Abstract
Inflammatory bowel diseases, comprising ulcerative colitis (UC) and Crohn’s disease, are chronic, immune-mediated and progressive inflammatory disorders affecting the gastrointestinal tract. Tofacitinib is the first oral small-molecule Janus kinase (JAK) inhibitor licensed and approved by the National Institute for Health and Care Excellence (NICE) for use in moderately-to-severely active UC after intolerance, inadequate response, or loss of response to conventional treatment or biologic therapy. The pivotal OCTAVE studies demonstrated the efficacy and safety of tofacitinib for the induction and maintenance of remission in UC. A growing body of evidence from real-world data supports the positive clinical and endoscopic benefits observed with tofacitinib treatment in the OCTAVE trials. This narrative review summarizes the current literature regarding the mechanism of action of tofacitinib, data from registrational trials, emerging real-world evidence, and an overview of the most recent safety evidence. We explore evolving treatment paradigms, including the use of tofacitinib in the COVID-19 era, pregnancy and extraintestinal manifestations, as well as the emerging concept of combining tofacitinib with biological therapy. We will also present a brief overview of the next generation of JAK inhibitors in the pipeline.
Keywords: Crohn’s disease, effectiveness, efficacy, inflammatory bowel diseases, Janus kinase inhibitors, ulcerative colitis, safety, tofacitinib
Introduction
Inflammatory bowel diseases (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), are chronic, immune-mediated and progressive inflammatory disorders of the gastrointestinal tract.1 Evolution in our understanding of the immunopathogenesis of IBD is reflected by the increase in our therapeutic armamentarium but is arguably still limited in comparison to other immune-mediated diseases.2 The era of biological treatment was spearheaded by the introduction of anti-TNF agents but novel therapeutic agents targeting distinct aspects of the immune cascade, namely IL-12/IL-23 inhibitors (ustekinumab (UST)) and anti-integrin agents (vedolizumab (VDZ)) are available, with others in development.3
Biologics have gradually transformed the treatment landscape,4 albeit with significant limitations such as primary and secondary loss of response, mechanistic failure, immunogenicity, associated economic costs with drug administration and increased infection risk.5 Furthermore, there are in fact several cytokine-driven inflammatory pathways that trigger and perpetuate the inflammatory cascade in IBD, underpinning the need to develop further therapeutic targets that overcome these associated drawbacks.6
The development of new molecules with the potential to target a myriad of cytokine targets, such as the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway, has been shown to be effective in IBD.7,8 Tofacitinib is a first-in-class JAK inhibitor with demonstrated efficacy in the treatment of rheumatoid arthritis (RA)9 and psoriatic arthritis10 and is now licensed for the treatment of UC.11
Methods
The aim of this narrative review is to summarize the current knowledge regarding tofacitinib in UC, for the practising clinician and to present a brief overview of next-generation JAK inhibitors in the pipeline.
An extensive review of literature available on PubMed, EMBASE, Medline and conference abstracts was conducted up to 31 October 2021. We used the following keywords in our search strategy: “inflammatory bowel disease”, “ulcerative colitis”, “Crohn’s disease”, “JAK inhibitors”, “JAK/STAT”, “tofacitinib”, “small molecules”, “filgotinib”, “upadacitinib”, “OCTAVE”, “real world” and “safety”.
Review
Mechanism of action
Many of the current biological options target the extracellular components of the immune cascade, namely cytokines and their associated cell-surface receptors.12 Tofacitinib differs from conventional biologics in that it is an oral targeted synthetic small molecule and operates at an intracellular level by reversibly and competitively inhibiting JAK.13
JAK pathways are involved in several key functions. They play a role in cell growth, survival, differentiation and migration as well as in modulating the innate and adaptive immune response and haemopoiesis.14,15
Being able to modulate JAK ultimately impacts the JAK–STAT signalling pathway, which has downstream effects on the above mentioned functions. There are four tyrosine kinase proteins: JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2), with each being activated by cytokines such as interleukins and interferons, as well as hormones such as thrombopoietin, growth hormone and erythropoietin.15
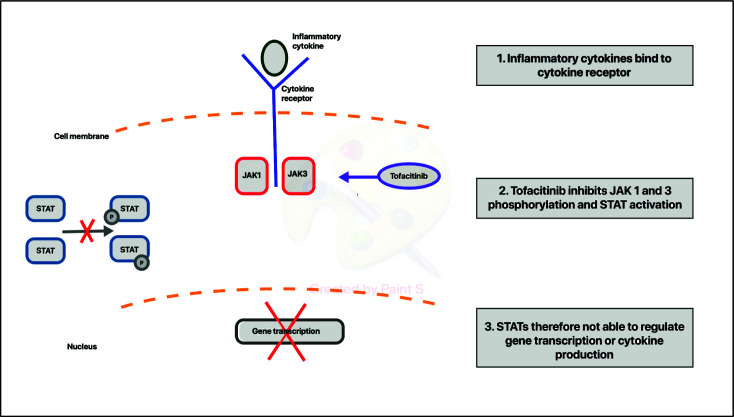
Each tyrosine kinase protein has specificity for certain cytokine receptors. Following the binding of a cytokine to a cytokine receptor, this leads to activation of the receptor-associated JAKs16 (Figure 1). The associated JAK receptor proteins are then involved in phosphorylating the associated intracellular receptor, turning them effectively into binding sites for STAT1–7 proteins.17 Once the STAT proteins bind to their associated receptor, they are phosphorylated by receptor-associated JAK proteins. The phosphorylated STAT proteins undergo dimerization after un-docking from the receptor subunit. Subsequently, they translocate into the nucleus of the cell and influence gene transcription, promoting further inflammation.14
Figure 1.
Schematic illustration of the mechanism of action of tofacitinib.
Tofacitinib is the first-in-class, reversible competitive JAK inhibitor, selectively inhibiting JAK1 and JAK 3 (Figure 1). Its structure mimics that of adenosine triphosphate (ATP) without the associated triphosphate group. This prevents the phosphorylation of JAK proteins, which prevents the triggering of the STAT pathway and downstream signalling of γ-chain cytokines IL-2, IL-4, IL-7, IL-15 and IL-21 and the synthesis of pro-inflammatory proteins that are implicated in mucosal inflammation.13
Tofacitinib is both rapidly absorbed after oral intake and rapidly eliminated due to a short half-life of 3 hours, thus has the potential dual benefit of quick effective onset of action and elimination.14 Furthermore, tofacitinib is not a monoclonal antibody and therefore has the additional advantage of no immunogenicity risk.14
Tofacitinib was approved by US Food and Drug Administration (FDA), the European Medical Agency (EMA) and National Institute for Health and Care Excellence (NICE) for use in moderately-to-severely active UC.11 Meanwhile, several selective JAK inhibitors are being developed that target specific JAK proteins.18
Tofacitinib in UC
The approval for tofacitinib was based on the efficacy data from initial phase II trials and three subsequent distinct phase III trials.19,20 In the phase II trial, 194 patients with moderately-to-severely active UC (Mayo score (MCS) 6–12, rectal bleeding subscore (RBS) 1–3 and Mayo endoscopic subscore (MES) 2–3) were assigned to receive placebo or oral tofacitinib 0.5, 3, 10 or 15 mg twice daily (BD) for 8 weeks. The primary endpoint of clinical response at 8 weeks was defined as ≥3 points absolute reduction from baseline MCS and a relative decrease of ≥30% from baseline along with a reduction of ≥1 point of the RBS, or an absolute RBS of 0 or 1. Clinical remission was defined as MCS ≤2, with no subscore of >1 at 8 weeks. The respective clinical response and remission rates at 8 weeks were 32% (p=0.39) and 13% (p=0.76) for the 0.5 mg arm, 48% (p=0.55) and 33% (p=0.01) for the 3 mg arm, 61% (p=0.10) and 48% (p<0.001) for the 10 mg arm, and 78% (p<0.001) and 41% (p<0.001) for the 15 mg arm as compared with 42% and 10% for patients receiving placebo. Furthermore, a dose–response relationship was noted for endoscopic response in the treatment group (higher for the 10 and 15 mg BD dose) compared to placebo or tofacitinib 5 mg BD.19
Three distinct phase III randomized controlled trials evaluated the efficacy and safety of tofacitinib: the identical OCTAVE 1 and 2 trials for induction therapy and OCTAVE Sustain for maintenance therapy in patients with moderately-to-severely active UC despite previous conventional therapy or treatment with an anti-TNF agent.20 Oral aminosalicylates and/or oral glucocorticoids (≤25 mg per day of prednisolone or equivalent) were permitted as concurrent medications provided they were continued at a stable dose throughout the induction trials and glucocorticoids were mandatorily tapered during maintenance.
