Key Points
Question
Does a home-based, walking exercise behavior change intervention delivered by physical therapists improve walking capacity compared with usual care in adults with peripheral artery disease (PAD) and intermittent claudication?
Findings
In this randomized clinical trial that included 190 participants with intermittent claudication due to PAD, receipt of the intervention, compared with usual care, resulted in a statistically significant adjusted difference in mean 6-minute walk distance of 16.7 m at 3 months.
Meaning
Among adults with PAD, a home-based, walking exercise behavior change intervention, compared with usual care, increased 6-minute walking distance at 3 months.
Abstract
Importance
Home-based walking exercise interventions are recommended for people with peripheral artery disease (PAD), but evidence of their efficacy has been mixed.
Objective
To investigate the effect of a home-based, walking exercise behavior change intervention delivered by physical therapists in adults with PAD and intermittent claudication compared with usual care.
Design, Setting, and Participants
Multicenter randomized clinical trial including 190 adults with PAD and intermittent claudication in 6 hospitals in the United Kingdom between January 2018 and March 2020; final follow-up was September 8, 2020.
Interventions
Participants were randomized to receive a walking exercise behavior change intervention delivered by physical therapists trained to use a motivational approach (n = 95) or usual care (n = 95).
Main Outcomes and Measures
The primary outcome was 6-minute walking distance at 3-month follow-up (minimal clinically important difference, 8-20 m). There were 8 secondary outcomes, 3 of which were the Walking Estimated Limitation Calculated by History (WELCH) questionnaire (score range, 0 [best performance] to 100), the Brief Illness Perceptions Questionnaire (score range, 0 to 80 [80 indicates negative perception of illness]), and the Theory of Planned Behavior Questionnaire (score range, 3 to 21 [21 indicates best attitude, subjective norms, perceived behavioral control, or intentions]); a minimal clinically important difference was not defined for these instruments.
Results
Among 190 randomized participants (mean age 68 years, 30% women, 79% White race, mean baseline 6-minute walking distance, 361.0 m), 148 (78%) completed 3-month follow-up. The 6-minute walking distance changed from 352.9 m at baseline to 380.6 m at 3 months in the intervention group and from 369.8 m to 372.1 m in the usual care group (adjusted mean between-group difference, 16.7 m [95% CI, 4.2 m to 29.2 m]; P = .009). Of the 8 secondary outcomes, 5 were not statistically significant. At 6-month follow-up, baseline WELCH scores changed from 18.0 to 27.8 in the intervention group and from 20.7 to 20.7 in the usual care group (adjusted mean between-group difference, 7.4 [95% CI, 2.5 to 12.3]; P = .003), scores on the Brief Illness Perceptions Questionnaire changed from 45.7 to 38.9 in the intervention group and from 44.0 to 45.8 in the usual care group (adjusted mean between-group difference, −6.6 [95% CI, −9.9 to −3.4]; P < .001), and scores on the attitude component of the Theory of Planned Behavior Questionnaire changed from 14.7 to 15.4 in the intervention group and from 14.6 to 13.9 in the usual care group (adjusted mean between-group difference, 1.4 [95% CI, 0.3 to 2.5]; P = .02). Thirteen serious adverse events occurred in the intervention group, compared with 3 in the usual care group. All were determined to be unrelated or unlikely to be related to the study.
Conclusions and Relevance
Among adults with PAD and intermittent claudication, a home-based, walking exercise behavior change intervention, compared with usual care, resulted in improved walking distance at 3 months. Further research is needed to determine the durability of these findings.
Trial Registrations
ISRCTN Identifier: 14501418; ClinicalTrials.gov Identifier: NCT03238222
Among adults with peripheral artery disease and intermittent claudication, this randomized clinical trial compares walking capacity at 3 months between patients who received a home-based, walking exercise behavior change intervention led by a physical therapist vs usual care.
Introduction
Lower extremity peripheral artery disease (PAD) is associated with reduced walking capacity and an increased risk of cardiovascular morbidity and mortality.1 Supervised exercise therapy is recommended to improve walking capacity in people with PAD, but participation rates are low.2,3 Barriers to participation include lack of time, requirements for transportation to supervised exercise sessions, motivation, and resources.2,4,5 Home-based exercise behavior change interventions that include regular support from a clinician or coach are an acceptable option and may help individuals adhere to walking exercise outside of a supervised setting, but evidence of their effect has been mixed.6,7
Important components of an intervention to support walking exercise behavior change include an individual’s knowledge and understanding of PAD, beliefs about walking as an effective therapy for PAD, confidence and ability to manage their symptoms, and guidance on appropriate walking dosage and environments.8,9 Targeting these factors using theory-based, behavioral change strategies and exercise advice may increase walking capacity among individuals with PAD.10,11
The Motivating Structured Walking Activity in People With Intermittent Claudication (MOSAIC) trial was a multicenter randomized clinical trial designed to determine whether a home-based, walking exercise behavior change intervention delivered by trained physical therapists improved walking capacity, compared with usual care, in people with PAD and intermittent claudication.
Methods
The National Research Ethics Committee London–Bloomsbury, United Kingdom approved the trial protocol. Participants provided written informed consent. This assessor-blinded, multicenter, randomized clinical trial with 2 parallel groups enrolled participants between January 2018 and March 2020 and conducted follow-up over a 6-month period (final follow-up was completed by September 8, 2020). The study protocol12 (Supplement 1) and statistical analysis plan (Supplement 2) are available online.
Participant Identification
Participants were recruited from vascular clinics of 6 public hospitals in southeast England, UK (Guy’s and St Thomas’ Hospital NHS Foundation Trust, King’s College Hospital NHS Foundation Trust, St George’s Hospital NHS Foundation Trust, Royal Free Hospital NHS Foundation Trust, Royal London Hospital, Barts Health NHS Trust, Ashford and St Peter’s Hospital NHS Foundation Trust).
Eligibility Criteria
Criteria for study inclusion comprised the following: (1) participants aged 50 years or older; (2) PAD determined by the consulting clinician based on ankle-brachial pressure index of 0.90 or less, radiographic evidence of PAD, or clinician-reported diagnosis of PAD (also, people with an ankle-brachial pressure index >0.90 were enrolled if there was other evidence of PAD [eg, clinical diagnosis or radiographic evidence of PAD determined by the consulting clinician]); (3) self-reported claudication identified using the San Diego Claudication Questionnaire13 and defined as calf pain during walking or atypical symptoms (eg, symptoms affecting the buttocks or thighs but not the calves); and (4) ability to participate in the trial and provide informed consent.
Criteria for study exclusion comprised the following: (1) unstable PAD, defined as self-reported change in symptoms during the previous 3 months in response to the question “Has there been any change in your symptoms during the past 3 months?”; (2) walking more than 90 minutes per week as self-reported on the Brief International Physical Activity Questionnaire14; (3) contraindications to exercise determined by the consulting clinician; and (4) completion of any medically prescribed supervised exercise in the previous 6 months or planned participation in prescribed supervised exercise in the next 6 months.
Randomization and Masking
Participants were randomly assigned in a 1:1 ratio to receive either a walking exercise behavior change intervention or usual care using a computer-generated randomization system, with randomly selected block sizes of 2 and 4 stratified by center (Figure 1). The outcome assessor and the trial statistician were masked to group allocation until analyses were completed. It was not possible to mask the participants or treating physical therapists to group allocation after randomization because of the nature of the interventions.
Figure 1. Flow of Participants Through the MOSAIC Randomized Trial of a Walking Exercise Behavior Change Intervention in Adults With Peripheral Artery Disease.
MOSAIC indicates Motivating Structured Walking Activity in People With Intermittent Claudication.
aRandomization took place after participants provided written consent and completed baseline testing.
