Abstract
Homoploid hybrid speciation (HHS) has been increasingly recognized as occurring widely during species diversification of both plants and animals. However, previous studies on HHS have mostly focused on closely-related species while it has been rarely reported or tested between ancestors of different genera. Here, we explore the likely HHS origin of Carpinus sect. Distegocarpus between sect. Carpinus and Ostrya in the family Betulaceae. We generate a chromosome-level reference genome for C. viminea of sect. Carpinus and re-sequence genomes of 44 individuals from the genera Carpinus and Ostrya. Our integrated analyses of all genomic data suggest that sect. Distegocarpus, which has three species, likely originates through HHS during the early divergence between Carpinus and Ostrya. Our study highlights the likelihood of an HHS event between ancestors of the extant genera during their initial divergences, which may have led to reticulate phylogenies at higher taxonomic levels.
Subject terms: Evolutionary genetics, Evolutionary biology, Ecological genetics, Plant evolution
Carpinus fangiana exhibits intermediate morphology between C. viminea and Ostrya rehderiana. Here, the authors report that Carpinus sect. Distegocarpus likely originate through homoploid hybrid speciation (HHS) during the early divergence between Carpinus and Ostrya through genomic analyses.
Introduction
Hybridization has been considered as a possible factor to drive biodiversity evolution at both low and high taxonomic level for a long time1. In the recent past, homoploid hybrid speciation (HHS) is repeatedly evidenced to be an important mechanism that generates new species and increases biodiversity without any change in chromosome number2. According to the strict definition3, HHS has to meet three criteria: genetic admixture from two parental lineages, distinct reproductive isolation (RI) between a stable hybrid lineage and its two parents, and RI resulting directly from a hybridization event. The genomic contributions of the two parents to the hybrid lineage may be equal when HHS arises from F1 hybrids, but unequal if it derives from backcrossing of hybrids4,5. Under the latter scenario, HHS is sometime confused with introgression (also called introgressive hybridization)2. All homoploid hybrids (from F1 and backcrossing) between distinct species can achieve partial intrinsic RI through fixing different allelic variations from one or other of the parents and sorting of genic incompatibilities6,7. In addition, introgression usually transfers adaptive alleles and helps the introgressed populations to colonize new niches where there is prezygotic RI distinct from that of the non-introgressed ones8, although introgression of non-RI alleles between species is likely (Supplementary Fig. 1). Both introgressed and HHS lineages may therefore experience hybrid recombination of the RI-related loci, leading directly to prezygotic5,8 and postzygotic RI7. However, HHS differs from introgression in that the former results in a stable lineage as a distinct taxonomic entity while the latter may be maintained as an intermittent and hybrid entity experiencing ongoing evolution, which may undergo further HHS or merge with one parent through repeated backcrossing9 (Supplementary Fig. 1). This crucial distinction between HHS and introgression is supported by ~150 case studies based on population genetic data (Supplementary Data 1) showing that introgression produces genetic admixture in a few individuals or populations, rather than in all hybrid offspring as does HHS2,5 (Supplementary Fig. 1).
Both HHS and incomplete lineage sorting (ILS) produce gene trees that are inconsistent between different loci across the genome, and this also results in it being difficult to distinguish between them10. However, ILS derives from random retention of ancestral polymorphisms across different contemporaneous species10. Using both outgroup and population genomic data, HHS and ILS can be discerned based on discordant site patterns and frequencies9. Another challenge in identifying an ancient HHS event is how to exclude effects of homoplasy11, which may derive from random and/or convergent nucleotide mutations during long-term evolutionary histories12,13. Long indels (≥5 bp) extracted from well-assembled genomes can be used to effectively exclude such evolutionary homoplasy effects11,12.
Most previous HHS events have been found to have occurred between closely-related species14–16; they have rarely been reported between ancestors of different genera during their initial and ancient divergences17 and efficiently tested by multiple genomic data11. The genera Carpinus L. (comprised of sect. Eucarpinus Sarg. [=Carpinus] and sect. Distegocarpus [Sieb. et Zucc.] Sarg.) and Ostrya Scop. belong to the family Betulaceae (also known as the birch family)18. They contain a total of ~60 species of trees and shrubs19. In Betulaceae, nutlet bract morphologies are used to distinguish different genera19,20. The nutlets of Ostrya and sect. Carpinus are completely or rarely enclosed by bracts; however, those of sect. Distegocarpus are intermediate between them19. Three species are recognized within sect. Distegocarpus: C. cordata Blume, C. fangiana Hu, and C. japonica Blume18,19,21. Phylogenetic relationships among these higher taxonomic groups have hitherto remained unclear and highly debated22–24, with sampled species of sect. Distegocarpus being identified as closely related to sect. Carpinus or Ostrya. These phylogenetic contradictions have suggested evolutionary complexities among the three groups, resulting from introgression, ILS, or HHS. With the rapid growth of genomics, genomic data are capable of resolving such evolutionary uncertainties9–12.
In this study, we focus on a likely ancient HHS event between ancestors of the two genera, Carpinus and Ostrya. We generate a high-quality chromosome-level reference genome for C. viminea Lindley (from sect. Carpinus) and re-sequence 44 individuals from the genera Carpinus and Ostrya (comprising ten species of sect. Carpinus, all three species of sect. Distegocarpus, and seven species of Ostrya) to test alternative hypotheses for the evolutionary relationships among the three groups.
Results
Genome features and comparative genomic analyses
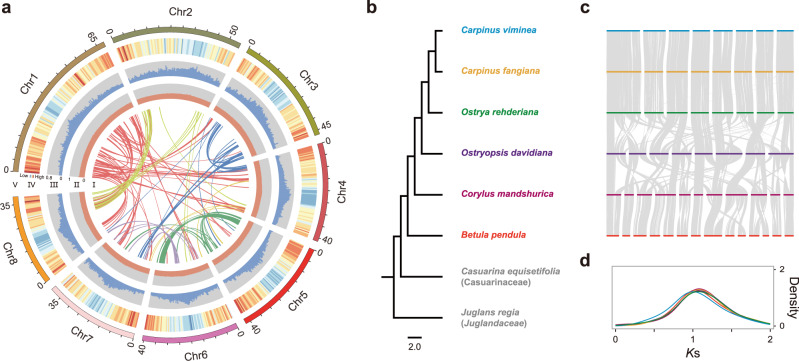
We assembled a chromosome-scale reference genome for C. viminea (Fig. 1a, Supplementary Figs. 2, 3, and Supplementary Tables 1–12; Supplementary Note 1) based on >110× Nanopore long reads, >150× Illumina short reads, and >170× Hi-C reads. We generated a high-continuity genome, with an assembly size of 372.7 Mb. A total of 242 contigs were anchored onto eight chromosomes, with contig and scaffold N50 of 4.3 Mb and 42.1 Mb respectively. A total of 26,621 protein-coding genes were predicted in the genome. We also improved our previously reported Ostrya rehderiana assembly25 to the chromosome-level using ~140× Hi-C reads (Supplementary Fig. 4 and Supplementary Tables 13–16; Supplementary Note 2). We firstly explored the phylogenetic relationship and chromosomal evolution of C. viminea, C. fangiana26, and O. rehderiana (as representatives of three lineages, sects. Carpinus and Distegocarpus, and Ostrya) with the other species in the Betulaceae (Supplementary Note 3). These three species formed a monophyletic clade, with Ostryopsis davidiana5, Corylus mandshurica27, and Betula pendula28 from other three genera as the other three separate clades (Fig. 1b). The genomes of these three species were found to be highly conserved with no change in chromosome number and independent whole-genome duplication (WGD) event and few structural variations (Fig. 1c, d and Supplementary Fig. 5). However, numerous structural variations were detected between Ostryopsis and these three species, suggesting their distinct relationship (Fig. 1c).
