Abstract
The current study was conducted to examine the in vitro anticancer potential of Cordia dichotoma (bark, leaves, pulp and seed). The plant material was collected from UT of J&K and methodical bioassays were carried out on ten human cancer cell lines (Michigan Cancer Foundation-7 (MCF-7), M.D. Anderson-Metastatic Breast (MDA-MB-231), Neuroblastoma-2a (N2A), SH-SY5Y, U-251, HCT-116, SW-620, A-549, MIA PaCa-2, Panc-1) from five different origins (breast, CNS, colon, lung, pancreas) respectively. Methanolic extracts were produced and fractions were then obtained from the extracts and evaluated for cytotoxicity. Mechanistic assays, HPLC, and GCMS profiling were performed on the highest active fraction. The Sulforhodamine B (SRB) assay determined the in vitro cytotoxicity. The findings revealed that the bark portion had in vitro cytotoxicity against the A-549 human lung cancer cell line. To our knowledge, this is the first study to show that the plant’s bark has anticancer properties and induced chromatin condensation, confirmed cell death via ROS generation, and significantly decreased colony formation in A-549 cell line from lung origin in a dose-dependent manner. Furthermore, HPLC and GCMS investigations indicated the presence of a number of bioactive molecules such as gallic acid (144,969.86) uV*sec, caffeic acid (104.26) uV*sec, ferulic acid (472.87) uV*sec, vanillic acid (13,775.39) uV*sec, palmitic acid (18.34%), cis vaccenic acid (28.81%), etc. and one of the compounds was reported for the first time from the bark. As a result of its promising efficacy, it may become an essential cancer chemopreventive or chemotherapeutic medication for patients with lung carcinoma.
Keywords: C. dichotoma, anticancer, apoptosis, ROS, HPLC, GCMS
1. Introduction
Cancer is one of the most persistent diseases in humans and there is a lot of scientific and economic attention in finding novel anticancer medicines in natural sources, including terrestrial and aquatic plants. Natural compounds originating from plants play an vital part in the expansion and marketing of pharmaceuticals [1,2]. The revelation of drugs from natural sources is associated with their ethno-pharmacological utilization and the heft of plant-inferred medicines are likewise connected to their conventional restorative use from quite some time ago [3,4]. Terpenoids, phenolics, flavonoids, and alkaloids are the principal types of plant-derived compounds with therapeutic effects [2]. These substances act on a variety of cellular components, causing alterations in the cell’s regular metabolism and growth. Although the majority of anticancer drugs isolated from different parts of the plants used in chemotherapy such as Vincristine and Vinblastime from Catharanthus roseus, Taxol from Taxus brevifolia, and camptothecin from Camptotheca acuminata [5,6,7,8] are recommended, which alter the basic biological processes [9,10] and impede nucleic acid synthesis, however the mechanisms of action differ significantly. A number of mechanisms [11,12], including mitochondrial pathways [13], can cause apoptosis. It is generally known that reactive oxygen species (ROS) may cause intracellular protein modification, lipid peroxidation, mitochondrial malfunction, and finally apoptosis [14,15]. ROS are important in cell signaling and the control of the primary apoptosis pathways mediated by mitochondria, death receptors, and the endoplasmic reticulum. Other controlled mechanisms of cell survival and death, such as autophagy and necroptosis, are also influenced by ROS [16]. The involvement of ROS in the intricate interaction and crosstalk between these diverse signaling pathways, in particular, will be investigated extensively in the coming years.
Cordia dichotoma (lasoda) is a member of the Boraginaceae family with around 2740 species divided into 148 genera and has been historically used to treat a number of human ailments in India. Cordia is a significant and prominent genus within this family. It is found in the Sub-Himalayan and outlying slopes of the Himalayas. It grows in a wide range of forests, from the Rajasthan to the India’s Western Ghats [17]. The species is common in the Philippines and can be found in thickets. The species is propagated by seeds. Leaves are elliptic-lanceolate to long ovate, simple, whole, and slightly dentate with a round and cordate base. The flowers have small bisexual stalks and vary in color from white to pink. The fruits with a sticky flesh mass are edible, immature fruits and are pickled/used as vegetables and the whole plant of C. dichotoma is edible/used as food [18]. According to ethnopharmacological studies, the plant has antimicrobial, antifungal [19], wound healing [20], antiinflammatory [21], and antibiofilm [22] properties.
The intention of this study was to determine the anticancer potential of different parts of C. dichotoma against ten human cancer cell lines from five different origins such as breast (MCF-7, MDA-MB-231), CNS (N2A, SH-SY5Y, U-251), colon (HCT-116, SW-620), lung (A-549), and pancreas (MIA PaCa-2, Panc-1), as well as to assess the potential for cellular oxidative stress at intracellular ROS levels, nuclear morphology, wound healing, and mitochondrial permeability. Furthermore, we investigated the bioactive compound responsible for the activity through GCMS and HPLC profiling.
2. Results
2.1. Screening for Cytotoxic Activity
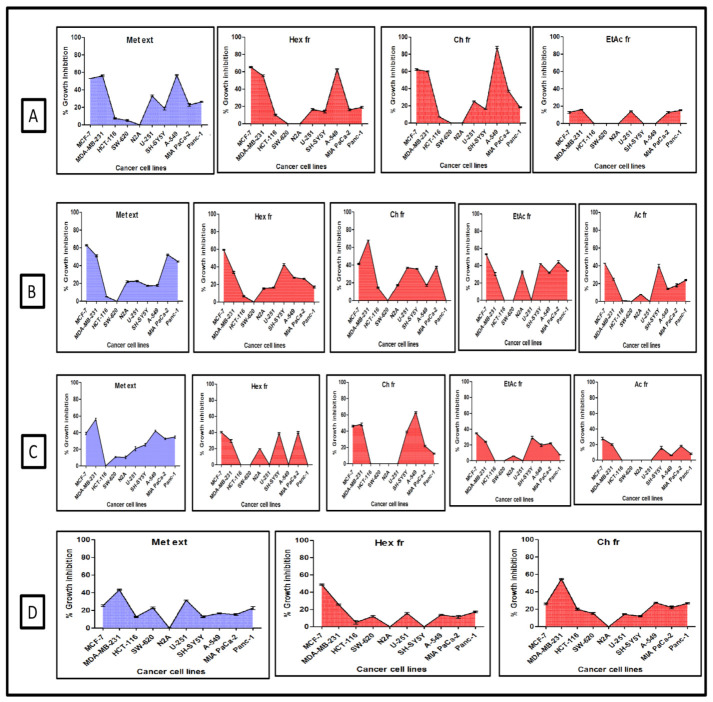
The SRB test was used to examine the cytotoxic impact of extracts and fractions from C. dichotoma bark, leaves, pulp, and seed on the development of various human cancer cell lines and the criteria for activity was a minimum of 70%. Figure 1 depicts the cell inhibitory activity of methanol extract and subsequent fractions (hexane, chloroform, ethyl acetate, and acetone).