The induction trials (to week 8) included 598 and 541 patients for OCTAVE 1 and 2, respectively, who were randomly assigned, in a 4:1 ratio, to receive induction therapy with oral tofacitinib (10 mg BD) or placebo for 8 weeks. The primary endpoint of clinical remission at 8 weeks (MCS ≤2, with no individual subscore >1) was noted more frequently in the active treatment arm compared to placebo (in OCTAVE 1: 18.2% versus 8.2% (p=0.007); OCTAVE 2: 16.6% versus 3.6% (p<0.001)). A key secondary endpoint of mucosal healing was noted more frequently in the active treatment arm compared to the placebo arm (OCTAVE 1: 31.3% versus 15.6% (p<0.001); OCTAVE 2: 28.4% versus 11.6% (p<0.001)). The treatment effects for both primary and secondary endpoints were similar in both TNF-naive and TNF-exposed patients.19 The dose selection was based on the dose-response data from the earlier phase II trial showing better treatment effects from the 10 and 15 mg doses. Originally, 16 and 6 patients were randomized to receive 15 mg doses of tofacitinib in the OCTAVE 1 and 2 trials, respectively. However, based on feedback received from regulatory authorities for RA, Pfizer discontinued further exploration of this dose in the trial. Limited analyses on the 15 mg dosing arm demonstrated clinical remission rates of 43.8% (7/16) in OCTAVE 1 and 50% (3/6) in OCTAVE 2 trials, providing avenues for further investigation of the 15 mg dose for induction therapy.19
A post hoc analysis of the OCTAVE 1 and 2 induction data showed a rapid onset of treatment effect. Significantly greater improvements in Mayo stool frequency subscore and RBS were noted in the tofacitinib arm compared to placebo by day 3 (9.2% versus 2.3% (p<0.01) and 14.4% versus 8.2% (p<0.05), respectively) with consistent effects thereafter until day 15. These effects were uniformly observed in all subgroups.21
Patients who had a clinical response to induction therapy (n=593) were rerandomized 1:1:1 to receive oral 5 mg tofacitinib BD, 10 mg tofacitinib BD or placebo in the maintenance trial for OCTAVE Sustain trial with a follow-up period of 52 weeks.20 Significantly higher clinical remission rates were noted in patients receiving tofacitinib 5 mg BD (34.3%) and tofacitinib 10 mg BD (40.6%) when compared with the placebo arm (11.1%) (p<0.001 for both comparisons with placebo). Similarly, for the key secondary endpoint of mucosal healing, significantly higher rates were noted in patients receiving tofacitinib 5 mg BD (37.4%) and tofacitinib 10 mg BD (45.7%) compared to placebo (13.1%) (p<0.001 for both comparisons with placebo). Sustained and corticosteroid-free clinical remission (CFCR) rates were also significantly higher in patients receiving tofacitinib 5 mg BD (35.4%) and tofacitinib 10 mg BD (47.3%) when compared with the placebo arm (5.1%) (p<0.001 for both comparisons with placebo).20
Statistically significant improvements in health-related quality of life were noted with tofacitinib in OCTAVE 1 and 2 compared with placebo and these improvements were maintained in the tofacitinib treatment arm for at least 52 weeks, irrespective of sex, baseline glucocorticoid use and previous anti-TNF exposure.22
Patients who completed OCTAVE Sustain were eligible to enrol into the long-term extension follow-on study (OCTAVE Open). Of the 142 patients in remission (defined as a total MCS ≤2, no individual subscore >1, and RBS of 0 at week 52) at the end of OCTAVE Sustain, efficacy endpoints were maintained with ongoing tofacitinib 5 mg BD dosing in OCTAVE Open, with 68.3% achieving remission, 77.5% clinical response and 73.9% endoscopic improvement at 12 months, and 50.4%, 56.0% and 55.3%, respectively, at 36 months.23
The final analysis of OCTAVE Open was recently published, demonstrating long-term efficacy of tofacitinib use for up to 7 years.24 A total of 944 patients entered OCTAVE Open; eligible patients were OCTAVE 1 or 2 non-responders, or OCTAVE Sustain completers or treatment failures. Patients in remission at baseline (81.5%) received 5 mg BD dosing and the remaining 18.5% received 10 mg BD dosing. At month 36 of OCTAVE Open (beyond week 52 of OCTAVE Sustain), 58.9% and 33.7% achieved or maintained clinical remission, 66.9% and 40.3% clinical response, and 64.6% and 37.1% endoscopic improvement, with tofacitinib 5 and 10 mg BD dosing, respectively.24
Outcomes of dose reduction and escalation
Efficacy increases with higher tofacitinib exposure, as demonstrated in OCTAVE, with average drug concentration during maintenance proportionately increasing with higher dosage in OCTAVE Sustain.20 However, adverse events (AEs) are also dose dependent, and manufacturer guidance is to use the lowest effective dose to maintain response where possible.25
In OCTAVE Open, patients in remission after 52 weeks of 10 mg BD dosing (n=66) were de-escalated to 5 mg BD dosing.26 After de-escalation, clinical response was maintained in 92.4% and 84.1%, and remission maintained in 80.3% and 74.6% at 2 months and 12 months, respectively. Tofacitinib induction responders who lost response on 5 mg maintenance dosing (n=57) were escalated to 10 mg BD; 57.9% and 64.9% recaptured clinical response, and 35.1% and 49.1% achieved remission again at months 2 and 12, respectively.26 OCTAVE Open data thus support the effectiveness of a dose-escalating strategy to recapture response in patients losing response on 5 mg BD maintenance. NICE also advocates dose escalation in cases of loss of response.11
The recently published primary analysis from RIVETING aimed to assess the outcomes of dose de-escalation in patients in stable remission.27 Patients on 10 mg BD dosing for ≥2 years were randomized 1:1 to de-escalate to 5 mg BD or continue 10 mg BD. Remission at 6 months (MES ≤1, Mayo stool frequency subscore ≤1, RBS 0) was 77.1% and 90.0% in the 5 and 10 mg BD subgroups, respectively, with an adjusted treatment difference between the two groups of 12.9% (95% CI 0.5–25.0).27 Deep endoscopic remission and no previous anti-TNF failure were factors predictive of maintenance of remission after de-escalation. Thus, endoscopic assessment and consideration of a patient’s prior treatment history should be considered before dose reduction. AEs were similar across both dosage groups and consistent with previously reported safety profiles.27
Real-world’ experience of tofacitinib in UC
A growing body of evidence from real-world data for tofacitinib supports the effectiveness of tofacitinib in the more complex and diverse patient populations seen in the OCTAVE studies (Table 1).
Table 1.
Real-world studies for tofacitinib.
| Study | n | Endpoint | Week 4 | Week 8 | Week 16 | Week 26 | Week 52 |
|---|---|---|---|---|---|---|---|
| Taxonera et al. 30 Systematic review and meta-analysis |
1162 (UC) | Clinical response | 62% | 64% (week 12–16) | 51% | 42% | |
| Clinical remission | 35% a | 47%a (week 12–16) | 38% a | ||||
| CFCR | 38% | 44% (week 12–16) | 34% | 31% | |||
| Mucosal healing | 42% | 35% (week 12–16) | |||||
| Honap et al.28 LEO IBD Research Consortium:Retrospective multicentre study |
134 (118 UC, 5 IBD-U) | Clinical response | 74% | 66% | 53% | ||
| Clinical remission | 57% | 51% | 45% | ||||
| CFCR | 48% | 49% | 44% | ||||
| Ungaro et al.29 Multicentre retrospective study |
123 (UC) 40.7% prior anti-TNF and VDZ, 28.5% biologic naive |
Clinical response | 61% a | 55%b | |||
| Clinical remission | 14%b | 49%b | |||||
| Mucosal healing | 65%b (within 6/12 of tofacitinib initiation) | ||||||
| Takada et al.31 Retrospective study |
66 (UC) (38 TOF, 28 VDZ) |
Clinical response | 50% TOF versus 36% VDZ (ns) (week 2) | 63% TOF versus 36% VDZ (week 6) | |||
| Clinical remission | 24% TOF versus 29% VDZ (ns) (week 2) | 39% TOF versus 32% VDZ (ns) (week 6) | |||||
| Biemans et al.32 ICC registry: Prospective multicentre study |
123 (UC) 95% prior anti-TNF, 62% prior VDZ, 59% prior VDZ and anti-TNF |
CFCR | 35% (week 12) | 29%a (week 24) | |||
| Clinical response | 56% (week 12) | 46% (week 24) | |||||
| Clinical remission | 41% (week 12) | 33% (week 24) | |||||
| Endoscopic response | 36% (week 12) | ||||||
| Endoscopic remission | 21% (week 12) | ||||||
| Chaparro et al.33 ENEIDA Registry: Prospective multicentre study |
113 (UC) 100% prior anti-TNF, 89% VDZ |
Clinical response | 40% | 60% | 57% | ||
| Clinical remission | 16% | 31% | 32% |
Study primary endpoint (in bold text);
Study secondary endpoint.
CFCR, corticosteroid-free clinical remission; ENEIDA, Spanish Team for Intercultural Studies on Academic Disclosure; LEO IBD, London, Exeter, Oxford IBD; IBD-U, IBD-unclassified; ICC registry, Dutch Initiative on Crohn and Colitis Registry; TOF, tofacitinib; TROPIC, Tofacitinib Real-world Outcomes in Patients with ulcerative colitis and Crohn’s disease.
In the largest real-world cohort to date, Honap et al. evaluated the efficacy of tofacitinib in 134 patients with UC from four centres in the United Kingdom.28 In contrast to the relatively lower rates of prior anti-TNF exposure included in the OCTAVE 1 and 2 cohorts (53% and 55%, respectively),20 the majority in the Honap et al. study cohort (80%) were previously treated with anti-TNF,27 reflecting the more complex and treatment refractory patients typically included in real-world data. By week 8, 74% achieved clinical response, and by week 26, 44% patients achieved CFCR.27 Younger age at initiation of tofacitinib treatment (p=0.014) and higher baseline C-reactive protein (CRP; median 9 mg/L (p=0.004)) were independently associated with primary non-response. Clinical remission or response was not affected by prior anti-TNF exposure.28 Even patients with prior first-line or second-line VDZ exposure had comparable response rates to tofacitinib.28 Dose escalation was successful in recapturing response in 47% of patients.28
A multicentre US study reported on 123 patients with UC treated with tofacitinib with clinical response observed in 55%, clinical remission in 49% and endoscopic healing in 65% at week 16.29 Bio-naive status was associated with an increased likelihood of week 16 clinical response (81%). No difference was noted between the prior anti-TNF group (36%) and the group with prior exposure to both anti-TNF and anti-integrin (35%; p<0.001). Conversely, endoscopic healing at 6 months was lower in the group with prior exposure to two biologic classes (17%), compared to exposure to one biologic class (57%) or bio-naive status (87%). Other variables associated with week 8 response included concomitant steroids at tofacitinib initiation (p=0.002) and male sex (p=0.007).29
In a systematic review of 17 real-world studies including 1162 patients with UC, the clinical response rate at week 8 was 62% (95% CI 55–69%) with similar week 12–16 response rates (64%; 95% CI 56–73%).30 Clinical remission was achieved by 35% (95% CI 24–45%) at week 8, and 47% (95% CI 40–54%) at weeks 12–16. Five studies assessed CFCR with 38% (95% CI 32–45%) achieving CFCR by week 8, and 31% (95% CI 20–42%) at 12 months, demonstrating sustained treatment efficacy.30 The results were comparable with the outcomes from OCTAVE, though notably, the real-world cohort analysed in the meta-analysis was a more refractory cohort, with 88% biologic-experienced and two-thirds failing both anti-TNF and VDZ, as compared to 51% of OCTAVE participants being anti-TNF experienced and none with prior VDZ exposure.20,30
Rates of mucosal healing reported in separate real-world studies show varying results (Table 1). Systematic review analysis from six studies (n=252) noted mucosal healing rates at week 8 of 42%, higher than those seen during OCTAVE 1 and 2 (31% and 28%).20,30 However, this mucosal healing data should be treated with caution given the small numbers as well as the lack of systematically scheduled endoscopic evaluations in a real-life cohort, with endoscopic assessments more likely to be performed for patients showing non-response.
