Abstract
Background:
Currently, there are no solutions to prevent congenital transmission of Chagas disease during pregnancy, which affects 1–40% of pregnant women in Latin America and is associated with a 5% transmission risk. With therapeutic vaccines under development, now is the right time to determine the economic value of such a vaccine to prevent congenital transmission.
Methods:
We developed a computational decision model that represented the clinical outcomes and diagnostic testing strategies for an infant born to a Chagas-positive woman in Mexico and evaluated the impact of vaccination.
Results:
Compared to no vaccination, a 25% efficacious vaccine averted 125 [95% uncertainty interval (UI): 122–128] congenital cases, 1.9 (95% UI: 1.6–2.2) infant deaths, and 78 (95% UI: 66–91) DALYs per 10,000 infected pregnant women; a 50% efficacious vaccine averted 251 (95% UI: 248–254) cases, 3.8 (95% UI: 3.6–4.2) deaths, and 160 (95% UI: 148–171) DALYs; and a 75% efficacious vaccine averted 376 (95% UI: 374–378) cases, 5.8 (95% UI: 5.5–6.1) deaths, and 238 (95% UI: 227–249) DALYs. A 25% efficacious vaccine was cost-effective (incremental cost-effectiveness ratio <3x Mexico’s gross domestic product per capita, <$29,698/DALY averted) when the vaccine cost ≤$240 and ≤$310 and cost-saving when ≤$10 and ≤$80 from the third-party payer and societal perspectives, respectively. A 50% efficacious vaccine was cost-effective when costing ≤$490 and ≤$615 and cost-saving when ≤$25 and ≤$160, from the third-party payer and societal perspectives, respectively. A 75% efficacious vaccine was cost-effective when ≤$720 and ≤$930 and cost-saving when ≤$40 and ≤$250 from the third-party payer and societal perspectives, respectively. Additionally, 13–42 fewer infants progressed to chronic disease, saving $0.41-$1.21 million to society.
Conclusion:
We delineated the thresholds at which therapeutic vaccination of Chagas-positive pregnant women would be cost-effective and cost-saving, providing economic guidance for decision-makers to consider when developing and bringing such a vaccine to market.
Keywords: Chagas disease, Vaccine, Pregnancy, Economics, Congenital transmission
Introduction
The prevalence of Chagas disease among pregnant women in Latin America is 1–40%[1–6], however, with no current solutions for preventing congenital transmission (5% risk[7]) once pregnant, pregnant women may be an ideal target population for vaccination. With vaccines currently under development, now is the time to determine the economic value of a therapeutic Chagas disease vaccine for pregnant women to prevent congenital transmission. While the majority of congenital infections are asymptomatic, Trypanosoma cruzi-infected infants can develop moderate to severe symptoms (e.g., respiratory distress syndrome after preterm birth, splenomegaly, hepatomegaly, and cardiomyopathy), with up to 6.5% of cases resulting in death.[1] Chagas disease is often difficult to diagnose even among symptomatic infants, as many other congenital infections have similar symptoms (e.g., TORCH: toxoplasmosis, other infections, rubella, cytomegalovirus, herpes simplex).[8] Diagnosis in infants is further complicated by requiring multiple diagnostic tests over the course of a year,[8] leading many Chagas-positive infants to be lost to follow-up and remain untreated.[9, 10] Additionally, congenitally-infected infants are thought to grow up with the same 20–30% chance of developing chronic disease that would occur if vectorially infected.[3]
As treatment is contraindicated in pregnancy[9, 11, 12], vaccination may be a viable option for preventing congenital transmission. As at least 12 vaccine candidates are in pre-clinical trials,[13] decision-makers (e.g., policy-makers, vaccine developers, manufacturers, third-party payers, and potential funders) need to understand the potential clinical and economic value of such a vaccine. With vaccines in the pipeline, evaluating the economic value prior to licensure can help guide development and implementation, desired efficacy profiles, and vaccine price points while there is still time to make adjustments.[14–16] Therefore, a computational simulation model was developed to evaluate the clinical and economic impact of a therapeutic vaccine given to pregnant women to prevent congenital Chagas infections in Mexico. Mexico served as a test case as it has one of the highest number of persons living with Chagas disease[17, 18] and a high number of congenital Chagas disease cases.[7, 19]
Methods
Model
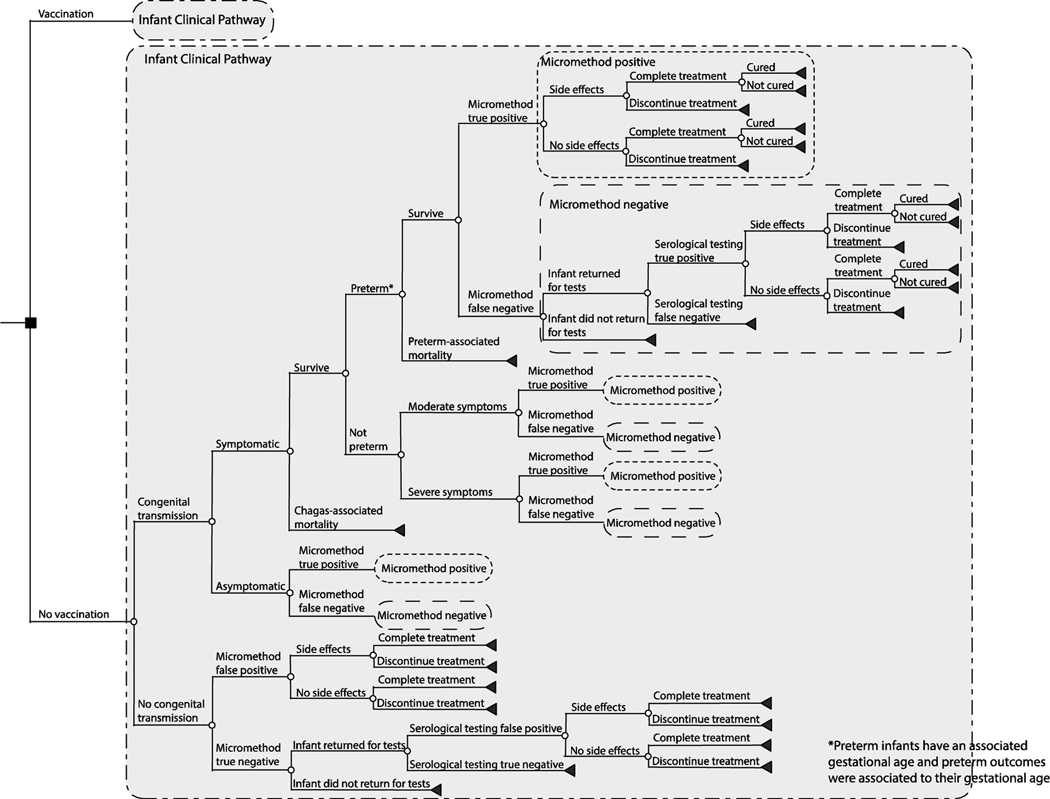
Using TreeAge Pro 2019 (Williamstown, MA), we developed a computational decision analytic model to represent the clinical outcomes and diagnostic testing strategies for an infant born to a Chagas-positive woman in Mexico and evaluated the impact of therapeutic vaccination from the third-party payer and societal perspectives. Figure 1 outlines the model structure. A Chagas-positive pregnant woman entered the model and could transmit the parasite to the fetus in utero at any point during the pregnancy or at birth based on the probability of transmission.[20, 21] Each woman in the model could either be vaccinated or not vaccinated. Vaccination, assumed to be administered during the first trimester, attenuated the probability of congenital transmission by its efficacy and had a probability of minor side effects.[22] We assumed vaccination efficacy and cost was for a full vaccination series, and that each woman was fully compliant with vaccination prior to giving birth.
