Abstract
Background
Currently, potent P2Y12 inhibition with the use of prasugrel or ticagrelor is the mainstay of treatment after an acute coronary syndrome (ACS). The 2020 European Society of Cardiology (ESC) Guidelines recommend the use of prasugrel over ticagrelor in patients with non-ST-elevation ACS (NSTE-ACS) intended to receive invasive management (class IIa recommendation), however there are contradictory views regarding this recommendation.
Aim
To compare oral P2Y12 inhibitors in NSTE-ACS in terms of efficacy and safety with a focus on patients intended to proceed to invasive management.
Methods
We systematically searched PubMed, Cochrane Central Register of Controlled Trials and Web of Science to identify studies that compared different oral P2Y12 inhibitors (clopidogrel, prasugrel and ticagrelor) in patients with NSTE-ACS. Efficacy outcomes included the major adverse cardiovascular events outcome and safety outcomes included minor and major bleedings. We performed a frequentist network meta-analysis.
Results
Nine studies (n=35 441 patients) were included in the systematic review. There was no difference between prasugrel and ticagrelor in the composite cardiovascular end point (prasugrel vs ticagrelor HR=0.80, 95% CI=0.61 to 1.06) in all patients with NSTE-ACS. In patients intended to receive invasive management, prasugrel resulted in a reduction of the composite cardiovascular end point both versus clopidogrel (HR=0.76, 95% CI=0.61 to 0.95) and ticagrelor (HR=0.74, 95% CI=0.56 to 0.98). Inconsistency was moderate and non-significant (I2=27%, total Q p=0.2). Prasugrel ranked as the most efficient treatment in the composite cardiovascular efficacy outcome, all-cause death, myocardial infarction and definite stent thrombosis, while clopidogrel ranked as safest in the bleeding outcomes.
Conclusion
In patients with NSTE-ACS intended to receive invasive management, an antiplatelet strategy based on prasugrel is more efficient than a similar strategy based on ticagrelor on a moderate level of evidence. This analysis supports the current recommendations by the ESC guidelines.
Keywords: acute coronary syndrome, meta-analysis, coronary artery disease
Key questions.
What is already known about this subject?
The initiation of dual antiplatelet therapy constituted by aspirin and a P2Y12 inhibitor, in the absence of indication for an oral anticoagulant, is the mainstay of antithrombotic therapy after an acute coronary syndrome (ACS).
What does this study add?
In patients with NSTE-ACS intended to receive invasive management, an antiplatelet strategy based on prasugrel is more efficient than a similar strategy based on ticagrelor on a moderate level of evidence.
How might this impact on clinical practice?
This analysis supports the current recommendations by the European Society of Cardiology guidelines.
Introduction
The management of antithrombotic therapy after an acute coronary syndrome (ACS) is getting increasingly complicated.1 Large outcome trials that test different strategies of antithrombotic therapies during the acute phase, in the immediate period after and long-term following an ACS are constantly being published altering the landscape of recommendations. However, to date, the initiation of dual antiplatelet therapy constituted by aspirin and a P2Y12 inhibitor, in the absence of indication for an oral anticoagulant, is the mainstay of antithrombotic therapy after an ACS.
Following the landmark Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 382 and Study of Platelet Inhibition and Patient Outcomes (PLATO)3 studies, the use of potent P2Y12 inhibitors (ie, prasugrel and ticagrelor) is recommended over clopidogrel as an integral part of antithrombotic therapy after an ACS, whether a revascularisation or a conservative management strategy is followed. The 2020 European Society of Cardiology (ESC) guidelines for the management of patients with non-ST-elevation ACS (NSTE-ACS) suggest that prasugrel should be considered in preference to ticagrelor for patients with NSTE-ACS who proceed to percutaneous coronary intervention (PCI) (class of recommendation IIa, level of evidence B).4 This recommendation is mainly based on the results of the 2019 Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 trial, an open-label, investigator-initiated randomised controlled trial (RCT), which showed that prasugrel had superior efficacy in the prevention of cardiovascular events over ticagrelor, without significantly increased rates of bleeding. However, given its design and methodological limitations, the study’s results and the subsequent guideline recommendation have received criticism.1 5
We aimed to synthesise the evidence across the literature regarding the use of oral P2Y12 inhibitors in patients with NSTE-ACS concentrating on their comparative efficacy and safety in this population and focusing in the subgroup of patients intended to receive invasive management. Because of the lack of numerous large trials that compare prasugrel and ticagrelor head-to-head, we aimed to incorporate direct and indirect evidence by conducting a network meta-analysis.
Methods
This systematic review and network meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement for Network Meta-analyses (online supplemental PRISMA Checklist).6 The protocol of this study is published in the PROSPERO registry (CRD42020211123).
openhrt-2021-001937supp001.pdf (23.7MB, pdf)
Search strategy
We used terms related to ‘P2Y12 inhibitors’, ‘acute coronary syndrome’ and an RCT search filter to search MEDLINE (via PubMed), the Cochrane Central Register of Controlled Trials and the Web of Science for RCTs that compared different oral P2Y12 inhibitors in patients with NSTE-ACS. For PubMed, a search string was created and modified accordingly to search in other databases. According to the snowball effect, all references from selected studies were retrieved and carefully examined. In addition, we looked for relevant abstracts from major cardiology conferences and combed through ClinicalTrials.gov for active research. There were no language limitations. Online supplemental material 1 contains the search string and search syntax.
