Abstract
Over 50% of breast tumors harbor alterations in one or more genes of the phosphatidylinositol 3-kinase (PI3K) pathway including PIK3CA mutations (31%), PTEN loss (34%), PTEN mutations (5%) and AKT1 mutations (3%). While PI3K and mTOR inhibitors are already approved in advanced breast cancer, AKT inhibitors have been recently developed as a new therapeutic approach. Capivasertib (AZD5363) is a novel, selective ATP-competitive pan-AKT kinase inhibitor that exerts similar activity against the three AKT isoforms, AKT1, AKT2, and AKT3. Preclinical studies demonstrated efficacy of capivasertib in breast cancer cell lines as a single agent or in combination with anti-HER2 agents and endocrine treatment, especially in tumors with PIK3CA or MTOR alterations. Phase I/II studies demonstrated greater efficacy when capivasertib was co-administered with paclitaxel, fulvestrant in hormone receptor (HR)-positive, HER2-negative breast cancer or olaparib. The recommended phase II dose of capivasertib as monotherapy was 480 mg bid on a 4-days-on, 3-days-off dosing schedule. Toxicity profile proved to be manageable with hyperglycemia (20–24%), diarrhea (14–17%) and maculopapular rash (11–16%) being the most common grade ≥3 adverse events. Ongoing Phase III trials of capivasertib in combination with fulvestrant (CAPItello-291), CDK4/6 inhibitor palbociclib (CAPItello-292) and paclitaxel (CAPItello- 290) will better clarify the therapeutic role of capivasertib in breast cancer.
Keywords: Capivasertib”, “Breast cancer”, “AKT inhibitor”, “PI3K/AKT/mTOR pathway”
Highlights
-
•
Phosphatidylinositol-3-kinase (PI3K)/Akt (PI3K/AKT) pathway is one of the most commonly altered pathways in breast cancer.
-
•
Capivasertib (AZD5363) is a highly potent Akt kinase inhibitor with activity against the three isoforms AKT1, AKT2, and AKT3.
-
•
Preclinical studies demonstrated efficacy of capivasertib either alone or in combination with anti-HER2 agents, chemotherapy and endocrine treatment.
-
•
Dose-limiting toxicities include hyperglycemia (20–24%), diarrhea (14–17%) and maculopapular rash (11–16%).
-
•
Capivasertib increased susceptibility to paclitaxel (PAKT, BEECH), fulvestrant (NCT01226316, FAKTION) or Olaparib (ComPAKT).
Abbreviations
- PI3K/AKT pathway
phosphatidylinositol-3-kinaseAkt pathway
- PTEN
phosphatase and tensin homolog
- mTOR
mammalian target of rapamycin
- FDA
Food and Drug Administration
- HR
hormone receptor
- HER2
human epidermal growth factor receptor 2
- RTK
receptor tyrosine kinase
- EGF
epidermal growth factor
- IGF
insulin-like growth factor
- PDGF
platelet-derived growth factor
- VEGF
vascular endothelial growth factor
- PH
pleckstrin homology
- TSC2
tuberos sclerosis complex 2
- GSK3β
glycogen synthase kinase-3β
- FoxO
forkhead box class O
- CDK4/6 inhibitors
cyclin-dependent kinase 4/6 inhibitors
- PRAS4
proline-rich Akt substrate of 40 kDa
- ATP
adenosine triphosphate
- IC50
half-maximal inhibitory concentration
- PKA/B/C
protein kinase A/B/C
- IHC
immunohistochemistry
- PK
pharmacokinetic
- AUC
Area Under the Curve
- Cmax
maximum Plasma Concentration
- tmax
time to maximum plasma concentration
- ILC
invasive lobular carcinoma
- IDC
invasive ductal carcinoma
- CBP
CREB-binding protein
- ER
estrogen receptor
- BET
bromodomain and extra-terminal domain
- BRD4
bromodomain-containing protein 4
- InsR
insulin receptor
- IGF-IR
insulin-like growth factor-I receptor
- HER3
human epidermal growth factor receptor 3
- FGFR
fibroblast growth factor receptor
- IGFBP-3
insulin-like growth factor-binding protein 3
- SGK1
glucocorticoid-regulated kinase 1
- PFS
progression-free survival
- TNBC
triple negative breast cancer
- CRPC
castration-resistant prostate cancer
- AR
androgen receptor
- PSA
prostate-specific antigen
- MTD
maximum tolerated dose
- ORR
objective response rate
- AE
adverse event
- CI
confidence interval
- ECG
electrocardiogram
- BRCA
breast cancer gene
- PR
partial response
- SD
stable disease
- OS
overall survival
- HR
hazard ratio
1. Introduction
The phosphatidylinositol-3-kinase (PI3K)/Akt (PI3K/AKT) pathway is the most commonly altered signaling pathway in human cancers [1]. Alterations in the PI3K/AKT/mTOR pathway were identified in 38% of cancer patients including mainly phosphatase and tensin homolog (PTEN) (30%), followed by mutations in PIK3CA (13%) and AKT1 (1%) [1]. In breast cancer, over 50% of patients exhibited alterations in one or more of these genes including PIK3CA mutations (31%), PTEN loss (34%), PTEN mutations (5%) and AKT1 mutations (3%) [1]. It is well-known that AKT pathway plays a pivotal role in cell growth, apoptosis suppression and neovascularization. Disruptions in the Akt signaling pathway have been associated with carcinogenesis and chemotherapy resistance. Apart from its role in cancer, AKT signaling pathway has a protective role in neural cell death in neurodegenerative diseases, in vessel remodeling and atherosclerosis and implicates in glucose metabolism and insulin resistance [2]. Given the importance of AKT cascade in malignancy, potential drug targets for directed therapy either upstream or downstream the AKT pathway have arisen. Identifying AKT inhibitors that could attenuate cancer growth has gained ground (see Table 1, Table 2, Fig. 1).
Table 1.
Ongoing clinical trials of capivasertib in breast cancer.
