Abstract
Insufficient dietary intake of micronutrients contributes to the onset of deficiencies termed hidden hunger—a global health problem affecting approximately 2 billion people. Vitamin B1 (thiamine) and vitamin B6 (pyridoxine) are essential micronutrients because of their roles as enzymatic cofactors in all organisms. Metabolic engineering attempts to biofortify rice endosperm—a poor source of several micronutrients leading to deficiencies when consumed monotonously—have led to only minimal improvements in vitamin B1 and B6 contents. To determine if rice germplasm could be exploited for biofortification of rice endosperm, we screened 59 genetically diverse accessions under greenhouse conditions for variation in vitamin B1 and vitamin B6 contents across three tissue types (leaves, unpolished and polished grain). Accessions from low, intermediate and high vitamin categories that had similar vitamin levels in two greenhouse experiments were chosen for in-depth vitamer profiling and selected biosynthesis gene expression analyses. Vitamin B1 and B6 contents in polished seeds varied almost 4-fold. Genes encoding select vitamin B1 and B6 biosynthesis de novo enzymes (THIC for vitamin B1, PDX1.3a–c and PDX2 for vitamin B6) were differentially expressed in leaves across accessions contrasting in their respective vitamin contents. These expression levels did not correlate with leaf and unpolished seed vitamin contents, except for THIC expression in leaves that was positively correlated with total vitamin B1 contents in polished seeds. This study expands our knowledge of diversity in micronutrient traits in rice germplasm and provides insights into the expression of genes for vitamin B1 and B6 biosynthesis in rice.
Keywords: rice, vitamin B1, vitamin B6, natural variation, germplasm, biofortification, micronutrient deficiency, hidden hunger
Introduction
Vitamin B1 (thiamine) and vitamin B6 (pyridoxine) are among the water-soluble vitamins that are essential micronutrients for humans and other animals. Several chemically related forms of vitamin B1 and B6 are found, termed vitamers, with thiamine diphosphate (TDP) and pyridoxal-5′-phosphate (PLP) the most well-known vitamers, respectively, owing to their known coenzyme functions in primary metabolism across all kingdoms (Colinas and Fitzpatrick, 2015). As humans obtain the bulk of vitamins from dietary sources, micronutrient deficiency disorders often arise when dietary intake is insufficient, either alone or in concert with congenital factors such as impaired vitamin metabolism and/or certain lifestyles (Bailey et al., 2015). Micronutrient deficiency (“hidden hunger”) is a global health problem, with over 2 billion people estimated to have one or more micronutrient deficiencies (Bailey et al., 2015). Monotonous consumption of staple crops with low dietary levels of vitamin B1 and B6 contributes to non-attainment of the recommended dietary allowance (RDA) of vitamin B1 (for adults: 1.1–1.3 mg/day) and B6 (for adults: 1.3–2.0 mg/day; Fitzpatrick et al., 2012; Titcomb and Tanumihardjo, 2019). Additional factors may further contribute to the inability to reach micronutrient RDAs, such as post-harvest deterioration, certain food processing and preparation practices, or consumption of foodstuffs containing vitamin-degrading enzymes (Van Der Straeten et al., 2020). Furthermore, the COVID-19 pandemic has reduced incomes in 63 low- and middle-income countries and increased the proportion of people unable to afford healthy diets, with implications for calorific and micronutrient deficiencies (Laborde et al., 2021).
Vitamin B1 deficiency disorders (also known as thiamine deficiency disorders) are endemic in several low- and middle-income countries in India, Asia, and Africa, in particular where polished rice is a major source of calories and interventions to improve dietary micronutrient intake are absent (Johnson et al., 2019; Whitfield et al., 2021). Deficiency in vitamin B1 may lead to a broad spectrum of neurological disorders including beriberi and tropical ataxia neuropathy (Whitfield et al., 2021), with Wernicke’s encephalopathy and Wernicke-Korsakoff syndrome prevalent in alcoholics (Isenberg-Grzeda et al., 2012; Latt and Dore, 2014; Dhir et al., 2019). Vitamin B1 deficiency also occurs in high income countries and may arise through diverse pathophysiological mechanisms beyond alcoholism (Gomes et al., 2021; Onishi et al., 2021). Congenital defects in vitamin B1 metabolism are also known to result in deficiency disorders (Ortigoza-Escobar et al., 2016). Although data at the population level is lacking for vitamin B1 deficiency compared to other micronutrients, studies on Chinese cohorts showed 91.8% of children and 81.7% of adults over 60 years do not reach their dietary estimated daily intake of vitamin B1 (Wang et al., 2017; Liu et al., 2019). Vitamin B6 deficiency may manifest with seizures and other neurological events, because of the role of PLP in neurotransmitter biosynthesis (Wilson et al., 2019; Akiyama et al., 2020; Rojo-Sebastián et al., 2020). Genetic defects in vitamin B6 metabolism could also lead to vitamin B6-dependent epilepsy (Wilson et al., 2019). Vitamin B6 deficiencies have been associated with low socioeconomic status (Liu et al., 2019; Zhu et al., 2020), certain medications (Lussana et al., 2003; Allen et al., 2015; Porter et al., 2019) and a range of diseases including inflammation (Paul et al., 2013), diabetes (Nix et al., 2015; Porter et al., 2019), cardiovascular disease (Dhalla et al., 2013; Wei and Ji, 2020), and certain cancers (Gylling et al., 2017). Lee (2021) reviews in detail vitamin B6 deficiency and women’s health issues. In countries where vitamin B6 status has been studied at the population level, 24% of Americans are at risk or deficient in vitamin B6 (Bird et al., 2017), rising to one-third in South Korea (Kim and Cho, 2014). Among a Chinese cohort, 95.1% of subjects aged 60 years and over were marginal or deficient for vitamin B6 status (Liu et al., 2019). Increased dietary intake of vitamin B1 and B6 is therefore likely to assist in mitigating respective deficiency disorders in a diverse range of populations (Titcomb and Tanumihardjo, 2019).
In addition to the well-established roles as enzymatic cofactors and the micronutrient deficiency disorders that may arise in turn when scarce, B1 and B6 vitamers are implicated in environmental stress responses in plants (Fitzpatrick, 2011; Fitzpatrick and Chapman, 2020). Both vitamins exhibit antioxidant activity in vitro (Gliszczyńska-Świgło, 2006; Czégény et al., 2019) and vitamin B6 can quench singlet oxygen in plants (Denslow et al., 2005) and fungi (Bilski et al., 2000). In cases where mutant alleles are non-lethal, downregulation or knock out of certain vitamin B1 and B6 metabolism genes typically results in stunting or increased susceptibility to various plant pathogens (Fudge et al., 2017; Fitzpatrick and Chapman, 2020). Protective effects of both vitamin B1 and B6 have been observed under abiotic stress, together with an induction of biosynthesis de novo (Ribeiro et al., 2005; Denslow et al., 2007; Rapala-Kozik et al., 2008; Tunc-Ozdemir et al., 2009; Rapala-Kozik et al., 2012; Huang et al., 2013; Moccand et al., 2014; Dell’Aglio et al., 2017). Exogenous vitamin B1 provision could induce expression of defense-related genes and “prime” plants for enhanced resistance upon subsequent pathogen challenge (Ahn et al., 2005, 2007; Boubakri et al., 2012; Huang et al., 2016). Mutants impaired in vitamin B6 metabolism are hyper-sensitive to abiotic stress and are susceptible to pathogen challenge (Vanderschuren et al., 2013; Zhang et al., 2015; Samsatly et al., 2020). Despite this body of evidence, relatively little is known about the molecular dialog between stress signaling and vitamin metabolism. Hanson et al. (2016) hypothesized that protective effects from exogenous vitamin supplementation complement acute cofactor deficiencies in planta upon stress, as opposed to simply having direct antioxidant properties. Taken together, crop varieties with high vitamin B1 and B6 contents may confer some resistance to stress through being poised to readily supply TDP and PLP to apoenzymes when demands on primary metabolism increase.
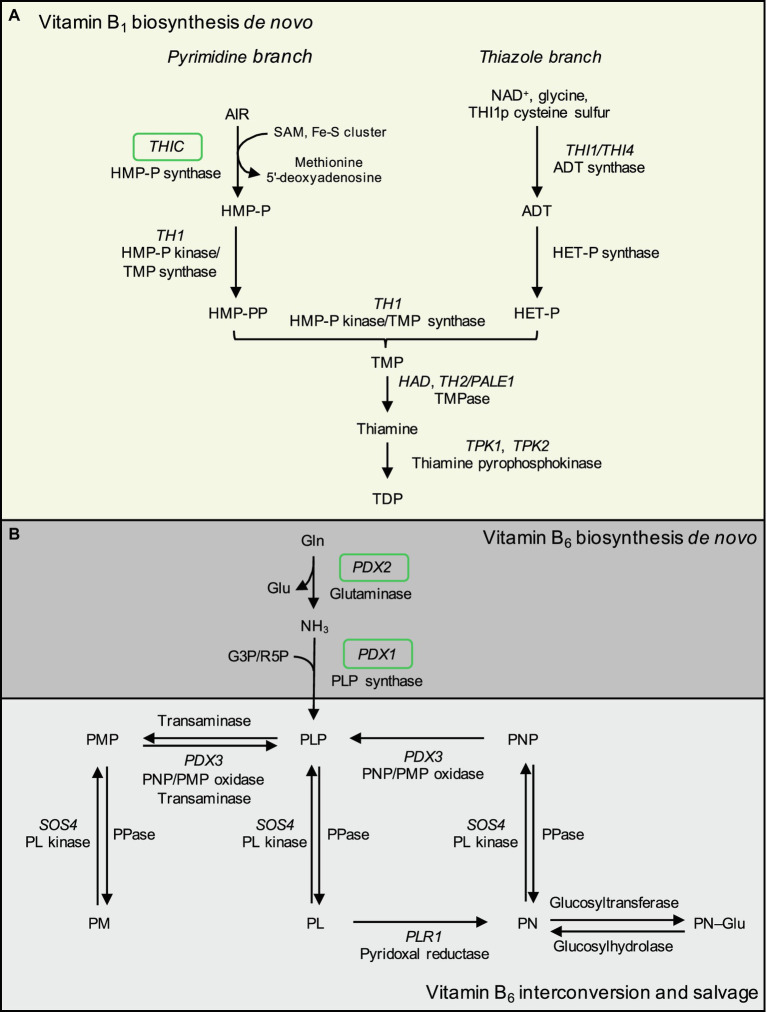
Biosynthesis de novo of vitamin B1 and B6 in plants (Figure 1) has largely been unraveled in model species, chiefly Arabidopsis (Arabidopsis thaliana, At), maize (Zea mays, Zm), and tobacco (Nicotiana tabacum, Nt; Colinas and Fitzpatrick, 2015). This has permitted rational design of metabolic engineering strategies toward biofortifying staple crops with enhanced levels of these vitamins in consumed tissues (Fudge et al., 2017; Goyer, 2017; Strobbe and Van Der Straeten, 2018). However, several limitations have been reported, including marginal increases in vitamin contents through upregulation of biosynthetic pathways, with limited over-accumulation of vitamins in the target tissues and organs (Dong et al., 2015, 2016; Mangel et al., 2019; Strobbe et al., 2021a,b). Further effort is therefore required to overcome bottlenecks to biofortify vitamin B1 and B6 in rice endosperm at meaningful levels to approach respective RDAs.
Figure 1.
Biosynthesis de novo of vitamin B1 and B6 in plants based on Arabidopsis. (A) Vitamin B1 biosynthesis de novo is predominantly localized to the chloroplast and comprises formation of pyrimidine and thiazole heterocycles linked by a methylene bridge. The pyrimidine moiety of vitamin B1 is supplied via conversion of 5-amino-imidazole ribotide (AIR) into 4-amino-5-hydroxymethyl-2-pyrimidine phosphate (HMP-P) by HMP-P synthase (encoded by THIC) in an iron-sulfur cluster and SAM-dependent reaction, with the release of methionine and a 5′-deoxyadenosine radical. Next, HMP-P is phosphorylated to HMP-PP by the bifunctional HMP-P kinase/TMP synthase (encoded by THIAMIN REQUIRING 1, TH1). The thiazole moiety of vitamin B1 is produced from nicotinamide adenine dinucleotide (NAD+), glycine, and a sulfur atom donated from a conserved cysteine residue from the single-turnover enzyme ADT synthase/THI4 (encoded by THI1 in plants, THI4 in yeast) to produce adenosine diphosphate thiazole (ADT). ADT is converted to hydroxyethylthiazole phosphate (HET-P) by HET-P synthase activity (loci encoding this activity currently unconfirmed). HET-P and HMP-PP are condensed by TMP synthase activity of TH1 to form thiamine monophosphate (TMP). TMP is dephosphorylated by TMP phosphatases (TMPase) including TH2/PALE1 (THIAMIN REQUIRING 2/PALE GREEN 1) and HALOACID DEHALOGENASE (HAD), forming free thiamine. The cofactor vitamer thiamine diphosphate (TDP) is generated by diphosphorylation of free thiamine at the hydroxyl position by thiamine pyrophosphokinases (encoded by AtTPK1–2, OsTPK1–3). (B) Vitamin B6 biosynthesis de novo is localized to the cytosol to form the cofactor vitamer pyridoxal 5′-phosphate (PLP). PDX2 is a glutaminase that yields glutamate and ammonia, the latter is used by PLP synthase (PDX1, encoded by PDX1.1–1.3 in Arabidopsis and PDX1.3a–c in rice) to form PLP with the addition of either ribose 5-phosphate (R5P) or glyceraldehyde 3-phosphate (G3P). B6 vitamers are interconverted in a salvage pathway with known components localized to the cytosol, mitochondria, and chloroplasts. Unknown phosphatases convert phosphorylated vitamers to non-phosphorylated vitamers. Pyridoxal kinase (encoded by SALT OVERLY SENSITIVE 4, SOS4) phosphorylates pyridoxal (PL), pyridoxine (PN), and pyridoxamine (PM) at the 5′ position. PL is converted to PN by pyridoxal reductase (encoded by PLR1). The PNP/PMP oxidase (encoded by PDX3) converts pyridoxine 5′-phosphate (PNP) and pyridoxamine 5′-phosphate (PMP) to PLP. Unknown glucosyltransferases and glucosylhydrolases convert PN to PN–Glu and vice versa. At: Arabidopsis thaliana. Os: Oryza sativa. Green boxes denote genes assayed by qRT-PCR in this study.
