et al. Irisin Mediates Effects on Bone and Fat via AV Integrin Receptors. Cell. 2018;175:1756–1768.e17. doi: 10.1016/j.cell.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Briganti S.I., Gaspa G., Tabacco G., Naciu A.M., Cesareo R., Manfrini S., Palermo A. Irisin as a Regulator of Bone and Glucose Metabolism. Minerva Endocrinol. 2018;43:489–500. doi: 10.23736/S0391-1977.17.02779-1. [DOI] [PubMed] [Google Scholar]
- 123.Kim O., Song J. The Role of Irisin in Alzheimer’s Disease. J. Clin. Med. 2018;7:407. doi: 10.3390/jcm7110407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maalouf G.E., el Khoury D. Exercise-Induced Irisin, the Fat Browning Myokine, as a Potential Anticancer Agent. J. Obes. 2019;2019:6561726. doi: 10.1155/2019/6561726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gannon N.P., Vaughan R.A., Garcia-Smith R., Bisoffi M., Trujillo K.A. Effects of the Exercise-Inducible Myokine Irisin on Malignant and Non-Malignant Breast Epithelial Cell Behavior in Vitro. Int. J. Cancer. 2015;136:E197–E202. doi: 10.1002/ijc.29142. [DOI] [PubMed] [Google Scholar]
- 126.Provatopoulou X., Georgiou G.P., Kalogera E., Kalles V., Matiatou M.A., Papapanagiotou I., Sagkriotis A., Zografos G.C., Gounaris A. Serum Irisin Levels Are Lower in Patients with Breast Cancer: Association with Disease Diagnosis and Tumor Characteristics. BMC Cancer. 2015;15:898. doi: 10.1186/s12885-015-1898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shao L., Li H., Chen J., Song H., Zhang Y., Wu F., Wang W., Zhang W., Wang F., Li H., et al. Irisin Suppresses the Migration, Proliferation, and Invasion of Lung Cancer Cells via Inhibition of Epithelial-to-Mesenchymal Transition. Biochem. Biophys. Res. Commun. 2017;485:598–605. doi: 10.1016/j.bbrc.2016.12.084. [DOI] [PubMed] [Google Scholar]
- 128.Kong G., Jiang Y., Sun X., Cao Z., Zhang G., Zhao Z., Zhao Y., Yu Q., Cheng G. Irisin Reverses the IL-6 Induced Epithelial-Mesenchymal Transition in Osteosarcoma Cell Migration and Invasion through the STAT3/Snail Signaling Pathway. Oncol. Rep. 2017;38:2647–2656. doi: 10.3892/or.2017.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu H., Liu M., Zhang N., Pan H., Lin G., Li N., Wang L., Yang H., Yan K., Gong F. Serum and Adipose Tissue MRNA Levels of ATF3 and FNDC5/Irisin in Colorectal Cancer Patients With or Without Obesity. Front. Physiol. 2018;9:1125. doi: 10.3389/fphys.2018.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu J., Song N., Huang Y., Chen Y. Irisin Inhibits Pancreatic Cancer Cell Growth via the AMPK-MTOR Pathway. Sci. Rep. 2018;8:15247. doi: 10.1038/s41598-018-33229-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gaggini M., Cabiati M., del Turco S., Navarra T., de Simone P., Filipponi F., Del Ry S., Gastaldelli A., Basta G. Increased FNDC5/Irisin Expression in Human Hepatocellular Carcinoma. Peptides. 2017;88:62–66. doi: 10.1016/j.peptides.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 132.Shi G., Tang N., Qiu J., Zhang D., Huang F., Cheng Y., Ding K., Li W., Zhang P., Tan X. Irisin Stimulates Cell Proliferation and Invasion by Targeting the PI3K/AKT Pathway in Human Hepatocellular Carcinoma. Biochem. Biophys. Res. Commun. 2017;493:585–591. doi: 10.1016/j.bbrc.2017.08.148. [DOI] [PubMed] [Google Scholar]