Fig. 1. Genome features of Carpinus viminea and comparative genomic analyses with other species of the same family Betulaceae.
a Genome features of the C. viminea assembly, including the synteny information (I), GC (guanine-cytosine) content (II), repeat density (III), gene density (IV), and genome chromosomes (V). b Phylogenomic tree of six Betulaceae species based on the concatenated 4DTv sites. The topology was generated by RAxML, with Casuarina equisetifolia and Juglans regia as outgroups. The bootstrap values for each node were all 100. c MCScanX identified synteny blocks between six Betulaceae species. The chromosome colors correspond to those in (b). d Ks distributions within each Betulaceae species. The colors of each species correspond to those in (b). Source data are provided as a Source Data file.
Phylogenetic analyses and highly inconsistent gene trees
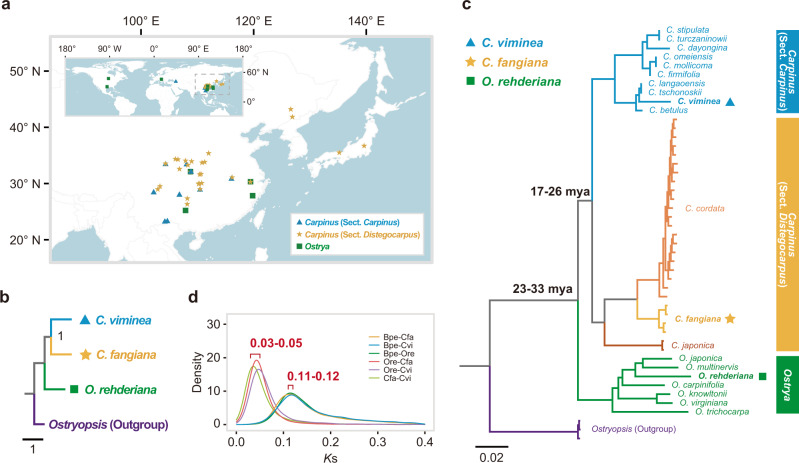
We then focused our study on three representative species (C. viminea, C. fangiana, and O. rehderiana) and further 44 re-sequenced individuals for ten species of sect. Carpinus, three species of sect. Distegocarpus, and seven species of Ostrya covering all major distributions of these three lineages (Fig. 2a and Supplementary Data 2). A total of 7468 strictly orthologous nuclear gene groups (1:1:1:1) were identified between C. viminea, C. fangiana, O. rehderiana, and the outgroup (Ostryopsis). After removing those with short aligned regions (<300 bp) or low alignment ratios (<50%), 6321 orthologous groups were used to generate a coalescent-based species tree, in which O. rehderiana was sister to a clade consisting of C. viminea and C. fangiana (Fig. 2b), consistent with the phylogenomic species tree based on the concatenated 4DTv sites (Fig. 1b). Population genomic data from the three lineages were then used to reconstruct their phylogeny. After assessment of the results, the C. viminea genome was used as the reference for producing more genetic variants (Supplementary Data 3). For each of the 44 re-sequenced samples, an average of ~11.0 Gb (>25×) clean bases were mapped to the reference genome, with an average mapping ratio of 89.3% and a > 20× mapping depth, covering >77% of the reference genome (Supplementary Data 4; Supplementary Note 5). Around 6.2 Mb high-quality biallelic SNPs and 443,792 indels were obtained (Supplementary Note 5). The maximum likelihood (ML) tree (Fig. 2c) of all re-sequenced individuals supported the monophyly of each lineage and agreed with the coalescent-based species tree (Fig. 2b). The times of divergence between the three lineages were dated from Ks distributions for the representative species (C. viminea, C. fangiana, O. rehderiana, and Betula pendula as outgroup) based on a secondary calibration (Fig. 2d; Supplementary Note 4). The genera Ostrya and Carpinus were estimated to have diverged at 23–33 million years ago (mya) and the divergence time between sects. Carpinus and Distegocarpus was dated to 17–26 mya (Fig. 2c, d).
Fig. 2. Sample locations and phylogenetic analyses.
a Geographic distributions of sampling locations for three lineages. b Coalescent-based species tree reconstructed using 6321 strictly orthologous nuclear gene groups by ASTRAL. The support values are estimated by local posterior probability. c Phylogeny of all re-sequenced samples based on analyses of nuclear SNPs. The bootstrap values for interspecific nodes were all 100. d Ks distributions between members of each pair of species. Betula pendula (Bpe) was used as the outgroup to date the times of divergence between it and Ostrya rehderiana (Ore), Carpinus viminea (Cvi), and C. fangiana (Cfa). The estimated divergence times are shown in (c). Source data are provided as a Source Data file.
