Abstract
Nonresolving inflammation contributes to many diseases, including COVID-19 in its fatal and long forms. Our understanding of inflammation is rapidly evolving. Like the immune system of which it is a part, inflammation can now be seen as an interactive component of a homeostatic network with the endocrine and nervous systems. This review samples emerging insights regarding inflammatory memory, inflammatory aging, inflammatory cell death, inflammatory DNA, inflammation-regulating cells and metabolites, approaches to resolving or modulating inflammation, and inflammatory inequity.
This review describes inflammation as part of an interacting system of homeostatic control and restoration along with the endocrine and nervous systems. A focus on recent advances in inflammation as they apply within an organism is joined with attention to the disproportionate burden of inflammation on some populations in society.
Introduction
A review in 2010 entitled “Nonresolving Inflammation” (Nathan and Ding, 2010) began, “Perhaps no single phenomenon contributes more to the medical burden in industrialized societies than nonresolving inflammation.” That view has not changed. In 2021, a leading thinker in the field wrote that “inflammation is associated with almost every major human disease” (Medzhitov, 2021). That same year, 13,905 review articles flagged “inflammation” as a key word and 1,284 of them included “inflammation” in their titles. This not only reflects that the topic is important but underscores that we are struggling to get a grip on it.
Since 2010, the toll of inflammation on human health has not subsided, despite major advances in understanding of the underlying biology, the tireless efforts of drug developers, and the clinical success of several interventions, such as biologics that block signaling by interleukin-1β (IL-1β) or tumor necrosis factor-α (TNF-α) (Netea et al., 2017). On the contrary, since 2020, inflammatory responses to COVID-19 (Casanova and Abel, 2021; Del Valle et al., 2020; Gruber et al., 2020; Karki et al., 2021) have driven the death toll from nonresolving inflammation to the highest level in the lifetime of anyone reading these words. Meanwhile, the striking but partial success of immuno-oncology has focused attention on the ability of intra-tumoral inflammation to either frustrate or assist the immunological control of cancer. Accordingly, efforts to resolve inflammation as a treatment for autoinflammatory and autoimmune diseases have been joined by efforts to modulate inflammation in the treatment of malignancies.
The goal here is not to repeat or replace other reviews, but to both enlarge our thinking and focus it. The enlargement offers an expanded definition of inflammation and directs attention to emerging topics, among them inflammatory memory, inflammatory modulation, and inflammatory inequity. The focus is on emerging knowledge that might lead to treatments for individuals and on the need for interventions to benefit communities and populations. Necessarily, for a subject and literature this vast, these topics are selective and the citations illustrative.
What is inflammation?
Definitions of inflammation are evolving as understanding of the biology grows and as more studies assert the involvement of inflammation without its classic signs—rubor (redness), calor (heat), humor (swelling), and dolor (pain). In the 20th century, inflammation was seen as a tissue reaction to an emergent stimulus (Table 1 ). The reaction was macroscopically visible on the outside of a host or within the host at endoscopy or surgery, and microscopically apparent in tissue from an affected site as the accumulation of neutrophils, monocytes, macrophages, and/or lymphocytes in a structure that might be disordered by edema, necrosis, fibrosis, lipidosis, malignancy, or infection.
Table 1.
Changing conceptions of inflammation over the past two decades
| Conception | Detection | Stimuli | Causes of non-resolution or recurrence | Participating cells | Overall function | Therapeutic concerns |
|---|---|---|---|---|---|---|
| 20th centurya,b | macroscopically or microscopically | emergent; usually evident; may be single | persistent stimulus | cells of the immune system | resolve problem or initiate an immune response | infection, trauma, cancer, asthma, atherosclerosis, diabetes, autoimmune disorders, etc. |
| 21st century V.0c,d | macroscopically, microscopically, or inferred from increased production of cytokines, chemokines, non-protein mediators and products they induce | dual stimuli, signaling infection plus injury; or inapparent but seemingly continual stimuli, implied by spontaneous inflammation being a phenotype of numerous gene deficiencies | persistent stimulus; emergent secondary stimulus, such as autoimmune response; excessive or prolonged initial response; subnormal initial response; defective switch of cells and mediators from pro- to anti-inflammatory, depending on context; loss of a constitutively operating anti-inflammatory mechanism | cells of the immune system | resolve problem or initiate an immune response | as for 20th century, with additional focus on metabolic and neurodegenerative diseases |
| 21st century V.1e | “any process involving signals and cells known to orchestrate the more familiar acute inflammatory response” | reaction to a perturbation, or participation “in normal homeostatic processes in the absence of any perturbations” | ||||
| 21st century V.2f | as for V.0 | as for V.0 plus air pollution, temperature extremes, dietary deficiencies, and stresses of poverty and discrimination | as for V.0, with additional recognition of inflammatory memory and inflammaging | any cells, including microbiota | as for V.1, with emphasis on joint participation with the endocrine and nervous systems in providing homeostatic control and restoration | as for V.0, with additional emphasis on inflammatory modulation in immuno-oncology and societal actions to reduce inflammatory inequity |
Cells in the table are unfilled when the topic was not a focus of the article cited.
This article
The early 21st century introduced a fundamentally different and still-evolving view, as summarized for versions V.0 (Nathan, 2002; Nathan and Ding, 2010), V.1 (Medzhitov, 2021), and V.2 (here) in Table 1. Versions V.0 and V.2 hold that in addition to inflammation arising in response to stimuli that are evident and new, multiple points of control act constitutively to restrain the onset of inflammation that otherwise emerges as if spontaneously, presumably in response to unnoticed contact with microbes, inanimate particulates, noxious gases, or aberrant cells. This is evidenced by the consequences of loss of function in any one of a vast number of genes (Nathan, 2002; Nathan and Ding, 2010), a number that continues to grow (Tyler et al., 2021). Inflammation is considered “nonresolving” not only when it is unremitting but when it is recurrent. Moreover, according to version V.0, inflammation can persist for reasons other than failure to remove an inciting stimulus (Nathan and Ding, 2010). COVID-19 illustrates each of these additional routes to nonresolving inflammation: an excessive or prolonged initial response, as in acute SARS-CoV-2 infection, which triggers inflammasomes (Rodrigues et al., 2021; Vora et al., 2021), elicits massive cytokine release (Del Valle et al., 2020), activates complement (Ma et al., 2021), and releases neutrophil extracellular traps (Veras et al., 2020); a subnormal initial response, as when autoantibodies or loss-of-function mutations prevent production of or response to type I interferons (Bastard et al., 2021; Zhang et al., 2020) or signaling through TLR7 (Asano et al., 2021); and emergence of secondary stimuli after viral clearance, such as when tissue damage sets up an autoimmune reaction (Wang et al., 2021). COVID-19 reminded us that perpetuation of inflammation by autoantibody formation in response to tissue damage may be operative in diverse states of nonresolving inflammation. It may contribute, for example, to atherosclerosis (Lorenzo et al., 2021), the leading cause of death before COVID-19 from a disease in which nonresolving inflammation plays a prominent part.
Recent years have revealed that immunity can involve any cells in the body, not just those of lymphohematopoietic origin, and, reciprocally, cells of lymphohematopoietic origin function in the development and homeostatic maintenance of other tissues and organs (Nathan, 2021). Medzhitov has ascribed similar features to inflammation, which is an integral part of immunity, namely, the participation in inflammation of more cells than were classically implicated, and the impact of inflammation on the development and homeostatic maintenance of tissues (Medzhitov, 2021). This is strikingly evident in the role of inflammation in normal fetal development and parturition, notwithstanding that inflammation can also contribute to fetal damage from infection (Megli and Coyne, 2022).
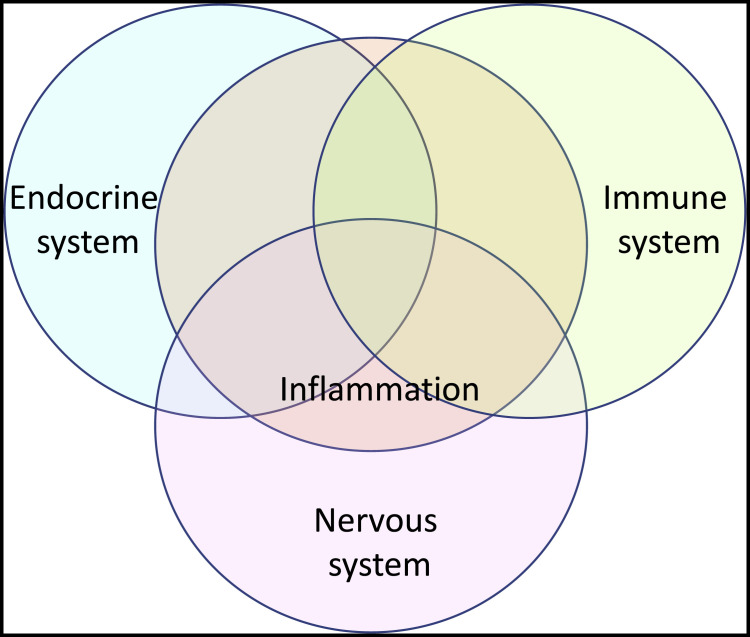
Definition V.2 (Table 1) is compatible with versions V.0 and V.1, but with additions in scope and changes in emphasis. According to V.2, inflammation, like the immune system of which it is a part, is a major module in a network of homeostatic bodily responses to perturbation that coordinates with the two other major systems of inter-organ, intra-tissue communication—the endocrine and nervous systems—and complements the limitations of each (Figure 1 ).
Figure 1.
Inflammation as a component of the homeostatic network
The immune system (with inflammation as a prominent part), the endocrine system, and the nervous system interact with each other in a meta-system of homeostatic control and restoration. Each member of the tripartite system complements the others with respect to their range of action in space and time, the diversity and nature of responses they command, and their exertion of control at levels of cells, tissues, organs, and organism.
Like the endocrine system, inflammation can send soluble signals throughout the body but can do so with molecules (cytokines) of greater molar potency than hormones, and more commonly takes advantage of the opportunity to release its signals in specific sites. The influence of the endocrine system on inflammation has long been appreciated. For example, endogenous stress-induced corticosteroids are anti-inflammatory and immunosuppressive, and corticosteroids in pharmacologic doses remain among the most powerful and widely used anti-inflammatory drugs. Many of the same hormones that regulate metabolism in other cells, such as insulin, do so in lymphohematopoietic cells as well. The reciprocal influence has also long been apparent, for example, with the recognition that inflammation is a major driver of insulin resistance in obesity (Rohm et al., 2022). Obesity, like atherosclerosis, is one of the most prevalent states of nonresolving inflammation.
