Abstract
Anorexia nervosa (AN) is a severe psychiatric disorder characterized by energy restriction, low body weight, a fear of gaining weight, and often excessive physical activity. Anxiety disorders appear to constitute a major risk factor for developing AN and are the most frequent comorbidity. Here, the influence of anxiety‐like behavior prior to food restriction on increased physical activity, leading to greater susceptibility to weight loss, was tested in rats. Furthermore, the possible anxiolytic effect of starvation itself was analyzed. A chronic starvation model activity‐based anorexia (ABA) was applied to mimic physiological and behavioral characteristics of AN. During the induction of starvation and acute starvation, food intake was reduced by 70% and the rats lost 25% of their body weight, which was kept stable to imitate chronic starvation. Anxiety‐like behavior was quantified before and after chronic starvation using the elevated plus maze, based on rodents’ aversion to open spaces. Anxiety‐related behavior before food restriction was associated with increased running‐wheel activity during habituation and during the induction of starvation, and predicted faster weight loss in ABA rats. Additionally, food‐restricted animals showed less anxiety‐like behavior after chronic starvation. Animals showing more anxiety‐like behavior appear to be more susceptible to weight loss, partially mediated by increased physical activity. Anxiety‐related behavior was associated with increased physical activity, which in turn was associated with more rapid weight loss. Our data let us assume that food restriction has an anxiolytic effect. These findings demonstrate the importance of considering anxiety disorders in patients with AN.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Anxiety disorders appear to constitute a major risk factor for developing anorexia nervosa (AN) and are the most frequent comorbidity. Food restriction seems to have an anxiolytic effect in rodents and anxiety‐like behavior was shown to be related with increased physical activity.
WHAT QUESTION DID THIS STUDY ADDRESS?
The influence of anxiety‐like behavior prior to food restriction on increased physical activity, leading to greater susceptibility to weight loss in the activity‐based anorexia (ABA) rat model was tested. Furthermore, the possible anxiolytic effect of starvation itself was analyzed.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The present animal study was able to show that anxiety‐like behavior prior to food starvation predicts weight loss in the ABA rats. Anxiety‐related behavior was associated with increased physical activity, which in turn was associated with more rapid weight loss. Our data let us assume that food restriction has an anxiolytic effect at least in rats.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
With regard to clinical consequences, our findings underline the relevance of considering anxiety disorders in patients with AN more intensively. Moreover, they could suggest the importance of using exercise as a therapeutic approach, because physical activity might be a means to regulate emotions, such as anxiety. Although excessive activity is counterproductive, controlled and moderate activity under therapeutic supervision could ease internal pressure, increase recovery rates, and prevent the development of osteoporosis. 1 , 2 Nevertheless, it should be considered that the present results are of observational nature and do not offer insight into mechanistic processes.
INTRODUCTION
Anorexia nervosa (AN) is a severe psychiatric disorder characterized by restricted energy intake, reduced body weight, body image disturbance, and a fear of gaining weight, primarily affecting adolescent girls and young women. 3 , 4 Anxiety disorders are the most frequent comorbidities of AN, and anxiety disorders (especially generalized anxiety disorder, social phobia, and obsessive‐compulsive disorder) prior to the onset of AN appear to constitute a major risk factor for the subsequent diagnosis of AN. 5 , 6 , 7 , 8 , 9 However, clear pathological mechanisms underlying this association remain unknown. Approximately 80% of patients with AN had at least one diagnosis of an anxiety disorder in their lifetime and, in 75% of the comorbid cases, it predated the eating disorder. 7 , 10 , 11 , 12 This observation and recently observed genetic correlations between AN and other psychiatric disorders (including anxiety disorders) indicate a sharing of genes across phenotypes and pleiotropy. 13 , 14 , 15 , 16
Excessive physical activity can be observed in up to 80% of patients with AN. 4 In an AN animal model, increased anxiety‐like behavior in mice was associated with higher levels of running‐wheel activity, 17 constituting a potential risk factor for more rapid weight loss.
The activity‐based anorexia (ABA) animal model combines limited food access with running‐wheel access, leading to body weight loss and increased physical activity. 18 Along with a behavioral test (elevated plus maze) to quantify anxiety‐like behavior, our experimental setting seems ideally suited to analyze the interaction among anxiety‐like behavior, physical activity, and starvation in a controlled experimental setting. The Elevated Plus Maze (EPM) has been widely used in the assessment of anxiety‐related behavior in rodents. 19 , 20 A previous study found an association between anxiety‐like behavior and running‐wheel activity (RWA) as well as reduced anxiety‐like behavior after short‐term food restriction in female mice using the EPM. 17
We used a chronic version of the ABA model and the EPM to quantify the influence of anxiety‐like behavior on weight loss and running activity in female adolescent rats. In the present study, we sought to determine whether animals showing more anxiety‐like behavior are more prone to weight loss and whether this is dependent on the physical activity levels. Moreover, we aimed to analyze whether starvation indeed has an anxiolytic effect in the animals. Therefore, we chose a longitudinal study design and extended the classical ABA model by experimental groups with and without food restriction combined with and without running‐wheel access, respectively.
