Abstract
The development and study of a simple copper-catalyzed reaction of nitroarenes with aryl boronic acids to form diarylamines that uses phenyl silane as the stoichiometric terminal reductant is described. This cross-coupling reaction requires as little as 2 mol % of CuX and 4 mol% of diphosphine for success and tolerates a broad range of functional groups on either the nitroarene or the aryl boronic acid with to afford the amine in good yield. Mechanistic investigations established that the cross-coupling reaction proceeds via a nitrosoarene intermediate and that copper is required to catalyze both the deoxygenation of the nitroarene to afford the nitrosoarene and C–NAr bond formation of the nitrosoarene with the aryl boronic acid.
Keywords: nitroarene, nitrosoarene, aryl boronic acid, cross-coupling, copper
Graphical Absract
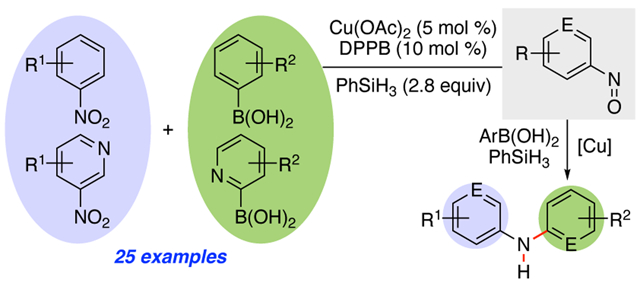
The ubiquitous nature of N,N-diarylamines in molecules that exhibit important biological- and material activities has spurred considerable research in developing efficient catalytic reactions to construct this important scaffold.1,2 While transition metal-catalyzed cross-coupling reactions that form C–N bonds are legion,3 few examples exist of their use to make secondary N,N-diarylamines from nitroarenes. Our laboratory has developed a series of transition metal-catalyzed reactions that exploit the reactivity embedded in nitroarenes for the construction of C–NAr bonds via nitrosoarene reactive intermediates,4 and we were curious if a nitroarene could serve as the nitrogen component of the cross-coupling reaction.5 In 2002, Sapountzis and Knochel reported that diarylamines 3 could be constructed from nitroarenes via the nucleophilic addition of Grignard reagents to nitrosoarenes followed by an iron-mediated reduction of the N-oxide product.6,7 After this seminal report, the development of reductive intermolecular cross-coupling reactions of nitroarenes lay dormant until 2015 when Baran and co-workers reported that 30 mol % of Fe(acac)3 catalyzed a reductive hydroamination reaction between a nitroarene and an olefin using superstoichmetric amounts of PhSiH3 and Zn(0) as the reductant (Scheme 1).8 The authors proposed that this reaction occurred through the addition of benzyl radical 5 to nitrosoarene 1. For this reaction, the transition metal catalyst was not involved in the formation of the C–NAr bond but functioned to facilitate deoxygenation of the nitroarene to the nitrosoarene. This report spurred a surge in interest in using nitroarenes to construct C–NAr bonds in reductive amination reactions.9 Recently, Radosevich and co-workers reported an organophosphorous-catalyzed reductive coupling of nitroarenes and aryl boronic acids using phenyl silane as the stoichiometric reductant.10 Critical to the success of this transformation was the strained nature of the phosphacyclobutane catalyst 6, which enables the P(III)-P(V)=O redox manifold.10 In 2019, a Mo-catalyzed reductive coupling of nitroarenes and boronic acids that used triphenylphosphine as the terminal reductant was reported by Suaréz-Pantiga, Sanz and co-workers.11 This transformation required only 5 mol % of MoO2Cl2(dmf)2 and bipyridine and a slight excess of either an aryl- or alkyl boronic acid for success and was proposed by the authors to proceed via metallocycle 10. We hoped to build on these results by developing a catalytic system that used a commercially available and earth abundant first row transition metal and ligand and a mild reductant. Herein, we report the development of a Cu-catalyzed cross-coupling reaction of nitroarenes and aryl boronic acids that uses phenyl silane as the stoichiometric reductant. Our data suggests that copper catalyzes both the deoxygenation of nitroarene to nitrosoarene and subsequent formation of the C–NAr bond.
Scheme 1.
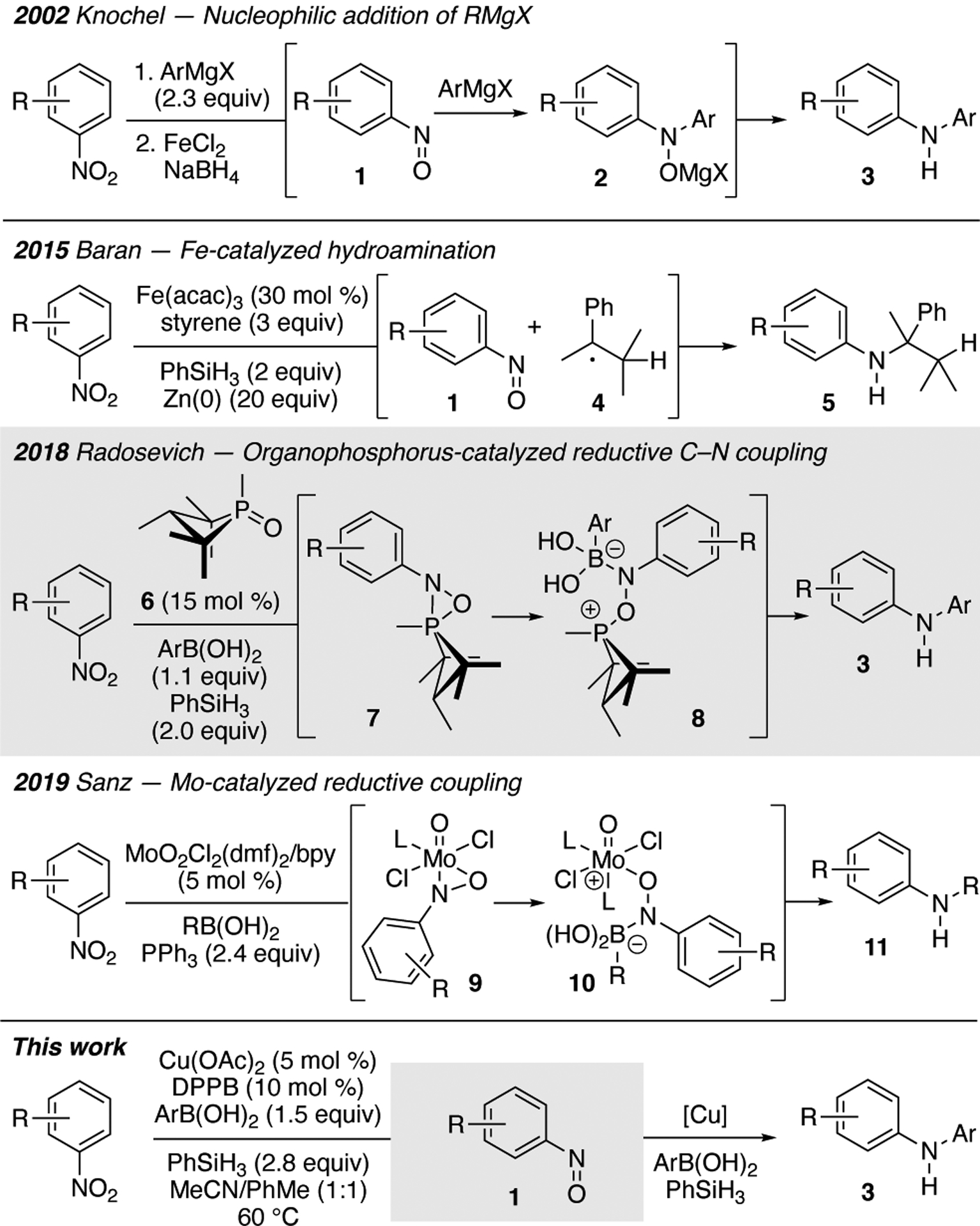
Progress towards the development of efficient catalysis of cross-coupling reactions of nitroarenes to access diarylamines.
