Key Points
Question
Is high-dose vitamin D supplementation safe for children aged 0 to 6 years?
Findings
In this systematic review and meta-analysis of 32 randomized clinical trials including 8400 unique children, high-dose vitamin D administered as a daily or bolus supplement was not associated with an increased risk of serious adverse events. Clinical adverse events associated with the supplementation were rare.
Meaning
This systematic review and meta-analysis suggests that vitamin D supplements in daily doses to 10 000 IU/d or bolus doses to 600 000 IU are well tolerated in children aged 0 to 6 years.
This systematic review and meta-analysis investigates the safety of high-dose vitamin D supplementation in children aged 0 to 6 years by examining potential clinical adverse events and biochemical changes reported in randomized clinical trials.
Abstract
Importance
Several health benefits of vitamin D have been suggested; however, the safety of high-dose supplementation in early childhood is not well described.
Objective
To systematically assess the risk of adverse events after high-dose supplementation with vitamin D reported in published randomized clinical trials.
Data Sources
PubMed and ClinicalTrials.gov were searched through August 24, 2021.
Study Selection
Randomized clinical trials of high-dose vitamin D supplementation in children aged 0 to 6 years, defined as greater than 1000 IU/d for infants (aged 0-1 year) and greater than 2000 IU/d for children aged 1 to 6 years.
Data Extraction and Synthesis
Following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline, 2 reviewers independently extracted the data from the eligible studies. Summary risk ratio (RR), 95% CI, and P values were derived from random-effects meta-analysis.
Main Outcomes and Measures
Adverse events, serious adverse events (SAEs), and/or levels of 25-hydroxyvitamin D, calcium, alkaline phosphatase, phosphate, parathyroid hormone, and/or the ratio of urine calcium to creatinine levels.
Results
A total of 32 randomized clinical trials with 8400 unique participants were included. Different clinical outcomes of children receiving high-dose vitamin D supplements ranging from 1200 to 10 000 IU/d and bolus doses from 30 000 IU/week to a single dose of 600 000 IU were evaluated. Eight studies with 4612 participants were eligible for meta-analysis using a control group receiving either low-dose vitamin D supplementation (≤400 IU/d) or placebo when investigating the risk of SAEs such as hospitalization or death. No overall increased risk of SAEs in the high-dose vitamin D vs control groups was found (RR, 1.01 [95% CI, 0.73-1.39]; P = .89, I2 = 0%). In addition, risk of hypercalcemia (n = 726) was not increased (RR, 1.18 [95% CI, 0.72-1.93]; P = .51). Clinical adverse events potentially related to the vitamin D supplementation reported in the studies were rare.
Conclusions and Relevance
This meta-analysis and systematic review found that high-dose vitamin D supplementation was not associated with an increased risk of SAEs in children aged 0 to 6 years, and that clinical adverse events potentially related to the supplementation were rare. These findings suggest that vitamin D supplementation in the dose ranges of 1200 to 10 000 IU/d and bolus doses to 600 000 IU to young children may be well tolerated.
Introduction
The influence of vitamin D on skeletal health is well known, but other biological nonskeletal effects have been discovered as the vitamin D receptor has been identified in cell types not involved in bone metabolism.1,2,3 Vitamin D deficiency is a common global problem,3 particularly in young children,4 and the insight about new biological nonskeletal actions of vitamin D demands revision of vitamin D supplementation policies,1 which have led to updated recommendations regarding clinical practice and recommended intake levels. Vitamin D is primarily obtained by cutaneous synthesis from sunlight exposure or as a nutrient from dietary sources such as fatty fish.5 However, as treatment and in prevention of deficiency, vitamin D supplements in the form of either cholecalciferol (vitamin D3) or ergocalciferol (vitamin D2) have been recommended.6 Supplementation of very high doses of vitamin D can cause hypercalcemia and subsequent kidney failure; therefore, an upper threshold concentration of serum 25-hydroxyvitamin D (25[OH]D) corresponding to 100 ng/mL (to convert to nanomoles per liter, multiply by 2.496) has been suggested by the Endocrine Society, at which the risk of developing hypercalcemia is considered minimal.6 Regarding intake, a tolerable upper intake level has been defined as 25 μg/d (1000 IU/d) for infants aged 0 to 12 months and 50 μg/d (2000 IU/d) for children aged 1 to 10 years by the European Food Safety Authority.3 In general, evidence from systematic reviews and meta-analyses evaluating the safety of high-dose vitamin D supplementation in early childhood is lacking. Therefore, the purpose of this study was to investigate the safety of high-dose vitamin D in children aged 0 to 6 years by examining potential clinical adverse events and biochemical changes reported in randomized clinical trials (RCTs).
Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. This study was registered with PROSPERO (CRD42020204074).
Search Strategy
A systematic literature search was conducted using the PubMed database to identify available studies until August 24, 2021. The search was restricted to English-language studies. Search terms were divided into 3 aspects using the following key terms: randomized controlled trials, child/infant, and vitamin D (eFigure 1 in the Supplement). The search protocol is provided in detail in eTable 1 in the Supplement. Both MeSH (Medical Subject Headings) and text word terms were used in the combined searches. Boolean operators were used to specify the search. All hits were screened based on title and abstract, and inclusion of the final studies was based on full-text reading and consensus with the coreviewers.
Study Selection and Quality Assessment
The study selection was based on the following inclusion criteria: (1) original RCT, (2) intervention with vitamin D2 or D3 supplementation greater than 1000 IU/d or bolus therapy, (3) children aged 0 to 6 years, and (4) clinical adverse events and/or biochemical levels of 25(OH)D, calcium, alkaline phosphatase (ALP), phosphate, parathyroid hormone (PTH), and/or ratio of urine calcium to creatinine levels (Ca:Cr ratio) must have been shown in figures or described in tables or the text. Exclusion criteria consisted of (1) secondary follow-up articles (ie, not primary RCT article), (2) topical administration of vitamin D, and (3) coadministration of calcium supplementation. All studies were quality assessed using the Cochrane Risk of Bias Tool.7
Data Extraction and Synthesis
Two authors (N.B. and S.Y.) extracted the following information from the selected studies: first author, publication year, outcomes, total sample size, description of interventions, race and ethnicity, and details of the study participants. Information on safety outcomes extracted included the number of children with 25(OH)D levels greater than 100 ng/mL; hypercalcemia; abnormal levels of ALP, phosphate, PTH, and/or Ca:Cr ratio; and clinical and serious adverse events (SAEs). The data were extracted directly from the selected articles and supported by information from the registration site (eg, ClinicalTrials.gov). If several follow-up periods with biochemical measures were presented in the articles, the follow-up period with the highest biochemical values regarding 25(OH)D, calcium, phosphate, and Ca:Cr ratio and the lowest ALP and PTH levels were extracted and used when assessing changes from baseline measurements.
