Abstract
Therapeutic IgG4 antibodies engage in Fab‐arm exchange with endogenous human immunoglobulin G4 (IgG4) to form monovalent hybrid molecules. A mechanistic population model was developed to quantitatively characterize the dynamic Fab‐arm exchange of tralokinumab, a human IgG4 monoclonal antibody currently being developed for the treatment of atopic dermatitis, with endogenous IgG4 in healthy volunteers. The estimated pharmacokinetic parameters for IgG4 were similar to those of immunoglobulin G1 or immunoglobulin G2 in humans. However, the mechanistically modeled clearance of half molecules is 21‐fold higher, likely due to the loss of avidity for the neonatal Fc receptor. Half molecules of tralokinumab randomly associate with those of endogenous IgG4 to form monovalent hybrid molecules, which became the dominant form of tralokinumab within 1 day postdose in healthy volunteers. As the potency of monovalent tralokinumab is comparable with that of bivalent tralokinumab, the IgG4 Fab‐arm exchange with endogenous IgG4 is not expected to affect the potency of neutralization of interleukin‐13 in vivo.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Therapeutic immunoglobulin G4 (IgG4) antibodies engage in dynamic Fab‐arm exchange with endogenous IgG4 to form monovalent hybrid molecules.
WHAT QUESTION DID THIS STUDY ADDRESS?
We developed a mechanistic pharmacokinetic (PK) model to characterize the in vivo Fab‐arm exchange of tralokinumab, a monoclonal IgG4 antibody, with endogenous IgG4 in healthy volunteers.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Based on the mechanistic modeling, half molecule (~75 kD) is cleared 21‐fold faster than IgG4. Half molecules of tralokinumab randomly associate with those of endogenous IgG4 to form monovalent hybrid molecules, which became the dominant form of tralokinumab within 1 day postdose. The IgG4 Fab‐arm exchange is not expected to affect the in vivo neutralization of interleukin‐13, as the potency of monovalent tralokinumab is comparable with that of bivalent tralokinumab.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
The mechanistic modeling approach facilitated evaluation of human PK predictability of therapeutic IgG4. Variation in Fab‐arm exchange related to interindividual differences in baseline endogenous IgG4 concentration was accounted for by the model.
INTRODUCTION
Tralokinumab (CAT‐354) is a human immunoglobulin G4 (IgG4) monoclonal antibody currently being developed for the treatment of moderate to severe atopic dermatitis (AD). 1 It potently and selectively neutralizes interleukin (IL)‐13, 2 a key cytokine associated with the severity of AD. 3 , 4 In a randomized, double‐blind, placebo‐controlled phase IIb study (NCT02347176), a significant improvement in Eczema Area and Severity Index (EASI) score was seen in adults with moderate to severe AD receiving 300 mg tralokinumab every 2 weeks, and a greater percentage of participants achieved an Investigator's Global Assessment Response. 1
As with some other therapeutic antibodies such as reslizumab, ibalizumab, and natalizumab, 5 , 6 , 7 tralokinumab was engineered as an IgG4 to avoid potential complement activation and antigen cross‐linking while retaining a long pharmacokinetic (PK) half‐life. 8 , 9 , 10 In healthy volunteers, the PK of tralokinumab was typical for an IgG, with mean systemic clearance (CL) of 0.188 L/d and a PK half‐life of 21.4 days. 11 After tralokinumab entered clinical development, it was reported that unlike other IgG subclasses, IgG4 antibodies are in a dynamic Fab‐arm exchange with each other. 12 In vitro and animal studies further demonstrated that therapeutic IgG4 antibodies engaged in Fab‐arm exchange with endogenous human IgG4, raising potential PK uncertainty concerns for these molecules. 13 The mechanism of IgG4 Fab‐arm exchange was further investigated by kinetic studies using a sensitive real‐time fluorescence resonance energy transfer assay. 14
To fully characterize the PK property of tralokinumab and the dynamic Fab‐arm exchange of IgG4 in humans, serum PK samples collected from healthy volunteers receiving a single intravenous (i.v.) infusion of 150 mg tralokinumab were analyzed using three different immunoassays. A mechanistic Fab‐arm exchange model was developed to describe the observed serum concentrations of intact (bivalent) tralokinumab, total (bivalent, half molecule, and monovalent hybrid) tralokinumab, and total IgG4 in healthy volunteers.
METHODS
Study design
In a randomized, single‐dose, open‐label PK study, 30 male healthy volunteers received a 30‐min i.v. infusion of 150 mg tralokinumab or a subcutaneous (s.c.) injection at the 150 or 300 mg dose level (n = 10 per group, NCT00638989). Blood samples were withdrawn predose, at the end of infusion or immediately following injection, and at various timepoints postdose up to Day 56 for PK evaluation. The study was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki, the International Conference on Harmonisation Guidance for Good Clinical Practice, and the US Code of Federal Regulations Title 21.
Bioanalysis
Serum PK samples from participants receiving a single i.v. infusion of tralokinumab were analyzed using three different assays developed and validated by MedImmune.
A double‐bridging immunoassay was used to quantify the intact (bivalent) tralokinumab in serum. Biotinylated CAT‐375 (antibody against the idiotypic region of tralokinumab) was used as both capturing and detecting reagent. The lower limit of quantitation of this double‐bridging assay was 1.0 µg/ml, with ≤20% coefficient of variation (CV) for both intra‐assay precision and interassay precision.
