Abstract
Functions of the neocortex depend on its bidirectional communication with the thalamus, via cortico-thalamo-cortical (CTC) loops. Recent work dissecting the synaptic connectivity in these loops is generating a clearer picture of their cellular organization. Here, we review findings across sensory, motor and cognitive areas, focusing on patterns of cell-type-specific synaptic connections between the major types of cortical and thalamic neurons. We outline simple and complex CTC loops, and note features of these loops that appear to be general versus specialized. CTC loops are tightly interlinked with local cortical and corticocortical (CC) circuits, forming extended chains of loops that are probably critical for communication across hierarchically organized cerebral networks. Such CTC–CC loop chains appear to constitute a modular unit of organization, serving as scaffolding for area-specific structural and functional modifications. Inhibitory neurons and circuits are embedded throughout CTC loops, shaping the flow of excitation. We consider recent findings in the context of established CTC and CC circuit models, and highlight current efforts to pinpoint cell-type-specific mechanisms in CTC loops involved in consciousness and perception. As pieces of the connectivity puzzle fall increasingly into place, this knowledge can guide further efforts to understand structure–function relationships in CTC loops.
Introduction
Brain regions operate not in isolation but through their interactions with other brain regions. These interactions are often strongly directional — for example, brainstem sensory nuclei typically send their output towards the thalamus, whereas motor nuclei send theirs to muscles; the basal ganglia and cerebellum participate in multi-regional loops that collect cortical input and funnel their output to the thalamus. What is striking and unusual about the relationship between the neocortex and the thalamus is that they form massive excitatory projections directly to each other. These thalamocortical and corticothalamic projections are enormous in their scale and scope, involving essentially all of the cortex and thalamus. At the cellular level, interactions between the two structures are implemented through the synaptic connections that link thalamic and cortical neurons. That these connections form looping circuits has long been assumed, and earlier studies made great progress towards delineating the anatomical pathways and neurophysiological activity in cortico-thalamo-cortical (CTC) loops1. Such loops have long been implicated in cortical rhythms, sleep, attention and corticocortical (CC) communication, and in disease states including absence epilepsy and tremor2. However, their detailed dissection at the level of cell-type-specific synaptic connections has only recently become routinely possible with the advent of tools for this purpose, particularly in mice. The recent flood of new findings relevant to CTC connectivity raises the question of how to make sense of all this new information. Here, we review results from such analyses across different cortical areas and pathways, in an effort to identify common versus specialized patterns. We focus on specific cell types and their synaptic connections (rather than only the anatomical projections of their axons), and use connectivity matrices as a graphical aid to represent and assimilate the burgeoning knowledge about the circuit connections. We highlight both what is known and what is not, not only about ‘who talks to whom’ but also ‘who’ does not — advocating the notion that the flow of activity and thus computational processing in these circuits is determined by the connections that are not made as well as those that are. Towards the end of the Review, we consider how CTC loops relate to CC circuits and to previously developed models incorporating those circuits, spotlight recent research relating CTC circuit architecture to conscious behaviour, and conclude by highlighting open questions and areas for further investigation.
The focus on synaptic connectivity and wiring-diagram descriptions of circuit organization precludes in-depth consideration of related topics important for understanding structure–function relationships in CTC loops, ranging from molecular determinants of cellular physiology to population activity, rhythms and behaviour; readers are referred to other sources covering these and related areas in the vast thalamocortical literature1,3-13.
Excitatory cell classes in the loop
CTC loops are formed by several distinct classes of excitatory projection neurons (Fig. 1). Much can be said about cellular classification, but as this Review focuses on connectivity, we pare this down to the bare essentials.
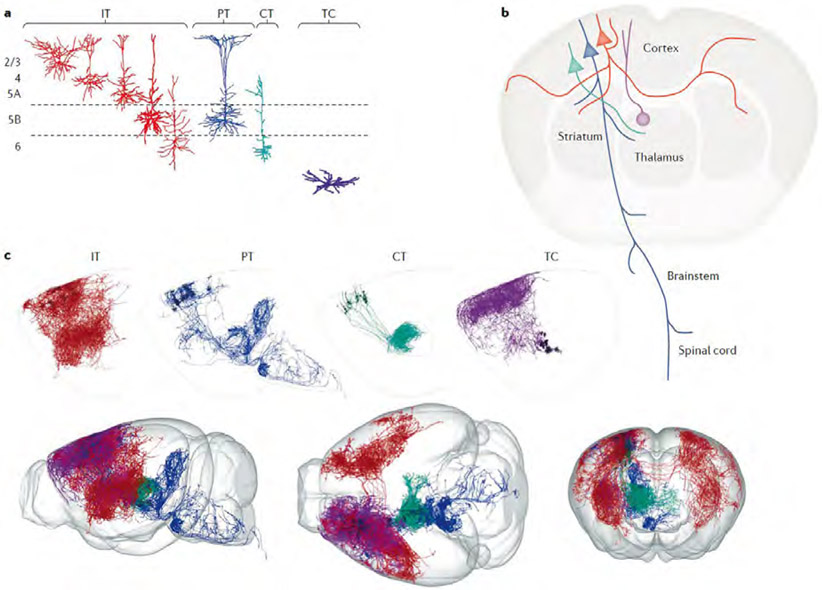
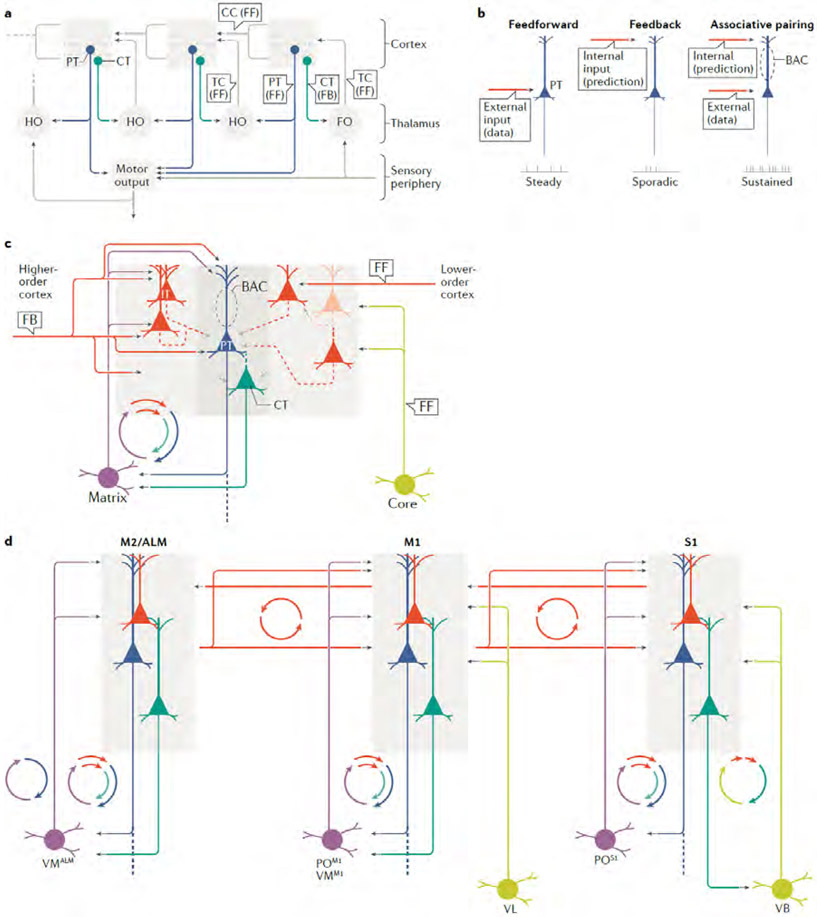
Fig. 1 ∣. Major classes of excitatory projection neurons in cortico-thalamo-cortical loops.
a ∣Reconstructed dendrites are shown for the major excitatory cell classes involved in forming cortico-thalamo-cortical loops — thalamocortical (TC) neurons in the thalamus (purple), and three broad classes of excitatory neurons in the neocortex: intratelencephalic (IT; red), pyramidal tract (PT; blue) and corticothalamic (CT; green) neurons. The IT neurons are a large and diverse class, comprising multiple subtypes across and within cortical layers, from layer 2 to layer 6. The PT neurons, sharing layer 5B with IT neurons, have large apical dendritic tufts in layer 1. The CT neurons, sharing layer 6 with IT neurons, have relatively small dendritic arbors. b ∣ The axonal projection patterns of the same four classes are depicted schematically, following the same colour scheme. The IT neurons project bilaterally to other cortical areas and striatum. The PT neurons form descending projections with branches to multiple subcortical and subcerebral destinations. The CT neurons are specialists with axons projecting almost entirely to the thalamus. The cortical projection to the thalamus is composed of the axons of both PT and CT neurons. c ∣ Axonal arbors of IT, PT and CT neurons in motor cortex, and of TC neurons projecting to motor cortex, are shown separately (top row; twelve examples of each) or together (bottom row, in side, top and front views). The axons are from the database of the MouseLight project, which developed methods for reconstructing axonal arbors in their entirety across the brain 31,197 The images were generated with the MouseLight interface (www.janelia.org/project-team/mouselight) by selecting multiple axon IDs for display (IT axons AA0662, AA0627, AA0622, AA0592, AA0582, AA0003, AA0656, AA0588, AA0442, AA0184, AA0102, AA0002; PT axons AA1050, AA0927, AA0926, AA0923, AA0617, AA0587, AA0584, AA0261, AA0135, AA0134, AA0132, AA0131; CT axons AA0863, AA0833, AA0770, AA0652, AA0640, AA0623, AA0548, AA0545, AA0185, AA0043, AA0041, AA0038, and, TC axons AA0692, AA0591, AA0586, AA0581, AA0391, AA0342, AA0809, AA0719, AA0675, AA0451, AA0176, AA0137). Reconstructions in part a adapted with permission from REFS. 74-76,78,196). Reconstructions in part c adapted with permission from REF.31.
Cortical projections to the thalamus are composed of axons from two of the three main classes of cortical projection neurons. Corticothalamic (CT) neurons in layer 6 project their axons almost exclusively to the thalamus, with branches in the thalamic reticular nucleus (TRN) but few branches intracortically1,28,37. (Layer 6, also called layer 6A, is distinct from the underlying thin layer 6B that harbours some neurons that, although projecting to the thalamus, appear not to be tightly integrated into CTC circuits14,15.) The other component of the cortical projection to thalamus arises as branches of ‘pyramidal tract’ type (PT) neurons, located in layer 5B and so named (loosely, by tradition16,17) for the most common route of their projection to subcerebral destinations in the brainstem and spinal cord. (The terminology of these neurons is a perennial source of confusion. In adult animals, not all PT neurons retain their pyramidal tract axon branch, owing to area-specific developmental pruning; for example, those in the primary visual cortex (V1) lose their pyramidal tract branches, but form branches to the midbrain and pons18. An equivalent term for PT neurons is ‘subcerebral projection neurons’19. The term ‘extratelencephalic’ (ET) has also been introduced20, but as both CT and PT neurons project outside the telencephalon this entails a further distinction between ET neurons of layers 5 and 6.) Recent findings define thalamus-projecting PT neurons as a distinct subtype tending to lack branches to the medulla and PT21-26. The CT and PT projection patterns to the thalamus from a cortical site tend to be similar, although they are not identical27-29, and both classes of neuron contain subtypes with distinct thalamic branching patterns and sublayer distributions, as discussed in more detail later. CT and PT axons project mainly to the ipsilateral thalamus, but they can also branch contralaterally30,31. Intratelencephalic (IT) neurons in the cortex, a broad class of neuron with diverse subtypes in layers 2–6, project to the cortex and striatum bilaterally but not to the thalamus.
Classifying thalamocortical (TC) neurons is complicated, with major classes not as clearly separable as they are in the cortex, although recent multi-omics studies are helping to establish relationships between TC neuron structure, function and gene expression28,32,33. As the laminar pattern of TC axonal branching in the cortex is a fundamental structural determinant of cellular connectivity in CTC loops, here we emphasize the distinction between TC axons that branch mainly in layer 1 (and secondarily, to a varying extent, in layer 5A) versus layer 4 (and secondarily, to a varying extent, around the layer 5B/6 border)34-38. Because this distinction conforms reasonably well to the classification of the thalamus into matrix-type (calbindin-expressing) nuclei and the core-type (parvalbumin-expressing (PV+), in primates) nuclei, respectively34, we use the matrix and core terminology as a proxy for the projection-defined TC classes. However, it is important to bear in mind that there is some overlap between matrix-type and core-type TC neurons, and there are clearly many variants for each type (subtypes), as discussed later. It may be useful to distinguish between the classically core-type TC projections to primary sensory cortices and the ‘core-like’ TC projections to other areas such as the primary motor cortex (M1) and the prefrontal cortex (PFC), where layer 4 is thinner or absent and the TC arborization includes branches in layer 1. Intralaminar TC neurons, a class characterized by axons projecting to deeper layers and implicated in loops with the basal ganglia, are less abundant and less well characterized, and they are not considered in detail here (see REF.36).
Next, we address how these major classes of cortical and thalamic excitatory projection neurons connect to form CTC loops.
