Abstract
Classic psychedelic drugs such as psilocybin and lysergic acid diethylamide (LSD) have recaptured the imagination of both science and popular culture, and may have efficacy in treating a wide range of psychiatric disorders. Human and animal studies of psychedelic drug action in the brain have demonstrated the involvement of the serotonin 2A (5-HT2A) receptor and the cerebral cortex in acute psychedelic drug action, but different models have evolved to try to explain the impact of 5-HT2A activation on neural systems.
Two prominent models of psychedelic drug action (the cortico-striatal thalamo-cortical, or CSTC, model and relaxed beliefs under psychedelics, or REBUS, model) have emphasized the role of different subcortical structures as crucial in mediating psychedelic drug effects. We describe these models and discuss gaps in knowledge, inconsistencies in the literature and extensions of both models. We then introduce a third circuit-level model involving the claustrum, a thin strip of grey matter between the insula and the external capsule that densely expresses 5-HT2A receptors (the cortico-claustro-cortical, or CCC, model).
In this model, we propose that the claustrum entrains canonical cortical network states, and that psychedelic drugs disrupt 5-HT2A-mediated network coupling between the claustrum and the cortex, leading to attenuation of canonical cortical networks during psychedelic drug effects.
Together, these three models may explain many phenomena of the psychedelic experience, and using this framework, future research may help to delineate the functional specificity of each circuit to the action of both serotonergic and non-serotonergic hallucinogens.
Keywords: claustrum, psilocybin, thalamus, default mode network, circuit models
Doss et al. review and discuss three different models that attempt to explain how psychedelic drugs work in the brain. The first model relates to perceptual gating and psychosis, the second to brain entropy and predictive coding, and the third to circuit-level control of cortical networks.
Introduction
Psychedelic drugs, which we define as agonists or partial agonists of the serotonin 2A receptor (5-HT2A)1 such as psilocybin (the main psychoactive constituent of Psilocybe mushrooms) and lysergic acid diethylamide (LSD), have recaptured the imagination of both science and popular culture. Recent studies have provided empirical evidence that psychedelic drugs evoke a unique array of alterations in perception and cognition,2-5 with subjective experiences in humans ranging from blissful and profound to anguished and paranoid.6–8 Psychedelics may also have efficacy in treating a wide range of medical indications, including mood,9–13 substance use14,15 and headache-related disorders.16,17 Understanding the neural circuit basis of psychedelic drug action has the potential to inform strategies for maximizing the therapeutic efficacy of these drugs, as well as minimizing their harm, while potentially elucidating novel functions of these circuits in both health and disease.
The cerebral cortex is thought to be central to the neural mechanisms underlying the psychedelic experience due to its high expression of these receptors. 5-HT2A receptor activation via the psychedelic 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) induces a substantial increase in the frequency and amplitude of excitatory postsynaptic current events in rodent prefrontal layer V pyramidal neurons, which are the major projectors and integrators in the cortex.18 DOI also increases c-fos expression in prefrontal and somatosensory GABA-releasing interneurons, which are important neuromodulators.19 Additional findings in rodents show that LSD induces gene expression changes in the cortex,20 and DOI increases extracellular GABA21 and glutamate in the cortex.22 Complementary work in humans has shown that psychedelic drugs acutely increase glutamate concentrations in the medial prefrontal cortex.23 Recent evidence from rodent studies suggests that a subset of 5-HT2A receptor-containing ‘trigger neurons’ in the medial prefrontal cortex and somatosensory cortex are responsible for the initial neural response to psychedelics.19 These neurons appear to recruit other neuronal populations, leading to cascading disruption of cortical and subcortical pathways in the development of psychedelic drug effects. In line with these observations, psychedelics generally attenuate the functional segregation of human cortical networks.24,25 Finally, 5-HT2A-mediated signalling pathways in the cortex are required for stereotyped animal behaviours produced by psychedelics such as the head-twitch response,26–28 and 5-HT2A receptor antagonism in humans blocks the subjective, cognitive and neural system effects of psilocybin,29–34 LSD35–37 and N, N-dimethyltryptamine (DMT).38
Although the role of cortical 5-HT2A receptors in psychedelic drug action is evident (primarily in prefrontal regions), the downstream effects of cortical psychedelic drug activation on subcortical targets and the activation of subcortical 5-HT2A receptors1 may also be crucial to psychedelic effects. Two leading models of the neural basis of psychedelic drug action differ on their views regarding the importance of different subcortical structures and extracortical 5-HT2A receptors. One model, the cortico-striatal thalamo-cortical loop model (CSTC; Fig. 1), proposes that psychedelic drugs modulate both the cortex and subcortical structures including the striatum and thalamus, resulting in aberrant thalamocortical coupling.39–41 This is thought to increase the flow of sensory information to the cortex, leading to perceptual alterations and effects on cognition, including diminished attention and sensorimotor gating. Another model, referred to as relaxed beliefs under psychedelics (REBUS; Fig. 2), asserts that cortical modulation by psychedelic drugs reduces the ability of higher-level cortical networks (i.e. the default mode network) to control lower-levels in the brain (e.g. ‘lower’ relative to the default mode network), specifically the hippocampus and parahippocampal gyrus (but also sensory cortices).42 This hypothesized reduction in top-down control is proposed to disrupt typical predictive coding processes and is expressed as increased entropy in measures of cortical function and network topology.
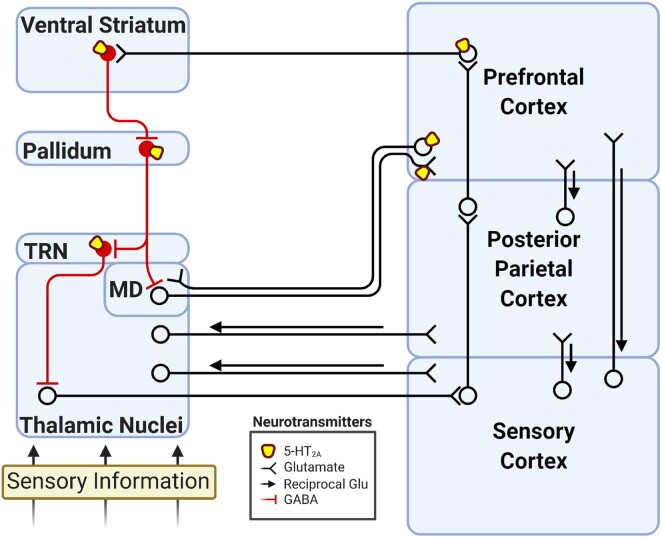
Figure 1.
Summary of the cortico-thalamo-cortical and CSTC loops. Disruption of this circuit by 5-HT2A activation is hypothesized to inundate the cortex with sensory information, contributing to the alterations of cognition and perception during psychedelic experiences.39–41 Glu = glutamate; TRN = thalamic reticular nucleus.
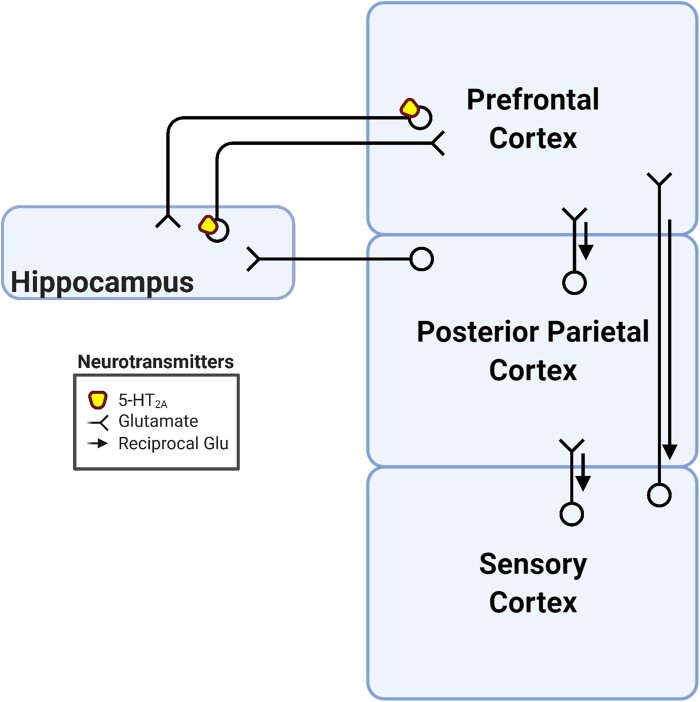
Figure 2.
Summary of the hippocampal-cortical processing loop implicated in the REBUS model. Psychedelic action is hypothesized to preferentially enhance bottom-up prediction error signalling to higher-level structures compared with top-down reciprocal signalling. This manifests as decreases in cortical synchrony in addition to relaxed priors. The REBUS model specifies hippocampus and the parahippocampal gyrus as originators of this bottom-up signalling, in concert with other unspecified structures.42 Glu = glutamate.
Here, we briefly review evidence for the CSTC and REBUS models, while describing potential gaps and extensions of each. Then, we introduce a third circuit-level model centering on the claustrum, a subcortical telencephalic nucleus that contains a sub-population of 5-HT2A-containing neurons. These neurons appear to be responsible for the initial neural response to psychedelics19 and have widespread, largely bidirectional cortical connections.43–49 This model, termed the cortico-claustro-cortical model (CCC; Fig. 3), emphasizes the function of the claustrum in the control of cortical network states, which arise from the synchronization of stereotyped sets of cortical regions across time. In the context of this new model, we highlight the potential role of psychedelic disruption of cortico-claustro-cortical circuits that may contribute to our understanding of the neural and subjective effects of psychedelic drugs.
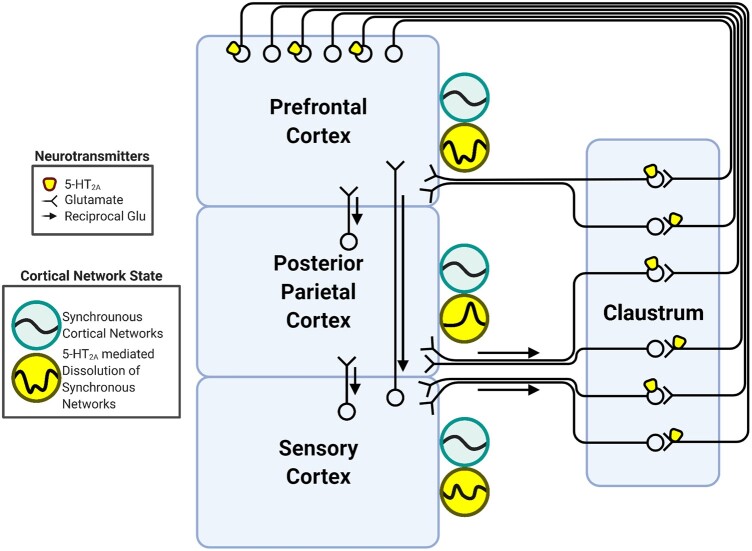
Figure 3.
Summary of the CCC model circuitry. In this model, disruption of prefronto-claustro-cortical circuitry by 5-HT2A activation dysregulates cortical network initiation, which contributes to the disruption of cortical network states (yellow circles). Turquoise and yellow circles depict two network states that arise from the dynamic synchronization of cortical regions across time. The first (turquoise circles) is a network state in which frontal, associative and sensory cortices are synchronized (represented by a similar wave form in each of the turquoise circles), and the second (yellow circles) is a state in which the network is disrupted across various cortical regions (represented by different waveforms in each of the yellow circles). Glu = glutamate.