The second type of immunoassay was developed to measure the serum concentration of total tralokinumab, which includes bivalent intact tralokinumab, half molecules, and monovalent hybrid with endogenous IgG4. The bioassay was performed on a Gyrolab assay platform (Gyros AB), with biotinylated CAT‐375 captured on streptavidin‐coated columns of the Gyros compact disc as the capture reagent, and a sheep anti‐human IgG4 antibody labeled with Alexa Fluor® 647 (Invitrogen) as the detecting reagent. The assay has a lower limit of quantification (LLOQ) of 0.30 µg/ml, with ≤20% CV for both intra‐assay precision and interassay precision.
The third assay quantified the total IgG4 in serum (Human IgG Subclass Kit, The Binding Site Group), including bivalent tralokinumab, endogenous IgG4, the half molecules, and hybrid monovalent IgG4. This was a commercial kit, with an LLOQ of 4 µg/ml with ≤2.5% CV for both intra‐assay precision and interassay precision.
Mechanistic IgG4 Fab‐arm exchange model
A mechanistic PK model was constructed to describe the disposition of intravenously administered tralokinumab, de novo production and degradation of endogenous IgG4, dissociation of IgG4 to single‐arm half molecules, and reassociation to form the monovalent hybrid molecule (Figure 3).
FIGURE 3.
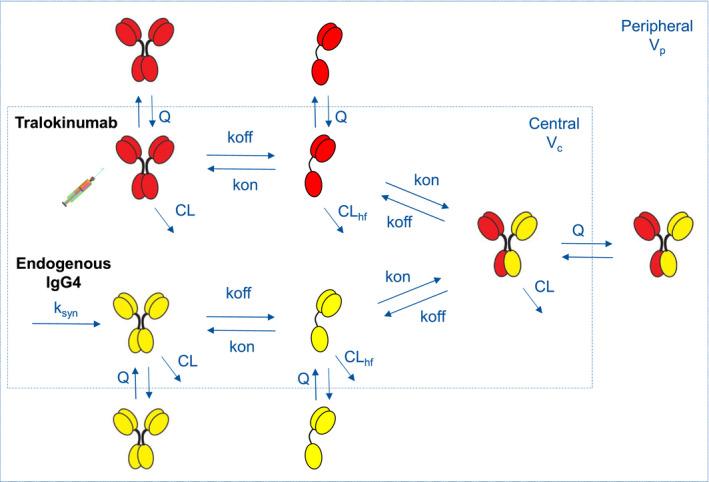
Mechanistic model structure for IgG4 Fab‐arm exchange in humans. CL, systemic clearance; CLhf, clearance of half IgG4 molecules; IgG4, immunoglobulin G4; k off, dissociation rate constant of IgG4 molecules; k on, association rate constant of IgG4 molecules; k syn, de novo production of endogenous IgG4; Q, intercompartment clearance; V c, central volume of distribution; V p, peripheral volume of distribution
The double‐bridging immunoassay quantified the intact, bivalent tralokinumab ( ) in serum, the central compartment. The second immunoassay with anti‐idiotypic capturing and sheep anti‐human IgG4 detection format measured the total serum concentration of bivalent tralokinumab (
) in serum, the central compartment. The second immunoassay with anti‐idiotypic capturing and sheep anti‐human IgG4 detection format measured the total serum concentration of bivalent tralokinumab ( ), dissociated single‐arm molecule (
), dissociated single‐arm molecule ( ), and monovalent hybrid IgG4 (
), and monovalent hybrid IgG4 ( ). Lastly, the universal IgG4 immunoassay detected all species in serum (
). Lastly, the universal IgG4 immunoassay detected all species in serum (



 ).
).
The equilibrium dissociation constant (K d) of IgG4 was fixed at 3.8 nM, a value determined in vitro at 37°C. 15 As such, k off is imputed as k on⋅K d, with the association rate constant k on to be estimated by modeling. The zero‐order production rate of endogenous IgG4 is computed from steady‐state constraints as
where and represent the baseline concentrations of endogenous IgG4 ( ) and half molecules (
) and half molecules ( ) in serum, respectively. This equation was derived from eq. 1 in the Supplement Materials under steady‐state condition assumption. Furthermore, as the half molecule concentration was not directly measured by these immunoassays, distribution parameters (peripheral volume of distribution ([V
p] and intercompartment clearance [Q]) of the half molecule were assumed to be the same as those of IgG4 to avoid model overparameterization. The differential equation system for the mechanistic model and initial conditions (including ) are provided in the Supplemental Material.
) in serum, respectively. This equation was derived from eq. 1 in the Supplement Materials under steady‐state condition assumption. Furthermore, as the half molecule concentration was not directly measured by these immunoassays, distribution parameters (peripheral volume of distribution ([V
p] and intercompartment clearance [Q]) of the half molecule were assumed to be the same as those of IgG4 to avoid model overparameterization. The differential equation system for the mechanistic model and initial conditions (including ) are provided in the Supplemental Material.
Data analysis
Serum concentration data of intact and total tralokinumab and total IgG4 from all participants receiving a single i.v. administration of 150 mg tralokinumab were log‐transformed and simultaneously modeled using the pharmacostatistical software package NONMEM (Version 7.2; ICON). The first‐order conditional estimation method with interaction, as implemented in NONMEM, was used for model development. An additive residual error model was used when the logarithm transformed concentration data were analyzed (it approximates a proportional residual error model in linear scale). Model stability and performance were assessed by bootstrapping and visual predictive check (VPC). Because of the small sample size, demographic covariate analysis was not conducted.
