Abstract
Main cause of severe illness and death in COVID-19 patients appears to be an excessive but ineffectual inflammatory immune response that may cause severe acute respiratory distress syndrome (ARDS). Vitamin D may favour an anti-inflammatory environment and improve cytotoxic response against some infectious diseases. A multicenter, single-blind, prospective, randomized clinical trial was approved in patients with COVID-19 pneumonia and levels of 25-hydroxyvitamin D (25(OH)D) of 14.8 ng/ml (SD: 6.18) to test antiviral efficacy, tolerance and safety of 10,000 IU/day of cholecalciferol (vitamin D3) for 14 days, in comparison with 2000 IU/day. After supplementation, mean serum 25(OH)D levels increased to 19 ng/ml on average in 2000 IU/day versus 29 ng/ml in 10,000 IU/day group (p < 0.0001). Although levels of inflammatory cytokines were not modified by treatment with 10,000 IU/day, there was an increase of anti-inflammatory cytokine IL-10 and higher levels of CD4+ T cells, with predominance of T central memory subpopulation. Cytotoxic response against pseudotyped SARS-CoV-2 infected cells was increased more than 4-fold in patients who received 10,000 IU/day. Moreover, levels of IFNγ were significantly higher in this group. Beneficial effect of supplementation with 10,000 IU/day was also observed in participants who developed ARDS and stayed at the hospital for 8.0 days, whereas those who received 2000 IU/day stayed for 29.2 days (p = 0.0381). Administration of high doses of vitamin D3 as adjuvant of the standard care treatment during hospitalization for COVID-19 may improve the inflammatory environment and cytotoxic response against pseudotyped SARS-CoV-2 infected cells, shortening the hospital stay and, possibly, improving the prognosis.
Keywords: COVID-19, SARS-CoV-2, Vitamin D supplementation, Immune cytotoxic response
Graphical Abstract
1. Introduction
In December 2019, several viral pneumonia cases of unknown origin were detected in Wuhan City (Hubei Province, China), associated with an outbreak in a seafood market. Sequencing of the pathogen genome identified a new member of Coronaviridae family which was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus rapidly spread all over the world and the Coronavirus Disease 2019 (COVID-19) was declared a global public health threat by the World Health Organization on March 11th, 2020 [1].
COVID-19 may cause different clinical manifestations, from asymptomatic disease to severe acute respiratory distress syndrome (ARDS), and ultimately death [2]. The host receptor for SARS‐CoV‐2 entry into intestinal and alveolar cells is angiotensin converting enzyme 2 (ACE2), and the subsequent dysregulation of the renin‐angiotensin system (RAS) may lead to a massive cytokine activation, known as cytokine storm. This is a life-threatening syndrome characterized by high-level activation of immune cells and excessive production of a large number of inflammatory cytokines and chemical mediators [3], [4], ultimately resulting in prospective fatal ARDS. Individuals with severe presentations of COVID-19 show high levels of proinflammatory cytokines such as interleukin 6 (IL-6) and tumour necrosis factor alpha (TNFα) that may interfere with the cytotoxic response of Natural Killer (NK) and CD8 + T cells by reducing the production of granzymes and perforins [5], [6], as well as lymphopenia, mainly of CD4 + T lymphocytes and NK cells that also show an exhausted phenotype [7], [8]. Moreover, individuals with severe COVID-19 present an impaired antiviral immune response as a consequence of reduced levels of cytokines with antiviral activity such as interferons (IFN) [9].
For a long time, there was no clinically proven specific treatment for COVID-19 [10]. Supportive treatment, including oxygen and conservative fluid therapy, remains the most important strategy for patient management [2], [11]. Drugs designed for other viral infections (e.g. remdesivir), used in rheumatology (e.g. baricitinib) and humanized monoclonal IL-6R antibodies (e.g. tocilizumab) were some of the options that were assayed, but none proved to have a decisive beneficial effect [12], [13], except for unspecific treatments to control the excessive inflammatory immune responses such as corticosteroids [14]. Recently, FDA authorized the oral administration of molnupiravir for the treatment of mild-to-moderate COVID-19 in adults, as well as nirmatrelvir and ritonavir for children and adults, but only in case of emergency for those individuals with high risk to progress to severe disease [15], [16].
It is still undetermined why some patients show an asymptomatic disease whereas other individuals require invasive mechanical ventilation and admission to the Intensive Care Unit (ICU). The development of these severe and critical forms of COVID-19 have been associated with some predisposing factors such as obesity, hypertension and diabetes [17]. Other factor that has also been related to an increased risk of dying from COVID-19 is vitamin D deficiency. In fact, several reports have described an association between serum levels of 25-hydroxyvitamin D [25(OH)D] below 20 ng/ml (50nmol/L), and concomitant diseases, including systemic infection [18], [19], [20], [21]. Vitamin D exerts an immunomodulatory role by promoting the development of an anti-inflammatory environment. Besides, it has been described that vitamin D stimulates ACE2 that may bind to SARS-CoV-2, preventing it from attaching to ACE2 receptors and infecting the target cells [22], [23], [24]. Moreover, some studies described that vitamin D insufficiency during COVID-19 may compromise the respiratory immune response, thereby increasing the risk of severity and mortality [25], and that there is a correlation between the vitamin D levels and the severity of the disease [26], [27]. However, there is still no clear evidence that vitamin D supplementation can prevent the severity and/or mortality of COVID-19.
In this study, we evaluated whether vitamin D supplementation to avoid its insufficiency during COVID-19 may influence positively the disease progression by reducing the levels of pro-inflammatory cytokines and increasing the cytotoxic antiviral response. Therefore, we analyzed the changes in the immune response of individuals with COVID-19 who were recruited for a randomized clinical trial to receive a moderate dose of 2000 International Units (IU)/daily of cholecalciferol or a higher dose of 10,000 IU/daily for 14 days, in combination with the standard drug therapeutic regimen. The efficacy of both treatments at reducing the duration and severity of COVID-19 was analyzed.
2. Materials and methods
2.1. Study design and participants
This study was a multicenter, single blind, prospective, randomized clinical trial to evaluate the effect of cholecalciferol (vitamin D) supplementation in combination with the standard drug regimen therapeutic in patients hospitalized with pneumonia due to COVID-19. For ethical reasons, it was not felt possible to treat one arm with placebo because a potentially effective treatment would be denied to the patients. This study was sponsored by the Hospital Universitario Severo Ochoa (Madrid, Spain) and was conducted in other four Tertiary Care Hospitals in Spain: Hospital Universitario Infanta Leonor (Madrid), Hospital Universitario Fundación Alcorcón (Madrid), Hospital Universitario Príncipe de Asturias (Madrid), and Hospital Universitario de Cabueñes (Asturias) between June 2020 and March 2021.
The inclusion criteria for the participants in the study were being adults (>18 years old) hospitalized for at least seven days from the onset of COVID-19 symptoms, which is when usually began the inflammatory phase, with a diagnosis of pneumonia due to COVID-19 based on clinical-radiological criteria and a laboratory confirmed SARS-CoV-2 infection diagnosis, oxygen saturation < 94% and 25(OH)D serum levels < 30 ng/ml. Participants were excluded if they were lactating, pregnant women, were participating in other clinical trials with drugs with potential antiviral action for COVID-19, were on treatment with digoxin, had evidence of Multiple Organ Dysfunction Syndrome (MODS), were requiring mechanical ventilation at the time of inclusion, had hypersensitivity to cholecalciferol or the excipient refined olive oil, had hypercalcemia or hypercalciuria, were diagnosed with hereditary fructose intolerance, sarcoidosis or hyperparathyroidism, had glucose-galactose malabsorption or sucrose insufficiency or had chronic kidney disease (stage 4; estimated glomerular filtration rate (eGFR) < 30). Participants who were expected to be transferred to another Hospital Care Centre in the following 96 h were also excluded. A sample size of 41 evaluable participants in each group was estimated to have 90% power, with a 0.05 two-sided significance level, to detect a difference of 15 ng/ml in 25(OH)D serum levels between groups before and after the cholecalciferol supplementation. The randomization was done by blocks of 5 using the block randomization-sampling module with the user command “rndseq block” included on the STATA 14.2 software (StataCorp LLC, College Station, TX). Eligible patients were assigned randomly in a 1:1 ratio to receive either a supplementation of a dose of 10,000 IU of cholecalciferol or a dose of 2000 IU once daily for fourteen days. In both groups, participants were given the oral solution Thorens 25,000 IU/2.5 ml (Grupo Italfarmaco, Madrid, Spain). Throughout the study, all patients received any concomitant medication deemed necessary to provide adequate standard care, according to local recommendations.