- 133.Li D.-J., Huang F., Lu W.-J., Jiang G.-J., Deng Y.-P., Shen F.-M. Metformin Promotes Irisin Release from Murine Skeletal Muscle Independently of AMP-Activated Protein Kinase Activation. Acta Physiol. 2015;213:711–721. doi: 10.1111/apha.12421. [DOI] [PubMed] [Google Scholar]
- 134.Haemmerle G., Moustafa T., Woelkart G., Büttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P.C., Zierler K., et al. ATGL-Mediated Fat Catabolism Regulates Cardiac Mitochondrial Function via PPAR-α and PGC-1. Nat. Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dillon L.M., Rebelo A.P., Moraes C.T. The Role of PGC-1 Coactivators in Aging Skeletal Muscle and Heart. IUBMB Life. 2012;64:231–241. doi: 10.1002/iub.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 137.Shen L., Wang H., Ye P. Effects of pioglitazone on myocardial peroxisome proliferator-activated receptor gamma co-activator lα expression in rats with myocardial ischemia/reperfusion injury. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:197–200. [PubMed] [Google Scholar]
- 138.Sun C.-K., Chang L.-T., Sheu J.-J., Wang C.-Y., Youssef A.A., Wu C.-J., Chua S., Yip H.-K. Losartan Preserves Integrity of Cardiac Gap Junctions and PGC-1 Alpha Gene Expression and Prevents Cellular Apoptosis in Remote Area of Left Ventricular Myocardium Following Acute Myocardial Infarction. Int. Heart J. 2007;48:533–546. doi: 10.1536/ihj.48.533. [DOI] [PubMed] [Google Scholar]
- 139.Martin A.R., Chung S., Koehler K. Is Exercise a Match for Cold Exposure? Common Molecular Framework for Adipose Tissue Browning. Int. J. Sports Med. 2020;41:427–442. doi: 10.1055/a-1100-7118. [DOI] [PubMed] [Google Scholar]
- 140.Rao R.R., Long J.Z., White J.P., Svensson K.J., Lou J., Lokurkar I., Jedrychowski M.P., Ruas J.L., Wrann C.D., Lo J.C., et al. Meteorin-like Is a Hormone That Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ruas J.L., White J.P., Rao R.R., Kleiner S., Brannan K.T., Harrison B.C., Greene N.P., Wu J., Estall J.L., Irving B.A., et al. A PGC-1α Isoform Induced by Resistance Training Regulates Skeletal Muscle Hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miao Z.-W., Hu W.-J., Li Z.-Y., Miao C.-Y. Involvement of the Secreted Protein Metrnl in Human Diseases. Acta Pharmacol. Sin. 2020;41:1525–1530. doi: 10.1038/s41401-020-00529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ushach I., Burkhardt A.M., Martinez C., Hevezi P.A., Gerber P.A., Buhren B.A., Schrumpf H., Valle-Rios R., Vazquez M.I., Homey B., et al. METEORIN-LIKE Is a Cytokine Associated with Barrier Tissues and Alternatively Activated Macrophages. Clin. Immunol. 2015;156:119–127. doi: 10.1016/j.clim.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ushach I., Arrevillaga-Boni G., Heller G.N., Pone E., Hernandez-Ruiz M., Catalan-Dibene J., Hevezi P., Zlotnik A. Meteorin-like/Meteorin-β Is a Novel Immunoregulatory Cytokine Associated with Inflammation. J. Immunol. 2018;201:3669–3676. doi: 10.4049/jimmunol.1800435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Buford T.W., Cooke M.B., Willoughby D.S. Resistance Exercise-Induced Changes of Inflammatory Gene Expression within Human Skeletal Muscle. Eur. J. Appl. Physiol. 2009;107:463–471. doi: 10.1007/s00421-009-1145-z. [DOI] [PubMed] [Google Scholar]