Intact IgG4 was used as the assay standard for the total IgG4 immunoassay, which detected the Fc component of a molecule. As such, molar concentration of the half molecule ( or
or  ) was halved for computation of the total IgG4 concentration (ie, 1 nM of half molecule is associated with an assay signal equivalent to 0.5 nM of intact IgG4). Similarly, the predicted molar concentration of half‐tralokinumab (
) was halved for computation of the total IgG4 concentration (ie, 1 nM of half molecule is associated with an assay signal equivalent to 0.5 nM of intact IgG4). Similarly, the predicted molar concentration of half‐tralokinumab ( ) from total tralokinumab assay was also halved. Molar concentration of the hybrid molecule (
) from total tralokinumab assay was also halved. Molar concentration of the hybrid molecule ( ) was unadjusted for the calculation of total IgG4 (
) was unadjusted for the calculation of total IgG4 (



 ) while a scaling factor was introduced to account for potential variation in total tralokinumab assay (
) while a scaling factor was introduced to account for potential variation in total tralokinumab assay (

 ).
).
RESULTS
Subjects and data set
In a randomized, single‐dose, open‐label PK study, 30 male healthy volunteers received a 30‐min i.v. infusion of 150 mg tralokinumab or an s.c. injection at the 150 or 300 mg dose level (n = 10 per group). All participants received the intended dose (150 mg i.v., 150 mg s.c., or 300 mg s.c.). 11
Serum PK samples from 10 participants in the i.v. dose group were analyzed using three different immunoassays measuring intact tralokinumab, total tralokinumab, and total IgG4. The PK data set contained 424 quantifiable tralokinumab or IgG4 concentration data points. The mean age of these 10 participants was 30 years, and the mean body weight was 78.7 kg. A majority (80%) of these participants were White. Baseline endogenous IgG4 concentration was unquantifiable in one subject. In nine other healthy volunteers, the median endogenous IgG4 at baseline was 304 µg/ml (range, 58–1383 µg/ml).
Total tralokinumab PK data from s.c. dose groups were previously reported. 11 Because of the potential flip‐flop kinetics of intact tralokinumab, greater variability, and confounding absorption process, PK data from participants who received an s.c. injection of tralokinumab were not used for mechanistic model development.
PK profiles of tralokinumab and IgG4
Mean serum concentration–time profiles of tralokinumab and IgG4 as measured by three different immunoassays are shown in Figure 1. To facilitate interpretation of the assay results, bivalent intact tralokinumab, half molecule, monovalent hybrid tralokinumab, and endogenous IgG4 are denoted hereafter as  ,
,  ,
,  , and
, and  , respectively. The PK profile of total tralokinumab (
, respectively. The PK profile of total tralokinumab (

 ) in nine participants with quantifiable endogenous IgG4 at baseline was typical for IgG (Figure 1a). The mean concentration of intact tralokinumab (
) in nine participants with quantifiable endogenous IgG4 at baseline was typical for IgG (Figure 1a). The mean concentration of intact tralokinumab ( ), as determined by a double‐bridging immunoassay, fell below the assay lower quantification limit (1.0 μg/ml) after Day 10. Serum concentrations of total IgG4 (
), as determined by a double‐bridging immunoassay, fell below the assay lower quantification limit (1.0 μg/ml) after Day 10. Serum concentrations of total IgG4 (



 ) as measured by a universal IgG4 assay were much higher. However, the observed tralokinumab and IgG4 profiles in one participant with unquantifiable endogenous IgG4 at baseline differed from those in other participants (Figure 1b).
) as measured by a universal IgG4 assay were much higher. However, the observed tralokinumab and IgG4 profiles in one participant with unquantifiable endogenous IgG4 at baseline differed from those in other participants (Figure 1b).
FIGURE 1.