More recent is the appreciation of functional similarities, complementary differences, and reciprocal interactions between inflammation and the nervous system (Kabata and Artis, 2019; Pavlov et al., 2018). Like the nervous system, inflammation can involve cell-cell contact, but is not limited to hard-wired, pre-determined contacts and has a greater number of cell-surface and secretory products that act on a larger repertoire of receptors and induce a wider range of responses. Like the nervous system, inflammation involves sensory and effector pathways. As in the nervous system, these involve some developmentally positioned cells, such as perivenular mast cells, skin-resident dendritic cells, liver-resident Kupffer cells, bone-resident osteoclasts, lung-resident alveolar macrophages, brain-resident microglia, and innate lymphoid cells resident in diverse tissues near neurons (Kabata and Artis, 2019). However, unlike nerves, inflammatory cells can migrate to any place in any tissue. The peripheral nervous system has a limited set of commands: it instructs cells to contract, relax, secrete, or excrete, and to do so within seconds or minutes. Inflammation instructs cells to change their transcriptome, metabolome, and secretome, and sometimes to die, grow, or proliferate, while commanding tissues to leak, swell, break down, or reconstruct, and issues these instructions over a period of hours to days, weeks, months, or years.
Increased understanding of interactions between inflammation on the one hand and the nervous system on the other hand represents one of the most important developments in inflammation biology of the last two decades. An explosion of insight followed the discovery that the inflammatory and nervous systems interact through the far-reaching, highly arborized vagus nerve in the “inflammatory reflex” (Tracey, 2002). T cells help induce inflammation through release of the neurotransmitter acetylcholine (Cox et al., 2019). Inflammatory cell autacoids and cytokines activate sensory neurons, while neuropeptides, neurotransmitters, and neuron-derived alarmins promote or restrain inflammation (Kabata and Artis, 2019; Moriyama et al., 2018; Nagashima et al., 2019; Yang et al., 2021). Certain neuronal guidance molecules prolong inflammation (Plant et al., 2020) or resolve it (Körner et al., 2021). Strikingly, the brain houses a form of inflammatory memory, as discussed in the following section.
Inflammatory memory
Appreciation has grown for forms of memory in the innate immune system, sometimes called “trained immunity” (Saeed et al., 2014). Likewise, evidence has mounted for an analogous form of inflammatory memory, namely, epigenetic changes that outlast a bout of inflammation and facilitate the recurrence or persistence of inflammation in the host or its emergence in the host’s offspring.
For example, inflammation can have an impact on hematopoietic precursors in the bone marrow that lasts long after inflammation has subsided. Inflammation can skew hematopoietic stem cells toward myelopoiesis, and increased numbers of myeloid cells can promote further inflammation (Chavakis et al., 2019). Inflammatory suppression of hematopoiesis can help select for the emergence of clones better able to withstand it (Avagyan et al., 2021; Caiado et al., 2021; Trowbridge and Starczynowski, 2021). Some of these clones may give rise to myelodysplastic syndrome or leukemia. However, nonmalignant clones that withstand the myelosuppression of inflammation can persist and expand in a manner that is markedly dependent on age (Jaiswal et al., 2014). In turn, this clonal hematopoiesis promotes inflammation, notably in the cardiovascular system (Libby and Ebert, 2018). In this sense, inflammation imprints a memory of itself in hematopoietic cells that is recalled and amplified in later years. Clonal hematopoeisis may be one of the major explanations for the association of age with an inflammatory diathesis, sometimes called “inflammaging,” other features of which are discussed further below.
Mature myeloid cells can also show lasting, epigenetically mediated effects of an inflammatory experience. For example, intraperitoneal injection of lipopolysaccharide (LPS) in mice induced long-lasting epigenetic changes in their microglia that impacted the responses of the microglia to inflammatory stimuli 6 months later (Wendeln et al., 2018). Pneumonia altered the epigenome of pulmonary alveolar macrophages, leading to a sustained defect in their phagocytic capacity (Roquilly et al., 2020).
Epithelial and mesenchymal cells can display inflammatory memory as well (Niec et al., 2021). For example, dermal inflammation induced by a toll-like receptor 7 (TLR7) agonist, abrasion, or fungal infection epigenetically altered epithelial stem cells such that they responded much faster to a long-delayed second insult (Naik et al., 2017). Injection of an agent that inflames the pancreas led to transcriptional and epigenetic changes in pancreatic acinar cells that reduced their inflammatory response to a subsequent challenge, while promoting their malignant transformation (Del Poggetto et al., 2021). Transient induction of inflammation in neonatal mice led to accumulation of Th2 cells alongside dermal fibroblasts, which led in turn to changes in fibroblastic responses to a subsequent injury (Boothby et al., 2021).
Prenatal inflammation can increase the incidence of inflammatory, neurodevelopmental, and behavioral disorders in adult progeny. For example, infection of pregnant mice induced IL-6, which altered the fetal stem cell epigenome in such a way as to predispose adult offspring to inflammation (Lim et al., 2021). Similarly, infection of pregnant mice induced IL-17A, which altered the maternal microbiota in such a way that offspring had epigenetic changes in their CD4+ T cells (Kim et al., 2022). Elevation of maternal IL-17A in response to injection of poly(I:C) altered their adult offspring’s behavior (Shin Yim et al., 2017). Exposure of pregnant mice to LPS altered the inflammatory responses of microglia in their adult offspring (Schaafsma et al., 2017).
Recently, Koren et al. (2021) identified specific neurons in the mouse posterior insular cortex that were activated during experimental colitis and other neurons that were activated during peritonitis. After the inflammation had subsided, the investigators activated the neurons that had responded to colitis, and this elicited a recurrence of lymphocyte accumulation in the colonic mucosa. When the investigators instead activated neurons that had responded to peritonitis, this elicited some of the inflammatory cell accumulations and cytokine elevations that characterized the original bout of peritonitis. These recall responses were mediated through the autonomic nervous system (Koren et al., 2021). This landmark study suggests the possibility that there is a fifth general route to nonresolving inflammation—understood here as recurring inflammation— besides the four routes described earlier (Nathan and Ding, 2010) and summarized in Table 1, namely, conscious or subconscious mental recall of a previous bout of inflammation. The signs of inflammation induced by activation of the neuronal “engram” were only a subset of what was seen after oral administration of dextran sodium sulfate or intraperitoneal injection of zymosan (Koren et al., 2021). However, it now seems possible that remembering the experience of inflammation might synergize with other factors to delay its resolution or contribute to flares, such as in relapsing-remitting multiple sclerosis or systemic lupus erythematosus. However, it will be difficult to establish whether this form of inflammatory memory occurs in people. And it will be important not to fault patients for reflecting on their illness. How could they not?
Inflammatory aging (“inflammaging”)
Aging promotes inflammation in additional ways besides through the inflammatory memory associated with the age-dependent expansion of clonal hematopoiesis. Aging-associated obesity is a major driver of inflammation (Rohm et al., 2022). Caloric restriction, which counteracts some effects of aging, reduced the expression of the platelet activating factor acetylhydrolase PLA2G7 in human and mouse myeloid cells, leading to decreased ceramide-dependent NLRP3 activation (Spadaro et al., 2022). Deletion of PLA2G7 led to lower amounts of circulating TNF-α and IL-1β in 2-year-old mice, implicating PLA2G7 as a mediator of inflammatory aging (Spadaro et al., 2022). Age-related de-repression of retrotransposable elements promotes type I IFN secretion (De Cecco et al., 2019). Mitochondrial function declines in aged T cells; T cells with an engineered mitochondrial deficiency drove a cytokine storm (Desdín-Micó et al., 2020). Epigenetic changes in T cells caused by residence in an aging host promoted clonal expansion of a subset of T cells that secreted granzyme K, which elicited inflammatory responses in other cells (Mogilenko et al., 2021). Decreased diurnal expression of the transcription factor Kruppel-like factor 4 accounted for functional defects in the macrophages of old mice (Blacher et al., 2022). Increased production of soluble VEGF receptor with age led to reduced VEGF signaling and impaired maintenance of microcapillaries, accompanied by an increase in circulating granulocytes, perivascular inflammatory cell infiltrates, elevated levels of monocyte chemoattractant-1 (MCP-1) and C-reactive protein (CRP), and immune cell infiltration of liver and fat (Grunewald et al., 2021). A study of leukocytes from 205,011 men in the UK biobank detected a markedly age-dependent loss of the Y chromosome (LOY) that reached a prevalence of 43.6% of men over 70 years of age (Thompson et al., 2019). Given the epidemiologic association between LOY and several diseases associated with nonresolving inflammation, such as obesity, cardiovascular disease, type II diabetes, and Alzheimer’s disease (Thompson et al., 2019), it deserves study whether LOY may contribute to inflammaging.
Anti-inflammatory functions of pro-inflammatory cells
A given type of cell can have a predominantly pro-inflammatory or anti-inflammatory impact, depending on context (Nathan and Ding, 2010) (Table 1). Recent evidence adds new examples and mechanisms. Regulatory T (Treg) cells are indispensable for resolution of inflammation (Hu et al., 2021), yet intradermal Treg cells promoted epidermal inflammation in wounded skin by driving keratinocytes to produce neutrophil-recruiting chemokines (Moreau et al., 2021). Platelets helped resolve the pulmonary inflammation associated with bacterial pneumonia in mice by physically trapping Treg cells in the lung and inducing a pro-resolution transcriptional program in macrophages (Rossaint et al., 2021). IgM+ B cells adhering within the pulmonary vasculature in the lungs of mice with pneumonitis induced by zymosan or Aspergillus produced lipoxin A4, reducing accumulation of neutrophils (Podstawka et al., 2021). Neutrophils helped restrain allergic inflammation in the lung by suppressing chemokine generation by type 2 innate lymphoid cells (ILC2s) (Patel et al., 2019). Neutrophil-like myeloid-derived suppressor cells appeared to be critical for suppressing inflammation in neonatal mice and human infants (He et al., 2018). Astrocytes drove inflammation in some contexts (Wheeler et al., 2020) and restrained it in others (Sanmarco et al., 2021).
Inflammatory cell death
Cell death from trauma, infection, intoxication, autoimmune attack, or the host’s inflammatory response can promote inflammation when cells dying by pyroptosis or necroptosis release IL-1α, IL-1β, IL-18, IL-33, HMGB1, galectin-1, or DNA (Newton et al., 2021; Orning et al., 2019; Russo et al., 2021). A kinase-caspase cascade readies gasdermins to form pores; the ensuing ionic imbalance activates cell surface NINJ1 protein to drill and rupture the plasma membrane (Kayagaki et al., 2021). Cells dying by apoptosis are considered non-inflammatory, but if they are not cleared, NINJ1 lyses them as well (Kayagaki et al., 2021). Clearance of apoptotic cells not only removes alarmins from the extracellular space, but helps trigger production of pro-resolving mediators, both processes being assisted by the protein developmental endothelial locus 1 (DEL-1) (Kourtzelis et al., 2019). Ferroptosis may also contribute to inflammation, including by fostering the generation and release of oxidized lipids and oxidized nucleosides (Chen et al., 2021b).