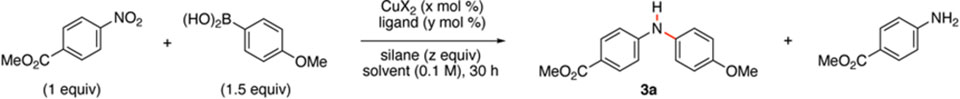
To determine if a first-row transition metal catalyzed reductive cross-coupling reaction of nitroarenes and aryl boronic acids could be achieved we examined the reactivity of 4-methoxyphenylboronic acid and methyl-4-nitrobenzoate (Table 1). While only aniline formation was observed using an iron- or nickel catalyst,12 we found that the combination of 10 mol % of Cu(OAc)2 and DPPB as the ligand afforded diarylamine 3a, but reduction to aniline was observed to be competitive (entry 1). While changing the oxidation state of copper resulted in no aniline formation, the yield of 3a was severely attenuated (entry 2). After control experiments established that both copper and the silane were required for the formation of diarylamine 3a,12 we turned our attention to improving the ratio of cross-coupling product 3a to aniline. Changing the ratio of the nitroarene to aryl boronic acid from 1.5:1 to 1:1.5 reduced the amount of aniline significantly (entry 3). A solvent screen was performed,12 and the amount of aniline was reduced in acetonitrile (entries 4 – 6). The catalyst loading was investigated using this reaction medium, and we observed that 5 mol % of Cu(OAc)2 could be used without attenuating the ratio of 3a to aniline (entry 7). The catalyst could be reduced to as little as 2 mol % without adversely affecting the reaction outcome (entry 8). While changing the counterion on copper did not have a positive effect on the cross-coupling reaction (entries 9 – 11),11 reducing the temperature of the reaction to 60 °C improved the ratio of 3a to aniline to almost 2:1 (entry 12). At this temperature, we surveyed a range of co-solvents,11 and using a 1:1 mixture of acetonitrile and toluene produced 76% of 3a, which was accompanied by only 18% of aniline (entry 13). Using this solvent mixture and temperature, we found that changing the identity of the silane or ligand had a negative effect on the reaction outcome and did not improve the amount diarylamine 3a formed (entries 14 – 18). The larger effect of changing the substituents on silane prompted us to examine the concentration of PhSiH3, and we found that using 2.8 equivalents of PhSiH3 afforded the highest ratio of diarylamine 3a to aniline (entries 19 and 20).
Table 1.
Development of optimal conditions.
| |||||||
|---|---|---|---|---|---|---|---|
| entry | Cu salt (mol %) | ligand (mol %) | silane (equiv) | solvent | T (°C) | yield 3a, %a | yield aniline, %a |
| 1b | Cu(OAc)2 (10) | DPPB (20) | PhSiH3 (3) | PhMe | 100 | 42 | 54 |
| 2b | CuCl (10) | DPPB (20) | PhSiH3 (3) | PhMe | 100 | 25 | 0 |
| 3 | Cu(OAc)2 (10) | DPPB (20) | PhSiH3 (3) | PhMe | 100 | 65 | 32 |
| 4 | Cu(OAc)2 (10) | DPPB (20) | PhSiH3 (3) | 1,4-dioxane | 100 | 56 | 40 |
| 5 | Cu(OAc)2 (10) | DPPB (20) | PhSiH3 (3) | DCE | 100 | 52 | 42 |
| 6 | Cu(OAc)2 (10) | DPPB (20) | PhSiH3 (3) | MeCN | 100 | 52 | 28 |
| 7 | Cu(OAc)2 (5) | DPPB (10) | PhSiH3 (3) | MeCN | 100 | 55 | 26 |
| 8 | Cu(OAc)2 (2) | DPPB (4) | PhSiH3 (3) | MeCN | 100 | 50 | 31 |
| 9 | CuCl2 (5) | DPPB (10) | PhSiH3 (3) | MeCN | 100 | 35 | 50 |
| 10 | Cu(tfacac)2 (5) | DPPB (10) | PhSiH3 (3) | MeCN | 100 | 45 | 28 |
| 11 | CuSO4 (5) | DPPB (10) | PhSiH3 (3) | MeCN | 100 | 36 | 34 |
| 12 | Cu(OAc)2 (5) | DPPB (10) | PhSiH3 (3) | MeCN | 60 | 63 | 33 |
| 13 | Cu(OAc)2 (5) | DPPB (10) | PhSiH3 (3) | MeCN/PhMe (1:1) | 60 | 76 | 18 |
| 14 | Cu(OAc)2 (5) | DPPB (10) | iPr3SiH (4) | MeCN/PhMe (1:1) | 60 | 0 | 0 |
| 15 | Cu(OAc)2 (5) | DPPB (10) | (MeO)2MeSiH (3) | MeCN/PhMe (1:1) | 60 | 27 | 48 |
| 16 | Cu(OAc)2 (5) | DPPP (10) | PhSiH3 (3) | MeCN/PhMe (1:1) | 60 | 55 | 27 |
| 17 | Cu(OAc)2 (5) | BINAP (10) | PhSiH3 (3) | MeCN/PhMe (1:1) | 60 | 33 | 37 |
| 18 | Cu(OAc)2 (5) | 1,1-phen (10) | PhSiH3 (3) | MeCN/PhMe (1:1) | 60 | 0 | 90 |
| 19 | Cu(OAc)2 (5) | DPPB (10) | PhSiH3 (2.8) | MeCN/PhMe (1:1) | 60 | 83 | 16 |
| 20 | Cu(OAc)2 (5) | DPPB (10) | PhSiH3 (2.6) | MeCN/PhMe (1:1) | 60 | 80 | 20 |
As determined using 1H NMR spectroscopy using CH2Br2 as the internal standard.
