Abstract
Objective:
Upper extremity (UE) deep vein thrombosis (DVT) is a common and increasing complication in hospitalized patients. The objective of the present study was to determine the prevalence, treatment strategies, complications, and outcomes of UE-DVT.
Methods:
We performed a retrospective single-institution study of patients with a diagnosis of UE-DVT from January 2016 through February 2018 (26 months). Patients aged ≥18 years who had been admitted to the hospital and who had had positive UE duplex ultrasound findings for acute UE-DVT were included in the present study. The outcomes were in-hospital mortality, major bleeding, pulmonary embolism (PE), and recurrent UE-DVT.
Results:
Among 63,045 patients admitted to the hospital, 1000 (1.6%) had been diagnosed with UE-DVT. Of 3695 UE venous duplex ultrasound examinations performed during the study period, almost one third (27.0%) were positive for acute UE-DVT. The mean age was 55.0 6 17.2 years, and most patients were men (58.3%), white (49.2%), and overweight (mean body mass index, 29.4 6 10.3 kg/m2). The most affected vein was the right internal jugular vein (54.8%). Most of the patients (96.9%) has been receiving venous thromboembolism prophylaxis or anticoagulation therapy at the diagnosis. Most patients (77.8%) had had an intravenous device (IVD) in place at the diagnosis. Most of the patients (84.4%) were treated with anticoagulation therapy in the hospital but only one half (54.5%) were discharged with anticoagulation therapy. In-hospital mortality was 12.1% unrelated to UE-DVT, major bleeding occurred in 47.6% of the patients during hospitalization (fatal bleeding, 1%), PE was diagnosed in 4.8% of the patients, and 0.7% were fatal. Recurrent UE-DVT occurred in 6.1% of the patients. On multivariable analysis, the risk of death was increased by older age, cancer, intensive care unit admission, concomitant lower extremity DVT, and bleeding before the UE-DVT diagnosis. The presence of an IVD increased the risk of PE and the risk of recurrent UE-DVT. The risk of major bleeding was increased by the presence of an IVD, female sex, and concomitant lower extremity DVT.
Conclusions:
UE-DVT is a common complication in hospitalized patients (1.6%). Consequent acute PE and recurrent DVT remain important complications, as does bleeding. It is unclear whether standard thromboprophylaxis effectively protects against UE-DVT. More studies dedicated to UE-DVT are required to provide appropriate guidance on prophylaxis and treatment.
Keywords: Anticoagulation, Catheter-associated thrombosis, Upper-extremity deep vein thrombosis
Upper extremity (UE) deep vein thrombosis (DVT) is a complication frequently seen in hospitalized patients.1 The prevalence of UE-DVT in intensive care unit (ICU) patients has been reported at 2% to 15%.2,3 UE-DVT is associated with the use of intravenous devices (IVDs) such as central venous catheters or wires, affecting 7% to 10% of patients with permanent catheters.4,5 Patients with non-IVD-associated UE-DVT frequently have a hypercoagulable state and malignancy as risk factors.6–8 The known complications of UE-DVT include pulmonary embolism (PE), post-thrombotic syndrome, and superior vena cava syndrome (recurrent and extensive UEDVT),9,10 and UE-DVT has been associated with an elevated mortality rate.11 However, these risks have not been systematically evaluated. The treatment guidelines are based on a low level of evidence (grade 2B or 2C) with data extrapolated from lower extremity DVT (LE-DVT) studies.12 Thus, uncertainty exists regarding the duration of anticoagulation therapy or how coexisting central venous catheters should be managed.13 Therefore, significant variability has resulted in the treatment decisions across hospitals, practices, and medical specialties.14 We analyzed information from hospitalized patients with UE-DVT in a tertiary center to determine the prevalence, treatment strategies, complications, and outcomes of this complication.
METHODS
Study protocol.
The institutional review board of the University of Maryland approved the protocol and granted exempt status, waiving the requirement for patient informed consent. All UE venous duplex ultrasound examinations at the University of Maryland, Baltimore hospital are performed at a central vascular laboratory. Patients with a diagnosis of an acute UE-DVT from January 2016 through February 2018 (26 months) were included in the present analysis. Those with chronic DVT alone and those who had undergone repeat studies were excluded. A convenience sample of the first 1000 consecutive patients with a diagnosis of UE-DVT was acquired from our vascular laboratory database. This was accomplished by searching the medical records for a 26-month period. In addition, information on demographics (eg, age, body mass index, sex, ethnicity), comorbidities (eg, diabetes, hypertension, hyperlipidemia, coronary artery disease, kidney disease, chronic liver disease), risk factors for venous thromboembolism (VTE), known inherited and acquired hypercoagulable states, pregnancy, and cancer were collected from the hospital medical records. The specific risk factors for UE-DVT included the presence of an IVD in the UE veins within 7 days before the diagnosis. IVDs were categorized as short-term catheters (peripherally inserted central catheters, extracorporeal membrane oxygenation [ECMO] cannulas, central venous catheters, SwanGanz catheters), long-term catheters (dialysis catheters, chemotherapy ports), and permanent devices (pacemaker wires).
Ultrasound protocol.
