Abstract
(1) Background: Increasing evidence indicates that lipid metabolism may influence the concentration of prostate-specific antigen (PSA). However, the association between triglycerides and PSA remains unclear and complicated. Hence, we evaluated the correlation between triglycerides and PSA based on the U.S. National Health and Nutrition Examination Survey (NHANES) database. (2) Methods: A total of 2910 participants out of 41,156 participants fit into our study after conducting the screening from the 2003 to 2010 NHANES survey. Serum triglycerides were the independent variable of our study, and PSA was the dependent variable; (3) Results: In our study, the average age of chosen participants was 59.7 years (±12.7). After adjusting for covariates, the result indicated that for each additional unit of serum triglyceride (mg/dL), the PSA concentrations were reduced by 0.0043 ng/mL (−0.0082, −0.0005) with a statistical difference. Furthermore, we used machine learning of the XGBoost model to determine the relative importance of selected variables as well as constructed a smooth curve based on the fully adjusted model to investigate the possible linear relationship between the triglyceride and PSA concentrations. (4) Conclusions: The serum triglyceride is independently and negatively correlated with PSA among American males, which may make it hard to detect asymptomatic prostate cancer and diagnose at an advance stage with higher triglycerides due to detection bias.
Keywords: prostate-specific antigen, triglycerides, National Health and Nutrition Examination Survey (NHANES), prostate cancer, machine learning
1. Introduction
In 2020, prostate cancer (PCa) was recorded as the most common cancer diagnosed in men, accounting for 26%, and was the second cause of the estimated death of 34,130 cases in males [1]. Detection of serum prostate-specific antigen (PSA) concentrations plays an essential part in screening prostate cancer at an early age amenable to curative treatment [2]. Widespread use of PSA concentration assays has significantly reduced the overall disease-specific mortality and improved the detection rate of asymptomatic, which highly differentiated prostate cancer [3,4]. Nevertheless, numerous studies have shown that PSA concentrations could be affected by various other factors, which may occur as detection bias in prostate cancer [5,6,7]. Inopportune and unnecessary treatment may take place due to overdiagnosis or missed diagnosis influenced by the various factors [8,9]. The U.S. Preventive Services Task Force (USPSTF) recently updated their recommendation, which changed from a grade D of guidance against PSA as based screening to a grade C of advocating for an individual screening [2,3,10]. Screening for prostate cancer by detecting PSA remains highly controversial because of the limitations of random trials, insufficient clinical evidence, and numerous factors affecting PSA levels [9,11]. Missing PSA data, hard to monitor, and the variety of decisions on patients between different areas may also contribute to practice variations [12].
An increasing number of studies strongly indicate that lipid and glucose metabolism play a critical role in prostate cancer (PCa) development [13]. Some studies have shown that cholesterol plays a possible role in prostate cancer development, aggressiveness, and progression through the effects of inflammation and steroidogenesis [14,15]. Some studies have also reported that higher serum low-density lipoprotein-cholesterol (LDL-C) levels correlated with a higher risk of prostate cancer. Meanwhile, higher levels of serum high-density lipoprotein-cholesterol (HDL-C) were corrected with the nonaggressive disease and lower risk of overall prostate cancer [16,17,18]. However, conflicting explanations exist on the function of triglycerides in the process of prostate cancer. Some studies have illustrated that triglycerides promote the malignant progression of prostate cancer [19]. However, some studies have considered triglycerides as a protection factor because of the higher triglyceride detection in a normal population [20,21]. In fact, the association may occur between triglyceride PSA metabolism, which could cause the detection bias in prostate cancer diagnosis. Moreover, we found this phenomenon has not been previously reported.
We hypothesized that serum triglycerides may influence the PSA concentrations among men without diagnosis of prostate cancer in the U.S. population. The presence of possible association could create a detection bias in observational studies of the exposures and prostate cancer. To test the hypothesis, we investigated the U.S. National Health and Nutrition Examination Survey (NHANES) for secondary data analysis. After controlling a vast array of influencing factors, we attempted to clarify the association between serum triglycerides and PSA concentrations among U.S. men without prostate cancer as well as try to explain the conflicting results
2. Materials and Methods
2.1. Data Availability
Since 1960, the National Health and Nutrition Examination Survey (NHANES) has been designed to estimate the health and nutritional status of adults and children in the United States, which is conducted by the National Centers for Disease Control (CDC) and Prevention National Health Statistics Center. Population and methodological details are available at the NHANES website (www.cdc.gov/nchs/nhanes, accessed on 7 October 2021). The National Center approved NHANES protocols for the Health Statistics research ethics review board.
2.2. Study Population
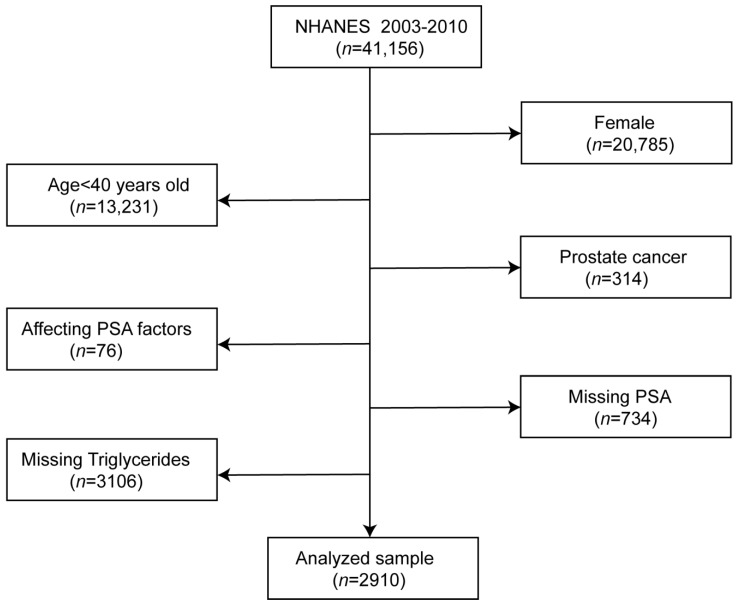
Four cycles’ two-year data of the NHANES survey from 2003 to 2010 were integrated into our study. These data included PSA concentrations, sociodemographic data, laboratory data, medical examination–personal life history, dietary, and comorbidities data for the second analysis. According to the following exclusion criteria, participants were left out of our study as follows: (1) female participants (n = 20,785); (2) aged < 40 years (n = 13,231); (3) participant diagnosed with prostate cancer (n = 314); (4) factors affecting PSA concentrations: diagnosed with prostatitis, stain drug user, received prostate biopsy within one week and had urinary system surgery within one month (n = 76); (5) missing PSA (734); and (6) missing serum triglycerides (n = 3106) [22]. There were 2910 participants out of 41,156 participants who fit into our study after conducting the screening (Figure 1). Furthermore, our study complied with the Helsinki Declaration of the World Medical Association in the process of study design and conduction. In our study, we used the data analysis based on NHANES.
