Abstract
Melanoma is a relentless type of skin cancer which involves myriad signaling pathways which regulate many cellular processes. This makes melanoma difficult to treat, especially when identified late. At present, therapeutics include chemotherapy, surgical resection, biochemotherapy, immunotherapy, photodynamic and targeted approaches. These interventions are usually administered as either a single-drug or in combination, based on tumor location, stage, and patients' overall health condition. However, treatment efficacy generally decreases as patients develop treatment resistance. Genetic profiling of melanocytes and the discovery of novel molecular factors involved in the pathogenesis of melanoma have helped to identify new therapeutic targets. In this literature review, we examine several newly approved therapies, and briefly describe several therapies being assessed for melanoma. The goal is to provide a comprehensive overview of recent developments and to consider future directions in the field of melanoma.
Keywords: cancer, melanoma, intervention, target, resistance
Introduction
Melanoma is best described as a relentless, aggressive, heterogeneous disease[1–2]. Unfortunately, the global prevalence of melanoma is also rapidly rising[3–4]. The American Cancer Society estimated that in 2021 in America alone there would be approximately 106 110 new melanoma cases, with 7180 people dying as a result of disease progression[5]. With an overall mortality of approximately 20%, melanoma is responsible for 80% of skin cancer-related deaths worldwide, largely attributed to its ability to evade treatment and metastasize to other organs[2,6]. Melanoma is more common in Caucasians[7], and occurs through pigment-producing cell mutations, known as melanocytes. These melanocytes are normally found in the inner ear, eye, leptomeninges, and skin[8–11] but can be found elsewhere. Early diagnosis is key to the success of interventions because once metastasizing, melanoma quickly becomes adept at evading targeted therapies[12–14]. In order to improve such dismal outcomes, Food and Drug Administration (FDA) has regularly assessed and approved a number of interventions for this insidious disease (Fig. 1). These treatments include surgical resection, radiotherapy, chemotherapy, immunotherapy, photodynamic therapy, or targeted therapies based on specific tumor stages, genetic profiles and location. Yet, for individuals at stages Ⅰ-Ⅲ, the most common treatment choice is surgical resection[4,15–16] and for solitary metastatic melanoma, metastasectomy remains the primary intervention. Of course, for some metastatic melanoma patients, chemotherapy is also considered[4,17]. Radiation therapy is on the other hand, seldom recommended for treating primary tumors, but it can be beneficial as an adjuvant intervention for those with brain, bone, or skin metastases[18]. The two main limitations in melanoma therapy appear to be adverse effects (AEs), which manifest as gastrointestinal and cutaneous toxicities, and there is a lack of tumor cell specificity[19]. A further limitation is resistance to chemotherapy, targeted treatments, immunotherapies, and intralesional therapies[17]. Genetic profiling has been adopted in order to address these limitations, and has helped to identify key molecules involved in the pathophysiology of malignant melanocyte transformation[19]. Here, we describe what is known about cutaneous melanoma pathogenesis and discuss recently approved interventions and other therapeutic advances in melanoma biology.
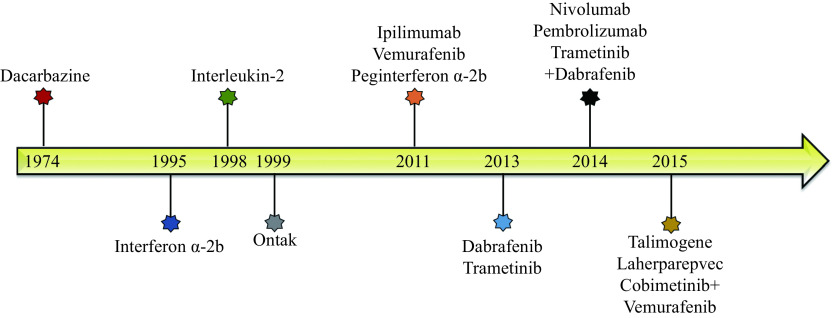
Figure 1.
Timeline of Food and Drug Administration approved melanoma interventions.
Pathogenesis of cutaneous melanoma
Neoplastic transformation of neural crest-derived melanocytes is believed to be responsible for melanoma[20]. Among other things, Caucasians are differentiated from intermittent or chronically solar-exposed individuals but melanoma can appear anywhere on the skin's surface. This suggests there is an evolutionary component although the location of melanoma also appears to be influenced by a patient's age and sex. We also know, approximately 20% of all melanomas in the neck and head area have a worse prognosis compared to melanomas in other regions. This may be the result of mutations in multiple genes including B-Raf proto-oncogene (BRAF), neurofibromin 1 (NF1), NRAS, phosphatase and tensin homolog (PTEN), cyclin-dependent kinase inhibitor 2A (CDKN2A), cyclin-dependent kinase 4 (CDK4), telomerase reverse transcriptase (TERT), and tumor protein p53 (TP53), which have been reported in melanoma[20–22]. Mutations in these genes principally affect two key pathways in melanoma,i.e., the phosphoinositol-3-kinase (PI3K)/AKT pathway, and the RAS/RAF/MEK/ERK signaling cascade, which is a mitogen-activated protein kinase pathway. The important ones are discussed here[20].
BRAF is a protooncogene that codes for serine/threonine-protein kinase and plays a crucial role in cell proliferation and growth. BRAF is also involved in the RAS-RAF-MEK-ERK kinase pathway[20] and according to reports, an active BRAF mutation can be found in 40% to 60% of all melanoma cases. In response to growth cues, BRAF normally forms homo- or hetero-dimers with another RAF kinase[20,23]. Activating mutations in BRAF creates self-sufficiency and constitutively active monomers which support uncontrolled cell proliferation and ultimately results in tumor development[24]. Among the many mutations that occur in BRAF, the missense mutation V600E, which causes valine to glutamic acid conversion, is the most frequently found mutation and counts for around ninety percent of all activating mutations in BRAF[25–26]. The second most commonly occurring mutation is V600K in which valine is converted to lysine. Additionally, research has shown that BRAF mutations such as V600E can be observed in 68% of all benign nevi suggesting that BRAF mutations may not play a role in melanoma carcinogenesis[27]. As nevi are generally stable after formation, some researchers believe that BRAF mutations have a role in the development of melanocytic neoplasia[27]. Recent research has demonstrated that the initial stages of melanoma development also known as the radial growth phase has a low BRAF mutation rate of around 10%. This supports the hypothesis that BRAF does not play a role in the initiation of melanoma[28]. On the other hand, 60% to 70% of vertical growth lesions and melanomas which have metastasized carry BRAF mutations, implicating these mutations in cancer progression[27–28]. Due to the high prevalence of BRAF V600E mutations in melanoma patients, researchers also consider it a prime target for anti-melanoma therapeutics and a large number of drugs have been developed targeting this mutation[20].
NRAS activating mutations are the second most common mutations and cause aberrant signaling through the MAPK pathway in melanoma[20]. In 15% to 30% of melanoma patients, NRAS is mutated, and the majority of these are missense mutations in codons 12, 13, or 61[29–29]. The NRAS-active GTP-bound state is therefore prolonged when these codons are mutated, which abnormally sustains NRAS signaling across both the PI3K and MAPK pathways[30–31]. Importantly, BRAF and NRAS mutations are thought to be mutually exclusive; yet, co-mutations do occur on occasion[20].
The tumor suppressor NF1 is altered in 10% to 15% of melanoma patients, making it the third most commonly mutated gene in melanoma[32–33]. The NF1 protein inhibits downstream RAS signaling by converting the active RAS-guanosine triphosphate (RAS-GTP) to the inactive RAS-guanosine diphosphate (RAS-GDP) form[34]. As a result, NF1 loss-of-function causes NRAS hyperactivation and enhances PI3K and MAPK pathway signaling[32]. NF1 genomic perturbations are more common in melanomas linked with continuous sun exposure and are frequently associated with a large variety of other genomic mutations, including that of NRAS and BRAF[35–36].
The receptor tyrosine kinase c-Kit has been implicated in melanoma proliferation and survival that is mediated via the RAS/RAF/MEK/ERK and the PI3K/AKT pathways[37–38]. The frequency of c-Kit mutations is around 2% to 5% in acral melanomas which are found on soles and palms[39]. Chromosomal duplications in the c-Kit gene have also been reported in 2% of cutaneous melanoma cases and 23% of acral melanomas[37]. Increased production of the KIT protein typically occurs due to point mutations and gene duplications in the c-Kit gene, which affects melanocyte proliferation, cell death, chemotaxis, adhesion, as well as contribute to tumorigenesis[40]. As a result, anti-melanoma therapies might be developed using c-Kit as a target[41].
The tumor suppressor gene P53 has been linked to a wide range of human cancers, including lung, breast, prostate, and colorectal cancers[42–50]. Cellular stress or DNA damage activates the p53 protein, which in turn causes cell death[51]. There is a large amount of research done on p53 but in melanoma, the role of this gene is not yet clear[52]. Immunohistochemistry studies reveal a wide range of p53 mutations, with researchers reporting expression rates of 11% to 85%[52]. The P53 gene locus does not appear to have mutations, and wild-type p53 expression is retained, according to sequence analyses[42]. Melanoma cells do not usually undergo apoptosis in response to chemotoxic drugs or gamma radiation, despite the lack of p53 mutations, suggesting that p53 may not be functioning effectively[41]. Another theory is that other proteins inactivate p53, preventing it from performing its tumor-suppressive actions[41].
The genes coding for p16INK4a and CDK4 have also been found to play a role in familial melanoma development[53]. There is a hereditary predisposition to develop melanoma in the case of familial melanoma and approximately 5% to 10% of melanoma patients have a family member who has also been diagnosed with the disease[54–55]. An autosomal dominant mode of inheritance has been observed for this predisposition with a 53% penetrance rate by the eighth decade[54]. CDK4 and p16INK4a govern cell cycle progression from G1 to S phase, together with other D-type cyclins, CDK6, and pRb[56]. CDK4 is a proto-oncogene that promotes the passage from G1 to S phase, enabling cell proliferation, when activated by cyclin D1, whereas p16INK4a inhibits CDK4 function, arresting cell proliferation[56]. According to studies, an inactivated p16INKA mutation on 9p21 can be detected in between 25% to 60% of melanoma patients with a family history of melanoma, although mutations in the CDK4 gene have been discovered at a significantly lower incidence[53–54,57]. Given these molecular alterations, inhibiting CDK4 would be a logical next step in preventing further cell cycle progression and limiting uncontrolled melanoma development[41].
PTEN is a tumor suppressor gene that regulates the cell cycle[22]. Dysregulation of PTEN has been found in 10% to 30% of cutaneous melanoma patients in the vertical growth phase and metastases[22]. The most common changes observed in PTEN are missense and frameshift mutations, as well as chromosomal deletions, however epigenetic mechanisms including microRNAs have also been found to regulate PTEN expression post-transcriptionally[58]. PTEN mutations and NRAS mutations are mutually exclusive; however, they commonly co-occur with BRAF gain-of-function[20]. The loss of PTEN is associated with increased PI3K/AKT pathway activation in melanoma[59]. Indeed, PTEN loss-of-function and BRAF mutations together activate both the PI3K and MAPK pathways, equating to NRAS activation alone[59–60]. PTEN deficiency is one of the mechanisms behind acquired resistance in BRAF mutant melanoma treated with BRAF inhibitors in the clinical context[61].
It is well-known that ultraviolet (UV) light radiation from sunlight is an environmental factors responsible for melanoma development[62–64]. Sun exposure does raise the risk of developing melanoma, which is linked to the UV intensity and, in particular, the UV-B spectrum[63]. Additionally, it has been reported that sun exposure patterns and timing have been linked to an elevated risk of melanoma[20]. When compared to a chronic continuous pattern of sun exposure, which is more typically linked to actinic keratosis and non-melanoma skin malignancies, severe and intermittent sun exposure is associated with a greater risk of developing melanoma[20,65]. Moreover, a history of sunburn during childhood or adolescence is linked to a higher chance of getting melanoma, whereas people who have had more than five incidents of severe sunburn are at a 2-fold greater risk of developing melanoma[65]. Individuals exposed to artificial sources of UV-A have been shown to be at a greater risk of melanoma development[20]. According to a number of reports, patients with psoriasis who had UV-A radiation phototherapy, as well as those who used sunbeds, had an elevated risk of melanoma development[66]. Several reports including meta-analyses have shown a positive link between the use of sunbeds and the risk of melanoma, particularly in children, presenting a serious public health concern[67]. Sunbed UV radiation has been designated as a human carcinogen[68]. Other lifestyle related determinants, such as tobacco/smoke addiction, have not been directly linked to melanoma[69].