Figure 1.
Model outline.
Infected infants had a probability of being symptomatic or asymptomatic. Symptomatic infants had a probability of Chagas-attributable mortality within 24 hours after birth. Those that survived, had a probability of Chagas-related preterm birth (<37 weeks gestation), with those preterm infants having an associated probability of mortality due to complications, varying with gestational age. Preterm infants stayed in the neonatal intensive care unit (NICU), with the duration based on their gestational age and mortality risk. Symptomatic full-term infants had a probability of moderate symptoms (e.g., hepatomegaly, splenomegaly, hepatosplenomegaly, anemia, petechiae, anasarca) or severe symptoms (e.g., respiratory distress syndrome, meningoencephalitis, and cardiomyopathy). All full-term infants with symptoms stayed in the NICU for the duration of their symptoms.
Care of infants born to Chagas-positive women followed recommendations by Carlier et al.[8] As all women were Chagas-positive in our model, all infants underwent a micromethod diagnostic test (i.e., direct parasitological detection by microscopic examination) at birth. Infants were deemed Chagas-positive based on the test’s associated sensitivity and specificity and if the infant was infected with T. cruzi. Those testing positive received treatment with benznidazole, regardless of their true infection status (i.e., true and false positives). Those testing negative had a follow-up clinic visit at 8 months of age (i.e., after maternal antibodies had disappeared) to perform serological testing, for which infants had a probability of returning for this testing. The serological testing consisted of an algorithm of 2 tests, an indirect hemagglutination assay (IHA) and an enzyme-linked immunosorbent assay (ELISA based IgG serology) test. Infants were deemed Chagas-positive based on the test sensitivity and specificity and if they were infected with T. cruzi. Again, those testing positive were treated with benznidazole, while a negative IgG serology result confirmed that the infant did not have Chagas.
Benznidazole treatment (5mg/kg/day for 60 days) was based on current recommendations[23, 24] and was associated with probabilities of minor side effects (e.g., allergic reaction), treatment completion (i.e., >80% of regimen), and being curative. As benznidazole is well tolerated and highly effective in children <1-year-old[9, 12, 25], only minor side effects were considered, which was associated with the cost of treatment (i.e., antihistamines).
For each simulation, the incremental cost-effectiveness ratio (ICER) was calculated as:
where health effects were measured in disability-adjusted life years (DALYs) and deaths. DALYs are the sum of the years of life lived with disability (YLD) and years of life lost (YLL) due to Chagas-related deaths. YLL and YLD are calculated as:
The ICER represents the difference in costs and effectiveness between vaccination and no vaccination. As DALYs measure years of perfect health lost (where 0 is perfect health and 1 is death), the ICER calculates the cost per DALY averted, thus uses a denominator in which no vaccination is first.[26]
The third-party payer or health system perspective included all direct medical costs of services and treatment (e.g., hospitalization, diagnostics, treatment), while the societal perspective included direct and indirect (e.g., productivity losses due to absenteeism and mortality) costs. The cost per NICU bed day and duration of the NICU stay were used to estimate NICU hospitalization costs. Daily wage served as a proxy for productivity losses associated with absenteeism and mortality (e.g., human capital approach).[27] A Chagas-specific premature death resulted in accruing the net present value (NPV) of that infant’s lifetime earnings, based on their life expectancy.[28] We assumed all infants with Chagas accrued productivity losses, as everyone is assumed to contribute to society. All costs are in 2019 $US, with all past and future values discounted with a 3% rate. Similarly, all future DALYs are presented in NPV, discounted with a 3% rate. Vaccination was considered highly cost-effective if ICERs were <$9,989 (Mexico’s gross domestic product, GDP, per capita[29]) per DALY averted; cost-effective if 1–3 times the GDP, and not cost-effective if >3 times the GDP.
Data Sources
Table 1 shows the model input parameters, values, and sources. All data were extracted from scientific literature or international databases and were age-specific and Mexico-specific when available. When converting costs between currencies, costs were discounted and then converted into US dollars using an exchange rate of 19.2 which was the average for 2019 as of August 1, 2019.[30] Life expectancy came from the World Health Organization (WHO) Global Health Observatory based on the current birth cohort in Mexico.[28] Daily income came from the reported national quarterly income, which includes other benefits (e.g., pensions, fringe benefits).[27] The risk of congenital transmission came from a systematic review and meta-analysis[7] and used the pooled risk of congenital transmission in endemic countries as the current estimate for this risk (modeled as a distribution with a median of 5%, range: 4% to 6%; Table 1). NICU bed day, clinic visits, and diagnostic tests costs came from Mexico-specific literature and national sources such as the Mexican Social Security Insititute.[31] Disability-weights came from the 2017 Global Burden of Disease.[32] Due to the lack of specific disability weights related to congenital Chagas, the model assumed that acute encephalitis served as a proxy for severe symptoms in infants and moderate anemia due to neglected tropical disease served as a proxy for moderate symptoms.
Table 1.
Model input parameters, values, and sources.