Patients had undergone ultrasound because of a clinical suspicion for DVT. Ultrasound was performed by a registered vascular technologist using a standardized protocol15 and was interpreted by trained physicians (Alliance for Physician Certification and Advancement, registered vascular technologist, or registered physician in vascular interpretation). Acute DVT was defined as the inability to occlude the vein lumen under compression during B-mode and/or the lack of flow within the vein on Doppler ultrasound imaging. A recurrent UE-DVT was defined as the presence of a new thrombus in a vein segment with a previously documented resolved or chronic thrombus. A thrombus was considered chronic if it was hyperechoic and non-occlusive with or without collateral circulation or if was a known thrombus >2 weeks old. Thrombus morphology was described as floating (tail of the thrombus moving freely inside the lumen), occlusive (lumen of the vein occupied by the thrombus with no blood flow detected), or non-occlusive (detectable flow in the lumen around the thrombus). The anatomic location was described according to the involved vein segment (brachial, axillary, subclavian, internal jugular, brachiocephalic, and superior vena cava) and laterality (left, right, unilateral, bilateral). The extent of the thrombus was described according to the number of vein segments involved (range, one to eight segments). Information about the presence of concomitant thrombosis in a different vascular bed was also collected. No additional radiologic imaging study was routinely performed to evaluate the UE-DVT.
Prophylaxis and treatment strategies.
Information about the type of DVT prophylaxis received at the diagnosis (pharmacologic or mechanical, or both) and whether the patient was receiving full dose anticoagulation therapy was collected. For most patients requiring ECMO, a low-intensity anticoagulation protocol was used to target a partial thromboplastin time of 40 to 60 seconds. Information on the treatment strategies implemented after the diagnosis was also collected (drug of choice and planned anticoagulation duration, management of the IVD [removed or left in place]). The concomitant presence of acute DVT in the LEs or a PE was also noted.
Outcomes.
In-hospital mortality, major bleeding events, the development of a PE, and recurrent UE-DVT within 90 days of the index event were recorded. The patients with UE-DVT were not all systematically evaluated for PE. If the patients had presented with symptoms suggestive of a PE, a complete evaluation was performed by the primary team that included spiral computed tomography angiography. A new PE was defined as a new filling defect in the pulmonary artery on computed tomography angiography or a new mismatch defect found by ventilation/perfusion scanning. A recurrent UE-DVT was defined as a new thrombus in the same arm.Major bleeding was defined as fatal bleeding, bleeding in a critical organ, bleeding causing a decrease in hemoglobin >2.0 g/dL requiring >2 U of blood, and bleeding requiring a surgical intervention.
Statistical analysis.
The baseline demographics, comorbidities, risk factors, prophylaxis, treatment modalities, and outcomes were summarized and tabulated using SAS, version 9.3, software (SAS Institute Inc, Cary, NC). Categorical variables are reported as frequencies and percentages. Continuous variables are reported as the mean ± standard deviation or the median and interquartile range, as appropriate. The patients were then allocated into the following groups: UE-DVT in the presence of an IVD and UE-DVT in the absence of an IVD. The characteristics of the two groups were compared using the Student t test. To evaluate the risk factors for the described outcomes for patients with UE-DVT, multivariable regression was performed for each outcome. The covariates incorporated into the model were the presence of an IVD, patient age, female sex, white race, active cancer, concomitant LE-DVT, bleeding before anticoagulation therapy, and ICU admission. The results are reported as odds ratios (ORs), 95% confidence intervals (CIs), and P values. A P value <.05 was considered statistically significant.
RESULTS
Patient population.
During the 26-month study period, there were 63,045 hospital admissions at the hospital. During the same period, 13,415 venous ultrasound examinations were performed in the vascular laboratory, of which 3693 (27.5%) were of the UEs. The indications for venous ultrasound were edema and pain of the UEs. Routine screening for UE-DVT was performed only for patients with ECMO. Of the ultrasound examinations, 1000 (7.45%) revealed acute and unique UE-DVT. The prevalence of acute UE-DVT among the hospitalized patients who had undergone venous duplex ultrasound was 1.6% in the 26-month period (Fig 1).
Fig 1.
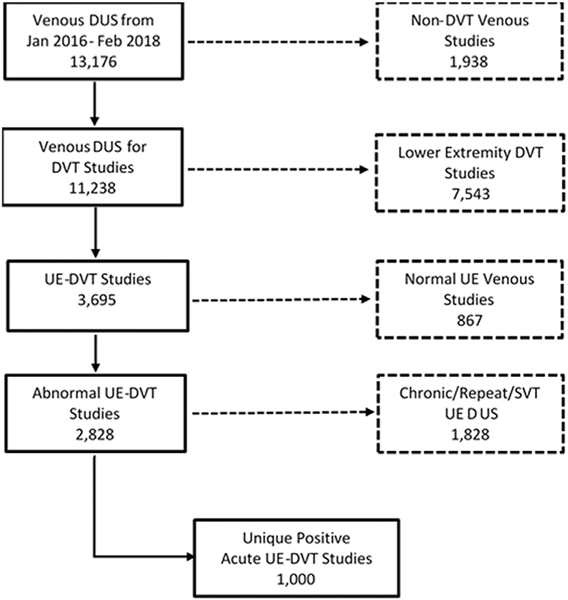
CONSORT (Consolidated Standards of Reporting Trials) diagram of patients admitted to the University of Maryland hospital who had developed upper extremity (UE) deep vein thrombosis (DVT). DUS, Duplex ultrasound; SVT, superficial vein thrombosis.
Patient characteristics.