Eligibility criteria
We included full-text RCTs that compared the different oral P2Y12 inhibitors (clopidogrel vs ticagrelor, clopidogrel vs prasugrel, ticagrelor vs prasugrel) in the setting of ACS and reported serious adverse cardiovascular events and/or bleeding events within the NSTE-ACS subgroup. We included RCTs which were designed to study the efficacy and safety of P2Y12 inhibitors in the entire ACS population and that reported outcomes on the NSTE-ACS population in a subgroup analysis. The efficacy composite end point of cardiovascular death, non-fatal myocardial infarction (MI) and non-fatal stroke was used as a primary end point, and the individual components of the primary efficacy composite end point, all-cause mortality and stent thrombosis were used as secondary efficacy end points. Major bleeding (as defined by each trial) and major or minor bleeding combined were the safety end points.
Studies with a primary pharmacokinetic/pharmacodynamic outcome, studies randomising patients based on genotype guidance or any other guidance, studies switching, by protocol definition, to an antiplatelet regimen at some point after ACS and studies with fewer than 100 patients in total were all excluded. Studies investigating short-acting intravenous P2Y12 inhibitors (such as cangrelor) were ruled out. When numerous texts with possibly overlapping populations were identified, we included the most current study.
Study selection, data extraction and quality assessment
All of the results of the search were entered into a reference management programme (Mendeley 1.19.3). All duplicates were deleted, and three reviewers (ID, AP and SZ) independently reviewed titles, abstracts and full texts for papers that were eligible. To resolve any discrepancies regarding research eligibility, a fourth review author (ITF) was consulted. We recorded all reasons for exclusion at the stage of full-text eligibility screening.
On a structured spreadsheet, two reviewers (AP, ITF) independently retrieved data on research design, efficacy and safety results. Before beginning, a pilot test was conducted to ensure coherence between authors, and any disagreements were settled through consensus. All data were extracted using standard procedures provided by the Cochrane Collaboration from full-texts, summary tables and figures or online supplemental information.7 Substudies related to the parent trials included in the analysis were retrieved to extract data from the NSTE-ACS and the invasively managed population (online supplemental material 13). Any missing data relevant to the analysis were obtained by contacting the authors.
Eligible studies were assessed for risk of bias (RoB) by two review authors (ITF, SZ) using the Cochrane collaboration RoB tool for RCTs (RoB 2).8 A sensitivity analysis was carried out to eliminate studies of poor quality. When relevant, the Egger’s test and visual assessment of funnel plot asymmetry were used to assess publication bias in the systematic review.
Strategy for data synthesis
To combine direct and indirect evidence across trials, we used a frequentist network meta-analysis with a random-effects model. To account for the time-to-event parameter, the effect estimate was the HR with the appropriate 95% CIs. Both global approaches, such as the Cochran’s Q and the I2 statistic (25% low, 25%–50% moderate and >50% high heterogeneity), and local ones, such as analysing consistency across direct and indirect comparisons with the node-splitting method, were used to measure consistency. The p-score was used to categorise interventions in a hierarchical order. This statistic has a range of 0–1, with values closer to 1 indicating better outcomes with the relevant intervention and values closer to 0 indicating worse outcomes. We used rankograms to visualise the probability of each treatment being at each possible rank.9 We explored the importance of each study to each comparison in the network by estimating the relative loss of precision if this study was left out of the network.10 An importance value of 1 means that the variance of the network effect becomes infinite if the study is removed from the network and, therefore, the study is an essential link to the specific comparison. We conducted a sensitivity analysis excluding patients that were treated conservatively (ie, without revascularisation).
All analyses were considered statistically significant if the p value was <0.05. The ‘netmeta’ package in the R Project for Statistical Computing was used for all analyses (V.3.6.3).11
Grading of evidence
We used the Confidence in Network Meta-analysis (CINeMA) framework, which was implemented in a web application available at http://cinema.ispm.ch, to assess the confidence in the network meta-analysis results. CINeMA is a network meta-analysis version of the Grading of Recommendations Assessment, Development, and Evaluation method.12
Patient and public involvement
There was no patient or public involvement during the completion of this paper.
Results
Search results, study characteristics and quality assessment
The search strategy yielded 6406 results after duplicates removal. Following the initial screening phase, 86 full-text studies were screened for eligibility and 77 studies were excluded with reasons. Ultimately, nine studies (n=35 441 patients) were included in our analysis. The study selection process can be seen in figure 1. Study characteristics for each individual study are presented in online supplemental material 2. The median percentage of patients with NSTE-ACS in the RCTs was 59%. The median follow-up period ranged from 9.2 to 17 months. Three studies compared prasugrel with clopidogrel,2 13 14 four studies compared ticagrelor with clopidogrel3 15–17 and two studies compared prasugrel with ticagrelor.18 19
Figure 1.
