Abstract
Background and Objectives
The Boston Puerto Rican Health Study (BPRHS) is a longitudinal study following self-identified Puerto Rican older adults living in the Greater Boston area. Studies have shown higher prevalence of hypertension (HTN) and type 2 diabetes (T2D) within this ethnic group compared to age-matched non-Hispanic White adults. In this study, we investigated the associations of HTN and T2D comorbidity on brain structural integrity and cognitive capacity in community-dwelling Puerto Rican adults and compared these measures with older adult participants (non-Hispanic White and Hispanic) from the Alzheimer's Disease Neuroimaging Initiative (ADNI) and National Alzheimer's Coordinating Center (NACC) databases.
Methods
BPRHS participants who underwent brain MRI and cognitive testing were divided into 4 groups based on their HTN and T2D status: HTN−/T2D−, HTN+/T2D−, HTN−/T2D+, and HTN+/T2D+. We assessed microstructural integrity of white matter (WM) pathways using diffusion MRI, brain macrostructural integrity using hippocampal volumes, and brain age using T1-weighted MRI and cognitive test scores. BPRHS results were then compared with results from non-Hispanic White and Hispanic participants from the ADNI and NACC databases.
Results
The prevalence of HTN was almost 2 times (66.7% vs 38.7%) and of T2D was 5 times (31.8% vs 6.6.%) higher in BPRHS than in ADNI non-Hispanic White participants. Diffusion MRI showed clear deterioration patterns in major WM tracts in the HTN+/T2D+ group and, to a lesser extent, in the HTN+/T2D− group compared to the HTN−/T2D− group. HTN+/T2D+ participants also had the smallest hippocampal volume and larger brain aging deviations. Trends toward lower executive function and global cognitive scores were observed in HTN+/T2D+ relative to HTN−/T2D− individuals. MRI measures and the Mini-Mental State Examination (MMSE) scores from the HTN+/T2D+ BPRHS group resembled those of ADNI White participants with progressive mild cognitive impairment (MCI), while the BPRHS HTN−/T2D− participants resembled participants with stable MCI. The BPRHS was not significantly different from the ADNI + NACC Hispanic cohort on imaging or MMSE measures.
Discussion
The effects of T2D and HTN comorbidity led to greater brain structural disruptions than HTN alone. The high prevalence of HTN and T2D in the Puerto Rican population may be a key factor contributing to health disparities in cognitive impairment in this group compared to non-Hispanic White adults in the same age range.
Trial Registration Information
ClinicalTrials.gov identifier: NCT01231958.
Hypertension (HTN) and type 2 diabetes (T2D) are the most common chronic diseases among older adults in the United States. Recent reports from the Centers for Disease Control and Prevention suggest that the prevalence of HTN is relatively equal between non-Hispanic White (43.6%) and Hispanic (43.7%) adult Americans, while T2D is more prevalent among Hispanic/Latino (17%) compared to non-Hispanic White (8%) adults in the United States.1,2 Although Hispanic individuals constitute >18% of the US population, they are underrepresented in large-scale epidemiologic studies and have usually been treated as a whole group instead of subgroups from different backgrounds (Puerto Rican, Cuban, Mexican, etc).3 An ongoing longitudinal study of 1,500 self-identified Puerto Rican older adults living in the Greater Boston area, the Boston Puerto Rican Health Study (BPRHS), has reported unique health profiles of this ethnic group. Notably, baseline prevalence of several health risk factors and disease conditions was high in this cohort (age 45–75 years at baseline). Almost 40% of BPRHS participants had T2D, 69% had HTN, and 57% were obese, considerably higher than in the general population.4 Findings have consistently shown a higher burden of adverse cardiovascular conditions in self-identified Puerto Rican than non-Hispanic White or Hispanic adults from other backgrounds (i.e., Mexican Americans).5
HTN, T2D, and other cardiometabolic risk factors have been implicated in accelerated cognitive aging. Mounting evidence supports that patients with T2D or HTN perform significantly lower on cognitive tests and may have higher risk of developing mild cognitive impairment (MCI) and Alzheimer disease (AD).6-8 Large-scale longitudinal neuroimaging databases such as the Alzheimer's Disease Neuroimaging Initiative (ADNI) and National Alzheimer's Coordinating Center (NACC) have enabled the exploration of brain atrophy and cognitive decline in relation to health risk factors in large samples across multiple time points. While most studies have examined the effects of T2D and HTN separately, the joint effect of both conditions on cognitive aging is less well characterized. These conditions are highly interrelated; up to 75% of adults with T2D also have HTN.9 An early study showed that participants with both conditions declined at a faster rate (determined by Mini-Mental State Examination [MMSE]) than those with only 1 condition.10 T2D combined with additional cardiovascular risk factors (e.g., HTN, high serum cholesterol, obesity, smoking) further elevates the risk of dementia, as shown in recent large-scale population-based studies.11,12 The relationship between T2D and HTN comorbidity and cognitive decline can be attributed to abnormal structural changes of the brain commonly found in patients with MCI and AD.13,14 However, most of these studies were with non-Hispanic White cohorts (or with unspecified ethnicity) and thereby do not reflect potential environmental, genetic, ethnic, or multicultural variabilities. A recent report from the Alzheimer's Association suggests that the risk of AD is 1.5 times higher in Hispanic than non-Hispanic White adults >65 years of age, largely accounted for by nongenetic factors (e.g., health-related behaviors, socioeconomic risk factors).15 More important, there is evidence that the prevalence of MCI and AD may differ across Hispanic ethnic groups with different geographic backgrounds.16,17 Therefore, more studies within this ethnic minority group may help to explain the co-occurring high prevalence of T2D, HTN, and cognitive impairment and ultimately reduce health disparities and improve the overall quality of life of this community.