| Trial | Registration no. | Phase | Study population | Study Size, n | Status | Treatment regimens | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| CAPItello-292 | NCT04862663 | 3 | Advanced or Metastatic HR-positive, HER2-Negative (HR+/HER2-) Breast Cancer | 628 | Recruiting | Capivasertib 320 mg bid 4-days-on/3-days-off Plus Palbociclib and Fulvestrant vs Placebo Plus Palbociclib and Fulvestrant | Not Reported | – |
| CAPItello-291 | NCT04305496 | 3 | Advanced or Metastatic HR-positive, HER2-Negative (HR+/HER2-) Breast Cancer | 834 | Recruiting | Capivasertib 400 mg bid 4-days-on/3-days-off plus Fulvestrant vs. Placebo plus Fulvestrant | Not Reported | – |
| CAPItello- 290 | NCT03997123 | 3 | Advanced or Metastatic Triple Negative Breast Cancer (TNBC) | 924 | Recruiting | Capivasertib 400 mg bid 4 days on/3 days off plus Paclitaxel weekly vs. Placebo plus Paclitaxel | Not Reported | – |
| PAKT | NCT02423603 | 2 | Triple-Negative Advanced or Metastatic Breast Cancer (TNBC) | 140 | Active, not Recruiting | Capivasertib 400 mg bid 4 days on/3 days off plus Paclitaxel vs. Placebo plus Paclitaxel | mPFS: 5.9 months vs. 4.2 months (HR: 0.74; 95% CI: 0.50–1.08; 1-sided P = 0.06) mOS:19.1 months vs. 13.5 months (HR: 0.70; 95% CI: 0.47–1.05; P = 0.085). In patients with PIK3CA/AKT1/PTEN-altered tumors: mPFS: 9.3 months vs. 3.7 months (HR: 0.30; 95% CI: 0.11–0.79; P = 0.01) AEs (97%): Diarrhea (72.1%), fatigue (44.1%), nausea (35.3%), rash (41.2%), neuropathy (25%), stomatitis (26.5%) AEs grade3/4 (54%): diarrhea (13.2%), fatigue (4.4%), rash (4.4%), infection (4.4%) |
[41,42] |
| SAFIR02_Breast | NCT02299999 | 2 | Metastatic Breast Cancer | 1460 | Active, not Recruiting | AZD2014 mTOR inhibitor, AZD4547 FGFR inhibitor, Capivasertib 480 mg bd, 4 days on/3 days off, AZD8931 Selumetinib, Vandetanib, Bicalutamide, Olaparib Vs. Standard maintenance treatment (Anthracyclines, Taxanes, cyclophosphamide, Capecitabine, 5-FU, Gemcitabine Methotrexate, Vinca alkaloids, Platinum-based chemotherapies, Bevacizumab, Mitomycin C Eribulin |
143 (22%) patients presented PIK3CA mutation. 104/364 (28%) of HR-positive, HER2-negative patients presented PIK3CA mutation Patients with PIK3CA mutation were less sensitive to chemotherapy. 27/255 (10%) of TNBC patients presented PIK3CA mutation No difference in chemosensitivity between the PIK3CA-mutated and wild-type cohort in mTNBC Patients with PIK3CA-mutated mTNBC presented a better OS compared with PIK3CA-WT mTNBC (24 versus vs. 14 months, P = 0.03) |
[44] |
| MATCH screening trial | NCT02465060 | 2 | Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma | 6452 | Recruiting | Targeted Therapy Directed by Genetic Testing (Capivasertib for AKT mutation, Afatinib for EGFR mutation, Crizotinib for MET amplification/mutation, ROS or ALK translocation, Dabrafenib for BRAF mutation etc) | Νot Reported | – |
| plasmaMATCH | NCT03182634 | 2 | Patients With Advanced Breast Cancer Where the Targetable Mutation is Identified Through ctDNA | 1150 | Recruiting | Cohort A: Fulvestrant, B: Neratinib, C: Capivasertib 480 mg bid 4 days on/3 days off, D: Capivasertib 400 mg bid 4 days on/3 days off plus Fulvestrant, E: Olaparib plus AZD6738 | Cohort C: 18/30 patients (60%) Most common mutations detected: Glu17Lys (94%), Leu52Arg (6%) 4 (22%) patients had confirmed PR, 4 patients had unconfirmed PR Median DOR: 7.5 months (IQR 4.1–9.8); mPFS: 10.2 months (95% CI: 3.2–18.2) Cohort D: 19 patients Mutations detected: Glu17Lys (26%), AKT1 Leu52Arg (5%), PTEN inactivating mutation (63%), PTEN homozygous deletion (5%) 2 (11%) patients had a confirmed PR (with AKT1 mutations) None of the patients with PTEN genomic alterations responded. Median DOR: 3.9 months (IQR 3.7–4.2) mPFS: 3.4 months (95% CI; 1.8–5.5) |
[45] |
| MATCH-Subprotocol Y | NCT04439123 | 2 | Advanced cancer with AKT1 E17K-mutations | 35 | Active, not recruiting | Capivasertib 480 mg bid 4-days-on/3-days-off | ORR: 28.6% (95% CI, 15%–46%). 1 patient had CR; 9 had PR; 4 had SD Median DOR: 4.4 (3.1 to ≥31.7) months Median PFS: 5.5 (0–35) months (95% CI, 4.6–11.3); Median OS: 14.5 months (95% CI, 10.2-NA) For breast cancer: Median PFS: 1.9 months (95% CI, 1.58-not applicable [NA]) AEs grade 1/2: diarrhea (17 [49%]), fatigue (15 [43%]), nausea (13 [37%]), proteinuria (10 [29%]), hyperglycemia (8 [23%]), and anorexia (7 [20%]) AEs grade 3: hyperglycemia (8 [23%]), maculopapular rash (4 [11%]), vomiting (2 [6%]), diarrhea (3 [9%]) |
[14] |
| BEECH | NCT01625286 | 1/2 | ER-positive, HER2-negative advanced or metastatic breast cancer | 148 | Active, not Recruiting | Capivasertib 400 mg b.i.d. 4 days on/3 days off plus Paclitaxel vs. Placebo plus Paclitaxel | mPFS = 10.9 months vs. 8.4 months (HR: 0.80; 80% CI 0.60–1.06; P = 0.308) In the PIK3CA + population: mPFS: 10.9 months vs. 10.8 months on placebo plus paclitaxel (HR 1.11; 80% CI 0.73–1.68; P = 0.760) AEs: diarrhea (76%), alopecia (52%), nausea (39%), anemia (33%), fatigue (30%), hyperglycemia (30%), vomiting (28%), stomatitis (28%), maculopapular rash (26%), sensory neuropathy (26%), pyrexia (26%) AEs grade3/4 (59%): diarrhea (22%), hyperglycemia (13%), neutropenia (11%), maculopapular rash (9%), peripheral neuropathy (6%), stomatitis (4%), ALT increased (4%) |
[12] |
| FAKTION | NCT01992952 | 1/2 | Postmenopausal Women with ER-positive, HER2-negative advanced or metastatic breast cancer | 149 | Active, not recruiting | Capivasertib 400 mg b.i.d. 4 days on/3 days off plus Fulvestrant vs Placebo plus Fulvestrant | mPFS = 10.3 months vs. 4.8 months (HR: 0.58; 95% CI: 0.39–0.84; p = 0·0044) ORR: 29% (20/69) vs 8% (6/71) (OR: 4.42; 95% CI: 1.65–11.84; p = 0·0031) Significantly longer PFS in the PI3K/PTEN pathway non-altered group (HR: 0.56, 95% CI: 0.33–0.96, p = 0.035), but not in the PI3K/PTEN pathway altered group (HR: 0.59, 95% CI: 0.34–1.03, p = 0.064) mOS: 26.0 months vs. 20.0 months (HR: 0.59; 95% CI: 0.34–1.05, p = 0.071) AEs grade3/4: hypertension (32%), diarrhea (14%), rash (20%), infection (6%), hyperglycemia (4%), fatigue (1%), vomiting (3%) |
[40] |
| BEGONIA | NCT03742102 | 1/2 | Metastatic Triple Negative Breast Cancer (TNBC) | 200 | Recruiting | Durvalumab plus Paclitaxel vs. Durvalumab plus Capivasertib or Oleclumab or Trastuzumab Deruxtecan or Datopotamab deruxtecan with or without paclitaxel | Not Reported | – |
| OLAPCO | NCT02576444 | 2 | Advanced Solid Tumors with PI3K/AKT/PTEN Pathway alterations | 64 | Active, not recruiting | Olaparib plus Capivasertib 640 mg bid two-days-on/five-days-off (2/7) | Not Reported | – |
| – | NCT01226316 | 1 | Advanced Solid Tumors | 285 | Active, Not Recruiting | Capivasertib 320, 480, 640 mg bid in a continuous, 4/7, and 2/7 dosing schedule | In AKT1 E17K-mutant breast cancer, mPFS:5.5 months (95% CI: 2.9–6.9 months) and ORR: 20%. Patients with concomitant PI3K pathway mutations has a better mPFS (mPFS: not reached vs. 4.3 months, HR: 0.21; 0.045) In PIK3CA-mutant breast cancer: 46% (12/26) showed a reduction in tumor size, 4% (1/28) achieved a RECIST response AEs: diarrhea (80%), nausea (56%), fatigue (41%), vomiting (44%), hyperglycemia (41%), maculopapular rash (30%) AEs grade 3/4: hyperglycemia (20–24%), diarrhea (14%), maculopapular rash (11–16%) |
[11,24] |
| SERENA-1 | NCT03616587 | 1 | Advanced/Metastatic ER-positive, HER2-negative Breast Cancer | 340 | Recruiting | AZD9833 oral SERD monotherapy (Parts A and B) or in combination with Palbociclib (Parts C and D) or Everolimus (Parts E and F) or Abemaciclib (Parts G and H) or Capivasertib (Parts I and J) | Not Reported for Capivasertib | – |
| MEDIPAC | NCT03772561 | 1 | Advanced or Metastatic Solid Tumors | 40 | Recruiting | Capivasertib 160 mg, 200 mg and 320 mg bid 4-days-on/3-days-off plus Olaparib 300 mg bid plus Durvalumab 1500 mg q28 days | Treatment was tolerable with mainly AEs grade 1-2 AEs grade ¾: anemia (4/22), hyperglycemia (3/22), raised lipase (3/22). No DLTs were observed at 160 and 200 mg bid; 1 DLT (G4 hyperglycemia) was observed at 320 mg bid. 1 pt with PIK3CA-mutant breast cancer had stable disease (SD) for 5 months, and 1 pt with PTEN loss and BRCA1-mutant breast cancer had SD of 6 months despite prior progression on PARP inhibitor. |
[46] |
| – | NCT03310541 | 1 | Advanced ER-positive breast cancer or prostate cancer with AKT1/2/3 mutations | 12 | Active, not recruiting | Capivasertib 400 mg bid 4-days- on/3-days-off plus Fulvestrant OR Capivasertib 400 mg bid 4-days- on/3-days-off plus Enzalutamide |
Not Reported | – |
| – | NCT02208375 | 1/2 | Recurrent Endometrial, TNBC, ovarian, primary peritoneal, or fallopian tube cancer | 159 | Active, not Recruiting | Olaparib 300 mg bid plus Capivasertib 400 mg or 320 mg bid 4 days on/3 days off (AZD5363) | 400 mg bid 4 days on/3 days off was the RP2D 19% (6/32) patients achieved PR; 22% (7/32) achieved SD for greater than 4 months TRAEs grade 3/4: anemia (23.7%), leukopenia (10.5%). |
[35] |
| DESTINY-Breast 08 | NCT04556773 | 1 | Metastatic HER2-negative, low expressing (IHC 2+/ISH- or IHC 1+) Advanced or Metastatic Breast Cancer | 185 | Recruiting | Trastuzumab Deruxtecan (T-DXd) 5.4 mg/kg in combination with other regimens (Capecitabine, Durvalumab plus Paclitaxel, Capivasertib 400 mg bid, Anastrozole, Fulvestrant) | Not Reported | – |
| NCT04958226 | 1 | Advanced Solid Tumors with PI3K/AKT/PTEN Pathway alterations | 23 | Recruiting | Capivasertib plus Midazolam | Not Reported | – |
HER2: human epidermal growth factor receptor 2; HR: hormone-receptor; bid: twice a day; AE: adverse event; TRAE: treatment-related adverse event; IHC: immunohistochemistry; TNBC: triple-negative breast cancer; PR: partial response; CR: complete response; SD: stable disease; ORR: overall response rate; DLT: dose-limiting toxixity; PFS: progression-free survival; HR: hazard ratio; CI: confidence interval; OS: overall survival; DOR: duration of response; SERD: selective estrogen receptor (ER) antagonist and degrader; vs.: versus; Ref: reference.