Furthermore, host risk factors such as frequency of congenital and acquired melanocytic nevi, genetic predisposition, and family history all play a role in melanoma development[70]. Melanoma develops on a pre-existing nevus in around 25% of cases. Not only the total number of nevi but also their size and type are linked to an increased risk of melanoma[71]. The polymorphisms of the melanocortin 1 receptor gene are responsible for the various human skin-color phenotypes in terms of genetic vulnerability[72]. Individuals with traits such as light eyes, light complexion, and red hair have poor pigmentation, makes them more sensitive to UV radiation[72]. Melanomas have also been reported to occur in families that are predisposed to certain types of malignancies such as melanoma-astrocytoma syndrome and familial atypical multiple mole-melanoma syndrome. Familial retinoblastoma, Xeroderma pigmentosum, Li-Fraumeni cancer syndrome, and Lynch syndrome type Ⅱ are other genetic disorders linked to an elevated risk of melanoma[20,73].
Surgery
The main treatment for melanoma is surgical resection, where the lesion is removed along with extra tissue to get rid of any cancerous cells present in the area[5]. Surgical procedures vary depending on the clinical and pathological characteristics of the tumor. For in situ melanoma, excision includes safety margins of 0.5 cm, tumors having a thickness of up to 2 mm, the excision safety margins is 1 cm and for tumors larger than 2 mm, the excision safety margin is 2 cm[15]. To improve overall survival, adjuvant therapies, like immunotherapy and targeted therapy are often administered[15,17].
Chemotherapy
For advanced melanoma, chemotherapy is the main treatment choice. Various chemotherapeutic combinations have been assessed to see whether they might improve clinical outcomes, but overall survival (OS) has not improved[74]. One of the principal reasons for drug resistance to chemotherapy in melanoma is resistance to apoptosis[75]. Chemotherapy still remains crucial in the palliative care of advanced, refractory, or relapsed melanomas despite supplementation by additional options[74].
Dacarbazine
Dacarbazine (DTIC) is an alkylating agent that has been used as a standard treatment for metastatic melanoma for more than thirty years. The mode of action occurs through DNA damage which results in cell growth arrest and cell death. Research has also shown that a full response using DTIC can be achieved in <5% of patients, with a 5-year OS rate of 2% to 6% of patients [76]. Multiple clinical trials using DTIC alone or together with several other chemotherapies, targeted therapies, and immunotherapies are ongoing (for details refer to the ClinicalTrials.gov). Treatment using DTIC is not without side effects, the most common adverse events are nausea and vomiting. It has also been reported that the use of DTIC leads to suppression of blood cell production in bone marrow leading to neutropenia and anemia. Further, flu-like symptoms and diarrhea have also been reported in relation to treatment with DTIC[77].
Temozolomide
Temozolomide (TMZ) is a lipophilic molecule belonging to the imidazotetrazine class of DNA alkylating agents and has been used for treating various types of solid tumors including brain tumors and advanced melanoma. TMZ is an oral prodrug which is a derivative of the alkylating agent DTIC and is prescribed as an active metabolite[76,78]. TMZ is a stable molecule at acidic pH values but has been shown to be labile above a pH of 7. A plasma half-life of 1.8 hours has been reported at pH 7.4 for TMZ [79]. In comparison to DTIC, TMZ appears to produce less improvement in median progression-free survival (PFS), but no changes were observed in OS or objective response rates[80]. TMZ has a distinct advantage over other medicaments used to treat advanced melanoma, such as Cisplatin and DTIC, because of molecular size and ability to pass the blood-brain barrier while still exhibiting anti-tumor activity. This is especially intriguing because substantial penetration into the central nervous system has been reported in advanced melanoma cases, which usually results in death[81].
Cisplatin
Cisplatin is one of the standard chemotherapeutic drugs used to manage diverse cancers, including advanced melanoma. It is a derivative of cis-diamminedichloroplatinum(Ⅱ) (CDDP)[82]. The mode of action is via inducing DNA damage leading to proliferative arrest or induction of mitochondrial apoptotic pathway. Recent reports indicate that the cytotoxic, as well as the cytostatic functions of CDDP, involve both cytoplasmic and nuclear mechanisms which promote oxidative stress eventually leading to direct cytotoxic functions or indirectly, enhance DNA damage[83–85].
5-Fluorouracil
5-Fluorouracil (5-FU) is another advocated chemotherapeutic drug used in the treatment of advanced melanoma. 5-FU targets the enzyme thymidine monophosphate and thymidylate synthase (TS). Inhibiting TS causes a thymidylate deficit, unbalances the nucleotide pool, and impairs DNA repair and replication. It is generally accepted that TS inhibition results in cell-cycle arrest and therefore DNA damage[86–87]. Unfortunately, intra-tumoral TS overexpression is extremely stimulated in melanoma patients in response to 5-FU and other TS inhibitors, limiting its usage[82].
Vinca alkaloids and taxanes
Apart from the use of alkylating agents to treat melanoma, many other chemotherapeutic drugs have also been tested for melanoma. For example, the vinca alkaloids were the very first class of agents discovered that targeted microtubules. These drugs prevent tubulin polymerization, causing cell arrest in metaphase subsequently leading to apoptosis. Several members of this class, including Vinorelbine[88], Vincristine[89], and Vinblastine, have been evaluated as single agents. The response rates to these drugs appear similar to those observed in DTIC and TMZ ranging from 10 to 20 percent, with a PFS of 2 to 4 months and no improvement in OS. Bone marrow suppression, neuropathy, and gastrointestinal toxicity have been more commonly observed AEs[90]. Another class of agents used in the treatment of melanoma are taxanes. Taxanes stabilize microtubules and interfere with their disassembly. Both Docetaxel and Paclitaxel have been studied as single treatments in patients with metastatic melanoma, and both have shown to have limited activity, similar to that of Vinca alkaloids[83].
Photodynamic therapy
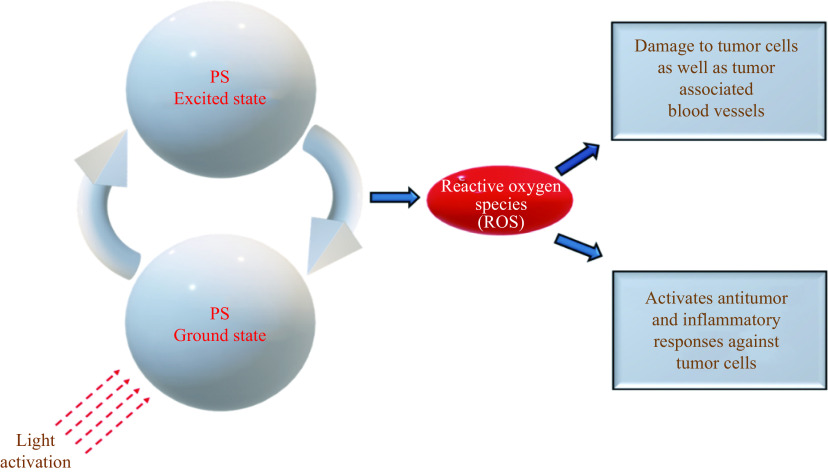
Photodynamic therapy (PDT) is a method used for metastatic melanoma patients in stages Ⅲ/Ⅳ[17,91]. PDT is a minimally invasive procedure which involves a photosensitizer (PS) and light of a certain wavelength to activate the PS[17]. When the PS is excited, it generates a reactive oxygen species (ROS)[92] and induces irreversible damage to tumor cells as well as blood vessels associated with these tumors (Fig. 2). Additionally, ROS activates antitumor and inflammatory responses. A novel PS used in melanoma cell lines as well as mouse models is Acai oil in nano emulsion. The results of this basic research have demonstrated that this PS causes 85% melanoma cell death by necrosis and late apoptosis, while preserving normal cells[93]. A combination of chemotherapy, e.g., DTIC and PDT, has also been found to be an effective therapeutic at reducing resistance in unpigmented and pigmented metastatic melanoma[94]. A combination of novel immunostimulatory therapies and PDT might be more effective at eradicating initial tumors and micro metastases. This intervention may also reduce melanoma recurrences[91] but further PDT trials are ongoing (NCT02685592) and we await findings.
Figure 2.
Activation of photosensitizer (PS) leads to production of reactive oxygen species (ROS), which damages tumor cells and brings about antitumor immune responses.
Electrochemotherapy
Electrochemotherapy (ECT) is a method where Cisplatin and Bleomycin are used along with high-intensity electrical pulses to facilitate the delivery of drugs to the cells[95–96]. Furthermore, ECT is beneficial in treating subcutaneous and cutaneous melanoma[97–98]. An overall response rate of 85% was reported in a review of the European Standard Operating Procedures for Electrochemotherapy and no major AEs were observed[98]. Another interesting feature of ECT is that normally treated nodules do not recur in the same location, presumably because the procedure destroys the lymphatic stream[95]. However, evidence is sparse and there is a need for further research before reaching any conclusion.
Immunotherapy
Melanoma is one cancer that responds reasonably well to immune modulation[99]. Multiple factors have been found to explain melanoma cell sensitivity to immune system activation. These include high tumor mutational burden owing to UV light exposure, production of cancer-testis antigens, and mimicry of melanocyte lineage proteins with pathogen-associated antigens[65,100–101]. Tumor-infiltrating lymphocytes (TILs) play an important role in the generation of an anti-tumor immune response, and a subset of TILs in melanoma has shown cytolytic activity against autologous tumors[102]. Their presence is also linked to a better prognosis and a lower likelihood of metastasis[99]. Multiple clinical studies using local or systemic immunomodulatory drugs such as interleukin-2 (IL-2), interferon-alpha (IFN-α)[103–104], adoptive cell transfer techniques[105], and cancer vaccines[106–107] have been conducted in recent experiments[99]. Despite some initial evidence of activity, these trials have not found a long-term benefit for patients with metastatic melanoma[99]. Recent research has also found that immune checkpoint inhibitors (ICIs) targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) have revolutionized the treatment of unresectable and metastatic melanoma, as well as those at high risk of recurrence following resection[108–109]. Unfortunately, ICI treatments are beset by issues such as primary and secondary resistance[110].
The goal of combining immunotherapies is to enhance responses and eliminate resistance, while biomarker identification is critical for patient selection optimization[99]. It was established for the first time in the 19th century that the immune system and cancer are associated, based on frequent observations of tumors at chronic inflammatory sites and the presence of immune cells in and around tumor tissues[111]. T-cells identify tumor-specific antigens during antitumor responses, then initiate activation, proliferate and differentiate. This enables T-cells to destroy cells expressing tumor-specific antigens. Further, inhibitory and stimulatory signaling pathways which restrict T-cell antitumoral responses and cancer cells also escape T-cell detection because they generally do not express B7 molecules[112]. In a number of cancers, complex interactions between the tumor and immune system play a crucial role in metastatic spread to different remote sites. As far as we know, the main reason for cancer-related deaths is metastasis, therefore, more precise prognostic markers are needed[113].
TILs are markers for lymph node metastasis[114]. TILs can modulate host immune responses against cancer cells and are linked to improved survival and positive outcome in malignant melanoma patients[114–115]. Immunotherapy appears to be a viable treatment for individuals with advanced-stage metastatic melanoma, based on these findings, compared to the prior standard of care therapies[114,116–118]. The tumor microenvironment (TME) along with various cellular effectors of inflammation and mediators, determine the success rate of immunotherapies[116]. Signal transduction pathways are also implicated in tumor-related inflammation and are now more understood. Therefore, more target molecules have been identified which might enhance early cancer diagnosis and treatment[115]. Regardless of positive results, relapse of cancers and differential success among various cancers are not surprising. The success rate of ICIs even in highly responsive cancers is only 50%[119]. Resistance to primary and acquired immunotherapy is common and might be because of the lack of recognition by T-cells. Furthermore, it may also include diverse components of cancer immune cycle (like M2 macrophages, myeloid-derived suppressor cells (MDSCs), and regulatory T-cells (Tregs), and interactions among numerous signaling molecules as well as pathways that inhibit immune cell infiltration or function within the TME[120–121]. Better knowledge of pathophysiology and a greater understanding of the role of the immune system in tumor evolution has led to the approval and development of various immunotherapies. Patients with melanoma of unknown primary site display higher survival rates and outcomes in comparison to stage-matched melanoma of known primary site due to higher immunogenicity as reflected in the immunologically mediated primary site regression[122].