In the current study, we used an analytical framework to investigate the potential impact of HTN and T2D comorbid conditions on cognitive aging within a unique community-dwelling sample of Puerto Rican adults from the Greater Boston area using a combination of biomarkers from different domains. Cross-ethnic comparison was done using shared databases containing age-matched cohorts with diverse ethnic backgrounds, i.e., ADNI and NACC. We identified HTN and T2D status and modeled quantitative associations with cognitive and MRI measures at ≈10-year follow-up of the BPRHS cohort to test the hypothesis that the comorbidity of HTN and T2D disrupts both microstructural white matter (WM) and macrostructural gray matter (GM) integrity of the brain, leading to greater age-related cognitive decline.
Methods
Study Cohort Description
The BPRHS is a longitudinal study initiated in 2004 with the primary goal of studying the effect of psychosocial stress on various health outcomes.4 Eligible participants from the BPRHS were recruited from areas of high Hispanic density in the Boston metropolitan area. All participants self-identified as Puerto Rican descent and were recruited through various methods, including door-to-door enumeration, community outreach, and referrals. Participants must self-identify as Puerto Rican, must be able to answer questions in English or Spanish, must be between 45 and 75 years of age, and must live in the Boston, MA, metropolitan region at the time of the study. The BPRHS protocol did not include collecting information on race. A total of 2,084 eligible participants were identified. Of these, 1,500 participants were invited and completed the baseline interviews.
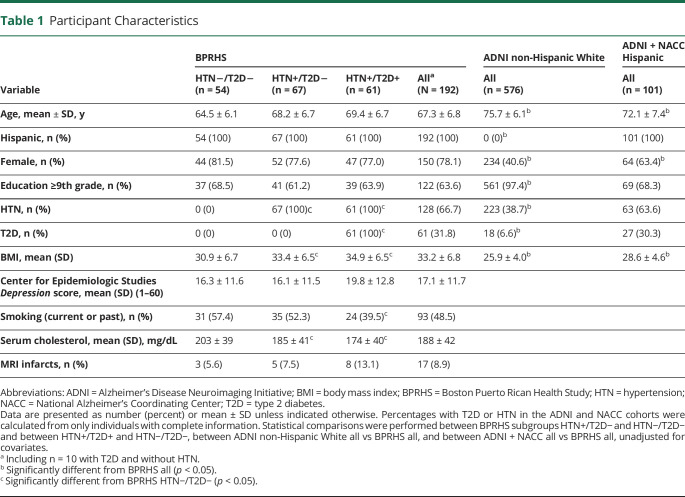
After baseline, participants were interviewed again at 2 years (n = 1,250), 5 years (n = 850), and ≈10 years (n = 550). Exclusion criteria included inability to answer questions due to serious health conditions, plans to move from the area, or MMSE score ≤10. All of the 550 participants were invited to have an MRI; of these, 59 refused, 5 were not cleared due to missing medical information, 14 were not able to complete MRI scanning, and 32 were ineligible due to medical conditions. We are continuing to recruit the remainder. At the time of writing, we have completed 192 MRIs. Multimodal MRI scans were obtained during the 10-year follow-up, performed on a 3T GE Signa HDx scanner (GE Healthcare, Chicago, IL) in Beth Israel Deaconess Medical Center. The previous report from BPRHS provides more details on the characteristics of this cohort.4 For the current analysis, we included n = 192 participants (age 55–87 years at 10-year visit, mean 67.4 years) with both MRI and cognitive assessment (Table 1).
Table 1.
Participant Characteristics
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the University of Massachusetts Lowell Institutional Review Board (No. 17-143). Both the Tufts and Beth Israel Deaconess Medical Center Institutional Review Boards ceded review to University of Massachusetts Lowell. Boston University determined that their roles were not considered human research. Written informed consent was obtained from all participants in the study. Authorization has been obtained for disclosure of any recognizable persons in photographs, videos, or other information that may be published in the journal, in derivative works by the American Academy of Neurology, or on the journal's website (when applicable). The clinical trials registration number for the parent study (BPRHS) is NCT01231958.
HTN and T2D
HTN was defined as systolic blood pressure (BP) ≥140 mm Hg or diastolic BP ≥90 mm Hg (confirmed at 2 or 3 different visit sessions) or use of antihypertensive medication.18 BP was measured following the International Society of Hypertension Global HTN practice guideline. T2D was determined by use of medication for diabetes or fasting blood glucose concentration ≥126 mg/dL on at least 2 different visits. We categorized participants into 4 groups: (1) HTN−/T2D− (n = 54), individuals without HTN or T2D; (2) HTN+/T2D− (n = 67), those with only HTN; (3) HTN−/T2D+ (n = 10), individuals with only T2D; and (4) HTN+/T2D+ (n = 61), individuals with both HTN and T2D. The HTN−/T2D+ participants were excluded from further analyses due to small sample size.
