Abstract
HIV-1 infection induces a chronic inflammatory environment not restored by suppressive antiretroviral therapy (ART). As of today, the effect of viral suppression and immune reconstitution in people living with HIV-1 (PLWH) has been well described but not completely understood. Herein, we show how PLWH who naturally control the virus (PLWHEC) have a reduced proportion of CD4+CCR6+ and CD8+CCR6+ cells compared to PLWH on suppressive ART (PLWHART) and HIV-1 negative controls (HC). Expression of CCR2 was reduced on both CD4+, CD8+ and classical monocytes in PLWHEC compared to PLWHART and HC. Longer suppressive therapy, measured in the same patients, decreased number of cells expressing CCR2 on all monocytic cell populations while expression on CD8+ T cells increased. Furthermore, the CD4+CCR6+/CCR6− cells exhibited a unique proteomic profile with a modulated energy metabolism in PLWHEC compared to PLWHART independent of CCR6 status. The CD4+CCR6+ cells also showed an enrichment in proteins involved in apoptosis and p53 signalling in PLWHEC compared to PLWHART, indicative of increased sensitivity towards cell death mechanisms. Collectively, this data shows how PLWHEC have a unique chemokine receptor profile that may aid in facilitating natural control of HIV-1 infection.
Subject terms: Infection, HIV infections
The expression profiles dynamics of several chemokine receptors are lower for people living with HIV-1 who naturally control the virus compared to those on suppressive antiretroviral therapy and HIV-negative controls, shedding light on the mechanisms of natural control of HIV-1 infection.
Introduction
Efficient antiretroviral therapy (ART) suppresses human immunodeficiency virus type 1 (HIV-1) replication to below the detection level of sensitive HIV RNA assays. Still, HIV-1 persists for decades despite efficient ART in presumably latently infected cells, making up the so-called latent viral reservoir. This reservoir resides in subtypes of memory T cells and cells from monocytic lineages, but also in some functional and naïve T cell subsets1. Eradication of latently infected cells, i.e. a sterilizing cure, has proven to be a major hurdle due to several factors such as heterogeneity in mechanisms governing latency and integration sites into the host genome2. Over the course of suppressive ART, persisting HIV-1 sustained by low levels of viral replication, homoeostatic proliferation, and cell-to-cell transmission, is a driver of chronic immune activation in the host3–5.
In HIV-1 infection, chemokine receptors are essential co-receptors needed for HIV-1 entry into the cell where the CC chemokine receptor 5 (CCR5) and CXC chemokine receptor 4 (CXCR4) are the main co-receptors used for HIV-1 entry, but alternative receptors such as CCR3 may play a role for macrophage-tropic viral strains6–8. T cells expressing the chemokine receptor CCR6 are also overrepresented amongst cell types infected by HIV-19. Furthermore, CD4+CCR6+ and CD4+CCR2+CCR5+ cells have been proposed to contribute to the HIV-1 reservoir10,11.
Alterations in chemokine receptor expression and chemokine levels modulate the activity of the immune response and inflammatory levels during HIV-1 infection12. In people living with HIV-1 (PLWH) on suppressive ART, these aberrations may contribute to (i) higher risk of age-associated co-morbidities together with elevated immune activation, (ii) microbiome dysbiosis leading to microbial translocation and (iii) dysregulated immune cell activation and function13–16. Consequently, HIV-1 pathogenesis and immune dysfunction promote chronic immune activation. This results in a vicious cycle as immune activation likely drives HIV-1 disease progression and inflammaging, defined as an age-related increase of inflammatory markers, that could possibly lead to earlier onset of age-related diseases. In our earlier study on a small group of 10 PLWH on ART for two decades, we reported normalisation of most of the plasma pro-inflammatory cytokines and chemokines to the level of HIV-negative controls17. Therefore, although there are several studies on immune reconstitution and viral suppression during short-term ART, the long-term effect of ART on the immune system is not known.
Eradication of latently infected cells, i.e., a sterilising cure, has proven to be a major hurdle. An alternative approach is a functional cure aimed at suppressing viral replication without ART. A model for functional cure studies is elite controllers (herein PLWHEC). PLWHEC constitute a small fraction (<0.5%) of HIV-1+ individuals able to naturally control viral replication in the absence of ART18,19. However, there is a population-based heterogeneity of how these individuals suppress viral replication which is likely caused by several factors such as viral and host genetic factors, variability of integration site in the human genome, and variation in the host response such as immunological factors20–23. In terms of immune cell activation, studies have shown how PLWHEC maintain lower levels of inflammatory markers compared to PLWH who progress in their disease24. However, as no consensus exists on how the PLWHEC phenotype is maintained, further studies comparing variability of immune function between PLWHEC and PLWH on suppressive ART (herein PLWHART) are required to understand the mechanisms of natural control of HIV-1.
In our recent study25, we hypothesised that an altered CCR6/CCL20 chemokine axis and CCR2-CCL7-CCL2 signalling may play a protective role in the PLWHEC phenotype. We identified a downregulation of CCR2 and CCR6 receptors on CD4+ and CD8+ lymphocytes and higher plasma abundance of CCL4, CCL7 and CCL20 in PLWHEC compared to the HIV-1-negative controls. This can provide natural resistance to HIV-1 infection although the expression profile during long-term suppressive ART is not known. In the present study, we extended our analysis to evaluate the expression profile dynamics of key chemokine receptors CCR2, CCR3, CCR5 and CCR6 in PLWHART. We also compared the proportion of integrated HIV-1 between PLWHEC and PLWHART. Furthermore, cell populations of interest were isolated for quantitative proteomics to evaluate specific characteristics regulating these cell populations and their potential role in HIV-1 persistence. Our study provides important understanding of the chemokine receptor dynamics and its role in HIV-1 persistence that differentiate PLWHEC from PLWHART.
Results