- 146.Phillips M.D., Mitchell J.B., Currie-Elolf L.M., Yellott R.C., Hubing K.A. Influence of Commonly Employed Resistance Exercise Protocols on Circulating IL-6 and Indices of Insulin Sensitivity. J. Strength Cond. Res. 2010;24:1091–1101. doi: 10.1519/JSC.0b013e3181cc2212. [DOI] [PubMed] [Google Scholar]
- 147.Della Gatta P.A., Garnham A.P., Peake J.M., Cameron-Smith D. Effect of Exercise Training on Skeletal Muscle Cytokine Expression in the Elderly. Brain Behav. Immun. 2014;39:80–86. doi: 10.1016/j.bbi.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 148.Quiles J.M., Klemp A., Dolan C., Maharaj A., Huang C.-J., Khamoui A.V., Trexler E.T., Whitehurst M., Zourdos M.C. Impact of Resistance Training Program Configuration on the Circulating Brain-Derived Neurotrophic Factor Response. Appl. Physiol. Nutr. Metab. 2020;45:667–674. doi: 10.1139/apnm-2019-0419. [DOI] [PubMed] [Google Scholar]
- 149.Tomeleri C.M., Ribeiro A.S., Souza M.F., Schiavoni D., Schoenfeld B.J., Venturini D., Barbosa D.S., Landucci K., Sardinha L.B., Cyrino E.S. Resistance Training Improves Inflammatory Level, Lipid and Glycemic Profiles in Obese Older Women: A Randomized Controlled Trial. Exp. Gerontol. 2016;84:80–87. doi: 10.1016/j.exger.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 150.Kazemi F. The Correlation of Resistance Exercise-Induced Myostatin with Insulin Resistance and Plasma Cytokines in Healthy Young Men. J. Endocrinol. Investig. 2016;39:383–388. doi: 10.1007/s40618-015-0373-9. [DOI] [PubMed] [Google Scholar]
- 151.Raue U., Slivka D., Jemiolo B., Hollon C., Trappe S. Myogenic Gene Expression at Rest and after a Bout of Resistance Exercise in Young (18–30 Yr) and Old (80–89 Yr) Women. J. Appl. Physiol. 2006;101:53–59. doi: 10.1152/japplphysiol.01616.2005. [DOI] [PubMed] [Google Scholar]
- 152.Bugera E.M., Duhamel T.A., Peeler J.D., Cornish S.M. The Systemic Myokine Response of Decorin, Interleukin-6 (IL-6) and Interleukin-15 (IL-15) to an Acute Bout of Blood Flow Restricted Exercise. Eur. J. Appl. Physiol. 2018;118:2679–2686. doi: 10.1007/s00421-018-3995-8. [DOI] [PubMed] [Google Scholar]
- 153.Kanzleiter T., Rath M., Görgens S.W., Jensen J., Tangen D.S., Kolnes A.J., Kolnes K.J., Lee S., Eckel J., Schürmann A., et al. The Myokine Decorin Is Regulated by Contraction and Involved in Muscle Hypertrophy. Biochem. Biophys. Res. Commun. 2014;450:1089–1094. doi: 10.1016/j.bbrc.2014.06.123. [DOI] [PubMed] [Google Scholar]
- 154.Bagheri R., Rashidlamir A., Motevalli M.S., Elliott B.T., Mehrabani J., Wong A. Effects of Upper-Body, Lower-Body, or Combined Resistance Training on the Ratio of Follistatin and Myostatin in Middle-Aged Men. Eur. J. Appl. Physiol. 2019;119:1921–1931. doi: 10.1007/s00421-019-04180-z. [DOI] [PubMed] [Google Scholar]
- 155.Negaresh R., Ranjbar R., Habibi A., Mokhtarzade M., Fokin A., Gharibvand M.M. The Effect of Resistance Training on Quadriceps Muscle Volume and Some Growth Factors in Elderly and Young Men. Adv. Gerontol. Uspekhi Gerontol. 2017;30:880–887. [PubMed] [Google Scholar]
