Background:
Because of limited accuracy of noninvasive tests, diastolic stress testing plays an important role in the diagnostic work-up of patients with heart failure with preserved ejection fraction (HFpEF). Exercise right heart catheterization is considered the gold standard and indicated when HFpEF is suspected but left ventricular filling pressures at rest are normal. However, performing exercise during right heart catheterization is not universally available. Here, we examined whether pulmonary capillary wedge pressure (PCWP) during a passive leg raise (PLR) could be used as simple and accurate method to diagnose or rule out occult-HFpEF.
Methods:
In our tertiary center for pulmonary hypertension and HFpEF, all patients who received a diagnostic right heart catheterization with PCWP-measurements at rest, PLR, and exercise were evaluated (2014–2020). The diagnostic value of PCWPPLR was compared with the gold standard (PCWPEXERCISE). Cut-offs derived from our cohort were subsequently validated in an external cohort (N=74).
Results:
Thirty-nine non-HFpEF, 33 occult-HFpEF, and 37 manifest-HFpEF patients were included (N=109). In patients with normal PCWPREST (<15 mmHg), PCWPPLR significantly improved diagnostic accuracy compared with PCWPREST (AUC=0.82 versus 0.69, P=0.03). PCWPPLR ≥19 mmHg (24% of cases) had a specificity of 100% for diagnosing occult-HFpEF, irrespective of diuretic use. PCWPPLR ≥11 mmHg had a 100% sensitivity and negative predictive value for diagnosing occult-HFpEF. Both cut-offs retained a 100% specificity and 100% sensitivity in the external cohort. Absolute change in PCWPPLR or V-wave derived parameters had no incremental value in diagnosing occult-HFpEF.
Conclusions:
PCWPPLR is a simple and powerful tool that can help to diagnose or rule out occult-HFpEF.
Keywords: diuretic; heart failure; hypertension, pulmonary; leg; pulmonary wedge pressure
WHAT IS NEW?
Passive leg raise (PLR) during right heart catheterization can be used as an extra simple maneuver to diagnose or rule out occult heart failure with preserved ejection fraction.
Pulmonary capillary wedge pressure (PCWP) during PLR (PCWPPLR) of below 11 mm Hg could be used to rule out occult heart failure with preserved ejection fraction, and PCWPPLR of 19 mm Hg and above could be used to diagnose occult heart failure with preserved ejection fraction with 100% accuracy.
WHAT ARE THE CLINICAL IMPLICATIONS?
Using the above described cut-offs of PCWPPLR to diagnose or rule out heart failure with preserved ejection fraction would allow for omission of exercise testing in approximately one-third of the patients with normal left ventricular filling pressures at rest.
PCWPPLR is especially of value in centers where exercise during a right heart catheterization is difficult to perform.
See Editorial by Cubero Salazar and Hsu
Heart failure with preserved ejection fraction (HFpEF) is a condition with a high prevalence, morbidity, and mortality.1 Stress testing is required to diagnose so-called “occult-HFpEF”, that is, patients with normal left ventricular (LV) filling pressures at rest but a pathological rise in filling pressures during exercise, as a consequence of LV diastolic dysfunction.2 Noninvasive tests (namely natriuretic peptides and [exercise] echocardiogram) have been proven to have a low sensitivity and/or low specificity to diagnose (occult) HFpEF.2–7 In case of intermediate pretest likelihood, it is recommended to perform a right heart catheterization (RHC) with exercise, the gold standard to diagnose HFpEF.8,9 Although RHC is proven safe in experienced centers,10,11 performing exercise during a RHC presents some technical challenges, requiring special equipment and expertise, and additional procedure time. This begs the question as to whether alternative provocative maneuvers may be used short of exercise to enhance diagnosis for some patients.
Passive leg raise (PLR) results in an acute increase in venous return to the heart as blood from the lower extremities is returned to the central circulation.12 In the current study, we investigated whether PLR during RHC could be used to increase diagnostic accuracy, potentially to allow deferment of exercise-RHC in some cases. Previous studies have shown that the subsequent increase in pulmonary capillary wedge pressure (PCWP) following PLR was more pronounced in (occult)-HFpEF patients, compared with controls.12–14 This augmented rise of LV filling pressures in HFpEF is attributed to a decreased compliance of the left ventricle,12,13 although restriction from the pericardium15 and/or a stiff left atrium are other potential causes. Thus far, no well-defined cut-offs have been established. The present study evaluates the diagnostic accuracy of PCWPPLR to diagnose or to rule out (occult-)HFpEF, using PCWP at peak exercise (PCWPEXERCISE) as reference, and then validate this cutoff in a separate cohort.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
This study analyzed all unique patients between 2014 and 2020, who received an exercise right heart catheterization at VU University Medical Center (VUmc), a tertiary center for pulmonary hypertension and HFpEF. All patients received their RHC as part of their diagnostic work-up for or follow-up of unexplained dyspnea or (suspected) pulmonary hypertension. All cases with recorded PCWPREST, PCWPPLR, and PCWPEXERCISE were included. Patients with elevated LV filling pressures because of causes other than HFpEF were excluded (LV ejection fraction ≤50%, cardiac ischemia, hypertrophic or infiltrative cardiomyopathy, relevant valvular pathology, congenital and/or pericardial disease). Cases with insufficient data or unreliable data quality were excluded. Patients were categorized as manifest-HFpEF (PCWPREST ≥15 mm Hg and PCWPEXERCISE ≥25 mm Hg), occult-HFpEF (PCWPREST <15 mm Hg and PCWPEXERCISE ≥25 mm Hg), or non-HFpEF (PCWPREST <15 mm Hg and PCWPEXERCISE <25 mm Hg) according to ESC-guidelines.8 Pretest probability of HFpEF was calculated for each patient using the H2FPEF score.16
The Medical Ethics Review Committee of the VU University Medical Center did not consider the current study to fall within the scope of Medical Research Involving Human Subjects act, since the diagnostic procedures were performed for clinical purposes, and granted a waiver of informed consent (approval number 2012.288).