Connectivity patterns under the loupe
In this section, we examine the cellular connectivity in CTC loops across diverse systems, ranging from primary sensory to motor and cognitive cortical areas. Much of the recent progress comes from studies of mice and rats, but it builds on decades of studies with diverse species.
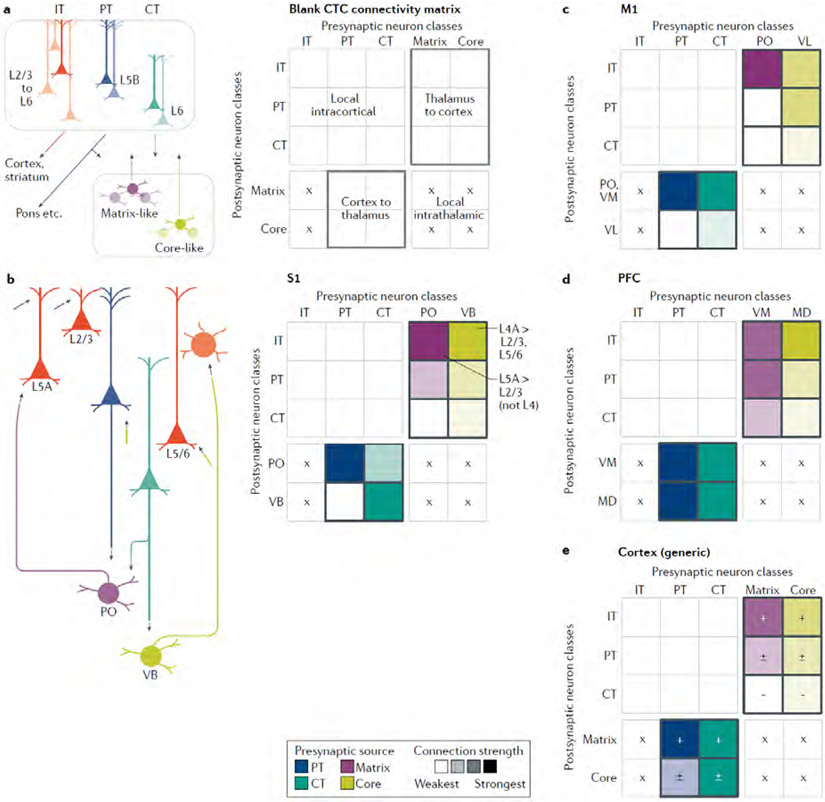
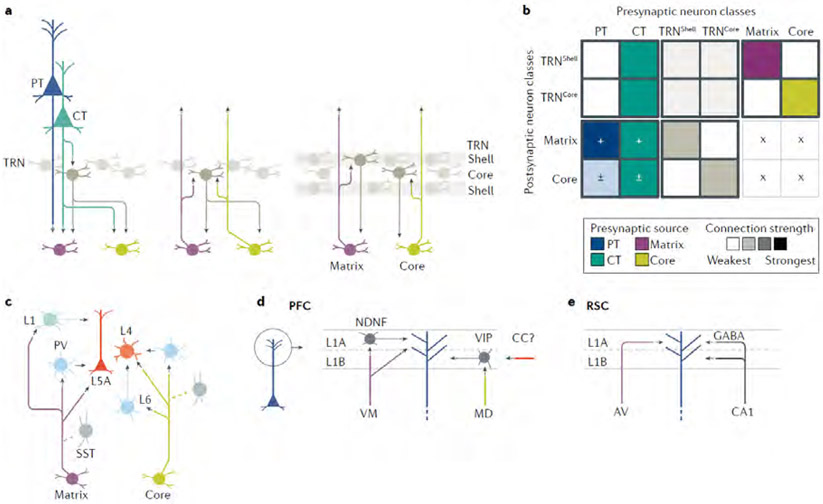
Along with describing the key observations, we incorporate them into connectivity matrix representations, as a graphical tool to facilitate comparisons. Connectivity matrices offer a compact way to depict how the presynaptic and postsynaptic cell types in CTC loops do or do not form synaptic connections, and thus hold promise as a way to pool, organize and distill results from across a large literature. Connectivity matrices are widely used in neural network models to specify synaptic weights, and are increasingly used to represent and analyze experimentally determined connection and projection patterns28,39-42. Although the matrices developed here are based on our qualitative assessments for combining quantitative results from multiple connectivity studies, they provide a general overview, a more structured alternative to wiring-diagram drawings, and a potential framework for future experimental and modelling efforts. Elements of the matrix represent the strengths of connections from presynaptic to postsynaptic neurons. In the cortex-to-thalamus direction, the presynaptic neurons are PT and CT (not IT, which do not project to the thalamus) and the possible postsynaptic neurons are matrix-type and core-type TC neurons (Fig. 2A). In the thalamus-to-cortex direction, matrix-type and core-type TC neurons are presynaptic and the possible postsynaptic neurons are IT, PT and CT neurons. We first fill in these two zones of the overall connectivity matrix, and in later sections add the local excitatory intracortical connections (upper left part of the matrix), and consider how inhibitory neurons fit in. Intrathalamic connections (lower right part of the matrix) are scarce and therefore omitted.
Fig. 2 ∣. Cell-type-specific cortico-thalamo-cortical connectivity matrices across areas.
a ∣ The major classes of excitatory projection neurons in the cortex and thalamus are drawn schematically (left), along with their ‘blank’ connectivity matrix (right), highlighting the boxes representing connections directly involved in cortico-thalamo-cortical (CTC) loops, from the thalamus to cortex (upper right) and the cortex to thalamus (lower left). Crosses (×) mark where connections cannot form owing to a lack of projections from the presynaptic source. b ∣ For the primary somatosensory cortex (S1), a wiring diagram (left) and connectivity matrix (right) are shown, incorporating findings for both vibrissal-related and forelimb-related S1, as discussed in detail in the main text46-51,53-64. The matrix was assembled by filling in each element based on a qualitative interpretation and weighting of experimental results, often from multiple studies, that have quantitatively measured the strengths of connections between specific presynaptic→postsynaptic combinations. For example, the matrix element in the upper right corner represents the strength of excitatory input from presynaptic ventrobasal (VB) axons to postsynaptic IT neurons (in this case primarily those in layer 4). The colour intensities represent the connection strengths (darker = stronger). The colours differ by column to represent the different types of presynaptic neurons. This colour scheme also emphasizes that most measurements have been experimentally determined by comparing the strength of input from a single presynaptic source to two or more postsynaptic targets, rather than vice versa. Accordingly, comparisons between matrix elements tend to be more robust along the columns than along the rows. For example, the relative strengths of VB inputs to different types of cortical neurons have been directly tested, whereas the relative strengths of VB and PO inputs to any one type of cortical neurons have not. c,d ∣ The same approach was taken to generate a CTC connectivity matrix for primary motor cortex (M1), incorporating findings for both vibrissal and forelimb related M1 31,33,48,68-70,72-78, and for the prefrontal cortex (PFC) 80-82 e ∣ An ‘average’ CTC connectivity matrix for cortical areas was assembled based on qualitative comparison across areas, including those considered above, ALM, A1, V1 and others, as discussed in the main text 25,52,75,84-94,101,102 Connections that tend to be found consistently are marked with ‘+’ and stronger colours, those that tend to be more variable with ‘±’ and intermediate colours, and those that tend to be weak or absent with ‘−’ and the lightest colours.
A caveat before we begin our survey across areas: we emphasize results from experiments using relatively high-precision, quantitative tools for cell-type-specific analysis of synaptic connectivity (for example, viral labelling, optogenetic photostimulation and whole-cell electrophysiology). The ‘strength’ of synaptic connectivity in a circuit can be measured in different ways, which have various inherent limitations and biases, and can furthermore be represented in different ways, from unitary to population-level connections43. In neuronal circuit science even ‘ground truth’ depends to some extent on how one defines it. The point is that one can get a different picture of circuit organization depending on which tools are used and how connection strength is defined. Although we emphasize quantitative findings, these have generally been reported in terms of relative rather than absolute connection strengths. Furthermore, these studies generally involve comparing how one presynaptic cell type connects to multiple different postsynaptic cell types, not vice versa (that is, how different presynaptic cell types connect to one postsynaptic cell type). For this reason, the matrices mainly reflect measurements made along each column, a point emphasized by colouring the columns according to presynaptic source. In addition, for all these reasons, the connectivity matrices shown here, although based on quantitative findings, should be viewed as mostly qualitative summary representations of overall connectivity patterns. We aim to point out more of the issues as we go along.
Primary somatosensory cortex.
We start with the whisker-barrel system of rodents, where much is now known about cell-type-specific connectivity in the CTC loops. Recent studies of the homologous circuits for the forelimb-related pathways confirm and extend those results. In this section, we treat the whisker-related and forelimb-related circuits together, noting differences where applicable.
We first consider CT axons (Fig. 2B). These project to the core-type ventral basal complex (VB) consisting of ventral posterior medial (whiskers) and lateral (limbs and trunk) nuclei. The CT axons synaptically excite VB neurons44-46, but do these connections ‘close’ CTC loops at the thalamic end? This is not entirely clear, as CT axons could innervate either reciprocally or non-reciprocally projecting VB neurons (or both)47. These patterns have different functional implications; for example, excitation of non-reciprocal VB neurons could mediate lateral excitation in the thalamus. Recent results from forelimb-related primary somatosensory cortex (S1)–CT circuits are illuminating in this regard48. When a single site in forelimb S1 is injected with both a virus (to anterogradely label the CT axons emanating from that particular site with an excitatory opsin) and a retrograde tracer (to label the thalamic neurons projecting to that particular site), the CT axons tend to co-localize with and strongly excite the S1-projecting VB (VBs1proj) neurons. Thus, these connections appear to close the potential CTC loop at the thalamic end. Moreover, the relative paucity of CT axonal projections to non-recurrently projecting VB subregions suggests that CTC loops tend to be spatially restricted not just to cortical areas and thalamic nuclei, but to paired subregions within those areas and nuclei. Whether this organization is a peculiarity of the CT→VB circuits of S1, which is extremely somatotopic, or applies more generally across CTC loops, is assessed as we consider other areas.
The CT axons also project to the matrix-type posterior nucleus (PO)44, in a pattern similar to the CT→VB loops described above: CT axons arising from a location in S1 tend to connect mainly to POs1proj neurons that project back to the same location48. The connection strengths are somewhat less than for CT excitation of VB neurons. Those CT neurons projecting to the PO also send branches to the VB and reside mainly in lower layer 6, whereas those that project exclusively to the VB reside in upper layer 6 (REFS49,50).
PT axons send axonal branches to the PO but not to the VB, en route to more caudal destinations51,52. Following injections of anterograde and retrograde tracers into a single cortical site in forelimb S1, the PT axons, similar to the CT axons as described above, overlap anatomically with and strongly excite POS1proj neurons, more so than non-recurrently projecting neurons48. Thus, the PT axons also tend to form CTC loops that are closed at the thalamic end, following a similar pattern of restriction to a pair of cortical and thalamic subregions.
Connectivity from the VB to the cortex has been investigated in detail (Fig. 2B). The VB axons project to layer 4 of S1, but also branch in deeper layers, around the layer 5B/6 border53. Functional analyses also show that the strongest VB input goes to layer 4, involving numerous albeit unitarily relatively weak connections, with additional inputs to pyramidal neurons in other layers54-58, such as to the basal dendrites of layer 2/3 neurons55. In terms of cortical cell types and projection classes, the VB axons excite mainly IT neurons (of which layer 4 neurons can be considered a subtype), with strongest input to layer 4 spiny stellate cells (which in the mouse are only abundant in layer 4 of S1, not other cortices) but also including moderately strong input to infragranular corticocortical neurons, and additional input to PT neurons55,56,59,60. Recent evidence indicates that thalamocortical drive from VB axons to PT neurons in S1 arises less from direct excitation and more from rapid and potent disynaptic excitation via layer 6 IT neurons61. The VB input to CT neurons is markedly weak50,60, consistent with the lack of CT responsiveness to whisker stimulation in vivo61, indicating that the core-type CTC loop is not closed at the cortical end through strong direct connections between TC and CT neurons. Instead, CT neurons probably get their main input from IT neurons60,62, implying that for the core-type nucleus, the CTC loop passes via IT neurons to close the loop through only CT (not PT) neurons.
The cortical connections of PO axons have also been examined and — consistent with the axonal arborization primarily in layers 1 and 5A – the strongest PO inputs are to IT neurons in layer 5A, with mostly weaker input to other IT neurons and PT neurons, and little or no input to layer 4 and CT (or unidentified layer 6) neurons55,57,60,63,64. Thus, for this matrix-type nucleus, the CTC loop at the cortical end appears mostly to bypass both PT and CT neurons, passing instead through IT neurons, particularly IT layer 5A neurons, to close the loop, in this case through both PT and CT neurons.
Primary motor cortex.