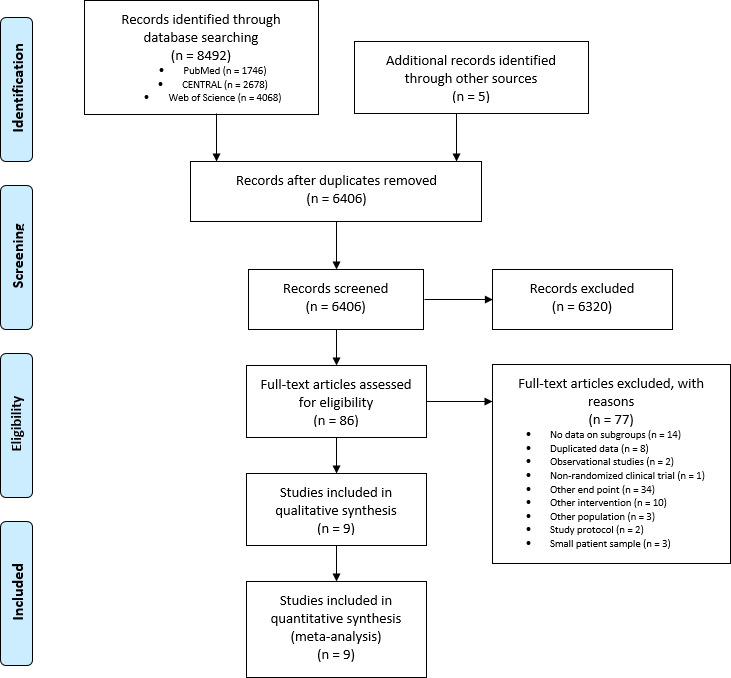
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of the study selection process.
Employing the RoB.2 tool, the RoB was assessed as low in five studies, with some concerns in three studies and high in one study (online supplemental material 3). After visual assessment of the funnel plots for each outcome, there was no evidence of publication bias.
Network meta-analysis
Primary efficacy outcome
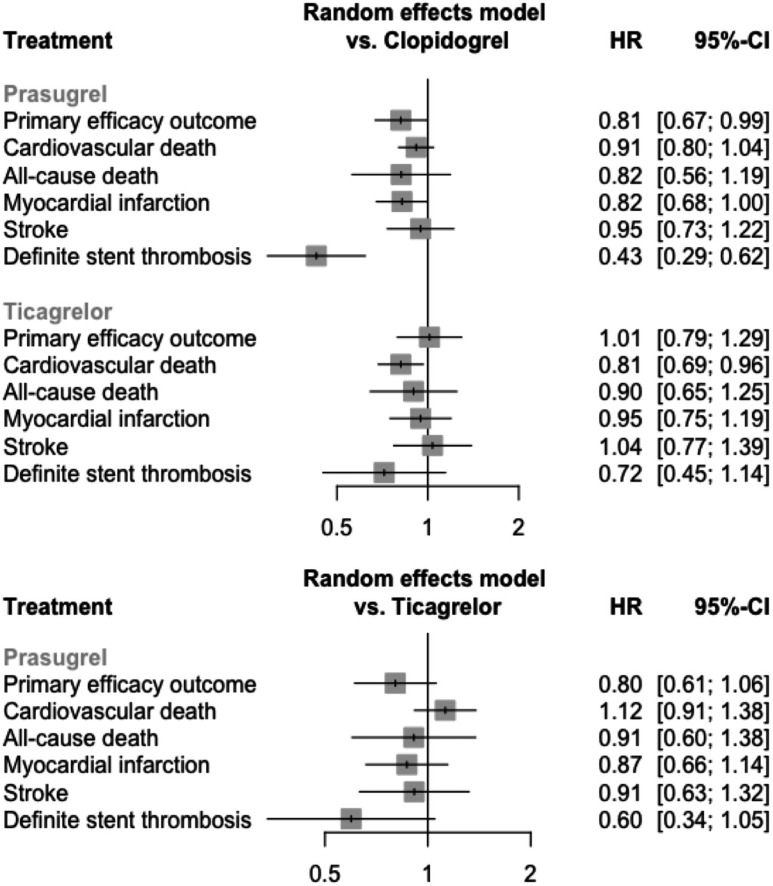
Eight studies (n=34 433 patients) contributed to the analysis.2 3 13–16 18 19 The network graph of interventions is presented in figure 2. With clopidogrel set as reference group, prasugrel showed a significant reduction of the primary efficacy end point (prasugrel vs clopidogrel HR=0.81, 95% CI=0.67 to 0.99), whereas ticagrelor did not (ticagrelor vs clopidogrel HR=1.01, 95% CI=0.79 to 1.29) (figure 3). In the prasugrel versus ticagrelor comparison the effect estimate favoured prasugrel, although the difference between the two treatments was not statistically significant (prasugrel vs ticagrelor HR=0.80, 95% CI=0.61 to 1.06). Prasugrel ranked best (p-score=0.96), followed by clopidogrel (p-score=0.28) and ticagrelor (p-score=0.26). The inconsistency was high in the model (I2=60%, total Q p=0.01). No disagreement was detected between direct and indirect evidence with the node-splitting method. Further details on inconsistency, the funnel plot and individual studies’ impact in the network of the primary outcome are presented in online supplemental material 4.