Peripheral blood samples and clinical data were collected at baseline (day 0), day 7 and day 14. Patients were required to fulfil the treatment and follow up visits even if the hospital discharge occurred before day 14. Patient data were registered at the Research Electronic Data Capture tool (REDCap) online platform (developed by Vanderbilt University, Nashville, TN), which included demographics, respiratory status, radiological score, biochemical parameters, coexisting comorbidities and treatments. Serious adverse events, the onset of the symptoms, length of stay at the hospital (LOS), ARDS, ICU admission and mortality were also collected. The primary endpoint was the increase of 25(OH)D serum level ≥ 30 ng/ml after 14 days of supplementation (end of supplementation), without any related adverse events. The secondary endpoints were the LOS and, in a subgroup of patients, the analysis of changes in the plasma inflammatory profile and the cytotoxic immune response from baseline to end of the supplementation.
2.2. Ethical statement
The protocol (ID 01052020) for this study was developed under the Declaration of Helsinki and previously reviewed and approved by the Spanish Agency for Medicines and Health Products (AEMPS) (EudraCT Number 2020–002312–43), as well as by the Ethics Committee for Research involving Medicines (CEIm) of the Hospital Universitario Severo Ochoa (Madrid). All patients recruited for the study gave their written informed consent to participate in the study. The Spanish and European Data Protection Laws in force guarantee the confidentiality of the participants.
2.3. Cells
Whole blood was collected in EDTA Vacutainer tubes (Becton Dickinson, Madrid, Spain) and then treated by Ficoll-Hypaque (Pharmacia Corporation, North Peapack, NJ) density gradient centrifugation to isolate peripheral blood mononuclear cells (PBMCs) and plasma that were cryopreserved until the analysis. PBMCs were cultured in RPMI 1640 medium supplemented with 10% (v/v) fetal calf serum (FCS), 2 mM L-glutamine, 100 µg/ml streptomycin, 100 IU/ml penicillin (Lonza, Basel, Switzerland). Cells viability after thawing was assessed by flow cytometry. K562 (Human Caucasian chronic myelogenous leukaemia) cell line (ECACC 89121407) was kindly provided by Dr Cristina Eguizabal (Basque Centre Transfusions and Human Tissue, Álava, Spain). These cells were also cultured in RPMI 1640 medium supplemented with 10% FCS, 2 mM L-glutamine, 100 µg/ml streptomycin, 100 IU/ml penicillin. Vero E6 (African green monkey kidney) cell line (ECACC 85020206) was kindly provided by Dr. Antonio Alcami (CBM Severo Ochoa, Madrid) and was cultured in DMEM supplemented with 10% FCS, 2 mM L-glutamine and 100 IU/ml penicillin and streptomycin (Lonza).
2.4. Vitamin D detection
The quantification of the level of 25(OH)D (ng/ml) was performed from serum samples with the automated immunoassays Liaison 25(OH) Vitamin D Total assay DiaSorin Liaison XL (DiaSorin, Italy) in the participating hospitals, according to the manufacturer’s instructions.
2.5. Luminex assay
The detection and quantification of cytokines in plasma samples of the participants was performed using a customized Human Magnetic Luminex Assay kit (R&D Systems) panel for IL1β, IL2R, IL6, IL8, IL10, sCD14, TNFα, MIP-1α, MIP-1β, GM-CSF, IFNα, IFNβ, and IFNγ according to the supplier’s guidelines and recommendations. The analysis was performed on a Bio-Plex 200 System (Bio-Rad).
2.6. Antibodies and flow cytometry
For staining of cell surface markers, the following conjugated antibodies were used: CD3-APC (BD Biosciences; San Jose, CA), CD4-PercP (BD), CD8-APC-H7 (BD), CD16-PercP (BD), CD56-FITC (BD), CD107a-PE-Cy7 (BD), TCRγδ-PE (BioLegend, London) and CD158f/KIR2DL5-BV421 (R&D Systems) for analysis of CD4 + conventional T-cells (CD4 + T), CD8 + conventional T-cells (CD8 + T), NK, and NKT cell populations. CD4-PercP, CD25-PE-Cy5 and CD127-FITC (R&D Systems) for CD4 + natural regulatory T-cells (Treg) and CCR7-FITC (Biosciences) and CD45RA-PE-Cy7 (Biosciences) for CD4 + , CD8 + T cell memory subpopulations that were determined as follows: naïve (CD45RA+CCR7 +), central memory (TCM) (CD45RA-CCR7 +), effector memory (TEM) (CD45RA-CCR7-) and terminally differentiated effector memory (TEMRA) (CD45RA+CCR7-) cells. Samples were acquired on BD LSRFortessa X-20 flow cytometer (BD Biosciences, San Jose, CA) and analyzed using Flow Jo software v10.0.7 (Tree Star Inc., Ashland, OR, USA).
2.7. Direct NK cell-mediated cytotoxicity assay
The analysis of the NK-mediated direct cell-mediated cytotoxicity (DCC) of PBMCs of the participants was performed using the NK-sensitive target K562 cell line as previously described [9]. K562 cells are lymphoblasts isolated from an individual with chronic myeloid leukemia that are used as specific targets of NK cells due to they do not express HLA Class I. Briefly, K562 cells were stained with PKH26 Red Fluorescence Cell Linker kit (Sigma Aldrich-Merck) and co-cultured 1:1 for 1 h with PBMCs isolated from the patients. Cells were then collected and Annexin V conjugated with FITC (Thermofisher) was used to measure early apoptosis by flow cytometry. Data were acquired on BD LSRFortessa X-20 flow cytometer (BD Biosciences) and analyzed using Flow Jo software v10.0.7 (Tree Star Inc.).
2.8. Direct cell-mediated cytotoxicity assay against pseudotyped SARS-CoV-2-infected cells
In order to evaluate the specific antiviral DCC against SARS-CoV-2-infected cells of PBMCs from the participants in the study, the single-cycle pseudotyped virus pNL4–3Δenv_SARS-CoV-2-SΔ19(G614)_Ren was used to infect a monolayer of sensitive Vero E6 cells. This virus encodes SARS-CoV-2 spike glycoprotein, within the HIV-1 genome, as well as the Renilla luciferase gene generated as previously described [28], [29]. Briefly, Vero E6 cells were infected with pNL4–3Δenv_ SARS-CoV-2-SΔ19(G614)_Ren (100 ng p24 Gag/well) for 48 h. Cells were then washed and co-cultured for 1 h with PBMC from the participants at a ratio of 1:2. PBMCs were collected and the cytotoxic cell populations were analyzed by flow cytometry. Vero E6 monolayer was dissociated from the plate with trypsin-EDTA solution (Sigma Aldrich-Merck, Darmstadt, Germany), and the caspase-3 activity was quantified by luminescence using Caspase-Glo 3/7 Analysis system (Promega) as a measurement of induction of apoptosis by the cytotoxic cells.
2.9. Statistical analysis
Categorical data were expressed as percentages, while the median was used to describe the central tendency of the non-normally distributed numerical data. Group comparisons were done using Mann–Whitney U-test or ordinary one-way ANOVA and Tukey’s multiple comparisons test for numerical data and Chi-square (χ2) or Fisher’s exact test for categorical data. All statistical analyses and graphics were performed using the GraphPad Prism software version 8.4.3 (GraphPad Software Inc., San Diego, California). P-values lower than 0.05 (two-tailed) were considered to be statistically significant.
3. Results
3.1. Demographics and clinical characteristics of the participants
A total of 86 patients were recruited for this study. Eighty-five participants fulfilled the inclusion criteria and agreed to participate in the study, and one patient was excluded from the study due to not being able to meet the study’s schedule. After randomization, 41 patients received the supplementation of 10,000 IU/day (high dose) and 44 patients received 2000 IU/day (moderate dose) of vitamin D. All patients in both groups completed the entire study. A summary of patient demographics and clinical data of the groups are described in Table 1. The median age of the participants was 65.0 years (interquartile range [IQR]: 53.0–74.0), and it took a median of seven days (IQR: 6–10) from the onset of the symptoms of COVID-19 to the hospitalization. Most of them (70.6%) were men and, according to the body mass index (BMI) classification, 54.1% of the patients were obese. Additionally, the main coexisting conditions were hypertension (48.2%), dyslipidemia (36.5%), and diabetes (22.3%). Most of the participants (85.9%) showed bilateral pneumonia at x-rays. The most common symptoms of COVID-19 were malaise (94.2%), fever (84.7%), cough (75.3%), and dyspnea (65.9%) (Supplemental Table 1). In agreement with adequate randomization, we did not observe differences at baseline in demographic characteristics, vital signs, blood biochemistry data, distribution of ordinal scale scores, or treatments by group, although the levels of haemoglobin and bilirubin were significantly higher (p = 0.006 and p = 0.010, respectively) in the group of participants who received 10,000 IU/day (Supplemental Table 1).
