Supplemental Digital Content is Available in the Text. The temporal nonrandom variation in pain tolerance and the associations with meteorological variables suggest that weather has a causal and dynamic effect on pain tolerance
Keywords: Meteorological variables, Weather, Pain tolerance
Abstract
It is a common belief that weather affects pain. Therefore, we hypothesized that weather can affect pain tolerance. This study used data from over 18,000 subjects aged 40 years or older from the general population, who participated in the Tromsø Study 7. They underwent a one-time assessment of cuff algometry pressure pain tolerance (PPT) and cold pain tolerance (CPT), tested with a cold pressor test. The results showed a clear seasonal variation in CPT. The rate of withdrawal in the cold pressor test was up to 75% higher in months in the warmer parts of the year compared with January 2016. There was no seasonal variation in PPT. The study not only found a nonrandom short-term variation in PPT but also indications of such a variation in CPT. The intrinsic timescale of this short-term variation in PPT was 5.1 days (95% % confidence interval 4.0-7.2), which is similar to the observed timescales of meteorological variables. Pressure pain tolerance and CPT correlated with meteorological variables, and these correlations changed over time. Finally, temperature and barometric pressure predicted future values of PPT. These findings suggest that weather has a causal and dynamic effect on pain tolerance, which supports the common belief that weather affects pain.
1. Introduction
It is a common belief that weather or constituents of weather, such as temperature, barometric pressure, and humidity, causes or aggravates episodes of pain.36,38 This effect has been suggested to apply to pain of diverse origins, ranging from musculoskeletal pain3 to headache24,41 and migraine.41 In a study of weather patterns and pain, chronic pain sufferers experienced the most pain on days characterized by below-normal barometric pressure, higher precipitation, above-normal relative humidity, and stronger winds.32 However, other studies have shown conflicting results; many authors have concluded that the effect of weather on pain is either nonexistent or very small.3,42 These conflicting results could be due to differing methodologies, the complexity underlying pain, and how we experience weather. Few existing studies neither have had sufficient power to address possible nonlinear associations nor did they use methodologies that could address the issue of nonlinearity. Studies on the association between temperature and mortality suggest a nonlinear relationship between weather and health.13 Another problem is that the effect of weather likely depends on the preceding weather; indeed, one experiences a temperature of 10°C differently when the preceding temperature was −5°C than when it was 25°C. Therefore, the effect of weather on pain may vary depending on the current, the preceding, and the change in weather.
Humans adapt physiologically to the climate they live in; they can show a reduced response to cold temperatures after being exposed to them only a few times.25 This adaptation could contribute to the differences observed in the association between temperature and mortality across cities, countries, and times of the year,13,23 as well as to different results regarding pain and weather. In addition, different meteorological variables might interact, eg, humidity and wind speed may alter the experienced temperature. Furthermore, adaptation to one stressor could affect the response to a novel stressor.6 This cross-adaptation and cross-sensitization could imply that the preceding temperature alters the effect of barometric pressure on the organism. This possible nonlinearity and state dependency is typical of biological systems and may occlude analyses and possible causal relationships, ie, weather and pain could be positively correlated, negatively correlated, or not correlated, depending on when, where, and over what period the associations are studied.35 Owing to these characteristics, traditional regression analyses are not suited to capture the actual association between weather and pain.
One way to study the effect of weather on pain is to use self-reported pain, which can be influenced by participants' beliefs regarding the connection between weather and health. Quantitative sensory testing is another way to assess the effect of weather on the sensory system. Different tests attempt to measure the amount of painful stimuli a person can tolerate.5,15,19 Although experimental pain tolerance is not the same as the experience of chronic pain, chronic pain sufferers have been reported to have a lower pain tolerance.5,15,19 We hypothesize that meteorological variables have an effect on pain tolerance and aim to investigate the seasonal variation and impact of weather on pain tolerance.
2. Methods
We used data from the seventh survey of the Tromsø Study (Tromsø 7). Tromsø is located at 69° north, with a mean temperature of −3.3°C in February as the coldest month, and a mean of 12.3°C in July. The westerlies give rise to frequent low pressure systems that affect the climate in the area. Tromsø 7 was conducted from March 2015 to November 2016. A total of 32,591 individuals aged 40 years or older were invited, 21,083 participated, 19,540 performed at least one test of pain tolerance, 18,987 performed the cuff algometry test, and 18,285 underwent the cold pressor test. Examination dates were randomly selected, and participants could choose another date if the given date was not suitable. During the examination, participants cycled through all research stations, normally starting with a physical examination station, followed by various questionnaire stations, and finally cuff algometry and the cold pressor test station. However, wait times did occur at the stations, and these times differed depending on the number of people attending at that moment. Acclimatization time was calculated as the time between the physical examination station and the cold pressor test station; we were unable to include any wait time that occurred before the physical examination.
2.1. Pressure pain tolerance
Pressure pain tolerance (PPT) was tested with computerized cuff algometry (NociTech, Aalborg, Denmark). Both legs were fitted with a cuff. Starting with one leg, the cuff was inflated by 1 kPa/s to the maximum pressure the participant could tolerate or to 100 kPa, whichever came first; then the procedure was repeated on the other leg. Pressure pain tolerance was calculated by taking the mean of the 2 inflations, one on each leg, for each participant. For amputees and those with a cast, the test was performed on one leg (ramp), and the single test results were used.
Participants were asked whether there was a reason not to undergo the test. Only those who stated no reason, were willing, and had no open sores were tested. Examples of reasons for not completing the test included hyperalgesia or problems with peripheral circulation. Individuals unable to understand instructions did not undergo cuff algometry.