M1 provides an interesting comparison to S1 CTC circuits, as it is a ‘motor’ rather than a ‘sensory’ area. (Many, perhaps all, cortical areas are arguably a mix of both; all areas possess PT neurons, the subcerebral projections of which directly innervate motor circuits, and sensory responses are often widespread65,66.) As such, M1 is perhaps more ‘output’ than ‘input’ oriented. However, it is also a ‘primary’ area rather than a ‘higher-order’ area — or perhaps not; the applicability of these concepts of hierarchical organization is more complicated in motor than in sensory areas67. Thalamic afferents to M1 arise from multiple nuclei in the ‘motor thalamus’, including the cerebellar recipient ventral lateral nucleus (VL), the basal ganglia recipient ventral anterior nucleus and ventral medial nucleus (VM), and the PO68-71. As seen in single-axon reconstructions31,33,72,73, axons from VL arborize in a mostly core-like manner in two laminar zones, one around layer 4 and adjacent layers and another around the layer 5B/6 border, plus a component in layer 1; axons from the VM, the ventral anterior nucleus and the PO arborize in a mostly matrix-type manner primarily to layers 1 and 5A, although the pattern in the middle layers is fuzzier than the sharply demarcated PO projections to layer 5A of S1.
The CTC-related connectivity patterns of M1 can be summarized as follows (Fig. 2C); again, we generally combine results for vibrissal and forelimb areas except where noted. Like the CTC circuits of S1, the CT and PT axons from M1 excite recurrently projecting neurons located in subregions of the matrix-type PO and VM48,74,75. Unlike those circuits, CT axons rather weakly excite recurrently projecting neurons in the core-like VL74. In the other direction, the VL innervation of M1 neurons largely resembles the pattern for VB→S1 in targeting layer 4, but it differs in that input to layer 4 (which is thin in motor cortex) is not dominant but comparable to the input to deeper-layer IT neurons at the layer 5B/6 border and PT neurons, and there is also some layer 1 input to PT neurons’ apical dendrites74,76,78. The PO projections are directed primarily to IT neurons in upper layers (layer 2/3 to layer 5A), and CT neurons again appear to be notably lacking in thalamic input, from either the VL or the PO74,77,78.
Prefrontal cortex.
Circuit investigations of the PFC, anatomically defined as the cortical projection territory of the mediodorsal nucleus (MD), include recent detailed dissections of excitatory connectivity in CTC loops79-82 (Fig. 2D). In this case, CT and PT axons from the PFC project to both the MD and VM and excite recurrently projecting neurons there. Both nuclei send projections to the PFC. Those from the MD follow a pattern that appears mainly core-like, in that the axonal branches to the PFC ramify in layer 3 (perhaps corresponding to layer 4, which therwise appears to be absent in the PFC), but with additional branching in layer 1, similar to the core-like VL projection to M1. The MD axons excite IT neurons most strongly (in layers 2/3 and 5), with moderate input to PTMDproj neurons as well — roughly comparable to the pattern of core-type input to S1 and core-like input to M1 neurons. The matrix-type VM axons ramify mainly in layer 1 of the PFC, exciting layer 2/3 IT and PTVMproj neurons in particular. Neither MD nor VM axons provide much if any input to CT neurons projecting to either nucleus.
Anterior lateral motor cortex.
The anterior lateral motor cortex (ALM) is a premotor-like area at the frontal pole that is involved in motor planning and execution83. Of particular relevance for CTC loops, reverberatory activity involving ALM and VM is implicated in anticipating and directing upcoming actions84. Results from recent analyses of cell-type-specific connectivity between the ALM and thalamus are summarized as follows75. The CT and PT axons from the ALM excite recurrently projecting neurons located in a subregion of the VM. The PT neurons with thalamic branches are more abundant in the upper than in the lower sublayers of layer 5B, and tend not to project more caudal than the pons25. In the other direction, VM axons project mostly to layer 1 of the ALM and excite both IT neurons in layer 2/3 and PTVMproj neurons. A disynaptically closed CTC loop is thereby formed by the PT→VM and VM→PT connections, which is probably augmented by the strong layer 2/3→PT connections in this cortical region85. The VM input to the PT neurons occurs through both apical-tuft and basal or perisomatic dendrites. The MD also receives some input from PT (and probably CT) axons, and may project weakly to the ALM. Nevertheless, the ALM appears to differ from M1 and S1 in that its main source of thalamic input is from a matrix-type nucleus (the VM).
Primary auditory cortex.
The primary auditory cortex (A1) receives a core-type projection from the ventral subdivision of the medial geniculate body (MGB). These axons form functional excitatory synaptic contacts mainly onto layer 4 pyramidal neurons, with additional weaker innervation of cortical pyramidal neurons across all layers86. This pattern resembles that of other core-type projections to the cortex, with branches ramifying most densely in layer 4, and to a lesser extent around the layer 5/6 border. The extent to which CT and PT neurons in A1 receive direct input from the auditory thalamus is not yet clear, but laminar profiles of MGB input suggest relatively weak excitation, consistent with patterns seen in other areas. In the other direction, the CT and PT innervation of TC neurons in the subdivisions of the MGB has also not been fully determined, but the available anatomical data indicate reciprocity, with auditory cortical projections overlapping extensively with auditory thalamic subregions projecting back to auditory cortical areas87,88.
Primary visual cortex.
The core-type projections from the dorsal lateral geniculate nucleus (dLGN) to V1 have been studied primarily using anatomical methods. Recent functional connectivity analyses86,89 confirm the anatomically suggested pattern of strongest input to pyramidal neurons in layer 4, with additional input to other layers, broadly similar to the pattern for MGB→A1 (discussed above). The input to infragranular layers has not yet been further dissected to assess the relative innervation of IT, CT and PT neurons. In addition to the main dLGN projection to layer 4 of V1, a subdivision of the dLGN has been identified that receives input from direction-selective ganglion cells and projects to superficial layers, particularly layer 1 (REF.90). Anatomically, the mouse’s homologue of the pulvinar, the lateral posterior nucleus (LP), projects to the visual cortex in a matrix-type pattern with axons ramifying in layer 1 (REFS91,92). The cell-type-specific connectivity has not yet been determined for these projections of LP to V1, but it has been determined for its projections to higher-order visual cortical areas, showing robust excitation of multiple types of IT neurons (projecting to V1, the striatum or the amygdala) with little or no input to corticotectal-type PT neurons92. In the other direction, the CT projection from V1 innervates dLGN neurons52,93. Anatomical evidence also indicates a projection from (lower) CT neurons to the LP52,91. The PT axons project not to dLGN but instead to LP51,52,94, forming transthalamic routes to higher-order cortex (discussed below).
A note on other species.
Our review emphasizes results from mice, in which functional synaptic connectivity in CTC circuits has been most extensively investigated, supplemented by rat data. Much anatomical work has been done on other species, particularly the visual pathways of primates and carnivores. Reviews and books are available that address various aspects of CTC circuits in diverse species (for examples, see REFS1,9,87,95-99). CTC circuits in non-rodent species are also under renewed scrutiny using optogenetic methods (for example, see REF.100). Here, we highlight two comparative aspects of CTC circuits. One concerns the TC→CT component: in cat V1, earlier indirect evidence had suggested strong direct LGN excitation of CT neurons, but ultrastructural analysis101 revealed relatively weak connectivity, a finding consistent with the several observations (reviewed above) of relatively weak TC input to CT neurons across mouse cortex. Another concerns CTC loops of primate V1: recent evidence indicates that both CT and PT axons from V1 project to the pulvinar, and pulvinar TC neurons project back to layer 1 of V1 (REF.102); thus, the same set of components described above for rodents appears to be present in primates. These examples underscore the value of studying diverse model organisms, which are essential to distinguish conserved versus species-specific features of the circuit architecture.
So, what’s generic, and what’s not?
A motivation for looking in some detail at the CTC loops across different areas and systems is that we can now try to assess their commonalities and differences (Fig. 2E, Fig. 3A,B).
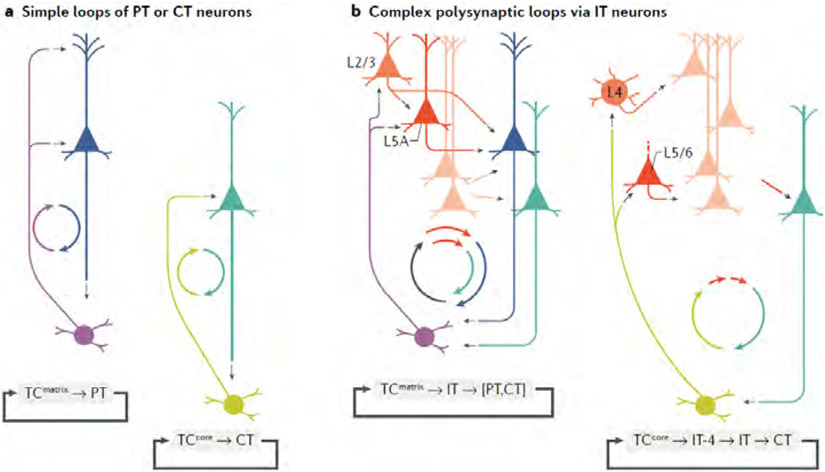
Fig. 3 ∣. Patterns and types of circuit connections in cortico-thalamo-cortical loops.
a ∣ Simple cortico-thalamo-cortical (CTC) loops involve direct connections between TC and PT or CT neurons. Such loops are relatively uncommon. b ∣ Complex loops involve IT neurons closing the cortical end of the loop. Complex loops, particularly of the matrix-type, appear to be ubiquitous. The smaller ‘cyclo-plots’ (circular circuit plots with coloured arrows) are included as concise representations of the basic cellular connectivity in each type of loop.
Types of CTC loops.
As a starting point, it may be useful to distinguish between simple and complex types of CTC loops. Simple loops (Fig. 3A) involve monosynaptic connections between recurrently projecting neurons in the cortex and the thalamus. The two classes of cortical neurons (CT and PT) and the two classes of thalamic neurons (core-type and matrix-type) can potentially form four distinct types of simple CTC loops: CT↔matrix, CT↔core, PT↔matrix and PT↔ore. None of these loops appears to be commonly found as a ‘strong’ connection across all CTC circuits. Some of these loops can be found, but they appear to be area or system specializations. The ALM PT↔VM loop is an example of a PT↔matrix loop. To the extent that the MD is a core-like nucleus, the PFC PT↔MD loop is an example of a PT↔ore loop.
Complex loops (Fig. 3B) additionally involve IT neurons to ‘close’ the cortical end of the loop, polysynaptically mediating the linkage between the input from the thalamus and the cortical output back to the thalamus. Both matrix-type and core-type neurons form complex loops, but a key difference is that they engage different subtypes of IT neurons with distinct local circuit connections (further discussed below). Simple and complex loops can both be present in a CTC circuit. In fact, complex loops appear to be ubiquitous.
Towards an outline of a basic CTC loop.
As alluded to above, there are different types of CTC loops, and most areas have multiple types. For example, in S1, the CT neurons are involved in both a core loop and a matrix loop, and the PT neurons are involved in a (similar) matrix loop. For any cortical area, it may be useful to think of the collection of individual types of loops (for example, of CT and/or PT neurons) as constituting a compound CTC loop. Can we identify elements common to the CTC loops of most cortical areas? An attempt to do so is as follows (Fig. 2E).
For the matrix-type nuclei in particular, several patterns stand out. First, in the thalamocortical direction, matrix-type TC axons mainly excite IT neurons — the one major class that does not project to the thalamus. Second, in the other direction, CT and PT axons from any cortical subregion mainly excite recurrently projecting TC neurons in a corresponding subregion in one and sometimes multiple matrix-type nuclei. Thus, dual [CT, PT]→matrix connections appear to be a standard feature in CTC loops. Third, within the cortex, IT neurons close the loop by linking TC inputs to PT and CT neurons through local-circuit connections (which are further discussed below). Another common feature in this type of loop is that CT neurons generally receive little or no direct excitation from the matrix-type axons. Thus, a conserved CTC loop is of the matrix-type polysynaptic IT-mediated variety (Fig. 3B). These loops are probably found across most if not all cortical areas, given the abundance of matrix-type nuclei and widespread distributions of their projections to cortex36,38.
Similar patterns are seen for core-type nuclei. The TC axons again mainly excite IT neurons, and the cortical axons (in this case, mainly just CT axons and not PT axons) again mainly excite recurrently projecting TC neurons in a corresponding thalamic subregion. Within the cortex, IT neurons via local circuits again link TC inputs to CT neurons, which again receive little or no direct excitation from core-type axons. These features collectively outline a CTC loop of the core-type polysynaptic IT-mediated variety (Fig. 3B). However, these loops probably have a restricted distribution across the cortex, as there are relatively fewer core-type nuclei (which include the classic sensory core-type nuclei such as the LGN and the VB, and ‘core-like’ nuclei such as the MD and the VL), and core-type axons tend to have ‘focused’ projections to a single subregion within an area36,38.
Other features of CTC circuits appear to be more variable, as well as being area-specific and system-specific, probably reflecting diverse computational processes and functions103,104. PT neurons receive matrix-type input that varies across areas (for example, stronger inputs in the PFC and ALM, but weaker inputs in S1, M1 and V1), and receive core-like input that also varies, in different patterns (for example, stronger in M1, weaker in PFC). In the other direction, PT axons usually do not innervate core-type nuclei, but an exception appears to be MD, which receives PT input from both the PFC and the ALM.
