Abstract
Michelia formosana (Kanehira) Masamune is a broad-leaved species widespread in East Asia; the wood extract and its constituents possess antifungal activity against wood-decay fungi. Antifungal activities of leaf essential oil and its constituents from M. formosana were investigated in the present study. Bioassay-guided isolation was applied to isolate the phytochemicals from leaf essential oil. 1D and 2D NMR, FTIR, and MS spectroscopic analyses were applied to elucidate the chemical structures of isolated compounds. Leaf essential oil displayed antifungal activity against wood decay fungi and was further separated into 11 fractions by column chromatography. Four sesquiterpenoids were isolated and identified from the active fractions of leaf essential oil through bioassay-guided isolation. Among these sesquiterpenoids, guaiol, bulnesol, and β-elemol have higher antifungal activity against brown-rot fungus Laetiporus sulphureus and white-rot fungus Lenzites betulina. Leaf essential oil and active compounds showed better antifungal activity against L. sulphureus than against L. betulina. The molecular structure of active sesquiterpenoids all contain the hydroxyisopropyl group. Antifungal sesquiterpenoids from M. formosana leaf essential oil show potential as natural fungicides for decay control of lignocellulosic materials.
Keywords: antifungal activity, Michelia formosana, sesquiterpenoids, wood-rotting fungi
1. Introduction
Lignocellulosic materials are organic polymeric biomaterials mainly composed of cellulose, hemicellulose, and lignin. They are easily degraded by biotic factors [1,2,3,4]. Biodegradation of lignocellulosic materials is a crucial issue for its utilization and product life cycle. Among the biodegradation of lignocellulosic materials, decay fungi cause the greatest financial losses of forest products; decay fungi include brown-rot fungi, white-rot fungi, and soft-rot fungi [3,4,5,6,7]. Traditionally, wood preservatives were applied to prevent the biodegradation of lignocellulosic materials, and most commercial preservatives are inorganic metal-containing agents. However, due to a growing focus on environmental consciousness, some highly toxic preservatives have been phased out and restricted from the global market [3,8,9].
Research and development in eco-benign fungicides for lignocellulosic materials are essential to achieve the optimal utilization of the lignocellulosic resources [10,11,12]. Many plant natural products have been proven to possess effective antifungal properties, including hinokitiol, trans-cinnamaldehyde, liriodenine, thymol, carvacrol, etc. [13,14,15,16,17].
Michelia formosana (Kanehira) Masamune, Formosan Michelia, belonging to the family Magnoliaceae, is a broad-leaved tree distributed in East Asia. Ogura et al. analyzed the natural products of M. formosana root extract and isolated 10 sesquiterpene lactones, including michelenolide, micheliolide, compressanolide, dihydroreynosin, parthenolide, dihydroparthenolide, costunolide, lanuginolide, reynosin, and santamarine, and one alkaloid, liriodenine [18]; Wu et al. also isolated the alkaloid compound, liriodenine, from M. formosana wood extract [16]. M. formosana extracts and its constituents possess the versatile bioactivities, including antifungal, anti-inflammatory, cytotoxic activities, etc. [16,18,19]. The aims of this study were to investigate the antifungal activity of M. formosana leaf essential oil against wood-decay fungi and to isolate and identify the constituents which possess antifungal activity from leaf essential oil.
2. Results and Discussion
2.1. Antifungal Activities of M. formosana Leaf Essential Oil and Its Fractions
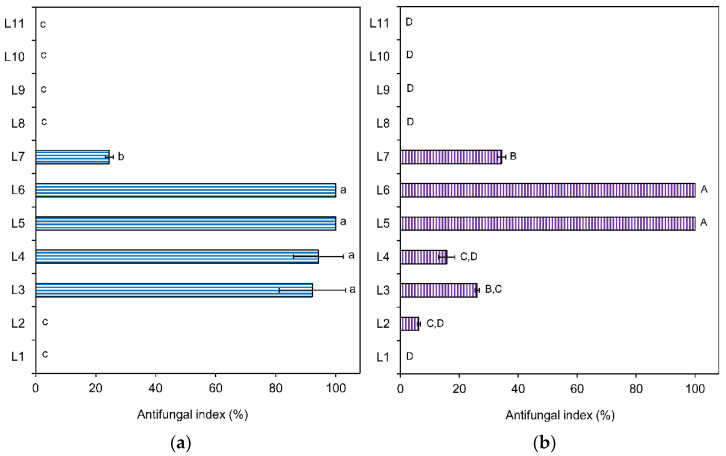
Brown-rot fungi selectively degrade polysaccharides, hemicellulose and cellulose, in wood and cause the oxidation of lignin; infected wood become a brownish color due to the high residual lignin. White-rot fungi degrade both lignin and cellulosic components of wood and change the color of wood to a little whitish [3,7]. Brown-rot fungus Laetiporus sulphureus (L. sulphureus) and white-rot fungus Lenzites betulina (L. betulina; Lenzites betulinus; Trametes betulina) are common fungal strains among the wood-rotting fungi [20,21]. Antifungal indexes of M. formosana leaf essential oil against fungi L. sulphureus and L. betulina were 100.00% and 94.19% at a concentration of 500 μg/mL, respectively, (Table 1); 67.44% and 25.97% at a concentration of 100 μg/mL. Leaf essential oil showed a better inhibition effect against brown-rot fungus L. sulphureus in comparison with white-rot fungus L. betulina. Antifungal activity of 11 fractions of leaf essential oil against wood-rotting fungi at a concentration of 200 μg/mL are shown in Figure 1. Fractions L5 and L6 had the highest antifungal activities with an antifungal index of 100%. The other fractions showed weak/no activity against examined wood-rotting fungi.