Germplasm of certain crops has proven a useful source to biofortify maize, cassava, and sweet potato with provitamin A (ß-carotene; Bouis and Saltzman, 2017; Bechoff et al., 2018; Govender et al., 2019; Foley et al., 2021). Several crop species including cassava, potato, quinoa, rice, maize, wheat, and pulses have been studied for variation in B complex vitamins (Villareal and Juliano, 1989; Sotelo et al., 1990; Kennedy and Burlingame, 2003; Goyer and Navarre, 2007; Dong et al., 2011, 2014; Goyer and Haynes, 2011; Goyer and Sweek, 2011; Shewry et al., 2011; Robinson et al., 2015; Nowak et al., 2016; Singh et al., 2016; Mangel et al., 2017; Zarei et al., 2017; Bali et al., 2018; Freitag et al., 2018; Granda et al., 2018; Li et al., 2018; Guo et al., 2019; Riaz et al., 2019; Robinson et al., 2019; Jha et al., 2020). However, only some of these studies have sought to understand the genetic basis underpinning such variation and/or carried out detailed vitamer profiling and quantification by analytical techniques such as HPLC. Knowledge of constituent vitamer profiles and molecular determinants for high vitamin content accessions could aid the introgression of desirable alleles into farmer-preferred crop varieties to deliver bioavailable vitamers to consumers most in need. Such detailed knowledge of rice germplasm is limited with respect to vitamin B1 (Villareal and Juliano, 1989; Sotelo et al., 1990; Kennedy and Burlingame, 2003), while vitamin B6 contents are known only for three rice varieties from the United States (Zarei et al., 2017). No information on the molecular mechanisms behind such variation in rice have been published to our knowledge.
Rice is a staple crop for 50% of the world’s population, including some of the world’s poorest (Bhullar and Gruissem, 2013). Cooked white, polished rice (lacking the seed coat and embryo) is a poor source of several micronutrients, including both vitamin B1 and B6, when considered as the source of 80% of the daily calorific intake (Fitzpatrick et al., 2012; de Pee, 2014). As much as 90–98% of grain vitamin B1 contents are lost during polishing (Dong et al., 2016; Strobbe et al., 2021a) and up to 85% of vitamin B6 (Mangel et al., 2019). Therefore, rice endosperm is a rational target for improvement of vitamin B1 and B6 contents. Toward obtaining the upper ranges of the respective RDA (for lactating women) from a 233 g serving of cooked polished rice, 32- and 13.6-fold increases in vitamins B1 and B6 are required in polished seeds (Fudge et al., 2017).
Here, toward determining if rice germplasm could be exploited for endosperm biofortification with vitamin B1 and B6, we performed an in-depth characterization of a genetically diverse panel of 59 rice accessions grown under greenhouse conditions. The selected accessions spanning three subspecies were sourced from geographically diverse countries and vary in their resistance and susceptibility to major rice diseases. Accessions with contrasting vitamin contents in three tissue types were selected for in-depth vitamer profiling by HPLC. RT-qPCR assays of selected vitamin biosynthesis de novo genes revealed differential expression across the accessions, which did not correlate with vitamin contents in leaves. Accessions profiled here with the highest polished seed vitamin B1 and B6 contents did not display sufficient levels of vitamin B1 and B6 to meet the respective RDAs. Future efforts ought to consider the use of substantially larger germplasm panels, alongside additional metabolic engineering strategies, toward combatting vitamin B1 and B6 deficiencies for rice consumers in greatest need.
Materials and Methods
Plant Material, Growth, and Sampling Conditions
Rice accessions (Supplementary Table S1) were obtained as dry seeds from the International Rice Research Institute, Philippines, and selected using the International Rice GeneBank Collection Information System.1 Dry seeds were dehusked and surface sterilized in 70% (v/v) ethanol for 30 s, followed by 30 min of agitation in 1.5% (v/v) sodium hypochlorite solution +0.01% (v/v) Tween-20. Seeds were then rinsed five times in sterile water and then transferred to sterile plastic jars containing full-strength MS media (Murashige and Skoog, 1962) supplemented with 3% (w/v) sucrose and 0.3% (w/v) Gelrite, at pH 5.8. Seeds were incubated in darkness at 28°C for 48 h, before transfer to a growth cabinet for 12 d under 16 h light and 8 h darkness at 28°C. Seedlings were then transferred to a greenhouse under controlled conditions (12 h artificial light at 30°C and 80% humidity, 12 h darkness at 22°C, and 60% humidity). Three seedlings of the same accession were planted in one pot (pot diameter 18 cm, 12 cm height).
For experiment 1, 49 accessions were propagated simultaneously in growth cabinets prior to transfer of the seedlings to a greenhouse (June–August 2013, Eschikon, Switzerland). In a second independent experiment (hereinafter called experiment 2), 21 selected accessions grown in experiment 1 were propagated for a second time under greenhouse conditions (August 2014–October 2014, Eschikon, Switzerland), together with 10 additional accessions from the Oryza SNP Sequencing Project (McNally et al., 2009), resulting in 31 accessions grown in experiment 2 and a total of 59 accessions screened across both experiments. Sampling was consistently performed in the morning (10.30 am–12 pm). Leaves were sampled from three different tillers of 50 days old vegetative plants in experiment 1 and 40 days old plants in experiment 2. Leaf tissue was pooled, snap frozen in liquid nitrogen, and stored at −80°C until further use. Fully ripened panicles (maturing at different times owing to variation in heading dates) were harvested and dried for 5 d at 37°C. Dried, mature seeds were dehusked (referred to hereinafter as unpolished seeds) and stored at −80°C until further use, or polished for 2 min in a PEARLEST polisher (Kett) to remove the embryo, aleurone layer, and seed coat (polished seeds), and stored at −80°C until further use.
Vitamin B1 and B6 Quantification
Vitamin B1
For vitamin B1, a Saccharomyces cerevisiae thi4 mutant deficient in vitamin B1 biosynthesis de novo was used for quantification of total vitamin B1 from rice tissues from plants grown in experiments 1 and 2 (Raschke et al., 2007). Fifty milligrams of frozen, ground rice leaves or seeds were used for extraction of vitamin B1 in 500 μl of 20 mM sulfuric acid in darkness at room temperature for 30 min, before heating to 100°C for 1 h. The solution was adjusted to pH 5.7 with 3 M sodium acetate and centrifuged. To convert phosphorylated B1 vitamers to free thiamine to permit uptake by yeast, supernatants were treated with acid phosphatase (0.2 U/10 μl per 50 μl of plant extract) overnight for 12–15 h at 37°C. Total vitamin B1 was calculated from the linear range of a standard curve prepared with 5–100 ng of thiamine hydrochloride provided to the thi4 yeast mutant in thiamine-deficient media.
Samples from candidate accessions with contrasting total vitamin B1 contents were selected for confirmation and vitamer profiling of thiochrome derivatives by HPLC using a method first described by Moulin et al. (2013). Fifty milligrams of frozen, ground rice leaves or seeds were used for extraction of soluble vitamin B1 in 100 μl in 1% (v/v) trichloroacetic acid by aggressive vortexing at room temperature for 30 min. Samples were centrifuged at full speed in a tabletop microcentrifuge for 10 min at room temperature. The clear supernatant was neutralized with 3 M sodium acetate to 10% of the final volume and oxidized to thiochrome derivatives using 15 μl of freshly prepared 30 mM potassium ferracyanide in 15% (w/v) NaOH, with 15 μl 1 M NaOH and 25 μl methanol according to Moulin et al. (2013). Samples were injected into an Agilent Technologies 1260 HPLC to determine vitamer profiles by separation of thiochrome derivatives on a Cosmosil π-NAP column (150 × 4.6 mm, 3 μm pore size) using a methanol gradient at 1 ml min−1 detailed in Moulin et al. (2013), with a 40 min run time. Peaks of fluorescence corresponding to the retention time of the commercial standards of B1 vitamers TDP (Sigma), thiamine monophosphate (TMP; Fluka), and thiamine (Fluka) were integrated and extrapolated against a standard curve for each vitamer. Peak area was integrated only from non-saturated peaks and in cases of peak saturation, samples were reinjected in lower volumes. Injection volumes ranged from 10 to 40 μl.
Data for leaf samples were normalized to fresh weight (FW) and seed samples to dry weight (DW).
Vitamin B6
For vitamin B6, a Saccharomyces pastorianus American Type Culture Collection 9080 strain was used (Tambasco-Studart et al., 2005) as reported (Mangel et al., 2019). Fifty milligrams of frozen, ground rice leaves or seeds were used for extraction of vitamin B6 in 500 μl of 20 mM sulfuric acid in darkness at room temperature for 30 min, before heating to 100°C for 1 h. The solution was adjusted to pH 5.7 with 3 M sodium acetate and centrifuged. To convert phosphorylated and glucosylated B6 vitamers to non-phosphorylated vitamers to permit uptake by yeast, supernatants were treated with acid phosphatase and β-glucosidase (0.2 U/10 μl of each enzyme per 50 μl of plant extract) overnight for 12–15 h at 37°C. Total vitamin B6 was calculated from the linear range of a standard curve prepared with 0.15–2.4 ng of pyridoxine hydrochloride provided to the yeast mutant in pyridoxine-deficient media.
Samples from candidate rice accessions with contrasting total vitamin B6 contents were selected for vitamer profiling by HPLC using an established protocol (Szydlowski et al., 2013). Fifty milligrams of frozen, ground rice leaves or seeds were used for extraction of vitamin B6 in 100 μl of 50 mM ammonium acetate pH 4.0 with aggressive vortexing for 10 min at room temperature. Samples were centrifuged at full speed in a tabletop microcentrifuge for 15 min at room temperature. The supernatant was incubated for 3 min at 99°C and again centrifuged for 15 min at room temperature before analysis. Extracts were injected into an Agilent Technologies 1200 HPLC to separate B6 vitamers on a Sunfire C18 column (Waters), 4.6 × 150 mm, 3.5 μm particle diameter, with post-column derivatization in 0.7 M potassium phosphate buffer with 1 g L−1 sodium bisulfite added freshly, flow rate 0.3 ml min−1. Samples were separated on an isocratic gradient of 50 mM ammonium acetate pH 4.0, flow rate 1 ml min−1 in a 40 min run time. Quantification was carried out using the linear range of a standard curve constructed with known amounts of standards (Colinas et al., 2016), with vitamin B6 glucoside (PN–Glu) determination calculated as PN equivalents (Mangel et al., 2019). Standards were prepared and injected into the HPLC with every set of extractions. Peak area was integrated only from non-saturated peaks and in cases of peak saturation, samples were reinjected in lower volumes. Injection volumes were typically 10–30 μl.
Data for leaf samples were normalized to fresh weight (FW) and seed samples to dry weight (DW).
RNA Isolation and Gene Expression Analyses
RNA was isolated from rice leaf samples using an established protocol (Chang et al., 1993) with Mangel et al. (2019). Frozen, homogenized tissue was mixed with 1 ml of extraction buffer (2% w/v polyvinylpyrrolidone K-30, 100 mM Tris-HCl pH 8.0, 25 mM ethylenediaminetetraacetic acid, 2 M NaCl, and 0.5 g L−1 spermidine) and 2% (v/v) β-mercaptoethanol added freshly. Samples were incubated at 50°C for 15 min with agitation at 400–500 rpm, before being extracted twice with 1 volume of chloroform:isoamylalcohol (24:1, pH 7.5–8.0). Nucleic acids were recovered from the aqueous phase by absolute ethanol precipitation for 30 min at −80°C followed by centrifugation at top speed in a microcentrifuge for 30 min at 4°C. The pellet was washed in 80% (v/v) ethanol and resuspended in diethyl pyrocarbonate (DEPC)-treated water. RNA was precipitated overnight at −20°C in 2 M lithium chloride and collected by centrifugation at top speed in a microcentrifuge for 30 min at 4°C. The RNA pellet was washed sequentially in 80% (v/v) and absolute ethanol, before vacuum drying and resuspension in DEPC-treated water. RNA was quantified spectrophotometrically (Nanodrop) and stored at −80°C until further use. Expression of selected genes encoding vitamin B1 and B6 biosynthesis de novo enzymes was assayed by RT-qPCR. Two micrograms of total RNA were converted to cDNA with random hexamer oligonucleotide primers using the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher) in accordance with the manufacturer’s instructions. Real-time qPCR reactions were prepared in 10 μl reaction volumes comprising 5 μl of 2x Fast SYBR Green master mix (Applied Biosystems), 2 μl of template cDNA diluted 5-fold in DEPC-treated water, 1 μl each of forward and reverse primer (1 μM working concentration), and 1 μl DEPC-treated water. A Roche LightCycler 480 II real-time qPCR machine was used with an initial denaturation at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 20 s, and extension at 72°C for 30 s. Target gene expression was normalized to the OsUBQ5 reference gene (Jain et al., 2006) and relative expression calculated using the delta-delta Ct method (Livak and Schmittgen, 2001). Samples for RT-qPCR were pipetted in duplicate and amplicon identity confirmed by melt curve analysis and Sanger sequencing. Primers for RT-qPCR were developed using Nipponbare genome sequences obtained from Phytozome v7_JGI (Goodstein et al., 2012). Sequences of oligonucleotide primers used for RT-qPCR are listed in Supplementary Table S2. Conservation of oligonucleotide primer binding sites in sequenced Oryza sativa accessions could be confirmed for Nipponbare (japonica), IR64 (indica), and I-Kung-Pao (indica), by search of the rice Molecular Breeding Knowledgebase.2 Multiple alignments were assembled for alleles belonging to these three accessions and the number of accessions from each subspecies belonging to a given GID group (i.e., bearing a given allele) are shown in Supplementary Figure S1. qRT-PCR threshold cycle (Ct) values for the reference gene UBQ5 are shown in Supplementary Table S3.