Interleukin-2
IL-2 is a growth factor used for in-vitro T cell propagation[123]. High-dose IL-2 has been shown to exhibit anti-tumor activity and, was approved by the FDA in 1998 for the treatment of metastatic melanoma[124]. In a recent meta-analysis, evidence suggests that the overall response rate for treatment with IL-2 treatment was 19.7%, partial response was 12.5%, and the complete response was only 4%.Intermediate and high dosages did not show complete response differences, and therefore the therapeutic dose should be reevaluated[125]. Fibronectin and serum vascular endothelial growth factor (VEGF) have been identified as biomarkers of interest for IL-2 therapeutic response; hence patients need to be assessed according to these biomarkers before undergoing IL-2 based therapies[126]. Tachycardia, hypotension, peripheral edema, cardiac arrhythmias, and reversible multisystem organ failure are the AEs[127]. Like interferons (IFNs), IL-2 is still under investigation through clinical trials, in combination with radiotherapy, chemotherapy, targeted therapies, and immunotherapy (for details refer to the ClinicalTrials.gov).
Peginterferon α-2b
In 2011, the FDA approved Peginterferon α-2b (Peg-IFN) as adjuvant therapy for stage Ⅲ melanoma[128]. Peginterferon is an amalgamation of IFN α-2b with a molecule of polyethylene glycol (PEG). This molecule has been reported to allow the compound to remain in the blood for longer, thereby improving the therapeutic effect[129]. Grade 1 liver toxicity, skin rash, anemia, and neutropenia were the most common AEs. However, the only observed grade 3/4 toxicities were hyponatremia and lymphopenia[7].
Inhibition of Tregs
Tregs have been illustrated to suppress activated effector T cells (Teffs) and inhibit antitumoral immune responses[127,130]. Tregs appear in TME and peripheral circulation in melanoma and are linked to poor clinical outcomes[131]. The therapeutic strategy involves Treg suppression and thereby enhances antitumoral immunity. In 1999, Ontak was approved by the FDA[132], which is the fusion of diphtheria toxin and IL-2 protein that selectively eradicates IL-2 receptor-expressing Tregs from the peripheral blood. In a phase-Ⅱ trial of stage-Ⅳ melanoma patients, a partial response of 16.7%, a mixed response of 15%, and 5% stable disease were observed[133]. On the other hand, another report suggested that metastatic melanoma patients administered with Ontak displayed no disease regression, no elimination of regulatory T cells, and no objective clinical response[134].
Blockade of cytotoxic T-lymphocyte-associated antigen 4
CTLA-4 is a cell surface inhibitory checkpoint receptor that blocks T-cell activation and leads to the induction of immune tolerance. In 2011, the FDA approved Ipilimumab, an anti-CTLA-4 antibody for treating advanced melanomas[135–136]. CTLA-4 antibodies block the inhibitory effect thereby enhancing the production of pro-inflammatory cytokines[137] and increasing T-cell clonal expansion and infiltration[138]. Multiple combination therapies have been studied but no improvement over Ipilimumab monotherapy has been demonstrated[139]. In a combined therapy study, Peg-IFN was administered along with Ipilimumab and showed a 40% overall response rate and 5.9 months of median PFS[140]. Likewise, in phase Ⅳ clinical trial, advanced melanoma patients were randomly administered Ipilimumab only, or Ipilimumab combined with gp100 peptide vaccine, or vaccine only. Ipilimumab monotherapy appeared to produce a superior response rate with a median OS of 10.1 months. Ipilimumab along with gp100 had a median OS of 10 months. These data suggest that Ipilimumab is useful because of good OS rates compared to vaccine monotherapy which had a median OS of 6.4 months[141]. The AEs related to Ipilimumab include endocrinopathies, autoimmune alterations such as colitis, dermatitis, hepatitis, and very rarely neuritis[142]. Intense immunosuppressive drugs or corticosteroids may be used to manage these AEs[4]. Currently, various clinical trials are ongoing with Ipilimumab in combination with immunotherapy, radiotherapy, chemotherapy, and targeted therapies (for details refer to the ClinicalTrials.gov). Tremelimumab is an antibody against CTLA-4 and is undergoing clinical trials as a monotherapy (NCT00378482) and in combination with other immunotherapies (NCT02535078/NCT02643303 and NCT01103635).
Programmed cell death protein 1/PD-1 ligand blockade
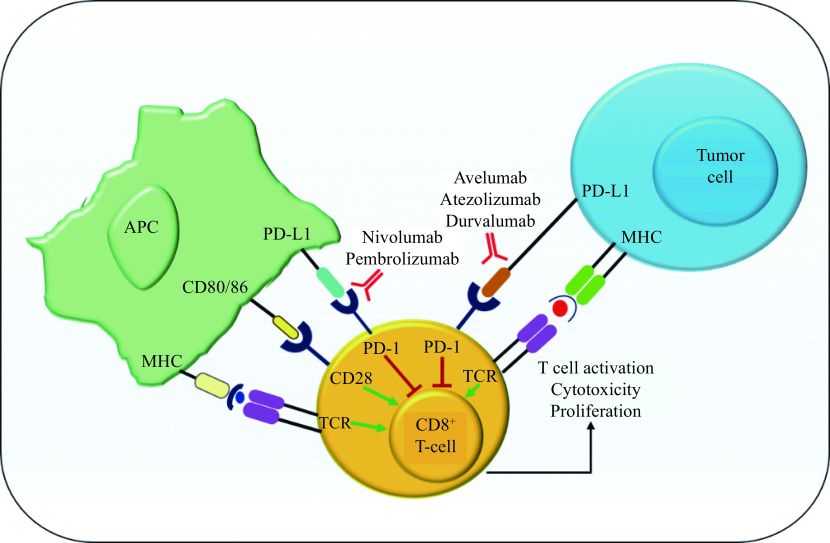
The PD-1 receptor serves as a co-inhibitory molecule for T-cells and suppresses activation of T-cells via binding to PD-1 ligands: PD-L1 and PD-L2. These ligands are expressed on antigen-presenting cells (APCs), in myriad human tumors as well as cells present in TME, in response to inflammatory stimuli. A recent report suggested that soluble PD-1 levels in the plasma can be utilized to predict prognosis and clinical outcomes in advanced melanoma patients receiving immunotherapy. As PD-1 is primarily expressed on activated TILs, we can hypothesize that low PD-1 levels in plasma indicate enrichment of TILs in the TME. Whereas, high plasmatic levels of this immune checkpoint may represent an impoverishment by the TME of lymphocytic infiltrate[143]. However, the utility of PD-L1 immunostaining for anti-PD-1 treatment as a predictive biomarker remains unclear[144]. Nivolumab a high-affinity anti-PD-1 monoclonal antibody was approved by FDA in 2014 for the treatment of metastatic melanoma patients[145]. Nivolumab inhibits the binding that occurs between PD-L1 and PD-L2 to its receptor PD-1 (Fig. 3)[146]. Blocking the interaction between PD-1 and its ligands initiates immune responses and activates an antitumor response which regresses tumor[147]. On the other hand, PD-L1-negative individuals may benefit from anti-PD-1 or anti-PD-L1 therapy. Indeed, objective responses in PD-L1-negative individuals have been recorded (typically between 11% to 20%)[148–149]. Nivolumab, with a PFS of 6.9 months, seems to be highly effective compared to Ipilimumab monotherapy which shows 2.9 months PFS or chemotherapy with 2.2 months median PFS[147]. Ipilimumab and Nivolumab combination therapy yielded an 11.5 months median PFS in PD-L1 negative patients which is far superior to monotherapies[147,150].
Figure 3.
Mechanism of action of PD-1/PD-L1.
APC: antigen presenting cell; MHC: major histocompatibility complex; TCR: T-cell receptor; PD-1: programmed cell death protein 1; PD-L1: programmed death ligand-1.
In 2015, Pembrolizumab was approved by the FDA for the treatment of melanoma in advanced stages and can be used as a standard treatment option for Ipilimumab refractory melanoma[151–153]. Pembrolizumab treatment led to the prolonged OS and PFS in advanced melanoma patients with low toxicity than Ipilimumab[151]. An important correlation was observed in a clinical trial where melanoma patients responding to Pembrolizumab treatment had an increase in several T-cell-inflamed genes including HLA-DRB1, HLA-DQA1, HLA-E, STAT1, TIGIT, CD8A, CXCR6, CXCL9, CCL5, LAG3, PSMB10, IDO1, CD27, CD276, CD274, NKG7, CMKLR1, and PDCD1LG2 compared to non-responders[154]. Combining Peg-IFN with Pembrolizumab is clinically active and well tolerated by advanced melanoma patients where surgical removal is not possible[155]. Diarrhea, rash, fatigue, headaches, nausea, arthralgia are the most frequent AEs related to this therapy[4]. There are multiple ongoing clinical trials (Table 1 ) using Pembrolizumab and Nivolumab as monotherapy or in combination with radiotherapy, immunotherapy, chemotherapy, and targeted therapies (for details refer to the ClinicalTrials.gov). JS100 is another anti-PD-1 molecule that is being investigated in clinical trials as a monotherapy (NCT03013101) and also in combination with targeted therapies (NCT03086174). Avelumab, Atezolizumab, Durvalumab (Fig. 3), PDR001, CK-301, and REGN2810 are some of the other drugs that are being investigated as monotherapies, or in combination with immunotherapy or targeted therapy.
Table 1. Top 10 ongoing clinical trials for metastatic melanoma and advanced metastatic melanoma.
| No. | Title | Status | Study results | Conditions | Interventions | Locations |
| PD-1: programmed cell death protein 1; PD-L1: programmed death ligand-1; TIL-ACT: Tumor-Infiltrating Lymphocyte-Adoptive Cell Therapy; NMA: non-myeloablative; IDO: Indoleamine 2,3-dioxygenase; IL-2: interleukin-2. | ||||||
| 1 | PET/CT whole-body dynamic acquisition at FDG to metastatic melanoma under immunotherapy | Recruiting | No results available | Metastatic melanoma | Diagnostic test: value of 4D body-to-whole dynamic acquisition in FDG | Hospital University ff Brest, Brest, Finistere, France |
| 2 | IN10018 monotherapy and combination therapy for metastatic melanoma | Recruiting | No results available | Metastatic melanoma | Drug: lN10018
Drug: Cobimetinib |
Sylvester Comprehensive Cancer Center, Miami, Florida, United States
Massachusetts General Hospital, Boston, Massachusetts, United States Dana-Farber Cancer Institute, Boston, Massachusetts, United States Columbia University Medical Center, New York, New York, United States MD Anderson, Houston, Texas, United States |
| 3 | Safety of AV-MEL-1 with anti-PD-1 therapy in metastatic melanoma | Recruiting | No results available | Metastatic melanoma | Drug: AV-MEL-l | Hoag Hospital - Irvine, Irvine, California, United States
Hoag Memorial Hospital Presbyterian, Newport Beach, California, United States |
| 4 | Ipilumumab and Nivolumab with or without hypofractionated radiotherapy in patients with metastatic melanoma | Recruiting | No results available | Metastatic melanoma | Radiation: hypofractionated radiation therapy
Drug: Nivolumab Drug: Ipilimumab |
Abramson Cancer Center, Philadelphia, Pennsylvania, United States |
| 5 | TIL-ACT after NMA Chemo with IL-2 and Nivo rescue in metastatic melanoma (mMEL) | Active, not recruiting | No results available | Metastatic melanoma | Other: TIL
Drug: Cyclophosphamide Drug: Fludarabine Drug: lnterleukin-2 Drug: Nivolumab |
CHUV Oncology department, Lausanne, Vaud, Switzerland |
| 6 | Combination therapy with Nivolumab and PD-L1/IDO peptide vaccine to patients with metastatic melanoma | Recruiting | No results available | Metastatic melanoma | Drug: Nivolumab
Biological: PD-L1/IDO peptide vaccine |
Center for Cancer Immune Therapy, Dept. of Oncology/Hematology, Herlev, Denmark
Herlev Hospital, Herlev, Denmark |
| 7 | A study to evaluate safety and therapeutic activity of RO6874281 in combination with Pembrolizumab, in participants with advanced or metastatic melanoma | Active, not recruiting | No results available | Metastatic melanoma | Drug: RO6874281
Drug: Pembrolizumab |
Yale University, New Haven, Connecticut, United States
University of Iowa, Iowa City, Iowa, United States Beth Israel Deaconess Med Ctr, Boston, Massachusetts, United States Dana Farber Cancer Institute, Boston, Massachusetts, United States Melanoma Institute Australia, North Sydney, New South Wales, Australia Peter Maccallum Cancer Institute; Clinical Trial Unit, Melbourne. Victoria, Australia UZ Antwerpen, Edegem, Belgium and 16 more… |
| 8 | Phase Ⅰ Clinical trial of Tremelimumab plus MEDI3617 in patients with unresectable stage Ⅲ or stage Ⅳ melanoma | Active, not recruiting | No results available | Metastatic melanoma | Drug: Tremelimumab
Drug: MEDI3617 |
Dana Farber Cancer Institute, Boston, Massachusetts, United States
Beth Israel Deaconess Medical Center Boston, Massachusetts, United States |
| 9 | Comparison of high-dose IL-2 and high-dose IL-2 with radiation therapy in patients with metastatic melanoma | Active, not recruiting | No results available | Metastatic melanoma | Other: radiation therapy and high-dose IL-2
Drug: High-dose IL-2 |
Providence Cancer Center Portland, Oregon, United States |
| 10 | Cabozantinib and Pembrolizumab for advanced metastatic melanoma | Recruiting | No results available | Advanced metastatic melanoma | Drug: Cabozantinib
Drug: Pembrolizumab |
University of Iowa Hospitals and Clinics, Iowa City, Iowa, United States |
Toll-like receptor agonists
Toll-like receptors (TLRs) are members of IL-1R superfamily and are type-Ⅰ membrane glycoproteins capable of inducing local cytokine production like IL-12 and IFN-α, which enhance local immune responses[156]. Furthermore, TLRs increase antitumor immunity[157]. Agonists of TLR are potent vaccine adjuvants and can stimulate the immune system in TME. Resiquimod is an agonist of TLR-7/8 and can stimulate plasmacytoid dendritic cells (pDCs, TLR7) as well as myeloid dendritic cells (mDCs, TLR8) in patients with advanced-stage melanoma. Patients receiving Resiquimod showed upregulation of IFN and IFN-γ at the site of vaccination, by activation of mDC/pDC and improvement in antitumor response with regression of in-transit melanoma metastases[158]. Clinical trials using TLR agonists in combination with immunotherapies (NCT00960752 and NCT02320305) and chemotherapy (NCT02650635) are ongoing.