MATERIALS AND METHODS
Forty‐nine adolescent 4‐week‐old female Wistar rats (RjHan:WI; Janvier, Hannover, Germany) arrived in the laboratory with an average body weight of 106.22 g ± 1.84 g. All animals were single‐housed in type IV, 1820 cm2 cages (Polysulfone, Tecniplast) with sawdust bedding, cotton wool pads as nesting material, and a running‐wheel. The rats were treated under standardized conditions with a 12 h day/night cycle (lights on at 7 a.m.). The room was constantly regulated to maintain a temperature of 22°C and 55% humidity. The rats were randomly split into four groups: (1) controls (ad libitum food, no running‐wheel, n = 12); (2) controls with running‐wheel (CRW, ad libitum food, running‐wheel, n = 12); (3) limited food (LF, starvation, no running‐wheel, n = 12), and (4) ABA (starvation, running‐wheel, n = 13). Note, that groups 3 and 4 differ in the absence and presence of the running‐wheel but the amount and the composition of food were identical. The sample size was calculated a priori using a two‐sided Satterthwaite t‐test leading to a power of at least 80% at a significance level of α = 0.05 (SAS Institute, Cary, NC, USA).
RWA, body weight and food intake were recorded daily at 12 a.m. The daily RWA was measured with a tachometer (Sigma BC 5.12). The animal trial was approved by the Governmental Animal Care and Use Committee (Approval no.: 84‐02‐04‐2017.A036). All experiments were performed in accordance with the German legislation governing animal studies, following the Guide for the Care and Use of Laboratory Animals (NIH, 8th edition, 2011) and the 2010/63/EU Directive on the Protection of Animals Used for Scientific Purposes (Official Journal of the European Union, 2010).
Activity‐based anorexia model
The ABA model is a well‐established and currently the most frequently used rodent model to induce physiological and behavioral characteristics of AN. 18 A modified concept of the ABA model was used here. 21 Instead of reducing the feeding time, as conducted in the initial model by Routtenberg and Kuzneshof, 22 a reduced amount of food equivalent to 30% of the ad libitum food intake in the habituation period was implemented in LF and ABA groups. Food‐restricted rats received food daily at 12 a.m. The target weight was set at 75% of the baseline body weight. Unlimited water was available for all groups during the entire experiment. The ABA group and the CRW group had unlimited access to a running‐wheel, whereas the running‐wheel was blocked in the cages of control and LF rats.
The experiment was divided into four phases. In the habituation period, all groups had ad libitum access to food. After 10 days, the induction of the starvation started followed by the acute starvation and the food intake was reduced to 30% in the LF and ABA groups. The maintenance period began once each individual rat had reached its target weight (−25% of its baseline body weight), imitating chronic starvation. In this phase, the amount of food was adjusted daily by increasing or decreasing the quantity of food pellets by 0.5–1.5 g depending on individual body weight, in order to keep the target weight stable. A reduced mortality rate has been shown using this protocol, 21 which allows an observation of chronic starvation that is often seen in patients with AN. 23 , 24 It also facilitates the analysis of influencing factors without body weight as a confounder.
Elevated plus maze
Behavioral testing was carried out twice: at the end of the habituation period (day 10, EPM1) and at the end of the chronic starvation period (day 30, EPM2). It was conducted in the morning before feeding to potentially further increase the effect of starvation. The animals arrived in the experimental room 30 min before testing to adapt to the environment. All tests were performed under red light. After every test, the animals were transferred back to their home cages and the maze was disinfected with Antifect N liquid (propanol 35% and ethanol 23.5%).
The EPM was used to quantify the influence of food restriction and RWA on anxiety‐like behavior. It consists of four arms arranged in a plus‐shape, two of which were open and two of which were enclosed by side walls that were 20 cm high. The arms were 50 cm long and were connected by an open center and positioned on 50 cm high table legs. Although rodents are prey animals which prefer to stay in the covered arms of the maze, they are also curious to explore new territories. 19 , 20 It is generally assumed that rats display higher levels of anxiety‐related behavior when they spend more time in the closed arms of the apparatus. 19
At the beginning, the animals were positioned at the center of the maze. The experiment was recorded for 10 min with a video camera (Basler, acA1300‐60gmNIR) and analyzed using ANY‐Maze Video Tracking System (Stoelting Co.). Each rat was tracked at the center of its body. The following parameters were recorded for further analysis: the average time the animal spent in the closed arms, the average number of entries in the closed arms, the average time the animal moved (time mobile), and the average number of entries in both the closed and open arms. Furthermore, two ratios were calculated to correct for general movement activity: the average time the animals spent in the closed arms divided by the total time the animals were active (time in closed arms/time mobile), and the average number of entries into the closed arms corrected for the average number of total entries in the open and closed arms (entries in closed arms/total entries). To evaluate differences between the two time points (before [EPM1] and after chronic starvation [EPM2]) delta (∆) values were determined.