Inflammatory DNA
One of the most important advances in inflammation biology of the last decade has been the discovery of cGAS as a sensor of cytosolic DNA that generates cGAMP as an activator of STING, a driver of type I interferon production (Sun et al., 2013). The inflammation characteristic of type I interferonopathies can result both from gain of function in DNA-sensing molecules like STING and MDA5 and loss of function in any of a large number of nucleic acid metabolizing enzymes or pathways controlling mitochondrial integrity (Crow and Stetson, 2021; Li and Chen, 2018).
Recent findings have revealed diverse cGAS-STING-dependent processes by which DNA can come to act as an inflammatory stimulus. In senescent cells or cells undergoing DNA damage from irradiation, chemotherapy, or deficiencies in DNA repair pathways, fragments of chromatin in cytosolic micronuclei can activate cGAS (Crow and Stetson, 2021; Dou et al., 2017; Li and Chen, 2018). In people with systemic lupus erythematosus, maturing erythrocytes tend to exhibit defective mitophagy. Autoantibodies can opsonize the erythrocytes for ingestion by macrophages. The ingested mitochondrial DNA can find its way to activating cGAS (Caielli et al., 2021). Mitochondrial DNA was a STING-dependent driver of inflammatory cytokine production and cell death in endothelial cells in skin lesions from patients with COVID-19, and ingestion of dying endothelial cells activated cGAS-STING-dependent type I IFN secretion in their macrophages (Domizio et al., 2022). Similarly, mitochondrial DNA triggered STING-dependent inflammation in exercising mice with Parkinson’s disease-associated mutations in the mitophagy regulators parkin and PINK1 (Sliter et al., 2018). In mice fed a high fat diet, the skin microbiota induced keratinocytes to increase their expression and reverse transcription of endogenous retroviruses, whose cDNA activated the cGAS-STING pathway, leading to accumulation of IL17A-producing T cells (Lima-Junior et al., 2021). In mouse models of macular degeneration, RNA from Alu retroelements, rather than leading to dsDNA, acted by an unclear mechanism to promote release of mitochondrial DNA, which activated cGAS (Kerur et al., 2018).
However, oxidized mitochondrial DNA can also contribute to inflammation in other ways, such as by binding and helping to activate NLRP3 (Zhong et al., 2018). Further, endogenous retroviruses can contribute to inflammation without acting through cGAS. For example, many patients with inflammatory bowel disease have a colonic deficiency of the histone methyltransferase SETBD1; in SETBD1-deficient mice, mobilization of retroviruses triggered necroptosis in intestinal stem cells in a ZBP-1-, RIP3-dependent manner (Wang et al., 2020). DNA released from dying cells can be taken up by macrophages via its attached histones, which are recognized by the C-type lectin Clec2d, and delivered to endosomes, where the DNA can trigger TLR9-driven cytokine and chemokine release (Lai et al., 2020). Extracellular histones themselves promote inflammation when they cause membrane disruption, leading to necrotic cell death (Silvestre-Roig et al., 2019).
Inflammation-regulating metabolites
Both the host and its microbiota are sources of metabolites with pro- or anti-inflammatory actions. Several intermediates in the host’s central carbon metabolism such as pyruvate and α-ketoglutarate are α-ketoacids that can act as antioxidants by undergoing oxidative decarboxylation upon encountering hydrogen peroxide (O’Donnell-Tormey et al., 1987). Those molecules and dicarboxylic acids can also acylate proteins, modifying their activity, and some of them bind G-protein coupled receptors. It is difficult to unravel which of these effects account for the anti-inflammatory activity of fumarate (given as a dimethyl ester) in multiple sclerosis (Liu et al., 2021) or pyruvate (given as an ethyl ester) in diverse preclinical models of inflammation (Koprivica et al., 2022). Among the acylation targets of succinate is gasdermin D, which when so modified, no longer supports pyroptosis (Humphries et al., 2020). The inflammation-resolving effects of IL-33 have been traced to its promotion of increased production of itaconate (Faas et al., 2021). Among the diverse effects of itaconate are inhibition of the NLRP3 inflammasome and activation of Nrf2 and ATF3, transcription factors with anti-inflammatory regulons (Peace and O’Neill, 2022).
Many microbiotal metabolites can affect inflammation, including through epigenetic regulation (Krautkramer et al., 2021). This branch of inflammation research took off with the discovery by Hazen and his colleagues that microbiotal metabolism of dietary phosphocholine and carnitine leads to trimethylamine-N-oxide, which is both a biomarker for and a driver of vascular inflammation, leading to both atherosclerosis (Koeth et al., 2013; Wang et al., 2011) and stroke (Zhu et al., 2021). Microbiotal metabolism of L-tyrosine to p-cresol reduced chemokine production by airway epithelial cells (Wypych et al., 2021). Microbiotal metabolism of tryptophan to indole and indoxyl sulfate suppressed miR-181 in white adipose tissue, preventing the tissue from becoming inflamed (Virtue et al., 2019), and generated ligands for the arylhydrocarbon receptor, suppressing inflammation (Hezaveh et al., 2022; Lamas et al., 2020).
While the responsible microbiotal products remain to identified in many cases, a voluminous literature documents the profound influence of the microbiota on host inflammatory responses in the gut, lung, brain, and skin (Agirman et al., 2021; Blander et al., 2017; Chen et al., 2018; Rosshart et al., 2019).
Suppressing inflammation
The list of potential targets for reducing nonresolving inflammation in one or more diseases has probably never been longer. The sampling that follows is biased toward agents not included in several wide-ranging reviews (Dinarello, 2010; Goldfine and Shoelson, 2017; Henderson et al., 2020; Rohm et al., 2022). Even so, it is far from complete. The selections are meant only to suggest the diversity of potential new approaches.
Enzyme targets include cGAS (Ablasser and Chen, 2019); the kinases JAK1/2 (Hoang et al., 2021), ephrin-B3 (Clark et al., 2021), and Fgr (Crainiciuc et al., 2022); the tyrosine phosphatase SHP2 (Paccoud et al., 2021); the proteases proprotein convertase subtilisin/Kexin 9 (PCSK 9) (Patriki et al., 2022) and the immunoproteasome (Ah Kioon et al., 2021; Kirk et al., 2021); the 8-oxoguanine DNA glycosylase OGG-1 (Visnes et al., 2018); and the histone 3 Lys27 trimethyltransferase Ezh2 (Zhang et al., 2018). Adaptor protein targets include STING (Ablasser and Chen, 2019) and NRLP3 (Wang et al., 2022). Among channel targets is transient receptor potential cation channel member A1 (TRPA1) (Balestrini et al., 2021). Targets that might reduce neutrophil accumulation in inflammatory sites include dipeptidyl peptidase 1 (a setting in which it acts non-enzymatically [Choudhury et al., 2019]) and the formation of potent heterodimers of particular chemokines (von Hundelshausen et al., 2017). Synthetic versions of natural product oleanane triterpenoids have multiple targets and resolve inflammation in a wide range of preclinical models, largely through reactions with cysteine residues (Liby and Sporn, 2012), as also seen with anti-inflammatory actions of itaconate and fumarate.
Antibody to the soluble axon guidance protein neogenin promoted formation of pro-resolving mediators (Schlegel et al., 2019). A retinoic acid receptor agonist suppressed the sterile inflammation of stroke, apparently by increasing the ability of inflammatory myeloid cells to clear alarmins (Shichita et al., 2017). An unusual addition to the list of receptors as targets is the endoplasmic reticulum sigma-1 receptor, whose agonistic engagement restrained inflammatory cytokine production (Rosen et al., 2019).
Exercise induced a circulating complement inhibitor, clusterin, with anti-inflammatory properties (De Miguel et al., 2021). Inhibition of complement activation accounts for much of the inflammation-resolving action of apolipoprotein E (Yin et al., 2019). High-density lipoprotein (HDL) can also reduce inflammation, in this case by suppressing TLR levels (De Nardo et al., 2014). HDL has been used to form nanoparticles that delivered inhibitors of TOR and of CD40 signaling to macrophages, leading to acceptance of allografts (Braza et al., 2018). Other kinds of nanoparticles have been used to deliver bilirubin to suppress inflammation in rodent models of colitis and pancreatitis (Vítek and Tiribelli, 2020).
Multiple cytokines have been targeted in efforts to reverse nonresolving inflammation. Less common are studies that demonstrate the benefit of infusing a cytokine. Delivery of VEGF counteracted inflammation in aging mice (Grunewald et al., 2021). GDF15, a TGFβ superfamily member, promoted the ability of mice to tolerate inflammation (Luan et al., 2019). miR-342 was found to mediate the anti-inflammatory response of Treg cells to glucocorticoids (Kim et al., 2020). miR-223 suppressed inflammation by inhibiting expression of the NLRP3 inflammasome (Neudecker et al., 2017). BCG vaccination reduced inflammatory biomarkers in the blood of volunteers tested 3 months later (Koeken et al., 2020). Anti-inflammatory effects of electrical stimulation of the vagus nerve have been documented in the clinic (Pavlov et al., 2020).
These examples teach us that despite the complexity of inflammation, the diversity of its causes, the wide range of tissues in which it can originate, and its propensity to exert systemic effects, there is a wide variety of molecular targets whose modulation can suppress it. Unfortunately, this does not mean that these targets are mutually non-redundant and universally involved. Each anti-inflammatory intervention has a partial effect. The broader the effect, the greater the risk of toxicity. As yet, we know of no single target for a magic bullet.
Modulating inflammation
There is intense interest in “re-programming” tumor-infiltrating myeloid cells so that instead of suppressing T cell-mediated tumor rejection, they contribute to control of the malignancy. Selected examples illustrate the breadth of approaches.
Administration of an inhibitor of inducible nitric oxide synthase or iNOS (NOS2) plus taxane induced tumor shrinkage in a high proportion of women with locally advanced breast cancer or metastatic triple-negative breast cancer, and some tumors regressed completely (Chung et al., 2021). Given that taxol induces macrophages to release TNFα (Ding et al., 1990), both of the experimental drugs in that study likely modulated inflammation. Macrophages from breast cancer patients’ pleural effusions were activated to kill tumor cells when exposed to IFN-γ and monophosphoryl lipid A (an LPS derivative) and administration of those agents to mice induced their tumor-associated macrophages to express iNOS, TNFα, and IL-12 in connection with improved responses to chemotherapy (Sun et al., 2021). The combination of IFN-γ and LPS is a powerful inducer of iNOS in mouse macrophages (Xie et al., 1992). Exosomes delivering anti-sense oligonucleotide to STAT6 drove TAMs in mouse models of colorectal and hepatocellular carcinoma toward expression of IL-1β, IL-12, TNFα, and iNOS, resulting in marked suppression of tumor growth (Kamerkar et al., 2022). The findings of Chung et al. (2021), Sun et al. (2021), and Kamerkar et al. (2022) recall a decades-old literature showing that iNOS can both suppress T cells and kill tumor cells. Which effect dominates in a tumor likely depends on the flux of reactive nitrogen species, but this will not be evident from measuring the level of iNOS mRNA. Output from iNOS will depend on the supply of its substrates (L-arginine, oxygen, and NADPH) and cofactor (tetrahydrobiopterin). Less obviously, iNOS, unlike other proteins made by the same macrophages, is selectively dependent for its synthesis and stability on levels of L-arginine, which can be depleted in an inflammatory site by another macrophage product, L-arginase (El-Gayar et al., 2003), whose expression was likely reduced by suppression of STAT6 (Kamerkar et al., 2022).