Oncolytic virus therapy
The use of oncolytic viruses to treat melanoma was allowed by the FDA in 2015; Talimogene laherparepvec (T-VEC) is a type-1 genetically modified herpes simplex virus[150,159–160]. This modified non-pathogenic viral strain is specifically inserted into metastatic melanoma nodules and, while it invades both healthy and malignant cells, it replicates only in melanoma cells. This contributes to tumor cell lysis and the release of tumor-specific antigens[159]. APCs identify these released antigens, thereby triggering melanoma-specific T-cell responses. In a phase Ⅱ clinical trial, T-VEC was administered to patients with unresectable stage Ⅲ or refractory stage Ⅳ melanoma, and an objective clinical response of 28% was reported[159]. This approach appears safe, with mild AEs, including nausea, chills, pyrexia, fatigue, pain at the injection site, and influenza-like illness[150]. T-VEC alone or in conjunction with radiotherapy, chemotherapy, targeted therapies, and immunotherapies are still undergoing clinical trials (for details refer to the ClinicalTrials.gov). CAVATAK or Coxsackievirus (CVA21) is a late-stage clinical oncolytic virus that has displayed lytic activity against melanoma in both in-vitro and in-vivo conditions[160–162]. CAVATAK is being evaluated in combination with Pembrolizumab in advanced melanomas in clinical trials. Moreover, CAVATAK is also being assessed in unresectable stage Ⅲ-Ⅳ melanoma in conjunction with Ipilimumab[161]. Other oncolytic viruses show promising results and under clinical trials are HF10 in conjunction with other immunotherapies (NCT03259425, NCT02272955, and NCT03153085) and monotherapy with GLONC1 prior to surgery (NCT002714374).
Adoptive T-cell therapy
In adoptive T-cell therapy (ACT), several melanoma-specific T-cells are infused into patients, but it is challenging and time-consuming to produce such cells. The antitumoral effect of ACT is not completely understood but may involve suppression of regulatory T-cells, elimination of host tumor immunosuppressive factors, and removal of cytokine sinks[163]. T-cells must be able to proliferate, complete effector roles, and form long-lived T-cells, which are essential for an effective immune response[164]. In-vitro studies have shown that more differentiated Teffs have increased antitumoral properties, but in in-vivo conditions, these T-cells are less effective[165]. T-cell metabolism control may be a beneficial tool for promoting T-cell memory formation rather than more differentiated Teffs. This is because memory T-cells exhibit a restricted uptake of glucose, and inhibiting glycolytic metabolism contributes to the development of memory precursor cells and thus improves antitumor functions[166]. In metastatic melanoma, this kind of immunotherapy seems to correlate with durable and complete responses as well as partial responses and sustained stabilization of disease[167]. ACT led to stable complete regression in 24% of the patients studied with metastatic melanoma, with a median survival >3 years [168].
Combination therapies are effective and studies have shown that metastasectomy in patients with progressive melanoma who undergo ACT therapy displayed a PFS of 11 months and a 5-year OS of 57%[167]. The AEs of this therapy include autoimmune changes, like the destruction of normal melanocytes in skin and eyes, and sometimes immunosuppression is necessary to manage these AEs[169]. Multiple clinical trials with ACT in combination with radiotherapy, immunotherapy, chemotherapy, and targeted therapies are ongoing (for details refer to the ClinicalTrials.gov). ACT with T-cells chimeric antigen receptors (CAR) is a novel treatment method for solid tumors like melanoma[170]. CARs consist of an extracellular domain, a single-chain antibody variable fragment that identifies a specific antigen (protein, carbohydrate antigens, or lipid), a transmembrane domain, and an intracellular signaling domain that is often the T-cell receptor CD3 ζ chain that activates T-cells to destroy tumor cells[171–172]. In this approach, patients are injected with pre-modified T-cells which are stimulated and genetically modified with retroviral or plasmid vectors to generate CAR-T cells for treatment[171,173]. It was recently demonstrated that Cas-9 based gene manipulation can enhance the efficacy of CAR-T cells[174]. Target antigen selection must have the maximum effect in tumor cells and the minimal effect in normal cells as a criterion[171,175]. Despite few studies in melanomas, ganglioside GD2 is an example of a molecule that is strongly expressed in melanoma cells and can be targeted by CARs[175–176]. In young adults and children with melanomas (NCT02107963), a phase Ⅰ study of T-cells expressing an anti-GD2 CAR was performed, but no definitive findings were observed. A phase Ⅰ CAR-T dose-escalation cell study in solid tumors, including melanomas, against antigen VEGF receptor 2, demonstrated one partial response (4%) in 24 patients[175]. Nonetheless, CAR-T cells are also subjected to inhibitory immune checkpoint signals of the TME. Therefore, combined therapies of CAR-T with CTLA-4 antibody or PD-1 antibody may overcome the TME features[171,175,177]. Clinical trials using CAR-T cells expressing cMET (NCT03060356) and anti-CD70 CAR-T (NCT02830724) are underway.
gp100 peptide vaccine
Glycoprotein gp100 is only expressed in the retina, healthy epidermal cells, and melanoma cells; it is not expressed in other healthy tissues[178–179]. gp100 is recognized by CTLs and stimulates their activity thereby making it a treatment option. However, gp100 as a monotherapy has showed poor and unsatisfactory performance in preclinical research therefore, it may be used only as adjuvant therapy[178]. The combination therapy of IL-2 and the gp100 peptide vaccine demonstrated an overall improvement in PFS and a complete clinical response of 5%[125]. When compared to IL-2 monotherapy, combination therapy showed a longer median OS. Various clinical trials are underway using gp100 as a monotherapy (NCT02889861 and NCT01744171) or in combination with different immunotherapies (NCT01176474, NCT00470015, NCT01176461, NCT02535078, and NCT00960752).
Biochemotherapy
Biochemotherapy (BCT) is an approach where chemotherapy and immunotherapy are combined to enhance clinical benefits. Many modern chemotherapies function in part by means of mechanisms stimulating the immune system[180]. One of the routinely used BCT procedures involves the use of Vinblastine, which targets microtubules[181] while Cisplatin enhances DNA damage[182] with IFN-α 2b or IL-2 as the immunotherapy. In comparison to chemotherapy alone, BCT resulted in a higher response rate and overall improvement in the median PFS. BCT, however, did not yield any improvement in OS and was related to significant toxicity and risk of brain metastases[183].
Therapies against the tumor microenvironment
One of the factors playing a key role in melanoma malignancy is TME. The TME is a complex network of cells, paracrine factors, molecules that support melanoma cells thereby, regulating their genesis, development and resistance to various therapeutic modalities. Considering the importance of the melanoma TME, targeting its components has become a treatment strategy with potential. Among various proteins in TME, the matrix metalloproteinases (MMPs) have been found to promote tumor progression by degrading surrounding tissues, membrane receptors, and modulating growth factors, as well as membrane receptors, inflammatory proteins, and chemo-attractive proteins[184]. Multiple studies have highlighted the role of MMPs in melanoma with MMP deregulation being linked to tumor cells and TME changes. Several MMP inhibitors have been developed and are divided into synthetic and endogenous inhibitors[184]. MMP activity is inhibited by a variety of ways, the most frequent of which includes binding molecules to the zinc atom of the protein's catalytic domain[184]. Rebimastat, Tanomastat, Prinomastat[185], Cipemastat, Marimastat, Doxycycline, 3-hydroxypyran-4-one, and Ro 28-2653 are some of the examples of synthetic MMP inhibitor compounds[184].
Endogenous MMP inhibitors include α2-macroglobulin and tissue inhibitors of metalloproteinases (TIMPs)[184,186]. The serum protein α2-macroglobulin binds to MMPs forming an inactive complex. In groundbreaking research, Kancha et al investigated protein levels for the α 2-macroglobulin receptor (LRP/2-MR). They observed that their levels were lower in invasive sub-clones derived from PC-3 and DU-145 human prostatic cells, as well as the A2058 melanoma cell line, compared to non-invasive ones[184]. This supports the theory that LRP/2-MR complex down-regulation might promote tumor cell invasiveness. TIMP inhibitors inhibit MMPs by binding to their catalytic site and blocking their proteolytic action. TIMP molecules feature 12 cysteine residues which create six loops via disulfide bonding, which is required for inhibitory effect against MMPs[184]. The N-terminal site of TIMPs can bind to the majority of MMPs whereas the C-terminal site of TIMP-1 and TIMP-2 bind to the hemopexin domain of pro-MMP-2 and pro-MMP-9, respectively[184]. In this way MMP inhibitors can be useful in the treatment of melanoma.
Targeted therapy
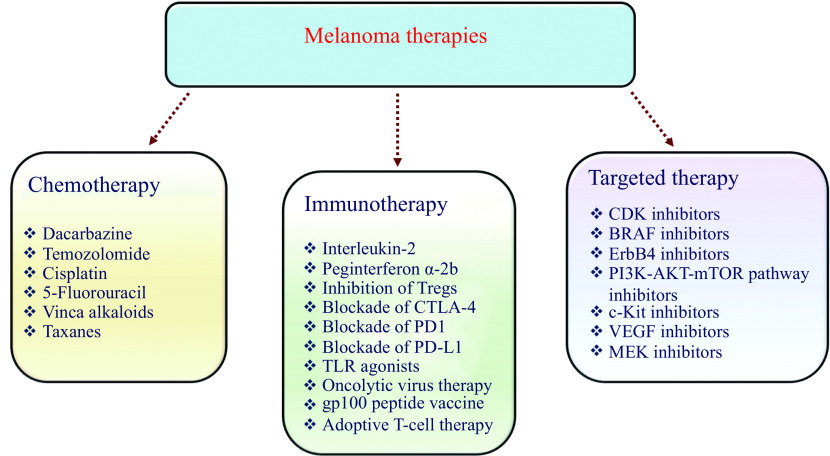
Almost 70% of cutaneous melanoma patients carry mutations in genes associated with key signaling pathways. These mutations are generally associated with the proliferation of melanoma cells and malignant phenotype[187]. The targeted therapy-based approach utilizes antibodies or small molecule inhibitors that alter these mutated proteins that are crucial for the disease progression (Fig. 4).
Figure 4.
Food and Drug Administration approved melanoma therapies.
CTLA-4: cytotoxic T-lymphocyte-associated antigen 4; PD-1: programmed cell death protein 1; PD-L1: programmed death ligand-1; CDK: cyclin-dependent kinase; TLR: Toll-like receptor; BRAF: serine/threonine-protein kinase B-raf; VEGF: vascular endothelial growth factor.