Figure 2.
Network graph of interventions for the primary outcome.
Figure 3.
Forest plot of the network estimates of the potent P2Y12 inhibitors against clopidogrel for the efficacy outcomes.
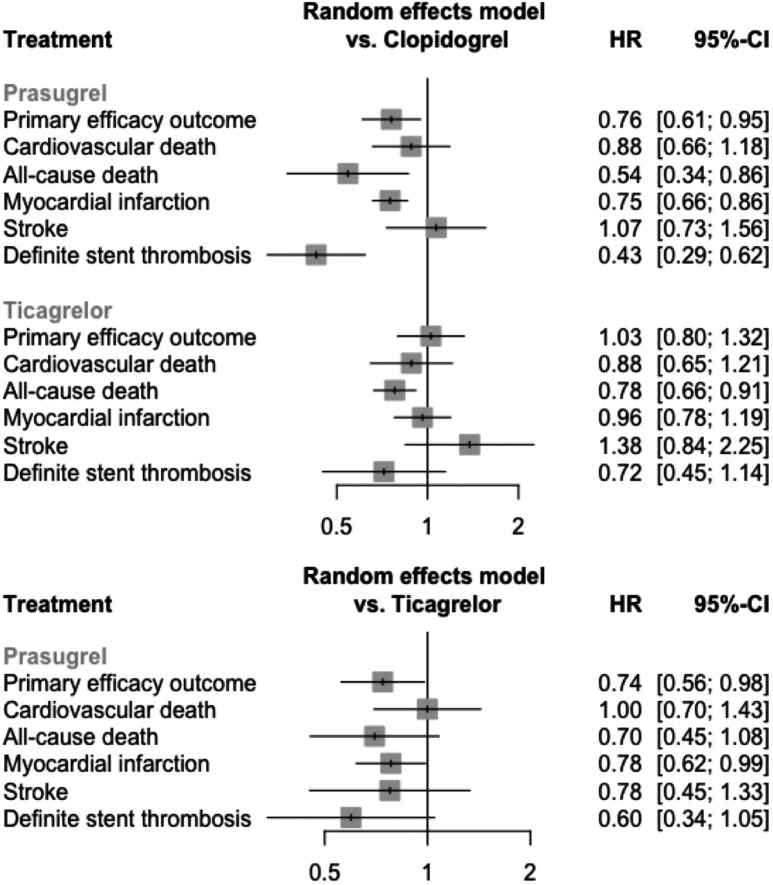
In the analysis of patients managed invasively (seven studies, n=19 049 patients), prasugrel resulted in a significant reduction in the primary end point versus clopidogrel (HR=0.76, 95% CI=0.61 to 0.95), as well as versus ticagrelor (HR=0.74, 95% CI=0.56 to 0.98). Inconsistency was moderate and non-significant (I2=27%, total Q p=0.2). The importance of ISAR-REACT 5 study to the prasugrel versus ticagrelor comparison (0.45) was slightly greater than PLATO (0.31) and TRITON-TIMI 38 (0.28). However, a sensitivity analysis excluding ISAR-REACT 5 resulted in an unchanged p-score ranking (prasugrel=0.96, clopidogrel=0.35, ticagrelor=0.19).
Secondary efficacy outcomes
Five studies (n=33 841) contributed to the network for the outcome of cardiovascular death (online supplemental material 5).2 3 13 17 19 Ticagrelor significantly reduced cardiovascular death compared with clopidogrel (HR=0.81, 95% CI=0.69 to 0.96), whereas prasugrel did not (HR=0.91, 95% CI=0.80 to 1.04), with no difference between prasugrel versus ticagrelor (HR=1.12, 95% CI=0.91 to 1.38) (I2=0%). However, in the sensitivity analysis of invasively managed patients none of the potent P2Y12 inhibitors resulted in significant reduction of the end point (online supplemental material 5). Four studies (n=23 773 patients) contributed to the network for the outcome of all-cause death (online supplemental material 6).3 13 17 19 None of the potent P2Y12 inhibitors resulted in significant reduction of the end point (I2=64%, total Q p=0.06), whereas in the sensitivity analysis both potent P2Y12 inhibitors led to reduction of all-cause mortality (HR=0.54 and 0.78, respectively, I2=0%).
Five studies (n=33 841) contributed to the network for the MI outcome (online supplemental material 7).2 3 13 17 19 There was no difference between the interventions in the main analysis (I2=60%, total Q p=0.05), however, in patients managed invasively prasugrel led to better outcomes when compared with clopidogrel (HR=0.75, 95% CI=0.66 to 0.86) and, also, when compared with ticagrelor (HR=0.78, 95% CI=0.62 to 0.99) (I2=0%). Five studies (n=33 841 patients) contributed to the network for the stroke outcome (online supplemental material 8).2 3 13 17 19 There was no difference between the interventions both in the main and the sensitivity analysis (I2=0%). Three studies (n=23 513) contributed to the network for the stent thrombosis outcome (online supplemental material 9).2 3 19 Prasugrel resulted in reduced rates of stent thrombosis compared with clopidogrel (HR=0.43, 95% CI=0.29 to 0.62), but not compared with ticagrelor (HR=0.60, 95% CI=0.34 to 1.05) (I2=0%).