Multiple receptors on tumor-associated macrophages (TAMs) have been targeted to improve tumor control. Agonistic anti-CD40 mAb plus gemcitabine led to regression of some human pancreatic carcinomas, and in mouse models, promoted infiltration of the tumors by tumoricidal macrophages (Beatty et al., 2011). Likewise, an agonistic anti-CD40 mAb combined with a CSF-1R inhibitor drove TAMs in a mouse melanoma model to secrete TNFα, IL-6, and IL-12 and suppressed tumor growth (Perry et al., 2018). Interference with the anti-phagocytic effect of tumor cell CD47 acting on macrophage SIRPα shrank tumors in mice and people and improved responses to chemo- and immunotherapy (Advani et al., 2018; Kosaka et al., 2021; Weiskopf et al., 2013). Prostate cancer cell-derived IL-1β increased MARCO expression on macrophages; MARCO engagement with lipids induced macrophages to make CCL6; CCL6 promoted prostate cancer cell metastasis; and anti-MARCO antibody reduced tumor growth (Masetti et al., 2022). A synthetic agonist of the CD206 mannose receptor reversed TAMs’ immunosuppressive phenotype and improved the response of mice to chemotherapy and immunotherapy (Jaynes et al., 2020).
TAMs that better support chemo- and immunotherapy were elicited in a mouse melanoma model by intravenous injection of nanoparticles of phospholipids, cholesterol, and apolipoprotein A that delivered a cargo of a synthetic, lipidated analog of the bacterial peptidoglycan subunit, muramyl dipeptide (Priem et al., 2020). Inhibition of CXCR2 reduced the accumulation of neutrophils and myeloid-derived suppressor cells (MDSCs) in pancreatic adenocarcinomas in mice and improved the response to checkpoint blockade (Steele et al., 2016). A diet that promoted microbial generation of STING ligands induced TAMs to produce type I IFN, and this was associated with improved responses to immunotherapy in mice, with correlative evidence in melanoma patients (Lam et al., 2021). Tumor cell-derived histamine influenced TAMs to suppress CD8+ T cell function; a blocker of histamine receptor HRH1 augmented responses to immunotherapy in mice (Li et al., 2022). Yet another approach to favorably modulating the inflammatory environment in a tumor is to irradiate the tumor (Brandmaier and Formenti, 2020).
Inflammatory inequity
People can develop nonresolving inflammation in response to their physical, economic, and psychosocial environments, including through the air they breathe, the temperatures they experience, the diet they access, and the stresses they endure from poverty, discrimination, or dysfunctional relationships (Furman et al., 2019). When these adverse influences fall disproportionately on a geographic community or a group of people whose shared geographic ancestry is associated with the biologically false social construct of “race,” we have “inflammatory inequity.” Though the supporting literature for inflammatory inequity is vast, causal influences are inter-related, mechanisms are complex, outcomes are long term, quantification is difficult, and controlled experiments are rare. Such methodologic limitations should not be taken to diminish the significance of inflammatory inequity.
For example, inhaling fine particulates causes inflammation that contributes to cardiovascular disease and insulin resistance (Bhatnagar, 2022). Metals and organic compounds borne on the particles may generate reactive oxygen species (Bhatnagar, 2022). The particles activate NLRP3 and trigger production of TNFα and IL-β (Cao et al., 2022; Zheng et al., 2018). Products of lipid and DNA (per)oxidation appear in the circulation (Bhatnagar, 2022). Sources of inflammatory particulates include power plants, automobile exhausts, forest fires (whose number and size are increasing with climate change), and unventilated indoor cooking. In the United States, communities with large Black and Hispanic contingents are disproportionately exposed to the first two sources. According to one study, “among zip codes with high levels of PM2.5 [fine particulate matter], 90% were predominantly African American” (Dey and Dominici, 2021). Mortality rates from COVID-19 have been strongly linked to levels of air pollution (Dey and Dominici, 2021; Frontera et al., 2020; Mendy et al., 2021; Pozzer et al., 2020), magnifying the inflammatory inequity.
Climate change brings many regions more days with difficult-to-tolerate temperatures. C-reactive protein, an inflammatory biomarker, rises with heat exposure (Kang et al., 2020).
Childhood under-nutrition and environmental enteric dysfunction are associated with biomarkers of inflammation (Victora et al., 2021). In Tanzania, adoption of a “western” diet in association with urbanization was associated with an inflammatory transcriptome in unstimulated whole blood (Temba et al., 2021). In industrialized countries, “food deserts” in impoverished communities lead to nutritional imbalances characteristic of the worst features of the “western diet.”
Mice stressed by social isolation, cage switching, or physical constraint increased their circulating IL-6 levels and became more likely to die when injected later with LPS (Qing et al., 2020). If a child’s family was poor when he/she was under 3 years of age, his/her IL-6 levels were likely to be higher when measured at age 9 (Kokosi et al., 2021). People who experienced early life adversity, including from poverty, had elevated production of inflammatory mediators and reduced responsiveness to the anti-inflammatory actions of glucocorticoids throughout their lifespans (Chen et al., 2021a).
Challenges
Despite an extensive preclinical and clinical anti-inflammatory pharmacopoeia (Dinarello, 2010; Goldfine and Shoelson, 2017; Henderson et al., 2020; Rohm et al., 2022), as yet there is no drug that abolishes nonresolving inflammation in the majority of people treated, in the sense that patients remain free of inflammation when they stop taking the drug. There is no single drug that benefits a substantial proportion of those treated for nonresolving inflammation no matter which inflammatory disease they have. Few drugs that afford substantial benefit by strongly mitigating nonresolving inflammation are free of the risk of major toxicities (e.g., Ytterberg et al., 2022). There is no way short of clinical trials to establish which of the diseases that nonresolving inflammation underpins will be most responsive to a given anti-inflammatory agent. We do not have a non-empirical basis for rationally designing combination anti-inflammatory therapies.
Though investigators have worked heroically and swiftly under difficult conditions to characterize and mitigate the lethal impact of acute inflammation in SARS-CoV-2 infection, it is still not clear how best to minimize tissue damage in COVID-19 through appropriately timed anti-inflammatory interventions that spare anti-viral responses. This challenge may fade if effective antivirals become widely available and vaccination and non-lethal infection increase population immunity.
However, another COVID-19 challenge is likely not to fade but to grow—post-acute sequelae of SARS-CoV-2 infection (PASC, or “long COVID”). PASC has reportedly afflicted 10%–30% of people who recovered from COVID-19 without hospitalization and 76% of those who were hospitalized (Phetsouphanh et al., 2022). Many of them show persistently elevated levels of IFN-β, IFN-γ, IFN-λ2/3, and IL-6 (Phetsouphanh et al., 2022). An increased risk of inflammation of the heart, pericardium, and arteries persists for at least a year after the diagnosis of COVID-19 (Xie et al., 2022). The hippocampi of some people who died from COVID-19 appeared free of virus but expressed IL-1β and IL-6 (Klein et al., 2021). Perhaps some of the neurological signs and symptoms in PASC reflect nonresolving inflammation in the central nervous system. Epstein-Barr virus infection precedes the onset of multiple sclerosis but can only be said to cause the disease in a minority of those infected (Bjornevik et al., 2022). One candidate for an additional and less widely distributed causal factor is the presence in the microbiota of strains of Clostridium perfringens that produce ε-toxin (Linden et al., 2015). We should be alert to the possibility that prior SARS-CoV-2 infection, in conjunction with the microbiota or other factors, might predispose to development of inflammatory neurodegenerative disease.
Immune checkpoint blockage has given hope to many cancer patients but left a greater number disappointed. “Cold tumors”—those lacking an IFN-γ-producing and -responding immune cell infiltrate—respond poorly to immune checkpoint blockade. Infiltration of a tumor by inflammatory cells can lead to regression (Agrawal et al., 2004; Mao et al., 2021), yet is also often associated with non-responsiveness to checkpoint blockade. What combination of intratumoral and systemic inflammatory and immune responses optimizes a patient’s chance of survival? How can we elicit the “right” form of inflammation when it is not present to begin with?
Despite these challenges, there is reason for optimism. Clinical advances pre-dating the period of focus in this review have been stunning, among them the impact of antagonists of IL-1β and TNF-α on autoinflammatory diseases, rheumatoid arthritis, and inflammatory bowel disease. The marked increase in basic research into inflammation gives hope for a knowledge roadmap that will identify practically actionable, highly effective, and safely addressable pathogenic pathways for patients suffering from atherosclerosis, obesity-related metabolic syndrome, asthma, rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus, scleroderma, non-alcoholic steatohepatitis, Alzheimer’s disease, multiple sclerosis, and other diseases in which nonresolving inflammation plays a major role. Future work will further unmask the microbiota’s storehouse of inflammation-regulating metabolites, leading to interventions based on diet, probiotics, and drugs. Electromedicine will take its place in the resolution of nonresolving inflammation refractory to molecular therapeutics.
Yet there is more we must do to reduce nonresolving inflammation that lies beyond biomedical research and the comfort zone of those who conduct it. Physicians and scientists must work to persuade voters and policymakers to act for communities and populations to prevent and reverse the degradation of neighborhoods, air, water, and climate, the sequelae of systematic discrimination, and the trans-generational perpetuation of poverty. Physicians and scientists are privileged with knowledge of the biological consequences of societal inaction. With that privilege comes a responsibility to help bring change.
Acknowledgments
Preparation of this review was supported by the Abby and Howard P. Milstein Program in Chemical Biology and Translational Medicine. The Department of Microbiology & Immunology is supported by the Randolph Hearst Trust.
Declaration of interests
The author is a co-inventor on patents related to immunoproteasome inhibitors; a scientific co-founder and equity holder in IpiNovyx, Inc; a member of the National Therapeutic Areas scientific advisory board for Pfizer External Sciences & Innovation; and a member or the scientific advisory board of Leap Therapeutics.