Table 1.
Main demographics and clinical data of the participants in this study at baseline (day 0), before the administration of 2000 IU/day or 10,000 IU/day of cholecalciferol.
|
Supplementation group |
||||
|---|---|---|---|---|
| Characteristics | All | 2000 IU/day | 10,000 IU/day | p-value |
| (n = 85) | (n = 44) | (n = 41) | ||
| Age at diagnosis, median (IQR)— yr. | 65.0 (53.0–74.0) | 65.3 (44.0–72.3) | 67.0 (58.0–75.0) | 0.0665 |
| Time from onset of symptoms to hospitalization, median (IQR) — days | 7 (6–10) | 7 (6–9) | 7 (6–10) | 0.5440 |
| Gender | ||||
| Male— no. (%) | 60 (70.6) | 30 (68.2) | 30 (73.2) | 0.7901 |
| Female— no. (%) | 25 (29.4) | 14 (31.2) | 11 (26.8) | |
| Classification by BMI (kg/m2) | ||||
| Normal Weight (18.5–24.9) — no. (%) | 7 (8.2) | 4 (9.1) | 3 (7.3) | 0.7747 |
| Overweight (25.0–25.9) — no. (%) | 32 (37.6) | 15 (34.1) | 17 (41.5) | |
| Obesity (>30) — no. (%) | 46 (54.1) | 25 (56.8) | 21 (51.2) | |
| Smoker | ||||
| No — no. (%) | 57 (67.1) | 30 (68.2) | 27 (65.8) | 0.9260 |
| Former Smoker— no. (%) | 25 (29.4) | 13 (29.5) | 12 (29.3) | |
| Yes— no. (%) | 3 (3.5) | 1 (2.3) | 2 (4.9) | |
| Alcohol consumption | ||||
| No— no. (%) | 72 (84.7) | 38 (86.4) | 34 (82.9) | 0.8973 |
| Former drinker— no. (%) | 4 (4.7) | 2 (4.5) | 2 (4.9) | |
| Yes— no. (%) | 9 (10.6) | 4 (9.1) | 5 (12.2) | |
| Coexisting conditions | ||||
| Hypertension— no. (%) | 41 (48.2) | 18 (40.1) | 23 (56.1) | 0.2368 |
| Dyslipidemia— no. (%) | 31 (36.5) | 12 (27.3) | 19 (46.3) | 0.1097 |
| Diabetes— no. (%) | 19 (22.3) | 8 (18.2) | 11 (26.8) | 0.4866 |
| Pneumonia at Rx | ||||
| Unilateral — no. (%) | 12 (14.1) | 7 (15.1) | 5 (12.2) | 0.8574 |
| Bilateral— no. (%) | 73 (85.9) | 37 (84.1) | 36 (87.8) | |
| Blood biochemistry data | ||||
| Leucocytes, mean (SD)— mil/µL | 8.1 (4.3) | 7.3 (3.8) | 8.9 (4.7) | 0.0815 |
| Neutrophils, mean (SD)— mil/µL | 6.3 (4.0) | 5.7 (3.6) | 7.0 (4.4) | 0.1614 |
| Lymphocytes, mean (SD)— mil/µL | 1.2 (0.9) | 1.1 (0.5) | 1.4 (1.2) | 0.0646 |
| Treatments— no. (%) | ||||
| Dexamethasone | 56 (65.9) | 29 (65.9) | 27 (65.8) | 1 |
| Methylprednisolone | 21 (24.7) | 9 (20.45) | 12 (29.3) | 0.4903 |
| Tocilizumab | 21 (24.7) | 9 (20.45) | 12 (29.3) | 0.4903 |
| LMWH | 73 (85.9) | 36 (81.8) | 37 (90.2) | 0.4219 |
| Ceftriaxone | 50 (58.9) | 24 (54.5) | 26 (59.1) | 0.5421 |
| Azithromycin | 36 (42.35) | 20 (45.5) | 16 (39) | 0.7040 |
BMI, body mass index; IQR, interquartile range; LMWH, Low-molecular-weight heparin; no., number; SD, standard deviation; yr., year.
3.2. Maintenance of vitamin D serum levels
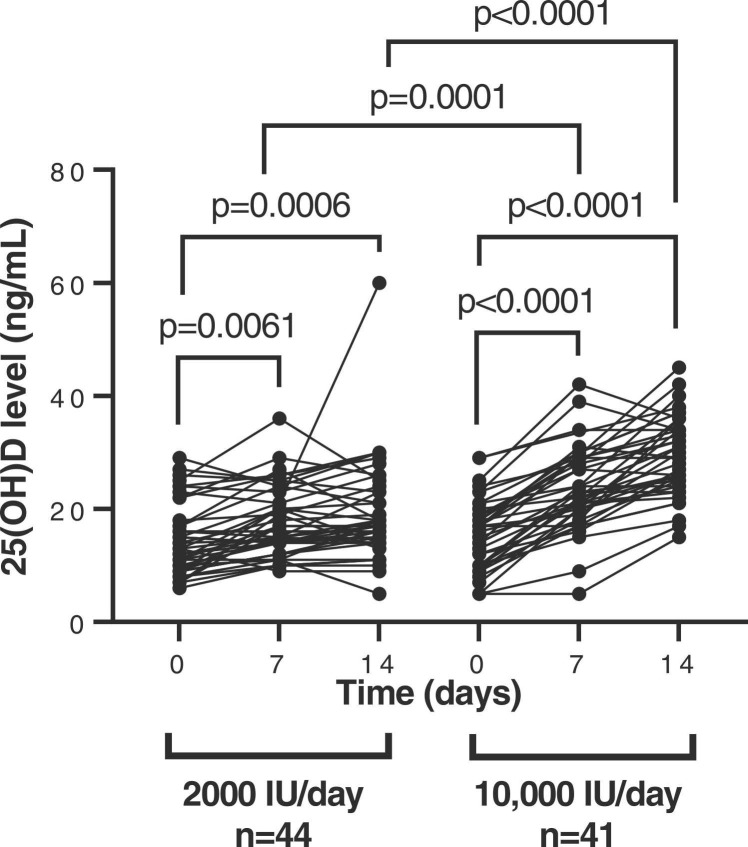
At the start of the study, mean serum 25(OH)D levels were 14 ng/ml (SD:6) in the group of patients who received 2000 IU/day and 15 ng/ml (SD: 6) in the group who received 10,000 IU/day. After seven days of supplementation, the increase in serum vitamin D levels was 1.31-fold (p = 0.0001) in the 10,000 IU/day group in comparison with the 2000 IU/day group, and after 14 days the levels were increased 1.53-fold (p < 0.0001) in the 10,000 IU/day group, in comparison with the 2000 IU group/day. Within the 2000IU/day group, serum vitamin D levels were increased 1.24- (p = 0.0061) and 1.33- (p = 0.0006) fold at days 7 and 14, respectively, whereas within the 10,000 IU/day group, serum vitamin D levels increased 1.51-fold (p < 0.0001) and 1.91-fold (p < 0.0001) after 7 and 14 days of supplementation, respectively ( Fig. 1). Ten (11.76%) participants of the study developed ARDS, six (13.64%) were assigned to the 2000 IU/day group (5 of 6 individuals of this group were admitted to the ICU) and four (9.76%) to the 10,000 IU/day group (2 of 4 individuals of this group were admitted to the ICU) but no significant differences were observed in the serum vitamin D levels of these participants between groups after seven and fourteen days of treatment. The quantification values of serum 25(OH)D of the participants measured at each time point of the study are shown in Table S2. After the supplementation, 9.09% (4/44) of the participants who received the moderate dose achieved serum 25(OH)D levels ≥ 30 ng/ml, while 39.02% (16/41) of the participants who received the high dose achieved the target 25(OH)D level of 30 ng/ml (p = 0.0027).
Fig. 1.
Quantification of 25(OH)D plasma levels. The levels of 25(OH)D (ng/ml) in serum samples of hospitalized patients with COVID-19 who received 2000 IU/day and 10,000IU/day of cholecalciferol was determined at baseline and after 7 and 14 days of treatment. Each dot corresponds to one sample. Statistical significance was calculated using Mann–Whitney U-test.