Figure 1.
Flowchart in selecting the studying participants.
In our study, serum triglyceride was the target-independent variable downloaded from the NHANES website. Collaborative Laboratory Services periodically refines these laboratory methods. More detailed information on the test principles and clinical relevance can be found on the NHANES website and articles elsewhere [23,24]. PSA concentration (ng/mL) was set as the dependent variable. Total prostate-specific antigen (PSA) from the participants’ serum were recorded by Beckman Access at the Department of Laboratory Medicine Immunology Division, and the Hybritech PSA method was used to record serum total PSA concentration (ng/mL) (https://wwwn.cdc.gov/nchs/data/nhanes/2003–2004/labmethods/l11psa_c_met_total_psa.pdf, accessed on 7 October 2021). In this study, we used total serum PSA as the result variable connected to serum triglycerides and we selected the following covariates, based on the previous articles regarding the connection to triglycerides, PSA, or prostate cancer and other covariates [25,26,27,28,29,30]. Covariates contained the sociodemographic data, laboratory data, medical examination–personal life history, dietary, and comorbidities data. Continuous variables included age (year), poverty income ratio (PIR), vitamin D (ng/mL), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), glycohemoglobin (%), C-reactive protein (mg/dL), BMI (kg/m2), alcohol (gm), and triglycerides (mg/dL). Categorical variables contained race/ethnicity, education, marital status, physical activity (MET-based rank), smoked at least 100 cigarettes in life, hypertension history, coronary heart disease history, diabetes history, and stroke. More information about the details of variables is available on the NHANES official website.
2.3. Statistical Analysis
Following the criteria of the CDC guidelines, we conducted a statistical analysis between the serum triglycerides and PSA level (https://www.cdc.gov/nchs/nhanes/index.htm, accessed on 7 October 2021). Serum triglyceride was expressed as the mean ± standard deviation as normally distributed. PSA concentrations and other continuous variables were expressed as the mean ± standard deviation (normal distribution). The categorical variables were expressed in percentage or frequency. Our aim was to research the potential relationship of particular participants between the serum triglycerides and PSA concentrations. First, we divided the continuous variables of serum triglycerides into four quartile concentrations. The weighted chi-square was used to calculate the categorical variable, and the weighted linear regression model was used to calculate the continuous variable between the quartile arrays which shown in the Table 1. Second, we constructed three weighted univariate and multiple linear regression models including a non-adjusted model, a minimally adjusted model, and a fully adjusted model, which are shown in Table 2 to figure out the linear relationship between the serum triglycerides and PSA concentrations. Third, subgroup analyses were performed to identify the stratified associations between triglyceride and PSA using stratified multivariate logistic regression. Furthermore, in the model-development phase, we constructed the XGBoost algorithm model to predict the relative importance of selected variables. We performed the XGBoost model to analyze the contribution (gain) of each variable to PSA concentration [31,32]. Finally, the penalty spline method constructed a fully adjusted model with a smooth curve fit to explore the potential linear relationship between the serum triglycerides and PSA concentrations. In order to prevent the bias caused by missing data, we curated the NHANES database to improve the accuracy of the analysis by using the MICE package to account for missing data [33]. The results showed that the original data represented no significant difference with the complete data. All in all, univariate and multiple analysis results were based on the calculated dataset as well as Rubin’s rules. All kinds of statistical analyses of analysis were performed by R software (Version 4.0.2) using the R package (http://www.R-project.org, The R Foundation, accessed on 7 October 2021) [34]. The software EmpowerStats also provided significant help in the process of analysis (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA). In our study, a p-value < 0.05 was considered statistically significant.
Table 1.
Baseline characteristics of the selected participants.