Statistical analysis
Body weight and RWA data were separated in different phases: habituation (days 1–10), induction of starvation (days 11–13), acute starvation (days 14–17), and chronic starvation (days 18–30). The induction of starvation was defined as the first days of food restriction (days 11–13) according to Wable et al. (2015), 17 when RWA significantly increases. The acute starvation period (days 14–17) was marked by body weight loss and declining activity levels, whereas chronic starvation (days 18–30) was characterized by a stable body weight and decreased activity levels. Alterations in body weight during the different phases were evaluated in a one‐way analysis of variance (ANOVA) with Bonferroni post hoc test. Group differences between control versus LF and CRW versus ABA were of particular interest in this study design. The days needed to reach the target weight were recorded for each rat to investigate its individual susceptibility to weight loss. Group differences for the days until the target weight was reached between LF and ABA were calculated with a two‐sided Student’s t‐test. Group comparisons (CRW vs. ABA) for RWA were calculated separately for the different phases using two‐sided Student’s t‐tests.
Differences in the EPM parameters were calculated by a repeated measure 2 × 2 ANOVA.
A two‐tailed Pearson correlation was calculated for anxiety‐like behavior before starvation (EPM1) and days needed to reach the target weight to explore the hypothesis that anxiety‐related behavior could predict body weight loss. To test whether increased RWA and/or increased anxiety‐like behavior more strongly predict increased body weight loss, a multiple regression analysis was performed. One‐tailed Pearson correlations were used to explore the hypothesis that anxiety‐like behavior predicts physical activity in the animals, as suggested before. 17
Effect sizes are displayed as Cohen’s d for Student’s t‐tests, η 2 for ANOVAs, and r 2 for Pearson correlations. Statistical analyses were performed with SPSS Statistics 25 (IBM, Chicago, IL, USA) and the significance level for all comparisons was set at 5%.
RESULTS
Body weight, days until target weight, and running‐wheel activity
As expected, during the entire starvation period, there were statistically significant differences in body weight between the control and food‐restricted groups (induction of starvation: F = 9.380, η 2 = 0.385, p < 0.0001, control vs. LF: p = 0.148, CRW vs. ABA: p = 0.016; acute starvation: F = 43.238, η 2 = 0.742, p < 0.0001, control vs. LF: p < 0.0001, CRW vs. ABA: p < 0.0001; chronic starvation: F = 104.684, η 2 = 0.875, p < 0.0001, control vs. LF: p < 0.0001, and CRW vs. ABA: p < 0.0001; Figure 1a).
FIGURE 1.
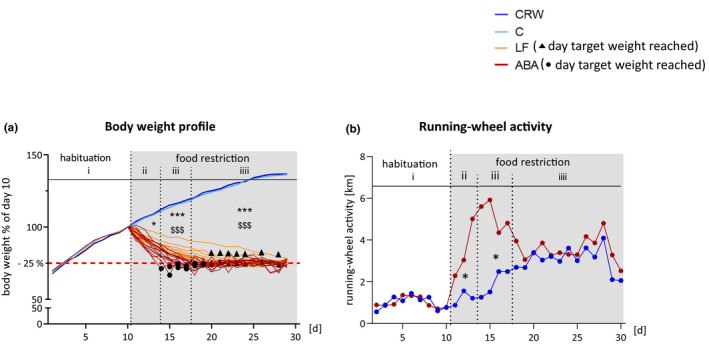
Body weight and running‐wheel activity profiles. (a) Average body weight in % of day 10. Individual body weight profiles are depicted for LF and ABA rats during the food restriction period. Black dots and rectangles represent the day target weight was reached in ABA and LF rats, respectively. (b) Mean RWA normalized to the average RWA of the habituation period: (i) habituation, (ii) induction of starvation, (iii) acute starvation, and (iv) chronic starvation. Differences between four groups were calculated with one‐way ANOVA and Bonferroni correction and between two groups with two‐sided Student’s t‐test. * Represents differences between control and LF, $ represents differences between CRW and ABA. ABA, activity‐based anorexia; C, control; CRW, control+running‐wheel; LF, limited food; RWA, running‐wheel activity. */$ = p ≤ 0.05, ***/$$$ = p ≤ 0.001
ABA animals needed significantly fewer days to reach the target weight (75% of baseline body weight) compared to the LF group (5.8 vs. 11.1 days; t = 5.73, Cohen’s d = 0.22, p < 0.0001), which is most likely the result of a higher caloric requirement due to physical exercise (see black rectangles and dots in Figure 1a).
RWA was significantly higher in ABA rats compared to CRW rats during the induction of starvation (t = −2.649, Cohen’s d = 1.07, p = 0.014) and the acute starvation period (t = −2.504, Cohen’s d = 1.07, p = 0.02, Figure 1b). During the chronic starvation, there was no difference in RWA (t = −0.218, Cohen’s d = 0.08, p = 0.832).