Cortico-striatial thalamo-cortical model and the disruption of thalamocortical gating
The thalamus is, in part, a collection of subcortical nuclei that ‘relay’ sensory information from sensory organs to the cortex. In this role of relaying sensory information, the thalamus acts as a ‘gate’, restricting the flow of information to permit only a subset of sensory information at any given time. All thalamic relay nuclei receive cortical inputs that control this gating function to support cognition and action, and the thalamic reticular nucleus may especially be important to gating via local inhibition of other thalamic nuclei.50 Apart from the reticular nucleus, all thalamic nuclei provide direct glutamatergic input to the cortex.51 CSTC loops have a rich history in the study of both neural development and the pathophysiology of a number of disorders,52 including psychotic disorders. In the context of psychedelic drugs as psychotomimetic substances, disruption of the gating of sensory information through thalamocortical circuits was hypothesized to underlie the acute alterations in perception and cognition that accompany psychedelic experiences.39–41 Here, we will review the basic conceptualization of the CSTC model as it pertains to psychedelics, discuss thalamic nuclei that are most likely to be involved, summarize the behavioural and neuroimaging evidence that supports this model and consider potential limitations and extensions of the model.
Cortico-striatal-thalamo-cortical loops
In the original conception of psychedelic disruption of CSTC loops,39 psychedelics are understood to activate medial prefrontal layer V 5-HT2A-containing pyramidal neurons projecting to GABAergic neurons of the ventral striatum, which in turn inhibit GABAergic pallido-thalamic neurons within these CSTC circuits (Fig. 1). Disruption of these GABAergic pallido-thalamic neurons results in disinhibition of the thalamus and inundation of the cortex with sensory information. Accordingly, independent groups have found that LSD administration in humans increases functional connectivity (i.e. the temporal coupling between the activity of two regions) between the thalamus and much of the cortex under task-free conditions.53–55 Moreover, directional modelling of human neuroimaging data has found evidence that LSD increases thalamic influence on posterior cingulate activity in a 5-HT2A-dependent fashion, but an accompanying decrease in striatal input to the thalamus was found to be independent of 5-HT2A activation.56 Although the CSTC model describes 5-HT2A receptors in regions within CSTC loops other than the thalamus and emphasizes specific thalamic nuclei such as the mediodorsal (MD) and reticular nuclei of the thalamus,39,40 it remains unclear how the model may account for the contribution of other subnuclei and extracortical 5-HT2A receptors to the effects of psychedelics.
Thalamic nuclei of interest
It is worth noting that human studies with acute psychedelic manipulations that report on changes in thalamic connectivity have not parcellated the thalamus into its constituent nuclei, despite differential 5-HT2A expression, functional specificity and anatomical connectivity of thalamic nuclei. One would expect those thalamic nuclei expressing 5-HT2A mRNA or receptor protein to be more important to consider when determining the basis of psychedelic drug action within the brain. However, only a subset of thalamic nuclei exhibits such receptor expression. For instance, the reticular nucleus expresses 5-HT2A57 receptors and was one of the thalamic nuclei originally proposed to be of importance to psychedelic effects in CSTC loops.39,40 Indeed, LSD increases burst firing in some reticular nucleus neurons, while suppressing burst firing in others.58 The reticular nucleus is thought to function as a ‘neuronal oscillator’,59 regulating the frequency of cortical rhythms, especially gamma waves.60–64 Oscillations of this kind are thought to support synchronization between brain regions and enable communication and information transfer.65,66 Some evidence indicates that presynaptic 5-HT2A receptors in the reticular nucleus are involved in GABA release,67 providing a potential mechanism by which psychedelics desynchronize low frequency bands in a 5-HT2A-dependent manner.34,38,68–74 The reticular nucleus also supports selective visual attention by controlling the gain of the lateral geniculate nucleus,75 the main target of the retina. Finally, neurons of the reticular nucleus are dependent on prefrontal interactions via the basal ganglia,76 suggesting a potential role for striatal nodes of CSTC circuitry in psychedelic-induced impairments of attention.30,77,78
The MD is a higher-order nucleus (i.e. not directly receiving sensory information), receiving inputs from the cortex, medial temporal lobe, pallidum and other thalamic nuclei79 that was originally proposed to be important to psychedelic action in CSTC loops39,40 (Fig. 1). Of relevance to psychedelics, the MD projects to the medial prefrontal cortex, thus completing a CSTC loop with a cortical region containing neurons that may be particularly sensitive to 5-HT2A-mediated activation by psychedelic drugs.19 Moreover, the MD expresses 5-HT2A receptors both within the thalamus, at the somatic level and on presynaptic projections in medial prefrontal cortex,80,81 suggesting multiple points within an MD to medial prefrontal cortex loop that could be targeted by 5-HT2A receptor activation. One study found DOI activation of the presynaptic 5-HT2A receptors of this circuit to increase excitatory postsynaptic currents in medial prefrontal cortex, and deletion of these receptors impaired episodic-like memory in mice.82 In another study, LSD increased action potential firing frequency, including burst firing, of neurons in a subregion of MD known to receive GABAergic input from the reticular nucleus and to project out to medial prefrontal cortex.58 This LSD-induced increase in MD firing, which was also met with increases in spontaneous firing activity in medial prefrontal cortex pyramidal neurons,58 lends further support to the notion that psychedelics increase thalamic activation of cortex. Interestingly, although psychedelics impair most aspects of cognition in humans, episodic memory encoding is relatively less impaired, even at high doses of psilocybin.5
Somatic and perceptual distortions often accompany the subjective effects of psychedelics.3 Thus, 5-HT2A receptor-containing sensory nuclei of the thalamus, such as the ventrobasal thalamus and pulvinar, may also play a role in psychedelic drug action. The ventrobasal thalamus is a first-order relay (i.e. a nucleus receiving direct sensory projections) that carries ascending tactile information to somatosensory cortex. Glutamatergic projections from the ventrobasal thalamus are activated by DOI and induce immediate early gene c-fos expression in layer V somatosensory neurons.22 Consistent with these findings, LSD increases functional connectivity between the thalamus and somatosensory cortex in humans,53–55 although it is unclear whether these effects play a role in specific psychedelic effects such as loss of perceptual boundaries between one’s body and the world.
Compared with somatosensation, psychedelics appear to have more reliable and complex effects on vision, perhaps in part due to modulation of the pulvinar, a nucleus that receives higher-order visual inputs from visual cortex. The pulvinar contains 5-HT2A protein without expressing 5-HT2A mRNA.83 This indicates that the pulvinar receives inputs that express the 5-HT2A receptor on axon terminals, and thus, psychedelics may alter pulvinar output to the cortex in a polysynaptic manner. Consistent with the pulvinar’s role in visual attention and object motion,84,85 psychedelics impair visual attention77,78 and motion perception,86 increase thalamic connectivity with visual cortex53–55 and increase activity in primary visual cortex during an attention task.78 However, pretreatment with ketanserin (a 5-HT2A/5-HT2C antagonist) did not block psilocybin effects on visual attention30 or binocular rivalry,31 suggesting other potential receptor targets of psilocybin [e.g. the serotonin 1B receptor (5-HT1B)] in mediating these effects.
Behavioural evidence, limitations and extensions
Behavioural models relevant to thalamocortical gating include pre-pulse inhibition (PPI) and inhibition of return (IOR), and some evidence from animal and human models suggests that psychedelics impact these behaviours. PPI is a phenomenon that occurs when a startle response is reduced by a preceding weaker, non-startling stimulus, and IOR refers to suppressed processing of a stimulus to which one has recently attended. PPI and IOR are thought to reflect pre-attentive sensory gating processes. Thalamic nuclei within thalamocortical circuits, including MD and sensory nuclei, facilitate these processes, and prefrontal cortical nodes are thought to control these processes.77,87,88 Animal models demonstrate that LSD and DOI reduce PPI of the acoustic startle response,89 but evidence for the presence or direction of an effect of psychedelics on PPI in humans is mixed. Some have reported that psilocybin dose-dependently reduces PPI when there are short intervals between pulses (30 ms) but has either no effect or increases PPI at intermediate and longer intervals (≥120 ms).90,91 Another study that used intermediate and longer intervals (100 and 240 ms) failed to show any effect of DMT on PPI.92 Although DMT has been found to attenuate IOR,93 DMT was also found to blunt neural responses in primary sensory cortices during sustained attention tasks in auditory and visual modalities.78 This is seemingly inconsistent with an attenuation of suppressed sensory processing and a general prediction of the CSTC model that cortical regions are inundated with sensory information under psychedelics. Alternatively, decreased activation of primary sensory cortices during the acute effects of psychedelics could represent increased noise and decreased sensitivity to experimentally controlled stimuli.
Other mixed evidence for the CSTC model comes from its prediction that psychedelics should produce increased activity within the prefrontal cortex. Activity within the prefrontal cortex might reasonably follow from disinhibition of the MD in CSTC loops58 and subsequent attempts by the prefrontal cortex to exert control over ungated sensory thalamic information.39–41 Diminished thalamic gating might also presume an accompanying increase in thalamic activity. However, both decreases24,94,95 and increases54–56,96,97 have been reported in overall prefrontal and thalamic activity in humans. This inconsistency could be a product of the unconstrained nature of task-free scans in which small samples of participants might not necessarily perform the same range of mental functions during relatively short scans. Such work could be improved through the use of task-based sensory paradigms and parcellation of the thalamus into its subnuclei.
Although the CSTC model focuses on psychedelics disrupting thalamo-cortical striatal-thalamic circuits, a recently revised view of thalamic anatomy suggests a potential role for other bidirectional thalamocortical projections in psychedelic drug action (Fig. 1; for reviews, see Sherman98,99). Layer V pyramidal neurons receiving first-order thalamic relays (e.g. lateral geniculate nucleus to primary visual cortex) project to higher-order thalamic nuclei (e.g. primary visual cortex to the inferior pulvinar). Interestingly, these projections back to the thalamus also branch to synapses on subcortical motor centres.100–103 Because of these branches, such corticothalamic projections have been proposed to carry ‘efference copies’, or motor messages sent to sensory areas that allow for prediction of the sensory consequences of self-generated movements.104 This bidirectional connectivity is not limited to early sensory processing. Thalamic nuclei receiving these putative efference copies also project back to higher levels of cortex (e.g. parietal association cortex), which in turn continue this pattern of branched projections to subcortical motor centres and subsequently higher-order thalamic nuclei (e.g. medial pulvinar). This neural scaffolding suggests that as information is processed through a sensory hierarchy, efference copies allow higher levels to predict the sensation of possible lower-level motor commands. Because 5-HT2A receptors are widely expressed both in thalamic nuclei and on layer V pyramidal neurons within these circuits, psychedelics may drive aberrant predictions via such efference copies, potentially explaining sensory distortions and a sense of surprise that is often encountered during psychedelic experiences. Interestingly, efference copies are also thought to be sent to the striatum from the cortex in CSTC circuits, suggesting that aberrant predictions from the cortex could also be involved in disinhibition of the thalamus.105 In the next section, we discuss a model that describes how psychedelics might break down functional hierarchies in the brain, resulting in predictions from lower levels exerting greater influence on processing at higher levels.
