Abstract
Background:
Older adults with multiple sclerosis (MS) experience mobility impairments, but conventional brain imaging is a poor predictor of walking abilities in this population.
Objective:
To test whether brain metabolites measured with Magnetic Resonance Spectroscopy (MRS) are associated with walking performance in older adults with MS.
Methods:
Fifteen older adults with MS (mean age: 60.9, SD: 5.1) and 22 age-matched healthy controls (mean age: 64.2, SD: 5.7) underwent whole-brain MRS and mobility testing. Levels of N-acetylaspartate (NAA), myo-inositol (MI), choline (CHO), and temperature in 47 brain regions were compared between groups and correlated with walking speed (Timed 25Foot Walk) and walking endurance (Six-Minute Walk).
Results:
Older adults with MS had higher MI in 23 areas, including the bilateral frontal (right: t(21.449)=−2.605, p=0.016; left: t(35)=−2.434, p=0.020), temporal (right: t(35)=−3.063, p=0.004; left: t(35)=−3.026, p=0.005), and parietal lobes (right: t(21.100)=−2.886, p=0.009; left: t(35)=− 2.507, p=0.017), and right thalamus (t(35)=−2.840, p=0.007). MI in eleven regions correlated with walking speed, and MI in twelve regions correlated with walking endurance. NAA was lower in MS in the bilateral thalami (right: t(35)=3.449, p<0.001; left: t(35)=2.061, p=0.047), caudate nuclei (right: t(33)=2.828, p=0.008; left: t(32)=2.132, p=0.041), and posterior cingulum (right: t(35)=3.077, p=0.004; left: t(35)=2.972, p=0.005). NAA in four regions correlated with walking speed and endurance. Brain temperature was higher in MS patients in four regions, but did not correlate with mobility measures. There were no group differences in CHO.
Conclusion:
MI and NAA may be useful imaging end-points for walking ability as a clinical outcome in older adults with MS.
Keywords: Older adults, Multiple sclerosis, Mobility, Magnetic Resonance Spectroscopy, Brain temperature
Introduction
Multiple sclerosis (MS) is an immune-mediated disease of the central nervous system (CNS) with a prevalence of one million adults in the United States.1 Older adults between 55–64 years of age now represent the largest group of those diagnosed with the condition.1 Mobility impairments are among the most disabling features of MS, particularly with aging,2 and are associated with poorer quality of life and participation.3, 4
Structural Magnetic Resonance Imaging (MRI) is routinely used to monitor disease progression in MS, as it can visualize white matter lesions and cortical thinning while being easily accessible in clinical settings. However, conventional MRI has limited ability to detect subtle tissue changes prior to lesion formation, and correlates poorly with disability.5 Whole- brain Magnetic Resonance Spectroscopy Imaging (MRSI) allows the measurement of brain metabolites, including N-acetylaspartate (NAA), myo-inositol (MI), choline-containing compounds (CHO), and creatine (CRE), across distinct brain regions and the entire brain. Alterations in these metabolites are linked to pathophysiological processes such as neuroinflammation, axonal degeneration, and glial proliferation, and are visible in early stages of MS.6–8 As such, MRSI provides a tool for probing the pathophysiological mechanisms leading to neurological decline in general, and mobility impairments in particular. For example, MI has been a reliable marker of glial proliferation, which increases in conditions involving white matter pathology, such as MS.9–14 It is unknown whether MI increases reflect detrimental or regenerative processes, or both, but it is conceivable that glial proliferation indicates active disease progression, which may interfere with walking performance. NAA is a marker of neuronal health, viability, and density, with decreases believed to indicate neuronal loss.15 In the context of neurodegenerative conditions such as MS, NAA decreases may reflect poorer structural integrity and functioning of the affected brain regions, which could presumably affect walking performance. CHO is a marker of cell membrane turnover. The evidence for CHO abnormalities in MS is less clear than for the other metabolites, as both increases and decreases have been reported, perhaps reflecting membrane synthesis and breakdown during different disease stages.16 Metabolic abnormalities throughout the brain may be able to account for clinical symptoms that conventional MRI cannot.5
Applications of single-voxel Magnetic Resonance Spectroscopy (MRS) have already uncovered associations between metabolic abnormalities in the MS brain and physical impairments.12, 17, 18 While useful, single-voxel techniques require a-priori selection of the brain region to be imaged, and contributions from surrounding areas are not assessed. Whole-brain MRSI studies are able to overcome this restriction, but those conducted in MS to date have typically restricted analyses to whole-brain white matter or lesions19, 20 rather than the entire brain.
Donadieu et al.21 were the first to apply atlas-based analyses of whole-brain metabolites and examine relationships with disability in a group of MS patients. The authors demonstrated that clusters of reduced NAA levels in subcortical gray matter structures and white matter tracts near the motor cortex were associated with performance on a functional composite score comprised of mobility, fine motor, and cognitive abilities (i.e., Multiple Sclerosis Functional Composite). To our knowledge, no study to date has investigated associations between metabolic abnormalities and mobility specifically in older adults with MS, and researchers have only recently focused on conventional MRI and clinical end-points is this segment of MS. Such investigation is important for understanding the metabolites and pathophysiological processes associated with mobility as possible latent mechanisms for inquiry in rehabilitation clinical trials of older adults with this disease.
The current study applied whole-brain 1H-MRSI to determine which metabolites and brain regions may be involved in mobility impairments among older adults with MS. Based on prior literature in the general MS population,22, 23 we hypothesized that older adults with MS would have increased levels of MI and CHO, and decreased levels of NAA, compared to age-matched healthy controls. Additionally, we hypothesized that higher MI and CHO, and lower NAA would be significantly correlated with impaired performance on measures of walking speed and walking endurance. We further calculated brain temperature, a proxy for neuroinflammation24, 25, and examined its relationship to mobility outcomes. The use of brain thermometry to assess neuroinflammation is still a novel concept, based on the idea that increased metabolic demands during inflammation overwhelm the brain’s cooling mechanisms and lead to localized increases in temperature. We included thermometry in the current analysis to further characterize its relationship to clinical outcomes in neuroinflammatory conditions, such as MS.
Methods
Participants
Twenty-nine adults with MS and 30 healthy age- and gender-matched healthy controls were recruited from the local Birmingham, AL community via flyers and word of mouth. Interested participants contacted the researchers via phone or e-mail and underwent phone screening to determine eligibility for the study. The following inclusion criteria applied to the MS group: (i) diagnosis of MS; (ii) relapse-free during the past 30 days; (iii) 55 years of age or older;1 (iv) able to walk with or without assistive device; and (v) score of 0–1 on the Physical Activity Readiness Questionnaire,26 indicating low risk of injury during strenuous exercise; this was necessary for controlling cardiovascular risk with the provision of maximal exercise testing and six-minute walk test performance. Control subjects met criteria (iii)-(v) above, were gender- and age-matched (within 5 years) to patients, and reported no neurological conditions. MRI contraindications, pregnancy, or lactation (for women of child-bearing potential) were exclusionary for all participants.
Mobility Assessment
Walking speed and endurance were measured with the Timed 25-Foot Walk (T25FW)27, 28and the Six-Minute Walk (6MW),29 respectively. The T25FW requires participants to walk from a standing position along a straight 25ft-long path indicated by floor markers. Instructions signaled participants to walk as quickly as possible, but safely. Participants completed two trials and performance was averaged across trials and converted to speed (ft/s), where higher values indicated faster walking speed. The 6MW requires participants to walk as far and as fast as possible, while safe, around a circular track, for six minutes. Distance walked in ft was recorded from a distance measuring wheel held by a research staff member, and higher values indicated greater walking endurance.
Imaging Acquisition
MRI data were acquired at the University of Alabama at Birmingham’s Civitan International Neuroimaging Laboratory on a 3-Tesla Siemens Magnetom Prisma (Siemens Healthineers AG, Erlangen, Germany) with 20-channel 1H head coil. A T1-weighted structural image was obtained with a magnetization-prepared rapid gradient echo sequence with TR: 2,400ms; TE: 2.22ms; flip angle: 8°; 208 slices, 0.8 mm thickness; and 256×256 matrix.