1.5 equiv of ArNO2 and 1.0 equiv of ArB(OH)2 used.
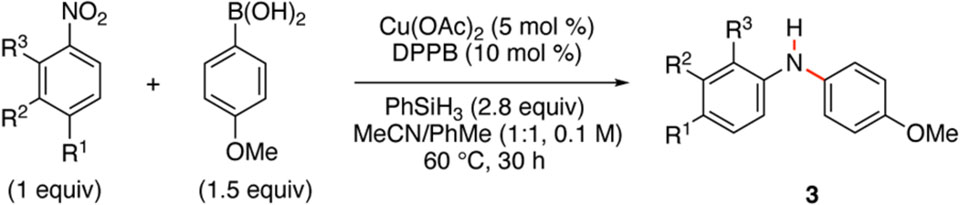
Using the optimal conditions, the scope and limitations of our Cu-catalyzed reductive cross-coupling reaction was investigated with respect to the nitroarene (Table 2). The influence of electronic nature of the nitroarene on the reaction outcome was examined by changing the identity of the R1-substituent (entries 1 – 6). We found the reaction worked best with electron-deficient nitroarenes, but diarylamines could be accessed from nitrobenzene albeit with a diminished yield. Electron-rich nitroarenes, however, such as 4-nitroanisole resulted in no reaction (entry 5).13 This result could be leveraged by using 1,4-dinitrobenzene, which resulted in coupling of the aryl boronic acid to only one of the two nitro groups to produce 3f, albeit in a slightly attenuated yield (entry 6). The effect of meta-substitution was investigated, and diarylamines 3g – 3i were formed in good yield irrespective of whether the R2-substituent was a F3C-, Me- or MeO-group (entries 7 – 10). To our surprise, the cross-coupling reaction exhibited a broad tolerance to the identity of the ortho-substituent (entries 10 – 13): high yields of diarylamine 3 were obtained with a potentially coordinating nitrile R3-substituent, an alkyl group and even an electron-donating methoxy R3-substituent, which inhibited the reaction when it was present in the para-position. The success of nitroarene 3j spurred us to examine other potentially coordinating heteroarenes, and we were delighted to see that 3-nitropyridines were competent substrates enabling access to 3o (entry 15). These results illustrate that diarylamines can be efficiently formed from a broad range of nitroarenes and heteroarenes.
Table 2.
Scope and limitations with regards to the nitroarene.
| |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | 3 | yield, %a |
| 1 | MeO2C | H | H | a | 83 (81)b |
| 2 | F3C | H | H | b | 84 |
| 3 | H | H | H | c | 66 |
| 4 | Cl | H | H | d | 85 |
| 5 | MeO | H | H | e | n.r. |
| 6 | O2N | H | H | f | 59 |
| 7 | H | F3C | H | g | 89 |
| 8 | H | Me | H | h | 73 |
| 9 | H | MeO | H | i | 85 |
| 10 | H | H | NC | j | 83 |
| 11 | H | H | Br | k | 89 |
| 12 | H | H | Et | l | 82 |
| 13 | H | H | OMe | m | 72 |
| 14 | −HC=CH−CH=CH− | H | n | 96 | |
| 15 |
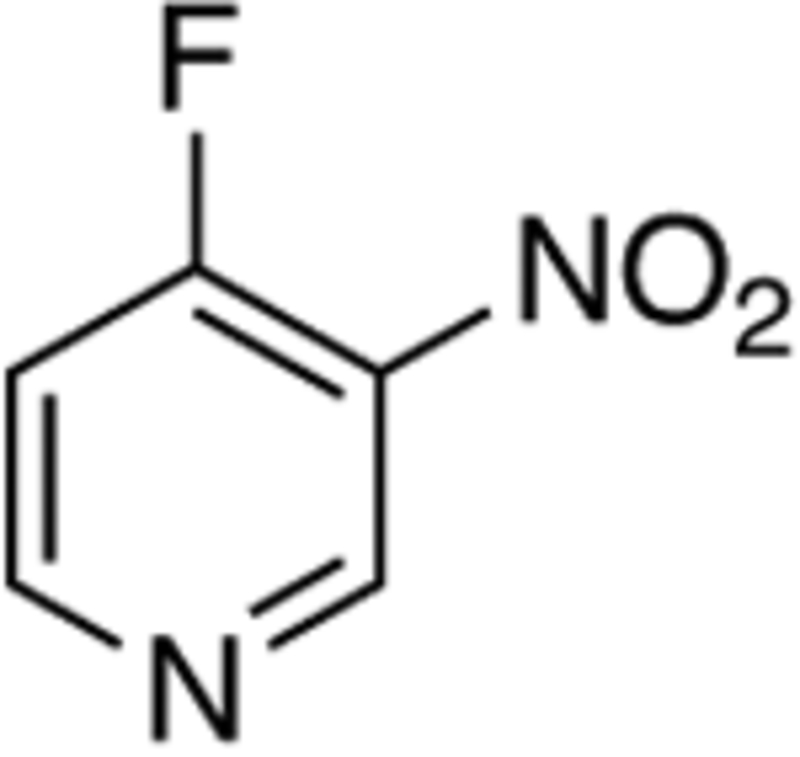
|
o | 75 | ||
Isolated after silica gel chromatography.