In summary, certain aspects of CTC loops exhibit variability across areas, but several appear relatively conserved. The matrix-type complex CTC loop appears to represent a particularly common and conserved aspect of circuit architecture, as does the core-type version for those cortical areas with an associated core-type nucleus.
Intertwining of CTC and cortical loops
CTC loops and excitatory local cortical circuits.
The passage of CTC loops through multiple cortical cell classes and particularly IT neurons raises the question of exactly how CTC loops and local circuits are interconnected. Addressing this question entails consideration of the local-circuit connectivity of excitatory neurons, a thumb-nail sketch of which can be summarized as a connectivity matrix that occupies the upper left part of the overall CTC matrix (Fig. 4A), with the following features (reviewed in REFS.37,105). All major neuron classes (IT, PT and CT) appear to form intra-class connections, albeit at rates that can vary within and across areas. Across-class connectivity, however, differs depending on the presynaptic and postsynaptic classes involved: IT and CT neurons connect bidirectionally, involving mainly IT neurons in deeper layers; IT and PT neurons connect mostly unidirectionally, from IT to PT; and, CT and PT neurons connect only weakly, if at all. Within the IT class, a major subtype is layer 4 neurons (comprising pyramidal, stellate, and other subtypes, in various areas), which is hodologically ‘upstream’ in the local circuit, in that layer 4 neurons form largely unidirectional connections, exciting layer 2/3 and other IT neurons but receiving few inputs back. The important implication for CTC loops, particularly in sensory cortices with an expanded layer 4, is that the loops that travel mainly via layer 4 (that is, core-type loops) generally traverse one additional layer than those via layer 2/3 or 5A (that is, matrix-type loops): core-type thalamus→4→2/3→[IT, PT, CT]→Thalamus, versus matrix-type thalamus→[2/3, 5A]→[IT, PT, CT]→Thalamus. However, in this regard, it is notable that direct thalamic input to PT neurons can arise from the core-type axons of the VB, but little or none from the matrix-type axons of the PO.
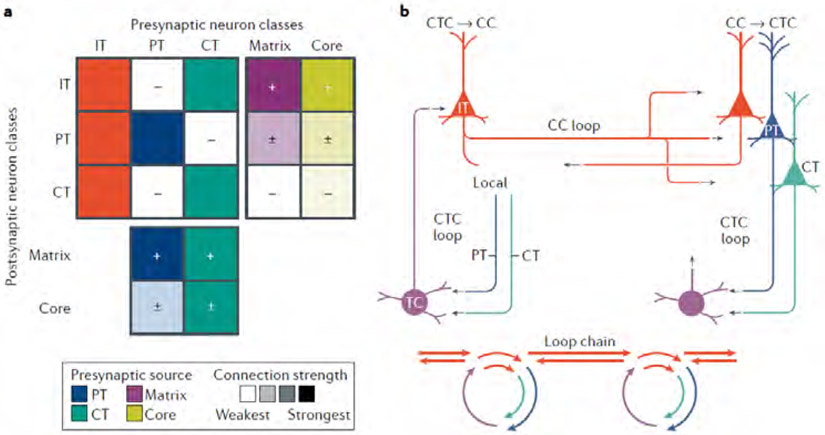
Fig. 4 ∣. Cortico-thalamo-cortical loops are interconnected with local cortical circuits and corticocortical circuits, via hub-like intratelencephalic neurons.
a ∣ The connectivity matrix combines the ‘average’ matrix for cortico-thalamo-cortical (CTC) connections (from Fig. 2e) and a matrix representing local excitatory connections among IT, PT and CT neurons in the cortex. The local-circuit portion of the matrix is a qualitative interpretation of data from many studies, as reviewed in REFS37,105. As a population, IT neurons are the strongest recipients of both matrix-type and core-type TC input, and they send their excitatory output broadly to IT, PT and CT classes. Output connections of PT and CT neurons are formed more selectively but, unlike the IT neurons, they include the thalamus. b ∣ The IT neurons (red) are hub-like components of CTC loops, local circuits and corticocortical (CC) circuits, which are also commonly organized as recurrent loops. The IT-mediated linking of CTC and CC loops forms an extended ‘loop chain’, also depicted in a simplified cyclo-plot below.
Another salient aspect of cortical circuits vis-à-vis CTC loops is that the local excitatory connections also form recurrent loops106-108. Recent evidence suggests that one important function of these local loops is to amplify thalamic signals6,104,109-111. In V1, for example, connected pairs of cortical neurons receive common LGN input, an architecture suggested to implement nonlinear classification involved in feature selectively89.
In summary, CTC loops feed right into, and right out of, local excitatory circuits in the cortex; the two are densely and inextricably interwoven.
CTC loops and excitatory CC circuits.
IT neurons — integral components of CTC loops and local cortical circuits — are also the main source of CC axonal projections28, which are the basis for direct inter-areal communication between cortical areas. Numerous connectivity analyses show that IT neurons are major sources and targets of excitatory CC connections55,112,76,77,113-117. This raises the possibility that a given cortical area’s CTC loops and CC circuits are fundamentally interconnected via IT neurons (Fig. 4B). Although multiple types of IT neurons can be involved, those in layers 2/3 and 5A appear to be particularly important for mediating links and cross-talk between CTC loops and CC circuits. The layer 2/3 IT neurons, as postsynaptic elements in CTC loops, receive direct input from matrix-type nuclei (although relatively stronger in frontal cortex than in primary sensory cortex) and mostly indirect input via layer 4 from core-type nuclei (reviewed above). At same time, layer 2/3 IT neurons are also postsynaptic elements in CC circuits, receiving input from presynaptic axons arising from other cortical areas. They are also major sources of CC axons, and thus important presynaptic elements in CC circuits. Similarly, layer 5A IT neurons serve as shared elements in CTC loops involving matrix-type nuclei and CC circuits. Layer 6 IT neurons can also be involved, receiving CC inputs from higher-order cortical areas and connecting to local CT neurons74,76,77,113,114,118.
By contrast, PT and CT neurons participate in CC circuits in a limited way. As presynaptic elements, PT neurons can form long-range projections between certain cortical areas26,119-122, but these projections are relatively uncommon and the terminal arbors appear less dense than those of the IT projections. CT neurons generally lack CC axons. As postsynaptic elements, PT neurons can receive input from CC axons at their apical tuft dendrites and/or basal dendrites55,76,77,115. CT neurons can also receive excitatory CC input from other areas, but to an extent that appears to vary across areas and systems77,114,115,118,123.
A key point is that CC circuits are themselves typically organized as loops76,113-115,117,118,124,125. Thus, concatenation of CTC and CC loops forms an extended CTC–CC ‘loop chain’. Coupling of CTC–CC loop chains across multiple areas forms an extended network (Fig. 4B).
The evidently ubiquitous interlinking of CTC and CC loops by IT neurons suggests that the extended CTC–CC loop constitutes a basic module of cerebral organization. This implies that activity in a CTC loop in a given cortical area can influence other interconnected CTC loops via CC loops. This chain of events can bridge the hemispheres through the callosal axons of the IT neurons at the heart of these extended loops.
In summary, CTC loops feed right into, and right out of, both CC and local cortical circuits, forming extended loop chains. In the next section, we look at the interlinked CTC and CC loops in the context of models addressing the hierarchical organization of the cortex.
Relating CTC loops to circuit models
The transthalamic model.
The transthalamic model describes a hierarchical organization across multiple cortical areas and thalamic nuclei, emphasizing divergent outputs from the cortex to the thalamus, including transthalamic pathways. This model was inspired by anatomical patterns in visual pathways and developed particularly by Sherman and Guillery; for example, see REFS9,10,97,126; for a related comparative anatomy review see REF.87. Its basic features as they pertain to a primary cortical area such as V1 are as follows (Fig. 5 A). The first stage of the circuit is the projection from a first-order thalamic nucleus to a first-order cortical area, as exemplified by dLGN→V1. The model does not explicitly distinguish among potential postsynaptic targets in the cortex (IT, PT, CT, etc.). A key distinction is in the two corticofugal outputs, which diverge: CT axons send feedback projections to the first-order thalamic nucleus, whereas PT axons send feedforward projections to a higher-order thalamic nucleus. The higher-order nucleus projects to a higher-order cortical area, constituting a transthalamic circuit from the first-order to the second-order cortical area, in addition to direct CC projections between areas. Accordingly, all thalamic nuclei receive CT input but only higher-order nuclei receive PT input. This model also embodies important concepts of functionally dichotomous ‘driver’ versus ‘modulator’ properties of the feedforward and feedback pathways, respectively, but here we focus on the connectivity patterns.
Fig. 5 ∣. Cortico-thalamo-cortical loops in the context of hierarchical models of cortical organization.
a ∣ In Sherman and Guillery’s model9,10,97,126 (sketched here based on REF.126, with the right-left orientation mirrored to match that of panel d below), CT and PT neurons send divergent output to thalamic nuclei. The PT axons form feedforward (FF) ‘transthalamic’ connections that disynaptically link lower-order to higher-order cortex, via higher-order (HO) thalamic nuclei, in parallel with direct corticocortical (CC) pathways. The CT axons form feedback (FB) connections back to the main thalamic nuclei supplying their cortical area, including the first-order (FO) nucleus, such as the LGN or VB. The transthalamic pathways of PT neurons are posited to carry efference copies of motor commands. b ∣ In Larkum’s model133, a simplified version of which is sketched here, an essential central element is a ‘backpropagation-activated coupling’ (BAC) firing mechanism that generates strong, sustained burst firing upon coincident excitation to apical and basal→perisomatic dendritic domains. On their own, the feedforward and feedback inputs generate only weak firing in the postsynaptic pyramidal neuron. The sketch omits additional aspects of the model (see REFS133,142). Various cortical and thalamic sources provide feedback and feedforward input, making the BAC mechanism a key activity-dependent integrative node in CTC circuits. The BAC firing model provides a compelling cellular mechanism for associative pairing of co-active neurons within large-scale CTC networks. c ∣ A similar model, incorporating additional connectivity details and emphasizing the polysynaptic routes of excitation to layer 5 neurons via IT neurons. d ∣ The CTC loops of S1, M1 and M2 are organized hierarchically, with ‘top-down’ M2→M1→S1 pathways running counter-current to ‘bottom-up’ S1→M1→M2 pathways. Areas additionally form ‘lateral’ connections with their contralateral counterparts and with other cortical regions, such as M1↔2 pathways (not shown). Area-associated subregions of matrix-type nuclei are indicated by superscripts (for example, VMALM). The layout is oriented with anterior/frontal to the left, both to follow the convention of standard atlases and to emphasize the bidirectionality of information flow in these loops. This three-area network offers a framework for further investigation of CTC loops in the context of hierarchical cortical circuits. Part a adapted with permission from REF.126. Part b adapted with permission from REF.133.
The CTC–CC loop model largely accords with the feedback CT projection to first-order thalamus and with PT projections to the higher-order thalamus posited in the transthalamic model. However, two observations that are not major features in the transthalamic model are the CT connections to higher-order thalamic nuclei, and the feedback-type connections from PT axons to recurrently projecting TC neurons. The important conceptual significance is that the CTC circuits form feedback loops driven by both PT and CT neurons via higher-order thalamus. This raises the question of whether the feedback loop is a prominent feature for CTC circuits, or co-exists with feedforward transthalamic circuits as proposed in the transthalamic model, which has also been demonstrated experimentally for V1→LP/pulvinar transthalamic pathways to higher-order visual areas as well as in some other systems10,94,114,127,128. A potential answer to this question can be gleaned from single-neuron reconstruction studies. These have shown that projections from matrix-type TC neurons can have multiareal branching, with each branch projecting to a different cortical area72,73,129-132. This points to the capacity of [CT, PT]→matrix connections to serve as both feedback and feedforward (transthalamic) connections. We return to this topic in a later section to consider further the potential significance of multiareal TC branching for CTC loop organization.
The backpropagation-activated coupling model.
Another important model concerned with CTC and CC circuits is the backpropagation-activated coupling (BAC) model developed particularly by Larkum, which emphasizes convergent input to cortical neurons generating nonlinear output via active dendritic mechanisms133. This model builds on Llinás’s influential thalamocortical model2 and Jones’ core–matrix framework34,38, emphasizing the concept that sensory perception involves the integration of ‘top-down’ and ‘bottom-up’ information (REF.134), and adding key features at the biophysical and network levels. Larkum’s model (Fig. 5B) attempts to explain how signalling along feedforward (bottom-up) and feedback (top-down) pathways, arising from lower-order and higher-order thalamus and the cortex, are bound together by layer 5 pyramidal (presumably PT) neurons in the given cortical area, and hypothesizes about its potential importance in generating perception and consciousness. The BAC model differs from the transthalamic model in focusing on cellular-level integration of information from TC and CC pathways rather than propagation of information along these pathways across areas. The feedback axons, arising from matrix-type thalamic nuclei and/or higher cortical areas, travel in layer 1 and contact the apical tuft dendrites of layer 5 pyramidal neurons. The feedforward axons from core-type nuclei and/or lower cortical areas travel in deeper layers and contact the basal dendrites of the same pyramidal neurons. Temporally coincident activity in both axons evokes burst-firing in layer 5 neurons through a BAC mechanism involving active properties of dendrites such as calcium-dependent spiking.