Cyclin-dependent kinase inhibitors
CDK4 germline mutations are associated with 2% of familial melanoma[188]. The oncogene CDK4 is involved in modulation of cell proliferation and is inhibited by p16[4,189–191]. Apart from CDK4, cyclins, and CDK6 (D1, D2, or D3) also control the G1 checkpoint[192]. Hyperactivation of cyclin D kinase, mutations associated with CDK4, cyclin D amplification or deletion of p16INK4a leads to an increased risk of melanoma development[193]. Abemaciclib, Ribociclib, and Palbociclib are inhibitors of CDK4/6, which selectively target tumors. These drugs have also been found to cause fewer AEs and improve effectiveness[191]. In Vemurafenib-resistant melanoma models, where reactivation of the MAPK pathway and high expression of cyclin D1 were observed, treatment with Abemaciclib led to tumor growth regression[194]. For an effective and successful therapeutic outcome, the appropriate selection of patients with CDK4 mutations seems to be critical[191]. Several inhibitors of CDK4/6 are being studied through clinical melanoma trials, including Abemaciclib monotherapy (NCT02308020) or in combination with immunotherapies (NCT02791334) and with chemotherapy (NCT02857270), Ribociclib in combination with targeted therapies (NCT01781572/NCT02159066), SHR6390 monotherapy (NCT02671513), Palbociclib monotherapy (NCT01037790) and in combination with targeted therapies (NCT02202200).
ErbB4 inhibitor
ErbB4 belongs to ErbB family of receptor tyrosine kinases. In melanoma, perturbations in ErbB4 have been identified and are associated with enhanced transformation ability and show increased kinase activity. Therefore, inhibition of ErbB4 receptors might be of therapeutic use in the treatment of melanomas. It has been found that the knockdown of ErbB4 in melanoma cells leads to reduced cell growth. Also, the treatment of cells with Lapatinib (inhibitor of ErbB) has proven to enhance the inhibitory effect[195].
PI3K-AKT-mTOR pathway inhibitors
mTOR plays a key function in tumor progression and development, and multiple inhibitors have been developed to downregulate the mTOR pathway[11,196]. mTOR forms two protein complexes, mTOR complex1, which is activated by the PI3K/AKT pathway, and mTOR complex 2[196]. In cutaneous melanoma, hyperactivation of the mTOR pathway has been found to be associated with BRAF mutations[197]. A study reported that combinations of PI-103 (PI3K inhibitor) and Rapamycin (mTOR inhibitor) could effectively induce autophagy as well as inhibit the growth of melanoma cells when compared to single drugs alone (Fig. 4)[11,198]. In one study of PI3K-AKT pathway inhibitors, there were enhanced apoptosis rates when compared to BRAF inhibitors[199]. There are other PI3K pathway inhibitors are under clinical investigation, such as BKM120 in conjunction with other targeting therapies (NCT02159066), INCB050465 in conjunction immunotherapies and other targeted therapies (NCT02646748), GSK2636771 in conjunction with immunotherapies (NCT03131908), and IPI-549 monotherapy compared to the combination with immunotherapies (NCT002637531). MK2206, an inhibitor of AKT, is also under clinical trial in conjunction with chemotherapy (NCT01480154).
When mTOR inhibition is combined with MAPK pathway inhibitors, an additive antitumor effect can be obtained. BRAF vector-transfected cells displayed an increased mTOR pathway activation, whereas BRAF-mutant melanoma cells are sensitive to mTOR inhibition[200]. Furthermore, inhibition of mTOR or AKT and combined inhibition of mTOR and PI3K have been shown to be effective alternative approaches at overcoming resistance to BRAF inhibitors[201–202]. In a clinical mTOR inhibitor trials, namely Temsirolimus or Everolimus are used in conjunction with a BRAF inhibitor (NCT01596140), also in another trial ASN003, an inhibitor of BRAF with enhanced selectivity against mTOR and PI3K kinases (NCT02961283). Combining low-dose inhibitors of mTOR with immunotherapy needs clinical validation because inhibition of mTOR can lead to immune activation or immune suppression depending on the mode of administration, timing, and dose[203].
c-Kit inhibitors
c-Kit mutations have been shown in different types of melanoma. For example, 36% of acral lentiginous melanoma, 28% of cutaneous melanoma, and 39% of mucosal melanoma show c-Kit mutations[204–205]. Mutations in c-Kit leading to gene amplifications cause constitutive ligand-independent activation of receptor thereby leading to the upregulation of the PI3K/AKT and MAPK pathway[206–207]. Mutations across several exons of KIT have been reported and are linked with the development of drug resistance[208]. Imatinib was found to produce significant activity in metastatic melanoma patients harboring aberrations in c-Kit with a response rate of 30% and a median PFS of 3 to 4 months[209–211]. Two Imatinib-based clinical trials are ongoing, in combination with immunotherapies (NCT02812693) and with chemotherapy (NCT00667953). Multi-kinase inhibitors such as Nilotinib, Sunitilib, and Dasatinib (Fig. 4) have also shown activity in melanoma patients having c-Kit mutations. Clinical trials are ongoing with these interventions, in conjunction with immunotherapies (NCT01876212) and chemotherapy (NCT01005472). Thus far, the AEs reported are fatigue, fluid retention, and myelosuppression[206].
Vascular endothelial growth factor inhibitors
VEGF, VEGF receptor-1, -2, and -3 are highly expressed in melanoma and they are associated with poor prognosis, growth of tumor neovasculature, and suppression of the immune system[212–213]. VEGF-promoted angiogenesis is critical for the progression of cancer[214]. Therefore, blockade of VEGF might be an effective therapeutic approach for melanoma therapy (Fig. 4). Bevacizumab is a monoclonal anti-VEGF antibody capable of targeting as well as neutralizing VEGF and inhibiting tumor growth[215]. The combined therapy of TMZ and Bevacizumab was used to treat patients with previously untreated metastatic melanomas in a single-arm phase-Ⅱ clinical trial[216]. A 16% objective response rate, 52% of overall disease control rate, 4.2 months of median PFS, 9.6 months of OS, and an improvement in OS in patients with BRAFV600E-mutated melanoma has been observed. Bevacizumab was given in combination with IFN-α2b in another single-arm phase-Ⅱ clinical trial. The median rate of progression-free was 4.8 months, and the rate of OS was 17 months. These studies illustrate the potential of VEGF as a therapeutic target but were unsuccessful in validating this therapy for melanoma. Clinical trials are underway, using Bevacizumab in combination with chemotherapy (NCT03175432) and with immunotherapies (NCT00790010, NCT2158520, NCT02681549, NCT01950390, and NCT03167177).
MEK inhibitors
Targeting signal-transducing modules downstream of oncogenes is a credible strategy to overcome resistance to BRAF inhibitors[217]. The downstream target of BRAF is MEK. MEK inhibitors have been found to enhance activity in melanoma with NRAS-mutations[218]. In 2013, Trametinib (MEK1/2 inhibitor) (Fig. 4) was approved as monotherapy for malignant melanoma or unresectable melanoma with BRAF mutations[206,219]. The blockade of MEK1/2 leads to growth factor/s mediated inhibition of cell signaling and a decrease in the proliferation of tumor cells. Compared to chemotherapy alone, Trametinib-treated metastatic melanoma patients encountered improved PFS, OS, and clinical response rates[217]. Peripheral edema, nausea, fatigue, diarrhea, and vomiting are some of the most common AEs of MEK inhibitors[206]. Combining Dabrafenib (a BRAF mutant inhibitor) with Trametinib has been found to produce a durable objective response in a multicenter, open-label, randomized study[23]. This combination was approved by the FDA in 2014 for the treatment of BRAF harboring unresectable metastatic melanoma[220]. Multiple clinical trials are ongoing with a combination of Dabrafenib and Trametinib. Further, these two drugs are also combined with immunotherapies, radiotherapy, and other targeted therapies (for details refer to the ClinicalTrials.gov). In 2015, a combination of Vemurafenib (BRAF-mutant inhibitor) and Cobimetinib (MEK inhibitor) was approved for treating melanomas with BRAF mutations that cannot be removed surgically or show metastization[220–221]. Nausea, chills, fatigue, pyrexia, vomiting, and diarrhea are some of the AEs of MEK and BRAF inhibitor combination therapy[206].
Conclusion
Understanding melanoma pathogenesis is a key factor in developing new therapeutic modalities. The analysis of oncogenic signaling pathways, as well as the intricate interactions between various signaling modules, will enable us to discover new therapeutic targets. In turn, these will enable us to develop effective treatments but each treatment encounters a number of challenges. Describing the nuances involved in each intervention or combination will help us to improve outcomes and progress our knowledge of unique mutations. One key factors is tolerability to treatments that can inhibit tumor growth. Combining interventions such as immunotherapy, chemotherapy, and targeted therapies appears to be an effective strategy to overcome these resistance. Yet, patient sample heterogeneity and indeed tumor differentiation correlate with different resistance mechanisms which adversely affect outcomes. Recently, there has been an increase in the number of approved immunotherapies. Favorable results can be explained by the triggering immune responses which initiate the T-cell repertoire. T-cells are able to adapt to heterogenous tumors and generate memory T-cells which can inhibit tumor recurrence. Based on molecular characteristics and individual differences between patients, as well as responses to therapy, personalized approaches are called for to improve outcomes. Further research is crucial to understand the complicated oncogenic signaling pathways and to delineate the role of TME in melanoma treatments.
Footnotes
CLC number: R739.5, Ducument code: A
The authors reported no conflict of interests.
References
- 1.Curtin JA, Fridlyand J, Kageshita T, et al Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 2.Maio M Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23(S8):viii10–viii14. doi: 10.1093/annonc/mds257. [DOI] [PubMed] [Google Scholar]
- 3.Abildgaard C, Guldberg P Molecular drivers of cellular metabolic reprogramming in melanoma. Trends Mol Med. 2015;21(3):164–171. doi: 10.1016/j.molmed.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Batus M, Waheed S, Ruby C, et al Optimal management of metastatic melanoma: Current strategies and future directions. Am J Clin Dermatol. 2013;14(3):179–194. doi: 10.1007/s40257-013-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epidemiology, and End Results Program (2021). Cancer Stat Facts: Melanoma of the Skin[EB/OL]. [2021-09-29]. https://seer.cancer.gov/statfacts/html/melan.html.
- 6.Gonzalez D, Fearfield L, Nathan P, et al BRAF mutation testing algorithm for vemurafenib treatment in melanoma: Recommendations from an expert panel. Br J Dermatol. 2013;168(4):700–707. doi: 10.1111/bjd.12248. [DOI] [PubMed] [Google Scholar]
- 7.Ferlay J, Soerjomataram I, Dikshit R, et al Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 8.Gray-Schopfer V, Wellbrock C, Marais R Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 9.Davids L. M. , Kleem B. The menace of melanoma: a photodynamic approach to adjunctive cancer therapy[M]//G. H. T. Duc. Melanoma - from early detection to treatment. London: IntechOpen, 2013: 319–328.