| Parameter | Distribution Type | Mean or Median | Standard Deviation or Range* | Source |
|---|---|---|---|---|
| Costs (2019 $US) | ||||
|
| ||||
| Minor vaccine side effects for the mother (paracetamol) | triangular | 2.17 | 1.31–2.51 | [22] |
| Serological testing algorithm | point estimate | 43.52 | [54] | |
| Micromethod diagnostic test | point estimate | 5.09 | [54] | |
| Benznidazole (100mg) | uniform | 0.128–0.3134 | [55] | |
| Minor benznidazole treatment side effects (antihistamines) | triangular | 2.19 | 0.77–5.25 | [22] |
| NICU bed day | gamma | 167.47 | 45.86 | [56] |
| Follow-up visit | point estimate | 40.69 | [31] | |
| Daily income§ | point estimate | 23.12 | [27] | |
| Lifetime cost per chronic Chagas disease case in Latin | ||||
| America | ||||
| Third-party payer perspective | triangular | 3,198 | 2,418 – 3,731 | [33] |
| Societal perspective | triangular | 29,818 | 14,051 | [33] |
|
| ||||
| Probabilities (annual) | ||||
|
| ||||
| Minor adverse reaction to the vaccine in pregnant women | point estimate | 0.27 | [57, 58] | |
| Congenital transmission (current estimate) | triangular | 0.05 | 0.04–0.06 | [7] |
| Symptomatic infant given Chagas infection | beta | 0.376 | 0.158 | [10, 12, 59–61] |
| Symptomatic infant has severe disease | point estimate | 0.371 | [10, 59–61] | |
| Symptomatic infant has moderate disease | point estimate | 0.629 | [59–61] | |
| Chagas-related mortality at birth (within 24 hours) | point estimate | 0.041 | [10, 59, 60] | |
| Preterm birth given infected infant | uniform | 0.125–0.194 | [59, 62] | |
| Preterm birth given non-infected infant (by gestational age) | ||||
| <28 weeks | point estimate | 0.05 | [63] | |
| 28–31 weeks | point estimate | 0.15 | [63] | |
| 32–33 weeks | point estimate | 0.2 | [63] | |
| 34–36 weeks | point estimate | 0.6 | [63] | |
| Preterm infant mortality (by gestational age) | ||||
| <28 weeks | triangular | 0.53 | 0.23–0.75 | [64] |
| 28–31 weeks | triangular | 0.26 | 0.08–0.51 | [64] |
| 32–33 weeks | triangular | 0.1 | 0.03–0.14 | [64] |
| 34–36 weeks | triangular | 0.0092 | 0.001–0.016 | [65] |
| Sensitivity of micromethod test | beta | 0.29 | 0.148 | [10, 59, 66–69] |
| Specificity of micromethod test | point estimate | 1 | [10, 59, 66–69] | |
| Infant returns for follow-up visit | gamma | 0.422 | 0.185 | [59, 67, 68, 70] |
| Sensitivity of IgG serology ≥8 months old | point estimate | 1 | [10, 71] | |
| Specificity of IgG serology ≥8 months old | triangular | 0.982 | 0.96–1.00 | [10, 71] |
| Minor adverse event related to benznidazole treatment | beta | 0.088 | 0.146 | [1, 12, 25, 69, 72, 73] |
| Infant completed >80% of benznidazole regimen | beta | 0.922 | 0.114 | [1, 12, 25, 68, 69, 72, 73] |
| Infant that completes treatment will be cured | beta | 0.975 | 0.044 | [1, 12, 25, 68, 69, 73] |
| Progressing to chronic Chagas disease | uniform | 0.2 – 0.3 | [34, 35] | |
|
| ||||
| Durations | ||||
|
| ||||
| Severe symptoms (not preterm) | point estimate | 30 days | Expert opinion | |
| Moderate symptoms (not preterm) | point estimate | 7 days | Expert opinion | |
| NICU stay for preterm infants who survive (by gestational age) | ||||
| <28 weeks | triangular | 116 | 97–138 | [64] |
| 28–31 weeks | triangular | 86 | 66–108 | [64] |
| 32–33 weeks | triangular | 49 | 40–60 | [64] |
| 34–36 weeks | triangular | 16 | 12–22 | [65] |
| NICU stay for preterm infants who die (by gestational age) | ||||
| <28 weeks | triangular | 16 | 7–39 | [64] |
| 28–31 weeks | triangular | 24 | 8–59 | [64] |
| 32–33 weeks | triangular | 21 | 2–73 | [64] |
| 34–36 weeks | triangular | 16 | 12–22 | [65] |
|
| ||||
| Disability Weights §§ | ||||
|
| ||||
| Severe symptoms | triangular | 0.133 | 0.088–0.190 | [32] |
| Moderate symptoms | triangular | 0.052 | 0.034–0.076 | [32] |
|
| ||||
| Infant Weight, Full Term (kg) | ||||
|
| ||||
| At birth | gamma | 3.157 | 0.477 | [74] |
| At 8 months for males | gamma | 8.91 | 1 | [75] |
| At 8 months for females | gamma | 8.49 | 1 | [75] |
Note: ELISA=enzyme-linked immunosorbent assay. NICU=neonatal intensive care unit.
Value includes fringe benefits and other income sources (e.g., pensions) as reported by the National Survey on Household Income and Expenditure (ENIGH) as it incorporates additional income beyond formal wages.
Due to the lack of specific disability weights related to congenital Chagas, we assumed that acute encephalitis served as a proxy for severe symptoms in infants and moderate anemia due to neglected tropical disease served as a proxy for moderate symptoms.
Variation in parameter values were based on data availability and variability reported in the literature.
Experiments and Sensitivity Analyses
Each simulation sent a Chagas-positive pregnant woman through the model 2,000 times. Each time she traveled through the model, Monte Carlo simulations (i.e., probabilistic sensitivity analyses) consisting of 2,000 trials simultaneously varied each parameter throughout their ranges (based on data availability and reported variability) in Table 1 (for a total of 4 million trials with unique outcomes). Multiway sensitivity analyses simultaneously varied vaccination cost ($10-$900) and vaccine efficacy (25%−75%) to help identify thresholds at which the economic value of vaccination would change. Vaccination cost was assumed to include the price of the vaccine itself, vaccine administration, and any other health systems costs. Additional multiway sensitivity analyses varied the congenital transmission risk (from 0.1% to 18%[7, 12]) to account for previous treatment and explore the extremes reported in the literature. We also estimated the lifetime costs of those infants that could progress to chronic disease by multiplying the number of cases that could progress by the lifetime cost per chronic Chagas disease case (Table 1), assuming 20–30% would progress to chronic Chagas disease[34, 35].
Results
Clinical and Economic Impact of Therapeutic Vaccination with the Current Transmission Risk Estimate
Table 2 shows the clinical (e.g., number of congenital cases and deaths) and economic (e.g., cost, DALYs) outcomes of Chagas vaccination per 10,000 infected pregnant women based on current estimates for the risk of congenital transmission (distribution with a median of 5%, range 4%−6%). Compared to no vaccination, a 25% efficacious therapeutic vaccination averted 125 (95% UI: 122–128) congenital cases, 1.9 (95% UI: 1.6–2.2) infant deaths, and 78 (95% UI: 66–91) DALYs per 10,000 infected pregnant women; a 50% efficacious vaccine averted 251 (95% UI: 248–254) congenital cases, 3.8 (95% UI: 3.6–4.2) infant deaths, and 160 (95% UI: 148–171) DALYs; and a 75% efficacious vaccine averted 376 (95% UI: 374–378) congenital cases, 5.8 (95% UI: 5.5–6.1) infant deaths, and 238 (95% UI: 227–249) DALYs. Vaccination also reduced the number of infants that can progress to chronic disease due to missing treatment opportunities (i.e., false-negative or lost to follow up) or those that did not successfully complete treatment (i.e., not cured or discontinued). Table 3 shows the number of these infants that progress to chronic disease and the resulting lifetime costs. Vaccination prevented 13–42 infants from later developing chronic Chagas disease, saving $42,487 (95% UI: $39,965-$45,103) to $131,452 (95% UI: $129,242-$133,661)6 from the third-party payer perspective and $403,083 (95% UI: $373,172-$432,995) to $1.22 million (95% UI: $1.19-$1.24 million) from the societal perspective, over the course of their lifetimes (Table 3).
Table 2.