The mean age of the patients with acute UE-DVT was 55 ± 17.23 years. Most of the patients were men (58.3%), and 49.2% were white and 44.2% were African American. The mean body mass index was 29.4 ± 10.3 kg/m2. Other pertinent demographics and comorbidities are described in Table I. Most of the patients had been admitted to an ICU (68.9%), telemetry (4.6%), or regular floor (21.8%) at the diagnosis, and the median length of hospital stay was 25 days (IQR, 13–45 days; range, 1–418 days). A more detailed distribution is provided in Fig 2.
Table I.
Demographic characteristics and risk factors
| Variable | Total | IVD related | P value | |
|---|---|---|---|---|
| Yes | No | |||
| Total | 1000 (100) | 778 (77.8) | 222 (22.2) | NA |
| Age, years | 55.2 ± 17.2 | 54.8 ± 17.0 | 56.8 ± 17.8 | .12 |
| BMI, kg/m2 | 29.2 ± 7.9 | 29.7 ± 8.0 | 27.4 ± 7.1 | < .0001 |
| Male sex | 584 (58.4) | 453 (58.2) | 131 (59.0) | .78 |
| White race | 493 (49.3) | 401 (51.5) | 95 (41.4) | .007 |
| African American race | 444 (444.4) | 327 (42.0) | 117 (52.7) | .005 |
| Current smoker | 212 (21.2) | 167 (21.5) | 45 (20.3) | .73 |
| Chronic kidney diseasea | 151 (15.1) | 118 (15.2) | 33 (14.9) | .95 |
| Hypertension | 558 (55.8) | 431 (55.4) | 127 (57.2) | .51 |
| Coronary artery disease | 182 (18.2) | 143 (18.4) | 39 (17.6) | .90 |
| Diabetes mellitus type 2 | 288 (28.8) | 217 (27.9) | 71 (32.0) | .20 |
| Hyperlipidemia | 279 (27.9) | 209 (26.9) | 70 (31.5) | .14 |
| History of cancer | 200 (20.0) | 147 (18.9) | 53 (23.9) | .08 |
| Active cancer | 64 (6.4) | 54 (6.9) | 10 (4.5) | .19 |
| History of UE-DVT | 145 (14.5) | 114 (14.7) | 31 (14.0) | .80 |
| History of LE-DVT | 84 (8.4) | 62 (8.0) | 22 (9.9) | .36 |
| History of PE | 80 (8.0) | 68 (8.7) | 12 (5.4) | .11 |
| Concomitant DVT | 176 (17.6) | 137 (17.6) | 39 (17.6) | .99 |
| ECMO | 104 (10.4) | 104 (13.4) | 0 (0) | < .0001 |
| Pregnancy | 3 (0.3) | 2 (0.3) | 1 (0.5) | .63 |
| Hypercoagulable stateb | 83 (8.3) | 58 (7.5) | 25 (11.3) | .07 |
BMI, Body mass index; DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; IVD, intravenous device; LE-DVT, lower extremity deep vein thrombosis; UE-DVT, upper extremity deep vein thrombosis.
Data presented as mean ± standard deviation or number (%).
Boldface P values represent statistical significance.
defined as glomerular filtration rate of ≤60 mL/min.
Including antiphospholipid antibody syndrome, factor V Leiden, prothrombin gene mutation, and protein C, S, and antithrombin deficiency
Fig 2.
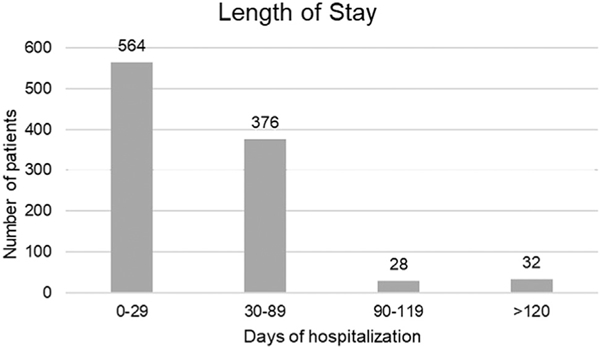
Distribution of length of hospitalization.
Thrombus description.
The thrombus was free floating in 33.3%, occlusive in 18.9%, nonocclusive in 11.7%, and not explicitly reported in 34.8% of the patients. In most cases, the thrombus involved a single vein segment (64.1%), with two (22.0%), three (8.2%), and four or more segments (5.7%) involved less frequently. The most commonly affected vein segment was the right internal jugular vein (Fig 3). Concomitant LE-DVT was found in 16.2% and a PE in 0.7% of the patients.
Fig 3.