2.2. Cold pain tolerance
Cold pain tolerance (CPT) was tested by the cold pressor test. Participants submerged their open and relaxed dominant hand and wrist into a 13-L plexi-glass vat containing circulating cold water (3.0°C). Temperature and continuous circulation of the water were controlled by an attached cooling circulator (Julabo FP40HE; Julabo Labortechnik GmbH, Seelbach, Germany, 22 L/min). Participants were asked to hold their hand and wrist in the water as long as possible, up to a maximum of 120 seconds. Time to withdrawal was used as the outcome of the test.
Participants were asked whether there was a reason not to perform the test. Only those who stated no reason and were willing underwent the cold pressor test. Examples of reasons for not performing the test included Raynaud syndrome or cold allergy that the participant believed to be an obstacle, bilateral loss of sensitivity, or breached skin affecting both hands. Individuals unable to understand instructions did not undergo the cold pressor test.
2.3. Meteorological variables
Data on daily mean temperature, barometric pressure, precipitation, relative humidity, and wind speed for the period 1990 to 2020 were obtained from the Meteorological Institute of Norway's web services (eKlima.net). As there is little geographical variation in weather within the municipality of Tromsø, we used the daily mean of meteorological observations from one station (Tromsø 90450). The station is located approximately 2.5 km from the test center of the Tromsø Study 7. A large majority of the inhabitants in Tromsø municipality live within 10 km from this station. To eliminate seasonal variation in meteorological data, we calculated meteorological anomalies as the difference between expected and observed meteorological variables. The expected meteorological variables for each specific date were determined by creating a 7-day moving average for the period 1990 to 2020 and calculating the mean of these averages for each date. We then determined the meteorological anomalies for each date by subtracting the expected from the observed values.
2.4. Chronic pain
Data on chronic pain were obtained with the question “Do you have persistent or recurrent pain lasting 3 months or more” (Yes/No).
2.5. Statistical analysis
2.5.1. Seasonal variation
To investigate the variation in pain tolerance throughout the study period, we categorized participants according to the month in which they were examined and calculated the range, median, and quartiles of PPT and CPT.
For CPT, we performed a Cox proportional hazard regression with the month of examination as the exposure and time to withdrawal as the survival time. We used January 2016 as the reference month and assessed the proportional hazard assumption with Schoenfeld residuals and log–log plots. The difference in hazard between sexes tended to decrease during the cold pressor test. However, stratified analysis or models allowing the effect of sex to vary over time had little effect on the estimates for months. Sex is therefore included as a covariate. To test the possible interaction between age, sex, and month of examination, we included interaction terms for age and sex in the regression model. The pretest hand temperature could bias the result, as the shock from the cold water might be less for a hand that was already cold. Therefore, we fitted an interaction term between the month of examination and acclimatization time. We also repeated the analysis in the subgroup of participants with an acclimatization time >60 minutes.
2.5.2. Short-term variation
To investigate the possible variation in shorter time periods, eg, days and weeks, we used daily mean PPT. Owing to right censoring in the data from the cold pressor test, we calculated daily CPT as the daily proportion of participants with a time to withdrawal >100 seconds in the cold pressor test. To illustrate the variation throughout the study period, we created 7-day moving averages of the daily measures of PPT and CPT. Because of a seasonal variation in CPT, we fitted a sinusoidal curve to the daily CPT and used the difference between the sinusoidal curve and the daily CPT to study the short-term variation in CPT. To identify any possible correlation from one day to the next, we calculated the autocorrelation for each time series. For time series with an autocorrelation, we assumed an exponential decay and used a generalized linear model with gamma distribution and a log-link function to estimate the average timescale of which the different measures of pain tolerance varied. We repeated the same procedure for meteorological anomalies. Assuming an exponential decay in the autocorrelation is an often used method for calculating the intrinsic timescales of different phenomena, for example, in neuroscience.27
To study if there was any difference in attendance by sex, age, or chronic pain, we calculated the proportion of women and participants reporting chronic pain at each date and used the daily mean of age. Owing to a drop in both age and proportion of women from July 2016, we repeated the time series analysis in a reduced data set, which included data from March 2015 to July 2016. We then used the reduced data set to calculate the autocorrelation for daily proportion women and participants reporting chronic pain, as well as for the daily mean of age. To further investigate if differences in mean age, proportion of women, day of week, or study technician rotation could introduce the observed autocorrelation in pain tolerance, we first conducted univariate analysis for each variable. We then made 500 randomly shuffled copies of the PPT time series. Using these copies, with no association to weather, we simulated the effect of sex, age, day of the week, and study technician rotation by adding twice the observed differences from the univariate analysis. For each randomly shuffled copy, we calculated the autocorrelation. Finally, we tested if the combination of sex, age, and study technician rotation could be the source of the observed autocorrelation.
2.5.3. Association between pain tolerance and meteorological variables
We created 3-day moving averages for PPT and the daily measures of CPT with the seasonality removed and used cross-correlation to investigate the possible association between pain tolerance and meteorological variables. We primarily used meteorological anomalies as they do not have any seasonal variation, but as the calculation of anomalies introduces noise in the time series, we repeated the analysis with the observed meteorological variables. As we expected to find different correlations in different periods, we first performed the cross-correlation for the whole period, then for each half year. To assess the likelihood of spurious correlations, we repeated this process for 500 randomly shuffled copies of PPT and CPT. One single correlation coefficient outside these random simulations would correspond to a P-value of approximately 0.002.