Clinical outcomes [mean (95% uncertainty interval)] and total costs (net present value; $US in millions) among 10,000 Chagas-infected pregnant women at current transmission risk estimates (distribution with a median of 5%, range 4% to 6%) for various vaccination strategies
| Number of Congenital Cases | Number of Infant Deaths | Number of Infants that Can Progress to Chronic Disease | Disability-Adjusted Life Years | Total Cost* |
Incremental Cost-Effectiveness Ratio (ICER)^ |
|||
|---|---|---|---|---|---|---|---|---|
| Third-Party Payer Perspective | Societal Perspective | Third-Party Payer Perspective | Societal Perspective | |||||
| No vaccination | 500 (405–600) | 8 (0–20) | 229 (145–330) | 313.8 (0.7–750.9) $50 vaccination |
1.0 (0.7–1.2) | 3.8 (0.9–11.4) | - | - |
| 25% efficacy | 375 (290–460) | 6 (0–20) | 172 (100–250) | 235.7 (0.5–600.8) | 1.3 (1.1–1.6) | 3.4 (1.3–6.8) | 4,648 | Dominant |
| 50% efficacy | 249 (185–320) | 4(0–15) | 115 (60–190) | 154.0 (0.2–450.4) | 1.2 (1.0–1.4) | 2.6 (1.1–5.4) | 1,438 | Dominant |
| 75% efficacy | 125 (80–170) | 2 (0–10) | 57 (20–110) | 75.7 (0.1–300.2) $100 vaccination |
1.0 (.9–1.2) | 1.8 (1.0–3.8) | 357 | Dominant |
| 25% efficacy | 374 (290–460) | 6 (0–20) | 172 (100–255) | 221.4 (0.5–600.6) | 1.8 (1.6–2.1) | 4.0 (1.8–7.4) | 11,175 | 2,071 |
| 50% efficacy | 250 (180–320) | 4(0–15) | 115 (60–190) | 153.8 (0.2–450.5) | 1.7 (1.5–1.9) | 3.1 (1.6–5.8) | 4,622 | Dominant |
| 75% efficacy | 126 (80–175) | 2 (0–10) | 58 (20–110) | 78.1 (0.1–300.2) $200 vaccination |
1.5 (1.4–1.7) | 2.3 (1.5–4.3) | 2,556 | Dominant |
| 25% efficacy | 376 (295–460) | 6 (0–20) | 173 (100–255) | 231.1 (0.5–600.6) | 2.8 (2.6–3.1) | 5.0 (2.8–8.4) | 24,341 | 13,859 |
| 50% efficacy | 249 (185–320) | 4(0–15) | 115 (55–190) | 151.3 (0.3–450.4) | 2.7 (2.5–2.9) | 4.1 (2.6–6.9) | 10,988 | 1,945 |
| 75% efficacy | 125 (75–175) | 2 (0–10) | 57 (20–115) | 74.5 (0.1–300.2) $500 vaccination |
2.5 (2.4–2.7) | 3.3 (2.5–5.4) | 6,981 | Dominant |
| 25% efficacy | 373 (320–550) | 6 (0–50) | 172 (100–255) | 228.6 (0.7–1,500.0) | 5.8 (5.6–6.1) | 8.0 (2.9–13.3) | 63,528 | 51,900 |
| 50% efficacy | 250 (205–460) | 4(0–25) | 115 (60–190) | 150.3 (0.4–900.0) | 5.7 (5.5–5.9) | 7.1 (2.7–10.5) | 29,040 | 20,811 |
| 75% efficacy | 125 (95–205) | 2 (0–20) | 58 (20–115) | 79.6 (0.1–600.0) | 5.5 (5.4–5.7) | 6.3 (2.5–9.2) | 19,352 | 10,742 |
In millions of $US; Cost includes only those incurred during infancy and excludes lifetime costs associated with chronic Chagas disease.
ICER is cost per disability adjusted life year averted; ICERs ≤$9,989/DALY averted are considered highly cost-effective, ICERs between $9,990–29,697/DALY averted are considered cost-effective, ICERs >$29,697/DALY averted are considered not cost-effective; dominant = less costly and provides health benefits.
Table 3.
Number of infants progressing to chronic Chagas disease per 10,000 infants born to Chagas-positive women [mean (95% uncertainty interval)] and their lifetime costs ($US) for various vaccination strategies (assuming the current transmission risk estimate, a distribution with a median of 5%, range 4% to 6%)
| Number of Infants that Can Progress to Chronic Disease | Number of Infants that Progress to Chronic Disease | Lifetime Third-Party Payer Cost | Lifetime Societal Cost | |
|---|---|---|---|---|
| No Vaccination | 229(145–330) | 56 (34–89) | 174,299 (103,270–279,162) |
1,610,458 (808,222–2,905,913) |
| Vaccination | ||||
| 25% efficacy | 172 (100–250) | 43 (24–67) | 131,765 (70,483–213,175) |
1,207,374 (580,993–2,212,298) |
| 50% efficacy | 115 (60–190) | 29 (14–50) | 88,231 (43,790–157,315) |
803,707 (357,043–1,627,632) |
| 75% efficacy | 57 (20–110) | 14 (5–29) | 42,847 (14,883–91,089) |
391,647 (128,841–911,751) |
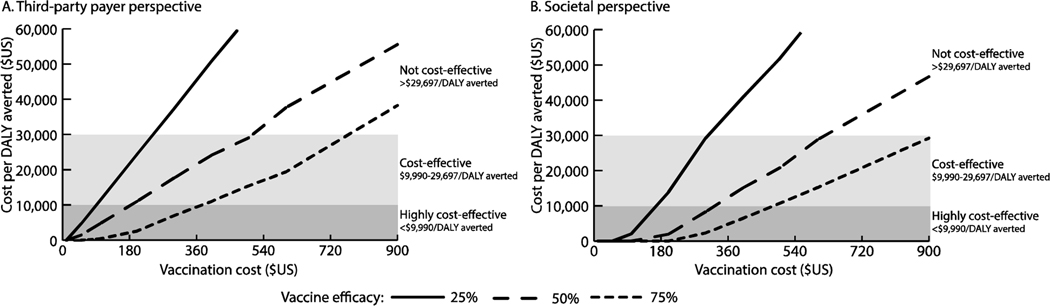
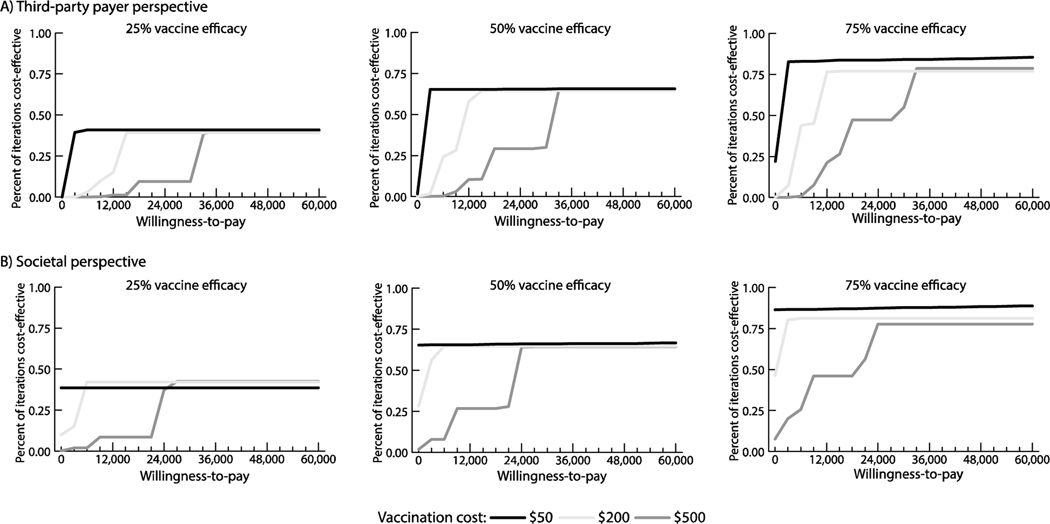
