Abstract
Vitamin D deficiency has a high worldwide prevalence, but actions to improve this public health problem are challenged by the heterogeneity of nutritional and clinical vitamin D guidelines, with respect to the diagnosis and treatment of vitamin D deficiency. We aimed to address this issue by providing respective recommendations for adults, developed by a European expert panel, using the Delphi method to reach consensus. Increasing the awareness of vitamin D deficiency and efforts to harmonize vitamin D guidelines should be pursued. We argue against a general screening for vitamin D deficiency but suggest 25-hydroxyvitamin D (25(OH)D) testing in certain risk groups. We recommend a vitamin D supplementation dose of 800 to 2000 international units (IU) per day for adults who want to ensure a sufficient vitamin D status. These doses are also recommended for the treatment of vitamin D deficiency, but higher vitamin D doses (e.g., 6000 IU per day) may be used for the first 4 to 12 weeks of treatment if a rapid correction of vitamin D deficiency is clinically indicated before continuing, with a maintenance dose of 800 to 2000 IU per day. Treatment success may be evaluated after at least 6 to 12 weeks in certain risk groups (e.g., patients with malabsorption syndromes) by measurement of serum 25(OH)D, with the aim to target concentrations of 30 to 50 ng/mL (75 to 125 nmol/L).
Keywords: vitamin D, recommendations, guidelines, supplementation, cholecalciferol, treatment
1. Introduction
Vitamin D is crucial for musculoskeletal health, as it plays an important role in the regulation of bone and mineral metabolism, and it can prevent and cure nutritional rickets and osteomalacia [1,2]. In addition, vitamin D receptor (VDR) expression in almost all human cells suggests, or even documents, a more widespread role of vitamin D for overall health, a notion that is supported by several experimental and epidemiological studies [1,3,4,5,6,7,8]. While there still exist knowledge gaps and controversy regarding potential extra-skeletal effects of vitamin D, there is a wide consensus that the high worldwide prevalence of vitamin D deficiency is of concern and requires actions to improve this situation [2,7,9]. Addressing this issue has to consider the unique metabolism of vitamin D, which is mainly synthesized in the skin stimulated by ultraviolet-B (UV-B) exposure, whereas nutrition is usually only a minor source of vitamin D [10]. Vitamin D from all different sources is metabolized to 25-hydroxyvitamin D (25(OH)D, calcifediol) in the liver, which is the main circulating vitamin D metabolite that is determined to assess vitamin D status. Further hydroxylation of 25(OH)D in the kidneys or certain extra-renal tissues results in the formation of 1,25-dihydroxyvitamin D (1,25(OH)2D, also called calcitriol), which exerts endocrine, autocrine, and paracrine effects as a steroid hormone [10]. Heterogeneous recommendations, regarding several issues in the practical management of vitamin D deficiency, represent a challenge for clinicians and health authorities on how to deal with this public health problem [11,12,13,14,15,16,17,18,19,20,21,22]. In this context, systematic evaluations of current vitamin D guidelines did, not only, observe a great heterogeneity of the recommendations, but it also reported a low quality score regarding the methodological processes for the majority of these vitamin D guidelines [17,18]. Table 1 and Table 2 provide an overview of selected guideline recommendations, with a focus on Central and Eastern European countries, for prevention and treatment of vitamin D deficiency, respectively.
Table 1.
Selected guideline recommendations for prevention of vitamin D deficiency in adults with a focus on Central and Eastern European countries, published since 2010.
| Authority and/or Country or Region (Year) |
Target Population | Age (Years) | Oral Vitamin D (IU) | Reference |
|---|---|---|---|---|
| Endocrine Society (2011) USA |
General population | 19–70 | 600–2000/day | Holick et al. [14] |
| >70 | 800–2000/day | |||
| Pregnant and lactating women | 600–2000/day | |||
| Obese individuals/Patients on anticonvulsants, glucocorticoids, antifungals, AIDS medications | 2–3 times more | |||
| DACH (2012) Germany/Austria/Switzerland |
General population | >18 | 800/day | DGE [23] |
| EVIDAS (2013) Central Europe |
General population | >18 | 800–2000/day | Płudowski et al. [21] |
| Obese individuals and elderly | 1600–4000/day | |||
| Prevention of pregnancy and fetal development complications |
>16 | 1500–2000/day | ||
| Night workers and dark skin pigmentation | 1000–2000/day | |||
| EFSA (2016) Europe |
General population | >18 | 600/day | EFSA [24] |
| Russia (2016) | General population | >18 | 800–1000/day | Pigarova et al. [25] |
| Pregnant women | 800–2000/day | |||
| Poland (2018) | General population | 19–75 | 800–2000/day | Rusińska, Płudowski et al. [26] |
| Obese individuals | 19–75 | 1600–4000/day | ||
| General population | >75 | 2000–4000/day | ||
| Obese individuals | >75 | 4000–8000/day | ||
| Pregnant and lactating women | 2000/day | |||
| Belarus (2013) | General population | >18 | 800–2000/day | Rudenko [27] |
| Hungary (2012) | General population | >18 | 1500–2000/day | Takács et al. [22] |
| Pregnant and lactating women | 1500–2000/day | |||
| Bulgaria (2019) | General population | >19 | 600–2000/day | Borisova et al. [28] |
| Pregnant and lactating women | 600–2000/day | |||
| Patients on anticonvulsants, glucocorticoids, antifungals | 2–3 times more | |||
| Slovakia (2018) | Postmenopausal osteoporosis patients | >50 | 800–1000/day | Payer et al. [29] |
Table 2.
Selected guideline recommendations for treatment of vitamin D deficiency in adults with a focus on Central and Eastern European countries, published since 2010.
| Authority and/or Country or Region (Year) |
Target Population | Oral Vitamin D for Treatment (IU) | Treatment Duration |
25(OH)D Target Concentration nmol/L (ng/mL) |
Oral Vitamin D for Maintenance (IU) |
Reference |
|---|---|---|---|---|---|---|
| Endocrine Society (2011) USA |
General population |
50,000/week or 6000/day |
8 weeks | 75 (30) |
1500–2000/day | Holick et al. [14] |
| Obese individuals/Patients on anticonvulsants, glucocorticoids, antifungals, AIDS medications | 2–3 times more; at least 6000–10,000/day | 3000–6000/day | ||||
| EVIDAS (2013) Central Europe |
General population |
50,000/week or 7000–10,000/day |
4–12 weeks | 75–125 (30–50) |
a maintenance dose may be instituted | Płudowski et al. [21] |
| Italy (2018) | General population |
50,000/week or 5000/day |
8 weeks | >75 (>30) |
50,000 IU twice per month or 1500–2000 IU/day |
Cesareo et al. [30] |
| Russia (2016) | General population |
25(OH)D < 50 nmol/L (<20 ng/mL): | >75 (>30) |
1000–2000/day or 6000–14,000/week |
Pigarova et al. [25] | |
| 50,000/week or | 8 weeks | |||||
| 200,000/month or | 2 months | |||||
| 150,000/month or | 3 months | |||||
| 6000–8000/day | 8 weeks | |||||
| 25(OH)D < 75 nmol/L (30 ng/mL): |
||||||
| 50,000/week or | 4 weeks | |||||
| 200,000 or | single dose | |||||
| 150,000 or | single dose | |||||
| 6000–8000/day | 4 weeks | |||||
| Poland (2018) | General population |
6000/day | 12 weeks or until a 25(OH)D concentration of 75 nmol/L (30 ng/mL) is reached |
>75–125 (>30–50) |
maintenance dose i.e., a prophylactic dose recommended for the general population (see Table 1) | Rusińska, Płudowski et al. [26] |
| Belarus (2013) | General population |
25(OH)D < 25 nmol/L (<10 ng/mL): 2000 to 10,000/day |
4–12 weeks | 75–200 (30–80) |
800–2000 IU/day | Rudenko [27] |
| 25(OH)D 25–50 nmol/L (10–20 ng/mL): 800 to 4000/day |
1 year | |||||
| Hungary (2012) | General population |
50,000/week or | 4–8 weeks | 75 (30) |
1500–2000/day | Takács et al. [22] |
| 30,000/week or | 6–12 weeks | |||||
| 2000/day | 12 weeks | |||||
| Bulgaria (2019) | General population |
To maintain bone health: 1000–2000/day |
- | 50 (20) |
maintenance dose i.e., a prophylactic dose recommended for the general population (see Table 1) | Borisova et al. [28] |
| For extra–skeletal effects: 2000–4000/day |
- | 75–110 (30–44) |
In clinical practice, a great variability and controversy is reported regarding vitamin D testing and supplementation, thus requiring an improved guidance for clinicians [31]. Therefore, we aimed to draft an expert consensus statement covering important aspects of the clinical practice in the prevention, diagnosis, and treatment of vitamin D deficiency of adults. Rather than covering all of the above-mentioned vitamin D issues in great and extensive detail, we aimed to provide a simple, easy-to-follow guidance for clinicians. Respective data and recommendations regarding these issues in children can be found elsewhere [14,21,26,32,33,34].
2. Consensus Development Process
A European expert panel with 10 members and a focus on physicians from Eastern Europe, who are usually underrepresented in such groups, was established by selecting clinicians and key opinion leaders with expertise in vitamin D and related topics from their respective countries. The first and senior authors (P.P. and S.P.) of this article served as the chairs of this expert panel, who selected and invited the other panel members. The consensus-reaching process itself was conducted by using the Delphi method, which is a widely accepted tool for clinical consensus statements [35,36]. The Delphi method was applied by using SurveyMonkey® for voting on various statements on a 9-point scale with the following numeric (and descriptive) anchors: 1 (strongly disagree), 3 (disagree), 5 (neutral), 7 (agree), and 9 (strongly agree). It was pre-specified that a consensus will be established, in the case that ≥75% of the participants agree with the statement, by a voting scale of 7 or above. In case of failing to reach a consensus according to this criterion, it was planned to repeat these surveys after group discussions and statement modifications, if necessary, until a consensus is reached.
