Abstract
Hepatitis is defined as inflammation of the liver; it can be acute or chronic. In chronic cases, the prolonged inflammation gradually damages the liver, resulting in liver fibrosis, cirrhosis, and sometimes liver failure or cancer. Hepatitis is often caused by viral infections. The most common causes of viral hepatitis are the five hepatitis viruses—hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus (HEV). While HAV and HEV rarely (or do not) cause chronic hepatitis, a considerable proportion of acute hepatitis cases caused by HBV (sometimes co-infected with HDV) and HCV infections become chronic. Thus, many medical researchers have focused on the treatment of HBV and HCV. It has been documented that host lipid metabolism, particularly cholesterol metabolism, is required for the hepatitis viral infection and life cycle. Thus, manipulating host cholesterol metabolism-related genes and proteins is a strategy used in fighting the viral infections. Efforts have been made to evaluate the efficacy of cholesterol-lowering drugs, particularly 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, in the treatment of hepatitis viral infections; promising results have been obtained. This review provides information on the relationships between hepatitis viruses and host cholesterol metabolism/homeostasis, as well as the discovery/development of cholesterol-lowering natural phytochemicals that could potentially be applied in the treatment of viral hepatitis.
Keywords: hepatitis virus, cholesterol metabolism, HMG-CoA reductase, phytochemicals
1. Introduction
Viral hepatitis is an inflammatory condition of the liver, caused by a viral infection. Although other viruses can sometimes cause liver inflammation, viral hepatitis is commonly caused by infections of hepatitis viruses. Five hepatitis viruses from distinct genetic backgrounds have been identified. Among these five, hepatitis A virus (HAV), a Picornaviridae virus; and hepatitis E virus (HEV), a member of Hepeviridae, can be transmitted via the fecal–oral route; these viruses are endemic in developing (low-income) countries [1]. They normally cause self-limiting hepatitis, and do not lead to fulminant liver failure. Chronic infections of these viruses only occur in rare cases in immune-compromised patients. On the other hand, the hepatitis B virus (HBV), a double-stranded DNA virus belonging to Hepadnaviridae; and the hepatitis C virus (HCV), a positive-sense single-stranded RNA virus belonging to Flaviviridae, cause acute and chronic hepatitis. Chronic viral hepatitis progressively results in liver fibrosis, cirrhosis, and, in many cases, hepatocellular carcinoma (HCC) [2]. The hepatitis D virus (hepatitis delta virus, HDV) is a negative-sense single-stranded RNA virus belonging to the genus Deltavirus. It is a satellite virus that has no independent life cycle and can only propagate depending on the function of HBV for its replication and expression. This means that HDV can only replicate in the host with a persistent HBV infection. The co-infection of HBV and HDV causes the most severe form of viral hepatitis [3]. Lipid metabolism in cells is highly related to inflammation and the development of cancers; host lipid metabolism has been linked to viral infection, replication, and assembly [4,5]. For hepatitis viruses that normally cause acute hepatitis only, altered lipid profiles have been observed in children with acute hepatitis induced by HAV infections [6]; decreased cholesterol levels and serum lipid concentrations were measured in HEV-infected cells, and in patients, respectively [7]. For viruses that cause chronic hepatitis, research shows that host lipid metabolism reprogramming is associated with HBV and HCV infections in the progression to severe liver disease [8,9]. Lipid droplets (LDs, also referred to as lipid bodies) are organelles that play important roles in lipid metabolism, energy homeostasis, and intracellular transport. They also have multiple roles in infections and inflammation. The LDs involve the storage of lipids and cholesterol in cells, and are associated with a variety of viruses, including Flaviviridae members dengue virus [10], rotavirus [11,12], reovirus [13], and Zika virus [4] for viral particle formations. It has been indicated that the replication and inflammatory mediator productions of other viruses, such as SARS-CoV-2, are fueled by LDs [14]. The interactions between HCV proteins and host cellular LDs have been determined as necessary for infectious particle productions [15,16,17] and the level of cellular cholesterol is important in viral life cycles [18]. In this review, the links between cholesterol metabolism and viral hepatitis are discussed. As research shows that a range of phytochemicals are effective at reducing cholesterol levels in the body and in the cells, it is possible to apply natural phytochemicals to manipulate cholesterol metabolism as a treatment strategy for hepatitis viral infections. The phytochemicals with cholesterol-lowering activities, as well as their identified antiviral effects, are also summarized in this review.
2. Cholesterol Homeostasis
Cholesterol is one of the major components of mammalian biological membranes. It is required for proper membrane functioning, such as permeability, fluidity, and organelle identity. It is also a crucial biomaterial in maintaining structures and controlling activities of membrane proteins. In addition, cholesterol is a precursor for almost all steroid hormones [19]. Cells maintain their cholesterol homeostasis through complicated transcriptional and posttranscriptional mechanisms [20]. It has been suggested that mammalian cells are able to synthesize cholesterol [21], even though a large quantity of cholesterol can be absorbed by the gastrointestinal tract [22]. On the other hand, only hepatocytes are capable of degrading and eliminating cholesterol in great quantities [22].
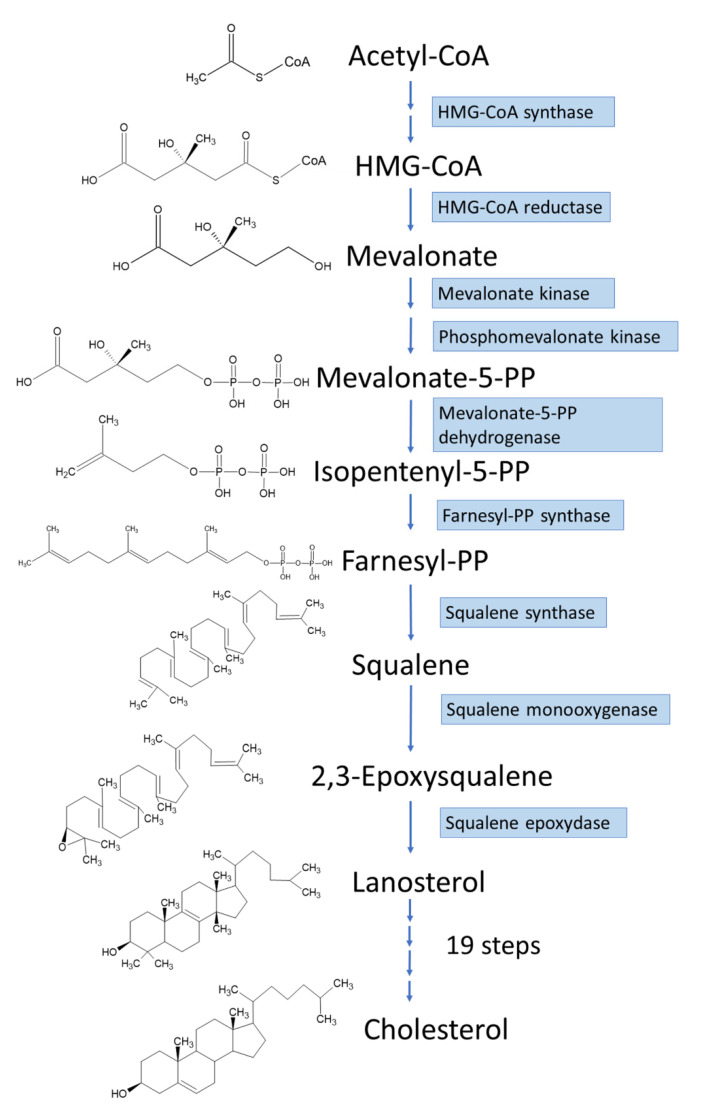
De novo biosynthesis of cholesterol in hepatic cells is a multistep biochemical process, starting from acetyl-CoA (Figure 1). The acetyl-CoA molecules are used as the substrate to synthesize 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) and the process is followed by the rate-limiting reaction in cholesterol biosynthesis catalyzed by HMG-CoA reductase to produce mevalonate, which is then processed into isopentenyl pyrophosphate. After that, the isopentenyl pyrophosphate is converted into lanosterol [23], which is further processed into cholesterol via a 19-step process [24]. Apart from de novo cholesterol biosynthesis, most cells acquire cholesterol from low-density lipoprotein (LDL) taken from the circulation via receptor-mediated endocytosis [25]. As cholesterol is an important component in the mammalian body, its homeostasis is crucial for proper cellular and systemic biological functions. Disturbance in cholesterol balance is suggested to be a significant factor, not only for cardiovascular disease [26,27], but also for a variety of other diseases, such as neurodegenerative diseases [28,29] and cancers [3,21,24]. In addition, cholesterol metabolism has great impact on the immune system and influence on antitumor immune responses [30].
Figure 1.
Cellular biosynthesis of cholesterol.
Lipid rafts are cholesterol- and sphingolipid-enriched membrane domains containing specific proteins for specialized cellular functions. Lipid rafts are involved in the signal transduction in cancer cell survival, cell death, and metastasis [31]. Alterations of lipid rafts are also linked to age-associated neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease [32]. In addition, research shows that lipid raft signaling is involved in the pathogenesis of a variety of conditions, such as cardiovascular diseases, prion diseases, systemic lupus erythematosus, and HIV infection [33].
LDs are reservoirs for cholesterol and lipids in mammalian cells. They are already linked to inflammatory responses through the synthesis and metabolism of eicosanoids and to metabolic disorders, such as obesity, cancer, and atherosclerosis [34,35]. The relationships between infectious diseases and LDs have been documented. Viruses, bacteria, and parasites obtain substrates (directly or indirectly) from LDs, for energy metabolism, replication compartments, infectious particle assembly, membrane building blocks, and tools for host colonization and/or evasion of anti-inflammatory responses [36]. As mentioned previously, cholesterol and its metabolism are crucial for the life cycles of a variety of viruses and are linked to host inflammation; in this article, we discuss the roles played by cholesterol metabolism in viral hepatitis.
3. Hepatitis Viruses and Cholesterol Metabolism
Viral hepatitis is a condition of inflammation in the liver induced by viral infections. The most common viruses causing viral hepatitis are hepatitis A, B, C, D, and E viruses (characteristics of hepatitis viruses are summarized in Table 1), while other types of viruses, such as cytomegalovirus [37], Epstein–Barr virus [38], yellow fever virus [39], and herpes simplex virus [40], can also cause liver inflammation. HAV and HEV are the leading causes of acute viral hepatitis worldwide. They are present in the feces of infected individuals and are typically transmitted by ingestion of contaminated food or water. In most cases, the symptoms caused by infections with HAV and HEV are relatively mild, with most infected patients making a full recovery. People in low-income countries, where sanitary and conditions are poor, are easily infected with these two viruses [41]. On the other hand, HBV and HCV can cause both acute and chronic hepatitis. HBV, HCV, and HDV are usually transmitted through parenteral contact with infected body fluids, including receiving contaminated blood or blood products, and invasive medical procedures using contaminated equipment. For HBV, transmissions from mother to baby at birth and through sexual contact are also frequently reported. On the contrary, the sexual transmission of HCV is much less common. HDV is identified as a satellite virus with infections only occurring in patients already infected with HBV. The dual infection of HDV and HBV very often results in a more serious illness and worse outcome. As HDV relies on HBV for its propagation and life cycle, HBV vaccines are able to offer protection from HDV infections.