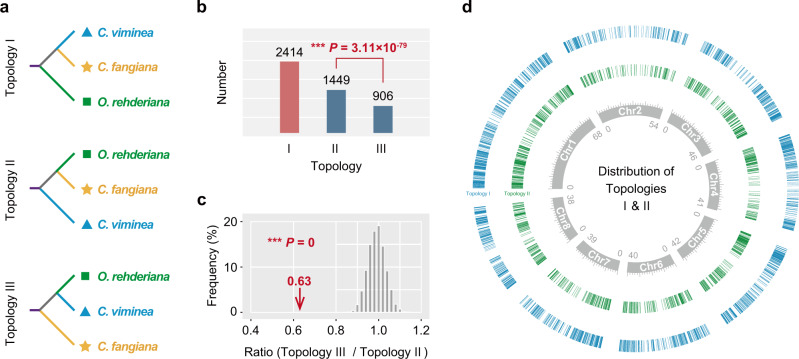
Then we explored the gene topologies of the three representative species, C. viminea, C. fangiana, and O. rehderiana. Phylogenies based on 4DTv sites from a total of 4769 ortholog groups with high-confidence support values (≥50) were obtained, comprised of three topologies (Fig. 3a, b; Supplementary Note 4). The most common tree (from 2414 ortholog groups) recovered C. fangiana as sister to C. viminea (Topology I). However, 1449 ortholog groups indicated that C. fangiana was sister to O. rehderiana (Topology II), significantly more (P = 3.11 × 10−79) than the number for topology III (906), in which C. fangiana clustered as a separate lineage. The unequal proportions of the three topologies suggest a likely hybrid origin for C. fangiana, since the latter two (Topologies II and III) would be expected to be nearly equal under a solely ILS scenario11,29. To further examine these significant differences, we simulated the gene trees and the proportion of each topology under the effects of ILS (Supplementary Note 4). The solely ILS hypothesis was strongly rejected due to a significant difference (P = 0) between observed and simulated ratios (Topology III/Topology II) (Fig. 3c). The genome-wide even distribution of Topologies I and II may further confirm the HHS hypothesis and exclude the solely introgression hypothesis (Fig. 3d and Supplementary Table 17).
Fig. 3. High incongruence in gene-tree phylogenies.
a, b Phylogenetic topologies (a) and the corresponding numbers (shown on the top of bar charts in (b) revealed from the phylogenies of 4769 strictly orthologous nuclear gene pairs with high-confidence support values (≥50). The number for Topology II was significantly more (P = 3.11 × 10−79) than that for Topology III. c Simulations under solely ILS scenario. The red arrow indicates the observed ratio (Topology III/Topology II) from 4769 ortholog groups (see in b). The gray bars are a histogram of the ratios obtained in 10,000 simulated datasets. The solely ILS hypothesis was strongly rejected due to a significant difference (P = 0) between observed and simulated ratios. d Genome-wide distribution of Topologies I (blue bars) and II (green bars). Statistical significance was determined by a two-tailed one-sample binomial test (b) and a two-tailed one-sample Student’s t test (c). Significant differences are indicated with asterisks (***P < 0.001). Source data are provided as a Source Data file.
HHS test based on long indels from population genomic data
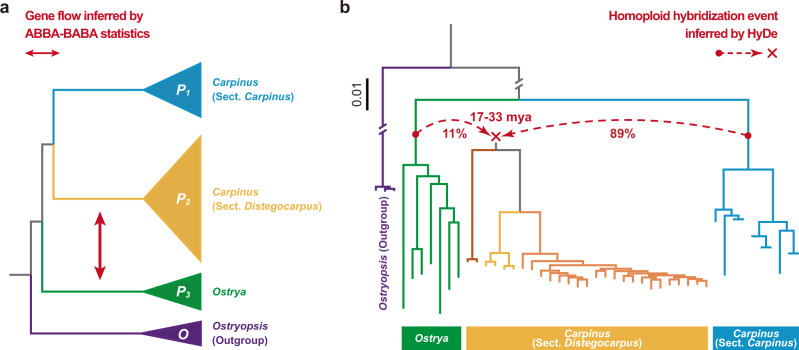
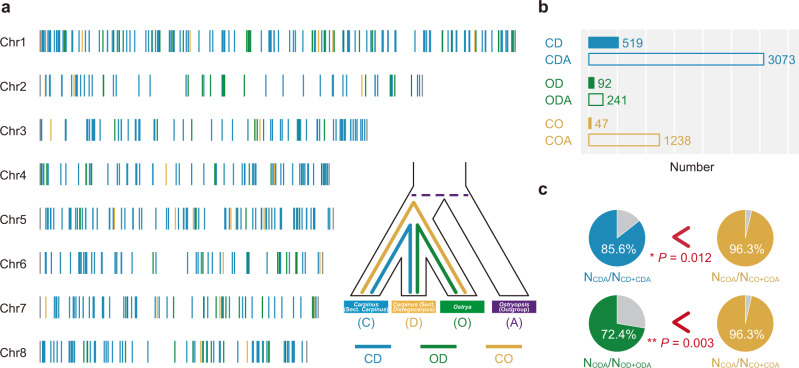
Introgression usually leads to genetic mixture in a few individuals or populations, rather than in all hybrid offspring as does HHS2,5 (Supplementary Fig. 1 and Supplementary Data 1). We used population genomic data from the three lineages to verify the occurrence of an HHS event between the ancestors of the two assumed parental lineages before species diversification in each lineage. According to previous studies, indels (especially those ≥5 bp) were a type of homoplasy-free markers, which could minimize the interference from random and convergent nucleotide mutations during the long-term evolutionary histories of the three lineages11–13. Based on long indels (≥5 bp) from population genomic data, ABBA-BABA test (D-statistic)30 and HyDe31 were used to examine the likelihood of ancient hybridization following two previous methods9,16 (Supplementary Note 6). These methods have been verified as being powerful for testing hybridization with the occurrence of ILS as a basis for shared polymorphisms29,31,32. When population genomic data are used, such methods can further distinguish HHS from introgression based on whether a hybridization signal is present in all hybrid offspring. The ABBA-BABA test indicated significant gene flow between sect. Distegocarpus and Ostrya (D = 0.16, Z = 15.68, P = 0; Fig. 4a) because of ancient hybridization rather than ILS only. HyDe also supported the hybrid origin of sect. Distegocarpus (Z = 4.00, P = 3.16 × 10−5), with a major genomic contribution (89%) from sect. Carpinus and a minor contribution (11%) from Ostrya (Fig. 4b). The hybridization event might have occurred around 17–33 mya, based on dating of the divergences of the representative species for ancestral lineages (Figs. 2c, d, 4b). We further extracted a total of 60,487 inter-group fixed long indels (≥5 bp) to exclude the ILS only and introgression hypotheses. Ancestral variations (AVs) and phylogenetically informative variations (PIVs) (reflecting the true evolutionary relationship) were detected between the members of each pair of selected lineages (Fig. 5a; Supplementary Note 6). If significant PIV signals could be detected in both the group consisting of sects. Carpinus and Distegocarpus (termed “CD”) and the group comprising Ostrya and sect. Distegocarpus (“OD”), the hybridization hypothesis would be significantly favored. We found that PIVs shared by “CD” were the most abundant (519), followed by those shared by “OD” (92), in both cases being evenly distributed across the genome (Fig. 5a, b). The significant PIV signals for both “CD” and “OD” at the population level clearly support the hybrid origin of sect. Distegocarpus (Fig. 5c; Supplementary Note 6). In addition, these abundant even-distributed long indels (≥5 bp) exclude the possibility of convergent homoplasy and introgression.
