Abstract
We previously reported that maternal PFBS, an emerging pollutant, exposure is positively associated with preeclampsia which can results from aberrant trophoblasts invasion and subsequent placental ischemia. In this study, we investigated the effects of PFBS on trophoblasts proliferation/invasion and signaling pathways. We exposed a human trophoblast line, HTR8/SVneo, to PFBS. Cell viability, proliferation, and cell cycle were evaluated by the MTS assay, Ki-67 staining, and flow cytometry, respectively. We assessed cell migration and invasion with live cell imaging-based migration assay and matrigel invasion assay, respectively. Signaling pathways were examined by Western blot, RNA-seq, and qPCR. PFBS exposure interrupted cell proliferation and invasion in a dose-dependent manner. PFBS(100 μM) did not cause cell death but instead significant cell proliferation without cell cycle disruption. PFBS(10 and 100 μM) decreased cell migration and invasion, while PFBS (0.1 μM) significantly increased cell invasion but not migration. Further, RNA-seq analysis identified dysregulated HIF-1α target genes which are relevant to cell proliferation/invasion and preeclampsia, while Western Blot data showed the activation of HIF-1α, but not Notch, ERK1/2, (PI3K)AKT, and P38 pathways.
In conclusions, PBFS exposure altered trophoblast cell proliferation/invasion which might be mediated by preeclampsia-related genes, suggesting a possible association between prenatal PFBS exposure and adverse placentation.
Keywords: PFBS, placenta cytotrophoblast, proliferation, invasion, preeclampsia
Introduction
Poly- and per-fluoroalkyl substances (PFAS) have attracted widespread attention in recent years due to their bioaccumulation, toxicity, and ubiquitous nature (1). PFAS are a group of compounds characterized by a hydrophobic poly-fluorinated alkyl chain and a polar hydrophilic terminal functional group. PFAS are used in a variety of industrial and consumer products such as surfactants for soil/stain resistance, textiles, paper and metals, firefighting foam, and pesticides (2, 3). Humans are exposed to PFAS through contaminated drinking water, food, outdoor air, indoor dust, and soil (4).
One of the most widely known PFAS is perfluorooctane sulfonic acid (PFOS), which has an eight-carbon backbone with a sulfonate. Due to strong carbon-fluorine bonds (C8), PFOS is extremely stable and persistent in the environment (USEPA, Document# 822R14002) and is not readily eliminated from humans due to its half-life of 5.4 years (5, 6) (7–16). Data from human and animal studies demonstrate numerous health and ecological risks resulting from PFOS exposure including increased risk of thyroid disease, blood cholesterol levels, and preeclampsia and decreased body’s response to vaccine, fertility in women, and birth weight, liver and immune system damage (17–29). Thus, beginning in 2002, most manufacturing of PFOS in the United States was discontinued voluntarily by 3M and DuPont in favor of shorter chain PFAS (C4 or C6, Toni Krasnic, the U. S. Environmental Protection Agency, April, 2011, https://www.oecd.org/env/ehs/risk-management/47643223.pdf) (30), such as perfluorobutane sulfonate (PFBS) (17, 31–35) (30).
PFBS, which has a four-carbon backbone (C4), has been used as an alternative to PFOS as it is less toxic (Toni Krasnic, the U. S. Environmental Protection Agency, April, 2011, https://www.oecd.org/env/ehs/risk-management/47643223.pdf). There are no current restrictions on the production and use of PFBS. However, although less so than PFOS, PFBS is 1) very resistant to degradation (36) and 2) bio-accumulative (37, 38). In addition, PFBS is highly soluble in water (42 g/L) (17), giving it higher mobility in the environment than PFOS (solubility: 591 mg/L). Water treatment processes using granular activated carbon can efficiently reduce long-chain PFAS, including PFOS, but have little effect on short-chain PFAS such as PFBS (39). Therefore, due to the higher mobility and increased ongoing emissions, and the fact that the greatest source of exposure is through drinking water, PFBS is now increasingly released into the environment. The median concentration of PFBS (1.21 ng/L) in drinking water was consistently higher than that of PFOS (0.25 ng/L) and perfluorooctanoic acid (PFOA, 0.74 ng/L) in a survey of 79 cities in China (40). In another study, PFOA and PFBS were the two most dominant compounds (median concentrations: 50.67 ng/L and 29.84 ng/L, respectively) identified in 39 surface water samples in Shanghai (30). Concentrations of PFOS were generally less than PFBS in these samples (30). In parallel, along with exposure via food and drinking water, humans are increasingly exposed to PFBS through dust inhalation and possible dermal contact with consumer products (41–43). With limited data about the health effects on humans, the public health impact of PFBS remains unclear and must be evaluated.
Researchers have demonstrated that exposure to PFBS may result in endocrine disruption (3, 44–47), toxicity in human placental trophoblasts (3) and neuronotypic cells (48), immunotoxicity (49, 50), and transcriptional effects (51). Recent studies indicate that exposure to PFBS may increase the risk of human female infertility due to endometriosis (52), reduce egg production and brood number in C. elegans (53), and decrease sperm motility in humans (54). Maternal serum PFBS can pass through the placental barrier and reach the fetus, evidenced by its detection in the umbilical cord blood of human newborns (55–57) and in a mother-fetus pair of killer whales (58). Recently we reported that cord blood levels of PFBS is positively associated with gestational hypertension and preeclampsia (57).
Preeclampsia is a pregnancy-specific disease that affects 5–8% of pregnant women. Preeclampsia is characterized by a new-onset of hypertension after twenty weeks of gestation and remains one of the major cause of adverse pregnancy and birth outcomes worldwide (59). Preeclampsia can cause fetal growth restriction and stillbirth. It is also one of the leading causes of premature births and its ensuing complications, including learning disabilities, epilepsy, cerebral palsy, and hearing and vision problems. In mothers-to-be, preeclampsia can cause serious complications that include stroke, seizure, pulmonary edema, heart failure, reversible blindness, bleeding from the liver, placental abruption, and hemorrhage. Despite the severe consequences of preeclampsia on maternal and fetal health, the pathogenesis is unclear. The syndrome is thought to begin with shallow trophoblastic invasion and abnormal placentation which leads to placental insufficiency and the release of various mediators into the maternal circulation (59). The processes of trophoblast invasion are highly controlled by numerous paracrine and autocrine factors which can be mediated through Mitogen-Activated Protein Kinases (MAPKs) (60), Phosphoinositide 3-Kinase (PI3K)/AKT (60), P38 (61, 62), Notch signaling pathways (63, 64), and cellular hypoxia conditions (65–67).
Within this context, the aim of this study is to determine the effects of PFBS exposure on the cytotoxicity and invasion of a human placental trophoblast cell line, HTR-8/SVneo. We hypothesize that PFBS impedes the invading trophoblast, which contributes to placental ischemia in preeclampsia. We will further explore PFBS-dysregulated signaling pathways governing trophoblast invasion by measuring the activation of key transcription factors of these pathways and performing RNA-seq analysis.
Materials and Methods
Chemicals
Potassium nonafluoro-1-butanesulfonate (K+PFBS, CAS No.: 29420–49-3, 98% purity) was purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions of PFBS at 100, 10, 1, 0.1, 0.01 mM were prepared by dissolving PFBS in ultrapure distilled water. These doses were chosen for cellular function studies based on LD10 (1000 μM). To ensure the effects on cellular functions are not due to cytotoxicity, we used doses at least 10 times lower than LD10 (100, 10, 1, 0.1, and 0.01 μM).
Cell culture
An immortalized first-trimester human cytotrophoblast cell line (HTR-8/SVneo; a gift from Dr. C.H. Graham, Queen’s University, Kingston, Ontario, Canada)(68) was cultured in RPMI 1640 supplemented with 5% fetal bovine serum (FBS) and maintained in a humidified, 5% CO2 incubator at 37°C. The cells were sub-cultured using 0.05% trypsin-EDTA (Gibco, Life Technologies, Carlsbad, California) for no more than five passages.
Cell viability assay (MTS assay)
HTR-8/SVneo cells were seeded at 2 × 104 cells/well in 48-well plates and incubated for 24 h. The cells were then treated with K+PFBS (0, 0.01, 0.1, 1, 10, 100 μM or 0, 0.1, 1, 2.5, 5, 10 mM) in quadruplicate for 24 h. Cell viability was measured using the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit per the manufacturer’s instructions (G5421; Promega, Madison, WI). Cell viability was compared between treatments by determining the optical density at 490 nm (OD490) after incubating the cells with MTS reagents for 4 h. These experiments were repeated five times (N=5).
Annexin V/PI flow cytometry
After the HTR-8/SVneo cells were treated with PFBS (0, 0.1, 10 mM) for 2, 6, and 18 h, the floating cells from the supernatants were collected by centrifugation. Attached cells were collected by brief trypsinization with 0.05% Trypsin-EDTA. Floating and attached cells were combined. The apoptotic and/or necrotic cells were measured with the Annexin V-FITC Early Apoptosis Detection Kit (P08758, Cell Signaling Technology, Danvers, MA) according to the manufacturer’s protocol. Briefly, after washing the cells with ice-cold PBS, the cells were suspended in Annexin V Binding buffer. Cells were then incubated with Annexin V-FITC Conjugate and Propidium Iodide (PI) for 10 min in the dark. The cells were immediately analyzed by the BD FACS Calibur analyzer. Experiments were repeated three times (N=3).
Cell cycle analysis
The distribution of HTR-8/SVneo cells in various phases of the cell cycle (G0/G1, S, G2/M) was evaluated by flow cytometry. Briefly, PFBS (0, 0.01, 0.1, 1, 10, 100 μM)-treated HTR-8/SVneo cells were harvested after 24 h of incubation and washed twice with cold PBS. The cells were then centrifuged at 3000 x g for 3 min at room temperature, followed by overnight fixation with 70% ethanol. Finally, the cells were incubated with staining solution which contained 50 μg/ml PI (Thermo Fisher Scientific, USA) and 0.1 mg/ml RNase A (Thermo Fisher Scientific, USA) at 37℃ for 30 min. The estimations of the percentage of cells in each phase of the cell cycle were analyzed using BD FACS Calibur analyzer and FlowJo software (FlowJo LLC, Ashland, OR). Experiments were repeated three times (N=3).
Immunofluorescence staining
HTR-8/SVneo cells were seeded at 2 × 104 cells/well in glass chamber slides (ibidi GmbH, Germany) and incubated for 24 h. Cells were then treated with K+PFBS (0, 0.01, 0.1, 1, 10, 100 μM) for 24 h. Cells were fixed with cold methanol (−20°C) for 10 min and blocked with 1% BSA, 5% normal goat serum and 0.1% tween-20 in PBS for 60 min at room temperature. After blocking, the cells were incubated with primary antibodies overnight at 4°C in humidified chambers. Primary rabbit anti-human Ki-67 antibody (Abcam, Cambridge, MA) was used at a 1:500 dilution. Anti-rabbit IgG antibodies were used as the negative control (R & D system, Minneapolis, MN). Goat anti-rabbit secondary antibodies and Alexa Fluo 594 (Life Technologies, Carlsbad, CA) were used at 1:500. Slides were mounted using mounting medium for fluorescence with DAPI (Vector Laboratories, Burlingame, CA) and examined with a Zeiss Axio Imager widefield fluorescence microscope. Images were taken from each quadrant per well, with an average of two images per quadrant (eight areas examined per treatment per repeat). The ratio of Ki-67 staining was determined by calculating the number of Ki-67 positive cells (red)/DAPI positive cells (blue). Experiments were repeated three times (N=3).
Cell migration assay
The cells were seeded at 1 × 106 cells/well in 6-well plates and grew into a 100% confluent monolayer in 24 h. The cell monolayer was then scraped in a straight line with a sterile P200 pipette tip, thus creating a wound area. Cells were then treated with K+PFBS (0, 0.01, 0.1, 1, 10, 100 μM). The wound area was filmed at three locations along the scratch using live imaging with the Zeiss Axio Observer microscope. Images were taken every 5 min for up to 18 h, with 216 images taken per location. The wound area at the different time points was determined using ImageJ analysis software (National Institutes of Health, Bethesda, MD) and relative cell migration rate was calculated from the slope of wound area over time as previously described (69). Experiments were repeated five times (N=5).