Figure 1.
Graphs represent in vitro cytotoxic potential of different parts of C. dichotoma Bark (A), Leaves (B), Pulp (C), and Seed (D) extracts and fractions at concentration 100 μg/mL. Values are the mean ± SD (n = 3). Met ext (Methanolic extract), Hex fr (Hexane fraction), Ch fr (Chloroform fraction), EtAc fr (Ethyl acetate fraction), and Ac fr (Acetone fraction).
In the preliminary investigation of methanolic extract obtained from the bark of Cordia dichotoma, against ten human cancer cell lines (MCF-7, MDA-MB-231, N2A, SH-SY5Y, U-251, HCT-116, SW-620, A-549, MIA PaCa-2, Panc-1) from five different origins (breast, CNS, colon, lung, pancreas) respectively, the results showed growth inhibition in the range of 0–57%. However, the chloroform fraction obtained from the abovementioned extract showed remarkable results as a growth inhibition of 88% was found against the A-549 cell line from lung tissue, whereas the same fraction inhibited the human cancer cell lines used in the present study in the range of 0–62%. While evaluating the cytotoxic potential of other fractions, the growth inhibition in case of n-hexane fraction was observed in the range of 0–65%, and for ethyl acetate fraction the growth inhibition range was 0–16% as shown in (Figure 1A). The acetone fraction was completely considered as inactive as growth inhibition was found as 0%.
The lasoda leaf extract (methanolic) and fractions were evaluated at the concentration of 100 µg/mL against ten human cancer cell lines used in the present study. The growth inhibition by extract was found in the range of 0–63%, whereas in case of fractions, the growth inhibition was in the range of 0–59% in n-hexane, 0–68% in chloroform, 0–53% in ethyl acetate, and 0–43% in acetone fraction as depicted in Figure 1B.
The pulp part of the lasoda fruit was assessed at the same concentration against ten human cancer cell lines used in the present investigation. The overall growth inhibition was seen in the range of 0–56% in methanolic extract, 0–40% in n-hexane fraction, 0–63% in chloroform fraction, 0–34% in ethyl acetate fraction, and 0–27% in acetone fraction as shown in Figure 1C. So, the pulp part of the lasoda fruit was considered as non-significant. The observations produced by the methanolic extract and subsequent fractions from the seed part of C. dichotoma are shown in Figure 1D and the data represented that the growth inhibition by the extract was observed in the range of 0–43%. The growth inhibition in case fractions, namely n-hexane and chloroform, was observed in the range of 0–48% and 0–54% respectively, which is considered non-significant. The ethyl acetate and acetone fractions were considered purely inactive as growth inhibition was found as 0%.
The chloroform fraction was further evaluated at lower concentrations (50, 40, 30, 20, 10 µg/mL) against the A-549 (lung) cancer cell line. Table 1 depicts the IC50 value of chloroform fraction and standard drug.
Table 1.
In vitro cytotoxicity IC50 of the chloroform fraction of bark and standard drug against human lung cancer cell line (A-549).
| Cell Line | Chloroform Fraction of Bark IC50 (µg/mL) |
Standard Drug (Paclitaxel) IC50 (nM) |
|---|---|---|
| A-549 | 37.978 | 4.8 |
2.2. Mechanistic Assays
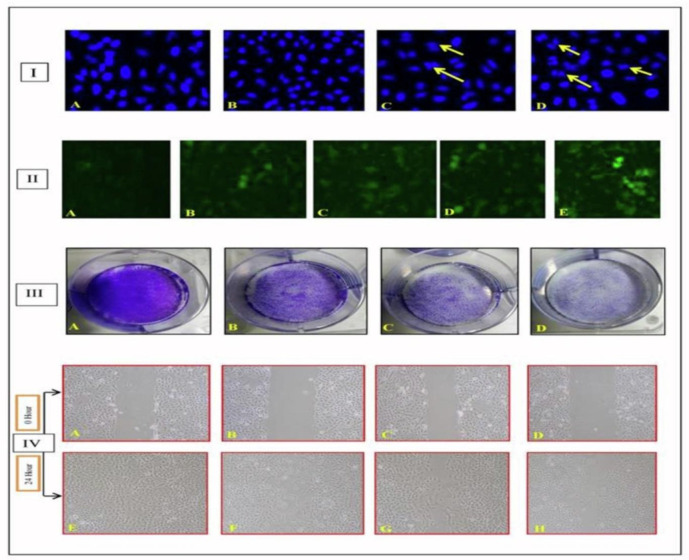
The chloroform fraction displayed a potent in vitro cytotoxic effect against human lung cancer cell line (A-549) with maximum growth inhibition and lower IC50 values. So, the fraction was chosen for several mechanistic experiments against the A-549 human lung cancer cell line as shown in Figure 2.
Figure 2.
Various mechanistic assays performed on human lung cancer cell line A-549. I. Nuclear morphological changes as indicated by arrow using DAPI staining with chloroform fraction of bark treatment. (A) Control (B) 50 µg/mL (C) 75 µg/mL and (D) 100 µg/mL II. Effect of chloroform fraction on A-549 in the production of reactive oxygen species evaluated by oxidation of DCFDA by hydrogen peroxide through fluoroscence microscopy at 24 h posttreatment. (A) Control (B) H2O2 0.5% (C) 50 µg/mL (D) 75 µg/mL (E) 100 µg/mL III. In vitro colony formation assay of bark chloroform fraction (A) Control (B) 50 µg/mL (C) 75 µg/mL and (D) 100 µg/mL IV. In vitro wound healing assay chloroform fraction. 0 h (A) Control (B) 50 µg/mL (C) 75 µg/mL and (D) 100 µg/mL. After 24 h (E) Control (F) 50 µg/mL (G) 75 µg/mL and (H) 100 µg/mL.