Table 1.
Characteristics of hepatitis viruses.
| HAV | HBV | HCV | HDV | HEV | |
|---|---|---|---|---|---|
| Family | Picornaviridae | Hepadnaviridae | Flaviviridae | Not applicable | Hepeviridae |
| Genus | Hepatovirus | Orthohepadnavirus | Hepacivirus | Deltavirus | Orthohepevirus |
| Genome | Positive-sense single-stranded linear RNA | Double-stranded DNA | Positive-sense single-stranded linear RNA | Negative-sense single-stranded circular RNA | Positive-sense single-stranded linear RNA |
| Transmission | Fecal–oral | Exposure to infected blood or body fluid/sexual/perinatal | Exposure to infected blood | Exposure to infected blood/body fluids | Fecal–oral/zoonotic/blood transfusion |
| Clinical outcome of infection | Self-limited | Self-limited and chronic | Self-limited and chronic | Self-limited and chronic | Self-limited |
3.1. Hepatitis A Virus and Hepatitis E Virus
HAV is a member of the Hepatovirus genus of Picornaviridae family. It is the only species known to infect humans in this genus [42,43]. HEV is classified as a member of the genus Orthohepevirus, in the family Hepeviridae. There are four species (A to D) in Orthohepevirus, yet only Orthohepevirus A infects humans [42]. HAV and HEV, despite significant differences in their evolutionary origins and genomic structures, tend to employ similar pathogenic strategies, and the diseases caused by these two viruses are clinically indistinguishable from each other. Both HAV and HEV are considered non-enveloped icosahedral viruses. As the lipid bilayer envelopes of viruses are easily disrupted in the environment, the lack of a lipid envelope offers both the viruses a more stable ability to spread in the environment. As a result, these two viruses frequently cause foodborne and waterborne outbreaks in many countries [42,44]. However, HAV and HEV isolated from the serum of patients suffering acute infections are “wrapped” with a hijacked layer of the host cell’s membrane, allowing the circulating virions to avoid host immune responses and attacks from neutralizing antibodies [45]. Hepatocytes are believed to be the principal sites of replication of HAV and HEV, and the virus particles are secreted to the intestinal tract via the biliary duct [46,47]. Experiments using cell culture and HAV-infected mice show that HAV is released across both the basolateral and apical membranes of polarized epithelial cells as enveloped HAV virions, and high concentrations of human bile acids (derivatives of cholesterol) convert quasi-enveloped HAV virions into naked, nonenveloped particles [47], as those found in the gut and stool of HAV infected patients.
The relationships between HAV/HEV and cholesterol metabolism have been investigated. Altering lipid metabolism can be an efficient mean applied by the host to drive antiviral responses. Upregulation of intracellular LDs was identified as a weapon of the host cells to fight against viral infections [48]. Following infections of cells with either RNA viruses (e.g., Zika, dengue, influenza A) or DNA viruses (e.g., herpes simplex virus-1), LDs were rapidly upregulated; this response was also induced following stimulations with viral mimic agonists [48]. Indeed, it was also observed in the serum of young patients with self-limited acute viral hepatitis A; triglyceride, cholesterol, LDL, and Apo B levels elevated, while Apo A-I levels decreased [6]. It was found that HEV infection reduced cholesterol levels in cells, and decreased serum-lipid concentrations in patients [7]. Thus, cholesterol metabolism can be possibly applied as a target in antiviral strategies against HEV. It has been indicated that simvastatin treatment, which reduces intracellular cholesterol, increases viral release in vitro [7]. On the other hand, elevation of intracellular cholesterol with LDL, 25-hydroxycholesterol, or a cholesterol-elevating drug significantly reduces viral release due to enhanced lysosomal degradation of HEV [7].
3.2. Hepatitis B Virus
HBV can cause both acute and chronic liver inflammations. HBV infection is one of the major causes of HCC. Approximately 350 million people are chronically infected with HBV worldwide, and chronic HBV infections account for at least 50% cases of HCC globally [49]. Accumulated research results suggest that HBV infections affect lipid metabolism and could promote fatty acid synthesis [50,51]. Phosphatidylcholine is a major component of mammalian membrane and is a precursor for the synthesis of a number of signaling molecules [52]. The enhancement of phosphatidylcholine biosynthesis in hepatocytes by HBV infection was reported [53]. Cholesterol and LDs are also heavily impacted/controlled by HBV infection. Research shows that HBV infection in hepatocyte cultures is dependent on the presence of cholesterol in the viral envelope. According to a previous study, the depletion of cholesterol from HBV purified from the plasma of infected patients led to a significantly reduced ability of infection, but the binding of the virus to the cells was not affected [54]. These data indicate that the cholesterol content in the viral envelope is important for later steps in viral uptake. An animal model study using HBV-infected humanized mice revealed a significant increase in human genes related to the uptake, biosynthesis, and transcriptional regulation of cholesterol. These genes include those expressing LDL receptor (LDLR), HMG-CoA reductase, and sterol regulatory element binding protein-2 (SREBP-2) [55]. The effects of HBV infection on host cellular lipid metabolism have also been investigated. Research shows that altered lipid metabolisms in host cells are associated with HBV proteins. Several reports have suggested the roles played by the HBV X protein regarding this issue. Kim et al. demonstrated lipid accumulation and expression of lipid metabolic genes in HBV X protein-expressing cells and transgenic mice [56]. The results indicated that overexpression of HBV X protein induced hepatic lipid accumulation and this effect was linked to transcriptional activation of SREBP-1 and PPAR-γ [56]. Liver fatty acid binding proteins (FABPs) are a family of cytoplasmic proteins involved in lipid uptake, transport, and metabolism [57]. Wolfrum et al. reported that overexpression of FABP1 significantly increased the rate of fatty acid uptake [58]. An investigation by Wu et al. found that the expression level of FABP1 was elevated in the HBV-producing hepatoma cell line, and this FABP1 regulation was mediated by the HBV X protein [59].
In 2013, Li et al. used adenovirus to send the HBV genome into hepatocyte HepG2 and observed an increased hepatic cholesterol accumulation. In addition, the infection of HBV-containing adenovirus was able to enhance the mRNA and protein levels of LDLR and HMG-CoA reductase in cells. These inductive effects were possibly due to suppression of TLR2 expression levels by siRNA [60]. Selitsky et al. applied a mixture of genomic, molecular, and biochemical approaches to identify key miRNAs that drive lipid phenotypes of chronic viral hepatitis and hepatitis-associated HCC. Several miRNAs were identified as the candidate master regulators of pathways mediating cholesterol homeostasis in chronic hepatitis induced by HBV or HCV, and virus-infection associated liver cancer [2]. Experiments in Huh-7 hepatocytes found that miR-21, miR-27, and miR-224 suppressed cholesterol synthesis, and predictions showed that all three of these miRNAs target the 3′-untranslated region (3′-UTR) of HMG-CoA reductase [2].
Cell death-inducing DNA fragmentation factor-α (DFFA)-like effector (CIDE) proteins have been identified as lipid droplet-associated proteins that regulate lipid metabolism [61]. Yasumoto et al. reported that CIDEB and CIDEC positively regulate cellular LD size. Overexpression of CIDEB or CIDEC enhanced HBV production in HBV-infected cells (HepG2.2.15), and deficiency in CIDEB or CIDEC impaired HBV production and reduced the size of single LDs [62]. Yasumoto et al. also suggested that impairment of CIDE expression might stabilize an appropriate production level of HBV and inhibit excessive HBV productions [62]. Experimental results from HBV-inducible Hep38.7-Tet cells incubated with HBV suggested that HBV production may affect the expression of CIDE proteins in hepatocytes, and CIDE proteins were found to significantly enhance the activity of HBV core promoter [62]. CIDEs and HBV proteins might directly or indirectly interact with each other, and the balance between CIDE proteins and HBV production may contribute to moderate reduction in LD size in HBV-producing cells [62]. Downregulation of CIDE proteins might be a mean adopted by HBV to optimize the virus production levels to avoid tissue/cell damages and host immune clearance for persistent infection [62]. However, the clinical links between chronic HBV infection and the lipid accumulation in liver has not yet been fully understood. In population studies, it was found that HBV infection is associated with a low prevalence of fatty liver, hypertriglyceridemia, and metabolic syndrome [63], and positive results in serum HBV surface antigen are associated with low prevalence of metabolic syndrome [64]. Bile acid is mainly synthesized from cholesterol in the liver and plays a crucial role in lipid digestion and absorption. Human sodium taurocholate co-transporting polypeptide (NTCP, also known as solute carrier family 10 member 1, SLC10A1) mediates the entry of bile salts from hepatic portal blood into hepatocytes [65]. NTCP has also been identified as the receptor mediating species-specific entry of HBV into hepatocytes [66]. Thus, the association between bile acid and HCV infection was suggested. Evidence showed that the preS1 lipopeptide of HBV envelope protein efficiently blocked the uptake of bile salts by NTCP [67], suggesting a role played by HBV infection in limiting NTCP functions. The blocking of bile acid uptake promotes compensatory upregulation of cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in bile acid synthesis and HMG-CoA reductase thus increase the synthesis of bile acid and cholesterol in cells [68].
3.3. Hepatitis C Virus
The interplays between host lipid metabolism and HCV infection are the most studied among the hepatitis viruses. HCV infection alters the lipid and cholesterol metabolism in cells. Experiments using HepG2 cells transfected with the HCV core protein revealed that the expression of several genes related to lipid metabolism, including peroxisome proliferator-activated receptor (PPAR) α, multidrug resistance protein (MDR) 3, and microsomal triglyceride transfer protein (MTP), acyl-CoA oxidase (ACO) 1, and carnitine palmitoyl transferase-1 (CPT-1), were modulated by the expression of the HCV core protein [69]. It was also found that transient expression of the HCV core protein in mice downregulated the expression of lipid metabolism-associated genes, MDR2, CPT, AOX, and PPARα, leading to triglyceride (TG) accumulation and induction of oxidative stress [69]. Significant increases in serum levels of alanine aminotransferase, free fatty acids, TG, as well as the accumulation of hepatic lipid droplets, were observed in mice infected with HCV NS5A-expressing lentivirus [70]. In vitro and in vivo experiments showed that HCV NS5A was able to inhibit phosphorylation of AMP-activated protein kinase (AMPK), a key player in controlling cellular energy homeostasis by activating glucose and fatty acid uptake and oxidation [71]. HCV NS5A can also increase the expression levels of SREBP-1c, acetyl-coenzyme A carboxylase 1 (ACC1) and fatty acid synthase (FASN) [70].