| Triglycerides (mg/dL) | Q1 | Q2 | Q3 | Q4 | p-Value |
|---|---|---|---|---|---|
| N | 718 | 719 | 744 | 729 | |
| PSA ng/ml | 1.56 ± 2.60 | 1.74 ± 3.11 | 1.40 ± 1.86 | 1.30 ± 1.62 | 0.0023 |
| Sociodemographic variables | |||||
| Age, mean ± SD (years) | 56.16 ± 11.94 | 55.84 ± 11.31 | 56.46 ± 11.36 | 54.48 ± 10.70 | 0.0041 |
| Poverty to income ratio, mean ± SD (years) | 3.31 ± 1.57 | 3.40 ± 1.55 | 3.32 ± 1.56 | 3.30 ± 1.61 | 0.6691 |
| Race/ethnicity (%) | <0.0001 | ||||
| Mexican American | 3.91 | 5.39 | 6.57 | 8.30 | |
| Other Hispanic | 2.47 | 2.69 | 3.72 | 4.16 | |
| Non-Hispanic White | 76.52 | 75.82 | 78.13 | 75.67 | |
| Non-Hispanic Black | 13.07 | 10.12 | 5.56 | 5.38 | |
| Other race/ethnicity | 4.02 | 5.98 | 6.02 | 6.50 | |
| Education (%) | 0.0335 | ||||
| Less than high school | 19.27 | 16.60 | 21.00 | 20.20 | |
| High school | 22.65 | 23.99 | 22.76 | 27.83 | |
| More than high school | 58.08 | 59.41 | 56.24 | 51.97 | |
| Marital status (%) | 0.2121 | ||||
| Married | 71.14 | 75.20 | 69.96 | 73.24 | |
| Single | 24.19 | 19.50 | 25.35 | 21.98 | |
| Living with a partner | 4.67 | 5.30 | 4.69 | 4.77 | |
| Variables of laboratory data | |||||
| VITD, mean ± SD (ng/mL) | 68.95 ± 21.51 | 60.86 ± 19.91 | 64.92 ± 19.99 | 60.63 ± 19.22 | <0.0001 |
| LDL-C, mean ± SD (mg/dL) | 112.74 ± 32.98 | 121.48 ± 31.45 | 123.05 ± 36.68 | 119.55 ± 36.91 | <0.0001 |
| HDL-C, mean ± SD (mg/dL) | 58.95 ± 15.70 | 51.76 ± 13.34 | 45.75 ± 9.90 | 40.82 ± 9.75 | <0.0001 |
| Glycohemoglobin (%) | 5.59 ± 0.80 | 5.68 ± 0.87 | 5.70 ± 0.89 | 5.90 ± 1.27 | <0.0001 |
| C-reactive protein, mean ± SD (mg/dL) | 0.37 ± 0.97 | 0.38 ± 1.07 | 0.47 ± 1.36 | 0.35 ± 0.40 | 0.0743 |
| Medical examination and personal life history | |||||
| Physical activity (MET-based rank) (%) | |||||
| Sits | 0.0013 | ||||
| Walks | 21.14 | 18.46 | 25.42 | 19.91 | |
| Light loads | 41.71 | 50.94 | 50.31 | 51.50 | |
| Heavy work | 22.80 | 24.21 | 15.82 | 20.17 | |
| Body mass index, mean ± SD (Kg/m2) | 14.35 | 6.40 | 8.45 | 8.41 | |
| Smoked at least 100 cigarettes in life | 27.38 ± 5.39 | 28.46 ± 6.47 | 29.58 ± 5.59 | 30.60 ± 5.17 | <0.0001 |
| Yes | 0.0253 | ||||
| No | 54.13 | 58.29 | 59.17 | 61.96 | |
| Dietary interview-individual foods | 45.87 | 41.71 | 40.83 | 38.04 | |
| Alcohol, mean ± SD (gm) | |||||
| Comorbidities (%) | 19.45 ± 36.73 | 14.22 ± 34.31 | 13.77 ± 29.79 | 15.30 ± 34.20 | 0.0079 |
| Hypertension history | |||||
| Yes | 0.0245 | ||||
| No | 35.25 | 36.41 | 46.94 | 46.49 | |
| Coronary heart disease | 64.75 | 63.59 | 53.06 | 53.51 | |
| Yes | 0.1771 | ||||
| No | 8.08 | 6.56 | 9.71 | 7.97 | |
| Diabetes history | 91.92 | 93.44 | 90.29 | 92.03 | |
| Yes | 0.0629 | ||||
| No | 9.35 | 11.41 | 13.16 | 13.39 | |
| Borderline | 88.81 | 86.27 | 84.55 | 83.16 | |
| Stroke | 1.84 | 2.31 | 2.29 | 3.45 | |
| Yes | 0.4934 | ||||
| No | 2.86 | 3.49 | 4.22 | 4.12 | |
| 97.14 | 96.51 | 95.78 | 95.88 |
Q1–Q4: Grouped by quartile according to the serum triglycerides. Our data included PSA concentrations, sociodemographic data, laboratory data, medical examination–personal life history, dietary, and comorbidities data for the second analysis.
Table 2.
Univariate and multivariate analyses by the weighted linear model.
| Exposure | Non-Adjusted Model | Minimally Adjusted Model | Fully Adjusted Model |
|---|---|---|---|
| Triglyceride | −0.0014 (−0.0023, −0.0005), 0.001309 | −0.0013 (−0.0022, −0.0004), 0.003832 | −0.0043 (−0.0082, −0.0005), 0.027856 |
| Triglyceride | |||
| Q1 Q2 Q3 Q4 |
Ref 0.1045 (−0.2349,0.4439), 0.546189 −0.3022 (−0.6387,0.0343), 0.078467 −0.4598 (−0.7980, −0.1216) 0.007755 |
Ref 0.0684 (−0.2852, 0.4220) 0.704653 −0.2621 (−0.6169, 0.0927) 0.147739 −0.4501 (−0.8093, −0.0909) 0.014117 |
Ref 0.2846 (−0.3559, 0.9250) 0.384057 −0.4040 (−1.0497, 0.2416) 0.220247 −0.5155 (−1.2396, 0.2085) 0.163151 |
| p for trend | <0.001 | 0.002 | 0.049 |
Non-adjusted model adjusts for none. Minimally adjusted model adjusts for race/ethnicity, education level, poverty income ration, and marital status. Fully adjusted model adjusts for race/ethnicity, education level, poverty income ration, marital status, VITD, LDL-C, total cholesterol, C-reactive protein, glycohemoglobin (%), BMI (kg/m2), physical activity (MET-based rank) (%), smoked at least 100 cigarettes in life, drinking alcohol (gm) first day, coronary heart disease, and stroke.