Invasion assay
Invasion of HTR-8/SVneo cytotrophoblast cells was measured in 24-well Matrigel invasion chamber plates (35–4480; Becton Dickinson Labware, Bedford, MA). The non-Matrigel-coated controls used were polyethylene terephthalate membranes (with 8.0 µm pore size) cell culture inserts (35–4578; Becton Dickinson Labware). To reconstitute the Matrigel, the Matrigel-coated inserts were allowed to warm to room temperature. Then, 500 µL of serum-free RPMI 1640 media was added to the interior of the inserts and bottom wells, and inserts were allowed to hydrate in a humidified, 5% CO2 incubator at 37°C for two hours. After rehydration, the media in the top and bottom chambers were removed and the cells were seeded at 1 × 104 cells/well in the upper chamber of the Matrigel-coated insert and the non-Matrigel-coated control inserts with a total volume of 500 µL of serum-free RPMI 1640 supplemented with 0.1 % fetal bovine serum (FBS). In the lower chamber, 750 µL of RMPI 1640 supplemented with 5% FBS was added to act as a chemoattractant. Cells were treated with K+PFBS (0, 0.01, 0.1, 1, 10, 100 μM) and incubated for 24 h. Non-invasive cells on the upper surface of the chambers were wiped away with a sterile cotton-tipped applicator and any remaining cells were removed from the rim of the chamber with a sterile P200 pipette tip. The invasion chambers were then stained with the Hema-3-stain kit (22–122911; Thermo Fisher Scientific, Waltham, MA). The stained chambers were later viewed and imaged with a Nikon Diaphot microscope. Percentage invasion was calculated by dividing the number of invading cells through Matrigel-coated inserts by the number of invading cells through non-Matrigel-coated inserts. Invasion index was calculated by dividing percent invasion of treatment group by percent invasion of control group (considered as “1”). Experiments were repeated six times (N=6).
Western blot
HTR-8/SVneo cells were seeded at 5 × 105 cells/well in 6-well plates and grew into a 70% confluent monolayer in 24 h. In the time course study, cells were treated with the K+PFBS (100 μM) for 0, 1, 3, and 6 h to examine the protein levels of HIF-1 α. In the dose response study, cells were treated with K+PFBS (0, 0.01, 0.1, 1, 10, 100 μM) for 1 h to examine the protein levels of HIF-1 α. For screening experiments, cells were treated with PFBS (0, 0.1 mM[=100 μM], 10 mM) for 15 min to examine the activation of the ERK1/2, (PI3K)/AKT, and P38 signaling pathways as manifested by the phosphorylation of ERK1/2, AKT, and P38, respectively. The time points for these pathways were optimized in a previously performed pilot study. In parallel, we exposed these cells to PFBS for 2, 6, and 18 h to investigate the activation of Notch signaling as manifested by the cleavage of Notch2 or Notch3.
After treatments, cell lysates were harvested using RIPA buffer (Sigma Aldrich, St. Louis, MO) with the complete mini-protease inhibitor cocktail (Roche, Mannheim, Germany). Protein concentration was determined with the Bradford assay (Bio-Rad Laboratories, Hercules, CA). Total protein samples (25μg) were loaded onto 10% sodium dodecyl sulfate polyacrylamide gels, separated, and then transferred onto a polyvinylidene difluoride membrane. The membranes were blocked with 5% milk in Tris-Buffered Saline and Tween 20 (TBST) buffer and probed in blocking buffer with primary antibodies overnight at 4°C. Primary antibodies used in this study included: rabbit anti-human HIF-1α antibody (1:1000 dilution) rabbit anti-human phosphor- ERK1/2 antibody (1:1000 dilution), rabbit anti-human phosphor-AKT antibody (1:1000 dilution), rabbit anti-human phosphor-P38 antibody (1:1000 dilution), rabbit anti-human Notch2 antibody (1:1000 dilution), rabbit anti-human Notch3 (1:1000 dilution) antibody, rabbit anti-human GAPDH antibody (1:20,000 dilution). The secondary antibody was used at a 1:2000 dilution. All antibodies were purchased from Cell Signaling Technology. The membranes were visualized and directly photographed using the ChemiDoc MP Imaging System with Image Lab Software (Bio-Rad, Berkeley, CA) and optimized to maintain bands within the linear range. Band intensity was measured using ImageJ analysis software (NIH, Bethesda, MD), and data are presented as ratios after being normalized to an internal control, GAPDH or actin. Experiments were repeated four times (N=4).
RNA-seq
HTR-8/SVneo cells were cultured in the absence (control) or presence of PFBS (0, 0.01, 1, 100 μM) for 24 h. Total RNA was extracted using the RNeasy Mini Kit Qiagen, CA, USA) following the manufacturer’s instructions. Extracted total RNA quality and concentration were assessed on a 2100 Bioanalyzer (Agilent Technologies) and Qubit 2.0 (ThermoFisher Scientific), respectively. Only extracts with RNA Integrity Number (RIN) greater than 7 were processed for sequencing. RNA-seq libraries were prepared using the commercially available KAPA Stranded mRNA-Seq Kit following the manufacturer’s protocol. In brief, mRNA transcripts were first captured using magnetic oligo-dT beads, fragmented using heat and magnesium, and reverse transcribed using random priming. During the 2nd strand synthesis, the cDNA:RNA hybrid was converted into to double-stranded cDNA (dscDNA) and dUTP incorporated into the 2nd cDNA strand, effectively marking the second strand. Illumina sequencing adapters were then ligated to the dscDNA fragments and amplified to produce the final RNA-seq library. The strand marked with dUTP was not amplified, allowing strand-specificity sequencing. Libraries were indexed using a dual indexing approach allowing for multiple libraries to be pooled and sequenced on the same sequencing lane on a HiSeq 4000 Illumina sequencing platform. Before pooling and sequencing, fragment length distribution and library quality was assessed on a 2100 Bioanalyzer using the High Sensitivity DNA Kit (Agilent Technologies). All libraries were then pooled in equimolar ratio and sequenced on one lane of HiSeq 4000 at 50bp Single-Read. Once generated, sequence data was demultiplexed and Fastq files generated using Illumina’s Bcl2Fastq2 conversion software. Three sets of experiments were conducted (N=3).
RNA-seq data was processed using the TrimGalore toolkit (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore) which employs Cutadapt (70) to trim low quality bases and Illumina sequencing adapters from the 3’ end of the reads. Only reads that were 20nt or longer after trimming were kept for further analysis. Reads were mapped to the GRCh37v75 version of the human genome and transcriptome (71) using the STAR RNA-seq alignment tool (72). Reads were kept for subsequent analysis if they mapped to a single genomic location. Gene counts were compiled using the HTSeq tool (http://www-huber.embl.de/users/anders/HTSeq/). Only genes with at least 10 reads in any given library were used in subsequent analyses. Normalization and differential expression were carried out using the edgeR (73) Bioconductor (74) package with the R statistical programming environment (www.r-project.org). A linear negative binomial mixed model was used with batch as a random intercept and dose as a factor. The false discovery rate was calculated using the Benjamini-Hochberg method to control for multiple hypothesis testing. Gene set enrichment analysis (75) was performed to identify gene ontology terms and pathways associated with altered gene expression for the comparison performed.
Quantitative RT-PCR
Following RNA-seq analysis, differentially expressed genes were validated using quantitative RT-PCR.
The reverse transcription reaction for first-strand cDNA synthesis was performed using the Reverse Transcription Kit (Bio-Rad). Primers used for all genes are listed in Table S1. Primers used for internal control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were forward (CATGAGAAGTATGACAACAGCCT) and reverse (AGTCCTTCCACGATACCAAAGT).
The PCR reaction was performed at 95°C for 3 min, followed by 40 cycles of 95°C for 30 s and 60°C for 40 s. Quantitative RT-PCR analysis was performed with the CFX Connect Real-Time system (Bio-Rad). The gene expression levels for each individual sample were normalized to GAPDH. The relative expression was analyzed using the 2−ΔΔCt method. Experiments were repeated four times (N=4)
Statistical analysis
Data are presented as means ± SEMs. One-way ANOVA with the post-hoc Dunnett’s test was used for multiple comparisons. All treatments were compared to control (PFBS, 0 μM). Results for which P < 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism 6.0 (La Jolla, CA).
Results
The cytotoxicity of PFBS in HTR-8/SVneo cells
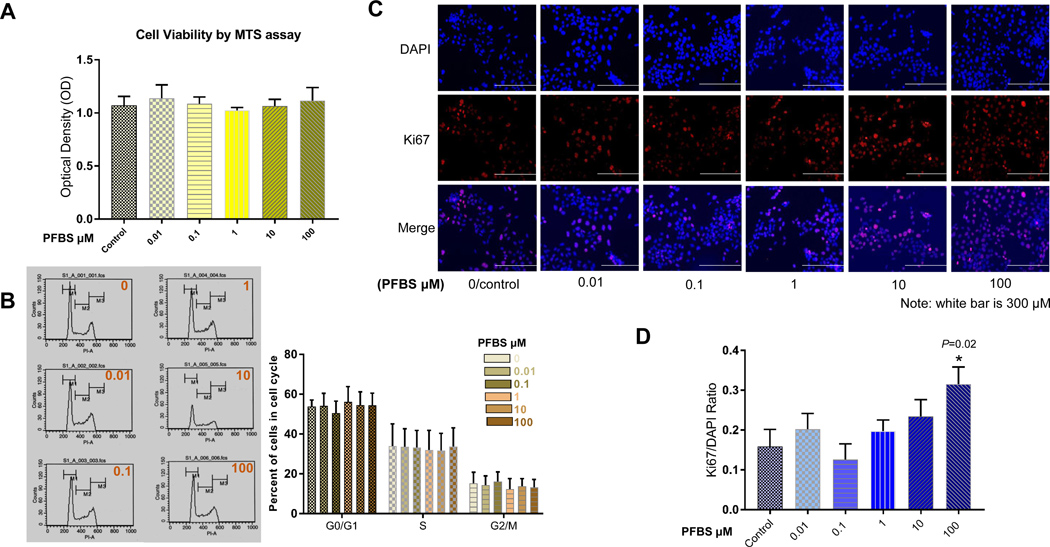
Treatment with PFBS (0, 0.01, 0.1, 1, 10, 100 μM) for 24 h did not result in cell death or disruption of the cell cycle in HTR-8/SVneo cells (Fig 1A and 1B). To evaluate the growth fraction of our cell population, we measured Ki-67. This protein, an excellent marker of cell proliferation, is present during all active phases of the cell cycle but absent in resting cells. Treatment with 100 μM PFBS significantly increased the proportion of Ki-67 staining in red to nuclei in blue vs. control cells. The ratio of Ki-67 staining to total nuclei staining was 0.32±0.04 (or 32%±4%) in cells treated with 100 μM PFBS and 0.16±0.05 (or 16%±5%) in control cells (P =0.02). Representative immunofluorescent images are present in Fig 1C. Fig 1D summarizes the comparison in numerical data.
Fig 1. PFBS promotes proliferation in HTR-8/SVneo cells in a dose-dependent fashion.
(1A) Cell viability of HTR-8/SVneo cells after PFBS (0, 0.01, 0.1, 1, 10, 100 µM) exposure as measured by MTS assay with an absorbance of 490 nm.
(1B) Cell cycle analysis in HTR-8/SVneo cells treated with PFBS (0, 0.01, 0.1, 1, 10, 100 µM) as measured by flow cytometry.
(1C) Representative immunofluorescent images showing DAPI, Ki-67, and DAPI+Ki-67 staining for HTR-8/SVneo cells treated with PFBS (0, 0.01, 0.1, 1, 10, 100 µM).
(1D) Ratio of Ki-67(+)/DAPI(+) in HTR-8/SVneo cells after PFBS (0, 0.01, 0.1, 1, 10, 100 µM) exposure.
To further evaluate the effects of PFBS on the cell growth, reproduction, and morphology of HTR-8/SVneo cells, we tested cytotoxic doses. At a concentration of 10 mM, PFBS significantly reduced the cell viability of HTR-8/SVneo to 27.2% relative to the controls (100%, N=5, P=0.0001, Supplementary Fig 1A). We identified both apoptotic and necrotic cell death induced by 10 mM PFBS in HTR-8/SVneo cells using Annexin V-FITC Early Apoptosis Detection Kit. This kit detects 1) the externalization of phosphatidylserine in apoptotic cells using recombinant annexin V conjugated to green-fluorescent FITC dye and 2) dead cells using propidium iodide (PI), which stains necrotic cells with red fluorescence. After being labeled with both probes, HTR-8/SVneo cells were sorted by flow cytometry into viable (annexin V and PI negative), early apoptotic (annexin V positive and PI negative), necrotic (annexin negative and PI positive), and necrotic/late apoptotic (annexin V and PI positive) cells (Supplementary Fig 1B). Treatment with 10 mM PFBS for 18 h consistently resulted in a decrease in viable cells. During the same incubation, there was a significant increase in the percentage of early and late apoptotic/necrotic cells. A representative dual-plot analysis of annexin V vs. PI staining at various times of treatment is shown in Supplementary Fig 1B. A live cell imaging (video) demonstrating apoptotic cell death morphology is presented in supplementary Fig 1C.