The BAC model constitutes an attractive candidate for a basic operational principle for cortical function. By detailing a cellular mechanism for associative pairing, it potentially explains how individual neurons within complex CTC–CC loops can be selectively activated by coincident activity. As further discussed below, it predicts this process to be fundamentally involved in associating external data with internal representations of the world, among other cortical functions. We conjecture about how this BAC model relates to the CTC–CC loop connectivity results reviewed above (Fig. 5C). Some of the details fit well, like anatomical projection of matrix-type thalamic axons to layer 1. In some cases where functional connectivity was also examined, such layer 1 targeting axons innervated the apical tuft dendrites of layer 5 neurons including PT neurons75,135. However, in other cases the matrix-type inputs, despite anatomically targeting layer 1, provide surprisingly little excitatory input to the apical tuft dendrites of PT neurons — notably in S1 (REF.55). Indeed, some of these matrix-type inputs (again, in S1 in particular) strongly innervate the basal dendrites — but of IT, rather than PT, neurons. More work is needed to explore this generally highly compelling model and understand how circuit organization in CTC–CC loops relates to it. The S1–M1–M2 network is particularly approachable as a model system for investigating models of hierarchical organization across areas, as much is already known about the cellular connectivity in the CTC and CC circuits in this system (Fig. 5D). Knowledge about the cellular organization of these CTC loops is beginning to be integrated into broader concepts about how multiple types of loops interact in the service of sensorimotor integration and motor control136-139.
Consciousness and perception
A longstanding idea about CTC circuit architecture is that it supports diverse consciousness-related aspects of behaviour, with layer 5 pyramidal neurons, and PT neurons in particular, proposed to serve in a critical capacity to integrate apical and basal inputs through active dendritic mechanisms, as described above133,140. As a fuller treatment of this complex subject is beyond the scope of this Review, we highlight current progress by focusing on recent findings from Larkum and colleagues (for in-depth coverage, see REFS141,142), and consider how these relate to some of the organizational features of cell-type-specific CTC loops outlined herein.
One line of research concerns how anaesthetics induce unconsciousness. Taking this approach, a recent study in mouse S1 found evidence that general anaesthesia dampens the coupling of apical dendritic activity to somatic firing in layer 5 pyramidal neurons, consistent with predictions of the BAC model143. The study left unresolved whether layer 5 IT or PT neurons (or both) may differentially contribute, but insight into this comes from another line of research concerning sensory perception. Using tools for selective access to layer 5 IT versus PT neurons in S1, a recent study showed that the apical dendrites of PT neurons, and not those of IT neurons, are indeed critical for tactile sensory perception144. Furthermore, PT connections to certain subcerebral targets including the PO are required for this function. These findings thus directly implicate PT neurons and their CTC connections in this form of perceptual processing. The involvement of thalamus-projecting PT neurons is intriguingly reminiscent of ALM circuits (reviewed earlier), in which thalamus-projecting PT neurons support preparatory activity in CTC loops with the VM.
Does this evidence relegate the other CTC loop elements to minor supportive roles in consciousness-related processes? The organization of complex polysynaptic CTC loops suggests instead that other cell types and their connections are also crucially involved, in distinct ways. For example, IT neurons of layer 5 may be especially important, given their prominent roles both in linking matrix-type TC input to PT neurons (Fig. 3B, Fig. 5C) and as hub-like circuit elements linking CTC and CC circuits into extended CTC–CC loops (Fig. 4B, Fig. 5D). The latest results144 do not exclude roles for these layer 5 IT neurons in general, only their apical dendrites — which are spare (‘thin-tufted’) and lack active mechanisms present in PT dendrites145. One challenge for future investigation is to further determine what each circuit element contributes to particular aspects of consciousness-related behaviours. A related challenge is to characterize the mechanisms that enable such functional differentiation within and across CTC and CC loops, despite the dense interlinkages via shared pools of IT neurons. Candidates include cell-type-specific neuromodulatory mechanisms, which are highly distinct for IT versus PT neurons (for reviews, see REFS146-148), as well as inhibitory microcircuits (discussed below). With the current pace of discovery in this area of research, a detailed cellular-level understanding of how CTC–CC loops mediate consciousness-related aspects of behaviour seems an increasingly realistic goal.
This brief treatment overlooks many aspects of this complex topic; for example, disorders of consciousness also accompany various forms of epilepsy, a topic that provides another important window onto CTC loops and their roles in normal and pathological rhythms, and one that underscores the importance of inhibitory mechanisms (discussed next)2,4,7,149.
Inhibitory mechanisms in CTC loops
This Review emphasizes CTC loop organization from the perspective of excitatory projection neurons, but the real picture is far more complicated, as GABAergic inhibitory neurons, embedded at each node in these loops, are also integral components and essential for the functions and multiple operational modes of these loops. We briefly highlight some of the major types and connections of inhibitory neurons implicated in CTC loops. For an in-depth review of thalamic inhibitory mechanisms, see REF.5.
Reticular nucleus.
The shell-like thalamic reticular nucleus (TRN) contains inhibitory neurons that project to TC neurons and, in return, receive projections from CT and TC neurons, but generally not PT neurons (Fig. 6A). The feedforward CT→TRN→TC and feedback TC→TRN→TC circuits are considered to be topographically aligned, and circuit analyses have delineated their functional connections and organization in sensory pathways45,46,93,150-152. However, many details about the TRN’s involvement in CTC loops remain to be elucidated, such as whether TC↔TRN connections are reciprocal at the level of individual pairs of neurons. The CT→TRN→TC circuits influence sensory processing in core-type thalamic nuclei in several ways, including potent disynaptic and lateral inhibition153-156. Feedforward TC→TRN→TC circuits both within and across thalamic nuclei have also been described157-160 (Fig. 6A). Recent evidence suggests that VP→ TRN and PO→TRN circuits are largely segregated within the somatosensory sector of the TRN161,162,198, indicating separate regulatory mechanisms for core-type and matrix-type CTC loops at the level of the TRN (Fig. 6A,B). TRN neurons are strongly coupled electrically, but GABAergic intra-TRN connectivity, present developmentally, is scarce in adulthood163-167. Another major function of TC↔TRN loops is thalamocortical rhythmogenesis, involving post-inhibitory rebound excitation in the TC neurons2,4,7,168,169. Cortical input is also involved, however; for example, input from S1 CT axons directly drives both TRN and TC neurons in the VB, which in turn drive TRN neurons through disynaptic excitation; genetic mutations that selectively impair CT→TRN signalling cause pathological rhythmogenesis152. This example underscores how TC↔TRN loops do not operate in isolation but are embedded within CTC loops.
Fig. 6 ∣. Inhibition in cortico-thalamo-cortical loops.
a ∣ The thalamic reticular nucleus (TRN) is a major inhibitory hub in CTC circuits. Connections of inhibitory TRN neurons include descending inputs from CT axons (left). One type of circuit thought to mediate inter-nuclear cross-talk is via the TRN (middle). Another type of circuit, based on recent evidence for segregated loops, links matrix-type thalamic nuclei with TRN ‘shell’ neurons, and core-type thalamic nuclei with TRN ‘core’ neurons (right). b ∣ The connectivity matrix qualitatively incorporates circuit connections of the TRN2,4,7,45,46,93,150-162,164-169. The two types of GABAergic neurons in the TRN have distinct connectivity patterns. c ∣ The diagram depicts the CTC-related connections of inhibitory neurons in the cortex that have been identified in the sensory cortex50,57,64,93,170-181. For both the core-type and matrix-type TC projections, the fast-spiking, parvalbumin-expressing interneurons are major targets, but the somatostatin-expressing neurons are not. Matrix-type projections excite additional classes of interneurons through their layer 1 axons. The circuit organization generates strong sensory-driven feedforward inhibition in sensory cortices. d ∣ In the PFC, dissection of CTC-related circuits of layer 1 (REF.183) shows stratification into parallel channels in two sublayers, with matrix-type VM axons targeting neuron-derived neurotrophic factor-expressing interneurons and pyramidal neuron dendrites in layer 1A, and core-like MD axons targeting vasoactive intestinal peptide-expressing interneurons in layer 1B, which are also likely targets of corticocortical (CC) input. Distinct forms of short-term plasticity at the various synapses generate complex dynamics to regulate pyramidal neuron excitability. e ∣ An unusual variant of layer 1 inhibition in CTC loops is found in the retrosplenial cortex (RSC), where apical tuft dendrites of pyramidal neurons receive both excitatory input from matrix-type axons of anteroventral (AV) thalamus and long-range GABAergic input from RSC-projecting CA1 neurons in hippocampus135. This apparently unique arrangement exemplifies how CTC circuit organization can be regionally specialized, presumably to mediate system-specific functions.
Cortical inhibitory neurons.
In sensory areas (Fig. 6C), core-type TC axons generate strong feedforward inhibition through monosynaptic excitation of cortical inhibitory neurons, particularly fast-spiking PV+ neurons in layer 4, which sharpen sensory responses both spatially and temporally170-175. These axons can also recruit inhibitory neurons in layer 6, including ‘translaminar’ PV+ neurons that also receive CT excitation, and send axons to multiple layers to modulate cortical gain 50,93,176, an organization that illustrates how the cell-type-specific connectivity of interneurons sets up complex inhibitory interactions within CTC loops.
Functional connections from matrix-type TC axons to inhibitory neurons in some ways resemble the core-type circuits, in that PO axons drive PV+ neurons in layer 5A of S1 more than other types of inhibitory neurons in the same or different layers57,64. A major difference from core-type circuits is innervation of inhibitory neurons in upper layer 2/3 and, particularly, layer 1, which harbours diverse inhibitory cell types177-179. For example, PO axons appear to engage feedforward inhibition in layer 2 but, in this case, via non-PV+ neurons, including neurons expressing serotonin receptor type 3a (a superclass of neurons that includes those expressing vasoactive intestinal peptide (VIP+ neurons)) at the layer 1/2 border64. The PO→VIP+ neuron circuits, however, also disinhibit layer 2/3 pyramidal neurons in vivo, via inhibition of upper-layer neurons that express somatostatin (SST+ neurons), thereby gating the integration of core-type and matrix-type TC inputs important for synaptic plasticity180. In A1, matrix-type MGB axons excite neurons in layer 1A that express neuron-derived neurotrophic factor (NDNF+ neurons), which in turn, inhibit the apical dendrites of layer 2/3 pyramidal neurons181. Layer 1A of A1 also receives matrix-type axons from the LP, which modulate auditory responses of layer 2/3 IT neurons through feedforward inhibition mediated by selective excitation of GABAergic neurons; multisensory input to the LP from the superior colliculus furthermore implicates this circuit in contextual and cross-modal modulation of auditory cortical processing182.
The extent to which these superficial-layer inhibitory mechanisms engaged by TC axons are common across different areas is beginning to be resolved as more areas and pathways are studied. In the PFC (Fig. 6D), the core-like MD axons branching in layer 3 produce feedforward inhibition in layer 2/3 pyramidal neurons via activation of fast-spiking PV+ neurons; however, unlike in S1, the relative input strength to excitatory and inhibitory neurons is more balanced80. MD axons branching in layer 1B, on the other hand, excite VIP+ and NDNF+ neurons with distinct local connectivity183. The VIP+ neurons selectively inhibit SST+ neurons; thus, MD→VIP+ neuron circuits may disinhibit layer 2/3 pyramidal neurons like PO→VIP+ neuron circuits in S1. By contrast, NDNF+ neurons selectively inhibit PV+, IT neurons in layer 2/3 and PT neurons but not SST+ neurons 183. The MD→ NDNF+ neuron circuits thus likely produce both feedforward inhibition and disinhibition in the simple and complex CTC loops found in this cortical area. Axons from VM project to layer 1A of the PFC where they preferentially activate late-spiking NDNF+ neurons81,183. The VM→NDNF neuron circuits inhibit apical tuft dendrites of PT neurons and regulate dendritic Ca2+ events (generated through back-propagation of action potentials from the soma), as well as integration of excitatory VM inputs directly innervating the same dendritic compartment.
In M1, comparison of core-type and matrix-type inputs to identified cortical inhibitory neurons is lacking, but two recent anatomical studies suggest reduced engagement of feedforward inhibition compared with other areas. In an electron microscopic study of M1 layer 4, TC terminals were found to synapse exclusively onto spiny and not smooth dendrites184. Another study showed fewer shaft synapses (putatively inhibitory contacts) for PO→M1 than PO→S1 or VB→S1 axons132, similarly indicating relatively weaker feedforward inhibition in the TC circuits of M1. However, M1 is not known to have fewer inhibitory neurons overall, suggesting instead an area-specific specialization in TC connections, biased towards excitatory rather than inhibitory neurons.