3. Results
3.1. Baseline Characteristics of Selected Participants Subsection
Weighted distribution of baseline characteristics is shown in Table 1 including sociodemographic data, laboratory data, medical examination–personal life history, dietary, and comorbidities data of chosen participants selected from the NHANES (2013–2010) survey. In this study, the average age of the chosen participants was 59.7 years (±12.7). Then, we divided different serum triglycerides into four quartiles (Q1–Q4). The distribution of poverty to income ratio, marital status, C-reactive protein, coronary heart disease history, diabetes history, and stroke history in Q1–Q4 of serum triglycerides showed no statistical difference with approximate similarities (p values > 0.05). Compared with the different groups in Table 1, the distribution of serum triglycerides showed an age difference, where younger participants had higher serum triglycerides than older ones; had more elevated LDL-C, higher glycohemoglobin, higher body mass index (BMI), more likely to have a lower education level, higher incidence of hypertension, and more likely smoked at least 100 cigarettes in life. On the other hand, participants with more elevated serum triglycerides had lower PSA concentrations, lower vitamin D, lower HDL-C, lower physical activity, and were more likely to drink less alcohol. In our study, non-Hispanic Whites were the main participants.
3.2. The Connection between PSA Concentrations and Serum Triglycerides
The results of the univariate and multivariate analyses by the weighted linear model are shown in Table 2. In the non-adjusted model, which adjusts for none, the PSA concentrations were reduced by 0.0014 (−0.0023, −0.0005) for each additional unit of serum triglyceride with p for a trend less than 0.05. In the minimally adjusted model, which adjusts for race/ethnicity, education level, poverty income ratio, and marital status, the PSA concentrations were reduced by 0.0013 (−0.0022, −0.0004) for each additional unit of serum triglyceride with p for the trend <0.05. The fully adjusted model that adjusts for race/ethnicity, education level, poverty income ratio, marital status, VITD, LDL-C, total cholesterol, C-reactive protein, glycohemoglobin (%), physical activity (MET-based rank) (%), BMI (kg/m2), smoked at least 100 cigarettes in life, drinking alcohol (gm) first day, coronary heart disease, and stroke indicated that the PSA concentrations were reduced by 0.0043 ng/mL for each additional unit of serum triglyceride (mg/dL).
3.3. Stratified Associations between PSA Concentrations and Serum Triglycerides
As shown in Table 3, we conducted stratified analysis by age, race, education, marital status, body mass index (BMI), and ratio of family income to assess the associations between triglycerides and PSA. It is likely that Non-Hispanic Whites, education level higher than high school, married status, and high group of ratios of family income had lower PSA concentrations, with increasing serum triglycerides displaying a significant trend (p for trend = 0.0036; p for trend = 0.0012; p for trend = 0.0004; and p for trend = 0.0002). In addition, we detected a significant difference by p for the interaction analyses. Variables of age and triglycerides may have an interaction effect associated with PSA concentrations (p for interaction < 0.0001).
Table 3.
Effect size of triglycerides on PSA in the prespecified and exploratory subgroup.
| Triglycerides (mg/dL) | N | β | 95% CI | p-Value | p for Interaction |
|---|---|---|---|---|---|
| Stratified by age | <0.0001 | ||||
| <60 | 1475 | −0.0012 | (−0.0028, 0.0003) | 0.1241 | |
| 60–80 | 1153 | −0.0038 | (−0.0090, 0.0014) | 0.1557 | |
| >80 | 282 | −0.0225 | (−0.0472, 0.0023) | 0.078 | |
| Stratified by race | 0.3315 | ||||
| Mexican American | 496 | −0.0012 | (−0.0054, 0.0031) | 0.5987 | |
| Other Hispanic | 213 | 0.0017 | (−0.0061, 0.0094) | 0.678 | |
| Non-Hispanic White | 1585 | −0.006 | (−0.0100, −0.0020) | 0.0036 | |
| Non-Hispanic Black | 498 | −0.0125 | (−0.0281, 0.0031) | 0.1184 | |
| Other race/ethnicity | 118 | −0.0092 | (−0.0228, 0.0044) | 0.1946 | |
| Stratified by education | 0.1640 | ||||
| Less than high school | 935 | −0.0082 | (−0.0173, 0.0009) | 0.0791 | |
| High school | 669 | −0.0055 | (−0.0124, 0.0014) | 0.1168 | |
| More than high school | 1306 | −0.0059 | (−0.0094, −0.0024) | 0.0012 | |
| Stratified by marital status | 0.8274 | ||||
| Married | 1981 | −0.0073 | (−0.0113, −0.0033) | 0.0004 | |
| Single | 789 | −0.0038 | (−0.0123, 0.0047) | 0.3763 | |
| Living with a partner | 136 | −0.0064 | (−0.0126, −0.0001) | 0.0527 | |
| Stratified by BMI | 0.1168 | ||||
| <25 | 710 | −0.0092 | (−0.0184, −0.0000) | 0.0504 | |
| 25–28 | 718 | −0.0052 | (−0.0172, 0.0068) | 0.3964 | |
| >28 | 1424 | −0.0029 | (−0.0056, −0.0003) | 0.0305 | |
| Stratified by ratio of family income | 0.5872 | ||||
| Low group | 896 | −0.0038 | (−0.0081, 0.0005) | 0.0873 | |
| Median group | 898 | −0.0066 | (−0.0157, 0.0026) | 0.1594 | |
| High group | 906 | −0.0093 | (−0.0142, −0.0044) | 0.0002 |
Note 1: Above adjusts for race/ethnicity, education level, poverty income ratio, marital status, VITD, LDL-C, total cholesterol, C-reactive protein, glycohemoglobin (%), BMI (kg/m2), physical activity (MET-based rank) (%), smoked at least 100 cigarettes in life, drinking alcohol (gm) first day, coronary heart disease, and stroke. Note 2: In each case, the model was not adjusted for the stratification variable itself.