Spectroscopy data were acquired with a fast 3D echoplanar spectroscopic imaging (EPSI) sequence covering the entire cerebrum with axial excitation and TR1: 1,500ms; TR2: 511ms; TE: 17.6ms; lipid inversion-nulling with TI: 198 ms; flip angle: 71°; FOV: 280 × 280 × 180mm; matrix: 50 × 50 × 18; GRAPPA factor: 1.3. Note that two TRs are needed to acquire the spectroscopy (TR1) and water reference (TR2) within the same acquisition. The FOV was kept in-plane and was not aligned to any anatomical landmarks. Automatic and interactive shimming was performed once each prior to the EPSI sequence in order to reduce magnetic field inconsistencies, with a target FWHM ≤ 30Hz. The acquisition time for EPSI was approximately 18 minutes.
Procedures
All procedures were approved by the Institutional Review Board of the University of Alabama at Birmingham (protocol # IRB-300002749). Participants gave written informed consent prior to enrollment and attended three visits at UAB. The first visit involved completion of a demographics questionnaire, the T25FW, and the 6MW. The Expanded Disability Status Scale (EDSS)30was derived for the MS group based on a neurological exam administered by a Neurostatus-certified examiner. The second study visit involved an exercise test (results not reported here), and the third visit consisted of MRI scanning, though the order of these two visits was reversed for some participants.
Image Processing
Whole-brain MRSI data were processed with the Metabolite Imaging and Data Analysis System (MIDAS).31 Preprocessing of the spectroscopy data involved reconstruction, corrections for magnetic field inhomogeneities, interpolation to a 64 × 64 × 32 matrix, and spatial smoothing with a 5mm-by-7mm Gaussian kernel, resulting in 5.6×5.6×5.0mm voxels. Metabolite peaks (NAA, CR, CHO, and MI) were fitted with a Gaussian line shape using the FITT2 module, and then normalized to institutional units by using the unsuppressed water signal as a reference, which is obtained during the EPSI acquisition. Metabolite maps were registered to the subject’s T1-weighted image. T1-weighted images were segmented using a three tissue compartment model in FSL/FAST32in order to determine the tissue distribution for the spectroscopy data. The metabolite and tissue distribution maps were then registered to Montreal Neurological Institute (MNI 2mm) space, and the inverse transform from this registration was applied to a modified version of the Automated Anatomical Labeling (AAL) atlas33containing 47 ROIs. As a result, AAL ROIs were present in subject space and spectral averaging in each ROI was carried out in the Map Integrated Spectrum (MINT) module. Only voxels meeting the following criteria were included in the integration: (i) linewidth of fitted metabolite ≤13 Hz; (ii) value within 2.5 standard deviations of all valid voxels; (iii) Cramér-Rao Lower Bounds for fitting CR ≤40%; and ≤30% CSF per voxel. For statistical analyses, metabolites were expressed as ratios, with CR as the reference metabolite. CR was chosen as the reference metabolite because it is generally expressed uniformly within the CNS, and the resultant analyses are comparable with existing literature. Prior to calculation of the metabolite ratios, we compared water-normalized CR levels between the two groups using independent t-tests in order to ensure that the reference metabolite was equivalent across groups. This ensured that any group differences in the ratios were attributable to the metabolite of interest and not due to differences in the CR reference.
Additionally, brain temperature was calculated in each ROI using the following fomula: T= −102.61 ×Δ water-CR + 206.1°C, where Δ water-CR is the difference between the water resonant frequency and the CR resonant frequency on the spectral plots.34
Statistical Analyses
Data were arranged in wide form and analyzed with IBM SPSS Statistics for Windows, version 25.0 (IBM Corp; 2017). Differences in baseline characteristics were assessed with independent-samples t-tests, as well as Chi-Square tests and Fisher’s exact test in case of categorical variables. Independent-samples t-tests were conducted to examine differences in mean metabolite levels and brain temperature in the 47 ROIs. Statistical significance was assumed at p<0.05. Due to the possibility of type I errors (false positive) when large numbers of tests are conducted, we applied an additional false discovery rate (FDR) of 0.01 to the metabolite analyses, equivalent to an uncorrected p<0.0023. To test whether metabolic abnormalities in MS patients were related to mobility, Pearson correlation coefficients were obtained for bivariate correlations between the metabolite ratios and T25FW and 6MW scores. Correlation coefficients were obtained only for regions with between-group differences in metabolite outcomes, and were considered significant at p<0.05.
Results
Twenty-nine patients with MS and 30 healthy controls were recruited. Imaging data from eleven MS patients were discarded due to user error during the MRSI acquisition, and one MS patient declined to provide MRSI data. Data from one healthy control was discarded due to scanner hardware issues, and three healthy control participants declined to provide MRSI data. Data from two additional MS patients and three healthy controls were discarded due to poor quality spectroscopy data, leaving a final dataset of 15 patients with MS and 22 healthy controls.
Sample Characteristics
Table 1 summarizes demographic and clinical characteristics of MS and healthy control samples. All patients included in the current analyses had the relapsing-remitting form of MS. The mean age in the MS group was 60.9 years, and the mean age of healthy controls was 64.2 years. The age difference was not significant (t(35)=1.780, p=0.084). The sex and racial makeup of the two groups was equivalent (sex: p=0.683, Fisher’s exact test; race: χ2(2, N=37)=1.886, p=0.389). Groups did not differ on body mass index (BMI; MS group: M=28.00, SD= 5.6; healthy controls: M= 27.9, SD= 6.1; t(34)=−0.050, p=0.960). As expected, the healthy control group walked faster (M=4.5, SD=1.5) than the MS group (M=6.1, SD=0.9; t(35)=4.084, p<0.001) during the T25FW and farther (M=1858.4, SD=300.8) than the MS group (M=1393.6, SD=426.7; t(35)=3.893, p<0.001) during the 6MW.
Table 1.
Baseline demographic and clinical characteristics in the two study groups.