Interventions
Walking Exercise Behavior Change Intervention
The walking exercise behavior change intervention was informed by 2 psychological models (Theory of Planned Behavior and the Common-Sense Model of Illness Representations).15,16 It consisted of two 60-minute in-person sessions (weeks 1 and 2) and two 20-minute telephone sessions (weeks 6 and 12) delivered by physical therapists over 3 months.12
Interventions were delivered by physical therapists who were trained to use a motivational interviewing approach guided by behavior change principles to increase participants’ intention and commitment to walking exercise. Each intervention session included mandatory components to facilitate accurate participant knowledge about PAD and positive beliefs about walking exercise as a treatment.12 Content was tailored to the participant’s knowledge and current walking exercise behavior; it helped participants to identify their current abilities and the goals participants wanted to achieve by increasing their walking capacity, their challenges to completing walking exercise, and strategies for overcoming these challenges.
Walking exercise goals and plans were agreed upon collaboratively with the physical therapist and included identifying progressive, individualized walking targets to achieve at least 30 minutes of walking per day, at a pace that elicited moderate leg symptoms, 3 times per week.3 Participants recorded where, when, and with whom they would walk17 and established ways to self-monitor their walking exercise (eg, recording steps with a pedometer or recording the distance or duration walked in an exercise diary). Participants received a pedometer (Yamax Digi-Walker SW-200) and an intervention manual that included an exercise diary, with goal setting, problem-solving, and action planning worksheets. The intervention was designed to enable participants to continue their walking exercise independently after the final intervention session.
All intervention sessions were audio recorded, and the physical therapists noted the intervention session components delivered on a checklist.
Sixteen physical therapists received 2 days of training, a physical therapy manual, and intervention session checklists. The training team met with the physical therapists at least every 3 months to provide feedback and advice to optimize fidelity of delivery.
Usual Care
Participants randomized to usual care received no study intervention and received standard care provided by their vascular specialists.
Measurement and Procedures
Medical History and Demographics
Self-reported information regarding medical history, racial or ethnic group, other demographics, and current symptoms (San Diego Claudication Questionnaire13) was obtained using questionnaires. Participants self-identified their racial or ethnic group from fixed categories on a questionnaire. This information was collected to assess the generalizability of the results. Body mass index and ankle-brachial pressure index were measured by the outcome assessor.18
Primary Outcome
The primary outcome was 6-minute walk distance at 3-month follow-up.19 Using a standardized protocol, participants walked as far as possible around 2 cones placed 30.48 m apart in a hospital corridor.20 The total distance (meters) walked after 6 minutes was recorded. The walk test was completed 2 times, at least 30 minutes apart, and the highest 6-minute walking distance was used for analysis. The minimum clinically important difference in people with PAD ranged between 8 m (small minimal clinically important difference) and 22 m (large minimal clinically important difference).21 It was not possible to repeat the 6-minute walking distance at 6 months due to funding constraints.
Secondary Outcomes
Secondary outcomes at 3- and 6-month follow-up were not consistent between the protocol and the statistical analysis plan (Item 6.2 in Supplement 2). Secondary outcomes (Item 5.3.3 in Supplement 2) consisted of the following: (1) perceived walking ability, measured by the Walking Estimated Limitation Calculated by History (WELCH; score range, 0 to 100 [100 indicates best]; no minimal clinically important difference defined)22; (2) the Self-Reported Maximum Walking Distance questionnaire (range, a small number of meters to greater than 500 [>500 indicates best]; no minimal clinically important difference defined)23; (3) activities of daily living, measured by the Nottingham Extended Activities of Daily Living scale (score range, 0 to 66 [66 indicates best]; no minimal clinically important difference defined)24; (4) health-related quality of life assessed with the Vascular Quality of Life Questionnaire-6 (score range, 6 to 24 [24 indicates best]; minimal clinically important difference score range between 1.7 and 2.2 points)25,26; (5) illness perceptions evaluated by the Brief Illness Perception Questionnaire (score range, 0 to 80 [indicates negative perception of illness]; no minimal clinically important difference defined)27; (6) walking treatment beliefs (attitude, subjective norms, perceived behavioral control, intentions) assessed by the Theory of Planned Behavior Questionnaire (score range for each construct, 3 to 21 [21 indicates best]; no minimal clinically important difference defined)28; (7) self-regulatory processes estimated using the action planning scale (score range, 4 to 16 [6 indicates best]) and the action control scale (score range, 6 to 24 [24 indicates best]; no minimal clinically important difference defined)17; and (8) physical activity estimated by the Brief International Physical Activity Questionnaire (defined as energy expenditure completed over the past 7 days [metabolic equivalent of task minutes per week]; higher scores indicate greater energy expenditure; no minimal clinically important difference defined).14 However, the statistical analysis plan did not prespecify the attitude, subjective norm, perceived behavioral control, and intentions constructs of the Theory of Planned Behavior Questionnaire. At 3-month follow-up, responses and results for all secondary outcome measures were collected.
Other Outcomes
Pain-free walking time and maximal walking capacity were measured during the 6-minute walk. Pain-free walking time was defined as the time (seconds) that the participant first experienced pain (no minimal clinically important difference was defined). Maximal walking capacity was defined as the time (seconds) that the participant stopped walking (no minimal clinically important difference was defined). Pain-free walking time and maximal walking capacity were measured during the 6-minute walk test. Maximal walking capacity was censored at 6 minutes if the participant was still walking at the 6-minute time point. At 3 and 6 months, exercise adherence was assessed by the Exercise Adherence Rating Scale (score range, 0 to 24 [24 indicates best]; minimal clinically important difference, 5.5 points [see Supplement 1 and Item 5.3.4 in Supplement 2]).29,30
Adverse Events
Adverse events were collected by the outcome assessor at 3- and 6-month follow-up as an exploratory outcome.
Fidelity of Intervention Delivery
Two trained assessors independently rated a 20% randomly selected sample of audio-recorded intervention sessions to assess the extent to which the mandatory components of each session were delivered as intended. The assessors compared their scores and agreed on a score for each intervention session component. High treatment fidelity was achieved if at least 80% of mandatory components were fully or partially delivered in each session.
Randomly selected 20-minute segments of the sampled intervention sessions were rated for motivational interviewing relational proficiency (3.5 on a 5-point scale indicates fair interpersonal style) and technical proficiency (a score of 3 on a 5-point scale indicates fair technique) using the Motivational Interviewing Treatment Integrity Scale.31
Specific aims to explore the participants’ experience of the intervention, assess the feasibility of collecting data on resource use and estimate the minimal clinically important difference for 5 clinical measures are not reported here.
Sample Size
When this study was designed, there was no established minimal clinically important difference for corridor-based 6-minute walking distance in people with PAD. Therefore, the power calculation used the mean (SD) 6-minute walking distance at 6-month follow-up from a similar trial32 (control group’s distance, 342.2 m [110.8 m] vs the intervention group’s distance, 399.8 m [101.6 m]; between-group difference, 58 m [111 m]). Based on this mean difference, statistical power of 90%, and a 2-sided significance level of .05, the minimum sample size necessary for the primary aim was 154 participants. Anticipating a drop-out rate of as much as 20% at 3-month follow-up, the desired total sample size was 192 participants.
Statistical Analyses
Participants were analyzed according to their assigned randomization group, even if they were nonadherent to their assigned intervention. Primary analyses were conducted using complete case data. The baseline characteristics were summarized using mean (SD) for continuous variables and frequency for categorical variables. Median IQRs were calculated if data were not normally distributed. The primary outcome was analyzed using multiple regression with the baseline 6-minute walking distance and the center, as stratification factor, included as covariates. Results for each outcome were reported as the adjusted between-group difference in mean 6-minute walking distance with 95% CI.