1 mmol reaction scale.
The scope with regards to the aryl boronic acid was also surveyed (Table 3). Modifying the electronic nature of the aryl boronic acid was investigated by examining the effect of a variety of R1- and R2-substituents on the cross-coupling reaction (entries 1 – 6), and we observed that the yield of 12 was high irrespective of whether the substituent was an electron-donating- or an electron-withdrawing group. In contrast, the cross-coupling reaction was sensitive to the identity of the ortho-substituent. While a methoxy substituent was tolerated, no diarylamine was formed from an aryl boronic acid bearing an ethyl R3-substituent (entries 8 and 9).14 While this effect could be attributed to the increased steric environment around the reaction center, we found 1-napthylboronic acid to be a good partner to afford diarylamine 12i (entry 9). Our cross-coupling reaction also tolerated a coordinating heterocycle in the boronic acid component to afford 2-pyrimidine amine 12j, albeit in an attenuated yield in comparison to pyridyl 3o (entry 10). While our method enables access to a broad range of N,N-diarylamines, neither nitroalkanes nor alkyl boronic acids are tolerated as substrates using our reaction conditions despite their use in Radosevich’s or Suaréz-Pantiga and Sanz’s methods.10,11
Table 3.
Scope and limitations with regards to the boronic acid.
| |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | 13 | yield, %a |
| 1 | F3C | H | H | a | 90 |
| 2 | F | H | H | b | 97 |
| 3 | Me | H | H | c | 90 |
| 4 | H | F | H | d | 88 |
| 5 | H | MeO | H | e | 92 |
| 6 | H | Me | H | f | 88 |
| 7 | H | H | MeO | g | 85 |
| 8 | H | H | Et | h | n.r. |
| 9 | H | −HC=CH−CH=CH− | i | 83 | |
| 10 |
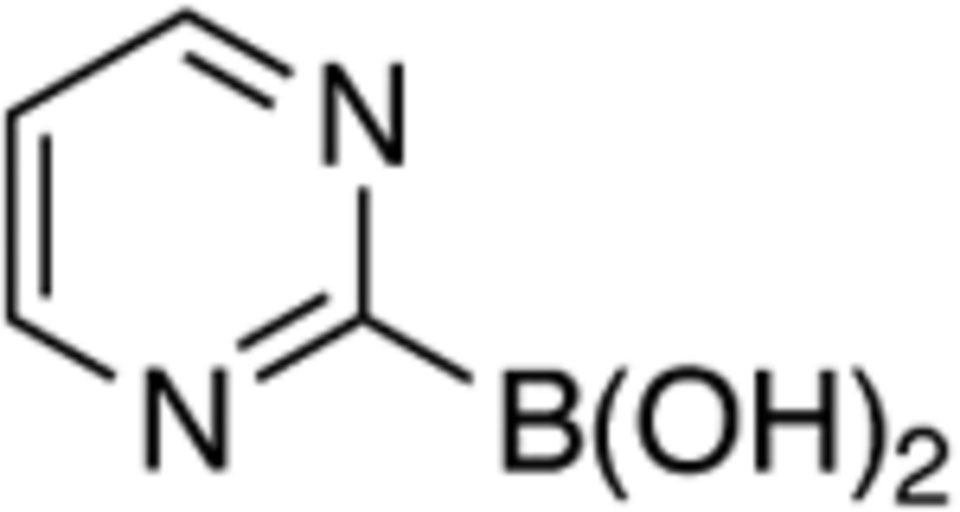
|
j | 59 | ||
Isolated after silica gel chromatography.
To provide insight into the mechanism of the reaction, several control experiments were performed (Scheme 2). To validate that the cross-coupling reactions proceeded via a nitrosoarene intermediate, an excess of 2,3-dimethylbutadiene was added to the reaction of 2-bromonitrobenzene and 4-methoxyphenyl boronic acid. Although the excess of diene inhibited this reaction, analysis of the reaction mixture after approximately 10% conversion using 1H NMR spectroscopy revealed that oxazine 13 and diarylamine 3k were both present.15,16 The observation of oxazine 13 indicates that cycloaddition of the nitrosoarene intermediate with 2,3-dimethylbutadiene is competitive with the cross-coupling process. Because anilines are well established as competent substrates for C–N bond cross-coupling reactions,17 we were curious to determine if C–NAr bond formation in our reaction occurred via a nitrosoarene or aniline. While no diarylamine formation was obtained using an aniline, submission of nitrosoarene 14 to reaction conditions afforded diarylamine 3p in 56%. Our data suggests that copper is required to trigger C–NAr bond formation. No reaction was observed when copper acetate was omitted from the reaction of nitrosoarene 14 and the aryl boronic acid. Our investigations also uncovered that the hydroxyl substituent of the aryl boronic acid plays a critical role for a successful reaction outcome. When an aryl boronic pinacolate ester or an aryl trifluoroborate were submitted to reaction conditions only reduction to aniline was observed.
Scheme 2.