After the alignment of the scope of this consensus document and recruitment of the 10 members of the expert panel, the detailed process of this work started with drafting various questions on practical issues regarding vitamin D, which were exclusively discussed and fine-tuned by the chairs. These questions were subsequently used in a first survey round in September 2021. All panel members were involved in this survey, and their answers, along with the existing literature on vitamin D, served as the basis to formulate respective statements by the chairs. At a hybrid meeting in Darmstadt, Germany on the 20 November 2021, the results of the first survey round, along with the subsequently formulated statements, were presented by the chairs. At the same meeting, each statement was subject to a group discussion and a Delphi voting round to reach consensus, which was finally successful for all statements. The 10 authors of this paper are the 10 experts who participated in the Delphi voting rounds. Each voting round was performed for the whole content of each Table or Figure, respectively. Group discussions before each voting round, however, were used to fine tune the statements according to the opinions and recommendations of the panel members. The work on this consensus document was funded and organized by Wörwag Pharma (Böblingen, Germany). The sponsor provided financial and logistic support but did not actively participate in the scientific discussions and consensus-reaching processes.
3. Consensus Recommendations
The final consensus statements with the level of agreement within the expert group are subsequently presented below. The evidence and considerations underpinning each statement are also outlined. It was our aim to base our work on the totality of available evidence by considering the established hierarchy of evidence levels. The expert panel group was encouraged to perform systematic literature reviews on the topics of this consensus document, but we have to acknowledge that this gathering of information did not follow a pre-specified structured process.
3.1. Current Situation of Vitamin D Deficiency Diagnosis, Prevention and Treatment
Epidemiological studies have documented a high prevalence of vitamin D deficiency worldwide [37]. Data from Europe showed that 25(OH)D concentrations below 20 ng/mL (50 nmol/L) and below 12 ng/mL (30 nmol/L) are observed in 40.4% and 13.0% of the general population, respectively [9]. Therefore, a huge gap exists between the recommendations of nutritional societies regarding dietary reference intakes, as well as target 25(OH)D concentrations, and the actual situation, as documented in large population surveys [38]. Public health actions are, therefore, required to improve the vitamin D status in the general population, but regional differences in vitamin D status, related to factors such as latitude, genetics, lifestyle, body composition, or dietary intake have to be considered [9,38,39,40,41]. A major issue to achieve this task is to harmonize the current heterogeneous efforts and guideline recommendations (Table 3).
Table 3.
Statement regarding the current situation of vitamin D deficiency diagnosis, prevention, and treatment.
| Consensus Statement | Consensus Voting Scale | Level of Agreement |
|---|---|---|
| To ensure an adequate screening, prevention and treatment of vitamin D deficiency in the clinical practice, it is necessary to increase the awareness and improve education in the public and medical community. Moreover, national and international guidelines/recommendations should be precise regarding the risk groups that need to be screened, the adequate dosages for prevention and treatment of vitamin D deficiency, the treatment regimen as well as the follow-up to allow transfer into clinical practice. |
9 (strongly agree) | 80% |
| 8 | 0% | |
| 7 (agree) | 20% | |
| 6 | 0% | |
| 5 (neutral) | 0% | |
| 4 | 0% | |
| 3 (disagree) | 0% | |
| 2 | 0% | |
| 1 (strongly disagree) | 0% | |
| Overall agreement 100%, consensus endorsed | ||
3.2. Screening of Vitamin D Deficiency in Adults
No published study evaluated the effects of a screening program for vitamin D deficiency in the general population, so the evidence is insufficient to balance the benefits and harms of such a screening [42,43]. Accordingly, we stress that it is currently not justified to recommend a general screening for vitamin D deficiency by measuring 25(OH)D concentrations in the whole general population. Nevertheless, considering that certain groups of individuals or patients are particularly prone to vitamin D deficiency and/or may particularly benefit from vitamin D treatment, we suggest, in line with the Endocrine Society, that 25(OH)D measurements should be considered in these groups, as listed in Table 4 [14].
Table 4.
Statement regarding screening of vitamin D deficiency in adults.
| Consensus Statement | Consensus Voting Scale | Level of Agreement |
|---|---|---|
| Screening of vitamin D deficiency should be considered in the following patients/individuals or conditions: Osteoporosis; Osteomalacia; Musculoskeletal pain; Chronic kidney disease; Hepatic failure; Malabsorption syndromes (e.g., cystic fibrosis, inflammatory bowel diseases, bariatric surgery, radiation enteritis); Hyperparathyroidism; Chronic treatment with medications that influence vitamin D metabolism (e.g., antiseizure medications, glucocorticoids, AIDS-medications, antifungal agents, cholestyramine); Chronic autoimmune diseases (e.g., multiple sclerosis, rheumatoid arthritis); Pregnant and lactating women; Institutionalized or hospitalized patients; Older adults (>65 years) in general; Older adults with history of falls or nontraumatic fractures; Granuloma-forming disorders (e.g., sarcoidosis, tuberculosis, histoplasmosis, berylliosis, coccidiomycosis); Obesity (BMI ≥ 30kg/m2); dark skin pigmentation. 25(OH)D is recommended as a laboratory marker for the diagnosis of vitamin D deficiency. In patients with diagnosed vitamin D deficiency (25(OH)D < 20 ng/mL (<50 nmol/L)) and suspected related health issues, serum calcium, phosphate, alkaline phosphatase, parathyroid hormone (PTH), creatinine, and serum magnesium levels should be considered for evaluation; in particular in individuals with a 25(OH)D concentration of <10 ng/mL (<25 nmol/L). A 25(OH)D concentration of <20 ng/mL (<50 nmol/L) is considered as vitamin D deficiency A 25(OH)D concentration of ≥20 ng/mL (≥50 nmol/L) and <30 ng/mL (<75 nmol/L) is considered as vitamin D insufficiency A 25(OH)D concentration of 30–50 ng/mL (75–125 nmol/L) is considered as vitamin D sufficiency A 25(OH)D concentration of >50–60 ng/mL (125–150 nmol/L) is considered as safe but not as a target level A 25(OH)D concentration of >60–100 ng/mL (150–250 nmol/L) is considered as area of uncertainty with potential benefits or risks. A 25(OH)D concentration of >100 ng/mL (250 nmol/L) is considered as oversupply/vitamin D toxicity |
9 (strongly agree) | 50% |
| 8 | 20% | |
| 7 (agree) | 30% | |
| 6 | 0% | |
| 5 (neutral) | 0% | |
| 4 | 0% | |
| 3 (disagree) | 0% | |
| 2 | 0% | |
| 1 (strongly disagree) | 0% | |
| Overall agreement 100%, consensus endorsed | ||
Total serum 25(OH)D concentration, i.e., the sum of 25(OH)D3 and 25(OH)D2, is the accepted marker for the assessment of vitamin D status, as it best reflects vitamin D supply by all different sources, i.e., endogenous vitamin D synthesis in the skin, diet, supplements, and mobilization from tissue stores. Previous reports on a relatively high inter-assay and inter-laboratory variability of 25(OH)D measurements underscore the need for assay standardization and laboratory quality assurance [44,45]. In patients with vitamin D deficiency and certain related health issues, e.g., bone diseases, it should be considered to measure additional laboratory parameters, including serum calcium, phosphate, alkaline phosphatase, parathyroid hormone (PTH), creatinine (to calculate the estimated glomerular filtration rate), and magnesium, as these laboratory markers may be useful to guide further diagnostics and treatment of these patients. Measurements of, e.g., serum calcium and creatinine are, however, also advised in patients with 25(OH)D concentrations above 100 ng/mL (250 nmol/L), as vitamin D oversupply/toxicity may lead to hypercalciuria, followed by hypercalcemia, potential acute kidney disease, and vascular calcification. Hypercalcemia does, however, usually not occur at 25(OH)D concentrations below 150 ng/mL (375 nmol/L) [46]. There are hardly any contraindications to correct vitamin D deficiency by vitamin D supplementation (e.g., kidney stones are per se no contraindication) except of rare conditions with an increased sensitivity to vitamin D treatment, such as inherited 24-hydroxylase-deficiency [47]. This is a rare genetic disorder in which catabolism of vitamin D metabolites is impaired, leading to hypercalcemia, low PTH concentrations, and relatively high serum 25(OH)D concentrations along with an increased risk of nephrolithiasis [47]. If such a disease is suspected, the measurement of 24,25-dihydroxyvitamin D, in a specialized laboratory, aids in the diagnosis as a high ratio of 25(OH)D to 24,25-dihydroxyvitamin D suggests this disease that is further confirmed by genetic analyses [47].
Classification of vitamin D status and its terminology, according to 25(OH)D concentration, remains a controversial issue in the scientific literature [7,16,21]. Being aware that it is an individual continuum from vitamin D deficiency to a sufficient and optimal vitamin D status, as well as to vitamin D toxicity, we suggest a classification system, as indicated in Table 4. It should be kept in mind that such a general classification of vitamin D status cannot take into account variations in the individual sensitivity to vitamin D effects that may be due to genetic polymorphisms, epigenetic or nutritional factors (e.g., magnesium status), as well as co-morbidities or medications [48,49,50,51,52].