Exercise Right Heart Catheterization
Ultrasound-guided catheterization was performed via the right internal jugular vein, using a fluid-filled 7-French Swan-Ganz catheter (131HF7, Baxter Healthcare), as previously described.17 The external pressure transducer was zeroed at mid-thoracic level in each patients, in accordance with latest consensus.18,19 RHC was performed in a supine position and in a nonfasting state, to prevent confounding of dehydration. In general, patients were advised not to take their loop-diuretics in the morning of RHC. All pressures were assessed end-expiratory and averaged over ≥3 cardiac cycles. The PCWP values were evaluated mid-A wave, and also the peak of the V-wave was recorded. Cardiac output was assessed using thermodilution, taking the average of ≥3 measurements (5 measurements in case of arrhythmia or more than 10% variance). Exercise was performed after all resting and PLR measurements were performed. All patients had an individualized stepped exercise protocol. All patients in the diagnostic work-up for unexplained dyspnea or HFpEF were required to exercise until maximum exertion. A few patients in follow-up of chronic thromboembolic pulmonary hypertension or gene carriers of pulmonary arterial hypertension performed submaximal exercise. Chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension-gene carriers with submaximal exercise with borderline elevated PCWPEXERCISE (20–25 mm Hg) were excluded, because of uncertainty about the diagnosis HFpEF.
Passive Leg Raise Maneuver
During the passive leg raise maneuver, the legs of the patients were raised by staff in an angle of ≈50 degrees. During this maneuver, the patient was explicitly instructed to relax his/her legs and not to help so as to avoid a Valsalva maneuver. After raising the legs, the legs were placed in the binders of the bicycle (remaining in a 50° angle), while continuing the PCWP recording for up to 3 minutes. The highest PCWP values during this maneuver and spontaneous breathing, averaged over ≥3 cardiac cycles, were reported as the PCWPPLR.
V-Wave Analysis
A large V-wave, defined as V-wave minus PCWP ≥10 mmHg,20,21 corresponds with elevated filling pressures during the passive filling of the left atrium. Although often associated with significant mitral valve pathology,22 a large V-wave can also be seen in HFpEF, which is then explained by impaired LV distensibility and/or HFpEF-related atrial dysfunction.20,23–26 In the present study, the diagnostic value of the V-wave were evaluated using receiver operating characteristics (ROC) curve analyses.
Validation Cohort
Cut-offs derived from the derivation cohort were externally validated in a cohort evaluated for HFpEF (N=74), as previously published by Obokata et al.2 This validation cohort exists of 24 non-HFpEF, 18 occult-HFpEF, and 32 manifest-HFpEF patients. Manifest-HFpEF was excluded for validation purposes, as these patients were not required to perform exercise for diagnostic purposes. Study procedures were similar to the derivation cohort.2 Conform derivation cohort, external pressure transducer was zeroed at mid-thoracic level in each patient.
Evaluation of Relationship Between Venous Return, Pericardial Restraint, and PCWPPLR
Rise in PCWP during PLR is considered to be more pronounced in HFpEF because of reduced LV compliance. However, increased venous return or pericardial restraint are other potential causes. To evaluate whether these factors were similarly increased in (occult-)HFpEF and non-HFpEF, rise in mean right atrial pressure (mRAP) during PLR was compared among groups: ΔmRAP can be considered a reflection of the interaction between change in preload and pericardial restraint, since the heart generally distended to the point of pericardial restraint.15 In addition, mRAP-to-PCWP ratio, another surrogate for pericardial restraint, was compared between groups.15
Statistics
Data were checked for normal distribution. Data are shown as mean±SD or median (interquartile range) in case of non-normal distribution. Data were log-transformed before statistical tests if applicable. Two-group comparisons of continuous variables were performed using a Student T-test, or Wilcoxon signed-rank test in case of a non-normal distribution. Three-group comparisons were performed using a 1-way ANOVA or Kruskal-Wallis test in case of a non-normal distribution, followed by a pairwise comparison with Bonferroni correction as a post hoc test. Categorical variables were compared using a chi-squared test or Fisher’s exact test in case of group sizes below 5. ROC analyses were used to evaluate the diagnostic performance and cut-off values of different variables. Cut-off values with 100% positive predictive value, 100% negative predictive value, and the Youden’s index were reported. DeLong’s method was used to compare areas under the curve (AUC). As diuretics may influence venous return by PLR, we separately studied the diagnostic value of PCWPPLR in patients chronically on diuretics. Pearson correlation analysis was performed to evaluate the relationship between ΔmRAPPLR and ΔPCWPPLR. Missing values were not imputed. Statistical significance was stated at P<0.05. R-Statistics (version 3.6.1.) was used for analyses.
Results
Baseline Characteristics
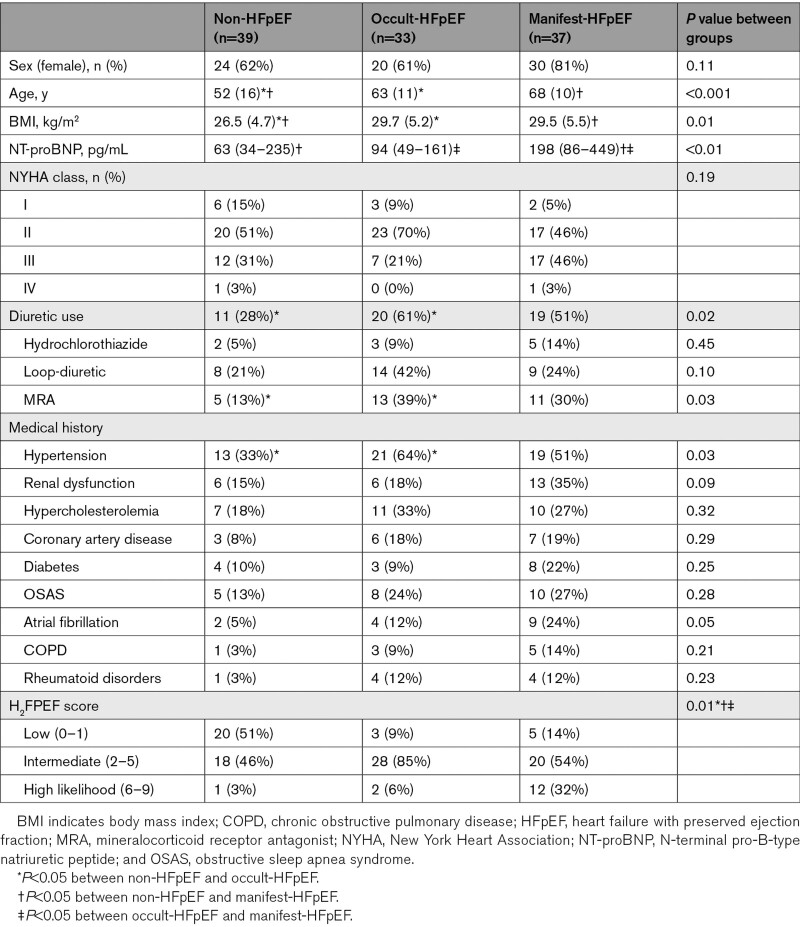
A total of 109 unique patients were included in this study (Figure S1). The derivation cohort consisted of 39 non-HFpEF patients, 33 occult-HFpEF patients, and 37 manifest-HFpEF patients. Patients in this cohort were predominantly women, with a median age of 64 years old. Baseline characteristics are shown in Table 1. The diagnoses of the non-HFpEF patients consisted mainly of pulmonary (artery) disease and patients without a pulmonary or cardiac diagnosis (Table S1). The majority of the patients had an intermediate H2FPEF score (Table 1). There was a stepwise increase in H2FPEF score between non-HFpEF, occult-HFpEF, and manifest-HFpEF, with a median score of 1, 3, and 4, respectively.