Statistical Analyses
Data were analyzed in GraphPad Prism (version 9) and R (R Core Team, 2013). To determine the effect of accession on vitamin contents and leaf biosynthetic gene expression levels, one-way ANOVA tests were carried out with post-hoc Tukey tests ( = 0.05) to correct for multiple comparisons. Statistically significant differences between accessions are denoted by different letters in respective figures and tables. For correlation analyses of total vitamin contents with leaf biosynthetic gene expression levels, Pearson’s correlation coefficients were determined in GraphPad Prism with two-tailed value of p tests and 95% confidence intervals. Standard deviations of ratios were estimated using the Taylor expansion formula.
Results
Vitamin B1 and B6 Contents in 59 Genetically Diverse Rice Accessions
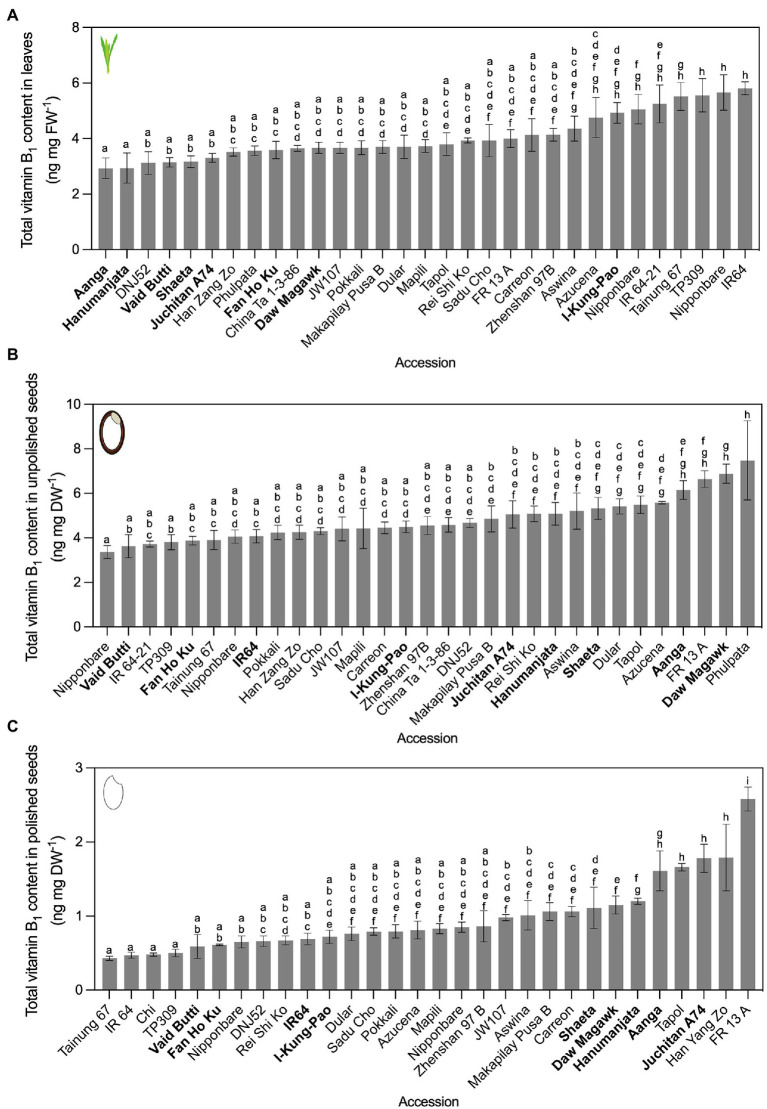
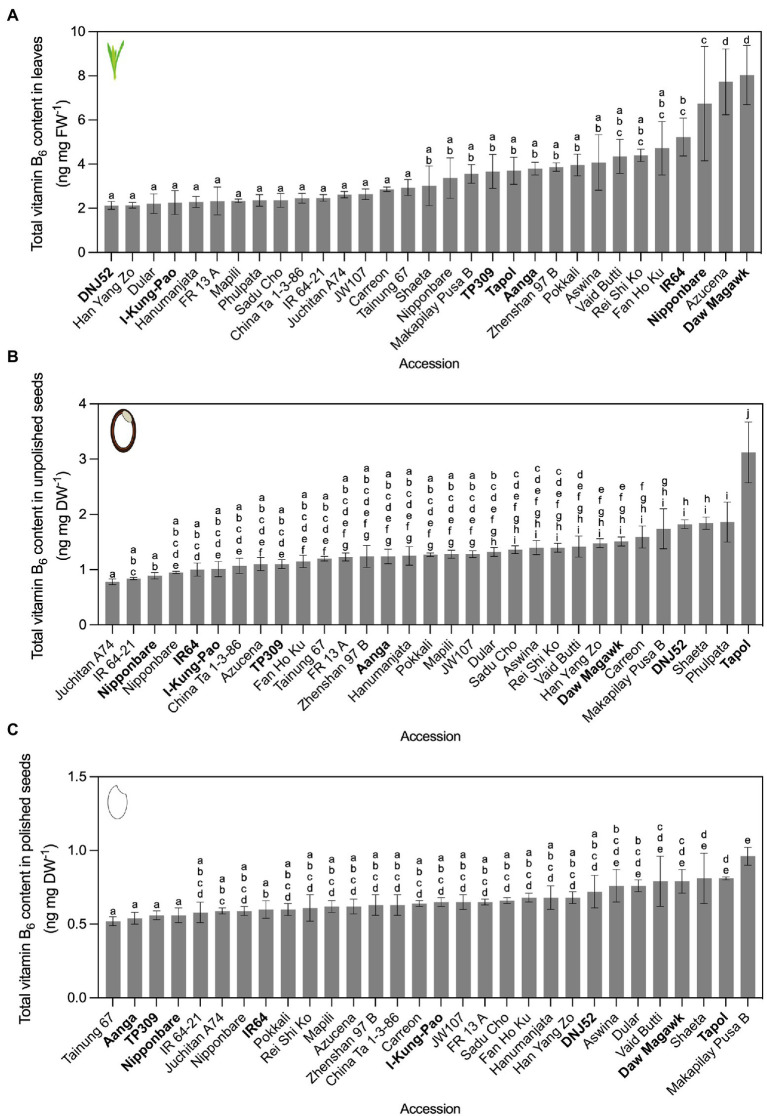
To obtain insights into the diversity of vitamin B1 and B6 contents in rice germplasm, we selected a total of 59 accessions for analysis from geographically diverse countries (Supplementary Table S1). Selected accessions included landraces and modern breeding lines spanning O. sativa subspecies japonica, indica, and javanica, ten accessions characterized in the OryzaSNP project (McNally et al., 2009), and with contrasting susceptibility and resistance to major rice diseases. These accessions were cultivated under greenhouse conditions across two independent plantings (experiment 1 and 2). To control for seasonal differences in greenhouse cultivation between the two independent plantings, plants were phenotyped in each experiment. The range of measured variation for accessions cultivated twice remained largely similar for plant height, number of panicles, and leaf dry weight (Supplementary Table S4). Total vitamin B1 and B6 contents in leaves, unpolished seeds, and polished seeds were first quantified using a yeast assay (Tambasco-Studart et al., 2005; Raschke et al., 2007; Figures 2, 3; Supplementary Tables S5, S6). Statistically significant differences in vitamin contents between accessions were determined in all cases by one-way ANOVA with multiple comparisons and Tukey’s post-hoc tests (α = 0.05). Accessions were placed into low, intermediate or high vitamin content groups based on their ranking below the 25th percentile, between the 25th and 75th percentile, or above the 75th percentile, respectively. Groups below the 25th percentile and above the 75th percentile were referred to as contrasting groups.
Figure 2.
Vitamin B1 contents quantified by yeast assay. Leaves (A), unpolished seeds (B), and polished seeds (C) in rice accessions grown under greenhouse conditions and quantified by yeast assay. Twenty-one accessions cultivated in experiment 1 were re-sown, alongside 10 additional accessions from the Oryza SNP Project. The accessions with vitamin B1 content below the 25th percentile of the distribution were considered as low vitamin B1 accessions and those with vitamin content above the 75th percentile were considered as high vitamin B1 accessions. Low, intermediate, and high vitamin B1 accessions selected for HPLC analysis are in bold. Data are mean ± SD of 3 biological replicates, except Nipponbare (n = 6), IR64 (n = 6) and TP309 (n = 6) for the three tissues; IR64-21 (n = 2) for leaves; Nipponbare and IR-64-21 are n = 2 for unpolished seeds; and Nipponbare (n = 2), IR64-21 (n = 2) and Phulpata (n = 0) for polished seeds. The effect of accession on total vitamin B1 contents in panels (A–C) was determined by one-way ANOVA (α = 0.05) with multiple comparisons and Tukey’s post-hoc test. Statistically significant differences between accessions are denoted by different letters.
Figure 3.
Vitamin B6 contents quantified by yeast assay. Leaves (A), unpolished seeds (B), and polished seeds (C) in contrasting rice accessions grown under greenhouse conditions and quantified by yeast assay. Twenty-one accessions cultivated in experiment 1 were re-sown, alongside 10 additional accessions from the Oryza SNP Project (experiment 2). The accessions with vitamin B6 content below the 25th percentile of the distribution were considered as low vitamin B6 accessions and those with vitamin content above the 75th percentile were considered as high vitamin B6 accessions. Low, intermediate, and high vitamin B6 accessions selected for HPLC analysis are bolded. Mean ± SD of 3 biological replicates, except Nipponbare (n = 6), IR64 (n = 6), and TP309 (n = 6) for the three tissues; IR 64–21 (n = 2) for leaves; Nipponbare (n = 2) and IR 64–21 (n = 2) for unpolished seeds; and Nipponbare (n = 2), IR 64–21 (n = 2) and Phulpata (n = 0) in polished seeds. The effect of accession on total vitamin B6 contents in panels (A–C) was determined by one-way ANOVA (α = 0.05) with multiple comparisons and Tukey’s post-hoc test. Statistically significant differences between accessions are denoted by different letters.
In experiment 1, 49 accessions were cultivated, with leaf tissues harvested from vegetative plants 50 days after germination (Supplementary Table S5A). Leaf vitamin B1 contents varied 3.32-fold, with Hanumanjata at 1.11 ng mg FW−1 as the lowest and Tapol with 3.68 ng mg FW−1 as the highest. Unpolished seed vitamin B1 contents varied 3.9-fold, from 2.35 ng mg DW−1 for Vaid Butti to Phulpata at 9.15 (± 1.11) ng mg DW−1 (Supplementary Table S5B). This was similar to previously reported ranges when converted to ng mg DW−1 (Villareal and Juliano, 1989; Sotelo et al., 1990; Kennedy and Burlingame, 2003), serving as benchmarks for six accessions assayed both here and previously. Polished seed vitamin B1 contents varied 2.72-fold, from 0.65 ng mg DW−1 for Hsinchu 56 to Hang Yang Zo at 1.76 ng mg DW−1 (Supplementary Table S5C). Twenty-one accessions across the three percentile groups from experiment 1 were cultivated a second time under the same greenhouse conditions for experiment 2, together with 10 additional accessions from the Oryza SNP Project (McNally et al., 2009). For vitamin B1 contents in experiment 2, leaf samples varied 1.98-fold, ranging from 2.93 ng mg FW−1 in Aanga to 5.81 ng mg FW−1 in IR64 (Figure 2A). Unpolished seed vitamin B1 contents varied 2.23-fold, from 3.36 ng mg DW−1 for Nipponbare to Phulpata at 7.48 ng mg DW−1 (Figure 2B). Polished seed vitamin B1 contents varied 6.06-fold, from 0.43 ng mg DW−1 for Tainung 67 to FR 13 A at 2.58 ng mg DW−1 (Figure 2C). Seed polishing led to a reduction in vitamin B1 content ranging between 57.9 and 89.3% (Supplementary Table S6). Although vitamin B1 contents showed some variation between experiment 1 and 2, the ranking of certain accessions based on vitamin B1 contents was not significantly different between the two experiments (Figure 2; Supplementary Table S5). Based on vitamin B1 contents, nine accessions from experiment 2 were selected for confirmation and in-depth vitamer profiling by HPLC and biosynthetic gene expression assays (see below).
Variation in vitamin B6 contents in rice germplasm was probed in an identical screening strategy as for vitamin B1. In experiment 1, vitamin B6 in leaves varied 4.64-fold, from 0.88 ng mg FW−1 in Hanumanjata to 4.07 ng mg FW−1 in Indane (Supplementary Table S7A). Unpolished seed vitamin B6 contents varied 3.9-fold, from 0.75 ng mg DW−1 for IR64 to Tapol at 2.49 ng mg DW−1 (Supplementary Table S7B). Polished seed vitamin B6 contents varied 2.94-fold, from 0.34 ng mg DW−1 for Fan Ho Ku to Phulpata at 1.01 ng mg DW−1 (Supplementary Table S7C). In experiment 2, vitamin B6 in leaves varied 3.77-fold, from 2.13 ng mg FW−1 in DNJ52 to 8.04 ng mg FW−1 in Daw Magawk (Figure 3A), with higher ranges compared to experiment 1. Seed samples were more stable in terms of variation between experiments compared to leaves, with unpolished seed vitamin B6 contents varying 4.0-fold, from 0.75 ng mg DW−1 for IR64 to Tapol at 3.12 ng mg DW−1 (Figure 3B). Consistent with our previous report (Mangel et al., 2019), polishing of rice seeds (that is, removal of the seed coat, embryo, and aleurone) results in losses of vitamin B6 contents in seeds for dietary intake, with a reduction in vitamin B6 content between unpolished and polished seeds ranging from 20% (Juchitan A74) to 76.5% (Aanga; Figure 3C; Supplementary Table S8). Similar to the vitamin B1 greenhouse screen results, vitamin B6 contents and fold changes also showed some variation between experiments. Several accessions from contrasting groups maintained similar vitamin B6 contents and eight were selected from experiment 2 for confirmation and vitamer profiling by HPLC and biosynthetic gene expression assays (see below).