References
- Ablasser A., Chen Z.J. cGAS in action: Expanding roles in immunity and inflammation. Science. 2019;363:eaat8657. doi: 10.1126/science.aat8657. [DOI] [PubMed] [Google Scholar]
- Advani R., Flinn I., Popplewell L., Forero A., Bartlett N.L., Ghosh N., Kline J., Roschewski M., LaCasce A., Collins G.P., et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirman G., Yu K.B., Hsiao E.Y. Signaling inflammation across the gut-brain axis. Science. 2021;374:1087–1092. doi: 10.1126/science.abi6087. [DOI] [PubMed] [Google Scholar]
- Agrawal N., Bettegowda C., Cheong I., Geschwind J.F., Drake C.G., Hipkiss E.L., Tatsumi M., Dang L.H., Diaz L.A., Jr., Pomper M., et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc. Natl. Acad. Sci. USA. 2004;101:15172–15177. doi: 10.1073/pnas.0406242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ah Kioon M.D., Pierides M., Pannellini T., Lin G., Nathan C.F., Barrat F.J. Noncytotoxic Inhibition of the Immunoproteasome Regulates Human Immune Cells In Vitro and Suppresses Cutaneous Inflammation in the Mouse. J. Immunol. 2021;206:1631–1641. doi: 10.4049/jimmunol.2000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Maglorius Renkilaraj M.R.L., Zhang P., Meertens L., Bolze A., Materna M., et al. COVID Human Genetic Effort. COVID-STORM Clinicians. COVID Clinicians. Imagine COVID Group. French COVID Cohort Study Group. CoV-Contact Cohort. Amsterdam UMC Covid- Biobank. NIAID-USUHS COVID Study Group X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 2021;6:eabl4348. doi: 10.1126/sciimmunol.abl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avagyan S., Henninger J.E., Mannherz W.P., Mistry M., Yoon J., Yang S., Weber M.C., Moore J.L., Zon L.I. Resistance to inflammation underlies enhanced fitness in clonal hematopoiesis. Science. 2021;374:768–772. doi: 10.1126/science.aba9304. [DOI] [PubMed] [Google Scholar]
- Balestrini A., Joseph V., Dourado M., Reese R.M., Shields S.D., Rougé L., Bravo D.D., Chernov-Rogan T., Austin C.D., Chen H., et al. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J. Exp. Med. 2021;218:e20201637. doi: 10.1084/jem.20201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., Michailidis E., Hoffmann H.H., Eto S., Garcia-Prat M., et al. HGID Lab. COVID Clinicians. COVID-STORM Clinicians. NIAID Immune Response to COVID Group. NH-COVAIR Study Group. Danish CHGE. Danish Blood Donor Study. St. James’s Hospital. SARS CoV2 Interest group. French COVID Cohort Study Group. Imagine COVID-Group. Milieu Intérieur Consortium. CoV-Contact Cohort. Amsterdam UMC Covid-19. Biobank Investigators. COVID Human Genetic Effort. CONSTANCES cohort. 3C-Dijon Study. Cerba Health-Care. Etablissement du Sang study group Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci. Immunol. 2021;6:eabl4340. doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty G.L., Chiorean E.G., Fishman M.P., Saboury B., Teitelbaum U.R., Sun W., Huhn R.D., Song W., Li D., Sharp L.L., et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A. Cardiovascular Effects of Particulate Air Pollution. Annu. Rev. Med. 2022;73:393–406. doi: 10.1146/annurev-med-042220-011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornevik K., Cortese M., Healy B.C., Kuhle J., Mina M.J., Leng Y., Elledge S.J., Niebuhr D.W., Scher A.I., Munger K.L., Ascherio A. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375:296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- Blacher E., Tsai C., Litichevskiy L., Shipony Z., Iweka C.A., Schneider K.M., Chuluun B., Heller H.C., Menon V., Thaiss C.A., Andreasson K.I. Aging disrupts circadian gene regulation and function in macrophages. Nat. Immunol. 2022;23:229–236. doi: 10.1038/s41590-021-01083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander J.M., Longman R.S., Iliev I.D., Sonnenberg G.F., Artis D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017;18:851–860. doi: 10.1038/ni.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby I.C., Kinet M.J., Boda D.P., Kwan E.Y., Clancy S., Cohen J.N., Habrylo I., Lowe M.M., Pauli M., Yates A.E., et al. Early-life inflammation primes a T helper 2 cell-fibroblast niche in skin. Nature. 2021;599:667–672. doi: 10.1038/s41586-021-04044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandmaier A., Formenti S.C. The Impact of Radiation Therapy on Innate and Adaptive Tumor Immunity. Semin. Radiat. Oncol. 2020;30:139–144. doi: 10.1016/j.semradonc.2019.12.005. [DOI] [PubMed] [Google Scholar]
- Braza M.S., van Leent M.M.T., Lameijer M., Sanchez-Gaytan B.L., Arts R.J.W., Pérez-Medina C., Conde P., Garcia M.R., Gonzalez-Perez M., Brahmachary M., et al. Inhibiting Inflammation with Myeloid Cell-Specific Nanobiologics Promotes Organ Transplant Acceptance. Immunity. 2018;49:819–828.e6. doi: 10.1016/j.immuni.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiado F., Pietras E.M., Manz M.G. Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J. Exp. Med. 2021;218:e20201541. doi: 10.1084/jem.20201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caielli S., Cardenas J., de Jesus A.A., Baisch J., Walters L., Blanck J.P., Balasubramanian P., Stagnar C., Ohouo M., Hong S., et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell. 2021;184:4464–4479.e19. doi: 10.1016/j.cell.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Wang X., Li J., Yan M., Chang C.H., Kim J., Jiang J., Liao Y.P., Tseng S., Kusumoputro S., et al. NLRP3 inflammasome activation determines the fibrogenic potential of PM2.5 air pollution particles in the lung. J. Environ. Sci. (China) 2022;111:429–441. doi: 10.1016/j.jes.2021.04.021. [DOI] [PubMed] [Google Scholar]
- Casanova J.L., Abel L. Mechanisms of viral inflammation and disease in humans. Science. 2021;374:1080–1086. doi: 10.1126/science.abj7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis T., Mitroulis I., Hajishengallis G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat. Immunol. 2019;20:802–811. doi: 10.1038/s41590-019-0402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.E., Fischbach M.A., Belkaid Y. Skin microbiota-host interactions. Nature. 2018;553:427–436. doi: 10.1038/nature25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.A., LeRoy A.S., Majd M., Chen J.Y., Brown R.L., Christian L.M., Fagundes C.P. Immune and Epigenetic Pathways Linking Childhood Adversity and Health Across the Lifespan. Front. Psychol. 2021;12:788351. doi: 10.3389/fpsyg.2021.788351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Kang R., Kroemer G., Tang D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021;218:e20210518. doi: 10.1084/jem.20210518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S.R., Babes L., Rahn J.J., Ahn B.Y., Goring K.R., King J.C., Lau A., Petri B., Hao X., Chojnacki A.K., et al. Dipeptidase-1 Is an Adhesion Receptor for Neutrophil Recruitment in Lungs and Liver. Cell. 2019;178:1205–1221.e17. doi: 10.1016/j.cell.2019.07.017. [DOI] [PubMed] [Google Scholar]
- Chung A.W., Anand K., Anselme A.C., Chan A.A., Gupta N., Venta L.A., Schwartz M.R., Qian W., Xu Y., Zhang L., et al. A phase 1/2 clinical trial of the nitric oxide synthase inhibitor L-NMMA and taxane for treating chemoresistant triple-negative breast cancer. Sci. Transl. Med. 2021;13:j5070. doi: 10.1126/scitranslmed.abj5070. [DOI] [PubMed] [Google Scholar]
- Clark I.C., Gutiérrez-Vázquez C., Wheeler M.A., Li Z., Rothhammer V., Linnerbauer M., Sanmarco L.M., Guo L., Blain M., Zandee S.E.J., et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science. 2021;372:eabf1230. doi: 10.1126/science.abf1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M.A., Duncan G.S., Lin G.H.Y., Steinberg B.E., Yu L.X., Brenner D., Buckler L.N., Elia A.J., Wakeham A.C., Nieman B., et al. Choline acetyltransferase-expressing T cells are required to control chronic viral infection. Science. 2019;363:639–644. doi: 10.1126/science.aau9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crainiciuc G., Palomino-Segura M., Molina-Moreno M., Sicilia J., Aragones D.G., Li J.L.Y., Madurga R., Adrover J.M., Aroca-Crevillén A., Martin-Salamanca S., et al. Behavioural immune landscapes of inflammation. Nature. 2022;601:415–421. doi: 10.1038/s41586-021-04263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow Y.J., Stetson D.B. The type I interferonopathies: 10 years on. Nat. Rev. Immunol. 2021 doi: 10.1038/s41577-021-00633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cecco M., Ito T., Petrashen A.P., Elias A.E., Skvir N.J., Criscione S.W., Caligiana A., Brocculi G., Adney E.M., Boeke J.D., et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature. 2019;566:73–78. doi: 10.1038/s41586-018-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel Z., Khoury N., Betley M.J., Lehallier B., Willoughby D., Olsson N., Yang A.C., Hahn O., Lu N., Vest R.T., et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature. 2021;600:494–499. doi: 10.1038/s41586-021-04183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nardo D., Labzin L.I., Kono H., Seki R., Schmidt S.V., Beyer M., Xu D., Zimmer S., Lahrmann C., Schildberg F.A., et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014;15:152–160. doi: 10.1038/ni.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Poggetto E., Ho I.L., Balestrieri C., Yen E.Y., Zhang S., Citron F., Shah R., Corti D., Diaferia G.R., Li C.Y., et al. Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis. Science. 2021;373:j0486. doi: 10.1126/science.abj0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdín-Micó G., Soto-Heredero G., Aranda J.F., Oller J., Carrasco E., Gabandé-Rodríguez E., Blanco E.M., Alfranca A., Cussó L., Desco M., et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020;368:1371–1376. doi: 10.1126/science.aax0860. [DOI] [PubMed] [Google Scholar]
- Dey T., Dominici F. COVID-19, Air Pollution, and Racial Inequity: Connecting the Dots. Chem. Res. Toxicol. 2021;34:669–671. doi: 10.1021/acs.chemrestox.0c00432. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Anti-inflammatory Agents: Present and Future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A.H., Porteu F., Sanchez E., Nathan C.F. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science. 1990;248:370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- Domizio J.D., Gulen M.F., Saidoune F., Thacker V.V., Yatim A., Sharma K., Nass T., Guenova E., Schaller M., Conrad C., et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022;603:145–151. doi: 10.1038/s41586-022-04421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z., Ghosh K., Vizioli M.G., Zhu J., Sen P., Wangensteen K.J., Simithy J., Lan Y., Lin Y., Zhou Z., et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gayar S., Thüring-Nahler H., Pfeilschifter J., Röllinghoff M., Bogdan C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J. Immunol. 2003;171:4561–4568. doi: 10.4049/jimmunol.171.9.4561. [DOI] [PubMed] [Google Scholar]