PLWHEC have reduced populations of CD4+CCR6+ and CD4+CCR2+ T lymphocytes compared to successfully long-term treated patients
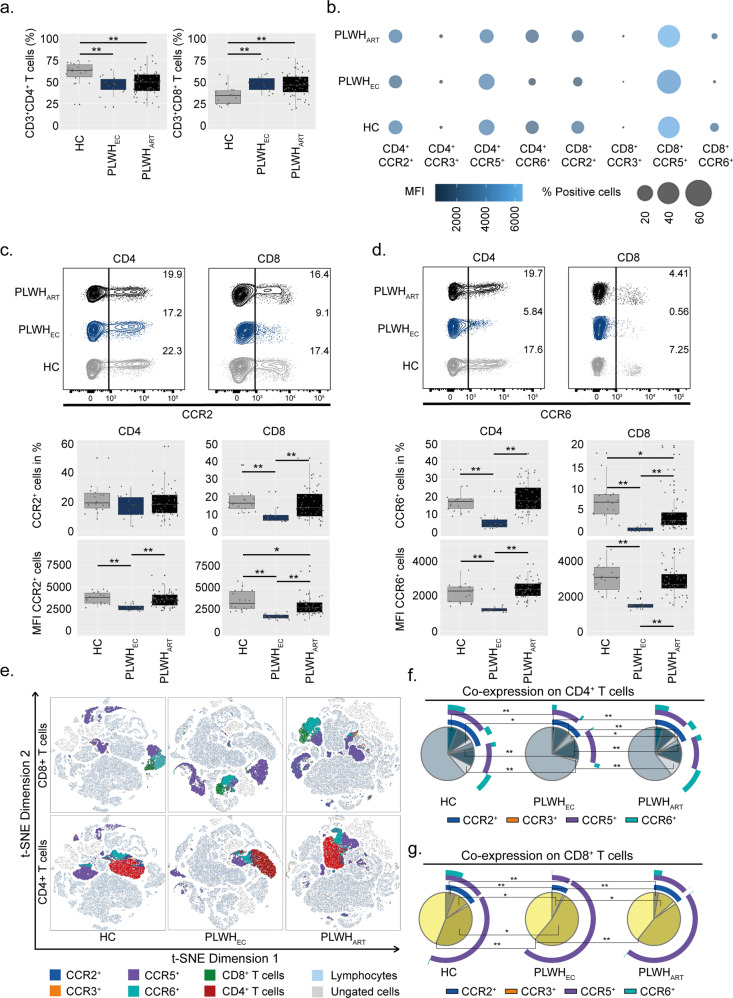
Herein, we wanted to evaluate if there are any differences in the expression profile of some key chemokine receptors between PLWH with natural control of the virus and those on ART. Therefore, we investigated the expression levels of CCR2, CCR3, CCR5 and CCR6 in peripheral blood mononuclear cells (PBMCs) from PLWHEC (n = 14), PLWHART (n = 54) and HIV-negative individuals (HC, n = 18) by flow cytometry (Supplementary Fig. 1). The clinical and demographic data are presented in Table 1 and Supplementary Table 1. All the PLWHART were successfully treated at the time of sample collection and plasma viral load was below the detection level (<40 copies/mL). Initial discrimination between CD4+ T cells (CD3+CD4+) and CD8+ T cells (CD3+CD8+) showed that both PLWHEC and PLWHART exhibited a reduced proportion of CD4+ T cells and elevated proportion of CD8+ T cells compared to HC (Fig. 1a). Single receptor expression in PLWHEC showed a distinct profile compared to both PLWHART and HC (Fig. 1b). Expression of CCR2 and CCR6 was reduced on both CD4+ and CD8+ T cells together with a decreased proportion of CD8+CCR2+, CD4+CCR6+ and CD8+CCR6+ in PLWHEC compared to both PLWHART and HC (Fig. 1c, d). The expression levels of CCR2 were also reduced on CD8+ T cells in PLWHART compared to HC, as well as the proportion of CD8+CCR6+ cells (Fig. 1c, d). Even as the total frequency of CD4+CCR3+ was low in all three groups, the expression of CCR3 was high in both PLWHEC and PLWHART compared to HC (Supplementary Fig. 2a). Furthermore, expression levels of CCR5 were lower on CD8+ T cells in PLWHEC compared to PLWHART (Supplementary Fig. 2b), while no difference was detected on CD4+ T cells. Collectively, these data show how PLWHEC have a unique expression profile of CCR2 and CCR6 while the expression signature of CCR3 is more similar to PLWHART on lymphocytic cells (Fig. 1e). Continuing, co-expression analysis on CD4+ cells showed that CCR2+CCR5+CCR6+, CCR2+CCR6+ and CCR5+CCR6+ were reduced in PLWHEC compared to both PLWHART and HC, while no differences were detected between PLWHART and HC (Fig. 1f). Furthermore, single receptor expression of CCR2 was reduced in PLWHEC compared to PLWHART and CCR6 expression was reduced in PLWHEC compared to both PLWHART and HC (Fig. 1f). Within the CD8+ T cell population, co-receptor expression of CCR2+CCR5+CCR6+ was reduced in both PLWHART and PLWHEC compared to HC while CCR2+CCR5+ was reduced in PLWHEC compared to HC and PLWHART (Fig. 1g). Single receptor expression analysis also showed that CCR5 was increased in PLWHEC compared to HC and CCR6 expression decreased in PLWHEC compared to both HC and PLWHART (Fig. 1g). As the major differences were detected on CCR2 and CCR6 expression, we measured the levels of their ligands, namely monocyte chemoattractant protein 1, MCP1 (CCL2) and macrophage inflammatory protein 3, MIP-3 (CCL20), respectively, in plasma from PLWHART (n = 49), PLWHEC (n = 12) and HC (n = 16). Plasma levels of CCL20 were increased in both PLWHEC and PLWHART compared to HC, while no significant difference was detected for CCL2 (Supplementary Fig. 2c, d). In summary, the receptor expression profile on lymphocytes in PLWHART resembles HC, while PLWHEC exhibits a distinct expression profile with decreased receptor expression of both CCR2 and CCR6.
Table 1.
Patient characteristics.
| Parameter | PLWHEC | PLWHART | HC | P-values |
|---|---|---|---|---|
| N | 14 | 54 | 18 | ND |
| Gender: Female, N (%) | 7 (50) | 19 (35) | 9 (50) | ND |
| Ethnicity, N (%) | ||||
| Black | 9 (64.2) | 21 (38.8) | 5 (27.7) | ND |
| Asian | – | 2 (3.7) | – | |
| Caucasian | 4 (28.6) | 31 (57.4) | 13 (72.2) | |
| Hispanic | 1 (7.1) | – | – | |
| At sampling | ||||
| Age in years, mean (SD) | 46 (10.5) | 50 (9.5) | 47 (9) | 0.2704a |
| CD4+ T cell count (cells/µL); median (IQR) | 680 (570–1060) | 660 (485–795) | NA | 0.5012b |
| CD8+ T cell count (cells/µL); median (IQR) | 900 (540–1330) | 600 (415–950) | NA | 0.0974b |
| CD4:CD8 ratio; median (IQR) | 1 (0.44–1.18) | 1 (0.7–1.45) | NA | 0.3295b |
| Duration of treatment in years; median (IQR) | NA | 8 (6.75–19) | NA | ND |
| Duration of suppressive treatment in years; median (IQR) | NA | 8 (7–15) | NA | ND |
| Years HIV positive; median (IQR) | 7 (4.5–11.7) | 12.5 (9–20) | NA | 0.0012b |
|
HIV RNA load; Copies/mL (IQR) |
<40 copies/mL | <40 copies/mL | NA | ND |
| Treatment regimen, N (%) | ||||
| 3rd drug, | ||||
| Boosted PI | 2 (3.7) | |||
| INSTI | 30 (55.5) | |||
| NNRTI | NA | 21 (38.8) | NA | NA |
| 1st drug, | ||||
| ABC | 34 (62.9) | |||
| TAF/TDF | 16 (29.6) | |||
| Other | 4 (7.4) | |||
| Initiation of treatment | ||||
| CD4+ T cell count at treatment initiation (cells/µL); median (IQR) | NA | 351 (167.5–522.5) | NA | ND |
| Viral Load at treatment initiation; Log10 copies/mL (IQR) | NA | 4.79 (4.06–5.44) | NA | ND |
ABC abacavir, IQR interquartile range, N number, NA not applicable, ND not done, PI protease inhibitor, INSTI integrase strand transfer inhibitor, NNRTI non-nucleoside reverse transcriptase inhibitor, SD standard deviation, TAF tenofovir alafenamide, TDF tenofovir disoproxil.
aOne-way ANOVA.
bMann–Whitney test.
Fig. 1. CCR2 and CCR6 are specifically depleted from T lymphocytes in PLWHEC.
Receptor expression on CD4+ and CD8+ T lymphocytes from PLWHEC (n = 14), PLWHART (n = 54) and HC (n = 18). a Frequencies of CD4+ T cells and CD8+ T cells in total peripheral blood mononuclear cells derived from CD3+ cells. b Bubble chart representing receptor expression of CCR2, CCR3, CCR5 and CCR6 on CD4+ and CD8+ T cells. Size of the bubble corresponds to percentage-positive cells while colour represents the median fluorescence intensity (MFI). c CCR2 receptor expression on CD4+ and CD8+ T cells (% cells and MFI). d CCR6 receptor expression on CD4+ and CD8+ T cells (% cells and MFI). e t-SNE plots showing receptor expression distribution on CD4+ and CD8+ T cells. f Co-receptor expression of CCR2, CCR3, CCR5 and CCR6 on CD4+ T cells. g Co-receptor expression of CCR2, CCR3, CCR5, and CCR6 on CD8+ T cells. c, d Contour plots show a representing sample from PLWHEC, PLWHART and HC corresponding to the median % of cells within each group. a, c, d, f, g Statistical significance was determined using two-tailed Mann–Whitney U-test (significance level p < 0.05, with *<0.05, **<0.001) and represented as pie charts or with median using 95% CI.