Table 1.
Baseline Characteristics
Diagnostic Value of Passive Leg Raise to Diagnose HFpEF (Occult- and Manifest-HFpEF Combined)
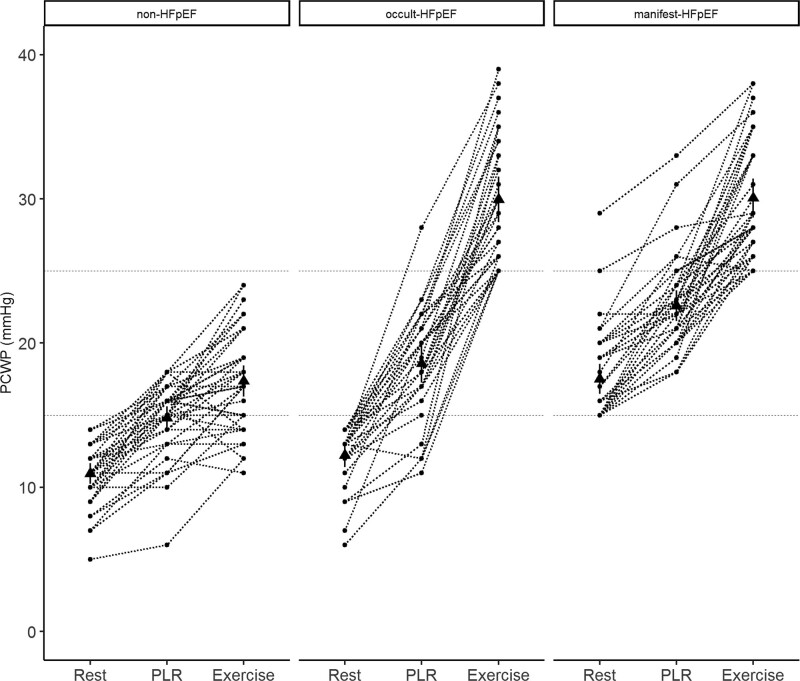
As shown in Figure 1 and Table 2, there was evident overlap in PCWPREST between non-HFpEF and occult-HFpEF. There was a clear and stepwise increase of PCWPPLR between non-HFpEF, occult-HFpEF, and manifest-HFpEF. The average rise of PCWP by PLR was 4 mm Hg in non-HFpEF, 6 mm Hg in occult-HFpEF, and 5 mm Hg in manifest-HFpEF. There was no significant difference in PCWPEXERCISE between occult and manifest-HFpEF. Importantly, all patients with PCWPREST ≥15 mm Hg had a PCWPEXERCISE ≥25 mm Hg, which was not the case for lower PCWPREST values. The average time to max PCWPPLR was 20±13 seconds (measured prospectively in 24 patients). After reaching max PCWPPLR, there was a short period of a steady state, followed by a slow decline in PCWP. All hemodynamic data are presented in Table 2.
Figure 1.
Pulmonary capillary wedge pressure (PCWP) at rest, passive leg raise (PLR), and exercise. Dots (●) are the individual data points. Triangles (▲) are the mean value of the group. HFpEF indicates heart failure with preserved ejection fraction.
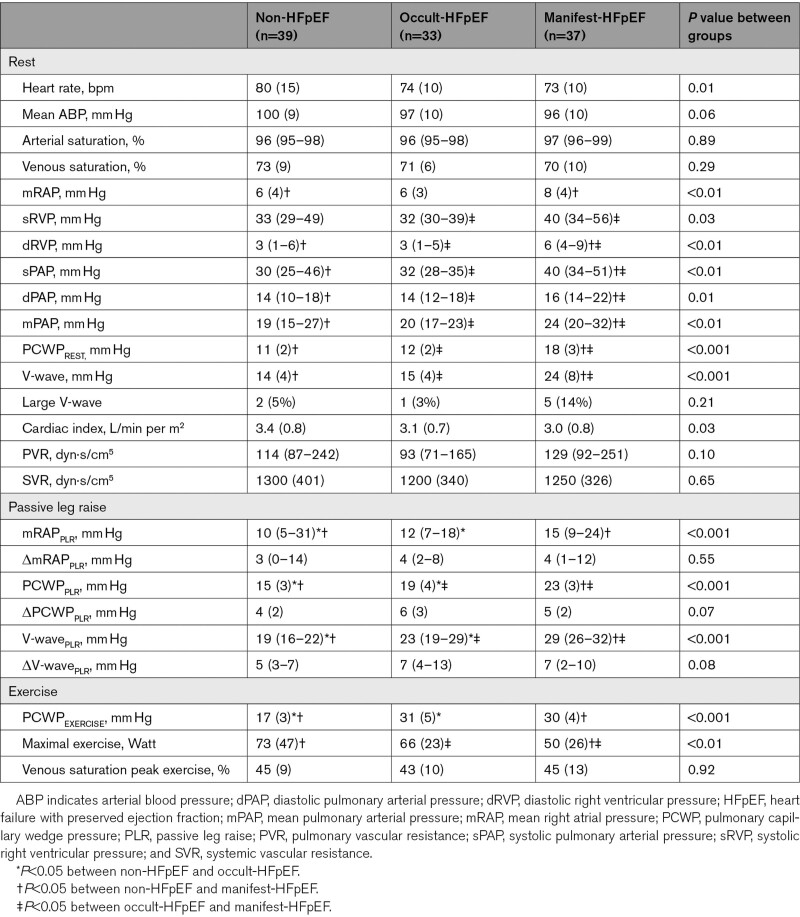
Table 2.