- Faas M., Ipseiz N., Ackermann J., Culemann S., Grüneboom A., Schröder F., Rothe T., Scholtysek C., Eberhardt M., Böttcher M., et al. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity. 2021;54:2531–2546.e5. doi: 10.1016/j.immuni.2021.09.010. [DOI] [PubMed] [Google Scholar]
- Frontera A., Cianfanelli L., Vlachos K., Landoni G., Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: The “double-hit” hypothesis. J. Infect. 2020;81:255–259. doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W., et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J., Goldstein I., Snyderman R., editors. Inflammation: Basic Principles and Clinical Correlates. Raven Press; NY: 1988. [Google Scholar]
- Goldfine A.B., Shoelson S.E. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J. Clin. Invest. 2017;127:83–93. doi: 10.1172/JCI88884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber C.N., Patel R.S., Trachtman R., Lepow L., Amanat F., Krammer F., Wilson K.M., Onel K., Geanon D., Tuballes K., et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C) Cell. 2020;183:982–995.e14. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald M., Kumar S., Sharife H., Volinsky E., Gileles-Hillel A., Licht T., Permyakova A., Hinden L., Azar S., Friedmann Y., et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. 2021;373:eabc8479. doi: 10.1126/science.abc8479. [DOI] [PubMed] [Google Scholar]
- He Y.M., Li X., Perego M., Nefedova Y., Kossenkov A.V., Jensen E.A., Kagan V., Liu Y.F., Fu S.Y., Ye Q.J., et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat. Med. 2018;24:224–231. doi: 10.1038/nm.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson N.C., Rieder F., Wynn T.A. Fibrosis: from mechanisms to medicines. Nature. 2020;587:555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezaveh K., Shinde R.S., Klötgen A., Halaby M.J., Lamorte S., Ciudad M.T., Quevedo R., Neufeld L., Liu Z.Q., Jin R., et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55:324–340.e8. doi: 10.1016/j.immuni.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T.N., Pino M., Boddapati A.K., Viox E.G., Starke C.E., Upadhyay A.A., Gumber S., Nekorchuk M., Busman-Sahay K., Strongin Z., et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184:460–475.e21. doi: 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Wang Z.M., Feng Y., Schizas M., Hoyos B.E., van der Veeken J., Verter J.G., Bou-Puerto R., Rudensky A.Y. Regulatory T cells function in established systemic inflammation and reverse fatal autoimmunity. Nat. Immunol. 2021;22:1163–1174. doi: 10.1038/s41590-021-01001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries F., Shmuel-Galia L., Ketelut-Carneiro N., Li S., Wang B., Nemmara V.V., Wilson R., Jiang Z., Khalighinejad F., Muneeruddin K., et al. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369:1633–1637. doi: 10.1126/science.abb9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B.G., Lindsley R.C., Mermel C.H., Burtt N., Chavez A., et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J.M., Sable R., Ronzetti M., Bautista W., Knotts Z., Abisoye-Ogunniyan A., Li D., Calvo R., Dashnyam M., Singh A., et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci. Transl. Med. 2020;12:eaax6337. doi: 10.1126/scitranslmed.aax6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabata H., Artis D. Neuro-immune crosstalk and allergic inflammation. J. Clin. Invest. 2019;129:1475–1482. doi: 10.1172/JCI124609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerkar S., Leng C., Burenkova O., Jang S.C., McCoy C., Zhang K., Dooley K., Kasera S., Zi T., Sisó S., et al. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci. Adv. 2022;8:j7002. doi: 10.1126/sciadv.abj7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Tang H., Jiang L., Wang S., Wang X., Chen Z., Zhang L., Zheng C., Wang Z., Huang G., Gao R., China CVD study investigators Air temperature variability and high-sensitivity C reactive protein in a general population of China. Sci. Total Environ. 2020;749:141588. doi: 10.1016/j.scitotenv.2020.141588. [DOI] [PubMed] [Google Scholar]
- Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.K.S., et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184:149–168.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N., Kornfeld O.S., Lee B.L., Stowe I.B., O’Rourke K., Li Q., Sandoval W., Yan D., Kang J., Xu M., et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591:131–136. doi: 10.1038/s41586-021-03218-7. [DOI] [PubMed] [Google Scholar]
- Kerur N., Fukuda S., Banerjee D., Kim Y., Fu D., Apicella I., Varshney A., Yasuma R., Fowler B.J., Baghdasaryan E., et al. cGAS drives noncanonical-inflammasome activation in age-related macular degeneration. Nat. Med. 2018;24:50–61. doi: 10.1038/nm.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Nguyen Q.T., Lee J., Lee S.H., Janocha A., Kim S., Le H.T., Dvorina N., Weiss K., Cameron M.J., et al. Anti-inflammatory Roles of Glucocorticoids Are Mediated by Foxp3+ Regulatory T Cells via a miR-342-Dependent Mechanism. Immunity. 2020;53:581–596.e5. doi: 10.1016/j.immuni.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Paik D., Ramirez R.N., Biggs D.G., Park Y., Kwon H.K., Choi G.B., Huh J.R. Maternal gut bacteria drive intestinal inflammation in offspring with neurodevelopmental disorders by altering the chromatin landscape of CD4+ T cells. Immunity. 2022;55:145–158.e7. doi: 10.1016/j.immuni.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk C.J., Muchamuel T., Wang J., Fan R.A. Discovery and Early Clinical Development of Selective Immunoproteasome Inhibitors. Cells. 2021;11:9. doi: 10.3390/cells11010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Soung A., Sissoko C., Nordvig A., Canoll P., Mariani M., Jiang X., Bricker T., Goldman J., Rosoklija G., et al. COVID-19 induces neuroinflammation and loss of hippocampal neurogenesis. Preprint at Res Sq. 2021 doi: 10.21203/rs.3.rs-1031824/v1. [DOI] [Google Scholar]
- Koeken V.A., de Bree L.C.J., Mourits V.P., Moorlag S.J., Walk J., Cirovic B., Arts R.J., Jaeger M., Dijkstra H., Lemmers H., et al. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. J. Clin. Invest. 2020;130:5591–5602. doi: 10.1172/JCI133935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokosi T., Flouri E., Midouhas E. The role of inflammation in the association between poverty and working memory in childhood. Psychoneuroendocrinology. 2021;123:105040. doi: 10.1016/j.psyneuen.2020.105040. [DOI] [PubMed] [Google Scholar]
- Koprivica I., Djedovic N., Stojanović I., Miljković Đ. Ethyl pyruvate, a versatile protector in inflammation and autoimmunity. Inflamm. Res. 2022;71:169–182. doi: 10.1007/s00011-021-01529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren T., Yifa R., Amer M., Krot M., Boshnak N., Ben-Shaanan T.L., Azulay-Debby H., Zalayat I., Avishai E., Hajjo H., et al. Insular cortex neurons encode and retrieve specific immune responses. Cell. 2021;184:5902–5915.e17. doi: 10.1016/j.cell.2021.10.013. [DOI] [PubMed] [Google Scholar]
- Körner A., Bernard A., Fitzgerald J.C., Alarcon-Barrera J.C., Kostidis S., Kaussen T., Giera M., Mirakaj V. Sema7A is crucial for resolution of severe inflammation. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2017527118. e2017527118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka A., Ishibashi K., Nagato T., Kitamura H., Fujiwara Y., Yasuda S., Nagata M., Harabuchi S., Hayashi R., Yajima Y., et al. CD47 blockade enhances the efficacy of intratumoral STING-targeting therapy by activating phagocytes. J. Exp. Med. 2021;218 doi: 10.1084/jem.20200792. e20200792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzelis I., Li X., Mitroulis I., Grosser D., Kajikawa T., Wang B., Grzybek M., von Renesse J., Czogalla A., Troullinaki M., et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat. Immunol. 2019;20:40–49. doi: 10.1038/s41590-018-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautkramer K.A., Fan J., Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021;19:77–94. doi: 10.1038/s41579-020-0438-4. [DOI] [PubMed] [Google Scholar]
- Lai J.J., Cruz F.M., Rock K.L. Immune Sensing of Cell Death through Recognition of Histone Sequences by C-Type Lectin-Receptor-2d Causes Inflammation and Tissue Injury. Immunity. 2020;52:123–135.e6. doi: 10.1016/j.immuni.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K.C., Araya R.E., Huang A., Chen Q., Di Modica M., Rodrigues R.R., Lopès A., Johnson S.B., Schwarz B., Bohrnsen E., et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184:5338–5356.e21. doi: 10.1016/j.cell.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas B., Hernandez-Galan L., Galipeau H.J., Constante M., Clarizio A., Jury J., Breyner N.M., Caminero A., Rueda G., Hayes C.L., et al. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci. Transl. Med. 2020;12:eaba0624. doi: 10.1126/scitranslmed.aba0624. [DOI] [PubMed] [Google Scholar]
- Li T., Chen Z.J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018;215:1287–1299. doi: 10.1084/jem.20180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xiao Y., Li Q., Yao J., Yuan X., Zhang Y., Yin X., Saito Y., Fan H., Li P., et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell. 2022;40:36–52.e9. doi: 10.1016/j.ccell.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ebert B.L. CHIP (Clonal Hematopoiesis of Indeterminate Potential): Potent and Newly Recognized Contributor to Cardiovascular Risk. Circulation. 2018;138:666–668. doi: 10.1161/CIRCULATIONAHA.118.034392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K.T., Sporn M.B. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol. Rev. 2012;64:972–1003. doi: 10.1124/pr.111.004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A.I., McFadden T., Link V.M., Han S.J., Karlsson R.M., Stacy A., Farley T.K., Lima-Junior D.S., Harrison O.J., Desai J.V., et al. Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Science. 2021;373:eabf3002. doi: 10.1126/science.abf3002. [DOI] [PubMed] [Google Scholar]
- Lima-Junior D.S., Krishnamurthy S.R., Bouladoux N., Collins N., Han S.J., Chen E.Y., Constantinides M.G., Link V.M., Lim A.I., Enamorado M., et al. Endogenous retroviruses promote homeostatic and inflammatory responses to the microbiota. Cell. 2021;184:3794–3811.e19. doi: 10.1016/j.cell.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J.R., Ma Y., Zhao B., Harris J.M., Rumah K.R., Schaeren-Wiemers N., Vartanian T. Clostridium perfringens Epsilon Toxin Causes Selective Death of Mature Oligodendrocytes and Central Nervous System Demyelination. MBio. 2015;6:e02513. doi: 10.1128/mBio.02513-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Liao Q., Wen H., Zhang Y. Disease modifying therapies in relapsing-remitting multiple sclerosis: A systematic review and network meta-analysis. Autoimmun. Rev. 2021;20:102826. doi: 10.1016/j.autrev.2021.102826. [DOI] [PubMed] [Google Scholar]