To describe the weather in periods with high or low pain tolerance, we chose the local maxima and minima that were above the 90th or below 10th percentile in the 3-day moving average of PPT and CPT (supplementary Figs. 1 and 2, available at http://links.lww.com/PAIN/B464). If 2 maxima or minima were closer than 6 days together, we defined them as being from the same maximum or minimum. We then calculated the mean of the 3-day moving averages of PPT and CPT for those days, as well as for 14 days before and after, and the mean of the 3-day moving averages of the meteorological anomalies for the same days.
To test if meteorological variables could predict future pain tolerance, we fitted a vector autoregressive model to the daily means of PPT, temperature, and barometric pressure. We used both meteorological anomalies and the observed temperature and barometric pressure. We chose the number of included lags (days) from the likelihood ratio test, Akaike information criterion, and Bayesian information sriterion, resulting in 6 different models, and performed a Granger causality test for all models.14 To further assess the fit of the different models, we calculated the autocorrelation of the residuals from the different models.
The autocorrelations and generalized models of them were performed in R.3.6.3. All the other analyses were performed in STATA 16.
2.5.4. Missing
Age and gender were collected from the official registry in the invitation process and are complete. Therefore, the Cox regression including only sex and age as covariates included all cold pressor tests performed. In the remaining analyses, we used daily measures of central tendency. These were calculated from all tests performed.
Out of the 19,540 performing at least one test of pain tolerance, 17,749 answered the question about chronic pain, and these answers were used to calculate the prevalence of chronic pain and its possible nonrandom variation over time. However, data from the chronic pain question were not included in any other analysis, and no participants were excluded from any other analysis because of a missing value on this question.
To further assess whether the missing values on the chronic pain question could be a potential source for a nonrandom variation over time, we calculated the autocorrelation of the daily proportion missing on the question. In addition, we performed multiple imputation with chained equations. To increase the probability of detecting any variation over time, we used all participants in Tromsø 7 (21,083). We included sex, age, chronic pain, CPT, and PPT. To improve prediction we also added education, pain the past 4 weeks from a computer-based questionnaire included in Tromsø 7,34 and 6 questions about musculoskeletal complaints lasting 3 months or more. We imputed 20 data sets and then calculated the prevalence of chronic pain, as well as the daily proportion having chronic pain and the autocorrelation of these daily proportions.
3. Results
Among the 19,540 performing at least one test of pain tolerance, the mean age was 56.9 years (Standard deviation: 11.1 years), 10,065 (51.5%) were women, and 17,749 answered the question about chronic pain, yielding a prevalence of 36.9% among the responders of the question. The prevalence in the imputed data was 37.7%. Pressure pain tolerance was measured in 18,987 participants. The distribution of PPT was right censored to some degree, as a proportion of participants reached the maximum pressure (100 kPa) in every month (Fig. 1A). Eighteen thousand two hundred eighty-five of the participants underwent the cold pressor test. Times to withdrawal were substantially right censored (Fig. 1B), as over 25% of participants reached the maximum time (120 seconds) in every month except July 2016, which was a month with few participants because of summer holidays.
Figure 1.
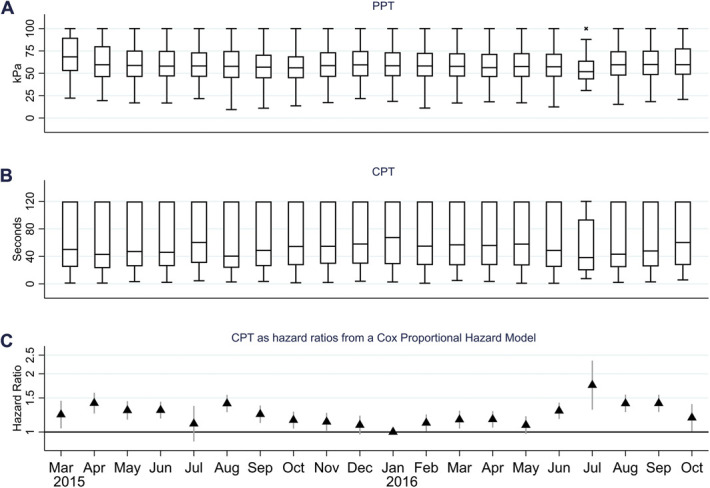
Monthly variation in pressure pain tolerance (PPT) and cold pain tolerance (CPT). (A) Box plot of monthly PPT, (B) CPT as time to withdrawal in the cold pressor test, and (C) CPT as hazard ratios from a Cox proportional hazard model using time to withdrawal in the cold pressor test as survival time and month of examination as exposure and adjusted for age and sex.
3.1. Seasonal variation
There was no clear seasonal variation in PPT (Fig. 1A). However, the median time to withdrawal in the cold pressor test tended to be highest around January 2016, and lowest in August 2015, July 2016, and August 2016 (Fig. 1B). A Cox proportional hazard model, in which month of examination was the exposure and the month of January 2016 was the reference, revealed a seasonal pattern in the hazard ratios, with a lower CPT in warmer parts of the year (Fig. 1C). We found no significant interaction between the month of examination and sex, age, or acclimatization time, meaning the effect of month did not differ by sex, age, or acclimatization time. The seasonal pattern was still evident in a model restricted to those with an acclimatization time of >60 minutes (supplementary Fig. 3, available at http://links.lww.com/PAIN/B464).