The results of the main efficacy outcomes analysis is collectively shown in figure 3 and the results of the sensitivity analysis of invasive management is shown in figure 4.
Figure 4.
Forest plot of the network estimates of the potent P2Y12 inhibitors against clopidogrel for the efficacy outcomes in patients managed invasively (sensitivity analysis).
Safety outcomes
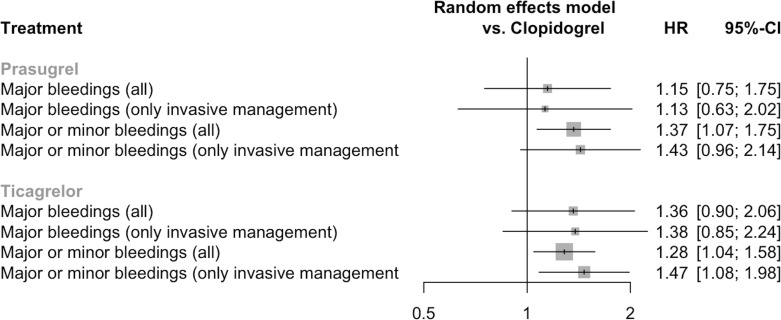
Six studies (n=33 702) contributed to the network for the major bleeding outcome (online supplemental material 10).2 3 13 17–19 The definition of major bleeding was not consistent across studies and it was defined by different criteria in each study (online supplemental material 2, column 7). Major bleeding did not differ between the interventions (prasugrel vs clopidogrel HR=1.15, 95% CI=0.75 to 1.75, ticagrelor vs clopidogrel HR=1.36, 95% CI=0.90 to 2.06, prasugrel vs ticagrelor HR=0.84, 95% CI=0.52 to 1.37) (figure 5). Inconsistency was high in the model (I2=73%) and significant (total Q p=0.005). The sensitivity analysis excluding patients with conservative management did not show any significant difference between the interventions (figure 5).
Figure 5.
Forest plot of the network estimates of the potent P2Y12 inhibitors against clopidogrel for the safety outcomes in both the main and the sensitivity analysis.
Five studies (n=31 870) contributed to the network for the major or minor bleeding outcome (online supplemental material 10).2 3 13 16 17 Both prasugrel and ticagrelor resulted in more major or minor bleeding than clopidogrel (HR=1.37, 95% CI=1.07 to 1.75 and HR=1.28, 95% CI=1.04 to 1.58, respectively) (figure 5). No difference was observed between prasugrel versus ticagrelor (HR=1.07, 95% CI=0.77 to 1.47). Inconsistency was moderate in the model (I2=46%) and non-significant (total Q p=0.14). Excluding conservative management patients, there was no longer difference in the prasugrel versus clopidogrel comparison (figure 5).
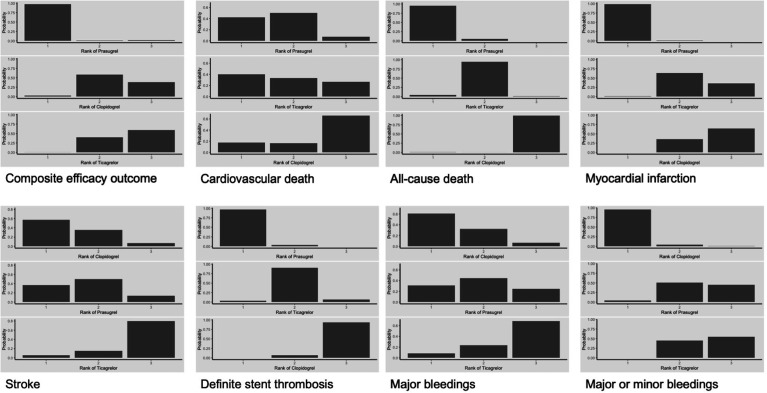
Treatment rankings
Rankograms of probabilities for each treatment to lie at each possible rank concerning the main analysis can be seen in online supplemental material 11. The corresponding rankograms for the patients managed invasively can be seen in figure 6. Prasugrel ranked with great probability as the most efficient treatment in the composite efficacy outcome, all-cause death, MI and definite stent thrombosis, while ticagrelor ranked as the most efficient treatment in cardiovascular death outcome, although with modest probability. Concerning the bleeding outcomes, clopidogrel ranked as the safest treatment with great probability.
Figure 6.
Rankograms—probabilities of each treatment to lie in each possible rank for every outcome in patients managed invasively (sensitivity analysis).
Grading of evidence
Details for the grading of evidence for all outcomes can be seen in online supplemental material 12. Grading of evidence in patients managed invasively (sensitivity analysis) can also be seen in online supplemental material 12.