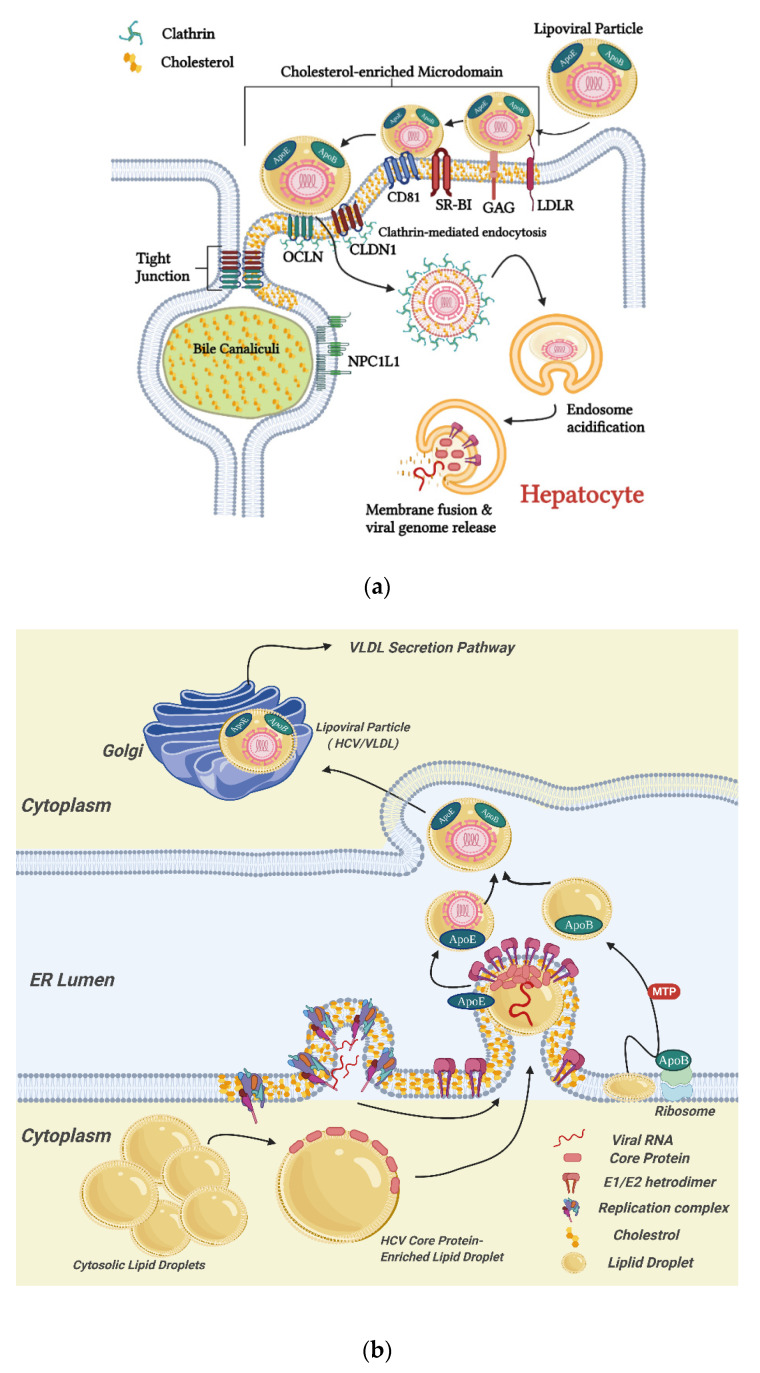
The importance of cellular lipid and cholesterol in the HCV life cycle has also been documented. HCV entry into hepatocytes, as well as the replication, assembly, and secretion of HCV, are illustrated in Figure 2. It has been indicated that cellular surface proteins CD81 and scavenger receptor B type I (SR-BI) are required elements for the entry of HCV into host cells [72,73,74]. As mentioned, cholesterol is a major component of lipid rafts. Many cellular proteins, including viral receptors, are preferentially localized in lipid rafts (Figure 2a). A study by Kapadia et al. demonstrated that cellular cholesterol content is crucial for HCV entry, and this might be due to the effects on cell surface expression and localization of CD81 [8]. Localization for both CD81 [75,76] and SR-BI [77] to lipid rafts has been demonstrated. Further, direct physical interaction between CD81 and cholesterol has been observed [78], and HCV pseudo-particle fusion with liposomes, an important step for virus entry, was enhanced with the presence of cholesterol in the target membranes [79]. In addition, the cellular Niemann–Pick C1-like 1 (NPC1L1) cholesterol uptake receptor has been identified as a HCV entry factor [80]. Research shows that NPC1L1 expression is required for HCV infection. Silencing or blocking of NPC1L1 impaired the initiation of HCV infection, and the application of NPC1L1 antagonist ezetimibe strongly blocked HCV uptake in vitro [80].
Figure 2.
Schematic diagrams of the HCV entry into hepatocyte (a) and the replication, assembly, and secretion of HCV (b). (a) HCV entry into hepatocytes is a sequential process in which multiple factors, including glycosaminoglycan (GAG), low-density lipoprotein receptor (LDLR), scavenger receptor class B type I (SR-BI), CD81, tight junction proteins, claudin-1 (CLDN1), and occludin (OCLN), as well as Niemann–Pick C1-like 1 (NPC1L1), are involved [73,87,88]. The process starts with the binding of the lipoviral particle (LVP) to GAG and LDLR, followed by the interactions with SR-BI, CD81, CLDN1, and OCLN, leading to viral internalization in cholesterol-enriched microdomains (lipid rafts) of the membranes via a clathrin-mediated endocytosis; (b) HCV viral proteins induce rearrangement of ER membranes and form replication complexes [15]. Localization of the HCV polyprotein to cholesterol-enriched membrane fractions is required for the polyprotein cleavage [89], and the replication complexes are associating with cholesterol-enriched domains [83]. New viral genomic RNA is synthesized by the replication complexes assembled by non-structural (NS) proteins of HCV, and the newly produced HCV core protein is translocated to LDs. Virion assembly occurs on core protein-enriched LDs and is associated by ApoE. Packaging of the capsid takes place with viral budding into the ER lumen at VLDL synthesis sites, mediated by microsomal triglyceride transfer protein (MTP). ApoB is lipidated by the MTP to generate VLDL precursors, which further fuse with ER luminal ApoE-bound LDs to form VLDL [87]. The matured HCV is associated with VLDL, in the form of LVP, and secreted through the VLDL secretion pathway. Graphics in this figure were created with BioRender.com (accessed on 26 February 2022).
It was suggested that HCV might also be able to enter the host cells via an CD81 independent route [81]. This entry pathway associates with the very-low-density lipoprotein receptor (VLDLR), which is important in cholesterol and apolipoprotein uptake [82]. Lipoproteins, lipids, and cholesterol play important roles in virus production and secretion (Figure 2b). HCV replication complex was found to associate with cholesterol-enriched lipid rafts in the cells [83]. Disruption of the cholesterol synthetic pathway by inhibiting HMG-CoA reductase, the enzyme catalyzing the rate limiting step, completely abolished HCV replication, according to [84]. Research shows that LDs are essential organelles involved in the production of infectious HCV particle assembly [15,85]. Disrupting the HCV core protein–lipid droplet associations leads to a loss in infectious virus production [16]. HCV core protein, responsible for viral capsid formation, is targeted to LDs by its relatively hydrophobic domain 2. A study comparing the HCV core protein sequences of two HCV strains, JFH1 and Jc1, differed in their virus production efficiencies in cultured human hepatoma cells, finding that the amino acid differences in the HCV core protein domain 2 could cause significant differences in the HCV core protein accumulation on LDs, leading to differences in the efficiency of virus production [17]. As the infectivity and productivity of HCV is highly related to lipid and cholesterol metabolism in host cells, these metabolic pathways are considered as drug targets in the treatment of HCV infection [86].
4. Therapeutic Potential of Natural Phytochemicals Targeting Cholesterol Metabolism for Treating Viral Infections
Chronic hepatitis, compared with acute hepatitis, can cause more serious problems, such as liver fibrosis, cirrhosis, and even HCC. As HBV and HCV often cause chronic hepatitis, research scientists have developed (are developing) strategies to deal with their infections. As discussed previously, lipid and cholesterol metabolism and homeostasis are crucial for hepatitis viral infection; cholesterol/lipid metabolism have been identified as potential antiviral targets [90]. Indeed, cellular cholesterol has been identified as a critical factor in a variety of viral infections [91,92,93,94,95,96]. Research shows that several FDA-approved cholesterol-lowering drugs exhibit antiviral abilities against flaviviruses, including dengue, Zika, yellow fever, and West Nile viruses [97]. Statins are fungal origin molecules capable of inhibiting HMG-CoA reductase, thus inhibiting cholesterol synthesis in cells [98,99]. Statins, with their strong cholesterol-lowering abilities, have been applied in the treatment of hypercholesterolemia [98]. There have been several attempts to use statins in the treatment of dengue viral infections. Cell-level experiments have shown that the use of lovastatin (Figure 3a), the first marketed statin drug used to treat high blood cholesterol and reduce the risk of cardiovascular disease, inhibited dengue virus RNA replication and viral secretion [100,101,102,103,104]. In a dengue virus-infected mouse model, lovastatin treatment was found to delay viral infection and increase the survival rates of infected mice [102]. However, a randomized, double-blind, placebo-controlled human clinical trial found that, although lovastatin is safe and well tolerated in adults with dengue viral infection, lovastatin treatment was unable to inhibit dengue viral infections in patients [105].