Table 2.
Completed clinical trials with capivasertib in breast cancer.
| Trial | Registration no. | Phase | Study population | Participants | Treatment regimens | Results | Ref. |
|---|---|---|---|---|---|---|---|
| STAKT | NCT02077569 | 2 | ER-positive invasive breast cancer | 48 | Capivasertib 480, 240, 360 mg bid | Significant percentage reductions in biomarkers −39% (P = 0.006) for pGSK3β and −50% (P < 0.0001) for pPRAS40 and percentage reduction in ki67 at 480 mg dose bid Milder dose- and concentration-dependent reductions in the biomarkers at doses of 240 and 360 mg bid |
[15] |
| NCT04712396 | 1 | Healthy Volunteers | 11 | Capivasertib plus Itraconazole | Not Reported | – | |
| [42] | NCT01353781 | 1 | Advanced Solid Tumors | 41 | Capivasertib 80, 240, 320, 400 mg bid continuously or 360, 480 mg bid 4-days-on/3-days-off or 640 mg bid 2-days-on/5-days-off | (2/37) had PR; 27% (10/37) had SD with duration of 46–360 days. TRAEs (97.6%): diarrhea (78%), hyperglycemia (68.3%), nausea (56.1%), maculopapular rash (56.1%), pyrexia (48.8%), stomatitis (41.5%) TRAEs grade3/4 (63.4%): hyperglycemia (39%), diarrhea (17.1%) |
[33] |
| ComPAKT | NCT02338622 | 1 | Advanced Solid Tumors | 64 | Capivasertib 320, 400, 480 mg bid 4-days-on/3-days-off and 480 mg, 560 mg and 640 mg bid 2-days-on/5-days-off plus Olaparib | 400 mg bid 4/3 and 640 mg bid 2/5 were chosen for the dose expansion phase 8/18 (44%) achieved clinical benefit; 6 patients had PR and 2 patients had SD. Median DOR: 38.2 (14.9–80.9) 5/7 of BRCA1/2 mutated patients achieved clinical benefit; Median DOR: 39.1 (14.9–80.9) TRAEs: nausea (67%), diarrhea (55%), vomiting (41%), fatigue (51%), anemia |
[37] |
| OAK trial | NCT01895946 | 1 | Advanced Solid Tumors | 33 | Capivasertib 480 mg bid 4-days-on/3-days-off tablet or capsule in a fast or fed state | Faster absorption from the tablet than from the capsule (tmax: 1.0 (0.6–2) vs 2.0 (1–4) Similar AUCτ and Cmax between the AZD5363 tablet and capsule Lower absorption rate in the fed vs fasted state (tmax: 2.0 (2–4.3) Vs 0.6 (0.5–4)) Lower and later peak concentrations in the fed vs fasted state |
[16] |
AE: adverse event; TRAE: treatment-related adverse event; bid: twice a day; PR: partial response; CR: complete response; SD: stable disease; HR: hazard ratio; CI: confidence interval; OS: overall survival; DOR: duration of response; Ref.:reference.
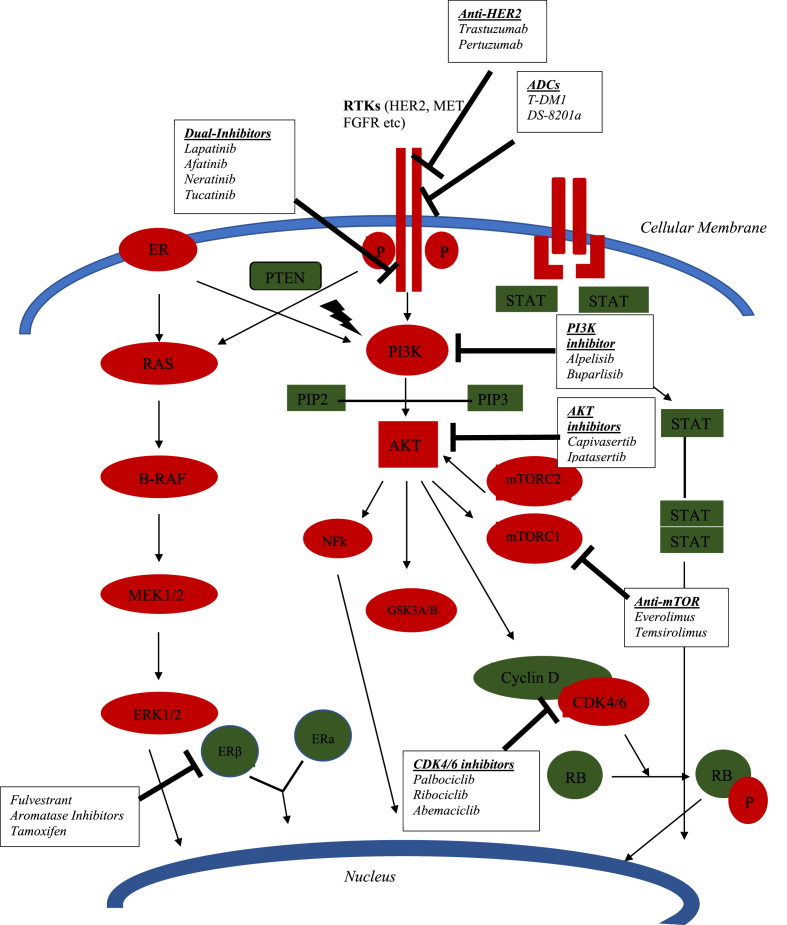
Fig. 1.
Therapeutic targets in breast cancer.
Several compounds have been designed to target AKT signaling in vitro and in vivo, although none of them has received FDA approval in solid tumors. Since PI3K is located upstream of AKT, either inhibitors of PI3K or mTOR could act on the AKT cascade as well. Recently, an α-specific PI3K inhibitor, Alpelisib, received FDA approval for the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutant, advanced or metastatic breast cancer providing evidence that the inhibition of the pathway could demonstrate clinical efficacy [3]. Synthetic and natural compounds targeting AKT have been tested in preclinical studies and some of them have entered clinical evaluation. Capivasertib (AZD5363) is a novel, selective ATP-competitive pan-AKT kinase inhibitor that exerted preclinical efficacy both in vivo and in vitro and is currently under investigation in clinical trials. Given its tolerable efficacy profile, Phase III trials have been designed to evaluate its efficacy in breast cancer. Our aim is to review all preclinical and clinical evidence regarding the antitumor activity of this promising agent in breast cancer.