A recent analysis of the CTC circuit delineates an unusual variant of inhibitory regulation in the retrosplenial cortex135 (Fig. 6E). A matrix-type projection from the anteroventral nucleus targets layer 1A, exciting the apical tuft dendrites of layer 5 (and likely additional) pyramidal neurons. This TC excitation can be directly suppressed by long-range inhibitory inputs arising from CA1 retrosplenial-projecting neurons in the dorsal hippocampus. Thus, unlike the within-loop feedforward and feedback inhibitory mechanism tightly linked to CTC loops described above, this form of inhibition arises outside of the CTC loop.
Summary.
The excitatory connections formed by IT, PT, CT and TC neurons to other excitatory neurons in CTC loops are almost always accompanied by connections to inhibitory neurons as well. An exception is PT neurons’ axons, which generally bypass the TRN without exciting inhibitory neurons. It is also intriguing that both PT and CT axons, particularly those arising from frontal areas, can branch into the contralateral thalamus30,31,185,186 (Fig. 1C). We speculate that these bypass the contralateral TRN, constituting another example of an inhibition-free connection, in this case involving an interhemispheric type of transthalamic CTC circuit. In complex CTC loops involving IT neurons, intracortical connections linking IT to PT and CT neurons are also intersected by inhibitory neurons105,176,187,188. Certain inhibitory mechanisms are evidently highly specialized for a particular system or area, with examples at the cortical level as mentioned above, and at the thalamic level such as nucleus-specific inhibition from diverse sources including basal ganglia, zona incerta, and more5. However, many of these inhibitory mechanisms are likely to be broadly conserved across areas, differing quantitatively rather than qualitatively. Few studies have directly compared CTC-related mechanisms between areas, but one recent report did so for MGB→A1 versus MD→PFC circuits, identifying multiple quantitative anatomical differences in axonal varicosity sizes, patterns and numbers of contacts onto PV+ neurons, and more79. These results underscore a basic principle of cortical circuit organization37: that cellular mechanisms tend to be serially homologous (that is, conserved across areas) but quantitative differences (for example, in sizes, positions, numbers of structures and physiological parameters) can generate functional diversification and specialization.
Significance of multiareal TC axons
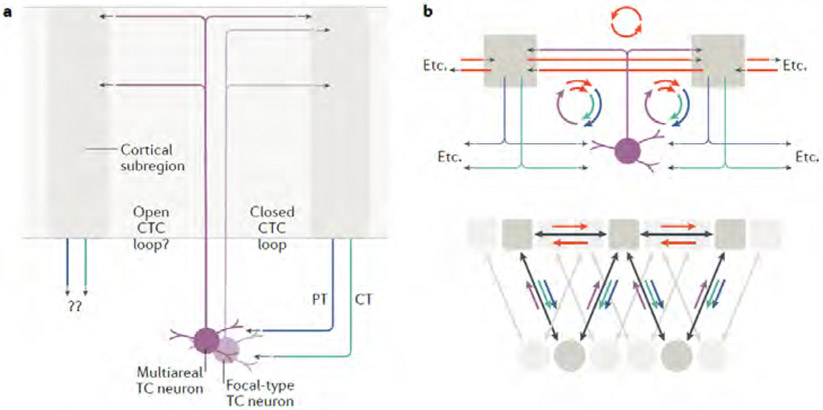
Alongside the loop-forming recurrent connections, divergent non-looping connections are also important, as these can link CTC loops to other circuits. Although CC axons can establish such a link, matrix-type TC axons may also contribute because their anatomy suggests dual functionality as both recurrent and divergent CTC circuit elements. The horizontal spread across the cortex of matrix-type arbors varies considerably, from focal to multiareal patterns36. The implication for CTC circuits is that although both types can participate in forming recurrent loops, only multiareal axons, through their divergent branches to other areas, can engage cortical neurons over a wider territory (Fig. 7A). Indeed, the idea that multiareal matrix axons in layer 1 can thereby serve to anatomically link and functionally ‘bind’ CTC modules across areas is a key feature of earlier CTC models, mentioned above38,133,140. Here, we highlight the further possibility that the divergent branches of multiareal matrix axons not only engage CTC circuits in those other areas but also, via CT and/or PT axons, loop back to innervate the same multiareal TC neurons (and possibly others, in other thalamic subregions). In this way, multiareal branching TC neurons in a given thalamic subregion could in principle serve to ‘anchor’ two or more CTC loops (Fig. 7B). By extension, this arrangement could interlink multiple CTC loops in even larger area-spanning networks (Fig. 7B). Moreover, the cortical regions that are interlinked by shared CTC loop connections could be directly interlinked by CC loops as well, forming large-scale networks while preserving specificity (Fig. 7B).
Fig. 7 ∣. Cortico-thalamo-cortical loop connectivity with the diverse types of matrix axon arborizations.
a ∣ TC neurons with focal-type projections (light purple) form a relatively focused, closed CTC loop with one cortical subregion (right), whereas those with divergently branching, multiareal-type projections (deep purple) arborize both within the same CTC loop and to one (or more) other areas (left). The targets of the CT and PT outputs of the second area are unclear. b ∣ Multiareal matrix projections can hypothetically form multiple CTC loops, the concatenation of which could form an extended network of cortical areas linked both by CC and CTC connections, forming widely distributed chains of loops.
Although such models are still largely hypothetical, they accord with several pieces of experimental evidence. For example, multiareal PO axons can arborize in both S1 and M1 (REFS132,189). As reviewed above, S1 and M1 form ‘closed’ CTC loops with the PO; if multiareal PO axons are relatively common, they are likely to be incorporated into these CTC loops. Consistent with this, there is evidence that S1-PT axons contact M1-projecting PO neurons to form an ‘open’ CTC circuit; that is, a transthalamic S1→PO→M1 pathway128. Moreover, the same cortical areas that receive multiareal axons from a particular thalamic nucleus, such as the PO, tend to be strongly interconnected through CC loops76,113,118. As reviewed above, CC loops are in turn interlinked with CTC loops. In the visual system, a recent analysis of anatomical projections to and from the LP indicated that transthalamic connections of visual cortical areas through the LP largely mirror direct CC pathways29, further supporting the idea of extended CTC–CC networks (Fig. 7B). Thus, there is considerable piece-wise evidence for the hypothetical schemes outlined above; what remains lacking is putting all the pieces together in a comprehensive connectivity-level analysis to determine whether CTC loops in separate cortical areas are indeed anchored by a shared group of multiareal TC neurons. More generally, the divergent projections of multiareal TC neurons need to be better understood in terms of their precise projection patterns, cortical targets, relative abundance, and the extent to which they receive converging inputs from all their cortical target regions. This information will help to clarify key aspects of CTC circuit organization, such as the balance between feedback (recurrent) and feedforward (for example, transthalamic) excitatory connections.
Conclusions and future directions
This Review has emphasized one fundamental aspect of CTC loops, that of cell-type-specific connectivity. This aspect concerns network organization — ‘who talks to whom’ — rather than the modes of operation43, which we have considered only through select examples. A more complete picture of the cellular organization of CTC loops entails synthesizing knowledge about the cellular connectivity with other major aspects and determinants of CTC circuit biology, including molecular specification, signaling dynamics, plasticity and neuromodulation. Development of more quantitative models will require incorporation of quantitative estimates of circuit-level parameters such as connection strengths, conduction velocities, and synaptic timing and dynamics (for examples, see REFS54,58,152,175,190,191). The mouse will surely continue to serve as a particularly useful model organism, but studies in other mammalian systems will only become more important to assess commonalities of circuit architecture across species and areas. The connectivity matrix approach may be helpful to qualitatively summarize and compare circuits, but it should be viewed as a starting point; such matrices need to be improved, not only by adding more detail (for example, at the crucial level of subcellular targeting of inputs to different dendritic domains) but by finding ways to test them and make them useful for guiding experiments and modeling. The broad survey and general framework provided here may be useful to facilitate future studies of CTC loops at multiple levels, from that of cellular connectivity to the in vivo modes of operation during behaviour, as well as informing and constraining computational models of CTC loops (for example, see REFS.168,192,193), and guiding investigation of CTC loop dysfunction in disease states (for example, see REFS149,152,194,195).
Acknowledgements
We thank L. Acsády, J. M. Barrett, S. Brown, J. Huguenard and L. Petreanu for comments and suggestions.
Glossary Terms:
- Hodology
The study of pathways
- Hierarchy
As applied to cerebral organization, refers to the concept of feedforward and feedback streams of information processing across areas and regions, reflected in distinct patterns of axonal projections
- Monosynaptic
A synaptic circuit connection from one neuron or set of neurons to another that is direct, with no other intervening neurons in the circuit
- Polysynaptic
A synaptic circuit connection from one neuron or set of neurons to another that is indirect, because it is via connections to other intervening neurons in the circuit
Footnotes
Competing interests statement
The authors declare no competing interests.
REFERENCES
- 1.Jones EG The Thalamus. 2 edn, (Cambridge University Press, 2007). [Google Scholar]
- 2.Llinás R, Urbano FJ, Leznik E, Ramirez RR & van Marle HJ Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 28, 325–333 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Halassa MM & Sherman SM Thalamocortical circuit motifs: a general framework. Neuron 103, 762–770 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fogerson PM & Huguenard JR Tapping the brakes: cellular and synaptic mechanisms that regulate thalamic oscillations. Neuron 92, 687–704 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halassa MM & Acsády L Thalamic inhibition: diverse sources, diverse scales. Trends Neurosci 39, 680–693 (2016). This review highlights how thalamic function is influenced by inhibition from the TRN and extrathalamic sources such the basal ganglia.
- 6.Halassa MM & Kastner S Thalamic functions in distributed cognitive control. Nat Neurosci 20, 1669–1679 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Huguenard JR & McCormick DA Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci 30, 350–356 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Llinás RR & Steriade M Bursting of thalamic neurons and states of vigilance. J Neurophysiol 95, 3297–3308 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Sherman SM & Guillery RW Exploring the thalamus. 2 edn, (MIT Press, 2006). [Google Scholar]
- 10. Sherman SM Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci 16, 533–541 (2016). This review considers thalamocortical and corticocortical interactions from the perspective of the driver/modulator framework and transthalamic pathway organization.
- 11.Pergola G et al. The Regulatory Role of the Human Mediodorsal Thalamus. Trends Cogn Sci 22, 1011–1025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff M & Vann SD The Cognitive Thalamus as a Gateway to Mental Representations. J Neurosci 39, 3–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shine JM The thalamus integrates the macrosystems of the brain to facilitate complex, adaptive brain network dynamics. Prog Neurobiol, 101951 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Hoerder-Suabedissen A et al. Subset of cortical layer 6b neurons selectively innervates higher order thalamic nuclei in mice. Cereb Cortex 28, 1882–1897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zolnik TA et al. Layer 6b is driven by intracortical long-range projection neurons. Cell reports 30, 3492–3505 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Wilson CJ The sensory striatum. Neuron 83, 999–1001 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Brodal A Neurological anatomy. 3 edn, (Oxford University Press, 1981). [Google Scholar]
- 18.Stanfield BB, O'Leary DD & Fricks C Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature 298, 371–373 (1982). [DOI] [PubMed] [Google Scholar]
- 19.Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H & Macklis JD Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci 14, 755–769 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saiki A et al. In vivo spiking dynamics of intra- and extratelencephalic projection neurons in rat motor cortex. Cereb Cortex 28, 1024–1038 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Jiang S et al. Anatomically revealed morphological patterns of pyramidal neurons in layer 5 of the motor cortex. Scientific reports 10, 7916 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oswald MJ, Tantirigama ML, Sonntag I, Hughes SM & Empson RM Diversity of layer 5 projection neurons in the mouse motor cortex. Front Cell Neurosci 7, 174 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueta Y, Otsuka T, Morishima M, Ushimaru M & Kawaguchi Y Multiple layer 5 pyramidal cell subtypes relay cortical feedback from secondary to primary motor areas in rats. Cereb Cortex 24, 2362–2376 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Rojas-Piloni G et al. Relationships between structure, in vivo function and long-range axonal target of cortical pyramidal tract neurons. Nature communications 8, 870 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Economo MN et al. Distinct descending motor cortex pathways and their roles in movement. Nature 563, 79–84 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z et al. Epigenomic diversity of cortical projection neurons in the mouse brain. bioRxiv, 10.1101/2020.1104.1101.019612 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong M et al. Comparative three-dimensional connectome map of motor cortical projections in the mouse brain. Scientific reports 6, 20072 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris JA et al. Hierarchical organization of cortical and thalamic connectivity. Nature 575, 195–202 (2019). This study of cell-type specific projectomics identifies systematic relationships in corticocortical, thalamocortical, and corticothalamic pathways.