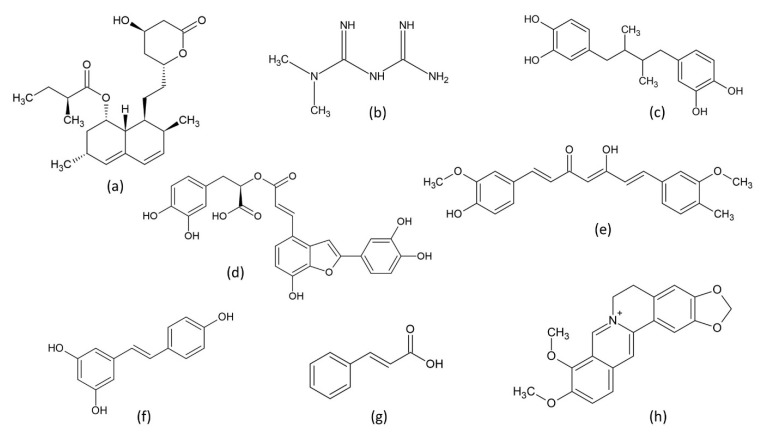
Figure 3.
Chemical structures of selected natural compounds with inhibitory activities on cholesterol biosynthesis. (a) lovastatin; (b) metformin; (c) nordihydroguaiaretic acid; (d) salvianolic acid C; (e) curcumin; (f) resveratrol; (g) cinnamic acid; (h) berberine.
Metformin (Figure 3b), a natural phytochemical originally found in the plant Galega officinalis [106], currently serves as a first-line medication for the treatment of type 2 diabetes [107]. Metformin is known to activate 5′ AMP-activated protein kinase (AMPK), which plays a key role in cellular energy homeostasis. Activation of AMPK results in downregulation of HMG-CoA reductase activity, consequently suppressing cholesterol biosynthesis [108]. The suppression of cholesterol synthesis might be linked to antiviral effects. This argument was supported by a study by Soto-Acosta et al. in which significant inhibition of the dengue virus replication was observed in cells treated with metformin [109]. Similar effects were also observed in experiments using other AMPK activating agents on other flaviviruses. Nordihydroguaiaretic acid (Figure 3c), a lignan found in Larrea tridentate with AMPK activation activity [110], and its derivative, were found to be effective against dengue [111], West Nile, and Zika viruses [112]. Another AMPK activator PF-06409577, an indole acid exhibiting cholesterol-lowering activity [113,114], was also found to inhibit flavivirus infection; this inhibition was through modification of host cell lipid metabolism [115]. Collectively, these results indicate that lowering cholesterol levels in the hosts can be a promising strategy for the treatment of flavivirus infections.
As HCV is also a flavivirus, the manipulation of cholesterol synthesis might also be a feasible strategy for treating HCV infection. As mentioned, statins are powerful inhibitors of cholesterol synthesis. The antiviral activity of lovastatin against HCV was reported by Ye et al. [84] and Kapadia and Chisari [116]. Ikeda and co-workers tested anti-HCV profiles of several statins, and found that atorvastatin, fluvastatin, and simvastatin exhibited stronger anti-HCV activity than lovastatin [86]. They also demonstrated that the combination treatment of interferon α and fluvastatin was a more effective treatment, as compared to that of interferon α with ribavirin [86]. Effects of metformin on HCV infection were also investigated. A randomized controlled clinical trial indicated that the combination of metformin with antiviral therapy increased the sustained virological response rate of patients infected with HCV genotype 1 and with insulin resistance [117].
In addition to HCV, statins were shown to be effective in the inhibition of HBV. Inhibition of cholesterol synthesis in HBV-producing cells using lovastatin resulted in impaired secretion of sub-viral particles, according to [118]. The antiviral activity of simvastatin was reported by Bader and Korba [119], and a combination of simvastatin with each of the FDA-approved nucleoside analogue inhibitors, including lamivudine, adefovir, tenofovir, and entecavir, showed synergistic antiviral activity [119]. As chronic HBV infection is highly related to the development of HCC, the inhibition of prolonged HBV infection might reduce the risk of HCC development in patients. A cohort study showed a dose-response relationship between statin use and the risk of HCC in the HBV cohort [120]. NB-598 is a potent inhibitor of squalene epoxidase [121], which is also a key enzyme in cholesterol synthesis. Cell experiments carried out by Bremer et al. demonstrated that infection with HBV from hepatocyte cultures treated with NB-598 showed a more than 60% decrease in infectivity as compared to HBV obtained from untreated cultures [54]. The indirect inhibitor of HMG-CoA reductase, metformin, was also found to inhibit HBV protein production and replication in human hepatoma cells [122].
Based on the mentioned previous studies, it is possible to apply drugs/molecules to alter cholesterol levels for the purposes of inhibition of viral infections. As hypercholesterolemia is a risk factor for cardiovascular diseases, efforts have been made to develop cholesterol-lowering drugs. HMG-CoA reductase, as a key enzyme in cholesterol synthesis, has been focused on in drug development. In silico approaches have been applied in the discovery of natural compounds exhibiting HMG-CoA reductase inhibitor activities [123], and in the investigations, concerning interactions between identified compounds and the enzyme [124,125]. Suganya et al., by using molecular docking and molecular dynamics simulations, identified rutin, a flavonoid found in a variety of plants, including citrus, to be a strong binder and inhibitor of HMG-CoA reductase [126]. Lin and co-workers applied structure-based screening to identify strong HMG-CoA reductase binding compounds from a traditional herbal medicine database [123]. They identified salvianolic acid C (Figure 3d), curcumin (Figure 3e), and docosanol, along with cell experiments, as possible strong inhibitors of HMG-CoA reductase [123]. Indeed, salvianolic acid C was found to impair structural formation of the envelope bound spike protein of SARS-CoV-2, inhibiting viral infections [127]. Curcumin was found to inhibit HCV entry into human hepatocytes by altering membrane fluidity, impairing virus binding and fusion [128]. The inhibitory effects of curcumin on HBV entry into host cells were also documented [129]. In addition, research shows that curcumin is capable of interfering with the infection processes of dengue [130] and Zika viruses [131].
A variety of natural compounds and extracts have been tested for their inhibitory activities on HMG-CoA reductase in animal models [132]. Experiments using LDLR (-/-) mice fed with high-cholesterol diets found that curcumin treatment lowered plasma and hepatic cholesterol comparable to the effects of lovastatin [133]. Resveratrol (Figure 3f), a stilbenoid and a phytoalexin, was found to attenuate the expression of HMG-CoA reductase mRNA in Syrian golden hamsters [134]. A study by Lin and co-workers found that resveratrol treatments were able to assist in the recovery from a fatty liver and protect against HBV-induced HCC in a mouse model [135]. Cinnamic acid (Figure 3g) is a phenolic compound found in plant materials, such as cinnamon oil, Shea nut (Vitellaria paradoxa) oil, and balsams. It is the foundation of substances for the biosynthesis of a variety of plant compounds, including flavonoids, lignols, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. In a rat model, dietary cinnamic acid can result in inhibited hepatic HMG-CoA reductase activity, leading to lower hepatic cholesterol content [136]. An investigation by Amano et al. demonstrated that several cinnamic acid derivatives exhibited inhibitory activity on HCV replication [137]. Berberine (Figure 3h) is a quaternary ammonium salt in plants, such as Berberis. Inhibitory effects on HMG-CoA reductase by administration of berberines have been observed in rats [138,139] and hamsters [140]. Research shows that berberine could specifically impede HCV attachment and entry steps of a viral infection [141]. Table 2 presents examples of natural cholesterol-lowering compounds tested to exhibit antiviral activities. As combating hypercholesteremia is one of the tasks involved in medical practice/research, it is believed that the development of novel cholesterol-lowering compounds will continue to grow. As downregulation of cholesterol synthesis can be effective, on its own or in combination with other antivirals, to inhibit viral infections, the development of novel cholesterol-lowering compounds will be beneficial in future treatments of viral hepatitis.
Table 2.
Examples of natural cholesterol-lowering compounds tested to exhibit antiviral activities.
| Compounds | Original Natural Sources | Functions on Cholesterol Metabolism | Viruses Studied to Be Affected |
|---|---|---|---|
| Statins | fungi | HMG-CoA reductase inhibitor | HBV [118,119], HCV [84,86,116], HEV [7], and other flaviviruses [97,100,101,102,104] |
| Metformin | Galega officinalis | AMPK activator | HBV [122], HCV [117], and other flaviviruses [109] |
| Nordihydroguaiaretic acid | Larrea tridentate | AMPK activator | Flaviviruses [111,112] |
| Resveratrol | Grapes, blueberries, raspberries, mulberries, and peanuts | HMG-CoA reductase mRNA expression inhibitor | HBV [135] |
| Salvianolic acid C | Salvia prionitis, Origanum vulgare, and others | HMG-CoA reductase inhibitor | SARS-CoV-2 [127] |
| Curcumin | Curcuma longa species | HMG-CoA reductase inhibitor | HCV [128], HBV [129], dengue virus [130], and Zika virus [131] |
| Docosanol | Docosanol is a saturated fatty alcohol found in plants | HMG-CoA reductase inhibitor | Lipid-enveloped viruses, including herpes simplex virus [142] |
| Cinnamic acid | Cinnamon oil, Vitellaria paradoxa oil, and balsams from plants | HMG-CoA reductase inhibitor | HCV [137] |
| Berberine | Berberis | HMG-CoA reductase inhibitor | HCV [141] |
As many pure, cholesterol-lowering compounds from natural sources exhibit antiviral activities, it can be implied that cholesterol-lowering foods or dietary patterns might also be beneficial as supplements or aids to prevent or reduce problems caused by viral hepatitis. With this logic, a high-cholesterol diet might further deteriorate the situation. These concepts have been tested in several studies. It was suggested in an animal model study that a high-cholesterol diet could indeed promote steatohepatitis and liver tumorigenesis in HCV core protein expressing transgenic mice [143]. On the other hand, it was found that black raspberry extract showed protective effects against hypercholesterolemia and subsequently reduced hepatitis in rat models fed with high-fat/high-choline diets [144]. A retrospective cohort analysis showed that chronic hepatitis C patients adhering to fish-rich dietary patterns had lower viral loads [145]. Red yeast rice is a Chinese food product in which rice is fermented with a fungus, Monascus purpureus. This product is claimed to have the ability to reduce blood cholesterol and triglyceride levels in humans, as it contains a considerable amount of statins [146]. One study indicated that Monascus pigment derivatives were able to inhibit HCV replication by interfering with the mevalonate biosynthesis, the reaction catalyzed by HMG-CoA reductase [147]. However, statins are powerful cholesterol-lowering compounds. An overdose of statins might cause adverse effects [146]. A case of acute liver injure induced in a patient taking a red yeast rice supplement for cholesterol-lowering purposes was reported [148]. Thus, caution is still required, even when following effective dietary patterns or taking natural compounds/extracts for specialized health purposes.