The reference ranges and comments from the authors of the included studies were used to categorize biochemical values as abnormal or within reference ranges. In this study, high-dose vitamin D supplementation was defined as greater than 1000 IU/d for children aged 0 to 1 year and greater than 2000 IU/d for children aged 1 to 6 years based on the European Food Safety Authority’s defined upper levels of tolerable intake.3
Statistical Analysis
We performed a meta-analysis of the extracted data from eligible articles with an intervention and a control group and calculated the summary risk ratio (RR), 95% CI, and P value from a random-effects meta-analysis supported by forest and funnel plots with a linear regression test of asymmetry. The presence of heterogeneity was measured with Cochran Q test. The variation in effect size due to between-study heterogeneity was considered using the I2 value. We performed an overall analysis of the risk of SAEs and subgroup analyses by intervention methods. All analyses were performed using the meta package in R, version 4.0.3 (R Program for Statistical Computing). The results were considered statistically significant with 2-sided P < .05.
Results
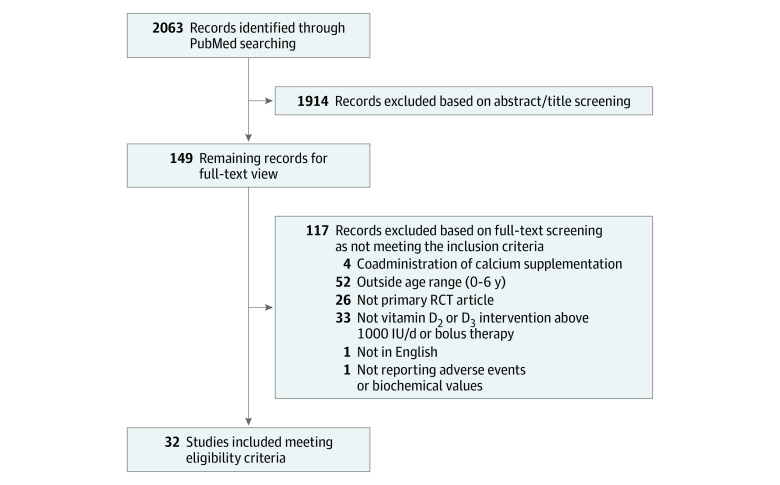
The main search resulted in 2063 studies potentially relevant for inclusion (Figure 1); of these, 32 studies8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39 (8400 unique participants) met our criteria and were included in this systematic review (Table 1). A subgroup of 21 studies8,9,10,18,19,20,21,22,23,24,25,28,29,30,31,32,33,34,35,36,37 (7358 unique participants) were eligible for meta-analysis because they included a control group receiving either low-dose vitamin D supplementation (≤400 IU/d) or placebo.
Figure 1. Study Flowchart.
RCT indicates randomized clinical trial.
Table 1. Study Characteristics.
| Source (country) | Study period | Outcomes | No. of participants | Intervention and follow-up | Race or ethnicity | Participant age | Diagnosis |
|---|---|---|---|---|---|---|---|
| Singh et al,8 2019 (India) | January 2013 to September 2014 | Recurrent pneumonia | 100 | Vitamin D bolus of 300 000 IU every 3 mo vs placebo for 1 y | Asian | 0-5 y | Pneumonia |
| Saleem et al,9 2018 (Pakistan) | June 2015 to November 2016 | Anthropometric measurements including weight gain and 25(OH)D, calcium, albumin, and prealbumin levels | 194 | Vitamin D3 bolus of 200 000 IU 2 and 4 wk vs placebo; follow-up at 8 wk | Asian | 6-59 mo | Severe acute malnutrition |
| Ducharme et al,10 2019 (Canada) | September 2014 to July 2016 | 25(OH)D levels, hypercalciuria, Ca:Cr ratio, asthma treatment, and hospitalizations | 47 | Vitamin D3 bolus of 100 000 IU vitamin D3 at baseline and after 3.5 mo vs placebo; follow-up until 7 mo | Multiracial with mostly White | 1-5 y | Asthma |
| Moslemi et al,11 2018 (Iran) | April to August 2016 | 25(OH)D levels and adverse effects including measurement of biochemical values | 108 | Vitamin D3, 50 000 IU twice per week for 3 wk vs single IM dose of 300 000 IU; follow-up at 3 wk | Asian | 30-72 mo | Vitamin D deficiency |
| Harnot et al,12 2017 (India) | July 2012 to June 2013 | Hypercalciuria, hypercalcemia, and 25(OH)D sufficiency | 60 | Single oral dose of vitamin D, 600 000 vs 300 000 IU; follow-up at 7-10 d | Asian | 3 mo to 3 y | Vitamin D deficiency |
| Mittal et al,13 2014 (India) | November 2010 to April 2012 | 25(OH)D levels, radiological findings, PTH and ALP levels, hypercalcemia, and Ca:Cr ratio | 76 | Single oral dose of Vitamin D3, 300 000 to 600 000 IU; follow-up at 12 wk | Asian | 6 mo to 5 y | Rickets |
| Mondal et al,14 2014 (India) | November 2009 to March 2011 | Hypercalcemia, hypercalciuria, and 25(OH)D, ALP, calcium, and phosphate levels | 71 | Single IM dose of vitamin D, 600 000 IU vs oral dose of 60 000 IU weekly for 10 wk; follow-up after 12 wk | Asian | 0.5 mo to 5 y | Rickets |
| Lubani et al,15 1989 (Kuwait) | June 1981 to August 1986 | Vitamin D deficiency | 250 | Dose of vitamin D, 600 000 IU plus 400 IU/d for 6 mo to 1 y vs 2000 IU/d for 4 wk plus 400 IU/d until age 2 y or 1 y after treatment start for older children; follow-up until 24-30 mo of age | Mixed | 1 mo to 2 y | Rickets |
| Zeghoud et al,16 1994 (Algeria) | 1991 to 1992 | 25(OH)D, ALP, calcium, and phosphorus levels | 30 | Single oral dose of vitamin D, 200 000 IU at birth vs 100 000 IU at birth and 3 and 6 mo; follow-up until 6-9 mo of age |
North African | Neonates | Healthy |
| Mittal et al,17 2018 (India) | NR | Rickets, hypercalcemia, Ca:Cr ratio, and 25(OH)D, PTH, and ALP levels | 110 | Single oral dose of vitamin D, 90 000 vs 300 000 IU; follow-up at 1 wk and 4 and 12 mo | Asian | 6 mo to 5 y | Rickets |
| Gupta et al,18 2016 (India) | August 2012 to January 2015 | Pneumonia and 25(OH)D and PTH levels | 324 | Single oral dose of vitamin D, 100 000 IU vs placebo; follow-up of blood level measurement at 2 wk | Asian | 6 mo to 5 y | Pneumonia |
| Somnath et al,19 2017 (India) | March 2013 to April 2014 | Length of hospital stay in children with acute lower respiratory tract infection and 25(OH)D levels | 156 | Single oral dose of vitamin D, 100 000 IU vs placebo; follow-up at 72 h for 25(OH)D level | Asian | 2 mo to 5 y | Acute lower respiratory infection |
| Huynh et al,20 2017 (Australia) | August 2013 to May 2014 | Vitamin D sufficiency, hypercalcemia, craniotabes, and bone development | 70 | Vitamin D3 of 400 IU/d for 4 mo vs single oral dose of 50 000 IU | Mixed | Newborn infants | Newborn infants of mothers with vitamin D deficiency |
| Moodley et al,21 2015 (Mexico) | July 2011 to July 2012 | Changes in 25(OH)D levels | 51 | Single oral dose of vitamin D, 50 000 IU vs placebo; follow-up until 6 mo | Hispanic | Infants | Healthy |
| Jensen et al,22 2016 (Canada) | November 2013 to August 2014 | Changes in 25(OH)D levels and vitamin D sufficiency | 22 | Oral single dose of vitamin D, 100 000 IU vs placebo; follow-up until 6 mo | Mixed with mostly White | 1-5 y | Asthma |
| Manaseki-Holland et al,23 2012 (Afghanistan) | November to May 2009 | Incidence and severity of pneumonia | 3046 | Vitamin D, 100 000 IU every 3 mo for 18 mo vs placebo | Asian | 1-11 mo | High-risk pneumonia |