A Japanese group carried out a real-world comparison of short-term efficacy and safety of tofacitinib and VDZ in UC.31 Clinical response rates at week 6 were 63% with tofacitinib versus 36% with VDZ and clinical remission rates at week 6 of 39% for tofacitinib versus 32% for VDZ. The authors also noted significantly higher response rates amongst the prior biologic failure tofacitinib group (59%) compared with VDZ (21%) (p=0.038), though the numbers included were small for firm conclusions to be drawn.31
The majority of real-world tofacitinib data have been limited by a retrospective design. The Dutch Initiative on Crohn and Colitis (ICC) Registry was the first prospective study of 123 patients with UC refractory to anti-TNF and VDZ treatment.32 Inclusion criteria were combined clinical criteria (short clinical colitis activity index (SCCAI) >2) and objective criteria (MES ≥1, CRP >5 mg/L, faecal calprotectin >250 μg/g). The primary endpoint was CFCR at week 24 (SCCAI ≤2), which was achieved by 29% of patients.32 Clinical response declined over time from 56% at week 12 to 33% at week 24. Furthermore, in contrast to Honap et al.,28 prior VDZ was associated with a significantly reduced week 24 clinical remission rate (OR 0.33, 95% CI 0.11–0.94; p=0.038).32 Association with prior anti-TNF could not be assessed due to the very low number of anti-TNF naive patients included.
The recently published prospective ENEIDA (Spanish Team for Intercultural Studies on Academic Discourse) registry reported data from 113 patients with highly refractory UC; 100% of patients had previously failed anti-TNF therapy, 89% failed VDZ, and 4% of patients had failed UST. Clinical response was achieved in 60% and clinical remission in 31% of patients by week 8.33 During follow-up, 38% of patients subsequently relapsed at a median of 44 weeks; however, response was successfully recaptured with dose escalation in about 50% of cases.33 Prior biologic exposure, baseline CRP levels and baseline corticosteroid use were not associated with likelihood of week 8 remission in line with OCTAVE results.33
TOUR (tofacitinib response in ulcerative colitis) is an ongoing real-world prospective multicentre study using an electronic system to capture SCCAI and patient-reported outcomes (PROs), including anxiety, depression and social satisfaction from patients commenced on tofacitinib therapy.34 Preliminary data from 65 patients published in abstract form showed that tofacitinib was associated with rapid clinical response. Over half of the study patients met criteria for clinical response at day 14 (54.2%) and 52.2% at week 6; overall mean disease activity reduced from baseline SCCAI 5.6 to SCCAI 3.1 on day 14, and 2.7 on week 14.34 Improvement in all PRO measures was also observed through week 14.
Tofacitinib in CD
Tofacitinib failed to show significant clinical benefit in patients with CD. The primary endpoint of clinical remission was not achieved in the induction and maintenance phase II trials.35,36 The initial 4-week multicentre phase II trial randomized 139 patients with moderately-to-severely active CD (Crohn’s Disease Activity Index (CDAI) score 220–450), 1:1:1:1 to receive oral tofacitinib 1 mg BD, 5 mg BD, 15 mg BD or placebo BD. The primary endpoint was the clinical response (≥70 points reduction in CDAI score from baseline) at week 4, and secondary endpoints included clinical remission (CDAI <150 points) at week 4. No statistically significant differences were noted in the proportion of patients achieving clinical response or remission between the tofacitinib and the placebo arms;35 of note, induction therapy was only for 4 weeks. This study was followed by two multicentre phase IIb studies (induction and maintenance) enrolling 280 patients with moderately-to-severely active CD. The results concluded minor treatment effects but did not demonstrate a statistically significant difference in primary endpoints between the treatment and placebo arms.36
A 48-week open-label extension study, investigating long-term safety in CD, reported worsening of CD in the treatment arm as the most frequent AE. This highlighted a lack of clinical efficacy of tofacitinib in CD, and further development of tofacitinib for CD was therefore discontinued.37 It is plausible that the failure to meet the primary endpoint in the phase II trial and the high placebo response and remission rates may be a result of the concomitant use of corticosteroids in a high proportion of patients, the slow and arguably prolonged taper during maintenance, the absence of blinded central endoscopy reading at recruitment, and the absence of PRO and objective biomarkers as markers of disease activity.
Safety profile
Adverse events
In the OCTAVE studies, nasopharyngitis, arthralgia and headache were the most commonly reported AEs in any treatment group during the maintenance phase of the trial.20 In the OCTAVE 1 trial, AEs were reported in 56.5% of patients receiving 10 mg BD tofacitinib and 59.8% receiving placebo; in OCTAVE 2, the corresponding figures were 54.1% and 52.7%, respectively. In OCTAVE Sustain, AEs were reported more frequently, with 72.2% of patients in the 5 mg group and 79.6% in the 10 mg group reporting AEs. The frequency of AEs reported in the placebo group was 75.3%.20
Serious AEs (SAEs) occurred at similar rates in treatment and placebo groups with the proportion of patients discontinuing treatment being similar in OCTAVE 1 and 2. A higher proportion of patients discontinued treatment due to AEs in the placebo group in OCTAVE Sustain (18.7% versus 9.1% in the 5 mg BD group, and 9.7% in the 10 mg group).20
The most recent safety data from 7.8 years of data from global clinical trials from 1157 patients reported that the incidence of AEs was comparable to placebo with worsening of UC (24%), nasopharyngitis (22%) and arthralgias (13%) being most frequently reported in both groups.38 The safety profile was generally consistent with other UC therapies, with the exception of herpes zoster discussed in the following.
Infections
The incidence of serious infections from the OCTAVE studies was higher in the induction group (0.9% for tofacitinib) versus none for placebo. Serious infections are those that meet the definition for a SAE, including infections that result in death, are considered life threatening, or result in significant disability or hospitalization. This trend seemed to be dose dependent with the highest risk noted in patients treated with 10 mg BD. Similar low infection rates were noted in the maintenance and overall cohorts irrespective of dosing schedules when compared to placebo (incidence rate (IR) 1.94 for placebo and 1.35 and 0.64 for tofacitinib 5 and 10 mg BD, respectively).39
Herpes zoster infection
Herpes zoster infection has been reported as the most common serious infection in patients with RA treated with tofacitinib.40 In the OCTAVE Sustain trial, the incidence of herpes zoster was higher in those patients treated with tofacitinib 10 mg (n=10, 5.1%) and 5 mg (n=3, 1.5%) than in the placebo group (n=1, 0.5%). None of the cases of herpes zoster seen were classed as severe AEs and treatment did not need to be discontinued. Furthermore, most cases were confined to only one or two adjacent dermatomes with none classed as severe or needing treatment discontinuation.20
When extrapolating and including all phase II/III/open-label extension studies involving patients receiving tofacitinib for UC, 65 (5.6%) patients were identified as having herpes zoster infection, 11 of whom had multidermatomal involvement with one patient developing herpes encephalitis.41 In the overall cohort, herpes zoster risk was generally similar between doses, mostly limited to 1–2 dermatomes, non-serious and resolved without the need to discontinue tofacitinib.42 Multivariate modelling identified older age, lower body weight, North American origin and prior anti-TNF failure as significant risk factors for herpes zoster infection. Patients of Asian origin had a higher herpes zoster risk on univariate but not in multivariate modelling.42
Thromboembolic risk
The FDA43 and EMA44 have raised concerns regarding the thromboembolic risk associated with tofacitinib use based on early data from an ongoing study (study A3921133) exploring tofacitinib use in patients over the age of 50 with RA and with at least one known cardiovascular risk factor. The study demonstrated a venous thromboembolism (VTE) risk fivefold that associated with anti-TNFs43 and highlighted an increased risk of thromboembolic events (deep vein thrombosis/pulmonary embolism) and an increase in all-cause mortality in patients receiving tofacitinib 10 mg BD dosing schedules compared to anti-TNFs and 5 mg tofacitinib.43
Accordingly, the Medicines and Healthcare products Regulatory Agency advised caution when considering tofacitinib in patients with known risk factors for thromboembolic disease, in addition to their underlying disease, adding that maintenance treatment at 10 mg BD is not recommended in patients with known risk factors for thromboembolic disease unless there is no suitable alternative.45
In contrast, a recently published population-based cohort study has found the occurrence of VTE in patients initiating both tofacitinib and a TNF inhibitor to be <1/100 person-years, with no increased risk of thromboembolic events in those taking tofacitinib in comparison to a TNF inhibitor.46 Furthermore, a 2020 systematic review and indirect meta-analysis has suggested that tofacitinib may have a protective effect against VTE.47 Further trial data are required to define this paradigm.