Relaxed beliefs under psychedelics and the disruption of cortico-medial temporal constraints
The REBUS model proposes that psychedelics disrupt the hierarchical nature of the brain by reducing the typical constraints that higher levels place on lower levels. This reduction in top-down control results in the increased influence of low-level prediction errors on higher levels of processing that encode or maintain prior beliefs. By allowing greater influence of incoming stimuli in prediction circuits, a greater number of cortical brain states may be expressed, thereby increasing cortical ‘entropy’ and putatively allowing updates or changes to prior beliefs. Although the hierarchies described in this model are stated to encompass sensory-centric circuit relationships (e.g. association to sensory cortices or secondary visual to primary visual cortex), the ‘lower levels’ are stated to include evolutionarily older subcortical structures, including the hippocampus, amygdala and thalamus. The REBUS model specifies the hippocampus (but also evolutionarily more recent cortical regions of the parahippocampal gyrus) as a structure involved in this psychedelic-mediated prediction error signalling.42
Whereas the CSTC model is less specific in terms of which cortical regions are of primary importance to psychedelic drug action (e.g. medial prefrontal cortex and sensory cortices,39 posterior cingulate and the superior temporal gyrus56), the major cortical focus of the REBUS model has been on higher-level cortical networks composed primarily of prefrontal and parietal association cortices such as the default mode, frontoparietal and salience networks (DMN, FPN and SN, respectively). Specifically, the DMN is the crux of this model and posited to be at the top of the brain’s hierarchy. In contrast to the CSTC model, and as described below, the REBUS model is rather vague regarding the subcortical structures of greatest importance to psychedelic drug effects or what constitutes a ‘lower’ level. Here we will review the basic conceptualization of REBUS, neuroimaging and behavioural evidence that supports this model and potential limitations and extensions of the model.
Default mode network centricity, egocentricity and medial temporal entropy release
The DMN is one of the most studied networks in the brain and its major hubs, the medial prefrontal and posterior cingulate cortices, densely express 5-HT2A receptors.106 The DMN is implicated in a number of high-level cognitive functions, including episodic memory, theory of mind, semantic processing and self-referential processing.107,108 The DMN’s role in self-referential processing has situated it at the top of the brain’s hierarchy in the REBUS model, with the DMN proposed to be the neurobiological substrate of the ‘ego’ (in the Freudian sense)109 and attenuations of DMN function have been interpreted as reflecting the ‘ego dissolution’ sometimes reported to be produced by psychedelics (but see Millière et al.110 for inconsistencies regarding post hoc neural correlates of psychedelic-mediated ego dissolution). Psychedelics have been shown to impact the activity or functional connectivity of regions belonging to the DMN, but whether such effects are increases or decreases, or even selective to the DMN, has varied by study. Some studies in humans found relatively selective decreases in activity in regions of the DMN under psychedelics compared with drug-free conditions,24,111 but most other studies have found both increases and decreases in regions within and outside the DMN.74,94–97,112 Although psychedelics consistently decrease functional connectivity within the DMN,23,24,74,111,113 equal or stronger decreases within other brain networks, including the FPN, SN and sensory networks,23,74,113 have also been reported during acute psychedelic effects. Rodent studies have failed to find selective decreases in activity or functional connectivity of the DMN during acute psychedelic drug effects, however this may be confounded by the isoflurane anaesthesia that was concurrent with drug administration and imaging procedures.114,115
One explicit hypothesis of the REBUS model is that psychedelics increase bottom-up, over reciprocal top-down, information flow from the hippocampus and paraphippocampal gyrus to higher-level cortical areas, especially the DMN42 (Fig. 2). The hippocampus and parahippocampal gyrus both express 5-HT2A receptors,106,116 and the entorhinal cortex of the parahippocampal gyrus is a particularly important site for serotonergic transmission.117 The entorhinal cortex is the principal input to the hippocampus and has been referred to as a ‘wall of inhibition’, due to its intrinsically inhibitory circuitry that appears to gate selective inputs to the hippocampus.118 In addition to episodic memory, the hippocampus has been shown to be involved in prediction that can determine the fate of episodic memories.119–121 Although speculative, psychedelics could attenuate entorhinal gating of information to the hippocampus and drive erroneous predictions that could subsequently flood higher-level cortical areas with aberrant mnemonic representations (analogous to disrupting thalamic gating). Empirical evidence, however, is contrary to this view. Psilocybin and LSD decrease functional connectivity of the hippocampus and parahippocampal gyrus with DMN regions.74,122 Moreover, perirhinal and parahippocampal cortices of the parahippocampal gyrus are considered higher levels of the ventral and dorsal visual processing streams, respectively, yet contrary to the REBUS model, directional modelling has found evidence for psychedelics to increase top-down information flow from the parahippocampal gyrus to visual cortex.123 These analyses suffer from a similar problem as analyses of thalamic connectivity, namely, treating the hippocampus and the parahippocampal gyrus as unitary structures when they are understood to have a more segregated functional anatomy. Future work may benefit from parsing the parahippocampal gyrus (i.e. entorhinal, perirhinal and parahippocampal cortices) and hippocampus (e.g. anterior and posterior or hippocampal subfields) into their subregions to hone in on potential increases in bottom-up modulation of higher-level cortices under psychedelics.
According to the REBUS model, psychedelic release of low-level information manifests as an increase in cortical entropy or complexity (in the information theoretic sense).42,122,124 Several studies have shown that psychedelics desynchronize scalp electrophysiological oscillations, especially in lower frequencies,34,38,68–74 while simultaneously increasing the complexity (i.e. decreasing the information compressibility) of these signals.125,126 Moreover, psychedelics increase various measures of entropy and variability within the DMN or between the hippocampus and cortex.122,127,128 However, these increases appear to be more widespread129 and greater in magnitude in the SN, FPN and even sensory and motor networks than in the DMN.122,127 Together, these findings have been interpreted as an expansion of ‘brain states’; although as described below, behavioural evidence for this assertion is lacking.
Behavioural evidence, limitations and extensions
A broad behavioural prediction put forth by the REBUS model is that ‘higher-level’ cognitive functions (i.e. processes later than initial sensory information processing such as attention and memory) should be more impaired than lower-level functions (e.g. perception).42 Consistent with this prediction, one study found psilocybin to impair motion perception but not contrast sensitivity.86 Moreover, due to a relaxation of top-down priors in favor of bottom-up signalling, some lower-level cognitive functions may even be enhanced. For example, noticing a unique stimulus with a salient feature in a visual array of otherwise similar objects (i.e. the ‘pop-out’ effect)130 appeared to be enhanced under the effects of the R-enantiomer of 3,4-methylenedioxyethylamphetamine (MDEA),131 a hallucinogenic 5-HT2A agonist like the R-enantiomer of its more commonly known analog 3,4-methylenedioxymethylamphetamine (MDMA; in contrast, the S-enantiomers of these drugs have entactogenic, stimulant and monoaminergic effects). Extinction learning in mice, a form of memory evolutionarily older relative to other types of memory such as episodic, has also been found to be enhanced by psilocybin and the psychedelic TCB-2.132,133 However, as previously described, psychedelics impair PPI and IOR, pre-attentive processes that are arguably even lower levels of sensory memory than extinction.89,90,93 Moreover, inconsistent with the neuroanatomy highlighted by the REBUS model, various forms of hippocampally-dependent learning are unaffected or even impaired by psychedelics. Specifically, in rodents, psilocybin does not enhance trace fear conditioning,132 DOI impairs the learning of a novel spatial location134 and TCB-2 impairs the encoding of hippocampal place fields.135 Similarly in humans, encoding of recollection memory, which is a form of episodic memory dependent on the hippocampus, is impaired by psilocybin5 and the entactogen-stimulant-psychedelic hybrid MDMA136 (the amnestic effects of the latter stemming from 5-HT2A activation).137
Although it has been claimed by proponents of the REBUS model that cognitive impairments under psychedelics are simply due to ‘generalized disengagement’,42 psilocybin has been found to more selectively impair working memory compared with episodic memory, while the opposite pattern was found with the hallucinogenic NMDA antagonist dextromethorphan5 (see also Lofwall et al.138 and Braun et al.139). Such double dissociations obfuscate claims of generalized disengagement (see also Doss et al.136 for disproportionate effects of MDMA on emotional compared to neutral memory). One interesting finding from these studies of episodic memory is that the encoding of familiarity, a mnemonic process dependent on the perirhinal cortex of the parahippocampal gyrus,140 was unaffected or even enhanced under psychedelics.5,136 Similarly in mice, post-encoding (i.e. during consolidation) administration of TCB-2 enhanced novel object recognition,132 a perirhinal-dependent analogue to familiarity.141 Together, these findings suggest that psychedelics could have a unique profile of effects on cognition via episodic memory hubs that the REBUS model proposes to drive cortical entropy. These findings also highlight the nuances of specific episodic memory processes and medial temporal subregions (for review see Ranganath and Ritchey142) compared with more generalized effects within the brain that may be elucidated by careful comparative pharmacology.
The functional outcomes of increased entropy and diversity of brain states under psychedelics remain unclear. Successful memory retrieval, arguably greater information content than the failure to retrieve a memory, is associated with less entropy in scalp electrophysiological signals,143 and GABAergic sedatives increase entropy during episodic memory retrieval143 but reduce resting state measures of complexity.144,145 Greater neural diversity has been suggested to be of relevance to cognitive flexibility,42,146,147 and 5-HT2A antagonism can attenuate models of cognitive flexibility148,149 in rats. However, in mice, psychedelics impair or have no effect on cognitive flexibility,150,151 and in humans, LSD was found to acutely impair cognitive flexibility.37 Furthermore, studies supporting psychedelic-induced increases in the number of brain states are based on task-free conditions and thus are under ambiguous control of cognitive operations. Increased cycling between brain states under task-free psychedelic conditions may simply be produced by participants inadvertently ‘performing cognitive tasks’ (e.g. related to visualization, memory retrieval, shifts in attention and theory of mind operations) instead of ‘resting.’ That is, under drug-free conditions, participants possess the capacity to cycle through multiple cognitive states despite not typically doing so, but under psychedelics, cognitive control is impaired, resulting in more cycling between cognitive states and less rest-like neural activity. In the next section, we discuss a model that describes how psychedelics might disrupt cognitive control and an associated neural interface that permits switching between cortical networks for complex cognition.
Cortico-claustro-cortical model and disruption of canonical cortical networks
The claustrum is a thin, ribbon-like telencephalic nucleus located lateral to the putamen, medial to the insula and in between the external and extreme capsules. The claustrum densely expresses 5-HT2A receptors and possesses bidirectional glutamatergic connections with the majority of the cortex,47,152 suggesting a role for the claustrum in psychedelic drug effects.1,153 This structure was famously proposed to mediate sensory binding and, therefore, generation of conscious percepts.152 However, functional assessments of the claustrum have pointed to a role for the claustrum in cognitive control functions rather than in sensory binding or the generation of consciousness (see also Bickel and Parvizi154). A synthesis of recent evidence points to a specific role of the claustrum as a brain structure of central importance in supporting cortical network states. Additional evidence supports a role of psychedelic drugs in disrupting higher-level cortical networks through claustro-cortical circuits, which may subsequently contribute to the neural and subjective effects observed during psychedelic experiences (Fig. 3). The claustrum also densely expresses κ-opioid receptors,153,155 the primary target of the atypical dissociative hallucinogen salvinorin A. Interestingly, some of the effects of salvinorin A on task-free brain function (e.g. reductions of DMN functional connectivity) resemble those of psychedelics (i.e. 5-HT2A activating hallucinogens),156 suggesting that the CCC model may also explain some effects of κ-opioid activating hallucinogens.