| Manaseki-Holland et al,24 2010 (Afghanistan) | December 2006 to May 2007 | Pneumonia length and risk of repeated episodes | 453 | Single oral dose of vitamin D, 100 000 IU vs placebo; follow-up until 90 d | Asian | 1-36 mo | Pneumonia |
| Shakiba et al,25 2010 (Iran) | January to September 2007 | Changes in 25(OH)D and calcium levels | 120 | Vitamin D, 200 vs 400 IU/d vs 50 000 IU every 2 mo for 6 mo | Asian | Infants | Healthy |
| Mawer et al,26 1986 (England) | NR | 25(OH)D levels | 38 | Vitamin D2, 1000 IU/d vs 3000 IU/d for 6 wk | European and Asian | Infants (gestational age 25-32 wk) | Premature |
| Moya et al,27 1977 | NR | 25(OH)D, calcium, phosphate, ALP, and urine pH levels | 35 | 25(OH)D, 6000 IU/d, vs vitamin D3, 6000 IU/d, vs 25(OH)D, 3000 IU/d; all participants received interventions in 20 d | European | 3-18 mo | Rickets |
| Rosendahl et al,28 2018 (Finland) | January 2013 to November 2017 | Bone strength and risk of infections | 975 | Vitamin D3, 1200 vs 400 IU/d, from 2 wk to 2 y of age | Scandinavian | Infants | Healthy |
| Choudhary et al,29 2012 (India) | NR | Length of severe pneumonia | 200 | Vitamin D, 1000 IU/d at <1 y and 2000 IU/d at >1 y vs placebo for 5 d | Asian | 2 mo to 5 y | Severe pneumonia |
| Gallo et al,30 2013 (Canada) | March 2007 to December 2011 | Vitamin D sufficiency (>300 ng/mL) | 132 | Vitamin D, 400 vs 800 vs 1200 vs 1600 IU/d; all participants received interventions for 11 mo with follow-up until 12 mo | Mixed | 1 mo | Healthy |
| Holmlund-Suila et al,31 2012 (Finland) | September 2010 to February 2011 | Vitamin D sufficiency (>320 ng/mL), calcium homeostasis, and skeletal parameters via peripheral quantitative computed tomography | 113 | Vitamin D, 400 vs 1200 vs 1600 IU/d from 2 wk to 3 mo of age | Scandinavian | 2 wk | Healthy |
| Evans et al,32 1989 (Canada) | NR | Risk of bone disease | 81 | Vitamin D2, 2000 vs 400 IU/d for 6 wk | NR | Infants | Very low birth weight |
| Aglipay et al,33 2017 (Canada) | September 2011 to June 2015 | Viral upper respiratory tract infections | 703 | Vitamin D, 2000 vs 400 IU/d for a minimum of 4 mo | Mixed | 1-5 y | Healthy |
| Zhou et al,34 2018 (China) | September 2016 to July 2018 | Prevention of influenza A and levels of 25(OH)D, calcium, and phosphorus | 400 | Vitamin D, 1200 vs 400 IU/d for 4 mo | Asian | 3-12 mo | Healthy |
| Tau et al,35 1986 (France) | October 1983 to October 1984 | Hypercalcemia and 25(OH)D and thyroid hormone levels in infants with hypothyroidism | 25 | Vitamin D2, 1200 IU/d vs placebo for 6 mo | NR | Infants | Infants with congenital hypothyroidism |
| Pacheco-Acosta et al,36 2020 (Chile) | August 2015 to July 2016 | 25(OH)D levels | 65 | Single oral dose of vitamin D, 100 000 IU vs 400 IU/d; follow-up at 6 mo of age | NR | Infants | Healthy |
| Aldaghi et al,37 2020 (Iran) | August to November 2018 | Atopic dermatitis | 81 | Vitamin D, 1400 vs 400 IU/d for 2 mo | NR | Infants | Atopic dermatitis |
| Chowdhury et al,38 2021 (Bangladesh) | June 2014 to June 2018 | Length of severe pneumonia | 197 | Oral single dose of vitamin D, 20 000 IU (<6 mo), 50 000 IU (6-12 mo), or 100 000 IU (13-59 mo) plus 10 000 IU/d after vs placebo plus 10 000 IU/d after for the next 4 d | NR | 2-59 mo | Severe pneumonia |
| Saluja et al,39 2021 (India) | November 2018 to April 2020 | 25(OH)D, calcium, phosphate, ALP, and PTH levels | 66 | Vitamin D, 2000 IU/d (3-12 mo) or 4000 IU/d (1-5 y) for 12 wk vs oral single dose of 60 000 IU (3-12 mo) or 150 000 (1-5 y) | NR | 3-5 mo | Rickets |
Abbreviations: ALP, alkaline phosphatase; Ca:Cr, calcium to creatinine; IM, intramuscular; NR, not reported; PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D.
SI conversion factor: To convert 25(OH)D to nanomoles per liter, multiply by 2.496.
Study Characteristics
Daily oral high-dose vitamin D intervention ranged from 1200 to 10 000 IU/d, whereas bolus doses ranged from 30 000 IU/week to 600 000 IU as a single dose. Twenty-five of the 32 RCTs8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,26,28,32,33,34,35,36,37 had 2 intervention groups, whereas the remaining 7 studies25,27,29,30,31,38,39 compared more than 2 groups. Only 1 study11 used 6 years as the upper age limit in their eligibility criteria. Fifteen studies16,20,21,23,25,26,28,30,31,32,34,35,36,37,39 included infants (aged 0-12 months) as their only eligible age group, when they were included. In 9 studies,8,9,11,12,13,14,15,16,17 at least 1 intervention group received a bolus dose of greater than 100 000 IU. Intervention doses of less than 2000 IU/d as highest vitamin D supplementation given were only seen among infants, which corresponds to a high-dose intervention in this age group. Most studies had a clinical diagnosis or vitamin D deficiency as inclusion criteria, including rickets in 6 studies,13,14,15,17,27,39 pneumonia in 6 studies,8,18,23,24,29,38 vitamin D deficiency in 2 studies,11,12 asthma in 2 studies,10,22 and congenital hypothyroidism,35 acute lower respiratory infection,19 malnutrition,9 atopic dermatitis,37 vitamin D–deficient mothers,20 prematurity,26 and very low birth weight32 each in 1 study. The remaining 9 studies16,21,25,28,30,31,33,34,36 included healthy participants. The most common race and ethnicity represented in the RCTs was Asian, in 14 studies.8,9,11,12,13,14,17,18,19,23,24,25,29,34
25(OH)D Level
Serum 25(OH)D levels above the threshold of 100 ng/mL were observed in 11 studies10,11,14,17,19,20,22,23,25,30,39 (Table 2). In 5 studies19,20,23,25,30 comparing high-dose vitamin D supplementation with a control condition (low dose of ≤400 IU/d or placebo), the total number of cases with levels greater than the 100-ng/mL threshold was 22 of 1682 (1.3%) in the high-dose groups vs 0 of 1702 in the control groups (P < .001). Any clinical adverse events possibly associated with the vitamin D intervention as described by the authors were reported in 3 studies20,25,29 and included mild symptoms such as diarrhea, vomiting, and irritability. Other clinical adverse events reported in the studies were considered associated with the diagnoses of the participants by the authors (ie, not associated with the vitamin D supplementation).