2. Mechanism of action
Akt is a key member of the AGC kinase family and comprises three isoforms that are encoded by three separate genes: Akt1 that is highly expressed in the majority of tissues; Akt2 that is mainly expressed in metabolic tissues, including the liver, skeletal muscle and adipose tissue; and Akt3 that is enriched in brain and testis [4]. Akt can be hyperactivated in cancer cells by multiple mechanisms, including PTEN loss, activating mutations of the PI3K catalytic subunit, AKT activating mutations etc. [4]. Apart from playing an essential role in cancer, AKT signaling is also crucial for normal cellular processes, like glucose homeostasis, cardiac function, coronary angiogenesis, endothelial nitric oxide synthesis and neural synaptic transmission. AKT activity is regulated by receptor tyrosine kinases (RTKs), such as EGF (epidermal growth factor), insulin-like growth factor (IGF), PDGF (platelet derived growth factor), and VEGF (vascular endothelial growth factor) families. RTKs activate class I phosphatidylinositol 3-kinases (PI3K) which results in Akt translocation to the cell membrane via its amino terminal pleckstrin homology (PH) domain. This translocation drives the activation of Akt via phosphorylation at Ser 473 and at Thr308 within the catalytic domain [5]. Upon activation, AKT phosphorylates its three main downstream substrates: tuberos sclerosis complex 2 (TSC2) that exerts an inhibitory effect on mTORC1, glycogen synthase kinase-3β (GSK3β) that is involved in the Wnt/β-catenin pathway and the forkhead box transcription factors (FOXO) that regulate many physiological processes. The activation of these downstream targets by AKT eventually promotes cell proliferation, tumor growth and progression.
Activation of AKT is associated with resistance to anticancer treatment and an adverse prognosis [6]. AKT activation has been shown to confer resistance to inhibitors of receptor tyrosine kinases such as anti-HER2 regimens, endocrine treatment and chemotherapy, including anthracyclines and taxanes in breast cancer [7]. Additionally, there is evidence that PTEN loss could mediate resistance to cyclin-dependent kinase (CDK) 4/6 inhibitors through increased AKT activation [8]. Mutations in the PI3K/AKT pathway are more frequently identified in hormone receptor–positive (34.5%) and HER2-positive (22.7%) breast cancer compared to basal-like tumors (8.3%) [9,10]. PIK3CA mutations occur mainly at somatic “hotspots” in exons 9 and 20 that encode parts of the helical and kinase domains of PI3K respectively [10]. Activation of the PI3K/AKT pathway can result from various mechanisms, including overexpression or amplification of HER2, activating mutations in PIK3CA, AKT mutation (2–4%) or amplification (5–10%) and PTEN loss of function (13–35%) [9]. A rationale therefore exists for targeting the key components of the PI3K/AKT pathway. While mTOR and PI3K inhibitors are already approved by the FDA for the treatment of advanced HR-positive breast cancer patients, AKT represents a novel druggable target.
Capivasertib (AZD5363) is a highly potent pan-Akt kinase inhibitor with similar activity against the three isoforms AKT1, AKT2, and AKT3. Capivasertib prevents substrate phosphorylation by AKT and down-regulates the phosphorylation levels of Akt downstream substrates GSK3β and PRAS40 in many cancer cells. AKT inhibitors can be categorized in two main subgroups: the ATP competitors that compete with ATP to associate with Akt kinase at the ATP binding site and include capivasertib (AZD5363), GSK2110183, GSK690693 and ipatasertib; and allosteric inhibitors that target the PH-domain and prevent the migration of AKT to the plasma membrane where activation by upstream kinases occurs, thus locking AKT in an inactive form. Capivasertib belongs to the category of ATP-competitive inhibitors that are the most widely studied. This group of inhibitors exerts no selectivity to the three subtypes of Akt and has poor selectivity to PKA, PKB and PKC kinases among AGC family. Capivasertib is a novel pyrrolopyrimidine-derived compound that inhibits all AKT isoforms with low nanomolar potency against Akt1, Akt2 and Akt3 with IC50 of 0.1 nM, 2 nM and 2.6 nM, respectively. It is mainly metabolized by the liver, as less than 10% of the dose is excreted in urine [11]. Plasma exposure is approximately dose dependent in the dose range 80–800 mg and terminal half life is approximately 10 h (range, 7–15) [11]. Most studies used the phosphorylation level of downstream effectors as an indicator of the efficacy of AKT inhibition, such as GSK3β, PRAS40 and S6 as measured by immunohistochemistry (IHC) [[11], [12], [13], [14]]. Significant decrease of GSK3β (>30%) and PRAS40 (>50%) phosphorylation is associated with increased capivasertib activity. These pharmacodynamic properties of capivasertib emerge from preclinical studies and data from in-human clinical trials. STAKT trial examined if capivasertib can reach its therapeutic levels after short-term exposure to either capivasertib 480 mg bid (stage 1) or capivasertib 360 or 240 mg bid (stage 2) [15]. Trial results showed that capivasertib 480 mg bid reached its therapeutic target after 4.5 days of treatment as shown by the statistically significant decreases from baseline of the biomarkers GSK3β, PRAS40 and pS6 and the proliferation marker ki67. Changes in the biomarkers were also observed at the lower doses of 240 and 360 mg bid although at a lesser extent indicating that capivasertib acts dose and concentration-dependently [15]. All doses of capivasertib should be taken in a fasted state at approximately the same time each day. Capivasertib was originally formulated as a capsule. Therefore, OAK Phase I trial was designed to compare the tablet and capsule forms of capivasertib in terms of pharmacokinetics and to explore potential differences between administration in a fasted and non-fasted state [16]. The pharmacokinetic (PK) analysis showed faster absorption from the tablet than from the capsule as median tmax was approximately 1 h earlier for the tablet [16]. However, AUCτ and Cmax at the steady state were comparable between the two forms indicating that exposure and safety profile were also comparable. Exposure and safety profile were also comparable between the fed and fasted states, although the absorption was delayed when administered with food [16].
3. Preclinical data
Several preclinical studies have investigated the efficacy of capivasertib in breast cancer. Davies BR et al. published one of the first studies presenting data on capivasertib activity in vitro and in vivo [17]. Capivasertib proved to be a pan-AKT inhibitor that inhibited all the three isoforms of AKT with an IC50 < 10 nmol/L and also exerted activity against 15 kinases, most of which were members of the AGC family. Capivasertib monotherapy was tested in vitro in 182 cell lines derived from solid and hematologic tumors [17]. HER2-positive and ER-positive breast cancer cell lines were persistently susceptible to treatment in contrast to lung and colorectal cancer cell lines that showed a lower frequency of response [17]. In vivo, capivasertib proved to be efficient in HER2-positive, PIK3CA-mutated breast cancer xenografts, while it synergized with anti-HER2 agents trastuzumab and lapatinib and treatment with docetaxel [17]. Moreover, capivasertib induced a dose-dependent inhibition of growth and survival in invasive lobular carcinoma (ILC) human and mouse breast cancer cell lines [18]. Indeed, mutations of PIK3CA (48%) and genetic loss of PTEN (13%) are more common in ILC compared to matched IDC (37% and 11%, respectively) tumors [18].
AKT inhibition leads to a reduction in ER-mediated transcription via attenuating the recruitment of ER and CREB-binding protein (CBP) to estrogen response elements [19]. The combination of capivasertib and endocrine treatment with fulvestrant, anastrozole or tamoxifen proved to be superior to either monotherapy in ER-positive endocrine-resistant breast cancer cell lines [19]. This effect was more pronounced in cells harboring mutations of PIK3CA or loss of PTEN function [19]. Of note, the combination of capivasertib with fulvestrant proved to be more effective not only in vitro but also in vivo in a patient-derived HR-positive breast cancer xenograft. This antitumor activity was confirmed in another study that capivasertib suppressed growth in three ER-positive breast cancer cell lines with acquired resistance to estrogen deprivation [20]. Fulvestrant significantly enhanced the growth-inhibitory effect of capivasertib both in vitro and in vivo in an ER-positive, PIK3CA-mutant breast cancer xenograft [20]. Additionally, the antitumor activity of capivasertib in luminal breast cancer cell lines could be enhanced through inhibition of BRD4/FOXO3a/CDK6 axis [21]. Treatment of four luminal breast cancer cells lines with capivasertib monotherapy did not induce great tumor regression, but when Akt inhibitors were combined with BET inhibitors the antitumor effect increased [21]. Indeed, BRD4 knockdown enhanced the antitumor efficacy of Akt inhibition indicating that BRD4 is involved in resistance to AKT inhibition [21]. Apart from BRD4/FOXO3a/CDK6 axis, resistance to AKT inhibition could emerge from feedback activation of RTKs, including IGF-IR, InsR, HER3 and FGFRs upon AKT inhibition [20]. Indeed, inhibition of AKT resulted in upregulation of ER- and FoxO-dependent IGF-IR, IGF-I, and IGF-II, while treatment with insulin-like growth factor-binding protein 3 (IGFBP-3) inhibited the capivasertib-induced phosphorylation of IGF-IR/InsR. This data provide the rationale for combinations of AKT and IGF-IR/InsR inhibitors in endocrine-resistant ER-positive breast cancer [20].