Figure 2 shows how the s varies with vaccination cost and vaccine efficacy from both perspectives and outlines cost-effectiveness thresholds. For example, from the third-party payer perspective, vaccination with a ≥25% efficacious vaccine was cost-effective when ≤$240 (≤$29,023/DALY averted), highly cost-effective when ≤$85 (≤$9,267/DALY averted), and economically dominant (i.e., saved costs and provided health benefits) when ≤$10 (saving ≥$4 per vaccinee) compared to no vaccination. A 75% efficacious vaccine was cost-effective up to $720 (≤$29,837/DALY averted) and up to $930 (≤$29,509/DALY averted) from the third-party payer and societal perspectives, respectively. From the societal perspective, a 25% efficacious vaccine was economically dominant when costing ≤$80 (saving ≥$44 per vaccinee); a 50% efficacious vaccine was dominant when costing ≤$160 (saving ≥$0.50 per vaccinee); and a 75% efficacious vaccine was dominant costing ≤$250 (saving ≥$48 per vaccinee). Figure 4 shows the probability that vaccination is cost-effective compared to no vaccination at various willingness-to-pay thresholds. For example, a 50% efficacious vaccine costing $200 was cost-effective in 58% of simulations and 65% of simulations at a willingness-to-pay threshold of $12,000/DALY averted from the third-party payer and societal perspectives, respectively.
Figure 2.
Impact of varying vaccination cost and vaccine efficacy on the incremental cost-effectiveness ratio (ICER) of a therapeutic vaccine given to pregnant women to prevent congenital transmission with current transmission estimates (distribution with a median of 5%, range: 4%−6%) from (A) the third-party payer perspective, and (B) the societal perspective. An ICER of 0 indicates that the vaccination strategy would be economically dominant (i.e., provides cost savings and positive health benefits); ICERs >$60,000/disability-adjusted life year (DALY) averted are not shown.
Figure 4.
Percent of simulation trials in which vaccination was cost-effective compared to no vaccination over a range of willingness-to-pay thresholds from the (A) third-party payer perspective and (B) societal perspective.
Clinical and Economic Impact of Therapeutic Vaccination when Varying Transmission Risk
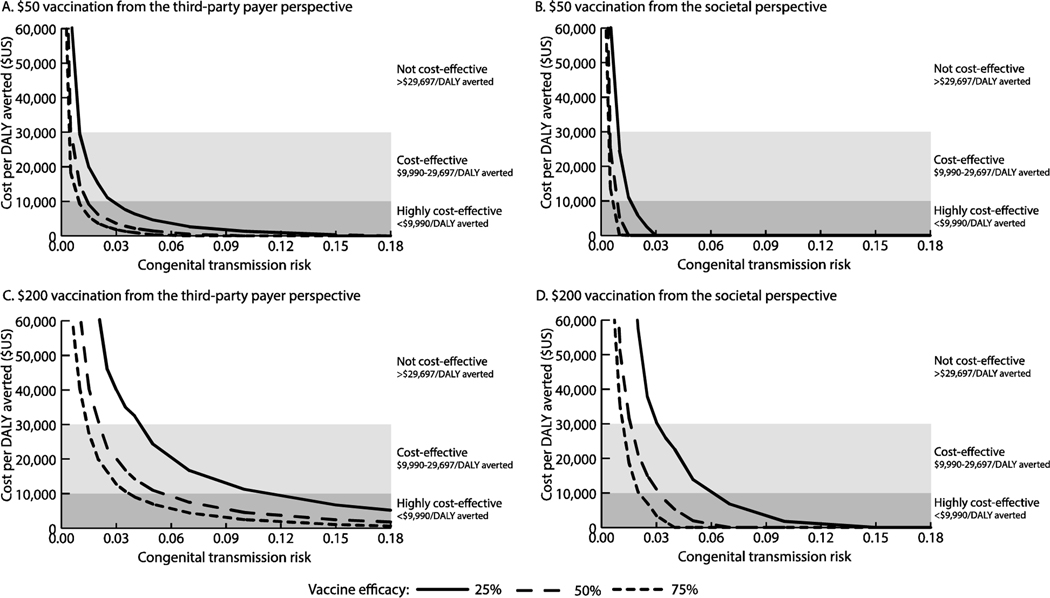
Figure 3 shows how the ICER varies with the risk of congenital transmission (from 0.1% to 18%) and vaccine efficacy. With lower risks for congenital transmission, the change in ICERs had a steeper slope than higher risks of transmission (Figure 3). Vaccination was cost-effective for risks ≥0.4% (≤$25,497/DALY averted) from the third-party payer perspective and for risks ≥0.3% (≤$24,196/DALY averted) from the societal perspective when ≥75% efficacious and costing ≤$50. Vaccination was dominant (saving ≥$3 per vaccinee) when then transmission risk was ≥1.1% and the vaccination cost $50 and was 75% efficacious from the societal perspective (Figure 3b). While a $200 vaccination was dominant when the risk was ≥4% and the vaccine was ≥75% efficacious. A 25% efficacious, $50 vaccine was highly cost-effective when the risk of transmission was ≥3% and dominant when ≥18% (third-party payer perspective).
Figure 3.
Impact of varying vaccine efficacy and congenital transmission risk on the incremental cost-effectiveness ratio (ICER) of a therapeutic vaccine given to pregnant women to prevent congenital transmission with (A) $50 vaccination from the third-party payer perspective, (B) $50 vaccination from the societal perspective, (C) $200 from the third-party payer perspective, and (D) $200 from the societal perspective. An ICER of 0 indicates that the vaccination strategy would be economically dominant (i.e., provides cost savings and positive health benefits); ICERs >$60,000/disability-adjusted life year (DALY) averted are not shown.
Discussion
This study defines circumstances and vaccine characteristics thresholds where a therapeutic Chagas disease vaccine for pregnant women to prevent congenital transmission would be cost-effective, highly cost-effective, and cost saving. Vaccination can avert up to 375 congenital cases and 5 infant deaths per 10,000 Chagas-infected pregnant women. This vaccine does not need to have highly favorable characteristics (e.g., 75% efficacy) in order to be beneficial. Vaccination can cost as high as $900 and still be cost-effective under certain conditions. Of course, this cost includes the vaccine, administration, supply chain and logistics, and service delivery costs, but a large percentage of this cost may be the price of the vaccine. The price of the vaccine can be a challenging issue. Showing that a higher vaccine price can be supported may encourage more manufacturers to develop and produce Chagas disease vaccines. However, the vaccine also needs to be affordable to be of broader use.