Table 2. Safety Outcomes.
| Source | Elevated 25(OH)D levels (>100 ng/mL)a | Other related biochemical changes | SAEs (death or hospitalization) | High-dose intervention vs control (≤400 IU/d or placebo) |
|---|---|---|---|---|
| Singh et al,8 2019 | NR | NR | Vitamin D vs placebo: 11 of 46 vs 15 of 45 hospitalized | Yes |
| Saleem et al,9 2018 | 0 | No difference in calcium, albumin, or prealbumin levels | 1 participant died due to gastroenteritis (before receiving any intervention) | Yes |
| Ducharme et al,10 2019 | Intervention group, 6 of 23 children with >90 ng/mL, 1 associated with Ca:Cr ratio of >1.0; placebo group, 0 of 24 | Abnormal urinary Ca:Cr ratio in 9 of 104 samples (intervention) vs 12 of 117 samples (placebo) | Vitamin D vs placebo: 0 of 23 vs 1 of 24 hospitalized | Yes |
| Moslemi et al,11 2018 | Total of 6 children, 4 in single-dose group and 2 in capsule group | NR | 0 | No |
| Harnot et al,12 2017 | 0 | Hypercalcemia and abnormal urinary Ca:Cr ratio (after 7-10 d) in 5 of 27 vs 3 of 28 and abnormal urinary Ca:Cr ratio in 5 of 27 vs 2 of 28 (after 3-5 d) | 0 | No |
| Mittal et al,13 2014 | 0 | Hypercalcemia: 1 (300 000 IU) vs 1 (600 000 IU), normal urinary Ca:Cr ratio | 0 | No |
| Mondal et al,14 2014 | Oral group, 2 of 30; IM group, 1 of 31 | Normal urinary Ca:Cr ratio and calcium level after intervention | NR | No |
| Lubani et al,15 1989 | NR | Normal calcium, phosphate, and ALP levels | 0 | No |
| Zeghoud et al,16 1994 | 0 | Normal calcium levels | NR | No |
| Mittal et al,17 2018 | Group A (90 000 IU), 0 of 55; group B (300 000 IU), 2 of 55 with >150 ng/mL | Abnormal Ca:Cr ratio: 3 of 55 in group A vs 5 of 55 in group B; hypercalcemia: 3 of 55 in group A vs 2 of 55 in group B | 0 | No |
| Gupta et al,18 2016 | NR | Normal calcium levels | Vitamin D vs placebo: 19 of 156 vs 20 of 159 | Yes |
| Somnath et al,19 2017 | Intervention group, 1 of 78; placebo group, 0 of 76 | NR | 0 | Yes |
| Huynh et al,20 2017 | High-dose group, 2 of 34; 400 IU/d group, 0 of 36 | Bolus vs daily low dose: 2 of 34 vs 7 of 36 with hypercalcemia | 3 children in all (groups not specified) | Yes |
| Moodley et al,21 2015 | NR | NR | NR | Yes |
| Jensen et al,22 2016 | Intervention group, 2 of 11 with >90 ng/mL; placebo group, 0 of 11 | Abnormal Ca:Cr ratio: 1 of 11 vs 1 of 11 | 0 | Yes |
| Manaseki-Holland et al,23 2012 | Vitamin D group, 2 of 1524; placebo group, 0 of 1522 | NR | Vitamin D vs placebo: 10 of 1524 vs 7 of 1522 | Yes |
| Manaseki-Holland et al,24 2010 | NR | NR | Vitamin D vs placebo: 2 of 224 vs 1 of 229 died | Yes |
| Shakiba et al,25 2010 | Bolus group (50 000 IU every 2 mo), 2 of 30; low-dose groups, 0 of 35 | Normal calcium levels | 0 | Yes |
| Mawer et al,26 1986 | NR | NR | NR | No |
| Moya et al,27 1977 | NR | NR | NR | No |
| Rosendahl et al,28 2018 | 0 | Hypercalcemia: 32 of 364 in 1200-IU vs 27 of 362 in 400-IU groups; mean PTH level: 16.5 vs 19 pg/mL (P = .004) | NR | Yes |
| Choudhary et al,29 2012 | NR | NR | Vitamin D vs placebo: 1 of 87 vs 1 of 86 died | Yes |
| Gallo et al,30 2013 | Group receiving 1600 IU/d, 15 of 16; group receiving 400 IU/d, 0 of 33 | Suspected hypercalcemia: 2 in the 800-IU/d, 2 in the 1200-IU/d, and 2 in the 1600-IU/d groups; suspected abnormal Ca:Cr ratio: 1 in the 800-IU/d, 1 in the 1200-IU/d, and 1 in the 1600-IU/d groups | 0 | Yes |
| Holmlund-Suila et al,31 2012 | Group receiving 1600 IU/d, 1 of 37; group receiving 1200 IU/d, 1 of 38; group receiving 400 IU/d, 0 of 38 with >90 ng/mL | No differences in calcium or PTH levels and urine Ca:Cr ratio | 0 | Yes |
| Evans et al,32 1989 | NR | No difference in calcium, ALP, or phosphate levels; increased urinary Ca:Cr ratio in control group (P < .001) | 4 of 45 in 2000-IU group vs 2 of 42 in 400-IU group died or had severe jaundice | Yes |
| Aglipay et al,33 2017 | NR | No differences in calcium, ALP, or PTH levels | 0 | Yes |
| Zhou et al,34 2018 | NR | NR | 7 of 200 in 1200-IU/d group vs 8 of 200 in 400-IU/d group secondary bacterial infection and hospitalized | Yes |
| Tau et al,35 1986 | NR | Increased calcium levels in intervention group; no differences in phosphorus or ALP levels | NR | Yes |
| Pacheco-Acosta et al,36 2020 | 0 | NR | NR | Yes |
| Aldaghi et al,37 2020 | NR | NR | 0 | Yes |
| Chowdhury et al,38 2021 | 0 | No differences in calcium and ALP levels | 1 of 97 in high-dose group vs 5 of 100 in placebo group died during hospitalization | No |
| Saluja et al,39 2021 | Daily group, 3 of 33; depot group, 1 of 33 | No hypercalcemia; hypophosphataemia: 1 of 33 vs 0 of 33 cases; ALP levels increased in 2 of 33 vs 2 of 33 cases; hyperparathyroidism in 3 of 33 vs 1 of 33 cases (no statistically significant differences) | NR | No |
Abbreviations: ALP, alkaline phosphatase; Ca:Cr, calcium to creatinine; IM, intramuscular; NR, not reported; PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D; SAE, serious adverse event.