3.3. Prevention of Vitamin D Deficiency in Adults
Most nutritional vitamin D guidelines conclude that vitamin D requirements are met for the vast majority (i.e., 97.5%) of the population when achieving a target 25(OH)D concentration of at least 20 ng/mL (50 nmol/L) [11,12]. Recommended dietary reference intakes for vitamin D usually range from 600 to 800 international units (IU) (40 IU are equal to 1 µg) per day and should ensure a sufficient vitamin D status under conditions of minimal-to-no sunlight exposure [11,12,13,16,53,54]. These vitamin D intake doses were calculated according to meta-regression analyses of so called “winter RCTs” to estimate the dose-response curve of vitamin D intakes and achieved serum 25(OH)D concentrations without relevant endogenous vitamin D synthesis in the skin [11]. It is a major limitation of most nutritional vitamin D guidelines that they performed meta-regression analyses based on aggregate data because such an approach does not adequately capture between person variability in the treatment response [53,54]. Using individual participant data instead of aggregate data for meta-regression analyses, as a superior methodological approach, results in significantly higher vitamin D intakes to achieve certain target 25(OH)D concentrations [53,54]. Individual participant data meta-regression analyses and single RCTs suggest that an overall vitamin D intake of about 1000 IU of vitamin D per day is required to maintain 25(OH)D concentrations of, at least, 20 ng/mL (50 nmol/L) in 97.5% of the population [54,55]. Therefore, we recommend a vitamin D supplement dose of at least 800 IU per day when targeting a sufficient vitamin D status, i.e., a 25(OH)D concentration of at least 20 ng/mL (50 nmol/L). We can, of course, improve and maintain vitamin D status by consuming natural or fortified food sources, but vitamin D intake by diet is usually in the range of about 100 to 200 IU per day in the general population [37,56].
In detail, we recommend a vitamin D supplementation dose of 800 to 2000 IU per day for adults who want to ensure a sufficient vitamin D status, with up to 4000 IU per day for certain groups, particularly for patients with obesity and malabsorption syndromes, as well as for individuals with a dark skin pigmentation (see Table 5). The relatively wide dose ranges for vitamin D account for various differences in the dose-response relationship for a given supplemental vitamin D dose and the achieved 25(OH)D concentration with higher dose requirements with increasing body weight and vice versa [57,58,59,60,61,62,63,64]. If a clinician is asked by a random individual which vitamin D dose is safe and very likely avoids vitamin D deficiency, a dose of 800 to 1000 IU per day should fulfill these criteria for the vast majority, even if individual characteristics, including the 25(OH)D status, is unknown. It should, however, also be noted that a few health authorities and experts consider a 25(OH)D concentration, of at least 10–12 ng/mL (25 to 30 nmol/L), as a reasonable treatment target that can be achieved by supplementation of 400 IU of vitamin D per day [11,13,16,53].
Table 5.
Statement regarding prevention of vitamin D deficiency in adults.
| Consensus Statement | Consensus Voting Scale | Level of Agreement |
|---|---|---|
| In healthy adults without other risk factors, a supplementation of 800–2000 IU/day, for those who want to achieve a targeted/measured 25(OH)D concentration, should be considered during wintertime (mainly November-April) due to insufficient endogenous dermal vitamin D synthesis and depending on the body weight. Due to decreased skin synthesis in elderly (>65 years), a supplementation of 800–2000 IU/day is recommended throughout the year. In hospitalized/institutionalized individuals, a supplementation of 800–2000 IU/day is recommended throughout the year. Women planning a pregnancy should start or maintain the vitamin D supplementation as recommended for healthy adults without other risk factors (800–2000 IU/day). The vitamin D supplementation should be continued throughout pregnancy and lactation. In certain patients/individuals or conditions 2–3 times higher vitamin D dosages, without using vitamin D doses above the UL of 4000 IU/day, are recommended for prevention compared to healthy adults without other risk factors: Malabsorption (e.g., cystic fibrosis, inflammatory bowel diseases, bariatric surgery, radiation enteritis) Obesity (BMI ≥ 30 kg/m2) Dark skin pigmentation As vitamin D metabolites are stored in fat and other tissues and gradually released into the blood circulation, a daily or weekly or monthly supplementation regimen is equally effective and safe, if monthly doses are not exceedingly high, for the prevention of vitamin D deficiency. A tailored approach for vitamin D administration, involving the patients’ preferences of the supplementation regimen (daily, weekly, monthly) might enhance the adherence to preventive vitamin D supplementation. For the prevention of vitamin D deficiency, the supplementation of oral cholecalciferol (vitamin D3) is recommended. |
9 (strongly agree) | 30% |
| 8 | 20% | |
| 7 (agree) | 50% | |
| 6 | 0% | |
| 5 (neutral) | 0% | |
| 4 | 0% | |
| 3 (disagree) | 0% | |
| 2 | 0% | |
| 1 (strongly disagree) | 0% | |
| Overall agreement 100%, consensus endorsed | ||
Daily, weekly, or monthly vitamin D supplementation, at equivalent doses, lead to similar increases in 25(OH)D serum concentrations, when measured after 2 to 3 months [65,66,67]. Adherence may be better with intermittent vitamin D dosing, but there are also concerns that high intermittent vitamin D doses may be less beneficial or might even be harmful in certain settings [67,68,69]. In view of the available evidence from clinical vitamin D trials and some pathophysiological considerations (e.g., altered vitamin D metabolism with high intermittent vitamin D doses), a daily vitamin D dosing schedule should rather be preferred, but when exceedingly high intermittent vitamin D doses are avoided, a weekly or monthly dosing schedule can also be applied [66,67]. The panel members could not reach a clear consensus on a clear cut-off for exceedingly high vitamin D doses, but single doses above about 50,000 IU of vitamin D should rather be avoided. Due to superior evidence regarding clinical benefits and dose-response, we rather prefer vitamin D3 (cholecalciferol) over vitamin D2 (ergocalciferol) for the prevention of vitamin D deficiency [51,70].
3.4. Treatment of Vitamin D Deficiency in Adults
Individuals with a measured 25(OH)D concentration below 20 ng/mL (50 nmol/L) should be treated with vitamin D supplementation, because their vitamin D requirements may not be met [11,12]. There is controversy in the scientific literature whether 25(OH)D concentrations between 20 ng/mL (50 nmol/L) and <30 ng/mL (75 nmol/L) justify vitamin D supplementation [71,72]. The recommended dose range of 800 to 2000 IU per day is a reflection of various considerations underlying such treatment goals (see Table 6). When aiming for a minimum 25(OH)D concentration of at least 20 ng/mL (50 nmol/L), a daily vitamin D supplement dose of about 800 IU per day is sufficient for almost all individuals, even during the winter season, in Europe [54,55]. Data are less clear on which vitamin D doses are required to achieve a 25(OH)D concentration of ≥30 ng/mL (75 nmol/L) in almost all patients, but doses may be in the range of about 1500 to 2000 IU per day or even higher [14,21,26,27,28]. The classic rule of thumb that 100 IU of vitamin D per day increases serum 25(OH)D concentrations by about 1 ng/mL (2.5 nmol/L) seems to be a useful approximation, but several factors modulate the individual treatment response [73,74]. For example, increases in 25(OH)D are relatively high at low vitamin D supplement doses and low baseline 25(OH)D concentrations, whereas the dose-response curve flattens with higher vitamin D supplement doses and higher baseline 25(OH)D concentrations [73,74]. Evaluations of treatment success, by measurements of 25(OH)D, may be considered in certain patients, such as those with e.g., malabsorption or questionable adherence, but this should not be done earlier than 6 to 12 weeks after starting vitamin D supplementation, as this is about the time that it takes to reach a steady-state in serum 25(OH)D concentrations [2]. Although there is, of course, a seasonal variation in serum 25(OH)D concentrations, usually with higher levels during summertime, as a consequence of endogenous vitamin D synthesis in the skin, we do, in general, recommend continuous and, usually, fixed doses of vitamin D supplementation throughout the year. The decrease in serum 25(OH)D during winter season is, in many patients, significant, but it is less than could be expected by the half-life of serum 25(OH)D concentrations of about 2 to 3 weeks because of a mobilization of vitamin D and its metabolites from various tissue stores (e.g., adipose tissue and muscle) [75].
Table 6.