The cortico-claustro-cortical model
In rodents, the claustrum receives dense projections from primarily contralateral areas of association cortices157–164 such as prefrontal (including regions of the DMN and FPN) and cingulate cortex (including the anterior cingulate cortex, a hub of the SN), while receiving comparatively sparser input from sensory and motor regions.163–166 Similarly, the human claustrum is functionally connected to much of the DMN, FPN and anterior cingulate cortex.48,49 Accordingly, claustrum neuron firing is primarily driven by input from prefrontal cortex.163,167 In contrast, the claustrum provides expansive ipsilateral projections back to prefrontal cortex, as well as to sensory and parietal association cortices.157–164 Claustrum input to the cortex powerfully and transiently suppresses cortical activity, followed by rebound excitation.168,169 Optogenetic activation of the claustrum results in widespread cortical synchronization.169 In the process of frontal regions initiating synchronous network activity,170–173 the CCC model proposes that a prefrontal cortico-claustro-cortical circuit configuration allows for prefrontal cortex-mediated instantiation of higher-level cortical networks that include prefrontal and posterior association cortices (Fig. 3). Thus, the CCC model proposes that the prefrontal cortex entrains canonical cortical network states through the activation of the claustrum, which in turn coordinates the recruitment of cortical networks in response to changing task demands. Claustrocortical neurons thereby provide a cortical network synchronization broadcast signal necessary for appropriate goal-directed or internally directed mental activity. We refer to these context-appropriate instantiations of cortical region synchrony as network states.
Supporting this role of the claustrum in instantiating network states, during performance of an attention task that elicits FPN responses in humans, the claustrum is activated along with the deactivation of the DMN.48 Moreover, in mice, the claustrum receives a preparatory signal from the anterior cingulate cortex, and this input, along with activation of the claustrum itself, are both required for accurate performance on the cognitively demanding five-choice serial reaction time task.167 Consistent with these findings, the mouse claustrum is also required for resilience to distraction.164
Psychedelic disruption of the cortico-claustro-cortical system
Interestingly, the claustrum expresses both 5-HT2A receptor mRNA and the 5-HT2A receptor protein, suggesting that it both contains 5-HT2A projection neurons and receives 5-HT2A-expressing projections.43,46,83,174 A large proportion of 5-HT2A receptor-containing psychedelic ‘trigger neurons’ were also found to be claustrum neurons, in addition to prefrontal cortical neurons.19 This finding led to the proposal that disruption of claustrum function by psychedelic drugs could amplify neuronal avalanches that eventually result in the destabilization of network states.175 We propose that, during psychedelic drug action, 5-HT2A activation causes a misalignment between the claustrum and cortex by driving prefrontal inputs to the claustrum and also by independently activating the claustrum. Thus, according to the CCC model, broad disruption of canonical network states may be effected by 5-HT2A-mediated decoupling of prefrontal activity from claustral activity, leading to aberrant cognitive control.176 This decoupling may ultimately lead to attenuation of cortical network (e.g. FPN and DMN) states (Fig. 3). While such states typically fluctuate depending on the goals, cues, cognitive load, prior cognitive state and upcoming states required for ongoing goal-directed behaviour, this natural fluctuation is altered during psychedelic drug action.
Supporting the CCC model account of psychedelic drug action, in humans, psilocybin decreases resting state claustrum activity, which was found to correlate with a sense of ineffability,49 a facet of ‘mystical experiences’ that closely aligns with a decrease in cognitive control. Moreover, psilocybin was found to decrease functional connectivity between the claustrum and cortical networks.49 Specifically, psilocybin decreased right claustrum connectivity with the DMN and auditory network and left claustrum connectivity with the FPN. Unexpectedly, psilocybin increased connectivity between the right claustrum and FPN. Although the CCC model might predict widespread decreases in connectivity of the claustrum with cortical networks, the laterality effects observed during acute effects of psilocybin may be a function of the claustrum’s laterality bias in structural connectivity.163,165,166 Considering the potential lateralization inherent in cognitive control processes,177 a task-based study may help to contextualize such idiosyncratic network states. Finally, some evidence supports the hypothesis that 5-HT2A-mediated disruption of prefrontal-claustrum coupling attenuates cortical network instantiation. During the acute effects of psilocybin, within-network connectivity of the DMN and FPN (which can be interpreted as ‘network integrity’) is correlated with their functional connectivity with the right and left claustrum, respectively.49
Together, these data provide support for the hypothesis that coordination between the claustrum and cortex is critical to establishing cortical network states necessary for cognitive control. In the context of prior evidence of psilocybin effects on prefrontal cortex,95,96,178 psilocybin may induce a ‘two-hit’ dysregulation of this system by disrupting both the prefrontal cortex and claustrum. In humans, claustrum activity was found to be prominently coupled with activity in the thalamus, striatum and the parahippocampal gyrus.48 Thus, while speculative, the claustrum may be positioned as a hub connecting key components inherent in all three circuit models of psychedelic drug action. While the human evidence of direct impingement of psychedelics on claustrum function is currently limited, new methods are allowing for this structure to be studied in humans.48,49 Given the integral involvement of the claustrum and prefrontal cortex with dynamic changes in goal-directed behaviour, psychedelic-induced disruption of this prefrontal cortico-claustro-cortical system may contribute to cognitive control dysfunction induced by psychedelic drugs.
Potential shared neural circuit mechanisms of 5-HT2A and κ-opioid hallucinogen effects
In addition to the claustrum densely expressing 5-HT2A receptors, it also densely expresses κ-opioid receptors,153,155 the primary target of atypical dissociative hallucinogens such as salvinorin A and enadoline. Compared with psychedelics, atypical dissociative hallucinogens seem to be more dysphoric,179 perhaps owing to their reduction in striatal dopamine transmission180–182 (but see Carlezon et al.180 and Braida et al.183 for no changes or even increased dopaminergic transmission at lower doses). Despite the sale of salvinorin A being unregulated in many states, it appears to be less commonly used than psychedelics, further suggesting fewer ‘good’ subjective effects and more frequent or stronger ‘bad’ subjective effects of salvinorin A compared with classic psychedelics. Nevertheless, atypical dissociative hallucinogens produce some subjective effects similar to classic psychedelics (i.e. 5-HT2A agonists) such as laughing, visual imagery and mystical-like experiences.184–187 Moreover, like psychedelics, there is increasing evidence that atypical dissociative hallucinogens may be useful in the treatment of addiction.188
Interestingly, some of the effects of salvinorin A on task-free brain function in humans resemble those of psychedelics. Similar to psilocybin, LSD and ayahuasca,70–72,74 salvinorin A was found to decrease power across frequency bands as measured using EEG.189 Also, in a recent functional MRI study,156 the largest effect of salvinorin A on human brain functional connectivity was a decrease of connectivity within the DMN, accompanied by trends for increased between-network connectivity of the DMN. Some of these effects were recently associated with changes in claustrum function during the acute effects of psilocybin.49 Finally, entropy was found to be increased across much of the brain during the acute effects of salvinorin A.156 However, despite a high, breakthrough dose of salvinorin A administered in the recent functional MRI study,156 changes in connectivity of task-positive networks during the acute effects of salvinorin A were not nearly as substantial as those observed during the acute effects of classic psychedelics.54,55,74,113,178,190
These data interpreted together suggest that claustrum disruption by both serotonergic psychedelics and κ-opioid receptor agonists may represent a polypharmacological mechanism underlying some ‘psychedelic-like’ effects. This interpretation must be tempered, though, as salvinorin A functional MRI data are limited to only one small-sample study, and claustrum functional MRI data during acute psychedelic effects are limited to only one separate small-sample study. Also, as noted earlier in this review, reductions in DMN connectivity and increases in entropy may be less specific than previously claimed and may be a direct result of participants cycling through various task-positive-like states, which has been understood to reduce DMN activation and connectivity for decades.108,191
κ-opioid receptors are also densely expressed in prefrontal cortex. It is possible that κ-opioid receptor-mediated disruption of the medial prefrontal cortex, or activation of striatal-projecting neurons in the medial prefrontal cortex, could result in disinhibition of the thalamus and a flooding of the cortex in line with the CSTC model, though of course this is speculative. Of note, decreases in DMN connectivity and increases in entropy are explicit features of psychedelic action in the brain that are proposed by REBUS. In fact, REBUS may be more applicable to salvinorin A, considering the largest effects of salvinorin A on human brain function were found in the DMN. Nevertheless, as described earlier, one has to be cautious when interpreting task-free connectivity. Increases in between-network connectivity and entropy may simply be an artefact of comparing a drug-free rest condition to a condition in which cognitive operations (that are able to be carried out while sober) are unconstrained (leading to inconsistent increases in specific between-network increases observed during psychedelic drug action).113
Conclusion
Here, we discussed three circuit models that may explain various effects of classic psychedelic drugs and highlight potential revisions to each. With bidirectional glutamatergic projections between the cortex and thalamus, the CSTC model’s role for the thalamus in psychedelic drug action is likely incomplete. Not only might psychedelics cause the thalamus to inundate the cortex with activity, psychedelics may also drive layer V cortical inputs to the thalamus (i.e. motor predictions or ‘efference copies’). However, similar to the REBUS model, there may be differences along the sensory processing hierarchies (e.g. under psychedelics, the thalamus may have more influence on association cortices than vice versa). Moreover, aberrant lower-level motor predictions that typically inform higher levels of cortex aligns with the REBUS model’s proposal that psychedelics can release lower-level predictions (due to less cortical constraints, however). The REBUS model also must be revised particularly regarding what constitutes a higher or lower level of the brain or cognition. Nevertheless, the brain regions specified and effects of psychedelics on behaviour suggest the possibility for unique effects of psychedelics on processes dependent on the medial temporal lobes such as episodic memory. Both the CSTC and REBUS models could benefit from more precise definitions in neuroanatomy (e.g. subnuclei of the thalamus and medial temporal lobe). Finally, the CCC model is in its infancy, and with newer technologies for probing the claustrum, future work will be needed to test its validity.
Although we discuss how disruption of claustrum function could contribute to the disruption of network states,49 cortical layer V pyramidal neurons18,19,192 and the thalamus22,53–55,94–96 could also be directly involved in such disruption. Network state disruption could take three possible forms: inhibition of sensory gating resulting in increased sensory information flow to higher cortical regions,39–41 a relaxation of predictive codes acquired from past experience,42 and disengagement of executive faculties and control of network states.49 Each of these cases could be used to explain why psychedelic drugs can allow new interpretations of familiar sensory cues, which may, in turn, promote novel behavioural responses. Modulation of maladaptive network states observed in clinical populations could provide clues to how psychedelics exhibit efficacy for brain disorders in which inelastic responses to cues foster repetitive behaviour or thoughts (e.g. addiction, depression, obsessive-compulsive disorder). Finally, differential modulation of one or all of these circuits might explain similarities among and differences between classic and atypical hallucinogenic compounds, specifically NMDA receptor antagonists (e.g. ketamine or dextromethorphan) and κ-opioid receptor agonists (e.g. salvinorin A) that have been shown to exhibit psychedelic-like effects.5,156,184,185,193,194 Pharmacological modulation of these other receptors is also being explored for therapeutic effects, and the claustrum, specifically, has a high density of both of these receptors. Mechanistic, hypothesis-driven work based on circuit models of cortical function coupled with the careful control of behaviour and cognition is needed to further establish evidence for and differentiate between the role of each model in explaining psychedelic drug effects.