Elevated plus maze
Food‐restricted rats show less anxiety‐like behavior
Using a repeated measure 2 × 2 ANOVA, a significant difference in the average number of entries in the closed arms was found comparing the two measurement time points before (EPM1) and after chronic starvation (EPM2; F = 11.577, η2 = 0.208, p = 0.001). Both food reduced groups showed significantly less anxiety‐like behavior after chronic starvation compared with controls (F = 4.924, η 2 = 0.101, p = 0.032; Figure 2a,b). Similarly, all food restricted rats spent significantly less time in the closed arms after chronic starvation, also indicating less anxiety‐related behavior (F = 11.517, η 2 = 0.207, p = 0.001; Figure 2c,d). No differences were seen due to the running‐wheel access. Thus, food restriction but not running‐wheel access led to a reduction of anxiety‐like behavior in the rats after chronic starvation. The change over time in the control groups probably represents a repetition effect as the rats generally move less at the second measure time point and preferably spent time in the closed arms. To exclude general movement as a confounder, the total numbers of entries in all arms and the total time the animals were mobile during the behavioral test were analyzed. For both parameters there was a statistically representative main effect for the time in all groups (total number of entries: F = 9.210, η 2 = 0.093, p = 0.004; Figure 2c; total time mobile: F = 75.387, η 2 = 0.560, p < .0001; Figure 2d). This finding could be explained by a repetition effect in the behavioral test, because all animals were generally less active during the second measurement time point. Correcting the number of entries in the closed arms for the number of total entries in all arms and correcting the time spent in the closed arms for the total time the rats were mobile led to similar effects as the uncorrected comparisons (F = 7.333, η 2 = 0.143, p = 0.01; Figure 2e; F = 7.533, η 2 = 0.146, p = 0.009; Figure 2f). Hence, we found a significant reduction of anxiety‐like behavior following food restriction (EPM2) that cannot be explained by increased general movement. See Table S1 for raw data of the EPM.
FIGURE 2.
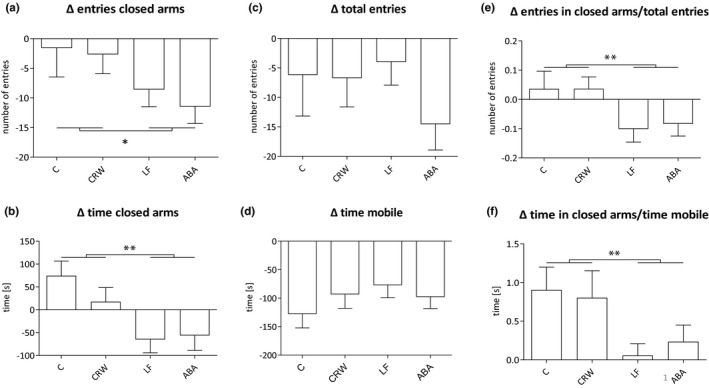
Entries and time in closed arms, total time mobile and total entries in the EPM. Differences of mean number of entries in the closed arms (a), mean time in the closed arms (b), total entries (c), and total time mobile (d) between EPM1 and EPM2. Ratio of mean entries in the closed arms and total entries (e) and mean time in the closed arm and total time mobile (f). Differences between groups were calculated with repeated measure ANOVA. The p values represent the interaction effect of time*food restriction. ABA, activity‐based anorexia; C, control; CRW, control+running‐wheel; EPM1/2, elevated plus maze at day 10/day 30; LF, limited food. *p ≤ 0.05, **p ≤ 0.01
Anxiety‐like behavior predicts weight loss
An explorative correlation analysis showed a strong relationship between anxiety‐like behavior in ABA animals before starvation (EPM1) and fewer days until they reached the respective target weight (r 2 = 0.609, p = 0.005; Figure 3). No such association was found in LF animals (r 2 = 0.003, p = 0.931; Figure 3), proposing a dissociation between anxiety‐related behavior and weight loss caused by the animals’ running‐wheel access. Anxiety‐like behavior before starvation (EPM1) and increased RWA during the induction of starvation significantly contribute to the explanation of body weight loss (F = 15.699, p = 0.001). Seventy‐one percent of the variance of faster body weight loss could be explained by these variables (see Table S2).
FIGURE 3.