2.2.1. Chloroform Fraction Triggered Chromatin Condensation
The fluorescent dye DAPI (4,6-diamidino-2-phenylindole) binds to the A-T region of DNA’s minor groove. The activation of apoptosis results in morphological changes such as chromatin condensation, cell shrinkage, nuclear distortion, and the development of apoptotic bodies and their elimination by neighboring cells. After being treated with different concentrations of chloroform fraction, namely 50, 75, and 100 µg/mL, A-549 cells were stained with DAPI dye and studied under a fluorescence microscope. Untreated cells exhibited a constant appearance of blue nuclei, indicating that they were healthy. The chloroform fraction-treated cells, on the other hand, demonstrated dose-dependent chromatin condensation of cell nuclei as shown in Figure 2I. After a 24-h incubation period, the size and shape of cell nuclei in drug-treated cells seemed to change. The fraction induced DNA fragmentation as well as a decrease in the number of cell nuclei in a concentration-dependent manner, according to the findings.
2.2.2. ROS Production by the Chloroform Fraction Causes Cell Death
DCFDA (2,7-dichlorofluorescein diacetate), a non-fluorescent dye that becomes green in the presence of ROS, was used to detect its presence. It is a hydrogen peroxide-detecting probe used to assess hydrogen peroxide levels in intact cells. It easily penetrates across the plasma membrane and oxidizes in the presence of free radicals to generate DCF (dichlorofluorescein), a green fluoroscent byproduct that is excited at 495 nm and emits at 520 nm. When treated with DCFDA dye, the positive control (0.5% H2O2) emitted green fluorescence, which was then compared with drug-treated cells. The results indicate a substantial dose-dependent increase in the fluorescence of drug-treated (chloroform fraction) cells, demonstrating that ROS generation is required for cell death as shown in Figure 2II.
2.2.3. Effect of Fraction on Colonies Development
It is an in vitro cell existence test that is based on the ability of a solitary cell to become a colony. This test evaluates the ability of each cell in a population to divide forever and is used to determine the effectiveness of cytotoxic drugs. It assists in assessing if cells have retained the potential to generate a large number of progenies or have perished as a result of various pharmacological treatments. Before seeding in the appropriate dilution, A-549 cells were treated with chloroform fraction at concentrations of 50, 75, and 100 µg/mL. The chloroform fraction of bark inhibited colony development in A-549 cells in a dose-dependent manner, according to the findings. As indicated in Figure 2III, the colony formation of the control cells was higher than that of the drug-treated cells.
2.2.4. Inhibition of Cell Migration by Chloroform Fraction
An in vitro wound healing assay was performed to evaluate the effect of chloroform fraction on the migration of A-549 cells. Following the drug treatment, images of cells moving into the scratched region were captured. The results indicated that wound closure was clearly evident in untreated cells after 24 h due to cell migration and cell proliferation, but cell migration was hindered in chloroform fraction treated cells with an increasing concentration of 100 µg/mL, where the scratch produced was clearly visible. As a result of the foregoing findings, the antiproliferative impact of fraction treatment resulted in impaired cell migration shown in Figure 2IV.
2.3. Identification of Anticancer Molecules
Motivated by the cytotoxicity findings and mechanistic assays, we tried to identify the positive molecules responsible for anticancer activity by suitable characterization investigations such as high-performance liquid chromatography (HPLC) and gas chromatography mass spectrometry (GCMS).
2.3.1. High-Performance Liquid Chromatography
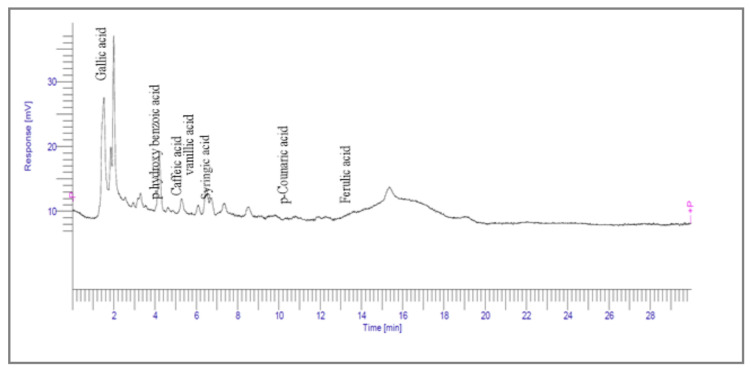
HPLC analysis was used to detect certain key pharmaceutical substances. Peaks were identified by comparing the retention period of the chloroform fraction of C. dichotoma bark with those of the reference substances. The generated peaks were proportioned to each constituent as shown in Figure 3.
Figure 3.
HPLC chromatogram of Chloroform fraction of bark of C. dichotoma.
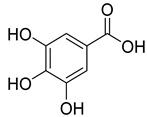
Phenolics are one of nature’s most diverse chemical families. Phenolic compounds are phytochemicals present in almost all plant tissues. They are secondary metabolites produced by the shikimic acid and phenylpropanoid pathways, respectively. They have a variety of bioactive characteristics [23]. HPLC quantification of the fraction identified the presence of gallic acid, syringic acid, p coumarin acid, caffeic acid, p-hydroxy benzoic acid, ferulic acid, and vanillic acid as depicted in (Table 2). Gallic acid and vanillic acid constituted the highest content observed with the areas of 144,969.86 and 13,775.39 uV*sec respectively.
Table 2.
HPLC analysis of chloroform fraction of bark of C. dichotoma.