Hemodynamics
Figure S2 shows the ROC-curve analyses of PCWP and V-wave at rest and during PLR. PCWPREST had an AUC of 0.85 to diagnose HFpEF (occult- and manifest-HFpEF combined), PCWPPLR had an incremental diagnostic value of borderline significance (AUC 0.91, P=0.05). The other parameters that we explored (ΔPCWPPLR, V-waveREST, V-wavePLR, ΔV-wavePLR) had no incremental diagnostic value. A “large V-wave” (n=8) had a low sensitivity, but a high specificity in diagnosing HFpEF (sensitivity: 9%, specificity: 95%, negative predictive value: 37%, and positive predictive value: 75%).
As shown in Figure S3, diuretic use showed no significant effect modification on PCWPREST, PCWPPLR, and ΔPCWPPLR (P=0.52, P=0.83, and P=0.22, respectively). ΔPCWPPLR and V-wave derived parameters showed no incremental value over PCWPREST or PCWPPLR in patients with or without diuretics (V-wave derived parameters were omitted from further figures to improve readability).
Diagnostic Value of Passive Leg Raise to Diagnose Occult-HFpEF (Manifest-HFpEF Excluded)
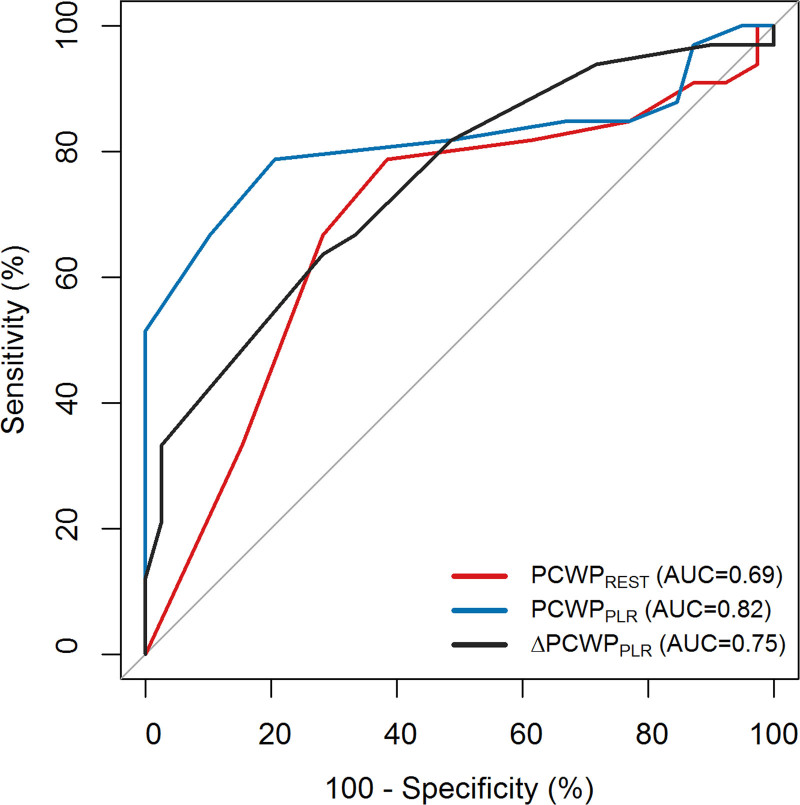
Since PCWPREST≥15 mm Hg is diagnostic for HFpEF (and no additional tests are required), we zoomed in on patients with PCWPREST <15 mm Hg (n=72), where diagnostic uncertainty remains and PLR may be most relevant. PCWPPLR had a significantly better predictive value to diagnose occult-HFpEF, compared with PCWPREST (Figure 2, AUC=0.82 versus 0.69, P=0.03). ΔPCWPPLR did not perform better than PCWPPLR or PCWPREST (Figure 2, P=0.21 and P=0.51, respectively).
Figure 2.
Receiver operating characteristics curve analysis to diagnose occult-heart failure with preserved ejection fraction (HFpEF; manifest-HFpEF excluded). AUC indicates area under the curve; PCWP, pulmonary capillary wedge pressure; and PLR, passive leg raise.
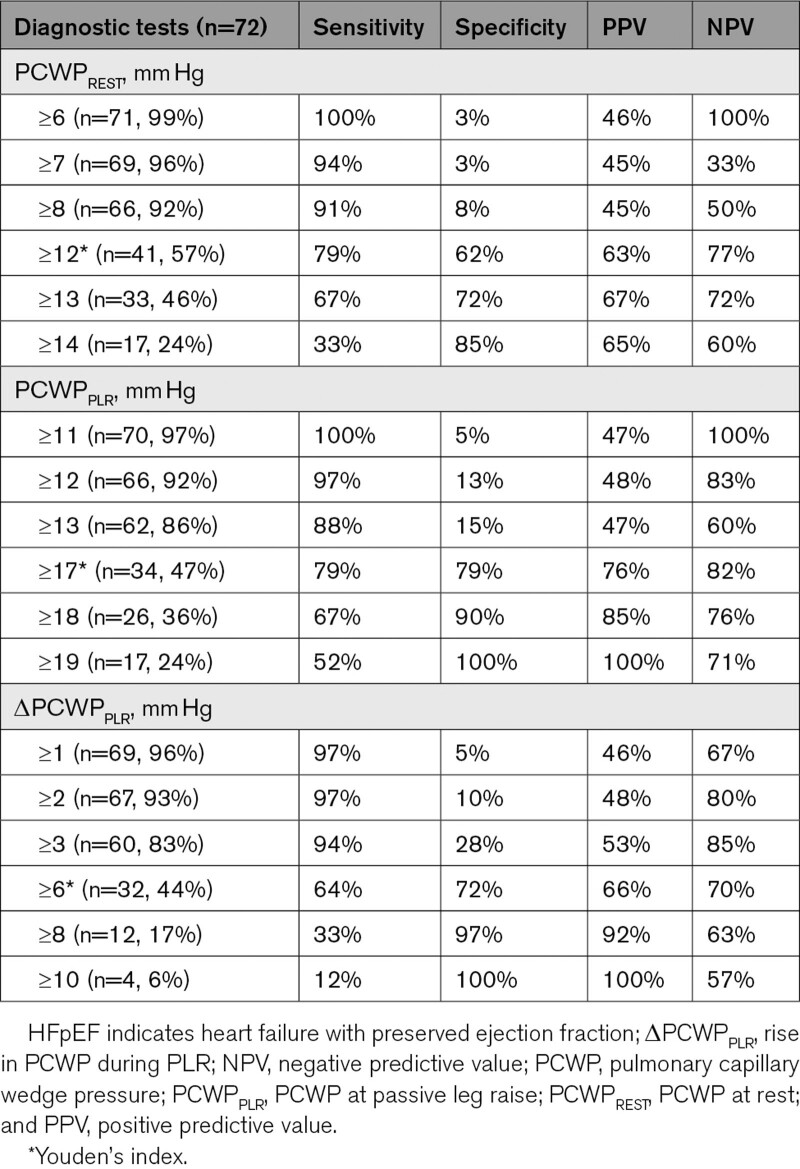
As shown in Table 3, PCWPPLR ≥19 mm Hg (24% of cases where PCWPREST was <15 mm Hg) could be used to diagnose patients with occult-HFpEF with a 100% specificity and positive predictive value. PCWPPLR ≥11 mm Hg had a 100% sensitivity and negative predictive value for diagnosing occult-HFpEF. Therefore, PCWPPLR <11 mm Hg (3% of cases) could be used to safely rule out occult-HFpEF (Table 3).