Cardiovascular risk and lipid profiles
IBD appears to be an independent risk factor for ischaemic heart disease and cerebrovascular disease.48 Tofacitinib has been shown to reversibly increase serum lipid levels; however, this has not been shown to correlate with an increase in cardiovascular risk in the RA or psoriasis cohort.49,50 An ongoing study (A3921133) is exploring the safety of tofacitinib in comparison to anti-TNFs in patients over the age of 50 with known cardiovascular risk factors.51
Amongst 1124 patients with UC receiving tofacitinib across the phase III/open-label extension tofacitinib UC clinical programme, LDL, HDL, total cholesterol and triglyceride elevation were noted. LDL to HDL ratios and total cholesterol to HDL ratios are strongly implicated in cardiovascular risk but these were relatively unchanged. Major adverse cardiovascular events were found to be infrequent.52
Whilst the exact mechanism for tofacitinib impacting lipid levels is not fully known, tofacitinib has been associated with decreasing the rate of cholesterol ester catabolism in patients with active RA.53 Current recommendations advise monitoring lipids levels 4–8 weeks after initiation of tofacitinib, with guidelines followed for the management of hyperlipidaemia contextualizing cardiovascular risk.54
Malignancy
The assessment of malignancy risk with tofacitinib in patients with UC is challenging given the heterogenous patient population and that many patients have previously received treatments which themselves are implicated in malignancy risk.55
In the tofacitinib UC clinical development programme, which includes trial data from 1124 patients with a tofacitinib treatment duration of up to 6.8 years, 20 patients developed malignancies (excluding non-melanoma skin cancer (NMSC)), with 17 of these occurring in patients receiving 10 mg BD. There was no clustering of malignancy type. Furthermore, the IR of malignancies was similar to that observed in the cohort of patients with RA and psoriasis.55 The authors also commented that the risk of malignancy was comparable to that of patients with UC on biologics and not on tofactinib.55
Across the OCTAVE studies, NMSC occurred in 5 patients receiving tofacitinib and 1 patient receiving placebo.20 NMSC occurred infrequently, being more likely to occur in patients with pre-existing risk factors for NMSC such as prior TNF failure, increasing age and prior NMSC.56 Furthermore, the risk of NMSC was higher in patients receiving 10 mg BD dosing.55
In the absence of more data on cancer risk with tofacitinib, extreme caution is advised in patients with a previous history of malignancy. We recommend discussion in multidisciplinary team panels with appropriate conversations regarding the associated risks from trial data at the time. This statement would need to be reviewed as more data become available over time.
Black box warning
Following the results of a large, randomized safety clinical trial evaluating tofacitinib in patients with RA, the FDA issued a black box warning for tofacitinib due to concerns regarding the increased risk of myocardial infarction, stroke, thromboembolic events and death in patients treated with tofacitinib in comparison to those treated with TNF blockers.57 A higher rate of lymphoma was also observed in patients treated with tofacitinib in comparison to those treated with TNF blockers. There was also a higher rate of lung cancer in smokers treated with tofacitinib.57
Safety checklist
As with current biological therapy, several considerations are required prior to initiating tofacitinib. We recommend referring to the prescriber treatment initiation checklist available on the electronic medicine compendium to ensure the relevant risks and screening tests are considered prior to prescribing tofacitinib (https://www.medicines.org.uk/emc/rmm/799/Document) (Box 1). Caution needs to be exercised in patients with known liver and renal disease whilst will having implications for drug metabolism. Furthermore, based on the evidence highlighted above, those with cardiovascular risk factors, stroke, hypertension, obesity and diabetes are also unlikely to be ideal candidates for tofacitinib use.
Box 1. Vaccinations prior to commencing tofacitinib58,59.
Herpes zoster vaccine (live): To be given at least 2 weeks (ideally 4 weeks) before starting treatment (test for VZV antibodies if concerns regarding lack of previous exposure). The non-live vaccine (Shingrix), should be preferred and is increasingly available58
Pneumococcal vaccine (live): Preferably before starting therapy. Repeated at 5 years
Influenza vaccination (inactive): To be given on an annual basis. (Intranasal preparation contains live virus)
BCG vaccination (live): Using clinical history, chest X-ray and IFNγ release assay testing to determine if vaccination required
Hepatitis B (inactive): Recommended for seronegative patients. Measure anti-hepatitis B antibodies following vaccination
Measles, mumps and rubella (live): If vaccination status is uncertain, serology should be checked and vaccination offered
Human Papilloma vaccination (inactive): Recommended for both males and females
The laboratory monitoring recommendations for patients taking tofacitinib include full blood count at baseline, after 4–8 weeks, and then 3-monthly thereafter. Lipid profile should be checked after 4–8 weeks, and liver enzymes should be monitored routinely.54
Tofacitinib in special circumstances
Use in acute severe UC
The rapid onset of action and short half-life have raised the prospect of using tofacitinib in the management of acute severe UC (ASUC). Data remain limited to a few case reports.30,60–64 In three case series, 14 of 15 patients received 3 days high-dose intravenous steroids prior to rescue tofacitinib with a history of anti-TNF failure.30 Eleven patients received standard induction dosing 10 mg BD, whereas the Michigan group used a higher dose of 10 mg three times daily.30,60 Following tofacitinib rescue therapy, 13% required colectomy during that index admission and 20% within 6 months, with no difference in colectomy rates according to tofacitinib dose.30 At the last follow-up, 47% remained in CFCR on maintenance therapy.30 Kotwani et al. reported endoscopic remission in addition to CFCR at last follow-up in 2 of the 4 patients included in their small case series.63 Overall, no major AEs were reported and notably no new thromboembolic events.30,61,64
More recently, a retrospective case–control study evaluated the efficacy of tofacitinib induction therapy in hospitalized patients with biologic-experienced ASUC.65 Compared to matched controls, there was a lower 90-day colectomy rate in the 40 patients who received tofacitinib with intravenous corticosteroids (hazard ratio 0.28, 95% CI 0.10–0.81; p=0.018). The benefit was only statistically significant at tofacitinib 10 mg three times daily and not at 10 mg BD dosing.65
Although these initial reports suggest a role for tofacitinib in ASUC in selected patients, randomized controlled trials are needed to help determine the efficacy, optimum dose and safety in ASUC before it can be considered in routine clinical practice.
Post-operative surgical outcomes
There are a few small retrospective reviews of surgical outcomes following preoperative tofacitinib exposure. Lightner reported 53 patients exposed to tofacitinib within 4 weeks of colectomy for medically refractory UC.66 They noted an increased risk of venous thromboembolism with 7 (13.2%) cases in their cohort.66 In contrast, in another study, no postoperative thrombotic complications were observed in 35 patients who underwent IBD-related surgery within 4 weeks of tofacitinib.67 The authors also reported postoperative complications in 20% of patients exposed to tofacitinib. These complications included postoperative surgical site infections, pneumonia, peri-anal abscess, and one case of reoperation for small bowel resection.67 These data are comparable to the rates of infectious complications reported in both anti-TNF exposed (20%) and unexposed (19.4%) patients in the prospective PUCCINI trial.68 Furthermore, Kani et al. concluded that preoperative tofacitinib exposure was not associated with an increased risk of 30-day postsurgical outcomes when compared with preoperative exposure to other biologics.69
Extraintestinal manifestations
Tofacitinib is also approved for use in RA and psoriatic arthritis, making it appealing for the treatment of these extraintestinal manifestations (EIMs) in patients with UC.9,10 In post hoc analysis of the OCTAVE data, 16.7% of patients with UC on 5 mg BD and 33% on 10 mg BD dosing reported improvement in IBD-related peripheral arthritis by week 52, whereas 18% on placebo reported worsening symptoms.70 Although not licensed yet, phase II studies have shown tofacitinib is more effective than placebo in ankylosing spondylitis,71 suggesting it may also be efficacious in axial spondyloarthropathy.
Tofacitinib proved effective in phase III studies in chronic plaque psoriasis.72 Upregulation of the JAK–STAT pathway has been observed in cutaneous EIMs, including pyoderma gangrenosum and erythema nodosum.73,74 To date, only small case series are available reporting improvement of pyoderma gangrenosum with tofacitinib therapy.75,76 The use of JAK inhibitors may be considered for cutaneous EIMs in the context of active UC, if anti-TNF fails or is contraindicated.73 Regarding ocular EIMs, there are only reports of successful treatment of refractory uveitis and scleritis with tofacitinib in two patients.74
Tofacitinib in COVID-19
The SECURE-IBD (Surveillance Epidemiology of Coronavirus Under Research Exclusion for IBD) registry reported outcomes of 37 patients with IBD on tofacitinib therapy during the COVID-19 pandemic.77 Overall, there was no difference in COVID-19 outcomes in the tofacitinib group compared with the non-tofacitinib treated cohort. None of the patients in the tofacitinib group were diagnosed with thromboembolic events.77 Notably, JAK inhibitors, including baricitinib and ruxolitinib, are being studied in clinical trials for their potential role in COVID-19 management by blunting the cytokine storm.78,79
Tofacitinib in pregnancy
There are limited safety data for tofacitinib use in pregnancy. As a small molecule, tofacitinib is likely to cross the placental barrier and be secreted in breast milk. Animal studies have shown teratogenic and foeticidal effects.25 Rheumatological data of 47 pregnancies in women treated with tofacitinib and 15 pregnancies in women with UC treated with tofacitinib suggest no increased risk of fetal malformations or neonatal death compared with the general population.80–82 Tofacitinib is not currently approved for use in pregnancy or breastfeeding, and the manufacturer advises effective contraception83 during and up to 6 weeks after tofacitinib use.25
Evolving paradigms
Positioning of tofacitinib in the UC therapeutic algorithm
Tofacitinib and other JAK inhibitors address an unmet need in the armamentarium of IBD therapy. Owing to a lack of head-to-head clinical trials or comparative effectiveness studies with tofacitinib, there is currently no clear guidance on its correct positioning in the UC therapeutic algorithm. A myriad of factors deserve consideration regarding its positioning and include disease activity, speed of onset of action, prior biologic exposure, immunogenicity and route of administration as well as patient age, clinical comorbidities and the safety profile.