SI conversion factors: To convert 25(OH)D to nanomoles per liter, multiply by 2.496; to convert PTH to nanograms per liter, multiply by 1.
Zero indicates no cases were reported in the study.
Serious Adverse Events
Serious adverse events (death or hospitalization) were observed in 10 studies,8,9,10,18,20,23,24,29,32,34 of which 8 studies8,10,18,23,24,29,32,34 were available for a meta-analysis because 1 study9 reported death before receiving any intervention and 1 study20 did not specify the group assignments of the 3 children experiencing SAEs. We unsuccessfully tried contacting the authors of the latter study for clarification. There was no increased risk of SAEs in the high-dose vitamin D vs control groups (Figure 2) (RR, 1.01 [95% CI, 0.73-1.39]; P = .89, I2 = 0%). A funnel plot of the eligible studies did not show publication bias (P = .65) (eFigure 2 in the Supplement). Also, we found no differences between the groups when stratifying the meta-analysis by intervention method (bolus or daily supplementation) (Figure 3). Further, most of the studies observing SAEs described those as not associated with the vitamin D intervention except for Evans et al,32 who did not comment on a possible association.
Figure 2. Summary Risk Ratio (RR) of the Association Between High-Dose Vitamin D Supplementation and Serious Adverse Events.
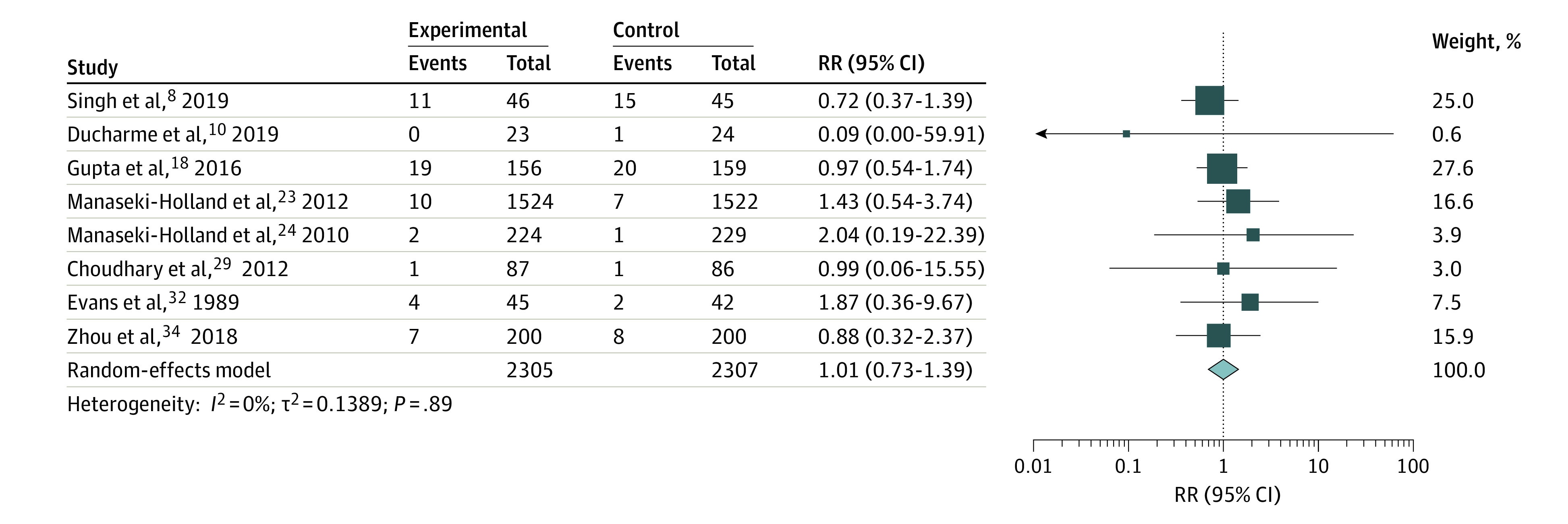
Different sizes of markers indicate weights of the studies; whiskers, 95% CIs.
Figure 3. Summary Risk Ratio (RR) of the Association Between High-Dose Vitamin D Supplementation and Serious Adverse Events Stratified by Bolus and Daily Supplementation.
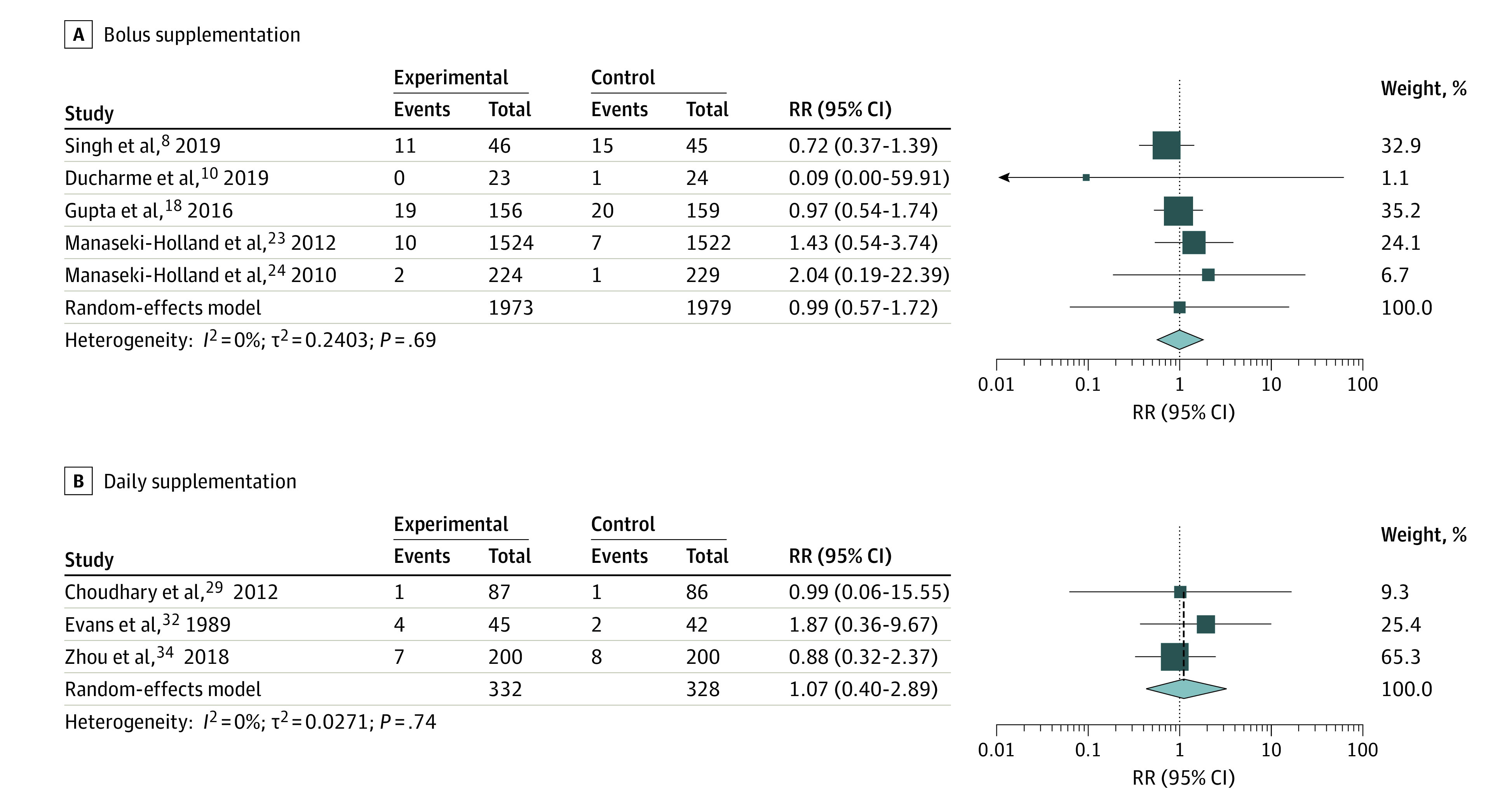
Different sizes of markers indicate weights of the studies; whiskers, 95% CIs.