- 29.Bennett C et al. Higher-order thalamic circuits channel parallel streams of visual information in mice. Neuron 102, 477–492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alloway KD, Olson ML & Smith JB Contralateral corticothalamic projections from MI whisker cortex: potential route for modulating hemispheric interactions. J Comp Neurol 510, 100–116 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winnubst J et al. Reconstruction of 1,000 projection neurons reveals new cell types and organization of long-range connectivity in the mouse brain. Cell 179, 268–281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh SW et al. A mesoscale connectome of the mouse brain. Nature 508, 207–214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips JW et al. A repeated molecular architecture across thalamic pathways. Nat Neurosci 22, 1925–1935 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones EG Viewpoint: the core and matrix of thalamic organization. Neuroscience 85, 331–345 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Herkenham M Laminar organization of thalamic projections to the rat neocortex. Science 207, 532–535 (1980). [DOI] [PubMed] [Google Scholar]
- 36. Clascá F, Rubio-Garrido P & Jabaudon D Unveiling the diversity of thalamocortical neuron subtypes. Eur J Neurosci 35, 1524–1532 (2012). This review develops a classification scheme for TC neurons in core and matrix nuclei based on axon morphology and other parameters.
- 37.Harris KD & Shepherd GM The neocortical circuit: themes and variations. Nat Neurosci 18, 170–181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones EG The thalamic matrix and thalamocortical synchrony. Trends Neurosci 24, 595–601 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Binzegger T, Douglas RJ & Martin KA A quantitative map of the circuit of cat primary visual cortex. J Neurosci 24, 8441–8453 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiler N, Wood L, Yu J, Solla SA & Shepherd GMG Top-down laminar organization of the excitatory network in motor cortex. Nat Neurosci 11, 360–366 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lefort S, Tomm C, Floyd Sarria JC & Petersen CC The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61, 301–316 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Hooks BM et al. Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas. PLoS Biol 9, e1000572 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Getting PA Emerging principles governing the operation of neural networks. Annu Rev Neurosci 12, 185–204 (1989). [DOI] [PubMed] [Google Scholar]
- 44.Landisman CE & Connors BW VPM and PoM nuclei of the rat somatosensory thalamus: intrinsic neuronal properties and corticothalamic feedback. Cereb Cortex 17, 2853–2865 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Cruikshank SJ, Urabe H, Nurmikko AV & Connors BW Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65, 230–245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crandall SR, Cruikshank SJ & Connors BW A corticothalamic switch: controlling the thalamus with dynamic synapses. Neuron 86, 768–782 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deschênes M, Veinante P & Zhang ZW The organization of corticothalamic projections: reciprocity versus parity. Brain Res Brain Res Rev 28, 286–308 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Guo K, Yamawaki N, Barrett JM, Tapies M & Shepherd GMG Cortico-thalamocortical circuits of mouse forelimb S1 are organized primarily as recurrent loops. J Neurosci 40, 2849–2858 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bourassa J, Pinault D & Deschênes M Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci 7, 19–30 (1995). [DOI] [PubMed] [Google Scholar]
- 50.Frandolig JE et al. The Synaptic Organization of Layer 6 Circuits Reveals Inhibition as a Major Output of a Neocortical Sublamina. Cell reports 28, 3131–3143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deschênes M, Bourassa J & Pinault D Corticothalamic projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res 664, 215–219 (1994). [DOI] [PubMed] [Google Scholar]
- 52.Bourassa J & Deschênes M Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer. Neuroscience 66, 253–263 (1995). [DOI] [PubMed] [Google Scholar]
- 53.Wimmer VC, Bruno RM, de Kock CP, Kuner T & Sakmann B Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex. Cerebral Cortex 20, 2265–2276 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruno RM & Sakmann B Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312, 1622–1627 (2006). [DOI] [PubMed] [Google Scholar]
- 55. Petreanu L, Mao T, Sternson SM & Svoboda K The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145 (2009). This study describes optogenetic methods for mapping inputs at the dendritic level, and provides maps of excitatory input to cortical neurons from diverse thalamic and cortical sources.
- 56.Constantinople CM & Bruno RM Deep cortical layers are activated directly by thalamus. Science 340, 1591–1594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sermet BS et al. Pathway-, layer- and cell-type-specific thalamic input to mouse barrel cortex. eLife 8, e52665 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu H & Agmon A Differential excitation of distally versus proximally targeting cortical interneurons by unitary thalamocortical bursts. J Neurosci 36, 6906–6916 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rah JC et al. Thalamocortical input onto layer 5 pyramidal neurons measured using quantitative large-scale array tomography. Front Neural Circuits 7, 177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crandall SR, Patrick SL, Cruikshank SJ & Connors BW Infrabarrels are layer 6 circuit modules in the barrel cortex that link long-range inputs and outputs. Cell reports 21, 3065–3078 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Egger R et al. Cortical output is gated by horizontally projecting neurons in the deep layers. Neuron 105, 122–137 (2020). This study illuminates the critical role of deeper-layer IT neurons in closing the CTC loop within S1.
- 62.Thomson AM Neocortical layer 6, a review. Front Neuroanat 4, 13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bureau I, von Saint Paul F & Svoboda K Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol 4, e382 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Audette NJ, Urban-Ciecko J, Matsushita M & Barth AL POm thalamocortical input drives layer-specific microcircuits in somatosensory cortex. Cereb Cortex 28, 1312–1328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diamond IT in Progress in Psychobiology and Physiological Psychology (eds Sprague JM & Epstein AN) 1–43 (Academic Press, 1979). [Google Scholar]
- 66.Hatsopoulos NG & Suminski AJ Sensing with the motor cortex. Neuron 72, 477–487 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shipp S The importance of being agranular: a comparative account of visual and motor cortex. Philos Trans R Soc Lond B Biol Sci 360, 797–814 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hunnicutt BJ et al. A comprehensive thalamocortical projection map at the mesoscopic level. Nat Neurosci 17, 1276–1285 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaneko T Local connections of excitatory neurons in motor-associated cortical areas of the rat. Front Neural Circuits 7, 75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bosch-Bouju C, Hyland BI & Parr-Brownlie LC Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and parkinsonian conditions. Frontiers in computational neuroscience 7, 163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitchell BD & Cauller LJ Corticocortical and thalamocortical projections to layer I of the frontal neocortex in rats. Brain Res 921, 68–77. (2001). [DOI] [PubMed] [Google Scholar]
- 72.Kuramoto E et al. Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cerebral Cortex 19, 2065–2077 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Kuramoto E et al. Ventral medial nucleus neurons send thalamocortical afferents more widely and more preferentially to layer 1 than neurons of the ventral anterior-ventral lateral nuclear complex in the rat. Cereb Cortex 25, 221–235 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Yamawaki N & Shepherd GMG Synaptic circuit organization of motor corticothalamic neurons. J Neurosci 35, 2293–2307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.*Guo K, Yamawaki N, Svoboda K & Shepherd GMG Anterolateral motor cortex connects with a medial subdivision of ventromedial thalamus through cell-type-specific circuits, forming an excitatory thalamo-cortico-thalamic loop via layer 1 apical tuft dendrites of layer 5B pyramidal tract type neurons. J Neurosci 38, 2849–2858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suter BA & Shepherd GMG Reciprocal interareal connections to corticospinal neurons in mouse m1 and s2. J Neurosci 35, 2959–2974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hooks BM et al. Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J Neurosci 33, 748–760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamawaki N, Borges K, Suter BA, Harris KD & Shepherd GMG A genuine layer 4 in motor cortex with prototypical synaptic circuit connectivity. eLife 3, e05422 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mukherjee A et al. Variation of connectivity across exemplar sensory and associative thalamocortical loops in the mouse. eLife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delevich K, Tucciarone J, Huang ZJ & Li B The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J Neurosci 35, 5743–5753 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cruikshank SJ et al. Thalamic control of layer 1 circuits in prefrontal cortex. J Neurosci 32, 17813–17823 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Collins DP, Anastasiades PG, Marlin JJ & Carter AG Reciprocal circuits linking the prefrontal cortex with dorsal and ventral thalamic nuclei. Neuron 98, 366–379 (2018). This study dissects the cell-type-specific excitatory connections between PFC and thalamus.
- 83.Li N, Chen TW, Guo ZV, Gerfen CR & Svoboda K A motor cortex circuit for motor planning and movement. Nature 519, 51–56 (2015). [DOI] [PubMed] [Google Scholar]
- 84. Guo ZV et al. Maintenance of persistent activity in a frontal thalamocortical loop. Nature 545, 181–186 (2017). This study implicates CTC loops in mediating the preparatory activity observed in a higher-order motor cortex area.
- 85.Qiu S, Anderson CT, Levitt P & Shepherd GMG Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated met receptor tyrosine kinase. J Neurosci 31, 5855–5864 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ji XY et al. Thalamocortical innervation pattern in mouse auditory and visual cortex: laminar and cell-type specificity. Cereb Cortex 26, 2612–2625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rouiller EM & Welker E A comparative analysis of the morphology of corticothalamic projections in mammals. Brain Res Bull 53, 727–741 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Winer JA, Diehl JJ & Larue DT Projections of auditory cortex to the medial geniculate body of the cat. J Comp Neurol 430, 27–55 (2001). [PubMed] [Google Scholar]
- 89.Morgenstern NA, Bourg J & Petreanu L Multilaminar networks of cortical neurons integrate common inputs from sensory thalamus. Nat Neurosci 19, 1034–1040 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Cruz-Martin A et al. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507, 358–361 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roth MM et al. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat Neurosci 19, 299–307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou N, Masterson SP, Damron JK, Guido W & Bickford ME The mouse pulvinar nucleus links the lateral extrastriate cortex, striatum, and amygdala. J Neurosci 38, 347–362 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olsen SR, Bortone DS, Adesnik H & Scanziani M Gain control by layer six in cortical circuits of vision. Nature 483, 47–52 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blot A et al. Visual intracortical and transthalamic pathways carry distinct information to cortical areas. bioRxiv, 10.1101/2020.1107.1106.189902. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Callaway EM Structure and function of parallel pathways in the primate early visual system. J Physiol 566, 13–19 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fitzpatrick D The functional organization of local circuits in visual cortex: insights from the study of tree shrew striate cortex. Cereb Cortex 6, 329–341 (1996). [DOI] [PubMed] [Google Scholar]
- 97.Usrey WM & Sherman SM Corticofugal circuits: Communication lines from the cortex to the rest of the brain. J Comp Neurol 527, 640–650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alitto HJ & Usrey WM Dissecting the dynamics of corticothalamic feedback. Neuron 86, 605–607 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Briggs F & Usrey WM Emerging views of corticothalamic function. Curr Opin Neurobiol 18, 403–407 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galvan A, Hu X, Smith Y & Wichmann T Effects of optogenetic activation of corticothalamic terminals in the motor thalamus of awake monkeys. J Neurosci 36, 3519–3530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.da Costa NM & Martin KA Selective targeting of the dendrites of corticothalamic cells by thalamic afferents in area 17 of the cat. J Neurosci 29, 13919–13928 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moore B et al. Cortical projections to the two retinotopic maps of primate pulvinar are distinct. J Comp Neurol 527, 577–588 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collins DP & Anastasiades PG Cellular specificity of cortico-thalamic loops for motor planning. J Neurosci 39, 2577–2580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Acsády L The thalamic paradox. Nat Neurosci 20, 901–902 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Adesnik H & Naka A Cracking the function of layers in the sensory cortex. Neuron 100, 1028–1043 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Douglas RJ, Koch C, Mahowald M, Martin KA & Suarez HH Recurrent excitation in neocortical circuits. Science 269, 981–985 (1995). [DOI] [PubMed] [Google Scholar]
- 107.Douglas RJ & Martin KA Recurrent neuronal circuits in the neocortex. Curr Biol 17, 496–500 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Shu Y, Hasenstaub A & McCormick DA Turning on and off recurrent balanced cortical activity. Nature 423, 288–293 (2003). [DOI] [PubMed] [Google Scholar]
- 109.Reinhold K, Lien AD & Scanziani M Distinct recurrent versus afferent dynamics in cortical visual processing. Nat Neurosci 18, 1789–1797 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Lien AD & Scanziani M Tuned thalamic excitation is amplified by visual cortical circuits. Nat Neurosci 16, 1315–1323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li YT, Ibrahim LA, Liu BH, Zhang LI & Tao HW Linear transformation of thalamocortical input by intracortical excitation. Nat Neurosci 16, 1324–1330 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Petreanu L, Huber D, Sobczyk A & Svoboda K Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10, 663–668 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Mao T et al. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 72, 111–123 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamawaki N, Radulovic J & Shepherd GM A corticocortical circuit directly links retrosplenial cortex to M2 in the mouse. J Neurosci 36, 9365–9374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Young H, Belbut B, Baeta M & Petreanu L Laminar-specific cortico-cortical loops in mouse visual cortex. eLife, 10:e59551. doi: 10.7554/eLife.59551 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu X & Carter AG Ventral Hippocampal Inputs Preferentially Drive Corticocortical Neurons in the Infralimbic Prefrontal Cortex. J Neurosci 38, 7351–7363 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anastasiades PG, Marlin JJ & Carter AG Cell-Type Specificity of Callosally Evoked Excitation and Feedforward Inhibition in the Prefrontal Cortex. Cell reports 22, 679–692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kinnischtzke AK, Fanselow EE & Simons DJ Target-specific M1 inputs to infragranular S1 pyramidal neurons. J Neurophysiol 116, 1261–1274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ueta Y, Hirai Y, Otsuka T & Kawaguchi Y Direction- and distance-dependent interareal connectivity of pyramidal cell subpopulations in the rat frontal cortex. Front Neural Circuits 7, 164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kita T & Kita H The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci 32, 5990–5999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nelson A et al. A circuit for motor cortical modulation of auditory cortical activity. J Neurosci 33, 14342–14353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Veinante P & Deschênes M Single-cell study of motor cortex projections to the barrel field in rats. J Comp Neurol 464, 98–103 (2003). [DOI] [PubMed] [Google Scholar]
- 123.Vélez-Fort M et al. The stimulus selectivity and connectivity of layer six principal cells reveals cortical microcircuits underlying visual processing. Neuron 83, 1431–1443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Johnson RR & Burkhalter A A polysynaptic feedback circuit in rat visual cortex. J Neurosci 17, 7129–7140 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Johnson RR & Burkhalter A Microcircuitry of forward and feedback connections within rat visual cortex. J Comp Neurol 368, 383–398 (1996). [DOI] [PubMed] [Google Scholar]
- 126.Sherman SM & Guillery RW Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol 106, 1068–1077 (2011). [DOI] [PubMed] [Google Scholar]
- 127.Theyel BB, Llano DA & Sherman SM The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci 13, 84–88 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mo C & Sherman SM A sensorimotor pathway via higher-order thalamus. J Neurosci 39, 692–704 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rockland KS, Andresen J, Cowie RJ & Robinson DL Single axon analysis of pulvinocortical connections to several visual areas in the macaque. J Comp Neurol 406, 221–250 (1999). [DOI] [PubMed] [Google Scholar]
- 130.Kuramoto E et al. Individual mediodorsal thalamic neurons project to multiple areas of the rat prefrontal cortex: A single neuron-tracing study using virus vectors. J Comp Neurol 525, 166–185 (2017). [DOI] [PubMed] [Google Scholar]
- 131.Ohno S et al. A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cereb Cortex 22, 2840–2857 (2012). [DOI] [PubMed] [Google Scholar]
- 132.Rodriguez-Moreno J et al. Area-specific synapse structure in branched posterior nucleus axons reveals a new level of complexity in thalamocortical networks. J Neurosci 40, 2663–2679 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Larkum M A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci 36, 141–151 (2013). This review develops a new conceptual framework about cortical and thalamocortical processing, hinging on the associative pairing of multiple inputs by layer 5 pyramidal neurons .