Statement regarding treatment of vitamin D deficiency in adults.
| Consensus Statement | Consensus Voting Scale | Level of Agreement |
|---|---|---|
| It is recommended to initiate a vitamin D deficiency treatment at a 25(OH)D concentration of <20 ng/mL (<50 nmol/L). At a concentration of <30 ng/mL (<75 nmol/L) a treatment may be considered. Individuals with diagnosed vitamin D deficiency can be initially treated with higher vitamin D dosages compared to the preventive dosages recommended for the general population, if a rapid correction of the 25(OH)D concentration is clinically indicated. As initial dose for the treatment of vitamin D deficiency in patients without other risk factors, a dosage of 6000 IU, equivalent to a daily dosage, is recommended. In certain individuals or conditions, higher vitamin D dosages, up to 10,000 IU, equivalent to a daily dosage, are recommended for treatment compared to healthy adults without other risk factors (Endocrine Society recommendation) [14]: -Malabsorption (e.g., cystic fibrosis, inflammatory bowel diseases, bariatric surgery, radiation enteritis) -Chronic treatment of medications that influence vitamin D metabolism (e.g., antiseizure medications, glucocorticoids, AIDS-medications, antifungal agents, cholestyramine) -Obesity (BMI ≥ 30 kg/m2) A treatment duration of 4–12 weeks is recommended, depending on the severity of vitamin D deficiency. A tailored approach for vitamin D administration, involving the patients’ preferences of the treatment regimen (daily, weekly, monthly) might enhance the adherence to the therapy. As soon as a 25(OH)D concentration of 30–50 ng/mL (75–125 nmol/L) is achieved, a maintenance dose of 800–2000 IU/day is recommended, that can also be used as an initial treatment dose if there is no requirement for a rapid correction of vitamin D deficiency. Approx. 6–12 weeks after start of the treatment, the effectiveness may be evaluated by measurement of the 25(OH)D concentration particularly in certain risk groups with e.g., malabsorption syndrome. For the treatment of vitamin D deficiency in adults, oral cholecalciferol (vitamin D3) is preferred. Calcifediol may be used instead of vitamin D in certain conditions, including obesity or malabsorption. Calcitriol and active vitamin D analogues may be considered in special patient groups. In certain risk groups (e.g., patients with severe malabsorption), parenteral vitamin D treatment can be considered. |
9 (strongly agree) | 60% |
| 8 | 10% | |
| 7 (agree) | 30% | |
| 6 | 0% | |
| 5 (neutral) | 0% | |
| 4 | 0% | |
| 3 (disagree) | 0% | |
| 2 | 0% | |
| 1 (strongly disagree) | 0% | |
| Overall agreement 100%, consensus endorsed | ||
If a rapid correction of vitamin D deficiency is clinically indicated, a regimen with a higher initial vitamin D dose, i.e., 6000 IU per day, and in certain cases, even up to 10,000 IU per day, followed by a maintenance dose with 800 to 2000 IU per day is recommended (Table 6). Such doses of 6000 IU, or even up to 10,000 IU, per day for several weeks are usually safe and ensure a more rapid correction of vitamin D deficiency compared to lower doses [14,76,77]. Daily vitamin D doses are generally preferred over intermittent dosing schedules [67]. The clinical indications for a rapid correction of vitamin D are, beyond osteomalacia, not clearly defined but may, according to our opinion, involve conditions such as extremely low 25(OH)D concentrations, osteoporosis patients with a very high fracture risk, patients with secondary hyperparathyroidism, and/or reduced serum calcium concentrations.
Regarding treatment of vitamin D deficiency and its prevention, we want to emphasize that promoting a healthy lifestyle by preventing or reducing obesity, regular physical activity with moderate (cautious) sunlight exposure, and a healthy balanced diet are also effective measures to improve both vitamin D status and overall health. Promoting such lifestyle measures is, of course, also highly recommended, and it should accompany any vitamin D treatment.
As for the prevention of vitamin D deficiency, we recommend vitamin D3 (cholecalciferol) over vitamin D2 (ergocalciferol) for its treatment. Although parenteral, particularly intramuscular, vitamin D treatment can be considered in patients with malabsorption, e.g., inflammatory bowel disease, we primarily suggest to increase the oral vitamin D dose in such settings [78]. Clinicians have to consider that patients with inflammatory bowel disease frequently require higher vitamin D doses, but with daily oral vitamin D supplementation of about 5000 to 10,000 IU, even these patients usually achieve their 25(OH)D target concentrations [78]. If intermittent intramuscular vitamin D injections (e.g., 100,000 IU all three months) are used, the doses are roughly similar and slightly more efficient for intramuscular compared to oral doses, in terms of raising serum 25(OH)D concentrations, but the increase in serum 25(OH)D is slower for intramuscular versus oral vitamin D supplementation [78,79,80,81].
Some experts argue that calcifediol (=25(OH)D3, calcidiol) may also be used to correct vitamin D deficiency in certain conditions. The use of calcifediol seems to be more justified in obese people, people with malabsorption syndromes, people with liver disease, patients suffering from chronic kidney disease (stage 3 or 4), and those in all conditions where rapid correction of vitamin D deficiency is required [82,83,84]. Furthermore, calcifediol use may also be beneficial in patients taking medications that disrupt the hepatic cytochrome P-450 enzyme system, including those taking glucocorticoids, anticonvulsants, anticancer drugs, or antiretroviral drugs [82,83,84,85]. The increase in serum 25(OH)D is markedly reduced in patients with obesity (high BMI) and in patients with malabsorption syndromes treated with cholecalciferol, but with calcifediol, the 25(OH)D increase is not significantly different according to BMI or according to the presence, or absence, of malabsorption syndromes. Moreover, the increase in serum 25(OH)D is faster, and the dose-response curve is more linear with the use of calcifediol versus vitamin D3, and when stopping treatment, the decline in 25(OH)D concentration is faster after calcifediol compared to vitamin D3 [75,82,83,84]. While accumulating evidence suggests that calcifediol may be an attractive alternative to “native” vitamin D, due to the lack of experience with this molecule in Central and Eastern European countries, at this stage, we continue to recommend vitamin D3 (cholecalciferol) [75]. Cholecalciferol and calcifediol appear, so far, as equal molecules in the fight with vitamin D deficiency. However, RCT data are still missing on the superior benefit of calcifediol versus vitamin D, with reference to hard clinical outcomes, but more data on this topic may be available in the future [75,84,85].
Calcitriol (=1,25(OH)2D) and its analogues are used at much lower doses compared to vitamin D3, have a relatively high risk of hypercalcemia and a relatively narrow therapeutic window, and are not recommended for the treatment of common vitamin D deficiency [64]. Therefore, active vitamin D treatment is only indicated in certain diseases, such as chronic hypoparathyroidism, chronic kidney disease, or mineral and bone disorders (CKD-MBD) [64,86].
3.5. Vitamin D in Musculoskeletal Disorders
While older meta-analyses of vitamin D RCTs showed a significant reduction in fractures by vitamin D supplementation at a daily dose of about 800 to 2000 IU per day, these data have recently been challenged by updated meta-analyses, suggesting no significant effect on fractures and falls [5,87,88]. Nevertheless, major osteoporosis guidelines recommend vitamin D treatment in osteoporosis patients, and some studies indicate that sufficient 25(OH)D concentrations are required for optimal bisphosphonate treatment efficacy [51,89]. While it is beyond the scope of our work to release detailed recommendations regarding calcium supplementation in osteoporosis patients, we wish to point out that a recent RCT with the bisphosphonate zoledronate showed excellent anti-fracture effects in patients using pure vitamin D supplementation without additional calcium supplements but consuming 1g of calcium daily by a usual diet [90]. These data may suggest that, even in osteoporosis patients, vitamin D treatment without additional calcium supplements may be sufficient in the case of adequate dietary calcium intake, but this issue is still not clarified in the scientific community, since it is challenging to disentangle the effects of vitamin D and calcium with regards to bone health [5,88,90,91,92]. Of note, increasing calcium and protein intake by milk, yoghurt, and cheese significantly reduces the risk of falls and fractures in aged care residents [93]. Calcium supplementation is, however, generally indicated in patients with osteoporosis and an insufficient dietary calcium intake. In this context, it should be stressed that most RCTs on osteoporosis drugs were conducted under the conditions of combined calcium plus vitamin D supplementation, thus supporting the use of calcium supplementation, in addition to vitamin D for osteoporosis treatment. Being aware of the uncertainties regarding vitamin D, in the context of osteoporosis, we nevertheless strongly argue to ensure a sufficient vitamin D status in patients with an increased risk of fractures and falls (Table 7). With reference to falls, some studies suggest that high-dose intermittent vitamin D supplementation may even be detrimental so that we prefer daily doses at the lower end of the dosing range of 800 to 2000 IU per day, in this setting [67,85,94]. Therefore, vitamin D overdosing must particularly be avoided in older and severely ill patients [94,95].
Table 7.
Statement regarding vitamin D in musculoskeletal disorders.
| Consensus Statement | Consensus Voting Scale | Level of Agreement |
|---|---|---|
| In osteoporosis patients, a supplementation of 800–2000 IU/day, with oral cholecalciferol (vitamin D3) is recommended in combination with calcium, if indicated. Vitamin D deficiency may impair the response to the osteoporosis treatment, thus screening of the 25(OH)D level and correction of vitamin D deficiency before starting the osteoporosis treatment with antiresorptive medications is recommended. In patients with an increased risk of falls or fractures, a supplementation of 800–2000 IU/day is recommended. |
9 (strongly agree) | 30% |
| 8 | 10% | |
| 7 (agree) | 60% | |
| 6 | 0% | |
| 5 (neutral) | 0% | |
| 4 | 0% | |
| 3 (disagree) | 0% | |
| 2 | 0% | |
| 1 (strongly disagree) | 0% | |
| Overall agreement 100%, consensus endorsed | ||
3.6. Extra-Skeletal Actions of Vitamin D in Adults
The expression of the VDR and of vitamin D-metabolizing enzymes in almost all human tissues and cells suggests a widespread role of vitamin D for overall human health [1,10]. In line with this, numerous epidemiological studies documented that low 25(OH)D concentrations are associated with an increased risk of all-cause mortality and major acute and chronic diseases, such as cancer, cardiovascular and autoimmune diseases, as well as infections (see Table 8) [1,3,4,6,8,92,96,97,98,99,100]. Confounding and reverse causation may, however, explain parts of these associations so that cause and effect relationships are not yet fully established for several of the above mentioned diseases [101].
Table 8.