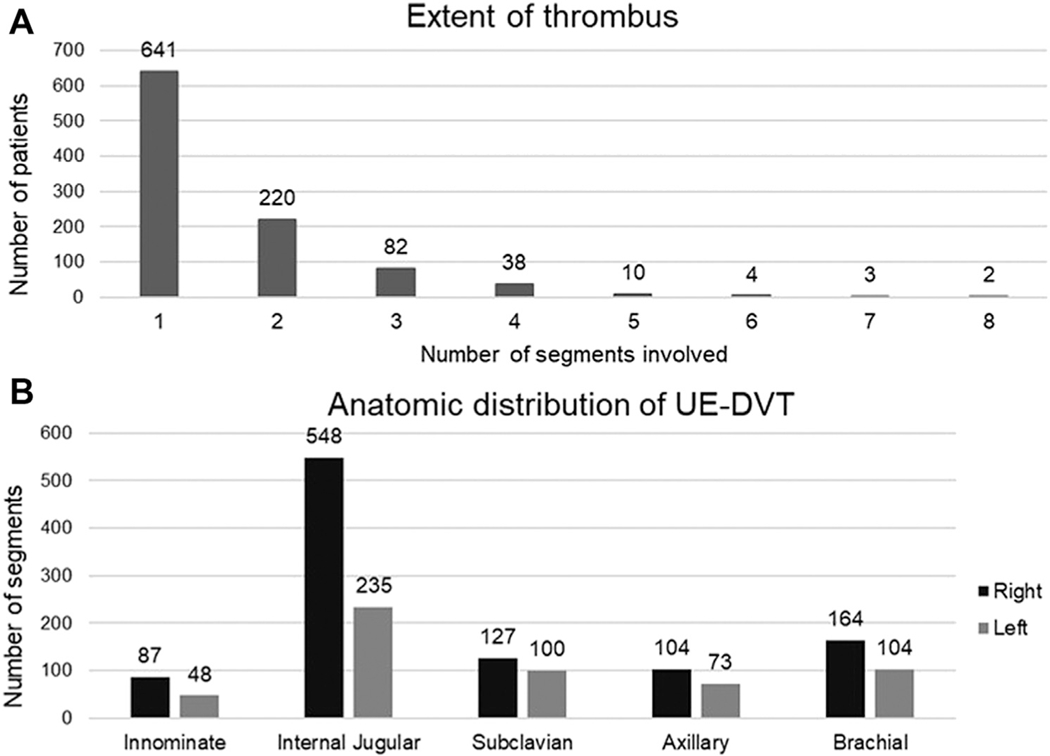
A, Extent of thrombus in 1000 patients with upper extremity (UE) deep vein thrombosis (DVT). B, Anatomic distribution of UE-DVT.
DVT prophylaxis.
Most of the patients had been receiving pharmacologic (68.1%) or mechanical (sequential compression device; 8.1%) DVT prophylaxis, with some patients receiving full-dose anticoagulation (20.7%) at the diagnosis of UE-DVT. A few patients (3.1%) had not been receiving either prophylaxis or anticoagulation therapy before the diagnosis of UE-DVT. The most common pharmacologic agents used for DVT prophylaxis were unfractionated heparin (47.9%), followed by low-molecular-weight heparin (LMWH; 7.1%). Of those receiving anticoagulation therapy, most were receiving warfarin (11.9%), followed by direct oral anticoagulant agents (DOACs; 4.9%), unfractionated heparin (4. 8%), LMWH (3.6%), fondaparinux (0.4%), argatroban (0.1%), and bivalirudin (0.1%). The indications for therapeutic anticoagulation were a history of DVT or PE (8.0%), atrial fibrillation (5.0%), mechanical heart valve (0.7%), the use of ECMO (0.4%), previous heart or vascular surgery (0.2%), heparin-induced thrombocytopenia (0.1%), acute coronary syndrome (0.1%), and other causes (6.5%).
Treatment strategies.
Most patients (84.4%) were treated with anticoagulation. If the patients were receiving anticoagulation therapy (20.7%) at the diagnosis, it was continued. The most common choice was unfractionated heparin (58.0%), followed by LMWH (17.6%), DOACs (4.1%), warfarin (3.4%) fondaparinux (0.8%), bivalirudin (0.5%), and argatroban (0.1%). For 15.6% of the patients, anticoagulation was not possible because of contraindications. Only four patients (0.4%) had undergone catheter-based thrombectomy or thrombolysis: one for superior vena cava syndrome and three for extensive UE-DVT.
Difference between IVD-related and non-IVD-related UE-DVT.
The baseline characteristics, comorbidities and risk factors were very similar between the IVD-related and non-IVD-related UE-DVT groups (Table I). White patients were more frequently diagnosed with IVD-related UE-DVT, and more African-American patients were observed with non-IVD-related UE-DVT. The presence of an IVD before the diagnosis of UE-DVT was observed in 77.8% of the patients. Only one patient was diagnosed with venous thoracic outlet syndrome in the non-IVD-related UE-DVT group. Most had had short-term catheters (70.1%), including central venous catheters (59.7%) and ECMO cannulas (10.4%). Some patients had had long-term catheters (7.4%), including dialysis catheters (5.3%) and permanent chemotherapy ports (1.8%). A few had permanent pacemaker wires (0.5%). Approximately one third of IVDs (31.4%) had been removed once UE-DVT had been diagnosed, including patients receiving ECMO (10.4%), in whom the cannula had been removed before the diagnosis of UE-DVT. Short-term catheters were removed more often than were long-term catheters (Fig 4). A new catheter was inserted in 100 patients (31.8%). Only 54.5% of the patients were discharged with anticoagulation therapy; 45.5% had had a contraindication or the primary team had not considered anticoagulation therapy necessary. The most frequent anticoagulant drugs prescribed were LMWH (20.3%), warfarin (20.3%), DOACs (13.7%), and fondaparinux (0.2%).
Fig 4.