Table 1.
Antifungal index of M. formosana leaf essential oil against wood-rotting fungi.
| Fungus | Antifungal Index (%) | |
|---|---|---|
| 100 μg/mL | 500 μg/mL | |
| L. sulphureus | 67.44 ± 3.29 b | 100.00 ± 0.00 a |
| L. betulina | 25.97 ± 0.67 c | 94.19 ± 1.16 a |
Different letters (a–c) in the Table are statistically different at p < 0.05 according to the Scheffe test.
Figure 1.
Antifungal activities of 11 fractions from leaf essential oil against wood-rotting fungi at a concentration of 200 μg/mL. (a) L. sulphureus; (b) L. betulina. Different letters (a–c; A–D) in the Figure are statistically different at p < 0.05 according to the Scheffe test.
2.2. Isolation and Identification of Constituents from M. formosana Leaf Essential Oil
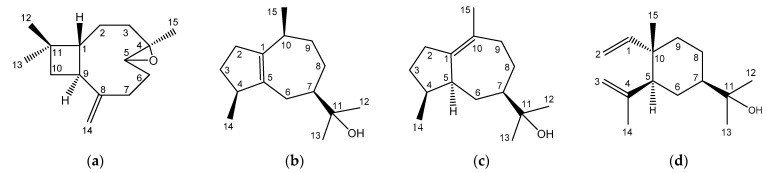
Four sesquiterpenoids including 4,5-epoxy-β-caryophyllene, guaiol, bulnesol, and β-elemol (Figure 2) were isolated from active fractions and identified by several spectral analyses. Guaiol and bulnesol were firstly identified from woody plant M. formosana. Through HPLC analysis, fraction L5 contained 5.62% 4,5-epoxy-β-caryophyllene and 54.78% guaiol, and fraction L6 contained 19.54% guaiol, 54.73% bulnesol, and 13.91% β-elemol.
Figure 2.
Chemical structures of sesquiterpenoids isolated from leaf essential oil. (a) 4,5-Epoxy-β-caryophyllene; (b) Guaiol; (c) Bulnesol; (d) β-Elemol.
4,5-Epoxy-β-caryophyllene: Colorless oil, EI-MS m/z: 79, 91, 105, 121, 145, 159, 173, 187, 202. Molecular formula: C15H24O. IR νmax cm−1: 2959 (C-H), 2920 (C-H), 1634 (C=C), 1458 (C-CH3) and 1383 (C-CH3). 1H NMR (CDCl3, 500 MHz): δ 0.94 (1H, m, H-3a), 0.98 (3H, s, H-12), 1.00 (3H, s, H-13), 1.20 (3H, s, H-15), 1.31 (1H, m, H-6a), 1.41 (1H, m, H-2a), 1.60 (1H, m, H-10a), 1.63 (1H, m, H-2b), 1.66 (1H, m, H-10b), 1.74 (1H, t, J = 10.0 Hz, H-1), 2.06 (1H, m, H-3b), 2.09 (1H, m, H-7a), 2.23 (1H, m, H-6b), 2.32 (1H, m, H-7b), 2.58 (1H, dt, J = 9.8, 9.2 Hz, H-9), 2.86 (1H, dd, J = 4.4, 10.8 Hz, H-5), 4.85 (1H, brs, H-14a), 4.97 (1H, brs, H-14b). 13C NMR (CDCl3, 125 MHz): δC 16.97 (t, C-15), 21.60 (q, C-13), 27.19 (t, C-2), 29.78 (t, C-7), 29.87 (q, C-12), 30.17 (t, C-6), 34.00 (s, C-11), 39.14 (t, C-3), 39.75 (t, C-10), 48.72 (d, C-9), 50.75 (d, C-1), 59.83 (s, C-4), 63.74 (d, C-5), 112.74 (q, C-14), 151.82 (s, C-8). 4,5-Epoxy-β-caryophyllene is a sesquiterpenoid with a structure based on the caryophyllane skeleton. NMR spectra were in agreement with the literature [22].