In a meta-analysis comparing efficacy and safety of first and second-line therapy in moderate-to-severe UC, tofacitinib ranked highest for induction of clinical remission (OR 11.88, 95% CI 2.32–60.89) and mucosal healing (OR 4.71, 95% CI 2.23–9.92) in patients with prior anti-TNF exposure.84 It should be noted that, whilst this may enable positioning of tofacitinib as first-line following conventional therapy, the FDA restricted use to after failure of anti-TNF therapy from July 2019, following concerns with VTE from patients with RA exposed to tofacitinib 10 mg BD.43 This risk has not been substantiated in the IBD literature. Direct head-to-head studies are needed to inform clinical decision-making and adequately position tofacitinib in the treatment paradigm.
Biomarkers predicting response
With the increasing armamentarium of IBD therapy and drive for personalized IBD therapy, predictive biomarkers are eagerly awaited to help predict treatment response and redefine our therapeutic algorithm. Kolar et al. identified an early decrease in IL-4 levels as a potential biomarker of tofacitinib response.85 In another study, a tofacitinib-specific mucosal genetic biomarker was identified that was significantly correlated with endoscopic response. The identified hub gene (p=1.5×10−9, fold change 2.3) had a predictive accuracy for the response of 100% (p<0.001).86 Most promising is the PROPHETIC prospective study (NCT04576000), currently recruiting, that will assess molecular determinants of response or non-response to tofacitinib in UC.87
Combining biologics with small molecule therapy
The concept of combining biologics in IBD management for incremental effect or indeed in the context of treatment refractoriness has gained interest in recent years. Data on the combined use of biologics with JAK inhibitors are limited. In one study of nine patients with UC treated with VDZ and tofacitinib, clinical response was observed in 62.5% of patients who were previously refractory to VDZ or tofacitinib monotherapy.88 In a retrospective study of 42 patients (63% previously failed ≥2 biologics) treated with combination tofacitinib and biologics (VDZ 64%, UST 19%, infliximab 14%) with a median follow-up of 5 months, 67% achieved a clinical response by weeks 8–16 of combination therapy with no additional safety signals compared to tofacitinib monotherapy.89 In another study of 50 patients, 15% were treated with tofacitinib and VDZ, 16% with anti-TNF and tofacitinib, and 6% with tofacitinib and UST.90 Significantly more patients on combination therapy were in clinical and endoscopic remission at end of follow-up compared to baseline (50% versus 14%, p=0.0018; and 34% versus 6%, p=0.0039). In view of the small number of patients treated, it was not possible to assess if any particular combination was associated with increased efficacy or less AEs. Of the eight SAEs reported in the cohort, none occurred in the tofacitinib combination group, and 57% occurred in patients on concomitant immunomodulator therapy.90 A recent systematic review and meta-analysis reported on dual biologic and combination tofacitinib therapy from 288 patients (76%) with CD with a median treatment duration of 24 weeks.91 Overall, 58/288 (20%) cases of combination therapy with tofacitinib were included: 11% tofacitinib and VDZ (32/288), 6% UST and tofacitinib (16/288), and 3% anti-TNF and tofacitinib (10/288).91 The pooled data demonstrated AE rates (31%) in line with previously reported rates seen with anti-TNF monotherapy. Pooled clinical and endoscopic remission rates were 59% (95% CI 42–74%) and 34% (95% CI 23–46%), respectively.91 Larger prospective trials are needed to assess the efficacy, safety and optimum strategy of combination therapy with JAK inhibitors.
Newer JAK inhibitors
See Table 2 for an overview of JAK inhibitors for UC and CD.
Table 2.
Overview of JAK inhibitors for UC and CD.
| Target | Name of the drug | Current status | Important completed clinical trials | Primary endpoint |
|---|---|---|---|---|
| UC | ||||
| Pan JAK inhibitor | Tofacitinib | Licensed for use in moderate-to-severe UC | Phase II (induction therapy)19 OCTAVE I and II (induction therapy)20 OCTAVE Sustain (maintenance therapy)20 |
Clinical response at 8 weeks Clinical remission at 8 weeks Clinical remission at 8 weeks |
| Peficitinib | Discontinued after phase IIb in view of inefficacy for moderate-to-severe UC | Phase IIb (induction therapy)105 | Clinical response at 8 weeks | |
| JAK1 selective inhibitor | Filgotinib | Received EMA license for use in moderate-to-severe UC | Phase IIb/III, SELECTION trial (induction therapy)97 Phase IIb/III, SELECTION trial (maintenance therapy)97 |
Clinical remission at 10 weeks Clinical remission at 10 weeks |
| Upadacitinib | Awaiting regulatory approvals from FDA and EMA for use in moderate-to-severe UC | Phase IIb, U-ACHIEVE program (induction therapy)99 Phase III U-ACCOMPLISH (induction therapy)101 Phase III (maintenance therapy)102 |
Clinical remission at 8 weeks Clinical remission at 8 weeks Clinical remission at 52 weeks |
|
| CD | ||||
| Pan JAK inhibitor | Tofacitinib | Failed to show significant benefit in moderate-to-severe CD | Phase II (induction therapy)35 Phase IIb (induction therapy)36 Phase IIb (maintenance therapy)36 |
Clinical response at 4 weeks Clinical remission at 8 weeks Clinical remission at 26 weeks |
| JAK1 selective inhibitor | Filgotinib | Results from multicentre phase III results in moderate-to-severe CD, awaited | Phase II, FITZROY trial (induction therapy)95 Phase III, DIVERSITY trial (induction therapy)96 Phase III, DIVERSITY trial (maintenance therapy)96 |
Clinical remission at 10 weeks Clinical remission at 10 weeks Clinical remission at 58 weeks |
| Upadacitinib | Phase III studies in moderate-to-severe CD, under way | Phase II, CELEST (induction therapy)100 Phase II, CELEST (maintenance therapy)100 |
Clinical remission at 16 weeks Clinical remission at 52 weeks |
|
CD, Crohn’s disease; EMA, European Medicines Agency; FDA, Food and Drug Administration; JAK, Janus kinase; UC, ulcerative colitis.
Filgotinib
Filgotinib is a selective JAK1 inhibitor with once-daily (OD) oral dosing and that has been evaluated for use in CD and UC.92 Safety and efficacy of filgotinib in moderate-to-severe CD and UC were reported in the FITZROY and SELECTION trials, respectively.93–95 The FITZROY study was a 20-week multicentre phase II trial, randomizing 174 moderately-to-severely active CD, TNF-naive and exposed patients, 3:1 to receive filgotinib 200 mg OD or placebo for 10 weeks.95 The primary endpoint was clinical remission (CDAI <150) at week 10. After 10 weeks, based on CDAI response status, investigators reassigned patients to receive filgotinib 200 mg OD, 100 mg OD, or placebo for an additional 10 weeks in the second part of the study. Clinical remission at 10 weeks was significantly higher in the filgotinib arm (47%) compared to placebo (23%; p=0.0077) with a safety profile comparable to placebo over 20 weeks.95 Recruitment for a multicentre phase III trial of filgotinib for induction and maintenance of remission in moderately-to-severely active CD (DIVERSITY trial, NCT02914561) has been completed and results are awaited.96
The results from the SELECTION study (phase IIb/III) with an induction and maintenance phase were recently published.97 In the induction trial, a total of 1348, biologic-naive (induction study A, n=659) or biologic-experienced (induction study B, n=689) patients with moderately-to-severely active UC were randomized and treated with filgotinib (200 or 100 mg OD) or placebo. A significantly higher proportion of patients achieved clinical remission at week 10 (primary endpoint) compared with placebo in both the biologic-naive (26.1% versus 15.3%, p=0.0157) and biologic-experienced (11.5% versus 4.2%; p=0.0103) arms.97 In the maintenance trial, patients achieving clinical response or remission at week 10 in the filgotinib arm (n=664), were rerandomized 2:1 to receive an induction dose of filgotinib or placebo and treated for a total of 58 weeks. At week 58, a significantly higher proportion of patients on filgotinib 100 mg (19.1% biologic naive and 9.5% biologic experienced) and 200 mg (26.1% biologic naive and 11.5% biologic experienced) were in clinical remission compared to placebo (15.3% biologic naive and 4.2% biologic experienced) and a significantly higher proportion of patients on 200 mg filgotinib achieved key secondary endpoints, including CFCR, sustained clinical remission, MCS remission and endoscopic and histological remission compared with placebo.97 Overall, the IR of AEs, SAEs and discontinuations due to AEs were similar in the filgotinib and placebo arms for both the induction and maintenance studies.