Hypercalcemia
Hypercalcemic values were observed in 7 studies12,13,17,20,28,30,35 based on the authors’ own definitions. Of these, only 1 study28 had a comparable control group and was available for the analysis of high-dose vitamin D (1200 IU/d) vs control (400 IU/d) on the risk of hypercalcemia. Rosendahl et al28 found that 32 of 364 children in the vitamin D group (8.8%) vs 27 of 362 in the control group (7.5%) developed mild hypercalcemia, defined as plasma ionized calcium levels of greater than 5.4 mg/dL (to convert to millimoles per liter, multiply by 0.25). This difference was not statistically significant according to our analysis (RR, 1.18 [95% CI, 0.72-1.93]; P = .51). In support, there was no statistically significant difference in mean ionized calcium concentrations between the 2 groups according to the authors, and no severe cases of hypercalcemia were registered.28
Mittal et al13,17 observed hypercalcemia cases (7 of 186) in single-bolus groups receiving 90 000, 300 000, and 600 000 IU, but no clinical adverse events were observed in children. Harnot et al12 reported similar results among bolus groups receiving 300 000 and 600 000 IU, with all children being asymptomatic despite hypercalcemia cases. Huynh et al20 reported hypercalcemia cases in both a low-dose group of 400 IU/d (7 of 36) vs a 50 000-IU bolus group (2 of 34) in which only 1 child receiving a bolus had 25(OH)D levels of greater than 100 ng/mL. Gallo et al30 reported that 15 of 16 children (93.7%) in a 1600-IU/d group developed 25(OH)D levels of greater than 100 ng/mL; however, no difference in total plasma calcium levels was found in this group compared with a low-dose group receiving 400 IU/d.
Urine Ca:Cr Ratio
The urine Ca:Cr ratio was reported as abnormal in 8 studies.10,12,14,17,22,30,31,32 Of these, 3 studies10,22,30 had a comparable control group. In the study by Gallo et al,30 suspected hypercalciuria was reported in 1 child in each of the high-dose groups (1200 and 1600 IU/d) vs 1 case in a low-dose group (800 IU/d), but with no cases in the lowest-dose group (400 IU/d). It was not clear how the term suspected was defined in the study, but clinical follow-up measurements were within reference range in all children. In one of the remaining studies,10 borderline abnormal urine Ca:Cr ratio was observed in 9 of 104 children in the vitamin D bolus group vs 12 of 117 in the placebo group, including baseline measurements. In a study by Jensen et al,22 1 of 11 children in the vitamin D bolus group vs 1 of 11 in the placebo group had a borderline abnormal urine Ca:Cr ratio.
Harnot et al12 reported abnormal urine Ca:Cr ratio in 300 000- and 600 000-IU bolus groups; these results are similar to those reported by Mittal et al17 in which cases of abnormal urine Ca:Cr ratios were reported in 90 000- and 300 000-IU bolus groups. In the study by Evans et al,32 increased mean urine Ca:Cr values were observed in the control group compared with the intervention group (400 vs 2000 IU/d; P < .001). The abnormal value reported by Mondal et al14 was observed before the intervention (ie, it was not associated with the intervention). Holmlund-Suila et al31 observed 39% of children with hypercalciuria, but with no differences between the intervention groups and no correlation with 25(OH)D levels.
ALP, Phosphate, and PTH Findings
In studies reporting levels of ALP, these levels were within the reference range and were similar between the intervention and control groups where applicable,15,30,32,33,35 except for 1 study39 in which children in the group receiving daily doses had elevated ALP levels (n = 2), hypophosphatemia (n = 1), and hyperparathyroidism (n = 3), and children in the bolus group had elevated ALP levels (n = 2) and hyperparathyroidism (n = 1) with no statistical differences between the groups. Phosphate levels within the reference range were reported by the remaining studies.15,32,35 In the study by Rosendahl et al,28 mean PTH levels were significantly lower in the 1200 vs 400 IU/d groups both at 1 and 2 years of age.
Risk of Bias
We used the Cochrane risk of bias tool (2019 version)7 to evaluate the qualities of the RCTs. Our overall risk of bias assessment showed low risk in 14 studies,9,10,11,12,18,23,24,28,30,31,33,37,38,39 some concerns in 4 studies,19,20,22,29 and high risk in 14 studies.8,13,14,15,16,17,21,25,26,27,32,34,35,36 This assessment is provided in detail in eTable 2 in the Supplement.
Discussion
Based on findings reported in the 32 included RCTs in this systematic review and meta-analysis, clinical adverse events were relatively rare, and most of the reported abnormal biochemical values were not described as raising serious concern or having a clear correlation with high-dose vitamin D supplementation and excess 25(OH)D levels. Most importantly, in the 8 studies available for meta-analysis,8,10,18,23,24,29,32,34 there was no increased risk of SAEs among patients assigned to a high-dose vitamin D intervention, either as a bolus or daily supplement, compared with a low-dose or a placebo group. Further, we found no statistical differences in the studies reporting hypercalcemia and abnormal urine Ca:Cr ratios. Thus, our findings suggest that vitamin D supplementation in the high-dose range of 1200 to 10 000 IU/d and bolus doses to 600 000 IU to infants and preschool children to 6 years of age may be safe in both healthy children and in children with various diseases.