- 10.Tolleson WH Human melanocyte biology, toxicology, and pathology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2005;23(2):105–161. doi: 10.1080/10590500500234970. [DOI] [PubMed] [Google Scholar]
- 11.Pópulo H, Soares P, Lopes JM Insights into melanoma: Targeting the mTOR pathway for therapeutics. Expert Opin Ther Targets. 2012;16(7):689–705. doi: 10.1517/14728222.2012.691472. [DOI] [PubMed] [Google Scholar]
- 12.Jiang B, Zhang L, Guo X, et al Poly(N-phenylglycine)-based nanoparticles as highly effective and targeted near-infrared photothermal therapy/photodynamic therapeutic agents for malignant melanoma . Small. 2017;13(8):1602496. doi: 10.1002/smll.201602496. [DOI] [PubMed] [Google Scholar]
- 13.Bombelli FB, Webster CA, Moncrieff M, et al The scope of nanoparticle therapies for future metastatic melanoma treatment. Lancet Oncol. 2014;15(1):e22–e32. doi: 10.1016/S1470-2045(13)70333-4. [DOI] [PubMed] [Google Scholar]
- 14.Dummer R, Hauschild A, Lindenblatt N, et al Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v126–v132. doi: 10.1093/annonc/mdv297. [DOI] [PubMed] [Google Scholar]
- 15.Van Zeijl MCT, Van Den Eertwegh AJ, Haanen JB, et al (Neo)adjuvant systemic therapy for melanoma. Eur J Surg Oncol. 2017;43(3):534–543. doi: 10.1016/j.ejso.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Miller KD, Siegel RL, Lin CC, et al Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 17.Austin E, Mamalis A, Ho D, et al Laser and light-based therapy for cutaneous and soft-tissue metastases of malignant melanoma: a systematic review. Arch Dermatol Res. 2017;309(4):229–242. doi: 10.1007/s00403-017-1720-9. [DOI] [PubMed] [Google Scholar]
- 18.Garbe C, Peris K, Hauschild A, et al Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline - Update 2016. Eur J Cancer. 2016;63:201–217. doi: 10.1016/j.ejca.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Domingues B, Lopes JM, Soares P, et al Melanoma treatment in review. ImmunoTargets Ther. 2018;7:35–49. doi: 10.2147/ITT.S134842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonardi GC, Falzone L, Salemi R, et al Cutaneous melanoma: From pathogenesis to therapy (Review) Int J Oncol. 2018;52(4):1071–1080. doi: 10.3892/ijo.2018.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krauthammer M, Kong Y, Ha BH, et al Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44(9):1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodis E, Watson IR, Kryukov GV, et al A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaherty KT, Infante JR, Daud A, et al Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solit D, Sawyers CL How melanomas bypass new therapy. Nature. 2010;468(7326):902–903. doi: 10.1038/468902a. [DOI] [PubMed] [Google Scholar]
- 25.Lovly CM, Dahlman KB, Fohn LE, et al Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS One. 2012;7(4):e35309. doi: 10.1371/journal.pone.0035309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinstein JC, Sznol M, Pavlick AC, et al Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010;8:67. doi: 10.1186/1479-5876-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock PM, Harper UL, Hansen KS, et al High frequency of BRAF mutations in nevi . Nat Genet. 2003;33(1):19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 28.Dong J, Phelps RG, Qiao R, et al BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. https://aacrjournals.org/cancerres/article/63/14/3883/510174/BRAF-Oncogenic-Mutations-Correlate-with. Cancer Res. 2003;63(14):3883–3885. [PubMed] [Google Scholar]
- 29.Jakob JA, Bassett RL Jr, Ng CS, et al NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118(16):4014–4023. doi: 10.1002/cncr.26724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giehl K Oncogenic Ras in tumour progression and metastasis. Biol Chem. 2005;386(3):193–205. doi: 10.1515/BC.2005.025. [DOI] [PubMed] [Google Scholar]
- 31.Fedorenko IV, Gibney GT, Smalley KSM NRAS mutant melanoma: biological behavior and future strategies for therapeutic management. Oncogene. 2013;32(25):3009–3018. doi: 10.1038/onc.2012.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maertens O, Johnson B, Hollstein P, et al Elucidating distinct roles for NF1 in melanomagenesis . Cancer Discov. 2013;3(3):338–349. doi: 10.1158/2159-8290.CD-12-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whittaker SR, Theurillat JP, Van Allen E, et al A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition . Cancer Discov. 2013;3(3):350–362. doi: 10.1158/2159-8290.CD-12-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nissan MH, Pratilas CA, Jones AM, et al Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res. 2014;74(8):2340–2350. doi: 10.1158/0008-5472.CAN-13-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krauthammer M, Kong Y, Bacchiocchi A, et al Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas . Nat Genet. 2015;47(9):996–1002. doi: 10.1038/ng.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibney GT, Smalley KSM An unholy alliance: cooperation between BRAF and NF1 in melanoma development and BRAF inhibitor resistance. Cancer Discov. 2013;3(3):260–263. doi: 10.1158/2159-8290.CD-13-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beadling C, Jacobson-Dunlop E, Hodi FS, et al KIT gene mutations and copy number in melanoma subtypes . Clin Cancer Res. 2008;14(21):6821–6828. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 38.Handolias D, Salemi R, Murray W, et al Mutations in KIT occur at low frequency in melanomas arising from anatomical sites associated with chronic and intermittent sun exposure. Pigment Cell Melanoma Res. 2010;23(2):210–215. doi: 10.1111/j.1755-148X.2010.00671.x. [DOI] [PubMed] [Google Scholar]
- 39.Bradford PT, Goldstein AM, McMaster ML, et al Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986–2005. Arch Dermatol. 2009;145(4):427–434. doi: 10.1001/archdermatol.2008.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivera RS, Nagatsuka H, Gunduz M, et al C-kit protein expression correlated with activating mutations in KIT gene in oral mucosal melanoma. Virchows Arch. 2008;452(1):27–32. doi: 10.1007/s00428-007-0524-2. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Sheikh MS Melanoma: Molecular pathogenesis and therapeutic management. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4346328/ Mol Cell Pharmacol. 2014;6(3):228. [PMC free article] [PubMed] [Google Scholar]
- 42.Stretch JR, Gatter KC, Ralfkiaer E, et al Expression of mutant p53 in melanoma. https://europepmc.org/article/MED/1933861. Cancer Res. 1991;51(21):5976–5979. [PubMed] [Google Scholar]
- 43.Nigro JM, Baker SJ, Preisinger AC, et al Mutations in the p53 gene occur in diverse human tumour types . Nature. 1989;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- 44.Prosser J, Thompson AM, Cranston G, et al Evidence that p53 behaves as a tumour suppressor gene in sporadic breast tumours. https://pubmed.ncbi.nlm.nih.gov/2250913/ Oncogene. 1990;5(10):1573–1579. [PubMed] [Google Scholar]
- 45.Iggo R, Bartek J, Lane D, et al Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-B. [DOI] [PubMed] [Google Scholar]
- 46.Baker SJ, Fearon ER, Nigro JM, et al Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas . Science. 1989;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- 47.Whibley C, Pharoah PDP, Hollstein M p53 polymorphisms: cancer implications. Nat Rev Cancer. 2009;9(2):95–107. doi: 10.1038/nrc2584. [DOI] [PubMed] [Google Scholar]
- 48.Easton DF, Pooley KA, Dunning AM, et al Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447(7148):1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eeles RA, Kote-Jarai Z, Giles GG, et al Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40(3):316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 50.Hunter DJ, Kraft P, Jacobs KB, et al A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer . Nat Genet. 2007;39(7):870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smalley KSM, Contractor R, Haass NK, et al An organometallic protein kinase inhibitor pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res. 2007;67(1):209–217. doi: 10.1158/0008-5472.CAN-06-1538. [DOI] [PubMed] [Google Scholar]
- 52.Albino AP, Vidal MJ, McNutt NS, et al Mutation and expression of the p53 gene in human malignant melanoma . Melanoma Res. 1994;4(1):35–45. doi: 10.1097/00008390-199402000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Liu L, Dilworth D, Gao L, et al Mutation of the CDKN2A 5′ UTR creates an aberrant initiation codon and predisposes to melanoma . Nat Genet. 1999;21(1):128–132. doi: 10.1038/5082. [DOI] [PubMed] [Google Scholar]
- 54.FitzGerald MG, Harkin DP, Silva-Arrieta S, et al Prevalence of germ-line mutations in p16, p19ARF, and CDK4 in familial melanoma: analysis of a clinic-based population. Proc Natl Acad Sci U S A. 1996;93(16):8541–8545. doi: 10.1073/pnas.93.16.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tucker MA, Bale SJ Clinical aspects of familial cutaneous malignant melanoma. https://pubmed.ncbi.nlm.nih.gov/3206237/ Semin Oncol. 1988;15(6):524–528. [PubMed] [Google Scholar]
- 56.Bartkova J, Lukas J, Guldberg P, et al The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. https://pubmed.ncbi.nlm.nih.gov/8968104/ Cancer Res. 1996;56(23):5475–5483. [PubMed] [Google Scholar]
- 57.Goldstein AM, Struewing JP, Chidambaram A, et al Genotype-phenotype relationships in U. S. melanoma-prone families with CDKN2A and CDK4 mutations. J Natl Cancer Inst. 2000;92(12):1006–1010. doi: 10.1093/jnci/92.12.1006. [DOI] [PubMed] [Google Scholar]
- 58.Mirmohammadsadegh A, Marini A, Nambiar S, et al Epigenetic silencing of the PTEN gene in melanoma . Cancer Res. 2006;66(13):6546–6552. doi: 10.1158/0008-5472.CAN-06-0384. [DOI] [PubMed] [Google Scholar]
- 59.Tsao H, Yang G, Goel V, et al Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma . J Invest Dermatol. 2004;122(2):337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nogueira C, Kim KH, Sung H, et al Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene. 2010;29(47):6222–6232. doi: 10.1038/onc.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi H, Hugo W, Kong X, et al Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4(1):80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilchrest BA, Eller MS, Geller AC, et al The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340(17):1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 63.Pennello G, Devesa S, Gail M Association of surface ultraviolet B radiation levels with melanoma and nonmelanoma skin cancer in United States blacks. https://pubmed.ncbi.nlm.nih.gov/10750668/ Cancer Epidemiol Biomarkers Prev. 2000;9(3):291–297. [PubMed] [Google Scholar]
- 64.Falzone L, Marconi A, Loreto C, et al Occupational exposure to carcinogens: Benzene, pesticides and fibers (Review) Mol Med Rep. 2016;14(5):4467–4474. doi: 10.3892/mmr.2016.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Candido S, Rapisarda V, Marconi A, et al Analysis of the B-RafV600E mutation in cutaneous melanoma patients with occupational sun exposure. Oncol Rep. 2014;31(3):1079–1082. doi: 10.3892/or.2014.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Archier E, Devaux S, Castela E, et al Carcinogenic risks ofpsoralen UV-A therapy and narrowband UV-B therapy inchronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(Suppl 3):22–31. doi: 10.1111/j.1468-3083.2012.04520.x. [DOI] [PubMed] [Google Scholar]
- 67.Wehner MR, Chren MM, Nameth D, et al International prevalence of indoor tanning: a systematic review and meta-analysis. JAMA Dermatol. 2014;150(4):390–400. doi: 10.1001/jamadermatol.2013.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The International Agency for Research on Cancer Working Group on Artificial Ultraviolet (UV) Light and Skin Cancer The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2007;120(5):1116–1122. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- 69.Ali Z, Yousaf N, Larkin J Melanoma epidemiology, biology and prognosis. EJC Suppl. 2013;11(2):81–91. doi: 10.1016/j.ejcsup.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawkes JE, Truong A, Meyer LJ Genetic predisposition to melanoma. Semin Oncol. 2016;43(5):591–597. doi: 10.1053/j.seminoncol.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Olsen CM, Zens MS, Stukel TA, et al Nevus density and melanoma risk in women: a pooled analysis to test the divergent pathway hypothesis. Int J Cancer. 2009;124(4):937–944. doi: 10.1002/ijc.24011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dessinioti C, Antoniou C, Katsambas A, et al Melanocortin 1 receptor variants: Functional role and pigmentary associations. Photochem Photobiol. 2011;87(5):978–987. doi: 10.1111/j.1751-1097.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 73.Goldstein AM, Tucker MA Genetic epidemiology of cutaneous melanoma: a global perspective. Arch Dermatol. 2001;137(11):1493–1496. doi: 10.1001/archderm.137.11.1493. [DOI] [PubMed] [Google Scholar]
- 74.Wilson MA, Schuchter LM. Chemotherapy for melanoma[M]//Kaufman H L, Mehnert J M. Melanoma. Cham: Springer, 2016: 209–229.