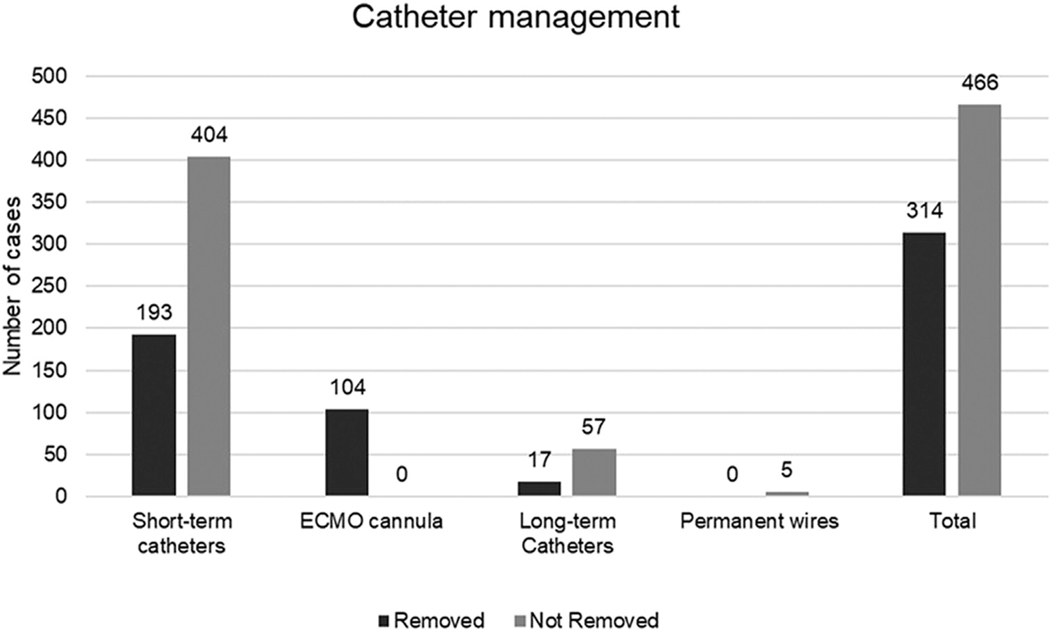
Catheter management in 770 patients with intravenous device (IVD)-associated upper extremity (UE) deep vein thrombosis (DVT). ECMO, Extracorporeal membrane oxygenation; long-term catheters, dialysis catheters, chemotherapy ports; short-term catheters, peripheral inserted central catheters, central venous catheters, Swan-Ganz catheters, angiography sheets.
Overall outcomes.
The in-hospital mortality was 12.1%. The most common cause of death was sepsis (2.9%), followed by heart disease (2.7%), pulmonary disease not related to PE (1.6%), major bleeding (1.0%; including intracranial, retroperitoneal, and hemorrhagic shock), traumatic brain injury (0.6%), cancer-related complications (0.5%), cerebrovascular disease (0.3%), end-stage liver disease (0.2%), PE (0.1%), and bowel ischemia (0.1%), with other causes recorded for 2.1% of the patients. Before the diagnosis of UE-DVT, a bleeding event had been reported for 26.7%, although anticoagulation therapy had been continued for many of these patients despite this (n = 61; 22.8%). Of the patients with a diagnosis of UE-DVT, a bleeding event had been reported after the initiation of treatment in 21.4%. The common bleeding sources were gastrointestinal (7.7%) surgical site (4.7%), vascular access (2.5%), intracranial (1.2%), intramuscular hematomas (0.9%), retroperitoneal (0.6%), and other (3.8%). PE after the diagnosis of UE-DVT occurred in 4.8% of patients during the hospitalization. Among these patients, only 0.6% had had a concomitant LE-DVT at the diagnosis. Seven patients (0.7%) had died in this group; however, only one death was attributed to PE. Recurrent UE-DVT occurred in 6.1% of the patients.
Outcomes stratified by IVD status.
Bleeding was more common in those with a previous IVD (5.7% vs 0.4%). All the patients with an IVD-related UE-DVT had experienced a recurrent event after they had received a second IVD.
Risk factors affecting outcomes in patients with UE-DVT.
Multivariable analysis was performed to determine the risk factors for the outcomes of death, PE, recurrent UE-DVT, and bleeding (Table II). The risk of death was increased for older patients (OR, 1.02; 95% CI, 1.00–1.03), those admitted to an ICU (OR, 3.65; 95% CI, 2.25–5.91), patients with active cancer (OR, 2.69; 95% CI, 1.22–5.93), patients with a concomitant LE-DVT (OR, 1.98; 95% CI, 1.23–3.17); and patients with an episode of bleeding before the diagnosis of UE-DVT (OR, 1.86; 95% CI, 1.23–2.81). The risk of bleeding was increased for patients with an IVD (OR, 1.61; 95% CI, 1.07–2.43), female patients (OR, 1.65; 95% CI, 1.21–2.25), and patients with a concomitant LE-DVT (OR, 2.39; 95% CI, 1.64–3.47). The risk of PE was increased for patients with an IVD (OR, 2.66; 95% CI, 1.03–6.85). Finally, the presence of an IVD increased the risk of recurrent UE-DVT (OR, 1.56; 95% CI, 1.00–2.45); however, the risk decreased if a concomitant LE-DVT was present (OR, 0.45; 95% CI, 0.25–0.79).
Table II.