Table 3.
PCWPREST, PCWPPLR, and ΔPCWPPLR to Diagnose or Rule Out HFpEF in Patients With Normal LV Filling Pressures at Rest
In patients evaluated for occult-HFpEF, diuretic use (43%) did not change the diagnostic value of PCWPPLR (AUC=0.82 if diuretics were used versus 0.81 if no diuretics were used, P=0.93). The PCWPPLR cut-off of ≥19 mm Hg, to diagnose occult-HFpEF with a 100% specificity, did not change whether patients used diuretics or not. A sub-analyses was performed without patients with chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension-gene carriers in the control group, which resulted in the same cut-off values of PCWPPLR to diagnose or rule out HFpEF. ΔPCWPPLR did not improve diagnostic accuracy. Also, V-wave-derived parameters had no incremental diagnostic value to diagnose occult-HFpEF. Of note, a large V-wave was only present in one occult-HFpEF patient and in 2 non-HFpEF patients.
External Validation of the Diagnostic Value of PCWPPLR
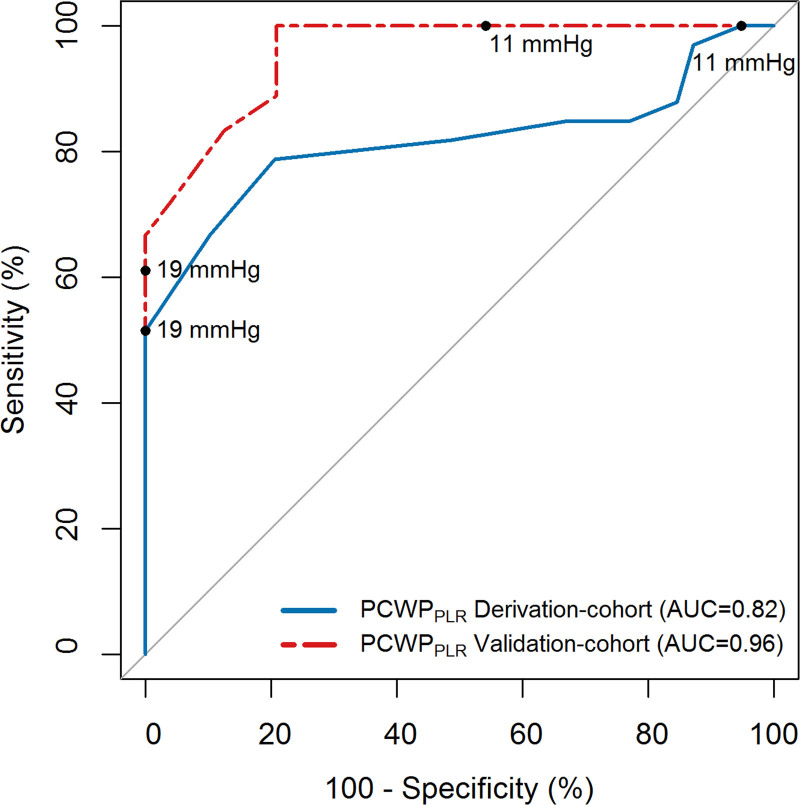
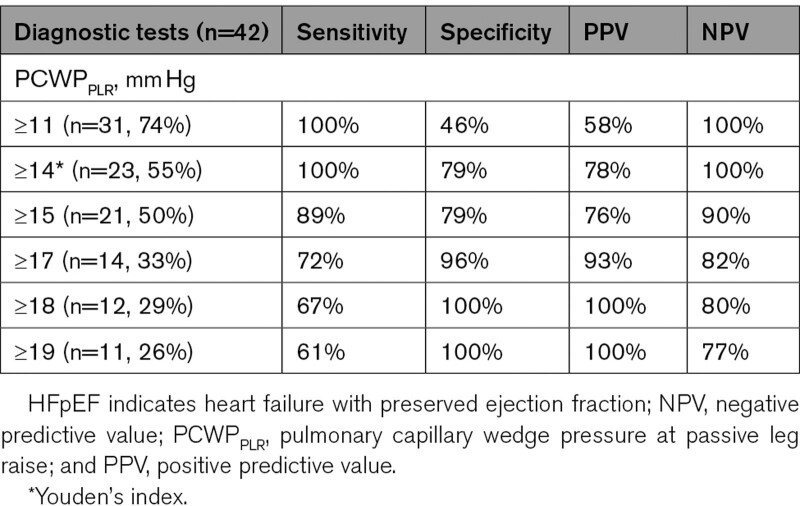
A well-described cohort of 18 occult-HFpEF and 24 non-HFpEF patients was used as validation cohort. Patients in this cohort were predominantly male (57%) with a mean age of 66 years old. A summary of patient characteristics and PCWP values are shown in Table S2, additional information is reported in the original article.2 PCWPREST and PCWPPLR were significantly lower in non-HFpEF compared with occult-HFpEF (8 versus 12 mm Hg and 12 versus 20 mm Hg, respectively). However, overlap was evident, especially in PCWPREST. As depicted in Figure 3, PCWPPLR had an even higher AUC (0.96) in the validation cohort. As shown in Table 4, PCWPPLR cut-offs derived from the derivation cohort (PCWPPLR ≥19 mm Hg to diagnose and PCWPPLR <11 mm Hg to rule out occult-HFpEF) retained a 100% specificity and 100% sensitivity in the validation cohort. These cut-offs could be used in 52% of the patients of the validation cohort to diagnose or rule out HFpEF with a 100% accuracy.