3.3. Differences in the length of hospital stay between groups
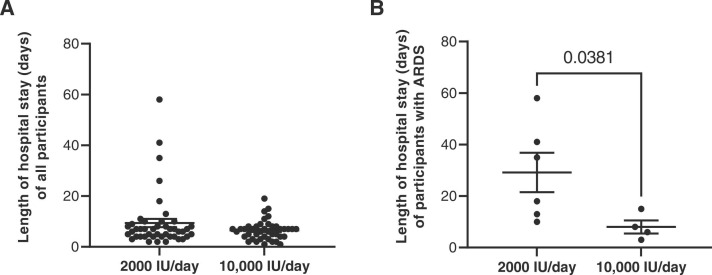
The participants who received 10,000 IU/day spent an average of 6.44 days in the hospital, while in the group of participants who received 2000 IU/day the average LOS was 9.36 days, but there was no significant difference between both groups ( Fig. 2A). The analysis of the LOS among the participants who developed ARDS showed that those participants (9.76%) who received the highest dose of vitamin D stayed at the hospital an average of 8.0 days (SD: 5.099), whereas those patients (13.6%) who received the moderate dose, stayed for an average of 29.2 days (SD: 18.76) (p = 0.0381) (Fig. 2B).
Fig. 2.
Length of hospital stay in patients treated with 2000 or 10,000 IU/day. (A) Total length of hospital stay (days) in hospitalized patients with COVID-19 who received 2000 IU/day and 10,000IU/day of cholecalciferol and (B) length of hospital stay (days) according to ARDS diagnosis. Each dot corresponds to one sample and lines represent mean ± standard error of the mean (SEM). Statistical significance was calculated using Mann–Whitney U-test.
3.4. Safety and mortality
During the study, 13 adverse effects were reported in the participants, of which 7 adverse effects occurred in the 2000 IU/day group and 8 adverse effects occurred in the 10,000 IU/day group. However, none of these adverse events was directly linked to the treatment with cholecalciferol. Nine participants (20.45%) experienced adverse events in the moderate dose group and 8 (19.51%) in the high dose group, with no statistically significant differences between both groups. One (2.27%) participant in the 2000 IU/day group and one (2.44%) participant in the 10,000 IU/day group died as a result of COVID-19 complications during the study.
3.5. Changes in the cytokine profile during treatment with vitamin D
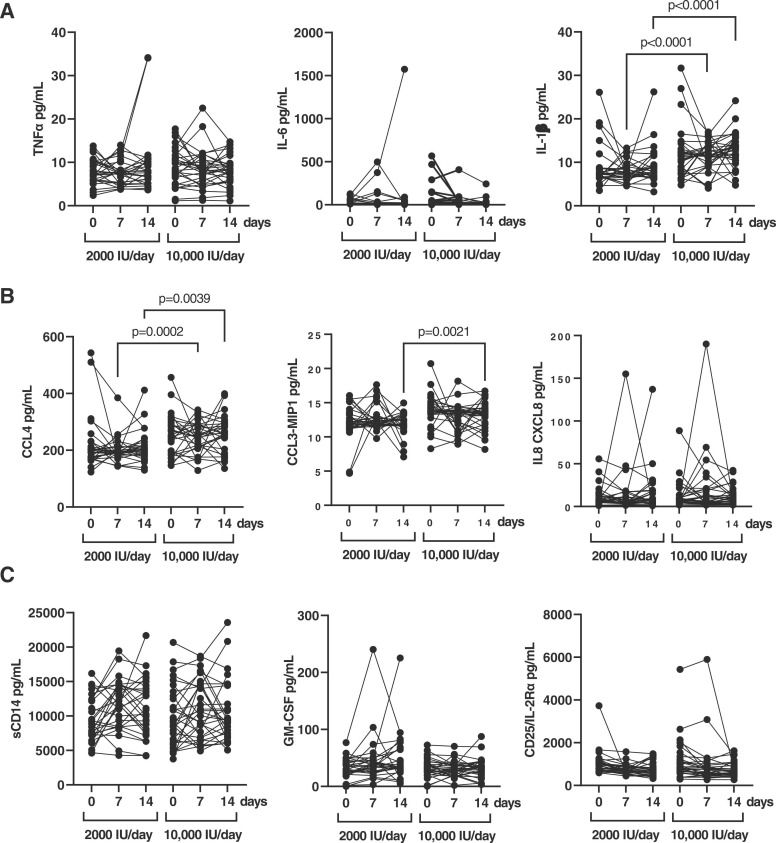
A subgroup of 30 patients of each supplementation group was randomly selected to analyze the profile of cytokines in plasma. The levels of pro-inflammatory cytokines TNFα and IL-6 were not significantly different between both groups of participants neither at 7 or 14 days after treatment, whereas the levels of IL-1β in the high dose group were increased 1.45- (p < 0.0001) and 1.44- (p < 0.0001) fold after 7 and 14 days, respectively ( Fig. 3A). The comparison between groups showed that the levels of the chemokine CCL4/MIP-1β were increased 1.29-fold (p = 0.0002) after 7 days of treatment, and 1.27-fold (p = 0.0039) after 14 days in the 10,000 IU/day group (Fig. 3B). Similarly, the levels of chemokine CCL3/MIP-1α were increased 1.12-fold (p = 0.0021) after 14 days in the 10,000 IU/day group. There were no significant differences between groups for IL8-CXCL8 levels and for cytokines related to cellular activation such as GM-CSF, sCD25/IL-2Rα and sCD14 (Fig. 3C). In the case of sCD14, the levels detected were higher than 20,000 pg/ml in some of the patients from both groups (normal values in the absence of infection <200 pg/ml).
Fig. 3.
Pro-inflammatory and activating cytokine profile in plasma samples of hospitalized patients with COVID-19 who received each dose of vitamin D. (A) Levels (pg/ml) of pro-inflammatory cytokines TNFα, IL-6, and IL-1β were quantified in plasma of patients hospitalized with COVID-19 who received 2000 IU/day and 10,000IU/day of cholecalciferol at baseline, 7 and 14 days of treatment. Levels (pg/ml) of chemokines CCL4, CCL3-MIP1, and IL8 CXCL8 (B), as well as cytokines related to cell activation sCD14, GM-CSF, and IL25/I2R-α (C) were also quantified in plasma of the same patients. Each dot corresponds to one sample. Statistical significance was calculated using Mann–Whitney U-test.
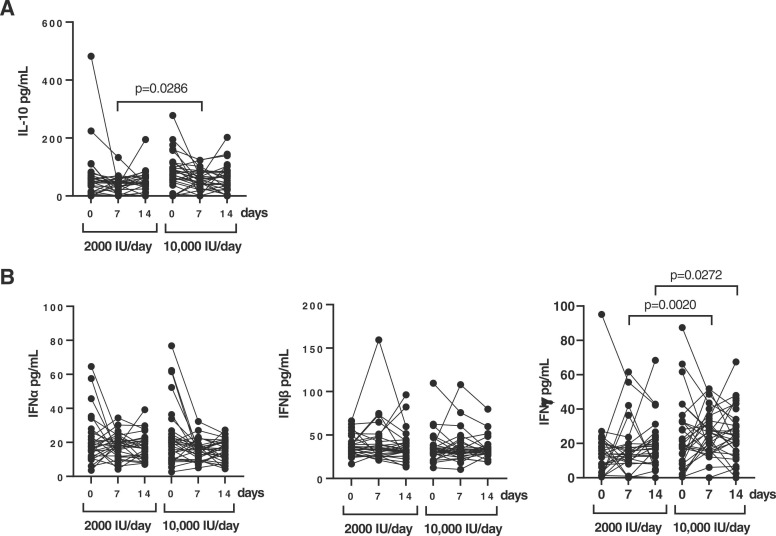
On the other hand, the level of the anti-inflammatory cytokine IL-10 was increased 1.48-fold (p = 0.0286) after 7 days in the 10,000 IU/day group in comparison with the 2000 IU/day group ( Fig. 4A). Regarding the antiviral cytokines, the level of IFNγ was significantly increased 1.54- (p = 0.0020) and 1.4- (p = 0.0272) fold at 7 and 14 days, respectively, in the 10,000 IU/day group (Fig. 4B). We did not find significant differences in the levels of IFN type I α and β between groups.
Fig. 4.