As far as sensitivity to AKT inhibition is concerned, several biomarkers that might be deployed to predict resistance or sensitivity to capivasertib have been proposed. Elevated serum- and glucocorticoid-regulated kinase 1 (SGK1) levels, an enzyme that is closely linked to AKT and is regulated by the same upstream components like PI3K and mTORC2 were associated with resistance of breast cancer cells to Akt inhibitors [22]. Indeed, Akt-inhibitor-resistant breast cancer cells displayed significantly higher SGK1 expression than sensitive ones, while SGK1 depletion sensitized these cells to AKT inhibition [22]. On the other hand, mutations in PIK3CA or AKT1 in the absence of mTORC1-activating alterations (e.g. MTOR mutations or TSC2 loss) were associated with sensitivity to capivasertib monotherapy [23]. In a Phase 1 study evaluating capivasertib in AKT1-mutated solid tumors the presence of synchronous PIK3CA or MTOR alterations was associated with improved progression-free survival (PFS) compared to patients with no mutations [24]. Another study also identified an association between susceptibility to capivasertib and the presence of activating PIK3CA mutations, PTEN loss or HER2 amplification, while the presence of a RAS mutation was linked to resistance to capivasertib [17]. Finally, AKT inhibition by capivasertib induces feedback activation of the upstream RTKs. Inhibition of the AKT pathway causes the activation of compensatory signaling pathways through feedback upregulation and activation of upstream receptor tyrosine kinases such as HER2 and HER3 [25]. Indeed, preclinical data showed that AKT inhibition results in feedback upregulation and phosphorylation of HER3 and to a lesser extent HER2 in HER2-amplified breast cancer cells [25]. This data provide a rationale of combining capivasertib with other kinase inhibitors that could limit the RTK feedback activation like AZD8931, an inhibitor of EGFR/HER2/HER3 signalling in HER2-amplified or TNBC EGFR-amplified breast cancer cells [25].
Similar evidence of preclinical efficacy has been reported with capivasertib as monotherapy or in combination with other agents in other solid tumors, including castrate-resistant prostate cancer (CRPC), PI3KCA-mutant gastric cancer, trastuzumab-resistant esophageal squamous cell carcinoma and non-small cell lung cancer [[26], [27], [28], [29], [30]]. In line with ER-positive breast cancer, capivasertib increased the efficacy of androgen receptor (AR)-antagonists bicalutamide and enzalutamide and resulted in greater apoptosis in CRPC cell lines by increasing the AR binding to androgen response element and thus AR transcriptional activity and the expression of AR-dependent genes such as PSA [26,28].
As far as dose is concerned, Yates J.W.T et al. constructed a mathematical model of pharmacokinetics, pharmacodynamics and anti-tumor effect based upon experimental data to explore the relative efficacy of continuous versus intermittent dosing schedules of capivasertib [31]. This model predicted that equivalent efficacy and better tolerability could be achieved at 1.3- and 1.7 times the continuous dose when capivasertib is given intermittently for 4 and 2 days per week, respectively [31].
4. Clinical trials
4.1. Data of capivasertib as monotherapy
The first in-human Phase I study (D3610C00001; NCT01226316) was designed to investigate the safety, tolerability and pharmacokinetics of capivasertib (AZD5363) in patients with advanced solid tumors [11,24]. Patients with metastatic breast cancer, gynecological or other solid tumors bearing either AKT1/PIK3CA or PTEN mutations were included. 90 patients were enrolled to receive capivasertib: 47 in the continuous, 21 in the 4-days-on, 3-days-off (4/7) and 22 in the 2-days-on, 5-days-off weekly schedule (2/7) [11]. The maximum tolerated doses (MTDs) were 320, 480, and 640 mg bid in the continuous, 4/7, and 2/7 schedules respectively [11]. Based on tolerability, pharmacokinetic profile and predictions of efficacy, the dose of 480 mg bid on a 4-days-on, 3-days-off dosing schedule was defined as the recommended phase II dose, while the 640 mg bid 2/7 dosing schedule proved to be also well-tolerated and PK efficient and could be of use in future studies [11].
Expansion cohorts were designed to evaluate capivasertib in PIK3CA-mutated breast and gynecologic cancers [11]. The recommended phase II dose of 480 mg bid 4-days-on, 3-days-off was assessed in 31 patients with PIK3CA-mutated breast cancer [11]. 12 of 26 (46%) demonstrated a reduction in tumor size while 4% (1/28) achieved response [11].
52 patients with AKT-mutant cancers received 480 mg AZD5363 twice daily for a 4-days-on/3-days-off dosing schedule [24]. The AKT E17K mutation results in a glutamic acid to lysine substitution at amino acid 17 and accounts for the 89% of the mutations found in this gene making it the most common mutation [32]. In patients with AKT1 E17K–mutant breast cancer, median progression-free survival (PFS) was 5.5 months (95% CI, 2.9–6.9 months) and objective response rate (ORR) was 20% [24]. Of note, patients with concomitant PI3K pathway mutations either upstream or downstream demonstrated an improved PFS (mPFS, not reached versus 4.3 months; HR, 0.21; P = 0.045) as discussed earlier [24]. Overall, the greatest efficacy was observed in ER-positive breast cancer as well as endometrial cancer. Another study also evaluated capivasertib in AKT1 E17K-mutated metastatic tumors [14]. Patients with breast cancer comprised approximately half of the overall population (18 of 35 [51%]), including 15 patients with HR-positive/HER2-negative disease and 3 with triple-negative breast cancer (TNBC) [14]. The ORR was 28.6% (10 of 35 patients; 95% CI, 15%–46%; P < 0.001) while the median PFS was 5.5 (0–35) months (95% CI, 4.6–11.3) in line with the previous study [14].
These studies evaluated the safety profile of capivasertib administered as a single agent [24]. Adverse events (AEs) of any grade included diarrhea (77.6–80%), nausea (51.7–56%), fatigue (39.7–41%), vomiting (39.7–44%), hyperglycemia (38–41%) and maculopapular rash (25–31%) in both studies. The most common grade ≥3 adverse events were hyperglycemia (20–24.1%), diarrhea (14–17.2%) and maculopapular rash (11–15.5%) [11,24]. Overall, 34% of patients required a dose reduction mainly due to diarrhea, maculopapular rash and hyperglycemia in the AKT-mutant cancer cohort and 23% in the cohort of PIK3CA-mutated gynecologic cancers [11,24]. 12% and 23% of patients permanently discontinued capivasertib respectively as a result of adverse events, the most common being diarrhea (8%) and maculopapular rash (8%) [11,24]. The safety and tolerability profile was common in other studies as well [14,33]. No deaths were attributed to capivasertib [11,14,33]. The risk of QT prolongation after treatment with capivasertib was also explored in another study [34]. There was no clinically significant risk for QT prolongation that is associated with pro-arrhythmic effects induced by capivasertib treatment [34]. In addition, no serious adverse events of sudden death, torsades de pointes, seizures or electrocardiogram (ECG) changes were reported [34]. Overall, capivasertib demonstrated a well-tolerated safety profile with self-limiting maculopapular rash and diarrhea that recovered when treatment stopped and hyperglycemia that was mainly treated with metformin. However, most studies excluded patients diagnosed with diabetes mellitus, so treatment-related hyperglycemia is not well determined in this population [11,24,33].