Risk factors associated with primary and secondary outcomes
| Risk factor | In-hospital mortality | PE | Recurrent UE-DVT | Bleeding after AC |
|---|---|---|---|---|
| IVD | 1.66 (0.98–2.9) | 2.66 (1.03–6.85) | 1.56 (1.00–2.45) | 1.61 (1.07– 2.43) |
| Older age | 1.02 (1.00–1.03) | 1.00 (0.98–1.02) | 0.99 (0.98–1.00) | 1.00 (0.99– 1.01) |
| Female sex | 0.70 (0.46–1.06) | 0.61 (0.33–1.15) | 1.02 (0.72–1.43) | 1.65 (1.21– 2.25) |
| White race | 1.00 (0.67–1.49) | 1.26 (0.7–2.29) | 0.91 (0.64–1.28) | 1.00 (0.73– 1.37) |
| Active cancer | 2.69 (1.22–5.93) | 0.24 (0.03–1.83) | 0.47 (0.2–1.13) | 0.48 (0.21– 1.08) |
| Concomitant LE-DVT | 1.98 (1.23–3.17) | 0.69 (0.29–1.67) | 0.45 (0.25–0.79) | 2.39 (1.64–3.47) |
| Bleeding before AC | 1.86 (1.23–2.81) | 0.67 (0.33–1.3) | 1.09 (0.74–1.59) | 1.03 (0.73– 1.45) |
| ICU admission | 3.65 (2.25–5.91) | 0.78 (0.43–1.41) | 1.03 (0.73–1.46) | 1.23 (0.89– 1.69) |
AC, Anticoagulation; ICU, intensive care unit; IVD, intravenous device; LE-DVT, lower extremity deep vein thrombosis; PE, pulmonary embolism; UE-DVT, upper extremity deep vein thrombosis.
Data presented as odds ratio (95% confidence interval).
DISCUSSION
To the best of our knowledge, the present study is one of the largest studies of patients with UE-DVT. The prevalence of UE-DVT was 1.6% of all hospitalizations. Most of the cases were associated with an IVD and admission to an ICU. Most of the UE-DVTs occurred in the internal jugular vein. Almost all the patients had been receiving DVT prophylaxis or full-dose anticoagulation therapy at the diagnosis. In-hospital treatment included anticoagulation; however, only one half of the patients were continued with anticoagulation therapy at discharge. The IVDs were removed in only one third of the patients. The in-hospital mortality was 12.1%; however, VTE-related mortality was rare. PE had been diagnosed in ≤4.8% of the patients. The risk factors associated with mortality were advanced age, ICU admission, cancer, concomitant LE-DVT, and bleeding before the diagnosis of UE-DVT. The presence of an IVD was associated with an increased risk of PE.
In a previous study, the prevalence was greater (15%) when ICU patients were systematically screened for DVT.2 However, that study is 30 years old. At present, minimally invasive procedures and the use of long-term catheters have increased in popularity. Therefore, it is evident that more updated studies are required to understand the prevalence of UE-DVT.
In our cohort, almost 80% of the patients with UE-DVT had had a concomitant IVD. Most devices were short-term central venous catheters, including ECMO cannulas, which are known to result in a high incidence of DVT.16 The most common complications associated with IVDs are thrombosis and infection. In 2011, the Centers for Disease Control and Prevention published a large and comprehensive guideline for the management of IVDs with the goal of decreasing the number of catheter-associated infections in the United States.17 However, no informed recommendations are available for reducing IVD-related UE-DVT. The American College of Chest Physicians (ACCP) did not address UE-DVT in their latest 2016 guidelines,13 and the 2012 guidelines were based on information obtained from LE-DVT studies.12
In the present study, most of the UE-DVTs had occurred in the right internal jugular vein and had involved a single segment, very similar to other reports.18 The internal jugular vein has been recommended as the preferred site for placement of a central venous device because it has been associated with fewer complications.19 Also, most physicians are right-handed. Up to one third of thrombi were described as free-floating. To the best of our knowledge, the present study is the first time a systematic observation of a “free floating” thrombus was made for the UEs. However, it is unclear whether its presence increases the risk of PE, because we had not systematically searched for PE in the present analysis.
Almost all our patients had been receiving VTE prophylaxis or full-dose anticoagulation therapy at the diagnosis of the UE-DVT. This implies that UE-DVT occurred despite this approach to prophylaxis. The 2016 ACCP guidelines do not provide guidance for the selection of prophylaxis for UE-DVT,20 primarily because few studies have addressed this question.4,5 The pathophysiology of UE-DVT is perhaps different from that of LE-DVT. The vein is injured at catheter placement, becoming thrombogenic owing to the release of tissue factor.21 Also, the catheter provokes a turbulent blood flow, activating the coagulation cascade, and the chemical action of the medicines might play an important role in the development of thrombosis.22
Therapeutic anticoagulation was given to most of the patients (84.4%) after the diagnosis of UE-DVT, even for those who had experienced a previous bleeding episode during the same hospitalization. Bleeding was frequently encountered in our cohort. Approximately one fourth of the patients had had a documented bleeding episode before the UE-DVT diagnosis, and these were included as part of the pre-existing comorbidities. Bleeding complications occurred in approximately one fifth of all patients treated with anticoagulation after a diagnosis of UE-DVT. Because invasive thrombus removal was rare in our cohort, we can only assume that the bleeding was secondary to the anticoagulation therapy. Therefore, the risks and benefits of anticoagulation therapy should be carefully considered for such patients and perhaps reserved for those with extensive thrombosis or additional indications for anticoagulation therapy.