- 134.Cauller L Layer I of primary sensory neocortex: where top-down converges upon bottom-up. Behav Brain Res 71, 163–170 (1995). [DOI] [PubMed] [Google Scholar]
- 135.Yamawaki N et al. Long-range inhibitory intersection of a retrosplenial thalamocortical circuit by apical tuft-targeting CA1 neurons. Nat Neurosci 22, 618–626 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Svoboda K & Li N Neural mechanisms of movement planning: motor cortex and beyond. Curr Opin Neurobiol 49, 33–41 (2018). [DOI] [PubMed] [Google Scholar]
- 137.Bizzi E & Ajemian RJ From Motor Planning to Execution: A Sensorimotor Loop Perspective. J Neurophysiol (2020). [DOI] [PubMed] [Google Scholar]
- 138.Edwards LL, King EM, Buetefisch CM & Borich MR Putting the "Sensory" Into Sensorimotor Control: The Role of Sensorimotor Integration in Goal-Directed Hand Movements After Stroke. Frontiers in integrative neuroscience 13, 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bostan AC & Strick PL The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 19, 338–350 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Llinás R & Ribary U Consciousness and the brain. The thalamocortical dialogue in health and disease. Ann N Y Acad Sci 929, 166–175 (2001). [PubMed] [Google Scholar]
- 141.Aru J, Suzuki M, Rutiku R, Larkum ME & Bachmann T Coupling the State and Contents of Consciousness. Front Syst Neurosci 13, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aru J, Suzuki M & Larkum ME Cellular mechanisms of conscious processing. Trends Cogn Sci 24, 814–825 (2020). [DOI] [PubMed] [Google Scholar]
- 143.Suzuki M & Larkum ME General anesthesia decouples cortical pyramidal neurons. Cell 180, 666–676 (2020). [DOI] [PubMed] [Google Scholar]
- 144.Takahashi N et al. Active dendritic currents gate descending cortical outputs in perception. Nat Neurosci 23, 1277–1285 (2020). [DOI] [PubMed] [Google Scholar]
- 145.Grewe BF, Bonnan A & Frick A Back-Propagation of Physiological Action Potential Output in Dendrites of Slender-Tufted L5A Pyramidal Neurons. Front Cell Neurosci 4, 13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shepherd GMG Corticostriatal connectivity and its role in disease. Nat Rev Neurosci 14, 278–291 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Radnikow G & Feldmeyer D Layer- and Cell Type-Specific Modulation of Excitatory Neuronal Activity in the Neocortex. Front Neuroanat 12, 1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baker A et al. Specialized Subpopulations of Deep-Layer Pyramidal Neurons in the Neocortex: Bridging Cellular Properties to Functional Consequences. J Neurosci 38, 5441–5455 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maheshwari A & Noebels JL Monogenic models of absence epilepsy: windows into the complex balance between inhibition and excitation in thalamocortical microcircuits. Prog Brain Res 213, 223–252 (2014). [DOI] [PubMed] [Google Scholar]
- 150.Lam YW & Sherman SM Functional organization of the thalamic input to the thalamic reticular nucleus. J Neurosci 31, 6791–6799 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lam YW & Sherman SM Functional topographic organization of the motor reticulothalamic pathway. J Neurophysiol 113, 3090–3097 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paz JT et al. A new mode of corticothalamic transmission revealed in the Gria4(−/−) model of absence epilepsy. Nat Neurosci 14, 1167–1173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Golshani P, Liu XB & Jones EG Differences in quantal amplitude reflect GluR4-subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci U S A 98, 4172–4177 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Temereanca S & Simons DJ Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system. Neuron 41, 639–651 (2004). [DOI] [PubMed] [Google Scholar]
- 155.Suga N & Ma X Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci 4, 783–794 (2003). [DOI] [PubMed] [Google Scholar]
- 156.Briggs F & Usrey WM Corticogeniculate feedback and visual processing in the primate. J Physiol 589, 33–40 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pinault D & Deschênes M Anatomical evidence for a mechanism of lateral inhibition in the rat thalamus. Eur J Neurosci 10, 3462–3469 (1998). [DOI] [PubMed] [Google Scholar]
- 158.Crabtree JW, Collingridge GL & Isaac JT A new intrathalamic pathway linking modality-related nuclei in the dorsal thalamus. Nat Neurosci 1, 389–394 (1998). [DOI] [PubMed] [Google Scholar]
- 159.Crabtree JW & Isaac JT New intrathalamic pathways allowing modality-related and cross-modality switching in the dorsal thalamus. J Neurosci 22, 8754–8761 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Crabtree JW Functional diversity of thalamic reticular subnetworks. Front Syst Neurosci 12, 41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Clemente-Perez A et al. Distinct thalamic reticular cell types differentially modulate normal and pathological cortical rhythms. Cell reports 19, 2130–2142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martinez-Garcia RI et al. Two dynamically distinct circuits drive inhibition in the sensory thalamus. Nature 583, 813–818 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Landisman CE et al. Electrical synapses in the thalamic reticular nucleus. J Neurosci 22, 1002–1009 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Deleuze C & Huguenard JR Distinct electrical and chemical connectivity maps in the thalamic reticular nucleus: potential roles in synchronization and sensation. J Neurosci 26, 8633–8645 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lam YW, Nelson CS & Sherman SM Mapping of the functional interconnections between thalamic reticular neurons using photostimulation. J Neurophysiol 96, 2593–2600 (2006). [DOI] [PubMed] [Google Scholar]
- 166.Parker PR, Cruikshank SJ & Connors BW Stability of electrical coupling despite massive developmental changes of intrinsic neuronal physiology. J Neurosci 29, 9761–9770 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hou G, Smith AG & Zhang ZW Lack of intrinsic GABAergic connections in the thalamic reticular nucleus of the mouse. J Neurosci 36, 7246–7252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Destexhe A, Contreras D & Steriade M Mechanisms underlying the synchronizing action of corticothalamic feedback through inhibition of thalamic relay cells. J Neurophysiol 79, 999–1016 (1998). [DOI] [PubMed] [Google Scholar]
- 169.Andersen P & Sears TA The role of inhibition in the phasing of spontaneous thalamo-cortical discharge. J Physiol 173, 459–480 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun QQ, Huguenard JR & Prince DA Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci 26, 1219–1230 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bruno RM & Simons DJ Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci 22, 10966–10975 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Agmon A & Connors BW Correlation between intrinsic firing patterns and thalamocortical synaptic responses of neurons in mouse barrel cortex. Journal of Neuroscience 12, 319–329 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Swadlow HA Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex 13, 25–32 (2003). [DOI] [PubMed] [Google Scholar]
- 174.Isaacson JS & Scanziani M How inhibition shapes cortical activity. Neuron 72, 231–243 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gabernet L, Jadhav SP, Feldman DE, Carandini M & Scanziani M Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48, 315–327 (2005). [DOI] [PubMed] [Google Scholar]
- 176.Bortone DS, Olsen SR & Scanziani M Translaminar inhibitory cells recruited by layer 6 corticothalamic neurons suppress visual cortex. Neuron 82, 474–485 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ibrahim LA, Schuman B, Bandler R, Rudy B & Fishell G Mining the jewels of the cortex's crowning mystery. Curr Opin Neurobiol 63, 154–161 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee AJ et al. Canonical organization of layer 1 neuron-led cortical inhibitory and disinhibitory interneuronal circuits. Cereb Cortex 25, 2114–2126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schuman B et al. Four unique interneuron populations reside in neocortical layer 1. J Neurosci 39, 125–139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Williams LE & Holtmaat A Higher-order thalamocortical inputs gate synaptic long-term potentiation via disinhibition. Neuron 101, 91–102 (2019). [DOI] [PubMed] [Google Scholar]
- 181.Abs E et al. Learning-related plasticity in dendrite-targeting layer 1 interneurons. Neuron 100, 684–699 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chou XL et al. Contextual and cross-modality modulation of auditory cortical processing through pulvinar mediated suppression. eLife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Anastasiades PG, Collins DP & Carter AG Mediodorsal and ventromedial thalamus engage distinct L1 circuits in the prefrontal cortex. Neuron, 109(2):314–330(2021). This study identifies layer 1 inhibitory circuits engaged by TC axons in PFC, suggesting how they function to regulate the activity of layer 5 pyramidal neurons.
- 184.Bopp R, Holler-Rickauer S, Martin KA & Schuhknecht GF An ultrastructural study of the thalamic input to layer 4 of primary motor and primary somatosensory cortex in the mouse. J Neurosci 37, 2435–2448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Goldman PS Contralateral projections to the dorsal thalamus from frontal association cortex in the rhesus monkey. Brain Res 166, 166–171 (1979). [DOI] [PubMed] [Google Scholar]
- 186.Sakai ST & Tanaka D Jr., Contralateral corticothalamic projections from area 6 in the raccoon. Brain Res 299, 371–375 (1984). [DOI] [PubMed] [Google Scholar]
- 187.Apicella A, Wickersham IR, Seung HS & Shepherd GMG Laminarly orthogonal excitation of fast spiking and low threshold spiking interneurons in mouse motor cortex. J Neurosci 32, 7021–7033 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pluta S et al. A direct translaminar inhibitory circuit tunes cortical output. Nat Neurosci 18, 1631–1640 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ohno S et al. A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cereb Cortex 22, 2840–2857 (2012). [DOI] [PubMed] [Google Scholar]
- 190.Stoelzel CR, Bereshpolova Y, Alonso JM & Swadlow HA Axonal conduction delays, brain state, and corticogeniculate communication. J Neurosci 37, 6342–6358 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gil Z, Connors BW & Amitai Y Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron 23, 385–397 (1999). [DOI] [PubMed] [Google Scholar]
- 192.Lumer ED, Edelman GM & Tononi G Neural dynamics in a model of the thalamocortical system. I. Layers, loops and the emergence of fast synchronous rhythms. Cereb Cortex 7, 207–227 (1997). [DOI] [PubMed] [Google Scholar]
- 193.Lin I-C, Okun M, Carandini M & Harris KD Equations governing dynamics of excitation and inhibition in the mouse corticothalamic network. bioRxiv, 10.1101/2020.1106.1103.132688 (2020). [DOI] [Google Scholar]
- 194.Acsády L Heartless beat or beatless heart? Nat Neurosci 21, 649–651 (2018). [DOI] [PubMed] [Google Scholar]
- 195.Llinás RR, Ribary U, Jeanmonod D, Kronberg E & Mitra PP Thalamocortical dysrhythmia:A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A 96, 15222–15227 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Scala F et al. Phenotypic variation of transcriptomic cell types in mouse motor cortex. Nature (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Economo MN et al. A platform for brain-wide imaging and reconstruction of individual neurons. eLife 5, e10566 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li Y et al. Distinct subnetworks of the thalamic reticular nucleus. Nature 583, 819–824, doi: 10.1038/s41586-020-2504-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]