4.2. Data of capivasertib in combination with olaparib
Capivasertib was evaluated in combination with Olaparib in 38 patients with recurrent TNBC, endometrial and ovarian cancer (NCT02208375) [35]. Of the patients evaluated, only 7 (18%, 5 ovarian, 2 breast) had known germline BRCA mutation. The recommended phase II dose (RP2D) of AZD5363 in combination with olaparib in gynecological cancer was 400 mg twice daily on a 4-days-on, 3-days-off (4/3) dosing schedule [35]. Dose-limiting toxicities observed were diarrhea and vomiting. Of 32 evaluable subjects, 6 (19%) had partial response (PR) while seven (22%) additional patients achieved stable disease.
ComPAKT trial Phase I trial (NCT02338622) also evaluated the combination of olaparib with capivasertib in two dosing schedules: either 4-days-on, 3-days-off (4/3) schedule with capivasertib at 320 mg, 400 mg or 480 mg bid or the 2-days-on, 5-days-off (2/5) schedule of capivasertib at 480 mg, 560 mg and 640 mg BID [36,37]. 64 patients with advanced solid tumors were enrolled in the study. Both the 400 mg bid 4/3 and 640 mg bid 2/5 dosing schedules were chosen for the dose expansion phase. 18 patients with advanced breast cancer were enrolled in the study, 8 (44%) of whom achieved clinical benefit. Six patients had PR and two had SD for at least 4 months. Median duration of response of patients who achieved clinical benefit was 38.2 weeks (14.9–80.9). Five (71.4%) out of 7 patients with BRCA1/2 mutated breast cancer achieved disease control; four had PR and one had SD [36,37]. Median duration of response was 39.1 weeks (range: 14.9–80.9). Treatment-related AEs included nausea (67%, [grade 3/4, 4%]), diarrhea (55%, [grade 3/4, 6%]), vomiting (41%, [grade 3/4, 5%]), fatigue (51%, [grade 3/4, 5%]) and anemia (grade 3: 10%).
4.3. Data of capivasertib in combination with fulvestrant
The dose-expansion cohort of the Phase I trial (NCT01226316) was the first to investigate the combination of capivasertib with fulvestrant in patients with AKT1 E17K–mutant ER-positive breast cancer [38]. 63 AKT1 E17K–mutant ER-positive metastatic breast cancer patients received capivasertib either as monotherapy (n = 20) or in combination with fulvestrant (n = 43) at a dose of 400 mg bid 4-days-on, 3-days-off [38]. Among patients who received the combination, 28 were previously treated with fulvestrant and 15 were fulvestrant naïve. ORR was 20% (95% CI: 8–58) in the monotherapy cohort versus 36% (95% CI: 19–56) in fulvestrant-pretreated patients and 20% (95% CI: 4–48) in patients that were not previously treated with fulvestrant [38]. All patients achieved a partial response with a median PFS of 5.6 (2–14 months) and 5 (3–8) months respectively. Although ORR appears to be higher in fulvestrant-pretreated patients, clinical benefit rate (CBR) at 24 weeks was similar in both subgroups (50%; 95% CI: 31–69 in fulvestrant-pretreated and 47%; 95% CI: 21–73 in fulvestrant-naïve patients) [38]. Response was durable lasting for over 6 months in 26% (11/43) of patients [38].
Another expansion cohort of this Phase I trial evaluated the combination of capivasertib with fulvestrant in patients with PTEN-mutant ER-positive metastatic breast cancer (NCT01226316) [39]. 31 PTEN-mutant ER-positive metastatic breast cancer patients (12 fulvestrant naïve and 19 fulvestrant pretreated) received capivasertib in combination with fulvestrant [39]. Median PFS was 2.7 months (95% CI 2–4) in patients with PTEN-mutant ER-positive metastatic breast cancer. ORR was 16% (95% CI: 6–34) in the overall population. 5 patients (16%) achieved stable disease for over 24 weeks. Both PFS (2.6 months versus 4.1 months) and ORR (8%; 1/12 versus 21%; 4/19) were improved in fulvestrant-pretreated patients compared to the fulvestrant-naïve population [39]. The association of co-existing PIK3CA mutations with PFS failed to reach statistical significance (P = 0.15) probably because of the small sample size. Safety profile of capivasertib and fulvestrant combination was comparable to monotherapy with capivasertib. The most common AEs reported in the combination cohort were diarrhea (59%), nausea (30%), maculopapular rash (21%), fatigue (18%) and hyperglycemia (18%). Treatment-related grade ≥3 AEs were reported in 21% of patients receiving capivasertib with fulvestrant versus 50% of patients receiving capivasertib as a single agent, although this difference is likely the result of the lower dose of capivasertib administered in the combination cohort (400 mg bid vs 480 mg bid 4 days on, 3 days off) [39].
FAKTION is a Phase I/II trial of capivasertib in combination with fulvestrant in aromatase-inhibitor-pretreated ER-positive, HER2-negative, metastatic or locally advanced breast cancer (NCT01992952) [40]. Median PFS was 10.3 months (95% CI 5·0–13·2) in the capivasertib group versus 4.8 months (3.1–7.7) in the placebo group (HR: 0.58; 95% CI 0.39–0.84). This significant prolongation in PFS seen with fulvestrant and capivasertib in the overall population was preserved in the PI3K/PTEN pathway non-altered group (HR: 0.56, 95% CI; 0.33–0.96, p = 0.035), but not in patients carrying PI3K/PTEN pathway alterations (HR: 0.59, 95%CI; 0.34–1.03, p = 0.064) [40]. Despite OS not being mature at the time of data cutoff, a survival difference in favor of the capivasertib group started to emerge after 12 months (26 months (95% CI 18·4–32·3) versus 20 months (15·1–21·2) (HR: 0.59, 95% CI; 0.34–1.05, p = 0.071).
4.4. Data of capivasertib in combination with chemotherapy
PAKT double-blind, randomized Phase II study evaluated the combination of Capivasertib and Paclitaxel as first line treatment of metastatic TNBC patients (NCT02423603) [41]. 140 patients with TNBC were randomly assigned (1:1) to receive paclitaxel with either capivasertib 400 mg twice daily for four consecutive days on a 7-day cycle or placebo [41]. Median PFS was 5.9 months for capivasertib plus paclitaxel versus 4.2 months for placebo plus paclitaxel (HR: 0.74; 95% CI: 0.50–1.08; P = 0.06). In patients with PIK3CA/AKT1/PTEN-altered tumors (n = 28), median PFS was 9.3 months with capivasertib plus paclitaxel and 3.7 months with placebo plus paclitaxel (HR, 0.30; 95% CI, 0.11 to 0.79; P = 0.01) [41]. Although the benefit of AKT inhibition was initially more pronounced in patients with PIK3CA/AKT1/PTEN-altered tumors, updated results after a median follow-up of 40 months revealed no significant differences between patients with or without alterations of PIK3CA/AKT1/PTEN [42]. Indeed, median OS was longer in the capivasertib arm in the overall population (19.1 vs 13.5 months; HR: 0.70, 95% CI 0.47–1.05, p = 0.085). The addition of capivasertib to paclitaxel favored both PIK3CA/AKT1/PTEN-altered group (HR: 0.58, p = 0.290) and PIK3CA/AKT1/PTEN non-altered subgroup (HR: 0.74, p = 0.207). As for the safety profile, 97% of patients receiving the combination of capivasertib and paclitaxel reported AEs of any grade, mainly including diarrhea (72.1%), fatigue (44.1%), nausea (35.3%), rash (41.2%), neuropathy (25%) and stomatitis (26.5%). Grade ≥3 AEs occurred in 54% (37/68) of patients and were diarrhea (13.2%), fatigue (4.4%), rash (4.4%) and infection (4.4%) [42].
BEECH Phase I/II trial (NCT01625286) investigated the combination of AZD5363 with paclitaxel as first-line treatment for advanced or metastatic ER-positive/HER2-negative breast cancer, stratified by PIK3CA mutation status [12,43]. Capivasertib 400 mg twice daily on a 4 days on/3 days off dosing schedule was selected as the recommended Phase II dose. Median PFS in the overall population was 10.9 months with capivasertib versus 8.4 months with placebo [HR: 0.80; P = 0.308]. In the PIK3CA-mutated sub-population, median PFS was 10.9 months with capivasertib versus 10.8 months with placebo (HR 1.11; P = 0.760) [12]. No significant prolongation of PFS was reported in either the overall population or the PIK3CA-mutated subgroup of ER-positive/HER2-negative metastatic breast cancer patients. Safety profile of the combination was similar to that reported in PAKT trial.