The effects of PFBS on HTR-8/SVneo cell migration and invasion
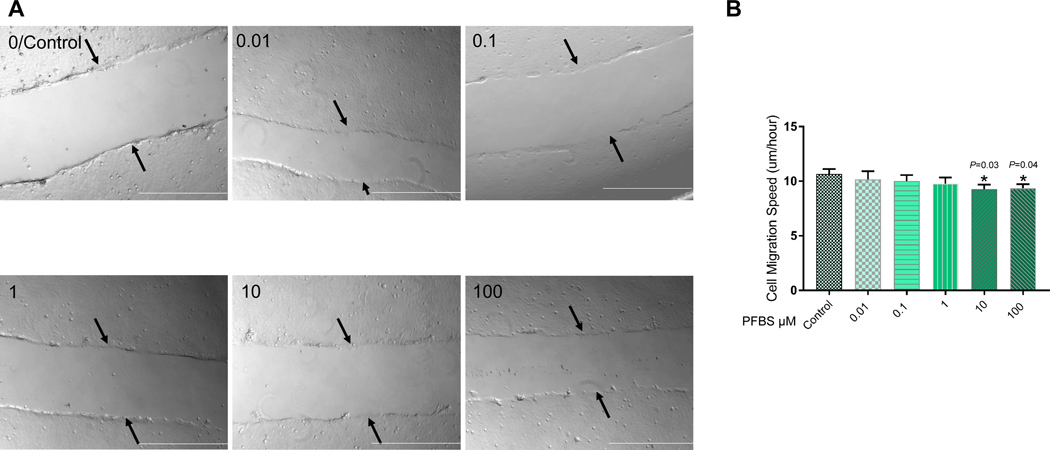
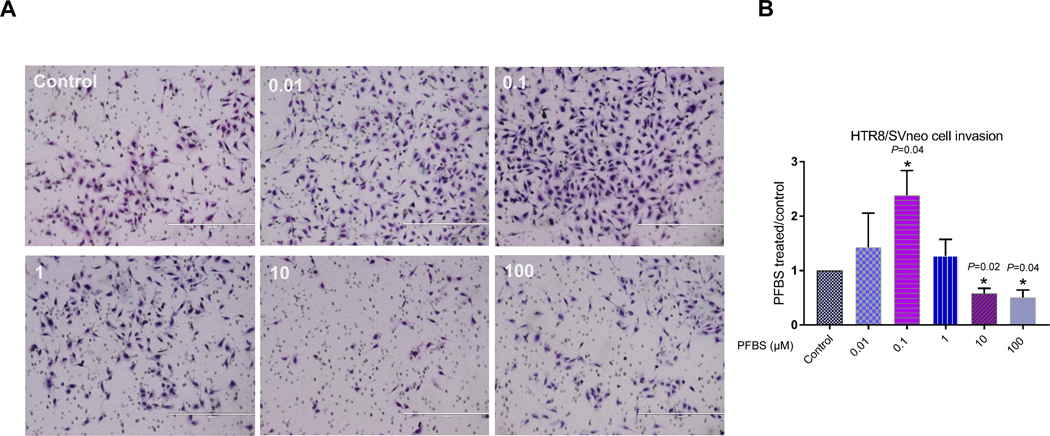
The ability to migrate and invade the maternal compartment is an important function of first trimester trophoblast cells during placenta formation. Therefore, we used a wound healing assay to evaluate the effect of PFBS on the ability of HTR-8/SVneo cells to migrate. In parallel, we tested the effect of PFBS on cell invasion in a trans-well assay. This assay has been used extensively to measure the ability of cells to invade an extracellular matrix (ECM) (76) and recapitulates the two main features that cells require to invade: migration and matrix remodeling. No statistically significant differences in migration were found between control and 0.01, 0.1, or 1 μM PFBS-treated cells. There was a slight decrease in cell migration in 10 and 100 μM PFBS-treated cells compared to control cells, and this was statistically significant (10 μM PFBS vs control: 9.28±0.41 vs. 10.66±0.46 µm/h, P=0.03; 100 μM PFBS vs. control: 9.36±0.46 vs. 10.66±0.46 µm/h, P=0.04, Fig 2B). Representative live cell imaging (video) is presented in Fig 2A. Accordantly, we showed that exposure to both 10 and 100 μM PFBS significantly reduced the invasiveness of HTR-8/SVneo cells. The invasion index of 10 and 100 μM PFBS-treated cells was remarkably lower compared to control cells (10 μM PFBS vs. control: 0.58±0.10 vs. 1±0, P=0.02; 100 μM PFBS vs. control: 0.50±0.14 vs. 1±0, P=0.04, Fig 3B). In contrast, treatment with 0.1 μM PFBS appeared to promote HTR-8/SVneo cell invasion. The invasion index of 0.1 μM PFBS-treated cells was significantly higher compared to control cells (0.1 μM PFBS vs. control: 2.38±0.46 vs. 1±0, P=0.04, Fig 3B). Representative images of invaded cells are presented in Fig 3A.
Fig 2. PFBS reduces cell migration in HTR-8/SVneo cells in a dose-dependent manner.
(2A) Representative videos of wound closure after PFBS (0, 0.01, 0.1, 1, 10, 100 µM) exposure in HTR-8/SVneo cells.
(2B) Rate of distance migrated over time (µm/h) across each wound after PFBS exposure (0, 0.01, 0.1, 1, 10, 100 µM) in HTR-8/SVneo cells.
Note: The black arrows point to the edge of the initial scratch. The play button is under each image. White bar is 500 μM
Fig 3. PFBS significantly disrupts HTR-8/SVneo cell invasion in a dose-dependent fashion.
(3A) Images of filters carrying invaded HTR-8/SVneo cells exposed to PFBS (0, 0.01, 0.1, 1, 10, 100 µM) in a Matrigel invasion assay.
(3B) Invasion index (relative to control; 0 µM PFBS) after PFBS exposure (0, 0.01, 0.1, 1, 10, 100 µM) in HTR-8/SVneo cells.
Note: white bar is 400 μM
PFBS dysregulates transcription factor HIF-1α but not AKT, ERK1/2, P38, or NOTCH
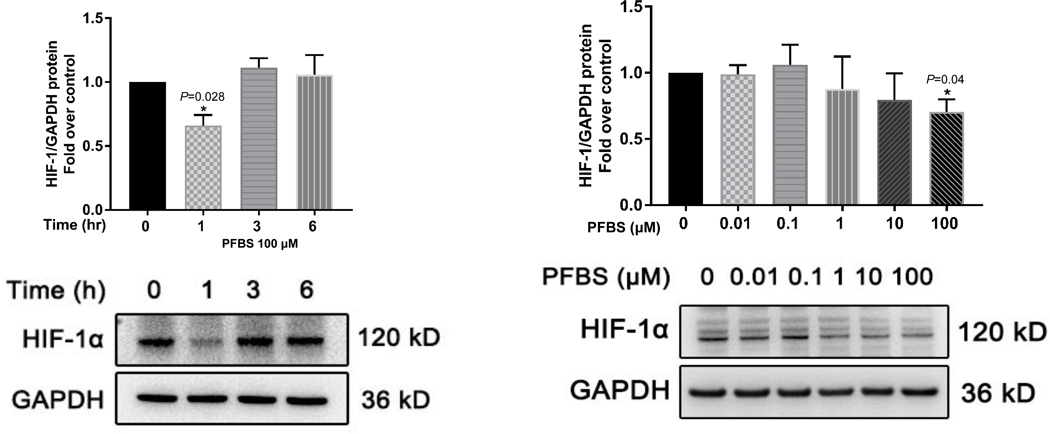
We first examined the time course of HIF-1α protein regulation in HTR-8/SVneo cells exposed to 100 μM PFBS. Treatment with PFBS for 1 h, but not 3 h or 6 h, significantly reduced HIF-1α protein levels (P=0.028 Fig 4A). We then examined the dose-responsiveness of HIF-1α protein regulation to 1 h exposure of PFBS at concentrations ranging from 0.01 μM to 100 μM PFBS. HIF-1α protein levels were mildly up-regulated by 0.1 μM PFBS exposure but not significant and significantly down-regulated by 100 μM PFBS exposure, as expected (P=0.04, Fig 4B). PFBS-induced regulation of HIF-1α appeared to correlate with the cell invasion activity. Specifically, 0.1 μM PFBS exposure increased HIF-1α production and invasion while 100 μM PFBS exposure decreased HIF-1α production and invasion.
Fig 4. PFBS significantly down-regulates hypoxia induced factor (HIF)-1α protein level in a time and dose-dependent fashion.
(4A) Protein levels of HIF-1α normalized to GAPDH and a representative Western blot image of HIF-1α and GAPDH in HTR-8/SVneo cells exposed to PFBS (100 µM) in 0, 1, 3, 6 h.
(4B) Protein levels of HIF-1α normalized to GAPDH and a representative Western blot image of HIF-1α and GAPDH in HTR-8/SVneo cells exposed to PFBS (0, 0.01, 0.1, 1, 10, 100 µM) in 1 h.
We then investigated the effects of PFBS exposure on the activation of AKT, ERK1/2, and P38 pathways, all of which have been previously reported to be related to trophoblast cell survival and cell invasion. In HTR-8/SVneo cells, phosphorylation of AKT, ERK1/2, and P38 usually reaches a maximum 5–15 min after stimulation. Treatment for 15 min with PFBS did not stimulate phosphorylation of AKT (P=0.9 for PFBS 0.1 mM [=100 μM]), P=0.09 for PFBS 10 mM, Supplementary Fig 2A and B); PFBS exposure did not induce phosphorylation of ERK1/2 (P=1.0 for PFBS 100 μM, P=0.9 for PFBS 10 mM, Supplementary Fig 2A and C). Exposure of cells to PFBS at 100 μM did not affect the phosphorylation of P38, but 10 mM PFBS treatment resulted in a significant activation (phosphorylation) of P38 (P=0.04, Supplementary Fig 2A and 2D). There were no differences in cleavage of Notch2 or Notch3 between the control and 100 μM PFBS-treated cells (P>0.1, Supplementary Fig 2E, 2F, and 2G). However, treatment with 10 mM PFBS resulted in a significant difference in Notch2 and Notch3 cleavage. We observed that the levels of cleaved-Notch2 and -Notch3 increased from 6 h to 18 h in both control and 100 μM PFBS-treated cells. This increased cleavage of Notch2 and Notch3 was diminished in 10 mM PFBS treated groups (P=0.05 for Notch2, P=0.005 for Notch3, Supplementary Fig 2E, 2F, and 2G).
Gene expression profiles in PFBS-treated cells
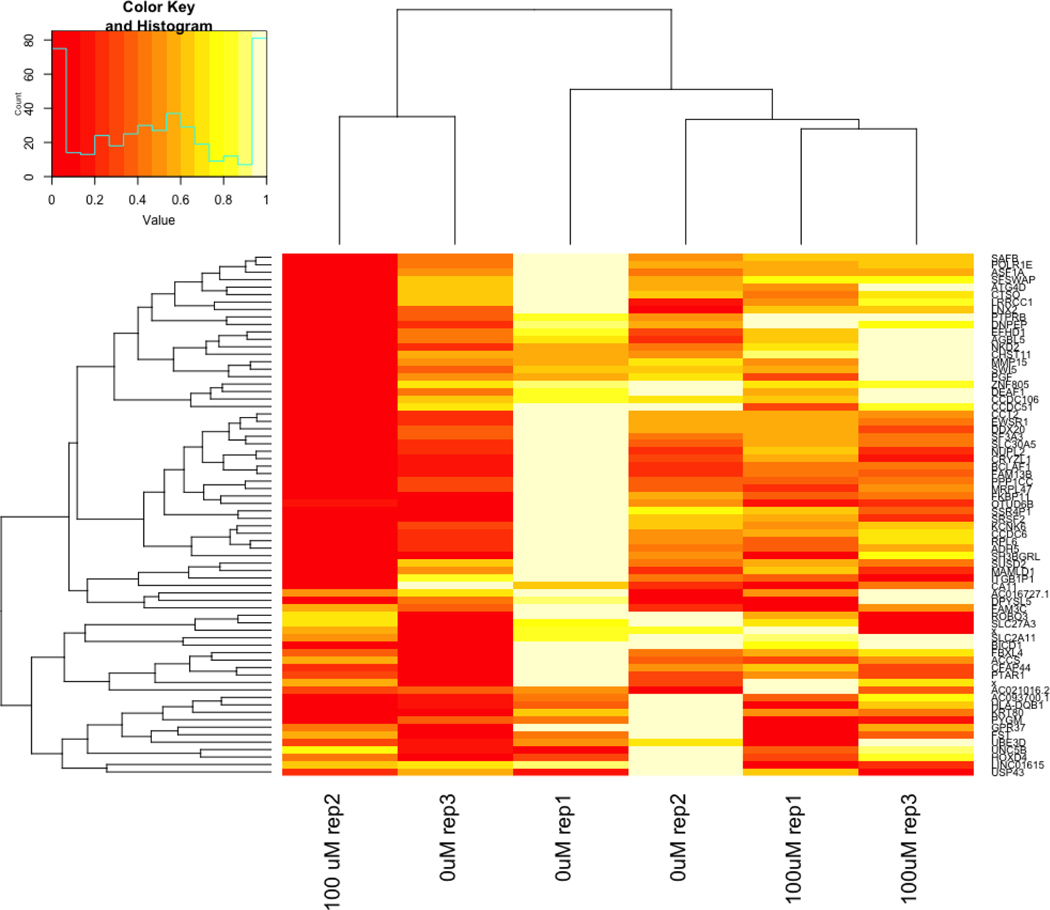
To further study the mechanisms affected by PFBS exposure, we performed a genome-wide mRNA-seq analysis of HTR-8/SVneo cells cultured in the absence (control) or presence of PFBS (100 μM) using a HiSeq 4000 Illumina sequencing platform. RNA-seq analysis identified seventy two significantly down-regulated genes and three overexpressed genes following PFBS treatment comparing to control cells (Table S2), the heatmap was presented in Fig 5. In particular, among the 72 genes, PFBS dysregulated the expression of 16 genes previously reported to be associated with preeclampsia. The functions of these genes are listed in Table 1.