Funding
This work was supported by grants from the National Institute on Drug Abuse T32DA007209 (M.K.D), the National Institute on Alcohol Abuse and Alcoholism R01AA024845 (B.N.M.), the Heffter Research Institute (R.R.G.) and by the financial support of the Johns Hopkins Center for Psychedelic and Consciousness Research by Tim Ferriss, Matt Mullenweg, Craig Nerenberg, Blake Mycoskie and the Stephen and Alexandra Cohen Foundation.
Competing interests
M.K.D. is a scientific advisor for Ocean Bio, Ltd. F.S.B. is a scientific advisor for Wavepaths, LLC. R.R.G. is a board member of the Heffter Research Institute. The other authors do not declare any competing interests.
Glossary
- 5-HT2A
serotonin 2A receptor
- CCC
cortico-claustro-cortical model
- CSTC
cortico-striatal thalamo-cortical
- DMN
default mode network
- FPN
frontoparietal network
- IOR
inhibition of return
- LSD
lysergic acid diethylamide
- MD
mediodorsal nucleus
- PPI
pre-pulse inhibition
- REBUS
relaxed beliefs under psychedelics model
- SN
salience network
References
- 1. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264–355. doi: 10.1124/pr.115.011478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Preller KH, Vollenweider FX.. Phenomenology, Structure, and Dynamic of Psychedelic States. Curr Top Behav Neurosci. Published online December 27, 2016; doi: 10.1007/7854_2016_459 [DOI] [PubMed] [Google Scholar]
- 3. Kometer M, Vollenweider FX.. Serotonergic Hallucinogen-Induced Visual Perceptual Alterations. Curr Top Behav Neurosci. 2018;36:257–282. doi: 10.1007/7854_2016_461 [DOI] [PubMed] [Google Scholar]
- 4. Barrett FS, Preller KH, Kaelen M.. Psychedelics and music: Neuroscience and therapeutic implications. International Review of Psychiatry. 2018;30(4):350–362. doi: 10.1080/09540261.2018.1484342 [DOI] [PubMed] [Google Scholar]
- 5. Barrett FS, Carbonaro TM, Hurwitz E, Johnson MW, Griffiths RR.. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: Effects on cognition. Psychopharmacology (Berl). Published online July 30, 2018;235(10):doi: 10.1007/s00213-018-4981-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffiths RR, Richards WA, McCann U, Jesse R.. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl.). 2006;187(3):268–283.discussion 284–292. doi: 10.1007/s00213-006-0457-5 [DOI] [PubMed] [Google Scholar]
- 7. Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R.. Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology (Berl.). 2011;218(4):649–665. doi: 10.1007/s00213-011-2358-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Studerus E, Kometer M, Hasler F, Vollenweider FX.. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J Psychopharmacol (Oxford.). 2011;25(11):1434–1452. doi: 10.1177/0269881110382466 [DOI] [PubMed] [Google Scholar]
- 9. Griffiths RR, Johnson MW, Carducci MA, et al. . Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol (Oxford.). 2016;30(12):1181–1197. doi: 10.1177/0269881116675513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ross S, Bossis A, Guss J, et al. . Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J Psychopharmacol (Oxford.). 2016;30(12):1165–1180. doi: 10.1177/0269881116675512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carhart-Harris RL, Bolstridge M, Rucker J, et al. . Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry. 2016;3(7):619–627. doi: 10.1016/S2215-0366(16)30065-7 [DOI] [PubMed] [Google Scholar]
- 12. Hibicke M, Landry AN, Kramer HM, Talman ZK, Nichols CD.. Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem Neurosci. 2020;11(6):864–871. doi: 10.1021/acschemneuro.9b00493 [DOI] [PubMed] [Google Scholar]
- 13. Davis AK, Barrett FS, May DG, et al. Published online. JAMA Psychiatry; 2020. Effects of psilocybin-assisted therapy for major depressive disorder: A randomized clinical trial. [DOI] [PMC free article] [PubMed]
- 14. Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR.. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol (Oxford). 2014;28(11):983–992. doi: 10.1177/0269881114548296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PCR, Strassman RJ.. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J Psychopharmacol (Oxford). 2015;29(3):289–299. doi: 10.1177/0269881114565144 [DOI] [PubMed] [Google Scholar]
- 16. Schindler EAD, Gottschalk CH, Weil MJ, Shapiro RE, Wright DA, Sewell RA.. Indoleamine Hallucinogens in Cluster Headache: Results of the Clusterbusters Medication Use Survey. J Psychoactive Drugs. 2015;47(5):372–381. doi: 10.1080/02791072.2015.1107664 [DOI] [PubMed] [Google Scholar]
- 17. Schindler EAD, Sewell RA, Gottschalk CH, et al. . Exploratory Controlled Study of the Migraine-Suppressing Effects of Psilocybin. Neurotherapeutics. Published online November 12, 2020;18(1):534–543. doi: 10.1007/s13311-020-00962-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aghajanian GK, Marek GJ.. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36(4-5):589–599. [DOI] [PubMed] [Google Scholar]
- 19. Martin DA, Nichols CD.. Psychedelics Recruit Multiple Cellular Types and Produce Complex Transcriptional Responses Within the Brain. EBioMedicine. 2016;11:262–277. doi: 10.1016/j.ebiom.2016.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nichols CD, Sanders-Bush E.. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology. 2002;26(5):634–642. doi: 10.1016/S0893-133X(01)00405-5 [DOI] [PubMed] [Google Scholar]
- 21. Abi-Saab WM, Bubser M, Roth RH, Deutch AY.. 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology. 1999;20(1):92–96. doi: 10.1016/S0893-133X(98)00046-3 [DOI] [PubMed] [Google Scholar]
- 22. Scruggs JL, Patel S, Bubser M, Deutch AY.. DOI-Induced activation of the cortex: Dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J Neurosci. 2000;20(23):8846–8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mason NL, Kuypers KPC, Müller F, et al. . Me, myself, bye: Regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology. Published online May 23, 2020;45(12):2003- 2009. doi: 10.1038/s41386-020-0718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carhart-Harris RL, Erritzoe D, Williams T, et al. . Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA. 2012;109(6):2138–2143. doi: 10.1073/pnas.1119598109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petri G, Expert P, Turkheimer F, et al. . Homological scaffolds of brain functional networks. J R Soc Interface. 2014;11(101):20140873- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gresch PJ, Barrett RJ, Sanders-Bush E, Smith RL.. 5-Hydroxytryptamine (serotonin)2A receptors in rat anterior cingulate cortex mediate the discriminative stimulus properties of d-lysergic acid diethylamide. J Pharmacol Exp Ther. 2007;320(2):662–669. doi: 10.1124/jpet.106.112946 [DOI] [PubMed] [Google Scholar]
- 27. González-Maeso J, Weisstaub NV, Zhou M, et al. . Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53(3):439–452. doi: 10.1016/j.neuron.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 28. Halberstadt AL, Geyer MA.. Characterization of the head-twitch response induced by hallucinogens in mice: Detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl). 2013;227(4):727–739. doi: 10.1007/s00213-013-3006-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D.. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9(17):3897–3902. [DOI] [PubMed] [Google Scholar]
- 30. Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX.. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005;17(10):1497–1508. doi: 10.1162/089892905774597191 [DOI] [PubMed] [Google Scholar]
- 31. Carter OL, Hasler F, Pettigrew JD, Wallis GM, Liu GB, Vollenweider FX.. Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology (Berl.). 2007;195(3):415–424. doi: 10.1007/s00213-007-0930-9 [DOI] [PubMed] [Google Scholar]
- 32. Quednow BB, Kometer M, Geyer MA, Vollenweider FX.. Psilocybin-Induced Deficits in Automatic and Controlled Inhibition are Attenuated by Ketanserin in Healthy Human Volunteers. Neuropsychopharmacology. 2012;37(3):630–640. doi: 10.1038/npp.2011.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX.. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. 2012;72(11):898–906. doi: 10.1016/j.biopsych.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 34. Kometer M, Schmidt A, Jäncke L, Vollenweider FX.. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci. 2013;33(25):10544–10551. doi: 10.1523/JNEUROSCI.3007-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Preller KH, Pokorny T, Hock A, et al. . Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U S A. 2016;113(18):5119–5124. doi: 10.1073/pnas.1524187113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barrett FS, Preller KH, Herdener M, Janata P, Vollenweider FX.. Serotonin 2A Receptor Signaling Underlies LSD-induced Alteration of the Neural Response to Dynamic Changes in Music. Cereb Cortex. Published online September 28, 2017;1–12. doi: 10.1093/cercor/bhx257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pokorny T, Duerler P, Seifritz E, Vollenweider FX, Preller KH.. LSD acutely impairs working memory, executive functions, and cognitive flexibility, but not risk-based decision-making. Psychol Med. Published Online September. 2019;10:1–10. doi: 10.1017/S0033291719002393 [DOI] [PubMed] [Google Scholar]
- 38. Valle M, Maqueda AE, Rabella M, et al. . Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. European Neuropsychopharmacology. 2016;26(7):1161–1175. doi: 10.1016/j.euroneuro.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 39. Vollenweider FX, Geyer MA.. A systems model of altered consciousness: Integrating natural and drug-induced psychoses. Brain Res Bull. 2001;56(5):495–507. [DOI] [PubMed] [Google Scholar]
- 40. Geyer MA, Vollenweider FX.. Serotonin research: Contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29(9):445–453. doi: 10.1016/j.tips.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 41. Vollenweider FX, Preller KH.. Psychedelic drugs: Neurobiology and potential for treatment of psychiatric disorders. Nature Reviews Neuroscience. 2020;21(11):611–624. doi: 10.1038/s41583-020-0367-2 [DOI] [PubMed] [Google Scholar]
- 42. Carhart-Harris RL, Friston KJ.. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol Rev. 2019;71(3):316–344. doi: 10.1124/pr.118.017160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pazos A, Cortés R, Palacios JM.. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985;346(2):231–249. doi: 10.1016/0006-8993(85)90857-1 [DOI] [PubMed] [Google Scholar]
- 44. Pompeiano M, Palacios JM, Mengod G.. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23(1-2):163–178. doi: 10.1016/0169-328x(94)90223-2 [DOI] [PubMed] [Google Scholar]
- 45. Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L.. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351(3):357–373. doi: 10.1002/cne.903510304 [DOI] [PubMed] [Google Scholar]
- 46. Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L.. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409(2):187–209. doi: [DOI] [PubMed] [Google Scholar]