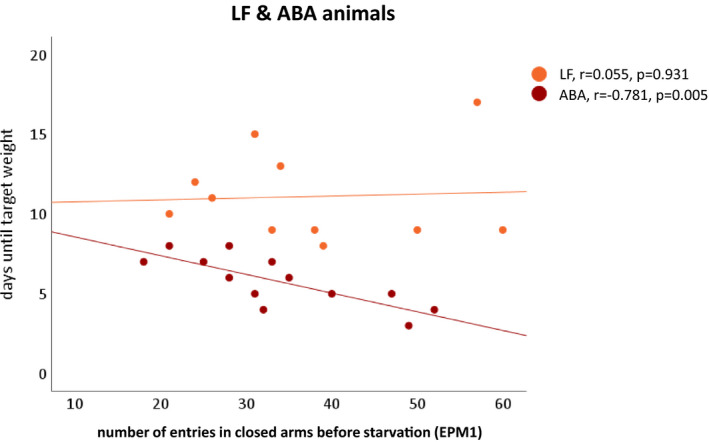
Association between body weight loss and anxiety‐like behavior in the EPM. Negative correlation between the days needed until target weight was reached and the mean number of entries in the closed arms before starvation (EPM1) in ABA rats (red). No such correlation in LF rats (orange). One value for LF animals is missing because this animal did not reach the target weight until the end of the protocol. Correlations were calculated by two‐tailed Pearson correlation. ABA, activity‐based anorexia; EPM1, elevated plus maze at day 10; LF, limited food
Anxiety‐like behavior is correlated with physical activity
Subsequently, a possible association between anxiety‐related behavior and physical activity as shown in an acute starvation mouse model was analyzed. 17 Correlation analyses suggest a similar effect in the present rat ABA model. Animals that show more anxiety‐like behavior before starvation (EPM1) showed higher RWA at the same time (r 2 = 0.254, p = 0.04; Figure 4a). Increased anxiety‐related behavior prior to starvation also predicted increasing RWA during the induction of starvation (r 2 = 0.290, p = 0.029; Figure 4b). However, no such correlation could be shown regarding the acute starvation and chronic starvation (all p > 0.05, data not shown).
FIGURE 4.
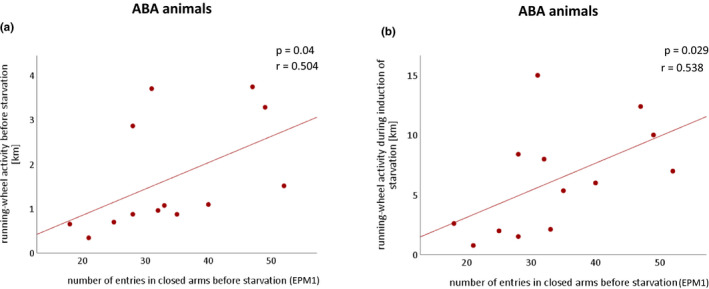
Associations between anxiety‐like behavior in the EPM and RWA. (a) Positive correlations between the mean number of entries in the closed arms before starvation (EPM1) in ABA rats with the RWA during habituation and (b) during induction of starvation. Correlations were calculated using one‐tailed Pearson correlations. ABA, activity‐based anorexia; EPM1, elevated plus maze at day 10; RWA, running‐wheel activity.
DISCUSSION
We examined whether high levels of anxiety‐like behavior measured by more and longer visits to the closed arms of the elevated plus maze lead to a greater susceptibility to weight loss in the ABA model, potentially via increased voluntary running. Furthermore, we wanted to confirm the potentially anxiolytic effect of starvation. Higher susceptibility to body weight loss could have important implications for a better understanding of the role of previously existing or comorbid anxiety in patients’ with AN pathogenesis.
Increased levels of anxiety‐like behavior before food starvation appeared to make rats more prone to faster body weight loss in the ABA model. These results could support the clinical hypothesis that high trait anxiety is a subsequent risk factor for the development of AN. 8 , 9 In the vast majority of cases, the first onset of an anxiety disorder predated the onset of AN. 5 , 7 Furthermore, findings suggest that anxiety disorders lead to a more severe clinical variant of AN 25 , 26 and that individuals with a genetic predisposition to pronounced anxiety identified by high scores in anxiety questionnaires (i.e., State Trait Anxiety Index) seem to be more susceptible to AN. 15 , 27 There is also proof that increased anxiety can complicate recovery from AN and lead to high relapse rates. 28 Our findings in the ABA paradigm thus support the clinical relevance of anxiety in AN. Because of the well‐controlled nature, animal studies can help to determine influencing factors of AN even though the ABA model does not mimic all aspects of AN psychopathology.
In our experiment, rats displaying more anxiety‐like behavior showed increased physical activity during normal feeding conditions. The same animals were additionally more susceptible to the typical increase in running‐wheel activity following food reduction. As exercise is associated with increased energy expenditure, this observation could partially explain how anxiety‐related behavior leads to rapid weight loss in the ABA model. These results fit well with a previous study showing increasing running levels in anxious mice, also tested in the EPM, under acute food restriction. 17 Mice carrying genes responsible for elevated trait anxiety similarly demonstrated greater hyperactivity when stressed. 29 Hyperactivity following food restriction in the ABA model is often interpreted as a foraging instinct. 30 The above results allow us to speculate that anxiety traits could be an additional cause. In support of this, there are indications that both anxiety and food restriction can induce hyperactivity in animal models and healthy humans. 31 Apart from the obvious motivation to exercise in order to lose weight, some patients with AN describe physical activity as a means to regulate emotions and desires. 32 Long‐distance runners commonly describe a feeling of excitement called the runner’s high, which, among others, leads to anxiolysis. It has been suggested that the runner’s high results from the stimulation of endorphins via opioid signaling during exercise. 33 In female ABA mice, transiently increasing levels of proopiomelanocortin RNA and peripheral β‐endorphin levels have been detected. 34 These findings could partially explain the association between running and reduced anxiety‐related behavior observed in the present study.