3.2. Short-term variation
Daily mean PPT and daily CPT are depicted with 7-day moving averages in Figure 2. There was a clear autocorrelation for daily mean PPT (Fig. 3), meaning the PPT on one day was correlated with the observations from preceding days. The autocorrelation for PPT had a mean lifetime of 5.1 days (95% confidence interval [CI] 4.0-7.2). This is within the range of mean lifetime for the meteorological anomalies, which varied from 2.6 days (95% CI 1.9-4.0) for precipitation to 6.2 days (95% CI 5.5-7.2) for barometric pressure (supplementary Table 1, available at http://links.lww.com/PAIN/B464). We found no autocorrelation for daily CPT after seasonality was removed (Fig. 3). However, there was a weak autocorrelation for weekly mean CPT, indicating nonrandom short-term variation (supplementary Fig. 4, available at http://links.lww.com/PAIN/B464). Owing to the lack of a clear systematic short-term variation in daily CPT, we present results from the analyses made with PPT, and present results from analyses of daily CPT in the supplementary materials when appropriate. Variations because of sex, age, day of the week, and study technician rotation is potential sources of systematic error and might theoretically contribute to the observed autocorrelation for PPT. Owing to lower proportion women and lower mean age of the sample towards the end of the study period, we repeated the time series analysis for PPT in a reduced data set, which included data from March 2015 to July 2016, and found an autocorrelation similar to that observed in the complete data set. Simulations of the effect of sex, age, day of the week, and study technician rotation on PPT in the reduced data set did not introduce autocorrelation as observed in the reduced data set (supplementary Figs. 5–7, available at http://links.lww.com/PAIN/B464). The reduced data set revealed some autocorrelation for the daily mean age, but no autocorrelation for the daily proportion of women or of participants reporting chronic pain (supplementary Fig. 8, available at http://links.lww.com/PAIN/B464). Furthermore, there was no autocorrelation in the daily proportion missing on the chronic pain question and no autocorrelation in the daily proportion having chronic pain in the imputed data (supplementary Fig. 8, available at http://links.lww.com/PAIN/B464).
Figure 2.
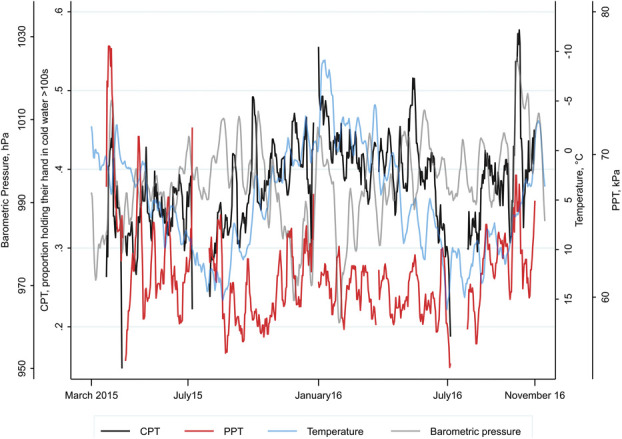
Seven-day moving averages for daily mean of pressure pain tolerance (PPT), daily proportions of participants who held their hand in cold water >100 seconds (CPT), barometric pressure, and temperature. The scale of temperature is inverted. The average from March 31, 2015 is not drawn, as the proportion of participants who held their hand in cold water >100 seconds on that date was 0. CPT, cold pain tolerance.
Figure 3.
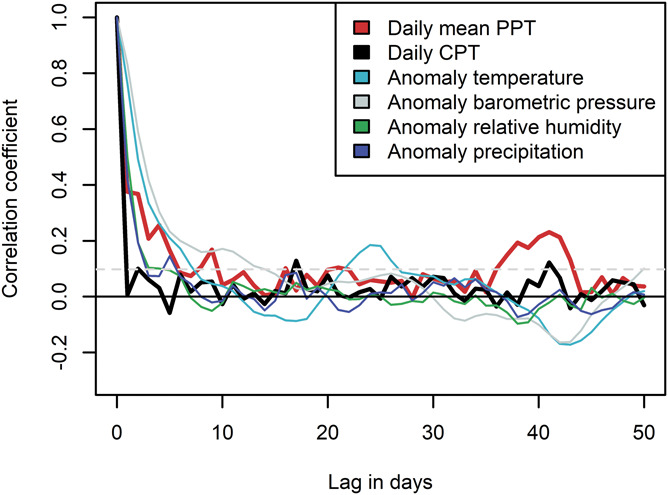
Autocorrelations for pressure pain tolerance (PPT), daily cold pain tolerance (CPT) after removal of seasonality, and meteorological anomalies.
3.3. Association between pain tolerance and meteorological variables
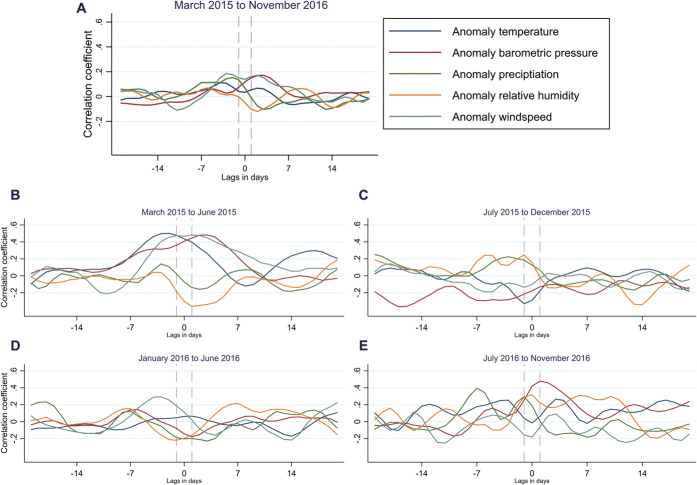
For the whole survey period, PPT correlated poorly with the investigated meteorological anomalies (Fig. 4A). However, the correlations varied depending on the time period for which they were calculated (Fig. 4B–E). For example, there was a small negative correlation between barometric pressure and PPT in the period from July to December 2015 (Fig. 4C) but a positive correlation from July to November 2016 (Fig. 4E). Similar results were seen for CPT. All cross-correlations for PPT and CPT and the assessment of the likelihood of the correlations to be random are presented in supplementary figures 9 to 18 (available at http://links.lww.com/PAIN/B464).