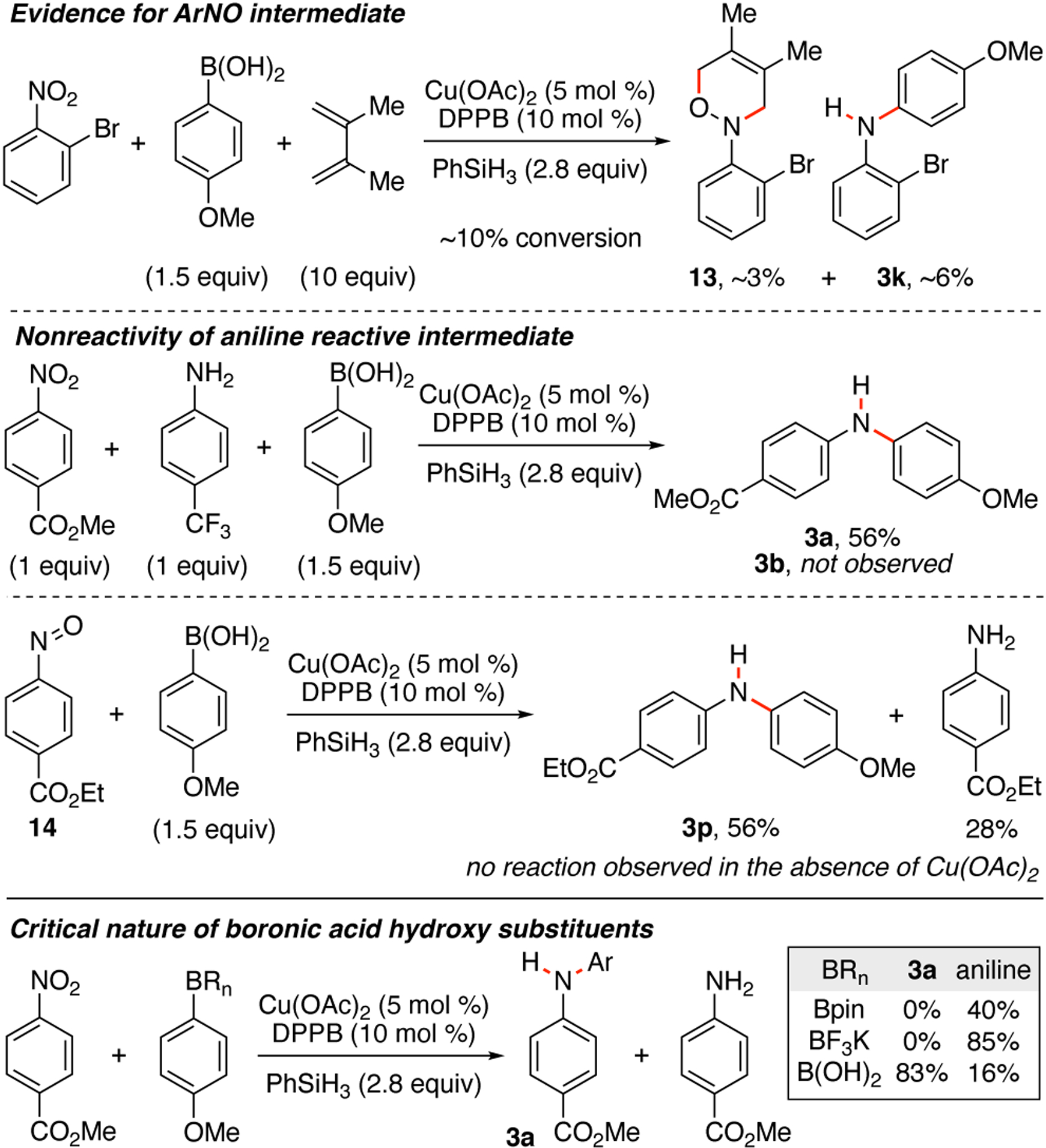
Mechanistic experiments.
Together these experiments suggest that the catalytic cycle for this cross-coupling reaction requires the copper catalyst for both the deoxygenation and C–NAr bond formation (Scheme 3). Deoxygenation of nitroarene by a copper hydride produces nitrosoarene and a copper hydroxide, which is reduced by silane.18 The observation of oxazine 13 when 2,3-dimethylbutadiene is present suggests that the nitrosoarene dissociates from the copper hydroxide complex before the subsequent C–NAr bond forming steps. If the copper remained coordinated to ArNO then only a nitroso-ene product would have been observed.19 Our control experiments indicate that copper also catalyzes the carbon–nitrogen bond formation. This could occur through coordination of copper to either the aryl boronic acid to form 15 or via copper nitrosoarene 16.19a,19b ,20 Reaction with the coupling partner—ArNO 1 or ArB(OH)2—forms metallocycle 17,21 which triggers a 1,2-aryl shift to establish the C–N bond and produce 18. Reduction of 18 with copper hydride produces the diarylamine and regenerates the copper catalyst.22,23
Scheme 3.
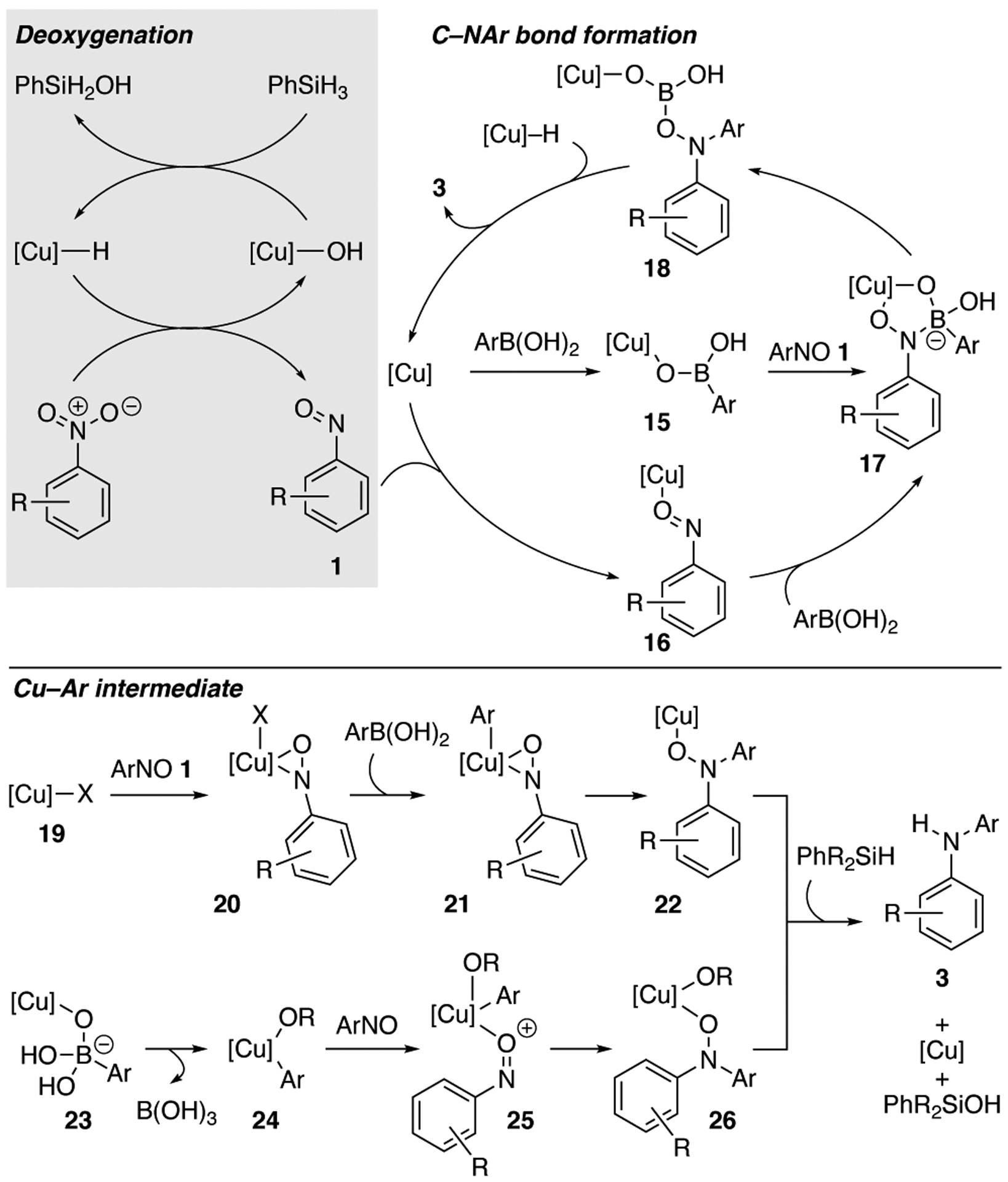
Potential catalytic cycle.