Anti-inflammatory and antiviral cytokine profile in plasma samples of hospitalized patients with COVID-19 who received each dose of vitamin D. Levels (pg/ml) of the anti-inflammatory cytokine IL-10 (A) and antiviral cytokines IFNα, IFNβ and IFNγ (B) were quantified in plasma of patients hospitalized with COVID-19 who received 2000 IU/day and 10,000IU/day of cholecalciferol at baseline, 7 and 14 days of treatment. Each dot corresponds to one sample. Statistical significance was calculated using Mann–Whitney U-test.
3.6. Effect of the dose of vitamin D on cytotoxic cell populations
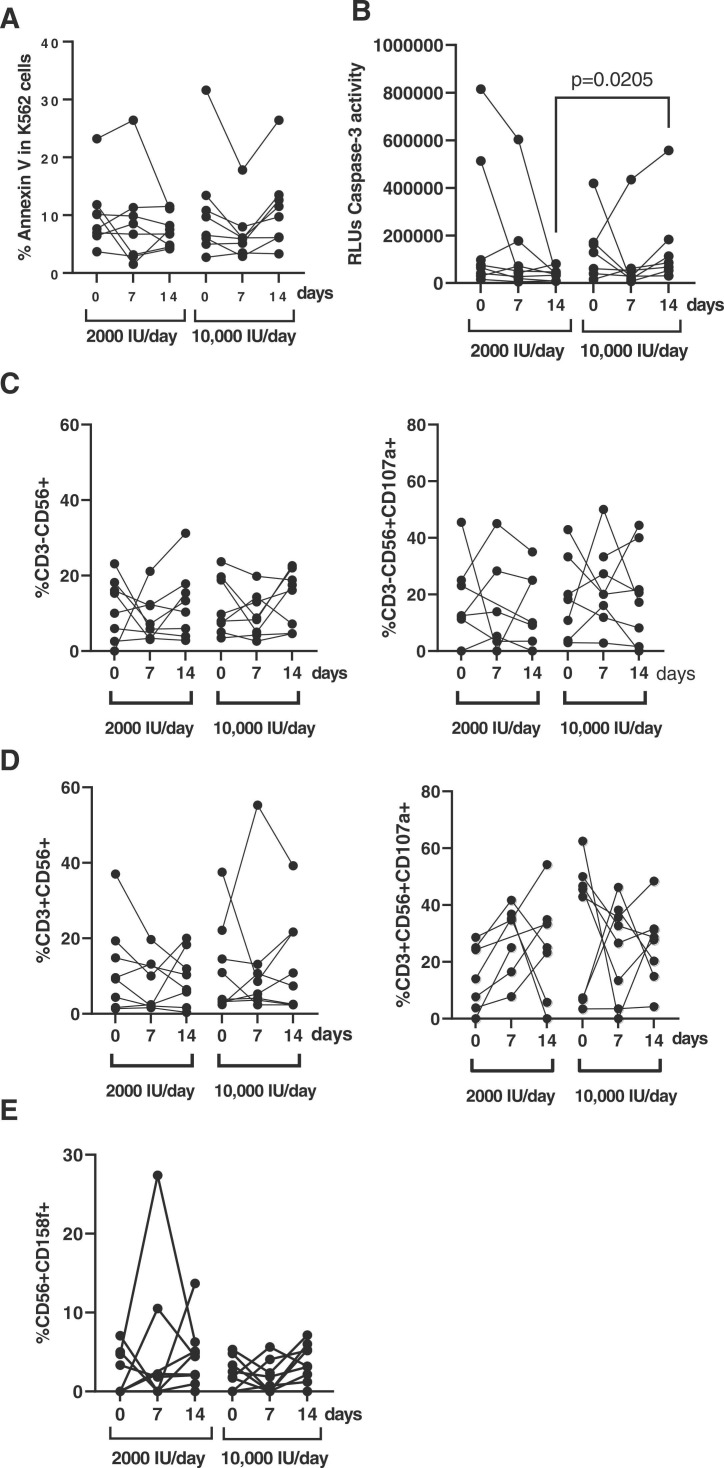
We randomly selected 8 participants from each group to evaluate the direct cytotoxic activity of PBMCs. When K562 cells were used as target, there was an increase of 1.5-fold in the cytotoxic activity of PBMCs from participants who received 10,000 IU/day after 14 days of treatment, in comparison with the 2000 IU/day group, but this was a non-significant trend ( Fig. 5A). However, when pseudotyped SARS-CoV-2-infected Vero E6 cells were used as target, there was a significant increase of 4.27-fold (p = 0.0205) in the activity of caspase-3 in these cells after co-culture with PBMCs isolated from patients supplemented for 14 days with 10,000 IU/day of vitamin D, in comparison with PBMCs from the 2000 IU/day group (Fig. 5B). The analysis of NK cells in these PBMCs revealed that although the levels of CD3-CD56 + cells were similar between both groups (Fig. 5C, left graph), the expression of the degranulation marker CD107a was reduced 1.2-fold (p = 0.0313) after 14 days of treatment in the group of 2000 IU/day of vitamin D, whereas in the group treated with 10,000 IU/day the expression of CD107a was increased 1.2-fold after 7 days of treatment (p = 0.0078) (Fig. 5C, right graph). No significant differences were found in total levels of NKT cells or in the expression of CD107a between both groups of treatment (Fig. 5D). Interestingly, the expression of the NK cells inhibitory marker CD158f/KIR2DL5 was increased 2.2-fold (p = 0.0078) in the PBMCs of participants from the group treated with 2000 IU/day after 14 days of treatment, whereas it was less increased in the PBMCs of participants treated with 10,000 IU/day (1.7-fold; p = 0.0078) (Fig. 5E).
Fig. 5.
Evaluation of the cytotoxic response in PBMCs of hospitalized patients with COVID-19 who received each dose of vitamin D. (A) Cytotoxicity of PBMCs from patients with COVID-19 who received 2000 IU/day and 10,000IU/day of cholecalciferol at baseline, 7 and 14 days was analyzed by quantifying early apoptosis in K562 cells as unspecific target after co-culture (1:1) for 1 h. (B) Direct antiviral cytotoxicity against SARS-CoV-2 infected cells was evaluated in the same PBMCs by quantifying caspase-3 activity in a monolayer of Vero E6 cells infected with pseudotyped SARS-CoV-2 virus G614 that were co-cultured with PBMCs (1:1) for 1 h. Both NK (C) and NKT (D) cell populations were analyzed by evaluating the expression of the activation marker CD56 and the degranulation marker CD107a. (D) The expression of the inhibitory marker CD158f/KIR2DL5 was evaluated in the same cells. Each dot corresponds to one sample. Statistical significance was calculated using Mann–Whitney U-test.
There were no significant differences between both groups in the total count of CD8 + T cells, the distribution of memory subpopulations, or the levels of CD3 +CD8 ± TCRδγ+ T cells (Fig. S1).
3.7. Distribution of CD4 + T cell subpopulations
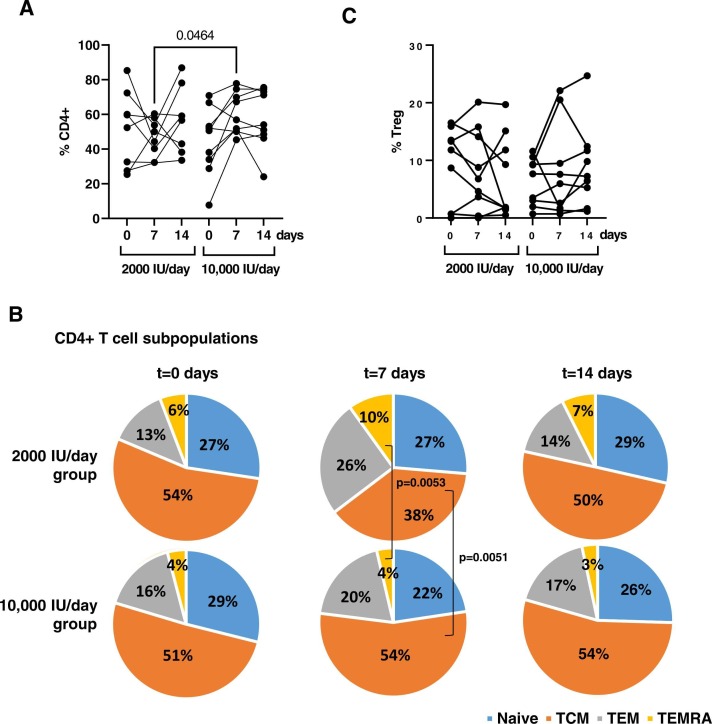
CD4 + T cell levels significantly increased 1.31-fold (p = 0.0464) after 7 days of supplementation with 10,000 IU/day of vitamin D ( Fig. 6A). In this group, TCM CD4 + T cells were increased 1.42-fold (p = 0.0053) whereas effector CD4 + T cells such as TEMRA were reduced 2.5-fold (p = 0.0051) after 7 days of treatment (Fig. 6B). No significant differences were found between both groups in the levels of Tregs (Fig. 6C).