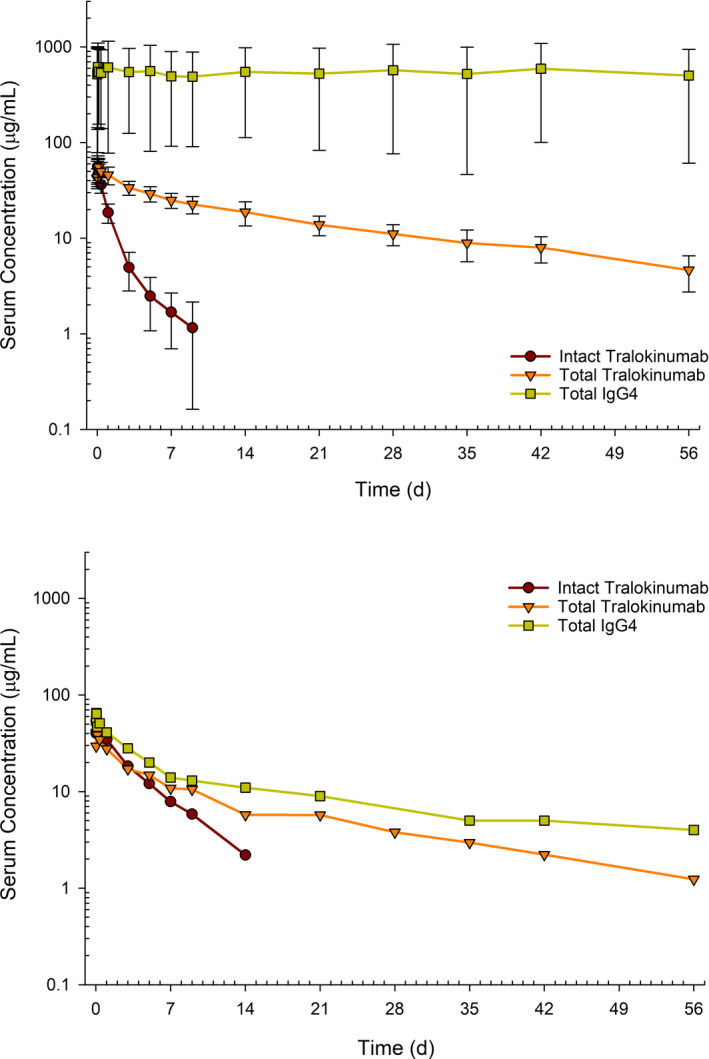
Observed serum concentration–time profiles of bivalent intact tralokinumab, total tralokinumab, and total IgG4 in healthy volunteers. The assay lower limit of quantification was 1.0 µg/ml for intact tralokinumab, 0.30 µg/ml for total tralokinumab, and 4 µg/ml for total IgG4. IgG4, immunoglobulin G4
Noncompartmental analysis
Noncompartmental PK parameter values are summarized in Table 1. The CL of bivalent intact tralokinumab ( ) increased with baseline endogenous IgG4 concentration (Figure 2).
) increased with baseline endogenous IgG4 concentration (Figure 2).
TABLE 1.
Noncompartmental pharmacokinetic parameter summary for tralokinumab and IgG4 in healthy volunteers receiving single intravenous infusion of 150 mg tralokinumab
| Intact tralokinumab | Total tralokinumab | Total IgG4 | ||||
|---|---|---|---|---|---|---|
| With quantifiable IgG4baseline | No quantifiable IgG4baseline | With quantifiable IgG4baseline | No quantifiable IgG4baseline | With quantifiable IgG4baseline | No quantifiable IgG4baseline | |
| n | 9 | 1 | 9 | 1 | 9 | 1 |
| Cmax (µg/ml) | 56.9 (10.8) | 54.4 | 60.2 (13.8) | 41.0 | 706 (555) | 65.0 |
| AUC a (µg × d/ml) | 81.2 (26.1) | 190 | 951 (236) | 367 | 29,900 (25,300) a | 552 a |
| CL (L/d) | 2.02 (0.67) | 0.79 | 0.166 (0.038) | 0.409 | NA | NA |
| V ss (L) | 5.35 (1.98) | 3.64 | 4.46 (0.78) | 8.26 | NA | NA |
| t 1/2 (d) | 3.93 (2.38) | 3.63 | 20.5 (2.67) | 16.6 | NA | NA |
Parameters are shown as mean (standard deviation).
Abbreviations: AUC, area under the concentration‐time curve; CL, systemic clearance; Cmax, maximum observed concentration; IgG4, immunoglobulin G4; NA, not applicable given flat terminal phase (continuous endogenous IgG4 production); n, number of subjects; t 1/2, half‐life; V ss, steady‐state volume of distribution.
AUC from time zero to 56 days postdose.
FIGURE 2.
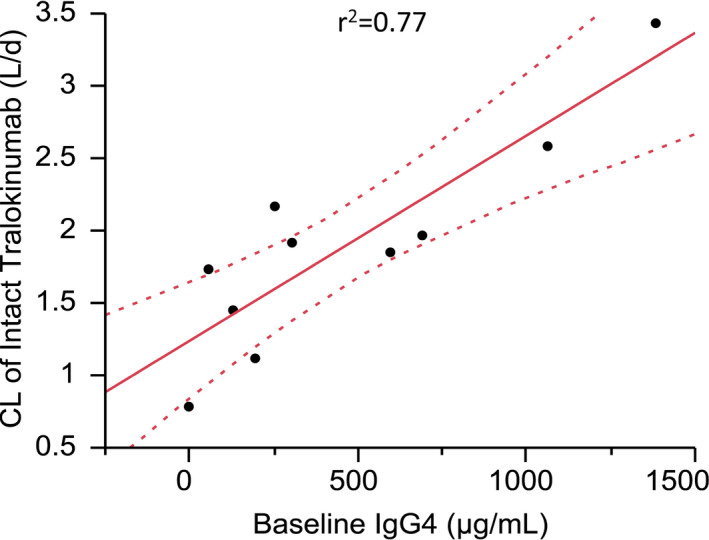
Correlations of systemic clearance of intact tralokinumab with baseline endogenous IgG4. The dotted curves represent 95th percentile confidence limits. CL, systemic clearance; IgG4, immunoglobulin G4
Mechanistic modeling of IgG4 Fab‐arm exchange
The structure of the IgG4 Fab‐arm exchange model is shown in Figure 3. Upon i.v. administration, bivalent intact tralokinumab  dissociates in blood to form half molecules
dissociates in blood to form half molecules that randomly associate with other half molecules to form either intact tralokinumab or a monovalent hybrid IgG4
that randomly associate with other half molecules to form either intact tralokinumab or a monovalent hybrid IgG4
 . Parameters k
on and k
off are the rate constants for association and dissociation of IgG4 molecules, respectively. CL and CLhf are the systemic clearance of intact IgG4 and single‐arm half molecules, respectively. In this diagram, the central compartment represents serum with a distribution volume (V
c). Peripheral tissue distribution of IgG4 and half molecules is characterized by V
p and Q. The de novo production of endogenous IgG4
. Parameters k
on and k
off are the rate constants for association and dissociation of IgG4 molecules, respectively. CL and CLhf are the systemic clearance of intact IgG4 and single‐arm half molecules, respectively. In this diagram, the central compartment represents serum with a distribution volume (V
c). Peripheral tissue distribution of IgG4 and half molecules is characterized by V
p and Q. The de novo production of endogenous IgG4
 is denoted by a zero‐order input function k
syn.