The studies included described high-dose vitamin D as being safe; however, many stated that their results were not sufficiently strong for making solid conclusions on safety owing to, for example, small sample sizes. The main strength of our study is the inclusion of several studies and a meta-analysis of an important safety outcome (SAEs), which allows for an overall safety assessment together with a descriptive review of reported safety end points when a meta-analysis could not be performed. Although abnormal biochemical values were still observed in some studies, and high-dose vitamin D supplementation may result in some adverse events, those reported in the studies were not considered SAEs or directly associated with the vitamin D intervention, because no excess in 25(OH)D levels was observed, which was also supported by our meta-analysis of SAEs showing no increased risk in the high-dose groups and no heterogeneity across the studies. Information about long-term clinical adverse events is generally lacking, and further RCTs with larger and more comparable study groups are needed to form a clearer image of the safety profile for high-dose vitamin D supplementation in different dose ranges.
A meta-analysis including RCTs conducted among adults given high-dose vitamin D for a minimum of 1 year showed that high-dose vitamin D supplementation did not increase the risk of total adverse events significantly, although the results showed borderline increased risk for hypercalcemia.40 This finding is consistent with our results and supports the notion that high-dose vitamin D intervention may be a safe treatment strategy without serious adverse events within the described range doses.
The safety outcomes assessed in this study are clinically relevant and should be investigated further because high-dose vitamin D administered to children seems to have beneficial effects on different health outcomes, such as preventing pneumonia8,24 and influenza.34 Exogenous vitamin D intoxication is a potential risk when administering high-dose supplements, and severe hypercalcemia is of greatest concern with symptoms such as vomiting, constipation, abdominal pain, dehydration, polyuria, concentration problems, and nephrocalcinosis.41 If the safety of high-dose vitamin D supplementation by bolus and/or daily administration is confirmed in future large-scale studies, it may be a new potential treatment strategy against several health outcomes and should result in a reevaluation of the definition of the upper levels of tolerable intake for vitamin D supplementation.
Limitations
The main limitations of this systematic review and meta-analysis are the lack of similar intervention doses, safety and end point definitions, similarity of the included children, focused safety outcome of the studies, and a broad variation in the intervention and follow-up periods. The daily vitamin D supplementation periods ranged from 5 days until approximately 1 year in the included RCTs, which is a limitation and reduces comparability between the studies. Further, the heterogeneity in the follow-up periods is a limitation when comparing the total number of adverse events given that short-term studies may not capture the same adverse events as long-term studies. The various diagnoses of the children may have played a role when assessing adverse effects or may have resulted in different metabolism patterns. For instance, among infants with hypothyroidism, as seen in the study by Tau et al,35 hypercalcemia often develops because of decreased metabolism of vitamin D or increased absorption.42 However, we investigated clinical adverse events and abnormal biochemical measures independent of the studied population and clinical outcomes of the trials to elucidate the overall safety profile of a high-dose vitamin D intervention, which allowed for a generalization of our results. In 7 of the 8 studies included in the meta-analysis investigating the risk of SAEs, children were diagnosed with asthma, pneumonia, or very low birth weight, which could influence the generalizability of the results given that these children constitute high-risk pediatric populations. However, because these children may be considered at an increased risk for developing SAEs due to high-dose vitamin D compared with healthy children, this should not change the overall safety assessment. Furthermore, factors such as skin pigmentation, latitude, clothing, and use of sunscreen all influence the endogenous synthesis of vitamin D3 in the skin.43 In our review, the predominant racial and ethnic population represented was Asian (14 studies8,9,11,12,13,14,17,18,19,23,24,25,29,34); therefore, RCTs conducted among other races and ethnicities such as White individuals are warranted.
In some studies, the high-dose vitamin D intervention was given simultaneously with other treatment, which potentially could mask whether the adverse events were due to vitamin D or not. For instance, in the study by Shakiba et al,25 in which vitamin D was given at the same time as a routine polio vaccination, it is difficult to assess whether the potential adverse events of diarrhea and agitation seen were due to vaccination or to high-dose vitamin D. Last, many studies lacked a comparable low-dose or placebo group to allow for a meta-analysis of safety outcomes, further limiting our findings.
Conclusions
This meta-analysis and systematic review found that adverse events occurring after vitamin D supplementation as assessed by clinical symptoms and biochemical changes were rare in the 32 RCTs included, for which high-dose vitamin D supplementation was characterized as being well tolerated overall. These results suggest that high-dose vitamin D supplementation to children aged 0 to 6 years may be a safe supplementation strategy against various health outcomes.
eFigure 1. Division of Search Terms
eFigure 2. Funnel Plot of Studies Included in the Meta-analysis of Serious Adverse Events by High-Dose Vitamin D Interventions
eTable 1. Search Protocol
eTable 2. Risk of Bias Score
eReferences
References
- 1.Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood). 2010;235(9):1034-1045. [DOI] [PubMed] [Google Scholar]
- 2.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8-F28. [DOI] [PubMed] [Google Scholar]
- 3.Nutrition and Allergies EFSA Panel on Dietetic Products . Scientific opinion on the tolerable upper intake level of vitamin D. EFSA J. 2012;10(7):2813. doi: 10.2903/j.efsa.2012.2813 [DOI] [Google Scholar]
- 4.Antonucci R, Locci C, Clemente MG, Chicconi E, Antonucci L. Vitamin D deficiency in childhood: old lessons and current challenges. J Pediatr Endocrinol Metab. 2018;31(3):247-260. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. ; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JPT, Savović J, Page MJ, Sterne JAC; RoB2 Development Group. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). August 22, 2019. Accessed December 11, 2020. https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2
- 8.Singh N, Kamble D, Mahantshetti NS. Effect of vitamin D supplementation in the prevention of recurrent pneumonia in under-five children. Indian J Pediatr. 2019;86(12):1105-1111. [DOI] [PubMed] [Google Scholar]
- 9.Saleem J, Zakar R, Zakar MZ, et al. High-dose vitamin D3 in the treatment of severe acute malnutrition: a multicenter double-blind randomized controlled trial. Am J Clin Nutr. 2018;107(5):725-733. [DOI] [PubMed] [Google Scholar]