BPRHS Cognitive Assessment
Cognitive function was assessed with a comprehensive battery of neuropsychological tests administered in the participant's preferred language (98% in Spanish) by a neuropsychologist-trained research assistant at baseline and 2 and 10 years. Tests included those from a cognitive battery designed and normed for a US Spanish-speaking population: a 16-word list learning test for verbal memory; the Stroop test for mental processing speed; the digit span forward and backward tests for attention and working memory; and verbal fluency for executive function with language.19 Our battery also included figure copying and clock drawing for visuospatial function and the MMSE as a measure of general function.20-22 We calculated a global cognitive score (GCS) by averaging the z scores for each of the cognitive scores generated from the tests. In addition, cognitive domain composite scores were derived through principal components analysis using the PROC FACTOR procedure in SAS (version 9.4; SAS Institute, Cary, NC). Two factors were identified and labeled executive and memory functions. A score for each factor was calculated by summing the test scores weighted by the factor loading. The GCS, executive, and memory scores from the 8-year follow-up were used in the current analyses, as well as the MMSE score for comparison with the ADNI cohort.
MRI and Cognitive Data From ADNI
For the ADNI-1 non-Hispanic White cohort, MRI and cognitive data for 576 non-Hispanic White participants (age 60–90 years, mean 75.7) were retrieved from the ADNI-1 database as the following: (1) cognitively normal controls (CN), n = 178; (2) those with MCI diagnosis for at least 5 years (MCI nonconverter [MCInc]), n = 63; (3) those with MCI at baseline who converted to AD during 5 years of follow-up (MCI converter [MCIc]), n = 173; and (4) those with AD at baseline, n = 162. Diabetes status for ADNI was determined as described for the BPRHS cohort.
For the ADNI + NACC Hispanic cohort, MRI and cognitive data were obtained from the ADNI-1 and NACC databases. We retrieved baseline cognitive and MRI data from 101 Hispanic individuals (age 47–95 years, mean 72.1 years) from these datasets.
Image Acquisition and Processing
T1-Weighted MRI
For BPRHS, structural imaging data were obtained with a GE 3T MRI scanner for each participant with the following imaging parameters: repetition time (TR) 7.6 milliseconds, echo time (TE) 3 milliseconds, flip angle (FA) 8°, inversion time (TI) 900 milliseconds, slice thickness (ST) 1.0 mm, total slices (TL) 164, field of view (FOV) 25.6 cm, and in-plane matrix 256 × 256. For ADNI, MRI was performed at 1.5T with a T1-weighted 3-dimensional magnetization-prepared rapid gradient echo sequence.23 The image parameters were TR/TE/TI 2,400/3/1,000 milliseconds, FA 8°, FOV 24 cm, 192 × 192 in-plane matrix, and ST 1.2 mm. Processing was performed on the downloaded images with FreeSurfer version 6.0. This includes (1) image reorientation, (2) cropping, (3) skull stripping, (4) image normalization to the Montreal Neurological Institute standard space, and (5) cortical parcellation.24 Detailed automated hippocampal segmentation was included in our processing pipeline with FreeSurfer version 6.0.25 From the above procedures, we extracted volumes of the left and right hippocampus and total intracranial volume.
Brain Age Modeling
From each participant's T1-weighted structural MRI scan, we extracted 1,118 brain imaging features, including cortical thickness, area, volume, and cerebellar-subcortical and cortical summary statistics, which were used to estimate brain age via a machine learning model.26 This model was previously established with 45,615 individuals (age 3–96 years of age) from multiple imaging databases and then tested on participants in the current study to generate brain age. The raw brain age deviation score (calculated as chronologic age − brain age) was used to estimate the rate of biological brain aging relative to chronologic age. The raw deviation values were then transformed to z scores to account for variations of age and sex between the training and testing cohorts.
Diffusion MRI
Diffusion MRI data were obtained from participants, including 49 gradient directions for b values of 1,000 and 2,000 s/mm2 and 60 gradient directions for b value of 3,000 s/mm2 (TR 2,650 milliseconds, TE 69.2 milliseconds, FA 90°, ST 2 mm, TL 69, FOV 24.0, matrix 120 × 120, multiband acceleration factor 3, arc accerleration factor 2). Microstructural diffusion measures were reconstructed from multishell diffusion MRI images containing 3 b value encodings using neurite density imaging (NDI).27,28 From the NDI model, we extracted the neurite density index that measures the fraction of tissue composed of neurites, which include axons/dendrites and tissue other than neurites. All NDI data were projected to a common space using the individual's fractional anisotropy images for nonlinear registration and skeletonization. NDI measures were extracted from 20 WM regions of interests according to the human diffusion tensor imaging tractography parcellation atlas.29
Statistical Analysis
Data within the BPRHS cohort were analyzed with MATLAB (version 2019b, MathWorks, Natick, MA). We conducted pairwise comparisons of imaging (hippocampal volume, brain age, and NDI), cognitive measures (MMSE score, executive, memory, GCS), and several demographic characteristics between the HTN−/T2D− group and the HTN+/T2D− and HTN+/T2D+ groups. Comparisons of demographic variables were conducted with either independent t tests (for continuous variables) or χ2 tests (for categorical variables) unadjusted for covariates. Analyses of neuroimaging and cognitive measures were conducted using multiple regression models adjusted for age and sex, as well as for total intracranial volume for hippocampal volume analyses (model 1). Because low education is common in the BPRHS cohort and may influence cognitive test performance, we additionally considered educational attainment (model 2). The relationship between cognitive function and each imaging measure was examined with Spearman nonparametric partial correlation coefficients (due to the skewed distribution of the cognitive scores) adjusted for age, sex, and education level. Significant features from group-level comparisons and correlation analyses are defined as p < 0.05 after correction for multiple comparisons using the Bonferroni-Holm30 method.