Fig 5. RNA-seq data revealed dysregulated genes by PFBS in HTR8/SVneo cells.
A heatmap and clustering of differentially expressed genes in control (0 µM)) and PFBS (100 µM) treated cells.
Note: rep is replicate
Table 1.
The 16 dysregulated preeclampsia associated genes by PFBS
| Gene Name | Functions |
|---|---|
| ADMATS1 | associated with various inflammatory process |
| ADM | vasodilation, angiogenesis, antimicrobial |
| ANGPTL4 | angiogenesis, proliferation, migration, invasion |
| CNTF | survival factors for various neuronal cells |
| CXCL12 | vasculogenic actions, regulate trophoblast function and uterine spiral artery remodeling |
| DDX10 | embryogenesis, cellular growth and division |
| FGF5 | embryonic development, cell growth and invasion; associated with hypertension |
| FILIP1L | angiogenesis activity, cell proliferation and migration |
| IGFBP5 | suppress trophoblast cell migration and invasion in Preeclampsia |
| IL7R | Immune regulation |
| KCTD11 | growth and proliferation |
| PAPPA | Inflammation, wound healing, associated with preeclampsia, female infertility. |
| PSG4 | Immune regulation |
| RUNX1 | an regulator of hematopoiesis in placenta, associated with preterm birth |
| SERPINE1 | cellular differentiation, modulation of apoptosis and steroidogenesis, associated with preeclampsia |
| SNAI2 | Focal adhesion, associated with placenta development and function and preeclampsia. |
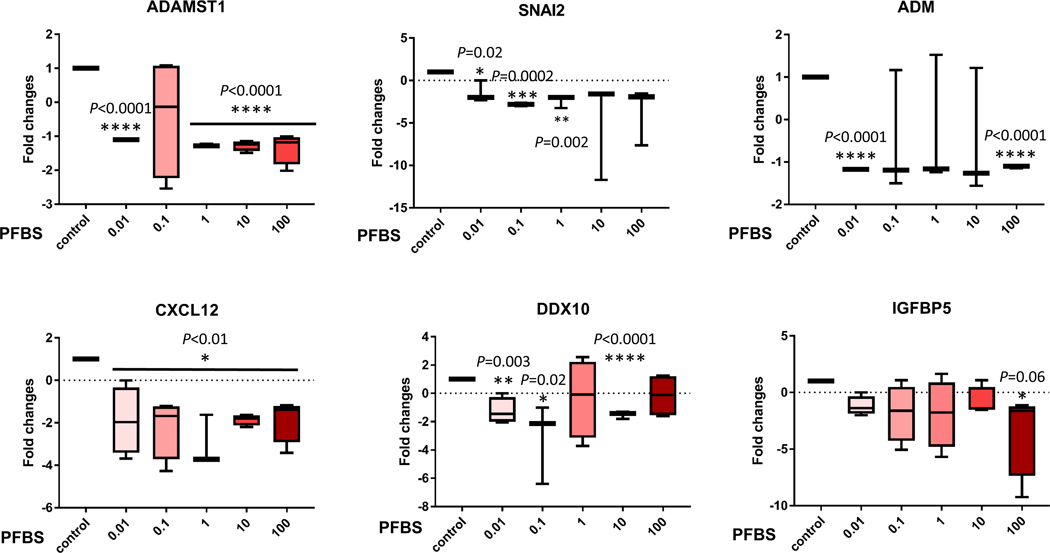
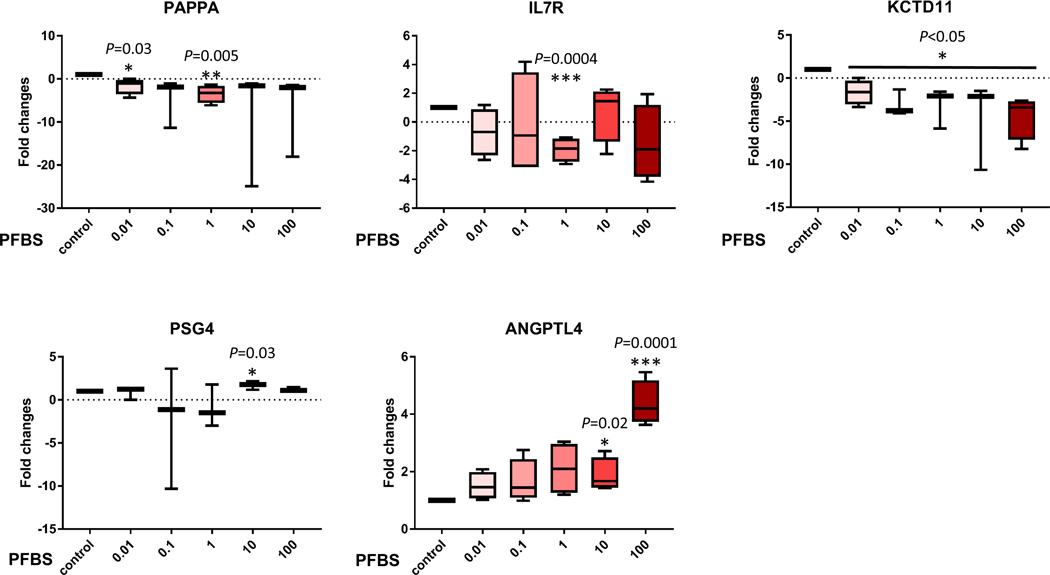
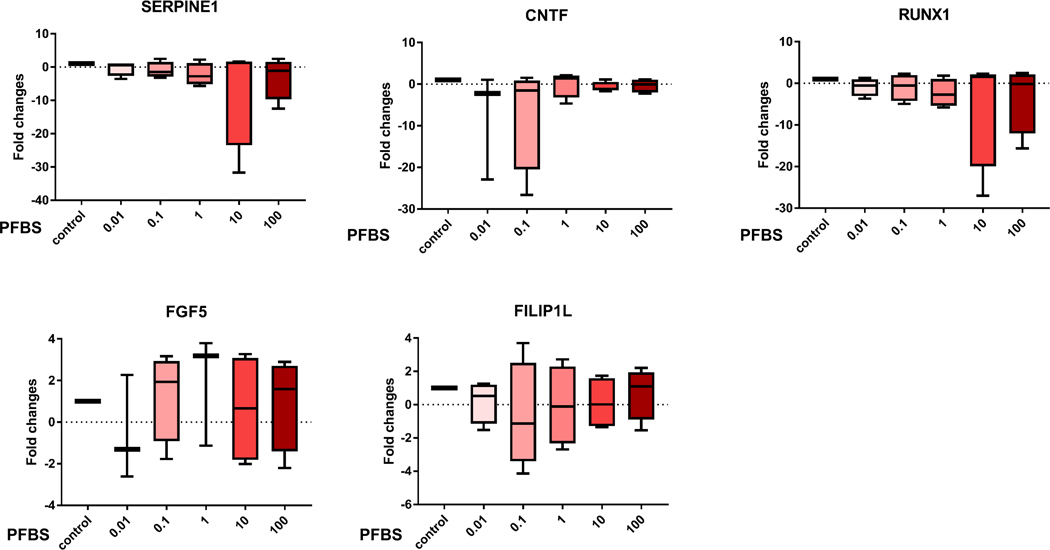
To confirm the gene chip data, we used quantitative real-time PCR to analyze transcripts of the 15 selected genes that are associated with preeclampsia. The expression patterns correlated with the RNA-seq profiling data (Fig 6). Eleven genes were confirmed to be significantly dysregulated by PFBS including Disintegrin and Metalloprotease Domains with Thrombospondins motifs (ADAMTS)1, Adrenomedullin (ADM), Snail Family Transcriptional Repressor 2 (SNAI2), Motif Chemokine Ligand 12 (CXCL12), DEAD-Box Helicase 10 (DDX10), Insulin Like Growth Factor Binding Protein 5 (IGFBP5), Pappalysin 1 (PAPPA), Interleukin 7 Receptor (IL7R), Potassium Channel Tetramerization Domain Containing 11 (KCTD11), Pregnancy Specific Beta-1-Glycoprotein 4 (PSG4), and Angiopoietin Like 4 (ANGPTL4). These genes are tightly linked to placental development and preeclampsia by regulating angiogenesis, cell proliferation and migration/invasion. In addition, these genes are HIF-1α targeted genes except PAPPA and PSG4.
Fig 6. qPCR data validated the dysregulation of genes identified by RNA-seq analyses.
Gene expression (fold changes) determined by quantitate-PCR analysis to compare the control (0 µM) and PFBS treatments (0.01, 0.1, 1, 10, 100 µM).
Discussion
Using a first trimester cell line, HTR-8/SVneo, we discovered that PFBS, an emerging environmental contaminant, can either promote cell growth or exert no influence on cell survival in a nM to μM dose range. PFBS exposure caused necrotic and apoptotic cell death at a concentration of 10 mM. These results demonstrate a low cytotoxic effect of PFBS on trophoblast cells. However, PFBS exposure in a μM dose range interrupted cell migration and invasion, a major cytotrophoblast cell function during early human placentation. We further demonstrated by Western blot that PFBS exposure in a μM dose range dysregulated HIF-1α expression, and RNA-seq analysis revealed genes relevant to angiogenesis, cell growth, and cell motility. Many of these genes are HIF-1α target genes. The expression of HIF-1α and the relevant genes are associated with preeclampsia (77, 78) and hypertension (79, 80). Taken together, these findings demonstrated that PBFS exposure interrupted trophoblast cell proliferation and invasion. The altered HIF-1α activation and gene expressions may provide a clue for the underlying mechanism.
Two major cell lineages of trophoblasts that arise during the early stages of human placental development are villous cytotrophoblasts (CTBs) and extravillous cytotrophoblasts (EVTs) (81). CTBs, trophoblast progenitor cells, follow one of the two existing differentiation pathways to form syncytiotrophoblasts (STBs) or EVTs. Endovascular and interstitial EVTs undergo epithelial–mesenchymal transition (EMT) to become motile and highly invasive cells (82, 83). Although primary cultures may be ideal for the study of trophoblast invasion, the limitations of these systems include the scarcity of first trimester placental tissue and low cell yield. Hence, trophoblastic cell lines have been widely used as surrogates to study EVT proliferation, invasion, and migration. Currently, two choriocarcinoma cell lines (JEG-3 and BeWo) and one EVT-derived cell line (HTR-8/SVneo) are commonly used. In the present study, we used HTR-8/SVneo cells, a standard in vitro model for early human placentation that has already been used to characterize the toxicity of diverse compounds such as Bisphenol A (BPA) (84), Methylmercury (85), atrazine, diethylstilbestrol (DES), and resveratrol (RES) (86) during gestation.