Figure 4.
Cross-correlation of 3-day moving averages of pressure pain tolerance (PPT) and meteorological anomalies. The correlation at day 0 should be interpreted as the correlation between the 3-day moving averages, of PPT, and the meteorological anomalies, centered at day 0. At day −7 (7 days before day 0), it is the correlation between the 3-day moving average of PPT centered at day 0 and the 3-day moving average of the meteorological anomaly centered at day −7. Calculated for the whole period, March 2015 to November 2016 (A), and in 4 different time periods (B–E). Dashed lines indicate the 3 days of PPT with which the anomalies are correlated.
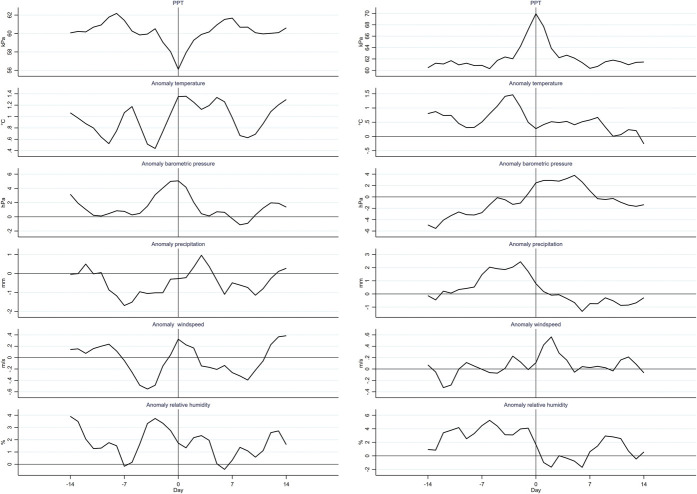
The local minima in PPT were preceded by a rise in temperature, barometric pressure, and wind speed and by a fall in relative humidity (Fig. 5). Precipitation went from below normal before the minima to above normal 3 days after the minima. However, the maxima in PPT coincided with falling temperature, precipitation, and relative humidity and a rising barometric pressure (Fig. 5). Similarly, the maxima in CPT coincided with a decrease in temperature and an increase in barometric pressure (supplementary Fig. 19, available at http://links.lww.com/PAIN/B464). The minima of CPT coincided with an increase in temperature and precipitation, a lower than normal barometric pressure, and a higher than normal relative humidity (supplementary Fig. 19, available at http://links.lww.com/PAIN/B464).
Figure 5.
Mean of 3-day moving averages of pressure pain tolerance (PPT) and meteorological anomalies at local minima and maxima of PPT, which were below 10th or above the 90th percentile, and in the 14 days before and after.
When fitting vector autoregressive models to PPT, temperature, and barometric pressure, the number of lags (days) identified as optimal differed depending on whether we used meteorological anomalies or observed weather, and whether the likelihood ratio test, the Akaike information criterion or Bayesian information criterion was used (supplementary Table 2, available at http://links.lww.com/PAIN/B464). However, 4 models that included temperature and barometric pressure predicted PPT significantly better than models without them. In some of the models, temperature was a significant predictor; in others, barometric pressure was significant (supplementary Table 2, available at http://links.lww.com/PAIN/B464). Bayesian information criterion opted for models using a 1-day lag; in these models, temperature or barometric pressure did not predict PPT. However, the residuals from the models using 1-day lag had more autocorrelation compared with the ones using more lags, indicating a poorer fit (supplementary Figs. 20 and 21, available at http://links.lww.com/PAIN/B464). Owing to the lack of autocorrelation in CPT, no vector autoregressive model was fitted.
4. Discussion
We found a clear seasonal variation in CPT and a nonrandom short-term variation in PPT. Furthermore, PPT and meteorological anomalies varied on similar timescales, and PPT and CPT correlated with meteorological variables. These correlations changed depending on the time period for which they were calculated. This could be a phenomenon called mirage correlation, meaning the sign and magnitude of the correlation changes with time. We also found that temperature and barometric pressure predict future values of PPT.
The seasonal variation in CPT, the correlation between temperature and CPT, and the pattern of falling or rising temperature at the maxima or minima make it likely that CPT is affected by temperature. Together with the lack of a distinct seasonal variation in PPT, these findings indicate that the seasonal variation in CPT is due to changes in temperature and not to other seasonal factors like variation in daylight.
Possible relevant effects of weather on the organism range from physiological responses, such as adaptations,25 increased blood pressure, and increased blood viscosity,22 to psychological responses.9 Molecular mechanisms reported in the literature include adaptations in cell lines1 and in gene expression of transient receptor potential melastatin 8 (TRPM8), a receptor for cold and pain.39 Interestingly, one study of repeated cold water immersion over 15 days found a decrease in pain experience in both hand and foot exposed to repeated immersions (trained) and in the hand and foot not exposed to these immersions,11 although the change was larger in the trained hand and foot. Thus, exposure to cold may induce local adaptations that affect how we experience temperature and simultaneously train the central nervous system to inhibit noxious cold stimuli.