| RT | Area (uV*sec) | Structure | Name |
|---|---|---|---|
| 1.988 | 144,969.86 |
|
Gallic acid |
| 4.842 | 2497.31 |
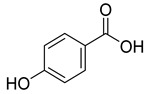
|
p-hydroxy benzoic acid |
| 5.908 | 104.26 |
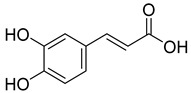
|
Caffeic acid |
| 6.093 | 13,775.39 |
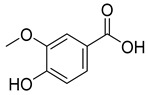
|
Vanillic acid |
| 7.130 | 2724.56 |
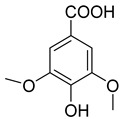
|
Syringic acid |
| 10.909 | 275.75 |
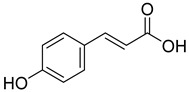
|
p-Counaric acid |
| 14.218 | 472.87 |
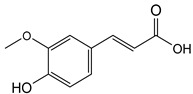
|
Ferulic acid |
2.3.2. Gas Chromatography Mass Spectroscopy Analysis
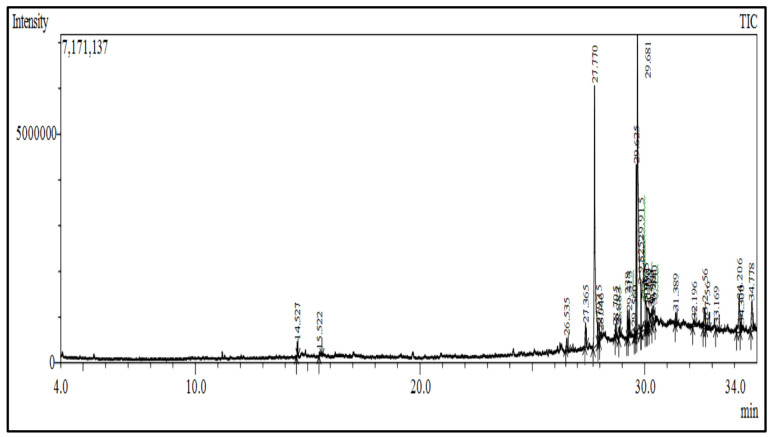
Chloroform fraction is composed of organic molecules that are volatile in nature, primarily fatty acids. Twenty six compounds were identified by GCMS as shown in the spectrum in Figure 4.
Figure 4.
GCMS chromatogram of the chloroform fraction of bark of C. dichotoma.
The compounds found in GCMS are presented in Table 3. Among all compounds, the highest percentage compound was cis-vaccenic acid followed by palmitic acid, palmitoleic acid, Octadecadienoic acid, and soon. Furthermore, to the best of our knowledge, it is the first time that cis-vaccenic acids, already described in other plants, were reported in the lasoda bark.
Table 3.
Compounds found in chloroform fraction of C. dichotoma bark by GC-MS.
| Compounds | Class of Compound | Area % | m/z | R. Time |
|---|---|---|---|---|
| 1,3-Di-tert-butyl benzene | Phenyl propanes | 0.68 | 175.10 | 14.527 |
| 4-Vinylguaiacol | Phenol | 0.51 | 150.15 | 15.522 |
| Diisobutyl phthalate | Ester | 0.83 | 149.05 | 26.535 |
| Methyl palmitate | Ester | 1.29 | 74.00 | 27.365 |
| Palmitic acid | Fatty acid | 18.34 | 73 | 27.770 |
| Benzylamine, 2-hydroxy-N,N-di[2-aminoethyl)amino]methyl)phenol | Phenol | 1.73 | 71.05 | 27.935 |
| 16-Hydroxyhexadecanoic acid | Fatty acid | 1.43 | 60.10 | 28.046 |
| Methyl linoleate | Fatty acid | 1.23 | 81.00 | 29.238 |
| Methyl cis-9,10-epoxystearate | Ester | 1.41 | 55.00 | 29.313 |
| Octadecadienoic acid | Fatty acids | 9.46 | 67.00 | 29.625 |
| cis-Vaccenic acid | Fatty acids | 28.81 | 55.00 | 29.681 |
| 1-Dimethyl(pentafluorophenyl)silyloxydodecane | NC | 1.82 | 79.05 | 29.825 |
| Palmitoleic acid | Fatty acids | 12.54 | 73.00 | 29.915 |
| 1-(beta.-d-Ribofuranosyl)-4-difluormethoxy-uracil | Dihydropyridine | 1.94 | 69.00 | 30.065 |
| Methylallyl Trifluoroacetate | Allyl acyls | 1.89 | 55.05 | 30.170 |
| Difluoroacetic acid | Monocarboxylic acid | 2.22 | 69.05 | 30.310 |
| Linoleic acid trimethylsilyl ester | Ester | 1.71 | 80.00 | 30.381 |
| (2-phenyl-1,3-dioxolan-4-yl)methyl (z)-octadec-9-enoate | NC | 0.85 | 73.00 | 30.440 |
| Tridecyl 2-Methoxyacetate | Ester | 0.70 | 57.00 | 31.389 |
| 6,9,12,15-Docosatetraenoic acid, methyl ester | Ester | 0.57 | 69.10 | 32.196 |
| Dotriacontane | Alkanes | 1.58 | 57.00 | 32.656 |
| (4-Allyloxy-3-methoxy-phenyl)-methanol | NC | 0.58 | 97.15 | 32.756 |
| 1-Propyltridecyl 4-bromobutanoate | Ester | 0.72 | 69.05 | 33.169 |
| 9-octylheptadecane | Alkanes | 3.39 | 57.00 | 34.206 |
| 1-[(1-Ethylundecyl)oxy]-1-methylsilinane | Phenylpyrazoles | 0.51 | 55.00 | 34.300 |
| Dioctyl phthalate | Benzoic acid | 2.66 | 148.95 | 34.778 |
NC—Not Classified.
3. Discussion
The burden of cancer rose to 18.1 million new cases and 9.6 million deaths in 2018 and with 36 different types, cancer mainly affects men in the form of colorectal, liver, lung, prostate, and stomach cancer and women in the form of breast, cervix, colorectal, lung, and thyroid cancer [24]. However, a projected 19.3 million new cancer cases and about 10.0 million cancer deaths occurred globally in 2020. With an anticipated 2.3 million new cases (11.7%), female breast cancer has surpassed lung cancer as the most often diagnosed malignancy, followed by lung (11.4%), colorectal (10.0%), prostate (7.3%), and stomach (5.6%). With a projected 1.8 million fatalities (18%), lung cancer remains the top cause of cancer mortality, followed by colorectal (9.4%), liver (8.3%), stomach (7.7%), and female breast (6.9%). Overall, the prevalence of both sexes was 2- to 3-fold greater in transitioned versus transitional nations, although death differed 2-fold for males and little for females [25], and treating cancer has become a whole new area of research. There are conventional as well as modern techniques applied against cancer and a variety of techniques like chemotherapy, radiation therapy, and surgery, which are used for treating the disease. However, all of them have some disadvantages [26] as the use of conventional chemicals bear side effects/toxicities [27], but as the problem persists, new approaches are needed for the control of disease, especially because of the failure of conventional chemotherapeutic approaches. Therefore, there is a need for new strategies for the prevention/cure of cancer to control the death rate and plant derived components has become a very safe, non-toxic and easily available source as botanicals are believed to neutralize the effects of diseases in a body because of their numerous medicinal properties [1,2,3,4]. In the present investigation, we evaluated the in vitro cytotoxic potential of Cordia dichotoma bark, leaves, pulp, and seed, collected from the Union Territory of Jammu & Kashmir. The extracts (methanolic) and subsequent fractions (n-hexane, chloroform, ethyl acetate, acetone, methanol soluble) were screened against ten human cancer cell lines (MCF-7, MDA-MB-231, N2A, SH-SY5Y, U-251, HCT-116, SW-620, A-549, MIA PaCa-2, Panc-1,) originated from five different tissues (breast, CNS, colon, lung, pancreas) respectively and the anticancer activity was produced by only chloroform fraction of bark as it fetched the IC50 value 37.978 µg/mL. The fraction was further used for mechanistic assays and it induced chromatin condensation, confirmed cell death via ROS generation, and significantly decreased colony formation in a dose-dependent manner in the A-549 cell line from lung origin.