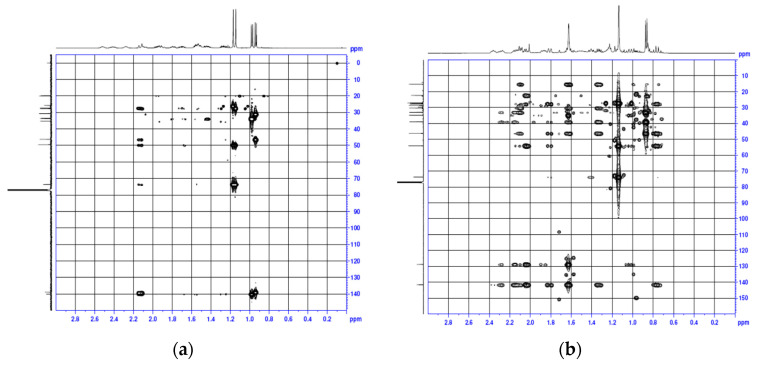
Guaiol: White needles, mp: 91–93 °C. EI-MS m/z: 79, 91, 105, 119, 133, 147, 161, 189, 204, 222 [M+]. Molecular formula: C15H26O. IR νmax cm−1: 3346 (OH), 2933 (C-H), 2856 (C-H), 1636 (C=C), 1458 (C-CH3), 1358 (C-CH3) and 918 (C-O). 1H NMR (CDCl3, 500 MHz): δ 0.91 (3H, s, H-14), 0.95 (3H, s, H-15), 1.11 (3H, s, H-12), 1.14 (3H, s, H-13), 1.25 (1H, m, H-3a), 1.42 (1H, m, H-8a), 1.51 (1H, m, H-7), 1.53 (1H, m, H-9a), 1.68 (1H, m, H-9b), 1.77 (1H, m, H-8b), 1.85 (1H, m, H-6a), 1.92 (1H, m, H-3b), 2.05 (1H, m, H-2a), 2.10 (1H, m, H-6b), 2.25 (1H, m, H-10), 2.38 (1H, m, H-2b), 2.49 (1H, m, H-4), 5.04 (1H, brs, -OH). 13C NMR (CDCl3, 125 MHz): δC 19.81 (q, C-15), 19.95 (q, C-14), 25.99 (q, C-12), 27.13 (t, C-8), 27.38 (q, C-13), 27.85 (t, C-6), 30.94 (t, C-3), 33.69 (d, C-10), 33.76 (t, C-9), 35.36 (t, C-2), 46.24 (d, C-4), 49.55 (d, C-7), 73.49 (s, C-11), 138.81 (s, C-5), 140.01 (s, C-1). Figure 3a is the HMBC spectrum of guaiol. Guaiol belongs to the guaiane skeleton which is a fused-bicyclic system with five- and seven-membered rings. NMR data of guaiol were in agreement with related literatures [23,24]. Guaiol has been reported to have antimicrobial and acaricidal activities [25].
Figure 3.
HMBC spectra of guaiol and bulnesol. (a) Guaiol; (b) Bulnesol.
Bulnesol (guai-1(10)-en-11-ol): Colorless oil, EI-MS m/z: 93, 105, 107, 119, 133, 161, 189, 204, 222 [M+]. Molecular formula: C15H26O. IR νmax cm−1: 3434 (OH), 2967 (C-H), 2933 (C-H), 1632 (C=C), 1458 (C-CH3) and 1370 (C-CH3). 1H NMR (CDCl3, 500 MHz): δ 0.77 (1H, dd, J = 11.7, 24.0 Hz, H-6a), 0.87 (3H, d, J = 7.0 Hz, H-14), 1.04 (1H, t, J = 11.0 Hz, H-8a), 1.14 (6H, s, H-12,13), 1.33 (1H, m, H-3a), 1.41 (1H, m, H-7), 1.61 (1H, m, H-3b), 1.63 (3H, s, H-15), 1.81 (1H, br.d, J = 12.5 Hz, H-6b), 1.87 (1H, m, H-8b), 2.05 (1H, m, H-9a), 2.11 (1H, m, H-4), 2.13 (1H, m, H-9b), 2.15 (1H, m, H-2a), 2.29 (1H, m, H-2b), 2.37 (1H, m, H-5). 13C NMR (CDCl3, 125 MHz): δC 15.29 (q, C-14), 22.29 (q, C-15), 27.07 (q, C-13), 27.16 (q, C-12), 27.67 (t, C-8), 28.67 (t, C-6), 30.28 (t, C-2), 32.99 (t, C-3), 34.81 (t, C-9), 38.97 (d, C-4), 46.23 (d, C-5), 54.06 (d, C-7), 73.75 (s, C-11), 128.80 (s, C-10), 141.61 (s, C-1). Figure 3b is the HMBC spectrum of bulnesol. Bulnesol also belongs to the guaiane skeleton. NMR spectra were consistent with those reported in the literature [23].