3.4. Machine Learning Using the XGBoost Algorithm Model
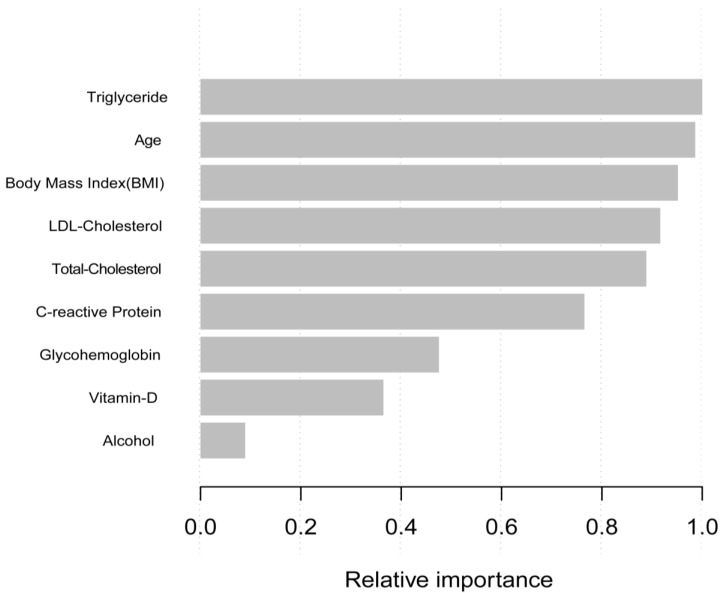
In the phase of model-development and validation, we used the machine learning of the XGBoost model to determine the relative importance of selected variables associated with the PSA. Variables included triglyceride, age, body mass index (BMI), LDL-cholesterol, total-cholesterol, C-relative protein, glycohemoglobin, vitamin-D, and alcohol. According to the results of each variables’ contribution by the XGBoost model, triglyceride, age, body mass index (BMI), LDL-cholesterol, and total-cholesterol were the top five most important variables of the dataset (Figure 2). Triglyceride, as the most relative variable, was included to construct smooth curve models in our study.
Figure 2.
Relative importance of the selected variables using XGBoost and the corresponding variable importance score. X-axis indicates the importance score, which is the relative number of a variable that is used to distribute the data, Y-axis indicates the selected variable.
3.5. Identification of Sensitivity Analysis
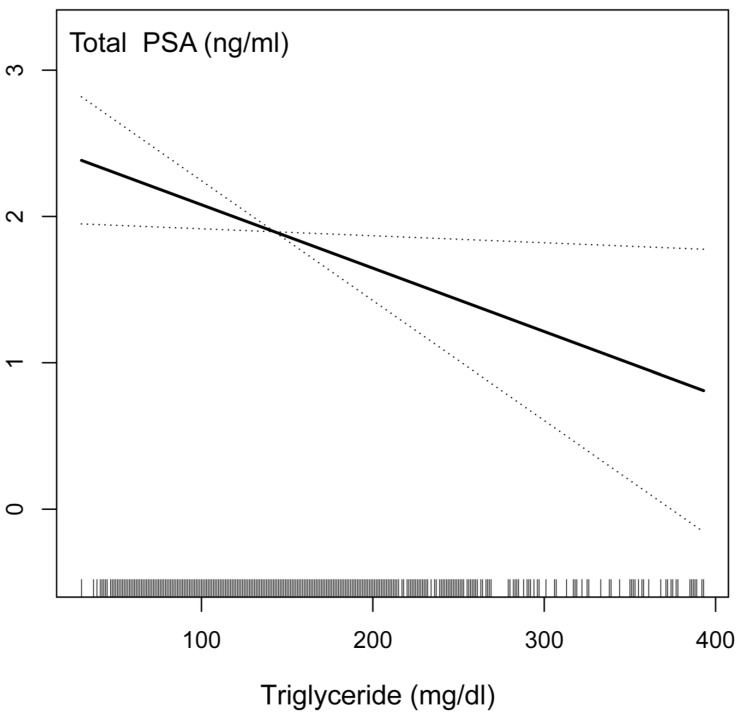
We conducted sensitivity analysis to confirm the accuracy and stability of the results. First, we converted the serum triglyceride as a continuous variable to the categorical variable in the quartile value, and then the p-value was calculated for trend. Surprisingly, the result of the categorical variable was consistent with the effect of the serum triglyceride as a continuous variable. To investigate the possible linear relationship between the serum triglycerides and PSA concentrations, we constructed a smooth curve based on the fully adjusted model. The relationship between the serum triglycerides and PSA concentrations was linear after adjusting for other covariates based on the fully adjusted model strategy (Figure 3). The results showed that if the serum triglyceride with each additional unit was mg/dL, the PSA concentrations were reduced by 0.0043 ng/mL. These results indicated a negative correlation between serum triglycerides and PSA concentrations.
Figure 3.
The relationship between serum triglyceride and prostate-specific antigen (PSA) connections.
4. Discussion
Our study is one of the most extensive cross-sectional studies to explore the potential association between the serum triglyceride and prostate-specific antigen (PSA) connections and is also the first to investigate this relationship in males without a history of malignant cancer in the United States based on the NHANES database. Up to now, there are no previous epidemiological studies that have reported on the association between triglyceride and PSA levels. Thus, after adjusting for the sociodemographic, laboratory, medical examination–personal life history, dietary, and comorbidities data, the serum triglyceride was negatively correlated with PSA. To avoid PSA detection bias in the process of diagnosis disease related to the prostate, it is necessary to understand that serum lipid may cause individual differences in PSA concentration and triglyceride is one aspect that needs further attention. Our study population was 2910 participants selected from the National Health and Nutrition Examination Survey (2003–2012). Machine learning of the XGBoost model was used to determine the relative importance of selected variables associated with the PSA, and triglyceride was the most relative variable. Our result shows that the PSA concentrations were reduced by 0.0043 ng/mL for each additional unit of serum triglyceride (mg/dL) and the result showed a statistical difference, which means that if 100 (mg/dL) of triglyceride is added, the PSA concentration will reduce by 4.3 ng/mL. Now, more and more of the population have dyslipidemia, which may aggravate PSA detection bias. Triglyceride, as the most relative variable, was included to construct smooth curve models to confirm the robustness of the result.