| Multiple Sclerosis (N=15) | Healthy Controls (N=22) | p | |
|---|---|---|---|
|
| |||
| Age (years; M, SD) | 60.9 (5.1) | 64.2 (5.7) | 0.084 |
| Sex | 0.683 | ||
| Women (n, %) | 13 (86.7) | 19 (86.4) | |
| Men (n, %) | 2 (13.3) | 3 (13.6) | |
| Race | 0.389 | ||
| White (n, %) | 13 (86.7) | 15 (68.2) | |
| Black (n, %) | 2 (13.3) | 6 (27.3) | |
| Hispanic (n, %) | 0 (0.0) | 1 (4.6) | |
| BMI | 28.00 (5.6) | 27.9 (6.1) | 0.960 |
| Disease Duration (years; M, SD) | 19.2 (5.0) | - | |
| EDSS (median, IQR) | 4.0 (1.0) | - | |
| T25FW (ft/s; M, SD) | 4.5 (1.5) | 6.1 (0.9) | <0.001 |
| 6MW (ft; M, SD) | 1393.6 (426.7) | 1858.4 (300.8) | <0.001 |
6MW=Six-Minute Walk; BMI=body mass index; IQR=interquartile range; EDSS=Expanded Disability Status Scale; M=mean; SD=standard deviations; T25FW=Timed 25-foot Walk
Metabolite Results
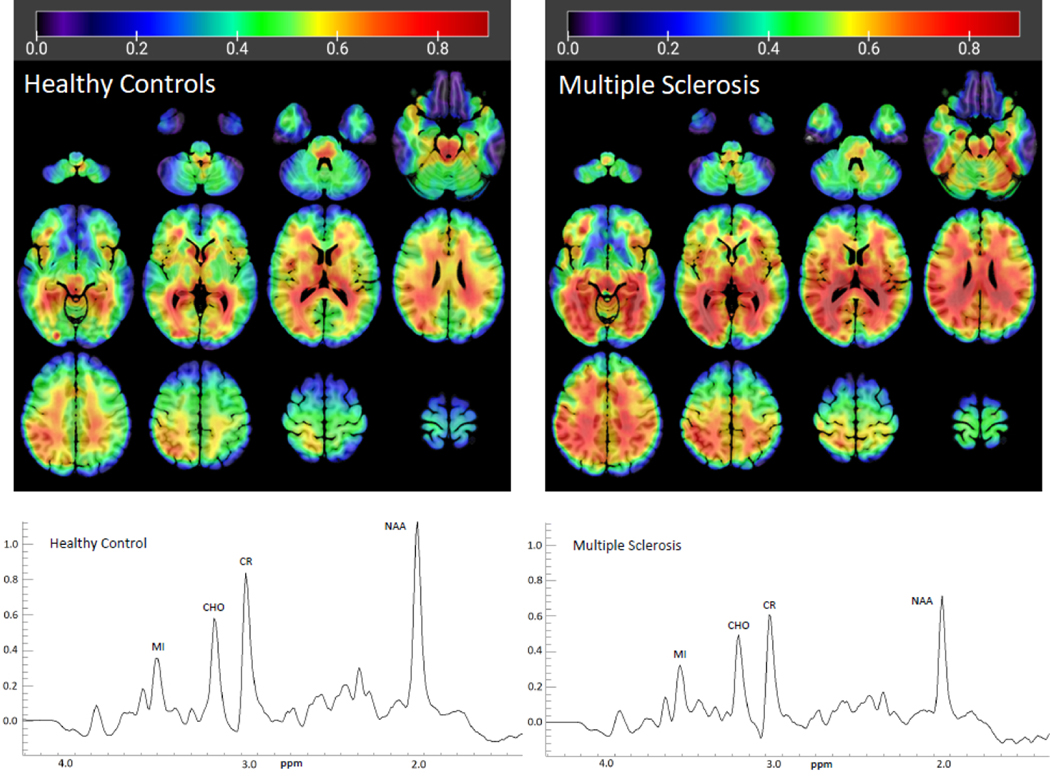
There were no differences in water-normalized CR levels in any of the 47 brain regions (all p>0.5), so we proceeded to use CR as the reference metabolite for all other analyses. The gray matter, white matter, and cerebrospinal fluid content in each brain region from each participant is included in the supplemental materials. Figure 1 displays sample integrated spectra from the right thalamus in an older adult with MS and an older healthy control, as well as group averages of MI/CR ratios. As shown in Table 2 and Figure 1, MI/CR was almost uniformly higher in the older MS group than in older healthy controls throughout the brain, with significant differences in the bilateral frontal (right: t(21.449)=−2.605, p=0.016; left: t(35)=−2.434, p=0.020), temporal (right: t(35)=−3.063, p=0.004; left: t(35)=−3.026, p=0.005), and parietal lobes (right: t(21.100)=−2.886, p=0.009; left: t(35)=−2.507, p=0.017), including the bilateral postcentral gyri (right: t(35)=−2.399, p=0.022; left: t(35)=−2.371, p=0.023), and bilateral Rolandic opercula at the fronto-parietal border (right: t(35)=−2.324, p=0.026; left: t(35)=−2.067, p=0.046); the bilateral middle cingulum (right: t(35)=−2.707, p=0.010; left: t(35)=−2.506, p=0.017), bilateral lingual (right: t(35)=−2.601, p=0.014; left: t(35)=−2.948, p=0.006) and fusiform gyri (right: t(35)=−2.329, p=0.026; left: t(35)=2.867, p=0.007), bilateral calcarine cortex (right: t(35)=−2.769, p=0.009; left: t(35)=−2.328, p=0.026), the left precuneus (t(35)=−2.109, p=0.042, subcortical regions comprising the right thalamus (t(35)=−2.840, p=0.007) and left caudate nucleus (t(32)=−2.139, p=0.040), and the cerebellum (t(20.259)=−2.287, p=0.033). There were no regions where MI/CR was significantly lower in MS patients than in the healthy controls.
Figure 1.
Average MI/CR ratios in the groups of older healthy controls (left) and older adults with multiple sclerosis (right). Metabolite maps are overlaid on an MNI standard brain for reference. Representative integrated spectra in the right thalamus from one participant in each group are displayed, showing decreased NAA in the Multiple Sclerosis patient. CHO=choline, CR=creatine, MI=myo-inositol, NAA=N-Acetylaspartate, ppm=parts per million
Table 2.
Differences in MI/CR ratios between MS patients and controls in 47 regions of interest.
| Multiple Sclerosis (N=15) | Healthy Controls (N=22) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | Mean | SD | t | df | p | |
|
| |||||||
| Anterior Cingulum L | 0.584 | 0.219 | 0.531 | 0.178 | −0.797 | 34 | 0.431 |
| Anterior Cingulum R | 0.573 | 0.178 | 0.451 | 0.212 | −1.836 | 35 | 0.075 |
| Calcarine L | 0.814 | 0.273 | 0.645 | 0.167 | −2.328 | 35 | 0.026* |
| Calcarine R | 0.730 | 0.269 | 0.549 | 0.123 | −2.769 | 35 | 0.009* |
| Caudate L | 0.712 | 0.241 | 0.545 | 0.211 | −2.139 | 32 | 0.040* |
| Caudate R | 0.605 | 0.262 | 0.641 | 0.616 | 0.202 | 33 | 0.841 |
| Cerebellum | 0.575 | 0.136 | 0.486 | 0.078 | −2.287 | 20.259† | 0.033* |
| Cuneus L | 0.772 | 0.284 | 0.618 | 0.133 | −1.951 | 18.242† | 0.067 |
| Cuneus R | 0.663 | 0.246 | 0.547 | 0.149 | −1.793 | 35 | 0.082 |
| Frontal L | 0.566 | 0.128 | 0.477 | 0.093 | −2.434 | 35 | 0.020* |
| Frontal R | 0.587 | 0.151 | 0.472 | 0.095 | −2.605 | 21.449† | 0.016* |
| Fusiform L | 0.724 | 0.246 | 0.551 | 0.118 | −2.867 | 35 | 0.007* |
| Fusiform R | 0.683 | 0.199 | 0.542 | 0.168 | −2.329 | 35 | 0.026* |
| Hippocampus L | 0.664 | 0.170 | 0.647 | 0.174 | −0.292 | 35 | 0.772 |
| Hippocampus R | 0.787 | 0.160 | 0.662 | 0.197 | −1.985 | 34 | 0.055 |
| Insula L | 0.592 | 0.151 | 0.550 | 0.159 | −0.813 | 35 | 0.422 |
| Insula R | 0.648 | 0.156 | 0.560 | 0.150 | −1.740 | 35 | 0.091 |
| Lingual L | 0.782 | 0.180 | 0.627 | 0.140 | −2.948 | 35 | 0.006* |
| Lingual R | 0.753 | 0.205 | 0.609 | 0.132 | −2.601 | 35 | 0.014* |
| Mid Cingulum L | 0.647 | 0.126 | 0.555 | 0.098 | −2.506 | 35 | 0.017* |
| Mid Cingulum R | 0.625 | 0.115 | 0.541 | 0.073 | −2.707 | 35 | 0.010* |
| Occipital L | 0.751 | 0.178 | 0.651 | 0.193 | −1.592 | 35 | 0.120 |
| Occipital R | 0.668 | 0.111 | 0.577 | 0.179 | −1.752 | 35 | 0.089 |
| Pallidum L | 0.356 | 0.233 | 0.419 | 0.322 | 0.632 | 34 | 0.532 |
| Pallidum R | 0.522 | 0.270 | 0.414 | 0.302 | −1.110 | 35 | 0.275 |
| Paracentral Lobule L | 0.478 | 0.176 | 0.421 | 0.112 | −1.214 | 35 | 0.233 |
| Paracentral Lobule R | 0.466 | 0.185 | 0.365 | 0.121 | −2.003 | 35 | 0.053 |
| Parietal L | 0.719 | 0.130 | 0.621 | 0.108 | −2.507 | 35 | 0.017* |
| Parietal R | 0.622 | 0.116 | 0.526 | 0.071 | −2.886 | 21.100† | 0.009* |
| Postcentral L | 0.609 | 0.117 | 0.525 | 0.096 | −2.371 | 35 | 0.023* |
| Postcentral R | 0.584 | 0.118 | 0.503 | 0.088 | −2.399 | 35 | 0.022* |
| Posterior Cingulum L | 0.702 | 0.139 | 0.626 | 0.138 | −1.648 | 35 | 0.108 |
| Posterior Cingulum R | 0.678 | 0.164 | 0.580 | 0.140 | −1.949 | 35 | 0.059 |
| Precentral L | 0.520 | 0.109 | 0.480 | 0.107 | −1.105 | 35 | 0.277 |
| Precentral R | 0.520 | 0.136 | 0.465 | 0.113 | −1.326 | 35 | 0.193 |