β-Elemol: Light yellow oil, EI-MS m/z: 79, 93, 105, 119, 133, 147, 161, 175, 189, 204. Molecular formula: C15H26O. IR νmax cm−1: 3424 (OH), 3083 (C=C-H), 2973 (C-H), 2936 (C-H), 2864 (C-H), 1636 (C=C), 1460 (C-CH3) and 1375 (C-CH3). 1H NMR (CDCl3, 500 MHz): δ 0.96 (3H, s, H-15), 1.18 (6H, s, H12, 13), 1.25 (1H, m, H-8a), 1.32 (1H, m, H-7), 1.40 (1H, m, H-6a), 1.42 (2H, m, H-9a, 9b), 1.56 (1H, m, H-6b), 1.63 (1H, m, H-8b), 1.69 (3H, brs, H-14), 1.94 (1H, dd, J = 12.0, 2.5 Hz, H-5), 4.56 (1H, brs, H-3a), 4.80 (1H, d, J = 1.5 Hz, H-3b), 4.86 (1H, dd, J = 11.0, 1.0 Hz, H-2 cis), 4.87 (1H, dd, J = 17.5, 1.0 Hz, H-2 trans), 5.78 (1H, dd, J = 17.5, 11.0 Hz, H-1). 13C NMR (CDCl3, 125 MHz): δC 16.57 (q, C-15), 22.53 (t, C-8), 24.77 (q, C-14), 27.13 (q, C-13), 27.15 (q, C-12), 28.47 (t, C-6), 39.69 (s, C-10), 39.85 (t, C-9), 49.32 (d, C-7), 52.68 (d, C-5), 72.75 (s, C-11), 109.88 (t, C-2), 112.03 (t, C-3), 147.89 (s, C-4), 150.22 (d, C-1). β-Elemol is an elemane-type skeleton sesquiterpenoid. NMR spectra were in agreement with those reported in the literature [23].
2.3. Antifungal Effect of Sesquiterpenoids from M. formosana Leaf Essential Oil
Antifungal activities of isolated sesquiterpenoids against wood-rotting fungi are presented in Table 2 below. 4,5-Epoxy-β-caryophyllene was not effective against both fungi; the other sesquiterpenoids possessed an inhibition effect with IC50 value less than 100 µg/mL. The compounds guaiol, bulnesol, and β-elemol showed better activities against L. sulphureus than against L. betulina comparing IC50 values of each specimen; the trend was similar to that of leaf essential oil, as described above. Among the three active sesquiterpenoids, bulnesol had the best inhibition effect with an IC50 value of 23.1 µg/mL (0.10 mM) against brown-rot fungus L. sulphureus. As for white-rot fungus L. betulina, guaiol and β-elemol were more active than bulnesol, with effective IC50 values of 44.1 µg/mL (0.20 mM) and 40.5 µg/mL (0.18 mM), which were lower than that of bulnesol (60.2 µg/mL; 0.27 mM). Active sesquiterpenoids belong to the guaiane and elemane-type skeletons; the molecular structure of these active compounds all contain the hydroxyisopropyl group.
Table 2.
IC50 values of compounds from leaf essential oil against wood-rotting fungi.
| Specimen | IC50 (μg/mL) | |
|---|---|---|
| L. Sulphureus | L. Betulina | |
| DDAC * | 0.37 ± 0.03 c ** (<0.01) *** |
3.24 ± 0.11 C
(0.01 ± 0.00) |
| 4,5-Epoxy-β-caryophyllene | >100 | >100 |
| Guaiol | 30.7 ± 2.8 a
(0.14 ± 0.01) |
44.1 ± 1.6 B
(0.20 ± 0.01) |
| Bulnesol | 23.1 ± 0.9 b
(0.10 ± 0.00) |
60.2 ± 2.6 A
(0.27 ± 0.01) |
| β-Elemol | 30.5 ± 2.3 a
(0.14 ± 0.01) |
40.5 ± 2.4 B
(0.18 ± 0.01) |
DDAC *: Positive control; **: Different letters (a–c; A–C) in the Table represent significantly different at the level of p < 0.05 according to Scheffe’s test; ***: (mM).