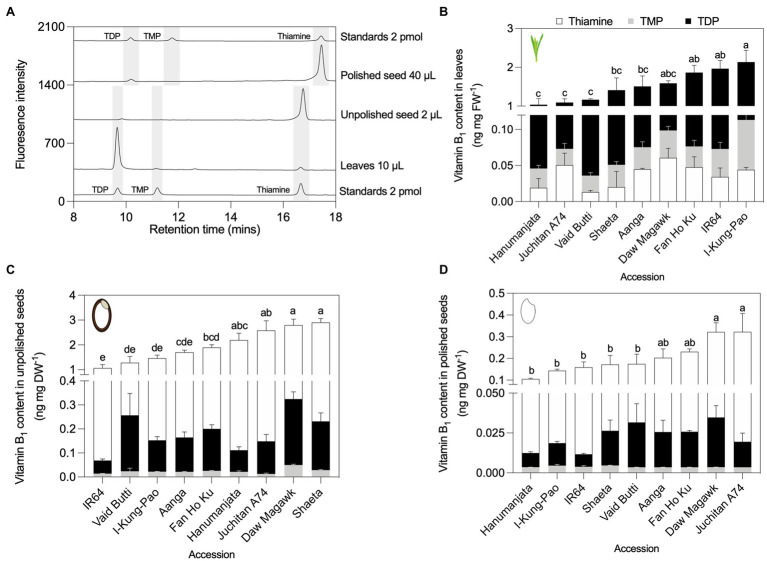
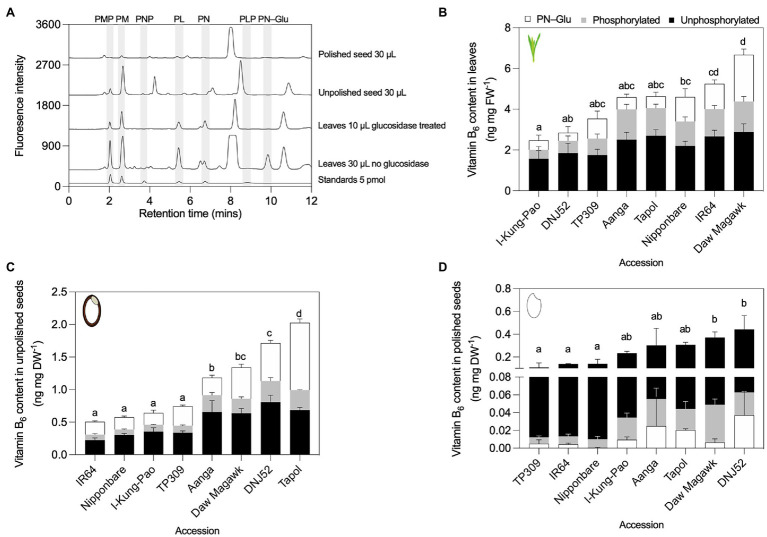
B1 and B6 Vitamer Profiling by HPLC of Three Tissues in Contrasting Rice Accessions
To validate the yeast assay screen data for respective total vitamin contents in three rice tissue types from selected accessions with low, intermediate and high vitamin contents, a precise quantification of their soluble constituent vitamers was performed by HPLC against known amounts of external standards (Moulin et al., 2013; Szydlowski et al., 2013). For B1 vitamers, profiles were obtained for TDP, TMP, and thiamine. B6 vitamers quantified were pyridoxal (PL), pyridoxamine (PM), pyridoxine (PN), and their phosphorylated esters. Since as much as 50% of vitamin B6 is found as glucosides in certain plant samples (Gregory, 1998), pyridoxine glucosides (PN–Glu) were determined as “PN equivalents” by treatment of samples with a ß-glucosidase and the increase in PN interpreted as PN–Glu (Mangel et al., 2019). HPLC assays revealed that the most abundant B1 vitamer in rice leaf tissues was TDP (94–97%), followed by minor but detectable pools of TMP and thiamine (Figures 4A,B; Supplementary Table S9A). This is consistent with previous observations in wild-type rice leaf samples (Dong et al., 2016; Hsieh et al., 2021), maize (Guan et al., 2014), Arabidopsis (Mimura et al., 2016; Hofmann et al., 2020), and cassava (Mangel et al., 2017). Total vitamin B1 (the sum of TDP, TMP, and thiamine) in the leaves of rice accessions assayed here showed statistically significant 2.07-fold variation, ranging from 1.04 in Hanumanjata to 2.13 ng mg FW−1 in I-Kung-Pao [F(6, 14) = 7.331, p = 0.0011, Figure 4B; Supplementary Table S9A]. In contrast to leaf samples, vitamin B1 contents in unpolished and polished rice seeds largely comprised free thiamine (80–95%), with low amounts of TDP and trace levels of TMP (Figures 4A,C,D; Supplementary Tables S9B,C). Unpolished seed samples varied 2.72-fold, ranging from 1.07 in IR64 to 2.90 ng mg DW−1 in Shaeta [F(8, 18) = 21.41, p < 0.0001; Figure 4C; Supplementary Table S9B]. As the seed coat, embryo, and aleurone cell layer are the rice seed compartments that store the bulk of vitamin B1, polishing rice grain is known to deplete grain thiamine contents by as much as 90–98% (Dong et al., 2016; Strobbe et al., 2021a). Here, total vitamin B1 in polished seed samples decreased approximately 10-fold compared to unpolished seeds, with 3.94-fold variation between the lowest and highest accessions, ranging from 0.1 in Hanumanjata to 0.3 ng mg DW−1 in Juchitan A74 [F(8, 18) = 8.3, p = 0.0001; Figure 4D; Supplementary Table S9C].
Figure 4.
HPLC profiling of B1 vitamers in leaves and seeds of rice accessions contrasting in total vitamin B1 contents. (A) Representative HPLC chromatograms of leaf and seed tissues. To facilitate visualization, chromatograms were offset from the baseline by 50 units for standards at bottom of the panel, 350 units for leaves, 950 units for unpolished seeds, 1,400 units for polished seeds, and 1,900 units for standards used for quantification of polished seed samples. Shifts in retention time can occur as a result of slight changes to the pH of HPLC running buffers between experiments. HPLC analysis of B1 vitamers in leaves (B), unpolished seeds (C), and polished seeds (D). Samples were obtained from plant material grown under greenhouse conditions in experiment 2. Leaves were sampled from 40-day-old plants. Seed samples were obtained from fully mature pannicles and dried before extraction. Data are the mean ± standard deviation of n = 3 biological replicates. The effect of accession on total vitamin B1 contents in panels (B–D) was determined by one-way ANOVA (α = 0.05) with multiple comparisons and Tukey’s post-hoc test. Statistically significant differences between accessions are denoted by different letters.
In contrasting accessions selected for HPLC analysis of vitamin B6 contents, leaf samples varied 2.24-fold between the selected accessions, from 2.44 in I-Kung-Pao to 5.48 ng mg FW−1 in Daw Magawk [F(7, 16) = 12.91, p < 0.001; Figures 5A,B; Supplementary Table S10]. Unphosphorylated vitamers (PL, PM, and PN) comprised the majority of vitamers in leaves of all accessions, with relative proportions of PN–Glu increasing in accessions with higher total vitamin B6 (Figure 5B; Supplementary Table S10A). Unphosphorylated B6 vitamers similarly comprised the majority of the vitamin B6 contents of Arabidopsis shoot material and is consistent with a previous observation for leaves of wild-type TP309 rice (Raschke et al., 2011; Mangel et al., 2019). For unpolished seeds, the total vitamin B6 contents varied 3.93-fold between the lowest and highest accessions, with 0.45 in IR64 to 1.78 ng mg DW−1 in Tapol [F(7, 16) = 65.63, p < 0.001; Figures 5A,C; Supplementary Table S10B]. In general, unphosphorylated B6 vitamers and PN–Glu comprised the bulk of vitamers in unpolished seeds (Figure 5C; Supplementary Table S9B). Similar to vitamin B1, polishing of rice grains depletes vitamin B6 stores by as much as 85% (Mangel et al., 2019). Polished seed vitamin B6 contents varied 3.95-fold between the lowest and highest accessions, corresponding to TP309 at 0.1 and 0.41 ng mg DW−1 for DNJ52 [F(7, 16) = 3.918, p = 0.0113]. Unphosphorylated vitamers comprised the bulk of polished seed vitamin B6 pools in all accessions, while phosphorylated vitamers (PLP, PMP, and PNP) and PN–Glu comprised relatively minor constituents or were below the detection limit (Figures 5A,D; Supplementary Table S10C).
Figure 5.
HPLC profiling of B6 vitamers in leaves and seeds of rice accessions contrasting in total vitamin B6 contents. (A) Representative HPLC chromatograms of leaf and seed tissues. To facilitate visualization, chromatograms were offset from the baseline of the standards by 300 units for leaves 30 μl injected no glucosidase, 1,200 units for leaves 10 μl injected glucosidase treated, 2,000 units for unpolished seed, and 2,700 units for polished seed. HPLC analysis of B6 vitamers in leaves (B), unpolished seeds (C), and polished seeds (D). Samples were obtained from plant material grown under greenhouse conditions in experiment 2. Leaves were sampled from 40-day-old plants. Seed samples were obtained from fully mature pannicles and dried before extraction. Data are the mean ± standard deviation of n = 3 biological replicates. The effect of accession on total vitamin B6 contents in panels (B–D) was determined by one-way ANOVA (α = 0.05) with multiple comparisons and Tukey’s post-hoc test. Statistically significant differences between accessions are denoted by different letters.
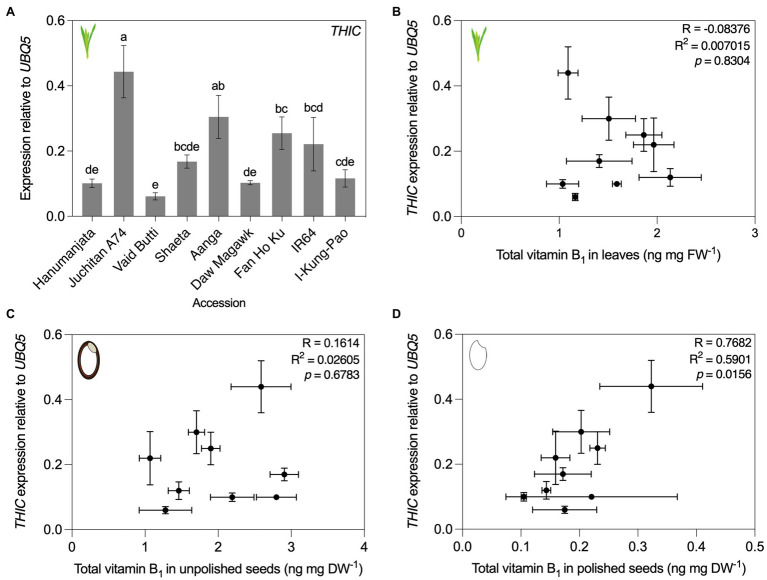
Vitamin B1 and B6 Biosynthesis de novo Gene Expression in Contrasting Accessions
Previous studies observed correlations between the expression of vitamin biosynthesis genes and vitamin B1 contents in cassava (Mangel et al., 2017) and vitamin B9 in potatoes (Robinson et al., 2019). To understand the molecular determinants underlying contrasting vitamin B1 and B6 contents of the rice accessions characterized here, we chose to quantify transcript levels of selected genes for biosynthesis enzymes (see Figure 1 for vitamin B1 and B6 metabolism schemes). RNA was isolated from leaf tissues sampled for vitamin analyses and converted to cDNA for RT-qPCR assays of THIC for vitamin B1, and PDX1.3a–c and PDX2 for vitamin B6 (Figures 6, 7). A single copy of THIC is encoded in Nipponbare and IR64 genomes (Mangel et al., 2017; Qin et al., 2021), with THIC expression quantified in Kitaake and Tainung 67 accessions (Dong et al., 2016; Hsieh et al., 2021). Consistent with the model that vitamin B1 biosynthesis de novo in plants (Figure 1A) is predominantly localized to photosynthetic tissue (Guan et al., 2014; Colinas and Fitzpatrick, 2015), THIC expression was detected in leaves of all assayed rice accessions. THIC was differentially expressed across the accessions sampled [F(8, 18) = 18.94, p < 0.0001; Figure 6A]. To determine if tissue total vitamin B1 contents correlated with differential expression of THIC in leaves, total vitamin B1 content means (as determined by HPLC analyses in Figures 4B–D) were plotted against expression level means of THIC for each accession. Pearson’s correlation coefficient tests indicated total vitamin B1 contents in leaves did not correlate with expression at the RNA level of THIC (Figure 6B, R = 0.045, R2 = 0.002, p = 0.895). No correlation was observed between THIC expression in leaves and unpolished seed vitamin B1 contents (Figure 4C, R = 0.1614, R2 = 0.02605, p = 0.6783). THIC expression in leaves did, however, show a statistically significant, positive correlation with polished seed total vitamin B1 contents (Figure 6D, R = 0.7682, R2 = 0.5901, p = 0.0156). The THIC RT-qPCR primers used here bind in the coding region of THIC and do not discriminate between splice variants in the THIC 3′ UTR which contains a TDP riboswitch (Bocobza et al., 2007; Wachter et al., 2007; Noordally et al., 2020).