is denoted by a zero‐order input function k
syn.
Intact tralokinumab, total tralokinumab, and total IgG4 data from 10 healthy volunteers were simultaneously modeled using a population approach. Estimated population PK parameters, interindividual variability, and residuals are listed in Table 2. The estimated CL of half molecules is 20.9‐fold higher than that of IgG4. Although one healthy volunteer had no quantifiable IgG4 at baseline, from population modeling the mechanistic model could be used to estimate the endogenous IgG4 concentration in this subject, 21.6 nM or 3.24 µg/ml, slightly below the LLOQ of total IgG4 assay (4.0 µg/ml). A scaling factor (0.685) was also incorporated in the model and estimated to account for monovalent hybrid and bivalent intact tralokinumab in the total tralokinumab assay.
TABLE 2.
Population pharmacokinetic structure and variance parameters estimated from mechanistic modeling
| Parameter | Original estimate | Bootstrap (n = 711) a | |
|---|---|---|---|
| Median | 95% CI | ||
| CL (L/d) | 0.151 | 0.151 | 0.102–0.185 |
| V c (L) | 2.94 | 2.95 | 2.63–3.28 |
| Q (L/d) | 0.559 | 0.547 | 0.514–0.588 |
| V p (L) | 3.18 | 3.20 | 2.58–4.07 |
| CLhf/CL | 20.9 | 20.8 | 10.0–43.7 |
| IgG4baseline (nM) | 2330 | 2290 | 1280–4,320 |
| IgG4baseline,ID10 (nM) | 21.6 | 20.9 | 20.1–21.7 |
| k on (nM−1d−1) | 0.260 | 0.260 | 0.226–0.283 |
| K d (nM) | 3.8 fixed b | – | – |
| Scaling factor (hybrid) | 0.685 | 0.680 | 0.633–0.735 |
| Interindividual variability c | |||
| ηCL | 15.0 | 13.4 | 2.0–20.1 |
| ηVc | 17.1 | 15.8 | 7.1–23.3 |
| ηVp | 32.4 | 32.5 | 0.3–43.3 |
| ηIgG4baseline | 91.2 | 86.0 | 57.9–108 |
| Residual variability c | |||
| Intact tralokinumab assay | 12.2 | 12.1 | 10.1–14.7 |
| Total tralokinumab assay | 15.0 | 15.0 | 11.7–19.0 |
| Total IgG4 assay | 15.5 | 15.3 | 12.6–18.4 |
Scaling factor (hybrid), a parameter to account for assay signals of bivalent and monovalent tralokinumab in the total tralokinumab assay.
Abbreviations: CI, confidence interval; CL, clearance; CLhf/CL, ratio of half molecule clearance and IgG4 clearance; IgG4, immunoglobulin G4; IgG4baseline, serum level of endogenous IgG4 at baseline; K d, equilibrium dissociation constant of IgG4; k on, association rate constant of half molecules; Q, intercompartmental clearance; V c, central volume of distribution; V p, peripheral volume of distribution.
Among 1000 bootstrapping runs, 711 converged with a number of significant figures ≥2.
Fixed to a value as determined by Förster resonance energy transfer assay. 15
Shown as percent coefficient of variation (%CV).
Except for baseline IgG4 concentration, the interindividual PK variability was relatively small in healthy volunteers (15%–32% CV). Furthermore, the estimated residual variability of these three immunoassays (12%–16% CV) agreed well with the parameters from the assay validation criterion.
Model evaluation
Predominantly, the medians of PK and variance parameters from bootstrapping runs were close to the values originally estimated from the model (Table 2). Basic goodness‐of‐fit plots and individual/typical profiles of tralokinumab and IgG4 are presented in Figures S1–S3. VPC plots are shown in Figure 4. Symbols represent the observed serum concentrations. The assay LLOQ was 1.0 μg/ml for intact tralokinumab. Concentrations below the LLOQ were not plotted. The shaded bands in VPC plots for nine participants with quantifiable baseline IgG4 concentrations reflect the interindividual and residual variability (Figure 4a). As there was only one subject with unquantifiable endogenous IgG4 at baseline, the shaded bands in Figure 4b only correspond to residual variability of assays.
FIGURE 4.