Fig. 6.
Analysis of CD4 + T cell subsets in PBMCs of hospitalized patients with COVID-19 who received each dose of vitamin D. Percentage of total CD4 + T cells (A), the distribution of CD4 subpopulations (B), and Tregs (C) was determined in PBMCs from hospitalized patients with COVID-19 who received 2000 IU/day or 10,000IU/day of cholecalciferol at baseline, 7 and 14 days. Each dot corresponds to one sample. Statistical significance was calculated using Mann–Whitney U-test.
4. Discussion
COVID-19 mortality has shown a decreasing North-South gradient that may be explained by the high prevalence of older people in Northern European populations, who are predisposed to a higher probability of cardio-pulmonary and metabolic co-morbidities [30]. However, countries such as Italy or Spain have also shown high mortality rates, suggesting that other risk factors might also contribute to COVID19 infection severity [31]. Due to the immunomodulatory effect of vitamin D, its deficiency may contribute to airway and gastrointestinal infectious diseases, and it has been suggested as an additional risk factor associated with COVID-19 severity and mortality [25], [27], although there is a great controversy about the effect of vitamin D supplementation during COVID-19 [32], [33], [34], [35]. Though Spain is a country with adequate sunlight, some studies describe that vitamin D intake is lower than the recommended levels of 10 µg/day [36], resulting in a high proportion of the Spanish population with deficient levels of vitamin D [37]. In our study, the majority (81.7%) of participants who were hospitalized due to severe COVID-19 showed 25(OH)D serum levels in a deficient range (<20 ng/ml) [38]. The daily supplementation of 10,000 IU cholecalciferol achieved the increase of 25(OH)D serum to levels close to sufficiency, whereas in those patients who received the 2000 IU/day dose only a slight increase was observed after 14 days. These results are in line with other randomized clinical trials on vitamin D supplementation in hospitalized COVID-19 patients in which one single dose of 200,000 IU or 5,000 IU/day for 14 days achieved 25(OH)D serum levels of sufficiency (≥30 ng/ml) [39], [40]. However, 90.90% of the participants who received the moderate dose and 53.66% of the participants who received the high dose did not reach the primary endpoint of 30 ng/ml 25(OH)D in serum. The reduction of 25(OH)D levels during COVID-19 are expected due to this prohormone is an acute phase reactant and it is usually reduced during acute inflammatory procedures [41], [42]. Moreover, although there is controversy about the possible role of vitamin D supplementation to improve the health status during COVID-19, the deficiency of vitamin D at hospital admission due to COVID-19 has been appointed as a potential prognostic marker of disease severity in these patients, as it would indicate the progression to the inflammatory form of the disease [43], [44]. Therefore, this inflammatory process that is developed during COVID-19 could be responsible for the impairment in achieving the primary endpoint of > 30 ng/ml in most participants, even with the higher dose of 10,000 IU/day.
The severe and critical presentations of COVID-19 are characterized by an enhanced inflammatory response [3], [6]. Consequently, the administration of corticosteroids has been one of the most effective treatments since the beginning of the pandemic [45]. Although with a much more modest effect, vitamin D has a immunomodulatory role that may favour an anti-inflammatory environment [46], mostly based on the inhibition of the production of interleukins responsible for Th1 and Th17 polarization, which is characterized by the release of pro-inflammatory cytokines such as IL-6 and TNFα [47]. In our pilot study, we did not find significantly increased levels of pro-inflammatory cytokines in the plasma of those individuals who developed ARDS, despite both events are usually related during COVID-19 [48] This could be due to the levels of cytokines are frequently higher in the lungs than in plasma and do not often correlate [49]. Therefore, the daily supplementation with a high dose of cholecalciferol did not reduce significantly the levels of inflammatory markers in plasma that had been already released, not even in individuals with ARDS, in accordance with previous studies [50]. However, the participants who received the supplementation of 10,000 IU/day of cholecalciferol showed a significant increase in plasma of the anti-inflammatory cytokine IL-10, which may contribute to reduce the signs and symptoms of the disease related to the inflammatory environment. This result corroborated other studies that also described a significant increase in IL-10 levels after the administration of vitamin D [51], [52]. Therefore, the supplementation with vitamin D could at least partially contribute to reducing the inflammatory environment characteristic of COVID-19, which may also be related with the shortening of the length of the hospital stay observed in those participants supplemented with 10,000 IU/day of vitamin D who developed ARDS during the study. Although the number of participants who developed ARDS was quite reduced in our cohort, the improvement in the clinical recovery and the shortening of the stay at the hospital has also been described for individuals who received 50,000 IU/day of cholecalciferol for five days [53].
On the other hand, COVID-19 is characterized by causing CD4 + T cell lymphopenia, which may be involved in the progression to more severe forms of the disease [54]. Although vitamin D may affect T-cell proliferation by interfering with the production of IL-2 [55], in our study, the CD4 count was significantly higher in patients treated with 10,000 IU/day for 7 days, which has also been described in patients with inflammatory diseases who receive vitamin D supplementation [56]. Moreover, those participants from our cohort who received 10,000 IU/day showed significantly enhanced levels of long-lived CD4 + TCM cell subpopulation and reduced levels of CD4 + TEMRA cells, which are short-lived cells that die upon activation [57] during protective immunity against pathogens [58]. Therefore, the reduced levels of CD4 + TEMRA cell subpopulation observed in those participants who received 10,000 IU/day may be a consequence of an increased antiviral activity. In fact, we also observed a significant increase in the production of IFNγ in the participants who received 10,000 IU/day after 7 days that was maintained after 14 days of treatment, which corroborated other studies that reported a significant increase of IFNγ levels after the administration of vitamin D [51], [52]. Therefore, supplementation with vitamin D could also be beneficial to induce a higher antiviral effect. Although vitamin D supplementation did not modify the proliferative capacity of cell populations with cytotoxic activity such as NK or CD8 + T cells, we observed a significant increase in the cytotoxic activity on SARS-CoV-2-infected cells exerted by PBMCs from those participants who received 10,000 IU/day for 14 days. This antiviral activity seemed to rely mostly on NK cell-mediated cytotoxic response, due to the degranulation capacity of these cells increased after 14 days of receiving 10,000 IU/day. Interestingly, an impairment in the expression of the degranulation marker CD107a has been described in NK cells from patients with the most severe forms of COVID-19 [59], which appeared to be counteracted by treatment with vitamin D. We cannot rule out that vitamin D was also promoting a similar effect on CD8 + T cells because there are precedents that deficiency in vitamin D receptor gene (Vdr-/-) may cause an impairment in the cytotoxic response against viral infections, reducing the diversity of effector CD8 + T cell repertoire and the survival of antigen-specific cytotoxic clones after viral clearance [60].