In prespecified analyses, the primary outcome was analyzed according to adherence to the protocol. The per-protocol analyses consisted of participants who attended both of the in-person sessions and at least 1 telephone session. Model assumptions were checked using a normal quantile-quantile plot to evaluate whether residuals followed a normal distribution. When this assumption was not met, a generalized linear model with appropriate distribution family and link was used. There was no prespecified plan to impute missing data.
Post Hoc Exploratory Analyses
Post hoc exploratory analyses were implemented by comparison of baseline characteristics in participants with and without primary outcome data, use of a linear mixed model for the primary outcome with center as a random effect, multiple imputation of the primary outcome using baseline data to predict missingness (eTable 1 in Supplement 3), and repeat primary analyses among participants with an ankle-brachial pressure index of 0.90 or less at the baseline study visit.
All analyses were 2-sided, and statistical significance was defined as a P value of less than .05. The statistical modeling used R package version 4.0.3 and Stata statistical software version 16.
Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be considered exploratory.
Trial Changes in Response to the COVID-19 Pandemic
Due to the COVID-19 pandemic, recruitment ceased on March 12, 2020, with 190 participants (less than the target number of 192 participants). It was also not possible to collect the 6-minute walking distance on 15 participants at 3-month follow-up.
Results
Among 190 participants randomized (mean age 68 years, 30% women, 79% White race), primary outcome data were complete for 148 (78%) at 3-month follow-up. Loss to follow-up was primarily due to the COVID-19 pandemic (Table 1, Figure 1). Self-reported outcomes were completed for 161 participants (85%) at 3-month follow-up. At 6-month follow-up, 166 of the 190 participants (87%) contributed data for 1 or more secondary outcomes (Figure 1).
Table 1. Baseline Characteristics of Participants With Peripheral Arterial Disease Randomized to Walking Exercise Behavior Change Intervention or Usual Care.
| No. (%) | ||
|---|---|---|
| Intervention (n = 95) | Usual care (n = 95) | |
| Age, mean (SD), y | 67.6 (8.7) | 68.2 (9.0) |
| Men | 66 (69) | 67 (71) |
| Women | 29 (31) | 28 (29) |
| Racial or ethnic groupa | ||
| Asian or Asian British | 2 (2.1) | 0 |
| Black, African, Caribbean, or Black British | 11 (12) | 7 (7) |
| Mixed or multiple ethnic groups | 4 (4.2) | 0 |
| White | 72 (76) | 78 (82) |
| Other ethnic groupb | 5 (5.3) | 6 (6.3) |
| Ankle-brachial pressure index, mean (SD)c | 0.63 (0.12) | 0.63 (0.15) |
| Body mass index, mean (SD)d | 26.7 (5.7) | 26.9 (5.8) |
| Comorbiditiese | ||
| High blood pressure | 56 (59) | 60 (63) |
| Cardiovascular disease | 46 (48) | 39 (41) |
| Diabetes | 34 (36) | 30 (32) |
| History of cardiac arrest | 19 (20) | 15 (16) |
| History of cerebrovascular accident | 13 (14) | 6 (6.3) |
| Kidney disease | 11 (12) | 6 (6.3) |
| Any other medical comorbidityf | 9 (9.5) | 9 (9.5) |
| Current or former smoker | 82 (86) | 85 (90) |
| History of lower extremity revascularization | 28 (30) | 24 (25) |
| Medication | ||
| Antiplatelet | 21 (22) | 20 (21) |
| Statin | 9 (9) | 13 (14) |
| Vasodilator | 1 (1) | 1 (1) |
| San Diego Claudication Questionnaireg | ||
| Exertional leg pain–stop (classic or atypical claudication) | 85 (89) | 89 (94) |
| Exertional leg pain (classic claudication) | 42 (44) | 57 (60) |
| Exertional leg pain–carry on (atypical claudication) | 8 (8.4) | 2 (2.1) |
| Pain at rest | 2 (2.1) | 4 (4.2) |
| No exertional leg pain | 0 | 0 |
| 6-min walk distance, mean (SD), mh | 352.9 (87.1) | 369.8 (77.8) |
| Walking Estimated Limitation Calculated by History score, median (IQR)i | 16 (8-24) | 20 (9-27) |
| Self-Reported Maximum Walking Distance score, median (IQR), mj | 100 (45-300) | 150 (69-255) |
| Nottingham Extended Activities of Daily Living score, median (IQR)k | 60 (51-66) | 60 (54-66) |
| Vascular Quality of Life Questionnaire-6 score, median (IQR)l | 13 (11-15) | 14 (11-16) |
| Brief Illness Perceptions Questionnaire score, mean (SD)m | 45.7 (11.5) | 44.0 (10.1) |
| Attitude score, mean (SD)n | 14.7 (3.1) | 14.6 (3.4) |
| Subjective norms score, mean (SD)n | 16.2 (4.9) | 15.8 (4.6) |
| Perceived behavioral control score, mean (SD)n | 17.5 (3.7) | 17.0 (3.8) |
| Intention score, mean (SD)n | 19.3 (2.8) | 19.0 (2.6) |
| Action planning score, mean (SD)o | 2.5 (1.2) | 2.3 (1.1) |
| Action control score, mean (SD)o | 2.4 (0.9) | 2.4 (1.0) |
| International Physical Activity Questionnaire, MET min/wk, mean energy expenditure (SD)p | 2846 (6359) | 2615 (5903) |
Abbreviation: MET min/wk, metabolic equivalent of task-minutes/week.
As reported in the UK Census (https://www.ethnicity-facts-figures.service.gov.uk/style-guide/ethnic-groups).
Indicates any self-reported group not described using the provided categories.
Calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures in each leg by the mean of all 4 brachial pressures.
Calculated as weight in kilograms divided by height in meters squared.
Indicates self-reported conditions from a predetermined list.
Indicates self-reported conditions not on the predetermined list.
Indicates participants’ evaluation of claudication pain based on location and extent and combined with activity level associated with pain.13
Indicates the maximum distance participants walked during the 6-minute walk test (range, small No. of meters to >500 m20; minimal clinically important distance, 8 m to 22 m).21
Measures participants’ reported walking limitation at different speeds compared with friends and relatives (ie, slower, same, or faster) on a 4-item questionnaire (score range, 0 [able to walk ≤30 seconds at slow speed] to 100 [able to walk ≥3 hours at fast speed]).22 A minimal clinically important difference has not been defined.
Measures self-reported walking distance in response to the question “What is the maximum distance (in meters) you can walk at your usual pace on a flat surface before leg pain forces you to stop?” Ranges from a small number of meters to more than 500 m (>500 indicates best).23 A minimal clinically important difference has not been defined.
Measures participants’ reported ease of completing activities of daily living on a 22-item questionnaire24 (score range, 0 to 66 [66 indicates best]). A minimal clinically important difference has not been defined.
Measures participants’ reported health-related quality of life on a 6-item questionnaire (score range, 6-24 [24 indicates best]25; minimal clinically important difference range, 1.7-2.2 points).26
Measures participants’ cognitive and emotional representations of their illness on a 9-item questionnaire27 (score range, 0 [indicates best] to 80; a minimal clinically important difference has not been defined).
The Theory of Planned Behavior Questionnaire measures 4 constructs: participants’ attitude, subjective normative beliefs, perceived behavioral control beliefs, and intention to walk on 12-item questionnaire.28 Score range for each construct, 3 to 21 (21 indicates best). A minimal clinically important difference has not been defined.
Measures participants’ ability to plan and self-regulate their walking behavior on a 4-item (score range, 4-16) and 6-item scale (score range, 6-24).17 Higher scores indicate best. A minimal clinically important difference has not been defined.
Measures participants’ reported energy expenditure (MET min/wk) completed over the past 7 days on a 7-item questionnaire. Higher scores indicate greater energy expenditure.14 A minimal clinically important difference has not been defined.