| Precuneus L | 0.662 | 0.163 | 0.577 | 0.082 | −2.109 | 35 | 0.042* |
| Precuneus R | 0.599 | 0.168 | 0.528 | 0.076 | −1.721 | 35 | 0.094 |
| Putamen L | 0.496 | 0.185 | 0.481 | 0.184 | −0.236 | 35 | 0.815 |
| Putamen R | 0.521 | 0.217 | 0.463 | 0.166 | −0.912 | 35 | 0.368 |
| Rolandic Operculum L | 0.626 | 0.128 | 0.539 | 0.126 | −2.067 | 35 | 0.046* |
| Rolandic Operculum R | 0.659 | 0.150 | 0.542 | 0.149 | −2.324 | 35 | 0.026* |
| Supplementary Motor Area L | 0.404 | 0.164 | 0.365 | 0.097 | −0.898 | 35 | 0.375 |
| Supplementary Motor Area R | 0.412 | 0.156 | 0.360 | 0.106 | −1.206 | 35 | 0.236 |
| Temporal L | 0.676 | 0.130 | 0.555 | 0.113 | −3.026 | 35 | 0.005* |
| Temporal R | 0.622 | 0.099 | 0.513 | 0.111 | −3.063 | 35 | 0.004* |
| Thalamus L | 0.646 | 0.262 | 0.533 | 0.162 | −1.622 | 35 | 0.114 |
| Thalamus R | 0.697 | 0.231 | 0.501 | 0.187 | −2.840 | 35 | 0.007* |
L=left; R=right; SD=standard deviation
df adjusted for unequal variances between the groups.
p<0.05
NAA/CR ratios were lower in the older MS group compared to older healthy controls throughout the brain (see Table 3), with significant differences in the bilateral thalami (right: t(35)=3.449, p=0.001; left: t(35)=2.061, p=0.047), caudate nuclei (right: t(33)=2.828, p=0.008; left: t(32)=2.132, p=0.041), and posterior cingulate cortices (right: t(35)=3.077, p=0.004; left: t(35)=2.972, p=0.005), the right occipital cortex (t(35)=2.546, p=0.015) and cuneus (t(35)=2.110, p=0.042), right insula (t(35)=2.732, p=0.010) and right Rolandic operculum (t(35)=2.301, p=0.027), left hippocampus (t(35)=3.226, p=0.003), and the left middle cingulum (t(35)=2.058, p=0.047). Increased NAA/CR in the right thalamus of MS patients survived the p-value correction for multiple comparisons. There were no regions where NAA/CR was significantly higher in MS patients than in controls.
Table 3.
Differences in NAA/CR ratios between MS patients and controls in 47 regions of interest.
| Multiple Sclerosis (N=15) | Healthy Controls (N=22) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Mean | SD | Mean | SD | t | df | p | |
|
| |||||||
| Anterior Cingulum L | 1.010 | 0.129 | 1.094 | 0.137 | 1.821 | 34 | 0.077 |
| Anterior Cingulum R | 1.032 | 0.198 | 1.045 | 0.126 | 0.241 | 35 | 0.811 |
| Calcarine L | 1.324 | 0.227 | 1.390 | 0.171 | 1.007 | 35 | 0.321 |
| Calcarine R | 1.295 | 0.175 | 1.336 | 0.191 | 0.652 | 35 | 0.519 |
| Caudate L | 1.048 | 0.206 | 1.237 | 0.282 | 2.132 | 32 | 0.041* |
| Caudate R | 1.062 | 0.178 | 1.226 | 0.160 | 2.828 | 33 | 0.008* |
| Cerebellum | 0.719 | 0.081 | 0.757 | 0.099 | 1.236 | 35 | 0.225 |
| Cuneus L | 1.337 | 0.177 | 1.470 | 0.242 | 1.824 | 35 | 0.077 |
| Cuneus R | 1.217 | 0.223 | 1.356 | 0.179 | 2.110 | 35 | 0.042* |
| Frontal L | 1.122 | 0.090 | 1.177 | 0.094 | 1.796 | 35 | 0.081 |
| Frontal R | 1.112 | 0.081 | 1.144 | 0.102 | 1.004 | 35 | 0.322 |
| Fusiform L | 1.073 | 0.106 | 1.097 | 0.121 | 0.622 | 35 | 0.538 |
| Fusiform R | 1.022 | 0.212 | 1.022 | 0.136 | −0.015 | 35 | 0.988 |
| Hippocampus L | 0.769 | 0.222 | 0.976 | 0.168 | 3.226 | 35 | 0.003* |
| Hippocampus R | 0.885 | 0.113 | 1.007 | 0.212 | 1.991 | 34 | 0.055 |
| Insula L | 1.059 | 0.113 | 1.086 | 0.120 | 0.699 | 35 | 0.489 |
| Insula R | 0.995 | 0.102 | 1.092 | 0.109 | 2.732 | 35 | 0.010* |
| Lingual L | 1.242 | 0.168 | 1.238 | 0.122 | −0.092 | 35 | 0.927 |
| Lingual R | 1.178 | 0.128 | 1.184 | 0.191 | 0.105 | 35 | 0.917 |
| Mid Cingulum L | 1.046 | 0.099 | 1.111 | 0.090 | 2.058 | 35 | 0.047* |
| Mid Cingulum R | 1.049 | 0.085 | 1.068 | 0.092 | 0.638 | 35 | 0.527 |
| Occipital L | 1.366 | 0.213 | 1.466 | 0.189 | 1.504 | 35 | 0.142 |
| Occipital R | 1.225 | 0.152 | 1.404 | 0.240 | 2.546 | 35 | 0.015* |
| Pallidum L | 0.956 | 0.227 | 1.126 | 0.272 | 1.942 | 34 | 0.060 |
| Pallidum R | 1.115 | 0.170 | 1.180 | 0.172 | 1.139 | 35 | 0.263 |
| Paracentral Lobule L | 1.171 | 0.149 | 1.237 | 0.154 | 1.299 | 35 | 0.203 |
| Paracentral Lobule R | 1.276 | 0.204 | 1.298 | 0.198 | 0.321 | 35 | 0.750 |
| Parietal L | 1.231 | 0.142 | 1.314 | 0.172 | 1.535 | 35 | 0.134 |
| Parietal R | 1.155 | 0.100 | 1.220 | 0.138 | 1.577 | 35 | 0.124 |
| Postcentral L | 1.211 | 0.155 | 1.296 | 0.134 | 1.781 | 35 | 0.084 |
| Postcentral R | 1.162 | 0.101 | 1.232 | 0.138 | 1.675 | 35 | 0.103 |
| Posterior Cingulum L | 1.163 | 0.195 | 1.353 | 0.188 | 2.972 | 35 | 0.005* |
| Posterior Cingulum R | 1.088 | 0.099 | 1.203 | 0.120 | 3.077 | 35 | 0.004* |
| Precentral L | 1.245 | 0.136 | 1.303 | 0.138 | 1.261 | 35 | 0.216 |
| Precentral R | 1.215 | 0.163 | 1.276 | 0.158 | 1.148 | 35 | 0.259 |
| Precuneus L | 1.155 | 0.083 | 1.220 | 0.127 | 1.755 | 35 | 0.088 |
| Precuneus R | 1.104 | 0.084 | 1.176 | 0.122 | 1.987 | 35 | 0.055 |
| Putamen L | 1.037 | 0.091 | 1.097 | 0.183 | 1.181 | 35 | 0.246 |
| Putamen R | 1.120 | 0.462 | 1.164 | 0.159 | 0.414 | 35 | 0.681 |
| Rolandic Operculum L | 1.144 | 0.122 | 1.212 | 0.151 | 1.454 | 35 | 0.155 |
| Rolandic Operculum R | 1.089 | 0.143 | 1.211 | 0.167 | 2.301 | 35 | 0.027* |
| Supplementary Motor Area L |
1.168 | 0.144 | 1.194 | 0.133 | 0.567 | 35 | 0.574 |
| Supplementary Motor Area R | 1.184 | 0.145 | 1.154 | 0.133 | −0.648 | 35 | 0.521 |
| Temporal L | 1.15 | 0.173 | 1.191 | 0.156 | 0.751 | 35 | 0.458 |
| Temporal R | 1.055 | 0.146 | 1.140 | 0.159 | 1.649 | 35 | 0.108 |
| Thalamus L | 0.937 | 0.265 | 1.084 | 0.171 | 2.061 | 35 | 0.047* |
| Thalamus R | 0.952 | 0.143 | 1.130 | 0.161 | 3.449 | 35 | 0.001** |
L=left; R=right; SD=standard deviation
p<0.05
p<0.0023
Although abnormalities in both MI/CR and NAA/CR ratios were observed in widespread regions throughout the brain, the distribution differed markedly, with only four out of 47 regions having both elevations in MI/CR and lower NAA/CR, namely the right thalamus and left caudate nucleus, right Rolandic operculum, and the left middle cingulum.