These results show that even a 25% efficacious vaccine could be cost-effective, suggesting that even with a lower efficacy, it still would have value. Expecting all vaccines to have efficacies above 50% is unrealistic and discounts the value that vaccines with lower efficacy may provide. If a candidate vaccine proves in preclinical/clinical trials to have a low efficacy, it does not mean this candidate should be disregarded as some vaccines have low to modest efficacies (e.g., the influenza[36], Bacille Calmette-Guerin (BCG)[37, 38], and RTS, S[39–42] vaccines). Thus, if the Chagas disease vaccine candidate proves to have an efficacy below 50%, it should not necessarily be a reason to discontinue development or prevent use.
Pregnant women represent an ideal target population for vaccination as once Chagas-positive and pregnant, it is too late to potentially prevent congenital transmission given current drug treatments (e.g., benznidazole and nifurtimox) are contraindicated in pregnancy[9, 11, 12]. This may be the case in many situations as many Chagas-infected individuals do not know they are infected, and many remain unidentified until experiencing symptoms associated with chronic disease. In this setting, the therapeutic vaccine would be a stand-alone antenatal intervention. However, the possibility remains that it might also find use in a vaccine-linked chemotherapy strategy, in which an antiparasitic drug is co-administered with the therapeutic vaccine. Potentially this approach could reduce the dose required for drug treatment, possibly increasing the safety profile of current antiparasitic drugs for use in pregnancy. Still another possible target population for vaccination is women prior to pregnancy (i.e., preconception).[43] This is based on the success of treating this population, wherein several studies have found that treating women prior to pregnancy will prevent congenital infection.[11, 12, 44, 45]
In each of these settings, both women’s health and antenatal care clinics present important opportunities to vaccinate those that are infected with T. cruzi. Thus, serving as a primary prevention strategy for infants and a complementary intervention to existing antiparasitic treatment and prevention.[43] An overall goal of linking a therapeutic Chagas disease vaccine to reproductive health would be to eliminate congenital Chagas disease. Even as vector control initiatives continue to be effective, congenital infections accounted for approximately 22% of all infections in 2010.[46]
The vaccine’s proposed mechanism of action would include both reducing the maternal parasite burden in target organs (including the heart, thereby reducing cardiac fibrosis) and possibly parasitemia, which is a key factor associated with congenital transmission. In fact, even if the risk of transmission was ≥0.3% (under the 5% currently estimated[7]), a vaccine would still be cost-effective. While observational studies suggest that in women previously treated with benznidazole the congenital transmission risk is 0%[11, 44, 45], randomized controlled trials have not been performed. Thus, a modest risk of transmission (e.g., lower than 5%) may be possible, thus, potentially leaving room for additional reduction in transmission by a vaccine.
However, economics is not the only thing to consider when determining if a vaccine is appropriate for pregnant women, as there are additional challenges, such as the type of vaccine, timing of immunization, and how to implement immunization. Live vaccines are not safe to administer during pregnancy[47]; yet, other vaccine formulations (killed or inactivated) serve as the basis for several routine vaccines given to pregnant women, such as influenza; tetanus, diphtheria, and pertussis (Tdap); hepatitis B; and polio.[48] Other considerations include the education of mothers and providers, healthcare services utilization, communications, and long-term surveillance and assessment of vaccine safety.[49] Developers and decision makers need to understand these key challenges for development and implementation, as well as thresholds of vaccination cost and efficacy that make this vaccine cost-effective. This study further builds the body of evidence showing the clinical and economic benefits of vaccination for Chagas disease.[50–52]
To be conservative, we did not include long-term outcomes of Chagas disease in infected, untreated children in the ICER calculations, as this would have increased the value of vaccination. However, we did estimate the costs these children could incur and the potential savings due to vaccination. The model did not include specific care for infants with mild symptoms as treatment is non-specific and it is often unknown that they are Chagas-positive. We also did not include any potential benefits of vaccination to the mother. For example, previous work has shown that therapeutic vaccination to reduce the progression of Chagas disease for patients with indeterminate disease and those with early cardiac symptoms is highly cost-effective and provides cost savings.[52] Including these would further increase the value of vaccination for a pregnant woman.
Limitations
All models are simplifications of real-life and therefore cannot account for every Chagas event or outcome. Current literature on congenital Chagas disease is limited and therefore, the model drew from data of varying quality, thus these results can be refined as new and better data become available. Additionally, the model did not distinguish between different treatments within Chagas symptom categories (moderate and severe) because the included symptoms are treated based on the underlying cause. The model did not include severe vaccine side effects as they are uncommon in currently existing vaccines.[53] We included only costs incurred during the NICU stay and those related to Chagas-diagnosis and treatment post-discharge. Therefore, we did not consider homecare costs such as pharmacy visits. We also did not include costs parents/caregivers may incur such as co-payments for treatment (which would vary based on insurance), transportation, food, lodging, or additional productivity losses which they may incur.
Conclusion
This study outline thresholds where therapeutic Chagas vaccination for an infected pregnant woman to prevent congenital transmission would be cost-effective, highly cost-effective, and cost saving for decision-makers such as developers, manufacturers, funders, and policy makers to consider when developing and implementing a therapeutic Chagas vaccine.