We investigated whether PFBS affects cytotrophoblast cell proliferation, migration, and invasion in vitro. We did not observe a cytotoxic effect of PFBS on HTR-8/SVneo cells until the 10 mM concentration was tested. This result is in agreement with reports by Gorrochategui (3) and Zhang (87), which suggest that in cytotrophoblast cells, shorter chain PFBS is less cytotoxic than the longer chain PFOS. The differences in chemical structures of PFOS and PFBS determine the differences in their water solubilities, which may affect their respective absorption by cells and hence cytotoxicity profile. PFBS is minimally absorbed by cytotrophoblast cells when compared to PFOS, which showed the highest intracellular concentration among eight PFASs tested in Gorrochategui’s study. It will be of scientific interest to further elucidate the cytotoxic mechanism of action of PFBS in HTR-8/SVneo cells; however, this is not relevant to public health as humans are unlikely to be exposed to high doses (10 mM).
Treatment of cells with PFBS at 10 or 100 μM decreased the HTR-8/SVneo cell migration and invasion, indicating that PFBS exposure resulted in the differentiation of HTR-8/SVneo cells from an invasive to a proliferative phenotype. A recent study using single-cell sequencing clearly demonstrated the upregulation of receptors, which are involved in invasion, in the process of CTBs differentiation into EVTs. In contrast, the expression of cell cycle genes decreased along the path from CTBs to EVTs and were no longer expressed towards the end of the trajectory that leads towards EVT differentiation nor where the EVT from the decidua are located (88). The simultaneous observations of increased cell proliferation and decreased cell invasion is in accordance with a previous study in BeWo and JAR cells (64). BeWo cell proliferation was dramatically increased, while migration and invasion were significantly inhibited, when Notch2 was downregulated. Similarly, JAR cell proliferation was significantly enhanced, but migration and invasion were suppressed, after Notch3 expression was silenced. Since these results mirrored our findings, we tested whether Notch signaling mediated the increased proliferation and decreased invasion following exposure of HTR-8/SVneo cells to 100 μM PFBS. Surprisingly, 100 μM PFBS exposure did not stimulate the Notch pathway in these cells. These results indicate that Notch signaling might not be involved in PFBS-induced inhibition of cell invasion.
In addition to the Notch pathway, hypoxia has been reported to regulate the phenotypes of EVTs in a similar fashion. Previous reports indicate that persistent hypoxia or failure to downregulate transforming growth factor β3 (TGF-β3) expression after nine weeks of gestation might result in failure of trophoblasts to differentiate from the proliferative to the invasive phenotype, thus resulting in shallow trophoblast invasion and malformation of placenta (89). Indeed, hypoxia-inducible factor (HIF)-1α, a marker of cellular oxygen deprivation, is expressed at high levels in trophoblasts. A growing body of literature supports HIF-1α as the molecular link between placental hypoxia and the downstream mediators of preeclampsia including endothelin-1 and endoglin (77, 90, 91). HIF-1 is a transcription factor complex stabilized under low oxygen tension to mediate cellular responses (92). HIF-1 is a heterodimeric protein consisting of the HIF-1β subunit that is constitutively active and the HIF-1α subunit that is rapidly inactivated and degraded by ubiquitination and subsequent passage via the proteasomal pathway, a process that is inhibited under hypoxic conditions (93). We observed that a decrease in HIF-1α expression correlated with a decrease in HTR-8/SVneo cell invasion following treatment with 10 or 100 μM PFBS.
In early pregnancy, EVTs form plugs that obstruct the maternal uterine spiral arteries and prevent maternal blood from quickly entering the intervillous space, which creates a physiologically low-oxygen environment (1–2%) (94, 95). Until around the 12th week of gestation, oxygen tension rises in the placenta after removal of the endovascular trophoblast plugs (96). This increasing oxygen level is an important signal for feto-placental development and trophoblast invasion (97). Although trophoblasts isolated from placental tissues have been reported to exhibit decreased invasiveness under low oxygen concentrations, such as 0.1% (Onogi et al. 2011) and 3% (Crocker et al. 2005), trophoblast-like cell lines, including HTR-8/SVneo and JEG3 cells, have been noted to exhibit increased invasiveness when exposed to 3% O2 (98) or 1% O2 (67). Therefore, the role of hypoxia in determining the invasive capacity of trophoblasts remains controversial and requires further elucidation. Our model supports the observation that decreased hypoxia conditions (i.e., lower HIF-1α expression) might limit trophoblast invasion. However, we understand that it is not ideal to study HIF-1 function under normoxia condition. The baseline level of HIF-1α could be due to some growth factors in the media or cell specific. Due to these limitations, further investigation is warrant.
Additional evidence of our observed phenomenon is the differentially regulated genes following exposure to 100 μM PFBS, as identified by RNA-seq analysis. These differentially regulated genes are involved in angiogenesis and cell growth and motility. ADAMTS play a role in events such as restructuring of tissue, coagulation, angiogenesis, degradation of the ECM and basal membrane, and tumoral cell invasion and metastasis (99). ADAMTS family proteases have been associated with reproductive disorders and pregnancy-related disorders such as preeclampsia including ADMATS1 (100, 101). ADM is a proangiogenic peptide hormone that is a potent vasodilator. Due to this property, ADM has been intensively studied in pregnancy and preeclampsia as a potential pathogenic factor in this disease (102–111). It is speculated to play a role in trophoblast implantation and angiogenesis (112). Indeed, we and others have shown that ADM mediates EVT growth, migration, invasion, and STB function (102, 108, 109). SNAI2 expression, stability and activity are under control of integrated and complex cellular signaling networks which can be furthermore affected by perturbations in the oxygen level (113, 114). Down-regulation of SNAI2 has been shown in preeclampsia (115). CXCL12 is a chemokine that are multifunctional with various functions including inflammatory response and angiogenesis. As an example, selective expression of CXCL12 in hypoxic tissues is associated with migration of adult stem cells recruitment and further tissue regeneration (116–118). Recently, it was reported that CXCL12 are variously expressed in preeclampsia (119). IGFBP5 serves as a cell survival and proliferation factor in cancers (120–123). Interestingly, IGFBP5 is localized in the syncytiotrophoblast layer of first trimester placental villi and attenuates the effects of insulin growth factor (IGF)-1 and IGF-2 on promoting HTR-8/SVneo cell migration (124). Additionally, IGFBP5 was over expressed in preeclamptic placenta (125). PAPPA is a placental glycoprotein which cleaves IGFBFs and positively regulating IGFs (126). PAPPA-mediated IGFs activity in very early pregnancy has been shown to be associated with pregnancy loss, hypertension, preeclampsia, preterm delivery, fetal growth restriction, and fetal death (127–132). Although the biological effects of ANGPTL4 in cancer cells are controversial, it has been shown to regulate cancer cell growth, angiogenesis, and metastasis (133). Recently, Liu et al. reported that ANGPTL4 might be associated with preeclampsia and promote HTR-8/SVneo cell proliferation and invasion (134). We determined that ANGPTL4 mRNA was specifically induced by treatment with 100 μM PFBS, which might be related to the regulation of cell growth and invasion following PFBS exposure. The promoted cell proliferation is consistent with the results from the Liu study, while the impeded cell invasion is in conflict with the study. Furthermore, these genes are directly or indirectly regulated by hypoxia and HIF-1α. Hatipoglu el at. found that ADAMTS1 is transiently induced by hypoxia in endothelial cells, and its transcription is mediated by HIF-1α binding (135). ADM promotes human endothelial cell proliferation via HIF-1α (136). SNAI2 belongs to a Snail family which is a direct target of HIF-1α in Hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells (137). IGF and IGFBPs are also regulated by HIF-1α in cancer cells to shift glucose metabolism from the more efficient oxidative phosphorylation to the less efficient glycolytic pathway in order to maintain their energy production (138). ANGPTL4 was reported to be induced by hypoxia/ischemia (139). Collectively, dysregulation of these genes and HIF-1α by PFBS exposure may mediate the disruption of HTR8/SVneo cell proliferation and motility. We observed a discrepancy between some genes expression and invasion phenotypes observed with exposure to different doses of PFBS. This could be that the invasion phenotype was the results of interactive regulation of genes instead of the regulation of individual gene.
In addition to the Notch and HIF-1α pathways, the Mitogen-Activated Protein Kinases (MAPKs) and Phosphoinositide 3-Kinase (PI3K)/AKT Signaling pathways are involved in trophoblast invasion and migration. MAPKs have four different families (140). Among them, ERK1/2 activation is known to mediate the regulation of HTR-8/SVneo cell invasion via numerous factors including endothelin (141), prostaglandin E2 (142), insulin-like growth factor-II (143), and epidermal growth factor (144). In particular, epidermal growth factor induces ECM remodeling through ERK1/2 and PI3K activation and promotes invasion in HTR-8/SVneo cells (145). However, no significant stimulation of these pathways was found in this current study.
Trophoblast invasion is a complex and tightly regulated process. For successful invasion, the trophoblast must perform a range of functions: transformation of the maternal spiral arteries, tolerance of hypoxia, cell survival, differentiation, adherence to and digestion of the extracellular matrix, and movement and interaction with the maternal immune system. Each of these functions has multiple overlapping control systems resulting in delicate balance among competing mechanisms. This sensitive and vulnerable physiological process is readily disturbed by chemical exposures. The newly identified detrimental effects of PFBS on trophoblast cell invasion are important first steps toward a comprehensive understanding of the toxicological effects of exposure to this emerging environmental chemical during pregnancy.
There are limitations to our present study. Due to the complexity of trophoblast invasion, our study is limited to in vitro observations and is unable to capture the functions of these cells in vivo. The dose range of PFBS (0.01–100 μM) was chosen by referencing cord blood levels of PFBS in previous in vitro studies including our study; these ranged from 0.009 to 1.48 ng/mL (0.027–4 μM) (57, 146–148). We included higher doses of PFBS in our current study after consideration of the following facts: 1) To date, no placental concentrations of PFBS have been reported in humans; 2) We and others found that PFBS does not internalized into the trophoblast cells in vitro (3), which seems not to be the case in vivo (149); 3) PFBS can bind to proteins in FBS which we used for cell cultures to reduce the free fraction of PFBS. Therefore, higher doses of PFBS are used in our study. We did not examine steroidogenesis using this cell model because steroidogenesis is more relevant to syncytiotrophoblasts. In fact, a recent study that examined steroidogenesis and the estrogen and androgen receptor activities in vitro suggested that PFBS does not exert endocrine effects (150).
Overall, our study is the first to examine how PFBS exerts its effects on human placental EVT cell function upon PFBS exposure. Our results highlight the ability of PFBS to alter placental EVT cell proliferation and invasion and relevant gene expressions and molecular pathways. The short chain PFBS, often considered one of the safe substitutes for PFOS, might be associated with adverse placentation. Future investigations in other cellular models and animal studies are warranted to understand additional effects of PFBS on placentas and birth outcomes.
Supplementary Material
Acknowledgements:
This work was supported by the Fogarty International Center of the National Institutes of Health under Award Number 1K01TW010828–01 (PI: Liping Feng) and the Ministry of Science and Technology of China under Award Number 2014CB943300 (PI: Jun J. Zhang). National Natural Science Foundation of China (81803246, PI: Rong Huang). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge Emi Yuan, a summer working student, performed the quantitate PCR to confirm RNA-seq results and Junjiao Song, a visiting scholar, performed Western Blot for HIF-1α.