Furthermore, when compared with the work of others, Hussain et al. [28] observed 69.4 and 72.1 percent growth inhibition in methanolic extract of bark against MDA-MB-231 and MCF-7 respectively. The methanolic extract of C. dichotoma leaves was tested for anticancer activity against human prostate carcinoma cell line (PC3) and human cervical cancer cell line (HeLa), and the results indicate that the extract exhibited good anti-cancerous activity against HeLa [29]. According to some publications, the antitumor and anticancer actions of C. dichotoma fruit pulp extract may be attributed to its antioxidant ability due to its high level of secondary metabolites [30]. Healthy cells maintain a correct equilibrium of ROS levels, but stress situations cause ROS levels to overwhelm the antioxidant capacity on defense, resulting in an oxidative stress state. Transcription factors play a significant part in the regulation of cellular growth cycle, tumor survival and angiogenesis [31]. The methanolic extract and aqueous portion of Cordia dichotoma seeds are cytotoxic to human cervical epitheloid (HeLa) [32]. The SRB test was used to assess the cytotoxic activity of Cordia myxa fruit extract and its micro and nano capsulated forms in vitro using THLE2 and HepG-2 cell lines. The fruit extract was more cytotoxic (IC50: 70 g/mL) than the micro and nanocapsules (IC50: 100 g/mL). The reduction in viability of hepatocellular carcinoma cells (HepG-2) treated with extract compared with treated normal cells (THLE2) demonstrated that the fruit extract was selective against only malignant cells. The extract inhibited cell proliferation less effectively against the HepG2 cell line. Further, micro and nanocapsules showed a very low cytotoxicity [33]. In our current study, the growth inhibition of various extracts and fractions deviated somewhat from that described in the literature. This might be due to regional differences, solvent polarities or extraction methods. The data regarding in vitro cytotoxicity of C. dichotoma bark against A-549, is not available in literature, made us unable to compare our results.
Our study further investigates the bioactive compound which might be responsible for the activity through phytochemical profiling via HPLC and GCMS. We investigate seven phenolic standards such as gallic acid, syringic acid, p-coumarin acid, caffeic acid, p-hydroxy benzoic acid, ferulic acid and vanillic acid. Ferulic acid, caffeic acid, gallic acid, and syringic acid have already been explored for their anticancer potential [34,35,36,37]. By regulating the JAK/STAT3 signaling pathway and downstream apoptotic molecules, some researchers revealed that GA had independent anticancer effects in non-small-cell lung cancer A549 cells and aided the anticancer effects of cisplatin. These findings might be used to justify more fundamental research and preclinical studies into GA’s anticancer properties and its auxiliary effects on cisplatin activity in non-small-cell lung cancer patients [38]. The results from the GCMS profiling depicted total 26 compounds. Some of these compounds such as palmitic acid, octadecadienoic acid, heptadecanoic acid, linoleic acid, cis vaccenic acid, and hexadecanoic acid have already been reported from bark extracts of other plant species, e.g., Quercus leucotrichophora [39]. Among the various compounds found, hexadeconic acid and vaccenic acid in particular have been investigated for their pharmacological potential, including anticancer [40,41] and anti-inflammatory properties [42]. Furthermore, the fraction’s activity might be attributed to the synergistic impact of these chemicals.
4. Materials and Methods
4.1. Chemicals and Reagents
Sigma Chemical Co. (St. Louis, MO, USA) provided the growth media for the cells, sulforhodamine B (SRB), crystal violet blue, Rhodamine 123, fetal bovine serum (FBS), and DCFDA (2,7-dichlorofluorescein diacetate). All additional chemicals were of analytical grade and obtained from standard sources.
4.2. Collection of Plant Material and Preparation of Extract and Fractions
A sufficient quantity of bark, leaves, and fruit of C. dichotoma was collected from district samba of UT J&K, India. To eliminate soil pollutants, the plant material was carefully rinsed under running tap water. The plant materials were chopped, dried in the shade, and powdered. The methanolic extract of the plant parts was obtained by impregnating the dried plant material in 95% methanol for 24 h with intermittent shaking. The mixture was filtered using filter paper and the residue was immersed in methanol for further 24 h. The filtrates were mixed and the mixture was concentrated to dryness under reduced pressure to produce a crude extract. Further fractionation of the crude (methanolic) extract was performed using n-hexane, chloroform, ethyl acetate and acetone solvents. All samples were concentrated and kept at −20 °C until further investigation.
4.3. Sample Preparation and Culturing of Cells
Stock solutions of 20 mg/mL were produced by dissolving the extract and subsequent fractions in DMSO. The human cancer cell lines A-549 (Lung), HCT-116 and SW-620 (Colon), MCF-7 and MDA-MB-231 (Breast), Mia PaCa-2 and Panc-1 (Pancreatic), SH-SY5Y, N2A, and U-251 (CNS) were acquired from the National Centre for Cell Science in Pune, India and the National Cancer Institute. These ten human cancer cell lines were developed and kept up with in RPMI-1640 media (A-549, SW-620, and MCF-7) and DMEM medium (HCT-116, MDA-MB-231, MIA PaCa-2, Panc-1, SH-SY5Y, N2A, and U-251).