Fig. 4. ABBA-BABA test and HyDe analysis using indels from population genomic data.
a ABBA-BABA test revealed the ancient gene flow between Ostrya and sect. Distegocarpus. Information for the lineages used was shown in the figure. b Homoploid hybrid origin of sect. Distegocarpus inferred by HyDe analysis. The red arrows (indicating the putative parents and hybrid lineages) and the red numbers (genomic contribution) indicate the results produced by HyDe. The time of homoploid hybrid origin was inferred by the previously dated divergence times between the three lineages (see in Fig. 2c, d). ABBA-BABA test and HyDe analysis were both performed at the population level, with the information from all individuals per population imputed together.
Fig. 5. PIVs across the genome.
a PIVs identified across the genome based on population genomic data. b Number of PIVs (“CD”, “OD”, and “CO”) and AVs (“CDA”, “ODA”, and “COA”) shared by different combinations of lineages. The exact numbers are shown on the right of bar charts. c Significant PIV signals were detected in both groups (P = 0.012 and 0.003 respectively), revealing the homoploid hybrid origin of sect. Distegocarpus. Statistical significance was determined by a Pearson’s Chi-square test with Yates’ correction for continuity. Significant differences are indicated with asterisks (*P < 0.05; **P < 0.01). Sect. Carpinus, sect. Distegocarpus, Ostrya, and Ostryopsis (outgroup) are identified as “C”, “D”, “O”, and “A”, respectively. Different combinations of the letters indicated PIVs (“CD”, “OD”, and “CO”) and AVs (“CDA”, “ODA”, and “COA”) shared by different combinations of the lineages. The different colors denote sect. Distegocarpus sharing indels (PIVs or AVs) with sect. Carpinus (“CD” or “CDA”, blue), Ostrya (“OD” or “ODA”, green), or sect. Carpinus sharing indels with Ostrya (“CO” or “COA”, yellow) as the graphic examples show. Source data are provided as a Source Data file.
Positively selected genes and hybrid signal
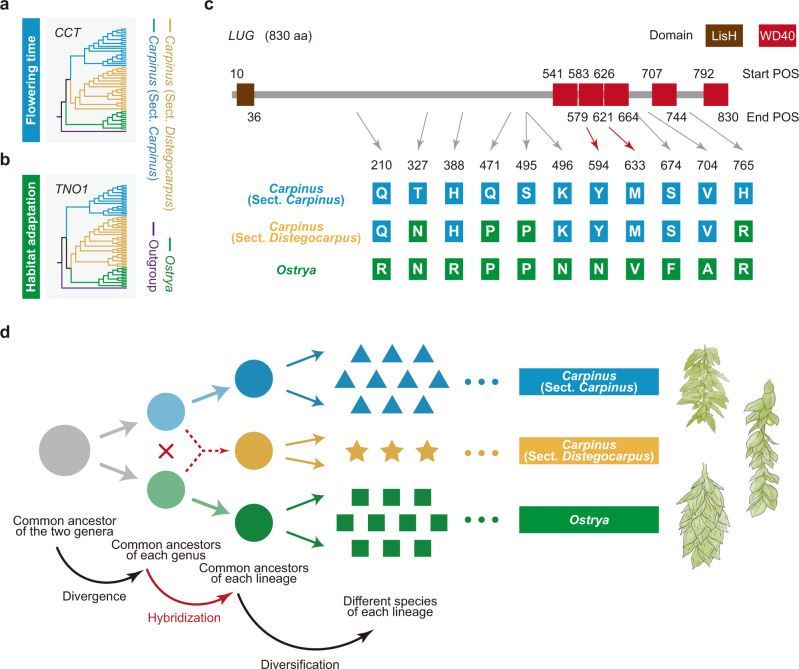
Finally, we explored whether hybridization contributed to RI and intermediate morphological trait of nutlet bract of sect. Distegocarpus. It has been suggested that the likely hybridization-contributed RI can further distinguish HHS from introgression an/or general genetic admixture3. Genes controlling prezygotic RI between parental lineages experienced rapid evolution because of natural selection and the diverged alleles might be alternately fixed in the hybrid offspring lineage5. We therefore identified positively selected genes (PSGs) in the hybrid lineage (which were shared and derived from each parental lineage) following Wang et al.5 (an integrated pipeline comprising HKA tests, non-synonymous mutations, and phylogenetic origin), which is the only pipeline hitherto developed to detect PSGs in HHS lineages and has been verified as effective by transgenic and common garden experiments (Supplementary Note 7). A total of 218 PSGs in sect. Distegocarpus were found to derive from sect. Carpinus, while 73 PSGs from Ostrya and functional analyses suggested that some of them are involved in flowering time and environmental adaptation, two crucial types of prezygotic RI in plant speciation process4,33,34 (Supplementary Data 5, 6). For example, a flowering time-related CCT gene35 in sect. Distegocarpus was derived from sect. Carpinus (Fig. 6a), while the TNO1 gene, which is involved in habitat adaptation36, originated from Ostrya (Fig. 6b). In addition, we identified 19 genes in sect. Distegocarpus, each of which contained alternative amino acid mutations fixed by each of the two parental lineages (Supplementary Data 7). One of such genes, LUG, was reported to regulate floral organ development37–39. The LUG gene of sect. Distegocarpus inherited respectively 7 or 4 amino acid mutations which have been fixed in sect. Carpinus or Ostrya (Fig. 6c). This is partly consistent with the intermediate nutlet bract of sect. Distegocarpus, because nutlets of Ostrya are completely enclosed by bracts, while those of sect. Carpinus are rarely enclosed by bracts19 (Fig. 6d). This recombinant LUG gene and others that experienced similar recombination due to hybridization may together have led to the intermediate nutlet bracts in sect. Distegocarpus.
Fig. 6. Selected PSGs involved in RI barriers and the LUG gene showing hybrid signals.
a, b Two selected RI-related PSGs of sect. Distegocarpus derived from sect. Carpinus (a) and Ostrya (b) respectively. ML trees were constructed based on haplotypes of protein sequences from all re-sequenced individuals. Each branch represents one identified haplotype. c The LUG gene showing hybrid signals because of hybrid recombination. All inter-lineage fixed amino acid differences were shown. d A simplified model of an ancient intergeneric HHS event between two genera and further species diversification. Representative morphologies of nutlet bracts from three lineages are shown on the right (Top: sect. Carpinus; middle: sect. Distegocarpus; bottom: Ostrya). In (a–c), the different background colors indicate the alleles (a, b) and mutations (c) of sect. Distegocarpus derived from sect. Carpinus (blue) and Ostrya (green).