We conducted pairwise comparisons of hippocampal volume, brain age z score, and MMSE scores to compare BPRHS results with each ADNI-1 non-Hispanic White group (CN, MCInc, MCIc, AD) and with ADNI + NACC Hispanic individuals, including the same covariates and Bonferroni-Holm corrections. We additionally included different scanner effects (3T or 1.5T MRI) as covariates for analyses involving imaging measures.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Study Population Characteristics
Within the BPRHS cohort, comparison across the 3 groups showed that those in the HTN+/T2D− and HTN+/T2D+ groups had significantly higher average body mass index (BMI) and higher serum cholesterol compared to those in the HTN−/T2D− (p < 0.05) (Table 1). There were no significant differences between the 3 groups in sex ratio, education level, Center for Epidemiologic Studies Depression score, or MRI infarcts. Compared with the ADNI-1 non-Hispanic White cohort, the BPRHS cohort had significantly lower mean age, higher percentage of females, lower average education level, higher prevalence of HTN and T2D, and higher mean BMI. The prevalence of HTN was almost 2 times and that of T2D was nearly 5 times higher in the BPRHS cohort compared to the ADNI non-Hispanic White cohort. In contrast, the BPRHS cohort and the ADNI + NACC Hispanic cohort were significantly different only in age, sex ratio, and BMI.
Associations of Comorbid T2D and HTN With Brain WM Connectional Loss in BPRHS
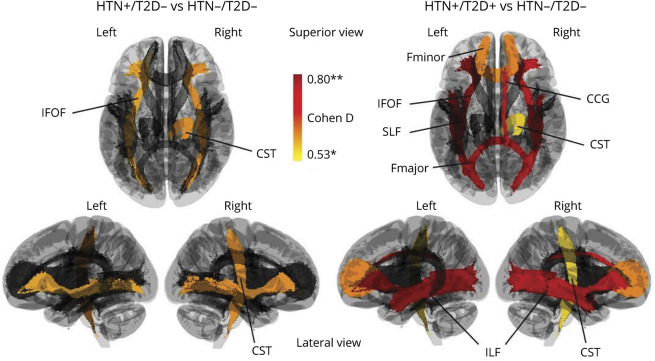
When we examined brain WM integrity using NDI, significant differences were found between BPRHS HTN−/T2D− and HTN+/T2D+ individuals in 8 of the 20 WM tracts (Figure 1, right). Specifically, significant structural disruptions were seen among the HTN+/T2D+ group in bilateral inferior longitudinal fasciculus (ILF) (left, Cohen d = 0.79; right, Cohen d = 0.76), bilateral inferior fronto-occipital fasciculus (IFOF) (left, Cohen d = 0.8; right, Cohen d = 0.74), forceps major (Cohen d = 0.77), forceps minor (Cohen d = 0.59), right corticospinal tract (Cohen d = 0.53), and right cingulum cingulate gyrus bundle (right, Cohen d = 0.68). Three of the features listed above were maintained when the HTN+/T2D− group was compared with the BPRHS HTN−/T2D− group, including bilateral IFOF (left, Cohen d = 0.56; right, Cohen d = 0.58) and right corticospinal tract (Cohen d = 0.58) (Figure 1, left). In summary, T2D and HTN comorbidity was significantly associated with WM structural deficits, especially in the long-range connectional fibers of the inferior and posterior regions of the brain, including IFOF and ILF. Detailed results of the statistical analysis performed can be found in eTable 1 (links.lww.com/WNL/B832).
Figure 1. Association of T2D and HTN With Brain WM Structure in Puerto Rican Adults.
(Left) Differences in anatomic connectivity of major WM tracts between HTN+/T2D− and HTN−/T2D− individuals. (Right) Differences in anatomic connectivity of major WM tracts between HTN+/T2D+ and HTN−/T2D− individuals. Colors indicate the effect size of the statistical differences (Cohen d), ranging from red (greater difference) to yellow (smaller difference). CCG = cingulum cingulate gyrus bundle; CST = corticospinal tract; Fmajor = corpus callosum forceps major; Fminor = corpus callosum forceps minor; HTN = hypertension; IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; SLF = superior longitudinal fasciculus; T2D = type 2 diabetes; WM = white matter. *p < 0.05, **p < 0.005.
Associations of Comorbid T2D and HTN With Brain GM Structure in BPRHS
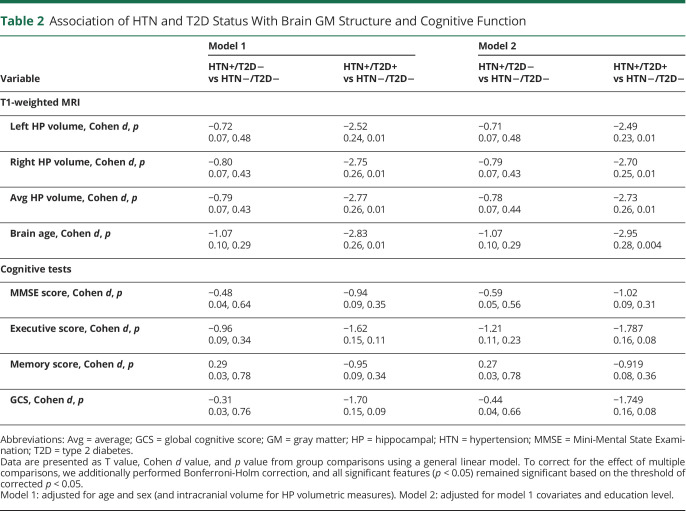
Compared to BPRHS HTN−/T2D− individuals, the HTN+/T2D+ group demonstrated significant differences in all 4 MRI measures, including smaller left, right, and average hippocampal volumes and more negative brain age z score (indicating larger deviation of estimated brain age from the chronologic age), with Cohen d values ranging from 0.24 to 0.26 (Table 2). None of these features were significantly different between HTN+/T2D− and BPRHS HTN−/T2D− individuals. The differences between HTN+/T2D+ and HTN−/T2D− individuals on hippocampal volume and brain age remained significant after controlling for education, as shown by models 1 and 2, with Cohen d values ranging from 0.23 to 0.28. This result showed that T2D and HTN comorbidity was significantly associated with bilateral hippocampal atrophy and larger deviation between brain aging and chronologic age.