Statement regarding extra-skeletal actions of vitamin D in adults.
| Consensus Statement | Consensus Voting Scale | Level of Agreement |
|---|---|---|
| Results from observational studies consider a low 25(OH)D concentration as a potential risk marker for several diseases such as cancer incidence and mortality, cardiovascular diseases, diabetes mellitus and its comorbidities, chronic autoimmune diseases, metabolic syndrome, acute respiratory tract infections, neurological diseases and total mortality. Results from meta-analyses of RCTs suggest that beyond musculoskeletal-effects, vitamin D supplementation may have beneficial extra-skeletal effects regarding acute respiratory tract infections and cancer mortality. In patients with or at risk of different types of cancer, certain cardiovascular diseases, diabetes mellitus and its comorbidities, chronic autoimmune diseases, certain neurological diseases and recurrent acute respiratory tract infections, a screening of vitamin D deficiency should be considered, and preventive vitamin D supplementation may be considered. |
9 (strongly agree) | 60% |
| 8 | 10% | |
| 7 (agree) | 30% | |
| 6 | 0% | |
| 5 (neutral) | 0% | |
| 4 | 0% | |
| 3 (disagree) | 0% | |
| 2 | 0% | |
| 1 (strongly disagree) | 0% | |
| Overall agreement 100%, consensus endorsed | ||
Meta-analyses of vitamin D RCTs suggest that vitamin D supplementation may reduce the incidence of acute respiratory infections, cancer mortality, as well as asthma and chronic obstructive pulmonary disease (COPD) exacerbations [5,96,102,103,104,105,106,107,108]. Considering the totality of evidence regarding vitamin D and a variety of extra-skeletal diseases, we are of the opinion that it is justified to consider screening and preventive vitamin D supplementation in certain populations at risk (see Table 8). The general dilemma with potential extra-skeletal health benefits of vitamin D in the context of vitamin D guidelines is that vitamin D requirements for skeletal health, such as for the prevention of rickets and osteomalacia, may be met at lower 25(OH)D concentrations than the requirements for certain extra-skeletal health benefits [1,14]. Of note, recent large vitamin D RCTs failed to document significant benefits regarding their primary outcomes, such as mortality, cancer, or cardiovascular diseases, but these trials enrolled populations that were, by the vast majority, not vitamin D deficient [109,110].
Numerous studies have been published on vitamin D, in the context of the Coronavirus Disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [111,112,113]. While there is accumulating evidence suggesting potential benefits of vitamin D or calcifediol, for the prevention and treatment of COVID-19, this hypothesis still requires confirmation in large clinical trials [111,112,113]. Fortunately, due to the efficacy of vaccines and natural immunity of individuals who recovered from SARS-CoV-2 infections, the COVID-19 pandemic has already been significantly mitigated in early 2022, with expected further improvements in the near future [114,115,116].
3.7. Development of a Vitamin D Deficiency Screening and Treatment Algorithm
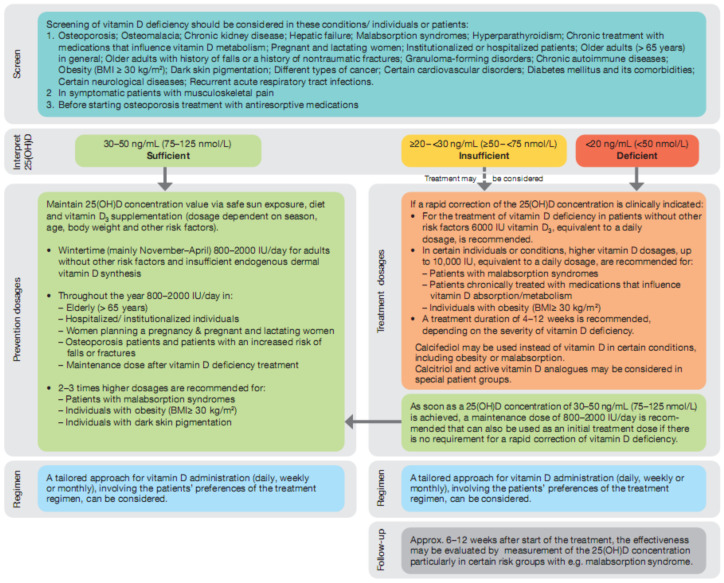
Based on our consensus statements, we developed an algorithm for the prevention and treatment of vitamin D deficiency that was also subject to a Delphi voting round, with the following point scale results: 9 points (20% of panel members), 8 points (30%), and 7 points (50%) (see Figure 1).
Figure 1.
Algorithm for vitamin D deficiency screening and treatment.
4. Strengths and Limitations
As a potential limitation, the financial and logistic support by an industry sponsor (Wörwag Pharma) must be acknowledged. Despite no involvement of the sponsor in the scientific group discussions and consensus reaching processes, we cannot totally exclude some sort of funding bias [17,18]. Moreover, this consensus document was not informed by an a priori structured and pre-registered systematic review of the evidence. A definite strength of our work is the a priori structured process for developing this document, based on the Delphi method for reaching consensus. The number of 10 participants for using the Delphi voting rounds may be considered as too low, but such a group size is not uncommon and, thus, is generally accepted in the scientific literature on this methodology [35]. The involvement of several colleagues from Eastern Europe, who are usually underrepresented in European expert groups, and the well-balanced gender distribution of this panel, may also be regarded as a strength of our work.
5. Conclusions
This consensus statement covers various statements with relevance for the clinical practice in the prevention, diagnosis, and treatment of vitamin D deficiency. We highlighted the relevance of vitamin D for public health and provided guidance regarding this issue by considering the totality of the available scientific evidence, including our personal experience and opinions.
We consider that our work adds to the existing literature by providing a useful and evidence-based guidance for clinicians and health-care workers regarding several relevant and partially controversial topics for the practical management, with reference to vitamin D. In addition, we also addressed various issues with relevance for public health authorities and individuals from the general population that will, hopefully, help to reduce the global health burden of vitamin D deficiency.
Author Contributions
Writing—original draft preparation, P.P. and S.P. Writing a final review and final editing, I.T., M.B., Z.B., C.C.D., T.M., N.Z., I.R., J.P., P.P. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Wörwag Pharma.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors received honoraria from Wörwag Pharma.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bouillon R., Marcocci C., Carmeliet G., Bikle D., White J.H., Dawson-Hughes B., Lips P., Munns C.F., Lazaretti-Castro M., Giustina A., et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019;40:1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilz S., Zittermann A., Trummer C., Theiler-Schwetz V., Lerchbaum E., Keppel M.H., Grubler M.R., Marz W., Pandis M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019;8:R27–R43. doi: 10.1530/EC-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maretzke F., Bechthold A., Egert S., Ernst J.B., Melo van Lent D., Pilz S., Reichrath J., Stangl G.I., Stehle P., Volkert D., et al. Role of Vitamin D in Preventing and Treating Selected Extraskeletal Diseases—An Umbrella Review. Nutrients. 2020;12:969. doi: 10.3390/nu12040969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zittermann A., Trummer C., Theiler-Schwetz V., Lerchbaum E., Marz W., Pilz S. Vitamin D and Cardiovascular Disease: An Updated Narrative Review. Int. J. Mol. Sci. 2021;22:2896. doi: 10.3390/ijms22062896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouillon R., Manousaki D., Rosen C., Trajanoska K., Rivadeneira F., Richards J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2021;18:96–110. doi: 10.1038/s41574-021-00593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilz S., Zittermann A., Obeid R., Hahn A., Pludowski P., Trummer C., Lerchbaum E., Perez-Lopez F.R., Karras S.N., Marz W. The Role of Vitamin D in Fertility and during Pregnancy and Lactation: A Review of Clinical Data. Int. J. Environ. Res. Public Health. 2018;15:2241. doi: 10.3390/ijerph15102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 8.Gaksch M., Jorde R., Grimnes G., Joakimsen R., Schirmer H., Wilsgaard T., Mathiesen E.B., Njolstad I., Lochen M.L., Marz W., et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE. 2017;12:e0170791. doi: 10.1371/journal.pone.0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cashman K.D., Dowling K.G., Skrabakova Z., Gonzalez-Gross M., Valtuena J., De Henauw S., Moreno L., Damsgaard C.T., Michaelsen K.F., Molgaard C., et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016;103:1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saponaro F., Saba A., Zucchi R. An Update on Vitamin D Metabolism. Int. J Mol. Sci. 2020;21:6573. doi: 10.3390/ijms21186573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilz S., Trummer C., Pandis M., Schwetz V., Aberer F., Grubler M., Verheyen N., Tomaschitz A., Marz W. Vitamin D: Current Guidelines and Future Outlook. Anticancer Res. 2018;38:1145–1151. doi: 10.21873/anticanres.12333. [DOI] [PubMed] [Google Scholar]
- 12.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buttriss J.L., Lanham-New S.A., Steenson S., Levy L., Swan G.E., Darling A.L., Cashman K.D., Allen R.E., Durrant L.R., Smith C.P., et al. Implementation strategies for improving vitamin D status and increasing vitamin D intake in the UK: Current controversies and future perspectives: Proceedings of the 2nd Rank Prize Funds Forum on vitamin D. Br. J. Nutr. 2021:1–21. doi: 10.1017/S0007114521002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M., Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 15.Giustina A., Bouillon R., Binkley N., Sempos C., Adler R.A., Bollerslev J., Dawson-Hughes B., Ebeling P.R., Feldman D., Heijboer A., et al. Controversies in Vitamin D: A Statement from the Third International Conference. JBMR Plus. 2020;4:e10417. doi: 10.1002/jbm4.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017;13:466–479. doi: 10.1038/nrendo.2017.31. [DOI] [PubMed] [Google Scholar]