- 156.Jang K.S., Kang S., Woo S.H., Bae J.Y., Shin K.O. Effects of Combined Open Kinetic Chain and Closed Kinetic Chain Training Using Pulley Exercise Machines on Muscle Strength and Angiogenesis Factors. J. Phys. Ther. Sci. 2016;28:960–966. doi: 10.1589/jpts.28.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hofmann M., Schober-Halper B., Oesen S., Franzke B., Tschan H., Bachl N., Strasser E.M., Quittan M., Wagner K.H., Wessner B. Effects of Elastic Band Resistance Training and Nutritional Supplementation on Muscle Quality and Circulating Muscle Growth and Degradation Factors of Institutionalized Elderly Women: The Vienna Active Ageing Study (VAAS) Eur. J. Appl. Physiol. 2016;116:885–897. doi: 10.1007/s00421-016-3344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ellefsen S., Vikmoen O., Slettaløkken G., Whist J.E., Nygaard H., Hollan I., Rauk I., Vegge G., Strand T.A., Raastad T., et al. Irisin and FNDC5: Effects of 12-Week Strength Training, and Relations to Muscle Phenotype and Body Mass Composition in Untrained Women. Eur. J. Appl. Physiol. 2014;114:1875–1888. doi: 10.1007/s00421-014-2922-x. [DOI] [PubMed] [Google Scholar]
- 159.Kim H.J., Lee H.J., So B., Son J.S., Yoon D., Song W. Effect of Aerobic Training and Resistance Training on Circulating Irisin Level and Their Association with Change of Body Composition in Overweight/Obese Adults: A Pilot Study. Physiol. Res. 2016;65:271–279. doi: 10.33549/physiolres.932997. [DOI] [PubMed] [Google Scholar]
- 160.Norheim F., Langleite T.M., Hjorth M., Holen T., Kielland A., Stadheim H.K., Gulseth H.L., Birkeland K.I., Jensen J., Drevon C.A. The Effects of Acute and Chronic Exercise on PGC-1α, Irisin and Browning of Subcutaneous Adipose Tissue in Humans. FEBS J. 2014;281:739–749. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 161.Nygaard H., Slettaløkken G., Vegge G., Hollan I., Whist J.E., Strand T., Rønnestad B.R., Ellefsen S. Irisin in Blood Increases Transiently after Single Sessions of Intense Endurance Exercise and Heavy Strength Training. PLoS ONE. 2015;10:e0121367. doi: 10.1371/journal.pone.0121367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pekkala S., Wiklund P.K., Hulmi J.J., Ahtiainen J.P., Horttanainen M., Pöllänen E., Mäkelä K.A., Kainulainen H., Häkkinen K., Nyman K., et al. Are Skeletal Muscle FNDC5 Gene Expression and Irisin Release Regulated by Exercise and Related to Health? J. Physiol. 2013;591:5393–5400. doi: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tibana R.A., Nascimento D.d.C., de Souza N.M.F., de Souza V.C., Neto I.V.d.S., Voltarelli F.A., Pereira G.B., Navalta J.W., Prestes J. Irisin Levels Are Not Associated to Resistance Training-Induced Alterations in Body Mass Composition in Older Untrained Women with and without Obesity. J. Nutr. Health Aging. 2017;21:241–246. doi: 10.1007/s12603-016-0748-4. [DOI] [PubMed] [Google Scholar]
- 164.Zhao J., Su Z., Qu C., Dong Y. Effects of 12 Weeks Resistance Training on Serum Irisin in Older Male Adults. Front. Physiol. 2017;8:171. doi: 10.3389/fphys.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.So B., Kim H.-J., Kim J., Song W. Exercise-Induced Myokines in Health and Metabolic Diseases. Integr. Med. Res. 2014;3:172–179. doi: 10.1016/j.imr.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Domínguez-Sanchéz M.A., Bustos-Cruz R.H., Velasco-Orjuela G.P., Quintero A.P., Tordecilla-Sanders A., Correa-Bautista J.E., Triana-Reina H.R., García-Hermoso A., González-Ruíz K., Peña-Guzmán C.A., et al. Acute Effects of High Intensity, Resistance, or Combined Protocol on the Increase of Level of Neurotrophic Factors in Physically Inactive Overweight Adults: The BrainFit Study. Front. Physiol. 