Discussion
In this paper, we report a de novo genome sequence for a Carpinus species and 44 re-sequenced genomes for representative individuals from two sections of this genus and Ostrya. Our analyses of multiple genomic datasets revealed highly discordant gene topologies (Fig. 3 and Supplementary Table 17) between sect. Distegocarpus and sect. Carpinus and Ostrya, which may have resulted from homoplasy, ILS, introgression, or HHS. We found that numerous long indels across the whole genome showed such inconsistent relationships and were shared by sect. Distegocarpus with sect. Carpinus or Ostrya, a result that seems unlikely to be explained on the basis of evolutionary homoplasy11,12. We excluded ILS as an explanation of observed patterns based on an ABBA-BABA test (D-statistic)30 and HyDe analysis31 (Fig. 4), both of which have been proved to be powerful methods for detecting hybridization29,31,32. Under both ancient and recent introgression scenarios, only a few hybrid offspring in sect. Distegocarpus would be expected to contain genomic admixture from the presumed parents9 (Supplementary Data 1). However, we found that long indels (PIVs) specific to sect. Carpinus and to sect. Ostrya were present together and fixed in all sampled species and individuals of sect. Distegocarpus (Fig. 5a). The hybridization that led to a genomic admixture in all samples of sect. Distegocarpus is therefore likely to have occurred earlier than the further diversification of this section into three current species. In addition, the major distinction between introgression and HHS is whether a stable and distinct lineage had been established9. These findings seem to suggest that sect. Distegocarpus has not only evolved as an independent and stable lineage but also diversified into three present-day species.
In addition to genetic admixture, we found that the ancient hybridization appears to have directly created RI in sect. Distegocarpus, initiating its independent evolution, based on the following findings. First, we found that some genes responsible for critical prezygotic RI (e.g., flowering time and habitat adaptation) were derived from either one or the other of the two parental lineages (Fig. 6a, b and Supplementary Data 5, 6), especially those genes that had experienced strong selection pressure and showed signals of positive evolution. The hybrid recombination of these RI alleles probably could have rapidly developed prezygotic RI in hybrids at the initial HHS stage by giving rise to differences in flowering time and habitat adaptation5, although further functional testing of these alleles to RI is needed in the future. Second, the alternatively fixed long indels (PIVs in Fig. 5a) from the two parents in numerous loci across the whole genome could have also created immediate postzygotic RI based on Bateson-Dobzhansky-Muller (BDM) genetic incompatibility7. With these initial RIs created by hybridization, the overall RI between sect. Distegocarpus and each parental lineage could have accumulated continuously and been reinforced in the course of its independent evolution. Thus the three strict criteria for the HHS hypothesis appear to be met3. In addition, recombinant genes formed during HHS may have accounted for some of the intermediate morphological traits of sect. Distegocarpus (Fig. 6c, d). The origin of sect. Distegocarpus was dated at around 17–26 mya, only a few million years after the divergence of the two parental lineages about 23–33 mya. Sect. Distegocarpus therefore likely originated through HHS during the early divergence between Carpinus and Ostrya.
Phylogenetic discordance based on different genetic datasets has been widely reported for plants at higher taxonomic levels, from genus to family, order and angiosperm clades40–43. Because WGD has been found to occur in numerous specific lineages (e.g., core eudicot), allopolyploid hybrid speciation has sometimes been invoked to explain such discordant phylogenies44–46. However, our results suggest that HHS without WGD during the initial divergence of these higher taxonomic lineages may also lead to phylogenetic discordance. In the future, this alternative explanation could be considered and tested based on the increasingly available genomic resources.
Methods
Genome sequencing and assembly of C. viminea
Fresh leaves of a wild C. viminea individual were collected from Ya’an, Sichuan Province, China (102°45′E, 30°23′N). Total genomic DNA was extracted using the cetyltrimethyl ammonium bromide (CTAB) method47. For sequencing, the Nanopore sequencing library, paired-end Illumina library, and Hi-C library were constructed and then sequenced by a PromethION DNA sequencer (Oxford Nanopore, Oxford, UK), a MGISEQ-2000 platform (Illumina, San Diego, CA, USA), and an Illumina HiSeq 4000 platform (Illumina, San Diego, CA, USA), respectively. We also collected four fresh tissue samples (leaf, flower, bud, and twig) from the same C. viminea individual for total RNA sequencing. The Nanopore long reads were corrected using NextDenovo (https://github.com/Nextomics/NextDenovo) and de novo assembled using SmartDenovo (https://github.com/ruanjue/smartdenovo). The contigs were corrected and polished with the Illumina reads using Pilon48 for three rounds. HiC-Pro49 was then used to analyzed and assessed the Hi-C data. LACHESIS50 was employed to cluster, reorder, and orientate the corrected contigs into pseudochromosomes.
Genome annotation of C. viminea
RepeatMasker v.4.0.751 and RepeatModeler v.1.0.10 (http://www.repeatmasker.org) were used to annotate the repeat sequences. For gene prediction, PASA v.2.1.0 (Program to Assemble Spliced Alignments)52, Augustus53, and GeneWise v.2.4.154 were performed, respectively. EVidenceModeler (EVM) v.1.1.155 was then used to combine these results, which generated the final protein-coding gene set. For functional annotation, the predicted genes were searched against Swiss-Prot56, TrEMBL56, and NCBI non-redundant protein (NR)57 databases. InterProScan v.5.25-64.058, blast2GO v.4.159, and KEGG (Kyoto Encyclopedia of Genes and Genomes) Automatic Annotation Server (KAAS)60 were also performed, respectively.
Genome improvement of O. rehderiana
To further extend the contiguity of the O. rehderiana genome assembly25, we collected a fresh sample of O. rehderiana from Tianmu Mountain, Zhejiang Province, China (119°27′E, 30°20′N) for Hi-C sequencing and performed chromosome anchoring following the same pipeline as those for the C. viminea genome assembly.
Genome features and comparative genomic analyses
For C. viminea assembly, genome features were summarized by a 500 Kb non-overlapping sliding-window and then visualized by Circos61. We performed the comparative genomic analyses with the representative species of different Betulaceae genera, including C. viminea, C. fangiana26, O. rehderiana, Ostryopsis davidiana5, Corylus mandshurica27, and Betula pendula28. Casuarina equisetifolia62 and Juglans regia63 were used as the outgroups. OrthoFinder64 and PRANK65 were used to identify the strictly orthologous nuclear gene groups between the eight species (1:1:1:1:1:1:1:1) and aligned their coding sequences (CDS) respectively. RAxML66 was then used to construct the maximum likelihood (ML) tree using a concatenated matrix of 4DTv (fourfold synonymous transversion) sites. MCScanX67 was employed to identify the synteny blocks between the six Betulaceae species (involving ≥20 collinear genes) and within each of them (involving ≥5 collinear genes). LAST68 was also used to perform the collinearity analysis between C. viminea, C. fangiana, and O. rehderiana based on their whole-genome sequences.