Figure 3.
Receiver operating characteristics curve analysis to diagnose occult heart failure with preserved ejection fraction (derivation-cohort and external validation). AUC indicates area under the curve; PCWP, pulmonary capillary wedge pressure; and PLR, passive leg raise.
Table 4.
PCWPPLR to Diagnose or Rule Out Occult-HFpEF (External Validation Cohort)
Relationship Between Venous Return, Pericardial Restraint, and PCWP During Leg Raise
The following analyses evaluate the influence of venous return or pericardial restraint on PCWPPLR. First of all, as shown in Table 2, there was no significant difference (P=0.55) in ΔmRAP (surrogate for the interaction between change in preload and pericardial restraint) during PLR between non-HFpEF, occult-HFpEF, and manifest-HFpEF. Furthermore, there was only a weak correlation between ΔmRAPPLR and ΔPCWPPLR (r=0.34, P<0.001), suggesting that the increase in PCWP was because of left heart properties and not due to venous return and pericardial interaction. In addition, mRAP-to-PCWP ratio (another surrogate for pericardial restraint) was not significantly different (P=0.50) between non-HFpEF, occult-HFpEF, and manifest-HFpEF, either. Moreover, mRAP-to-PCWP ratio did not correlate with ΔPCWPPLR (r=−0.08, P=0.40).
Discussion
This study evaluated the diagnostic value of passive leg raise in diagnosing (occult) HFpEF. Performing PLR during RHC improved the diagnostic accuracy significantly over measurement at rest. PCWPPLR was particularly useful in the patient group with normal LV filling pressures at rest, where diagnostic uncertainty is the highest. In addition, we found that a high PCWPPLR (≥19 mmHg) could be used to diagnose occult-HFpEF with 100% specificity and positive predictive value. In addition, PCWPPLR <11 mm Hg could be used to safely rule out HFpEF, although number of observations in this category was low. External validation confirmed these findings. More specifically, the cut-off value of PCWPPLR (≥19 mm Hg) was relatively common (26%), and 100% specific for the diagnoses of occult HFpEF in the external validation cohort. Similarly, the cut-off value of PCWPPLR <11 mmHg was also relatively common (26%), and 100% accurate to rule out occult HFpEF. The validation cohort was even suggestive for a PCWP <14 mm Hg and ≥18 mm Hg to rule out or diagnose HFpEF. The difference in cut-off values found in the validation cohort was considered a play of chance. Using these cut-offs would have led to misclassification in the derivation cohort and will result in a diminished diagnostic accuracy. Therefore, the more conservative and more accurate cut-off values of the derivation cohort (PCWP <11 mm Hg and PCWP ≥19 mm Hg) are advised.
Importantly, the diagnostic value of PCWPPLR was not influenced by diuretic use. Furthermore, we observed that PCWP increases rapidly with leg raise, reaching a maximum in less than 1 minute. ΔPCWPPLR or V-wave derived parameters had no additional value compared with (absolute) PCWPPLR. Furthermore, we found that PCWPREST of ≥15 mm Hg had 100% positive predictive value for PCWPEXERCISE ≥25 mm Hg, which was not the case for lower PCWPREST values. Confirming the PCWPREST cut-off of 15 mmHg currently used to manifest HFpEF.8,9 In addition, PCWPREST ≥6 mm Hg had a 100% negative predictive value for HFpEF. However, as the number of patients below this cut-off were small, we could not safely identify a low threshold of PCWPREST that provided 100% certainty to rule out occult HFpEF. However, we could conclude that low-normal PCWPREST values (6–14 mm Hg) cannot be used to decide not to perform a PLR and/or exercise.
The results of our study are in accordance with other studies underlining the incremental diagnostic value of PLR. Borlaug et al12 found a similar value of PCWPPLR in occult-HFpEF (average of 18 mmHg) but had lower PCWPPLR values in their control group (11 mm Hg), resulting in an even higher AUC of PCWPPLR of 0.94. Unfortunately, no cut-off values were reported. Tossavainen et al13 reported a sensitivity and specificity of 91 and 92% of PCWPPLR ≥15 mm Hg to predict HFpEF. However, half of these HFpEF patients had manifest-HFpEF, consequently, this cut-off value would have less diagnostic value to predict occult-HFpEF. In addition, for a cut-off value assessed with an invasive test, a sensitivity or specificity of 90% may be less desirable. As a side note, although RHC is preferable, PLR has also been shown to be feasible during left heart catheterization.14,27
Additional analyses show that the V-wave derived parameters did not have incremental value over PCWP in diagnosing occult-HFpEF. This could be explained by the low prevalence of large V-waves in occult-HFpEF patients (n=1). In contrast, the large V-wave was present in 14% of manifest-HFpEF and showed a high positive predictive value for manifest-HFpEF. The observation that the V-wave is more prominent in patients with HFpEF with elevated LV filling pressures at rest is also described by Manouras et al,21 reporting a prevalence of the large V-waves of 24% in patients with HFpEF, but mainly in patients with higher PCWPREST.
Analysis of right atrial pressures suggest that the distinct response in PCWPPLR between HFpEF and non-HFpEF is due to the reduced left heart (LA and LV) compliance in HFpEF and not due to increased venous return or exaggerated pericardial restraint.15,28
Strength and Limitations
This study is the first to investigate cut-off values of PCWPPLR to diagnose occult-HFpEF and elucidate its potential to complement exercise-RHC. Another strength is the patient selection, having excluded all other causes for elevated LV filling pressure, testing the diagnostic performance of PLR where diagnostic uncertainty remains. Also, all patients underwent exercise-RHC to invasively confirm or exclude the diagnosis HFpEF. However, our study has some limitations. The derivation cohort comes from a retrospective study, although the external validation cohort was prospectively enrolled. Although study results were externally validated, the total sample size remains relatively small (N=114); therefore, a larger prospective cohort is warranted to improve the external validity of this study.