Figure 6.
THIC expression in leaves and correlation of accessions with contrasting vitamin B1 contents. (A) RNA was isolated from plant material used in Figure 4B for qRT-PCR assay of THIC expression. Expression was normalized to UBQ5 and data are the mean ± standard deviation of n = 3 biological replicates. The effect of accession on gene expression was determined by one-way ANOVA (α = 0.05) with multiple comparisons and Tukey’s post-hoc test. Statistically significant differences between accessions are denoted by different letters. Accessions are ordered according to leaf total vitamin B1 content as determined in Figure 4B. (B–D) Correlation of THIC gene expression from panel (A) with leaf (B) total vitamin B1 contents in Figure 4B by Pearson’s correlation coefficient test (p < 0.05), unpolished seed from Figure 4C (C), and polished seed total vitamin B1 contents from Figure 4D (D).
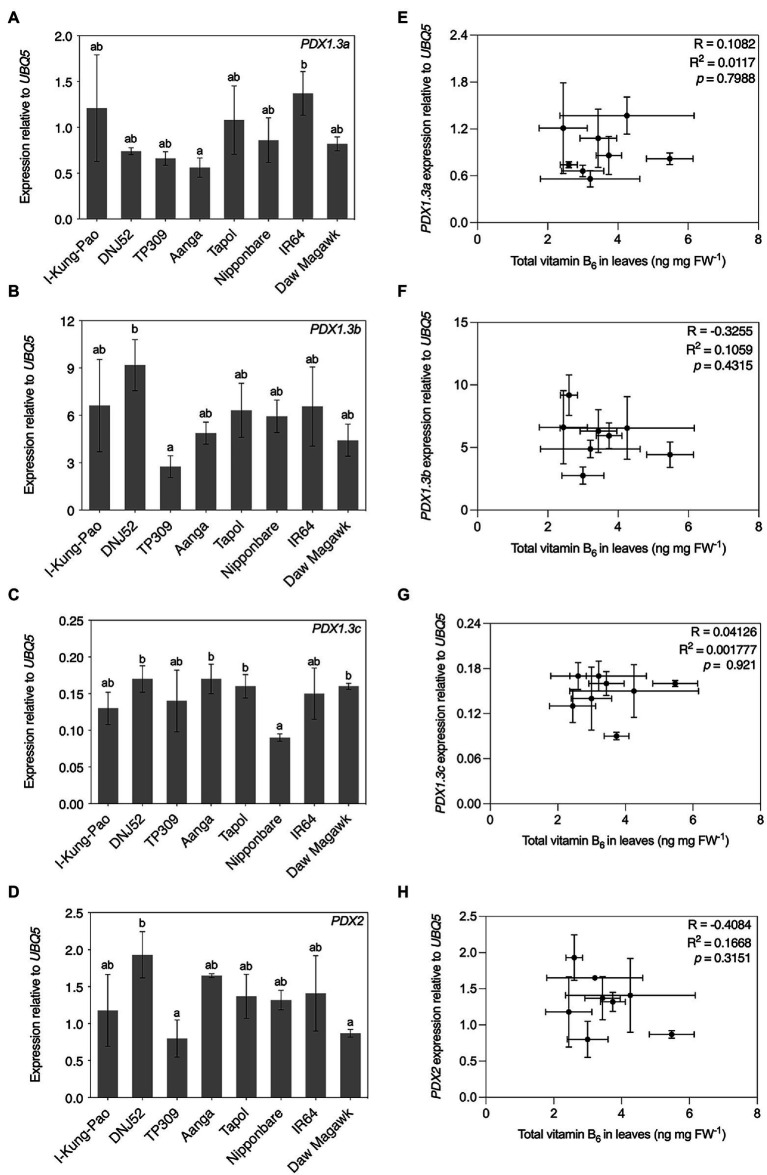
Figure 7.
Expression and correlation of select genes for vitamin B6 biosynthesis de novo enzymes in leaves of accessions with contrasting vitamin B6 contents. RNA was isolated from plant material used in Figure 5B for qRT-PCR assays of PDX1.3a (A), PDX1.3b (B), PDX1.3c (C), and PDX2 (D) expression. Expression was normalized to UBQ5 and data are the mean ± standard deviation of n = 3 biological replicates. Accessions are ordered according to leaf total vitamin B6 content as determined in Figure 5B. The effect of accession on gene expression was determined by one-way ANOVA (α = 0.05) with multiple comparisons and Tukey’s post-hoc test. Statistically significant differences between accessions are denoted by different letters. Correlation of PDX1.3a (E), PDX1.3b (F), PDX1.3c (G), and PDX2 (H) gene expression from panels (A–D), respectively, with leaf total vitamin B6 contents in Figure 3B by Pearson’s correlation coefficient test (p < 0.05).
Vitamin B6 biosynthesis de novo in plants (Figure 1B) is considered to take place ubiquitously throughout plant tissues (Titiz et al., 2006). Three PDX1.3 orthologs (PDX1.3a–c) are expressed in rice accessions Nipponbare and TP309 (Dell’Aglio et al., 2017; Mangel et al., 2019) and the encoded enzymes are predicted to be catalytically active (Moccand et al., 2014). A single PDX2 locus is encoded in the Nipponbare and IR64 genomes and is expressed in TP309 (Mangel et al., 2019; Qin et al., 2021). Expression levels of these rice PDX genes were assayed by RT-qPCR using RNA samples isolated from leaf samples also used for HPLC quantification of vitamin B6 presented in Figure 5B. All four assayed PDX genes were expressed in leaves and are differentially expressed between the accessions PDX1.3a: [F(7, 16) = 17.29, p = 0.0301]; PDX1.3b: [F(7, 16) = 3.65, p = 0.0152]; PDX1.3c: [F(7, 16) = 3.946, p = 0.0109]; and PDX2: [F(7, 16) = 4.425, p = 0.0066; Figures 7A–D]. PDX1.3b was the most abundant PDX1.3 transcript in leaves under these conditions, reaching levels over 50-fold higher than PDX1.3c for DNJ52 (Figures 7B,C). The mean of total vitamin B6 content was plotted against PDX gene expression for each accession for Pearson’s correlation coefficient tests (Figures 7E–H). PDX1.3a–c and PDX2 expression levels showed no correlations with total vitamin B6 contents in leaves of the rice accessions sampled here (p > 0.05; Figures 7E–H). Unpolished and polished seed total vitamin B6 contents from Figures 5C,D were similarly plotted against PDX expression levels in leaves. No statistically significant correlations were observed between PDX expression levels in leaves and seed vitamin B6 contents (Supplementary Figure S2).
Discussion
Previous studies reported natural variation for seed contents of total vitamin B1 across 121 accessions in rice germplasm (Villareal and Juliano, 1989; Sotelo et al., 1990; Kennedy and Burlingame, 2003), but such information is limited to three rice accessions for vitamin B6 contents in rice germplasm (Zarei et al., 2017). We profiled B1 and B6 vitamers using microbiological and HPLC assays in three tissue types (leaves, unpolished and polished grain) across a panel of 59 genetically diverse rice accessions, thereby expanding knowledge on these traits and providing insight into vitamer proportionality. The knowledge was complemented with an investigation of a select set of key genes involved in biosynthesis de novo of vitamin B1 and B6. This resource has the potential to inform on strategies for biofortification purposes as well as providing insight into possible regulatory pathways for vitamin content.
First for vitamin B1, seven accessions within our panel had been measured previously for unpolished and polished seed total vitamin B1 contents (Villareal and Juliano, 1989; Sotelo et al., 1990; Kennedy and Burlingame, 2003) and served as benchmarks in our screens (Figures 2, 4C,D; Supplemental Table 4). Similar values were obtained to those reported previously, despite employing different vitamin quantification techniques. Further, the relative distribution of the different B1 vitamers quantified here by HPLC across the three rice tissue types was consistent with previous studies (Dong et al., 2016; Strobbe et al., 2021a). Our study expands the list of accessions profiled for vitamin B1 contents and reinforces evidence that the genetic diversity of this trait in rice seeds is limited in the surveyed rice germplasm. Data collected so far indicate that immediate exploitation of rice germplasm is likely impractical for a breeding-based vitamin B1 biofortification strategy. The molecular basis underpinning vitamin B1 variation in staple crop germplasm has been investigated in cassava, wheat, and potato (Goyer and Haynes, 2011; Goyer and Sweek, 2011; Mangel et al., 2017; Li et al., 2018). In certain cassava accessions bearing duplications of THI1 and THIC genes, leaf vitamin B1 contents are negatively correlated with transcript levels of MeTHIC2 (containing a functional TDP riboswitch) and MeTHI1b (Mangel et al., 2017). Based on this finding, and similar observations for vitamin B9 in potato germplasm (Bali et al., 2018; Robinson et al., 2019), we sought to determine if this was also the case in rice as a potential molecular determinant underpinning vitamin B1 diversity (Figure 6). Clear differential expression of THIC transcripts in rice leaves was observed across the rice accessions contrasting in vitamin B1 contents (Figure 6A). Leaves are the principal site of vitamin B1 biosynthesis de novo (Fitzpatrick and Chapman, 2020). The expression pattern of THIC did not correlate with leaf total vitamin B1 contents under greenhouse conditions (Figure 6B). Yet, further correlation analyses with total vitamin B1 contents revealed a statistically significant, positive correlation between THIC expression in leaves and total vitamin B1 contents in polished seeds (Figure 6D). THIC encodes the chloroplast-localized HMP-P synthase and is the first enzyme committed to pyrimidine moiety biosynthesis de novo of vitamin B1 in plants (Figure 1A; Raschke et al., 2007; Coquille et al., 2013; Palmer and Downs, 2013). THIC is functional in rice (Dong et al., 2016; Strobbe et al., 2021a). The promoter activity of Arabidopsis THIC is positively regulated by light (Raschke et al., 2007) and negatively regulated by the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) transcription factor that phases time of day circadian expression (Bocobza et al., 2013; Noordally et al., 2020). A TDP riboswitch is conserved in the 3′ UTR of THIC in higher plants and in Arabidopsis is subject to alternative mRNA 3′ processing in response to high or low nuclear TDP concentration (Raschke et al., 2007; Noordally et al., 2020), which produces stable or unstable transcripts through use of alternative polyadenylation sites (Bocobza et al., 2007; Wachter et al., 2007). The TDP riboswitch regulation of THIC is thought to be conserved in the green lineage (Bocobza et al., 2007; Croft et al., 2007; Wachter et al., 2007) and has been experimentally validated in both cassava THIC genes (Mangel et al., 2017). Arabidopsis THIC mRNA alternative 3′ processing is considered to fine-tune intracellular TDP supply in response to changes in demand for TDP-dependent enzymes throughout the day (Bocobza et al., 2013), independently of the circadian control of the THIC promoter (Bocobza et al., 2013; Noordally et al., 2020). A CCA1 binding site is present 28 bp upstream of the Nipponbare OsTHIC 5′ UTR (Supplementary Figure S3), suggesting the OsTHIC promoter could be subject to circadian regulation by CCA1. How high THIC expression in leaves of rice varieties might correspond to higher endosperm thiamine contents in the presence of a presumably functional TDP riboswitch in rice requires further investigation. With additional functional evidence, OsTHIC relative expression levels in leaves might serve as a useful indicator of vitamin B1 contents in rice endosperm. TDP riboswitch activity in OsTHIC needs to be confirmed by base editing, alongside analysis of thiC null mutant vitamer and developmental phenotypes. Based on Arabidopsis, rice, and maize models of vitamin B1 metabolism in plants, future work could characterize expression patterns of other genes encoding enzymes for vitamin B1 biosynthesis de novo in leaves (the main organ of biosynthesis de novo activity). For example, research efforts in vitamin B1 biofortification could explore the expression of the TH1 gene responsible for condensation of the pyrimidine and thiazole heterocycles (Figure 1A) in rice endosperm, or candidate genes involved in transport or salvage pathways in tissues with minimal vitamin B1 biosynthesis de novo activity, such as seeds (Yazdani et al., 2013; Guan et al., 2014; Zallot et al., 2014; Dong et al., 2016). THI1, which encodes a single-turnover enzyme in thiazole moiety biosynthesis (Figure 1A), is functional in rice (Wang et al., 2006) and is sufficient to over-accumulate HET and vitamin B1 when ectopically expressed in transgenic rice (Dong et al., 2016; Strobbe et al., 2021a). THI1 therefore represents an additional candidate gene and awaits characterization in diverse rice germplasm. Alternative sources of the thiazole moiety have been investigated (Sun et al., 2019) and could be exploited for biofortification purposes. It is clear nonetheless that an enhanced understanding of vitamin B1 metabolism is required in crops, including in rice, to permit successful endosperm biofortification at useful levels either by metabolic engineering or through identification of higher vitamin B1 content accessions other than those profiled here and previously (Villareal and Juliano, 1989; Sotelo et al., 1990; Kennedy and Burlingame, 2003; Dong et al., 2015; Hanson et al., 2018; Strobbe et al., 2021a). Given the high prevalence of suboptimal vitamin B1 status and deficiency disorders around the world, together with the wide consumption of vitamin B1-poor polished rice (Bhullar and Gruissem, 2013; Dhir et al., 2019; Johnson et al., 2019; Titcomb and Tanumihardjo, 2019; Whitfield et al., 2021), vitamin B1 biofortification of rice should be considered as a priority in assisting to combat micronutrient deficiency.