- Lorenzo C., Delgado P., Busse C.E., Sanz-Bravo A., Martos-Folgado I., Bonzon-Kulichenko E., Ferrarini A., Gonzalez-Valdes I.B., Mur S.M., Roldán-Montero R., et al. ALDH4A1 is an atherosclerosis auto-antigen targeted by protective antibodies. Nature. 2021;589:287–292. doi: 10.1038/s41586-020-2993-2. [DOI] [PubMed] [Google Scholar]
- Luan H.H., Wang A., Hilliard B.K., Carvalho F., Rosen C.E., Ahasic A.M., Herzog E.L., Kang I., Pisani M.A., Yu S., et al. GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell. 2019;178:1231–1244.e11. doi: 10.1016/j.cell.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Sahu S.K., Cano M., Kuppuswamy V., Bajwa J., McPhatter J., Pine A., Meizlish M., Goshua G., Chang C.H., et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Preprint at bioRxiv. 2021 doi: 10.1101/2021.02.22.432177. 2021.02.22.432177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C., Xu X., Ding Y., Xu N. Optimization of BCG Therapy Targeting Neutrophil Extracellular Traps, Autophagy, and miRNAs in Bladder Cancer: Implications for Personalized Medicine. Front. Med. (Lausanne) 2021;8:735590. doi: 10.3389/fmed.2021.735590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masetti M., Carriero R., Portale F., Marelli G., Morina N., Pandini M., Iovino M., Partini B., Erreni M., Ponzetta A., et al. Lipid-loaded tumor-associated macrophages sustain tumor growth and invasiveness in prostate cancer. J. Exp. Med. 2022;219:e20210564. doi: 10.1084/jem.20210564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374:1070–1075. doi: 10.1126/science.abi5200. [DOI] [PubMed] [Google Scholar]
- Megli C.J., Coyne C.B. Infections at the maternal-fetal interface: an overview of pathogenesis and defence. Nat. Rev. Microbiol. 2022;20:67–82. doi: 10.1038/s41579-021-00610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A., Wu X., Keller J.L., Fassler C.S., Apewokin S., Mersha T.B., Xie C., Pinney S.M. Long-term exposure to fine particulate matter and hospitalization in COVID-19 patients. Respir. Med. 2021;178:106313. doi: 10.1016/j.rmed.2021.106313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilenko D.A., Shpynov O., Andhey P.S., Arthur L., Swain A., Esaulova E., Brioschi S., Shchukina I., Kerndl M., Bambouskova M., et al. Comprehensive Profiling of an Aging Immune System Reveals Clonal GZMK+ CD8+ T Cells as Conserved Hallmark of Inflammaging. Immunity. 2021;54:99–115.e12. doi: 10.1016/j.immuni.2020.11.005. [DOI] [PubMed] [Google Scholar]
- Moreau J.M., Dhariwala M.O., Gouirand V., Boda D.P., Boothby I.C., Lowe M.M., Cohen J.N., Macon C.E., Leech J.M., Kalekar L.A., et al. Regulatory T cells promote innate inflammation after skin barrier breach via TGF-β activation. Sci. Immunol. 2021;6:eabg2329. doi: 10.1126/sciimmunol.abg2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama S., Brestoff J.R., Flamar A.L., Moeller J.B., Klose C.S.N., Rankin L.C., Yudanin N.A., Monticelli L.A., Putzel G.G., Rodewald H.R., Artis D. β2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359:1056–1061. doi: 10.1126/science.aan4829. [DOI] [PubMed] [Google Scholar]
- Nagashima H., Mahlakõiv T., Shih H.Y., Davis F.P., Meylan F., Huang Y., Harrison O.J., Yao C., Mikami Y., Urban J.F., Jr., et al. Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation. Immunity. 2019;51:682–695.e6. doi: 10.1016/j.immuni.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S., Larsen S.B., Gomez N.C., Alaverdyan K., Sendoel A., Yuan S., Polak L., Kulukian A., Chai S., Fuchs E. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature. 2017;550:475–480. doi: 10.1038/nature24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Nathan C. Rethinking immunology. Science. 2021;373:276–277. doi: 10.1126/science.abj5637. [DOI] [PubMed] [Google Scholar]
- Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Netea M.G., Balkwill F., Chonchol M., Cominelli F., Donath M.Y., Giamarellos-Bourboulis E.J., Golenbock D., Gresnigt M.S., Heneka M.T., Hoffman H.M., et al. A guiding map for inflammation. Nat. Immunol. 2017;18:826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudecker V., Haneklaus M., Jensen O., Khailova L., Masterson J.C., Tye H., Biette K., Jedlicka P., Brodsky K.S., Gerich M.E., et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J. Exp. Med. 2017;214:1737–1752. doi: 10.1084/jem.20160462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K., Dixit V.M., Kayagaki N. Dying cells fan the flames of inflammation. Science. 2021;374:1076–1080. doi: 10.1126/science.abi5934. [DOI] [PubMed] [Google Scholar]
- Niec R.E., Rudensky A.Y., Fuchs E. Inflammatory adaptation in barrier tissues. Cell. 2021;184:3361–3375. doi: 10.1016/j.cell.2021.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell-Tormey J., Nathan C.F., Lanks K., DeBoer C.J., de la Harpe J. Secretion of pyruvate. An antioxidant defense of mammalian cells. J. Exp. Med. 1987;165:500–514. doi: 10.1084/jem.165.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning P., Lien E., Fitzgerald K.A. Gasdermins and their role in immunity and inflammation. J. Exp. Med. 2019;216:2453–2465. doi: 10.1084/jem.20190545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccoud R., Saint-Laurent C., Piccolo E., Tajan M., Dortignac A., Pereira O., Le Gonidec S., Baba I., Gélineau A., Askia H., et al. SHP2 drives inflammation-triggered insulin resistance by reshaping tissue macrophage populations. Sci. Transl. Med. 2021;13:eabe2587. doi: 10.1126/scitranslmed.abe2587. [DOI] [PubMed] [Google Scholar]
- Patel D.F., Peiró T., Bruno N., Vuononvirta J., Akthar S., Puttur F., Pyle C.J., Suveizdytė K., Walker S.A., Singanayagam A., et al. Neutrophils restrain allergic airway inflammation by limiting ILC2 function and monocyte-dendritic cell antigen presentation. Sci. Immunol. 2019;4:eaax7006. doi: 10.1126/sciimmunol.aax7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriki D., Saravi S.S.S., Camici G.G., Liberale L., Beer J.H. PCSK 9: A Link Between Inflammation and Atherosclerosis. Curr. Med. Chem. 2022;29:251–267. doi: 10.2174/0929867328666210707192625. [DOI] [PubMed] [Google Scholar]
- Pavlov V.A., Chavan S.S., Tracey K.J. Molecular and Functional Neuroscience in Immunity. Annu. Rev. Immunol. 2018;36:783–812. doi: 10.1146/annurev-immunol-042617-053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A., Chavan S.S., Tracey K.J. Bioelectronic Medicine: From Preclinical Studies on the Inflammatory Reflex to New Approaches in Disease Diagnosis and Treatment. Cold Spring Harb. Perspect. Med. 2020;10:a034140. doi: 10.1101/cshperspect.a034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace C.G., O’Neill L.A. The role of itaconate in host defense and inflammation. J. Clin. Invest. 2022;132:e148548. doi: 10.1172/JCI148548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C.J., Muñoz-Rojas A.R., Meeth K.M., Kellman L.N., Amezquita R.A., Thakral D., Du V.Y., Wang J.X., Damsky W., Kuhlmann A.L., et al. Myeloid-targeted immunotherapies act in synergy to induce inflammation and antitumor immunity. J. Exp. Med. 2018;215:877–893. doi: 10.1084/jem.20171435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phetsouphanh C., Darley D.R., Wilson D.B., Howe A., Munier C.M.L., Patel S.K., Juno J.A., Burrell L.M., Kent S.J., Dore G.J., et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022;23:210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- Plant T., Eamsamarng S., Sanchez-Garcia M.A., Reyes L., Renshaw S.A., Coelho P., Mirchandani A.S., Morgan J.M., Ellett F.E., Morrison T., et al. Semaphorin 3F signaling actively retains neutrophils at sites of inflammation. J. Clin. Invest. 2020;130:3221–3237. doi: 10.1172/JCI130834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podstawka J., Sinha S., Hiroki C.H., Sarden N., Granton E., Labit E., Kim J.H., Andonegui G., Lou Y., Snarr B.D., et al. Marginating transitional B cells modulate neutrophils in the lung during inflammation and pneumonia. J. Exp. Med. 2021;218:e20210409. doi: 10.1084/jem.20210409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzer A., Dominici F., Haines A., Witt C., Münzel T., Lelieveld J. Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc. Res. 2020;116:2247–2253. doi: 10.1093/cvr/cvaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priem B., van Leent M.M.T., Teunissen A.J.P., Sofias A.M., Mourits V.P., Willemsen L., Klein E.D., Oosterwijk R.S., Meerwaldt A.E., Munitz J., et al. Trained Immunity-Promoting Nanobiologic Therapy Suppresses Tumor Growth and Potentiates Checkpoint Inhibition. Cell. 2020;183:786–801.e19. doi: 10.1016/j.cell.2020.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing H., Desrouleaux R., Israni-Winger K., Mineur Y.S., Fogelman N., Zhang C., Rashed S., Palm N.W., Sinha R., Picciotto M.R., et al. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell. 2020;182:372–387.e14. doi: 10.1016/j.cell.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues T.S., de Sá K.S.G., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., Gonçalves A.V., Perucello D.B., Andrade W.A., Castro R., et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021;218:e20201707. doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohm T.V., Meier D.T., Olefsky J.M., Donath M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquilly A., Jacqueline C., Davieau M., Mollé A., Sadek A., Fourgeux C., Rooze P., Broquet A., Misme-Aucouturier B., Chaumette T., et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 2020;21:636–648. doi: 10.1038/s41590-020-0673-x. [DOI] [PubMed] [Google Scholar]