CCR2 expression is reduced in PLWHEC compared to PLWHART on monocytic cell populations
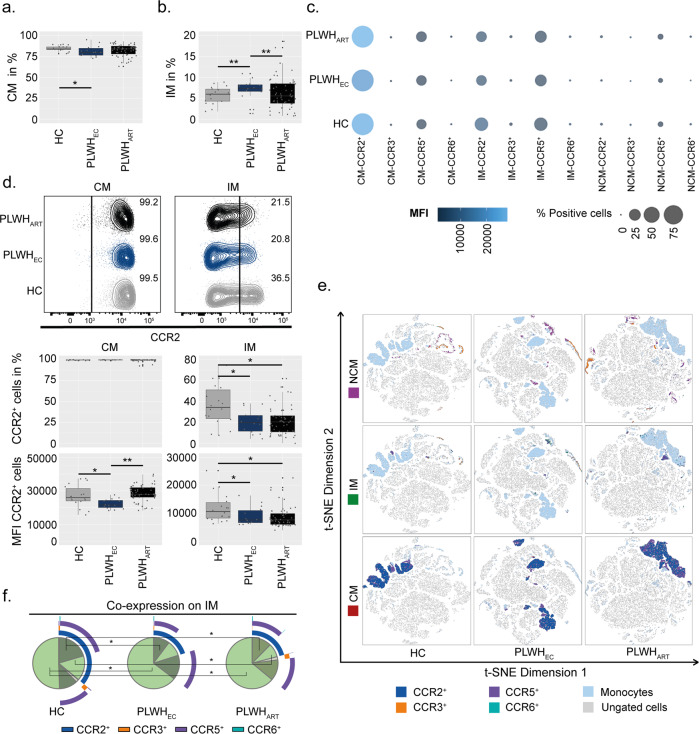
In HIV-1 infection there is a chronic activation of monocytes contributing to organ-specific inflammatory events that are poorly understood in virologically suppressed patients. Our group and others have previously shown persistent low-level inflammation in PLWHEC24–26. Furthermore, chemokine receptors like CCR2 and CCR5 play a major role in inflammatory trafficking of monocytes27. Therefore, we determined the CCR2, CCR3, CCR5 and CCR6 receptor expression in monocytic cell populations; classical (CM, CD14+CD16−), intermediate (IM, CD14+CD16+) and non-classical (NCM, CD14−CD16+). Herein, PLWHEC exhibited reduced numbers of CM compared to HC and elevated levels of IM compared to both HC and PLWHART (Fig. 2a, b and Supplementary Fig. 3a). Generally, the receptor expression of CCR3 and CCR6 was low on all three monocytic cell subsets (Fig. 2c). The major difference was a reduced expression of CCR2 on CM and IM in PLWHEC compared to HC and on CM compared to PLWHART (Fig. 2d). In PLWHART, both the frequency of IM-CCR2+ cells and expression of CCR2 on IM cells were reduced compared to HC (Fig. 2d). Although the proportion of CM-CCR6+ cells was decreased in PLWHEC compared to PLWHART, CCR6 expression was increased in PLWHEC compared to HC and PLWHART, while PLWHART showed reduced expression compared to HC (Supplementary Fig. 3b). Furthermore, NCM-CCR6+ cells were lower in PLWHEC compared to PLWHART and HC, and the frequency of CM-CCR3+ cells was decreased in PLWHEC compared to PLWHART (Supplementary Fig. 3b, c). The receptor expression of CCR5 was reduced on IM in PLWHART compared to HC and on NCM in PLWHEC compared to both PLWHART and HC (Supplementary Fig. 3d). This data indicates that the main difference in the monocytic cell subsets is a reduced expression of CCR2 in PLWHEC compared to the other groups (Fig. 2e). The IM were the only monocytic cell population which exhibited differences in co-receptor expression. For these cells, the largest variation was a reduction of CCR2+CCR5+ expressing cells in both PLWHEC and PLWHART compared to HC. An increase in cells lacking all four receptors was found in both PLWHEC and PLWHART compared to HC (Fig. 2f). Furthermore, PLWHART exhibited reduced numbers of cells expressing only CCR2 compared to HC (Fig. 2f). Collectively, this data shows that the largest variation occurred on IM where PLWHART and PLWHEC showed a similar trend compared to HC.
Fig. 2. CCR2 is depleted from intermediate monocytes of PLWH.
Receptor expression on monocytic subpopulations (classical monocytes (CM); CD14+CD16−, intermediate monocytes (IM) CD14+CD16+ and non-classical monocytes (NCM); CD14−CD16+) in PLWHEC (n = 14), PLWHART (n = 54) and HC (n = 18). a, b Percentages of CM (a) and IM (b) from CD3− cells in peripheral blood mononuclear cells. c Bubble chart representing receptor expression of CCR2, CCR3, CCR5 and CCR6 on CM, IM and NCM. Size of the bubble corresponds to percentage-positive cells while colour represents the median fluorescent intensity (MFI). d CCR2 receptor expression on CM and IM (% cells and MFI). e t-SNE plots showing receptor expression distribution of CCR2, CCR3, CCR5 and CCR6 on CM, IM and NCM. f Co-receptor expression of CCR2, CCR3, CCR5 and CCR6 on IM. d Contour plot shows a representing sample from PLWHEC, PLWHART and HC corresponding to the median % of cells within each group. a, b, d, f Statistical significance was determined using two-tailed Mann–Whitney U-test (significance level p < 0.05, with *<0.05, **<0.001) and represented as pie charts or with median using 95% CI.
Longer suppressive therapy reduces expression of CCR2 on monocytes
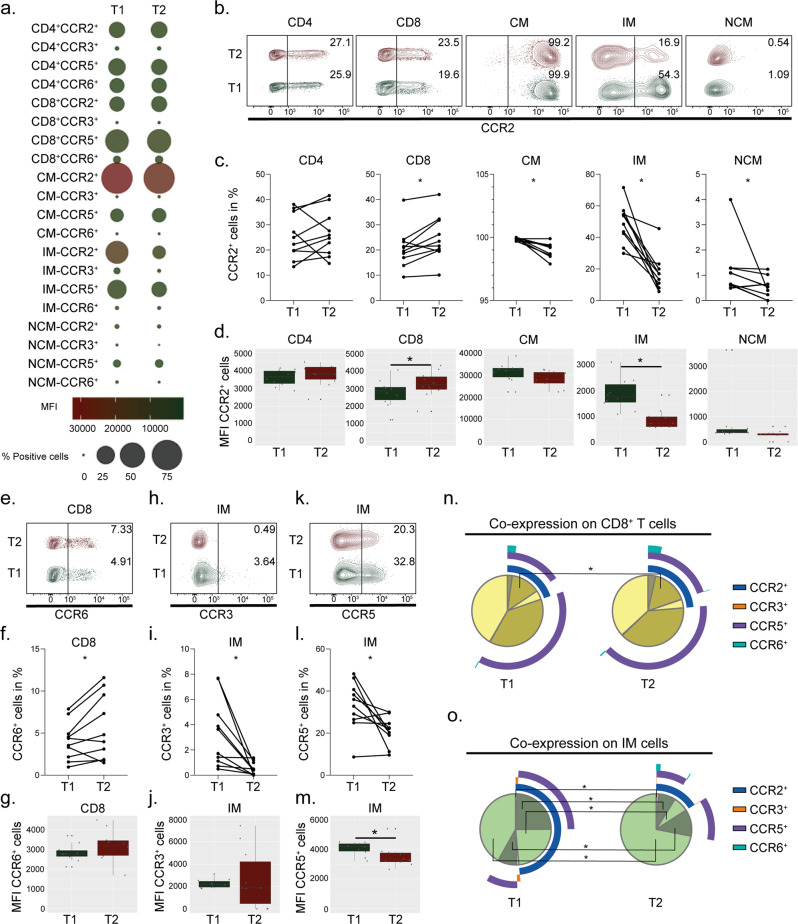
The chronic inflammatory condition due to HIV-1 persistence and an activated immune response persists during suppressive ART. Therefore, to evaluate if longer treatment duration affects the receptor expression, we included longitudinal samples (timepoint 1 (T1) n = 10 and timepoint 2 (T2) n = 10) where the same patients were followed-up after 3 years of additional suppressive therapy (clinical parameters can be viewed in Supplementary Table 2). Treatment duration did not affect frequencies of CD4+, CD8+ T cells or monocytic cell populations (Supplementary Fig. 4a, b). For the detected receptor expressions, most of the differences were detected in the monocytic cell populations while no significant variations were seen on CD4+ T cells (Fig. 3a). Longer treatment duration increased the frequency of CD8+CCR2+ cells, while all three monocytic cell populations exhibited reduced proportions of cells expressing CCR2 in T2 (Fig. 3b, c). Following a similar trend, CCR2 expression was increased on CD8+ T cells and IM exhibited reduced expression of CCR2 in T2 while CM and NCM showed no difference (Fig. 3d). The proportion of CD8+CCR6+ cells was increased in T2, without showing any significant effect on CCR6 expression levels (Fig. 3e–g). Furthermore, the proportions of IM-CCR3+ and IM-CCR5+ cells decreased over time while only expression levels of CCR5 decreased in T2 (Fig. 3h–m). Investigations of co-receptor expression on the lymphocytic cell populations showed that the only difference was an increase in CCR2+CCR5+ in T2 on CD8+ T cells (Fig. 3n). Within the monocytic cell populations, the only differences of co-expression of the receptors were seen on IM where T2 exhibited higher percentage of cells negative for all four receptors while CCR2+, CCR2+CCR3+ and CCR2+CCR5+ were decreased in T2 (Fig. 3o). Conclusively, the effect induced by longer suppressive treatment mainly reduces expression levels of these chemokine receptors on IM.
Fig. 3. In time, CCR2 and CCR6 increase on CD8 cells while CCR2 decreases on monocytes in PLWHART.
