Abstract
Background:
Higher circulating carotenoids are associated with lower breast cancer risk. The underlying biology remains under-explored.
Methods:
We profiled 293 pre-diagnostic plasma metabolites in a nested case-control study (n=887 cases) within the Nurses’ Health Studies. Associations between circulating carotenoids and metabolites were identified using linear-mixed models (FDR≤0.05), and we further selected metabolites most predictive of carotenoids with LASSO. Metabolic signatures for carotenoids were calculated as weighted sums of LASSO selected metabolites. We further evaluated the metabolic signatures in relation to breast cancer risk using conditional logistic-regression.
Results:
We identified 48–110 metabolites associated with plasma levels of α-carotene, β-carotene, β-cryptoxanthin, estimated-vitamin-A-potential, lutein/zeaxanthin, and lycopene, which included primarily positively associated metabolites implicated in immune regulation (tryptophan), redox balance (plasmalogens, glutamine), epigenetic regulations (acetylated-/methylated-metabolites), and primarily inversely associated metabolites involved in β-oxidation (carnitines) (FDR≤0.05). The metabolomic signatures derived for β-carotene (Q4 vs. Q1 relative risk RR=0.74, p-trend=0.02), and estimated-vitamin-A-potential (Q4 vs. Q1 RR=0.74, p-trend=0.02)—measured ≥10 years before diagnosis—were associated with lower breast cancer risk. Modest attenuations of RR for measured levels of β-carotene and estimated-vitamin-A-potential were seen when we adjusted for their corresponding metabolic signatures.
Conclusions:
Metabolites involved in immune regulation, redox balance, membrane signaling and β-oxidation were associated with plasma carotenoids. While some metabolites may reflect shared common food sources or compartmental co-localization with carotenoids, others may signal the underlying pathways of carotenoids-associated lowered breast cancer risk.
Impact:
Consumption of carotenoid-rich diet is associated with a wide-range of metabolic changes which may help to reduce breast cancer risk.
Keywords: circulating carotenoids, metabolomics, breast cancer risk
INTRODUCTION
Carotenoids, a family of lipophilic micronutrients in fruit and vegetables that play a role in photosynthesis, represent one of few modifiable factors that have been associated with lower breast cancer risk.(1–4) In pooled analyses from large prospective cohorts, higher dietary and circulating levels of carotenoids were associated with lower breast cancer risk.(1, 4) In the Nurses’ Health Studies (NHS), women with higher circulating levels of carotenoids, measured up to 20 years prior to diagnosis, were at reduced breast cancer risk,(2) and the associations were in general suggestively stronger for distant blood measures (>10 years before diagnosis).(2) Experimental evidence reveals that carotenoids have direct free-radical quenching properties (5, 6) and provitamin A activities,(7) in which retinoic acids (derived from α- and β-carotenes, and β-cryptoxanthin) bind their nuclear receptors to modulate over 500 DNA response elements and initiate transcriptional regulation of genes involved in lipid metabolism,(8) insulin sensitivity,(9) energy balance,(10) and epigenetic tuning.(7, 11) Carotenoids may also interact with vitamin D (12) and thyroid hormone (13) receptors, and modulate inflammation and immune response.(14–17) However, despite the compelling laboratory evidence, few population based studies have explored pathways through which carotenoids may lower breast cancer risk. Due the controversies arising from previous high-dose α-tocopherol, β-carotene, and retinyl palmitate supplementation trials (i.e., a higher risk of lung cancer was observed for heavy smokers in the supplementation arm),(18–20) a better understanding of the mechanisms by which carotenoids modulate biological pathways is important to guide future prevention strategies in humans.