2018;9:741. doi: 10.3389/fphys.2018.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Forti L.N., van Roie E., Njemini R., Coudyzer W., Beyer I., Delecluse C., Bautmans I. Dose-and Gender-Specific Effects of Resistance Training on Circulating Levels of Brain Derived Neurotrophic Factor (BDNF) in Community-Dwelling Older Adults. Exp. Gerontol. 2015;70:144–149. doi: 10.1016/j.exger.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 168.Lodo L., Moreira A., Bacurau R.F.P., Capitani C.D., Barbosa W.P., Massa M., Schoenfeld B.J., Aoki M.S. Resistance Exercise Intensity Does Not Influence Neurotrophic Factors Response in Equated Volume Schemes. J. Hum. Kinet. 2020;74:227–236. doi: 10.2478/hukin-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jørgensen M.L.K., Kjølhede T., Dalgas U., Hvid L.G. Plasma Brain-Derived Neurotrophic Factor (BDNF) and Sphingosine-1-Phosphat (S1P) Are NOT the Main Mediators of Neuroprotection Induced by Resistance Training in Persons with Multiple Sclerosis—A Randomized Controlled Trial. Mult. Scler. Relat. Disord. 2019;31:106–111. doi: 10.1016/j.msard.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 170.Marston K.J., Newton M.J., Brown B.M., Rainey-Smith S.R., Bird S., Martins R.N., Peiffer J.J. Intense Resistance Exercise Increases Peripheral Brain-Derived Neurotrophic Factor. J. Sci. Med. Sport. 2017;20:899–903. doi: 10.1016/j.jsams.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 171.Marston K.J., Brown B.M., Rainey-Smith S.R., Bird S., Wijaya L., Teo S.Y.M., Laws S.M., Martins R.N., Peiffer J.J. Twelve Weeks of Resistance Training Does Not Influence Peripheral Levels of Neurotrophic Growth Factors or Homocysteine in Healthy Adults: A Randomized-Controlled Trial. Eur. J. Appl. Physiol. 2019;119:2167–2176. doi: 10.1007/s00421-019-04202-w. [DOI] [PubMed] [Google Scholar]
- 172.Roh H., Cho S., So W. A Cross-Sectional Study Evaluating the Effects of Resistance Exercise on Inflammation and Neurotrophic Factors in Elderly Women with Obesity. J. Clin. Med. 2020;9:842. doi: 10.3390/jcm9030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Urzi F., Marusic U., Ličen S., Buzan E. Effects of Elastic Resistance Training on Functional Performance and Myokines in Older Women—A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2019;20:830–834.e2. doi: 10.1016/j.jamda.2019.01.151. [DOI] [PubMed] [Google Scholar]
- 174.Vega S.R., Knicker A., Hollmann W., Bloch W., Strüder H.K. Effect of Resistance Exercise on Serum Levels of Growth Factors in Humans. Horm. Metab. Res. Horm.—Stoffwechs. Horm. Metab. 2010;42:982–986. doi: 10.1055/s-0030-1267950. [DOI] [PubMed] [Google Scholar]