The similar weather patterns, a decrease in temperature, and an increase in barometric pressure at the maxima in PPT and CPT also indicate central mechanisms that are not necessarily specific to the test stimuli. Indeed, innocuous stimuli activate nociceptive fibres,16,37 and nociception could be interpreted as “homeoception.” In such a case, it may be that we experience pain if and when the homeostasis of the organism is threatened4,8 and that the feeling of pain is meant to induce a behavioral response to a homeostatic threat. Temperature, barometric pressure, precipitation, and humidity, alone or in combination, have a direct effect on homeostasis. A change in these meteorological variables will therefore alter the input to many structures used in the processing of pain.8 Experimental studies have found that lowering barometric pressure increased pain behavior in rats12,30 and induced neural activation in superior vestibular nucleus in mice.31 Therefore, a possible explanation for the observed association between meteorological variables and pain tolerance could be that these stimuli change the state in the parts of the brain that are involved in processing pain.
Weather might also affect people's mental status, which likely affects the capacity to endure pain.40 There is some support for a seasonal variation in clinical depression.28 However, the observed lack of seasonal variation in PPT, higher CPT in winter, and inconsistencies in the literature regarding seasonal affective disorder and mental distress20,28 make it difficult to describe the possible role of mental status in explaining our results. In summary, it is unlikely that one singular mechanism can explain the variations in pain tolerance observed in the current study; it is more probable that this is the net result of many, possibly antagonistic, mechanisms.
Earlier research has found that chronic pain sufferers experience more pain when meteorological variables fall outside of normal ranges, ie, when barometric pressure is lower and precipitation, relative humidity, and wind speed are higher than normal.10,32 Such observations are in line with our result of local minima in CPT coinciding with below-normal barometric pressure and above-normal relative humidity. Earlier research has suggested that chronic pain sufferers have a lower pain tolerance.5,19 The hypothesized effect of weather on parts of the brain that are involved in processing pain could explain why some chronic pain sufferers experience more pain in certain weather conditions. Indeed, these individuals might have a sensory system that is already “off balance,” ie, have disturbed bodily representation,26 sensitized nociceptors, and reduced descending inhibition.2 These changes can reduce their ability to adequately adapt to a changing environment.
A large proportion of people with chronic pain report that changes in weather affects their pain.18 Therefore, participants' own beliefs about this topic could have been of interest. However, we consider it a strength that participants were not informed of the purpose of this particular study, and thus, expectation bias is reduced. Another strength of the current study is that Tromsø 7 was conducted over 20 months, so the study period provided data from all seasons and from more than one complete seasonal cycle.
One limitation of our study is that PPT and CPT were measured only once. Thus, we studied the average pain tolerance of a population and were unable to include possible individual variation or adaptation over time. Previous studies on musculoskeletal pain, headache, and migraine have suggested that only a portion of patients is sensitive to changes in weather.33,41 Thus, we might have underestimated the effect of weather on pain tolerance if only a proportion of the population is affected. A lower attendance rate among the youngest and oldest invitees could limit the generalizability. However, it is unlikely that this selection bias or selection into pain tolerance tests should differ over time and thus introduce any systematic variation over time. The data were collected 4 to 6 years ago, but changes in climate or characteristics of the population are unlikely to be of such a magnitude that they greatly limit the external validity of the results.
Other limitations were mitigated by performing additional analyses. We tried to minimize the influence of pretest hand temperature by repeating the Cox regression analysis among participants with an acclimatization time >60 minutes; seasonal variation was still evident in that analysis. The limitation of nonrandom attendance of participants was examined through simulations on shuffled data sets. These simulations did not introduce an autocorrelation similar to that observed for PPT, and therefore, the likelihood of nonrandom attendance giving rise to the results are considered small.
Cross-correlation is a crude analysis with no method for adjusting for possible confounders. Furthermore, as the timescale of PPT was substantially shorter than months, there might be mirage correlation within these periods. Therefore, the correlation coefficients must be interpreted with caution.
Empirical analysis of dynamic nonlinear systems is inherently difficult, and although we found that barometric pressure and temperature can predict future PPT in the models that showed the best fit, the analysis might be flawed. An important assumption in the Granger causality test is that variables in the model should be separable.14 However, since temperature and barometric pressure are closely associated, and probably pain tolerance as well, past values of one of these will also contain information about the others. Furthermore, the appropriateness of the Granger causality test for use in dynamic nonlinear systems continues to be debated.35 Several findings from our analysis and in the literature makes us believe that we are studying a dynamic, nonlinear system: the possible mirage correlation between meteorological anomalies and PPT and CPT, the habituation and physiological acclimatization to temperature, an dynamic effect of TRPM8 in pain and on vascular tone,17,21,29 and the fact that neural networks behave in a dynamical nonlinear way.7 However, the individual differences in the study sample from day to day probably introduce a lot of noise in the time series. In addition, the lack of tests on Sundays or holidays limits the power in the time-series analyses. Together, this decreases the likelihood of capturing the dynamics of the association between weather and pain tolerance, which in turn decreases the likelihood of arriving at a better description of the causal structures involved.
Although the climate in Tromsø is cold compared with many areas, the winters are relatively mild. The mean difference in other meteorological variables such as barometric pressure, wind speed, and relative humidity are less pronounced. Although it is possible that cold temperatures limit the generalizability of the results, patients' belief of weather affecting pain conditions is prevalent also in other climates.18,36,38 In addition, there seems to be a day-to-day variation in PPT and CPT at temperatures above 10°C, and PPT often correlates strongest with meteorological variables from preceding or succeeding days, indicating that changes are as important as absolute values. The dynamic relationship over time in Tromsø indicates that there are spatial and temporal differences in these relationships.
In this study of the general population, there was a clear seasonal variation in CPT and a nonrandom short-term variation in PPT. The PPT and meteorological factors varied on similar timescales, PPT and CPT correlated with meteorological anomalies, and temperature and barometric pressure predicted future values of PPT. These observations, especially those for CPT, should be considered when planning future studies on pain tolerance. Although observational, these findings suggest that weather has a causal, nonlinear, dynamic effect on pain tolerance.