Gong et al. reported that garlic essential oil and its compounds diallyl disulfide and diallyl trisulfide showed high toxicity against brown-rot fungus L. sulphureus with IC50 values of 44.6, 73.2, and 31.6 µg/mL, respectively [26]. Cinnamaldehyde is a well-known natural antifungal agent; IC50 values of cinnamaldehyde were 0.17 and 0.65 mM against L. sulphureus and L. betulina, respectively [11]. Wu et al. investigated antifungal activity of sesquiterpenoids from Taiwania cryptomerioides heartwood essential oil and derivatives against wood-rotting fungi; active antifungal compounds were α-cadinol (0.13 mM), 3β-ethoxy-T-muurolol (0.15 mM), and 15-oxo-α-cadinol (0.20 mM) against white-rot fungus L. betulina [27]. Present results revealed that M. formosana leaf essential oil and the active sesquiterpenoids, guaiol, bulnesol, and β-elemol, exhibited potent antifungal activity against wood-rotting fungi.
3. Materials and Methods
3.1. Plant Materials
Leaves of Michelia formosana, around 70 years old, were collected from the Experimental Forest of National Taiwan University in Nantou County, Taiwan. The voucher specimen was deposited in the Lab of Chemical Utilization of Biomaterials, School of Forestry and Resource Conservation, National Taiwan University.
3.2. Hydrodistillation of Leaf Essential Oil
Fresh leaves (100 g) of M. formosana were hydrodistilled in a Clevenger-type apparatus (1 L) with 600 mL of distilled water for 8 h to obtain essential oil [28,29,30,31]. Yield of leaf essential oil was 0.87% (w/w). The obtained leaf essential oil was stored in dark glass vials at 4 °C until used.
3.3. Antifungal Assay
Antifungal activity of each specimen was evaluated by using the agar plate test. The wood-rotting fungi were brown-rot fungus Laetiporus sulphureus Karst. (BCRC 35305, L. sulphureus) and white-rot fungus Lenzites betulina Fr. (BCRC 35296, L. betulina) bought from Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan). Specimens were dissolved in 90 μL (1%) of ethanol, then added into 9 mL PDA (potato dextrose agar) and mixed well in a 60 mm Petri dish. After the agar became solid, mycelial plugs (5 mm in diameter) from the edges of the blank dish were incubated in the center of each plate and cultured at 26 °C and 70% RH for 8–12 days until the fungal mycelia covered the entire control dish (1% ethanol). All experiments were repeated in triplicate. Antifungal index was calculated as the following: Antifungal index (%) = (1 − Dt/Dc) × 100, where Dt is the diameter of growth zone in the test dish and Dc is the diameter of growth zone in the control dish. IC50 values, half maximal inhibitory concentration, of specimens were graphically obtained from the dose response curves derived from five concentrations [16,32,33]. The positive control, didecyl dimethyl ammonium chloride (DDAC), is a commercial fungicide used in wood preservatives.
3.4. Bioassay-Guided Isolation by Various Chromatographies
Leaf essential oil was subjected to silica gel column chromatography (CC) with a gradient elution of n-hexane and ethyl acetate of increasing polarity, then separated into 11 fractions (L1-L11) by thin layer chromatography (TLC). The yields of each fraction were 35.3% (L1, elution with 100% n-hexane), 5.5% (L2, elution with 3% ethyl acetate/97% n-hexane), 11.2% (L3, elution with 5% ethyl acetate/95% n-hexane), 7.6% (L4, elution with 10% ethyl acetate/90% n-hexane), 6.9% (L5, elution with 30% ethyl acetate/70% n-hexane), 28.7% (L6, elution with 50% ethyl acetate/50% n-hexane), 2.7% (L7, elution with 50% ethyl acetate/50% n-hexane), 0.9% (L8, elution with 100% ethyl acetate), 0.3% (L9, elution with 100% ethyl acetate), 0.3% (L10, elution with 100% ethyl acetate), and 0.6% (L11, elution with 100% ethyl acetate). Pure compounds were obtained from active fractions by high-performance liquid chromatography (HPLC, L-2130, Hitachi, Tokyo, Japan) with a preparative 9.4 × 250 mm Zorbax Sil column (5 μm). The isocratic mobile phase consisted of n-hexane (90%) and ethyl acetate (10%), at a flow rate of 2 mL/min; elution peaks were detected by the refractive index (RI) detector [34,35,36].
3.5. Structural Elucidation
The structural determination of isolated compounds was performed by spectral analyses, including 1D NMR (Nuclear magnetic resonance spectroscopy) (1H-NMR, 500 MHz; 13C-NMR, 125 MHz) and 2D NMR (HSQC, HMBC, COSY, and NOESY) measured on a Bruker AVIII NMR spectrometer (Bruker Avance, Rheinstetten, Germany), FTIR (Fourier transform infrared spectroscopy, FTS-40, Bio-rad, Hercules, CA, USA), and MS (mass spectroscopy, MAT-958, Finnigan, MA, USA) [37,38,39,40].