Previously, some prospective studies have provided evidence showing a potential role for lipid metabolism in PCa development, which suggests that a higher serum total cholesterol may relate to a higher grade or more aggressive prostate cancer [18,35,36,37,38,39]. The relationship between the triglyceride and the prognostic outcomes of prostate cancer have been seldomly reported and are unclear. One study showed evidence that triglycerides may influence the aggressiveness and severity of prostate cancer [25]. However, one study considered triglycerides as a protective factor because of the higher triglyceride detection in a normal population [20,21]. This result was also reported by a study from a Chinese investigation that revealed the inverse association between serum triglycerides and PSA levels [40]. Research is still needed to assess the relationship between the serum lipids and PSA level as most studies involve the population of relatively low-risk Asian men. Thus, we hypothesized that triglycerides may influence the PSA concentrations and cause detection bias, leading to this conflict in explanation. Additionally, we need to conduct further cohort experiments to understand the function of triglyceride as a protective or dangerous factor in the process of prostate cancer
The possible explanation of the inverse function of triglycerides with the PSA concentrations may be due to the detection bias between the process of prostate cancer, as shown in several recent studies [41,42,43], requires consideration. As we observed, higher concentrations of triglyceride populations are associated with lower PSA (Figure 3), so the possibility of detecting asymptomatic prostate cancer might be lower among high triglycerides. Meanwhile, if prostate cancer is already present, people with higher triglycerides may be more likely to be diagnosed at an advanced stage by PSA testing. Thus, our results considered that detection bias due to negative association between triglycerides and PSA does not explain the inverse association between the serum triglycerides and advanced prostate cancer than previously reported.
Our results support a negative association between triglycerides and PSA that may lead to detection bias, which can have implications for prostate cancer screening. Because triglycerides preferentially decrease PSA concentration in males without prostate cancer, the specificity of the PSA test for prostate cancer screening might be improved in males with high triglycerides. Thus, it is necessary to adjust the PSA threshold for further examination with various triglycerides if triglycerides can decrease PSA production by prostate tumor or change the ability of tumor-derived PSA to enter thee serum. Further studies are needed to explore the mechanism by which triglycerides influence PSA concentration and the influence on prostate cancer screening. Moreover, prospective cohort studies are still needed to confirm the causality, and serum triglyceride is involved in the occurrence and development of PCa, which needs to be verified via in vitro and in vivo experiments.
This study had several advantages compared with previously published articles. First, a large sample with a total of 2910 participants was utilized in our research. Second, machine learning of the XGBoost algorithm model was used to assess each variable’s contribution to PSA. Then, we performed a sensitivity analysis that considered and evaluated the impact of other factors, which may influence the result. Finally, a smooth curve was constructed based on the fully adjusted model to investigate the possible linear relationship between the serum triglycerides and PSA concentrations. Nevertheless, our study had some limitations in interpreting the results, which need to be considered. First, in our study, it was hard to distinguish the causality because of the intrinsic limitations of the NHANES database as a cross-sectional survey. Therefore, prospective cohort studies are still needed to confirm the causality. Additionally, we excluded participants diagnosed with prostate cancer, with factors affecting PSA concentrations and missing data. Thus, our results cannot explain the aforementioned populations. Finally, our investigation was based on the NHANES database, which is limited to the American people. Therefore, generalizability is geographically restricted. All of the above points require further evaluation and investigation in the future.
5. Conclusions
This nationally representative study showed that serum triglyceride is independently and negatively correlated with PSA among adult American males without a history of tumors. People with higher triglycerides would be more likely to be diagnosed with prostate cancer at an advanced stage in the future. This detection bias is unlikely to explain the inverse association between triglycerides and aggressive prostate cancer.
Acknowledgments
The NHANES protocol was approved by the NCHS Research Ethics Review Board and thanks so much for little bear who supported us the most.