At baseline, 173 participants had an ankle-brachial pressure index of 0.90 or less, and 17 participants had an ankle-brachial pressure index of greater than 0.90.
Eighty-two of 95 participants (85%) attended at least 3 intervention sessions, and 63 of 95 participants (66%) attended all intervention sessions (eTable 2 in Supplement 3).
Primary Outcome
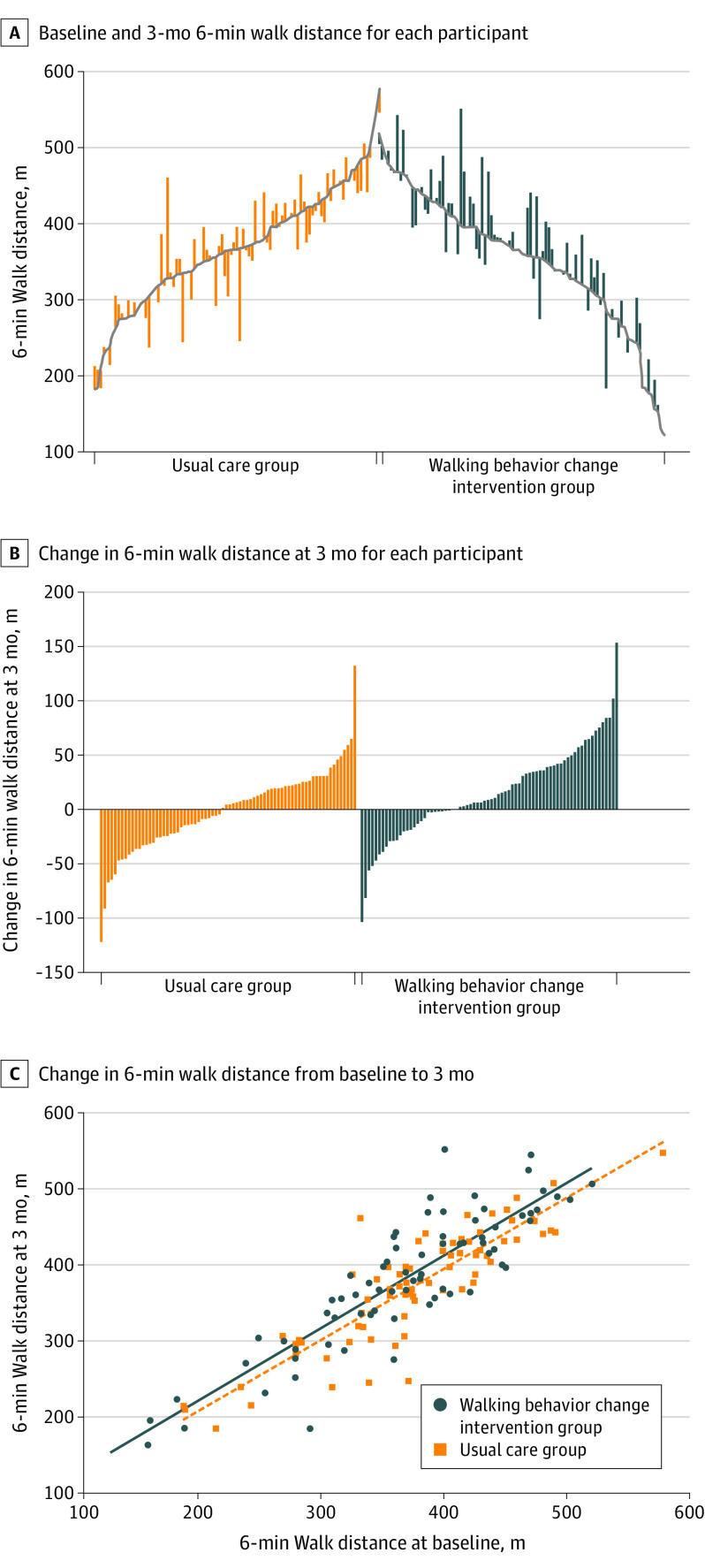
At 3-month follow-up, compared with usual care, the 6-minute walking distance was significantly improved in the walking exercise behavior change group. The distance changed from 352.9 m at baseline to 380.6 m in the intervention group and from 369.8 m at baseline to 372.1 m at 3 months in the usual care group (adjusted between-group difference, 16.7 m [95% CI, 4.2 m to 29.2 m]; P = .009) (Table 2, Figure 2).
Table 2. Effects of Home-Based, Walking Exercise Behavior Change Intervention on Primary and Secondary Outcomes at 3-Month or 6-Month Follow-up.
| Walking exercise behavior change intervention | Usual care | Walking exercise behavior change vs usual care | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) [No.]a | Within-group change, mean (95% CI)b | Mean (SD) [No.]a | Within-group change, mean (95% CI)b | Adjusted between-group difference, mean (95% CI)c | P value | |||
| Baseline (n = 95) | 3-mo follow-up | Baseline (n = 95) | 3-mo follow-up | |||||
| Primary outcome | ||||||||
| 6-min walk distance, md | 352.9 (87.1) | 380.6 (87.7) [74] | 22.3 (0.5 to 44.2) | 369.8 (77.8) | 372.1 (77.3) [74] | 9.2 (−15.2 to 33.6) | 16.7 (4.2 to 29.2) | .009 |
| Secondary outcomes | Baseline | 6-mo follow-up | Baseline | 6-mo follow-up | ||||
| Walking Estimated Limitation Calculated by History scoree | 18.0 (12.6) [94] | 27.8 (18.5) [71] | 6.6 (2.4 to 10.8) | 20.7 (13.9) | 20.7 (14.2) [72] | −1.4 (−4.8 to 2.1) | 7.4 (2.5 to 12.3) | .003 |
| Self-Reported Maximum Walking Distance test, mf | 199 (241) | 586 (1430) [83] | 378 (72 to 685) | 275 (549) | 305 (588) [83] | 71 (−44 to 185) | 104 (−56 to 264) | .20 |
| Nottingham Extended Activities of Daily Living scoreg | 51.3 (15.7) | 56.3 (13.1) [74] | −0.6 (−1.4 to 0.2) | 54.3 (11.0) | 58.4 (8.5) [73] | −0.3 (−1.0 to 0.3) | −1.4 (−4.4 to 1.6) | .37 |
| Vascular Quality of Life Questionnaire-6 scoreh | 13.3 (3.5) [94] | 15.2 (3.9) [83] | 1.5 (0.7 to 2.2) | 13.9 (3.1) | 14.6 (3.9) [82] | 0.8 (0.1 to 1.6) | 0.5 (−0.5 to 1.5) | .33 |
| Brief Illness Perceptions Questionnaire scorei | 45.7 (11.5) | 38.9 (11.3) [72] | −4.3 (−6.9 to −1.7) | 44.0 (10.1) | 45.8 (12.2) [73] | 2.0 (−0.2 to 4.2) | −6.6 (−9.9 to −3.4) | <.001 |
| Attitude scorej | 14.7 (3.1) | 15.4 (3.7) [70] | 0.7 (−0.3 to 1.7) | 14.6 (3.4) | 13.9 (3.6) [72] | −0.6 (−1.4 to 0.2) | 1.4 (0.3 to 2.5) | .02 |
| Subjective norms scorej | 16.2 (4.9) | 16.7 (4.9) [69] | −0.0 (−1.3 to 1.2) | 15.8 (4.6) | 16.0 (4.4) [72] | 0.0 (−1.0 to 1.0) | 0.3 (−1.1 to 1.7) | .67 |
| Perceived behavioral control scorej | 17.5 (3.7) | 16.8 (3.4) [70] | −0.8 (−1.8 to 0.3) | 17.0 (3.8) | 16.8 (3.9) [72] | −0.2 (−1.2 to 0.9) | −0.2 (−1.4 to 1.0) | .78 |
| Intention scorej | 19.3 (2.8) | 18.0 (3.5) [70] | −1.1 (−2.1 to −0.1) | 19.0 (2.6) | 18.3 (3.7) [72] | −0.8 (−1.7 to 0.1) | −0.3 (−1.5 to 0.9) | .64 |
| Action planning scorek | 2.5 (1.2) | 3.0 (0.9) [70] | 0.5 (0.2 to 0.7) | 2.3 (1.1) | 2.8 (1.0) [72] | 0.4 (0.1 to 0. 7) | 0.2 (−0.1 to 0.5) | .16 |
| Action control scorek | 2.4 (0.9) | 3.1 (0.8) [70] | 0.7 (0.4 to 0.9) | 2.4 (1.0) | 3.0 (0.8) [71] | 0.6 (0.3 to 0.8) | 0.1 (−0.1 to 0.4) | .36 |
| International Physical Activity Questionnaire, MET min/wkl | 2846 (6359) | 2764 (4198) [82] | −57 (−877 to 992) | 2615 (5903) | 2599 (5534) [82] | −110 (−599 to 819) | −2.0 (−1034 to 1029) | >.99 |
Abbreviation: MET min/wk, metabolic equivalent of task-minutes/week.