Table 2.
Association of HTN and T2D Status With Brain GM Structure and Cognitive Function
Associations of Comorbid T2D and HTN With Cognitive Functions in BPRHS
No significant differences were found between either the HTN+/T2D+ or HTN+/T2D− group and the HTN−/T2D− group for any comparisons of cognitive function when the model was adjusted only for age and sex (model 1). When we included education level as a covariate, differences were found between the HTN+/T2D+ and HTN−/T2D− individuals in executive function and GCS with marginal significance based on p < 0.05. Specifically, HTN+/T2D+ participants scored lower on the executive function test (p = 0.08) and GCS (p = 0.08).
Associations of Cognitive Functions and Brain Imaging Measures in BPRHS
Correlation analysis between cognitive and GM imaging measures showed that MMSE score was positively associated with brain age (ρ = 0.16) and with left hippocampal volume (ρ = 0.15) and that executive function score was positively associated with left hippocampal volume (ρ = 0.17) on the basis of raw p < 0.05. Correlation analysis between cognitive and WM imaging measures showed that MMSE score was positively associated with neurite density index in right cingulum-angular bundle (ρ = 0.19), right ILF (ρ = 0.16), and right superior longitudinal fasciculus (ρ = 0.17); between executive function and right ILF (ρ = 0.24); and between GCS and right ILF (ρ = 0.16) on the basis of raw p < 0.05. All features remained statistically significant after 10,000 permutation tests. Detailed results are presented in eTable 2 (links.lww.com/WNL/B832).
Comparison of Brain Age, Hippocampal Volume, and Cognitive Function Between BPRHS and ADNI/NACC Cohorts
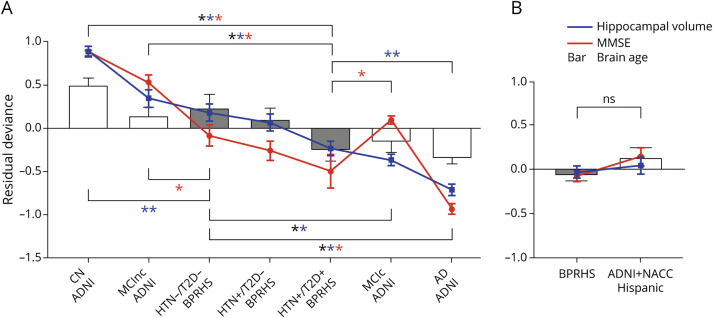
BPRHS participants were compared with the (1) ADNI-1 non-Hispanic White and (2) ADNI + NACC Hispanic groups on the measures of brain age z score, hippocampal volume (average), and cognitive function (MMSE sum score) (Figure 2). For imaging measures, compared to ADNI non-Hispanic White participant subgroups, BPRHS HTN−/T2D− participants were not significantly different from ADNI CN individuals in brain age and not significantly different from those classified as MCInc in both brain age and hippocampal volume, but they presented with significantly smaller hippocampal volume than ADNI CN and larger hippocampal volume and higher brain age z score than ADNI participants with MCIc or AD. In contrast, BPRHS HTN+/T2D+ participants were not significantly different from ADNI participants with MCIc in brain age and hippocampal volume and not significantly different from those with AD in brain age z score, but they showed significantly smaller hippocampal volume and smaller brain age z score compared to ADNI CN participants and those with MCInc and larger hippocampal volume than the ADNI participants with AD. The BPRHS HTN+/T2D− participants were not significantly different from those with MCInc in both brain age and hippocampal volume, but they showed lower brain age z score and hippocampal volume than ADNI CN individuals and larger hippocampal volume and brain age than the ADNI participants with MCIc and AD (not depicted in Figure 2 due to its similarly to HTN−/T2D−). For the comparison of MMSE scores, BPRHS HTN−/T2D− individuals were not different from ADNI participants with MCIc, but they scored lower than CN individuals and those with MCInc and higher than participants with AD. Both HTN+/T2D− and HTN+/T2D+ individuals scored significantly lower than CN participants and all with MCI but higher than those with AD. We additionally compared the HTN−/T2D− and HTN+/T2D− groups in ADNI with matching groups in BPRHS. The analysis highlighted patterns similar to those of the whole group comparison between ADNI and BPRHS (eTable 3, links.lww.com/WNL/B832). In summary, the BPRHS HTN−/T2D− individuals were similar to the ADNI participants with MCInc in overall rate of brain aging, hippocampal volume, and cognitive decline, while BPRHS participants with T2D and HTN comorbidity were similar to the ADNI-1 participants with MCIc.
Figure 2. Comparisons of Imaging and Cognitive Measures Between Puerto Rican Adults (BPRHS) and Other Cohorts.