- 17.Fraile Navarro D., Lopez Garcia-Franco A., Nino de Guzman E., Rabassa M., Zamanillo Campos R., Pardo-Hernandez H., Ricci-Cabello I., Canelo-Aybar C., Meneses-Echavez J.F., Yepes-Nunez J.J., et al. Vitamin D recommendations in clinical guidelines: A systematic review, quality evaluation and analysis of potential predictors. Int. J. Clin. Pract. 2021;75:e14805. doi: 10.1111/ijcp.14805. [DOI] [PubMed] [Google Scholar]
- 18.Dai Z., McKenzie J.E., McDonald S., Baram L., Page M.J., Allman-Farinelli M., Raubenheimer D., Bero L.A. Assessment of the Methods Used to Develop Vitamin D and Calcium Recommendations—A Systematic Review of Bone Health Guidelines. Nutrients. 2021;13:2423. doi: 10.3390/nu13072423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen C.J., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., Kovacs C.S., et al. IOM committee members respond to Endocrine Society vitamin D guideline. J. Clin. Endocrinol. Metab. 2012;97:1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pludowski P., Holick M.F., Grant W.B., Konstantynowicz J., Mascarenhas M.R., Haq A., Povoroznyuk V., Balatska N., Barbosa A.P., Karonova T., et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018;175:125–135. doi: 10.1016/j.jsbmb.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Pludowski P., Karczmarewicz E., Bayer M., Carter G., Chlebna-Sokol D., Czech-Kowalska J., Debski R., Decsi T., Dobrzanska A., Franek E., et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—Recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013;64:319–327. doi: 10.5603/EP.2013.0012. [DOI] [PubMed] [Google Scholar]
- 22.Takacs I., Benko I., Toldy E., Wikonkal N., Szekeres L., Bodolay E., Kiss E., Jambrik Z., Szabo B., Merkely B., et al. Hungarian consensus regarding the role of vitamin D in the prevention and treatment of diseases. Orv. Hetil. 2012;153((Suppl. 2)):5–26. doi: 10.1556/OH.2012.29410. [DOI] [PubMed] [Google Scholar]
- 23.German Nutrition Society (DGE) New reference values for vitamin D. Ann. Nutr. Metab. 2012;60:241–246. doi: 10.1159/000337547. [DOI] [PubMed] [Google Scholar]
- 24.EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016;14:4547. doi: 10.2903/j.efsa.2016.4547. [DOI] [Google Scholar]
- 25.Pigarova E.A., Rozhinskaya L.Y., Belaya J.E., Dzeranova L.K., Karonova T.L., Ilyin A.V., Melnichenko G.A., Dedov I.I. Russian Association of Endocrinologists recommendations for diagnosis, treatment and prevention of vitamin D deficiency in adults. Probl. Endocrinol. 2016;62:60–84. doi: 10.14341/probl201662460-84. (In Russian) [DOI] [Google Scholar]
- 26.Rusinska A., Pludowski P., Walczak M., Borszewska-Kornacka M.K., Bossowski A., Chlebna-Sokol D., Czech-Kowalska J., Dobrzanska A., Franek E., Helwich E., et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland-Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel with Participation of National Specialist Consultants and Representatives of Scientific Societies—2018 Update. Front. Endocrinol. 2018;9:246. doi: 10.3389/fendo.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudenko E. National Regulation about Methods of Diagnosis, Prevention and Differentiated Treatment of Vitamin D Deficiency. Ministry of Health of the Republic of Belarus; Minsk, Belarus: 2013. [Google Scholar]
- 28.Borissova A.-M., Boyanov M.A., Popivanov P.R., Kolarov Z., Petranova T.P., Shinkov A.D. Recommendation for Diagnosis, Prevention and Treatment of Vitamin D Deficiency. Bulgarian Society of Endocrinology; Sofia, Bulgaria: 2019. [Google Scholar]
- 29.Payer J., Killinger Z., Jackuliak P., Kužma M. Postmenopausal osteoporosis: Standard diagnostic and therapeutic practice. Clin. Osteol. 2018;23:18–27. [Google Scholar]
- 30.Cesareo R., Attanasio R., Caputo M., Castello R., Chiodini I., Falchetti A., Guglielmi R., Papini E., Santonati A., Scillitani A., et al. Italian Association of Clinical Endocrinologists (AME) and Italian Chapter of the American Association of Clinical Endocrinologists (AACE) Position Statement: Clinical Management of Vitamin D Deficiency in Adults. Nutrients. 2018;10:546. doi: 10.3390/nu10050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockwell M., Kraak V., Hulver M., Epling J. Clinical Management of Low Vitamin D: A Scoping Review of Physicians’ Practices. Nutrients. 2018;10:493. doi: 10.3390/nu10040493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zittermann A., Pilz S., Berthold H.K. Serum 25-hydroxyvitamin D response to vitamin D supplementation in infants: A systematic review and meta-analysis of clinical intervention trials. Eur. J. Nutr. 2020;59:359–369. doi: 10.1007/s00394-019-01912-x. [DOI] [PubMed] [Google Scholar]
- 33.Munns C.F., Shaw N., Kiely M., Specker B.L., Thacher T.D., Ozono K., Michigami T., Tiosano D., Mughal M.Z., Makitie O., et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016;101:394–415. doi: 10.1210/jc.2015-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grossman Z., Hadjipanayis A., Stiris T., Del Torso S., Mercier J.C., Valiulis A., Shamir R. Vitamin D in European children-statement from the European Academy of Paediatrics (EAP) Eur. J. Pediatr. 2017;176:829–831. doi: 10.1007/s00431-017-2903-2. [DOI] [PubMed] [Google Scholar]
- 35.Diamond I.R., Grant R.C., Feldman B.M., Pencharz P.B., Ling S.C., Moore A.M., Wales P.W. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J. Clin. Epidemiol. 2014;67:401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfeld R.M., Nnacheta L.C., Corrigan M.D. Clinical Consensus Statement Development Manual. Otolaryngol. Head Neck Surg. 2015;153:S1–S14. doi: 10.1177/0194599815601394. [DOI] [PubMed] [Google Scholar]
- 37.Cashman K.D. Global differences in vitamin D status and dietary intake: A review of the data. Endocr. Connect. 2022;11:e210282. doi: 10.1530/EC-21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lips P., Cashman K.D., Lamberg-Allardt C., Bischoff-Ferrari H.A., Obermayer-Pietsch B., Bianchi M.L., Stepan J., El-Hajj Fuleihan G., Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019;180:P23–P54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 39.Płudowski P., Ducki C., Konstantynowicz J., Jaworski M. Vitamin D status in Poland. Pol. Arch. Med. Wewn. 2016;126:530–539. doi: 10.20452/pamw.3479. [DOI] [PubMed] [Google Scholar]
- 40.Avdeeva V.A., Suplotova L.A., Pigarova E.A., Rozhinskaya L.Y., Troshina E.A. Vitamin D deficiency in Russia: The first results of a registered, non-interventional study of the frequency of vitamin D deficiency and insufficiency in various geographic regions of the country. Probl. Endokrinol. 2021;67:84–92. doi: 10.14341/probl12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolova M.G., Boyanov M.A., Tsakova A.D. Correlations of Serum Vitamin D with Metabolic Parameters in Adult Outpatients with Different Degrees of Overweight/Obesity Coming from an Urban Community. Acta Endocrinol. 2018;14:375–383. doi: 10.4183/aeb.2018.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnett-Bowie S.M., Cappola A.R. The USPSTF 2021 Recommendations on Screening for Asymptomatic Vitamin D Deficiency in Adults: The Challenge for Clinicians Continues. JAMA. 2021;325:1401–1402. doi: 10.1001/jama.2021.2227. [DOI] [PubMed] [Google Scholar]
- 43.US Preventive Services Task Force. Krist A.H., Davidson K.W., Mangione C.M., Cabana M., Caughey A.B., Davis E.M., Donahue K.E., Doubeni C.A., Epling J.W., Jr., et al. Screening for Vitamin D Deficiency in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:1436–1442. doi: 10.1001/jama.2021.3069. [DOI] [PubMed] [Google Scholar]
- 44.Macova L., Bicikova M. Vitamin D: Current Challenges between the Laboratory and Clinical Practice. Nutrients. 2021;13:1758. doi: 10.3390/nu13061758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Binkley N., Dawson-Hughes B., Durazo-Arvizu R., Thamm M., Tian L., Merkel J.M., Jones J.C., Carter G.D., Sempos C.T. Vitamin D measurement standardization: The way out of the chaos. J. Steroid Biochem. Mol. Biol. 2017;173:117–121. doi: 10.1016/j.jsbmb.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Marcinowska-Suchowierska E., Kupisz-Urbanska M., Lukaszkiewicz J., Pludowski P., Jones G. Vitamin D Toxicity—A Clinical Perspective. Front. Endocrinol. 2018;9:550. doi: 10.3389/fendo.2018.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cappellani D., Brancatella A., Morganti R., Borsari S., Baldinotti F., Caligo M.A., Kaufmann M., Jones G., Marcocci C., Cetani F. Hypercalcemia due to CYP24A1 mutations: A systematic descriptive review. Eur. J. Endocrinol. 2021;186:137–149. doi: 10.1530/EJE-21-0713. [DOI] [PubMed] [Google Scholar]
- 48.Carlberg C. Vitamin D: A Micronutrient Regulating Genes. Curr. Pharm. Des. 2019;25:1740–1746. doi: 10.2174/1381612825666190705193227. [DOI] [PubMed] [Google Scholar]