Abbreviations
- PFAS
Poly- and per-fluoroalkyl substances
- PFOS
Perfluorooctane sulfonic acid
- PFBS
Perfluorobutane sulfonate
- BPA
Bisphenol A
- DES
Diethylstilbestrol
- RES
Resveratrol
- CTBs
Cytotrophoblasts
- EVTs
Extravillous cytotrophoblasts
- STBs
Syncytiotrophoblasts
- EMT
Epithelial–mesenchymal transition
- ECM
Extracellular matrix
- MTS
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- RT-PCR
Reverse transcription polymerase chain reaction
- PI
Propidium Iodide
- DAPI
4′,6-diamidino-2-phenylindole
- RIPA
Radioimmunoprecipitation assay
- FITC
Fluorescein isothiocyanate
- RIN
RNA Integrity Number
- USEPA
The United States Environmental Protection Agency
- MAPKs
Mitogen-Activated Protein Kinases
- PI3K
Phosphoinositide 3-Kinase
- TGF–β3
Transforming growth factor β3
References
- 1.Pelch KE, Reade A, Wolffe TAM, and Kwiatkowski CF (2019) PFAS health effects database: Protocol for a systematic evidence map. Environment international 130, 104851. [DOI] [PubMed] [Google Scholar]
- 2.Zushi Y, Hogarh JN, and Masunaga S (2012) Progress and perspective of perfluorinated compound risk assessment and management in various countries and institutes. Clean Technologies and Environmental Policy 14, 9–20 [Google Scholar]
- 3.Gorrochategui E, Perez-Albaladejo E, Casas J, Lacorte S, and Porte C (2014) Perfluorinated chemicals: differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol Appl Pharmacol 277, 124–130 [DOI] [PubMed] [Google Scholar]
- 4.Fromme H, Tittlemier SA, Volkel W, Wilhelm M, and Twardella D (2009) Perfluorinated compounds--exposure assessment for the general population in Western countries. International journal of hygiene and environmental health 212, 239–270 [DOI] [PubMed] [Google Scholar]
- 5.Parsons JR, Sáez M, Dolfing J, and de Voogt P (2008) Biodegradation of Perfluorinated Compounds. In Reviews of Environmental Contamination and Toxicology Vol 196 (Whitacre DM, ed) pp. 53–71, Springer US, New York, NY: [DOI] [PubMed] [Google Scholar]
- 6.Smart BE (1994) Characteristics of C-F Systems. In Organofluorine Chemistry: Principles and Commercial Applications (Banks RE, Smart BE, and Tatlow JC, eds) pp. 57–88, Springer US, Boston, MA [Google Scholar]
- 7.Giesy JP, and Kannan K (2002) Peer Reviewed: Perfluorochemical Surfactants in the Environment. Environmental Science & Technology 36, 146A–152A [DOI] [PubMed] [Google Scholar]
- 8.Giesy JP, and Kannan K (2001) Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environmental Science & Technology 35, 1339–1342 [DOI] [PubMed] [Google Scholar]
- 9.Kannan K, Tao L, Sinclair E, Pastva SD, Jude DJ, and Giesy JP (2005) Perfluorinated Compounds in Aquatic Organisms at Various Trophic Levels in a Great Lakes Food Chain. Archives of Environmental Contamination and Toxicology 48, 559–566 [DOI] [PubMed] [Google Scholar]
- 10.Houde M, Martin JW, Letcher RJ, Solomon KR, and Muir DCG (2006) Biological Monitoring of Polyfluoroalkyl Substances: A Review. Environmental Science & Technology 40, 3463–3473 [DOI] [PubMed] [Google Scholar]
- 11.Benskin JP, Phillips V, St. Louis VL, and Martin JW (2011) Source Elucidation of Perfluorinated Carboxylic Acids in Remote Alpine Lake Sediment Cores. Environmental Science & Technology 45, 7188–7194 [DOI] [PubMed] [Google Scholar]
- 12.Butt CM, Berger U, Bossi R, and Tomy GT (2010) Levels and trends of poly- and perfluorinated compounds in the arctic environment. Science of The Total Environment 408, 2936–2965 [DOI] [PubMed] [Google Scholar]
- 13.Campo J, Lorenzo M, Pérez F, Picó Y, Farré M. l., and Barceló D (2016) Analysis of the presence of perfluoroalkyl substances in water, sediment and biota of the Jucar River (E Spain). Sources, partitioning and relationships with water physical characteristics. Environmental Research 147, 503–512 [DOI] [PubMed] [Google Scholar]
- 14.Castiglioni S, Valsecchi S, Polesello S, Rusconi M, Melis M, Palmiotto M, Manenti A, Davoli E, and Zuccato E (2015) Sources and fate of perfluorinated compounds in the aqueous environment and in drinking water of a highly urbanized and industrialized area in Italy. Journal of Hazardous Materials 282, 51–60 [DOI] [PubMed] [Google Scholar]
- 15.Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S, and Lindström G (2007) Exposure of Perfluorinated Chemicals through Lactation: Levels of Matched Human Milk and Serum and a Temporal Trend, 1996–2004, in Sweden. Environmental Health Perspectives 115, 226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl T, Mattern D, and Brunn H (2011) Toxicology of perfluorinated compounds. Environmental Sciences Europe 23, 38 [Google Scholar]
- 17.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, and Seed J (2007) Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicological Sciences 99, 366–394 [DOI] [PubMed] [Google Scholar]
- 18.Houde M, De Silva AO, Muir DCG, and Letcher RJ (2011) Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review. Environmental Science & Technology 45, 7962–7973 [DOI] [PubMed] [Google Scholar]
- 19.Lindstrom AB, Strynar MJ, Delinsky AD, Nakayama SF, McMillan L, Libelo EL, Neill M, and Thomas L (2011) Application of WWTP Biosolids and Resulting Perfluorinated Compound Contamination of Surface and Well Water in Decatur, Alabama, USA. Environmental Science & Technology 45, 8015–8021 [DOI] [PubMed] [Google Scholar]
- 20.Chen T, Zhang L, Yue J. q., Lv Z. q., Xia W, Wan Y. j., Li Y. y., and Xu S. q. (2012) Prenatal PFOS exposure induces oxidative stress and apoptosis in the lung of rat off-spring. Reproductive Toxicology 33, 538–545 [DOI] [PubMed] [Google Scholar]
- 21.Conder JM, Hoke RA, Wolf W. d., Russell MH, and Buck RC (2008) Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environmental Science & Technology 42, 995–1003 [DOI] [PubMed] [Google Scholar]
- 22.Fromme H, Tittlemier SA, Völkel W, Wilhelm M, and Twardella D (2009) Perfluorinated compounds – Exposure assessment for the general population in western countries. International Journal of Hygiene and Environmental Health 212, 239–270 [DOI] [PubMed] [Google Scholar]
- 23.Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, and Stevenson LA (2003) Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicological sciences : an official journal of the Society of Toxicology 74, 382–392 [DOI] [PubMed] [Google Scholar]
- 24.Lau C, Butenhoff JL, and Rogers JM (2004) The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicology and applied pharmacology 198, 231–241 [DOI] [PubMed] [Google Scholar]
- 25.Grandjean P, Andersen E, Budtz-Jørgensen E, and et al. (2012) Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307, 391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorrochategui E, Pérez-Albaladejo E, Casas J, Lacorte S, and Porte C (2014) Perfluorinated chemicals: Differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicology and Applied Pharmacology 277, 124–130 [DOI] [PubMed] [Google Scholar]
- 27.Darrow LA, Stein CR, and Steenland K (2013) Serum Perfluorooctanoic Acid and Perfluorooctane Sulfonate Concentrations in Relation to Birth Outcomes in the Mid-Ohio Valley, 2005–2010. Environmental Health Perspectives 121, 1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein CR, Savitz DA, and Dougan M (2009) Serum Levels of Perfluorooctanoic Acid and Perfluorooctane Sulfonate and Pregnancy Outcome. American Journal of Epidemiology 170, 837–846 [DOI] [PubMed] [Google Scholar]
- 29.Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, and Goldman LR (2007) Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environmental health perspectives 115, 1670–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun R, Wu M, Tang L, Li J, Qian Z, Han T, and Xu G (2018) Perfluorinated compounds in surface waters of Shanghai, China: Source analysis and risk assessment. Ecotoxicology and environmental safety 149, 88–95 [DOI] [PubMed] [Google Scholar]
- 31.Ritter SK (2010) Fluorochemicals Go Short. Chem Eng News 88, 12–17 [Google Scholar]
- 32.Xie S, Lu Y, Wang T, Liu S, Jones K, and Sweetman A (2013) Estimation of PFOS emission from domestic sources in the eastern coastal region of China. Environment International 59, 336–343 [DOI] [PubMed] [Google Scholar]
- 33.Xie S, Wang T, Liu S, Jones KC, Sweetman AJ, and Lu Y (2013) Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environment International 52, 1–8 [DOI] [PubMed] [Google Scholar]
- 34.Renner R (2006) The long and the short of perfluorinated replacements. Environmental Science & Technology 40, 12–13 [DOI] [PubMed] [Google Scholar]
- 35.Zhao P, Xia X, Dong J, Xia N, Jiang X, Li Y, and Zhu Y (2016) Short- and long-chain perfluoroalkyl substances in the water, suspended particulate matter, and surface sediment of a turbid river. Science of The Total Environment 568, 57–65 [DOI] [PubMed] [Google Scholar]
- 36.Quinete N, Orata F, Maes A, Gehron M, Bauer KH, Moreira I, and Wilken RD (2010) Degradation studies of new substitutes for perfluorinated surfactants. Archives of environmental contamination and toxicology 59, 20–30 [DOI] [PubMed] [Google Scholar]
- 37.Quinete N, Orata F, Maes A, Gehron M, Bauer K-H, Moreira I, and Wilken R-D (2010) Degradation Studies of New Substitutes for Perfluorinated Surfactants. Archives of Environmental Contamination and Toxicology 59, 20–30 [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Cousins IT, Scheringer M, and Hungerbuehler K (2015) Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions. Environment International 75, 172–179 [DOI] [PubMed] [Google Scholar]
- 39.Rahman MF, Peldszus S, and Anderson WB (2014) Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review. Water research 50, 318–340 [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Li J, Zhang L, Huang Z, Liu Y, Wu N, He J, Zhang Z, Zhang Y, and Niu Z (2018) Perfluoroalkyl acids in drinking water of China in 2017: Distribution characteristics, influencing factors and potential risks. Environment international 123, 87–95 [DOI] [PubMed] [Google Scholar]
- 41.Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, and Darnerud PO (2012) Perfluorinated Alkyl Acids in Blood Serum from Primiparous Women in Sweden: Serial Sampling during Pregnancy and Nursing, And Temporal Trends 1996–2010. Environmental Science & Technology 46, 9071–9079 [DOI] [PubMed] [Google Scholar]
- 42.Fromme H, Tittlemier SA, Volkel W, Wilhelm M, and Twardella D (2009) Perfluorinated compounds—exposure assessment for the general population in western countries. Int J Hyg Environ Health 212 [DOI] [PubMed] [Google Scholar]
- 43.Gyllenhammar I, Benskin JP, Sandblom O, Berger U, Ahrens L, Lignell S, Wiberg K, and Glynn A (2018) Perfluoroalkyl Acids (PFAAs) in Serum from 2–4-Month-Old Infants: Influence of Maternal Serum Concentration, Gestational Age, Breast-Feeding, and Contaminated Drinking Water. Environmental science & technology 52, 7101–7110 [DOI] [PubMed] [Google Scholar]
- 44.Lou Q-Q, Zhang Y-F, Zhou Z, Shi Y-L, Ge Y-N, Ren D-K, Xu H-M, Zhao Y-X, Wei W-J, and Qin Z-F (2013) Effects of perfluorooctanesulfonate and perfluorobutanesulfonate on the growth and sexual development of Xenopus laevis. Ecotoxicology 22, 1133–1144 [DOI] [PubMed] [Google Scholar]
- 45.Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, and Stevenson LA (2003) Exposure to Perfluorooctane Sulfonate during Pregnancy in Rat and Mouse. II: Postnatal Evaluation. Toxicological Sciences 74, 382–392 [DOI] [PubMed] [Google Scholar]
- 46.Lau C, Butenhoff JL, and Rogers JM (2004) The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicology and Applied Pharmacology 198, 231–241 [DOI] [PubMed] [Google Scholar]
- 47.Feng X, Cao X, Zhao S, Wang X, Hua X, Chen L, and Chen L (2017) Exposure of Pregnant Mice to Perfluorobutanesulfonate Causes Hypothyroxinemia and Developmental Abnormalities in Female Offspring. Toxicological Sciences 155, 409–419 [DOI] [PubMed] [Google Scholar]
- 48.Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, and Seidler FJ (2008) Developmental Neurotoxicity of Perfluorinated Chemicals Modeled in Vitro. Environmental Health Perspectives 116, 716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corsini E, Sangiovanni E, Avogadro A, Galbiati V, Viviani B, Marinovich M, Galli CL, Dell’Agli M, and Germolec DR (2012) In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs). Toxicology and Applied Pharmacology 258, 248–255 [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y, Qin X-D, Zeng X-W, Paul G, Morawska L, Su M-W, Tsai C-H, Wang S-Q, Lee YL, and Dong G-H (2016) Associations of serum perfluoroalkyl acid levels with T-helper cell-specific cytokines in children: By gender and asthma status. Science of The Total Environment 559, 166–173 [DOI] [PubMed] [Google Scholar]