Alternatively, diarylamine 3 could form via a copper aryl intermediate. This species could be generated following a mechanism proposed by Liebeskind and co-workers for the cross-coupling of nitrosoarenes with aryl boronic acids:24 reaction of copper(I) complex 19 with ArNO 1 to afford side-on formal copper(III) complex 20.25 Transmetalation by the aryl boronic acid would form 21, and reductive elimination would generate the C–NAr bond. Reduction of 22 by silane would form diarylamine 3 and generate the copper catalyst.
The C–NAr bond could also be formed through a mechanism similar to the Chan–Evans–Lam reaction. Coordination of the aryl boronic acid to the copper hydroxide substituent affords 23, which triggers transmetalation to produce Cu(II) intermediate 24 and boric acid. Coordination of nitrosoarene 1 affords 25, which undergoes a 1,2 insertion to produce 26. Reduction of 26 with silane produces diarylamine 3 and regenerates the copper catalyst. This mechanism appears less likely because formation of copper aryl species 24 should occur for aryl boronic pinacolate esters or trifluoroborate salts.26,27
In conclusion, we have discovered a mild copper-catalyzed reaction that couples nitroarenes or nitroheteroarenes and aryl boronic acids using phenyl silane as the stoichiometric reductant. Our reaction requires as little as 2 mol % of copper and tolerates a broad range of functionality on both the nitroarene and aryl boronic acid to furnish the amine product. Our preliminary mechanistic investigations reveal that the cross-coupling reaction proceeds via a nitrosoarene intermediate and that copper is required for to catalyzed both the deoxygenation and the C–NAr bond forming steps.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to the National Institutes of Health, NIGMS (R01GM138388) and the National Science Foundation (CHE-1564959) for their generous financial support. We thank Mr. Furong Sun (UIUC) for high resolution mass spectrometry data.
Footnotes
Supporting Information
Experimental procedures, spectroscopic and analytical data for the products (PDF) are available free of charge via the Internet at http://pubs.acs.org.
Experimental details and spectral data (PDF)
REFERENCES
- 1.(a) Knölker H-J; Reddy KR; The Alkaloids; Cordell GA, Ed.; Academic Press: Amsterdam, 2008, Vol 65; p 1.18499945 [Google Scholar]; (b) Welsch ME; Snyder SA; Stockwell BR Privileged Scaffolds for Library Design and Drug Discovery. Curr. Opin. Chem. Biol 2010, 14, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257. [DOI] [PubMed] [Google Scholar]
- 2.(a) Liang M; Chen J Arylamine Organic Dyes for Dye-Sensitized Solar Cells. Chem. Soc. Rev 2013, 42, 3453. [DOI] [PubMed] [Google Scholar]; (b) Wang J; Liu K; Ma L; Zhan X Triarylamine: Versatile Platform for Organic, Dye-Sensitized, and Perovskite Solar Cells. Chem. Rev 2016, 116, 14675. [DOI] [PubMed] [Google Scholar]
- 3.(a) Jiang L; Buchwald SL In Metal-Catalyzed Cross-Coupling Reactions 2004, p 699. [Google Scholar]; (b) Hartwig JF Carbon–Heteroatom Bond Formation Catalysed by Organometallic Complexes. Nature 2008, 455, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bariwal J; Van der Eycken E C–N Bond Forming Cross-Coupling Reactions: An Overview. Chem. Soc. Rev 2013, 42, 9283. [DOI] [PubMed] [Google Scholar]; (d) Sambiagio C; Marsden SP; Blacker AJ; McGowan PC Copper Catalysed Ullmann Type Chemistry: From Mechanistic Aspects to Modern Development. Chem. Soc. Rev 2014, 43, 3525. [DOI] [PubMed] [Google Scholar]; (e) Corcoran EB; Pirnot MT; Lin S; Dreher SD; DiRocco DA; Davies IW; Buchwald SL; MacMillan DWC Aryl Amination Using Ligand-Free Ni(II) Salts and Photoredox Catalysis. Science 2016, 353, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ruiz-Castillo P; Buchwald SL Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev 2016, 116, 12564. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Dorel R; Grugel CP; Haydl AM The Buchwald–Hartwig Amination After 25 Years. Angew. Chem. Int. Ed 2019, 58, 17118. [DOI] [PubMed] [Google Scholar]; (h) West MJ; Fyfe JWB; Vantourout JC; Watson AJB Mechanistic Development and Recent Applications of the Chan–Lam Amination. Chem. Rev 2019, 119, 12491. [DOI] [PubMed] [Google Scholar]
- 4.(a) Jana N; Zhou F; Driver TG Promoting Reductive Tandem Reactions of Nitrostyrenes with Mo(CO)6 and a Palladium Catalyst To Produce 3H-Indoles. J. Am. Chem. Soc 2015, 137, 6738. [DOI] [PubMed] [Google Scholar]; (b) Zhou F; Wang D-S; Guan X; Driver TG Nitroarenes as the Nitrogen Source in Intermolecular Palladium-Catalyzed Aryl C–H Bond Aminocarbonylation Reactions. Angew. Chem. Int. Ed 2017, 56, 4530. [DOI] [PubMed] [Google Scholar]; (c) Shevlin M; Guan X; Driver TG Iron-Catalyzed Reductive Cyclization of o-Nitrostyrenes Using Phenylsilane as the Terminal Reductant. ACS Catal. 2017, 7, 5518. [Google Scholar]; (d) Ford RL; Alt I; Jana N; Driver TG Intramolecular Pd-Catalyzed Reductive Amination of Enolizable sp3-C–H Bonds. Org. Lett 2019, 21, 8827. [DOI] [PubMed] [Google Scholar]