Hybridization test based on de novo genome sequences
We performed the hybridization tests between the three species, C. viminea, C. fangiana26, and O. rehderiana, with Ostryopsis davidiana5 as outgroup. OrthoFinder64 was employed to identify the strictly orthologous nuclear gene groups (1:1:1:1) based on the protein-coding sequences. For each group of genes, PRANK65 was used to align their coding sequences (CDS). Then, we extracted their 4DTv sites, neutral sites of a widely used type which are capable of minimizing interference due to biased selection. RAxML66 was employed to construct the ML tree for each gene group based on their concatenated 4DTv sites. ASTRAL69 was used to estimate the species tree under a multi-species coalescent model. The branch lengths of the species tree so generated were in coalescent units. According to the coalescent-based species tree produced, DendroPy70 was then applied to simulate the gene trees under the effects of ILS. Finally, to infer the time scale of the hybridization event, we estimated the times of divergence based on the distributions of Ks (synonymous substitutions per synonymous site) values and a secondary calibration.
Population materials
To explore more genetic detail, we sampled 47 individuals (including a total of 21 species) from all of three lineages for population genomic resequencing, including ten individuals (from ten species) of sect. Carpinus, 27 individuals (from three species) of sect. Distegocarpus, seven individuals (from seven species) of Ostrya, and three individuals (from one species) of Ostryopsis as the outgroup. Except for the outgroup, all sampled individuals were selected from different populations (one individual per population). The samples of sect. Carpinus and Ostrya covered all acknowledged species of these two lineages, respectively. The samples of sect. Distegocarpus covered all three species described for this lineage and almost all samples were collected in natural field.
Genome resequencing, read mapping, and variants calling
For each sample, total genomic DNA was extracted by the CTAB method47. Illumina paired-end reads were produced by Illumina HiSeq platform (Illumina, San Diego, CA, USA). The filtered reads were then mapped to the reference genome, the genome of C. viminea, by BWA-MEM71 with recommended parameters. SAMtools71 was used to sort the mapped reads and further remove the duplicated ones. The generated BAM files were used for variant calling based on the pipeline corresponding to GATK best practice. GATK HaplotypeCaller72 was first performed to detect the variants for each sample. Then, the variants identified for each sample were merged by GATK GenotypeGVCFs72. After a set of robust filtering, we obtained a high-quality SNP set, containing 6,244,030 biallelic SNPs with the outgroup and 6,302,136 biallelic SNPs without the outgroup. We also identified a total of 443,792 long indels (≥5 bp) (with the outgroup) for subsequent analyses.
Hybridization test based on population genomic data
To explore the hybridization event in greater depth, we employed hybridization tests using the population genomic data. The population-level phylogeny was constructed using RAxML66 using the previously generated 6,244,030 biallelic SNPs. The other analyses were performed based on the previously identified 443,792 long indels (≥5 bp). First, HyDe31 was performed to test whether a hybrid origin scenario was supported. The individuals were classified into four groups: sect. Carpinus, sect. Distegocarpus, Ostrya, and Ostryopsis (as the outgroup). Then, Dsuit73 was used to perform the ABBA-BABA test (D-statistic)30, with sect. Carpinus, sect. Distegocarpus, Ostrya, and Ostryopsis as P1, P2, P3, and O, respectively. HyDe and ABBA-BABA tests were performed at the population level, with the information from all individuals per population being input together. Finally, to test the ancient HHS scenario, we modified the approach developed by Jiang et al.11 and applied it to the population-level data (Supplementary Note 6). We identified the AVs and the PIVs across the genome and further detected the significant PIV signals. AVs and PIVs were classified based on their times of occurrence11. AVs occurred before the differentiation of all species. PIVs occurred after the first species differentiated and before the last one. If significant PIV signals can be detected between the assumed hybrid species and each of the parental species, the HHS assumption is validated and introgression and ILS can be excluded.
Positively selected genes and hybrid signals detected on genes
We identified PSGs and genes harboring hybrid signals because of hybrid recombination based on the re-sequenced individuals (using the previously identified 6,302,136 biallelic SNPs without an outgroup), respectively. PSGs were identified following the methods in Wang et al.5 (Supplementary Note 7), where all final PSGs must conformed to three criteria: (1) significant P values (≤0.01) in HKA tests74; (2) the number of fixed non-synonymous mutation sites ranked in the top 2.5% of all genes tested; (3) phylogenies with sect. Distegocarpus individuals deriving mainly from one parental lineage. The hybrid signals were detected according to whether the genes were positively selected with both grouping methods: sects. Distegocarpus (hybrid lineage) and Carpinus as one group compared with Ostrya, and sect. Distegocarpus and Ostrya as one group compared with sect. Carpinus. When detecting hybrid signals, only two criteria (a significant P value in an HKA test and the number of fixed non-synonymous mutation sites ranking in the top 2.5%) were considered, taking no account of phylogenies.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported equally by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31000000), National Natural Science Foundation of China (grant numbers 31590821 and 91731301) and the National Key Research and Development Program of China (2017YFC0505203), and also by Fundamental Research Funds for the Central Universities (SCU2019D013 and 2020SCUNL207), and National High-Level Talents Special Support Plan (10 Thousand of People Plan).
Source data
Author contributions
J.Liu designed and led the project. Z.W. conducted the research. C.C. collected the materials. Z.W. prepared DNA for sequencing and analyzed the data. M.K. contributed to genome assembling and prediction. J. Li, Z.Z., Y.W., and Y.Y. provided technical support. Z.W. wrote the manuscript. J.Liu revised the paper. Z.W. and M.K. contributed equally.
Peer review
Peer review information
Nature Communications thanks Richard Buggs and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All the sequencing data have been deposited in the National Genomics Data Center (https://ngdc.cncb.ac.cn). The sequencing data for the de novo genome of Carpinus viminea (including the ONT long reads, Illumina reads of WGS, Hi-C reads, and transcriptomes) have been deposited under the accession number PRJCA005724. The Hi-C sequencing data for Ostrya rehderiana have been deposited under the accession number PRJCA005717. The resequencing data for all individuals have been deposited under the accession numbers PRJCA003130 and PRJCA005842. The genome assemblies and annotations of C. viminea and O. rehderiana have been uploaded to figshare [10.6084/m9.figshare.14988777]. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zefu Wang, Minghui Kang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29643-4.
References
- 1.Lotsy, J. P. Evolution by Means of Hybridization (Martinus Nijhoff, 1916).