Author Contributions
Conceptualization and design, C.W. and L.T.; Acquisition and analysis of the data, C.W., B.H. and B.J.; Interpretation of the data, C.W. and M.W.; Writing—original draft, C.W., M.X. and Z.C.; Writing—review and editing and critical analysis of the results, C.W., T.H., X.Y., Y.H., C.D. and Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available at NHANES website https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 7 October 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Grossman D.C., Curry S.J., Owens D.K., Bibbins-Domingo K., Caughey A.B., Davidson K.W., Doubeni C.A., Ebell M., Epling J.W., Jr., Kemper A.R., et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901–1913. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 3.Vickers A.J. Prostate Cancer Screening: Time to Question How to Optimize the Ratio of Benefits and Harms. Ann. Intern. Med. 2017;167:509–510. doi: 10.7326/M17-2012. [DOI] [PubMed] [Google Scholar]
- 4.Gudmundsson J., Sigurdsson J.K., Stefansdottir L., Agnarsson B.A., Isaksson H.J., Stefansson O.A., Gudjonsson S.A., Gudbjartsson D.F., Masson G., Frigge M.L., et al. Genome-wide associations for benign prostatic hyperplasia reveal a genetic correlation with serum levels of PSA. Nat. Commun. 2018;9:4568. doi: 10.1038/s41467-018-06920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristal A.R., Chi C., Tangen C.M., Goodman P.J., Etzioni R., Thompson I.M. Associations of demographic and lifestyle characteristics with prostate-specific antigen (PSA) concentration and rate of PSA increase. Cancer. 2006;106:320–328. doi: 10.1002/cncr.21603. [DOI] [PubMed] [Google Scholar]
- 6.Buddingh K.T., Maatje M.G.F., Putter H., Kropman R.F., Pelger R.C.M. Do antibiotics decrease prostate-specific antigen levels and reduce the need for prostate biopsy in type IV prostatitis? A systematic literature review. Can. Urol. Assoc. J. 2018;12:E25–E30. doi: 10.5489/cuaj.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Xiao G., Zhou J.W., Yang J.K., Lu L., Bian J., Zhong L., Wei Q.Z., Zhou Q.Z., Xue K.Y., et al. Optimal Starting Age and Baseline Level for Repeat Tests: Economic Concerns of PSA Screening for Chinese Men-10-Year Experience of a Single Center. Urol. Int. 2020;104:230–238. doi: 10.1159/000503733. [DOI] [PubMed] [Google Scholar]
- 8.Tan G.H., Nason G., Ajib K., Woon D.T.S., Herrera-Caceres J., Alhunaidi O., Perlis N. Smarter screening for prostate cancer. World J. Urol. 2019;37:991–999. doi: 10.1007/s00345-019-02719-5. [DOI] [PubMed] [Google Scholar]
- 9.Han P.K., Kobrin S., Breen N., Joseph D.A., Li J., Frosch D.L., Klabunde C.N. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann. Fam. Med. 2013;11:306–314. doi: 10.1370/afm.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenton J.J., Weyrich M.S., Durbin S., Liu Y., Bang H., Melnikow J. Prostate-Specific Antigen-Based Screening for Prostate Cancer: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018;319:1914–1931. doi: 10.1001/jama.2018.3712. [DOI] [PubMed] [Google Scholar]
- 11.Misra-Hebert A.D., Hu B., Klein E.A., Stephenson A., Taksler G.B., Kattan M.W., Rothberg M.B. Prostate cancer screening practices in a large, integrated health system: 2007–2014. BJU Int. 2017;120:257–264. doi: 10.1111/bju.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin R.M., Donovan J.L., Turner E.L., Metcalfe C., Young G.J., Walsh E.I., Lane J.A., Noble S., Oliver S.E., Evans S., et al. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. JAMA. 2018;319:883–895. doi: 10.1001/jama.2018.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamnagerwalla J., Howard L.E., Allott E.H., Vidal A.C., Moreira D.M., Castro-Santamaria R., Andriole G.L., Freeman M.R., Freedland S.J. Serum cholesterol and risk of high-grade prostate cancer: Results from the REDUCE study. Prostate Cancer Prostatic Dis. 2018;21:252–259. doi: 10.1038/s41391-017-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz P.M., Mo H., McConathy W.J., Sabnis N., Lacko A.G. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: A review of scientific findings, relevant to future cancer therapeutics. Front. Pharmacol. 2013;4:119. doi: 10.3389/fphar.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zadra G., Photopoulos C., Loda M. The fat side of prostate cancer. Biochim. Biophys. Acta. 2013;1831:1518–1532. doi: 10.1016/j.bbalip.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farwell W.R., D’Avolio L.W., Scranton R.E., Lawler E.V., Gaziano J.M. Statins and prostate cancer diagnosis and grade in a veterans population. J. Natl. Cancer Inst. 2011;103:885–892. doi: 10.1093/jnci/djr108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kok D.E., van Roermund J.G., Aben K.K., den Heijer M., Swinkels D.W., Kampman E., Kiemeney L.A. Blood lipid levels and prostate cancer risk; a cohort study. Prostate Cancer Prostatic Dis. 2011;14:340–345. doi: 10.1038/pcan.2011.30. [DOI] [PubMed] [Google Scholar]
- 18.Van Hemelrijck M., Walldius G., Jungner I., Hammar N., Garmo H., Binda E., Hayday A., Lambe M., Holmberg L. Low levels of apolipoprotein A-I and HDL are associated with risk of prostate cancer in the Swedish AMORIS study. Cancer Causes Control CCC. 2011;22:1011–1019. doi: 10.1007/s10552-011-9774-z. [DOI] [PubMed] [Google Scholar]
- 19.YuPeng L., YuXue Z., PengFei L., Cheng C., YaShuang Z., DaPeng L., Chen D. Cholesterol Levels in Blood and the Risk of Prostate Cancer: A Meta-analysis of 14 Prospective Studies. Cancer Epidemiol. Prev. Biomark. 2015;24:1086–1093. doi: 10.1158/1055-9965.EPI-14-1329. [DOI] [PubMed] [Google Scholar]