5. Conclusions
Viral hepatitis is commonly caused by five hepatitis viruses, including HAV, HBV, HCV, HDV, and HEV. Among these five viruses, HBV and HCV have attracted the most attention in biomedical research, as they often cause chronic infections and inflammation, leading to liver fibrosis, cirrhosis, and sometimes HCC. The infections and life cycles of the hepatitis viruses are heavily associated with the metabolism of lipids, particularly cholesterol. Cholesterol levels in the host play a major role in viral infections; research shows that inhibition of cholesterol synthesis could reduce the infections of a variety of human viruses, including HBV and HCV. Therefore, manipulation of cholesterol biosynthesis by cholesterol-lowering drugs are proposed as a strategy to treat hepatitis viral infections. In the investigations, for this purpose, HMG-CoA reductase, the rate-limiting enzyme in cholesterol biosynthesis, is the most studied target. Owing to the great need to solve health problems caused by hypercholesterolemia, extensive efforts have been made into the discovery/development of cholesterol-lowering medications. Plants are natural sources of therapeutic compounds. Natural phytochemicals with desired cholesterol-lowering activities have been identified, and their efficacies at inhibiting viral infections have been tested. It is expected that, with the increasing number of cholesterol-lowering natural compounds being discovered, more choices could be provided for the design/development of antiviral strategies that will treat/prevent viral hepatitis in the future.
Acknowledgments
The authors would like to thank Ji-Hshiung Chen and Alex Yong Kwang Tan for the valuable suggestions in this manuscript. The schematic diagrams in Figure 2 were prepared using BioRender.com (accessed on 26 February 2022).
Author Contributions
Conceptualization, J.-W.L. and J.-H.Y.; investigation, J.-W.L. and J.-H.Y.; resources, J.-W.L. and J.-H.Y.; data curation, H.M., J.-W.L. and J.-H.Y.; writing—original draft preparation, J.-W.L., H.M. and J.-H.Y.; writing—review and editing, J.-W.L. and J.-H.Y.; visualization, J.-W.L. and H.M.; supervision, J.-W.L. and J.-H.Y.; funding acquisition, J.-W.L. and J.-H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology, Taiwan (MOST 110-2221-E-320-002 (J.-W.L.); MOST 108-2320-B-320-002-MY3 (J.-H.Y.)), Buddhist Compassion Relief Tzu Chi Foundation (TCIRP106001-01 (J.-W.L.); TCU-B02 (J.-W.L.)), Buddhist Tzu Chi Medical Foundation TCMF-SP 110-01 (J.-H.Y.), and a collaborative grant by Tzu Chi Medical Foundation, Tzu Chi University, and Academia Sinica (TCAS-111-03 (J.-W.L.)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is a review paper and there are no raw data.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stanaway J.D., Flaxman A.D., Naghavi M., Fitzmaurice C., Vos T., Abubakar I., Abu-Raddad L.J., Assadi R., Bhala N., Cowie B., et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selitsky S.R., Dinh T.A., Toth C.L., Kurtz C.L., Honda M., Struck B.R., Kaneko S., Vickers K.C., Lemon S.M., Sethupathy P. Transcriptomic Analysis of Chronic Hepatitis B and C and Liver Cancer Reveals MicroRNA-Mediated Control of Cholesterol Synthesis Programs. mBio. 2015;6:e01500–e01515. doi: 10.1128/mBio.01500-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringehan M., McKeating J.A., Protzer U. Viral hepatitis and liver cancer. Phil. Trans. R. Soc. B. 2017;372:20160274. doi: 10.1098/rstb.2016.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambroggio E.E., Costa Navarro G.S., Perez Socas L.B., Bagatolli L.A., Gamarnik A.V. Dengue and Zika virus capsid proteins bind to membranes and self-assemble into liquid droplets with nucleic acids. J. Biol. Chem. 2021;297:101059. doi: 10.1016/j.jbc.2021.101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton N.S., Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8:422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selimoglu M.A., Caner I., Yildiz L. Lipid profile in children with acute viral hepatitis A. Pediatr. Int. 2007;49:215–219. doi: 10.1111/j.1442-200X.2007.02341.x. [DOI] [PubMed] [Google Scholar]
- 7.Glitscher M., Martin D.H., Woytinek K., Schmidt B., Tabari D., Scholl C., Stingl J.C., Seelow E., Choi M., Hildt E. Targeting Cholesterol Metabolism as Efficient Antiviral Strategy Against the Hepatitis E Virus. Cell. Mol. Gastroenterol. Hepatol. 2021;12:159–180. doi: 10.1016/j.jcmgh.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapadia S.B., Barth H., Baumert T., McKeating J.A., Chisari F.V. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J. Virol. 2007;81:374–383. doi: 10.1128/JVI.01134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haberl E.M., Weiss T.S., Peschel G., Weigand K., Kohler N., Pauling J.K., Wenzel J.J., Horing M., Krautbauer S., Liebisch G., et al. Liver Lipids of Patients with Hepatitis B and C and Associated Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021;22:5297. doi: 10.3390/ijms22105297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samsa M.M., Mondotte J.A., Iglesias N.G., Assuncao-Miranda I., Barbosa-Lima G., Da Poian A.T., Bozza P.T., Gamarnik A.V. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009;5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaunt E.R., Cheung W., Richards J.E., Lever A., Desselberger U. Inhibition of rotavirus replication by downregulation of fatty acid synthesis. J. Gen. Virol. 2013;94:1310–1317. doi: 10.1099/vir.0.050146-0. [DOI] [PubMed] [Google Scholar]
- 12.Cheung W., Gill M., Esposito A., Kaminski C.F., Courousse N., Chwetzoff S., Trugnan G., Keshavan N., Lever A., Desselberger U. Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication. J. Virol. 2010;84:6782–6798. doi: 10.1128/JVI.01757-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffey C.M., Sheh A., Kim I.S., Chandran K., Nibert M.L., Parker J.S. Reovirus outer capsid protein micro1 induces apoptosis and associates with lipid droplets, endoplasmic reticulum, and mitochondria. J. Virol. 2006;80:8422–8438. doi: 10.1128/JVI.02601-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dias S.S.G., Soares V.C., Ferreira A.C., Sacramento C.Q., Fintelman-Rodrigues N., Temerozo J.R., Teixeira L., Nunes da Silva M.A., Barreto E., Mattos M., et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020;16:e1009127. doi: 10.1371/journal.ppat.1009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyanari Y., Atsuzawa K., Usuda N., Watashi K., Hishiki T., Zayas M., Bartenschlager R., Wakita T., Hijikata M., Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 16.Boulant S., Targett-Adams P., McLauchlan J. Disrupting the association of hepatitis C virus core protein with lipid droplets correlates with a loss in production of infectious virus. J. Gen. Virol. 2007;88:2204–2213. doi: 10.1099/vir.0.82898-0. [DOI] [PubMed] [Google Scholar]
- 17.Shavinskaya A., Boulant S., Penin F., McLauchlan J., Bartenschlager R. The lipid droplet binding domain of hepatitis C virus core protein is a major determinant for efficient virus assembly. J. Biol. Chem. 2007;282:37158–37169. doi: 10.1074/jbc.M707329200. [DOI] [PubMed] [Google Scholar]
- 18.Glitscher M., Hildt E. Endosomal Cholesterol in Viral Infections—A Common Denominator? Front. Physiol. 2021;12:750544. doi: 10.3389/fphys.2021.750544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper D.R., Kraemer W.J., Focht B.C., Volek J.S., DuPont W.H., Caldwell L.K., Maresh C.M. Endocrinological Roles for Testosterone in Resistance Exercise Responses and Adaptations. Sports Med. 2017;47:1709–1720. doi: 10.1007/s40279-017-0698-y. [DOI] [PubMed] [Google Scholar]
- 20.Espenshade P.J., Hughes A.L. Regulation of sterol synthesis in eukaryotes. Annu. Rev. Genet. 2007;41:401–427. doi: 10.1146/annurev.genet.41.110306.130315. [DOI] [PubMed] [Google Scholar]
- 21.Huang B., Song B.L., Xu C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020;2:132–141. doi: 10.1038/s42255-020-0174-0. [DOI] [PubMed] [Google Scholar]
- 22.Cohen D.E. Balancing cholesterol synthesis and absorption in the gastrointestinal tract. J. Clin. Lipidol. 2008;2:S1–S3. doi: 10.1016/j.jacl.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerqueira N.M., Oliveira E.F., Gesto D.S., Santos-Martins D., Moreira C., Moorthy H.N., Ramos M.J., Fernandes P.A. Cholesterol Biosynthesis: A Mechanistic Overview. Biochemistry. 2016;55:5483–5506. doi: 10.1021/acs.biochem.6b00342. [DOI] [PubMed] [Google Scholar]
- 24.Lasuncion M.A., Martin-Sanchez C., Canfran-Duque A., Busto R. Post-lanosterol biosynthesis of cholesterol and cancer. Curr. Opin. Pharmacol. 2012;12:717–723. doi: 10.1016/j.coph.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein J.L., Brown M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo J., Yang H., Song B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020;21:225–245. doi: 10.1038/s41580-019-0190-7. [DOI] [PubMed] [Google Scholar]
- 27.Mc Auley M.T. Modeling cholesterol metabolism and atherosclerosis. WIREs Mech. Dis. 2021;2021:e1546. doi: 10.1002/wsbm.1546. [DOI] [PubMed] [Google Scholar]
- 28.Benarroch E.E. Brain cholesterol metabolism and neurologic disease. Neurology. 2008;71:1368–1373. doi: 10.1212/01.wnl.0000333215.93440.36. [DOI] [PubMed] [Google Scholar]