Approximately one third of all catheters were removed in our cohort. This number is similar to that found by our nationwide survey, in which 32% of the physicians would remove the catheter in the presence of an UE-DVT.14 Long-term catheters, such as peripherally inserted central catheters, dialysis catheters, and chemotherapy ports, were less likely to be removed, although it is possible that some treating physicians might have also elected not to remove short-term catheters. This is a reflection of the lack of evidence to guide the decision. The 2012 ACCP guidelines recommended retaining a functional intravenous catheter in place in the presence of an UE-DVT and continuing anticoagulation therapy for as long as the catheter is in place.12 In other cohorts, the removal of a catheter was associated with symptomatic relief and complete thrombus resolution; however, the use of anticoagulation therapy was not.23 Additionally, the immediate placement of a new catheter after catheter removal was associated with a high rate of recurrent UE-DVT (86%).18 For pacemaker wires, the decision for removal could be associated with significant complications.24 An isolated small thrombus in a central vein might resolve spontaneously if it was related to a central vein catheter, although the frequency with which this occurs has not been quantified. The ACCP guideline has not made a distinction between single-vs multiple-segment UE-DVT and should be addressed in future clinical trials.
PE complications from UE-DVT are few and almost never fatal, owing to the small clot burden. Our results have confirmed this often-stated, yet not adequately quantified, belief.9,25,26 However, the more important finding from our study was the degree of complexity in managing this complication. To the best of our knowledge, we have shown, for the first time, the frequency with which UE-DVT is associated with placement of a central venous device. This raises the concern of whether to remove the device (which might be lifesaving), provide anticoagulation therapy (which might lead to systemic bleeding), or ignore the thrombus (which could result in downstream complications)27 dall in generally very ill patients. Also, the chronic complications of UE-DVT have not been established. The current guidelines do not offer a clear approach to this problem because all recommendations have been extrapolated from LEDVT studies, which we consider to be quite a different disease. The present study has offered information on how this struggle is being addressed in a large clinical cohort.
In our analysis, the presence of an IVD was associated with an increased risk of PE. Also, the risk of PE decreased when a patient had a concomitant LE-DVT. Although surprising, a likely explanation could be that such patients had received more consistent longer term intense anticoagulation therapy and, thereby, were protected. The questions regarding whether to routinely remove IVDs in the presence of an UE-DVT and whether to offer routine long-term anticoagulation therapy to patients who have developed an IVD-related thrombus remain unanswered and require further clinical trials.
STUDY LIMITATIONS
The true prevalence of UE-DVT is unknown, because the data from our cohort were based on an evaluation of symptomatic patients only because screening UE venous ultrasound was not routinely offered to our hospitalized patients. Although this selection bias might have underestimated the incidence of UE-DVT, our findings do provide important information on the strong relationship between IVD usage and UE-DVT and insight into the relevant risk factors for developing thrombosis and its subsequent complications. Our study was subject to the known limitations and selection bias of a retrospective, nonrandomized study. We reported the in-hospital mortality only, which explains the lower mortality for our cohort compared with other studies that reported the 2-month and 1-year mortality.11,25 Also, we had recorded recurrent DVTs in all the patients who had returned to our hospital but not for those who had followed up with different providers, explaining the lower rate of recurrent events compared with that for other cohorts.28,29 Other important chronic complications of UE-DVT such as post-thrombotic syndrome were not addressed in our study because our follow-up was limited to hospital discharge. Additional analyses of symptomatic patients with a negative venous duplex ultrasound examination will facilitate an assessment of the additional risk factors and confounders for UE-DVT.
CONCLUSIONS
UE-DVT is a common complication in hospitalized patients and mainly associated with the use of an IVD. Thromboprophylaxis or anticoagulation therapy seems ineffective in preventing UE-DVT. Also, PE needs to be studied, because it might occur more often than previously suspected. Further studies are required to understand the pathophysiology, risk factors, and appropriate treatment of UE-DVT to reduce a preventable comorbidity in hospitalized patients. A more standardized approach to prophylaxis and treatment is required, based on studies of UE-DVT rather than extrapolating recommendations from the LE-DVT experience.
ARTICLE HIGHLIGHTS.
Type of Research: A single-center, retrospective cohort study
Key Findings: A vascular laboratory review identified 1000 patients among 63,045 hospitalizations (1.6%) during a 26-month period with upper extremity deep vein thrombosis, with 77.8% associated with intravenous devices. Anticoagulation therapy was provided to 84.4% of patients at diagnosis and 54.5% at discharge. The rate of in-hospital mortality, major bleeding, and pulmonary embolism was 12%, 21.4%, and 4.8%, respectively.
Take Home Message: Upper extremity deep vein thrombosis is common among hospitalized patients, with most cases associated with intravenous devices.Treatment remains challenging.
Acknowledgments
The present study was supported by the Department of Veterans Affairs (merit awards RX000995 and CX001621), the National Institutes of Health (grants NS080168, NS097876, and AG000513 to B.K.L. and grants AG028747 and DK072488 to J.D.S.), and Baltimore Veterans Affairs Medical Center (GRECC to J.D.S.).
Footnotes
Author conflict of interest: none.