Acknowledgments
This work was supported by the Carlos Slim Foundation, the Agency for Healthcare Research and Quality (AHRQ) via grant R01HS023317, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) via grant U01HD086861 and R01HD086013, the NICHD and Office of Behavioral and Social Sciences Research (OBSSR) under award number U54HD070725, and the United States Agency for International Development (USAID) under agreement number AID-OAA-A-15-0064. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Salas N, Cot M, Schneider D, Mendoza B, Santalla J, Postigo J, et al. Risk factors and consequences of congenital Chagas disease in Yacuiba, south Bolivia. Tropical Medicine & International Health. 2007;12:1498–505. [DOI] [PubMed] [Google Scholar]
- [2].Clavijo NS, Postigo J, Schneider D, Santalla J, Brutus L, Chippaux J-P. Prevalence of Chagas disease in pregnant women and incidence of congenital transmission in Santa Cruz de la Sierra, Bolivia. Acta Tropica. 2012;124:87–91. [DOI] [PubMed] [Google Scholar]
- [3].Rendell VR, Gilman RH, Valencia E, Galdos-Cardenas G, Verastegui M, Sanchez L, et al. Trypanosoma cruzi-infected pregnant women without vector exposure have higher parasitemia levels: implications for congenital transmission risk. PLoS One. 2015;10:e0119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cucunuba ZM, Florez AC, Cardenas A, Pavia P, Montilla M, Aldana R, et al. Prevalence and risk factors for Chagas disease in pregnant women in Casanare, Colombia. Am J Trop Med Hyg. 2012;87:837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mendoza Ticona CA, Cordova Benzaquen E, Ancca Juarez J, Saldana Diaz J, Torres Choque A, Velasquez Talavera R, et al. [The prevalence of Chagas’ disease in puerperal women and congenital transmission in an endemic area of Peru]. Rev Panam Salud Publica. 2005;17:147–53. [DOI] [PubMed] [Google Scholar]
- [6].Montes-Rincon LM, Galaviz-Silva L, Gonzalez-Bravo FE, Molina-Garza ZJ. Trypanosoma cruzi seroprevalence in pregnant women and screening by PCR and microhaematocrit in newborns from Guanajuato, Mexico. Acta Trop 2016;164:100–6. [DOI] [PubMed] [Google Scholar]
- [7].Howard EJ, Xiong X, Carlier Y, Sosa-Estani S, Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: A systematic review and meta-analysis. BJOG: An International Journal of Obstetrics & Gynaecology. 2014;121:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carlier Y, Torrico F, Sosa-Estani S, Russomando G, Luquetti A, Freilij H, et al. Congenital Chagas disease: Recommendations for diagnosis, treatment and control of newborns, siblings and pregnant women. PLoS Neglected Tropical Diseases. 2011;5:e1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Messenger LA, Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Current Opinion in Infectious Diseases. 2018;31:415–21. [DOI] [PubMed] [Google Scholar]
- [10].Messenger LA, Gilman RH, Verastegui M, Galdos-Cardenas G, Sanchez G, Valencia E, et al. Toward improving early diagnosis of congenital Chagas disease in an endemic setting. Clinical Infectious Diseases. 2017;65:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fabbro DL, Danesi E, Olivera V, Codebó MO, Denner S, Heredia C, et al. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Neglected Tropical Diseases. 2014;8:e3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Murcia L, Simón M, Carrilero B, Roig M, Segovia M. Treatment of infected women of childbearing age prevents congenital Trypanosoma cruzi infection by eliminating the parasitemia detected by PCR. The Journal of Infectious Diseases. 2017;215:1452–8. [DOI] [PubMed] [Google Scholar]
- [13].Beaumier CM, Gillespie PM, Strych U, Hayward T, Hotez PJ, Bottazzi ME. Status of vaccine research and development of vaccines for Chagas disease. Vaccine. 2016;34:2996–3000. [DOI] [PubMed] [Google Scholar]
- [14].Lee BY, Bartsch SM. How to determine if a model is right for neglected tropical disease decision making. PLoS Negl Trop Dis. 2017;11:e0005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee BY, Mueller LE, Tilchin CG. A systems approach to vaccine decision making. Vaccine. 2017;35 Suppl 1:A36–A42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010;28:2806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hotez PJ, Damania A, Naghavi M. Blue Marble Health and the Global Burden of Disease Study 2013. PLoS Negl Trop Dis. 2016;10:e0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chagas disease in Latin America: An epidemiological update based on 2010 estimates. Weekly Epidemiological Record. 2015;90:33–43. [PubMed] [Google Scholar]
- [19].Arnal A, Waleckx E, Rico-Chávez O, Herrera C, Dumonteil E. Estimating the current burden of Chagas disease in Mexico: A systematic review and meta-analysis of epidemiological surveys from 2006 to 2017. PLoS Neglected Tropical Diseases. 2019;13:e0006859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carlier Y, Truyens C. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta and fetuses. Acta Tropica. 2015;151:103–15. [DOI] [PubMed] [Google Scholar]
- [21].Cevallos AM, Hernández R. Chagas’ disease: pregnancy and congenital transmission. BioMed Research International. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Management Sciences for Health. International Medical Products Price Guide. 2016. [Google Scholar]
- [23].Carlier Y, Truyens C. Maternal–fetal transmission of Trypanosoma cruzi. American Trypanosomiasis: Elsevier; 2010. p. 539–81. [Google Scholar]
- [24].Edwards MS, Stimpert KK, Montgomery SP. Chagas Disease. Neonatal Infections: Springer; 2018. p. 75–82. [Google Scholar]
- [25].Chippaux JP, Clavijo ANS, Santalla JA, Postigo JR, Schneider D, Brutus L. Antibody drop in newborns congenitally infected by Trypanosoma cruzi treated with benznidazole. Tropical Medicine & International Health. 2010;15:87–93. [DOI] [PubMed] [Google Scholar]
- [26].Lee BY, Bartsch SM, Gorham KM. Economic and financial evaluation of neglected tropical diseases. Advances in Parasitology. 2015;87:329–417. [DOI] [PubMed] [Google Scholar]
- [27].Instituto Nacional de Estadistica y Geografia. Encuesta Nacional de Ingresos y Gastos de los Hogares (ENIGH). [National Household Income and Expenditure Survey] In Spanish. 2016. [Google Scholar]
- [28].World Health Organization. Global Health Observatory: Country Statistics. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- [29].The World Bank. World Development Indicators, 1960–2019. The World Bank; 2019. [Google Scholar]
- [30].Bloomberg Markets. USDMXN Spot Exchange Rate. New York City, NY: Bloomberg L.P.; 2018. [Google Scholar]
- [31].Gobjerno de Mexico. Instituto Mexicano del Seguro Social. In Spanish. 2019. [Google Scholar]
- [32].Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2017 (GBD 2017) Disability Weights. Seattle, United States: Institute for Health Metrics and Evaluation (IHME); 2018. [Google Scholar]
- [33].Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. Global economic burden of Chagas disease: a computational simulation model. The Lancet Infectious Diseases. 2013;13:342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Perez-Molina JA, Molina I. Chagas disease. Lancet. 2018;391:82. [DOI] [PubMed] [Google Scholar]
- [35].Bern C. Chagas’ Disease. N Engl J Med. 2015;373:456–66. [DOI] [PubMed] [Google Scholar]
- [36].Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. CDC Seasonal Flu Vaccine Effectiveness Studies. [Google Scholar]
- [37].Barreto ML, Pereira SM, Ferreira AA. BCG vaccine: efficacy and indications for vaccination and revaccination. J Pediatr (Rio J). 2006;82:S45–54. [DOI] [PubMed] [Google Scholar]
- [38].Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].RTS S, Clinical Trials Partnership, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. [DOI] [PubMed] [Google Scholar]