- 29.Chang T.Y., Yamauchi Y., Hasan M.T., Chang C. Cellular cholesterol homeostasis and Alzheimer’s disease. J. Lipid Res. 2017;58:2239–2254. doi: 10.1194/jlr.R075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King R.J., Singh P.K., Mehla K. The cholesterol pathway: Impact on immunity and cancer. Trends Immunol. 2022;43:78–92. doi: 10.1016/j.it.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B., Qin Y., Yu X., Xu X., Yu W. Lipid raft involvement in signal transduction in cancer cell survival, cell death and metastasis. Cell Prolif. 2021;55:e13167. doi: 10.1111/cpr.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesa-Herrera F., Taoro-Gonzalez L., Valdes-Baizabal C., Diaz M., Marin R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int. J. Mol. Sci. 2019;20:3810. doi: 10.3390/ijms20153810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michel V., Bakovic M. Lipid rafts in health and disease. Biol. Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg A.S., Coleman R.A., Kraemer F.B., McManaman J.L., Obin M.S., Puri V., Yan Q.W., Miyoshi H., Mashek D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Investig. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bozza P.T., Viola J.P. Lipid droplets in inflammation and cancer. Prostaglandins Leukot. Essent. Fat. Acids. 2010;82:243–250. doi: 10.1016/j.plefa.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Bosch M., Sweet M.J., Parton R.G., Pol A. Lipid droplets and the host-pathogen dynamic: FATal attraction? J. Cell Biol. 2021;220:e202104005. doi: 10.1083/jcb.202104005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Cunha T., Wu G.Y. Cytomegalovirus Hepatitis in Immunocompetent and Immunocompromised Hosts. J. Clin. Transl. Hepatol. 2021;9:106–115. doi: 10.14218/JCTH.2020.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinedi T.B., Koff R.S. Cholestatic hepatitis induced by Epstein-Barr virus infection in an adult. Dig. Dis. Sci. 2003;48:539–541. doi: 10.1023/A:1022592801060. [DOI] [PubMed] [Google Scholar]
- 39.Denis B., Chirio D., Ponscarme D., Brichler S., Colin de Verdiere N., Simon F., Molina J.M. Hepatitis Rebound after Infection with Yellow Fever Virus. Emerg. Infect. Dis. 2019;25:1248–1249. doi: 10.3201/eid2506.190069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman B., Gandhi S.A., Louie E., Rizzi R., Illei P. Herpes simplex virus hepatitis: Case report and review. Clin. Infect. Dis. 1997;24:334–338. doi: 10.1093/clinids/24.3.334. [DOI] [PubMed] [Google Scholar]
- 41.Migueres M., Lhomme S., Izopet J. Hepatitis A: Epidemiology, High-Risk Groups, Prevention and Research on Antiviral Treatment. Viruses. 2021;13:1900. doi: 10.3390/v13101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb G.W., Kelly S., Dalton H.R. Hepatitis A and Hepatitis E: Clinical and Epidemiological Features, Diagnosis, Treatment, and Prevention. Clin. Microbiol. Newsl. 2020;42:171–179. doi: 10.1016/j.clinmicnews.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drexler J.F., Corman V.M., Lukashev A.N., van den Brand J.M., Gmyl A.P., Brunink S., Rasche A., Seggewibeta N., Feng H., Leijten L.M., et al. Evolutionary origins of hepatitis A virus in small mammals. Proc. Natl. Acad. Sci. USA. 2015;112:15190–15195. doi: 10.1073/pnas.1516992112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purcell R.H., Emerson S.U. Hepatitis E: An emerging awareness of an old disease. J. Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Feng Z., Hirai-Yuki A., McKnight K.L., Lemon S.M. Naked Viruses That Aren’t Always Naked: Quasi-Enveloped Agents of Acute Hepatitis. Annu. Rev. Virol. 2014;1:539–560. doi: 10.1146/annurev-virology-031413-085359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Himmelsbach K., Bender D., Hildt E. Life cycle and morphogenesis of the hepatitis E virus. Emerg. Microbes Infect. 2018;7:196. doi: 10.1038/s41426-018-0198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirai-Yuki A., Hensley L., Whitmire J.K., Lemon S.M. Biliary Secretion of Quasi-Enveloped Human Hepatitis A Virus. mBio. 2016;7:e01998-16. doi: 10.1128/mBio.01998-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monson E.A., Helbig K.J. Host upregulation of lipid droplets drives antiviral responses. Cell Stress. 2021;5:143–145. doi: 10.15698/cst2021.09.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie Y. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Adv. Exp. Med. Biol. 2017;1018:11–21. doi: 10.1007/978-981-10-5765-6_2. [DOI] [PubMed] [Google Scholar]
- 50.Yang F., Yan S., He Y., Wang F., Song S., Guo Y., Zhou Q., Wang Y., Lin Z., Yang Y., et al. Expression of hepatitis B virus proteins in transgenic mice alters lipid metabolism and induces oxidative stress in the liver. J. Hepatol. 2008;48:12–19. doi: 10.1016/j.jhep.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Hajjou M., Norel R., Carver R., Marion P., Cullen J., Rogler L.E., Rogler C.E. cDNA microarray analysis of HBV transgenic mouse liver identifies genes in lipid biosynthetic and growth control pathways affected by HBV. J. Med. Virol. 2005;77:57–65. doi: 10.1002/jmv.20427. [DOI] [PubMed] [Google Scholar]
- 52.Yalcin A., Clem B., Makoni S., Clem A., Nelson K., Thornburg J., Siow D., Lane A.N., Brock S.E., Goswami U., et al. Selective inhibition of choline kinase simultaneously attenuates MAPK and PI3K/AKT signaling. Oncogene. 2010;29:139–149. doi: 10.1038/onc.2009.317. [DOI] [PubMed] [Google Scholar]
- 53.Li H., Zhu W., Zhang L., Lei H., Wu X., Guo L., Chen X., Wang Y., Tang H. The metabolic responses to hepatitis B virus infection shed new light on pathogenesis and targets for treatment. Sci. Rep. 2015;5:8421. doi: 10.1038/srep08421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bremer C.M., Bung C., Kott N., Hardt M., Glebe D. Hepatitis B virus infection is dependent on cholesterol in the viral envelope. Cell. Microbiol. 2009;11:249–260. doi: 10.1111/j.1462-5822.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 55.Oehler N., Volz T., Bhadra O.D., Kah J., Allweiss L., Giersch K., Bierwolf J., Riecken K., Pollok J.M., Lohse A.W., et al. Binding of hepatitis B virus to its cellular receptor alters the expression profile of genes of bile acid metabolism. Hepatology. 2014;60:1483–1493. doi: 10.1002/hep.27159. [DOI] [PubMed] [Google Scholar]
- 56.Kim K.H., Shin H.J., Kim K., Choi H.M., Rhee S.H., Moon H.B., Kim H.H., Yang U.S., Yu D.Y., Cheong J. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARgamma. Gastroenterology. 2007;132:1955–1967. doi: 10.1053/j.gastro.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 57.Hertzel A.V., Bernlohr D.A. The mammalian fatty acid-binding protein multigene family: Molecular and genetic insights into function. Trends Endocrinol. Metab. 2000;11:175–180. doi: 10.1016/S1043-2760(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 58.Wolfrum C., Buhlmann C., Rolf B., Borchers T., Spener F. Variation of liver-type fatty acid binding protein content in the human hepatoma cell line HepG2 by peroxisome proliferators and antisense RNA affects the rate of fatty acid uptake. Biochim. Biophys. Acta. 1999;1437:194–201. doi: 10.1016/S1388-1981(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 59.Wu Y.L., Peng X.E., Zhu Y.B., Yan X.L., Chen W.N., Lin X. Hepatitis B Virus X Protein Induces Hepatic Steatosis by Enhancing the Expression of Liver Fatty Acid Binding Protein. J. Virol. 2016;90:1729–1740. doi: 10.1128/JVI.02604-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y.J., Zhu P., Liang Y., Yin W.G., Xiao J.H. Hepatitis B virus induces expression of cholesterol metabolism-related genes via TLR2 in HepG2 cells. World J. Gastroenterol. 2013;19:2262–2269. doi: 10.3748/wjg.v19.i14.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slayton M., Gupta A., Balakrishnan B., Puri V. CIDE Proteins in Human Health and Disease. Cells. 2019;8:238. doi: 10.3390/cells8030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yasumoto J., Kasai H., Yoshimura K., Otoguro T., Watashi K., Wakita T., Yamashita A., Tanaka T., Takeda S., Moriishi K. Hepatitis B virus prevents excessive viral production via reduction of cell death-inducing DFF45-like effectors. J. Gen. Virol. 2017;98:1762–1773. doi: 10.1099/jgv.0.000813. [DOI] [PubMed] [Google Scholar]
- 63.Wong V.W., Wong G.L., Chu W.C., Chim A.M., Ong A., Yeung D.K., Yiu K.K., Chu S.H., Chan H.Y., Woo J., et al. Hepatitis B virus infection and fatty liver in the general population. J. Hepatol. 2012;56:533–540. doi: 10.1016/j.jhep.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 64.Choi J.S., Han K.J., Lee S., Chun S.W., Kim D.J., Kim H.C., Kim H.M. Serum HBV surface antigen positivity is associated with low prevalence of metabolic syndrome in Korean adult men. J. Epidemiol. 2015;25:74–79. doi: 10.2188/jea.JE20140053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb. Exp. Pharmacol. 2011;2011:205–259. doi: 10.1007/978-3-642-14541-4_5. [DOI] [PubMed] [Google Scholar]
- 66.Ni Y., Lempp F.A., Mehrle S., Nkongolo S., Kaufman C., Falth M., Stindt J., Koniger C., Nassal M., Kubitz R., et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Yan H., Peng B., Liu Y., Xu G., He W., Ren B., Jing Z., Sui J., Li W. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J. Virol. 2014;88:3273–3284. doi: 10.1128/JVI.03478-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geier A. Hepatitis B virus: The “metabolovirus” highjacks cholesterol and bile acid metabolism. Hepatology. 2014;60:1458–1460. doi: 10.1002/hep.27224. [DOI] [PubMed] [Google Scholar]
- 69.Yamaguchi A., Tazuma S., Nishioka T., Ohishi W., Hyogo H., Nomura S., Chayama K. Hepatitis C virus core protein modulates fatty acid metabolism and thereby causes lipid accumulation in the liver. Dig. Dis. Sci. 2005;50:1361–1371. doi: 10.1007/s10620-005-2788-1. [DOI] [PubMed] [Google Scholar]
- 70.Meng Z., Liu Q., Sun F., Qiao L. Hepatitis C virus nonstructural protein 5A perturbs lipid metabolism by modulating AMPK/SREBP-1c signaling. Lipids Health Dis. 2019;18:191. doi: 10.1186/s12944-019-1136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hardie D.G., Ross F.A., Hawley S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorner M., Horwitz J.A., Robbins J.B., Barry W.T., Feng Q., Mu K., Jones C.T., Schoggins J.W., Catanese M.T., Burton D.R., et al. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208–211. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colpitts C.C., Tsai P.L., Zeisel M.B. Hepatitis C Virus Entry: An Intriguingly Complex and Highly Regulated Process. Int. J. Mol. Sci. 2020;21:2091. doi: 10.3390/ijms21062091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang C.C., Hsu H.J., Yen J.H., Lo S.Y., Liou J.W. A Sequence in the loop domain of hepatitis C virus E2 protein identified in silico as crucial for the selective binding to human CD81. PLoS ONE. 2017;12:e0177383. doi: 10.1371/journal.pone.0177383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cherukuri A., Shoham T., Sohn H.W., Levy S., Brooks S., Carter R., Pierce S.K. The tetraspanin CD81 is necessary for partitioning of coligated CD19/CD21-B cell antigen receptor complexes into signaling-active lipid rafts. J. Immunol. 2004;172:370–380. doi: 10.4049/jimmunol.172.1.370. [DOI] [PubMed] [Google Scholar]