Receptor expression of longitudinal data on lymphocytes and monocytes at arbitrary timepoint 1 (T1) (n = 10) and three years later at timepoint 2 (T2) (n = 10) from total peripheral blood mononuclear cells. a Bubble chart representing receptor expression of CCR2, CCR3, CCR5 and CCR6 on CD4+ and CD8+ T cells together with classical (CM; CD14+CD16−), intermediate (IM; CD14+CD16+), and non-classical (NCM; CD14−CD16+) monocytes. Size of the bubble corresponds to percentage-positive cells while colour represents the median fluorescent intensity (MFI). b, c Receptor expression of CCR2 on CD4+, CD8+, CM, IM and NCM. d MFI of CCR2 on CD4+, CD8+, CM, IM and NCM. e, f Receptor expression of CCR6 on CD8+ T cells. g MFI of CCR6 on CD8+ T cells. h, i Receptor expression of CCR3 on IM. j MFI of CCR3 on IM. k, l Receptor expression of CCR5 on IM. m MFI of CCR5 on IM. n, o Co-receptor expression of CCR2, CCR3, CCR5 and CCR6 on CD8+ T cells (n) and IM (o). b, e, h, k Contour plot shows a representing sample from T1 and T2 corresponding to the median % of cells within each group. c, d, f, g, i, j, l–o Statistical significance was determined using two-tailed Wilcoxon matched-pairs signed-rank test (significance level p < 0.05, with *<0.05, **<0.001) and represented as paired before and after plot, median using 95% CI or as pie charts.
The HIV-1 reservoir is lower in PLWHEC compared to PLWHART
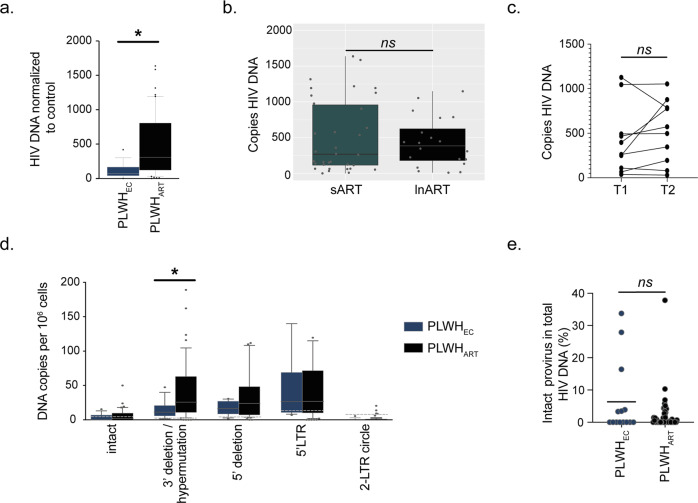
To evaluate the proportion of HIV-1 infected cells in our cohort, we quantified total HIV-1 DNA in the PLWHEC (n = 14) and PLWHART (n = 54). Herein, we observed a higher level of total HIV-1 DNA in PLWHART compared to PLWHEC (Fig. 4a) despite a median of eight years of successful treatment. No significant difference was observed between long-term ART (lnART; >10 yrs (median 20 yrs, Interquartile range (IQR); 18–22.75), n = 20) and short-term ART (sART; <10 yrs (median 7 yrs, IQR; 6–8), n = 34) nor was the reservoir affected by longer suppressive treatment between T1 and T2 within the same individuals (Fig. 4b, c). This data indicates that after the initial two phases of HIV DNA decline the size of the reservoir is not affected during successful suppressive ART. However, PLWHEC exhibited reduced proportions of HIV-1 infected cells presumably due to their natural control of HIV-1. Next, to evaluate the proportion of integrated HIV-1 that was intact/defective, we employed the intact provirus DNA assay (IPDA)28 together with quantification of the 5′LTR and 2-LTR circles on the genomic DNA from PBMCs. A significant increase of 3′deletion/hypermutation was detected in PLWHART compared to PLWHEC, but no significant differences in intact provirus, 5′deletion, 5′LTR or 2-LTR circles between the two groups (Fig. 4d). Moreover, percentages of intact provirus out of total HIV-1 DNA did not differ between the groups (Fig. 4e). The CCL20 ligand for CCR6 can facilitate nuclear integration of HIV-1 by remodelling the actin cytoskeleton, but also directly inhibits infection in the female reproductive tract29,30. Therefore, to evaluate if the ligands for CCR2 and CCR6 might have an effect on the HIV-1 reservoir we performed correlation analysis between plasma levels of CCL20 CCL2 and the HIV-1 reservoir. No significant correlation between HIV-1 DNA and CCL20 plasma levels was revealed in either PLWHART or PLWHEC (Supplementary Fig. 5a). For CCL2, a negative correlation was detected in PLWHEC but not in PLWHART (Supplementary Fig. 5b). Collectively, this data indicates that although PLWHART have higher proportion of infected cells compared to PLWHEC, a larger fraction of the integrated HIV-1 carries hypermutations or 3′deletions within the viral genome. Furthermore, the frequency of intact provirus out of total HIV-1 DNA is low in both PLWHEC and PLWHART.
Fig. 4. The HIV-1 reservoir in PLWHEC is distinct from PLWHART.
a Quantified total HIV-1 DNA in peripheral blood mononuclear cells from PLWHEC (n = 14) and PLWHART (n = 54). b Total HIV-1 DNA in short-term ART (sART, <10 yrs, n = 34) compared to long-term ART (lnART, >10 yrs, n = 20) in people living with HIV-1 on suppressive therapy. c Total HIV-1 DNA in longitudinal data from HIV-1 infected individuals (timepoint 1 (T1), n = 10 and timepoint 2 (T2), n = 10). d Box and Whisker plot show the distribution of integrated HIV-1 DNA copies per 106 PBMCs in PLWHART (n = 54) and PLWHEC (n = 14) with whiskers representing the 10–90 percentile. The median is represented by the line inside the box. The dotted line marks the background represented by HC samples. e Percentage of intact provirus out of total HIV-1 DNA detected. Statistical significance was determined using two-tailed Mann–Whitney U-test (significance level p < 0.05, with *<0.05, **<0.001) and represented as median using 95% CI. All individual samples were run in technical duplicates.
Altered metabolic pathways in CD4+CCR6+ cells are characterised by an upregulation in p53 signalling and apoptosis in PLWHEC
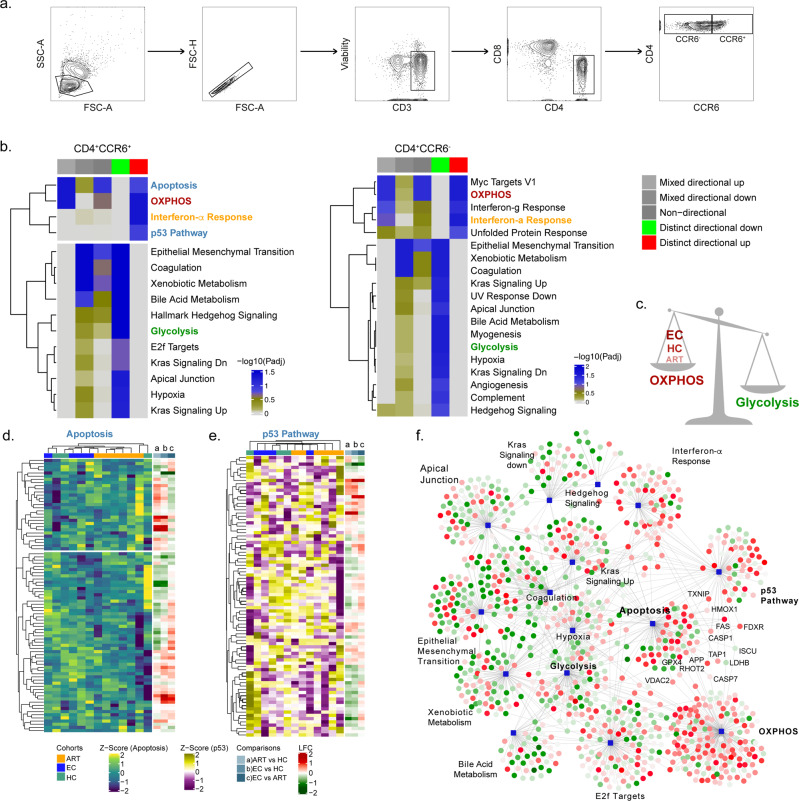
The subpopulation of CD4+CCR6+ cells has been described as more permissive towards HIV-1 and targets for productive infection31,32. To further characterise the CD4+ T cells that express or lack the CCR6 receptor we sorted out these cell populations using fluorescence-activated cell sorting (FACS) from HC (n = 3), PLWHEC (n = 6) and PLWHART (n = 6) (Fig. 5a). The PLWHART samples were selected from PLWH on long-term suppressive ART (median 20 years, IQR; 17.5–22.25). First, as the HIV-1 reservoir has been proposed to reside in CD4+CCR6+ T cells, we measured the amount of integrated HIV-1 in PLWHEC (n = 5) and PLWHART (n = 3). However, the variation within the data and the small sample size made the comparison inconclusive (Supplementary Fig. 6a). To evaluate the specific proteomic profile of CD4+CCR6− and CD4+CCR6+ cell populations we isolated these cell populations by FACS (15,000–30,000 cells) and performed mass spectrometry-based proteomics using a quantitative tandem mass tags (TMT) labelling approach. Due to low protein detection by mass spectrometry four PLWHEC samples, two samples out of each of the CCR6+ and CCR6− cell populations were excluded from the analysis. The principal component analysis (PCA) identified heterogeneity among the samples (Supplementary Fig. 6b). Differential expression analysis was unable to find statistically significant regulated proteins due to heterogeneity among samples and low sample size. Therefore, functional analysis was performed using a consensus scoring approach based on multiple protein set analysis (PSA) run by incorporating the directionality of protein abundance. Using the group-specific consensus scores (PLWHEC vs PLWHART) and directionality classes, we identified distinct upregulation of oxidative phosphorylation (OXPHOS) (adj p < 0.05) and interferon-α response (adj p < 0.05) and distinct downregulation of glycolysis (adj p < 0.05) in both CD4+CCR6+ and CD4+CCR6− cells while using the MSigDB hallmark gene set (Fig. 5b). Similarly, the KEGG gene set also identified a distinct upregulation of OXPHOS (adj p < 0.1), and a distinct downregulation of other pathways of amino acid and carbohydrate metabolism (Supplementary Fig. 6c). This data indicates that the metabolic trade-off of increased OXPHOS and reduced glycolysis is elevated in PLWHEC compared to PLWHART (Fig. 5c). The relative abundance of the OXPHOS proteins was higher in PLWHEC compared to HC while lower in PLWHART compared to HC (Supplementary Fig. 7a, b). Interestingly, only p53 signalling and apoptosis were distinctly upregulated in CD4+CCR6+ (adj p < 0.05) (Fig. 5b) and both pathways had unique features in PLWHEC (Fig. 5d, e). The majority of the PLWHART clustered together. There were two downregulated proteins in PLWHEC (ISCU and LDHB) that were common between OXPHOS and p53 signalling. Furthermore, four proteins (CASP7, VDAC2, RHOT2 and GPX4) were common between OXPHOS and apoptosis, while seven proteins (FAS, TAP1, FDXR, TXNIP, CASP1, APP and HMOX1) were common between p53 signalling and apoptosis among the proteins upregulated in PLWHEC compared to PLWHART (Fig. 5f). Taken together, this data shows how the CD4+CCR6+ cell population have a unique profile in PLWHEC. The co-regulation of OXPHOS, p53 signalling and apoptosis may be a contributing factor to control HIV-1 infection.