Vertical axis is the residual deviance of each measure after adjusting for covariates (i.e., age + sex for imaging, age + sex + education for MMSE) in the general linear regression model. Blue line shows average hippocampal volume; red line, MMSE sum score; gray bar, brain age z-score for BPRHS; white bar, brain-age z-score for ADNI-1 or non-Hispanic White/ADNI + NACC Hispanic individuals; black asterisk, significant difference in brain age; blue asterisk, significant difference in hippocampal volume; and red asterisk, significant difference in MMSE score. Data are presented as mean (error bars show SEM). AD = Alzheimer disease; ADNI = Alzheimer's Disease Neuroimaging Initiative; BPRHS = Boston Puerto Rican Health Study; CN = cognitively normal control; HTN = hypertension; MCIc = mild cognitive impairment converter; MCInc = mild cognitive impairment nonconverter; MMSE = Mini-Mental State Examination; NACC = National Alzheimer's Coordinating Center; NS = not significantly different on any measures; T2D = type 2 diabetes.
Discussion
Through our investigation of the BPRHS brain imaging and cognitive data, we presented 3 main findings. (1) Compared to the ADNI non-Hispanic White cohort of similar age range, the BPRHS Hispanic cohort had almost double the prevalence of HTN and 5 times higher diabetes, but this prevalence was similar in the ADNI + NACC Hispanic cohort. (2) Compared to the BPRHS HTN−/T2D− group, those with comorbid T2D and HTN showed significant bilateral hippocampal atrophy, reduced integrity of long-range connectional fibers in the inferior regions of the brain, larger deviation between predicted brain aging and chronologic age, and a trend of declines in executive function and global cognitive function, while those with HTN but without T2D did not differ from the HTN−/T2D− individuals except in WM NDI measures. (3) The levels of brain structural changes and cognitive decline in individuals with T2D and HTN comorbidity were comparable to those observed in ADNI MCIc.
Participants of the BPRHS experience high prevalence of chronic health conditions, including T2D (40%), HTN (69%), and obesity (57%), according to previous reports.4 These estimates are consistent with the 192 participants in our current analysis who underwent MRI, with nearly twice the HTN, 5 times the T2D, and significantly higher mean BMI compared with the ADNI-1 non-Hispanic White elderly cohort. The BPRHS cohort also showed a slightly higher prevalence of T2D and HTN than the ADNI + NACC Hispanic cohort (Table 1). This is consistent with previous studies showing a high prevalence of T2D and HTN in the Hispanic population, especially for Puerto Ricans compared with individuals from other Hispanic backgrounds.31,32 In contrast, a recent large-scale UK Biobank study (n = 22,059, mean age 62 years) with mainly non-Hispanic European participants showed only 11% HTN and 5% T2D prevalence.33 Health disparities between Hispanic and non-Hispanic populations may be associated with socioeconomic and nutritional factors; previous reports from the BPRHS showed that large proportions had education below ninth grade (48%), fell below the poverty line (59%), and had less healthy dietary habits compared to the general population.4 Moreover, Hispanic Americans were reported to have more poorly controlled T2D and more associated medical complications.34,35 An earlier investigation of BPRHS showed that lower overall dietary quality was associated with poor longitudinal glycemic control.36 Among participants with diabetes, investigators observed significant interactions between low income and food insecurity and lower intake of whole fruit and vegetables, which led to the subsequent elevation of hemoglobin A1c.
As mentioned previously, both animal models and limited human studies showed that comorbid T2D and HTN were associated with lower WM and GM integrity. For instance, ADNI non-Hispanic White cohort demonstrated an indirect relationship between T2D and cognitive decline, which was mediated by the reduced cortical thickness.13 Another study using a mouse model showed that while HTN alone had minimal effect on GM volume, both type 1 diabetes and T2D were associated with GM atrophy. Furthermore, comorbid diabetes and HTN led to significant hippocampal neuronal loss.14 These findings support the effect of diabetes comorbidity on the brain and cognition but are hampered by the lack of large-scale, stratified sampling. Our analysis of the BPRHS brain imaging data revealed that HTN+/T2D+ was associated with more severe neurologic abnormalities than HTN+/T2D−. Specifically, T2D comorbidity was most significantly associated with reduced WM connectivity of the inferior longitudinal tracts (i.e., ILF and IFOF) of the brain. These tracts have been shown to be correlated with cognitive impairment and other neurologic diseases.37,38 An early study using the rhesus monkey model suggested that WM abnormality occurs during aging as a result of degeneration of oligodendrocytes (demyelination), which disrupted the integrity of neural circuits underlying cognitive performances.39 A recent large non-Hispanic White sample–based study (UK Biobank) showed that the cerebrovascular risk score (derived from age, antihypertensive medication use, diabetes, and APOE ε4 status) was associated with disrupted WM integrity in the frontoparietal cortical network, which has a major role in executive function.30 The different regions highlighted by our study compared to the UK Biobank study may be attributed to different cohort characteristics, that is, age range and ethnicity. Our results from GM structural measures showed significant hippocampal atrophy and accelerated brain aging only in those with comorbid T2D and HTN. This is consistent with a previous animal study that showed that T2D and HTN comorbidity led to hippocampal neuronal loss, while either T2D or HTN led to synaptic loss.14 Hippocampal atrophy and accelerated brain aging are also well-established diagnostic markers for MCI and AD.