No. of participants (shown in brackets) may differ in some rows.
Within-group difference includes only participants with values at baseline and at 3 or 6 months (as appropriate), whereas baseline values include all participants.
Multiple regression using complete cases with baseline value, trial randomization group, and center as covariates; generalized linear model with same covariates was used for nonnormal outcomes.
See footnote h in Table 1.
See footnote i in Table 1.
See footnote j in Table 1.
See footnote k in Table 1.
See footnote l in Table 1.
See footnote m in Table 1.
See footnote n in Table 1.
See footnote o in Table 1.
See footnote p in Table 1.
Figure 2. Baseline, 3-Month Follow-up, and Change in 6-Minute Walk Distance at 3 Months Among Adults With Peripheral Artery Disease.
A, Each vertical line represents an individual participant, with participants ordered by baseline value and the vertical line extending up (improvement) or down (deterioration) to the 3-month value.
B, Vertical lines extending up denote the degree of improvement in 6-minute walk distance at 3-month follow-up. Vertical lines extending down denote the degree of decline in 6-minute walk distance.
C, Each point represents an individual participant. The vertical distance between the 2 regression lines represents the estimated difference between the groups from the analysis of covariance between baseline and 3 months.
In per-protocol analyses, the intervention group significantly improved the 6-minute walk distance compared with the usual care group (between-group difference, 19.2 m [95% CI, 6.3 m to 32.1 m] P = .004) (eTable 3 in Supplement 3).
Secondary Outcomes
At 6-month follow-up, compared with usual care, the intervention group had a significantly greater WELCH score (between-group difference, 7.4 [95% CI, 2.5 to 12.3]; P = .003), Brief Illness Perceptions Questionnaire score (between-group difference, −6.6 [95% CI, −9.9 to −3.4]; P < .001), and attitude score (between-group difference, 1.4 [95% CI, 0.3 to 2.5]; P = .02) (Table 2).
There were no significant between-group differences in the Self-Reported Maximum Walking Distance questionnaire (104 m [95% CI, −56 m to 264 m]; P = .20), Nottingham Extended Activities of Daily Living score (−1.4 [95% CI, −4.4 to 1.6], P = .37), Vascular Quality of Life Questionnaire-6 score (0.5 [95% CI, −0.5 to 1.5]; P = .33), subjective norms score (0.3 [95% CI, −1.1 to 1.7]; P = .67), perceived behavioral control score (−0.2 [95% CI, −1.4 to 1.0]; P = .78), intention score (−0.3 [95% CI, −0.5 to 0.9]; P = .64), action planning score (0.2 [95% CI, −0.1 to 0.5]; P = .16), action control score (0.1 [95% CI, −0.1 to 0.4]; P = .36), or International Physical Activity Questionnaire score (−2.0 [95% CI, −1034 to 1029]; P > .99) (Table 2).
Other Outcomes
At 3-month follow-up, compared with usual care, the intervention group significantly improved the WELCH score (between-group difference, 10.2 [95% CI, 5.7 to 14.7]; P < .001), the Self-Reported Maximum Walking Distance score (between-group difference, 181.0 m [95% CI, 60 m to 302 m]; P = .003), Nottingham Extended Activities of Daily Living score (between-group difference, 2.8 [95% CI, 0.1 to 5.4]; P = .04), pain-free walking time (between-group difference, 30.3 seconds [95% CI, 5.4 to 55.3]; P = .02), Brief Illness Perceptions Questionnaire score (between-group difference, −5.8 [95% CI, −8.6 to −2.9]; P < .001), attitude score (between-group difference, 1.1 [95% CI, 0.2 to 2.0]; P = .02), subjective norms score (between-group difference, 1.3 [95% CI, 0.1 to 2.6]; P = .03), intention score (between-group difference, 0.9 [95% CI, 0.0 to 1.9]; P = .048), action planning score (between-group difference, 0.5 [95% CI, 0.2 to 0.8]; P = .001), action control score (between-group difference, 0.7 [95% CI, 0.5 to 1.0]; P < .001), and Exercise Adherence Rating Scale score (between-group difference, 2.0 [95% CI, 0.5 to 3.5]; P = .01) (Table 3).
Table 3. Effects of Home-Based, Walking Exercise Behavior Change Intervention on Other Outcomes at 3-Month or 6-Month Follow-up.