Conflict of interest statement
The authors have no conflicts of interest to declare.
The Regional Committee of Research Ethics approved the seventh survey of The Tromsø Study and this particular analysis.
Informed consent was obtained from all individual participants included in the study.
All data are available by applying to The Tromsø Study http://tromsoundersokelsen.uit.no/tromso.
The code is available at https://github.com/erlendhfarbu/To-tolerate-weather-and-to-tolerate-pain.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B464.
Acknowledgments
This particular study was funded by UiT—The Arctic University of Norway. The seventh survey of the Tromsø Study is a collaboration between the Northern Norway Regional Health Authority, UiT—The Arctic University of Norway, the Norwegian Ministry of Health and Care Services, Troms County, and the Norwegian Institute of Public Health.
Author contributions: E.H. Farbu conducted the data analysis. M. Rypdal assisted in the analysis. E.H. Farbu wrote the manuscript with the assistance of A.C. Höper, M. Skandfer, and T. Brenn. All authors contributed to the interpretation and revised the manuscript. C.S. Nielsen, O.A. Steingrímsdóttir, and A. Stubhaug contributed to the data acquisition in the seventh survey of the Tromsø Study. All authors read and approved the final manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Martin Rypdal, Email: martin.rypdal@uit.no.
Morten Skandfer, Email: morten.skandfer@uit.no.
Ólöf Anna Steingrímsdóttir, Email: OlofAnna.Steingrimsdottir@fhi.no.
Tormod Brenn, Email: tormod.brenn@uit.no.
Audun Stubhaug, Email: astubhau@ous-hf.no.
Christopher Sivert Nielsen, Email: ChristopherSivert.Nielsen@fhi.no.
Anje Christina Höper, Email: anje.hoeper@uit.no.
References
- [1].Alfaqaan S, Yoshida T, Imamura H, Tsukano C, Takemoto Y, Kakizuka A. PPARα-Mediated positive-feedback loop contributes to cold exposure memory. Sci Rep 2019;9:4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arendt-Nielsen L, Graven-Nielsen T. Translational musculoskeletal pain research. Best Pract Res Clin Rheumatol 2011;25:209–26. [DOI] [PubMed] [Google Scholar]
- [3].Beukenhorst AL, Schultz DM, McBeth J, Sergeant JC, Dixon WG. Are weather conditions associated with chronic musculoskeletal pain? Review of results and methodologies. PAIN 2020;161:668–83. [DOI] [PubMed] [Google Scholar]
- [4].Brodal P. A neurobiologist's attempt to understand persistent pain. Scand J Pain 2017;15:140–7. [DOI] [PubMed] [Google Scholar]
- [5].Carli G, Suman AL, Biasi G, Marcolongo R. Reactivity to superficial and deep stimuli in patients with chronic musculoskeletal pain. PAIN 2002;100:259–69. [DOI] [PubMed] [Google Scholar]
- [6].Chauhan E, Bali A, Singh N, Jaggi AS. Cross stress adaptation: phenomenon of interactions between homotypic and heterotypic stressors. Life Sci 2015;137:98–104. [DOI] [PubMed] [Google Scholar]
- [7].Chialvo DR. Emergent complex neural dynamics. Nat Phys 2010;6:744–50. [Google Scholar]
- [8].Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci 2003;26:303–7. [DOI] [PubMed] [Google Scholar]
- [9].Denissen JJ, Butalid L, Penke L, van Aken MA. The effects of weather on daily mood: a multilevel approach. Emotion 2008;8:662–7. [DOI] [PubMed] [Google Scholar]
- [10].Dixon WG, Beukenhorst AL, Yimer BB, Cook L, Gasparrini A, El-Hay T, Hellman B, James B, Vicedo-Cabrera AM, Maclure M, Silva R, Ainsworth J, Pisaniello HL, House T, Lunt M, Gamble C, Sanders C, Schultz DM, Sergeant JC, McBeth J. How the weather affects the pain of citizen scientists using a smartphone app. NPJ digital Med 2019;2:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Daanen HA, Koedam J, Cheung SS. Trainability of cold induced vasodilatation in fingers and toes. Eur J Appl Physiol 2012;112:2595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Funakubo M, Sato J, Obata K, Mizumura K. The rate and magnitude of atmospheric pressure change that aggravate pain-related behavior of nerve injured rats. Int J Biometeorol 2011;55:319–26. [DOI] [PubMed] [Google Scholar]
- [13].Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, Tobias A, Tong S, Rocklöv J, Forsberg B, Leone M, De Sario M, Bell ML, Guo YL, Wu CF, Kan H, Yi SM, de Sousa Zanotti Stagliorio Coelho M, Saldiva PH, Honda Y, Kim H, Armstrong B. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 2015;386:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica 1969;37:424–38. [Google Scholar]
- [15].Graven-Nielsen T, Vaegter HB, Finocchietti S, Handberg G, Arendt-Nielsen L. Assessment of musculoskeletal pain sensitivity and temporal summation by cuff pressure algometry: a reliability study. PAIN 2015;156:2193–202. [DOI] [PubMed] [Google Scholar]
- [16].Green BG, Roman C, Schoen K, Collins H. Nociceptive sensations evoked from 'spots' in the skin by mild cooling and heating. PAIN 2008;135:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harrington AM, Hughes PA, Martin CM, Yang J, Castro J, Isaacs NJ, Blackshaw AL, Brierley SM. A novel role for TRPM8 in visceral afferent function. PAIN 2011;152:1459–68. [DOI] [PubMed] [Google Scholar]