Discussion
In this study, we generated a network meta-analysis to assess the comparative efficacy and safety of P2Y12 inhibitors in patients with NSTE-ACS, focusing on patients receiving an invasive course of management. Our results suggest that, in patients with invasive management, prasugrel was associated with lower rates of the composite cardiovascular efficacy end point by 26% compared with ticagrelor on a moderate level of evidence. Prasugrel ranked as the most effective treatment in the composite efficacy outcome, all-cause death, MI and definite stent thrombosis, without an excessive risk of major bleeding compared with clopidogrel.
The sole largest RCT powered for clinical outcomes to directly compare ticagrelor with prasugrel, which also included a fair percentage of patients with NSTE-ACS, is the ISAR-REACT 5 study (the Comparison of Prasugrel and Ticagrelor in the Treatment of Acute Myocardial Infarction (PRAGUE) 18 trial included a very small proportion of patients with NSTE-ACS). In NSTE-ACS, higher rates of the primary end point (all-cause death, non-fatal MI, non-fatal stroke) were observed with ticagrelor. As a consequence, a recommendation was included in the latest 2020 NSTE-ACS ESC guidelines with a preference for prasugrel over ticagrelor in patients managed invasively. However, the trial has received criticism mainly because of its open-label design, modest sample size and slightly higher rates of ticagrelor discontinuation, and therefore some suggest that its results should not be overinterpreted and cannot form the basis of a recommendation for prasugrel over ticagrelor in that setting. On the other hand, the PLATO trial has been subject to criticism because approximately half of its patients were treated with a conservative treatment, therefore making difficult an extrapolation exclusively to the invasively managed population, whereas the TRITON-TIMI 38 and ISAR-REACT 5 trials were designed to have PCI as first-line treatment. Our results suggest that prasugrel ranked as most effective in ischaemic event reduction and mortality over ticagrelor and in particular the impact of the ISAR-REACT study on the prasugrel versus ticagrelor in the network was not dominant over the other two large RCTs, PLATO and TRITON-TIMI 38. In addition, the sensitivity analysis excluding the ISAR-REACT 5 trial did not alter the ranking of treatments.
The ISAR-REACT 5 study required the administration of prasugrel after knowledge of the coronary anatomy, whereas ticagrelor was routinely administered pretreatment and thus, the observed efficacy of prasugrel over ticagrelor in the NSTE-ACS population may confirm that there is no apparent benefit for pretreatment. The decision of invasively treating the NSTE-ACS patient is an important one, requiring robust risk stratification and has direct implications on the use of a P2Y12 inhibitor prior to the revascularisation. Following the Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction (ACCOAST) trial, pretreatment with prasugrel, before knowledge of the coronary anatomy, is prohibited because of the lack of any ischaemic benefit and increased risk of bleeding.20 A recent meta-analysis suggests that pretreatment with oral P2Y12 inhibitors prior to angiography, compared with after knowledge of coronary anatomy at the time of PCI, is associated with no difference in cardiovascular outcomes and with increased bleeding risk, irrespective of the P2Y12 inhibitor type and, thus, routine pretreatment in NSTE-ACS is not supported.21
A network meta-analysis on the overall ACS population was recently conducted.22 A significant reduction in cardiovascular death and all-cause mortality was found only with ticagrelor compared with clopidogrel, while prasugrel effectively reduced MI compared with clopidogrel. Notably, in none of the explored outcomes there was a difference between ticagrelor and prasugrel, but they both increased major bleeding compared with clopidogrel. Other meta-analyses of the total ACS population have focused solely on the prasugrel versus ticagrelor comparison by analysing RCTs and observational studies together or by including studies not designed to primarily explore clinical outcomes.23 24 Yet, they found no difference in cardiovascular outcomes between prasugrel and ticagrelor. Our meta-analysis differs from the already published evidence in focusing on the NSTE-ACS population and especially in those managed invasively.
Of note, ticagrelor and not prasugrel reduced the cardiovascular death outcome compared with clopidogrel. The PLATO trial showed an impressive 23% significant reduction of death due to vascular causes with ticagrelor compared with clopidogrel in patients with NSTE-ACS, which had a major impact in the network analysis, while there was not such a significant effect of prasugrel in the TRITON-TIMI 38 trial.25 26
In contrast, non-fatal MIs were effectively reduced with prasugrel compared with both clopidogrel and ticagrelor in patients managed invasively. Our analysis included all MIs, both spontaneous and periprocedural and it was not possible to conduct a separate analysis for each type of MI. In the ISAR-REACT 5 study, MIs of all types were numerically higher in the ticagrelor group than in the prasugrel group, although there was no significant difference between groups.27 However, the impact of ISAR-REACT 5 to the network for the prasugrel versus ticagrelor comparison in the MI outcome was lower than the impact of PLATO, implying that the results are not exclusively driven by the ISAR-REACT 5 trial.