The link between T2D and accelerated cognitive decline in the aging population is well supported by a large body of evidence.6-8,10 As mentioned previously, the presence of T2D in combination with several other cardiovascular risk factors (e.g., HTN, elevated serum cholesterol) has been associated with increased risk of developing dementia later in life. While our result from a within-BPRHS group comparison showed only a trend of difference in cognitive function between HTN+/T2D+ and HTN−/T2D−, the comparison between BPRHS and ADNI subgroups suggested a downward shift for MMSE scores in all BRPHS participants (Figure 2). For instance, the BRPHS controls without HTN or T2D scored significantly lower than the ADNI CN controls on the MMSE even with adjustment for education level. Considering the smaller hippocampal volumes shown in BPRHS HTN−/T2D− participants compared to ADNI CN participants, a similar pattern in MMSE performance may support the association between cognitive decline and abnormal biological changes in the brain. The BPRHS HTN−/T2D− individuals also demonstrated lower but nonsignificant brain age z score than ADNI CN individuals, which may suggest that cultural/ethnic influence on cognitive aging is related more to focal change (i.e., hippocampus) than to global changes across the whole brain. In addition, the presence of health risk factors (i.e., HTN and T2D comorbidity) contributes further to global changes in the brain during aging. As we showed in this study, combining biomarkers in different domains such as neuroimaging and cognitive markers provides a more integrated model for understanding the aging process.
The disparities of cognitive function between Hispanic and non-Hispanic White participants are in line with recent surveys conducted by the Alzheimer's Association, which showed that the prevalence of AD varies significantly across ethnicities. AD is the most common type of dementia, currently affecting >6 million Americans and projected to affect 12.7 million by 2050.40 Compared to non-Hispanic White elders, the rate is nearly 2 times higher among older Black individuals and one and half times higher in older Hispanic populations.15 Previous reports also showed that AD is the fourth leading cause of death on the island of Puerto Rico.41 Moreover, there is a greater chance of misdiagnosis among these ethnic minority groups due to lack of awareness.
Our study examined well-established objective AD biomarkers highlighted by the A/T/N diagnostic framework, including hippocampal atrophy and impaired performance on neuropsychological testing.42 We showed that the decline in brain health (measured by regional and global structural changes) and cognitive capacity in HTN+/T2D+ BPRHS participants was comparable to that in ADNI MCI participants who progressed to AD within 5 years of follow-up. In addition, we showed significant disruption of WM tract integrity among HTN+/T2D+ individuals, which is consistent with recent human diffusion tensor imaging studies suggesting that WM abnormalities correlate with memory impairment and MCI-to-AD conversion.43,44 Our result suggests that the high prevalence of T2D and HTN in Puerto Ricans may contribute to the higher risk of AD within this population.
Strengths of our study include the use of robust statistical models with adjustment for relevant covariates and multiple comparisons and defining groups with clinical variables (i.e., HTN and T2D) collected at multiple time points. Nonetheless, several limitations should be addressed in future analyses. The current analysis is limited to BPRHS participants with both cognitive and MRI data available, and due to the small number with T2D but without HTN (n = 10), we were unable to evaluate this group in the current study. Because BPRHS is an ongoing study that has already enrolled 1,500 participants, more MRI scans, including longitudinal follow-up data, will be available for follow-up analyses using larger sample sizes. Interpretation of our findings is also limited by the lack of clinical diagnostic information on MCI from the BPRHS study. Although BPRHS participants demonstrated overall lower performances on the MMSE compared to age-matched non-Hispanic White ADNI participants, formal clinical diagnoses were not performed. Because our results suggested that those with comorbid T2D and HTN had brain and cognitive capacity resembling MCIc, tracking changes in their neurologic disease profiles may be important. Our results highlight important health disparities in the understudied Puerto Rican population and call for future large-scale investigations that incorporate ethnic and cultural diversity.
Glossary
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- BMI
body mass index
- BP
blood pressure
- BPRHS
Boston Puerto Rican Health Study
- CN
cognitively normal
- FA
flip angle
- FOV
field of view
- GCS
global cognitive score
- GM
gray matter
- HTN
hypertension
- IFOF
inferior fronto-occipital fasciculus
- ILF
inferior longitudinal fasciculus
- MCI
mild cognitive impairment
- MCIc
MCI converter
- MCInc
MCI nonconverter
- MMSE
Mini-Mental State Examination
- NACC
National Alzheimer's Coordinating Center
- NDI
neurite density imaging
- ST
slice thickness
- TE
echo time
- TI
inversion time
- TL
total slices
- TR
repetition time
- T2D
type 2 diabetes
- WM
white matter
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
Editorial, page 609
Study Funding
This analysis was supported by R01AG055948 and W81XWH2010236 (B.-B.K. and C,-h.C.). The BPRHS was also supported by P50 HL105185 and P01 AG023394. Data collection and sharing for this project were funded by the ADNI (NIH grant U01 AG024904) and DOD ADNI (Department of Defense award W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging and the National Institute of Biomedical Imaging and Bioengineering and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc; Biogen; Bristol-Myers Squibb Co; CereSpir, Inc; Cogstate; Eisai Inc; Elan Pharmaceuticals, Inc; Eli Lilly and Co; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc; Fujirebio; GE Healthcare; IXICO Ltd; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co, Inc; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corp; Pfizer Inc; Piramal Imaging; Servier; Takeda Pharmaceutical Co; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the NIH (fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. The NACC database is funded by National Institute on Aging (NIA)/NIH grant U01 AG016976. NACC data are contributed by the NIA-funded Alzheimer’s Disease Research Centers: P30 AG019610 (principal investigator [PI] Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017-2018. NCHS Data Brief. 2020(364):1-8. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 3.Vega WA, Rodriguez MA, Gruskin E. Health disparities in the Latino population. Epidemiologic Rev. 2009;31(1):99-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucker KL, Mattei J, Noel SE, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daviglus ML, Talavera GA, Avilés-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308(17):1775-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knopman D, Boland LL, Mosley T, et al. ; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42-48. [DOI] [PubMed] [Google Scholar]