- 75.Soengas MS, Lowe SW Apoptosis and melanoma chemoresistance. Oncogene. 2003;22(20):3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 76.Kim C, Lee CW, Kovacic L, et al Long-term survival in patients with metastatic melanoma treated with DTIC or temozolomide. Oncologist. 2010;15(7):765–771. doi: 10.1634/theoncologist.2009-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang G, Li RH, Sun C, et al Dacarbazine combined targeted therapy versus dacarbazine alone in patients with malignant melanoma: A meta-analysis. PLoS One. 2014;9(12):e111920. doi: 10.1371/journal.pone.0111920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agarwala SS, Kirkwood JM Temozolomide, a Novel Alkylating Agent with Activity in the Central Nervous System, May Improve the Treatment of Advanced Metastatic Melanoma. Oncologist. 2000;5(2):144–151. doi: 10.1634/theoncologist.5-2-144. [DOI] [PubMed] [Google Scholar]
- 79.Zhang JH, Stevens MFG, Bradshaw TD Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol. 2012;5(1):102–114. doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 80.Middleton MR, Grob JJ, Aaronson N, et al Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 81.Quirt I, Verma S, Petrella T, et al Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist. 2007;12(9):1114–1123. doi: 10.1634/theoncologist.12-9-1114. [DOI] [PubMed] [Google Scholar]
- 82.Mattia G, Puglisi R, Ascione B, et al Cell death-based treatments of melanoma: conventional treatments and new therapeutic strategies review-Article. Cell Death Dis. 2018;9(2):112. doi: 10.1038/s41419-017-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luke JJ, Schwartz GK Chemotherapy in the management of advanced cutaneous malignant melanoma. Clin Dermatol. 2013;31(3):290–297. doi: 10.1016/j.clindermatol.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sperka T, Wang JW, Rudolph KL DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13(9):579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- 85.Jordan P, Carmo-Fonseca M Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci. 2000;57(8-9):1229–1235. doi: 10.1007/pl00000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chu E, Callender MA, Farrell MP, et al Thymidylate synthase inhibitors as anticancer agents: from bench to bedside. Cancer Chemother Pharmacol. 2003;52(Suppl 1):S80–S89. doi: 10.1007/s00280-003-0625-9. [DOI] [PubMed] [Google Scholar]
- 87.Wilson PM, Danenberg PV, Johnston PG, et al Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol. 2014;11(5):282–298. doi: 10.1038/nrclinonc.2014.51. [DOI] [PubMed] [Google Scholar]
- 88.Whitehead RP, Moon J, McCachren SS, et al A Phase II trial of vinorelbine tartrate in patients with disseminated malignant melanoma and one prior systemic therapy: a Southwest Oncology Group study. Cancer. 2004;100(8):1699–1704. doi: 10.1002/cncr.20183. [DOI] [PubMed] [Google Scholar]
- 89.Bedikian AY, Papadopoulos NE, Kim KB, et al A pilot study with vincristine sulfate liposome infusion in patients with metastatic melanoma. Melanoma Res. 2008;18(6):400–404. doi: 10.1097/CMR.0b013e328311aaa1. [DOI] [PubMed] [Google Scholar]
- 90.Maverakis E, Cornelius LA, Bowen GM, et al Metastatic melanoma - a review of current and future treatment options. Acta Derm Venerol. 2015;95(5):516–524. doi: 10.2340/00015555-2035. [DOI] [PubMed] [Google Scholar]
- 91.Baldea I, Filip AG Photodynamic therapy in melanoma - An update. J Physiol Pharmacol. 2012;63(2):109–118. [PubMed] [Google Scholar]
- 92.Yin R, Wang M, Huang Y, et al Photodynamic therapy with decacationic [60]fullerene monoadducts: effect of a light absorbing electron-donor antenna and micellar formulation. Nanomedicine. 2014;10(4):795–808. doi: 10.1016/j.nano.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monge-Fuentes V, Muehlmann LA, Longo JPF, et al Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma . J Photochem Photobiol B Biol. 2017;166:301–310. doi: 10.1016/j.jphotobiol.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Biteghe FN, Davids LM A combination of photodynamic therapy and chemotherapy displays a differential cytotoxic effect on human metastatic melanoma cells. J Photochem Photobiol B Biol. 2017;166:18–27. doi: 10.1016/j.jphotobiol.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 95.Testori A, Ribero S, Bataille V Diagnosis and treatment of in-transit melanoma metastases. Eur J Surg Oncol. 2017;43(3):544–560. doi: 10.1016/j.ejso.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 96.Miklavčič D, Serša G, Brecelj E, et al Electrochemotherapy: Technological advancements for efficient electroporation-based treatment of internal tumors. Med Biol Eng Comput. 2012;50(12):1213–1225. doi: 10.1007/s11517-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matthiessen LW, Chalmers RL, Sainsbury DCG, et al Management of cutaneous metastases using electrochemotherapy. Acta Oncol (Madr) 2011;50(5):621–629. doi: 10.3109/0284186X.2011.573626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marty M, Sersa G, Garbay JR, et al Electrochemotherapy - An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer Suppl. 2006;4(11):3–13. doi: 10.1016/j.ejcsup.2006.08.002. [DOI] [Google Scholar]
- 99.Leonardi GC, Candido S, Falzone L, et al Cutaneous melanoma and the immunotherapy revolution (Review) Int J Oncol. 2020;57(3):609–618. doi: 10.3892/ijo.2020.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawakami Y, Rosenberg SA T-cell recognition of self peptides as tumor rejection antigens. Immunol Res. 1996;15(3):179–190. doi: 10.1007/BF02918248. [DOI] [PubMed] [Google Scholar]
- 101.Faramarzi S, Ghafouri-Fard S Melanoma: a prototype of cancer-testis antigen-expressing malignancies. Immunotherapy. 2017;9(13):1103–1113. doi: 10.2217/imt-2017-0091. [DOI] [PubMed] [Google Scholar]
- 102.Chua MMJ, Ortega CE, Sheikh A, et al CK2 in cancer: Cellular and biochemical mechanisms and potential therapeutic target. Pharmaceuticals. 2017;10(1):E18. doi: 10.3390/ph10010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wheatley K, Ives N, Hancock B, et al Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev. 2003;29(4):241–252. doi: 10.1016/S0305-7372(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 104.Kirkwood JM, Ibrahim JG, Sosman JA, et al High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: Results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19(9):2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 105.Rohaan MW, Van Den Berg JH, Kvistborg P, et al Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: A viable treatment option. J Immunother Cancer. 2018;6(1):102. doi: 10.1186/s40425-018-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klebanoff CA, Acquavella N, Yu Z, et al Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239(1):27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schwartzentruber DJ, Lawson DH, Richards JM, et al gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ascierto PA, McArthur GA, Dréno B, et al Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial . Lancet Oncol. 2016;17(9):1248–1260. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 109.Weber JS, D’Angelo SP, Minor D, et al Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 110.Christofi T, Baritaki S, Falzone L, et al Current perspectives in cancer immunotherapy. Cancers (Basel) 2019;11(10):1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balkwill F, Mantovani A Inflammation and cancer: Back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 112.Sharma P, Allison JP The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 113.Jemal A, Siegel R, Ward E, et al Cancer Statistics, 2009. CA A Cancer J Clinic. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 114.Gata VA, Lisencu CI, Vlad CI, et al Tumor infiltrating lymphocytes as a prognostic factor in malignant melanoma. Review of the literature. https://pubmed.ncbi.nlm.nih.gov/28730761/ J BUON. 2017;22(3):592–598. [PubMed] [Google Scholar]
- 115.Mantovani A, Allavena P, Sica A, et al Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 116.Gasser S, Lim LHK, Cheung FSG The role of the tumour microenvironment in immunotherapy. Endocr Relat Cancer. 2017;24(12):T283–T295. doi: 10.1530/ERC-17-0146. [DOI] [PubMed] [Google Scholar]
- 117.Delitto D, Wallet SM, Hughes SJ Targeting tumor tolerance: A new hope for pancreatic cancer therapy? Pharmacol Ther. 2016;166:9–29. doi: 10.1016/j.pharmthera.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 118.Schreiber RD, Old LJ, Smyth MJ Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 119.Chen DS, Mellman I Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 120.Gide TN, Wilmott JS, Scolyer RA, et al Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res. 2018;24(6):1260–1270. doi: 10.1158/1078-0432.CCR-17-2267. [DOI] [PubMed] [Google Scholar]
- 121.Sharma P, Hu-Lieskovan S, Wargo JA, et al Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boussios S, Rassy E, Samartzis E, et al Melanoma of unknown primary: New perspectives for an old story. Crit Rev Oncol Hematol. 2021;158:103208. doi: 10.1016/j.critrevonc.2020.103208. [DOI] [PubMed] [Google Scholar]
- 123.Nelson BH IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172(7):3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 124.Krieg C, Létourneau S, Pantaleo G, et al Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A. 2010;107(26):11906–11911. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bright R, Coventry BJ, Eardley-Harris N, et al Clinical response rates from interleukin-2 therapy for metastatic melanoma over 30 Years' experience: a meta-analysis of 3312 patients. J Immunother. 2017;40(1):21–30. doi: 10.1097/CJI.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 126.Sabatino M, Kim-Schulze S, Panelli MC, et al Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol. 2009;27(16):2645–2652. doi: 10.1200/JCO.2008.19.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Han Y, Guo Q, Zhang M, et al CD69+ CD4+ CD25− T cells, a new subset of regulatory T Cells, suppress T cell proliferation through membrane-bound TGF-β1 . J Immunol. 2009;182(1):111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- 128.Eggermont AM, Suciu S, Santinami M, et al Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372(9633):117–126. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 129.Harris JM, Chess RB Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 130.De Lafaille MAC, Lafaille JJ Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? . Immunity. 2009;30(5):626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 131.Jacobs JFM, Nierkens S, Figdor CG, et al Regulatory T cells in melanoma: The final hurdle towards effective immunotherapy? Lancet Oncol. 2012;13(1):e32–e42. doi: 10.1016/S1470-2045(11)70155-3. [DOI] [PubMed] [Google Scholar]
- 132.Bobo D, Robinson KJ, Islam J, et al Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 133.Telang S, Rasku MA, Clem AL, et al Phase II trial of the regulatory T cell-depleting agent, denileukin diftitox, in patients with unresectable stage IV melanoma. BMC Cancer. 2011;11:515. doi: 10.1186/1471-2407-11-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Attia P, Maker AV, Haworth LR, et al Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma . J Immunother. 2005;28(6):582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brunet JF, Denizot F, Luciani MF, et al A new member of the immunoglobulin superfamily-CTLA-4. Nature. 1987;328(6127):267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 136.Waterhouse P, Penninger JM, Timms E, et al Lymphoproliferative disorders with early lethality in mice deficient in ctla-4. Science. 2011;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 137.Hanson DC, Canniff PC, Primiano MJ, et al Preclinical in vitro characterization of anti-CTLA4 therapeutic antibody CP-675, 206 . Am Assoc Cancer Res. 2004;64(7):87. [Google Scholar]
- 138.Ribas A, Comin-Anduix B, Economou JS, et al Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2, 3-Dioxygenase in Patients with Melanoma undergoing CTLA4 blockade. Clin Cancer Res. 2009;15(1):390–399. doi: 10.1158/1078-0432.CCR-08-0783. [DOI] [PubMed] [Google Scholar]
- 139.Weide B, Martens A, Wistuba-Hamprecht K, et al Combined treatment with ipilimumab and intratumoral interleukin-2 in pretreated patients with stage IV melanoma—safety and efficacy in a phase II study. Cancer Immunol Immunother. 2017;66(4):441–449. doi: 10.1007/s00262-016-1944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brohl AS, Khushalani NI, Eroglu Z, et al A phase ib study of ipilimumab with peginterferon alfa-2b in patients with unresectable melanoma. J Immunother Cancer. 2016;4(1):85. doi: 10.1186/s40425-016-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hodi FS, O'Day SJ, McDermott DF, et al Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wolchok JD, Neyns B, Linette G, et al Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 143.Incorvaia L, Badalamenti G, Rinaldi G, et al Can the plasma PD-1 levels predict the presence and efficiency of tumor-infiltrating lymphocytes in patients with metastatic melanoma? Ther Adv Med Oncol. 2019;11:1758835919848872. doi: 10.1177/1758835919848872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cho J, Ahn S, Yoo KH, et al Treatment outcome of PD-1 immune checkpoint inhibitor in Asian metastatic melanoma patients: correlative analysis with PD-L1 immunohistochemistry. Invest New Drugs. 2016;34(6):677–684. doi: 10.1007/s10637-016-0373-4. [DOI] [PubMed] [Google Scholar]
- 145.Raedler LA Opdivo (Nivolumab): second PD-1 inhibitor receives FDA approval for unresectable or metastatic melanoma. https://pubmed.ncbi.nlm.nih.gov/26629287/ Am Health Drug Benefits. 2015;8(Spec Feature):180–183. [PMC free article] [PubMed] [Google Scholar]
- 146.Melero I, Grimaldi AM, Perez-Gracia JL, et al Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19(5):997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 147.Specenier P Nivolumab in melanoma. Expert Rev Anticancer Ther. 2016;16(12):1247–1261. doi: 10.1080/14737140.2016.1249856. [DOI] [PubMed] [Google Scholar]