There were no differences in CHO/CR ratios between the groups in any of the assessed brain regions (all p>0.05). Results from the analyses are available in the supplemental materials.
Brain Temperature
Brain temperature was generally higher in older adults with MS compared to older healthy controls (see the supplemental materials for all between-group tests), with significantly elevated temperatures in the left frontal lobe (t(30.320)=−2.314, p=0.028), anterior cingulum (t(34)=−2.525, p=0.016), and Rolandic operculum (t(26.216)=−3.029, p=0.005), as well as in the right cuneus (t(35)=−2.077, p=0.045). None of the temperature results survived corrections for multiple comparisons. There was little overlap between temperature and metabolic abnormalities. The left frontal lobe and left Rolandic operculum had both elevated MI/CR and elevated temperature, and the right cuneus had decreased NAA/CR in addition to elevated temperature. No brain regions were characterized by abnormalities in all three outcomes.
Correlations between brain metabolites and walking ability
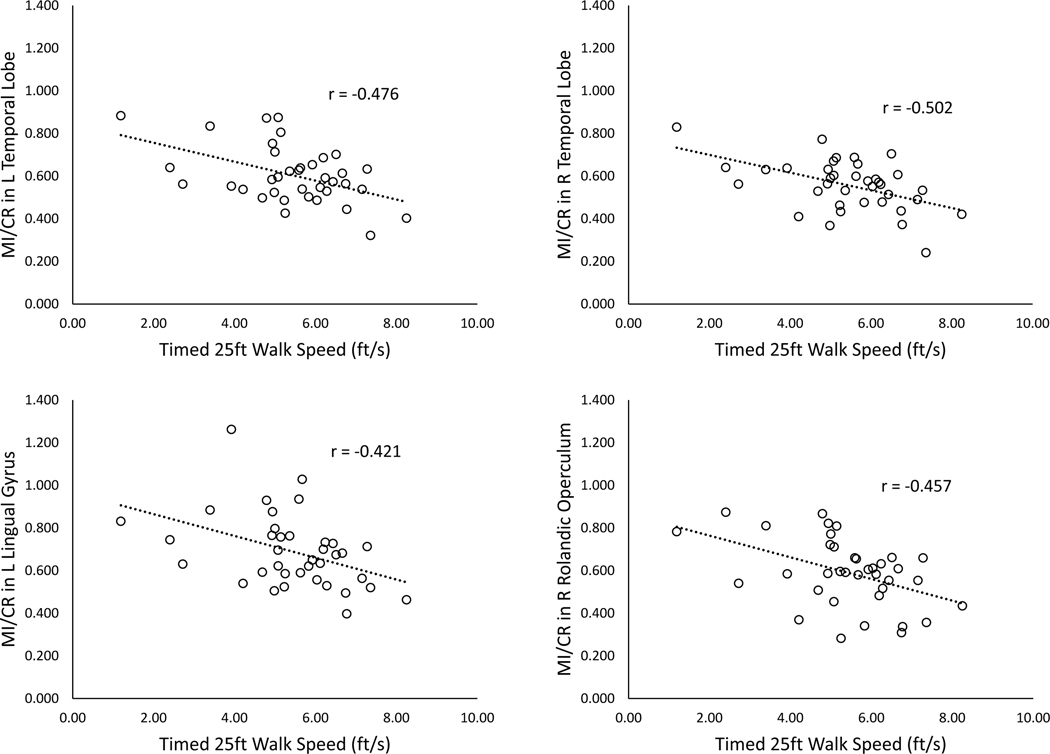
Several brain regions with metabolic abnormalities demonstrated statistically significant correlations with walking performance (Table 4 and Figure 2). MI/CR in eleven of the 23 regions tested was negatively correlated with walking speed (T25FW), namely in the bilateral temporal (right: r=−0.502, p=0.002; left: r=−0.476, p=0.003) and parietal lobes (right: r=−0.405, p=0.013; left: r=−0.362, p=0.028), bilateral fusiform gyri (right: r=−0.361, p=0.028, left: r=−0.387, p=0.018), left lingual (r=−0.421, p=0.010) and postcentral (r=−0.346, p=0.036) gyri, the right calcarine cortex (r=−0.357, p=0.030) and right Rolandic operculum (r=−0.457, p=0.004), and the cerebellum (r=−0.369, p=0.025). MI/CR was further negatively correlated with walking endurance (6MW) in the bilateral temporal (right: r=−0.506, p=0.001; left: r=−0.503, p=0.001) and parietal lobes (right: r=−0.340, p=0.040; left: r=−0.353, p=0.032), bilateral postcentral (right: r=−0.346, p=0.036; left: r=−0.394, p=0.016) and fusiform (right: r=−0.377, p=0.021; left: r=− 0.341, p=0.039) gyri, bilateral Rolandic opercula (right: r=−0.444, p=0.006; left: r=−0.385, p=0.019), left lingual gyrus (r=−0.369, p=0.025), and the cerebellum (r=−0.411, p=0.012). NAA/CR levels had significant positive correlations with walking speed in the left hippocampus (r=0.376, p=0.022) and right caudate(r=0.360, p=0.034), and with walking endurance in the right thalamus (r=0.337, p=0.041), left hippocampus (r=0.360, p=0.029), and left middle cingulum (r=0.327, p=0.048). There were no significant correlations between brain temperature and the mobility measures in the four ROIs tested.
Table 4.