In contrast to vitamin B1, natural variation in vitamin B6 contents has not been extensively studied in rice germplasm with only three varieties quantified to date (Zarei et al., 2017), but no information is published on the molecular basis of such variation in rice. Vitamin B6 contents in germplasm of other crops have also received minimal attention, except for analyses in potatoes (Mooney et al., 2013; Goyer et al., 2019), wheat (Shewry et al., 2011; Freitag et al., 2018; Granda et al., 2018), and a small number of accessions of barley (Freitag et al., 2018; Granda et al., 2018), field beans (Freitag et al., 2018), and quinoa (Granda et al., 2018). Furthermore, regulation of vitamin B6 metabolism has not been widely investigated in monocot species compared to eudicots (Dell’Aglio et al., 2017; Yang et al., 2017; Mangel et al., 2019; Suzuki et al., 2020). Rice PDX1 and PDX2 genes are active (Dell’Aglio et al., 2017; Mangel et al., 2019) and are differentially expressed in transgenic lines over-expressing MALATE DEHYDROGENASE 1, concomitant with alterations to B6 vitamer profiles (Nan et al., 2020). Maize PDX2 is functional (Yang et al., 2017; Suzuki et al., 2020) and is required for proper embryo development (Yang et al., 2017). Contrasting results from vitamin B6 biofortification efforts in model and crop plants that aimed at increasing vitamin B6 in target tissues through metabolic engineering indicates further research is needed to understand the regulation of vitamin B6 metabolism and sequestration, particularly in cereal endosperm (Chen and Xiong, 2009; Raschke et al., 2011; Li et al., 2015; Fudge et al., 2017; Mangel et al., 2019). Our results show that moderate natural variation does exist for vitamin B6 contents in rice germplasm (Figures 3, 5; Supplementary Tables S6, S9). Similar to vitamin B1, accessions profiled here with the highest vitamin B6 contents in polished seeds fall well below a practical level to justify introgression of such a trait for biofortification purposes (Figures 3, 5; Supplementary Table S9). Although no statistically significant correlations were observed between the expression in leaves of genes encoding for biosynthesis de novo enzymes and vitamin B6 contents under greenhouse conditions, future studies should expand such analyses to include analyses of vitamin B6 salvage pathway genes in leaves or expression analyses in the endosperm. Given the prevalence of vitamin B6 deficiency in certain populations, combined with the wide consumption of vitamin B6-poor polished rice, biofortification of rice with vitamin B6 also remains a priority in combatting micronutrient deficiency (Kim and Cho, 2014; Bird et al., 2017; Liu et al., 2019; Zhu et al., 2020).
A growing abundance of rice genetic resources such as the rice 3,000 genomes project (The 3000 rice genomes project, 2014; Wang et al., 2018), single-nucleotide polymorphism databases (McNally et al., 2009; Chebotarov et al., 2016), together with contemporary genome or base editing techniques (Zhu et al., 2017; Jin et al., 2019; Lu et al., 2020), remain alternative avenues to explore in order to biofortify rice endosperm with enhanced micronutrient contents. Redesigning the energetically costly vitamin B1 biosynthesis de novo pathway (Nelson et al., 2014) has also been proposed (Hanson et al., 2018; Sun et al., 2019) and could be drawn on for biofortification purposes.
In conclusion, our screen of diverse rice accessions under controlled greenhouse conditions advances our understanding of micronutrient trait diversity in rice germplasm by combining quantifications of total vitamin B1 and B6 levels as well as vitamer partition, over three tissue types of rice with insights into biosynthetic gene expression patterns. Further analyses using larger germplasm panels together with in silico genetic analyses such as genome-wide association studies (GWAS) might be useful for identifying accessions and loci to exploit for biofortification.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
NM propagated and sampled the rice accessions, conducted yeast assays and RT-qPCR experiments, performed data analysis, and drafted figures and prepared tables. JBF performed the HPLC measurements and data analysis, prepared figures and tables, and wrote the manuscript. WG, TBF, and HV conceived the study, obtained funding, contributed to the analysis of the data, and edited manuscript drafts for submission. NM, JBF, WG, TBF, and HV commented and agreed on the final version of the manuscript and contributed to the article and approved the submitted version. The authors wish it to be known that NM and JBF are equal first authors and that WG, TBF, and HV are equal last and corresponding authors. For the purpose of their CVs, the respective authors can list their name as the first or as the last author. All authors contributed to the article and approved the submitted version.
Funding
We gratefully acknowledge financial support from the Swiss National Science Foundation (grants 31003A-141117/1 and 31003A-162555 to TBF; grant 31003A-140911 to WG, HV, and TBF), the VELUX Foundation (WG), the Université de Genève (TBF), and ETH Zurich (WG). WG is supported by a Yushan Scholarship of the Ministry of Education in Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Irene Zurkirchen (ETH Zurich) for support of the greenhouse work. JBF wishes to thank Michael Moulin (Université de Genève) for advice with HPLC analyses.
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fpls.2022.856880/full#supplementary-material
References
- Ahn I. P., Kim S., Lee Y. H. (2005). Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol. 138, 1505–1515. doi: 10.1104/pp.104.058693, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn I. P., Kim S., Lee Y. H., Suh S. C. (2007). Vitamin B1-induced priming is dependent on hydrogen peroxide and the NPR1 gene in Arabidopsis. Plant Physiol. 143, 838–848. doi: 10.1104/pp.106.092627, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Toda S., Kimura N., Mogami Y., Hanaoka Y., Tokorodani C., et al. (2020). Vitamin B6 in acute encephalopathy with biphasic seizures and late reduced diffusion. Brain Dev. 42, 402–407. doi: 10.1016/j.braindev.2020.02.002, PMID: [DOI] [PubMed] [Google Scholar]
- Allen L. H., Hampel D., Shahab-Ferdows S., York E. R., Adair L. S., Flax V. L., et al. (2015). Antiretroviral therapy provided to HIV-infected Malawian women in a randomized trial diminishes the positive effects of lipid-based nutrient supplements on breast-milk B vitamins. Am. J. Clin. Nutr. 102, 1468–1474. doi: 10.3945/ajcn.114.105106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R. L., West K. P., Jr., Black R. E. (2015). The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 66, 22–33. doi: 10.1159/000371618 [DOI] [PubMed] [Google Scholar]
- Bali S., Robinson B. R., Sathuvalli V., Bamberg J., Goyer A. (2018). Single nucleotide polymorphism (SNP) markers associated with high folate content in wild potato species. PLoS One 13:e0193415. doi: 10.1371/journal.pone.0193415, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechoff A., Chijioke U., Westby A., Tomlins K. I. (2018). ‘Yellow is good for you’: consumer perception and acceptability of fortified and biofortified cassava products. PLoS One 13:e0203421. doi: 10.1371/journal.pone.0203421, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar N. K., Gruissem W. (2013). Nutritional enhancement of rice for human health: the contribution of biotechnology. Biotechnol. Adv. 31, 50–57. doi: 10.1016/j.biotechadv.2012.02.001, PMID: [DOI] [PubMed] [Google Scholar]
- Bilski P., Li M. Y., Ehrenshaft M., Daub M. E., Chignell C. F. (2000). Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem. Photobiol. 71, 129–134. doi: , PMID: [DOI] [PubMed] [Google Scholar]
- Bird J. K., Murphy R. A., Ciappio E. D., McBurney M. I. (2017). Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients 9:655. doi: 10.3390/nu9070655, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocobza S., Adato A., Mandel T., Shapira M., Nudler E., Aharoni A. (2007). Riboswitch-dependent gene regulation and its evolution in the plant kingdom. Genes Dev. 21, 2874–2879. doi: 10.1101/gad.443907, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocobza S. E., Malitsky S., Araujo W. L., Nunes-Nesi A., Meir S., Shapira M., et al. (2013). Orchestration of thiamin biosynthesis and central metabolism by combined action of the thiamin pyrophosphate riboswitch and the circadian clock in Arabidopsis. Plant Cell 25, 288–307. doi: 10.1105/tpc.112.106385, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubakri H., Wahab M. A., Chong J., Bertsch C., Mliki A., Soustre-Gacougnolle I. (2012). Thiamine induced resistance to Plasmopara viticola in grapevine and elicited host-defense responses, including HR like-cell death. Plant Physiol. Biochem. 57, 120–133. doi: 10.1016/j.plaphy.2012.05.016 [DOI] [PubMed] [Google Scholar]
- Bouis H. E., Saltzman A. (2017). Improving nutrition through biofortification: a review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 12, 49–58. doi: 10.1016/j.gfs.2017.01.009, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Puryear J., Cairney J. (1993). A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Report. 11, 113–116. doi: 10.1007/BF02670468 [DOI] [Google Scholar]
- Chebotarov D., Borja F. N., Detras J., Abriol-Santos J. M., McNally K. L., Mansueto L., et al. (2016). Rice SNP-seek database update: new SNPs, indels, and queries. Nucleic Acids Res. 45, D1075–D1081. doi: 10.1093/nar/gkw1135, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Xiong L. (2009). Enhancement of vitamin B6 levels in seeds through metabolic engineering. Plant Biotechnol. J. 7, 673–681. doi: 10.1111/j.1467-7652.2009.00433.x, PMID: [DOI] [PubMed] [Google Scholar]
- Colinas M., Eisenhut M., Tohge T., Pesquera M., Fernie A. R., Weber A. P., et al. (2016). Balancing of B6 vitamers is essential for plant development and metabolism in Arabidopsis. Plant Cell 28, 439–453. doi: 10.1105/tpc.15.01033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinas M., Fitzpatrick T. B. (2015). Natures balancing act: examining biosynthesis de novo, recycling and processing damaged vitamin B metabolites. Curr. Opin. Plant Biol. 25, 98–106. doi: 10.1016/j.pbi.2015.05.001, PMID: [DOI] [PubMed] [Google Scholar]
- Coquille S., Roux C., Mehta A., Begley T. P., Fitzpatrick T. B., Thore S. (2013). High-resolution crystal structure of the eukaryotic HMP-P synthase (THIC) from Arabidopsis thaliana. J. Struct. Biol. 184, 438–444. doi: 10.1016/j.jsb.2013.10.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M. T., Moulin M., Webb M. E., Smith A. G. (2007). Thiamine biosynthesis in algae is regulated by riboswitches. Proc. Natl. Acad. Sci. U. S. A. 104, 20770–20775. doi: 10.1073/pnas.0705786105, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czégény G., Kőrösi L., Strid Å., Hideg É. (2019). Multiple roles for vitamin B(6) in plant acclimation to UV-B. Sci. Rep. 9:1259. doi: 10.1038/s41598-018-38053-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pee S. (2014). Proposing nutrients and nutrient levels for rice fortification. Ann. N. Y. Acad. Sci. 1324, 55–66. doi: 10.1111/nyas.12478, PMID: [DOI] [PubMed] [Google Scholar]
- Dell’Aglio E., Boycheva S., Fitzpatrick T. B. (2017). The pseudoenzyme PDX1.2 sustains vitamin B6 biosynthesis as a function of heat stress. Plant Physiol. 174, 2098–2112. doi: 10.1104/pp.17.00531, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow S. A., Rueschhoff E. E., Daub M. E. (2007). Regulation of the Arabidopsis thaliana vitamin B6 biosynthesis genes by abiotic stress. Plant Physiol. Biochem. 45, 152–161. doi: 10.1016/j.plaphy.2007.01.007, PMID: [DOI] [PubMed] [Google Scholar]
- Denslow S. A., Walls A. A., Daub M. E. (2005). Regulation of biosynthetic genes and antioxidant properties of vitamin B6 vitamers during plant defense responses. Physiol. Mol. Plant Pathol. 66, 244–255. doi: 10.1016/j.pmpp.2005.09.004 [DOI] [Google Scholar]
- Dhalla N. S., Takeda S., Elimban V. (2013). Mechanisms of the beneficial effects of vitamin B6 and pyridoxal 5-phosphate on cardiac performance in ischemic heart disease. Clin. Chem. Lab. Med. 51, 535–543. doi: 10.1515/cclm-2012-0553, PMID: [DOI] [PubMed] [Google Scholar]
- Dhir S., Tarasenko M., Napoli E., Giulivi C. (2019). Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Front. Psych. 10:207. doi: 10.3389/fpsyt.2019.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Cheng Z., Wang X., Wang B., Zhang H., Su N., et al. (2011). Determination of folate content in rice germplasm (Oryza sativa L.) using tri-enzyme extraction and microbiological assays. Int. J. Food Sci. Nutr. 62, 537–543. doi: 10.3109/09637486.2011.555476, PMID: [DOI] [PubMed] [Google Scholar]
- Dong W., Cheng Z. J., Xu J. L., Zheng T. Q., Wang X. L., Zhang H. Z., et al. (2014). Identification of QTLs underlying folate content in milled rice. J. Integr. Agric. 13, 1827–1834. doi: 10.1016/S2095-3119(13)60537-7 [DOI] [Google Scholar]