- 51.Naile JE, Wiseman S, Bachtold K, Jones PD, and Giesy JP (2012) Transcriptional effects of perfluorinated compounds in rat hepatoma cells. Chemosphere 86, 270–277 [DOI] [PubMed] [Google Scholar]
- 52.Wang B, Zhang R, Jin F, Lou H, Mao Y, Zhu W, Zhou W, Zhang P, and Zhang J (2017) Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environment International 102, 207–212 [DOI] [PubMed] [Google Scholar]
- 53.Chen F, Wei C, Chen Q, Zhang J, Wang L, Zhou Z, Chen M, and Liang Y (2018) Internal concentrations of perfluorobutane sulfonate (PFBS) comparable to those of perfluorooctane sulfonate (PFOS) induce reproductive toxicity in Caenorhabditis elegans. Ecotoxicology and environmental safety 158, 223–229 [DOI] [PubMed] [Google Scholar]
- 54.Song X, Tang S, Zhu H, Chen Z, Zang Z, Zhang Y, Niu X, Wang X, Yin H, Zeng F, and He C (2018) Biomonitoring PFAAs in blood and semen samples: Investigation of a potential link between PFAAs exposure and semen mobility in China. Environment international 113, 50–54 [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Lin Z, Hu M, Wang X, Lian Q, Lin K, Dong Q, and Huang C (2011) Perfluorinated chemicals in blood of residents in Wenzhou, China. Ecotoxicology and Environmental Safety 74, 1787–1793 [DOI] [PubMed] [Google Scholar]
- 56.Wang B, Chen Q, Shen L, Zhao S, Pang W, and Zhang J (2016) Perfluoroalkyl and polyfluoroalkyl substances in cord blood of newborns in Shanghai, China: Implications for risk assessment. Environment International 97, 7–14 [DOI] [PubMed] [Google Scholar]
- 57.Huang R, Chen Q, Zhang L, Luo K, Chen L, Zhao S, Feng L, and Zhang J (2019) Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environmental health : a global access science source 18, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gebbink WA, Bossi R, Rigét FF, Rosing-Asvid A, Sonne C, and Dietz R (2016) Observation of emerging per- and polyfluoroalkyl substances (PFASs) in Greenland marine mammals. Chemosphere 144, 2384–2391 [DOI] [PubMed] [Google Scholar]
- 59.Young BC, Levine RJ, and Karumanchi SA (2010) Pathogenesis of preeclampsia. Annu Rev Pathol 5, 173–192 [DOI] [PubMed] [Google Scholar]
- 60.Gupta SK, Malhotra SS, Malik A, Verma S, and Chaudhary P (2016) Cell Signaling Pathways Involved During Invasion and Syncytialization of Trophoblast Cells. American Journal of Reproductive Immunology 75, 361–371 [DOI] [PubMed] [Google Scholar]
- 61.Appel S, Ankerne J, Appel J, Oberthuer A, Mallmann P, and Dotsch J (2014) CNN3 Regulates Trophoblast Invasion and Is Upregulated by Hypoxia in BeWo Cells. PloS one 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szabo S, Mody M, Romero R, Xu Y, Karaszi K, Mihalik N, Xu Z, Bhatti G, Fule T, Hupuczi P, Krenacs T, Rigo J, Tarca AL, Hassan SS, Chaiworapongsa T, Kovalszky I, Papp Z, and Than NG (2015) Activation of Villous Trophoblastic p38 and ERK1/2 Signaling Pathways in Preterm Preeclampsia and HELLP Syndrome. Pathol Oncol Res 21, 659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hunkapiller NM, Gasperowicz M, Kapidzic M, Plaks V, Maltepe E, Kitajewski J, Cross JC, and Fisher SJ (2011) A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development (Cambridge, England) 138, 2987–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao W-X, Wu Z-M, Liu W, and Lin J-H (2017) Notch2 and Notch3 suppress the proliferation and mediate invasion of trophoblast cell lines. Biology Open 6, 1123–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arimoto-Ishida E, Sakata M, Sawada K, Nakayama M, Nishimoto F, Mabuchi S, Takeda T, Yamamoto T, Isobe A, Okamoto Y, Lengyel E, Suehara N, Morishige K, and Kimura T (2009) Up-regulation of alpha5-integrin by E-cadherin loss in hypoxia and its key role in the migration of extravillous trophoblast cells during early implantation. Endocrinology 150, 4306–4315 [DOI] [PubMed] [Google Scholar]
- 66.Zhang T, Kawaguchi N, Hayama E, Furutani Y, and Nakanishi T (2018) High expression of CXCR4 and stem cell markers in a monocrotaline and chronic hypoxia-induced rat model of pulmonary arterial hypertension. Experimental and therapeutic medicine 15, 4615–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Highet AR, Khoda SM, Buckberry S, Leemaqz S, Bianco-Miotto T, Harrington E, Ricciardelli C, and Roberts CT (2015) Hypoxia induced HIF-1/HIF-2 activity alters trophoblast transcriptional regulation and promotes invasion. European journal of cell biology 94, 589–602 [DOI] [PubMed] [Google Scholar]
- 68.Graham CH, Hawley TS, Hawley RC, MacDougall JR, Kerbel RS, Khoo N, and Lala PK (1993) Establishment and Characterization of First Trimester Human Trophoblast Cells with Extended Lifespan. Experimental Cell Research 206, 204–211 [DOI] [PubMed] [Google Scholar]
- 69.Jonkman JEN, Cathcart JA, Xu F, Bartolini ME, Amon JE, Stevens KM, and Colarusso P (2014) An introduction to the wound healing assay using live-cell microscopy. Cell Adhesion & Migration 8, 440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haiminen N, Kuhn DN, Parida L, and Rigoutsos I (2011) Evaluation of Methods for De Novo Genome Assembly from High-Throughput Sequencing Reads Reveals Dependencies That Affect the Quality of the Results. PloS one 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kersey PJ, Staines DM, Lawson D, Kulesha E, Derwent P, Humphrey JC, Hughes DS, Keenan S, Kerhornou A, Koscielny G, Langridge N, McDowall MD, Megy K, Maheswari U, Nuhn M, Paulini M, Pedro H, Toneva I, Wilson D, Yates A, and Birney E (2012) Ensembl Genomes: an integrative resource for genome-scale data from non-vertebrate species. Nucleic acids research 40, D91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Love MI, Huber W, and Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, Gottardo R, Hahne F, Hansen KD, Irizarry RA, Lawrence M, Love MI, MacDonald J, Obenchain V, Oles AK, Pages H, Reyes A, Shannon P, Smyth GK, Tenenbaum D, Waldron L, and Morgan M (2015) Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods 12, 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, and Groop LC (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature genetics 34, 267–273 [DOI] [PubMed] [Google Scholar]
- 76.Albini A (2016) Extracellular Matrix Invasion in Metastases and Angiogenesis: Commentary on the Matrigel “Chemoinvasion Assay”. Cancer Research 76, 4595–4597 [DOI] [PubMed] [Google Scholar]
- 77.Tal R (2012) The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biology of reproduction 87, 134. [DOI] [PubMed] [Google Scholar]
- 78.Rath G, Aggarwal R, Jawanjal P, Tripathi R, and Batra A (2016) HIF-1 Alpha and Placental Growth Factor in Pregnancies Complicated With Preeclampsia: A Qualitative and Quantitative Analysis. Journal of clinical laboratory analysis 30, 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bishop T, and Ratcliffe PJ (2015) HIF hydroxylase pathways in cardiovascular physiology and medicine. Circulation research 117, 65–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, and Semenza GL (1999) Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. The Journal of clinical investigation 103, 691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, and Fisher SJ (2004) Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. The Journal of clinical investigation 114, 744–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vicovac L, and Aplin JD (1996) Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat (Basel) 156, 202–216 [DOI] [PubMed] [Google Scholar]
- 83.DaSilva-Arnold SC, Zamudio S, Al-Khan A, Alvarez-Perez J, Mannion C, Koenig C, Luke D, Perez AM, Petroff M, Alvarez M, and Illsley NP (2018) Human trophoblast epithelial-mesenchymal transition in abnormally invasive placenta. Biology of reproduction 99, 409–421 [DOI] [PubMed] [Google Scholar]
- 84.Lan X, Fu LJ, Zhang J, Liu XQ, Zhang HJ, Zhang X, Ma MF, Chen XM, He JL, Li LB, Wang YX, and Ding YB (2017) Bisphenol A exposure promotes HTR-8/SVneo cell migration and impairs mouse placentation involving upregulation of integrin-beta 1 and MMP-9 and stimulation of MAPK and PI3K signaling pathways. Oncotarget 8, 51507–51521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tucker EK, and Nowak RA (2018) Methylmercury alters proliferation, migration, and antioxidant capacity in human HTR8/SV-neo trophoblast cells. Reproductive toxicology 78, 60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bechi N, Sorda G, Spagnoletti A, Bhattacharjee J, Vieira Ferro EA, de Freitas Barbosa B, Frosini M, Valoti M, Sgaragli G, Paulesu L, and Ietta F (2013) Toxicity assessment on trophoblast cells for some environment polluting chemicals and 17beta-estradiol. Toxicology in vitro : an international journal published in association with BIBRA 27, 995–1000 [DOI] [PubMed] [Google Scholar]
- 87.Zhang N, Wang WS, Li WJ, Liu C, Wang Y, and Sun K (2015) Reduction of progesterone, estradiol and hCG secretion by perfluorooctane sulfonate via induction of apoptosis in human placental syncytiotrophoblasts. Placenta 36, 575–580 [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Fan X, Wang R, Lu X, Dang YL, Wang H, Lin HY, Zhu C, Ge H, Cross JC, and Wang H (2018) Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell research 28, 819–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M, and Lye SJ (1999) Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. The Journal of clinical investigation 103, 1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nova A, Sibai BM, Barton JR, Mercer BM, and Mitchell MD (1991) Maternal plasma level of endothelin is increased in preeclampsia. American journal of obstetrics and gynecology 165, 724–727 [DOI] [PubMed] [Google Scholar]
- 91.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, and Granger JP (2001) Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension 37, 485–489 [DOI] [PubMed] [Google Scholar]
- 92.Haddad JJ (2002) Oxygen-sensing mechanisms and the regulation of redox-responsive transcription factors in development and pathophysiology. Respiratory research 3, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ziello JE, Jovin IS, and Huang Y (2007) Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 80, 51–60 [PMC free article] [PubMed] [Google Scholar]
- 94.Anin SA, Vince G, and Quenby S (2004) Trophoblast invasion. Human fertility 7, 169–174 [DOI] [PubMed] [Google Scholar]
- 95.James JL, Stone PR, and Chamley LW (2006) The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Human reproduction update 12, 137–144 [DOI] [PubMed] [Google Scholar]
- 96.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, and Burton GJ (2000) Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. The American journal of pathology 157, 2111–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hiden U, Eyth CP, Majali-Martinez A, Desoye G, Tam-Amersdorfer C, Huppertz B, and Ghaffari Tabrizi-Wizsy N (2018) Expression of matrix metalloproteinase 12 is highly specific for non-proliferating invasive trophoblasts in the first trimester and temporally regulated by oxygen-dependent mechanisms including HIF-1A. Histochem Cell Biol 149, 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lash GE, Hornbuckle J, Brunt A, Kirkley M, Searle RF, Robson SC, and Bulmer JN (2007) Effect of low oxygen concentrations on trophoblast-like cell line invasion. Placenta 28, 390–398 [DOI] [PubMed] [Google Scholar]
- 99.Tortorella MD, Malfait F, Barve RA, Shieh HS, and Malfait AM (2009) A review of the ADAMTS family, pharmaceutical targets of the future. Current pharmaceutical design 15, 2359–2374 [DOI] [PubMed] [Google Scholar]
- 100.Russell DL, Brown HM, and Dunning KR (2015) ADAMTS proteases in fertility. Matrix Biol 44–46, 54–63 [DOI] [PubMed] [Google Scholar]
- 101.Namli Kalem M, Kalem Z, Yuce T, and Soylemez F (2018) ADAMTS 1, 4, 12, and 13 levels in maternal blood, cord blood, and placenta in preeclampsia. Hypertension in pregnancy 37, 9–17 [DOI] [PubMed] [Google Scholar]
- 102.Li HS, Dakour J, Kaufman S, Guilbert LJ, Winkler-Lowen B, and Morrish DW (2003) Adrenomedullin is decreased in preeclampsia because of failed response to epidermal growth factor and impaired syncytialization. Hypertension 42, 895–900 [DOI] [PubMed] [Google Scholar]