In conclusion, the efficacy of vitamin D supplementation in patients with COVID-19 is still controversial [32], [33], [34], [35], likely due to variations in the clinical presentations of the disease and the design of the different studies. In our pilot clinical trial, the supplementation of 10,000 IU/day for 14 days in patients with severe outcomes of COVID-19 was safe and well-tolerated and it appeared to have a beneficial clinical effect, despite the serum levels of vitamin D did not reach the primary clinical endpoint in all individuals. However, the participants showed improved biochemical and haematological parameters in plasma, such as vitamin D levels close to sufficiency and enhanced CD4 count, as well as increased levels of the anti-inflammatory cytokine IL-10, which may contribute to decreasing the inflammatory environment characteristic of the severest forms of COVID-19. Besides, our results are consistent with previous observations that vitamin D may enhance the lytic activity of NK cells [61], [62], [63], although this is the first description that this vitamin D-mediated lytic activity may be directed against cells expressing the spike protein of SARS-CoV-2 on the surface after infection with pseudotyped virus. We cannot rule out that early administration of vitamin D, just after being hospitalized due to severe COVID-19, would have been more beneficial than waiting for the inflammatory phase to begin. However, due to the controversy, more studies are needed to establish a solid association between vitamin D and clinical recovery of COVID-19.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgements and funding
We greatly appreciate all the patients for their participation in this study. We thank the excellent secretarial assistance of Mrs Olga Palao at the Centro Nacional de Microbiología (CNM, Instituto de Salud Carlos III). The authors also acknowledge María C. de la Cruz at Unidad Central de Apoyo a la Investigación Clínica y Ensayos Clínicos (Instituto de Investigación Sanitaria Gregorio Marañon; IiSGM) for her advice and assistance related to the clinical research with medicines. This work was supported by the Coordinated Research Activities at CNM (Instituto de Salud Carlos III) (COV20_00679) to promote an integrated response against SARS-CoV-2 in Spain (Spanish Ministry of Science and Innovation) that is coordinated by Dr Inmaculada Casas (WHO National Influenza Center of the CNM); the Spanish Ministry of Science and Innovation (PID2019–110275RB-I00); the Spanish AIDS Research Network RD16CIII/0002/0001 that is included in Acción Estratégica en Salud, Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica 2016–2020, Instituto de Salud Carlos III, European Region Development Fund (ERDF) and Fundación Universidad Alfonso X el Sabio (FUAX, Madrid, Spain; Reference 1012010) . The work of Montserrat Torres is financed by the Coordinated Research Activities at the CNM (Instituto de Salud Carlos III) (COV20_00679). The work of María Rosa López-Huertas and Sara Rodríguez-Mora is financed by NIH grant R01AI143567. The work of Lorena Vigón is supported by a pre-doctoral grant from Instituto de Salud Carlos III (FIS PI16CIII/00034-ISCIII-FEDER). The work of Fernando Ramos Martín is financed by the Spanish Ministry of Science and Innovation (PID2019–110275RB-I00). Drug Cholecalciferol (vitamin D) used in the study was donated by Italfarmaco Group (Cholecalciferol 25,000IU/2,5 ml oral solution). Italfarmaco Group had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript.
Author Contributions
MCer and MCo conceptualized the project. MT, GC and MCo wrote the manuscript. MT, GC and LV performed the study of cytotoxicity. MT, GC and SRM performed the analysis of cell populations by flow cytometry with technical assistance from EM. MRLH and GC performed the analysis of cytokines in plasma. FR and EM processed and stored all blood samples. DLW, MNM, PRM, MLTM and MCer identified, selected, and recruited the patients, and also collected the blood samples. MT, MCer and MRLH collected and analyzed the clinical data. All co-authors read and approved the final version of the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.biopha.2022.112965.
Contributor Information
Contributing members of the Multidisciplinary Group of Study of COVID-19 (in alphabetical order):
David Alonso-Menchén, Sandra Arévalo Camacho, Cristina Avila Calzada, José Antonio Barbado Albaladejo, Natalia Blanca López, Irene Cañamares Orbis, Gema Carrillo Blanco, Almudena Cascajero Díaz, María Teresa Chica Burguillo, Ana Corrochano García, Sara Corredera García, Victor Díez Viñas, Marta Gómez-Alvarez Domínguez, Claudia Patricia Fernández Fernández, Yanira Fernández Mondelo, Eva Fonseca Aizpuri, Concepción García Lacalle, Javier García-Pérez, Cristina Helguera Amezua, Francisco José Hidalgo Correas, Amparo Lucena Campillo, Mariano Matarranz del Amo, Oriol Martín Sagarra, Emilio José Martínez Martín, José Javier Martínez Simón, María Novella-Mena, Virginia Pardo Guimera, María Luisa Pinillos Pardo, Fr`ancisca Ramírez Fuentes, Daniel Renuncio García, María Angeles Rodríguez Dávila, Almudena Roger Revilla, Lourdes Sampablo Valverde, José Sanz Moreno, Rafael Torres Perea, Jorge Valencia La Rosa, María Velasco Arribas, and Ana Villanueva Fernández-Ardavín
Appendix A. Supplementary material
Supplementary material.
.
Supplementary material.
.
Supplementary material.
.
References
- 1.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020;19:1–14. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet Lond. Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration U. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Lond. Engl. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., Xiong W., Yang D., Chen R., Lu F., Lu Y., Liu X., Chen Y., Li X., Li Y., Summah H.D., Lin H., Yan J., Zhou M., Lu H., Qu J. COVID-19 with different severities: a multicenter study of clinical features. Am. J. Respir. Crit. Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cifaldi L., Prencipe G., Caiello I., Bracaglia C., Locatelli F., De Benedetti F., Strippoli R. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheuma. 2015;67:3037–3046. doi: 10.1002/art.39295. [DOI] [PubMed] [Google Scholar]
- 6.Gustine J.N., Jones D. Immunopathology of hyperinflammation in COVID-19. Am. J. Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni L., Cheng M.L., Feng Y., Zhao H., Liu J., Ye F., Ye Q., Zhu G., Li X., Wang P., Shao J., Deng Y.Q., Wei P., Chen F., Qin C.F., Wang G., Li F., Zeng H., Dong C. Impaired Cellular Immunity to SARS-CoV-2 in Severe COVID-19 Patients. Front. Immunol. 2021;12:59. doi: 10.3389/fimmu.2021.603563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigón L., Fuertes D., García-Pérez J., Torres M., Rodríguez-Mora S., Mateos E., Corona M., Saez-Marín A.J., Malo R., Navarro C., Murciano-Antón M.A., Cervero M., Alcamí J., García-Gutiérrez V., Planelles V., López-Huertas M.R., Coiras M. Impaired Cytotoxic Response in PBMCs From Patients With COVID-19 Admitted to the ICU: Biomarkers to Predict Disease Severity. Front. Immunol. 2021;12:1901. doi: 10.3389/fimmu.2021.665329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra S.K., Tripathi T. One year update on the COVID-19 pandemic: Where are we now? Acta Trop. 2021;214 doi: 10.1016/j.actatropica.2020.105778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iturricastillo G., Ávalos Pérez-Urría E., Couñago F., Landete P. Scientific evidence in the COVID-19 treatment: A comprehensive review. World J. Virol. 2021;10:217–228. doi: 10.5501/wjv.v10.i5.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., Santoro A., Di Gaetano M., Puzzolante C., Carli F., Bedini A., Corradi L., Fantini R., Castaniere I., Tabbì L., Girardis M., Tedeschi S., Giannella M., Bartoletti M., Pascale R., Dolci G., Brugioni L., Pietrangelo A., Cossarizza A., Pea F., Clini E., Salvarani C., Massari M., Viale P.L., Mussini C. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheuma. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RECOVERY Collaborative Group, et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parums D.V. Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Patients. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022;28 doi: 10.12659/MSM.935952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couzin-Frankel J. Antiviral pills could change pandemic’s course. Science. 2021;374:799–800. doi: 10.1126/science.acx9605. [DOI] [PubMed] [Google Scholar]
- 17.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. IJID . Publ. Int. Soc. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouillon R., Marcocci C., Carmeliet G., Bikle D., White J.H., Dawson-Hughes B., Lips P., Munns C.F., Lazaretti-Castro M., Giustina A., Bilezikian J. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019;40:1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dankers W., Colin E.M., van Hamburg J.P., Lubberts E. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front. Immunol. 2017;7 doi: 10.3389/fimmu.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Infante M., Ricordi C., Sanchez J., Clare-Salzler M.J., Padilla N., Fuenmayor V., Chavez C., Alvarez A., Baidal D., Alejandro R., Caprio M., Fabbri A. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients. 2019;11:2185. doi: 10.3390/nu11092185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., Grant C.C., Griffiths C.J., Janssens W., Laaksi I., Manaseki-Holland S., Mauger D., Murdoch D.R., Neale R., Rees J.R., Simpson S Jr, Stelmach I., Kumar G.T., Urashima M., Camargo CA Jr. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fakhoury H., Kvietys P.R., Shakir I., Shams H., Grant W.B., Alkattan K. Lung-Centric Inflammation of COVID-19: Potential Modulation by Vitamin D. Nutrients. 2021;13:2216. doi: 10.3390/nu13072216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishop E., Ismailova A., Dimeloe S.K., Hewison M., White J.H. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR. 2020 doi: 10.1002/jbm4.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss S.T., Litonjua A.A. Vitamin D in host defense: implications for future. Res. Am. J. Respir. Cell Mol. Biol. 2017;56:692–693. doi: 10.1165/rcmb.2017-0064ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368:m810. doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- 26.Hastie C.E., Pell J.P., Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2021;60:545–548. doi: 10.1007/s00394-020-02372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020;32:1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Perez J., Sanchez-Palomino S., Perez-Olmeda M., Fernandez B., Alcami J. A new strategy based on recombinant viruses as a tool for assessing drug susceptibility of human immunodeficiency virus type 1. J. Med. Virol. 2007;79:127–137. doi: 10.1002/jmv.20770. [DOI] [PubMed] [Google Scholar]
- 29.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorci G., Faivre B., Morand S. Explaining among-country variation in COVID-19 case fatality rate. Sci. Rep. 2020;10:18909. doi: 10.1038/s41598-020-75848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes J.M., Subramanian S., Laird E., Griffin G., Kenny R.A. Perspective: Vitamin D deficiency and COVID-19 severity – plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J. Intern. Med. 2021;289:97–115. doi: 10.1111/joim.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Rocha A.P., Atallah A.N., Aldrighi J.M., Pires A., Dos Santos Puga M.E., Pinto A. Insufficient evidence for vitamin D use in COVID-19: A rapid systematic review. Int. J. Clin. Pract. 2021;e14649:14649. doi: 10.1111/ijcp.14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae J.H., Choe H.J., Holick M.F., Lim S. Association of vitamin D status with COVID-19 and its severity. Rev. Endocr. Metab. Disord. 2022 doi: 10.1007/s11154-021-09705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annweiler G., Corvaisier M., Gautier J., Dubée V., Legrand E., Sacco G., Annweiler C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients. 2020;12 doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raisi-Estabragh Z., Martineau A.R., Curtis E.M., Moon R.J., Darling A., Lanham-New S., Ward K.A., Cooper C., Munroe P.B., Petersen S.E., Harvey N.C. Vitamin D and coronavirus disease 2019 (COVID-19): rapid evidence review. Aging Clin. Exp. Res. 2021;33:2031–2041. doi: 10.1007/s40520-021-01894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olza J., Aranceta-Bartrina J., González-Gross M., Ortega R., Serra-Majem L., Varela-Moreiras G., Gil Á. Reported Dietary Intake, Disparity between the Reported Consumption and the Level Needed for Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D in the Spanish Population: Findings from the ANIBES Study †. Nutrients. 2017;9:168. doi: 10.3390/nu9020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarro Valverde C., Quesada Gómez J.M. Deficiencia de vitamina D en España: ¿realidad o mito? Rev. Osteoporos. Metab. Min. 2014;6:5–10. [Google Scholar]
- 38.Recomendaciones de la SEIOMM en la prevención y tratamiento del déficit de vitamina D. Revista de Osteoporosis y Metabolismo Mineral·Publicación Oficial SEIOMM, 2021. 〈http://revistadeosteoporosisymetabolismomineral.com/2021/07/08/recomendaciones-la-seiomm-la-prevencion-tratamiento-del-deficit-vitamina-d/〉.