| Walking exercise behavior change intervention | Usual care | Walking exercise behavior change intervention vs usual care | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) [No.]a | Within-group change, mean (95% CI)b | Mean (SD) [No.]a | Within-group change, mean (95% CI)b | Adjusted between-group difference, mean (95% CI)c | P value | |||
| Baseline (n = 95) | 3-mo follow-up | Baseline (n = 95) | 3-mo follow-up | |||||
| Walking Estimated Limitation Calculated by History scored | 18.0 (12.6) [94] | 29.5 (18.3) [n = 81] | 9.7 (5.6 to 13.9) | 20.7 (13.9) | 20.0 (13.5) [n = 80] | −1.0 (−3.8 to 1.7) | 10.2 (5.7 to 14.7) | <.001 |
| Self-Reported Maximum Walking Distance test, me | 199 (241) | 500 (1140) [n = 90] | 298 (75 to 521) | 275 (549) | 277 (402) [n = 89] | 51 (−40 to 141) | 181 (60 to 302) | .003 |
| Nottingham Extended Activities of Daily Living scoref | 51.3 (15.7) | 58.3 (8.4) [n = 81] | 0.2 (−0.3 to 0.7) | 54.3 (11.0) | 56.2 (12.6) [n = 83] | −0.8 (−1.5 to −0.0) | 2.8 (0.1 to 5.4) | .04 |
| Vascular Quality of Life Questionnaire-6 scoreg | 13.3 (3.5) | 14.8 (3.8) [n = 91] | 1.4 (0.7 to 2.0) | 13.9 (3.1) | 14.6 (3.5) [n = 90] | 0.6 (0.1 to 1.2) | 0.6 (−0.2 to 1.4) | .17 |
| Pain-free walking time, sh | 159.0 (77.2) [n = 87] | 208.0 (86.4) [n = 65] | 52.8 (33.7 to 72.0) | 163.0 (83.8) [n = 91] | 173.0 (81.4) [n = 68] | 22.7 (4.2 to 41.3) | 30.3 (5.4 to 55.3) | .02 |
| Maximum walking capacity, si | 301.5 (93.3) | 324.4 (82.6) [n = 74] | 10.9 (−7.6 to 29.5) | 289.7 (100.0) | 308.7 (87.9) [n = 74] | 21.7 (1.9 to 41.5) | 12.0 (−16.9 to 40.8) | .42 |
| Brief Illness Perceptions Questionnaire scorej | 45.7 (11.5) | 40.4 (11.5) [81] | −4.2 (−6.6 to −1.7) | 44.0 (10.1) | 45.6 (10.7) [n = 82] | 1.7 (−0.2 to 3.6) | −5.8 (−8.6 to −2.9) | <.001 |
| Attitude scorek | 14.7 (3.1) | 15.7 (2.9) [n = 81] | 1.0 (0.2 to 1.7) | 14.6 (3.4) | 14.5 (4.0) [n = 82] | −0.1 (−0.8 to 0.5) | 1.1 (0.2 to 2.0) | .02 |
| Subjective norms scorek | 16.2 (4.9) | 17.2 (4.1) [n = 81] | 0.9 (−0.1 to 1.8) | 15.8 (4.6) | 15.6 (4.9) [n = 82] | 0.0 (−1.0 to 1.0) | 1.3 (0.1 to 2.6) | .03 |
| Perceived behavioral control scorek | 17.5 (3.7) | 17.2 (3.5) [n = 81] | −0.5 (−1.4 to 0.4) | 17.0 (3.8) | 17.3 (3.5) [n = 82] | 0.3 (−0.7 to 1.3) | −0.3 (−1.3 to 0.8) | .60 |
| Intention scorek | 19.3 (2.8) | 19.5 (2.2) [n = 81] | 0.3 (−0.4 to 1.0) | 19.0 (2.6) | 18.5 (3.8) [n = 82] | −0.5 (−1.3 to 0.3) | 0.9 (0.0 to 1.9) | .048 |
| Action planning scorel | 2.5 (1.2) | 3.2 (0.9) [n = 81] | 0.8 (0.5 to 1.1) | 2.3 (1.1) | 2.7 (1.1) [n = 82] | 0.4 (0.1 to 0.6) | 0.5 (0.2 to 0.8) | .001 |
| Action control scorel | 2.4 (0.9) | 3.5 (0.6) [n = 81] | 1.1 (0.9 to 1.3) | 2.4 (1.0) | 2.7 (1.0) [n = 82] | 0.3 (0.1 to 0.5) | 0.7 (0.5 to 1.0) | <.001 |
| International Physical Activity Questionnaire, MET min/wkm | 2874 (6387) [n = 94] | 3846 (6192) [n = 91] | 838 (−500 to 2175) | 2615 (5903) | 3207 (5035) [n = 90] | 424 (−658 to 1506) | 532 (−855 to 1919) | .45 |
| Exercise Adherence Rating Scale score at 3 mon | 13.9 (5.8) | 17.3 (5.1) [n = 81] | 3.3 (2.0 to 4.7) | 13.6 (5.7) | 15.3 (5.7) [n = 82] | 1.4 (0.0 to 2.7) | 2.0 (0.5 to 3.5) | .01 |
| Exercise Adherence Rating Scale score at 6 mon | 13.9 (5.8) | 16.0 (5.4) [n = 70]n | 1.7 (0.1 to 3.3) | 13.6 (5.7) | 14.7 (6.3) [n = 72]n | 0.6 (−1.0 to 2.3) | 1.2 (−0.7 to 3.1) | .21 |
Abbreviation: MET min/wk, metabolic equivalent of task-minutes/week.
See footnote a in Table 2.
See footnote b in Table 2.
See footnote c in Table 2.
See footnote i in Table 1.
See footnote j in Table 1.
See footnote k in Table 1.
See footnote l in Table 1.
Represents the time in seconds that participants first experienced pain during 6-minute walk test. Ranges from small number of seconds to 360 seconds; 360 seconds indicates best. A minimal clinically important difference has not been defined.
Represents time in seconds that participants stopped walking during 6-minute walk test. Ranges from small number of seconds to 360 seconds; 360 seconds indicates best. A minimal clinically important difference has not been defined.
See footnote m in Table 1.
See footnote n in Table 1.
See footnote o in Table 1.
See footnote p in Table 1.
At 3-month follow-up, compared with usual care, there was no effect of the walking exercise behavior change intervention on the maximal walking capacity (12.0 [95% CI, −16.9 to 40.8]; P = .42), Vascular Quality of Life Questionnaire-6 score (0.6 [95% CI, −0.2 to 1.4]; P = .17), perceived behavioral control score (−0.3 [95% CI, −1.3 to 0.8]; P = .60), or International Physical Activity Questionnaire score (532 [95% CI, −855 to 1919]; P = .45) (Table 3).
At 6-month follow-up, compared with usual care, there was no significant effect of the intervention on the Exercise Adherence Rating Scale score (1.2 [95% CI, −0.7 to 3.1]; P = .21) (Table 3).
Post Hoc Exploratory Analyses
Compared with participants who did not complete the 6-minute walk test at 3-month follow-up, those who completed the test at 3 months had significantly greater baseline 6-minute walking distance (mean [SD], 369.5 m [77.5 m] vs 332.8 m [94.7 m]; P = .01) (eTable 4 in Supplement 3). Results for the primary outcome (16.7 m [95% CI, 4.2 m to 29.2 m]; P = .009) did not meaningfully change when the analyses were repeated using a mixed model with center modeled as a random effect (16.3 m [95% CI, 3.9 m to 28.6 m]; P = .01) or when analyses were repeated using multiple imputation (18.9 m [5.5 m to 32.3 m]; P = .006) (eTable 1 in Supplement 3). Results did not meaningfully change when analyses were limited to those with a baseline study visit ankle-brachial pressure index of 0.90 or less (15.9 [95% CI, 2.6 to 29.2]; P = .02).
Fidelity of Intervention Delivery
Fifteen physical therapists delivered the walking exercise behavior change intervention. Sixty-two (21%) randomly selected intervention sessions were rated. Overall, 79% of sessions were delivered with fidelity. High fidelity was achieved in the 2 in-person sessions (session 1, 100%; session 2, 88%), but fidelity was lower in the telephone sessions (session 3, 67%; session 4, 54%). Fair technical motivational interviewing proficiency was achieved in all sessions (3.2-3.9 on a scale of 5) and fair relational motivational interviewing proficiency in both in-person sessions (3.5 on a scale of 5 for both sessions), but the telephone sessions did not attain at least fair relational motivational interviewing proficiency (session 1 [3.1 on a scale of 5]; session 2 [3.2 on a scale of 5]; eTable 5 in Supplement 3).
Adverse Events
There were 37 adverse events (25 in the intervention group; 12 in the usual care group). Twenty-one nonserious adverse events were reported by 19 participants (12 in the intervention group; 9 in the usual care group) (eTable 6 in Supplement 3). Falls were the most common nonserious adverse event (3 in the intervention group; 3 in the usual care group; eTable 7 in Supplement 3). None of the nonserious adverse events were judged to be related to the study. Sixteen serious adverse events, due to hospitalization, were reported by 15 participants (13 in the intervention group; 3 in the usual care group) (eTable 8 in Supplement 3). All serious adverse events were judged to be either unrelated or unlikely to be related to the study by the trial steering/data monitoring and ethics committee.
Discussion
In this trial of 190 participants with PAD and symptoms of intermittent claudication, a walking exercise behavior change intervention delivered by physical therapists significantly improved mean 6-minute walk distance, compared with usual care, at 3-month follow-up. Out of 8 secondary outcomes, 3 improved significantly more at 6-month follow-up in the intervention group: self-reported walking capacity measured by the WELCH score, illness perceptions measured by the Brief Illness Perceptions Questionnaire, and walking treatment beliefs (attitude score) measured by the Theory of Planned Behavior Questionnaire, compared with usual care.