- 47. Mathur BN. The claustrum in review. Front Syst Neurosci. 2014;8:48-doi: 10.3389/fnsys.2014.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krimmel SR, White MG, Panicker MH, Barrett FS, Mathur BN, Seminowicz DA.. Resting state functional connectivity and cognitive task-related activation of the human claustrum. Neuroimage. 2019;196:59–67. doi: 10.1016/j.neuroimage.2019.03.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barrett FS, Krimmel SR, Griffiths RR, Seminowicz DA, Mathur BN.. Psilocybin acutely alters the functional connectivity of the claustrum with brain networks that support perception, memory, and attention. Neuroimage. 2020;218:116980-doi: 10.1016/j.neuroimage.2020.116980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Halassa MM, Sherman SM.. Thalamocortical Circuit Motifs: A General Framework. Neuron. 2019;103(5):762–770. doi: 10.1016/j.neuron.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sherman SM, Guillery RW.. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357(1428):1695–1708. doi: 10.1098/rstb.2002.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18(1):7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tagliazucchi E, Roseman L, Kaelen M, et al. . Increased Global Functional Connectivity Correlates with LSD-Induced Ego Dissolution. Curr Biol. 2016;26(8):1043–1050. doi: 10.1016/j.cub.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 54. Müller F, Lenz C, Dolder P, et al. . Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr Scand. 2017;136(6):648–657. doi: 10.1111/acps.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Preller KH, Burt JB, Ji JL, et al. . Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife. 2018;7:doi: 10.7554/eLife.35082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Preller KH, Razi A, Zeidman P, Stämpfli P, Friston KJ, Vollenweider FX.. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc Natl Acad Sci USA. 2019;116(7):2743–2748. doi: 10.1073/pnas.1815129116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rodríguez JJ, Noristani HN, Hoover WB, Linley SB, Vertes RP.. Serotonergic projections and serotonin receptor expression in the reticular nucleus of the thalamus in the rat. Synapse. 2011;65(9):919–928. doi: 10.1002/syn.20920 [DOI] [PubMed] [Google Scholar]
- 58. Inserra A, De Gregorio D, Rezai T, Lopez-Canul MG, Comai S, Gobbi G.. Lysergic acid diethylamide differentially modulates the reticular thalamus, mediodorsal thalamus, and infralimbic prefrontal cortex: An in vivo electrophysiology study in male mice. J Psychopharmacol. Published online March 1, 2021;35(4):469–482. doi: 10.1177/0269881121991569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Steriade M, Deschenes M.. The thalamus as a neuronal oscillator. Brain Res. 1984;320(1):1–63. doi: 10.1016/0165-0173(84)90017-1 [DOI] [PubMed] [Google Scholar]
- 60. Macdonald KD, Fifkova E, Jones MS, Barth DS.. Focal stimulation of the thalamic reticular nucleus induces focal gamma waves in cortex. J Neurophysiol. 1998;79(1):474–477. doi: 10.1152/jn.1998.79.1.474 [DOI] [PubMed] [Google Scholar]
- 61. Clemente-Perez A, Makinson SR, Higashikubo B, et al. . Distinct Thalamic Reticular Cell Types Differentially Modulate Normal and Pathological Cortical Rhythms. Cell Rep. 2017;19(10):2130–2142. doi: 10.1016/j.celrep.2017.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vantomme G, Osorio-Forero A, Lüthi A, Fernandez LMJ.. Regulation of Local Sleep by the Thalamic Reticular Nucleus. Front Neurosci. 2019;13:576-doi: 10.3389/fnins.2019.00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Halassa MM, Chen Z, Wimmer RD, et al. . State-dependent architecture of thalamic reticular sub-networks. Cell. 2014;158(4):808–821. doi: 10.1016/j.cell.2014.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lewis LD, Voigts J, Flores FJ, et al. . Thalamic reticular nucleus induces fast and local modulation of arousal state. eLife. 2015;4: doi: 10.7554/eLife.08760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Buzsáki G, Draguhn A.. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- 66. Buzsáki G, Watson BO.. Brain rhythms and neural syntax: Implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci. 2012;14(4):345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goitia B, Rivero-Echeto MC, Weisstaub NV, et al. . Modulation of GABA release from the thalamic reticular nucleus by cocaine and caffeine: Role of serotonin receptors. J Neurochem. 2016;136(3):526–535. doi: 10.1111/jnc.13398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Don NS, McDonough BE, Moura G, et al. . Effects of Ayahuasca on the human EEG. Phytomedicine. 1998;5(2):87–96. doi: 10.1016/S0944-7113(98)80003-2 [DOI] [PubMed] [Google Scholar]
- 69. Riba J, Anderer P, Morte A, et al. . Topographic pharmaco-EEG mapping of the effects of the South American psychoactive beverage ayahuasca in healthy volunteers. Br J Clin Pharmacol. 2002;53(6):613–628. doi: 10.1046/j.1365-2125.2002.01609.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Riba J, Anderer P, Jané F, Saletu B, Barbanoj MJ.. Effects of the South American psychoactive beverage ayahuasca on regional brain electrical activity in humans: A functional neuroimaging study using low-resolution electromagnetic tomography. Neuropsychobiology. 2004;50(1):89–101. doi: 10.1159/000077946 [DOI] [PubMed] [Google Scholar]
- 71. Kometer M, Pokorny T, Seifritz E, Volleinweider FX.. Psilocybin-induced spiritual experiences and insightfulness are associated with synchronization of neuronal oscillations. Psychopharmacology (Berl.). 2015;232(19):3663–3676. doi: 10.1007/s00213-015-4026-7 [DOI] [PubMed] [Google Scholar]
- 72. Muthukumaraswamy SD, Carhart-Harris RL, Moran RJ, et al. . Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci. 2013;33(38):15171–15183. doi: 10.1523/JNEUROSCI.2063-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schenberg EE, Alexandre JFM, Filev R, et al. . Acute Biphasic Effects of Ayahuasca. PLoS One. 2015;10(9):e0137202-doi: 10.1371/journal.pone.0137202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Carhart-Harris RL, Muthukumaraswamy S, Roseman L, et al. . Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci USA. 2016;113(17):4853–4858. doi: 10.1073/pnas.1518377113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM.. Thalamic control of sensory selection in divided attention. Nature. 2015;526(7575):705–709. doi: 10.1038/nature15398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nakajima M, Schmitt LI, Halassa MM.. Prefrontal Cortex Regulates Sensory Filtering through a Basal Ganglia-to-Thalamus Pathway. Neuron. 2019;103(3):445–458.e10. doi: 10.1016/j.neuron.2019.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Daumann J, Heekeren K, Neukirch A, Thiel CM, Möller-Hartmann W, Gouzoulis-Mayfrank E.. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl.). 2008;200(4):573–583. doi: 10.1007/s00213-008-1237-1 [DOI] [PubMed] [Google Scholar]
- 78. Daumann J, Wagner D, Heekeren K, Neukirch A, Thiel CM, Gouzoulis-Mayfrank E.. Gouzoulis-Mayfrank E. Neuronal correlates of visual and auditory alertness in the DMT and ketamine model of psychosis. J Psychopharmacol (Oxford.). 2010;24(10):1515–1524. doi: 10.1177/0269881109103227 [DOI] [PubMed] [Google Scholar]
- 79. Mitchell AS. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neuroscience & Biobehavioral Reviews. 2015;54:76–88. doi: 10.1016/j.neubiorev.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 80. Wai MSM, Lorke DE, Kwong WH, Zhang L, Yew DT.. Profiles of serotonin receptors in the developing human thalamus. Psychiatry Res. 2011;185(1-2):238–242. doi: 10.1016/j.psychres.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 81. Jakab RL, Goldman-Rakic PS.. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. PNAS. 1998;95(2):735–740. doi: 10.1073/pnas.95.2.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Barre A, Berthoux C, De Bundel D, et al. . Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc Natl Acad Sci U S A. 2016;113(10):E1382–1391. doi: 10.1073/pnas.1525586113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. López-Giménez JF, Vilaró MT, Palacios JM, Mengod G.. Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100,907 autoradiography and in situ hybridization studies. J Comp Neurol. 2001;429(4):571–589. doi:10.1002/1096-9861(20010122)429:4<571::aid-cne5>3.0.co;2-x [DOI] [PubMed] [Google Scholar]
- 84. Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S.. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337(6095):753–756. doi: 10.1126/science.1223082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bennett C, Gale SD, Garrett ME, et al. . Higher-Order Thalamic Circuits Channel Parallel Streams of Visual Information in Mice. Neuron. 2019;102(2):477–492.e5. doi: 10.1016/j.neuron.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Carter OL, Pettigrew JD, Burr DC, Alais D, Hasler F, Vollenweider FX.. Psilocybin impairs high-level but not low-level motion perception. Neuroreport. 2004;15(12):1947–1951. [DOI] [PubMed] [Google Scholar]
- 87. Mayer AR, Seidenberg M, Dorflinger JM, Rao SM.. An event-related fMRI study of exogenous orienting: Supporting evidence for the cortical basis of inhibition of return? J Cogn Neurosci. 2004;16(7):1262–1271. doi: 10.1162/0898929041920531 [DOI] [PubMed] [Google Scholar]
- 88. Mayer AR, Dorflinger JM, Rao SM, Seidenberg M.. Neural networks underlying endogenous and exogenous visual-spatial orienting. Neuroimage. 2004;23(2):534–541. doi: 10.1016/j.neuroimage.2004.06.027 [DOI] [PubMed] [Google Scholar]
- 89. Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR.. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology (Berl.). 2001;156(2-3):117–154. doi: 10.1007/s002130100811 [DOI] [PubMed] [Google Scholar]
- 90. Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB.. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32(9):1876–1887. doi: 10.1038/sj.npp.1301324 [DOI] [PubMed] [Google Scholar]
- 91. Gouzoulis-Mayfrank E, Heekeren K, Thelen B, et al. . Effects of the hallucinogen psilocybin on habituation and prepulse inhibition of the startle reflex in humans. Behav Pharmacol. 1998;9(7):561–566. [DOI] [PubMed] [Google Scholar]
- 92. Heekeren K, Neukirch A, Daumann J, et al. . Prepulse inhibition of the startle reflex and its attentional modulation in the human S-ketamine and N,N-dimethyltryptamine (DMT) models of psychosis. J Psychopharmacol (Oxford.). 2007;21(3):312–320. doi: 10.1177/0269881107077734 [DOI] [PubMed] [Google Scholar]
- 93. Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, et al. . Inhibition of return in the human 5HT2A agonist and NMDA antagonist model of psychosis. Neuropsychopharmacology. 2006;31(2):431–441. doi: 10.1038/sj.npp.1300882 [DOI] [PubMed] [Google Scholar]
- 94. Hermle L, Fünfgeld M, Oepen G, et al. . Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: Experimental psychosis as a tool for psychiatric research. Biol Psychiatry. 1992;32(11):976–991. doi: 10.1016/0006-3223(92)90059-9 [DOI] [PubMed] [Google Scholar]
- 95. Gouzoulis-Mayfrank E, Schreckenberger M, Sabri O, et al. . Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology. 1999;20(6):565–581. doi: 10.1016/S0893-133X(98)00089-X [DOI] [PubMed] [Google Scholar]
- 96. Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J.. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16(5):357–372. doi: 10.1016/S0893-133X(96)00246-1 [DOI] [PubMed] [Google Scholar]