- 39.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., Silva C.B.R., Franco A.S., Macedo M.B., Dalmolin H.H.H., Baggio J., Balbi G.G.M., Reis B.Z., Antonangelo L., Caparbo V.F., Gualano B., Pereira R.M.R. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabico S., Enani M.A., Sheshah E., Aljohani N.J., Aldisi D.A., Alotaibi N.H., Alshingetti N., Alomar S.Y., Alnaami A.M., Amer O.E., Hussain S.D., Al-Daghri N.M. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients. 2021;13:2170. doi: 10.3390/nu13072170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonelli M., Kushner I. Low Serum Levels of 25-Hydroxyvitamin D Accompany Severe COVID-19 Because it is a Negative Acute Phase Reactant. Am. J. Med. Sci. 2021;362:333–335. doi: 10.1016/j.amjms.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louw J.A., Werbeck A., Louw M.E., Kotze T.J., Cooper R., Labadarios D. Blood vitamin concentrations during the acute-phase response. Crit. Care Med. 1992;20:934–941. doi: 10.1097/00003246-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Borsche L., Glauner B., von Mendel J. COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 ng/ml 25(OH)D3: Results of a Systematic Review and Meta-Analysis. Nutrients. 2021;13:3596. doi: 10.3390/nu13103596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiodini I., Gatti D., Soranna D., Merlotti D., Mingiano C., Fassio A., Adami G., Falchetti A., Eller-Vainicher C., Rossini M., Persani L., Zambon A., Gennari L. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.736665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Group T.R.C. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baeke F., Takiishi T., Korf H., Gysemans C., Mathieu C. Vitamin D: modulator of the immune system. Curr. Opin. Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen A.W., Holmstrøm K., Jensen S.S., Fuchs D., Rasmussen S., Kvistborg P., Claesson M.H., Zocca M.B. Phenotypic and functional markers for 1α,25-dihydroxyvitamin D3-modified regulatory dendritic cells. Clin. Exp. Immunol. 2009;157:48–59. doi: 10.1111/j.1365-2249.2009.03961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen R., Lan Z., Ye J., Pang L., Liu Y., Wu W., Qin X., Guo Y., Zhang P. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.589095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kvietys P.R., Fakhoury H., Kadan S., Yaqinuddin A., Al-Mutairy E., Al-Kattan K. COVID-19: Lung-Centric Immunothrombosis. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.679878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolf L., Muris A.H., Bol Y., Damoiseaux J., Smolders J., Hupperts R. Vitamin D3 supplementation in multiple sclerosis: Symptoms and biomarkers of depression. J. Neurol. Sci. 2017;378:30–35. doi: 10.1016/j.jns.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Karonova T., Stepanova A., Bystrova A., Jude E.B. High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients. Nutrients. 2020;12:2518. doi: 10.3390/nu12092518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos-Martínez E., López-Vancell M.R., Fernández de Córdova-Aguirre J.C., Rojas-Serrano J., Chavarría A., Velasco-Medina A., Velázquez-Sámano G. Reduction of respiratory infections in asthma patients supplemented with vitamin D is related to increased serum IL-10 and IFNγ levels and cathelicidin expression. Cytokine. 2018;108:239–246. doi: 10.1016/j.cyto.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Ohaegbulam K.C., Swalih M., Patel P., Smith M.A., Perrin R. Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series. Am. J. Ther. 2020;27:e485–e490. doi: 10.1097/MJT.0000000000001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;0 doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cantorna M.T., Snyder L., Lin Y.-D., Yang L. Vitamin D and 1,25(OH)2D Regulation of T cells. Nutrients. 2015;7:3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bendix-Struve M., Bartels L.E., Agnholt J., Dige A., Jørgensen S.P., Dahlerup J.F. Vitamin D3 treatment of Crohn’s disease patients increases stimulated T cell IL-6 production and proliferation. Aliment. Pharmacol. Ther. 2010;32:1364–1372. doi: 10.1111/j.1365-2036.2010.04463.x. [DOI] [PubMed] [Google Scholar]
- 57.Sani, M. et al. Reduced CD4+ terminally differentiated effector memory T cells in moderate-severe house dust mites sensitized allergic rhinitis patients. Asian Pac J Allergy Immunol 9. [DOI] [PubMed]
- 58.Tian Y., Babor M., Lane J., Schulten V., Patil V.S., Seumois G., Rosales S.L., Fu Z., Picarda G., Burel J., Zapardiel-Gonzalo J., Tennekoon R.N., De Silva A.D., Premawansa S., Premawansa G., Wijewickrama A., Greenbaum J.A., Vijayanand P., Weiskopf D., Sette A., Peters B. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat. Commun. 2017;8:1473. doi: 10.1038/s41467-017-01728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuzefpolskiy Y., Baumann F.M., Penny L.A., Studzinski G.P., Kalia V., Sarkar S. Vitamin D Receptor Signals Regulate Effector and Memory CD8 T Cell Responses to Infections in Mice. J. Nutr. 2014;144:2073–2082. doi: 10.3945/jn.114.202895. [DOI] [PubMed] [Google Scholar]
- 61.Chen X., Diao L., Lian R., Qi L., Yu S., Liu S., Lin S., Xue Z., Zeng Y. Potential impact of maternal vitamin D status on peripheral blood and endometrium cellular immunity in women with recurrent implantation failure. Am. J. Reprod. Immunol. 2020;84 doi: 10.1111/aji.13243. [DOI] [PubMed] [Google Scholar]
- 62.Al-Jaderi Z., Maghazachi A.A. Effects of Vitamin D3, Calcipotriol and FTY720 on the Expression of Surface Molecules and Cytolytic Activities of Human Natural Killer Cells and Dendritic Cells. Toxins. 2013;5:1932–1947. doi: 10.3390/toxins5111932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee G.Y., Park C.Y., Cha K.S., Lee S.E., Pae M., Han S.N. Differential effect of dietary vitamin D supplementation on natural killer cell activity in lean and obese mice. J. Nutr. Biochem. 2018;55:178–184. doi: 10.1016/j.jnutbio.2018.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Supplementary material.
Supplementary material.