Fig. 5. CD4+CCR6+ are enriched in proteins from p53 and apoptosis signalling in PLWHEC compared to PLWHART.
Characteristics of CD4+CCR6+ and CD4+CCR6− cell populations isolated by FACS from PLWHEC (n = 4), PLWHART (n = 6) and HC (n = 3). a Gating strategy for isolation of CD4+CCR6+ and CD4+CCR6− cell populations from total peripheral blood mononuclear cells, exhibited by an HC sample. b Heatmap representation of significant pathways found deregulated in CD4+CCR6+ PLWHEC compared to CD4+CCR6+ PLWHART and in CD4+CCR6− PLWHEC compared to CD4+CCR6− PLWHART using MSigDBv. Colour gradient corresponds to the negative log scaled adjusted p-values. Each column represents p-values of various directionality classes, calculated for the pathways. Non-directional p-values are calculated based on gene-level statistics regardless of the direction of expression. Mixed directional up and mixed directional down p-values are calculated using the subset of the gene statistics that are upregulated and downregulated, respectively. Distinct directional up and distinct directional down p-values are calculated from gene statistics with expression direction. c Schematic representation of metabolic trade-off between OXPHOS and glycolysis detected in the cells. d, e Heatmap representation of proteins part of apoptosis (d) and p53 pathway (e). Colour gradient corresponds to the z-score scaled normalized expression values. Column annotation represents the cohorts and row annotation visualizes the log2 transformed fold change values in each of the differential expression analysis of PLWHART compared with HC, PLWHEC compared with HC and PLWHEC compared with PLWHART. f Network visualization of significant pathways enriched in CD4+CCR6+ PLWHEC compared to CD4+CCR6+ PLWHART. Each edge represents association of the protein with the corresponding pathways. Circular nodes denote proteins and square-shaped nodes are pathways. Red colour gradient and green colour gradient represent upregulation and downregulation of the proteins in CD4+CCR6+ PLWHEC compared to CD4+CCR6+ PLWHART.
Discussion
In our study, a unique cell type expression profile of CCR2 and CCR6 was identified in PLWHEC, while PLWHART exhibited an expression profile more like HC. CCR6+ cells were thus decreased on both CD4+ and CD8+ T cells in PLWHEC but not in PLWHART. Furthermore, the CD4+ T cells exhibited a protein profile of modulated metabolic activity towards OXPHOS, and reduced protein levels involved in glycolysis in PLWHEC, irrespective of CCR6 status. Collectively, as CD4+CCR6+ cells have been reported to be highly permissive to HIV-131,32, and substantial contributors to the HIV-1 reservoir, low levels of CD4+CCR6+ cells, metabolic modulation, and enrichment of proteins involved in cell death, could potentially contribute to natural control of HIV-1.
Within the CD4+ T cell subpopulation, CCR6 expression is a marker of some memory T cells subsets, namely Th17 cells, natural killer T (NKT) cells, and γδ T cells33. Of these cell populations, the Th17 cells promote a strong pro-inflammatory response and are drivers of chronic inflammation and autoimmune diseases34,35. Earlier studies have shown how memory CD4+CCR6+ T cells express a Th17 lineage profile and high susceptibility towards HIV-136. In our cohort, the proportion of CCR6+ cells was reduced in PLWHEC compared to both PLWHART and HC. CCR6 is also a homing marker for gut-associated lymphoid tissue (GALT)37. Therefore, the causative factor of reduced CD4+CCR6+ cells in peripheral blood in PLWHEC could be tissue residency in GALT to a higher extent than in PLWHART. Although initiation of ART restores the intestinal CD4+ T cell compartments, the severe depletion induced during the early stages of infection cannot be fully recovered38, which contributes to exacerbation of HIV-1 through microbial translocation and hyperactivation of the immune system39–41. Collectively, an increased gut homing potential of CCR6+ cells in PLWHEC, could help to restore the imbalance of CD4+ T cells in GALT and reduce systemic chronic inflammation among these patients.
We have earlier shown that low expression of CCR6 and high levels of its ligand, CCL20, may result in low levels of inflammation in PLWHEC25. Herein, we detected increased plasma levels of CCL20 in PLWH, independent of HIV-1 status compared to HC, confirming earlier observations42. CCL20 has previously been described to have an effect on the infection steps of HIV-1 and migration/function of immune cells33. However, we did not see any difference in CCL20 levels between PLWHEC and PLWHART. Therefore, most likely it is the CD4+CCR6+ cells, rather than levels of CCL20, that in PLWHEC could contribute to natural control of HIV-1.
Furthermore, CD4+CCR6+ and CD8+CCR6+ cells decline in untreated patients with HIV-143 since the CCR6+ cells are more susceptible towards apoptosis and therefore involved in the depletion of memory T cells. However, we did not detect any alterations in CD4+CCR6+ compartments in PLWHART compared to HC, although for CD8+CCR6+ cells there was a reduction in both patient groups. We identified the CD4+CCR6+ cells as having increased proteins involved in apoptosis and p53 signalling in PLWHEC, indicating an increased sensitivity towards apoptosis, possibly as a result of HIV-1 proteins, activation-induced cell death or induced expression of death receptors, e.g., Fas and TRAIL-RI/TRAIL-RII44. Since these cells are not restored in the GALT during ART, it can be postulated that the CD4+CCR6+ cell population is a major target for HIV-1 infection that in PLWH contributes to the inflammatory environment in both GALT and peripheral blood. In PLWHEC, these cells may be more susceptible towards apoptosis, possibly through expression of pro-apoptotic HIV-1 proteins during infection or reactivation from latency. Furthermore, p53 is a tumour suppressor gene that during HIV-1 infection is activated by interferon α and β stimulation of immune cells45. Activity of p53 can interfere with HIV-1 replication by inhibiting reverse transcription of the virus and suppressing the activity of the transactivator tat, needed for viral transcription46. Therefore, an enrichment of p53 signalling in PLWHEC could contribute to natural control of HIV-1 infection in CD4+CCR6+ T cells by reducing replication of HIV-1 and inducing apoptosis upon latency reversal. However, the sample size included in this study is relatively small and further studies validating these results are warranted. In addition, PLWHEC exhibited an enrichment of proteins involved in OXPHOS together with decreased number of proteins involved in glycolysis, irrespective of CCR6 expression. Increased glycolysis is a hallmark for T cell activation but also upregulated during HIV-1 infection for cells to sustain the energy-demanding process of virus production47,48. Generally, activation of a T cell induces a shift from OXPHOS to aerobic glycolysis in a similar fashion as the metabolic reprogramming occurring during activation from latency49,50. Therefore, the metabolic state of the CD4+ T cell compartment in PLWHEC, independently of CCR6 expression indicates a unique proportion of CD4 T cell subsets and metabolic reprogramming.