- Dong W., Stockwell V. O., Goyer A. (2015). Enhancement of thiamin content in Arabidopsis thaliana by metabolic engineering. Plant Cell Physiol. 56, 2285–2296. doi: 10.1093/pcp/pcv148, PMID: [DOI] [PubMed] [Google Scholar]
- Dong W., Thomas N., Ronald P. C., Goyer A. (2016). Overexpression of thiamin biosynthesis genes in rice increases leaf and unpolished grain thiamin content but not resistance to Xanthomonas oryzae pv. Oryzae. Frontiers. Plant Sci. 7:616. doi: 10.3389/fpls.2016.00616, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick T. B. (2011). “Vitamin B6 in plants: more than meets the eye,” in Advances in Botanical Research. eds. Rebeille F., Douce R. (Amsterdam: Academic Press; ), 1–38. [Google Scholar]
- Fitzpatrick T. B., Basset G. J., Borel P., Carrari F., DellaPenna D., Fraser P. D., et al. (2012). Vitamin deficiencies in humans: can plant science help? Plant Cell 24, 395–414. doi: 10.1105/tpc.111.093120, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick T. B., Chapman L. M. (2020). The importance of thiamine (vitamin B1) in plant health: From crop yield to biofortification. J. Biol. Chem. 295, 12002–12013. doi: 10.1074/jbc.REV120.010918, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. K., Michaux K. D., Mudyahoto B., Kyazike L., Cherian B., Kalejaiye O., et al. (2021). Scaling up delivery of biofortified staple food crops globally: paths to nourishing millions. Food Nutr. Bull. 42, 116–132. doi: 10.1177/0379572120982501, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag S., Verrall S. R., Pont S. D. A., McRae D., Sungurtas J. A., Palau R., et al. (2018). Impact of conventional and integrated management systems on the water-soluble vitamin content in potatoes, field beans, and cereals. J. Agric. Food Chem. 66, 831–841. doi: 10.1021/acs.jafc.7b03509, PMID: [DOI] [PubMed] [Google Scholar]
- Fudge J., Mangel N., Gruissem W., Vanderschuren H., Fitzpatrick T. B. (2017). Rationalising vitamin B6 biofortification in crop plants. Curr. Opin. Biotechnol. 44, 130–137. doi: 10.1016/j.copbio.2016.12.004, PMID: [DOI] [PubMed] [Google Scholar]
- Gliszczyńska-Świgło A. (2006). Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 96, 131–136. doi: 10.1016/j.foodchem.2005.02.018 [DOI] [Google Scholar]
- Gomes F., Bergeron G., Bourassa M. W., Fischer P. R. (2021). Thiamine deficiency unrelated to alcohol consumption in high-income countries: a literature review. Ann. N. Y. Acad. Sci. 1498, 46–56. doi: 10.1111/nyas.14569, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodstein D. M., Shu S., Howson R., Neupane R., Hayes R. D., Fazo J., et al. (2012). Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40, D1178–D1186. doi: 10.1093/nar/gkr944, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govender L., Pillay K., Siwela M., Modi A. T., Mabhaudhi T. (2019). Consumer perceptions and acceptability of traditional dishes prepared with provitamin A-biofortified maize and sweet potato. Nutrients 11:1577. doi: 10.3390/nu11071577, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer A. (2017). Thiamin biofortification of crops. Curr. Opin. Biotechnol. 44, 1–7. doi: 10.1016/j.copbio.2016.09.005, PMID: [DOI] [PubMed] [Google Scholar]
- Goyer A., Haynes K. G. (2011). Vitamin B1 content in potato: effect of genotype, tuber enlargement, and storage, and estimation of stability and broad-sense heritability. Am. J. Potato Res. 88, 374–385. doi: 10.1007/s12230-011-9203-6 [DOI] [Google Scholar]
- Goyer A., Navarre D. A. (2007). Determination of folate concentrations in diverse potato germplasm using a trienzyme extraction and a microbiological assay. J. Agric. Food Chem. 55, 3523–3528. doi: 10.1021/jf063647x, PMID: [DOI] [PubMed] [Google Scholar]
- Goyer A., Picard M., Hellmann H. A., Mooney S. L. (2019). Effect of low temperature storage on the content of folate, vitamin B6, ascorbic acid, chlorogenic acid, tyrosine, and phenylalanine in potatoes. J. Sci. Food Agric. 99, 4842–4848. doi: 10.1002/jsfa.9750 [DOI] [PubMed] [Google Scholar]
- Goyer A., Sweek K. (2011). Genetic diversity of thiamin and folate in primitive cultivated and wild potato (Solanum) species. J. Agric. Food Chem. 59, 13072–13080. doi: 10.1021/jf203736e, PMID: [DOI] [PubMed] [Google Scholar]
- Granda L., Rosero A., Benesova K., Pluhackova H., Neuwirthova J., Cerkal R. (2018). Content of selected vitamins and antioxidants in colored and nonpigmented varieties of quinoa, barley, and wheat grains. J. Food Sci. 83, 2439–2447. doi: 10.1111/1750-3841.14334, PMID: [DOI] [PubMed] [Google Scholar]
- Gregory J. F., III. (1998). Nutritional properties and significance of vitamin glycosides. Annu. Rev. Nutr. 18, 277–296. doi: 10.1146/annurev.nutr.18.1.277, PMID: [DOI] [PubMed] [Google Scholar]
- Guan J. C., Hasnain G., Garrett T. J., Chase C. D., Gregory J., Hanson A. D., et al. (2014). Divisions of labor in the thiamin biosynthetic pathway among tissues of maize. Front. Plant Sci. 5:370. doi: 10.3389/fpls.2014.00370, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Lian T., Wang B., Guan J., Yuan D., Wang H., et al. (2019). Genetic mapping of folate QTLs using a segregated population in maize. J. Integr. Plant Biol. 61, 675–690. doi: 10.1111/jipb.12811, PMID: [DOI] [PubMed] [Google Scholar]
- Gylling B., Myte R., Schneede J., Hallmans G., Häggström J., Johansson I., et al. (2017). Vitamin B-6 and colorectal cancer risk: a prospective population-based study using 3 distinct plasma markers of vitamin B-6 status. Am. J. Clin. Nutr. 105, 897–904. doi: 10.3945/ajcn.116.139337, PMID: [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Amthor J. S., Sun J., Niehaus T. D., Gregory J. F., Bruner S. D., et al. (2018). Redesigning thiamin synthesis: prospects and potential payoffs. Plant Sci. 273, 92–99. doi: 10.1016/j.plantsci.2018.01.019 [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Beaudoin G. A., McCarty D. R., Gregory J. F. (2016). Does abiotic stress cause functional B vitamin deficiency in plants? Plant Physiol. 172, 2082–2097. doi: 10.1104/pp.16.01371, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Loubéry S., Fitzpatrick T. B. (2020). On the nature of thiamine triphosphate in Arabidopsis. Plant Direct 4:e00258. doi: 10.1002/pld3.258, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P. H., Chung Y. H., Lee K. T., Wang S. Y., Lu C. A., Hsieh M. H. (2021). The rice PALE1 homolog is involved in the biosynthesis of vitamin B1. Plant Biotechnol. J. 19, 218–220. doi: 10.1111/pbi.13465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. K., Ji H. L., Gheysen G., Kyndt T. (2016). Thiamine-induced priming against root-knot nematode infection in rice involves lignification and hydrogen peroxide generation. Mol. Plant Pathol. 17, 614–624. doi: 10.1111/mpp.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Zhang J., Wang L., Huang L. (2013). Effect of abiotic stress on the abundance of different vitamin B6 vitamers in tobacco plants. Plant Physiol. Biochem. 66, 63–67. doi: 10.1016/j.plaphy.2013.02.010, PMID: [DOI] [PubMed] [Google Scholar]
- Isenberg-Grzeda E., Kutner H. E., Nicolson S. E. (2012). Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics 53, 507–516. doi: 10.1016/j.psym.2012.04.008 [DOI] [PubMed] [Google Scholar]
- Jain M., Nijhawan A., Tyagi A. K., Khurana J. P. (2006). Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 345, 646–651. doi: 10.1016/j.bbrc.2006.04.140, PMID: [DOI] [PubMed] [Google Scholar]
- Jha A. B., Gali K. K., Zhang H., Purves R. W., Tar’an B., Vandenberg A., et al. (2020). Folate profile diversity and associated SNPs using genome wide association study in pea. Euphytica 216, 18. doi: 10.1007/s10681-020-2553-8 [DOI] [Google Scholar]
- Jin S., Zong Y., Gao Q., Zhu Z., Wang Y., Qin P., et al. (2019). Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 364, 292–295. doi: 10.1126/science.aaw7166 [DOI] [PubMed] [Google Scholar]
- Johnson C. R., Fischer P. R., Thacher T. D., Topazian M. D., Bourassa M. W., Combs G. F., Jr. (2019). Thiamin deficiency in low- and middle-income countries: disorders, prevalences, previous interventions and current recommendations. Nutr. Health 25, 127–151. doi: 10.1177/0260106019830847, PMID: [DOI] [PubMed] [Google Scholar]
- Kennedy G., Burlingame B. (2003). Analysis of food composition data on rice from a plant genetic resoruces perspective. Food Chem. 80, 589–596. doi: 10.1016/S0308-8146(02)00507-1 [DOI] [Google Scholar]
- Kim Y. N., Cho Y. O. (2014). Evaluation of vitamin B6 intake and status of 20- to 64-year-old Koreans. Nutr. Res. Pract. 8, 688–694. doi: 10.4162/nrp.2014.8.6.688, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde D., Herforth A., Headey D., de Pee S. (2021). COVID-19 pandemic leads to greater depth of unaffordability of healthy and nutrient-adequate diets in low- and middle-income countries. Nat. Food 2, 473–475. doi: 10.1038/s43016-021-00323-8 [DOI] [PubMed] [Google Scholar]
- Latt N., Dore G. (2014). Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern. Med. J. 44, 911–915. doi: 10.1111/imj.12522 [DOI] [PubMed] [Google Scholar]
- Lee A. S. D. (2021). The role of vitamin B6 in women's health. Nurs. Clin. N. Am. 56, 23–32. doi: 10.1016/j.cnur.2020.10.002, PMID: [DOI] [PubMed] [Google Scholar]
- Li J., Liu J., Wen W. E., Zhang P., Wan Y., Xia X., et al. (2018). Genome-wide association mapping of vitamins B1 and B2 in common wheat. Crop J. 6, 263–270. doi: 10.1016/j.cj.2017.08.002 [DOI] [Google Scholar]
- Li K. T., Moulin M., Mangel N., Albersen M., Verhoeven-Duif N. M., Ma Q., et al. (2015). Increased bioavailable vitamin B6 in field-grown transgenic cassava for dietary sufficiency. Nat. Biotechnol. 33, 1029–1032. doi: 10.1038/nbt.3318, PMID: [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhao L., Man Q., Wang J., Zhao W., Zhang J. (2019). Dietary micronutrients intake status among Chinese elderly people living at home: data from CNNHS 2010–2012. Nutrients 11:1787. doi: 10.3390/nu11081787, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu Y., Tian Y., Shen R., Yao Q., Wang M., Chen M., et al. (2020). Targeted, efficient sequence insertion and replacement in rice. Nat. Biotechnol. 38, 1402–1407. doi: 10.1038/s41587-020-0581-5, PMID: [DOI] [PubMed] [Google Scholar]
- Lussana F., Zighetti M. L., Bucciarelli P., Cugno M., Cattaneo M. (2003). Blood levels of homocysteine, folate, vitamin B6 and B12 in women using oral contraceptives compared to non-users. Thromb. Res. 112, 37–41. doi: 10.1016/j.thromres.2003.11.007, PMID: [DOI] [PubMed] [Google Scholar]
- Mangel N., Fudge J. B., Fitzpatrick T. B., Gruissem W., Vanderschuren H. (2017). Vitamin B1 diversity and characterization of biosynthesis genes in cassava. J. Exp. Bot. 68, 3351–3363. doi: 10.1093/jxb/erx196, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel N., Fudge J. B., Li K. T., Wu T. Y., Tohge T., Fernie A. R., et al. (2019). Enhancement of vitamin B6 levels in rice expressing Arabidopsis vitamin B6 biosynthesis de novo genes. Plant J. 99, 1047–1065. doi: 10.1111/tpj.14379, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally K. L., Childs K. L., Bohnert R., Davidson R. M., Zhao K., Ulat V. J., et al. (2009). Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc. Natl. Acad. Sci. U. S. A. 106, 12273–12278. doi: 10.1073/pnas.0900992106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura M., Zallot R., Niehaus T. D., Hasnain G., Gidda S. K., Nguyen T. N. D., et al. (2016). Arabidopsis TH2 encodes the orphan enzyme thiamin monophosphate phosphatase. Plant Cell 28, 2683–2696. doi: 10.1105/tpc.16.00600, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moccand C., Boycheva S., Surriabre P., Tambasco-Studart M., Raschke M., Kaufmann M., et al. (2014). The pseudoenzyme PDX1.2 boosts vitamin B6 biosynthesis under heat and oxidative stress in Arabidopsis. The journal of Bological. Chemistry 289, 8203–8216. doi: 10.1074/jbc.M113.540526, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney S., Chen L., Kuhn C., Navarre R., Knowles N. R., Hellmann H. (2013). Genotype-specific changes in vitamin B6 content and the PDX family in potato. Biomed. Res. Int. 2013:389723. doi: 10.1155/2013/389723, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin M., Nguyen G. T., Scaife M. A., Smith A. G., Fitzpatrick T. B. (2013). Analysis of Chlamydomonas thiamin metabolism in vivo reveals riboswitch plasticity. Proc. Natl. Acad. Sci. U. S. A. 110, 14622–14627. doi: 10.1073/pnas.1307741110, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- Nan N., Wang J., Shi Y., Qian Y., Jiang L., Huang S., et al. (2020). Rice plastidial NAD-dependent malate dehydrogenase 1 negatively regulates salt stress response by reducing the vitamin B6 content. Plant Biotechnol. J. 18, 172–184. doi: 10.1111/pbi.13184, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. J., Alexova R., Jacoby R. P., Millar A. H. (2014). Proteins with high turnover rate in barley leaves estimated by proteome analysis combined with in planta isotope labeling. Plant Physiol. 166, 91–108. doi: 10.1104/pp.114.243014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix W. A., Zirwes R., Bangert V., Kaiser R. P., Schilling M., Hostalek U., et al. (2015). Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res. Clin. Pract. 107, 157–165. doi: 10.1016/j.diabres.2014.09.058, PMID: [DOI] [PubMed] [Google Scholar]