Results of prior randomized clinical trials of home-based exercise therapy for people with PAD have been mixed, with multiple prior clinical trials showing benefits of home-based exercise for PAD32,33,34,35 but at least 2 showing no effect of a home-based exercise intervention for PAD.36,37
The difference in 6-minute walking distance following this intervention was greater than a small minimal clinically important difference for people with PAD but did not meet the threshold for a large minimal clinically important difference in people with PAD.21 Three factors may have contributed to the success of the intervention in the current trial. First, it was designed to address theory-based, psychological factors that influenced walking exercise behavior in people with PAD.8,9,11 Previous work reported that positive walking beliefs, defined by the Theory of Planned Behavior, are positively associated with motivation to walk.8,38 An individual’s accurate understanding of their illness and perceptions about the causes and ability to control PAD were previously associated with greater motivation and with better 6-minute walking distance.8 Second, the intervention included evidence-based, behavior change principles that may have helped participants translate intention to walk for exercise into actual behavior.10,17,39 Third, the intervention was tailored to each participant’s knowledge, skills, and environment.
Compared with prior effective home-based walking exercise interventions for PAD that improved the 6-minute walk distance by 45 m to 53 m when compared with the control group,32,34,35 the effect size for the current intervention was smaller (ie, 16.7 m). A possible reason for the smaller effect size could be because social restrictions during the COVID-19 pandemic hindered participants’ planned walking. However, most participants completed the primary outcome prior to the start of the pandemic. Alternatively, the smaller effect size may have been because the participants did not walk at sufficient intensity to produce large improvements in 6-minute walking distance35 or because there was an insufficient number of intervention sessions to support large intervention effects. However, a prior highly effective 12-week home-based exercise intervention had a similar number of intervention sessions.34 Further study is needed to determine whether, for the current intervention, more sessions would have had a more potent effect.
Another potential explanation for the lower potency of the current intervention, compared with prior home-based exercise interventions in PAD,32,34,35 may be the relatively low fidelity of delivery and motivational interviewing proficiency in the telephone sessions of the current trial. Higher levels of treatment fidelity are associated with better treatment outcomes.40 There are several possible explanations for this. First, at the start of this trial, it was not typical for physical therapy to be delivered remotely. Lack of familiarity with remote interventions may have affected the therapists’ confidence and ability to deliver the mandatory components.
Second, the telephone consultations, which lasted for a mean duration of 20 minutes, may have been too brief to deliver the mandatory components. However, another highly effective home-based exercise intervention for PAD used intervention telephone calls that were shorter than 20 minutes.35
Third, during the trial, some therapists may have drifted from the motivational interviewing approach. However, regular meetings with the training team should have mitigated this.
Fourth, low participant recruitment at some sites may have compromised therapist proficiency and effectiveness, due to less experience delivering the intervention over time.
Fifth, therapist training may have been suboptimal. Training may not have differentiated between mandatory and optional components sufficiently or the therapists may not have understood the importance of delivering the mandatory components at every intervention session. Further study is needed to determine whether better interventionist training, monitoring, and feedback could improve the potency of the intervention.
Despite the positive effect of the intervention on 6-minute walking distance, most secondary outcomes did not improve at 6 months compared with usual care. Improving quality of life is a key clinical and health priority. The lack of change in quality of life at 6-month follow-up may be because only 2 of the items in the VacsuQol-6 assessed physical health, and improvement in mental health may lag behind improvements in physical function. Alternatively, people may underestimate changes in quality of life following exercise programs because improvements are slower and less noticeable than with other interventions (such as revascularization), and other comorbidities can also affect quality of life.
Limitations
This study has several limitations. First, it was not possible to collect the primary outcome on all participants, which contributed to a large loss to follow-up for the 6-minute walking distance (22%). Post hoc power calculations showed this had negligible effect on power (88% vs 90% planned). Second, participants without 6-minute walking distance at follow-up had a lower baseline 6-minute walking distance than those with the primary outcome. The post hoc multiple imputation models for the 6-minute walking distance showed a greater magnitude of improvement between the intervention and the control groups in analyses that included multiple imputation. Third, most participants were White men, which limits the generalizability of these results. Fourth, the 3-month follow-up period is relatively short. The durability of the intervention is unknown. Fifth, the comparator to the intervention was not an attention control group. Sixth, relatively low fidelity of delivery in the telephone intervention sessions may have limited the intervention effect. Seventh, no actual walking activity data were collected.
Conclusions
Among adults with PAD and intermittent claudication, a home-based, walking exercise behavior change intervention, compared with usual care, improved walking distance at 3 months. Further research is needed to determine the durability of these findings.
Trial Protocol
Statistical Analysis Plan
eTable 1. The Effects of a Walking Exercise Behavior Change Intervention Compared to Usual Care on Primary Outcome: Post Hoc Exploratory Analyses
eTable 1. The Effects of a Home-Based, Physical Therapist–Led Walking Exercise Behavior Change Intervention Compared to Usual Care on Primary Outcome: Post Hoc Exploratory Analyses
eTable 2. Participants’ Attendance at Physical Therapist Consultations in a Home-Based, Walking Exercise Behavior Change Intervention
eTable 3. Per-Protocol Analysis of Primary Outcome in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
eTable 4. Baseline Characteristics of Participants With and Without Primary Outcome Data in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
eTable 5. Fidelity of Delivery of Physical Therapist Intervention Sessions in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
eTable 6. Summary of Reported Adverse Events by Randomized Group in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
eTable 7. Reported Nonserious Adverse Events by Randomized Group in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
eTable 8. Reported Serious Adverse Events by Randomized Group in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Polonsky TS, McDermott MM. Lower extremity peripheral artery disease without chronic limb-threatening ischemia: a review. JAMA. 2021;325:2188-2198. doi: 10.1001/jama.2021.2126 [DOI] [PubMed] [Google Scholar]
- 2.Harwood AE, Smith GE, Cayton T, Broadbent E, Chetter IC. A systematic review of the uptake and adherence rates to supervised exercise programs in patients with intermittent claudication. Ann Vasc Surg. 2016;34:280-289. doi: 10.1016/j.avsg.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 3.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e686-e725. doi: 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2012;44(6):569-575. doi: 10.1016/j.ejvs.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 5.Harwood AE, Pymer S, Ibeggazene S, Ingle L, Caldow E, Birkett ST. Provision of exercise services in patients with peripheral artery disease in the United Kingdom. Vascular. 2021;17085381211035259. doi: 10.1177/17085381211035259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golledge J, Singh TP, Alahakoon C, et al. Meta-analysis of clinical trials examining the benefit of structured home exercise in patients with peripheral artery disease. Br J Surg. 2019;106(4):319-331. doi: 10.1002/bjs.11101 [DOI] [PubMed] [Google Scholar]
- 7.Pymer S, Ibeggazene S, Palmer J, et al. An updated systematic review and meta-analysis of home-based exercise programs for individuals with intermittent claudication. J Vasc Surg. 2021;74(6):2076-2085.e20. doi: 10.1016/j.jvs.2021.03.063 [DOI] [PubMed] [Google Scholar]