Adding evidence, we found that anxiety‐like behavior and hyperactivity explain a great variance of faster body weight loss. This possibly clarifies how anxiety‐like behavior associated with increased RWA leads to faster weight loss in the ABA model. Obviously, greater energy expenditure due to increased physical exercise has been repeatedly shown in patients with AN, with some studies describing a disturbed energy homoeostasis. 32 Of notice, hyperactivity in patients with AN has been linked to a worse prognosis. 35
In our experimental setting, both LF and ABA groups showed a reduction of anxiety‐like behavior after chronic starvation regardless of RWA. This corresponds well to similar results studying short‐term food reduction in mice. 17 Independently from AN animal research, an anxiolytic effect of mild to moderate calorie restriction in mice was previously observed. 36 , 37 This effect also seems to persist during chronic starvation in the present study. We speculate that food‐restricted animals are more likely to venture into dangerous areas as a result of their foraging instinct and predominant struggle for survival. However, in preclinical studies, it has been reported that starving animals in the ABA model show GABAergic alterations within the hippocampus that are central to regulating anxiety‐related behavior and influence susceptibility to ABA. 38 Moreover, dietary restriction has been suspected to improve anxiety‐related behavior via reduced brain levels of serotonin, noradrenalin, and its precursors, which are known to modulate anxiety‐like behavior. 39 , 40 , 41 Weight loss in humans can be initially rewarding, goal‐driven, and positively reinforced. 42 , 43 The reduction of anxiety can thus be considered as a decisive factor leading to the maintenance of self‐starvation. 44 , 45 , 46 Although the irrational fear of excessive body mass persists or even grows regardless of body weight and weight loss during starvation, 47 some aspects of anxiety (e.g., blushing during public speaking) seem to diminish during acute starvation. 48 , 49 , 50 This may be caused by a shift of attention toward eating and body weight concerns and away from social issues. 51 , 52 Thus, the anxiolytic effect of starvation seen in the animal model can only partly be confirmed in clinical observations.
Strengths and limitations
ABA is a well‐controlled experimental model that has been widely proved to mimic physiological and behavioral characteristics of AN. We were able to assess the effects of starvation, RWA, and anxiety‐like behavior separately using different experimental groups. Furthermore, we examined not only acute but also chronic starvation creating a realistic clinical course of AN. Using an animal study, cognitive components that possibly influence the process of weight loss were excluded. To assess the clinical reality of AN even more accurately, it may be beneficial to define anxiety categorically by using rodent strains bred for both high and low anxiety‐like behavior. The association between anxiety‐like behavior and physical activity was most prominent during the induction of starvation, whereas this association was not well observable in the acute and chronic starvation period. RWA probably decreases during prolonged food restriction to prevent exhaustion.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
C.S. wrote the manuscript. B.H.D., C.B., J.S., and S.T. designed the research. C.S., C.V., V.K., A.S., and S.T. performed the research. C.S., J.S., and S.T. analyzed the data.[Correction added on 2 March 2022, after first online publication: The author contributions has been modified in this version.]
Supporting information
Supplementary Material
ACKNOWLEDGEMENT
The authors would like to acknowledge the technical support of Helga Helten, Petra Ibold, and Uta Zahn at the Institute of Neuroanatomy.
Schwenzer C, Voelz C, Kogel V, et al. Fear and food: Anxiety‐like behavior and the susceptibility to weight loss in an activity‐based anorexia rat model. Clin Transl Sci. 2022;15:889–898. doi: 10.1111/cts.13196
Funding information
This research was funded by the University Hospital Aachen, RWTH Aachen University (START 108/17), by the Swiss Anorexia Nervosa Foundation (Project #80‐17) and by the Federal Ministry of Education and Research (BMBF) together with the European Union (ERA‐NET NEURON, 01EW1906A). Open Access funding enabled and organized by Projekt DEAL.