- 5.For related work in cross-coupling reactions of nitroarenes, see:; (a) Li G; Yang L; Liu J-J; Zhang W; Cao R; Wang C; Zhang Z; Xiao J; Xue D Light-Promoted C–N Coupling of Aryl Halides with Nitroarenes. Angew. Chem. Int. Ed 2021, 60, 5230.; [DOI] [PubMed] [Google Scholar]; (b) Kashihara M; Nakao Y Cross-Coupling Reactions of Nitroarenes. Acc. Chem. Res 2021, 54, 2928. [DOI] [PubMed] [Google Scholar]
- 6.Sapountzis I; Knochel P A New General Preparation of Polyfunctional Diarylamines by the Addition of Functionalized Arylmagnesium Compounds to Nitroarenes. J. Am. Chem. Soc 2002, 124, 9390. [DOI] [PubMed] [Google Scholar]
- 7.Niggemann and co-workers demonstrated that secondary aryl amines could be formed through the addition of alkyl- or aryl zinc reagents to a nitrenoid generated in situ from a B2pin2-mediated reduction of nitroarenes. See:; Rauser M; Ascheberg C; Niggemann M Electrophilic Amination with Nitroarenes. Angew. Chem. Int. Ed 2017, 56, 11570. [DOI] [PubMed] [Google Scholar]
- 8.Gui J; Pan C-M; Jin Y; Qin T; Lo JC; Lee BJ; Spergel SH; Mertzman ME; Pitts WJ; La Cruz TE; Schmidt MA; Darvatkar N; Natarajan SR; Baran PS Practical Olefin Hydroamination with Nitroarenes. Science 2015, 348, 886. [DOI] [PubMed] [Google Scholar]
- 9.(a) Cheung CW; Ploeger ML; Hu X Direct Amidation of Esters with Nitroarenes. Nat. Commun 2017, 8, 14878. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cheung CW; Ploeger ML; Hu X Nickel-Catalyzed Reductive Transamidation of Secondary Amides with Nitroarenes. ACS Catal. 2017, 7, 7092. [Google Scholar]
- 10.(a) Nykaza TV; Cooper JC; Li G; Mahieu N; Ramirez A; Luzung MR; Radosevich AT Intermolecular Reductive C–N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV═O Catalysis. J. Am. Chem. Soc 2018, 140, 15200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li G; Nykaza TV; Cooper JC; Ramirez A; Luzung MR; Radosevich AT An Improved PIII/PV═O-Catalyzed Reductive C–N Coupling of Nitroaromatics and Boronic Acids by Mechanistic Differentiation of Rate- and Product-Determining Steps. J. Am. Chem. Soc 2020, 142, 6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suárez-Pantiga S; Hernández-Ruiz R; Virumbrales C; Pedrosa MR; Sanz R Reductive Molybdenum-Catalyzed Direct Amination of Boronic Acids with Nitro Compounds. Angew. Chem. Int. Ed 2019, 58, 2129. [DOI] [PubMed] [Google Scholar]
- 12. Refer to the Supporting Information for more details.
- 13.Raising the temperature of the reaction did not result in the formation of diarylamine 3e.
- 14.Visualization of the reaction progress using thin layer chromatography suggested that no reaction had occurred. The nitroarene and 2-ethylboronic acid were the only observable species.
- 15.For leading reports using a diene to trap a nitrosoarene reactive intermediate, see:; (a) Oikawa E; Tsubaki S The Structure of the Crystalline Adduct of Nitrosobenzene and 2,3-Dimethyl-1,3-butadiene. Bull. Chem. Soc. Jpn 1973, 46, 1819. [Google Scholar]; (b) Taylor EC; Tseng CP; Rampal JB Conversion of a Primary Amino Group into a Nitroso Group. Synthesis of Nitroso-Substituted Heterocycles. J. Org. Chem 1982, 47, 552. [Google Scholar]
- 16.The nitrosoarene intermediate from 4-methylcarboxylate-nitrobenzene was also trapped using 2,3-dimethylbutadiene. Analysis of the reaction mixture using 1H NMR spectroscopy revealed the presence of an oxazine, whose peaks were consistent with those reported by Ragaini and co-workers:; El-Atawy MA; Formenti D; Ferretti F; Ragaini F Synthesis of 3,6-Dihydro-2H-[1, 2]-Oxazines from Nitroarenes and Conjugated Dienes, Catalyzed by Palladium/Phenanthroline Complexes and Employing Phenyl Formate as a CO Surrogate. ChemCatChem 2018, 10, 4707–4717. [Google Scholar]; See the Supporting Information for additional details.
- 17.cf.; (a) Ullmann F Ueber Eine Neue Bildungsweise von Diphenylaminderivaten. Ber. Dtsch. Chem. Ges 1903, 36, 2382. [Google Scholar]; (b) Wolfe JP; Wagaw S; Buchwald SL An Improved Catalyst System for Aromatic Carbon−Nitrogen Bond Formation: The Possible Involvement of Bis(Phosphine) Palladium Complexes as Key Intermediates. J. Am. Chem. Soc 1996, 118, 7215. [Google Scholar]; (c) Driver MS; Hartwig JF A Second-Generation Catalyst for Aryl Halide Amination: Mixed Secondary Amines from Aryl Halides and Primary Amines Catalyzed by (DPPF)PdCl2. J. Am. Chem. Soc 1996, 118, 7217. [Google Scholar]; (d) Chan DMT; Monaco KL; Wang R-P; Winters MP New N- and O-arylations with Phenylboronic Acids and Cupric Acetate. Tetrahedron Lett 1998, 39, 2933. [Google Scholar]; (e) Yoo W-J; Tsukamoto T; Kobayashi S Visible-Light-Mediated Chan–Lam Coupling Reactions of Aryl Boronic Acids and Aniline Derivatives. Angew. Chem. Int. Ed 2015, 54,6587. [DOI] [PubMed] [Google Scholar]
- 18. Control experiments established that no nitrosoarene was formed in the absence of copper acetate or phenylsilane, see the Supporting Information for more details.