- 148.Revythis A, Shah S, Kutka M, et al Unraveling the wide spectrum of melanoma biomarkers. Diagnostics. 2021;11(8):1341. doi: 10.3390/diagnostics11081341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Larkin J, Chiarion-Sileni V, Gonzalez R, et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Franklin C, Livingstone E, Roesch A, et al Immunotherapy in melanoma: Recent advances and future directions. Eur J Surg Oncol. 2017;43(3):604–611. doi: 10.1016/j.ejso.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 151.Robert C, Schachter J, Long G V, et al Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 152.Robert C, Ribas A, Wolchok JD, et al Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 153.Ribas A, Puzanov I, Dummer R, et al Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hamid O, Robert C, Daud A, et al Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588. doi: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zarour HM, Tawbi H, Tarhini AA, et al Study of anti-PD-1 antibody pembrolizumab and pegylated-interferon alfa-2b (Peg-IFN) for advanced melanoma. https://ascopubs.org/doi/abs/10.1200/jco.2015.33.15_suppl.e20018 J Clin Oncol. 2015;33(Suppl 15):e20018. [Google Scholar]
- 156.Kanzler H, Barrat FJ, Hessel EM, et al Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13(5):552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 157.Barton GM, Kagan JC A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9(8):535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Royal RE, Vence LM, Wray T, et al A toll-like receptor agonist to drive melanoma regression as a vaccination adjuvant or by direct tumor application. J Clin Oncol. 2017;35(S 15):9582. doi: 10.1200/JCO.2017.35.15_suppl.9582. [DOI] [Google Scholar]
- 159.Pol J, Kroemer G, Galluzzi L First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2016;5(1):e1115641. doi: 10.1080/2162402X.2015.1115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hersey P, Gallagher S Intralesional immunotherapy for melanoma. J Surg Oncol. 2014;109(4):320–326. doi: 10.1002/jso.23494. [DOI] [PubMed] [Google Scholar]
- 161.Mandalà M, Tondini C, Merelli B, et al Rationale for new checkpoint inhibitor combinations in melanoma therapy. Am J Clin Dermatol. 2017;18(5):597–611. doi: 10.1007/s40257-017-0282-0. [DOI] [PubMed] [Google Scholar]
- 162.Andtbacka RHI, Kaufman H, Daniels GA, et al CALM study: a phase II study of intratumoral coxsackievirus A21 in patients with stage IIIc and stage IV malignant melanoma. https://ascopubs.org/doi/abs/10.1200/jco.2013.31.15_suppl.tps3128 J Clin Oncol. 2013;31(Suppl 15):TPS3128. [Google Scholar]
- 163.Dudley ME, Yang JC, Sherry R, et al Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chang CH, Pearce EL Emerging concepts of T cell metabolism as a target of immunotherapy. Nat Immunol. 2016;17(4):364–368. doi: 10.1038/ni.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gattinoni L, Klebanoff CA, Palmer DC, et al Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells . J Clin Invest. 2005;115(6):1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sukumar M, Liu J, Ji Y, et al Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function . J Clin Invest. 2013;123(10):4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Klemen ND, Feingold PL, Goff SL, et al Metastasectomy following immunotherapy with adoptive cell transfer for patients with advanced melanoma. Ann Surg Oncol. 2017;24(1):135–141. doi: 10.1245/s10434-016-5537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Goff SL, Dudley ME, Citrin DE, et al Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol. 2016;34(20):2389–2397. doi: 10.1200/JCO.2016.66.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dudley ME, Wunderlich JR, Yang JC, et al Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang EH, Xu HM A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. J Hematol Oncol. 2017;10(1):1. doi: 10.1186/s13045-016-0379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jackson HJ, Rafiq S, Brentjens RJ Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13(6):370–383. doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yu SN, Li AP, Liu Q, Li T, et al Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10(1):78. doi: 10.1186/s13045-017-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Almåsbak H, Aarvak T, Vemuri MC CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602. doi: 10.1155/2016/5474602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rupp LJ, Schumann K, Roybal KT, et al CRISPR/Cas9-mediated PD-1 disruption enhances anti-Tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Merhavi-Shoham E, Itzhaki O, Markel G, et al Adoptive cell therapy for metastatic melanoma. Cancer J. 2017;23(1):48–53. doi: 10.1097/PPO.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 176.Yvon E, DelVecchio M, Savoldo B, et al Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Cancer Res. 2009;15(18):5852–5860. doi: 10.1158/1078-0432.CCR-08-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.John LB, Devaud C, Duong CPM, et al Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19(20):5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 178.Panelli MC, Wunderlich J, Jeffries J, et al Phase 1 study in patients with metastatic melanoma of immunization with dendritic cells presenting epitopes derived from the melanoma-associated antigens MART-1 and gp100. J Immunother. 2000;23(4):487–498. doi: 10.1097/00002371-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 179.Yuan J, Ku GY, Gallardo HF, et al Safety and immunogenicity of a human and mouse gp100 DNA vaccine in a phase I trial of patients with melanoma. https://pubmed.ncbi.nlm.nih.gov/19496531/ Cancer Immun. 2009;9:5. [PMC free article] [PubMed] [Google Scholar]
- 180.Galluzzi L, Buqué A, Kepp O, et al Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 181.Castle BT, McCubbin S, Prahl LS, et al Mechanisms of kinetic stabilization by the drugs paclitaxel and vinblastine. Mol Biol Cell. 2017;28(9):1238–1257. doi: 10.1091/mbc.e16-08-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Siddik ZH Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 183.Samlowski WE, Moon J, Witter M, et al High frequency of brain metastases after adjuvant therapy for high-risk melanoma. Cancer Med. 2017;6(11):2576–2585. doi: 10.1002/cam4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Napoli S, Scuderi C, Gattuso G, et al Functional roles of matrix metalloproteinases and their inhibitors in melanoma. Cells. 2020;9(5):1151. doi: 10.3390/cells9051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Winer A, Adams S, Mignatti P Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther. 2019;17(6):1147–1155. doi: 10.1158/1535-7163.MCT-17-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Raeeszadeh-Sarmazdeh M, Do LD, Hritz BG Metalloproteinases and their inhibitors: potential for the development of new therapeutics. Cells. 2020;9(5):1313. doi: 10.3390/cells9051313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Flaherty KT Targeting metastatic melanoma. Annu Rev Med. 2012;63(1):171–183. doi: 10.1146/annurev-med-050410-105655. [DOI] [PubMed] [Google Scholar]
- 188.Soura E, Eliades PJ, Shannon K, et al Hereditary melanoma: genetics of familial atypical multiple mole melanoma syndrome. J Am Acad Dermatol. 2016;74(3):395–407. doi: 10.1016/j.jaad.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bennett DC Human melanocyte senescence and melanoma susceptibility genes. Oncogene. 2003;22(20):3063–3069. doi: 10.1038/sj.onc.1206446. [DOI] [PubMed] [Google Scholar]
- 190.Goldstein AM, Chidambaram A, Halpern A, et al Rarity of CDK4 germline mutations in familial melanoma. Melanoma Res. 2002;12(1):51–55. doi: 10.1097/00008390-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 191.O'Leary B, Finn RS, Turner NC Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 192.Ranade K, Hussussian CJ, Sikorski RS, et al Mutations associated with familial melanoma impair p16INK4 function . Nat Genet. 1995;10(1):114–116. doi: 10.1038/ng0595-114. [DOI] [PubMed] [Google Scholar]
- 193.Prével C, Pellerano M, González-Vera JA, et al Fluorescent peptide biosensor for monitoring CDK4/cyclin D kinase activity in melanoma cell extracts, mouse xenografts and skin biopsies. Biosens Bioelectron. 2016;85:371–380. doi: 10.1016/j.bios.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 194.Yadav V, Burke TF, Huber L, et al The CDK4/6 inhibitor LY2835219 overcomes vemurafenib resistance resulting from MAPK reactivation and cyclin D1 upregulation. Mol Cancer Ther. 2014;13(10):2253–2263. doi: 10.1158/1535-7163.MCT-14-0257. [DOI] [PubMed] [Google Scholar]
- 195.Prickett TD, Agrawal NS, Wei X, et al Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4 . Nat Genet. 2009;41(10):1127–1132. doi: 10.1038/ng.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pópulo H, Lopes JM, Soares P, et al The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13(2):1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pópulo H, Soares P, Faustino A, et al mTOR pathway activation in cutaneous melanoma is associated with poorer prognosis characteristics. Pigment Cell Melanoma Res. 2011;24(1):254–257. doi: 10.1111/j.1755-148X.2010.00796.x. [DOI] [PubMed] [Google Scholar]
- 198.Li X, Wu D, Shen J, et al Rapamycin induces autophagy in the melanoma cell line M14 via regulation of the expression levels of Bcl-2 and Bax . Oncol Lett. 2012;5(1):167–172. doi: 10.3892/ol.2012.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kwong LN, Davies MA Targeted therapy for melanoma: Rational combinatorial approaches. Oncogene. 2014;33(1):1–9. doi: 10.1038/onc.2013.34. [DOI] [PubMed] [Google Scholar]
- 200.Pópulo H, Tavares S, Faustino A, et al GNAQ and BRAF mutations show differential activation of the mTOR pathway in human transformed cells. PeerJ. 2013;1:e104. doi: 10.7717/peerj.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Atefi M, Von Euw E, Attar N, et al Reversing melanoma cross-resistance to BRAF and MEK inhibitors by Co-targeting the AKT/mTOR pathway. PLoS One. 2011;6(12):e28973. doi: 10.1371/journal.pone.0028973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Deng W, Vashisht Gopal YN, Scott A, et al Role and therapeutic potential of PI3K-mTOR signaling in de novo resistance to BRAF inhibition. Pigment Cell Melanoma Res. 2012;25(2):248–258. doi: 10.1111/j.1755-148X.2011.00950.x. [DOI] [PubMed] [Google Scholar]
- 203.Gotwals P, Cameron S, Cipolletta D, et al Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17(5):286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 204.Willmore-Payne C, Holden JA, Tripp S, et al Human malignant melanoma: Detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol. 2005;36(5):486–493. doi: 10.1016/j.humpath.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 205.Curtin JA, Busam K, Pinkel D, et al Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 206.Livingstone E, Zimmer L, Vaubel J, et al BRAF, MEK and KIT inhibitors for melanoma: Adverse events and their management. Chinese Clin Oncol. 2014;3(3):29. doi: 10.3978/j.issn.2304-3865.2014.03.03. [DOI] [PubMed] [Google Scholar]
- 207.Carlino MS, Todd JR, Rizos H Resistance to c-Kit inhibitors in melanoma: Insights for future therapies. Oncoscience. 2014;1(6):423–426. doi: 10.18632/oncoscience.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Heinrich MC, Corless CL, Demetri GD, et al Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 209.Hodi FS, Corless CL, Giobbie-Hurder A, et al Imatinib for melanomas harboring mutationally activated or amplified kit arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31(26):3182–3190. doi: 10.1200/JCO.2012.47.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hodi FS, Friedlander P, Corless CL, et al Major response to imatinib mesylate in KIT-mutated melanoma . J Clin Oncol. 2008;26(12):2046–2051. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- 211.Guo J, Si L, Kong Y, et al Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification . J Clin Oncol. 2011;29(21):2904–2909. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]
- 212.Tas F, Duranyildiz D, Oguz H, et al Circulating serum levels of angiogenic factors and vascular endothelial growth factor receptors 1 and 2 in melanoma patients. Melanoma Res. 2006;16(5):405–411. doi: 10.1097/01.cmr.0000222598.27438.82. [DOI] [PubMed] [Google Scholar]
- 213.Mehnert JM, McCarthy MM, Jilaveanu L, et al Quantitative expression of VEGF, VEGF-R1, VEGF-R2, and VEGF-R3 in melanoma tissue microarrays. Hum Pathol. 2010;41(3):375–384. doi: 10.1016/j.humpath.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Folkman J, Klagsbrun M Angiogenic factors. Science. 1987;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 215.Kim KJ, Li B, Winer J, et al Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo . Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 216.Von Moos R, Seifert B, Simcock M, et al First-line temozolomide combined with bevacizumab in metastatic melanoma: A multicentre phase II trial (SAKK 50/07) Ann Oncol. 2012;23(2):531–536. doi: 10.1093/annonc/mdr126. [DOI] [PubMed] [Google Scholar]
- 217.Flaherty KT, Robert C, Hersey P, et al Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 218.Ascierto PA, Schadendorf D, Berking C, et al MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: A non-randomised, open-label phase 2 study . Lancet Oncol. 2013;14(3):249–256. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- 219.Wright CJM, McCormack PL Trametinib: First global approval. Drugs. 2013;73(11):1245–1254. doi: 10.1007/s40265-013-0096-1. [DOI] [PubMed] [Google Scholar]
- 220.Niezgoda A, Niezgoda P, Czajkowski R Novel approaches to treatment of advanced melanoma: A review on targeted therapy and immunotherapy. Biomed Res Int. 2015;2015:851387. doi: 10.1155/2015/851387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hoeflich KP, Merchant M, Orr C, et al Intermittent administration of MEK inhibitor GDC-0973 plus pi3k inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72(1):210–219. doi: 10.1158/0008-5472.CAN-11-1515. [DOI] [PubMed] [Google Scholar]