- 7.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64(4):570-575. [DOI] [PubMed] [Google Scholar]
- 8.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484-491. [DOI] [PubMed] [Google Scholar]
- 9.Long AN, Dagogo-Jack S. Comorbidities of diabetes and hypertension: mechanisms and approach to target organ protection. J Clin Hypertens. 2011;13(4):244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassing LB, Hofer SM, Nilsson SE, et al. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age Ageing. 2004;33(4):355-361. [DOI] [PubMed] [Google Scholar]
- 11.Samieri C, Perier MC, Gaye B, et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320(7):657-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peloso GM, Beiser AS, Satizabal CL, et al. Cardiovascular health, genetic risk, and risk of dementia in the Framingham Heart Study. Neurology. 2020;95(10):e1341-e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran C, Beare R, Wang W, Callisaya M, Srikanth V. Alzheimer's Disease Neuroimaging Initiative (ADNI). Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology. 2019;92(8):e823-e830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devisser A, Yang C, Herring A, et al. Differential impact of diabetes and hypertension in the brain: adverse effects in grey matter. Neurobiol Disretraction Neurobiol Dis. 2014;4468(2):161229-161273. [DOI] [PubMed] [Google Scholar]
- 15.2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327-406. [DOI] [PubMed] [Google Scholar]
- 16.González HM, Tarraf W, Fornage M, et al. A research framework for cognitive aging and Alzheimer's disease among diverse US Latinos: design and implementation of the Hispanic Community Health Study/Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA). Alzheimers Dement. 2019;15(12):1624-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72-83. [DOI] [PubMed] [Google Scholar]
- 18.Unger T, Borghi C, Charchar F, et al. International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;75(6):1334-1357. [DOI] [PubMed] [Google Scholar]
- 19.Artiola Fortuny L, Romo HD, Heaton RK, Pardee RE. Manual de Normas y Procedimientos para la Baterıa Neuropsicologica en Espanol. Swets & Zeitlinger; 2000. [Google Scholar]
- 20.Beery K. The Developmental Test of Visual-Motor Integration Manual, Revised Edition. Modern Curriculum Press; 1989. [Google Scholar]
- 21.Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS. Screening for Alzheimer's disease by clock drawing. J Am Geriatr Soc. 1989;37:730-734. [DOI] [PubMed] [Google Scholar]
- 22.Karno M, Burnam A, Escobar JI, Hough RL, Eaton WW. Development of the Spanish-language version of the National Institute of Mental Health Diagnostic Interview Schedule. Arch Gen Psychiatry. 1983;40:1183-1188. [DOI] [PubMed] [Google Scholar]
- 23.Jack CR Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iglesias JE, Augustinack JC, Nguyen K, et al. , Alzheimer's Disease Neuroimaging Initiative. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufmann T, van der Meer D, Doan NT, et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat Neurosci. 2019;22(10):1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CH, Koo BB, Calderazzo S, et al. Alterations in high-order diffusion imaging in veterans with Gulf War illness is associated with chemical weapons exposure and mild traumatic brain injury. Brain Behav Immun. 2020;89:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000-1016. [DOI] [PubMed] [Google Scholar]
- 29.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65-70. [Google Scholar]
- 31.Schneiderman N, Llabre M, Cowie CC, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care. 2014;37(8):2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elfassy T, Zeki Al Hazzouri A, Cai J, et al. Incidence of hypertension among US HISPANICS/LATINOS: the Hispanic Community Health Study/Study of Latinos, 2008 to 2017. J Am Heart Assoc. 2020;9(12):e015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veldsman M, Tai XY, Nichols T, et al. Cerebrovascular risk factors impact frontoparietal network integrity and executive function in healthy ageing. Nat Commun. 2020;11(1):4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendel CS, Shah JH, Duckworth WC, Hoffman RM, Mohler MJ, Murata GH. Racial and ethnic disparities in the control of cardiovascular disease risk factors in southwest American veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study. BMC Health Serv Res. 2006;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13(6):814-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berkowitz SA, Gao X, Tucker KL. Food-insecure dietary patterns are associated with poor longitudinal glycemic control in diabetes: results from the Boston Puerto Rican Health Study. Diabetes Care. 2014;37(9):2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20(2):209-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashtari M, Cottone J, Ardekani BA, et al. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007;64(11):1270-1280. [DOI] [PubMed] [Google Scholar]
- 39.Peters A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Front Neuroanat. 2009;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40 .Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41 .Friedman DB, Gibson A, Torres W, et al. Increasing community awareness about Alzheimer's disease in Puerto Rico through coffee shop education and social media. J Community Health. 2016;41(5):1006-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42 .Jack CR Jr, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji F, Pasternak O, Ng KK, et al. White matter microstructural abnormalities and default network degeneration are associated with early memory deficit in Alzheimer's disease continuum. Sci Rep. 2019;9(1):4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maier-Hein KH, Westin CF, Shenton ME, et al. Widespread white matter degeneration preceding the onset of dementia. Alzheimers Dement. 2015;11(5):485-493.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.