- 19.For reports of copper nitrosoarene complexes, see:; (a) Srivastava RS; Khan MA; Nicholas KM Nitrosoarene−Cu(I) Complexes Are Intermediates in Copper-Catalyzed Allylic Amination. J. Am. Chem. Soc 2005, 127, 7278. [DOI] [PubMed] [Google Scholar]; (b) Srivastava RS; Tarver NR; Nicholas KM Mechanistic Studies of Copper(I)-Catalyzed Allylic Amination. J. Am. Chem. Soc 2007, 129, 15250. [DOI] [PubMed] [Google Scholar]; (c) Lee J; Chen L; West AH; Richter-Addo GB Interactions of Organic Nitroso Compounds with Metals. Chem. Rev 2002, 102, 1019. [DOI] [PubMed] [Google Scholar]
- 20.Coordination of the aryl boronic acid to a copper hydroxide substituent has been proposed in Cu-catalyzed Chan–Evans–Lam reaction, see:; (a) King AE; Ryland BL; Brunold TC; Stahl SS Kinetic and Spectroscopic Studies of Aerobic Copper(II)-Catalyzed Methoxylation of Arylboronic Esters and Insights into Aryl Transmetalation to Copper(II). Organometallics 2012, 31, 7948. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Vantourout JC; Miras HN; Isidro-Llobet A; Sproules S; Watson AJB Spectroscopic Studies of the Chan–Lam Amination: A Mechanism-Inspired Solution to Boronic Ester Reactivity. J. Am. Chem. Soc 2017, 139, 4769. [DOI] [PubMed] [Google Scholar]; (c) Hardouin Duparc V; Bano GL; Schaper F Chan–Evans– Lam Couplings with Copper Iminoarylsulfonate Complexes: Scope and Mechanism. ACS Catal. 2018, 8, 7308. [Google Scholar]
- 21. Coordination of the aryl boronic acid to the nitrogen of the nitrosobenzene was proposed as a catalytic intermediate by both Radosevich and Sanz, see: ref 9b and ref 10.
- 22. Exposure of an N-hydroxydiarylamine to reaction conditions (1.4 equiv of PhSiH3, 2 mol % of Cu(OAc)2, and 4 mol % of DPPB in a 1:1 mixture of PhMe and MeCN at 60 °C) produced N,N-diarylamine in 59% yield. No reaction was observed when Cu(OAc)2 was omitted from the reaction. Please refer to the Supporting Information for more details.
- 23.For copper-catalyzed reduction of Ar2N–OH, see:; Golubev VA; Sen’ VD Preparative Syntheses of Bis(4-tert-butylphenyl)aminoxyl. Russ. J. Org. Chem 2013, 49, 555. [Google Scholar]
- 24.Yu Y; Srogl J; Liebeskind LS Cu(I)-Mediated Reductive Amination of Boronic Acids with Nitroso Aromatics. Org. Lett 2004, 6, 2631. [DOI] [PubMed] [Google Scholar]
- 25.(a) Otsuka S; Aotani Y; Tatsuno Y; Yoshida T Aromatic Nitroso Compounds as π Acids in the Zerovalent Nickel Triad Metal Complexes and the Metal-Assisted Atom-Transfer Reactions with Donor Reagents. Inorg. Chem 1976, 15, 656. [Google Scholar]; (b) Ittel SD Electron Donor-Acceptor Properties in the Bonding of Olefins and Other Unsaturated Molecules to Zerovalent Nickel. Inorg. Chem 1977, 16, 2589. [Google Scholar]; (c) Wiese S; Kapoor P; Williams KD; Warren TH Nitric Oxide Oxidatively Nitrosylates Ni(I) and Cu(I) C-Organonitroso Adducts. J. Am. Chem. Soc 2009, 131, 18105. [DOI] [PubMed] [Google Scholar]
- 26.For the use of ArBpin or ArBF3K reagents in Chan–Evans–Lam reactions, see:; (a) Quach TD; Batey RA Ligand- and Base-Free Copper(II)-Catalyzed C−N Bond Formation: Cross-Coupling Reactions of Organoboron Compounds with Aliphatic Amines and Anilines. Org. Lett 2003, 5, 4397. [DOI] [PubMed] [Google Scholar]; (b) Tzschucke CC; Murphy JM; Hartwig JF Arenes to Anilines and Aryl Ethers by Sequential Iridium-Catalyzed Borylation and Copper-Catalyzed Coupling. Org. Lett 2007, 9, 761. [DOI] [PubMed] [Google Scholar]; (c) Sueki S; Kuninobu Y Copper-Catalyzed N- and O-Alkylation of Amines and Phenols using Alkylborane Reagents. Org. Lett 2013, 15, 1544. [DOI] [PubMed] [Google Scholar]; (d) Joliton A; Carreira EM Novel SF5-Anilines and SF5-Aryl Ethers from SF5-Substituted Potassium Aryl Trifluorobo-rates. Synlett 2015, 26, 737. [Google Scholar]; (e) Vantourout JC; Law RP; Isidro-Llobet A; Atkinson SJ; Watson AJB Chan–Evans–Lam Amination of Boronic Acid Pinacol (BPin) Esters: Overcoming the Aryl Amine Problem. J. Org. Chem 2016, 81, 3942. [DOI] [PubMed] [Google Scholar]
- 27.While the Chan–Evans–Lam reaction of aryl amines with boronic pinacolate esters requires modified conditions for high yields, Watson and co-workers reported in ref 25e that using Cu(OAc)2 in combination with Et3N in MeCN at 60 °C produced diarylamine from 4-phenylbenzene boronic pinacolate ester and aniline, albeit in a reduced yield. Consequently, some diarylamine 3 would be expected using ArBpin as a coupling partner.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