- Rosen D.A., Seki S.M., Fernández-Castañeda A., Beiter R.M., Eccles J.D., Woodfolk J.A., Gaultier A. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 2019;11:eaau5266. doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossaint J., Thomas K., Mersmann S., Skupski J., Margraf A., Tekath T., Jouvene C.C., Dalli J., Hidalgo A., Meuth S.G., et al. Platelets orchestrate the resolution of pulmonary inflammation in mice by T reg cell repositioning and macrophage education. J. Exp. Med. 2021;218:e20201353. doi: 10.1084/jem.20201353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosshart S.P., Herz J., Vassallo B.G., Hunter A., Wall M.K., Badger J.H., McCulloch J.A., Anastasakis D.G., Sarshad A.A., Leonardi I., et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science. 2019;365:eaaw4361. doi: 10.1126/science.aaw4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A.J., Vasudevan S.O., Méndez-Huergo S.P., Kumari P., Menoret A., Duduskar S., Wang C., Pérez Sáez J.M., Fettis M.M., Li C., et al. Intracellular immune sensing promotes inflammation via gasdermin D-driven release of a lectin alarmin. Nat. Immunol. 2021;22:154–165. doi: 10.1038/s41590-020-00844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed S., Quintin J., Kerstens H.H., Rao N.A., Aghajanirefah A., Matarese F., Cheng S.C., Ratter J., Berentsen K., van der Ent M.A., et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmarco L.M., Wheeler M.A., Gutiérrez-Vázquez C., Polonio C.M., Linnerbauer M., Pinho-Ribeiro F.A., Li Z., Giovannoni F., Batterman K.V., Scalisi G., et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature. 2021;590:473–479. doi: 10.1038/s41586-020-03116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma W., Basterra L.B., Jacobs S., Brouwer N., Meerlo P., Schaafsma A., Boddeke E.W.G.M., Eggen B.J.L. Maternal inflammation induces immune activation of fetal microglia and leads to disrupted microglia immune responses, behavior, and learning performance in adulthood. Neurobiol. Dis. 2017;106:291–300. doi: 10.1016/j.nbd.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Schlegel M., Körner A., Kaussen T., Knausberg U., Gerber C., Hansmann G., Jónasdóttir H.S., Giera M., Mirakaj V. Inhibition of neogenin fosters resolution of inflammation and tissue regeneration. J. Clin. Invest. 2019;129:2165. doi: 10.1172/JCI128681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T., Ito M., Morita R., Komai K., Noguchi Y., Ooboshi H., Koshida R., Takahashi S., Kodama T., Yoshimura A. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat. Med. 2017;23:723–732. doi: 10.1038/nm.4312. [DOI] [PubMed] [Google Scholar]
- Shin Yim Y., Park A., Berrios J., Lafourcade M., Pascual L.M., Soares N., Yeon Kim J., Kim S., Kim H., Waisman A., et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature. 2017;549:482–487. doi: 10.1038/nature23909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre-Roig C., Braster Q., Wichapong K., Lee E.Y., Teulon J.M., Berrebeh N., Winter J., Adrover J.M., Santos G.S., Froese A., et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 2019;569:236–240. doi: 10.1038/s41586-019-1167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliter D.A., Martinez J., Hao L., Chen X., Sun N., Fischer T.D., Burman J.L., Li Y., Zhang Z., Narendra D.P., et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 2018;561:258–262. doi: 10.1038/s41586-018-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro O., Youm Y., Shchukina I., Ryu S., Sidorov S., Ravussin A., Nguyen K., Aladyeva E., Predeus A.N., Smith S.R., et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science. 2022;375:671–677. doi: 10.1126/science.abg7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C.W., Karim S.A., Leach J.D.G., Bailey P., Upstill-Goddard R., Rishi L., Foth M., Bryson S., McDaid K., Wilson Z., et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2016;29:832–845. doi: 10.1016/j.ccell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Kees T., Almeida A.S., Liu B., He X.Y., Ng D., Han X., Spector D.L., McNeish I.A., Gimotty P., et al. Activating a collaborative innate-adaptive immune response to control metastasis. Cancer Cell. 2021;39:1361–1374.e9. doi: 10.1016/j.ccell.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temba G.S., Kullaya V., Pecht T., Mmbaga B.T., Aschenbrenner A.C., Ulas T., Kibiki G., Lyamuya F., Boahen C.K., Kumar V., et al. Urban living in healthy Tanzanians is associated with an inflammatory status driven by dietary and metabolic changes. Nat. Immunol. 2021;22:287–300. doi: 10.1038/s41590-021-00867-8. [DOI] [PubMed] [Google Scholar]
- Thompson D.J., Genovese G., Halvardson J., Ulirsch J.C., Wright D.J., Terao C., Davidsson O.B., Day F.R., Sulem P., Jiang Y., et al. International Lung Cancer Consortium (INTEGRAL-ILCCO) Breast Cancer Association Consortium. Consortium of Investigators of Modifiers of BRCA1/2. Endometrial Cancer Association Consortium. Ovarian Cancer Association Consortium. Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) Consortium. Kidney Cancer GWAS Meta-Analysis Project. eQTLGen Consortium. Biobank-based Integrative Omics Study (BIOS) Consortium. 23andMe Research Team Genetic predisposition to mosaic Y chromosome loss in blood. Nature. 2019;575:652–657. doi: 10.1038/s41586-019-1765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Trowbridge J.J., Starczynowski D.T. Innate immune pathways and inflammation in hematopoietic aging, clonal hematopoiesis, and MDS. J. Exp. Med. 2021;218:e20201544. doi: 10.1084/jem.20201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler P.M., Bucklin M.L., Zhao M., Maher T.J., Rice A.J., Ji W., Warner N., Pan J., Morotti R., McCarthy P., et al. Human autoinflammatory disease reveals ELF4 as a transcriptional regulator of inflammation. Nat. Immunol. 2021;22:1118–1126. doi: 10.1038/s41590-021-00984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetité D., Tavares L.A., Paiva I.M., et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., Christian P., Vidaletti L.P., Gatica-Domínguez G., Menon P., Black R.E. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. 2021;397:1388–1399. doi: 10.1016/S0140-6736(21)00394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue A.T., McCright S.J., Wright J.M., Jimenez M.T., Mowel W.K., Kotzin J.J., Joannas L., Basavappa M.G., Spencer S.P., Clark M.L., et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci. Transl. Med. 2019;11:eaav1892. doi: 10.1126/scitranslmed.aav1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visnes T., Cázares-Körner A., Hao W., Wallner O., Masuyer G., Loseva O., Mortusewicz O., Wiita E., Sarno A., Manoilov A., et al. Small-molecule inhibitor of OGG1 suppresses proinflammatory gene expression and inflammation. Science. 2018;362:834–839. doi: 10.1126/science.aar8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vítek L., Tiribelli C. Bilirubin, Intestinal Integrity, the Microbiome, and Inflammation. N. Engl. J. Med. 2020;383:684–686. doi: 10.1056/NEJMcibr2013250. [DOI] [PubMed] [Google Scholar]
- von Hundelshausen P., Agten S.M., Eckardt V., Blanchet X., Schmitt M.M., Ippel H., Neideck C., Bidzhekov K., Leberzammer J., Wichapong K., et al. Chemokine interactome mapping enables tailored intervention in acute and chronic inflammation. Sci. Transl. Med. 2017;9:eaah6650. doi: 10.1126/scitranslmed.aah6650. [DOI] [PubMed] [Google Scholar]
- Vora S.M., Lieberman J., Wu H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021;21:694–703. doi: 10.1038/s41577-021-00588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Li H., Wu J., Cai Z.Y., Li B., Ni H., Qiu X., Chen H., Liu W., Yang Z.H., et al. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature. 2020;580:386–390. doi: 10.1038/s41586-020-2127-x. [DOI] [PubMed] [Google Scholar]
- Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., et al. Yale IMPACT Team Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- Wang L., Crackower M.A., Wu H. Advances toward structure-based drug discovery for inflammasome targets. J. Exp. Med. 2022;219:e20211147. doi: 10.1084/jem.20211147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf K., Ring A.M., Ho C.C., Volkmer J.P., Levin A.M., Volkmer A.K., Ozkan E., Fernhoff N.B., van de Rijn M., Weissman I.L., Garcia K.C. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeln A.C., Degenhardt K., Kaurani L., Gertig M., Ulas T., Jain G., Wagner J., Häsler L.M., Wild K., Skodras A., et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. 2018;556:332–338. doi: 10.1038/s41586-018-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M.A., Clark I.C., Tjon E.C., Li Z., Zandee S.E.J., Couturier C.P., Watson B.R., Scalisi G., Alkwai S., Rothhammer V., et al. MAFG-driven astrocytes promote CNS inflammation. Nature. 2020;578:593–599. doi: 10.1038/s41586-020-1999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wypych T.P., Pattaroni C., Perdijk O., Yap C., Trompette A., Anderson D., Creek D.J., Harris N.L., Marsland B.J. Microbial metabolism of L-tyrosine protects against allergic airway inflammation. Nat. Immunol. 2021;22:279–286. doi: 10.1038/s41590-020-00856-3. [DOI] [PubMed] [Google Scholar]
- Xie Q.W., Cho H.J., Calaycay J., Mumford R.A., Swiderek K.M., Lee T.D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Zeng Q., Silverman H.A., Gunasekaran M., George S.J., Devarajan A., Addorisio M.E., Li J., Tsaava T., Shah V., et al. HMGB1 released from nociceptors mediates inflammation. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2102034118. e2102034118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C., Ackermann S., Ma Z., Mohanta S.K., Zhang C., Li Y., Nietzsche S., Westermann M., Peng L., Hu D., et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat. Med. 2019;25:496–506. doi: 10.1038/s41591-018-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ytterberg S.R., Bhatt D.L., Mikuls T.R., Koch G.G., Fleischmann R., Rivas J.L., Germino R., Menon S., Sun Y., Wang C., et al. ORAL Surveillance Investigators Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022;386:316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang Y., Yuan J., Li N., Pei S., Xu J., Luo X., Mao C., Liu J., Yu T., et al. Macrophage/microglial Ezh2 facilitates autoimmune inflammation through inhibition of Socs3. J. Exp. Med. 2018;215:1365–1382. doi: 10.1084/jem.20171417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. COVID-STORM Clinicians. COVID Clinicians. Imagine COVID Group. French COVID Cohort Study Group. CoV-Contact Cohort. Amsterdam UMC Covid-19 Biobank. COVID Human Genetic Effort. NIAID-USUHS/TAGC COVID Immunity Group Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R., Tao L., Jian H., Chang Y., Cheng Y., Feng Y., Zhang H. NLRP3 inflammasome activation and lung fibrosis caused by airborne fine particulate matter. Ecotoxicol. Environ. Saf. 2018;163:612–619. doi: 10.1016/j.ecoenv.2018.07.076. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Liang S., Sanchez-Lopez E., He F., Shalapour S., Lin X.J., Wong J., Ding S., Seki E., Schnabl B., et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018;560:198–203. doi: 10.1038/s41586-018-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Romano K.A., Li L., Buffa J.A., Sangwan N., Prakash P., Tittle A.N., Li X.S., Fu X., Androjna C., et al. Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe. 2021;29:1199–1208.e5. doi: 10.1016/j.chom.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach B.W., Grant L., McCluskey R.T. Academic Press; New York: 1965. The Inflammatory Process. [Google Scholar]