Relationship between walking ability and MI/CR and NAA/CR in regions of interest demonstrating between-group differences in metabolite levels. Pearson correlation coefficients (r) and corresponding p-values are displayed.
| Metabolite | ROI | T25FW speed (r) | p | 6MW (r) | p |
|---|---|---|---|---|---|
|
| |||||
| MI/CR | Calcarine R | −0.357 | 0.030* | −0.285 | 0.087 |
| Cerebellum | −0.369 | 0.025* | −0.411 | 0.012* | |
| Fusiform L | −0.387 | 0.018* | −0.341 | 0.039* | |
| Fusiform R | −0.361 | 0.028* | −0.377 | 0.021* | |
| Lingual L | −0.421 | 0.010** | −0.369 | 0.025* | |
| Parietal L | −0.362 | 0.028* | −0.353 | 0.032* | |
| Parietal R | −0.405 | 0.013* | −0.340 | 0.040* | |
| Postcentral L | −0.346 | 0.036* | −0.394 | 0.016* | |
| Postcentral R | −0.322 | 0.052 | −0.346 | 0.036* | |
| Rolandic Operculum L | −0.283 | 0.090 | −0.385 | 0.019* | |
| Rolandic Operculum R | −0.457 | 0.004** | −0.444 | 0.006** | |
| Temporal L | −0.476 | 0.003** | −0.503 | 0.001** | |
| Temporal R | −0.502 | 0.002** | −0.506 | 0.001** | |
|
| |||||
| NAA/CR | Caudate R | 0.360† | 0.034* | 0.299 | 0.081 |
| Hippocampus L | 0.376 | 0.022* | 0.360 | 0.029* | |
| Mid Cingulum L | 0.303 | 0.068 | 0.327 | 0.048* | |
| Thalamus R | 0.303 | 0.069 | 0.337 | 0.041* | |
CR=creatine, MI=myo-inositol, NAA=N-Acetylaspartate, ROI=region of interest, T25FW=Timed 25-foot Walk, 6MW=Six-minute Walk
p<0.01
<0.05,
N=37
Figure 2.
Relationships between MI/CR and walking speed on the Timed 25-foot Walk (T25FW) in four distinct brain regions.
Discussion
The current study applied MRSI for examining brain metabolites and regions that may be involved in mobility impairments in older adults with MS. As hypothesized, older adults with MS had increased MI/CR and decreased NAA/CR in widespread brain regions when compared with healthy older adults. MI/CR was consistently elevated throughout the brain, and the difference reached significance in almost half of the assessed regions, including the bilateral frontal, temporal, parietal lobes, and middle cingulum, subcortical regions comprising the right thalamus and left caudate nucleus, and the cerebellum. MI is primarily expressed in astrocytes and is a validated marker of glial proliferation, a process that occurs during neuroinflammation 9, 10. MI/CR increases in MS are a well-replicated finding, but the current study is the first to demonstrate both increases in MI/CR and associations with walking performance in older adults with MS. The brain regions most strongly associated with walking performance were the temporal lobes and the Rolandic operculum. The temporal lobes are associated with auditory processing, language, and encoding of declarative memories, but are not conventionally thought to support motor functioning. The correlation may reflect the observation of cognitive-motor coupling in MS, whereby cognitive dysfunction often co-occurs with loss of mobility. Similarly, the Rolandic Operculum is critical for interoceptive awareness and language, and has not been implicated in motor functioning. It appears unlikely that inflammatory processes in these particular regions drive mobility impairments in MS patients, but it is possible that their location within the brain makes them particularly susceptible to neuroinflammation. However, given our results, future research might further examine changes in MI/CR in those regions with therapeutic neurorehabilitation programs and interventions. Such studies may probe whether metabolic abnormalities in these regions are related to impairments in language or memory deficits.
NAA/CR was lower in the MS group in the bilateral thalami and caudate nuclei, posterior cingulate cortices, and many other cortical regions throughout the brain. NAA is a CNS-specific metabolite and considered a marker of neuronal health, viability, and density, with decreases believed to indicate neuronal loss.15 Although MS was long considered a disease of the white matter, involvement of gray matter pathology is now recognized as a central feature of MS pathophysiology.21, 35–37 Our findings of reduced NAA/CR in widespread brain regions in the older MS group are consistent with the emerging evidence regarding gray matter pathology in MS, which has been demonstrated using both conventional MRI38–40as well as MRS.20, 41 Gray matter pathology may be a result of demyelination, may precede white matter abnormalities, or both. NAA/CR decreases in the right thalamus are particularly notable because this was the only result to survive corrections for multiple comparisons in our sample, and is consistent with prior studies that have demonstrated thalamic NAA reductions.21, 42–44
The right thalamus, left caudate nucleus, right Rolandic operculum, and the left middle cingulum were the only regions to demonstrate both decreases in NAA/CR and increases in MI/CR. The lack of overlap between metabolic abnormalities in most brain areas indicates that several disease processes may occur in different parts of the brain at different times, perhaps reflecting relatively larger contributions from neuronal and glial abnormalities in those regions. The discrepancies further support a comprehensive assessment of brain metabolites in the diagnosis and monitoring of MS, rather than an exclusive focus on one particular metabolite.
Some of the regions with metabolic abnormalities, such as the caudate nuclei, thalami, and the cerebellum, are known to be involved in motor functioning, and metabolites in many of these regions demonstrated correlations with walking performance. Interestingly though, the primary motor cortices (precentral gyri) and supplementary motor areas, which are central to motor planning and execution, did not have metabolic abnormalities or correlations with walking ability. It is likely that metabolic abnormalities in older adults with MS do not selectively impair walking performance. Rather, these may affect other essential cognitive functions, such as memory, executive function, and attention, which are impaired in MS, but which were not included in the current study.45–47
There were few brain regions with significant differences in brain temperature between the groups, yet it is notable that temperature throughout the brain demonstrated abnormalities in the expected directions (i.e., higher brain temperature in MS patients). This observation is consistent with increased neuroinflammation and/or metabolic demands in this neurological disorder. Given the consistency of these results, it is possible that the lack of differences meeting statistical thresholds was due to limited statistical power in our small sample. Due to the limited overlap between the brain temperature results and metabolic abnormalities, brain temperature may yield additional information about disease processes in MS that is not apparent from the metabolite data, and this should be considered in future research among older adults with MS.
We did not identify any abnormalities in CHO. Although several studies have previously reported CHO increases in MS,43, 48–50 others have reported decreases,51–55 and no study has been able to resolve this discrepancy.16 It is possible that CHO abnormalities in MS are limited to certain stages of the disease, or to areas of active demyelination.56
It is important to note that comparisons between our results and prior studies is limited because the current study is the first to assess brain metabolites specifically in older adults with the disease. Studies suggest that aging itself may involve metabolic changes due to increased oxidative stress, inflammation, cell membrane turnover, and endogenous neuroprotection during aging.57 One cross-sectional study demonstrated decreased NAA and increased CHO in older compared to younger healthy adults,58 but longitudinal studies are still needed to address this issue. Because our healthy control sample was age-matched to the MS patients, metabolic abnormalities in older adults with MS appear to surpass changes related to aging alone.
Limitations
There are limitations to consider when interpreting the present results. First, we reported group differences in brain metabolites and temperature using both the traditional significance threshold (p<0.05) as well as an adjusted p-value. Adjustments for multiple comparisons can result in over-correction in case of small sample sizes, and we report both results here so that readers may consider all available information. Because only one of the results (lower NAA/CR in the right thalamus of MS patients) met the adjusted threshold for significance, we caution against over-interpreting metabolic differences in older adults with MS until these findings are replicated with larger sample sizes.
We did not assess the relationship between metabolic abnormalities and the extent or location of brain lesions because MRSI has previously been applied for this purpose, and novel diagnostic tools for MS should not merely duplicate conventional lesion imaging. However, it would have been interesting to consider metabolic abnormalities in the spinal cord and the possible contribution to walking ability in older adults with MS, because spinal cord integrity is essential to normal motor performance, and metabolic abnormalities in the spinal cord appear have been linked to disability in the general MS population.59
Third, there are additional MRSI metabolites, such as lactate, gamma-Aminobutyric acid (GABA), and glutamate, that could be related to walking performance in older adults with MS due to their associations with metabolic stress and excitotoxicity, but the quantification requires specialized MRS sequences and was outside the scope of the current study.