In November 2021, filgotinib received a European license for the treatment of adult patients with moderately-to-severely active UC who have failed or are intolerant to conventional treatment or biologic therapy.98
Upadacitinib
Upadacitinib (UPA) is another selective JAK1 inhibitor being evaluated for the management of patients with UC and CD.99,100 The phase II CELEST study assessed the safety and efficacy of UPA in patients with moderately-to-severely active CD either failing or intolerant to immunosuppressants or anti-TNF therapy.100 It randomized 220 patients in 1:1:1:1:1:1 to receive immediate-release UPA 3 mg BD, 6 mg BD, 12 mg BD, 24 mg BD, 24 mg OD or placebo as induction therapy for 16 weeks, followed by rerandomization 1:1:1 to receive immediate-release UPA 3 mg BD, 12 mg BD or 24 mg OD as maintenance therapy for 36 weeks. Significantly higher clinical remission rates were noted in the 6 mg BD arm compared with placebo (27% versus 11%; p<0.1), and a significant dose–response relationship was observed for endoscopic remission between the UPA versus placebo arms with efficacy maintained in the treatment arm for most endpoints till week 52. Numerically higher AEs were noted in the UPA arm compared with placebo but there was no relationship noted with the dose.100
The safety and efficacy of UPA was reported from a multicentre phase IIb study, part of an overarching U-ACHIEVE programme.99 The trial randomized 250 patients with moderately-to-severely active UC with inadequate response, loss of response or intolerance to conventional or biologic therapies to a treatment arm (7.5, 15, 30 or 45 mg OD extended-release UPA) or placebo for 8 weeks. At week 8, significantly higher clinical remission rates were noted in the treatment arm compared with none in the placebo arm (7.5 mg: 8.5%, p=0.052; 15 mg: 14.3%, p=0.013; 30 mg: 13.5%, p=0.011; and 45 mg: 19.6%, p=0.002). Compared with the placebo arm (2.2%), the treatment arm reported a significantly higher endoscopic improvement at week 8 (7.5 mg: 14.9%, p=0.033; 15 mg: 30.6%, p<0.001; 30 mg: 26.9%, p<0.001; and 45 mg: 35.7%, p<0.001). Similar incidences of AEs and discontinuation related to AEs were noted across the UPA groups with numerically higher events in the placebo group.99
In the phase III U-ACCOMPLISH study, a significantly higher proportion of patients receiving UPA 45 mg daily achieved clinical remission (33.5% versus placebo 4.1%) at 8 weeks.101 Likewise, all ranked secondary endpoints (symptomatic, endoscopic histological improvements) were achieved in a significantly higher proportion of patients receiving UPA 45 mg daily versus placebo (p<0.001).101
The efficacy and safety of UPA maintenance therapy were reported recently from the randomized phase III study.102 Both UPA 15 and 30 mg met the primary endpoint of clinical remission at week 52, and all secondary endpoints. Significantly greater percentages of patients receiving 15 mg and 30 mg versus placebo achieved clinical remission (42.3% and 51.7%, versus 12.1%), endoscopic improvement (48.7% and 61.6%, versus 14.5%), maintenance of clinical remission (59.2% and 69.7%, versus 22.2%), CFCR (57.1% and 68.0%, versus 22.2%), maintenance of endoscopic improvement (61.6% and 69.5%, versus 18.9%), endoscopic remission (24.2% and 25.9%, versus 5.6%), maintenance of clinical response (63.0% and 76.6%, versus 18.8%) and histo-endoscopic mucosal improvement (34.8% and 49.3%, versus 11.8%) (p<0.001 for all endpoints).102 UPA 15 and 30 mg were both well tolerated and no new safety signals were observed.
The use of UPA in the management of UC is not yet approved. Regulatory applications have been submitted to the FDA and EMA for approval of UPA for the treatment of moderately-to-severely active UC, and the outcome is awaited.103
Conclusion
Tofacitinib is the first-in-class and currently the only approved JAK inhibitor licensed for the treatment of moderately-to-severely active UC. Being a small molecule, its oral administration, rapid onset of action (as early as 3 days) and short half-life with high intestinal bioavailability are unique advantages. Furthermore, the lack of immunogenicity implicates a lower risk of loss of response over time typical of anti-TNF agents. Efficacy and effectiveness data from clinical trials and real-world evidence have demonstrated that tofacitinib can be effective even following corticosteroid, thiopurine, and anti-TNF and VDZ failure.20
There is an increased dose-related risk of venous thromboembolism with tofacitinib and it should therefore be used very cautiously in patients at high risk.44,45 Caution also needs to be exercised in patients with cardiovascular risk factors, history of strokes, hypertension, obesity and diabetes. However, overall, the safety profile has been reassuring. Recent literature has also allayed anxieties regarding heightened risks of herpes zoster but vaccination prior to treatment is recommended as indeed with other biologic and immunomodulator agents. Economic modelling has demonstrated tofacitinib to be cost effective.104
The development of agents targeting the JAK–STAT pathway represents the dawn of a new era in abrogation of the immune–inflammatory pathway that triggers and perpetuates IBD. Whilst tofacitinib has established its place in UC therapy, we are at the threshold of a plethora of newer JAK inhibitors seeking imminent licensing and others in development, aiming to complement our therapeutic armamentarium and the promise of a better future.
Acknowledgements
None.
Footnotes
Contributions: EL, NA, GN and JKL coauthored the initial draft of the manuscript. JKL subsequently revised and edited the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICJME) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: EL and JKL have a non-pharmaceutical investigator-initiated sponsored research grant from Galapagos. EL has received speaker fees from Janssen. JKL has received speaker and consultancy fees from Abbvie, Arena, MSD, Galapagos, Janssen, Takeda, Pfizer and Tillots, and research support from Galapagos and Takeda. EL has received speaker fees from Janssen. The authors declare that they have no other conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/03/dic.2021-11-4-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2022 Liu E, Aslam N, Nigam G, Limdi JK. https://doi.org/10.7573/dic.2021-11-4. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Cosnes J, Gower–Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Gravallese EM. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. 2021;21(10):680–686. doi: 10.1038/s41577-021-00603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Amico F, Baumann C, Rousseau H, et al. Phase I, II and III trials in inflammatory bowel diseases: a practical guide for the non-specialist. J Crohn’s Colitis. 2020;14(5):710–718. doi: 10.1093/ecco-jcc/jjz214. [DOI] [PubMed] [Google Scholar]
- 4.Vogelaar L, Spijker AV, van der Woude CJ. The impact of biologics on health-related quality of life in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2009;2:101–109. doi: 10.2147/ceg.s4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholapranee A, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45(10):1291–1302. doi: 10.1111/apt.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383(27):2652–2664. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 7.D’Amico F, Fiorino G, Furfaro F, et al. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin Investig Drugs. 2018;27(7):595–599. doi: 10.1080/13543784.2018.1492547. [DOI] [PubMed] [Google Scholar]
- 8.Olivera P, Danese S, Peyrin-Biroulet L. Next generation of small molecules in inflammatory bowel disease. Gut. 2017;66(2):199–209. doi: 10.1136/gutjnl-2016-312912. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence (NICE) Overview: Tofacitinib for moderate to severe rheumatoid arthritis. [Accessed November 2, 2021]. https://www.nice.org.uk/guidance/ta480 .
- 10.National Institute for Health and Care Excellence (NICE) Recommendations: Tofacitinib for treating active psoriatic arthritis after inadequate response to DMARDs. [Accessed November 2, 2021]. https://www.nice.org.uk/guidance/ta543/chapter/1-Recommendations .
- 11.National Institute for Health and Care Excellence (NICE) Tofacitinib for moderately to severely active ulcerative colitis. [TA547] 2018. [Accessed November 2, 2021]. https://www.nice.org.uk/guidance/ta547 .
- 12.de Mattos BRR, Garcia MPG, Nogueira JB, et al. Inflammatory bowel disease: an overview of immune mechanisms and biological treatments. Mediators Inflamm. 2015;2015:e493012. doi: 10.1155/2015/493012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodge JA, Kawabata TT, Krishnaswami S, et al. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. [Accessed November 2, 2021];Clin Exp Rheumatol. 2016 34(2):318–328. http://www.ncbi.nlm.nih.gov/pubmed/26966791 . [PubMed] [Google Scholar]
- 14.Lefevre PLC, vande Casteele N. Clinical pharmacology of Janus kinase inhibitors in inflammatory bowel disease. J Crohns Colitis. 2020;14(Suppl 2):S725–S736. doi: 10.1093/ecco-jcc/jjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magro F, Estevinho MM. Is tofacitinib a game-changing drug for ulcerative colitis? U Eur Gastroenterol J. 2020;8(7):755–763. doi: 10.1177/2050640620935732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virtanen AT, Haikarainen T, Raivola J, et al. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. BioDrugs. 2019;33(1):15–32. doi: 10.1007/s40259-019-00333-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Shea JJ, Schwartz DM, Villarino A, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Ann Rev Med. 2015;66(1):311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danese S, Argollo M, le Berre C, et al. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut. 2019;68(10):1893–1899. doi: 10.1136/gutjnl-2019-318448. [DOI] [PubMed] [Google Scholar]
- 19.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367(7):616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 20.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–1736. doi: 10.1056/NEJMoa1606910. [DOI] [PubMed] [Google Scholar]
- 21.Hanauer S, Panaccione R, Danese S, et al. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17(1):139–147. doi: 10.1016/j.cgh.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Panés J, Vermeire S, Lindsay JO, et al. Tofacitinib in patients with ulcerative colitis: health-related quality of life in phase 3 randomised controlled induction and maintenance studies. J Crohns Colitis. 2018;12(2):145–156. doi: 10.1093/ecco-jcc/jjx133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombel JF, Osterman MT, Thorpe AJ, et al. Maintenance of remission with tofacitinib therapy in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20:116–125e5. doi: 10.1016/j.cgh.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Sandborn WJ, Lawendy N, Danese S, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. 2022;55(4):464–478. doi: 10.1111/apt.16712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfizer Inc. Xeljanz prescribing information. [Accessed November 2, 2021]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203214s024,208246s010lbl.pdf .