- 8.Galea Holmes MN, Weinman JA, Bearne LM. Are walking treatment beliefs and illness perceptions associated with walking intention and 6-minute walk distance in people with intermittent claudication? a cross-sectional study. J Aging Phys Act. 2019;27(4):473-481. doi: 10.1123/japa.2018-0245 [DOI] [PubMed] [Google Scholar]
- 9.Galea Holmes MN, Weinman JA, Bearne LM. ‘You can’t walk with cramp!’ a qualitative exploration of individuals’ beliefs and experiences of walking as treatment for intermittent claudication. J Health Psychol. 2017;22(2):255-265. doi: 10.1177/1359105315600238 [DOI] [PubMed] [Google Scholar]
- 10.Galea MN, Weinman JA, White C, Bearne LM. Do behaviour-change techniques contribute to the effectiveness of exercise therapy in patients with intermittent claudication? a systematic review. Eur J Vasc Endovasc Surg. 2013;46(1):132-141. doi: 10.1016/j.ejvs.2013.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galea Holmes MN, Weinman JA, Bearne LM. A randomized controlled feasibility trial of a home-based walking behavior-change intervention for people with intermittent claudication. J Vasc Nurs. 2019;37(2):135-143. doi: 10.1016/j.jvn.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Bearne L, Galea Holmes M, Bieles J, et al. Motivating Structured walking Activity in people with Intermittent Claudication (MOSAIC): protocol for a randomised controlled trial of a physiotherapist-led, behavioural change intervention versus usual care in adults with intermittent claudication. BMJ Open. 2019;9(8):e030002. doi: 10.1136/bmjopen-2019-030002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1(1):65-71. doi: 10.1177/1358863X9600100112 [DOI] [PubMed] [Google Scholar]
- 14.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 15.Leventhal H, Meyer D, Nerenz D. The common sense representation of illness danger. In: Rachman S, ed. Contributions to Medical Psychology. Pergamon Press;1980:17-30. [Google Scholar]
- 16.Ajzen I. From intentions to actions: a theory of planned behavior. In: Kuhl J, Beckmann J, eds. Action Control. Springer; 1985:11-39. doi: 10.1007/978-3-642-69746-3_2 [DOI] [Google Scholar]
- 17.Sniehotta FF, Scholz U, Schwarzer R. Bridging the intention–behaviour gap: planning, self-efficacy, and action control in the adoption and maintenance of physical exercise. Psychol Health. 2005;20(2):143-160. doi: 10.1080/08870440512331317670 [DOI] [Google Scholar]
- 18.Johnston KW, Hosang MY, Andrews DF. Reproducibility of noninvasive vascular laboratory measurements of the peripheral circulation. J Vasc Surg. 1987;6(2):147-151. doi: 10.1067/mva.1987.avs0060147 [DOI] [PubMed] [Google Scholar]
- 19.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130(1):61-68. doi: 10.1161/CIRCULATIONAHA.114.007002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 21.McDermott MM, Tian L, Criqui MH, et al. Meaningful change in 6-minute walk in people with peripheral artery disease. J Vasc Surg. 2021;73(1):267-276.e1. doi: 10.1016/j.jvs.2020.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tew GA, Nawaz S, Humphreys L, Ouedraogo N, Abraham P. Validation of the English version of the Walking Estimated-Limitation Calculated by History (WELCH) questionnaire in patients with intermittent claudication. Vasc Med. 2014;19(1):27-32. doi: 10.1177/1358863X14520870 [DOI] [PubMed] [Google Scholar]
- 23.Tew G, Copeland R, Le Faucheur A, Gernigon M, Nawaz S, Abraham P. Feasibility and validity of self-reported walking capacity in patients with intermittent claudication. J Vasc Surg. 2013;57(5):1227-1234. doi: 10.1016/j.jvs.2012.02.073 [DOI] [PubMed] [Google Scholar]
- 24.Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clin Rehabil. 1987;1(4):301-305. doi: 10.1177/026921558700100409 [DOI] [Google Scholar]
- 25.Nordanstig J, Wann-Hansson C, Karlsson J, Lundström M, Pettersson M, Morgan MB. Vascular Quality of Life Questionnaire-6 facilitates health-related quality of life assessment in peripheral arterial disease. J Vasc Surg. 2014;59(3):700-707. doi: 10.1016/j.jvs.2013.08.099 [DOI] [PubMed] [Google Scholar]
- 26.Nordanstig J, Pettersson M, Morgan M, Falkenberg M, Kumlien C. Assessment of minimum important difference and substantial clinical benefit with the vascular quality of life questionnaire-6 when evaluating revascularisation procedures in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2017;54(3):340-347. doi: 10.1016/j.ejvs.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 27.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631-637. doi: 10.1016/j.jpsychores.2005.10.020 [DOI] [PubMed] [Google Scholar]
- 28.Ajzen I. Constructing a theory of planned behaviour questionnaire: conceptual and methodological considerations. Published 2006. Accessed August 8, 2021. http://people.umass.edu/aizen/pdf/tpb.measurement.pdf
- 29.Newman-Beinart NA, Norton S, Dowling D, et al. The development and initial psychometric evaluation of a measure assessing adherence to prescribed exercise: the Exercise Adherence Rating Scale (EARS). Physiotherapy. 2017;103(2):180-185. doi: 10.1016/j.physio.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 30.de Lira MR, de Oliveira AS, França RA, Pereira AC, Godfrey EL, Chaves TC. The Brazilian Portuguese version of the Exercise Adherence Rating Scale (EARS-Br) showed acceptable reliability, validity and responsiveness in chronic low back pain. BMC Musculoskelet Disord. 2020;21(1):294. doi: 10.1186/s12891-020-03308-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moyers TB, Manuel JK, Ernst D. Motivational Interviewing Treatment Integrity Coding Manual 4.2.1. Unpublished manual, 2014. https://casaa.unm.edu/download/miti4_2.pdf [Google Scholar]
- 32.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310(1):57-65. doi: 10.1001/jama.2013.7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. J Vasc Surg. 2012;55(5):1346-1354. doi: 10.1016/j.jvs.2011.11.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc. 2014;3(5):e001107. doi: 10.1161/JAHA.114.001107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDermott MM, Spring B, Tian L, et al. Effect of low-intensity vs high-intensity home-based walking exercise on walk distance in patients with peripheral artery disease: the LITE randomized clinical trial. JAMA. 2021;325(13):1266-1276. doi: 10.1001/jama.2021.2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319(16):1665-1676. doi: 10.1001/jama.2018.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins TC, Lu L, Ahluwalia JS, et al. Efficacy of community-based exercise therapy among African-American patients with peripheral artery disease: a randomized clinical trial. JAMA Netw Open. 2019;2(2):e187959. doi: 10.1001/jamanetworkopen.2018.7959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galea MN, Bray SR. Predicting walking intentions and exercise in individuals with intermittent claudication: an application of the theory of planned behavior. Rehabil Psychol. 2006;51(4):299-305. doi: 10.1037/0090-5550.51.4.299 [DOI] [Google Scholar]
- 39.Meade LB, Bearne LM, Sweeney LH, Alageel SH, Godfrey EL. Behaviour change techniques associated with adherence to prescribed exercise in patients with persistent musculoskeletal pain: systematic review. Br J Health Psychol. 2019;24(1):10-30. doi: 10.1111/bjhp.12324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent. 2011;71(s1):S52-S63. doi: 10.1111/j.1752-7325.2011.00233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. The Effects of a Walking Exercise Behavior Change Intervention Compared to Usual Care on Primary Outcome: Post Hoc Exploratory Analyses
eTable 1. The Effects of a Home-Based, Physical Therapist–Led Walking Exercise Behavior Change Intervention Compared to Usual Care on Primary Outcome: Post Hoc Exploratory Analyses
eTable 2. Participants’ Attendance at Physical Therapist Consultations in a Home-Based, Walking Exercise Behavior Change Intervention
eTable 3. Per-Protocol Analysis of Primary Outcome in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
eTable 4. Baseline Characteristics of Participants With and Without Primary Outcome Data in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
eTable 5. Fidelity of Delivery of Physical Therapist Intervention Sessions in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
eTable 6. Summary of Reported Adverse Events by Randomized Group in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
eTable 7. Reported Nonserious Adverse Events by Randomized Group in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
eTable 8. Reported Serious Adverse Events by Randomized Group in a Randomized Clinical Trial Comparing the Effect of a Physical Therapist–Led, Home-Based, Walking Exercise Behavior Change Intervention vs Usual Care on Walking Capacity
Nonauthor Collaborators
Data Sharing Statement