5. Conclusion
The PI3K/AKT pathway is one of the most frequently altered signaling pathways in breast cancer since 50% of HR-positive breast cancer and about 25% of TNBC present PI3K/AKT pathway hyperactivation, mainly emerging from PIK3CA mutations in HR-positive tumors and by PTEN loss in TNBC [9]. Identifying AKT inhibitors that can block PI3K/AKT signaling could attenuate cancer growth and increase susceptibility to endocrine treatment or chemotherapy. Since the introduction of GSK690693 ATP-competitive panAKT kinase inhibitor in clinical trials that were terminated prematurely due to severe hyperglycemia, several AKT inhibitors have been developed with a more favorable pharmacokinetic and toxicity profile. Capivasertib emerged as a novel oral highly potent pan-Akt kinase inhibitor that demonstrated promising results in preclinical studies suppressing tumor proliferation and reducing the phosphorylation of biomarkers including PRAS40, GSK3b and S6. Phase I/II trials evaluating capivasertib as monotherapy (NCT01226316) or in combination with other antineoplastic agents like paclitaxel (PAKT, BEECH), fulvestrant in HR-positive, HER2-negative breast cancer (NCT01226316, FAKTION) or Olaparib (NCT02208375, ComPAKT) demonstrated greater efficacy with the combination treatment along with an acceptable toxicity profile. In monotherapy trials, discontinuation rate ranged from 12 to 23% mainly due to diarrhea (8%) and maculopapular rash (8%). The main grade ≥3 adverse events encountered included hyperglycemia, diarrhea and maculopapular rash. The safety profile was consistent in combination studies as well. More results of the ongoing Phase III trials of capivasertib in combination with fulvestrant (CAPItello-291), Palbociclib CDK4/6 inhibitor (CAPItello-292) and paclitaxel (CAPItello- 290) are anticipated.
Declaration of competing interest
ML has received honoraria from Roche, Astra Zeneca, Astellas, MSD, Janssen, Bristol-Myers-Squibb and IPSEN. MAD has received honoraria from participation in advisory boards from Amgen, Bristol-Myers-Squibb, Celgene, Janssen, Takeda. FZ has received honoraria for lectures and has served in an advisory role for Astra Zeneca, Daiichi, Eli-Lilly, Merck, Novartis, Pfizer, and Roche. The remaining authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
Not applicable.
Contributor Information
Angeliki Andrikopoulou, Email: aggelikiandrikop@gmail.com.
Spyridoula Chatzinikolaou, Email: s.d.chatzinikolaou@gmail.com.
Evangelia Panourgias, Email: epanourgias@yahoo.com.
Maria Kaparelou, Email: mkaparelou@yahoo.com.
Michalis Liontos, Email: mliontos@gmail.com.
Meletios-Athanasios Dimopoulos, Email: mdimop@med.uoa.gr.
Flora Zagouri, Email: florazagouri@yahoo.co.uk.
References
- 1.Millis S.Z., Ikeda S., Reddy S., Gatalica Z., Kurzrock R. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19 784 diverse solid tumors. JAMA Oncol. 2016;2:1565–1573. doi: 10.1001/JAMAONCOL.2016.0891. [DOI] [PubMed] [Google Scholar]
- 2.Nitulescu G.M., Van De Venter M., Nitulescu G., Ungurianu A., Juzenas P., Peng Q., et al. The Akt pathway in oncology therapy and beyond (Review) Int J Oncol. 2018;53:2319–2331. doi: 10.3892/IJO.2018.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA approves alpelisib for metastatic breast cancer | FDA n.d. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-alpelisib-metastatic-breast-cancer (accessed January 30, 2022).
- 4.Wang Q., Chen X., Hay N. Akt as a target for cancer therapy: more is not always better (lessons from studies in mice) Br J Cancer. 2017;117:159. doi: 10.1038/BJC.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao Y., Hung M.C. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19. [PMC free article] [PubMed] [Google Scholar]
- 6.Altomare D.A., Testa J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/SJ.ONC.1209085. [DOI] [PubMed] [Google Scholar]
- 7.Dong C., Wu J., Chen Y., Nie J., Chen C. Activation of PI3K/AKT/mTOR pathway causes drug resistance in breast cancer. Front Pharmacol. 2021;12 doi: 10.3389/FPHAR.2021.628690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa C., Ye W., Ly A., Hosono Y., Murchi E., Walmsley C.S., et al. PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov. 2020;10:72–85. doi: 10.1158/2159-8290.CD-18-0830. [DOI] [PubMed] [Google Scholar]
- 9.Koboldt D.C., Fulton R.S., McLellan M.D., Schmidt H., Kalicki-Veizer J., McMichael J.F., et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/NATURE11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stemke-Hale K., Gonzalez-Angulo A.M., Lluch A., Neve R.M., Kuo W.L., Davies M., et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerji U., Dean E.J., Alejandro Perez-Fidalgo J., Batist G., Bedard P.L., You B., et al. A phase I open-label study to identify a dosing regimen of the pan-AKT inhibitor AZD5363 for evaluation in solid tumors and in PIK3CA-mutated breast and gynecologic cancers. Clin Cancer Res. 2018;24 doi: 10.1158/1078-0432.CCR-17-2260. 2050–9. [DOI] [PubMed] [Google Scholar]
- 12.Turner N.C., Alarcón E., Armstrong A.C., Philco M., López Chuken Y.A., Sablin M.P., et al. BEECH: a dose-finding run-in followed by a randomised phase II study assessing the efficacy of AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in patients with estrogen receptor-positive advanced or metastatic breast cancer, and in a PIK3CA mutant sub-population. Ann Oncol. 2019;30:774. doi: 10.1093/ANNONC/MDZ086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi A.R., Kim J.H., Woo Y.H., Cheon J.H., Kim H.S., Yoon S. Co-treatment of LY294002 or MK-2206 with AZD5363 attenuates azd5363-induced increase in the level of phosphorylated AKT. Anticancer Res. 2016;36:5849–5858. doi: 10.21873/ANTICANRES.11170. [DOI] [PubMed] [Google Scholar]
- 14.Kalinsky K., Hong F., McCourt C.K., Sachdev J.C., Mitchell E.P., Zwiebel J.A., et al. Effect of capivasertib in patients with an AKT1 E17K-mutated tumor: NCI-match subprotocol EAY131-Y nonrandomized trial. JAMA Oncol. 2021;7:271–278. doi: 10.1001/JAMAONCOL.2020.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson J.F.R., Coleman R.E., Cheung K.L., Evans A., Holcombe C., Skene A., et al. Proliferation and AKT activity biomarker analyses after capivasertib (AZD5363) treatment of patients with ER + invasive breast cancer (STAKT) Clin Cancer Res. 2020;26:1574–1585. doi: 10.1158/1078-0432.CCR-19-3053. [DOI] [PubMed] [Google Scholar]
- 16.Dean E., Banerji U., Schellens J.H.M., Krebs M.G., Jimenez B., van Brummelen E., et al. A Phase 1, open-label, multicentre study to compare the capsule and tablet formulations of AZD5363 and explore the effect of food on the pharmacokinetic exposure, safety and tolerability of AZD5363 in patients with advanced solid malignancies: OAK. Cancer Chemother Pharmacol. 2018;81:873–883. doi: 10.1007/S00280-018-3558-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies B.R., Greenwood H., Dudley P., Crafter C., Yu D.H., Zhang J., et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Therapeut. 2012;11:873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 18.Teo K., Gómez-Cuadrado L., Tenhagen M., Byron A., Rätze M., van Amersfoort M., et al. E-cadherin loss induces targetable autocrine activation of growth factor signalling in lobular breast cancer. Sci Rep. 2018;8 doi: 10.1038/S41598-018-33525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribas R., Pancholi S., Guest S.K., Marangoni E., Gao Q., Thuleau A., et al. AKT antagonist AZD5363 influences estrogen receptor function in endocrine-resistant breast cancer and synergizes with fulvestrant (ICI182780) in vivo. Mol Cancer Therapeut. 2015;14:2035–2048. doi: 10.1158/1535-7163.MCT-15-0143. [DOI] [PubMed] [Google Scholar]