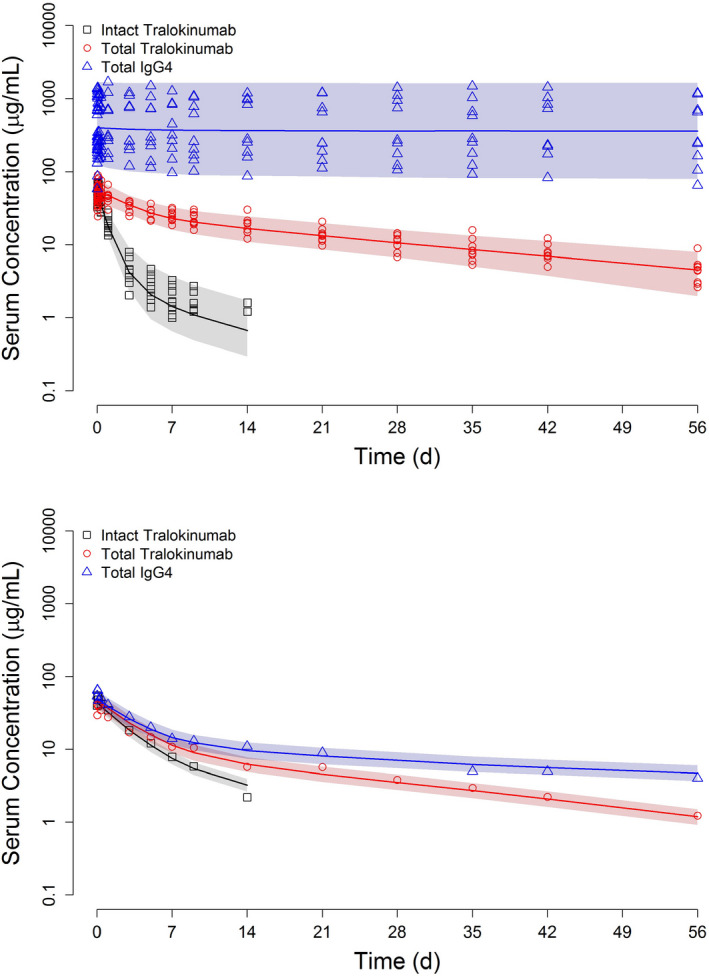
Visual predictive check for IgG4 Fab‐arm exchange model: symbols, observed concentrations; solid curve, median of 1000 simulated profiles; and shaded area, range between the 5th and 95th percentiles. The assay lower limits of quantification were 1.0 µg/ml for intact tralokinumab, 0.30 µg/ml for total tralokinumab, and 4 µg/ml for total IgG4
DISCUSSION
IL‐13 is secreted predominantly by cluster of differentiation 4+ (CD4+) T helper‐2 (Th2) cells, which may play a central role in the pathogenesis of AD. 3 , 4 , 16 , 17 , 18 Tralokinumab is a recombinant fully human monoclonal antibody that potently and specifically neutralizes IL‐13. It was engineered as an IgG4 antibody to avoid complement system activation and sensitization of mast cells. In vitro and in vivo animal studies demonstrated potent inhibition of IL‐13 induced effects and no evidence of drug‐related toxicity. 2 With a 3‐week half‐life, the PK of tralokinumab was typical for an IgG in healthy volunteers. 11 A phase II clinical trial demonstrated that tralokinumab treatment achieved clinically significant improvements in EASI scores in adults with moderate to severe AD. 1
When tralokinumab had just entered clinical development, it became known that IgG4 molecules engaged in a dynamic Fab‐arm exchange. 12 In this process, bivalent IgG4 molecules dissociate to form half molecules, which then randomly combine with other half molecules to form monovalent hybrid IgG4. Such IgG4 Fab‐arm exchange was further characterized in vitro and in animal studies, with kinetic models built to delineate the random dissociation–association process. 13 , 14 There had been concerns, however, with the implications of IgG4 Fab‐arm exchange for clinical efficacy and human PK predictability of therapeutic IgG4 molecules. 13 In this investigation, three different immunoassays were deployed to analyze PK samples from healthy volunteers receiving a single 150 mg intravenous infusion of tralokinumab. A mechanistic population PK model was developed to characterize the in vivo Fab‐arm exchange of tralokinumab with endogenous IgG4 in humans (Figure 3).
The PK profile of total tralokinumab (bivalent, half molecule, and monovalent) was typical for an IgG (Figure 1a), with a mean CL of 0.166 L/d and an elimination half‐life of 20.5 days from noncompartmental analysis (Table 1). The faster clearance of bivalent tralokinumab, as measured by a double‐bridging immunoassay, reflected the conversion/Fab‐arm exchange into monovalent molecules. Serendipitously, one of these 10 subjects had no quantifiable endogenous IgG4 at baseline, and the PK profiles in this subject were dramatically different from others (Figure 1b). The apparent CL of bivalent intact tralokinumab increased with baseline endogenous IgG4, reflecting augmented formation of hybrid monovalent IgG4 in subjects with high endogenous IgG4 (Figure 2).
The Fab‐arm exchange of tralokinumab with endogenous IgG4 in healthy volunteers was adequately described by a mechanistic PK model (Figure 3). The structure and variance parameter estimates from the population model were close to the medians of bootstrapping (Table 2), and there was no apparent trend in conditional weighted residuals when plotted against population predicted concentrations or time (Figure S1). In VPC plots, the observed concentrations were evenly distributed across the simulated median curves and enclosed within the shaded 5th–95th percentile range (Figure 4).