Persistent HIV-1 is a driver of chronic immune activation and inflammation. In our cohort, PLWHEC had a reduced amount of integrated HIV-1 in total PBMCs compared to PLWHART, correlating to earlier observations23. On the other hand, treatment duration did not significantly alter the proportion of latently infected cells probably due to the stability of the reservoir during long-term ART. During suppressive therapy, persisting HIV-1 contributes to low levels of viral replication and cell-to-cell spread driving the chronic immune cell activation and inflammaging that ultimately sustains the HIV-1 reservoir in the body, together with homoeostatic proliferation51,52. However, evaluation of the intact provirus by IPDA showed a decrease of 3′deletion/hypermutation in the provirus from PLWHEC compared to PLWHART. Earlier studies have shown that 3′ deletion clones decline during suppressive therapy53. These specific mutated proviruses may be under negative selection due to their capacity to stimulate the immune system53,54. With a reduced proportion of provirus in PLWHEC compared to PLWHART, it could be hypothesised that this negative selection is stronger in PLWHEC, explaining the lower proportion of integrated HIV-1.
Monocytes produce pro-inflammatory cytokines where IM are efficient producers of IL6 and IL8 in response to microbial pathogens together with CM, whereas NCM produce TNFα, CCL3 and IL-1β in response to viral elements55. In our cohort, elevated levels of IM were detected in PLWHEC, indicative of an increased pro-inflammatory response. However, the expression profile of CCR2 on IM was similar between PLWHEC and PLWHART. Furthermore, expression of CCR2 decreased on IM during longer suppressive therapy in our longitudinal PLWHART cohort and a similar trend was seen in PLWHEC and PLWHART compared to HC. Monocytes are targets of HIV-1 infection and drivers of inflammation and comorbidities during HIV-1 infection56. Therefore, the expansion of IM could indicate a role in a heightened pathogen defence in PLWHEC.
Overall, our study showed that the chemokine receptor profile in PLWHART is more similar towards HC on lymphocytic cell populations, and PLWHEC on monocytic cell populations. PLWHEC have a reduced proportion of CD4+CCR6+ cells in peripheral blood compared to PLWHART. Based on our proof of principle proteomic analysis we hypothesise that the CD4+CCR6+ cells are more susceptible to apoptosis, while CD4+ T cells in PLWHEC (CCR6−/CCR6+) exhibit decreased metabolic activation, indicative of a reduced activation state. Collectively, these lymphocytic cell populations may aid in facilitating the unique characteristics of PLWHEC and contribute to chronic inflammation and HIV-1 persistence during suppressive therapy.
Methods
Patient material
The clinical cohort consisted of HIV-1+ individuals on suppressive therapy (PLWHART, n = 54), elite controllers (PLWHEC, n = 14) collected from the InfCareHIV cohort at the Department of Infectious Diseases, Karolinska University Hospital, Sweden, and HIV-1 negative individuals (HC, n = 18). The cohort was age-matched and the median duration of treatment was 8 years (IQR; 6.75–19). Cohort characteristics can be viewed in Table 1 and treatment regimen for PLWHART in Supplementary Table 1. The cohort also consisted of longitudinal data within the PLWHART group (T1, n = 10 and T2, n = 10) (Supplementary Table 2). At the time of sampling, none of the study participants had any co-infections. Patient material consisted of plasma and PBMCs collected from whole blood using Ficoll-Paque (Cytiva).
This study was approved by the Regional Ethics Committee of Stockholm. Informed consent was given from all participants prior to inclusion, and data were anonymized and delinked before analysis.
Flow cytometry and FACS
PBMCs were stained for cell surface markers for 20 min at room temperature (RT) using 5 µL/106 PBMCs of CD3 (OKT3, FITC, Biolegend #317306), CD4 (SK3, BUV395, BDbioscience #563550), CD8 (RPA-T8, APC, Biolegend #301014), CD14 (M5E2, BV510, Biolegend #301842), CD16 (3G8, BV786, BDbioscience #563690), CCR2 (1D9, BB700, BDbioscience #747847), CCR3 (5E8, BV421, Biolegend #310714), CCR5 (2D7, PE-CF594, BDbioscience #562456), CCR6 (G034E3, BV711, Biolegend #353436), and Near IR viability dye (Invitrogen #L10119). All antibodies were used at a volume of 5 µL/100 µL reaction and Near IR viability dye at a concentration of 1:100. Cells were subsequently washed two times using FACS buffer (PBS with 2% FBS and 2 mM EDTA) and fixed 15 min at RT using 2% paraformaldehyde. Results were acquired on BD FACS Symphony (BD Bioscience, USA) using laser and filter settings as indicated for BUV395, BV421, BV510, BV711, BV786, FITC, BB700, PE-CF594, APC and Near IR, respectively; 355 nm UV (100 mW) 379/28, 405 nm violet (100 mW), 450/50, 525/50 (505 LP), 710/50 (685 LP), 810/40 (770 LP), 488 nm blue (200 mW) 530/30 (505 LP), 710/50 (685 LP), 561 nm Y/G (200 mW), 610/20 (600LP) and 637 nm Red (140 mW), 670/30 and 780/60 (750LP). Cell expression analysis and t-SNE were performed using FlowJo 10.6 (Tree Star Inc). Co-expression analysis was performed using the Boolean gating function and the visualisation of complex data using Spice57. Data from flow cytometry detection in PLWHEC (n = 14) and HC (n = 8) samples were used from our earlier publication25.
Cryopreserved PBMCs were used for fluorescent activated cell sorting (FACS) for subsequent proteomic analysis and DNA extraction. Cells were thawed and stained using anti-human CD3 (OKT3, FITC, Biolegend #317306), CD4 (SK3, PE-Cy5, Biolegend # 344654), CD8 (RPA-T8, BV570, Biolegend #301038), CD14 (M5E2, BV510, Biolegend #301842), CD16 (3G8, BV786, BDbioscience #563690), CCR6 (G034E3, BV711, Biolegend #353436) for 30 min at 4 °C and subsequent FVS450 viability dye (BDbioscience #562247) for 10 min at RT. All antibodies were used at a volume of 5 µL/100 µL reaction and FVS450 was used at 1:1000. CD4+CCR6− and CD4+CCR6+ cell was collected for downstream analysis. Cells were sorted using SONY MA900 (SONY cooperation, Tokyo, Japan).
DNA extraction
Total DNA was extracted from whole PBMCs or collected cell populations by QIAamp Mini or QIAamp Micro DNA isolation kit (Qiagen), according to the manufacturers protocol.
HIV-1 DNA quantification
Patient PBMCs or sorted cells were used for quantification of total HIV-1 DNA by Internally Controlled qPCR (IC-qPCR) developed by Vicenti et al.58. In brief, total HIV-1 DNA was quantified using Takara Universal mastermix on the ABI 7500F and normalized to β-Actin levels, primer and probe details are described in Supplementary Table 3. For each sample, 200–500 ng were run per reaction in duplicates and data were normalised to background detection in HC.
Digital droplet PCR and intact proviral DNA assay (IPDA)
Digital droplet PCR (ddPCR) was performed using the QX200 Droplet Digital qPCR System (RioRad). Samples consisting of 10 µL Supermix for probes (no dUTPs) (Bio-Rad), 900 nM primers, 250 nM probe (labelled with either HEX or FAM) or 500 ng of genomic sample DNA and 50 ng of DNA for RPP30 quantification for shearing analysis and normalization, were emulsified with the QX200 droplet generator. PCR reactions were subsequently performed on a C1000 thermal cycler (Bio-Rad) using the following thermal cycling protocol: 10 min at 95 °C for enzyme activation followed by 45 annealing/extension cycles of 30 s at 94 °C and 60 s at 57 °C and a final enzyme deactivation step at 98 °C for 10 min. Subsequently, samples were read with the QX200 Droplet Reader (Bio-Rad) and analysed with the QuantaSoft software version 1.5 (Bio-Rad).