- 2.Abbott RJ, et al. Hybridization and speciation. J. Evol. Biol. 2013;26:229–246. doi: 10.1111/j.1420-9101.2012.02599.x. [DOI] [PubMed] [Google Scholar]
- 3.Schumer M, Rosenthal GG, Andolfatto P. How common is homoploid hybrid speciation? Evolution. 2014;68:1553–1560. doi: 10.1111/evo.12399. [DOI] [PubMed] [Google Scholar]
- 4.Payseur BA, Rieseberg LH. A genomic perspective on hybridization and speciation. Mol. Ecol. 2016;25:2337–2360. doi: 10.1111/mec.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang ZF, et al. Hybrid speciation via inheritance of alternate alleles of parental isolating genes. Mol. Plant. 2021;14:208–222. doi: 10.1016/j.molp.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Müntzing A. Outlines to a genetic monograph for the genus Galeopsis: with special reference to the nature and inheritance of partial sterility. Hereditas. 1930;13:185–341. doi: 10.1111/j.1601-5223.1930.tb02522.x. [DOI] [Google Scholar]
- 7.Schumer M, Cui R, Rosenthal GG, Andolfatto P. Reproductive isolation of hybrid populations driven by genetic incompatibilities. Plos. Genet. 2015;11:e1005041. doi: 10.1371/journal.pgen.1005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor SA, Larson EL. Insights from genomes into the evolutionary importance and prevalence of hybridization in nature. Nat. Ecol. Evol. 2019;3:170–177. doi: 10.1038/s41559-018-0777-y. [DOI] [PubMed] [Google Scholar]
- 9.Kong S, Kubatko LS. Comparative performance of popular methods for hybrid detection using genomic data. Syst. Biol. 2021;70:891–907. doi: 10.1093/sysbio/syaa092. [DOI] [PubMed] [Google Scholar]
- 10.Goulet BE, Roda F, Hopkins R. Hybridization in plants: old ideas, new techniques. Plant Physiol. 2016;173:65–78. doi: 10.1104/pp.16.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang YF, et al. Differentiating homoploid hybridization from ancestral subdivision in evaluating the origin of the D lineage in wheat. N. Phytol. 2020;228:409–414. doi: 10.1111/nph.16578. [DOI] [PubMed] [Google Scholar]
- 12.Rokas A, Holland P. Rare genomic changes as a tool for phylogenetics. Trends Ecol. Evol. 2000;15:454–459. doi: 10.1016/S0169-5347(00)01967-4. [DOI] [PubMed] [Google Scholar]
- 13.Bapteste E, Philippe H. The potential value of indels as phylogenetic markers: position of trichomonads as a case study. Mol. Biol. Evol. 2002;19:972–977. doi: 10.1093/oxfordjournals.molbev.a004156. [DOI] [PubMed] [Google Scholar]
- 14.Mavárez J, et al. Speciation by hybridization in Heliconius butterflies. Nature. 2006;441:868–871. doi: 10.1038/nature04738. [DOI] [PubMed] [Google Scholar]
- 15.Lamichhaney S, et al. Rapid hybrid speciation in Darwin’s finches. Science. 2018;359:224–228. doi: 10.1126/science.aao4593. [DOI] [PubMed] [Google Scholar]
- 16.Zhang BW, et al. Phylogenomics reveals an ancient hybrid origin of the Persian walnut. Mol. Biol. Evol. 2019;36:2451–2461. doi: 10.1093/molbev/msz112. [DOI] [PubMed] [Google Scholar]
- 17.Guo X, Thomas DC, Saunders RMK. Gene tree discordance and coalescent methods support ancient intergeneric hybridisation between Dasymaschalon and Friesodielsia (Annonaceae) Mol. Phylogenet. Evol. 2018;127:14–29. doi: 10.1016/j.ympev.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Winkler, H. Betulaceae. In: Pflanzenreich IV (Verlag von Wilhelm Engelmann, 1904).
- 19.Li, P. Q. & Skvortsov, A. K. Betulaceae. In: Flora of China (Science Press & Missouri Botanical Garden Press, 1999).
- 20.Crane PR. Betulaceous leaves and fruits from the British Upper Palaeocene. Bot. J. Linn. Soc. 1981;83:103–136. doi: 10.1111/j.1095-8339.1981.tb01224.x. [DOI] [Google Scholar]
- 21.Li, P. Q. & Cheng, S. X. Betulaceae. In: Flora Reipublicae Popularis Sinicae (Science Press, 1979).
- 22.Yoo KO, Wen J. Phylogeny and biogeography of Carpinus and subfamily Coryloideae (Betulaceae) Int. J. Plant Sci. 2002;163:641–650. doi: 10.1086/340446. [DOI] [Google Scholar]
- 23.Li JH. Sequences of low-copy nuclear gene support the monophyly of Ostrya and paraphyly of Carpinus (Betulaceae) J. Sys. Evol. 2008;46:333–340. [Google Scholar]
- 24.Yang XY, et al. Plastomes of Betulaceae and phylogenetic implications. J. Sys. Evol. 2019;57:508–518. doi: 10.1111/jse.12479. [DOI] [Google Scholar]
- 25.Yang YZ, et al. Genomic effects of population collapse in a critically endangered ironwood tree Ostrya rehderiana. Nat. Commun. 2018;9:5449. doi: 10.1038/s41467-018-07913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang XY, et al. A chromosome-level reference genome of the hornbeam, Carpinus fangiana. Sci. Data. 2020;7:24. doi: 10.1038/s41597-020-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, et al. The Corylus mandshurica genome provides insights into the evolution of Betulaceae genomes and hazelnut breeding. Hortic. Res. 2021;8:54. doi: 10.1038/s41438-021-00495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salojärvi J, et al. Genome sequencing and population genomic analyses provide insights into the adaptive landscape of silver birch. Nat. Genet. 2017;49:904–912. doi: 10.1038/ng.3862. [DOI] [PubMed] [Google Scholar]
- 29.Tajima F. Evolutionary relationship of DNA-sequences in finite populations. Genetics. 1983;105:437–460. doi: 10.1093/genetics/105.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durand EY, Patterson N, Reich D, Slatkin M. Testing for ancient admixture between closely related populations. Mol. Biol. Evol. 2011;28:2239–2252. doi: 10.1093/molbev/msr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blischak PD, Chifman J, Wolfe AD, Kubatko LS. HyDe: a Python package for genome-scale hybridization detection. Syst. Biol. 2018;67:821–829. doi: 10.1093/sysbio/syy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubatko LS, Chifman J. An invariants-based method for efficient identification of hybrid species from large-scale genomic data. BMC Evol. Biol. 2019;19:112. doi: 10.1186/s12862-019-1439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baack E, Melo MC, Rieseberg LH, Ortiz-Barrientos D. The origins of reproductive isolation in plants. N. Phytol. 2015;207:968–984. doi: 10.1111/nph.13424. [DOI] [PubMed] [Google Scholar]
- 34.Sobel JM, Chen GF. Unification of methods for estimating the strength of reproductive isolation. Evolution. 2014;68:1511–1522. doi: 10.1111/evo.12362. [DOI] [PubMed] [Google Scholar]
- 35.Imura Y, et al. CRYPTIC PRECOCIOUS/MED12 is a novel flowering regulator with multiple target steps in Arabidopsis. Plant Cell Physiol. 2012;53:287–303. doi: 10.1093/pcp/pcs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S-J, Bassham DC. TNO1 is involved in salt tolerance and vacuolar trafficking in Arabidopsis. Plant Physiol. 2011;156:514–526. doi: 10.1104/pp.110.168963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F, et al. Control of leaf blade outgrowth and floral organ development by LEUNIG, ANGUSTIFOLIA3 and WOX transcriptional regulators. N. Phytol. 2019;223:2024–2038. doi: 10.1111/nph.15921. [DOI] [PubMed] [Google Scholar]