Lastly, stent thrombosis rates were significantly reduced with prasugrel compared with clopidogrel, but not with ticagrelor and there was not a significant difference between the potent P2Y12 inhibitors. A network meta-analysis in the overall ACS population reports similar results.28
Studies focusing on the effect of P2Y12 inhibitors on the platelet function and endothelial function could also be relevant. A prespecified substudy of the ISAR-REACT 5 study compared the pharmacodynamic effects of prasugrel and ticagrelor through the use of platelet function testing.29 Prasugrel resulted in lower platelet aggregation both at the first and second 24-hour interval after loading dose administration. Lower platelet aggregation values significantly predicted lower incidence of the primary end point, denoting the clinical value of the observation. In a recent RCT of invasively managed patients with ST-elevation myocardial infarction and NSTE-ACS, treatment with prasugrel before stenting resulted in stronger platelet inhibition, improved endothelial function and reduced inflammation than both clopidogrel and ticagrelor.30 In contrast to the aforementioned studies, a meta-analysis of 14 pharmacodynamic studies revealed increased platelet reactivity with prasugrel than ticagrelor.31 These discrepancies between studies imply the inconsistency between testing methods and arrays in the studies and render difficult the translation to clinical value.
Strengths and limitations
To our knowledge, this is the first study to generate a network meta-analysis of randomised and outcome-driven trials and to assess the comparative efficacy and safety profiles of oral P2Y12 inhibitors in a NSTE-ACS context. A previous meta-analysis in the NSTE-ACS population jointly assessed the potent P2Y12 inhibitors in comparison to clopidogrel.32 However, given the relative small number of RCTs to directly compare ticagrelor and prasugrel, a network approach has certain advantages as it allows the incorporation of indirect evidence to the overall effect. In the current study, there was no difference between direct and indirect source of evidence for any of the comparisons in all outcomes, enhancing thus the robustness of our results. Nevertheless, certain limitations should be noted. The results are based on trial-level data and not on individual patient-level data. Randomisation in the included RCTs was not performed based on the ACS status, therefore undiscovered confounders may exist. Other confounders, such as differences in loading regimens and timing of P2Y12 administration (pretreatment or not), differences in access sites and stent preferences may have also played a role. In the sensitivity analysis, some smaller studies had a mixed population of revascularisation and conservative management but were not able to be selectively excluded from the analysis due to lack of individual patient data reporting. However, the vast majority of patients in these studies received revascularisation. There was not a unified approach to the reporting of bleeding outcomes across studies. All studies reported the composite primary outcome of cardiovascular death, non-fatal MI or non-fatal stroke, but there may be variability in the exact definitions.
Conclusion
In patients with NSTE-ACS intended to receive invasive management, an antiplatelet strategy with prasugrel was superior to ticagrelor on a moderate level of evidence, mainly due to more favourable rates in the composite cardiovascular efficacy outcome, all-cause death, MI and definite stent thrombosis. This analysis supports the current recommendations by the ESC guidelines.
Acknowledgments
This research has been co‐financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH–CREATE–INNOVATE (project code: T1EDK-04005).
Footnotes
Twitter: @itfarmakis
Contributors: ITF, SZ, ID, GK and GG contributed to the conception or design of the work. SZ, ID, AP and ITF contributed to the acquisition, analysis or interpretation of data for the work. ITF, SZ and ID drafted the manuscript. EK, DVM, NS, LKM, GK, HK and GG critically revised the manuscript. ITF acts as guarantor of the study. All gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Funding: This research has been co‐financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH–CREATE–INNOVATE (project code: T1EDK-04005).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data are available from the first and the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Rodriguez F, Harrington RA. Management of antithrombotic therapy after acute coronary syndromes. N Engl J Med 2021;384:452–60. 10.1056/NEJMra1607714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–15. 10.1056/NEJMoa0706482 [DOI] [PubMed] [Google Scholar]
- 3.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 4.Collet J-P, Thiele H, Barbato E. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of cardiology (ESC). Eur Heart J 2021:1289–367. [DOI] [PubMed] [Google Scholar]
- 5.Angoulvant D, Sabouret P, Savage MP. NSTE-ACS ESC guidelines recommend prasugrel as the preferred P2Y12 inhibitor: a Contrarian view. Am J Cardiovasc Drugs 2021;21:483–6. 10.1007/s40256-021-00471-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 7.Higgins J, Thomas J, Chandler J. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020). Cochrane, 2020. [Google Scholar]
- 8.Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 9.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 10.Rücker G, Nikolakopoulou A, Papakonstantinou T, et al. The statistical importance of a study for a network meta-analysis estimate. BMC Med Res Methodol 2020;20:190. 10.1186/s12874-020-01075-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rücker G, Krahn U, König J. netmeta: network meta-analysis using Frequentist methods, 2020. [Google Scholar]