- 103.Gratton RJ, Gluszynski M, Mazzuca DM, Nygard K, and Han VKM (2003) Adrenomedullin messenger ribonucleic acid expression in the placentae of normal and preeclamptic pregnancies. J Clin Endocr Metab 88, 6048–6055 [DOI] [PubMed] [Google Scholar]
- 104.Al-Ghafra A, Gude NM, Brennecke SP, and King RG (2006) Increased adrenomedullin protein content and mRNA expression in human fetal membranes but not placental tissue in pre-eclampsia. Molecular human reproduction 12, 181–186 [DOI] [PubMed] [Google Scholar]
- 105.Dikensoy E, Balat O, Pence S, Balat A, Cekmen M, and Yurekli M (2009) The Changes of Plasma Malondialdehyde, Nitric Oxide, and Adrenomedullin Levels in Patients with Preeclampsia. Hypertension in pregnancy 28, 383–389 [DOI] [PubMed] [Google Scholar]
- 106.Boc-Zalewska A, Seremak-Mrozikiewicz A, Barlik M, Kurzawinska G, and Drews K (2011) The possible role of adrenomedullin in the etiology of gestational hypertension and preeclampsia. Ginekologia polska 82, 178–184 [PubMed] [Google Scholar]
- 107.Boc-Zalewska A, Seremak-Mrozikiewicz A, Barlik M, Bogacz A, Przemyslaw M, Grzeskowiak E, and Drews K (2011) Adrenomedullin mRNA expression in placenta of preeclamptic women. Ginekologia polska 82, 585–591 [PubMed] [Google Scholar]
- 108.Beiswenger TR, Feng LP, Brown HL, Heine RP, Murtha AP, and Grotegut CA (2012) The Effect of Cigarette Smoke Extract on Trophoblast Cell Viability and Migration: The Role of Adrenomedullin. Reproductive sciences 19, 526–533 [DOI] [PubMed] [Google Scholar]
- 109.Kraus DM, Feng LP, Heine RP, Brown HL, Caron KM, Murtha AP, and Grotegut CA (2014) Cigarette Smoke-Induced Placental Adrenomedullin Expression and Trophoblast Cell Invasion. Reproductive sciences 21, 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lenhart PM, Nguyen T, Wise A, Caron KM, Herring AH, and Stuebe AM (2014) Adrenomedullin Signaling Pathway Polymorphisms and Adverse Pregnancy Outcomes. American journal of perinatology 31, 327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Whigham CA, MacDonald TM, Walker SP, Pritchard N, Hannan NJ, Hastie R, De Alwis N, Cannon P, Nguyen TV, Tong S, and Kaitu’u-Lino T (2019) Circulating adrenomedullin mRNA is decreased in women destined to develop term preeclampsia. Pregnancy Hypertension-an International Journal of Womens Cardiovascular Health 16, 16–25 [DOI] [PubMed] [Google Scholar]
- 112.Grill S, Rusterholz C, Zanetti-Dallenbach R, Tercanli S, Holzgreve W, Hahn S, and Lapaire O (2009) Potential markers of preeclampsia--a review. Reproductive biology and endocrinology : RB&E 7, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lopez-Novoa JM, and Nieto MA (2009) Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO molecular medicine 1, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nieto MA (2011) The ins and outs of the epithelial to mesenchymal transition in health and disease. Annual review of cell and developmental biology 27, 347–376 [DOI] [PubMed] [Google Scholar]
- 115.Fedorova L, Gatto-Weis C, Smaili S, Khurshid N, Shapiro JI, Malhotra D, and Horrigan T (2012) Down-regulation of the transcription factor snail in the placentas of patients with preeclampsia and in a rat model of preeclampsia. Reproductive biology and endocrinology : RB&E 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, and Penn MS (2003) Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362, 697–703 [DOI] [PubMed] [Google Scholar]
- 117.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, and Asahara T (2003) Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 107, 1322–1328 [DOI] [PubMed] [Google Scholar]
- 118.Wang C, Chen W, and Shen J (2018) CXCR7 Targeting and Its Major Disease Relevance. Frontiers in pharmacology 9, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Darakhshan S, Hassanshahi G, Mofidifar Z, Soltani B, and Karimabad MN (2019) CXCL9/CXCL10 angiostasis CXC-chemokines in parallel with the CXCL12 as an angiogenesis CXC-chemokine are variously expressed in pre-eclamptic-women and their neonates. Pregnancy hypertension 17, 36–42 [DOI] [PubMed] [Google Scholar]
- 120.Butt AJ, Dickson KA, McDougall F, and Baxter RC (2003) Insulin-like growth factor-binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. The Journal of biological chemistry 278, 29676–29685 [DOI] [PubMed] [Google Scholar]
- 121.Xu XL, Lee TC, Offor N, Cheng C, Liu A, Fang Y, Jhanwar SC, Abramson DH, and Cobrinik D (2010) Tumor-associated retinal astrocytes promote retinoblastoma cell proliferation through production of IGFBP-5. The American journal of pathology 177, 424–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Johnson SK, and Haun RS (2009) Insulin-like growth factor binding protein-5 influences pancreatic cancer cell growth. World journal of gastroenterology : WJG 15, 3355–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miyake H, Pollak M, and Gleave ME (2000) Castration-induced up-regulation of insulin-like growth factor binding protein-5 potentiates insulin-like growth factor-I activity and accelerates progression to androgen independence in prostate cancer models. Cancer research 60, 3058–3064 [PubMed] [Google Scholar]
- 124.Crosley EJ, Dunk CE, Beristain AG, and Christians JK (2014) IGFBP-4 and −5 are expressed in first-trimester villi and differentially regulate the migration of HTR-8/SVneo cells. Reproductive biology and endocrinology : RB&E 12, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nishizawa H, Pryor-Koishi K, Suzuki M, Kato T, Kogo H, Sekiya T, Kurahashi H, and Udagawa Y (2008) Increased levels of pregnancy-associated plasma protein-A2 in the serum of pre-eclamptic patients. Molecular human reproduction 14, 595–602 [DOI] [PubMed] [Google Scholar]
- 126.Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR 3rd, and Conover CA (1999) The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proceedings of the National Academy of Sciences of the United States of America 96, 3149–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, and Connor JM (2002) Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. The Journal of clinical endocrinology and metabolism 87, 1762–1767 [DOI] [PubMed] [Google Scholar]
- 128.Pihl K, Larsen T, Krebs L, and Christiansen M (2008) First trimester maternal serum PAPP-A, beta-hCG and ADAM12 in prediction of small-for-gestational-age fetuses. Prenatal diagnosis 28, 1131–1135 [DOI] [PubMed] [Google Scholar]
- 129.Barrett SL, Bower C, and Hadlow NC (2008) Use of the combined first-trimester screen result and low PAPP-A to predict risk of adverse fetal outcomes. Prenatal diagnosis 28, 28–35 [DOI] [PubMed] [Google Scholar]
- 130.Krantz D, Goetzl L, Simpson JL, Thom E, Zachary J, Hallahan TW, Silver R, Pergament E, Platt LD, Filkins K, Johnson A, Mahoney M, Hogge WA, Wilson RD, Mohide P, Hershey D, Wapner R, First Trimester Maternal Serum, B., and Fetal Nuchal Translucency Screening Study, G. (2004) Association of extreme first-trimester free human chorionic gonadotropin-beta, pregnancy-associated plasma protein A, and nuchal translucency with intrauterine growth restriction and other adverse pregnancy outcomes. American journal of obstetrics and gynecology 191, 1452–1458 [DOI] [PubMed] [Google Scholar]
- 131.De Villiers CP, Hedley PL, Placing S, Wojdemann KR, Shalmi AC, Carlsen AL, Rode L, Sundberg K, Tabor A, and Christiansen M (2017) Placental protein-13 (PP13) in combination with PAPP-A and free leptin index (fLI) in first trimester maternal serum screening for severe and early preeclampsia. Clinical chemistry and laboratory medicine 56, 65–74 [DOI] [PubMed] [Google Scholar]
- 132.Duan H, Zhao G, Xu B, Hu S, and Li J (2017) Maternal Serum PLGF, PAPPA, beta-hCG and AFP Levels in Early Second Trimester as Predictors of Preeclampsia. Clinical laboratory 63, 921–925 [DOI] [PubMed] [Google Scholar]
- 133.Tan MJ, Teo Z, Sng MK, Zhu P, and Tan NS (2012) Emerging roles of angiopoietin-like 4 in human cancer. Molecular cancer research : MCR 10, 677–688 [DOI] [PubMed] [Google Scholar]
- 134.Liu L, Zhuang X, Jiang M, Guan F, Fu Q, and Lin J (2017) ANGPTL4 mediates the protective role of PPARgamma activators in the pathogenesis of preeclampsia. Cell death & disease 8, e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hatipoglu OF, Hirohata S, Cilek MZ, Ogawa H, Miyoshi T, Obika M, Demircan K, Shinohata R, Kusachi S, and Ninomiya Y (2009) ADAMTS1 is a unique hypoxic early response gene expressed by endothelial cells. The Journal of biological chemistry 284, 16325–16333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen L, Qiu JH, Zhang LL, and Luo XD (2012) Adrenomedullin promotes human endothelial cell proliferation via HIF-1alpha. Molecular and cellular biochemistry 365, 263–273 [DOI] [PubMed] [Google Scholar]
- 137.Xu X, Tan X, Tampe B, Sanchez E, Zeisberg M, and Zeisberg EM (2015) Snail Is a Direct Target of Hypoxia-inducible Factor 1alpha (HIF1alpha) in Hypoxia-induced Endothelial to Mesenchymal Transition of Human Coronary Endothelial Cells. The Journal of biological chemistry 290, 16653–16664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Masoud GN, and Li W (2015) HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5, 378–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koyama S, Ohtani K, Fukuzawa J, Yao N, Fukuda M, Jang SJ, Hasebe N, Kikuchi K, Itabe H, Yoshida I, Suzuki Y, and Wakamiya N (2011) The induction of human CL-P1 expression in hypoxia/reoxygenation culture condition and rat CL-P1 after ischemic/reperfusion treatment. Biochimica et biophysica acta 1810, 836–842 [DOI] [PubMed] [Google Scholar]
- 140.Kyriakis JM, and Avruch J (2001) Mammalian Mitogen-Activated Protein Kinase Signal Transduction Pathways Activated by Stress and Inflammation. Physiological Reviews 81, 807–869 [DOI] [PubMed] [Google Scholar]
- 141.Chakraborty C, Barbin YP, Chakrabarti S, Chidiac P, Dixon SJ, and Lala PK (2003) Endothelin-1 promotes migration and induces elevation of [Ca2+]i and phosphorylation of MAP kinase of a human extravillous trophoblast cell line. Molecular and Cellular Endocrinology 201, 63–73 [DOI] [PubMed] [Google Scholar]
- 142.Nicola C, Chirpac A, Lala PK, and Chakraborty C (2008) Roles of Rho Guanosine 5′-Triphosphatase A, Rho Kinases, and Extracellular Signal Regulated Kinase (1/2) in Prostaglandin E2-Mediated Migration of First-Trimester Human Extravillous Trophoblast. Endocrinology 149, 1243–1251 [DOI] [PubMed] [Google Scholar]
- 143.McKinnon T, Chakraborty C, Gleeson LM, Chidiac P, and Lala PK (2001) Stimulation of Human Extravillous Trophoblast Migration by IGF-II Is Mediated by IGF Type 2 Receptor Involving Inhibitory G Protein(s) and Phosphorylation of MAPK. The Journal of Clinical Endocrinology & Metabolism 86, 3665–3674 [DOI] [PubMed] [Google Scholar]
- 144.Qiu Q, Yang M, Tsang BK, and Gruslin A (2004) Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. MHR: Basic science of reproductive medicine 10, 677–684 [DOI] [PubMed] [Google Scholar]
- 145.Qiu Q, Yang M, Tsang BK, and Gruslin A (2004) EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction 128, 355–363 [DOI] [PubMed] [Google Scholar]
- 146.Zhang W, Lin Z, Hu M, Wang X, Lian Q, Lin K, Dong Q, and Huang C (2011) Perfluorinated chemicals in blood of residents in Wenzhou, China. Ecotoxicology and environmental safety 74, 1787–1793 [DOI] [PubMed] [Google Scholar]
- 147.Wang B, Zhang R, Jin F, Lou H, Mao Y, Zhu W, Zhou W, Zhang P, and Zhang J (2017) Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environment international 102, 207–212 [DOI] [PubMed] [Google Scholar]
- 148.Wang B, Chen Q, Shen L, Zhao S, Pang W, and Zhang J (2016) Perfluoroalkyl and polyfluoroalkyl substances in cord blood of newborns in Shanghai, China: Implications for risk assessment. Environment international 97, 7–14 [DOI] [PubMed] [Google Scholar]
- 149.Wang Y, Han W, Wang C, Zhou Y, Shi R, Bonefeld-Jorgensen EC, Yao Q, Yuan T, Gao Y, Zhang J, and Tian Y (2019) Efficiency of maternal-fetal transfer of perfluoroalkyl and polyfluoroalkyl substances. Environmental science and pollution research international 26, 2691–2698 [DOI] [PubMed] [Google Scholar]
- 150.Behr AC, Lichtenstein D, Braeuning A, Lampen A, and Buhrke T (2018) Perfluoroalkylated substances (PFAS) affect neither estrogen and androgen receptor activity nor steroidogenesis in human cells in vitro. Toxicology letters 291, 51–60 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.