- 20.Guo H., Jia X., Liu H. Based on biomedical index data: Risk prediction model for prostate cancer. Medicine. 2021;100:e25602. doi: 10.1097/MD.0000000000025602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrido M.M., Marta J.C., Ribeiro R.M., Pinheiro L.C., Guimarães J.T. Serum lipids and prostate cancer. J. Clin. Lab. Anal. 2021;35:e23705. doi: 10.1002/jcla.23705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang S.L., Harshman L.C., Presti J.C., Jr. Impact of common medications on serum total prostate-specific antigen levels: Analysis of the National Health and Nutrition Examination Survey. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28:3951–3957. doi: 10.1200/JCO.2009.27.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moshfegh A.J., Rhodes D.G., Baer D.J., Murayi T., Clemens J.C., Rumpler W.V., Paul D.R., Sebastian R.S., Kuczynski K.J., Ingwersen L.A., et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008;88:324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 24.Zipf G., Chiappa M., Porter K.S., Ostchega Y., Lewis B.G., Dostal J. National health and nutrition examination survey: Plan and operations, 1999–2010. [(accessed on 7 October 2021)];Vital Health Stat. Ser. 1 Programs Collect. Proced. 2013 :1–37. Available online: https://stacks.cdc.gov/view/cdc/21304. [PubMed] [Google Scholar]
- 25.Arthur R., Møller H., Garmo H., Holmberg L., Stattin P., Malmstrom H., Lambe M., Hammar N., Walldius G., Robinson D., et al. Association between baseline serum glucose, triglycerides and total cholesterol, and prostate cancer risk categories. Cancer Med. 2016;5:1307–1318. doi: 10.1002/cam4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y., Zhang Y., Wang X., Lin D., Chen Z. Relationship between body mass index and concentrations of prostate specific antigen: A cross-sectional study. Scand. J. Clin. Lab. Investig. 2020;80:162–167. doi: 10.1080/00365513.2019.1703217. [DOI] [PubMed] [Google Scholar]
- 27.Mydlo J.H., Tieng N.L., Volpe M.A., Chaiken R., Kral J.G. A pilot study analyzing PSA, serum testosterone, lipid profile, body mass index and race in a small sample of patients with and without carcinoma of the prostate. Prostate Cancer Prostatic Dis. 2001;4:101–105. doi: 10.1038/sj.pcan.4500514. [DOI] [PubMed] [Google Scholar]
- 28.Capitanio U., Perrotte P., Hutterer G.C., Suardi N., Jeldres C., Shariat S.F., Duclos A., Arjane P., Montorsi F., Karakiewicz P.I. Effect of body mass index on prostate-specific antigen and percentage free prostate-specific antigen: Results from a prostate cancer screening cohort of 1490 men. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2009;16:91–95. doi: 10.1111/j.1442-2042.2008.02192.x. [DOI] [PubMed] [Google Scholar]
- 29.Barqawi A.B., Golden B.K., O’Donnell C., Brawer M.K., Crawford E.D. Observed effect of age and body mass index on total and complexed PSA: Analysis from a national screening program. Urology. 2005;65:708–712. doi: 10.1016/j.urology.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 30.Bañez L.L., Hamilton R.J., Partin A.W., Vollmer R.T., Sun L., Rodriguez C., Wang Y., Terris M.K., Aronson W.J., Presti J.C., Jr., et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275–2280. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z., Ho K.M., Hong Y. Machine learning for the prediction of volume responsiveness in patients with oliguric acute kidney injury in critical care. Crit. Care. 2019;23:112. doi: 10.1186/s13054-019-2411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livne M., Boldsen J.K., Mikkelsen I.K., Fiebach J.B., Sobesky J., Mouridsen K. Boosted Tree Model Reforms Multimodal Magnetic Resonance Imaging Infarct Prediction in Acute Stroke. Stroke. 2018;49:912–918. doi: 10.1161/STROKEAHA.117.019440. [DOI] [PubMed] [Google Scholar]
- 33.Hsu C.H., Yu M. Cox regression analysis with missing covariates via nonparametric multiple imputation. Stat. Methods Med. Res. 2019;28:1676–1688. doi: 10.1177/0962280218772592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leroux A., Di J., Smirnova E., McGuffey E.J., Cao Q., Bayatmokhtari E., Tabacu L., Zipunnikov V., Urbanek J.K., Crainiceanu C. Organizing and analyzing the activity data in NHANES. Stat. Biosci. 2019;11:262–287. doi: 10.1007/s12561-018-09229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Hemelrijck M., Garmo H., Holmberg L., Walldius G., Jungner I., Hammar N., Lambe M. Prostate cancer risk in the Swedish AMORIS study: The interplay among triglycerides, total cholesterol, and glucose. Cancer. 2011;117:2086–2095. doi: 10.1002/cncr.25758. [DOI] [PubMed] [Google Scholar]
- 36.Mondul A.M., Clipp S.L., Helzlsouer K.J., Platz E.A. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21:61–68. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platz E.A., Till C., Goodman P.J., Parnes H.L., Figg W.D., Albanes D., Neuhouser M.L., Klein E.A., Thompson I.M., Jr., Kristal A.R. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol. Prev. Biomark. 2009;18:2807–2813. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morote J., Celma A., Planas J., Placer J., de Torres I., Olivan M., Carles J., Reventós J., Doll A. Role of serum cholesterol and statin use in the risk of prostate cancer detection and tumor aggressiveness. Int. J. Mol. Sci. 2014;15:13615–13623. doi: 10.3390/ijms150813615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs E.J., Stevens V.L., Newton C.C., Gapstur S.M. Plasma total, LDL, and HDL cholesterol and risk of aggressive prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Causes Control. 2012;23:1289–1296. doi: 10.1007/s10552-012-0006-y. [DOI] [PubMed] [Google Scholar]
- 40.Liu M., Wang J.Y., Zhu L., Wan G. Body mass index and serum lipid profile influence serum prostate-specific antigen in Chinese men younger than 50 years of age. Asian J. Androl. 2011;13:640–643. doi: 10.1038/aja.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Wong E.W., Chang F., Lehmann B., Terrian D.M., Milella M., Tafuri A., et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekine Y., Koike H., Nakano T., Nakajima K., Takahashi S., Suzuki K. Remnant lipoproteins induced proliferation of human prostate cancer cell, PC-3 but not LNCaP, via low density lipoprotein receptor. Cancer Epidemiol. 2009;33:16–23. doi: 10.1016/j.canep.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Sekine Y., Koike H., Nakano T., Nakajima K., Suzuki K. Remnant lipoproteins stimulate proliferation and activate MAPK and Akt signaling pathways via G protein-coupled receptor in PC-3 prostate cancer cells. Clin. Chim. Acta Int. J. Clin. Chem. 2007;383:78–84. doi: 10.1016/j.cca.2007.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available at NHANES website https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 7 October 2021).