4.4. SRB Assay for Cytotoxicity
The samples were examined for anticancer potential against ten distinct human cancer cell lines from five distinct origins: lung (A-549), colon (HCT-116, SW-620), breast (MCF-7, MDA-MB-231), pancreas (MIA PaCa-2 and Panc-1), and CNS (SHSY-5Y, U-251, and N2A). Cell suspensions of optimum cell density for A-549 (7500), HCT-116 (7000), SW-620 (13,000), MCF-7 (8000), MDA-MB-231 (8000), MIA PaCa-2 (10,000), Panc-1 (10,000), SH-SY5Y (15,000), U-251 (15,000), and N2A (10,000) were seeded in 96-well flat-bottom plates (NUNC). Cell suspension was added in an amount of 100 μL/well and the cells were allowed to proliferate for 24 h. Cells were subsequently grown for 48 h at 37 °C with test material (extract and fractions) including full growth medium (100 μL/well). Cell development was halted by carefully stacking 50 μL of chilled TCA in each well and incubating at 4 °C for one hour to fix the cells connected to the lower part of the wells. Each well’s liquid was then carefully pipetted out and disposed. The wells were cleaned and air-dried five times with distilled water. Each well received 100 μL of SRB and was incubated for 30 min at room temperature. Unbound SRB was removed from the cells by washing them five times with 1 percent v/v acetic acid. After drying at room temperature, tris buffer (100 μL) was added to each well to solubilize the color and plates were delicately swirled for few minutes on a mechanical stirrer. A microplate reader was used to measure the optical density at 540 nm [43].
4.5. Mechanistic Assays
4.5.1. Evaluation of Cell Apoptosis
In a six-well plate, cells were planted at a density of 2 × 105 cells/mL and incubated for 24 h. The chloroform fraction was added at concentrations of 50, 75, and 100 µg/mL for an additional 24 h. Following the incubation period, cells were splashed with PBS and fixed in 4 percent paraformaldehyde for 15 min at 4 °C. The fixed cells were washed again and stained with DAPI for 15 min in the dark. The stained cells were again splashed with PBS and analysed for nuclear morphological alterations using a fluorescent microscope (Olympus-1X53 magnification) [44].
4.5.2. Estimation of Reactive Oxygen Species Production
Cells were seeded in a six-well plate (2 × 105) and treated for 24 h with chloroform fraction (50, 75, and 100 µg/mL). 2 h before termination, H2O2 was introduced to the well-holding medium. The cells were then incubated for 30 min at 37 °C in the dark with 10 µM DCFDA (2, 7-dichlorofluorescein diacetate). The cells were washed with PBS before being inspected with a fluorescence microscope (Olympus-1X 53 magnifications). The fluorescence intensity was measured at 480/530 nm emission and excitation wavelengths [45].
4.5.3. Colony Formation Assay
In a six-well plate, cells were seeded at a density of (2 × 105) and cultured for 24 h. Following the incubation time, cells were treated with chloroform fraction at concentrations of 50, 75, and 100 µg/mL and incubated for another 24 h. The treated cells were then trypsinized, counted, and reseeded in a new six-well plate at 1000 cells per well. The plate was placed in an incubator for at least 6 cell divisions. The cells were rinsed with PBS after the medium was removed. After that, the cells were fixed in 1% formaldehyde for 20–25 min. The plate was kept at room temperature for 30 min after the fixed cells were stained with crystal violet dye. Following the incubation period, the crystal violet was removed, the plate was gently washed with water, and colonogenic survival was evaluated [46].
4.5.4. Wound Healing Assay
Cells with density (3 × 105)/well were seeded in a 6-well plate for 24 h. Then, using 200 µL tips at the top of the well, a horizontal scratch was formed on the cell monolayer and it was treated with chloroform fraction (50, 75 and 100 µg/mL) for the following 24 h. Cells were seen under the microscope at 0 h. After 24 h, the medium was aspirated, cleaned with PBS, and examined using a phase contrast microscope.
4.6. Analysis of Phytochemicals by HPLC and GCMS
In vitro cytotoxicity of extracts and fractions were tested against human cancer cell lines used in the present investigation. Based on the cytotoxicity results, chloroform fraction (ch fr) was picked for additional phytochemical portrayal by HPLC and GCMS.
Qualitative and quantitative analysis of phenols was performed by reverse-phase high-performance liquid chromatography (HPLC) using the method reported by Tarnawaski et al. [47]. A liquid chromatography system (Perkin Elmer, Boston, MA, USA) comprising of an HPLC pump, auto sampler, diode array detector (LC 200 D series), and Peltier Column oven (200 series) Perkin Elmer reverse phase column (150 × 4.6 mm, 5 μm C18) was used for analysis of phenols using 20 µL of samples. For analysis and data collection, total chrome workstation software (version 6.3.1.0504) was utilized. Mobile phase was comprised of acetic acid (2% v/v) in water and methanol (82:15, v/v) and a flow rate was adjusted at 1 mL/min. Analysis of phenolic acids chromatograms was performed at 280 nm on 30 min retention time frame. Standard phenolic compounds (gallic acid, ferulic acid, syringic acid, caffeic acid, vanillic acid, p-coumarin acid, p-hydroxy benzoic acid) at concentration of 1 mg/mL prepared in methanol were used. Phenolic compounds in samples were identified by comparing retention time of standard phenolic compounds with retention time of each phenolic compound.
Gas chromatography and mass spectroscopy were used to analyze the active fraction. The GC-MS 4000 (Varian, Atlanta, GA, USA) system was employed, along with an HP-5 MS agilent column (30 m × 0.25 mm i.d., 0.25 film thickness). The temperature of the injector was set at 280 degree Celsius. After holding the column at 50 degree Celsius for 5 min, the temperature was steadily raised by 3 degrees Celsius each minute up to 280 degree Celsius and then held constant at 280 degree Celsius for 7 min. Helium was used as the carrier gas at a constant flow rate of 1.0 mL/min. A sample solution of 0.2 L was put onto the column and examined. The MS scan settings comprised a 70 Ev electron impact ionization voltage, a mass range of 40–500 m/z, and the identification of the sample’s components was based on a comparison of their relative indexes.
4.7. Statistical Analysis
All experiments were conducted in triplicate and the findings were computed as mean ± standard deviation (SD). GraphPAD prism 5.0 was used to calculate IC50.