- 12.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. Cinema: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med 2020;17:e1003082. 10.1371/journal.pmed.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roe MT, Armstrong PW, Fox KAA, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297–309. 10.1056/NEJMoa1205512 [DOI] [PubMed] [Google Scholar]
- 14.Saito S, Isshiki T, Kimura T, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J 2014;78:1684–92. 10.1253/circj.CJ-13-1482 [DOI] [PubMed] [Google Scholar]
- 15.Goto S, Huang C-H, Park S-J, et al. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome -- randomized, double-blind, phase III PHILO study. Circ J 2015;79:2452–60. 10.1253/circj.CJ-15-0112 [DOI] [PubMed] [Google Scholar]
- 16.Park D-W, Kwon O, Jang J-S, et al. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation 2019;140:1865–77. 10.1161/CIRCULATIONAHA.119.041766 [DOI] [PubMed] [Google Scholar]
- 17.Gimbel M, Qaderdan K, Willemsen L, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (popular age): the randomised, open-label, non-inferiority trial. Lancet 2020;395:1374–81. 10.1016/S0140-6736(20)30325-1 [DOI] [PubMed] [Google Scholar]
- 18.Motovska Z, Hlinomaz O, Kala P, et al. 1-Year Outcomes of Patients Undergoing Primary Angioplasty for Myocardial Infarction Treated With Prasugrel Versus Ticagrelor. J Am Coll Cardiol 2018;71:371–81. 10.1016/j.jacc.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 19.Schüpke S, Neumann F-J, Menichelli M, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med 2019;381:1524–34. 10.1056/NEJMoa1908973 [DOI] [PubMed] [Google Scholar]
- 20.Montalescot G, Bolognese L, Dudek D, et al. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med 2013;369:999–1010. 10.1056/NEJMoa1308075 [DOI] [PubMed] [Google Scholar]
- 21.Dawson LP, Chen D, Dagan M, et al. Assessment of pretreatment with oral P2Y12 inhibitors and cardiovascular and bleeding outcomes in patients with non-ST elevation acute coronary syndromes: a systematic review and meta-analysis. JAMA Netw Open 2021;4:e2134322. 10.1001/jamanetworkopen.2021.34322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarese EP, Khan SU, Kołodziejczak M, et al. Comparative Efficacy and Safety of Oral P2Y12 Inhibitors in Acute Coronary Syndrome: Network Meta-Analysis of 52 816 Patients From 12 Randomized Trials. Circulation 2020;142:150–60. 10.1161/CIRCULATIONAHA.120.046786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray A, Najmi A, Khandelwal G, et al. Prasugrel versus ticagrelor in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a systematic review and meta-analysis of randomized trials. Cardiovasc Drugs Ther 2021;35:561–74. 10.1007/s10557-020-07056-z [DOI] [PubMed] [Google Scholar]
- 24.Ullah W, Ali Z, Sadiq U, et al. Meta-Analysis comparing the safety and efficacy of prasugrel and ticagrelor in acute coronary syndrome. Am J Cardiol 2020;132:22–8. 10.1016/j.amjcard.2020.07.017 [DOI] [PubMed] [Google Scholar]
- 25.Lindholm D, Varenhorst C, Cannon CP, et al. Ticagrelor vs. clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: results from the Plato trial. Eur Heart J 2014;35:2083–93. 10.1093/eurheartj/ehu160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Servi S, Goedicke J, Schirmer A, et al. Clinical outcomes for prasugrel versus clopidogrel in patients with unstable angina or non-ST-elevation myocardial infarction: an analysis from the TRITON-TIMI 38 trial. Eur Heart J Acute Cardiovasc Care 2014;3:363–72. 10.1177/2048872614534078 [DOI] [PubMed] [Google Scholar]
- 27.Valina C, Neumann F-J, Menichelli M, et al. Ticagrelor or prasugrel in patients with non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol 2020;76:2436–46. 10.1016/j.jacc.2020.09.584 [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Zhang C, Zhao J, et al. Effects of clopidogrel, prasugrel and ticagrelor on prevention of stent thrombosis in patients underwent percutaneous coronary intervention: a network meta-analysis. Clin Cardiol 2021;44:488–94. 10.1002/clc.23536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer K, Bongiovanni D, Karschin V, et al. Ticagrelor or prasugrel for platelet inhibition in acute coronary syndrome patients: the ISAR-REACT 5 trial. J Am Coll Cardiol 2020;76:2569–71. 10.1016/j.jacc.2020.09.586 [DOI] [PubMed] [Google Scholar]
- 30.Schnorbus B, Daiber A, Jurk K, et al. Effects of clopidogrel vs. prasugrel vs. ticagrelor on endothelial function, inflammatory parameters, and platelet function in patients with acute coronary syndrome undergoing coronary artery stenting: a randomized, blinded, parallel study. Eur Heart J 2020;41:3144–52. 10.1093/eurheartj/ehz917 [DOI] [PubMed] [Google Scholar]
- 31.Wen M, Li Y, Qu X, et al. Comparison of platelet reactivity between prasugrel and ticagrelor in patients with acute coronary syndrome: a meta-analysis. BMC Cardiovasc Disord 2020;20:430. 10.1186/s12872-020-01603-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bavishi C, Panwar S, Messerli FH, et al. Meta-analysis of comparison of the newer oral P2Y12 inhibitors (prasugrel or ticagrelor) to clopidogrel in patients with non-ST-elevation acute coronary syndrome. Am J Cardiol 2015;116:809–17. 10.1016/j.amjcard.2015.05.058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2021-001937supp001.pdf (23.7MB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are available from the first and the corresponding author on reasonable request.