The population estimates of PK parameters for IgG4 were similar to those of immunoglobulin G1 (IgG1) or immunoglobulin G2 (IgG2) in humans, with a typical CL of 0.151 L/d. However, the estimated CL of half molecules is 21‐fold higher (Table 2). The low CL of IgG by the reticuloendothelial system is due to neonatal Fc receptor (FcRn)–mediated intracellular recycling of endocytosed IgG. The effective FcRn‐mediated recycling requires bivalency of the Fc dimer to decelerate the dissociation in the endosome. 19 Without such avidity effect, the monomeric form of Fc fused with nonspecific Fab fragment was cleared 30‐fold faster than keyhole limpet hemocyanin–derived antibody in mice. 15 As such, the estimated substantially faster clearance of IgG4 half molecules in healthy volunteers may be associated with the loss of avidity for FcRn in endosomes, resulting in enhanced intracellular degradation in lysosomes. The smaller molecular size of IgG4 half molecules may also contributed to the rapid clearance, as demonstrated in studies of antibody fragments in FcRn knockout mice. 20
The observed rapid decline of intact (bivalent) tralokinumab concentration in serum is according to the model due to its dissociation to half molecules with subsequent hybrid molecule formation. The K d of IgG4 was fixed to 3.8 nM, as determined by in vitro monitoring of Förster resonance energy transfer (FRET) assay signals. 14 From mechanistic modeling, the estimated association rate constant k on was 0.260 nM−1 d−1, corresponding to a k off of 0.988 d−1 or a dissociation half‐life of 0.70 days for IgG4 in humans (Table 2). The k off of IgG4 from the in vitro FRET assay was 1.2 × 10−3 s−1 (1.7 d−1). 14 The slower dissociation of IgG4 in vivo compared with the in vitro system could be attributed to the variation in local redox potentials.
One subject had no quantifiable endogenous IgG4 concentration at baseline. The estimated CL of IgG4 at 0.149 L/d from mechanistic modeling was not much different from that in other subjects. After the faster clearance of half molecules and endogenous IgG4‐dependent formation of hybrid molecules were taken into account, the interindividual variability in the CL and V c of IgG4 were low in healthy volunteers (15% CV for CL and 17% CV for V c). On the contrary, the endogenous IgG4 was highly variable among these subjects (91% CV).
Circulating IL‐13 is a monomeric Th2 cytokine with a molecular weight of approximately 15 kD. As such, the avidity effect is irrelevant in the neutralization of IL‐13 signaling by tralokinumab. Indeed, from reporter gene assay or an in vitro assay using human umbilical vein endothelial cells, the bioactivity of tralokinumab was not impacted by the Fab exchange. 2 , 21 Extensive preclinical and clinical studies have demonstrated potent and specific IL‐13 neutralizing activity of tralokinumab. Despite the dynamic Fab‐arm exchange with endogenous IgG4, in the absence of target‐mediated clearance, the reported PK half‐lives of therapeutic IgG4 were 3–4 weeks, similar to those of IgG1 and IgG2. 11 , 22 , 23 Based on overall evaluation of PK, in vitro potency, and clinical efficacy, the Fab‐arm exchange of tralokinumab with endogenous IgG4 is not expected to affect its potential use for the treatment of AD. Given the smaller molecular size, the peripheral distribution, in particular the intercompartmental clearance of the half molecules, could be different from IgG4. 23 In the absence of half‐molecule PK data, distribution parameter values (V p and Q) of half molecules were assumed the same as IgG4 to avoid model overparameterization. Although the overall data fitting was reasonably well, this assumption poses a main limitation of the mechanistic model.
In summary, a mechanistic model was developed to characterize the Fab‐arm exchange of tralokinumab with endogenous IgG4 in healthy volunteers. Upon i.v. administration, bivalent tralokinumab dissociates into half molecules with a dissociation half‐life of 0.7 days. The clearance of half molecules is 21‐fold higher than IgG4, due to less efficient FcRn‐mediated intracellular recycling. Half molecules of tralokinumab randomly associate with those of endogenous IgG4 to form monovalent hybrid molecules, which became the dominant form of tralokinumab within 1 day post‐i.v. administration in healthy volunteers. As the potency of monovalent tralokinumab is comparable with that of bivalent tralokinumab, the IgG4 Fab‐arm exchange with endogenous IgG4 is not expected to affect the potency of in vivo neutralization of IL‐13 in patients with asthma.
CONFLICT OF INTEREST
B.W. is a former employee of AstraZeneca. L.K.R. and J.G. are current employees of AstraZeneca. All authors own AstraZeneca stocks.
AUTHOR CONTRIBUTIONS
B.W., J.G., and L.K.R. designed the research, performed the research, analyzed the data, and wrote the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Tim Meyers (formerly of AstraZeneca) and the bioanalysis group at AstraZeneca Cambridge for performing clinical PK sample analysis, and Yvonne (Yi‐Yang) Lau (formerly of AstraZeneca) for contribution to the initial PK model development. The authors also thank Paolo Vicini, Chris Kell, and Ed Piper (AstraZeneca) for critical review of this manuscript. The authors thank Lauren Smith, BA (QXV Comms, an Ashfield business, part of UDG Healthcare plc, Macclesfield, UK), who provided editing assistance funded by AstraZeneca.