3.6. Statistical Analysis
The Scheffe multiple comparison test of the SAS 9.3 statistical program (Cary, NC, USA) was employed to evaluate differences for the antifungal assay. The confidence interval was set at 95%.
4. Conclusions
Antifungal activities of M. formosana leaf essential oil and its constituents against wood-rotting fungi were assessed in the present study. Antifungal indexes of leaf essential oil against brown-rot fungus L. sulphureus and white-rot fungus L. betulina were 100.00% and 94.19% at a concentration of 500 μg/mL, respectively. Through the bioassay guided isolation, four sesquiterpenoids, including 4,5-epoxy-β-caryophyllene, guaiol, bulnesol, and β-elemol, were obtained from active fractions of leaf essential oil. Among the examined sesquiterpenoids, guaiol, bulnesol, and β-elemol had the best inhibition effect against wood-rotting fungi. Results indicated these sesquiterpenoids from M. formosana leaf essential oil have promising potential as eco-benign fungicides for decay control of lignocellulosic materials.
Acknowledgments
The authors are grateful to the Experimental Forest of National Taiwan University (NTU), the NMR experiments of the Instrumentation Center at NTU, and the National Center for High-Performance Computing (NCHC) for the chemistry database search.
Author Contributions
Conceptualization, H.-T.C. and C.-C.W.; Methodology, S.-L.H., C.-H.K. and C.-C.W.; Software, C.-H.K. and S.-L.H.; Formal Analysis and Investigation, C.-C.W. and H.-T.C.; Writing—Original Draft Preparation, H.-T.C. and C.-H.K.; Writing—Review and Editing, H.-T.C. and C.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Montegut D., Indictor N., Koestler R.J. Fungal deterioration of cellulosic textiles: A review. Int. Biodeterior. 1991;28:209–226. doi: 10.1016/0265-3036(91)90043-Q. [DOI] [Google Scholar]
- 2.Martínez A.T., Speranza M., Ruiz-Dueñas F.J., Ferreira P., Camarero S., Guillén F., Martínez M.J., Gutiérrez A., del Río J.C. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 2005;8:195–204. [PubMed] [Google Scholar]
- 3.Shmulsky R., Jones P.D. Forest Products and Wood Science: An Introduction. 6th ed. Wiley-Blackwell; Ames, IA, USA: 2011. Durability and protection; pp. 229–252. [Google Scholar]
- 4.Aleinikovas M., Varnagirytė-Kabašinskienė I., Povilaitienė A., Šilinskas B., Škėma M., Beniušienė L. Resistance of wood treated with iron compounds against wood-destroying decay and mould fungi. Forests. 2021;12:645. doi: 10.3390/f12050645. [DOI] [Google Scholar]
- 5.Suzuki M.R., Hunt C.G., Houtman C.J., Dalebroux Z.D., Hammel K.E. Fungal hydroquinones contribute to brown rot of wood. Environ. Microbiol. 2006;8:2214–2223. doi: 10.1111/j.1462-2920.2006.01160.x. [DOI] [PubMed] [Google Scholar]
- 6.Bari E., Daniel G., Yilgor N., Kim J.S., Tajick-Ghanbary M.A., Singh A.P., Ribera J. Comparison of the decay behavior of two white-rot fungi in relation to wood type and exposure conditions. Microorganisms. 2020;8:1931. doi: 10.3390/microorganisms8121931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broda M. Natural compounds for wood protection against fungi—A review. Molecules. 2020;25:3538. doi: 10.3390/molecules25153538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preston A.F. Wood preservation: Trends of today that will influence the industry tomorrow. For. Prod. J. 2000;50:13–19. [Google Scholar]
- 9.Morais S., Fonseca H.M.A.C., Oliveira S.M.R., Oliveira H., Gupta V.K., Sharma B., de Lourdes Pereira M. Environmental and health hazards of chromated copper arsenate-treated wood: A review. Int. J. Environ. Res. Public Health. 2021;18:5518. doi: 10.3390/ijerph18115518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voda K., Boh B., Vrtacnik M., Pohleven F. Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot Trametes versicolor and the brown-rot Coniophora puteana. Int. Biodeter. Biodegrad. 2003;51:51–59. doi: 10.1016/S0964-8305(02)00075-6. [DOI] [Google Scholar]