- 76.Soldaini E., Wack A., D’Oro U., Nuti S., Ulivieri C., Baldari C.T., Abrignani S. T cell costimulation by the hepatitis C virus envelope protein E2 binding to CD81 is mediated by Lck. Eur. J. Immunol. 2003;33:455–464. doi: 10.1002/immu.200310021. [DOI] [PubMed] [Google Scholar]
- 77.Rhainds D., Bourgeois P., Bourret G., Huard K., Falstrault L., Brissette L. Localization and regulation of SR-BI in membrane rafts of HepG2 cells. J. Cell Sci. 2004;117:3095–3105. doi: 10.1242/jcs.01182. [DOI] [PubMed] [Google Scholar]
- 78.Charrin S., Manie S., Thiele C., Billard M., Gerlier D., Boucheix C., Rubinstein E. A physical and functional link between cholesterol and tetraspanins. Eur. J. Immunol. 2003;33:2479–2489. doi: 10.1002/eji.200323884. [DOI] [PubMed] [Google Scholar]
- 79.Lavillette D., Bartosch B., Nourrisson D., Verney G., Cosset F.L., Penin F., Pecheur E.I. Hepatitis C virus glycoproteins mediate low pH-dependent membrane fusion with liposomes. J. Biol. Chem. 2006;281:3909–3917. doi: 10.1074/jbc.M509747200. [DOI] [PubMed] [Google Scholar]
- 80.Sainz B., Jr., Barretto N., Martin D.N., Hiraga N., Imamura M., Hussain S., Marsh K.A., Yu X., Chayama K., Alrefai W.A., et al. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat. Med. 2012;18:281–285. doi: 10.1038/nm.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ujino S., Nishitsuji H., Hishiki T., Sugiyama K., Takaku H., Shimotohno K. Hepatitis C virus utilizes VLDLR as a novel entry pathway. Proc. Natl. Acad. Sci. USA. 2016;113:188–193. doi: 10.1073/pnas.1506524113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nimpf J., Schneider W.J. From cholesterol transport to signal transduction: Low density lipoprotein receptor, very low density lipoprotein receptor, and apolipoprotein E receptor-2. Biochim. Biophys. Acta. 2000;1529:287–298. doi: 10.1016/S1388-1981(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 83.Aizaki H., Lee K.J., Sung V.M., Ishiko H., Lai M.M. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology. 2004;324:450–461. doi: 10.1016/j.virol.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 84.Ye J., Wang C., Sumpter R., Jr., Brown M.S., Goldstein J.L., Gale M., Jr. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc. Natl. Acad. Sci. USA. 2003;100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ogawa K., Hishiki T., Shimizu Y., Funami K., Sugiyama K., Miyanari Y., Shimotohno K. Hepatitis C virus utilizes lipid droplet for production of infectious virus. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009;85:217–228. doi: 10.2183/pjab.85.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ikeda M., Abe K., Yamada M., Dansako H., Naka K., Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117–125. doi: 10.1002/hep.21232. [DOI] [PubMed] [Google Scholar]
- 87.Felmlee D.J., Hafirassou M.L., Lefevre M., Baumert T.F., Schuster C. Hepatitis C virus, cholesterol and lipoproteins--impact for the viral life cycle and pathogenesis of liver disease. Viruses. 2013;5:1292–1324. doi: 10.3390/v5051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fafi-Kremer S., Habersetzer F., Baumert T.F. Hepatitis C virus entry and glucocorticosteroids. J. Hepatol. 2010;53:1148–1150. doi: 10.1016/j.jhep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 89.Moriishi K., Matsuura Y. Exploitation of lipid components by viral and host proteins for hepatitis C virus infection. Front. Microbiol. 2012;3:54. doi: 10.3389/fmicb.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farfan-Morales C.N., Cordero-Rivera C.D., Reyes-Ruiz J.M., Hurtado-Monzon A.M., Osuna-Ramos J.F., Gonzalez-Gonzalez A.M., De Jesus-Gonzalez L.A., Palacios-Rapalo S.N., Del Angel R.M. Anti-flavivirus Properties of Lipid-Lowering Drugs. Front. Physiol. 2021;12:749770. doi: 10.3389/fphys.2021.749770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chung C.S., Huang C.Y., Chang W. Vaccinia virus penetration requires cholesterol and results in specific viral envelope proteins associated with lipid rafts. J. Virol. 2005;79:1623–1634. doi: 10.1128/JVI.79.3.1623-1634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Danthi P., Chow M. Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry. J. Virol. 2004;78:33–41. doi: 10.1128/JVI.78.1.33-41.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang H., Li Y., Sadaoka T., Tang H., Yamamoto T., Yamanishi K., Mori Y. Human herpesvirus 6 envelope cholesterol is required for virus entry. J. Gen. Virol. 2006;87:277–285. doi: 10.1099/vir.0.81551-0. [DOI] [PubMed] [Google Scholar]
- 94.Nayak D.P., Barman S. Role of lipid rafts in virus assembly and budding. Adv. Virus Res. 2002;58:1–28. doi: 10.1016/s0065-3527(02)58001-5. [DOI] [PubMed] [Google Scholar]
- 95.Sun X., Whittaker G.R. Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 2003;77:12543–12551. doi: 10.1128/JVI.77.23.12543-12551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wielgosz M.M., Rauch D.A., Jones K.S., Ruscetti F.W., Ratner L. Cholesterol dependence of HTLV-I infection. AIDS Res. Hum. Retrovir. 2005;21:43–50. doi: 10.1089/aid.2005.21.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osuna-Ramos J.F., Reyes-Ruiz J.M., Del Angel R.M. The Role of Host Cholesterol During Flavivirus Infection. Front. Cell. Infect. Microbiol. 2018;8:388. doi: 10.3389/fcimb.2018.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferri N., Corsini A. Clinical Pharmacology of Statins: An Update. Curr. Atheroscler. Rep. 2020;22:26. doi: 10.1007/s11883-020-00844-w. [DOI] [PubMed] [Google Scholar]
- 99.Sirtori C.R. The pharmacology of statins. Pharmacol. Res. 2014;88:3–11. doi: 10.1016/j.phrs.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 100.Rothwell C., Lebreton A., Young Ng C., Lim J.Y., Liu W., Vasudevan S., Labow M., Gu F., Gaither L.A. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. 2009;389:8–19. doi: 10.1016/j.virol.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 101.Martinez-Gutierrez M., Castellanos J.E., Gallego-Gomez J.C. Statins reduce dengue virus production via decreased virion assembly. Intervirology. 2011;54:202–216. doi: 10.1159/000321892. [DOI] [PubMed] [Google Scholar]
- 102.Martinez-Gutierrez M., Correa-Londono L.A., Castellanos J.E., Gallego-Gomez J.C., Osorio J.E. Lovastatin delays infection and increases survival rates in AG129 mice infected with dengue virus serotype 2. PLoS ONE. 2014;9:e87412. doi: 10.1371/journal.pone.0087412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Soto-Acosta R., Mosso C., Cervantes-Salazar M., Puerta-Guardo H., Medina F., Favari L., Ludert J.E., del Angel R.M. The increase in cholesterol levels at early stages after dengue virus infection correlates with an augment in LDL particle uptake and HMG-CoA reductase activity. Virology. 2013;442:132–147. doi: 10.1016/j.virol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 104.Bryan-Marrugo O.L., Arellanos-Soto D., Rojas-Martinez A., Barrera-Saldana H., Ramos-Jimenez J., Vidaltamayo R., Rivas-Estilla A.M. The antidengue virus properties of statins may be associated with alterations in the cellular antiviral profile expression. Mol. Med. Rep. 2016;14:2155–2163. doi: 10.3892/mmr.2016.5519. [DOI] [PubMed] [Google Scholar]
- 105.Whitehorn J., Nguyen C.V.V., Khanh L.P., Kien D.T.H., Quyen N.T.H., Tran N.T.T., Hang N.T., Truong N.T., Hue Tai L.T., Cam Huong N.T., et al. Lovastatin for the Treatment of Adult Patients With Dengue: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2016;62:468–476. doi: 10.1093/cid/civ949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bailey C.J. Metformin: Historical overview. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 107.Crofford O.B. Metformin. N. Engl. J. Med. 1995;333:588–589. doi: 10.1056/NEJM199508313330910. [DOI] [PubMed] [Google Scholar]
- 108.Foretz M., Viollet B. Regulation of hepatic metabolism by AMPK. J. Hepatol. 2011;54:827–829. doi: 10.1016/j.jhep.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 109.Soto-Acosta R., Bautista-Carbajal P., Cervantes-Salazar M., Angel-Ambrocio A.H., Del Angel R.M. DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: A potential antiviral target. PLoS Pathog. 2017;13:e1006257. doi: 10.1371/journal.ppat.1006257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee M.S., Kim D., Jo K., Hwang J.K. Nordihydroguaiaretic acid protects against high-fat diet-induced fatty liver by activating AMP-activated protein kinase in obese mice. Biochem. Biophys. Res. Commun. 2010;401:92–97. doi: 10.1016/j.bbrc.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 111.Soto-Acosta R., Bautista-Carbajal P., Syed G.H., Siddiqui A., Del Angel R.M. Nordihydroguaiaretic acid (NDGA) inhibits replication and viral morphogenesis of dengue virus. Antivir. Res. 2014;109:132–140. doi: 10.1016/j.antiviral.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 112.Merino-Ramos T., Jimenez de Oya N., Saiz J.C., Martin-Acebes M.A. Antiviral Activity of Nordihydroguaiaretic Acid and Its Derivative Tetra-O-Methyl Nordihydroguaiaretic Acid against West Nile Virus and Zika Virus. Antimicrob. Agents Chemother. 2017;61:e00376-17. doi: 10.1128/AAC.00376-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Esquejo R.M., Salatto C.T., Delmore J., Albuquerque B., Reyes A., Shi Y., Moccia R., Cokorinos E., Peloquin M., Monetti M., et al. Activation of Liver AMPK with PF-06409577 Corrects NAFLD and Lowers Cholesterol in Rodent and Primate Preclinical Models. EBioMedicine. 2018;31:122–132. doi: 10.1016/j.ebiom.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cameron K.O., Kung D.W., Kalgutkar A.S., Kurumbail R.G., Miller R., Salatto C.T., Ward J., Withka J.M., Bhattacharya S.K., Boehm M., et al. Discovery and Preclinical Characterization of 6-Chloro-5-[4-(1-hydroxycyclobutyl)phenyl]-1H-indole-3-carboxylic Acid (PF-06409577), a Direct Activator of Adenosine Monophosphate-activated Protein Kinase (AMPK), for the Potential Treatment of Diabetic Nephropathy. J. Med. Chem. 2016;59:8068–8081. doi: 10.1021/acs.jmedchem.6b00866. [DOI] [PubMed] [Google Scholar]