- [18].Jamison RN, Anderson KO, Slater MA. Weather changes and pain: perceived influence of local climate on pain complaint in chronic pain patients. PAIN 1995;61:309–15. [DOI] [PubMed] [Google Scholar]
- [19].Jespersen A, Dreyer L, Kendall S, Graven-Nielsen T, Arendt-Nielsen L, Bliddal H, Danneskiold-Samsoe B. Computerized cuff pressure algometry: a new method to assess deep-tissue hypersensitivity in fibromyalgia. PAIN 2007;131:57–62. [DOI] [PubMed] [Google Scholar]
- [20].Johnsen MT, Wynn R, Bratlid T. Is there a negative impact of winter on mental distress and sleeping problems in the subarctic: the Tromsø Study. BMC Psychiatry 2012;12:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 2009;296:H1868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Keatinge WR, Coleshaw SR, Cotter F, Mattock M, Murphy M, Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br Med J (Clinical Res ed) 1984;289:1405–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee M, Nordio F, Zanobetti A, Kinney P, Vautard R, Schwartz J. Acclimatization across space and time in the effects of temperature on mortality: a time-series analysis. Environ Health 2014;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maini K, Schuster NM. Headache and barometric pressure: a narrative review. Curr Pain Headache Rep 2019;23:87. [DOI] [PubMed] [Google Scholar]
- [25].Makinen TM. Different types of cold adaptation in humans. Front Biosci (Schol Ed 2010;2:1047–67. [DOI] [PubMed] [Google Scholar]
- [26].Moseley LG. I can't find it! Distorted body image and tactile dysfunction in patients with chronic back pain. PAIN 2008;140:239–43. [DOI] [PubMed] [Google Scholar]
- [27].Murray JD, Bernacchia A, Freedman DJ, Romo R, Wallis JD, Cai X, Padoa-Schioppa C, Pasternak T, Seo H, Lee D, Wang X-J. A hierarchy of intrinsic timescales across primate cortex. Nat Neurosci 2014;17:1661–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Øverland S, Woicik W, Sikora L, Whittaker K, Heli H, Skjelkvåle FS, Sivertsen B, Colman I. Seasonality and symptoms of depression: a systematic review of the literature. Epidemiol Psychiatr Sci 2019;29:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol 2006;16:1591–605. [DOI] [PubMed] [Google Scholar]
- [30].Sato J. Weather change and pain: a behavioral animal study of the influences of simulated meteorological changes on chronic pain. Int J Biometeorol 2003;47:55–61. [DOI] [PubMed] [Google Scholar]
- [31].Sato J, Inagaki H, Kusui M, Yokosuka M, Ushida T. Lowering barometric pressure induces neuronal activation in the superior vestibular nucleus in mice. PLoS One 2019;14:e0211297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schultz DM, Beukenhorst AL, Yimer BB, Cook L, Pisaniello HL, House T, Gamble C, Sergeant JC, McBeth J, Dixon WG. Weather patterns associated with pain in chronic-pain sufferers. Bull Am Meteorol Soc 2020;101:E555–66. [Google Scholar]
- [33].Smedslund G, Hagen KB. Does rain really cause pain? A systematic review of the associations between weather factors and severity of pain in people with rheumatoid arthritis. Eur J Pain 2011;15:5–10. [DOI] [PubMed] [Google Scholar]
- [34].Steingrímsdóttir ÓA, Engdahl B, Hansson P, Stubhaug A, Nielsen CS. The Graphical Index of Pain: a new web-based method for high-throughput screening of pain. PAIN 2020;161:2255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sugihara G, May R, Ye H, Hsieh CH, Deyle E, Fogarty M, Munch S. Detecting causality in complex ecosystems. Science 2012;338:496–500. [DOI] [PubMed] [Google Scholar]
- [36].Timmermans EJ, van der Pas S, Schaap LA, Sánchez-Martínez M, Zambon S, Peter R, Pedersen NL, Dennison EM, Denkinger M, Castell MV, Siviero P, Herbolsheimer F, Edwards MH, Otero A, Deeg DJ. Self-perceived weather sensitivity and joint pain in older people with osteoarthritis in six European countries: results from the European Project on OSteoArthritis (EPOSA). BMC Musculoskelet Disord 2014;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Van Hees J, Gybels J. C nociceptor activity in human nerve during painful and non painful skin stimulation. J Neurol Neurosurg Psychiatry 1981;44:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].von Mackensen S, Hoeppe P, Maarouf A, Tourigny P, Nowak D. Prevalence of weather sensitivity in Germany and Canada. Int J Biometeorol 2005;49:156–66. [DOI] [PubMed] [Google Scholar]
- [39].Wang XP, Yu X, Yan XJ, Lei F, Chai YS, Jiang JF, Yuan ZY, Xing DM, Du LJ. TRPM8 in the negative regulation of TNFα expression during cold stress. Sci Rep 2017;7:45155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Willoughby SG, Hailey BJ, Mulkana S, Rowe J. The effect of laboratory-induced depressed mood state on responses to pain. Behav Med 2002;28:23–31. [DOI] [PubMed] [Google Scholar]
- [41].Yang AC, Fuh J-L, Huang NE, Shia B-C, Wang S-J. Patients with migraine are right about their perception of temperature as a trigger: time series analysis of headache diary data. The J headache pain 2015;16:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zebenholzer K, Rudel E, Frantal S, Brannath W, Schmidt K, Wöber-Bingöl C, Wöber C. Migraine and weather: a prospective diary-based analysis. Cephalalgia 2011;31:391–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B464.