REFERENCES
- 1.Czihal M, Hoffmann U. Upper extremity deep venous thrombosis. Vasc Med 2011;16:191–202. [DOI] [PubMed] [Google Scholar]
- 2.Lamontagne F, McIntyre L, Dodek P, Heels-Ansdell D, Meade M, Pemberton J, et al. Nonleg venous thrombosis in critically ill adults. JAMA Intern Med 2014;174:689. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch DR, Ingenito EP, Goldhaber SZ. Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA 1995;274:335–7. [PubMed] [Google Scholar]
- 4.Young AM, Billingham LJ, Begum G, Kerr DJ, Hughes AI, Rea DW, et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet 2009;373:567–74. [DOI] [PubMed] [Google Scholar]
- 5.Lavau-Denes S, Lacroix P, Maubon A, Preux PM, Genet D, Labourey JL, et al. Prophylaxis of catheter-related deep vein thrombosis in cancer patients with low-dose warfarin, low molecular weight heparin, or control: a randomized, controlled, phase III study. Cancer Chemother Pharmacol 2013;72:65–73. [DOI] [PubMed] [Google Scholar]
- 6.Mansour A, Saadeh SS, Abdel-Razeq N, Khozouz O, Abunasser M, Taqash A. Clinical course and complications of catheter and non-catheter-related upper extremity deep vein thrombosis in patients with cancer. Clin Appl Thromb 2018;24:1234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Héron E, Lozinguez O, Alhenc-Gelas M, Emmerich J, Fiessinger JN. Hypercoagulable states in primary upper-extremity deep vein thrombosis. Arch Intern Med 2000;160:382–6. [DOI] [PubMed] [Google Scholar]
- 8.Hendler MF, Meschengieser SS, Blanco AN, Alberto MF, Salviú MJ, Gennari L, et al. Primary upper-extremity deep vein thrombosis: high prevalence of thrombophilic defects. Am J Hematol 2004;76:330–7. [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Albuquerque F, Pfeifer JD. Low incidence of pulmonary embolism associated with upper-extremity deep venous thrombosis. Ann Vasc Surg 2012;26:964–72. [DOI] [PubMed] [Google Scholar]
- 10.Kucher N Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861–9. [DOI] [PubMed] [Google Scholar]
- 11.Hingorani A, Ascher E, Markevich N, Yorkovich W, Schutzer R, Mutyala M, et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg 2005;41:476–8. [DOI] [PubMed] [Google Scholar]
- 12.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141 (Suppl):e419S-96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 14.Cires-Drouet R, Sharma J, McDonald T, Sorkin JD, Lal BK. Variability in the management of line-related upper extremity deep vein thrombosis. Phlebology 2019;34:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Prior SJ, Kampmann M, Sorkin JD, Caldwell K, Braganza M, et al. Measurement of thrombus resolution using three-dimensional ultrasound assessment of deep vein thrombosis volume. J Vasc Surg Venous Lymphat Disord 2014;2:140–7. [DOI] [PubMed] [Google Scholar]
- 16.Menaker J, Tabatabai A, Rector R, Dolly K, Kufera J, Lee E, et al. Incidence of cannula-associated deep vein thrombosis after veno-venous extracorporeal membrane oxygenation. ASAIO J 2017;63:588–91. [DOI] [PubMed] [Google Scholar]
- 17.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52:e162–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones MA, Lee DY, Segall JA, Landry GJ, Liem TK, Mitchell EL, et al. Characterizing resolution of catheter-associated upper extremity deep venous thrombosis. J Vasc Surg 2010;51:108–13. [DOI] [PubMed] [Google Scholar]
- 19.McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med 2003;348:1123–33. [DOI] [PubMed] [Google Scholar]
- 20.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, et al. Prevention of VTE in nonsurgical patients. Chest 2012;141(Suppl): e195S-226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens AP, Mackman N. Tissue factor and thrombosis: the clot starts here. Thromb Haemost 2010;104:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hathcock JJ. Flow effects on coagulation and thrombosis. Arterioscler Thromb Vasc Biol 2006;26:1729–37. [DOI] [PubMed] [Google Scholar]
- 23.Malinoski DJ, Ewing T, Patel MS, Nguyen D, Le T, Cui E, et al. The natural history of upper extremity deep venous thromboses in critically ill surgical and trauma patients: what is the role of anticoagulation? J Trauma 2011;71:312–6. [DOI] [PubMed] [Google Scholar]
- 24.Complications Bracke F. and lead extraction in cardiac pacing and defibrillation. Neth Heart J 2008;16(Suppl 1):S28–31. [PMC free article] [PubMed] [Google Scholar]
- 25.Cote LP, Greenberg S, Caprini JA, Tafur A, Choi C, Muñoz FJ, et al. Comparisons between upper and lower extremity deep vein thrombosis: a review of the RIETE registry. Clin Appl Thromb Hemost 2017;23:748–54. [DOI] [PubMed] [Google Scholar]
- 26.Spencer FA, Emery C, Lessard D, Goldberg RJ. Upper extremity deep vein thrombosis: a community-based perspective. Am J Med 2007;120:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masding A, Preston SD, Toshner M, Barnett J, Harries C, Dimopoulos K, et al. Chronic thromboembolic pulmonary hypertension following long-term peripherally inserted central venous catheter use. Pulm Circ 2019;9: 2045894019859474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton DH, Monreal Bosch M, Amendola M, Wolfe L, Perez Ductor C, Lecumberri R, et al. Analysis of non-catheter-associated upper extremity deep venous thrombosis from the RIETE registry. J Vasc Surg Venous Lymphat Disord 2017;5:18–24.e1. [DOI] [PubMed] [Google Scholar]
- 29.Lechner D, Wiener C, Weltermann A, Eischer L, Eichinger S, Kyrle PA. Comparison between idiopathic deep vein thrombosis of the upper and lower extremity regarding risk factors and recurrence. J Thromb Haemost 2008;6:1269–74. [DOI] [PubMed] [Google Scholar]