5. Conclusions
The assessment of anticancer activity of different parts of C. dichotoma against human cancer cell lines used in the study suggested that bark part of C. dichotoma might be a significant natural source for anticancer drugs. This is the first study to show that bark has anticancer properties against the A-549 cell line from lung origin. The chloroform fraction of bark has anticancer potential and causes cell death against A-549 human cancer cell line from lung origin via apoptosis induction mediated by excessive ROS production. Furthermore, HPLC and GCMS analyses revealed the presence of a number of bioactive molecules such as gallic acid, vanillic acid, ferulic acid, caffeic acid, palmitic acid, cis vaccenic acid, and others. We reported, for the first time, cis vaccenic acid from the bark through GC-MS. As a result of its promising efficacy, the fraction might be an essential cancer chemopreventive or chemotherapeutic drug for patients with lung carcinoma. To better comprehend their capacity to manage illnesses that have a significant impact on quality of life, more research into the separation of responsible anticancer components, as well as their mechanism of action, is required.
6. Patents
To best of our knowledge, this is the first study to show that the plant’s bark has anticancer properties against the A-549 human cancer cell line and induced chromatin condensation, confirmed cell death via ROS generation and significantly decreased colony formation in a dose dependent manner in A-549 cell line from lung origin. Furthermore, GC-MS investigations indicated the presence of the compound that was reported from the bark i.e., cis vaccenic acid, for the first time.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz 684 University, Jeddah, under grant No. (DF-002-130-1441). The authors, therefore, gratefully 685 acknowledge DSR technical and financial support.
Author Contributions
Conceptualization, V.S. and S.R.; methodology, S.R. and V.S.; software, S.R.; validation, V.S. and S.R.; formal analysis, S.R. and V.S. investigation, S.R.; resources, S.R. and K.R.H.; data curation, V.S., H.F.A.; T.A.Z., A.Z. and S.R.; writing—original draft preparation, S.R. and K.R.H.; writing—review and editing, S.R., V.S., Z.N.S. and N.K.; visualization, V.S. and S.R; supervision, V.S. and S.K.S.; project administration, H.F.A.; A.Z.; T.A.Z., K.R.H.; funding acquisition, H.F.A.; A.Z.; T.A.Z., K.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz 684 University, Jeddah, under grant No. (DF-002-130-1441). The authors, therefore, gratefully acknowledge DSR technical and financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data, tables and figures are original.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Greenwell M., Rahman P.K.S.M. Medicinal plants: Their use in anticancer treatment. Int. J. Pharm. Sci. 2015;6:4103–4112. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dias D.A., Urban S., Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslam M.S., Ahmad M.S. Worldwide importance of medicinal plants: Current and historical perspectives. Recent. Adv. Biol. Med. 2016;2:88–93. [Google Scholar]
- 4.Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rybak S.M., Pearson J.W., Fogler W.E., Volker K., Spence S.E., Newton D.L., Longo D.L. Enhancement of vincristine cytotoxicity in drug-resistant cells by simultaneous treatment with onconase, an antitumor ribonuclease. J. Natl. Cancer Inst. 1996;88:747–753. doi: 10.1093/jnci/88.11.747. [DOI] [PubMed] [Google Scholar]
- 6.Silvestri R. New prospects for vinblastine analogues as anticancer agents. J. Med. Chem. 2013;56:625–627. doi: 10.1021/jm400002j. [DOI] [PubMed] [Google Scholar]
- 7.Son Y.O., Choi K.C., Lee J.C., Kook S.H., Lee S.K., Takada K., Jang Y.S. Involvement of caspase activation and mitochondrial stress in taxol-induced apoptosis of Epstein–Barr virus-infected Akata cells. Biochim. Biophys. Acta. 2006;1760:1894–1902. doi: 10.1016/j.bbagen.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Dallavalle S., Giannini G., Alloatti D., Casati A., Marastoni E., Musso L., Zunino F. Synthesis and cytotoxic activity of polyamine analogues of camptothecin. J. Med. Chem. 2006;49:5177–5186. doi: 10.1021/jm060285b. [DOI] [PubMed] [Google Scholar]
- 9.Benderoth M., Pfalz M., Kroymann J. Methylthioalkylmalate synthases: Genetics, ecology and evolution. Phytochem. Rev. 2009;8:255–268. [Google Scholar]
- 10.Ham S.L., Nasrollahi S., Shah K.N., Soltisz A., Paruchuri S., Yun Y.H., Tavana H. Phytochemicals potently inhibit migration of metastatic breast cancer cells. Integr Biol. 2015;7:792–800. doi: 10.1039/c5ib00121h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuominen E.K., Zhu K., Wallace C.J., Lewis C.I., Craig D.B., Rytoomaa M., Kinnunen P.K. ATP induces a conformational change in lipid-bound cytochrome c. J. Biol. Chem. 2011;276:19356–19362. doi: 10.1074/jbc.M100853200. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q., Gong B., Almasan A. Distinct stages of cytochrome c release from mitochondria: Evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 2000;7:227–233. doi: 10.1038/sj.cdd.4400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haridas V., Higuchi M., Jayatilake G.S., Bailey D., Mujoo K., Blake M.E., Gutterman J.U. Avicins: Triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proc. Natl. Acad. Sci. USA. 2001;98:5821–5826. doi: 10.1073/pnas.101619098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korsmeyer S.J. Regulators of cell death. Trends Genet. 1995;11:101–105. doi: 10.1016/S0168-9525(00)89010-1. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Liu Y., Fu Y., Wei T., Guyader L., Gao G., Chen C. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials. 2012;33:402–411. doi: 10.1016/j.biomaterials.2011.09.091. [DOI] [PubMed] [Google Scholar]
- 16.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Jamkhande P.G., Barde S.R., Patwekar S.L., Tidke P.S. Plant profile, phytochemistry and pharmacology of Cordia dichotoma (Indian cherry): A review. Asian Pac. J. Trop. Biomed. 2013;3:1009–1012. doi: 10.1016/S2221-1691(13)60194-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain N., Kakoti B.B. Review on ethnobotany and phytopharmacology of C. dichotoma. J. Drug. Deliv. Ther. 2013:3. [Google Scholar]