- 175.Walsh J.J., D’Angiulli A., Cameron J.D., Sigal R.J., Kenny G.P., Holcik M., Doucette S., Alberga A.S., Prud’homme D., Hadjiyannakis S., et al. Changes in the Brain-Derived Neurotrophic Factor Are Associated with Improvements in Diabetes Risk Factors after Exercise Training in Adolescents with Obesity: The Hearty Randomized Controlled Trial. Neural Plast. 2018;2018:7169583. doi: 10.1155/2018/7169583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goldfield G.S., Kenny G.P., Prud’homme D., Holcik M., Alberga A.S., Fahnestock M., Cameron J.D., Doucette S., Hadjiyannakis S., Tulloch H., et al. Effects of Aerobic Training, Resistance Training, or Both on Brain-Derived Neurotrophic Factor in Adolescents with Obesity: The Hearty Randomized Controlled Trial. Physiol. Behav. 2018;191:138–145. doi: 10.1016/j.physbeh.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 177.Alberga A.S., Goldfield G.S., Kenny G.P., Hadjiyannakis S., Phillips P., Prud’homme D., Tulloch H., Gougeon R., Wells G.A., Sigal R.J. Healthy Eating, Aerobic and Resistance Training in Youth (HEARTY): Study Rationale, Design and Methods. Contemp. Clin. Trials. 2012;33:839–847. doi: 10.1016/j.cct.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 178.Alberga A.S., Prud’homme D., Kenny G.P., Goldfield G.S., Hadjiyannakis S., Gougeon R., Phillips P., Malcolm J., Wells G., Doucette S., et al. Effects of Aerobic and Resistance Training on Abdominal Fat, Apolipoproteins and High-Sensitivity C-Reactive Protein in Adolescents with Obesity: The HEARTY Randomized Clinical Trial. Int. J. Obes. 2015;39:1494–1500. doi: 10.1038/ijo.2015.133. [DOI] [PubMed] [Google Scholar]
- 179.Walsh J.J., Scribbans T.D., Bentley R.F., Kellawan J.M., Gurd B., Tschakovsky M.E. Neurotrophic Growth Factor Responses to Lower Body Resistance Training in Older Adults. Appl. Physiol. Nutr. Metab. 2016;41:315–323. doi: 10.1139/apnm-2015-0410. [DOI] [PubMed] [Google Scholar]
- 180.Wens I., Keytsman C., Deckx N., Cools N., Dalgas U., Eijnde B.O. Brain Derived Neurotrophic Factor in Multiple Sclerosis: Effect of 24 Weeks Endurance and Resistance Training. Eur. J. Neurol. 2016;23:1028–1035. doi: 10.1111/ene.12976. [DOI] [PubMed] [Google Scholar]
- 181.Thompson W.R., Sallis R., Joy E., Jaworski C.A., Stuhr R.M., Trilk J.L. Exercise Is Medicine. Am. J. Lifestyle Med. 2020;14:511–523. doi: 10.1177/1559827620912192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Senesi P., Luzi L., Terruzzi I. Adipokines, Myokines, and Cardiokines: The Role of Nutritional Interventions. Int. J. Mol. Sci. 2020;21:8372. doi: 10.3390/ijms21218372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Duan Y., Li F., Li Y., Tang Y., Kong X., Feng Z., Anthony T.G., Watford M., Hou Y., Wu G., et al. The Role of Leucine and Its Metabolites in Protein and Energy Metabolism. Amino Acids. 2016;48:41–51. doi: 10.1007/s00726-015-2067-1. [DOI] [PubMed] [Google Scholar]
- 184.Nieman D.C., Lila M.A., Gillitt N.D. Immunometabolism: A Multi-Omics Approach to Interpreting the Influence of Exercise and Diet on the Immune System. Annu. Rev. Food Sci. Technol. 2019;10:341–363. doi: 10.1146/annurev-food-032818-121316. [DOI] [PubMed] [Google Scholar]
- 185.Nieman D.C., Pence B.D. Exercise Immunology: Future Directions. J. Sport Health Sci. 2020;9:432–445. doi: 10.1016/j.jshs.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Spiering B.A., Kraemer W.J., Anderson J.M., Armstrong L.E., Nindl B.C., Volek J.S., Maresh C.M. Resistance Exercise Biology: Manipulation of Resistance Exercise Programme Variables Determines the Responses of Cellular and Molecular Signalling Pathways. Sports Med. 2008;38:527–540. doi: 10.2165/00007256-200838070-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.