- 26.Sands BE, Armuzzi A, Marshall JK, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther. 2020;51(2):271–280. doi: 10.1111/apt.15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermeire S, Su C, Lawendy N, et al. Outcomes of tofacitinib dose reduction in patients with ulcerative colitis in stable remission from the randomized RIVETING trial. J Crohns Colitis. 2020;15(7):1130–1141. doi: 10.1093/ecco-jcc/jjaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honap S, Chee D, Chapman TP, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. 2020;14(10):1385–1393. doi: 10.1093/ecco-jcc/jjaa075. [DOI] [PubMed] [Google Scholar]
- 29.Ungaro R, Fenster M, Dimopoulos C, et al. P344 Real-world effectiveness of tofacitinib in ulcerative colitis: a multi-centre study. J Crohns Colitis. 2019;13(Suppl 1):S274–S275. doi: 10.1093/ecco-jcc/jjy222.468. [DOI] [Google Scholar]
- 30.Taxonera C, Olivares D, Alba C. Real-World effectiveness and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflam Bowel Dis. 2022;28(1):32–40. doi: 10.1093/ibd/izab011. [DOI] [PubMed] [Google Scholar]
- 31.Takada Y, Naganuma M, Mutaguchi M, et al. P093 The comparison of short-term efficacy of treatments between tofacitinib and vedolizumab in patients with ulcerative colitis. Am J Gastroenterol. 2019;114(1):S24–S25. doi: 10.14309/01.ajg.0000613340.02177.3d. [DOI] [Google Scholar]
- 32.Biemans VBC, Sleutjes JAM, de Vries AC, et al. Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51(9):880–888. doi: 10.1111/apt.15689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in ulcerative colitis: real-world evidence from the ENEIDA registry. J Crohns Colitis. 2021;15(1):35–42. doi: 10.1093/ecco-jcc/jjaa145. [DOI] [PubMed] [Google Scholar]
- 34.Long M, Abdalla M, Afzali A, et al. S0668 Time to improvement in patient-reported outcomes with tofactinib in ulcerative colitis: initial results from a real world prospective multicenter study (TOUR) Am J Gastroenterol. 2020;115(1):S335. doi: 10.14309/01.ajg.0000704720.99780.b3. [DOI] [Google Scholar]
- 35.Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12(9):1485–1493. doi: 10.1016/j.cgh.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66(6):1049–1059. doi: 10.1136/gutjnl-2016-312735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panés J, D’Haens GR, Higgins PDR, et al. Long-term safety and tolerability of oral tofacitinib in patients with Crohn’s disease: results from a phase 2, open-label, 48-week extension study. Aliment Pharmacol Ther. 2019;49(3):265–276. doi: 10.1111/apt.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandborn J, D’Haens GR, Sands BE, et al. Tofacitinib for the treatment of ulcerative colitis: up to 7.8 years of safety data from global clinical trials. UEG J. 2021;9(S1) doi: 10.1002/ueg2.12142. [DOI] [Google Scholar]
- 39.Winthrop KL, Loftus EV, Baumgart DC, et al. Tofacitinib for the treatment of ulcerative colitis: analysis of infection rates from the ulcerative colitis clinical programme. J Crohns Colitis. 2021;15(6):914–929. doi: 10.1093/ecco-jcc/jjaa233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curtis JR, Xie F, Yun H, et al. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(10):1843–1847. doi: 10.1136/annrheumdis-2016-209131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winthrop KL, Melmed GY, Vermeire S, et al. Herpes Zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflam Bowel Dis. 2018;24(10):2258–2265. doi: 10.1093/ibd/izy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winthrop KL, Melmed GY, Vermeire S, et al. OP197 Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib: results from the phase 2 and phase 3 clinical programs. UEG J. 2021;9(S1) doi: 10.1002/ueg2.12142. [DOI] [Google Scholar]
- 43.US Food and Drug Administration; Drug Safety Communications. Safety trial finds risk of blood clots in the lungs and death with higher dose of tofacitinib (Xeljanz, Xeljanz XR) in rheumatoid arthritis patients; FDA to investigate. [Accessed November 6, 2021]. https://www.fda.gov/media/120485/download .
- 44.European Medicines Agency. Increased risk of blood clots in lungs and death with higher dose of Xeljanz (tofacitinib) for rheumatoid arthritis. [Accessed November 3, 2021]. https://www.ema.europa.eu/en/documents/press-release/increased-risk-blood-clots-lungs-death-higher-dose-xeljanz-tofacitinib-rheumatoid-arthritis_en.pdf .
- 45.Medicines and Healthcare Products Regulatory Agency. Tofacitinib (Xeljanz): new measures to minimise risk of venous thromboembolism and of serious and fatal infections. [Accessed November 3, 2021]. https://www.gov.uk/drug-safety-update/tofacitinib-xeljanz-new-measures-to-minimise-risk-of-venous-thromboembolism-and-of-serious-and-fatal-infections .
- 46.Desai RJ, Pawar A, Khosrow-Khavar F, et al. Risk of venous thromboembolism associated with tofacitinib in patients with rheumatoid arthritis: a population-based cohort study. Rheumatology. 2021;61(1):121–130. doi: 10.1093/rheumatology/keab294. [DOI] [PubMed] [Google Scholar]
- 47.Giménez Poderós T, Gallardo Borge S, Vazquez-Ferreiro P. Risk of venous thromboembolism associated with tofacitinib and baricitinib: a systematic review and indirect meta-analysis. Pharmacotherapy. 2020;40(12):1248–1264. doi: 10.1002/phar.2472. [DOI] [PubMed] [Google Scholar]
- 48.Singh S, Singh H, Loftus EV, et al. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12(3):382–393. doi: 10.1016/j.cgh.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Charles-Schoeman C, Wicker P, Gonzalez-Gay MA, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum. 2016;46(3):261–271. doi: 10.1016/j.semarthrit.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Gladman DD, Charles-Schoeman C, McInnes IB, et al. Changes in lipid levels and incidence of cardiovascular events following tofacitinib treatment in patients with psoriatic arthritis: a pooled analysis across phase III and long-term extension studies. Arthritis Care Res. 2019;71(10):1387–1395. doi: 10.1002/acr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.US NIH National Library of Medicine ClinicalTrials gov. Safety study of tofacitinib versus tumor necrosis factor (TNF) inhibitor in subjects with rhuematoid arthritis. [Accessed November 7, 2021]. https://clinicaltrials.gov/ct2/show/NCT02092467 .
- 52.Sands BE, Colombel JF, Ha C, et al. Lipid profiles in patients with ulcerative colitis receiving tofacitinib—implications for cardiovascular risk and patient management. Inflam Bowel Dis. 2020;27(6):797–808. doi: 10.1093/ibd/izaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charles-Schoeman C, Fleischmann R, Davignon J, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67(3):616–625. doi: 10.1002/art.38974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfizer. Dosing and administration in UC. Lab Monitoring. [Accessed November 2, 2021]. https://www.xeljanzhcp.com/uc/dosing/lab-monitoring .
- 55.Lichtenstein GR, Rogler G, Ciorba MA, et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancy (excluding nonmelanoma skin cancer) events across the ulcerative colitis clinical program. Inflam Bowel Dis. 2021;27:816–825. doi: 10.1093/ibd/izaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sands BE, Reinisch W, Panés J, et al. S0787 An update on the analysis of non-melanoma skin cancer in the tofacitinib ulcerative colitis clinical program as of May 2019. Am J Gastroenterol. 2020;115:S402. doi: 10.14309/01.ajg.0000705196.89572.d4. [DOI] [Google Scholar]
- 57.US Food and Drug Administration; Drug Safety Communication. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. [Accessed February 18, 2022]. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death .