For the quantification of intact, 5′ deleted, and 3′ deleted/hypermutated proviruses, the Intact Proviral DNA Assay was performed as previously described28. Briefly, a first ddPCR was performed as described above with primer/probe combinations for the packaging signal (Ψ), which frequently shows small mutations. The second reaction targets the HIV-1 Rev response element, present in the Env coding region. Two hydrolysis probes were used in this reaction: a HEX-labelled probe specific for the intact proviral sequence and an unlabelled probe specific for the APOBEC-3G hypermutation, which competes with the HEX-labelled probe and results in no signal in the case of APOBEC hypermutations. Duplicate wells for IPDA were merged during analysis. All primer and probe details can be viewed in Supplementary Table 3.
Targeted proteomic profiling
Plasma levels of CCL20 were measured using Human Quantikine ELISA kits (R&D Systems) according to the manufacturers protocol. Data for plasma CCL2 was used from our earlier publication18. In brief, plasma levels were analysed using proximity extension assay with the OLINK® immune-oncology panel (Olink, Sweden). Proteins are reported as normalised protein expression levels (NPX) where Ct values are normalised by the subtraction of the extension control and inter-plate control.
Mass spectrometry-based proteomics
Proteins were extracted by adding 8 M urea in 100 mM ammonium bicarbonate lysis buffer. Samples were vortexed and sonicated in cold bath for 10 min. Protein concentration was estimated with Pierce Micro BCA assay (ThermoFisher Scientific). Extracted proteins were reduced with 10 mM dithiothreitol for 60 min at 37 °C and alkylated with 40 mM chloroacetamide (CAM) for 30 min at room temperature. Proteins were digested with trypsin (sequencing grade from Promega) with a 1:20 ratio enzyme:protein overnight at 37 °C. Resulting peptides were cleaned-up using C-18 stage tips (ThermoFisher Scientific) according to manufacturer’s instructions and dried in a vacuum concentrator (Eppendorf). Cleaned peptides were labelled with TMT11plex™ tags (ThermoFisher Scientific) following manufacturer’s instructions. Briefly, peptides were resuspended in 100 mM triethylammonium bicarbonate and individual TMT tags were dissolved in dry acetonitrile (ACN). Peptides were labelled by adding the respective TMT tag solution, the mix was incubated for 1 h at room temperature, the reaction was quenched by adding 5% hydroxylamine and incubated for 15 min at RT. Labelled peptides were mixed in three different batches according to the scheme described in Supplementary Table 4, a pool of samples was used across the three batches to normalize and evaluate batch effect (TMT channel 131C).
Labelled peptides were fractionated using Pierce High pH Reversed-Phase Peptide Fractionation Kit columns (ThermoFisher Scientific) following manufacturer’s instructions. Briefly, columns were washed with 100% ACN and then conditioned with 0.1% trifluoroacetic acid (TFA). Peptides were dissolved in 0.1% TFA and loaded into the column by centrifugation at 3000 × g for 2 min. Columns were washed with 5% ACN in 0.1% triethanolamine (TEA), and peptides were eluted in eight different fractions by centrifugation at 3000 × g according to the ACN concentration in 0.1% TEA solutions: 10, 12.5, 15, 17.5, 20, 22.5, 25 and 50%. All fractions were dried down in a vacuum concentrator (Eppendorf) prior to LC-MS/MS analysis.
Fractionated peptides were analysed on an Ultimate 3000 UHPLC (ThermoFisher Scientific) hyphenated to an Orbitrap™ Lumos™ mass spectrometer (ThermoFisher Scientific). Peptides were loaded in an Acclaim PepMap trap column, 2 μm × 75 μm ID × 2 cm (Thermo Scientific) and separated in an EASY-Spray™ HPLC Column, 2 μm × 75 μm ID × 500 mm (Thermo Scientific) using a 120 min linear gradient. Data were acquired in data-dependent acquisition (DDA) mode, isolating the top 20 most intense precursors at 120,000 mass resolution in the mass range of m/z 375–1400, maximum injection time (IT) of 50 ms and dynamic exclusion of 30 s, precursors were isolated with 0.7 Th width. MS/MS scans were obtained using high collision energy of 34%, resolution of 45,000 and maximum IT of 86 ms.
Proteins were searched against both SwissProt human and HIV-1 databases using the search engine Mascot Server v2.5.1 (MatrixScience Ltd) in Proteome Discoverer v2.5 (ThermoFisher Scientific) software environment allowing maximum two missed cleavages. Oxidation of methionine, deamidation of asparagine and glutamine, TMT6plex modification of lysine and N-termini were set as variable modifications, while carbamidomethylation of cysteine was used as fixed modification. The false discovery rate (FDR) was set to 1%.
Bioinformatics analysis
The data were normalized using quantile method from the R/Bioconductor package NormalyzerDE v1.4.0. Missing data were imputed by employing the nearest-neighbour averaging method using impute.knn function from R package impute v1.60.0. Default setting was used for the data imputation. Technical variations in the data due to batch effect were removed using R function Combat from the package sva v3.34.0. Differential expressions analysis was performed with R/Bioconductor package Limma v3.42.2. Gene-set enrichment analysis was performed using R/Bioconductor package piano v2.2.0. Gene-level t-statistics given by Limma, and hallmark gene set downloaded from MSigDB were used to find significantly enriched gene-sets. Gene-sets with Benjamini–Hochberg adjusted p-values <0.2 were considered as significantly enriched. Volcano and bubble plots were created using R package ggplot2 v3.3.2.
Statistics and reproducibility
Statistical analysis was performed using two-tailed Mann–Whitney U-test, and Wilcoxon ranked sum test or paired t-test for longitudinal data, based on the distribution of the data in Prism v8 (GraphPad Prism Software). Statistical significance was p < 0.05 for all analysis. Data were visualized using the package ggplot2 v.3.3.2 in Rstudio (v.1.3.1056). Correlation analysis was performed using Spearman’s correlation (p < 0.05) in Rstudio (v.1.3.1056). All assays were performed in technical duplicates except for the flow cytometry evaluation that was performed in one replicate per sample.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This study was funded by the Swedish Research Council Grants (2017-01330 to U.N. and 2017-05848 to A.S.) and Karolinska Institutet Stiftelser och Fonder. U.N. was also supported by the Swedish Research Council Interdisciplinary Grant (2018-06156). We would like to thank Assistant professor Robert van Domselaar for providing intellectual input on immunological aspects of the manuscript. Furthermore, we would like to acknowledge Dr. Sivasankaran Munusamy Ponnan for performing the t-SNE plots.
Author contributions
U.N. conceived the study; A.S. initiated, designed, and governed the Swedish Elite controller cohort; U.N. and S.S.A. planned the experiments; S.S.A., S.K., S.G., B.J., and M.S. performed the research; J.E.R. and A.V. performed the proteomic analysis; A.T.A. performed the bioinformatics analysis. A.S., P.N. and J.V contributed with patient material. S.S.A. and U.N. analysed the data. S.G. and P.S. contributed with intellectual input. S.S.A. and U.N. wrote the manuscript. All authors critically reviewed and revised the manuscript.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Deborah H. Fuller and Manuel Breuer. Peer reviewer reports are available.
Funding
Open access funding provided by Karolinska Institute.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD027749. Raw data for FACS and flow cytometry are available from the corresponding authors upon request. The source data underlying most graphs used in this manuscript are provided as a Supplementary data file (Supplementary Data 1, excel).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sara Svensson Akusjärvi, Email: sara.svensson.akusjarvi@ki.se.
Ujjwal Neogi, Email: ujjwal.neogi@ki.se.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-03315-x.