- 11.Hsu F.L., Chang H.T., Chang S.T. Evaluation of antifungal properties of octyl gallate and its synergy with cinnamaldehyde. Bioresour. Technol. 2007;98:734–738. doi: 10.1016/j.biortech.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Vek V., Balzano A., Poljanšek I., Humar M., Oven P. Improving fungal decay resistance of less durable sapwood by impregnation with scots pine knotwood and black locust heartwood hydrophilic extractives with antifungal or antioxidant properties. Forests. 2020;11:1024. doi: 10.3390/f11091024. [DOI] [Google Scholar]
- 13.Baya M., Soulounganga P., Gelhaye E., Gerardin P. Fungicidal activity of beta-thujaplicin analogues. Pest Manag. Sci. 2001;57:833–838. doi: 10.1002/ps.379. [DOI] [PubMed] [Google Scholar]
- 14.Venalainen M., Harju A.M., Saranpaa P., Kainulainen P., Tiitta M., Velling P. The concentration of phenolics in brown-rot decay resistant and susceptible Scots pine heartwood. Wood Sci. Technol. 2004;38:109–118. doi: 10.1007/s00226-004-0226-8. [DOI] [Google Scholar]
- 15.Barrera-Necha L.L., Garduno-Pizana C., Garcia-Barrera L.J. In vitro antifungal activity of essential oils and their compounds on mycelial growth of Fusarium oxysporum f. sp. gladioli (Massey) Snyder and Hansen. Plant Pathol. J. 2009;8:17–21. doi: 10.3923/ppj.2009.17.21. [DOI] [Google Scholar]
- 16.Wu C.C., Wu C.L., Huang S.L., Chang H.T. Antifungal activity of liriodenine from Michelia formosana heartwood against wood-rotting fungi. Wood Sci. Technol. 2012;46:737–747. doi: 10.1007/s00226-011-0428-9. [DOI] [Google Scholar]
- 17.Passos J.L., Barbosa L.C.A., Demuner A.J., Alvarenga E.S., Silva C.M.d., Barreto R.W. Chemical characterization of volatile compounds of Lantana camara L. and L. radula Sw. and their antifungal activity. Molecules. 2012;17:11447–11455. doi: 10.3390/molecules171011447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogura M., Cordell G.A., Farnsworth N.R. Anticancer sesquiterpene lactones of Michelia compressa (Magnoliaceae) Phytochemistry. 1978;17:957–961. doi: 10.1016/S0031-9422(00)88656-2. [DOI] [Google Scholar]
- 19.Chan Y.Y., Juang S.H., Huang G.J., Liao Y.R., Chen Y.F., Wu C.C., Chang H.T., Wu T.S. The constituents of Michelia compressa var. formosana and their bioactivities. Int. J. Mol. Sci. 2014;15:10926–10935. doi: 10.3390/ijms150610926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girometta C.E., Bernicchia A., Baiguera R.M., Bracco F., Buratti S., Cartabia M., Picco A.M., Savino E. An Italian research culture collection of wood decay fungi. Diversity. 2020;12:58. doi: 10.3390/d12020058. [DOI] [Google Scholar]
- 21.Cartabia M., Girometta C.E., Milanese C., Baiguera R.M., Buratti S., Branciforti D.S., Vadivel D., Girella A., Babbini S., Savino E., et al. Collection and characterization of wood decay fungal strains for developing pure mycelium mats. J. Fungi. 2021;7:1008. doi: 10.3390/jof7121008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alejandro F.B., Molina J., Oltra J.E., Altarejos J., Barragán A., Lara A., Segura M. Stereochemistry of 14-hydroxy-β-caryophyllene and related compounds. Tetrahedron. 1995;51:3813–3822. [Google Scholar]
- 23.Faure R., Raharivelomanana P., Bianchini J.P., Cambon A., Azzaro M. Two-dimensional NMR of sesquiterpenes. 8-complete assignment of 1H and 13C NMR spectra of seven sequiterpene alcohols from Neocallitropsis pancheri. Magn. Reson. Chem. 1995;33:233–235. [Google Scholar]
- 24.Benovit S.C., Silva L.L., Salbego J., Loro V.L., Mallmann C.A., Baldisserotto B., Flores E.M., Heinzmann B.M. Anesthetic activity and bio-guided fractionation of the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. in silver catfish Rhamdia quelen. An. Acad. Bras. Cienc. 2015;87:1675–1689. doi: 10.1590/0001-3765201520140223. [DOI] [PubMed] [Google Scholar]
- 25.Sadgrove N.J., Senbill H., Van Wyk B.E., Greatrex B.W. New labdanes with antimicrobial and acaricidal activity: Terpenes of Callitris and Widdringtonia (Cupressaceae) Antibiotics. 2020;9:173. doi: 10.3390/antibiotics9040173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong X., Su X., Liu H. Diallyl trisulfide, the antifungal component of garlic essential oil and the bioactivity of its nanoemulsions formed by spontaneous emulsification. Molecules. 2021;26:7186. doi: 10.3390/molecules26237186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C.L., Chien S.C., Wang S.Y., Kuo Y.H., Chang S.T. Structure-activity relationships of cadinane-type sesquiterpene derivatives against wood-decay fungi. Holzforschung. 2005;59:620–627. doi: 10.1515/HF.2005.100. [DOI] [Google Scholar]