- 20.Fox E.M., Kuba M.G., Miller T.W., Davies B.R., Arteaga C.L. Autocrine IGF-I/insulin receptor axis compensates for inhibition of AKT in ER-positive breast cancer cells with resistance to estrogen deprivation. Breast Cancer Res. 2013;15 doi: 10.1186/bcr3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Duan Z, Guo W, Zeng L, Wu Y, Chen Y, et al. Targeting the BRD4/FOXO3a/CDK6 axis sensitizes AKT inhibition in luminal breast cancer n.d. 10.1038/s41467-018-07258-y. [DOI] [PMC free article] [PubMed]
- 22.Sommer E.M., Dry H., Cross D., Guichard S., Davies B.R., Alessi D.R. Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochem J. 2013;452:499–508. doi: 10.1042/BJ20130342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gris-Oliver A., Palafox M., Monserrat L., Braso-Maristany F., Odena A., Sanchez-Guixe M., et al. Genetic alterations in the PI3K/AKT pathway and baseline AKT activity define AKT inhibitor sensitivity in breast cancer patient-derived xenografts. Clin Cancer Res. 2020;26:3720–3731. doi: 10.1158/1078-0432.CCR-19-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyman D.M., Smyth L.M., Donoghue M.T.A., Chang M.T., Reichel J.B., Bouvier N., et al. AKT inhibition in solid tumors with AKT1 mutations. J Clin Oncol. 2017;35:2251. doi: 10.1200/JCO.2017.73.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crafter C., Vincent J.P., Tang E., Dudley P., James N.H., Klinowska T., et al. Combining AZD8931, a novel EGFR/HER2/HER3 signalling inhibitor, with AZD5363 limits AKT inhibitor induced feedback and enhances antitumour efficacy in HER2-amplified breast cancer models. Int J Oncol. 2015;47:446–454. doi: 10.3892/IJO.2015.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toren P., Kim S., Cordonnier T., Crafter C., Davies B.R., Fazli L., et al. Combination AZD5363 with enzalutamide significantly delays enzalutamide-resistant prostate cancer in preclinical models. Eur Urol. 2015;67:986–990. doi: 10.1016/J.EURURO.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Li J., Davies B.R., Han S., Zhou M., Bai Y., Zhang J., et al. The AKT inhibitor AZD5363 is selectively active in PI3KCA mutant gastric cancer, and sensitizes a patient-derived gastric cancer xenograft model with PTEN loss to Taxotere. J Transl Med. 2013;11 doi: 10.1186/1479-5876-11-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas C., Lamoureux F., Crafter C., Davies B.R., Beraldi E., Fazli L., et al. Synergistic targeting of PI3K/AKT pathway and androgen receptor axis significantly delays castration-resistant prostate cancer progression in vivo. Mol Cancer Therapeut. 2013;12:2342–2355. doi: 10.1158/1535-7163.MCT-13-0032. [DOI] [PubMed] [Google Scholar]
- 29.Wu X., Zhang J., Zhen R., Lv J., Zheng L., Su X., et al. Trastuzumab anti-tumor efficacy in patient-derived esophageal squamous cell carcinoma xenograft (PDECX) mouse models. J Transl Med. 2012;10:180. doi: 10.1186/1479-5876-10-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puglisi M., Thavasu P., Stewart A., de Bono J.S., O'Brien M.E.R., Popat S., et al. AKT inhibition synergistically enhances growth-inhibitory effects of gefitinib and increases apoptosis in non-small cell lung cancer cell lines. Lung Cancer. 2014;85:141–146. doi: 10.1016/J.LUNGCAN.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Yates J.W.T., Dudley P., Cheng J., D'Cruz C., Davies B.R. Validation of a predictive modeling approach to demonstrate the relative efficacy of three different schedules of the AKT inhibitor AZD5363. Cancer Chemother Pharmacol. 2015;76:343–356. doi: 10.1007/S00280-015-2795-7. [DOI] [PubMed] [Google Scholar]
- 32.COSMIC | Catalogue of Somatic Mutations in Cancer n.d. https://cancer.sanger.ac.uk/cosmic (accessed January 30, 2022).
- 33.Tamura K., Hashimoto J., Tanabe Y., Kodaira M., Yonemori K., Seto T., et al. Safety and tolerability of AZD5363 in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;77:787–795. doi: 10.1007/S00280-016-2987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voronova V., Cullberg M., Delff P., Parkinson J., Dota C., Schiavon G., et al. Concentration-QT modelling shows no evidence of clinically significant QT interval prolongation with capivasertib at expected therapeutic concentrations. Br J Clin Pharmacol. 2021;88(2):858–864. doi: 10.1111/BCP.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westin S.N., Labrie M., Litton J.K., Blucher A., Fang Y., Vellano C.P., et al. Phase ib dose expansion and translational analyses of olaparib in combination with capivasertib in recurrent endometrial, triple-negative breast, and ovarian cancer. Clin Cancer Res. 2021;27:6354–6365. doi: 10.1158/1078-0432.CCR-21-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalarea V., Lorente D., Lopez J., Carreira S., Hassam H., Parmar M., et al. Abstract CT323: accelerated phase I trial of two schedules of the combination of the PARP inhibitor olaparib and AKT inhibitor AZD5363 using a novel intrapatient dose escalation design in advanced cancer patients. Cancer Res. 2015;75 doi: 10.1158/1538-7445.AM2015-CT323. CT323–CT323. [DOI] [Google Scholar]
- 37.Yap T.A., Kristeleit R., Michalarea V., Pettitt S.J., Lim J.S.J., Carreira S., et al. Phase I trial of the poly(ADP-ribose) polymerase (PARP) inhibitorolaparib and AKT inhibitor capivasertib in patients withBRCA1/2 and non-BRCA1/2 mutantcancers. Cancer Discov. 2020;10:1528. doi: 10.1158/2159-8290.CD-20-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smyth L.M., Tamura K., Oliveira M., Ciruelos E.M., Mayer I.A., Sablin M.P., et al. Capivasertib, an AKT kinase inhibitor, as monotherapy or in combination with fulvestrant in patients with AKT1E17K-mutant, ER-positive metastatic breast cancer. Clin Cancer Res. 2020;26:3947–3957. doi: 10.1158/1078-0432.CCR-19-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smyth L.M., Batist G., Meric-Bernstam F., Kabos P., Spanggaard I., Lluch A., et al. Selective AKT kinase inhibitor capivasertib in combination with fulvestrant in PTEN-mutant ER-positive metastatic breast cancer. NPJ Breast Cancer. 2021;7 doi: 10.1038/S41523-021-00251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones R.H., Casbard A., Carucci M., Cox C., Butler R., Alchami F., et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020;21:345–357. doi: 10.1016/S1470-2045(19)30817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid P., Abraham J., Chan S., Wheatley D., Brunt A.M., Nemsadze G., et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol. 2020;38:423–433. doi: 10.1200/JCO.19.00368. [DOI] [PubMed] [Google Scholar]
- 42.Schmid P., Abraham J., Chan S., Brunt A.M., Nemsadze G., Baird R.D., et al. Abstract PD1-11: mature survival update of the double-blind placebo-controlled randomised phase II PAKT trial of first-line capivasertib plus paclitaxel for metastatic triple-negative breast cancer. Cancer Res. 2021;81:PD1–11. doi: 10.1158/1538-7445.SABCS20-PD1-11. [DOI] [Google Scholar]
- 43.Investigating Safety, Tolerability and Efficacy of AZD5363 When Combined With Paclitaxel in Breast Cancer Patients - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT01625286 (accessed January 30, 2022).
- 44.Mosele F., Stefanovska B., Lusque A., Tran Dien A., Garberis I., Droin N., et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann Oncol. 2020;31:377–386. doi: 10.1016/J.ANNONC.2019.11.006/ATTACHMENT/7ABE1A73-A7FB-4F17-B2FB-2B05861C18ED/MMC1.DOCX. [DOI] [PubMed] [Google Scholar]
- 45.Turner N.C., Kingston B., Kilburn L.S., Kernaghan S., Wardley A.M., Macpherson I.R., et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020;21:1296. doi: 10.1016/S1470-2045(20)30444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.A phase I trial of durvalumab (Durv) in combination with olaparib (Ola) and capivasertib (Cap) in patients (pts) with advanced or metastatic cancer… | OncologyPRO n.d. https://oncologypro.esmo.org/meeting-resources/esmo-congress/a-phase-i-trial-of-durvalumab-durv-in-combination-with-olaparib-ola-and-capivasertib-cap-in-patients-pts-with-advanced-or-metastatic-cancer (accessed March 22, 2022).