- 38.Liu ZC, Franks RG, Klink VP. Regulation of gynoecium marginal tissue formation by LEUNIG and AINTEGUMENTA. Plant Cell. 2000;12:1879–1891. doi: 10.1105/tpc.12.10.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sitaraman J, Bui M, Liu Z. LEUNIG_HOMOLOG and LEUNIG perform partially redundant functions during Arabidopsis embryo and floral development. Plant Physiol. 2008;147:672–681. doi: 10.1104/pp.108.115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CL, et al. Phylotranscriptomics reveals extensive gene duplication in the subtribe Gentianinae (Gentianaceae) J. Sys. Evol. 2021;59:1198–1208. doi: 10.1111/jse.12651. [DOI] [Google Scholar]
- 41.Morales-Briones DF, et al. Disentangling sources of gene tree discordance in phylogenomic data sets: testing ancient hybridizations in Amaranthaceae s.l. Syst. Biol. 2021;70:219–235. doi: 10.1093/sysbio/syaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YZ, et al. Prickly waterlily and rigid hornwort genomes shed light on early angiosperm evolution. Nat. Plants. 2020;6:215–222. doi: 10.1038/s41477-020-0594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stull GW, et al. Gene duplications and phylogenomic conflict underlie major pulses of phenotypic evolution in gymnosperms. Nat. Plants. 2021;7:1015–1025. doi: 10.1038/s41477-021-00964-4. [DOI] [PubMed] [Google Scholar]
- 44.Luo X, et al. Chasing ghosts: allopolyploid origin of Oxyria sinensis (Polygonaceae) from its only diploid congener and an unknown ancestor. Mol. Ecol. 2017;26:3037–3049. doi: 10.1111/mec.14097. [DOI] [PubMed] [Google Scholar]
- 45.Grover CE, et al. Re-evaluating the phylogeny of allopolyploid Gossypium L. Mol. Phylogenet. Evol. 2015;92:45–52. doi: 10.1016/j.ympev.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Edger PP, McKain MR, Bird KA, VanBuren R. Subgenome assignment in allopolyploids: challenges and future directions. Curr. Opin. Plant Biol. 2018;42:76–80. doi: 10.1016/j.pbi.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochem. Bull. 1987;19:11–15. [Google Scholar]
- 48.Walker BJ, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. Plos ONE. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Servant N, et al. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 2015;16:259. doi: 10.1186/s13059-015-0831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burton JN, et al. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat. Biotechnol. 2013;31:1119–1125. doi: 10.1038/nbt.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinf. 2004;5:4.10.1–4.10.14. doi: 10.1002/0471250953.bi0410s05. [DOI] [PubMed] [Google Scholar]
- 52.Haas BJ, et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 2003;31:5654–5666. doi: 10.1093/nar/gkg770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanke M, et al. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006;34:W435–W439. doi: 10.1093/nar/gkl200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birney E, Clamp M, Durbin R. GeneWise and genomewise. Genome Res. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haas BJ, et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008;9:R7. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bairoch A, Apweiler R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 2000;28:45–48. doi: 10.1093/nar/28.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marchler-Bauer A, et al. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225–D229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hunter S, et al. InterPro: the integrative protein signature database. Nucleic Acids Res. 2009;37:D211–D215. doi: 10.1093/nar/gkn785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conesa A, Götz S. Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genomics. 2008;2008:619832. doi: 10.1155/2008/619832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35:W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krzywinski M, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye G, et al. De novo genome assembly of the stress tolerant forest species Casuarina equisetifolia provides insight into secondary growth. Plant J. 2019;97:779–794. doi: 10.1111/tpj.14159. [DOI] [PubMed] [Google Scholar]
- 63.Marrano A, et al. High-quality chromosome-scale assembly of the walnut (Juglans regia L.) reference genome. GigaScience. 2020;9:giaa050. doi: 10.1093/gigascience/giaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emms DM, Kelly S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20:238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Löytynoja, A. Phylogeny-aware alignment with PRANK. In: Multiple Sequence Alignment Methods, Methods in Molecular Biology (Humana Press, 2014). [DOI] [PubMed]
- 66.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang YP, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kielbasa SM, Wan R, Sato K, Horton P, Frith MC. Adaptive seeds tame genomic sequence comparison. Genome Res. 2011;21:487–493. doi: 10.1101/gr.113985.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang C, Rabiee M, Sayyari E, Mirarab S. ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinform. 2018;19:153. doi: 10.1186/s12859-018-2129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sukumaran J, Holder MT. DendroPy: a Python library for phylogenetic computing. Bioinformatics. 2010;26:1569–1571. doi: 10.1093/bioinformatics/btq228. [DOI] [PubMed] [Google Scholar]
- 71.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McKenna A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malinsky M, Matschiner M, Svardal H. Dsuite—Fast D-statistics and related admixture evidence from VCF files. Mol. Ecol. Resour. 2021;21:584–595. doi: 10.1111/1755-0998.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hudson RR, Kreitman M, Aguadé M. A test of neutral molecular evolution based on nucleotide data. Genetics. 1987;116:153–159. doi: 10.1093/genetics/116.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All the sequencing data have been deposited in the National Genomics Data Center (https://ngdc.cncb.ac.cn). The sequencing data for the de novo genome of Carpinus viminea (including the ONT long reads, Illumina reads of WGS, Hi-C reads, and transcriptomes) have been deposited under the accession number PRJCA005724. The Hi-C sequencing data for Ostrya rehderiana have been deposited under the accession number PRJCA005717. The resequencing data for all individuals have been deposited under the accession numbers PRJCA003130 and PRJCA005842. The genome assemblies and annotations of C. viminea and O. rehderiana have been uploaded to figshare [10.6084/m9.figshare.14988777]. Source data are provided with this paper.