- 49.Hanel A., Neme A., Malinen M., Hamalainen E., Malmberg H.R., Etheve S., Tuomainen T.P., Virtanen J.K., Bendik I., Carlberg C. Common and personal target genes of the micronutrient vitamin D in primary immune cells from human peripheral blood. Sci. Rep. 2020;10:21051. doi: 10.1038/s41598-020-78288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krasniqi E., Boshnjaku A., Wagner K.H., Wessner B. Association between Polymorphisms in Vitamin D Pathway-Related Genes, Vitamin D Status, Muscle Mass and Function: A Systematic Review. Nutrients. 2021;13:3109. doi: 10.3390/nu13093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilezikian J.P., Formenti A.M., Adler R.A., Binkley N., Bouillon R., Lazaretti-Castro M., Marcocci C., Napoli N., Rizzoli R., Giustina A. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all? Rev. Endocr. Metab. Disord. 2021;22:1201–1218. doi: 10.1007/s11154-021-09693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kupisz-Urbanska M., Pludowski P., Marcinowska-Suchowierska E. Vitamin D Deficiency in Older Patients—Problems of Sarcopenia, Drug Interactions, Management in Deficiency. Nutrients. 2021;13:1247. doi: 10.3390/nu13041247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cashman K.D. Vitamin D Requirements for the Future-Lessons Learned and Charting a Path Forward. Nutrients. 2018;10:533. doi: 10.3390/nu10050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cashman K.D., Ritz C., Kiely M., Odin C. Improved Dietary Guidelines for Vitamin D: Application of Individual Participant Data (IPD)-Level Meta-Regression Analyses. Nutrients. 2017;9:469. doi: 10.3390/nu9050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pilz S., Hahn A., Schon C., Wilhelm M., Obeid R. Effect of Two Different Multimicronutrient Supplements on Vitamin D Status in Women of Childbearing Age: A Randomized Trial. Nutrients. 2017;9:30. doi: 10.3390/nu9010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calvo M.S., Whiting S.J. Public health strategies to overcome barriers to optimal vitamin D status in populations with special needs. J. Nutr. 2006;136:1135–1139. doi: 10.1093/jn/136.4.1135. [DOI] [PubMed] [Google Scholar]
- 57.Mo M., Wang S., Chen Z., Muyiduli X., Wang S., Shen Y., Shao B., Li M., Chen D., Chen Z., et al. A systematic review and meta-analysis of the response of serum 25-hydroxyvitamin D concentration to vitamin D supplementation from RCTs from around the globe. Eur. J. Clin. Nutr. 2019;73:816–834. doi: 10.1038/s41430-019-0417-x. [DOI] [PubMed] [Google Scholar]
- 58.Bacha D.S., Rahme M., Al-Shaar L., Baddoura R., Halaby G., Singh R.J., Mahfoud Z.R., Habib R., Arabi A., El-Hajj Fuleihan G. Vitamin D3 Dose Requirement That Raises 25-Hydroxyvitamin D to Desirable Level in Overweight and Obese Elderly. J. Clin. Endocrinol. Metab. 2021;106:e3644–e3654. doi: 10.1210/clinem/dgab296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zittermann A., Ernst J.B., Gummert J.F., Borgermann J. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: A systematic review. Eur. J. Nutr. 2014;53:367–374. doi: 10.1007/s00394-013-0634-3. [DOI] [PubMed] [Google Scholar]
- 60.Autier P., Gandini S., Mullie P. A systematic review: Influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J. Clin. Endocrinol. Metab. 2012;97:2606–2613. doi: 10.1210/jc.2012-1238. [DOI] [PubMed] [Google Scholar]
- 61.Cashman K.D., Ritz C., Adebayo F.A., Dowling K.G., Itkonen S.T., Ohman T., Skaffari E., Saarnio E.M., Kiely M., Lamberg-Allardt C. Differences in the dietary requirement for vitamin D among Caucasian and East African women at Northern latitude. Eur. J. Nutr. 2019;58:2281–2291. doi: 10.1007/s00394-018-1775-1. [DOI] [PubMed] [Google Scholar]
- 62.Cashman K.D., Kiely M.E., Andersen R., Gronborg I.M., Tetens I., Tripkovic L., Lanham-New S.A., Lamberg-Allardt C., Adebayo F.A., Gallagher J.C., et al. Individual participant data (IPD)-level meta-analysis of randomised controlled trials to estimate the vitamin D dietary requirements in dark-skinned individuals resident at high latitude. Eur. J. Nutr. 2021;61:1015–1034. doi: 10.1007/s00394-021-02699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazahery H., von Hurst P.R. Factors Affecting 25-Hydroxyvitamin D Concentration in Response to Vitamin D Supplementation. Nutrients. 2015;7:5111–5142. doi: 10.3390/nu7075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramasamy I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin. Biochem. Rev. 2020;41:103–126. doi: 10.33176/AACB-20-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ish-Shalom S., Segal E., Salganik T., Raz B., Bromberg I.L., Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J. Clin. Endocrinol. Metab. 2008;93:3430–3435. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 66.Takacs I., Toth B.E., Szekeres L., Szabo B., Bakos B., Lakatos P. Randomized clinical trial to comparing efficacy of daily, weekly and monthly administration of vitamin D3. Endocrine. 2017;55:60–65. doi: 10.1007/s12020-016-1137-9. [DOI] [PubMed] [Google Scholar]
- 67.Mazess R.B., Bischoff-Ferrari H.A., Dawson-Hughes B. Vitamin D: Bolus Is Bogus—A Narrative Review. JBMR Plus. 2021;5:e10567. doi: 10.1002/jbm4.10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jorde R., Grimnes G. Serum cholecalciferol may be a better marker of vitamin D status than 25-hydroxyvitamin D. Med. Hypotheses. 2018;111:61–65. doi: 10.1016/j.mehy.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 69.Rothen J.P., Rutishauser J., Walter P.N., Hersberger K.E., Arnet I. Oral intermittent vitamin D substitution: Influence of pharmaceutical form and dosage frequency on medication adherence: A randomized clinical trial. BMC Pharmacol. Toxicol. 2020;21:51. doi: 10.1186/s40360-020-00430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tripkovic L., Lambert H., Hart K., Smith C.P., Bucca G., Penson S., Chope G., Hypponen E., Berry J., Vieth R., et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012;95:1357–1364. doi: 10.3945/ajcn.111.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bjelakovic G., Gluud L.L., Nikolova D., Whitfield K., Wetterslev J., Simonetti R.G., Bjelakovic M., Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev. 2008:CD007470. doi: 10.1002/14651858.CD007470. [DOI] [PubMed] [Google Scholar]
- 72.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012;97:1153–1158. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 73.Heaney R.P., Armas L.A. Quantifying the vitamin D economy. Nutr. Rev. 2015;73:51–67. doi: 10.1093/nutrit/nuu004. [DOI] [PubMed] [Google Scholar]
- 74.Pludowski P., Socha P., Karczmarewicz E., Zagorecka E., Lukaszkiewicz J., Stolarczyk A., Piotrowska-Jastrzebska J., Kryskiewicz E., Lorenc R.S., Socha J. Vitamin D supplementation and status in infants: A prospective cohort observational study. J. Pediatr. Gastroenterol. Nutr. 2011;53:93–99. doi: 10.1097/MPG.0b013e318216920f. [DOI] [PubMed] [Google Scholar]
- 75.Vieth R. Vitamin D supplementation: Cholecalciferol, calcifediol, and calcitriol. Eur. J. Clin. Nutr. 2020;74:1493–1497. doi: 10.1038/s41430-020-0697-1. [DOI] [PubMed] [Google Scholar]
- 76.Billington E.O., Burt L.A., Rose M.S., Davison E.M., Gaudet S., Kan M., Boyd S.K., Hanley D.A. Safety of High-Dose Vitamin D Supplementation: Secondary Analysis of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2020;105:1261–1273. doi: 10.1210/clinem/dgz212. [DOI] [PubMed] [Google Scholar]
- 77.Vieth R. Vitamin D and cancer mini-symposium: The risk of additional vitamin D. Ann. Epidemiol. 2009;19:441–445. doi: 10.1016/j.annepidem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Nielsen O.H., Hansen T.I., Gubatan J.M., Jensen K.B., Rejnmark L. Managing vitamin D deficiency in inflammatory bowel disease. Frontline Gastroenterol. 2019;10:394–400. doi: 10.1136/flgastro-2018-101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masood M.Q., Khan A., Awan S., Dar F., Naz S., Naureen G., Saghir S., Jabbar A. Comparison of Vitamin D Replacement Strategies with High-Dose Intramuscular or Oral Cholecalciferol: A Prospective Intervention Study. Endocr. Pract. 2015;21:1125–1133. doi: 10.4158/EP15680.OR. [DOI] [PubMed] [Google Scholar]
- 80.Wylon K., Drozdenko G., Krannich A., Heine G., Dolle S., Worm M. Pharmacokinetic Evaluation of a Single Intramuscular High Dose versus an Oral Long-Term Supplementation of Cholecalciferol. PLoS ONE. 2017;12:e0169620. doi: 10.1371/journal.pone.0169620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gupta N., Farooqui K.J., Batra C.M., Marwaha R.K., Mithal A. Effect of oral versus intramuscular Vitamin D replacement in apparently healthy adults with Vitamin D deficiency. Indian J. Endocrinol. Metab. 2017;21:131–136. doi: 10.4103/2230-8210.196007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sosa Henriquez M., Gomez de Tejada Romero M.J. Cholecalciferol or Calcifediol in the Management of Vitamin D Deficiency. Nutrients. 2020;12:1617. doi: 10.3390/nu12061617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quesada-Gomez J.M., Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos. Int. 2018;29:1697–1711. doi: 10.1007/s00198-018-4520-y. [DOI] [PubMed] [Google Scholar]