- 19.Nariya P.B., Bhalodia N.R., Shukla V.J., Acharya R.N. Antimicrobial and antifungal activities of Cordia dichotoma (Forster F.) bark extracts. Ayu. 2011;32:585. doi: 10.4103/0974-8520.96138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuppast I.J., Nayak P.V. Wound healing activity of Cordia dichotoma Forst. f. fruits. Indian. J. Nat. Prod. Resour. 2006;5:99–102. [Google Scholar]
- 21.Sharma U.S., Sharma U.K., Sutar N., Singh A., Shukla D.K. Anti-inflammatory activity of Cordia dichotoma forst f. seeds extracts. Int. J. Pharm. Anal. 2010;2:1. [Google Scholar]
- 22.Bharathi D., Vasantharaj S., Bhuvaneshwari V. Green synthesis of silver nanoparticles using Cordia dichotoma fruit extract and its enhanced antibacterial, anti-biofilm and photo catalytic activity. Mater. Res. Express. 2018;5:055404. [Google Scholar]
- 23.Del-Toro-Sanchez C.L., Rodríguez-Felix F., Cinco-Moroyoqui F.J., Juárez J., Ruiz-Cruz S., Wong-Corral F.J., Tapia-Hernández J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021;45:e15765. [Google Scholar]
- 24.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 25.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 26.Karpuz M., Silindir G.M., Ozer A.Y. Current and future approaches for effective cancer imaging and treatment. Cancer Biother. Radiopharm. 2018;33:39–51. doi: 10.1089/cbr.2017.2378. [DOI] [PubMed] [Google Scholar]
- 27.Nobili S., Lippi D., Witort E., Donnini M., Bausi L., Mini E., Capaccioli S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. Commun. 2009;59:365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Hussain N., Kakoti B.B., Rudrapal M., Sarwa S.S. Anticancer and antioxidant activities of Cordia dichotoma Forst. Biomed. Pharmacol. J. 2020;13:2093–2099. [Google Scholar]
- 29.Rahman M.A., Akhtar J. Evaluation of anticancer activity of Cordia dichotoma leaves against a human prostate carcinoma cell line, PC3. J. Tradit. Complement. Med. 2017;7:315–321. doi: 10.1016/j.jtcme.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim A.Y., El-Newary S.A., Ibrahim G.E. Antioxidant, cytotoxicity and anti-tumor activity of Cordia dichotoma fruits accompanied with its volatile and sugar composition. Ann. Agric. Sci. 2019;64:29–37. [Google Scholar]
- 31.Anantharaju P.G., Gowda P.C., Vimalambike M.G., Madhunapantula S.V. An overview of the role of dietary phenolics for the treatment of cancers. J. Nutr. 2016;15:99–106. doi: 10.1186/s12937-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usmani S., Ahmad M., Hussain A., Arshad M., Ali M. Cellular oxidative stress and antiproliferative effects of Cordia dichotoma (Linn.) seeds extract and their fractions on human cervix epitheloid (HeLa) and human lung (A549) carcinoma cells. Eur. J. Integr. Med. 2018;21:1–10. [Google Scholar]
- 33.Massry K.F., Farouk A., Mahmoud K.F., Ghorab A.H., Musa A., Mostafa E.M., Abdelgawad M.A. Chemical characteristics and targeted encapsulated Cordia myxa fruits extracts nanoparticles for antioxidant and cytotoxicity potentials. Saudi J. Biol. Sci. 2021;28:5349–5358. doi: 10.1016/j.sjbs.2021.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moheb A., Grondin M., Ibrahim R.K., Roy R., Sarhan F. Selective anticancer potential of several methylated phenolic compounds. J. Nat. Pharm. 2013;4:44. [Google Scholar]
- 35.Zhang Y.X., Yu P.F., Gao Z.M., Yuan J., Zhang Z. Caffeic acid n-butyl ester-triggered necrosis-like cell death in lung cancer cell line A549 is prompted by ROS mediated alterations in mitochondrial membrane potential. Eur. Rev. Med. Pharm. Sci. 2017;21:1665–1671. [PubMed] [Google Scholar]
- 36.Maurya D.K., Nandakumar N., Devasagayam T.P.A. Anticancer property of gallic acid in A549, a human lung adenocarcinoma cell line, and possible mechanisms. J. Clin. Biochem. Nutr. 2010;48:85–90. doi: 10.3164/jcbn.11-004FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gheena S., Ezhilarasan D. Syringic acid triggers reactive oxygen species–mediated cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2019;38:694–702. doi: 10.1177/0960327119839173. [DOI] [PubMed] [Google Scholar]
- 38.Semwal P., Painuli S., Badoni H., Bacheti R.K. Screening of phytoconstituents and antibacterial activity of leaves and bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya. Clin. Phytoscience. 2018;4:1–6. [Google Scholar]
- 39.Bharath B., Perinbam K., Devanesan S., AlSalhi M.S., Saravanan M. Evaluation of the anticancer potential of Hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J. Mol. Struct. 2021;1235:130229. [Google Scholar]
- 40.Field C.J., Blewett H.H., Proctor S., Vine D. Human health benefits of vaccenic acid. Appl. Physiol. Nutr. Metab. 2009;34:979–991. doi: 10.1139/H09-079. [DOI] [PubMed] [Google Scholar]
- 41.Aparna V., Dileep K.V., Mandal P.K., Karthe P., Sadasivan C., Haridas M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012;80:434–439. doi: 10.1111/j.1747-0285.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- 42.Monks A., Scudiero D., Skehan P., Shoemaker R., Paull K., Vistica D., Boyd M. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 43.Zhang T., Ma L., Wu P., Li W., Li T., Gu R., Xiao Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019;41:1779–1788. doi: 10.3892/or.2019.6976. [DOI] [PubMed] [Google Scholar]
- 44.Chashoo G., Singh S.K., Sharma P.R., Mondhe D.M., Hamid A., Saxena A., Andotra S.S., Shah B.A., Qazi N.A., Taneja S.C., et al. Apropionyloxy derivative of 11-keto -boswellic acid induces apoptosis in HL-60 cells mediated through topoisomerase I & II inhibition. Chem. Biol. Interact. 2011;189:60–71. doi: 10.1016/j.cbi.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Banskota S., Regmi S.C., Kim J.A. NOX1 to NOX2 switch deactivates AMPK and induces invasive phenotype in colon cancer cells through overexpression of MMP-7. Mol. Cancer. 2015;14:1–14. doi: 10.1186/s12943-015-0379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franken N.A.P., Rhodermond H.M., Stap J., Haveman J., Bree C.V. Clonogenic assay of cells in vitro. Nat. Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 47.Tarnawski M., Depta K., Grejciun D., Szelepin B. HPLC determination of phenolic acids and antioxidant activity in concentrated peat extract—A natural immunomodulator. J. Pharm. Biomed. Anal. 2006;41:182–188. doi: 10.1016/j.jpba.2005.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data, tables and figures are original.