- 97. Riba J, Romero S, Grasa E, Mena E, Carrió I, Barbanoj MJ.. Increased frontal and paralimbic activation following ayahuasca, the pan-Amazonian inebriant. Psychopharmacology (Berl.). 2006;186(1):93–98. doi: 10.1007/s00213-006-0358-7 [DOI] [PubMed] [Google Scholar]
- 98. Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nature Neuroscience. 2016;19(4):533–541. doi: 10.1038/nn.4269 [DOI] [PubMed] [Google Scholar]
- 99. Sherman SM. Functioning of Circuits Connecting Thalamus and Cortex. Compr Physiol. 2017;7(2):713–739. doi: 10.1002/cphy.c160032 [DOI] [PubMed] [Google Scholar]
- 100. Deschênes M, Bourassa J, Pinault D.. Corticothalamic projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res. 1994;664(1-2):215–219. doi: 10.1016/0006-8993(94)91974-7 [DOI] [PubMed] [Google Scholar]
- 101. Bourassa J, Pinault D, Deschênes M.. Corticothalamic Projections from the Cortical Barrel Field to the Somatosensory Thalamus in Rats: A Single-fibre Study Using Biocytin as an Anterograde Tracer. European Journal of Neuroscience. 1995;7(1):19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x [DOI] [PubMed] [Google Scholar]
- 102. Bourassa J, Deschênes M.. Corticothalamic projections from the primary visual cortex in rats: A single fiber study using biocytin as an anterograde tracer. Neuroscience. 1995;66(2):253–263. doi: 10.1016/0306-4522(95)00009-8 [DOI] [PubMed] [Google Scholar]
- 103. Veinante P, Lavallé P, Deschênes M.. Corticothalamic projections from layer 5 of the vibrissal barrel cortex in the rat. Journal of Comparative Neurology. 2000;424(2):197–204. doi: [DOI] [PubMed] [Google Scholar]
- 104. Bridgeman B. A review of the role of efference copy in sensory and oculomotor control systems. Ann Biomed Eng. 1995;23(4):409–422. doi: 10.1007/BF02584441 [DOI] [PubMed] [Google Scholar]
- 105. Fee MS. The role of efference copy in striatal learning. Current Opinion in Neurobiology. 2014;25:194–200. doi: 10.1016/j.conb.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Beliveau V, Ganz M, Feng L, et al. . A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J Neurosci. 2017;37(1):120–128. doi: 10.1523/JNEUROSCI.2830-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Andrews-Hanna JR, Smallwood J, Spreng RN.. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Raichle ME. The Brain’s Default Mode Network. Annual Review of Neuroscience. 2015;38(1):433–447. doi: 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- 109. Carhart-Harris RL, Friston KJ.. The default-mode, ego-functions and free-energy: A neurobiological account of Freudian ideas. Brain. 2010;133(Pt 4):1265–1283. doi: 10.1093/brain/awq010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Millière R, Carhart-Harris RL, Roseman L, Trautwein F-M, Berkovich-Ohana A.. Psychedelics, Meditation, and Self-Consciousness. Front Psychol. 2018;9:1475-doi: 10.3389/fpsyg.2018.01475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Palhano-Fontes F, Andrade KC, Tofoli LF, et al. . The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. PLoS ONE. 2015;10(2):e0118143-doi: 10.1371/journal.pone.0118143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lewis CR, Preller KH, Kraehenmann R, Michels L, Staempfli P, Vollenweider FX.. Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. Neuroimage. 2017;159:70–78. doi: 10.1016/j.neuroimage.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 113. Müller F, Dolder PC, Schmidt A, Liechti ME, Borgwardt S.. Altered network hub connectivity after acute LSD administration. Neuroimage Clin. 2018;18:694–701. doi: 10.1016/j.nicl.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Spain A, Howarth C, Khrapitchev AA, Sharp T, Sibson NR, Martin C.. Neurovascular and neuroimaging effects of the hallucinogenic serotonin receptor agonist psilocin in the rat brain. Neuropharmacology. 2015;99:210–220. doi: 10.1016/j.neuropharm.2015.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Grandjean J, Buehlmann D, Buerge M, et al. . Psilocybin exerts distinct effects on resting state networks associated with serotonin and dopamine in mice. NeuroImage. 2021;225:117456-doi: 10.1016/j.neuroimage.2020.117456 [DOI] [PubMed] [Google Scholar]
- 116. Bombardi C. Neuronal localization of 5-HT2A receptor immunoreactivity in the rat hippocampal region. Brain Res Bull. 2012;87(2-3):259–273. doi: 10.1016/j.brainresbull.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 117. Deng P-Y, Lei S.. Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K+ channels. Molecular and Cellular Neuroscience. 2008;39(2):273–284. doi: 10.1016/j.mcn.2008.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. de Curtis M, Paré D.. The rhinal cortices: A wall of inhibition between the neocortex and the hippocampus. Prog Neurobiol. 2004;74(2):101–110. doi: 10.1016/j.pneurobio.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 119. Lisman J, Redish AD.. Prediction, sequences and the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1193–1201. doi: 10.1098/rstb.2008.0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kim G, Lewis-Peacock JA, Norman KA, Turk-Browne NB.. Pruning of memories by context-based prediction error. PNAS. 2014;111(24):8997–9002. doi: 10.1073/pnas.1319438111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sherman BE, Turk-Browne NB.. Statistical prediction of the future impairs episodic encoding of the present. Proc Natl Acad Sci U S A. 2020;117(37):22760–22770. doi: 10.1073/pnas.2013291117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Carhart-Harris RL, Leech R, Hellyer PJ, et al. . The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20-doi: 10.3389/fnhum.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kaelen M, Roseman L, Kahan J, et al. . LSD modulates music-induced imagery via changes in parahippocampal connectivity. Eur Neuropsychopharmacol. 2016;26(7):1099–1109. doi: 10.1016/j.euroneuro.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 124. Carhart-Harris RL. The entropic brain - Revisited. Neuropharmacology. Published Online March 13. 2018;142:167–178. doi: 10.1016/j.neuropharm.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 125. Schartner MM, Carhart-Harris RL, Barrett AB, Seth AK, Muthukumaraswamy SD.. Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci Rep. 2017;7:46421-doi: 10.1038/srep46421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Timmermann C, Roseman L, Schartner M, et al. . Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep. 2019;9(1):16324-doi: 10.1038/s41598-019-51974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lebedev AV, Kaelen M, Lövdén M, et al. . LSD-induced entropic brain activity predicts subsequent personality change. Hum Brain Mapp. 2016;37(9):3203–3213. doi: 10.1002/hbm.23234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tagliazucchi E, Carhart-Harris R, Leech R, Nutt D, Chialvo DR.. Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp. 2014;35(11):5442–5456. doi: 10.1002/hbm.22562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Viol A, Palhano-Fontes F, Onias H, de Araujo DB, Viswanathan GM.. Shannon entropy of brain functional complex networks under the influence of the psychedelic Ayahuasca. Sci Rep. 2017;7(1):7388-doi: 10.1038/s41598-017-06854-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Treisman AM, Gelade G.. A feature-integration theory of attention. Cognitive Psychology. 1980;12(1):97–136. doi: 10.1016/0010-0285(80)90005-5 [DOI] [PubMed] [Google Scholar]
- 131. Spitzer M, Franke B, Walter H, et al. . Enantio-selective cognitive and brain activation effects of N-ethyl-3,4-methylenedioxyamphetamine in humans. Neuropharmacology. 2001;41(2):263–271. doi: 10.1016/S0028-3908(01)00060-0 [DOI] [PubMed] [Google Scholar]
- 132. Zhang G, Ásgeirsdóttir HN, Cohen SJ, Munchow AH, Barrera MP, Stackman RW.. Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology. 2013;64:403–413. doi: 10.1016/j.neuropharm.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Catlow BJ, Song S, Paredes DA, Kirstein CL, Sanchez-Ramos J.. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res. 2013;228(4):481–491. doi: 10.1007/s00221-013-3579-0 [DOI] [PubMed] [Google Scholar]
- 134. Kant GJ, Wylie RM, Chu K, Ghosh S.. Effects of the Serotonin Agonists 8-OH-DPAT, Buspirone, and DOI on Water Maze Performance. Pharmacology Biochemistry and Behavior. 1998;59(3):729–735. doi: 10.1016/S0091-3057(97)00553-4 [DOI] [PubMed] [Google Scholar]
- 135. Zhang G, Cinalli D, Stackman RW.. Effect of a hallucinogenic serotonin 5-HT2A receptor agonist on visually guided, hippocampal-dependent spatial cognition in C57BL/6J mice. Hippocampus. 2017;27(5):558–569. doi: 10.1002/hipo.22712 [DOI] [PubMed] [Google Scholar]
- 136. Doss MK, Weafer J, Gallo DA, de Wit H.. MDMA Impairs Both the Encoding and Retrieval of Emotional Recollections. Neuropsychopharmacology. 2018;43(4):791–800. doi: 10.1038/npp.2017.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. van Wel JHP, Kuypers KPC, Theunissen EL, Bosker WM, Bakker K, Ramaekers JG.. Blockade of 5-HT 2 Receptor Selectively Prevents MDMA-Induced Verbal Memory Impairment. Neuropsychopharmacology. 2011;36(9):1932–1939. doi: 10.1038/npp.2011.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lofwall MR, Griffiths RR, Mintzer MZ.. Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults. Exp Clin Psychopharmacol. 2006;14(4):439–449. doi: 10.1037/1064-1297.14.4.439 [DOI] [PubMed] [Google Scholar]
- 139. Braun U, Schäfer A, Bassett DS, et al. . Dynamic brain network reconfiguration as a potential schizophrenia genetic risk mechanism modulated by NMDA receptor function. Proc Natl Acad Sci USA. 2016;113(44):12568–12573. doi: 10.1073/pnas.1608819113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yonelinas AP, Aly M, Wang W-C, Koen JD.. Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus. 2010;20(11):1178–1194. doi: 10.1002/hipo.20864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Winters BD, Saksida LM, Bussey TJ.. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32(5):1055–1070. doi: 10.1016/j.neubiorev.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 142. Ranganath C, Ritchey M.. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13(10):713–726. doi: 10.1038/nrn3338 [DOI] [PubMed] [Google Scholar]
- 143. Talebi N, Nasrabadi AM, Curran T.. Investigation of changes in EEG complexity during memory retrieval: The effect of midazolam. Cogn Neurodyn. 2012;6(6):537–546. doi: 10.1007/s11571-012-9214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Weber AM, Soreni N, Noseworthy MD.. A preliminary study on the effects of acute ethanol ingestion on default mode network and temporal fractal properties of the brain. MAGMA. 2014;27(4):291–301. doi: 10.1007/s10334-013-0420-5 [DOI] [PubMed] [Google Scholar]
- 145. Sarasso S, Boly M, Napolitani M, et al. . Consciousness and Complexity during Unresponsiveness Induced by Propofol, Xenon, and Ketamine. Curr Biol. 2015;25(23):3099–3105. doi: 10.1016/j.cub.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 146. Dajani DR, Uddin LQ.. Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends Neurosci. 2015;38(9):571–578. doi: 10.1016/j.tins.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Douw L, Wakeman DG, Tanaka N, Liu H, Stufflebeam SM.. State-dependent variability of dynamic functional connectivity between frontoparietal and default networks relates to cognitive flexibility. Neuroscience. 2016;339:12–21. doi: 10.1016/j.neuroscience.2016.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Boulougouris V, Glennon JC, Robbins TW.. Dissociable Effects of Selective 5-HT 2A and 5-HT 2C Receptor Antagonists on Serial Spatial Reversal Learning in Rats. Neuropsychopharmacology. 2008;33(8):2007–2019. doi: 10.1038/sj.npp.1301584 [DOI] [PubMed] [Google Scholar]