References
- 1.Cohn, L. B., Chomont, N. & Deeks, S. G. The biology of the HIV-1 latent reservoir and implications for cure strategies. Cell Host Microbe27, 519–530 (2020). [DOI] [PMC free article] [PubMed]
- 2.Khanal, S., Schank, M., El Gazzar, M., Moorman, J. P. & Yao, Z. Q. HIV-1 latency and viral reservoirs: existing reversal approaches and potential technologies, targets, and pathways involved in HIV latency studies. Cells10, 475 (2021). [DOI] [PMC free article] [PubMed]
- 3.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedro KD, Henderson AJ, Agosto LM. Mechanisms of HIV-1 cell-to-cell transmission and the establishment of the latent reservoir. Virus Res. 2019;265:115–121. doi: 10.1016/j.virusres.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massanella M, Fromentin R, Chomont N. Residual inflammation and viral reservoirs: alliance against an HIV cure. Curr. Opin. HIV AIDS. 2016;11:234–241. doi: 10.1097/COH.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe H, et al. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/S0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 7.Alkhatib G, Berger EA, Murphy PM, Pease JE. Determinants of HIV-1 coreceptor function on CC chemokine receptor 3. Importance of both extracellular and transmembrane/cytoplasmic regions. J. Biol. Chem. 1997;272:20420–20426. doi: 10.1074/jbc.272.33.20420. [DOI] [PubMed] [Google Scholar]
- 8.He J, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JL, et al. Human immunodeficiency virus (HIV)-infected CCR6+ rectal CD4+ T cells and HIV persistence on antiretroviral therapy. J. Infect. Dis. 2020;221:744–755. doi: 10.1093/infdis/jiz509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosselin A, et al. HIV persists in CCR6+CD4+ T cells from colon and blood during antiretroviral therapy. AIDS. 2017;31:35–48. doi: 10.1097/QAD.0000000000001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Packard TA, Herzig E, Roan NR, Greene WC. Establishing the HIV reservoir: HIV-susceptible cells and the signals that recruit them. J. Immunol. 2017;198:125.121. [Google Scholar]
- 12.Wang Z, Shang H, Jiang Y. Chemokines and chemokine receptors: accomplices for human immunodeficiency virus infection and latency. Front. Immunol. 2017;8:1274–1274. doi: 10.3389/fimmu.2017.01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zicari, S. et al. Immune activation, inflammation, and non-AIDS Co-morbidities in HIV-infected patients under long-term ART. Viruses11, 200 (2019). [DOI] [PMC free article] [PubMed]
- 14.Nakayama K, et al. Imbalanced production of cytokines by T cells associates with the activation/exhaustion status of memory T cells in chronic HIV type 1 infection. AIDS Res. Hum. Retroviruses. 2011;28:702–714. doi: 10.1089/aid.2011.0073. [DOI] [PubMed] [Google Scholar]
- 15.Babu, H. et al. Systemic inflammation and the increased risk of inflamm-aging and age-associated diseases in people living with HIV on long term suppressive antiretroviral therapy. Front. Immunol.10, 1965 (2019). [DOI] [PMC free article] [PubMed]
- 16.Babu H, et al. Plasma metabolic signature and abnormalities in HIV-infected individuals on long-term successful antiretroviral therapy. Metabolites. 2019;9:210. doi: 10.3390/metabo9100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperk M, Zhang W, Nowak P, Neogi U. Plasma soluble factor following two decades prolonged suppressive antiretroviral therapy in HIV-1-positive males: a cross-sectional study. Medicine. 2018;97:e9759–e9759. doi: 10.1097/MD.0000000000009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, et al. Transcriptomics and targeted proteomics analysis to gain insights into the immune-control mechanisms of HIV-1 infected elite controllers. EBioMedicine. 2018;27:40–50. doi: 10.1016/j.ebiom.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson AD, et al. An evaluation of HIV elite controller definitions within a large seroconverter cohort collaboration. PLoS ONE. 2014;9:e86719–e86719. doi: 10.1371/journal.pone.0086719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poropatich K, Sullivan DJ. Human immunodeficiency virus type 1 long-term non-progressors: the viral, genetic and immunological basis for disease non-progression. J. Gen. Virol. 2011;92:247–268. doi: 10.1099/vir.0.027102-0. [DOI] [PubMed] [Google Scholar]
- 21.International HIVCS. et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaren PJ, et al. Fine-mapping classical HLA variation associated with durable host control of HIV-1 infection in African Americans. Hum. Mol. Genet. 2012;21:4334–4347. doi: 10.1093/hmg/dds226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang C, et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature. 2020;585:261–267. doi: 10.1038/s41586-020-2651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hocini H, et al. HIV controllers have low inflammation associated with a strong HIV-specific immune response in blood. J. Virol. 2019;93:e01690–01618. doi: 10.1128/JVI.01690-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperk, M. et al. Distinct lipid profile, low-level inflammation and increased antioxidant defense signature in HIV-1 elite control status. iScience24, 102111 (2021). [DOI] [PMC free article] [PubMed]
- 26.Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM, Palmer CS. Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J. Immunol. Res. 2014;2014:569819–569819. doi: 10.1155/2014/569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaworowski A, Hearps AC, Angelovich TA, Hoy JF. How monocytes contribute to increased risk of atherosclerosis in virologically-suppressed HIV-positive individuals receiving combination antiretroviral therapy. Front. Immunol. 2019;10:1378–1378. doi: 10.3389/fimmu.2019.01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruner KM, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature. 2019;566:120–125. doi: 10.1038/s41586-019-0898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron PU, et al. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc. Natl Acad. Sci. USA. 2010;107:16934–16939. doi: 10.1073/pnas.1002894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh M, et al. CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am. J. Reprod. Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gosselin A, et al. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J. Immunol. 2010;184:1604–1616. doi: 10.4049/jimmunol.0903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Planas, D. et al. HIV-1 selectively targets gut-homing CCR6+CD4+ T cells via mTOR-dependent mechanisms. JCI Insight2, e93230 (2017). [DOI] [PMC free article] [PubMed]
- 33.Lee AYS, Korner H. CCR6/CCL20 chemokine axis in human immunodeficiency virus immunity and pathogenesis. J. Gen. Virol. 2017;98:338–344. doi: 10.1099/jgv.0.000691. [DOI] [PubMed] [Google Scholar]
- 34.Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019;41:283–297. doi: 10.1007/s00281-019-00733-8. [DOI] [PubMed] [Google Scholar]
- 35.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 36.Monteiro P, et al. Memory CCR6+CD4+T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin β7. J. Immunol. 2011;186:4618. doi: 10.4049/jimmunol.1004151. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guadalupe M, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat. Rev. Microbiol. 2012;10:655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 40.Chun T-W, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J. Infect. Dis. 2008;197:714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 41.Valverde-Villegas JM, Matte MCC, Medeiros RMD, Chies JAB. New insights about treg and Th17 cells in HIV infection and disease progression. J. Immunol. Res. 2015;2015:647916. doi: 10.1155/2015/647916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fontaine J, Poudrier J, Roger M. Short Communication: Persistence of high blood levels of the chemokines CCL2, CCL19, and CCL20 during the course of HIV infection. AIDS Res. Hum. Retroviruses. 2010;27:655–657. doi: 10.1089/aid.2010.0261. [DOI] [PubMed] [Google Scholar]
- 43.Lécureuil C, et al. Trapping and apoptosis of novel subsets of memory T lymphocytes expressing CCR6 in the spleen of HIV-infected patients. Blood. 2007;109:3649–3657. doi: 10.1182/blood-2006-01-035717. [DOI] [PubMed] [Google Scholar]
- 44.Cummins NW, Badley AD. Mechanisms of HIV-associated lymphocyte apoptosis: 2010. Cell Death Dis. 2010;1:e99–e99. doi: 10.1038/cddis.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imbeault M, Ouellet M, Tremblay MJ. Microarray study reveals that HIV-1 induces rapid type-I interferon-dependent p53 mRNA up-regulation in human primary CD4+ T cells. Retrovirology. 2009;6:5–5. doi: 10.1186/1742-4690-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi B, et al. Inhibition of HIV early replication by the p53 and its downstream gene p21. Virol. J. 2018;15:53–p53. doi: 10.1186/s12985-018-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hegedus A, Kavanagh Williamson M, Huthoff H. HIV-1 pathogenicity and virion production are dependent on the metabolic phenotype of activated CD4+ T cells. Retrovirology. 2014;11:98–98. doi: 10.1186/s12977-014-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer CS, et al. Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. AIDS. 2014;28:297–309. doi: 10.1097/QAD.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shytaj IL, et al. Glycolysis downregulation is a hallmark of HIV-1 latency and sensitizes infected cells to oxidative stress. EMBO Mol. Med. 2021;13:e13901. doi: 10.15252/emmm.202013901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Massanella M, Fromentin R, Chomont N. Residual inflammation and viral reservoirs: alliance against an HIV cure. Curr. Opin. HIV AIDS. 2016;11:234–241. doi: 10.1097/COH.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinzone MR, et al. Longitudinal HIV sequencing reveals reservoir expression leading to decay which is obscured by clonal expansion. Nat. Commun. 2019;10:728. doi: 10.1038/s41467-019-08431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venanzi Rullo, E. et al. Persistence of an intact HIV reservoir in phenotypically naive T cells. JCI Insight5, 10.1172/jci.insight.133157 (2020). [DOI] [PMC free article] [PubMed]
- 55.Cros J, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong, M. E., Jaworowski, A. & Hearps, A. C. The HIV reservoir in monocytes and macrophages. Front. Immunol.10, 1435 (2019). [DOI] [PMC free article] [PubMed]
- 57.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytom. A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vicenti I, et al. Development of an internally controlled quantitative PCR to measure total cell-associated HIV-1 DNA in blood. Clin. Chem. Lab Med. 2018;56:e75–e77. doi: 10.1515/cclm-2017-0587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD027749. Raw data for FACS and flow cytometry are available from the corresponding authors upon request. The source data underlying most graphs used in this manuscript are provided as a Supplementary data file (Supplementary Data 1, excel).