- 10.Ducharme FM, Jensen M, Mailhot G, et al. Impact of two oral doses of 100 000 IU of vitamin D3 in preschoolers with viral-induced asthma: a pilot randomised controlled trial. Trials. 2019;20(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moslemi L, Esmaeili Dooki M, Moghadamnia AA, et al. Stoss therapy using fortified biscuit for vitamin D-deficient children: a novel treatment. Pediatr Res. 2018;84(5):662-667. [DOI] [PubMed] [Google Scholar]
- 12.Harnot J, Verma S, Singhi S, Sankhyan N, Sachdeva N, Bharti B. Comparison of 300 000 and 600 000 IU oral vitamin-D bolus for vitamin-D deficiency in young children. Indian J Pediatr. 2017;84(2):111-116. [DOI] [PubMed] [Google Scholar]
- 13.Mittal H, Rai S, Shah D, et al. 300 000 IU or 600 000 IU of oral vitamin D3 for treatment of nutritional rickets: a randomized controlled trial. Indian Pediatr. 2014;51(4):265-272. [DOI] [PubMed] [Google Scholar]
- 14.Mondal K, Seth A, Marwaha RK, et al. A randomized controlled trial on safety and efficacy of single intramuscular versus staggered oral dose of 600 000 IU vitamin D in treatment of nutritional rickets. J Trop Pediatr. 2014;60(3):203-210. [DOI] [PubMed] [Google Scholar]
- 15.Lubani MM, al-Shab TS, al-Saleh QA, et al. Vitamin-D–deficiency rickets in Kuwait: the prevalence of a preventable disease. Ann Trop Paediatr. 1989;9(3):134-139. [DOI] [PubMed] [Google Scholar]
- 16.Zeghoud F, Ben-Mekhbi H, Djeghri N, Garabédian M. Vitamin D prophylaxis during infancy: comparison of the long-term effects of three intermittent doses (15, 5, or 2.5 mg) on 25-hydroxyvitamin D concentrations. Am J Clin Nutr. 1994;60(3):393-396. [DOI] [PubMed] [Google Scholar]
- 17.Mittal M, Yadav V, Khadgawat R, Kumar M, Sherwani P. Efficacy and safety of 90 000 IU versus 300 000 IU single dose oral vitamin D in nutritional rickets: a randomized controlled trial. Indian J Endocrinol Metab. 2018;22(6):760-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta P, Dewan P, Shah D, et al. Vitamin D supplementation for treatment and prevention of pneumonia in under-five children: a randomized double-blind placebo controlled trial. Indian Pediatr. 2016;53(11):967-976. [DOI] [PubMed] [Google Scholar]
- 19.Somnath SH, Biswal N, Chandrasekaran V, Jagadisan B, Bobby Z. Therapeutic effect of vitamin D in acute lower respiratory infection: a randomized controlled trial. Clin Nutr ESPEN. 2017;20:24-28. [DOI] [PubMed] [Google Scholar]
- 20.Huynh J, Lu T, Liew D, et al. Vitamin D in newborns: a randomised controlled trial comparing daily and single oral bolus vitamin D in infants. J Paediatr Child Health. 2017;53(2):163-169. [DOI] [PubMed] [Google Scholar]
- 21.Moodley A, Spector SA. Single high-dose vitamin D at birth corrects vitamin D deficiency in infants in Mexico. Int J Food Sci Nutr. 2015;66(3):336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen ME, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;17(1):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manaseki-Holland S, Maroof Z, Bruce J, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet. 2012;379(9824):1419-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manaseki-Holland S, Qader G, Isaq Masher M, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health. 2010;15(10):1148-1155. [DOI] [PubMed] [Google Scholar]
- 25.Shakiba M, Sadr S, Nefei Z, Mozaffari-Khosravi H, Lotfi MH, Bemanian MH. Combination of bolus dose vitamin D with routine vaccination in infants: a randomised trial. Singapore Med J. 2010;51(5):440-445. [PubMed] [Google Scholar]
- 26.Mawer EB, Stanbury W, Robinson MJ, James J, Close C. Vitamin D nutrition and vitamin D metabolism in the premature human neonate. Clin Endocrinol (Oxf). 1986;25(6):641-649. [DOI] [PubMed] [Google Scholar]
- 27.Moya M, Beltran J, Colomer J. Therapeutic and collateral effects of 25-hydroxycholecalciferol in vitamin D deficiency. Eur J Pediatr. 1977;127(1):49-55. [DOI] [PubMed] [Google Scholar]
- 28.Rosendahl J, Valkama S, Holmlund-Suila E, et al. Effect of higher vs standard dosage of vitamin D3 supplementation on bone strength and infection in healthy infants: a randomized clinical trial. JAMA Pediatr. 2018;172(7):646-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhary N, Gupta P. Vitamin D supplementation for severe pneumonia—a randomized controlled trial. Indian Pediatr. 2012;49(6):449-454. [DOI] [PubMed] [Google Scholar]
- 30.Gallo S, Comeau K, Vanstone C, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA. 2013;309(17):1785-1792. [DOI] [PubMed] [Google Scholar]
- 31.Holmlund-Suila E, Viljakainen H, Hytinantti T, Lamberg-Allardt C, Andersson S, Mäkitie O. High-dose vitamin d intervention in infants—effects on vitamin D status, calcium homeostasis, and bone strength. J Clin Endocrinol Metab. 2012;97(11):4139-4147. [DOI] [PubMed] [Google Scholar]
- 32.Evans JR, Allen AC, Stinson DA, et al. Effect of high-dose vitamin D supplementation on radiographically detectable bone disease of very low birth weight infants. J Pediatr. 1989;115(5, pt 1):779-786. [DOI] [PubMed] [Google Scholar]
- 33.Aglipay M, Birken CS, Parkin PC, et al. ; TARGet Kids! Collaboration . Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. 2017;318(3):245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Du J, Huang L, Wang Y, Shi Y, Lin H. Preventive effects of vitamin D on seasonal influenza A in infants: a multicenter, randomized, open, controlled clinical trial. Pediatr Infect Dis J. 2018;37(8):749-754. [DOI] [PubMed] [Google Scholar]
- 35.Tau C, Garabedian M, Farriaux JP, Czernichow P, Pomarede R, Balsan S. Hypercalcemia in infants with congenital hypothyroidism and its relation to vitamin D and thyroid hormones. J Pediatr. 1986;109(5):808-814. [DOI] [PubMed] [Google Scholar]
- 36.Pacheco-Acosta J, Pizarro F. Effect of vitamin D supplementation as a single dose on the nutritional status of vitamin D [in Spanish and English]. Rev Chil Pediatr. 2020;91(5):684-690. [DOI] [PubMed] [Google Scholar]
- 37.Aldaghi M, Tehrani H, Karrabi M, Abadi FS, Sahebkar M. The effect of multistrain synbiotic and vitamin D3 supplements on the severity of atopic dermatitis among infants under 1 year of age: a double-blind, randomized clinical trial study. J Dermatolog Treat. Published online June 26, 2020. [DOI] [PubMed] [Google Scholar]
- 38.Chowdhury F, Shahid ASMSB, Tabassum M, et al. Vitamin D supplementation among Bangladeshi children under-five years of age hospitalised for severe pneumonia: a randomised placebo controlled trial. PLoS One. 2021;16(2):e0246460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saluja RK, Dewan P, Gomber S, Madhu SV, Bhat S, Gupta P. Low dose depot oral vitamin D3v. daily oral vitamin D3 for treating nutritional rickets: a randomised clinical trial. Br J Nutr. Published online July 19, 2021. doi: 10.1017/S0007114521002713 [DOI] [PubMed] [Google Scholar]
- 40.Malihi Z, Wu Z, Lawes CMM, Scragg R. Adverse events from large dose vitamin D supplementation taken for one year or longer. J Steroid Biochem Mol Biol. 2019;188:29-37. [DOI] [PubMed] [Google Scholar]
- 41.Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P, Jones G. Vitamin D toxicity—a clinical perspective. Front Endocrinol (Lausanne). 2018;9:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozkan B, Hatun S, Bereket A. Vitamin D intoxication. Turk J Pediatr. 2012;54(2):93-98. [PubMed] [Google Scholar]
- 43.Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115-124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Division of Search Terms
eFigure 2. Funnel Plot of Studies Included in the Meta-analysis of Serious Adverse Events by High-Dose Vitamin D Interventions
eTable 1. Search Protocol
eTable 2. Risk of Bias Score
eReferences