- Noordally Z. B., Trichtinger C., Dalvit I., Hofmann M., Roux C., Zamboni N., et al. (2020). The coenzyme thiamine diphosphate displays a daily rhythm in the Arabidopsis nucleus. Commun. Biol. 3, 209. doi: 10.1038/s42003-020-0927-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak V., Du J., Charrondiere U. R. (2016). Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 193, 47–54. doi: 10.1016/j.foodchem.2015.02.111, PMID: [DOI] [PubMed] [Google Scholar]
- Onishi H., Sato I., Uchida N., Takahashi T., Furuya D., Ebihara Y., et al. (2021). High proportion of thiamine deficiency in referred cancer patients with delirium: a retrospective descriptive study. Eur. J. Clin. Nutr. 75, 1499–1505. doi: 10.1038/s41430-021-00859-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortigoza-Escobar J. D., Molero-Luis M., Arias A., Marti-Sanchez L., Rodriguez-Pombo P., Artuch R., et al. (2016). Treatment of genetic defects of thiamine transport and metabolism. Expert. Rev. Neurother. 16, 755–763. doi: 10.1080/14737175.2016.1187562, PMID: [DOI] [PubMed] [Google Scholar]
- Palmer L. D., Downs D. M. (2013). The thiamine biosynthetic enzyme ThiC catalyzes multiple turnovers and is inhibited by S-adenosylmethionine (AdoMet) metabolites. J. Biol. Chem. 288, 30693–30699. doi: 10.1074/jbc.M113.500280, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul L., Ueland P. M., Selhub J. (2013). Mechanistic perspective on the relationship between pyridoxal 5′-phosphate and inflammation. Nutr. Rev. 71, 239–244. doi: 10.1111/nure.12014, PMID: [DOI] [PubMed] [Google Scholar]
- Porter K. M., Ward M., Hughes C. F., O'Kane M., Hoey L., McCann A., et al. (2019). Hyperglycemia and metformin use are associated with B vitamin deficiency and cognitive dysfunction in older adults. J. Clin. Endocrinol. Metab. 104, 4837–4847. doi: 10.1210/jc.2018-01791 [DOI] [PubMed] [Google Scholar]
- Qin P., Lu H., Du H., Wang H., Chen W., Chen Z., et al. (2021). Pan-genome analysis of 33 genetically diverse rice accessions reveals hidden genomic variations. Cell 184, 3542–3558.e16. doi: 10.1016/j.cell.2021.04.046, PMID: [DOI] [PubMed] [Google Scholar]
- R Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rapala-Kozik M., Kowalska E., Ostrowska K. (2008). Modulation of thiamine metabolism in Zea mays seedlings under conditions of abiotic stress. J. Exp. Bot. 59, 4133–4143. doi: 10.1093/jxb/ern253, PMID: [DOI] [PubMed] [Google Scholar]
- Rapala-Kozik M., Wolak N., Kujda M., Banas A. K. (2012). The upregulation of thiamine (vitamin B1) biosynthesis in Arabidopsis thaliana seedlings under salt and osmotic stress conditions is mediated by abscisic acid at the early stages of this stress response. BMC Plant Biol. 12:2. doi: 10.1186/1471-2229-12-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke M., Boycheva S., Crèvecoeur M., Nunes-Nesi A., Witt S., Fernie A. R., et al. (2011). Enhanced levels of vitamin B6 increase aerial organ size and positively affect stress tolerance in Arabidopsis. Plant J. 66, 414–432. doi: 10.1111/j.1365-313X.2011.04499.x, PMID: [DOI] [PubMed] [Google Scholar]
- Raschke M., Burkle L., Muller N., Nunes-Nesi A., Fernie A. R., Arigoni D., et al. (2007). Vitamin B1 biosynthesis in plants requires the essential iron sulfur cluster protein, THIC. Proc. Natl. Acad. Sci. U. S. A. 104, 19637–19642. doi: 10.1073/pnas.0709597104, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz B., Liang Q., Wan X., Wang K., Zhang C., Ye X. (2019). Folate content analysis of wheat cultivars developed in the North China plain. Food Chem. 289, 377–383. doi: 10.1016/j.foodchem.2019.03.028, PMID: [DOI] [PubMed] [Google Scholar]
- Ribeiro D. T., Farias L. P., de Almeida J. D., Kashiwabara P. M., Ribeiro A. F., Silva-Filho M. C., et al. (2005). Functional characterization of the thi1 promoter region from Arabidopsis thaliana. J. Exp. Bot. 56, 1797–1804. doi: 10.1093/jxb/eri168, PMID: [DOI] [PubMed] [Google Scholar]
- Robinson B. R., Garcia Salinas C., Ramos Parra P., Bamberg J., Diaz de la Garza R. I., Goyer A. (2019). Expression levels of the γ-glutamyl hydrolase I gene predict vitamin B9 content in potato tubers. Agronomy 9:734. doi: 10.3390/agronomy9110734 [DOI] [Google Scholar]
- Robinson B., Sathuvalli V., Bamberg J., Goyer A. (2015). Exploring folate diversity in wild and primitive potatoes for modern crop improvement. Genes 6, 1300–1314. doi: 10.3390/genes6041300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo-Sebastián A., González-Robles C., García de Yébenes J. (2020). Vitamin B6 deficiency in patients with Parkinson disease treated with levodopa/carbidopa. Clin. Neuropharmacol. 43, 151–157. doi: 10.1097/wnf.0000000000000408, PMID: [DOI] [PubMed] [Google Scholar]
- Samsatly J., Bayen S., Jabaji S. H. (2020). Vitamin B6 is under a tight balance during disease development by rhizoctonia solani on different cultivars of potato and on Arabidopsis thaliana mutants. Front. Plant Sci. 11:875. doi: 10.3389/fpls.2020.00875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewry P. R., Van Schaik F., Ravel C., Charmet G., Rakszegi M., Bedo Z., et al. (2011). Genotype and environment effects on the contents of vitamins B1, B2, B3, and B6 in wheat grain. J. Agric. Food Chem. 59, 10564–10571. doi: 10.1021/jf202762b, PMID: [DOI] [PubMed] [Google Scholar]
- Singh J., Srivastava R. P., Gupta S., Basu P. S., Kumar J. (2016). Genetic variability for vitamin B9 and total dietary fiber in lentil (Lens culinaris L.) cultivars. Int. J. Food Prop. 19, 936–943. doi: 10.1080/10942912.2015.1048353 [DOI] [Google Scholar]
- Sotelo A., Sousa V., Montalvo I., Hernandez M., and Hernandez-Aragon (1990). Chemical composition of different fractions of 12 Mexican varieties of rice obtained during milling. Cereal Chem. 67, 209–212. [Google Scholar]
- Strobbe S., Van Der Straeten D. (2018). Toward eradication of B-vitamin deficiencies: considerations for crop biofortification. Front. Plant Sci. 9:443. doi: 10.3389/fpls.2018.00443, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobbe S., Verstraete J., Stove C., Van Der Straeten D. (2021a). Metabolic engineering of rice endosperm towards higher vitamin B1 accumulation. Plant Biotechnol. J. 19, 1253–1267. doi: 10.1111/pbi.13545, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobbe S., Verstraete J., Stove C., Van Der Straeten D. (2021b). Metabolic engineering provides insight into the regulation of thiamin biosynthesis in plants. Plant Physiol. 186, 1832–1847. doi: 10.1093/plphys/kiab198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Sigler C. L., Beaudoin G. A. W., Joshi J., Patterson J. A., Cho K. H., et al. (2019). Parts-prospecting for a high-efficiency thiamin thiazole biosynthesis pathway. Plant Physiol. 179, 958–968. doi: 10.1104/pp.18.01085, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Wu S., Mimura M., Alseekh S., Fernie A. R., Hanson A. D., et al. (2020). Construction and applications of a B vitamin genetic resource for investigation of vitamin-dependent metabolism in maize. Plant J. 101, 442–454. doi: 10.1111/tpj.14535 [DOI] [PubMed] [Google Scholar]
- Szydlowski N., Burkle L., Pourcel L., Moulin M., Stolz J., Fitzpatrick T. B. (2013). Recycling of pyridoxine (vitamin B6) by PUP1 in Arabidopsis. Plant J. 75, 40–52. doi: 10.1111/tpj.12195, PMID: [DOI] [PubMed] [Google Scholar]
- Tambasco-Studart M., Titiz O., Raschle T., Forster G., Amrhein N., Fitzpatrick T. B. (2005). Vitamin B6 biosynthesis in higher plants. Proc. Natl. Acad. Sci. U. S. A. 102, 13687–13692. doi: 10.1073/pnas.0506228102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 3000 rice genomes project (2014). The 3,000 rice genomes project. GigaScience 3, 7. doi: 10.1186/2047-217X-3-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titcomb T. J., Tanumihardjo S. A. (2019). Global concerns with B vitamin statuses: biofortification, fortification, hidden hunger, interactions, and toxicity. Compr. Rev. Food Sci. Food Saf. 18, 1968–1984. doi: 10.1111/1541-4337.12491, PMID: [DOI] [PubMed] [Google Scholar]
- Titiz O., Tambasco-Studart M., Warzych E., Apel K., Amrhein N., Laloi C., et al. (2006). PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J. 48, 933–946. doi: 10.1111/j.1365-313X.2006.02928.x, PMID: [DOI] [PubMed] [Google Scholar]
- Tunc-Ozdemir M., Miller G., Song L., Kim J., Sodek A., Koussevitzky S., et al. (2009). Thiamin confers enhanced tolerance to oxidative stress in Arabidopsis. Plant Physiol. 151, 421–432. doi: 10.1104/pp.109.140046, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Straeten D., Bhullar N. K., De Steur H., Gruissem W., MacKenzie D., Pfeiffer W., et al. (2020). Multiplying the efficiency and impact of biofortification through metabolic engineering. Nat. Commun. 11, 5203. doi: 10.1038/s41467-020-19020-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren H., Boycheva S., Li K. T., Szydlowski N., Gruissem W., Fitzpatrick T. B. (2013). Strategies for vitamin B6 biofortification of plants: a dual role as a micronutrient and a stress protectant. Front. Plant Sci. 4:143. doi: 10.3389/fpls.2013.00143, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal C. P., Juliano B. O. (1989). Variability in contents of thiamine and riboflavin in brown rice, crude oil in brown rice and bran-polish, and silicon in hull of IR Rices. Plant Foods Hum. Nutr. 39, 287–297. doi: 10.1007/BF01091939, PMID: [DOI] [PubMed] [Google Scholar]
- Wachter A., Tunc-Ozdemir M., Grove B. C., Green P. J., Shintani D. K., Breaker R. R. (2007). Riboswitch control of gene expression in plants by splicing and alternative 3′ end processing of mRNAs. Plant Cell 19, 3437–3450. doi: 10.1105/tpc.107.053645, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Ding X., Yuan M., Qiu D., Li X., Xu C., et al. (2006). Dual function of rice OsDR8 gene in disease resistance and thiamine accumulation. Plant Mol. Biol. 60, 437–449. doi: 10.1007/s11103-005-4770-x, PMID: [DOI] [PubMed] [Google Scholar]
- Wang W., Mauleon R., Hu Z., Chebotarov D., Tai S., Wu Z., et al. (2018). Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557, 43–49. doi: 10.1038/s41586-018-0063-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wang D., Ouyang Y., Huang F., Ding G., Zhang B. (2017). Do Chinese children get enough micronutrients? Nutrients 9, 397. doi: 10.3390/nu9040397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Ji J. S. (2020). Modification of vitamin B6 on the associations of blood lead levels and cardiovascular diseases in the US adults. BMJ Nutr. Preven. Health 3, 180–187. doi: 10.1136/bmjnph-2020-000088, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield K. C., Smith T. J., Rohner F., Wieringa F. T., Green T. J. (2021). Thiamine fortification strategies in low- and middle-income settings: a review. Ann. N. Y. Acad. Sci. 1498, 29–45. doi: 10.1111/nyas.14565, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. P., Plecko B., Mills P. B., Clayton P. T. (2019). Disorders affecting vitamin B(6) metabolism. J. Inherit. Metab. Dis. 42, 629–646. doi: 10.1002/jimd.12060 [DOI] [PubMed] [Google Scholar]
- Yang Y. Z., Ding S., Wang Y., Li C. L., Shen Y., Meeley R., et al. (2017). Small kernel2 encodes a glutaminase in vitamin B6 biosynthesis essential for maize seed development. Plant Physiol. 174, 1127–1138. doi: 10.1104/pp.16.01295, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani M., Zallot R., Tunc-Ozdemir M., de Crecy-Lagard V., Shintani D. K., Hanson A. D. (2013). Identification of the thiamin salvage enzyme thiazole kinase in Arabidopsis and maize. Phytochemistry 94, 68–73. doi: 10.1016/j.phytochem.2013.05.017, PMID: [DOI] [PubMed] [Google Scholar]
- Zallot R., Yazdani M., Goyer A., Ziemak M. J., Guan J. C., McCarty D. R., et al. (2014). Salvage of the thiamin pyrimidine moiety by plant TenA proteins lacking an active-site cysteine. Biochem. J. 463, 145–155. doi: 10.1042/bj20140522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei I., Brown D. G., Nealon N. J., Ryan E. P. (2017). Rice bran metabolome contains amino acids, vitamins & cofactors, and phytochemicals with medicinal and nutritional properties. Rice 10:24. doi: 10.1186/s12284-017-0157-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Jin X., Ouyang Z., Li X., Liu B., Huang L., et al. (2015). Vitamin B6 contributes to disease resistance against Pseudomonas syringae pv. Tomato DC3000 and Botrytis cinerea in Arabidopsis thaliana. J. Plant Physiol. 175, 21–25. doi: 10.1016/j.jplph.2014.06.023, PMID: [DOI] [PubMed] [Google Scholar]
- Zhu C., Bortesi L., Baysal C., Twyman R. M., Fischer R., Capell T., et al. (2017). Characteristics of genome editing mutations in cereal crops. Trends Plant Sci. 22, 38–52. doi: 10.1016/j.tplants.2016.08.009, PMID: [DOI] [PubMed] [Google Scholar]
- Zhu Y., Minović I., Dekker L. H., Eggersdorfer M. L., van Zon S. K. R., Reijneveld S. A., et al. (2020). Vitamin status and diet in elderly with low and high socioeconomic status: the lifelines-MINUTHE study. Nutrients 12, 2659. doi: 10.3390/nu12092659, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.