- 84.Perez-Castrillon J.L., Duenas-Laita A., Brandi M.L., Jodar E., Del Pino-Montes J., Quesada-Gomez J.M., Cereto Castro F., Gomez-Alonso C., Gallego Lopez L., Olmos Martinez J.M., et al. Calcifediol is superior to cholecalciferol in improving vitamin D status in postmenopausal women: A randomized trial. J. Bone Miner. Res. 2021;36:1967–1978. doi: 10.1002/jbmr.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bischoff-Ferrari H.A., Dawson-Hughes B., Orav E.J., Staehelin H.B., Meyer O.W., Theiler R., Dick W., Willett W.C., Egli A. Monthly High-Dose Vitamin D Treatment for the Prevention of Functional Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2016;176:175–183. doi: 10.1001/jamainternmed.2015.7148. [DOI] [PubMed] [Google Scholar]
- 86.Bollerslev J., Rejnmark L., Zahn A., Heck A., Appelman-Dijkstra N.M., Cardoso L., Hannan F.M., Cetani F., Sikjaer T., Formenti A.M., et al. European Expert Consensus on Practical Management of Specific Aspects of Parathyroid Disorders in Adults and in Pregnancy: Recommendations of the ESE Educational Program of Parathyroid Disorders. Eur. J. Endocrinol. 2022;186:R33–R63. doi: 10.1530/EJE-21-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bischoff-Ferrari H.A., Willett W.C., Orav E.J., Lips P., Meunier P.J., Lyons R.A., Flicker L., Wark J., Jackson R.D., Cauley J.A., et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N. Engl. J. Med. 2012;367:40–49. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 88.Bolland M.J., Grey A., Avenell A. Effects of vitamin D supplementation on musculoskeletal health: A systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6:847–858. doi: 10.1016/S2213-8587(18)30265-1. [DOI] [PubMed] [Google Scholar]
- 89.Carmel A.S., Bockman R.S. Vitamin D and bisphosphonate response. Osteoporos. Int. 2014;25:2155. doi: 10.1007/s00198-014-2711-8. [DOI] [PubMed] [Google Scholar]
- 90.Reid I.R., Horne A.M., Mihov B., Stewart A., Garratt E., Wong S., Wiessing K.R., Bolland M.J., Bastin S., Gamble G.D. Fracture Prevention with Zoledronate in Older Women with Osteopenia. N. Engl. J. Med. 2018;379:2407–2416. doi: 10.1056/NEJMoa1808082. [DOI] [PubMed] [Google Scholar]
- 91.Li S., Xi C., Li L., Long Z., Zhang N., Yin H., Xie K., Wu Z., Tian J., Wang F., et al. Comparisons of different vitamin D supplementation for prevention of osteoporotic fractures: A Bayesian network meta-analysis and meta-regression of randomised controlled trials. Int. J. Food Sci. Nutr. 2021;72:518–528. doi: 10.1080/09637486.2020.1830264. [DOI] [PubMed] [Google Scholar]
- 92.Ganmaa D., Enkhmaa D., Nasantogtokh E., Sukhbaatar S., Tumur-Ochir K.E., Manson J.E. Vitamin D, respiratory infections, and chronic disease: Review of meta-analyses and randomized clinical trials. J. Intern. Med. 2021 doi: 10.1111/joim.13399. [DOI] [PubMed] [Google Scholar]
- 93.Iuliano S., Poon S., Robbins J., Bui M., Wang X., De Groot L., Van Loan M., Zadeh A.G., Nguyen T., Seeman E. Effect of dietary sources of calcium and protein on hip fractures and falls in older adults in residential care: Cluster randomised controlled trial. BMJ. 2021;375:n2364. doi: 10.1136/bmj.n2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Appel L.J., Michos E.D., Mitchell C.M., Blackford A.L., Sternberg A.L., Miller E.R., 3rd, Juraschek S.P., Schrack J.A., Szanton S.L., Charleston J., et al. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults: A Response-Adaptive, Randomized Clinical Trial. Ann. Intern. Med. 2021;174:145–156. doi: 10.7326/M20-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zittermann A., Ernst J.B., Prokop S., Fuchs U., Dreier J., Kuhn J., Knabbe C., Birschmann I., Schulz U., Berthold H.K., et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): A 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur. Heart J. 2017;38:2279–2286. doi: 10.1093/eurheartj/ehx235. [DOI] [PubMed] [Google Scholar]
- 96.Gnagnarella P., Muzio V., Caini S., Raimondi S., Martinoli C., Chiocca S., Miccolo C., Bossi P., Cortinovis D., Chiaradonna F., et al. Vitamin D Supplementation and Cancer Mortality: Narrative Review of Observational Studies and Clinical Trials. Nutrients. 2021;13:3285. doi: 10.3390/nu13093285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Charoenngam N., Holick M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients. 2020;12:2097. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hajhashemy Z., Shahdadian F., Moslemi E., Mirenayat F.S., Saneei P. Serum vitamin D levels in relation to metabolic syndrome: A systematic review and dose-response meta-analysis of epidemiologic studies. Obes. Rev. 2021;22:e13223. doi: 10.1111/obr.13223. [DOI] [PubMed] [Google Scholar]
- 99.Han J., Guo X., Yu X., Liu S., Cui X., Zhang B., Liang H. 25-Hydroxyvitamin D and Total Cancer Incidence and Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutrients. 2019;11:2295. doi: 10.3390/nu11102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hahn J., Cook N.R., Alexander E.K., Friedman S., Walter J., Bubes V., Kotler G., Lee I.M., Manson J.E., Costenbader K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. 2022;376:e066452. doi: 10.1136/bmj-2021-066452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Autier P., Boniol M., Pizot C., Mullie P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 102.Jolliffe D.A., Camargo C.A., Jr., Sluyter J.D., Aglipay M., Aloia J.F., Ganmaa D., Bergman P., Bischoff-Ferrari H.A., Borzutzky A., Damsgaard C.T., et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9:276–292. doi: 10.1016/S2213-8587(21)00051-6. [DOI] [PubMed] [Google Scholar]
- 103.Sluyter J.D., Manson J.E., Scragg R. Vitamin D and Clinical Cancer Outcomes: A Review of Meta-Analyses. JBMR Plus. 2021;5:e10420. doi: 10.1002/jbm4.10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scragg R., Sluyter J.D. Is There Proof of Extraskeletal Benefits from Vitamin D Supplementation from Recent Mega Trials of Vitamin D? JBMR Plus. 2021;5:e10459. doi: 10.1002/jbm4.10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jolliffe D.A., Greenberg L., Hooper R.L., Mathyssen C., Rafiq R., de Jongh R.T., Camargo C.A., Griffiths C.J., Janssens W., Martineau A.R. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax. 2019;74:337–345. doi: 10.1136/thoraxjnl-2018-212092. [DOI] [PubMed] [Google Scholar]
- 106.Li X., He J., Yu M., Sun J. The efficacy of vitamin D therapy for patients with COPD: A meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2020;9:286–297. doi: 10.21037/apm.2020.02.26. [DOI] [PubMed] [Google Scholar]
- 107.Forno E., Bacharier L.B., Phipatanakul W., Guilbert T.W., Cabana M.D., Ross K., Covar R., Gern J.E., Rosser F.J., Blatter J., et al. Effect of Vitamin D3 Supplementation on Severe Asthma Exacerbations in Children with Asthma and Low Vitamin D Levels: The VDKA Randomized Clinical Trial. JAMA. 2020;324:752–760. doi: 10.1001/jama.2020.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zittermann A., Pilz S., Hoffmann H., Marz W. Vitamin D and airway infections: A European perspective. Eur. J. Med. Res. 2016;21:14. doi: 10.1186/s40001-016-0208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pilz S., Trummer C., Theiler-Schwetz V., Grubler M.R., Verheyen N.D., Odler B., Karras S.N., Zittermann A., Marz W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients. 2022;14:303. doi: 10.3390/nu14020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neale R.E., Baxter C., Romero B.D., McLeod D.S.A., English D.R., Armstrong B.K., Ebeling P.R., Hartel G., Kimlin M.G., O’Connell R., et al. The D-Health Trial: A randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol. 2022;10:120–128. doi: 10.1016/S2213-8587(21)00345-4. [DOI] [PubMed] [Google Scholar]
- 111.Zemb P., Bergman P., Camargo C.A., Jr., Cavalier E., Cormier C., Courbebaisse M., Hollis B., Joulia F., Minisola S., Pilz S., et al. Vitamin D deficiency and the COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2020;22:133–134. doi: 10.1016/j.jgar.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smaha J., Kuzma M., Jackuliak P., Payer J. Vitamin D supplementation as an important factor in COVID-19 prevention and treatment: What evidence do we have? Vnitr. Lek. 2020;66:494–500. doi: 10.36290/vnl.2020.146. [DOI] [PubMed] [Google Scholar]
- 113.Stroehlein J.K., Wallqvist J., Iannizzi C., Mikolajewska A., Metzendorf M.I., Benstoem C., Meybohm P., Becker M., Skoetz N., Stegemann M., et al. Vitamin D supplementation for the treatment of COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021;5:CD015043. doi: 10.1002/14651858.CD015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu Q., Qin C., Liu M., Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: A systematic review and meta-analysis. Infect. Dis. Poverty. 2021;10:132. doi: 10.1186/s40249-021-00915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pilz S., Chakeri A., Ioannidis J.P., Richter L., Theiler-Schwetz V., Trummer C., Krause R., Allerberger F. SARS-CoV-2 re-infection risk in Austria. Eur. J. Clin. Investig. 2021;51:e13520. doi: 10.1111/eci.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pilz S., Theiler-Schwetz V., Trummer C., Krause R., Ioannidis J.P.A. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022;209:112911. doi: 10.1016/j.envres.2022.112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.