- 58.Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021;15(6):879–913. doi: 10.1093/ecco-jcc/jjab052. [DOI] [PubMed] [Google Scholar]
- 59.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berinstein JA, Steiner CA, Regal RE, et al. Efficacy of induction therapy with high-intensity tofacitinib in 4 patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17(5):988–990. doi: 10.1016/j.cgh.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honap S, Pavlidis P, Ray S, et al. Tofacitinib in acute severe ulcerative colitis – a real-world tertiary center experience. Inflam Bowel Dis. 2020;26(11):e147–e149. doi: 10.1093/ibd/izaa157. [DOI] [PubMed] [Google Scholar]
- 62.Bercier B, Niland B. S2282 Use of tofacitinib as rescue therapy in a biologic-experienced patient hospitalized with acute severe ulcerative colitis. Am J Gastroenterol. 2020;115(1):pS1287. doi: 10.14309/01.ajg.0000711176.22796.b5. [DOI] [Google Scholar]
- 63.Kotwani P, Terdiman J, Lewin S. Tofacitinib for rescue therapy in acute severe ulcerative colitis: a real-world experience. J Crohns Colitis. 2020;14(7):1026–1028. doi: 10.1093/ECCO-JCC/JJAA018. [DOI] [PubMed] [Google Scholar]
- 64.Chen C, Davis E, White L, et al. Tofacitinib-induced remission of infliximab-refractory acute severe ulcerative colitis: a case report. J Gastroenterol Hepatol. 2020;35(S1):130–131. doi: 10.1111/jgh.15271. [DOI] [Google Scholar]
- 65.Berinstein JA, Sheehan JL, Dias M, et al. Tofacitinib for biologic-experienced hospitalized patients with acute severe ulcerative colitis: a retrospective case-control study. Clin Gastroenterol Hepatol. 2021;19(10):2112–2120e1. doi: 10.1016/j.cgh.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lightner AL, Vaidya P, Holubar S, et al. Perioperative safety of tofacitinib in surgical ulcerative colitis patients. Colorectal Dis. 2021;23(8):2085–2090. doi: 10.1111/codi.15702. [DOI] [PubMed] [Google Scholar]
- 67.Deepak P, Alayo QA, Khatiwada A, et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;19(8):1592–1601e3. doi: 10.1016/j.cgh.2020.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen BL, Fleshner P, Kane SV, et al. 415a – Anti-tumor necrosis factor therapy is not associated with post-operative infection: results from prospective cohort of ulcerative colitis and Crohn’s disease patients undergoing surgery to identify risk factors for postoperative infection I (Puccini) Gastroenterology. 2019;156(6):S-80. doi: 10.1016/S0016-5085(19)36987-2. [DOI] [Google Scholar]
- 69.Kani HT, Esen E, Chang S, et al. Su1857 Safety of tofacitinib on postoperative outcomes after subtotal colectomy in ulcerative colitis. Gastroenterology. 2020;158(6):S-677. [Google Scholar]
- 70.Rubin D, Reinisch W, Greuter T, et al. Extraintestinal manifestations at baseline, and effect of tofacitinib in patients with moderate to severe ulcerative colitis in the OCTAVE program. Am J Gastroenterol. 2019;114(1):S21. doi: 10.14309/01.ajg.0000613288.54649.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2017;76(8):1340–1347. doi: 10.1136/annrheumdis-2016-210322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bachelez H, van de Kerkhof PCM, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552–561. doi: 10.1016/S0140-6736(14)62113-9. [DOI] [PubMed] [Google Scholar]
- 73.Greuter T, Rieder F, Kucharzik T, et al. Emerging treatment options for extraintestinal manifestations in IBD. Gut. 2021;70(4):796–802. doi: 10.1136/gutjnl-2020-322129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siegmund B. Janus kinase inhibitors in the new treatment paradigms of inflammatory bowel disease. J Crohns Colitis. 2020;14(Suppl 2):S761–S766. doi: 10.1093/ecco-jcc/jjaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gregory MH, Ciorba MA, Deepak P, et al. Successful treatment of pyoderma gangrenosum with concomitant tofacitinib and infliximab. Inflam Bowel Dis. 2019;25(7):e87–e88. doi: 10.1093/ibd/izz015. [DOI] [PubMed] [Google Scholar]
- 76.Kochar B, Herfarth N, Mamie C, et al. Tofacitinib for the treatment of pyoderma gangrenosum. Clin Gastroenterol Hepatol. 2019;17(5):991–993. doi: 10.1016/j.cgh.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 77.Agrawal M, Brenner EJ, Zhang X, et al. Characteristics and outcomes of IBD patients with COVID-19 on tofacitinib therapy in the SECURE-IBD Registry. Inflam Bowel Dis. 2021;27(4):585–589. doi: 10.1093/ibd/izaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146(1):137–146. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clowse MEB, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39(8):755–762. doi: 10.1007/s40264-016-0431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Selinger CP, Nelson-Piercy C, Fraser A, et al. IBD in pregnancy: recent advances, practical management. Frontline Gastroenterol. 2021;12(3):214–224. doi: 10.1136/flgastro-2019-101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahadevan U, Baumgart D, Dubinsky M, et al. Pregnancy outcomes in the tofacitinib ulcerative colitis OCTAVE studies: an update as of February 2020. Am J Gastroenterol. 2020;115(1):S437–S438. doi: 10.14309/01.ajg.0000705436.64452.7d. [DOI] [Google Scholar]
- 83.Limdi JK, Farraye J, Cannon R, et al. Contraception, venous thromboembolism, and inflammatory bowel disease: what clinicians (and patients) should know. Inflam Bowel Dis. 2019;25(10):1603–1612. doi: 10.1093/ibd/izz025. [DOI] [PubMed] [Google Scholar]
- 84.Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther. 2018;47(2):162–175. doi: 10.1111/apt.14422. [DOI] [PubMed] [Google Scholar]
- 85.Kolar M, Lukas M, Malíčková K, et al. P368 Tofacitinib induction efficiency and intracellular cytokine dynamics in ulcerative colitis: results from clinical practice. J Crohns Colitis. 2020;14(Suppl 1):S348. doi: 10.1093/ecco-jcc/jjz203.497. [DOI] [Google Scholar]
- 86.Verstockt B, Verstockt S, Alsoud D, et al. Predicting tofacitinib induced endoscopic response in ulcerative colitis through a mucosal biomarker. U Eur Gastroenterol J. 2020;8(S8):358–359. doi: 10.1177/2050640620927345. [DOI] [Google Scholar]
- 87.US NIH National Library of Medicine ClinicalTrials gov. Identification of biomarkers of janus kinase inhibitor therapy in patients with ulcerative colitis (PROPHETIC) [Accessed November 7, 2021]. https://clinicaltrials.gov/ct2/show/NCT04576000 .
- 88.Scheinberg A, Dauer R, Damas O, et al. Is two better than one? Combination therapy with vedolizumab and tofacitinib for the treatment of patients with inflammatory bowel disease. Am J Gastroenterol. 2020;115(1):S460–S461. doi: 10.14309/01.ajg.0000705628.04061.db. [DOI] [Google Scholar]
- 89.Khatiwada A, Alayo Q, Patel A, et al. Efficacy and safety of combining tofacitinib with a biologic in patients with refractory inflammatory bowel diseases. Am J Gastroenterol. 2020;115(1):S461–S462. doi: 10.14309/01.ajg.0000705636.81939.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glassner K, Oglat A, Duran A, et al. The use of combination biological or small molecule therapy in inflammatory bowel disease: a retrospective cohort study. J Dig Dis. 2020;21(5):264–271. doi: 10.1111/1751-2980.12867. [DOI] [PubMed] [Google Scholar]
- 91.Ahmed W, Galati J, Kumar A, et al. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;20(3):E361–E379. doi: 10.1016/j.cgh.2021.03.034. [DOI] [PubMed] [Google Scholar]
- 92.Namour F, Diderichsen PM, Cox E, et al. Pharmacokinetics and pharmacokinetic/pharmacodynamic modeling of filgotinib (GLPG0634), a selective JAK1 inhibitor, in support of phase IIB dose selection. Clin Pharmacokinet. 2015;54(8):859–874. doi: 10.1007/s40262-015-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feagan BG, Loftus EV, Jr, Danese S, et al. A15 Efficacy and safety of filgotinib as induction therapy for patients with moderately to severely active ulcerative colitis: results from the Phase 2B/3 SELECTION study. J Can Assoc Gastroenterol. 2021;4(Suppl 1):18–20. doi: 10.1093/jcag/gwab002.014. [DOI] [Google Scholar]
- 94.Peyrin-Biroulet L, Loftus EV, Jr, Danese S, et al. A17 Efficacy and safety of filgotinib as maintenance therapy for patients with moderately to severely active ulcerative colitis: results from the phase 2B/3 SELECTION study. J Can Assoc Gastroenterol. 2021;4(Suppl 1):21–23. doi: 10.1093/jcag/gwab002.016. [DOI] [Google Scholar]
- 95.Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389(10066):266–275. doi: 10.1016/S0140-6736(16)32537-5. [DOI] [PubMed] [Google Scholar]
- 96.US NIH National Library of Medicine ClinicalTrials gov. Filgotinib in the induction and maintenance of remission in adults with moderately to severely active Crohn’s disease (DIVERSITY1) [Accessed November 7, 2021]. https://clinicaltrials.gov/ct2/show/NCT02914561?term=NCT02914561&draw=2&rank=1 .
- 97.Feagan BG, Danese S, Loftus EV, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397(10292):2372–2384. doi: 10.1016/S0140-6736(21)00666-8. [DOI] [PubMed] [Google Scholar]
- 98.European Medicines Agency. Jyseleca license publication. 2021. [Accessed February 20, 2022]. https://www.ema.europa.eu/en/medicines/human/EPAR/jyseleca .
- 99.Sandborn WJ, Ghosh S, Panes J, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. 2020;158(8):2139–2149. doi: 10.1053/j.gastro.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 100.Sandborn WJ, Feagan BG, Loftus EV, et al. Efficacy and safety of upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterology. 2020;158(8):2123–2138e8. doi: 10.1053/j.gastro.2020.01.047. [DOI] [PubMed] [Google Scholar]
- 101.Vermeire S, Danese S, Zhou W, et al. OP23 Efficacy and safety of upadacitinib as induction therapy in patients with moderately to severely active ulcerative colitis: results from phase 3 U-ACCOMPLISH study. J Crohns Colitis. 2021;15(Suppl 1):S021–S022. doi: 10.1093/ecco-jcc/jjab075.022. [DOI] [Google Scholar]
- 102.Panaccione R, Hebuterne X, Lindsay J, et al. Efficacy and safety of upadacitinib maintenance therapy in patients with moderately to severely active ulcerative colitis: results from a randomized phase 3 study. [Accessed November 7, 2021];U Eur Gastroenterol J. 2021 9(Suppl 1) https://ueg.eu/library/efficacy-and-safety-of-upadacitinib-maintenance-therapy-in-patients-with-moderately-to-severely-active-ulcerative-colitis-results-from-a-randomized-phase-3-study/243733 . [Google Scholar]
- 103.Abbvie. AbbVie submits regulatory applications to FDA and EMA for upadacitinib (RINVOQ®) for the treatment of adults with moderately to severely active ulcerative colitis. [Accessed February 20, 2022]. https://news.abbvie.com/news/press-releases/abbvie-submits-regulatory-applications-to-fda-and-ema-for-upadacitinib-rinvoq-for-treatment-adults-with-moderately-to-severely-active-ulcerative-colitis.htm .
- 104.Milev S, daCosta DiBonaventura M, Quon P, et al. An economic evaluation of tofacitinib for the treatment of moderately-to-severely active ulcerative colitis: modeling the cost of treatment strategies in the United States. J Med Econ. 2019;22(9):859–868. doi: 10.1080/13696998.2019.1609481. [DOI] [PubMed] [Google Scholar]
- 105.Sands BE, Sandborn WJ, Feagan BG, et al. Peficitinib-UC Study Group Peficitinib, an oral Janus kinase inhibitor, in moderate-to-severe ulcerative colitis: results from a randomized, phase 2 study. J Crohns Colitis . 2018:1158–1169. doi: 10.1093/ecco-jcc/jjy085. [DOI] [PubMed] [Google Scholar]