Clinical Implications
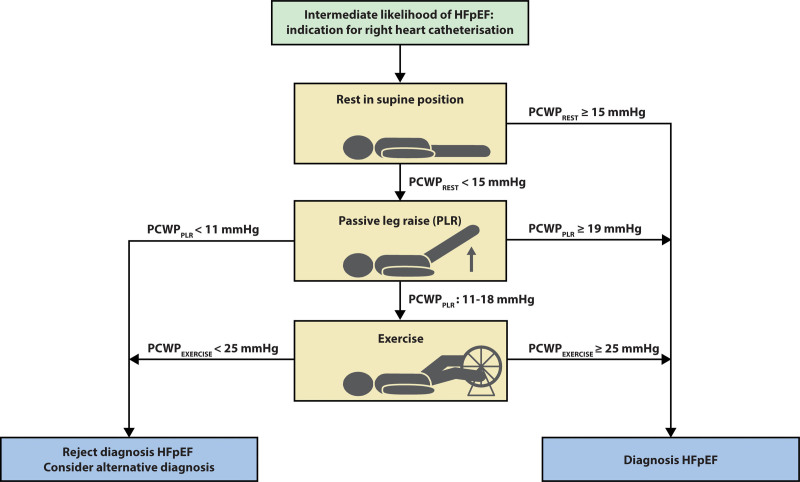
In this sample, use of PCWPPLR ≥19 mmHg to diagnose HFpEF and PCWPPLR <11 mmHg to rule out HFpEF would allow for omission of exercise testing in 36% of the patients with normal resting PCWP. A proposed diagnostic pathway with implementation of the passive leg raise is depicted in Figure 4. This would be most useful in centers where equipment and expertise for exercise testing is not available, although it is important to acknowledge that additional important physiological data are available from exercise testing, even if these diagnostic thresholds are met, including evaluation of cardiac output reserve with stress,29 and increases in pulmonary vascular pressures with respect to cardiac output, which have been shown to have prognostic implications.30,31 Others have utilized fluid challenge to facilitate diagnosis of HFpEF.32,33 While this is also an option and may provide value, one potential strength of PLR as compared with fluid loading is greater simplicity and shorter duration. In addition, PLR only temporarily increases venous return, compared with an absolute increase in volume in fluid loading, consequently, there is no risk of decompensation with PLR. Of note, this study used mid-thoracic zero-leveling, conform the guidelines, hence cut-off values derived from this study should only be used if mid-thoracic zero-leveling is used. In this study, the diagnostic value of PCWPPLR was demonstrated. Whether PCWPPLR is also of prognostic value remains unclear and is of interest for future studies. Nevertheless, increased PCWPPLR reflects elevated PCWPEXERCISE, which has been shown to be of prognostic relevance in HFpEF.34
Figure 4.
Implementation of passive leg raise test during right heart catheterization. HFpEF indicates heart failure with preserved ejection fraction; PCWP, pulmonary capillary wedge pressure; and PLR, passive leg raise.
Conclusions
There is no clear lower threshold of PCWP at rest where occult-HFpEF can be excluded, without performing a diastolic stress test. Elevation in PCWP to 19 mm Hg or higher with passive leg raise can be used to diagnose occult-HFpEF. Conversely, a low PCWP with leg raise below 11 mm Hg could be used to rule out (occult-)HFpEF.
Article Information
Sources of Funding
Dr Noordegraaf is supported by the Netherlands Organization for Scientific Research (NWO; 918.16.610). Dr de Man is supported by NWO (917.18.338) and the Dutch Heart Foundation (DHF; 2018T059). Drs Noordegraaf, Bogaard, and de Man are supported by the Netherlands CardioVascular Research Initiative (2017-10, 2018-29). Dr Handoko is supported by DHF (2020T058).
Disclosures
Dr Handoko received educational/speaker/consultancy fees from Novartis, Boehringer Ingelheim, Daiichi Sankyo, Vifor Pharma, AstraZeneca, Bayer, MSD, and Quin, but all is not related to this work. The other authors report no conflicts.
Supplemental Material
Tables S1 and S2
Figures S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AUC
- area under the curve
- CTEPH
- chronic thromboembolic pulmonary hypertension
- HFpEF
- heart failure with preserved ejection fraction
- LV
- left ventricular
- mRAP
- mean right atrial pressure
- PCWP
- pulmonary capillary wedge pressure
- PCWPPLR
- PCWP during PLR
- PLR
- passive leg raise
- RHC
- right heart catheterization
- ROC
- receiver operating characteristic
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.121.008935.
For Sources of Funding and Disclosures, see page 329.
Contributor Information
Arno A. van de Bovenkamp, Email: ac.vrossum@amsterdamumc.nl.
Niels Wijkstra, Email: n.wijkstra@amsterdamumc.nl.
Frank P.T. Oosterveer, Email: f.oosterveer@amsterdamumc.nl.
Harm Jan Bogaard, Email: hj.bogaard@amsterdamumc.nl.
Albert C. van Rossum, Email: ac.vrossum@amsterdamumc.nl.
Frances S. de Man, Email: fs.deman@amsterdamumc.nl.
Barry A. Borlaug, Email: borlaug.barry@mayo.edu.