- [41].Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, et al. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N Engl J Med. 2016;374:2519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.[] RTS S, Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11:e1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dumonteil E, Herrera C, Buekens P. A therapeutic preconceptional vaccine against Chagas disease: A novel indication that could reduce congenital transmission and accelerate vaccine development. PLoS Neglected Tropical Diseases. 2019;13:e0006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moscatelli G, Moroni S, García-Bournissen F, Ballering G, Bisio M, Freilij H, et al. Prevention of congenital Chagas through treatment of girls and women of childbearing age. Memórias do Instituto Oswaldo Cruz. 2015;110:507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Álvarez MG, Vigliano C, Lococo B, Bertocchi G, Viotti R. Prevention of congenital Chagas disease by Benznidazole treatment in reproductive-age women. An observational study. Acta Tropica. 2017;174:149–52. [DOI] [PubMed] [Google Scholar]
- [46].World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Weekly Epidemiological Record. 2015;90:33–44. [PubMed] [Google Scholar]
- [47].National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1–64. [PubMed] [Google Scholar]
- [48].Center for Disease Control and Prevention. Guidelines for Vaccinating Pregnant Women. Toolkit for Prenatal Care Providers. cdc.gov; 2016. [Google Scholar]
- [49].Munoz FM. Current Challenges and Achievements in Maternal Immunization Research. Front Immunol. 2018;9:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lee BY, Bacon KM, Connor DL, Willig AM, Bailey RR. The potential economic value of a Trypanosoma cruzi (Chagas Disease) vaccine in Latin America. PLoS Neglected Tropical Diseases. 2010;4:e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lee BY, Bacon KM, Wateska AR, Bottazzi ME, Dumonteil E, Hotez PJ. Modeling the economic value of a Chagas’ disease therapeutic vaccine. Hum Vaccin Immunother. 2012;8:1293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bartsch SM, Bottazzi ME, Asti L, Strych U, Meymandi S, Falcon-Lezama JA, et al. Economic value of a therapeutic Chagas vaccine for indeterminate and Chagasic cardiomyopathy patients. Vaccine. 2019;37:3704–14. [DOI] [PubMed] [Google Scholar]
- [53].Spencer JP, Trondsen Pawlowski RH, Thomas S. Vaccine Adverse Events: Separating Myth from Reality. Am Fam Physician. 2017;95:786–94. [PubMed] [Google Scholar]
- [54].Secretaria de Hacienda y Credito Publico, Unidad de Políticas de Ingresos No Tributarios. Anexo I: Aprovechamientos Autorizados al Intituto de Ddiagnostico y Referencia Epidemiologicos. In Spanish. 2018. [Google Scholar]
- [55].Pinheiro E, Brum-Soares L, Reis R, Cubides JC. Chagas disease: review of needs, neglect, and obstacles to treatment access in Latin America. Rev Soc Bras Med Trop. 2017;50:296–300. [DOI] [PubMed] [Google Scholar]
- [56].Martínez-Valverde S, Castro-Ríos A, Salinas-Escudero G, Villasis-Keever MA, Garduño-Espinosa J, Muñoz-Hernández O. Direct medical costs of neonatal respiratory distress syndrome in two specialized public hospitals in Mexico. Salud Pública de México. 2014;56:612–8. [DOI] [PubMed] [Google Scholar]
- [57].Diez-Domingo J, Weinke T, de Lomas JG, Meyer CU, Bertrand I, Eymin C, et al. Comparison of intramuscular and subcutaneous administration of a herpes zoster live-attenuated vaccine in adults aged≥ 50 years: a randomised non-inferiority clinical trial. Vaccine. 2015;33:789–95. [DOI] [PubMed] [Google Scholar]
- [58].Sari T, Tulek N, Bulut C, Oral B, Ertem GT. Adverse events following rabies post-exposure prophylaxis: A comparative study of two different schedules and two vaccines. Travel Medicine and Infectious Disease. 2014;12:659–66. [DOI] [PubMed] [Google Scholar]
- [59].Blanco SB, Segura EL, Cura EN, Chuit R, Tulián L, Flores I, et al. Congenital transmission of Trypanosoma cruzi: An operational outline for detecting and treating infected infants in north-western Argentina. Tropical Medicine & International Health. 2000;5:293–301. [DOI] [PubMed] [Google Scholar]
- [60].Torrico F, Alonso-Vega C, Suarez E, Rodriguez P, Torrico M-C, Dramaix M, et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. The American Journal of Tropical Medicine and Hygiene. 2004;70:201–9. [PubMed] [Google Scholar]
- [61].Freilij H, Altcheh J. Congenital Chagas’ disease: diagnostic and clinical aspects. Clinical Infectious Diseases. 1995;21:551–5. [DOI] [PubMed] [Google Scholar]
- [62].Negrette OS, Mora MC, Basombrio MA. High prevalence of congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics. 2005;115:e668–e72. [DOI] [PubMed] [Google Scholar]
- [63].Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. The Lancet. 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Profit J, Lee D, Zupancic JA, Papile L, Gutierrez C, Goldie SJ, et al. Clinical benefits, costs, and cost-effectiveness of neonatal intensive care in Mexico. PLoS Medicine. 2010;7:e1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ancel P-Y, Goffinet F, Kuhn P, Langer B, Matis J, Hernandorena X, et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: Results of the EPIPAGE-2 cohort study. JAMA Pediatrics. 2015;169:230–8. [DOI] [PubMed] [Google Scholar]
- [66].Mora MC, Negrette OS, Marco D, Barrio A, Ciaccio M, Segura MA, et al. Early diagnosis of congenital Trypanosoma cruzi infection using PCR, hemoculture, and capillary concentration, as compared with delayed serology. Journal of Parasitology. 2005;91:1468–74. [DOI] [PubMed] [Google Scholar]
- [67].Bern C, Verastegui M, Gilman RH, LaFuente C, Galdos-Cardenas G, Calderon M, et al. Congenital Trypanosoma cruzi transmission in Santa Cruz, Bolivia. Clinical Infectious Diseases. 2009;49:1667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Alonso-Vega C, Billot C, Torrico F. Achievements and challenges upon the implementation of a program for national control of congenital Chagas in Bolivia: Results 2004–2009. PLoS Neglected Tropical Diseases. 2013;7:e2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Russomando G, De Tomassone M, De Guillen I, Acosta N, Vera N, Almiron M, et al. Treatment of congenital Chagas’ disease diagnosed and followed up by the polymerase chain reaction. The American journal of tropical medicine and hygiene. 1998;59:487–91. [DOI] [PubMed] [Google Scholar]
- [70].De Rissio AM, Riarte AR, García MM, Esteva MI, Quaglino M, Ruiz AM. Congenital Trypanosoma cruzi infection. Efficacy of its monitoring in an urban reference health center in a non-endemic area of Argentina. The American Journal of Tropical Medicine and Hygiene. 2010;82:838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin MJ, et al. Aetiological treatment of congenital Chagas’ disease diagnosed and monitored by the polymerase chain reaction. Journal of Antimicrobial Chemotherapy. 2003;52:441–9. [DOI] [PubMed] [Google Scholar]
- [72].Altcheh J, Moscatelli G, Moroni S, Garcia-Bournissen F, Freilij H. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics. 2011;127:e212. [DOI] [PubMed] [Google Scholar]
- [73].Murcia L, Carrilero B, Munoz-Davila MJ, Thomas MC, López MC, Segovia M. Risk factors and primary prevention of congenital Chagas disease in a nonendemic country. Clinical Infectious Diseases. 2012;56:496–502. [DOI] [PubMed] [Google Scholar]
- [74].Gonzalez F, Kumar S. Prenatal Care and Birthweight in Mexico (Vol 50, pg 1156, year 2017). Applied Economics. 2018;50. [Google Scholar]
- [75].Botton J, Heude B, Maccario J, Ducimetière P, Charles M-A, Group FS. Postnatal weight and height growth velocities at different ages between birth and 5 y and body composition in adolescent boys and girls. The American Journal of Clinical Nutrition. 2008;87:1760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]