REFERENCES
- 1. Kolnes LJ. Exercise and physical therapy help restore body and self in clients with severe anorexia nervosa. J Bodywork Movement Therapies. 2017;21(3):481‐494. [DOI] [PubMed] [Google Scholar]
- 2. Legenbauer . Bewegung in der Behandlung der Magersucht. Aktuelle Medizin. 2014;156(20). [DOI] [PubMed] [Google Scholar]
- 3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychological Association; 2013. [Google Scholar]
- 4. Herpertz‐Dahlmann B. Adolescent eating disorders: update on definitions, symptomatology, epidemiology, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2015;24(1):177‐196. [DOI] [PubMed] [Google Scholar]
- 5. Bulik CM, Sullivan PF, Fear JL, Joyce PR. Eating disorders and antecedent anxiety disorders: a controlled study. Acta Psychiatr Scand. 1997;96(2):101‐107. [DOI] [PubMed] [Google Scholar]
- 6. Strober M, Freeman R, Lampert C, Diamond J. The association of anxiety disorders and obsessive compulsive personality disorder with anorexia nervosa: evidence from a family study with discussion of nosological and neurodevelopmental implications. Int J Eat Dis. 2007;40(Suppl):S46‐S51. [DOI] [PubMed] [Google Scholar]
- 7. Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161(12):2215‐2221. [DOI] [PubMed] [Google Scholar]
- 8. Lloyd EC, Sallis HM, Verplanken B, Haase AM, Munafò MR. Understanding the nature of association between anxiety phenotypes and anorexia nervosa: a triangulation approach. BMC Psychiatry. 2020;20(1):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meier SM, Bulik CM, Thornton LM, Mattheisen M, Mortensen PB, Petersen L. Diagnosed anxiety disorders and the risk of subsequent anorexia nervosa: a Danish population register study. Eur Eat Dis Rev. 2015;23(6):524‐530. [DOI] [PubMed] [Google Scholar]
- 10. Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry. 2006;63(3):305‐312. [DOI] [PubMed] [Google Scholar]
- 11. Thornton LM, Dellava JE, Root TL, Lichtenstein P, Bulik CM. Anorexia nervosa and generalized anxiety disorder: further explorations of the relation between anxiety and body mass index. J Anxiety Disord. 2011;25(5):727‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Godart NT, Flament MF, Lecrubier Y, Jeammet P. Anxiety disorders in anorexia nervosa and bulimia nervosa: co‐morbidity and chronology of appearance. Eur Psychiatry. 2000;15(1):38‐45. [DOI] [PubMed] [Google Scholar]
- 13. Silberg JL, Bulik CM. The developmental association between eating disorders symptoms and symptoms of depression and anxiety in juvenile twin girls. J Child Psychol Psychiatry. 2005;46(12):1317‐1326. [DOI] [PubMed] [Google Scholar]
- 14. Lulé D, Schulze UME, Bauer K, et al. Anorexia nervosa and its relation to depression, anxiety, alexithymia and emotional processing deficits. Eat Weight Dis. 2014;19(2):209‐216. [DOI] [PubMed] [Google Scholar]
- 15. Watson HJ, Yilmaz Z, Thornton LM, et al. Genome‐wide association study identifies eight risk loci and implicates metabo‐psychiatric origins for anorexia nervosa. Nat Genet. 2019;51(8):1207‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duncan L, Yilmaz Z, Gaspar H, et al. Significant locus and metabolic genetic correlations revealed in genome‐wide association study of anorexia nervosa. Am J Psychiatry. 2017;174(9):850‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wable GS, Min JY, Chen YW, Aoki C. Anxiety is correlated with running in adolescent female mice undergoing activity‐based anorexia. Behav Neurosci. 2015;129(2):170‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schalla MA, Stengel A. Activity based anorexia as an animal model for anorexia nervosa‐a systematic review. Front Nutr. 2019;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety‐related behavior in rodents. Nat Protoc. 2007;2(2):322‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus‐maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149‐167. [DOI] [PubMed] [Google Scholar]
- 21. Frintrop L, Trinh S, Liesbrock J, et al. Establishment of a chronic activity‐based anorexia rat model. J Neurosci Methods. 2018;293:191‐198. [DOI] [PubMed] [Google Scholar]
- 22. Routtenberg A, Kuznesof AW. Self‐starvation of rats living in activity wheels on a restricted feeding schedule. J Comparative Physiol Psychol. 1967;64(3):414‐421. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez A, Kohn MR, Clarke SD. Eating disorders in adolescents. Aust Fam Physician. 2007;36(8):614‐619. [PubMed] [Google Scholar]
- 24. Steinhausen HC. Outcome of eating disorders. Child Adolesc Psychiatr Clin N Am. 2009;18(1):225‐242. [DOI] [PubMed] [Google Scholar]
- 25. Brand‐Gothelf A, Leor S, Apter A, Fennig S. The impact of comorbid depressive and anxiety disorders on severity of anorexia nervosa in adolescent girls. J Nerv Ment Dis. 2014;202(10):759‐762. [DOI] [PubMed] [Google Scholar]
- 26. Raney TJ, Thornton LM, Berrettini W, et al. Influence of overanxious disorder of childhood on the expression of anorexia nervosa. Int J Eat Dis. 2008;41(4):326‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Root TL, Szatkiewicz JP, Jonassaint CR, et al. Association of candidate genes with phenotypic traits relevant to anorexia nervosa. Eur Eat Dis Rev. 2011;19(6):487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holtkamp K, Müller B, Heussen N, Remschmidt H, Herpertz‐Dahlmann B. Depression, anxiety, and obsessionality in long‐term recovered patients with adolescent‐onset anorexia nervosa. Eur Child Adolesc Psychiatry. 2005;14(2):106‐110. [DOI] [PubMed] [Google Scholar]