References
- 1.Lewis EF, Lamas GA, O’Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, et al. ; CHARM Investigators. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;9:83–91. doi: 10.1016/j.ejheart.2006.10.012 [DOI] [PubMed] [Google Scholar]
- 2.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation. 2017;135:825–838. doi: 10.1161/CIRCULATIONAHA.116.024822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifov OF, Gupta H. What is the evidence that the tissue doppler index E/e’ reflects left ventricular filling pressure changes after exercise or pharmacological intervention for evaluating diastolic function? A systematic review. J Am Heart Assoc. 2017;6:e004766. doi: 10.1161/JAHA.116.004766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Bovenkamp AA, Enait V, de Man FS, Oosterveer FTP, Bogaard HJ, Vonk Noordegraaf A, van Rossum AC, Handoko ML. Validation of the 2016 ASE/EACVI guideline for diastolic dysfunction in patients with unexplained dyspnea and a preserved left ventricular ejection fraction. J Am Heart Assoc. 2021;10:e021165. doi: 10.1161/JAHA.121.021165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hummel YM, Liu LCY, Lam CSP, Fonseca-Munoz DF, Damman K, Rienstra M, van der Meer P, Rosenkranz S, van Veldhuisen DJ, Voors AA, et al. Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: a study utilizing simultaneous echocardiography and invasive measurements. Eur J Heart Fail. 2017;19:1651–1660. doi: 10.1002/ejhf.957 [DOI] [PubMed] [Google Scholar]
- 6.Remmelzwaal S, van Ballegooijen AJ, Schoonmade LJ, Dal Canto E, Handoko ML, Henkens MTHM, van Empel V, Heymans SRB, Beulens JWJ. Natriuretic peptides for the detection of diastolic dysfunction and heart failure with preserved ejection fraction-a systematic review and meta-analysis. BMC Med. 2020;18:290. doi: 10.1186/s12916-020-01764-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 9.Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
- 10.Hoeper MM, Lee SH, Voswinckel R, Palazzini M, Jais X, Marinelli A, Barst RJ, Ghofrani HA, Jing ZC, Opitz C, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48:2546–2552. doi: 10.1016/j.jacc.2006.07.061 [DOI] [PubMed] [Google Scholar]
- 11.Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:Cd006962. doi: 10.1002/14651858.CD006962.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tossavainen E, Wikström G, Henein MY, Lundqvist M, Wiklund U, Lindqvist P. Passive leg-lifting in heart failure patients predicts exercise-induced rise in left ventricular filling pressures. Clin Res Cardiol. 2020;109:498–507. doi: 10.1007/s00392-019-01531-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, Kocka V. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure-volume loop analysis. J Am Coll Cardiol. 2010;55:1701–1710. doi: 10.1016/j.jacc.2009.11.076 [DOI] [PubMed] [Google Scholar]
- 15.Borlaug BA, Reddy YNV. The role of the pericardium in heart failure: implications for pathophysiology and treatment. JACC Heart Fail. 2019;7:574–585. doi: 10.1016/j.jchf.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. doi: 10.1161/CIRCULATIONAHA.118.034646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huis In’t Veld AE, Oosterveer FPT, De Man FS, Marcus JT, Nossent EJ, Boonstra A, Van Rossum ACB, Vonk Noordegraaf A, Bogaard HJ, Handoko ML. Hemodynamic effects of pulmonary arterial hypertension-specific therapy in patients with heart failure with preserved ejection fraction and with combined post- and precapillay pulmonary hypertension. J Card Fail. 2020;26:26–34. doi: 10.1016/j.cardfail.2019.07.547 [DOI] [PubMed] [Google Scholar]
- 18.Kovacs G, Avian A, Olschewski A, Olschewski H. Zero reference level for right heart catheterisation. Eur Respir J. 2013;42:1586–1594. doi: 10.1183/09031936.00050713 [DOI] [PubMed] [Google Scholar]
- 19.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. ; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 20.Pichard AD, Diaz R, Marchant E, Casanegra P. Large V waves in the pulmonary capillary wedge pressure tracing without mitral regurgitation: the influence of the pressure/volume relationship on the V wave size. Clin Cardiol. 1983;6:534–541. doi: 10.1002/clc.4960061104 [DOI] [PubMed] [Google Scholar]
- 21.Manouras A, Johnson J, Lund LH, Nagy AI. Optimizing diastolic pressure gradient assessment. Clin Res Cardiol. 2020;109:1411–1422. doi: 10.1007/s00392-020-01641-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi H, Abe Y, Morita Y, Nakane E, Haruna Y, Haruna T, Inoko M. The accuracy of a large V wave in the pulmonary capillary wedge pressure waveform for diagnosing current mitral regurgitation. Cardiology. 2018;141:46–51. doi: 10.1159/000493007 [DOI] [PubMed] [Google Scholar]
- 23.Purga SL, Karas MG, Horn EM, Torosoff MT. Contribution of the left atrial remodeling to the elevated pulmonary capillary wedge pressure in patients with WHO Group II pulmonary hypertension. J Echocardiogr. 2019;17:187–196. doi: 10.1007/s12574-018-0410-8 [DOI] [PubMed] [Google Scholar]
- 24.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050 [DOI] [PubMed] [Google Scholar]
- 25.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–15. doi: 10.1161/CIRCIMAGING.108.813071 [DOI] [PubMed] [Google Scholar]
- 26.Morris DA, Gailani M, Vaz Pérez A, Blaschke F, Dietz R, Haverkamp W, Ozcelik C. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24:651–662. doi: 10.1016/j.echo.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 27.Choi S, Shin JH, Park WC, Kim SG, Shin J, Lim YH, Lee Y. Two distinct responses of left ventricular end-diastolic pressure to leg-raise exercise in euvolemic patients with exertional dyspnea. Korean Circ J. 2016;46:350–364. doi: 10.4070/kcj.2016.46.3.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyberg JV, Taichman GC, Smith ER, Douglas NW, Smiseth OA, Keon WJ. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation. 1986;73:428–432. doi: 10.1161/01.cir.73.3.428 [DOI] [PubMed] [Google Scholar]
- 29.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, et al. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail. 2018;11:e004750. doi: 10.1161/CIRCHEARTFAILURE.117.004750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho JE, Zern EK, Lau ES, Wooster L, Bailey CS, Cunningham T, Eisman AS, Hardin KM, Farrell R, Sbarbaro JA, et al. Exercise pulmonary hypertension predicts clinical outcomes in patients with dyspnea on effort. J Am Coll Cardiol. 2020;75:17–26. doi: 10.1016/j.jacc.2019.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. 2015;8:41–48. doi: 10.1161/CIRCHEARTFAILURE.114.001731 [DOI] [PubMed] [Google Scholar]
- 33.D’Alto M, Badesch D, Bossone E, Borlaug BA, Brittain E, Humbert M, Naeije R. A fluid challenge test for the diagnosis of occult heart failure. Chest. 2021;159:791–797. doi: 10.1016/j.chest.2020.08.019 [DOI] [PubMed] [Google Scholar]
- 34.Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B, Neumann FJ. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–3112. doi: 10.1093/eurheartj/ehu315 [DOI] [PubMed] [Google Scholar]