- 149. Furr A, Lapiz-Bluhm MD, Morilak DA.. 5-HT2A receptors in the orbitofrontal cortex facilitate reversal learning and contribute to the beneficial cognitive effects of chronic citalopram treatment in rats. International Journal of Neuropsychopharmacology. 2012;15(9):1295–1305. doi: 10.1017/S1461145711001441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Amodeo DA, Hassan O, Klein L, Halberstadt AL, Powell SB.. Acute serotonin 2A receptor activation impairs behavioral flexibility in mice. Behav Brain Res. 2020;395:112861-doi: 10.1016/j.bbr.2020.112861 [DOI] [PubMed] [Google Scholar]
- 151. Odland AU, Kristensen JL, Andreasen JT.. The selective 5-HT2A receptor agonist 25CN-NBOH does not affect reversal learning in mice. Behav Pharmacol. Published online February 15, 2021; doi: 10.1097/FBP.0000000000000626 [DOI] [PubMed] [Google Scholar]
- 152. Crick FC, Koch C.. What is the function of the claustrum? Philos Trans R Soc Lond, B, Biol Sci. 2005;360(1458):1271–1279. doi: 10.1098/rstb.2005.1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Stiefel KM, Merrifield A, Holcombe AO.. The claustrum’s proposed role in consciousness is supported by the effect and target localization of Salvia divinorum. Front Integr Neurosci. 2014;8:20-doi: 10.3389/fnint.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bickel S, Parvizi J.. Electrical stimulation of the human claustrum. Epilepsy Behav. 2019;97:296–303. doi: 10.1016/j.yebeh.2019.03.051 [DOI] [PubMed] [Google Scholar]
- 155. Patru MC, Reser DH.. A New Perspective on Delusional States - Evidence for Claustrum Involvement. Front Psychiatry. 2015;6:158-doi: 10.3389/fpsyt.2015.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Doss MK, May DG, Johnson MW, et al. . The Acute Effects of the Atypical Dissociative Hallucinogen Salvinorin A on Functional Connectivity in the Human Brain. Sci Rep. 2020;10(1):doi: 10.1038/s41598-020-73216-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Norita M. Demonstration of bilateral claustro-cortical connections in the cat with the method of retrograde axonal transport of horseradish peroxidase. Arch Histol Jpn. 1977;40(1):1–10. doi: 10.1679/aohc1950.40.1 [DOI] [PubMed] [Google Scholar]
- 158. Squatrito S, Battaglini PP, Galletti C, Riva Sanseverino E.. Projections from the visual cortex to the contralateral claustrum of the cat revealed by an anterograde axonal transport method. Neurosci Lett. 1980;19(3):271–275. doi: 10.1016/0304-3940(80)90272-4 [DOI] [PubMed] [Google Scholar]
- 159. Olson CR, Graybiel AM.. Sensory maps in the claustrum of the cat. Nature. 1980;288(5790):479–481. doi: 10.1038/288479a0 [DOI] [PubMed] [Google Scholar]
- 160. Li ZK, Takada M, Hattori T.. Topographic organization and collateralization of claustrocortical projections in the rat. Brain Res Bull. 1986;17(4):529–532. doi: 10.1016/0361-9230(86)90220-0 [DOI] [PubMed] [Google Scholar]
- 161. Smith JB, Alloway KD.. Functional specificity of claustrum connections in the rat: Interhemispheric communication between specific parts of motor cortex. J Neurosci. 2010;30(50):16832–16844. doi: 10.1523/JNEUROSCI.4438-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Smith JB, Radhakrishnan H, Alloway KD.. Rat claustrum coordinates but does not integrate somatosensory and motor cortical information. J Neurosci. 2012;32(25):8583–8588. doi: 10.1523/JNEUROSCI.1524-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. White MG, Cody PA, Bubser M, Wang H-D, Deutch AY, Mathur BN.. Cortical hierarchy governs rat claustrocortical circuit organization. J Comp Neurol. 2017;525(6):1347–1362. doi: 10.1002/cne.23970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Atlan G, Terem A, Peretz-Rivlin N, et al. . The Claustrum Supports Resilience to Distraction. Curr Biol. 2018;28(17):2752–2762.e7. doi: 10.1016/j.cub.2018.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wang Q, Ng L, Harris JA, et al. . Organization of the connections between claustrum and cortex in the mouse. J Comp Neurol. 2017;525(6):1317–1346. doi: 10.1002/cne.24047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zingg B, Dong H-W, Tao HW, Zhang LI.. Input-output organization of the mouse claustrum. J Comp Neurol. 2018;526(15):2428–2443. doi: 10.1002/cne.24502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. White MG, Mathur BN.. Claustrum circuit components for top-down input processing and cortical broadcast. Brain Struct Funct. 2018;223(9):3945–3958. doi: 10.1007/s00429-018-1731-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Jackson J, Karnani MM, Zemelman BV, Burdakov D, Lee AK.. Inhibitory Control of Prefrontal Cortex by the Claustrum. Neuron. 2018;99(5):1029–1039.e4. doi: 10.1016/j.neuron.2018.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Narikiyo K, Mizuguchi R, Ajima A, et al. . The claustrum coordinates cortical slow-wave activity. Nat Neurosci. 2020;23(6):741–753. doi: 10.1038/s41593-020-0625-7 [DOI] [PubMed] [Google Scholar]
- 170. Ayodeji O, Baker HW.. Is there a specific abnormality of sperm morphology in men with varicoceles? Fertil Steril. 1986;45(6):839–842. doi: 10.1016/s0015-0282(16)49403-3 [DOI] [PubMed] [Google Scholar]
- 171. Grent-'t-Jong T, Woldorff MG.. Timing and sequence of brain activity in top-down control of visual-spatial attention. PLoS Biol. 2007;5(1):e12-doi: 10.1371/journal.pbio.0050012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gregoriou GG, Gotts SJ, Zhou H, Desimone R.. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324(5931):1207–1210. doi: 10.1126/science.1171402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Buschman TJ, Miller EK.. Goal-direction and top-down control. Philos Trans R Soc Lond B Biol Sci. 2014. 1655;369: doi: 10.1098/rstb.2013.0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Pasqualetti M, Nardi I, Ladinsky H, Marazziti D, Cassano GB.. Comparative anatomical distribution of serotonin 1A, 1D alpha and 2A receptor mRNAs in human brain postmortem. Brain Res Mol Brain Res. 1996;39(1-2):223–233. doi: 10.1016/0169-328x(96)00026-5 [DOI] [PubMed] [Google Scholar]
- 175. Nichols DE, Johnson MW, Nichols CD.. Psychedelics as Medicines: An Emerging New Paradigm. Clin Pharmacol Ther. 2017;101(2):209–219. doi: 10.1002/cpt.557 [DOI] [PubMed] [Google Scholar]
- 176. Schmidt A, Müller F, Lenz C, et al. . Acute LSD effects on response inhibition neural networks. Psychol Med. 2018;48(9):1464–1473. doi: 10.1017/S0033291717002914 [DOI] [PubMed] [Google Scholar]
- 177. Serrien DJ, Sovijärvi-Spapé MM.. Cognitive control of response inhibition and switching: Hemispheric lateralization and hand preference. Brain Cogn. 2013;82(3):283–290. doi: 10.1016/j.bandc.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 178. Preller KH, Duerler P, Burt JB, et al. . Psilocybin Induces Time-Dependent Changes in Global Functional Connectivity. Biol Psychiatry. 2020;88(2):197–207. doi: 10.1016/j.biopsych.2019.12.027 [DOI] [PubMed] [Google Scholar]
- 179. Walsh SL, Strain EC, Abreu ME, Bigelow GE.. Enadoline, a selective kappa opioid agonist: Comparison with butorphanol and hydromorphone in humans. Psychopharmacology. 2001;157(2):151–162. doi: 10.1007/s002130100788 [DOI] [PubMed] [Google Scholar]
- 180. Carlezon WA, Béguin C, DiNieri JA, et al. . Depressive-Like Effects of the κ-Opioid Receptor Agonist Salvinorin A on Behavior and Neurochemistry in Rats. J Pharmacol Exp Ther. 2006;316(1):440–447. doi: 10.1124/jpet.105.092304 [DOI] [PubMed] [Google Scholar]
- 181. Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ.. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: Agonist actions at kappa opioid receptors. Psychopharmacology (Berl.). 2005;179(3):551–558. doi: 10.1007/s00213-004-2087-0 [DOI] [PubMed] [Google Scholar]
- 182. Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH.. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl.). 2010;210(2):241–252. doi: 10.1007/s00213-010-1836-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Braida D, Limonta V, Capurro V, et al. . Involvement of κ-Opioid and Endocannabinoid System on Salvinorin A-Induced Reward. Biological Psychiatry. 2008;63(3):286–292. doi: 10.1016/j.biopsych.2007.07.020 [DOI] [PubMed] [Google Scholar]
- 184. Johnson MW, MacLean KA, Reissig CJ, Prisinzano TE, Griffiths RR.. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug Alcohol Depend. 2011;115(1-2):150–155. doi: 10.1016/j.drugalcdep.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. MacLean KA, Johnson MW, Reissig CJ, Prisinzano TE, Griffiths RR.. Dose-related effects of salvinorin A in humans: Dissociative, hallucinogenic, and memory effects. Psychopharmacology (Berl.). 2013;226(2):381–392. doi: 10.1007/s00213-012-2912-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Maqueda AE, Valle M, Addy PH, et al. . Salvinorin-A Induces Intense Dissociative Effects, Blocking External Sensory Perception and Modulating Interoception and Sense of Body Ownership in Humans. Int J Neuropsychopharmacol. 2015;18(12):pyv065- doi: 10.1093/ijnp/pyv065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Addy PH. Acute and post-acute behavioral and psychological effects of salvinorin A in humans. Psychopharmacology. 2012;220(1):195–204. doi: 10.1007/s00213-011-2470-6 [DOI] [PubMed] [Google Scholar]
- 188. Kivell BM, Ewald AWM, Prisinzano TE.. Salvinorin A analogs and other κ-opioid receptor compounds as treatments for cocaine abuse. Adv Pharmacol. 2014;69:481–511. doi: 10.1016/B978-0-12-420118-7.00012-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ranganathan M, Schnakenberg A, Skosnik PD, et al. . Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist Salvinorin A in humans. Biol Psychiatry. 2012;72(10):871–879. doi: 10.1016/j.biopsych.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Roseman L, Leech R, Feilding A, Nutt DJ, Carhart-Harris RL.. The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front Hum Neurosci. 2014;8:204-doi: 10.3389/fnhum.2014.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL.. A default mode of brain function. PNAS. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Béïque J-C, Imad M, Mladenovic L, Gingrich JA, Andrade R.. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci USA. 2007;104(23):9870–9875. doi: 10.1073/pnas.0700436104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Reissig CJ, Carter LP, Johnson MW, Mintzer MZ, Klinedinst MA, Griffiths RR.. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology (Berl.). 2012;223(1):1–15. doi: 10.1007/s00213-012-2680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Carbonaro TM, Johnson MW, Hurwitz E, Griffiths RR.. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: Similarities and differences in subjective experiences. Psychopharmacology (Berl.). 2018;235(2):521–534. doi: 10.1007/s00213-017-4769-4 [DOI] [PMC free article] [PubMed] [Google Scholar]