Finally, we stress that our analyses were correlational, and that these results alone cannot demonstrate that brain metabolites and walking performance are causally related. Longitudinal studies tracking the relationship between metabolites and walking performance over time could provide additional support that such a relationship exists, and this could be confirmed in clinical trials of rehabilitation.
Conclusion
The current study is the first to assess whole-brain metabolites related to mobility impairments in older adults with MS. We have demonstrated that increased MI and decreased NAA throughout widespread brain regions were related to lower walking speed and walking endurance in this population, although primary and secondary motor cortices appear not to be involved. The metabolic abnormalities may not selectively impair mobility, but are likely related to systemic brain changes that would also affect other cognitive functions. Despite accumulating evidence that MRS yields important information about disease progression in MS, the technique has not been adopted as a first-line diagnostic tool in this condition, perhaps due to the technical expertise required for acquisition and analysis of spectroscopy data. However, as MRSI becomes more widely accessible, we hope that the modality could be used to predict mobility issues and other clinical variables of interest in older adults with MS.
Supplementary Material
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National MS Society [grant number CA-1708].
Footnotes
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
References
- 1.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States. A population-based estimate using health claims data. 2019;92(10):e1029–e1040. doi: 10.1212/wnl.0000000000007035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird JF, Cederberg KLJ, Sikes EM, et al. Physical activity and walking performance across the lifespan among adults with multiple sclerosis. Mult Scler Relat Disord. Oct 2019;35:36–41. doi: 10.1016/j.msard.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 3.LaRocca NG. Impact of Walking Impairment in Multiple Sclerosis. The Patient: Patient-Centered Outcomes Research. 2011/09/01 2011;4(3):189–201. doi: 10.2165/11591150-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 4.Pike J, Jones E, Rajagopalan K, Piercy J, Anderson P. Social and economic burden of walking and mobility problems in multiple sclerosis. BMC Neurology. 2012/09/18 2012;12(1):94. doi: 10.1186/1471-2377-12-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol. Jun 2002;15(3):239–45. doi: 10.1097/00019052-200206000-00003 [DOI] [PubMed] [Google Scholar]
- 6.Chard DT, Griffin CM, McLean MA, et al. Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsing–remitting multiple sclerosis. Brain. 2002;125(10):2342–2352. doi: 10.1093/brain/awf240 [DOI] [PubMed] [Google Scholar]
- 7.Tiberio M, Chard DT, Altmann DR, et al. Metabolite changes in early relapsing-remitting multiple sclerosis. A two year follow-up study. J Neurol. Feb 2006;253(2):224–30. doi: 10.1007/s00415-005-0964-z [DOI] [PubMed] [Google Scholar]
- 8.Van Au Duong M, Audoin B, Le Fur Y, et al. Relationships between gray matter metabolic abnormalities and white matter inflammation in patients at the very early stage of MS : a MRSI study. J Neurol. Jul 2007;254(7):914–23. doi: 10.1007/s00415-006-0474-7 [DOI] [PubMed] [Google Scholar]
- 9.Kim H, McGrath BM, Silverstone PH. A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorders--focus on magnetic resonance spectroscopy (MRS) studies. Hum Psychopharmacol. Jul 2005;20(5):309–26. doi: 10.1002/hup.693 [DOI] [PubMed] [Google Scholar]
- 10.Hock A, Wilm B, Zandomeneghi G, et al. Neurochemical profile of the human cervical spinal cord determined by MRS. 10.1002/nbm.3589. NMR in Biomedicine. 2016/10/01 2016;29(10):1464–1476. doi: 10.1002/nbm.3589 [DOI] [PubMed] [Google Scholar]
- 11.Hnilicová P, Kantorová E, Poláček H, et al. Altered hypothalamic metabolism in early multiple sclerosis - MR spectroscopy study. J Neurol Sci. Dec 15 2019;407:116458. doi: 10.1016/j.jns.2019.116458 [DOI] [PubMed] [Google Scholar]
- 12.Llufriu S, Kornak J, Ratiney H, et al. Magnetic resonance spectroscopy markers of disease progression in multiple sclerosis. JAMA Neurol. Jul 1 2014;71(7):840–7. doi: 10.1001/jamaneurol.2014.895 [DOI] [PubMed] [Google Scholar]
- 13.Kirov II, Liu S, Tal A, et al. Proton MR spectroscopy of lesion evolution in multiple sclerosis: Steady-state metabolism and its relationship to conventional imaging. Hum Brain Mapp. Aug 2017;38(8):4047–4063. doi: 10.1002/hbm.23647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. May 2005;128(Pt 5):1016–25. doi: 10.1093/brain/awh467 [DOI] [PubMed] [Google Scholar]
- 15.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Progress in neurobiology. 2007;81(2):89–131. doi: 10.1016/j.pneurobio.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanberg KM, Landheer K, Pitt D, Juchem C. Quantifying the Metabolic Signature of Multiple Sclerosis by in vivo Proton Magnetic Resonance Spectroscopy: Current Challenges and Future Outlook in the Translation From Proton Signal to Diagnostic Biomarker. Review. Frontiers in Neurology. 2019-November-15 2019;10(1173)doi: 10.3389/fneur.2019.01173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cawley N, Solanky BS, Muhlert N, et al. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain. 2015;138(9):2584–2595. doi: 10.1093/brain/awv209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Stefano N, Matthews PM, Fu L, et al. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain. Aug 1998;121 ( Pt 8):1469–77. doi: 10.1093/brain/121.8.1469 [DOI] [PubMed] [Google Scholar]
- 19.Rahimian N, Saligheh Rad H, Firouznia K, et al. Magnetic resonance spectroscopic findings of chronic lesions in two subtypes of multiple sclerosis: primary progressive versus relapsing remitting. Iran J Radiol. Sep 2013;10(3):128–32. doi: 10.5812/iranjradiol.11336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donadieu M, Le Fur Y, Maarouf A, et al. Metabolic counterparts of sodium accumulation in multiple sclerosis: A whole brain 23Na-MRI and fast 1H-MRSI study. Multiple Sclerosis Journal. 2019/01/01 2017;25(1):39–47. doi: 10.1177/1352458517736146 [DOI] [PubMed] [Google Scholar]
- 21.Donadieu M, Le Fur Y, Lecocq A, et al. Metabolic voxel-based analysis of the complete human brain using fast 3D-MRSI: Proof of concept in multiple sclerosis. J Magn Reson Imaging. 2016;44(2):411–419. doi: 10.1002/jmri.25139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rovira A, Alonso J. 1H magnetic resonance spectroscopy in multiple sclerosis and related disorders. Neuroimaging Clin N Am. Aug 2013;23(3):459–74. doi: 10.1016/j.nic.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 23.Sajja BR, Wolinsky JS, Narayana PA. Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimaging Clin N Am. Feb 2009;19(1):45–58. doi: 10.1016/j.nic.2008.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen HL, Yamada K, Sakai K, Lu CH, Chen MH, Lin WC. Alteration of brain temperature and systemic inflammation in Parkinson’s disease. Neurol Sci. May 2020;41(5):1267–1276. doi: 10.1007/s10072-019-04217-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Childs C. Human brain temperature: regulation, measurement and relationship with cerebral trauma: Part 1. British Journal of Neurosurgery. 2008/01/01 2008;22(4):486–496. doi: 10.1080/02688690802245541 [DOI] [PubMed] [Google Scholar]