- 28.Chang H.T., Lin C.Y., Hsu L.S., Chang S.T. Thermal degradation of linalool-chemotype Cinnamomum osmophloeum leaf essential oil and its stabilization by microencapsulation with β-cyclodextrin. Molecules. 2021;26:409. doi: 10.3390/molecules26020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C.Y., Yeh T.F., Hsu F.L., Lin C.Y., Chang S.T., Chang H.T. Xanthine oxidase inhibitory activity and thermostability of cinnamaldehyde-chemotype leaf oil of Cinnamomum osmophloeum microencapsulated with β-cyclodextrin. Molecules. 2018;23:1107. doi: 10.3390/molecules23051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang H.T., Chang M.L., Chen Y.T., Chang S.T., Hsu F.L., Wu C.C., Ho C.K. Evaluation of motor coordination and antidepressant activities of Cinnamomum osmophloeum ct. linalool leaf oil in rodent model. Molecules. 2021;26:3037. doi: 10.3390/molecules26103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheljazkov V.D., Cantrell C.L., Semerdjieva I., Radoukova T., Stoyanova A., Maneva V., Kačániová M., Astatkie T., Borisova D., Dincheva I., et al. Essential oil composition and bioactivity of two Juniper species from Bulgaria and Slovakia. Molecules. 2021;26:3659. doi: 10.3390/molecules26123659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang H.T., Cheng Y.H., Wu C.L., Chang S.T., Chang T.T., Su Y.C. Antifungal activity of essential oil and its constituents from Calocedrus macrolepis var. formosana Florin leaf against plant pathogenic fungi. Bioresour. Technol. 2008;99:6266–6270. doi: 10.1016/j.biortech.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Merad N., Andreu V., Chaib S., de Carvalho Augusto R., Duval D., Bertrand C., Boumghar Y., Pichette A., Djabou N. Essential oils from two Apiaceae species as potential agents in organic crops protection. Antibiotics. 2021;10:636. doi: 10.3390/antibiotics10060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dymek A., Widelski J., Wojtanowski K.K., Vivcharenko V., Przekora A., Mroczek T. Fractionation of Lycopodiaceae alkaloids and evaluation of their anticholinesterase and cytotoxic activities. Molecules. 2021;26:6379. doi: 10.3390/molecules26216379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santonocito D., Granata G., Geraci C., Panico A., Siciliano E.A., Raciti G., Puglia C. Carob seeds: Food waste or source of bioactive compounds? Pharmaceutics. 2020;12:1090. doi: 10.3390/pharmaceutics12111090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen G.R., Chang M.L., Chang S.T., Ho Y.T., Chang H.T. Cytotoxicity and apoptosis induction of 6,7-dehydroroyleanone from Taiwania cryptomerioides bark essential oil in hepatocellular carcinoma cells. Pharmaceutics. 2022;14:351. doi: 10.3390/pharmaceutics14020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W.H., Chang S.T., Chang S.C., Chang H.T. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Nat. Prod. Res. 2008;22:1085–1093. doi: 10.1080/14786410802267510. [DOI] [PubMed] [Google Scholar]
- 38.Dziwornu G.A., Caira M.R., Mare J.A.d.l., Edkins A.L., Bolton J.J., Beukes D.R., Sunassee S.N. Isolation, characterization and antiproliferative activity of new metabolites from the South African endemic red algal species Laurencia alfredensis. Molecules. 2017;22:513. doi: 10.3390/molecules22040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvestre G.F.G., Lucena R.P., Oliveira G.D., Pereira H.N., Dias J.A.B., Souza I.A., Alves H.S. Anti-tumor and anti-inflammatory activity in vivo of Apodanthera congestiflora Cogn. (Cucurbitaceae) Pharmaceutics. 2021;13:743. doi: 10.3390/pharmaceutics13050743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C.Y., Liu I.H., Huang X.Z., Chen H.J., Chang S.T., Chang M.L., Ho Y.T., Chang H.T. Antimelanogenesis effects of leaf extract and phytochemicals from ceylon olive (Elaeocarpus serratus) in zebrafish model. Pharmaceutics. 2021;13:1059. doi: 10.3390/pharmaceutics13071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author on reasonable request.