- 29. Gelegen C, Pjetri E, Campbell IC, Collier DA, Oppelaar H, Kas MJ. Chromosomal mapping of excessive physical activity in mice in response to a restricted feeding schedule. Eur Neuropsychopharmacol. 2010;20(5):317‐326. [DOI] [PubMed] [Google Scholar]
- 30. Adan RA, Hillebrand JJ, Danner UN, Cardona Cano S, Kas MJ, Verhagen LA. Neurobiology driving hyperactivity in activity‐based anorexia. Curr Top Behav Neurosci. 2011;6:229‐250. [DOI] [PubMed] [Google Scholar]
- 31. Guisinger S. Adapted to flee famine: adding an evolutionary perspective on anorexia nervosa. Psychol Rev. 2003;110(4):745‐761. [DOI] [PubMed] [Google Scholar]
- 32. Lehmann C, Hofmann T, Elbelt U, et al. The role of objectively measured, altered physical activity patterns for body mass index change during inpatient treatment in female patients with anorexia nervosa. J Clin Med. 2018;7(9):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hicks SD, Jacob P, Perez O, Baffuto M, Gagnon Z, Middleton FA. The transcriptional signature of a runner’s high. Med Sci Sports Exerc. 2019;51(5):970‐978. [DOI] [PubMed] [Google Scholar]
- 34. Daimon CM, Hentges ST. β‐endorphin differentially contributes to food anticipatory activity in male and female mice undergoing activity‐based anorexia. Physiol Rep. 2021;9(5):e14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scheurink AJ, Boersma GJ, Nergårdh R, Södersten P. Neurobiology of hyperactivity and reward: agreeable restlessness in anorexia nervosa. Physiol Behav. 2010;100(5):490‐495. [DOI] [PubMed] [Google Scholar]
- 36. Riddle MC, McKenna MC, Yoon YJ, et al. Caloric restriction enhances fear extinction learning in mice. Neuropsychopharmacology. 2013;38(6):930‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamamoto Y, Tanahashi T, Kawai T, et al. Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol Genomics. 2009;39(3):227‐235. [DOI] [PubMed] [Google Scholar]
- 38. Aoki C, Chowdhury TG, Wable GS, Chen YW. Synaptic changes in the hippocampus of adolescent female rodents associated with resilience to anxiety and suppression of food restriction‐evoked hyperactivity in an animal model for anorexia nervosa. Brain Res. 2017;1654:102‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chuang J‐C, Perello M, Sakata I, et al. Ghrelin mediates stress‐induced food‐reward behavior in mice. J Clin Investig. 2011;121(7):2684‐2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaye WH, Barbarich NC, Putnam K, et al. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int J Eat Dis. 2003;33(3):257‐267. [DOI] [PubMed] [Google Scholar]
- 41. Lutter M, Sakata I, Osborne‐Lawrence S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11(7):752‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Godier LR, Park RJ. Compulsivity in anorexia nervosa: a transdiagnostic concept. Front Psychol. 2014;5:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park RJ, Godier LR, Cowdrey FA. Hungry for reward: How can neuroscience inform the development of treatment for Anorexia Nervosa? Behav Res Ther. 2014;62:47‐59. [DOI] [PubMed] [Google Scholar]
- 44. Lloyd EC, Frampton I, Verplanken B, Haase AM. How extreme dieting becomes compulsive: A novel hypothesis for the role of anxiety in the development and maintenance of anorexia nervosa. Med Hypotheses. 2017;108:144‐150. [DOI] [PubMed] [Google Scholar]
- 45. Fiore F, Ruggiero GM, Sassaroli S. Emotional dysregulation and anxiety control in the psychopathological mechanism underlying drive for thinness. Front Psychiatry. 2014;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pop‐Jordanova N, Zorcec T, Demerdzieva A. Anorexia: abnormal phobia of normal weight. PRILOZI. 2017;38(2):45‐53. [DOI] [PubMed] [Google Scholar]
- 47. Mattar L, Thiébaud MR, Huas C, Cebula C, Godart N. Depression, anxiety and obsessive‐compulsive symptoms in relation to nutritional status and outcome in severe anorexia nervosa. Psychiatry Res. 2012;200(2–3):513‐517. [DOI] [PubMed] [Google Scholar]
- 48. Het S, Vocks S, Wolf JM, Hammelstein P, Herpertz S, Wolf OT. Blunted neuroendocrine stress reactivity in young women with eating disorders. J Psychosom Res. 2015;78(3):260‐267. [DOI] [PubMed] [Google Scholar]
- 49. Schmalbach I, Herhaus B, Pässler S, et al. Cortisol reactivity in patients with anorexia nervosa after stress induction. Transl Psychiat. 2020;10(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zonnevylle‐Bender MJ, van Goozen SH, Cohen‐Kettenis PT, Jansen LM, van Elburg A, Engeland H. Adolescent anorexia nervosa patients have a discrepancy between neurophysiological responses and self‐reported emotional arousal to psychosocial stress. Psychiatry Res. 2005;135(1):45‐52. [DOI] [PubMed] [Google Scholar]
- 51. Oldershaw A, Startup H, Lavender T. Anorexia nervosa and a lost emotional self: a psychological formulation of the development, maintenance, and treatment of anorexia nervosa. Front Psychol. 2019;10:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Startup H, Lavender A, Oldershaw A, et al. Worry and rumination in anorexia nervosa. Behav Cogn Psychother. 2013;41(3):301‐316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