- 26.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci. Dec 1992;17(4):338–45. [PubMed] [Google Scholar]
- 27.Kieseier BC, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler. Jul 2012;18(7):914–24. doi: 10.1177/1352458512444498 [DOI] [PubMed] [Google Scholar]
- 28.Motl RW, Cohen JA, Benedict R, et al. Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Mult Scler. Apr 2017;23(5):704–710. doi: 10.1177/1352458517690823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. Apr 2008;14(3):383–90. doi: 10.1177/1352458507082607 [DOI] [PubMed] [Google Scholar]
- 30.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. Nov 1983;33(11):1444–52. doi: 10.1212/wnl.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 31.Maudsley AA, Domenig C, Govind V, et al. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn Reson Med. Mar 2009;61(3):548–59. doi: 10.1002/mrm.21875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. Jan 2001;20(1):45–57. doi: 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. Jan 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 34.Maudsley AA, Goryawala MZ, Sheriff S. Effects of tissue susceptibility on brain temperature mapping. Neuroimage. Feb 1 2017;146:1093–1101. doi: 10.1016/j.neuroimage.2016.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messina S, Patti F. Gray matters in multiple sclerosis: cognitive impairment and structural MRI. Mult Scler Int. 2014;2014:609694–609694. doi: 10.1155/2014/609694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haider L, Simeonidou C, Steinberger G, et al. Multiple sclerosis deep grey matter: the relation between demyelination, neurodegeneration, inflammation and iron. J Neurol Neurosurg Psychiatry. 2014;85(12):1386–1395. doi: 10.1136/jnnp-2014-307712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. Lancet Neurol. Sep 2008;7(9):841–51. doi: 10.1016/s1474-4422(08)70191-1 [DOI] [PubMed] [Google Scholar]
- 38.Hulst HE, Geurts JJG. Gray matter imaging in multiple sclerosis: what have we learned? BMC Neurology. 2011/12/12 2011;11(1):153. doi: 10.1186/1471-2377-11-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messina S, Patti F. Gray Matters in Multiple Sclerosis: Cognitive Impairment and Structural MRI. Mult Scler Int. 2014/01/22 2014;2014:609694. doi: 10.1155/2014/609694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honce JM. Gray Matter Pathology in MS: Neuroimaging and Clinical Correlations. Mult Scler Int. 2013/06/25 2013;2013:627870. doi: 10.1155/2013/627870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narayana PA, Wolinsky JS, Rao SB, He R, Mehta M. Multicentre proton magnetic resonance spectroscopy imaging of primary progressive multiple sclerosis. Mult Scler. Jun 2004;10 Suppl 1:S73–8. doi: 10.1191/1352458504ms1035oa [DOI] [PubMed] [Google Scholar]
- 42.Wylezinska M, Cifelli A, Jezzard P, Palace J, Alecci M, Matthews PM. Thalamic neurodegeneration in relapsing-remitting multiple sclerosis. Neurology. Jun 24 2003;60(12):1949–54. doi: 10.1212/01.wnl.0000069464.22267.95 [DOI] [PubMed] [Google Scholar]
- 43.Inglese M, Liu S, Babb JS, Mannon LJ, Grossman RI, Gonen O. Three-dimensional proton spectroscopy of deep gray matter nuclei in relapsing-remitting MS. Neurology. Jul 13 2004;63(1):170–2. doi: 10.1212/01.wnl.0000133133.77952.7c [DOI] [PubMed] [Google Scholar]
- 44.Geurts JJ, Reuling IE, Vrenken H, et al. MR spectroscopic evidence for thalamic and hippocampal, but not cortical, damage in multiple sclerosis. Magn Reson Med. Mar 2006;55(3):478–83. doi: 10.1002/mrm.20792 [DOI] [PubMed] [Google Scholar]
- 45.Jongen PJ, Ter Horst AT, Brands AM. Cognitive impairment in multiple sclerosis. Minerva Med. Apr 2012;103(2):73–96. [PubMed] [Google Scholar]
- 46.Staffen W, Zauner H, Mair A, et al. Magnetic resonance spectroscopy of memory and frontal brain region in early multiple sclerosis. J Neuropsychiatry Clin Neurosci. Summer 2005;17(3):357–63. doi: 10.1176/jnp.17.3.357 [DOI] [PubMed] [Google Scholar]
- 47.Foong J, Rozewicz L, Davie CA, Thompson AJ, Miller DH, Ron MA. Correlates of Executive Function in Multiple Sclerosis. The Journal of Neuropsychiatry and Clinical Neurosciences. 1999/02/01 1999;11(1):45–50. doi: 10.1176/jnp.11.1.45 [DOI] [PubMed] [Google Scholar]
- 48.Anik Y, Demirci A, Efendi H, Bulut SSD, Celebi I, Komsuoglu S. Evaluation of Normal Appearing White Matter in Multiple Sclerosis. Clinical Neuroradiology. 2011/12/01 2011;21(4):207–215. doi: 10.1007/s00062-011-0091-4 [DOI] [PubMed] [Google Scholar]
- 49.Kocevar G, Stamile C, Hannoun S, et al. Weekly follow up of acute lesions in three early multiple sclerosis patients using MR spectroscopy and diffusion. Journal of Neuroradiology. 2018/03/01/2018;45(2):108–113. doi: 10.1016/j.neurad.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 50.Marliani AF, Clementi V, Albini Riccioli L, et al. Quantitative Cervical Spinal Cord 3T Proton MR Spectroscopy in Multiple Sclerosis. American Journal of Neuroradiology. 2010;31(1):180–184. doi: 10.3174/ajnr.A1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Hanson LG, Skimminge A, et al. Cortical N-acetyl aspartate is a predictor of long-term clinical disability in multiple sclerosis. Neurological Research. 2014/08/01 2014;36(8):701–708. doi: 10.1179/1743132813Y.0000000312 [DOI] [PubMed] [Google Scholar]
- 52.Mathiesen HK, Tscherning T, Sorensen PS, et al. Multi-slice echo-planar spectroscopic MR imaging provides both global and local metabolite measures in multiple sclerosis. 10.1002/mrm.20407. Magnetic Resonance in Medicine. 2005/04/01 2005;53(4):750–759. doi: 10.1002/mrm.20407 [DOI] [PubMed] [Google Scholar]
- 53.Bellenberg B, Busch M, Trampe N, Gold R, Chan A, Lukas C. 1H-Magnetic Resonance Spectroscopy in diffuse and focal cervical cord lesions in Multiple Sclerosis. European Radiology. 2013/12/01 2013;23(12):3379–3392. doi: 10.1007/s00330-013-2942-7 [DOI] [PubMed] [Google Scholar]
- 54.Sijens PE, Mostert JP, Irwan R, Potze JH, Oudkerk M, De Keyser J. Impact of fluoxetine on the human brain in multiple sclerosis as quantified by proton magnetic resonance spectroscopy and diffusion tensor imaging. Psychiatry Research: Neuroimaging. 2008/12/30/2008;164(3):274–282. doi: 10.1016/j.pscychresns.2007.12.014 [DOI] [PubMed] [Google Scholar]
- 55.Gustafsson MC, Dahlqvist O, Jaworski J, Lundberg P, Landtblom A-ME. Low Choline Concentrations in Normal-Appearing White Matter of Patients with Multiple Sclerosis and Normal MR Imaging Brain Scans. American Journal of Neuroradiology. 2007;28(7):1306–1312. doi: 10.3174/ajnr.A0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arnold DL, Matthews PM, Francis GS, O’Connor J, Antel JP. Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol. Mar 1992;31(3):235–41. doi: 10.1002/ana.410310302 [DOI] [PubMed] [Google Scholar]
- 57.Harris JL, Yeh H-W, Swerdlow RH, Choi I-Y, Lee P, Brooks WM. High-field proton magnetic resonance spectroscopy reveals metabolic effects of normal brain aging. Neurobiol Aging. 2014;35(7):1686–1694. doi: 10.1016/j.neurobiolaging.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitz B, Wang X, Barker PB, et al. Effects of Aging on the Human Brain: A Proton and Phosphorus MR Spectroscopy Study at 3T. J Neuroimaging. Jul 2018;28(4):416–421. doi: 10.1111/jon.12514 [DOI] [PubMed] [Google Scholar]
- 59.Bjartmar C, Kidd G, Mörk S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. Dec 2000;48(6):893–901. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.