Wang B, Goodman J, Roskos LK. Mechanistic modeling of a human IgG4 monoclonal antibody (tralokinumab) Fab‐arm exchange with endogenous IgG4 in healthy volunteers. CPT Pharmacometrics Syst Pharmacol. 2022;11:438–446. doi: 10.1002/psp4.12738
Funding information
No funding was received for this work.
Contributor Information
Bing Wang, Email: Bing.Wang@AmadorBio.com.
Lorin K. Roskos, Email: LKR001@gmail.com, Email: Bing.Wang@AmadorBio.com.
REFERENCES
- 1. Wollenberg A, Howell MD, Guttman‐Yassky E, et al. Treatment of atopic dermatitis with tralokinumab, an anti‐IL‐13 mAb. J Allergy Clin Immunol. 2019;143:135‐141. [DOI] [PubMed] [Google Scholar]
- 2. May RD, Monk PD, Cohen ES, et al. Preclinical development of CAT‐354, an IL‐13 neutralizing antibody, for the treatment of severe uncontrolled asthma. Br J Pharmacol. 2012;166:177‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ungar B, Garcet S, Gonzalez J, et al. An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol. 2017;137:603‐613. [DOI] [PubMed] [Google Scholar]
- 4. Khattri S, Shemer A, Rozenblit M, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salfeld JG. Isotype selection in antibody engineering. Nat Biotechnol. 2007;25:1369‐1372. [DOI] [PubMed] [Google Scholar]
- 6. Rispens T, Leeuwen AV, Vennegoor A, et al. Measurement of serum levels of natalizumab, an immunoglobulin G4 therapeutic monoclonal antibody. Anal Biochem. 2011;411:271‐276. [DOI] [PubMed] [Google Scholar]
- 7. Dumet C, Pottier J, Gouilleux‐Gruart V, Watier H. Insights into the IgG heavy chain engineering patent landscape as applied to IgG4 antibody development. mAbs. 2019;11:1341‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Zee JS, van Sweiten P, Aalberse RC. Inhibition of complement activation by igg4 antibodies. Clin Exp Immunol. 1986;64:415‐422. [PMC free article] [PubMed] [Google Scholar]
- 9. Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther. 2007;7:1401‐1413. [DOI] [PubMed] [Google Scholar]
- 10. Schuurman J, Van Ree R, Perdok GJ, Van Doorn HR, Tan KY, Aalberse RC. Normal human immunoglobulin G4 is bispecific: it has two different antigen‐combining sites. Immunology. 1999;97:693‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oh CK, Faggioni R, Jin F, et al. An open‐label, single‐dose bioavailability study of the pharmacokinetics of cat‐354 after subcutaneous and intravenous administration in healthy males. Brit J Clin Pharmacol. 2010;69:645‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Neut Kolfschoten M, Schuurman J, Losen M, et al. Anti‐inflammatory activity of human IgG4 antibodies by dynamic fab arm exchange. Science. 2007;317:1554‐1557. [DOI] [PubMed] [Google Scholar]
- 13. Labrijn AF, Buijsse AO, van den Bremer ETJ, et al. Therapeutic IgG4 antibodies engage in fab‐arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol. 2009;27:767‐771. [DOI] [PubMed] [Google Scholar]
- 14. Rispens T, Ooijevaar‐de Heer P, Bende O, Aalberse RC. Mechanism of immunoglobulin G4 fab‐arm exchange. J Am Chem Soc. 2011;133:10302‐10311. [DOI] [PubMed] [Google Scholar]
- 15. Ishino T, Wang M, Mosyak L, et al. Engineering a monomeric Fc domain modality by N‐glycosylation for the half‐life extension of biotherapeutics. J Biol Chem. 2013;288:16529‐16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moreno AS, McPhee R, Arruda LK, Howell MD. Targeting the T helper 2 inflammatory axis in atopic dermatitis. Int Arch Allergy Immunol. 2016;171:71‐80. [DOI] [PubMed] [Google Scholar]
- 17. Hamilton JD, Ungar B, Guttman‐Yassky E. Drug evaluation review: dupilumab in atopic dermatitis. Immunotherapy. 2015;7:1043‐1058. [DOI] [PubMed] [Google Scholar]
- 18. Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti‐IL‐13 monoclonal antibody) in adults with moderate‐to‐severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo‐controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78:863‐871. [DOI] [PubMed] [Google Scholar]
- 19. Tesar DB, Tiangco NE, Bjorkman PJ. Ligand valency affects transcytosis, recycling and intracellular trafficking mediated by the neonatal Fc receptor. Traffic. 2006;7:1127‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Z, Krippendorff B‐F, Shah DK. Influence of Molecular size on the clearance of antibody fragments. Pharm Res. 2017;34:2131‐2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carlsson M, Braddock M, Li Y, et al. Evaluation of antibody properties and clinically relevant immunogenicity, anaphylaxis, and hypersensitivity reactions in two phase III trials of tralokinumab in severe, uncontrolled asthma. Drug Saf. 2019;42:769‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Industries TP. Cinqair (reslizumab) injection prescribing information; 2016.
- 23. van Hartingsveldt B, Nnane IP, Bouman‐Thio E, et al. Safety, tolerability and pharmacokinetics of a human anti‐interleukin‐13 monoclonal antibody (CNTO 5825) in an ascending single‐dose first‐in‐human study. Br J Clin Pharmacol. 2013;75:1289‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