- 115.Jimenez de Oya N., Blazquez A.B., Casas J., Saiz J.C., Martin-Acebes M.A. Direct Activation of Adenosine Monophosphate-Activated Protein Kinase (AMPK) by PF-06409577 Inhibits Flavivirus Infection through Modification of Host Cell Lipid Metabolism. Antimicrob. Agents Chemother. 2018;62:e00360-18. doi: 10.1128/AAC.00360-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kapadia S.B., Chisari F.V. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc. Natl. Acad. Sci. USA. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu J.W., Sun L.J., Zhao Y.H., Kang P., Yan B.Z. The effect of metformin on the efficacy of antiviral therapy in patients with genotype 1 chronic hepatitis C and insulin resistance. Int. J. Infect. Dis. 2012;16:e436–e441. doi: 10.1016/j.ijid.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 118.Lin Y.L., Shiao M.S., Mettling C., Chou C.K. Cholesterol requirement of hepatitis B surface antigen (HBsAg) secretion. Virology. 2003;314:253–260. doi: 10.1016/S0042-6822(03)00403-3. [DOI] [PubMed] [Google Scholar]
- 119.Bader T., Korba B. Simvastatin potentiates the anti-hepatitis B virus activity of FDA-approved nucleoside analogue inhibitors in vitro. Antivir. Res. 2010;86:241–245. doi: 10.1016/j.antiviral.2010.02.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsan Y.T., Lee C.H., Wang J.D., Chen P.C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J. Clin. Oncol. 2012;30:623–630. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 121.Horie M., Tsuchiya Y., Hayashi M., Iida Y., Iwasawa Y., Nagata Y., Sawasaki Y., Fukuzumi H., Kitani K., Kamei T. NB-598: A potent competitive inhibitor of squalene epoxidase. J. Biol. Chem. 1990;265:18075–18078. doi: 10.1016/S0021-9258(17)44716-8. [DOI] [PubMed] [Google Scholar]
- 122.Xun Y.H., Zhang Y.J., Pan Q.C., Mao R.C., Qin Y.L., Liu H.Y., Zhang Y.M., Yu Y.S., Tang Z.H., Lu M.J., et al. Metformin inhibits hepatitis B virus protein production and replication in human hepatoma cells. J. Viral Hepat. 2014;21:597–603. doi: 10.1111/jvh.12187. [DOI] [PubMed] [Google Scholar]
- 123.Lin S.H., Huang K.J., Weng C.F., Shiuan D. Exploration of natural product ingredients as inhibitors of human HMG-CoA reductase through structure-based virtual screening. Drug. Des. Dev. Ther. 2015;9:3313–3324. doi: 10.2147/DDDT.S84641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marahatha R., Basnet S., Bhattarai B.R., Budhathoki P., Aryal B., Adhikari B., Lamichhane G., Poudel D.K., Parajuli N. Potential natural inhibitors of xanthine oxidase and HMG-CoA reductase in cholesterol regulation: In silico analysis. BMC Complement. Med. Ther. 2021;21:1. doi: 10.1186/s12906-020-03162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Islam B., Sharma C., Adem A., Aburawi E., Ojha S. Insight into the mechanism of polyphenols on the activity of HMGR by molecular docking. Drug Des. Dev. Ther. 2015;9:4943–4951. doi: 10.2147/DDDT.S86705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suganya S., Nandagopal B., Anbarasu A. Natural Inhibitors of HMG-CoA Reductase-An Insilico Approach Through Molecular Docking and Simulation Studies. J. Cell. Biochem. 2017;118:52–57. doi: 10.1002/jcb.25608. [DOI] [PubMed] [Google Scholar]
- 127.Yang C., Pan X., Xu X., Cheng C., Huang Y., Li L., Jiang S., Xu W., Xiao G., Liu S. Salvianolic acid C potently inhibits SARS-CoV-2 infection by blocking the formation of six-helix bundle core of spike protein. Signal Transduct. Target. Ther. 2020;5:220. doi: 10.1038/s41392-020-00325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Colpitts C.C., Schang L.M., Rachmawati H., Frentzen A., Pfaender S., Behrendt P., Brown R.J., Bankwitz D., Steinmann J., Ott M., et al. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut. 2014;63:1137–1149. doi: 10.1136/gutjnl-2012-304299. [DOI] [PubMed] [Google Scholar]
- 129.Thongsri P., Pewkliang Y., Borwornpinyo S., Wongkajornsilp A., Hongeng S., Sa-Ngiamsuntorn K. Curcumin inhibited hepatitis B viral entry through NTCP binding. Sci. Rep. 2021;11:19125. doi: 10.1038/s41598-021-98243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Padilla S.L., Rodriguez A., Gonzales M.M., Gallego G.J., Castano O.J. Inhibitory effects of curcumin on dengue virus type 2-infected cells in vitro. Arch. Virol. 2014;159:573–579. doi: 10.1007/s00705-013-1849-6. [DOI] [PubMed] [Google Scholar]
- 131.Mounce B.C., Cesaro T., Carrau L., Vallet T., Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017;142:148–157. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 132.Mahdavi A., Bagherniya M., Fakheran O., Reiner Z., Xu S., Sahebkar A. Medicinal plants and bioactive natural compounds as inhibitors of HMG-CoA reductase: A literature review. Biofactors. 2020;46:906–926. doi: 10.1002/biof.1684. [DOI] [PubMed] [Google Scholar]
- 133.Shin S.K., Ha T.Y., McGregor R.A., Choi M.S. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 2011;55:1829–1840. doi: 10.1002/mnfr.201100440. [DOI] [PubMed] [Google Scholar]
- 134.Cho I.J., Ahn J.Y., Kim S., Choi M.S., Ha T.Y. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem. Biophys. Res. Commun. 2008;367:190–194. doi: 10.1016/j.bbrc.2007.12.140. [DOI] [PubMed] [Google Scholar]
- 135.Lin H.C., Chen Y.F., Hsu W.H., Yang C.W., Kao C.H., Tsai T.F. Resveratrol helps recovery from fatty liver and protects against hepatocellular carcinoma induced by hepatitis B virus X protein in a mouse model. Cancer Prev. Res. (Phila) 2012;5:952–962. doi: 10.1158/1940-6207.CAPR-12-0001. [DOI] [PubMed] [Google Scholar]
- 136.Lee J.S., Jeon S.M., Park E.M., Huh T.L., Kwon O.S., Lee M.K., Choi M.S. Cinnamate supplementation enhances hepatic lipid metabolism and antioxidant defense systems in high cholesterol-fed rats. J. Med. Food. 2003;6:183–191. doi: 10.1089/10966200360716599. [DOI] [PubMed] [Google Scholar]
- 137.Amano R., Yamashita A., Kasai H., Hori T., Miyasato S., Saito S., Yokoe H., Takahashi K., Tanaka T., Otoguro T., et al. Cinnamic acid derivatives inhibit hepatitis C virus replication via the induction of oxidative stress. Antivir. Res. 2017;145:123–130. doi: 10.1016/j.antiviral.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 138.Wu N., Sarna L.K., Siow Y.L., Karmin O. Regulation of hepatic cholesterol biosynthesis by berberine during hyperhomocysteinemia. Am. J. Physiol. Integr. Comp. Physiol. 2011;300:R635–R643. doi: 10.1152/ajpregu.00441.2010. [DOI] [PubMed] [Google Scholar]
- 139.Chang X.X., Yan H.M., Xu Q., Xia M.F., Bian H., Zhu T.F., Gao X. The effects of berberine on hyperhomocysteinemia and hyperlipidemia in rats fed with a long-term high-fat diet. Lipids Health Dis. 2012;11:86. doi: 10.1186/1476-511X-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hsieh Y.L., Yeh Y.H., Lee Y.T., Hsieh C.H. Ameliorative effect of Pracparatum mungo extract on high cholesterol diets in hamsters. Food Funct. 2014;5:149–157. doi: 10.1039/C3FO60445D. [DOI] [PubMed] [Google Scholar]
- 141.Hung T.C., Jassey A., Liu C.H., Lin C.J., Lin C.C., Wong S.H., Wang J.Y., Yen M.H., Lin L.T. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine. 2019;53:62–69. doi: 10.1016/j.phymed.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 142.Pope L.E., Marcelletti J.F., Katz L.R., Lin J.Y., Katz D.H., Parish M.L., Spear P.G. The anti-herpes simplex virus activity of n-docosanol includes inhibition of the viral entry process. Antivir. Res. 1998;40:85–94. doi: 10.1016/S0166-3542(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 143.Wang X., Tanaka N., Hu X., Kimura T., Lu Y., Jia F., Sato Y., Nakayama J., Moriya K., Koike K., et al. A high-cholesterol diet promotes steatohepatitis and liver tumorigenesis in HCV core gene transgenic mice. Arch. Toxicol. 2019;93:1713–1725. doi: 10.1007/s00204-019-02440-7. [DOI] [PubMed] [Google Scholar]
- 144.Lim T., Ryu J., Lee K., Park S.Y., Hwang K.T. Protective Effects of Black Raspberry (Rubus occidentalis) Extract against Hypercholesterolemia and Hepatic Inflammation in Rats Fed High-Fat and High-Choline Diets. Nutrients. 2020;12:2448. doi: 10.3390/nu12082448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ojeda-Granados C., Panduro A., Gonzalez-Aldaco K., Rivera-Iniguez I., Campos-Medina L., Roman S. Adherence to a Fish-Rich Dietary Pattern Is Associated with Chronic Hepatitis C Patients Showing Low Viral Load: Implications for Nutritional Management. Nutrients. 2021;13:3337. doi: 10.3390/nu13103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Klimek M., Wang S., Ogunkanmi A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. Pharm. Ther. 2009;34:313–327. [PMC free article] [PubMed] [Google Scholar]
- 147.Sun J.M., Kim S.J., Kim G.W., Rhee J.K., Kim N.D., Jung H., Jeun J., Lee S.H., Han S.H., Shin C.S., et al. Inhibition of hepatitis C virus replication by Monascus pigment derivatives that interfere with viral RNA polymerase activity and the mevalonate biosynthesis pathway. J. Antimicrob. Chemother. 2012;67:49–58. doi: 10.1093/jac/dkr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Loubser L., Weider K.I., Drake S.M. Acute liver injury induced by red yeast rice supplement. BMJ Case Rep. 2019;12:e227961. doi: 10.1136/bcr-2018-227961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review paper and there are no raw data.