The metabolomic profile integrates low-molecular-weight compounds (< 1 kDa) of the human body, and provides a systematic readout of the physiological status of an individual to reflect the complex interplay between genetics and both endogenous and exogenous environmental influences (21–24). Among 2,188 cases and 2,188 matched controls in the NHS, we have previously shown that women with higher circulating levels of carotenoids, measured up to 20 years prior to diagnosis, were at lower breast cancer risk.(2) In the present analysis, we measured plasma metabolomics in a subset of participants from the same nested case-control study (877 cases and 877 matched controls). Our goal was to identify plasma metabolites that may, in part, explain the associations of circulating carotenoids with reduced breast cancer risk. Specifically, our goals were to: (1) select metabolites associated with plasma levels of α-carotene, β-carotene, β-cryptoxanthin, estimated vitamin A potential, lutein/zeaxanthin and lycopene; (2) derive a metabolomic signature for each individual carotenoid; (3) examine whether the carotenoid metabolomic signatures were associated with subsequent breast cancer risk; and (4) evaluate whether the carotenoid—breast cancer associations were attenuated when we control for the metabolomic signatures. We considered distant (≥10 years prior to breast cancer diagnosis) and proximate (<10 years) carotenoids measures separately with the underlying assumption that distant blood samples were more likely to capture cancer initiation processes, while proximate blood samples may reflect progression.
MATERIALS AND METHODS
Study population
This study includes participants from the NHS, a prospective cohort of registered female nurses in the United States, described previously.(25) In brief, the NHS was established in 1976, when 121,701 women aged 30–55 years returned an initial questionnaire. Participants have been followed via questionnaires mailed biennially to update information on sociodemographic characteristics, dietary and lifestyle factors, medical history, medication use, as well as to ascertain outcomes.(25) The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T. H. Chan School of Public Health, and those of participating registries as required. This study is not a randomized trial.
Nested case-control selection
Blood was collected at two time points, first in 1989–1990 from 32,826 women aged 43–69 years,(26) and again in 2000–2002 from a subset of 18,743 of the same women.(26) Eligible cases with no prior report of cancer (other than nonmelanoma skin) before blood collection and diagnosed with breast cancer before June 1, 2010 were selected.(2, 27) Cases were matched to controls (1:1) on age at blood draw (± 2 years), menopausal status and hormone therapy (HT) use (premenopausal, postmenopausal and not taking HT, postmenopausal and taking HT, and unknown), month (± 1 month), time of day (± 2 hours) and fasting status (≥ 8 hours after a meal or <8 hours after a meal and unknown).(2, 27) This study included 883 case-control pairs with circulating carotenoids and blood metabolomics measured ≥10 years prior to breast cancer diagnosis (distant), and 545 pairs with measurements <10 years prior to diagnosis (proximate); 531 pairs had two blood measurements. A complete study flow is in Supplementary Figure 1. This study included 887 unique case-control pairs, of which 531 case-control pairs had two blood measurements. Of those, 883 pairs had circulating carotenoids and blood metabolomics measured ≥10 years prior to breast cancer diagnosis; while 545 pairs only had measurements <10 years prior to diagnosis.
Carotenoid measurements
Circulating levels of plasma carotenoids were measured with reversed-phase high-performance liquid chromatography at the Micronutrient Analysis Laboratory at the Harvard T. H. Chan School of Public Health.(2) Carotenoids were assayed in 13 batches, and coefficient of variation (CVs) were in general ≤15%.(2) Intraclass correlations at two blood collections ~10 years apart were ranges from 0.30 to 0.54.(2)
Plasma metabolomics assessment
Liquid chromatography tandem mass spectrometry (LC-MS) metabolomic assays were conducted at the Broad Institute in the laboratory of Dr. Clish. Samples were analyzed using hydrophilic interaction chromatography (HILIC) and reversed phase chromatography coupled with high resolution and accurate mass MS to profile polar metabolites and lipids, respectively, as described in detail previously.(28) We used reference standards for each metabolite to determine chromatographic retention time and MS multiple reaction monitoring transitions, delustering potentials and collision energies.(29) Reference samples were obtained from synthetic mixtures of reference metabolites (Sigma) and lipid extracts from a pooled human plasma stock (Bioreclamation).(29)” For quality control (QC), internal standards were introduced during sample extraction and monitored throughout the analyses to ensure analytic performance. In addition to blinded QC samples, pairs of pooled plasma reference samples were analyzed at intervals of approximately twenty study samples and one sample from each pair was used to correct for drift in instrument sensitivity using nearest-neighbor scaling. Reference standards were used to confirm identities of polar metabolites. Lipid identities were determined based on comparison to representative lipid standards from each lipid class and reference plasma extracts. Lipids were denoted by total number of carbons and double bonds in the lipid acyl chain(s). We received peak areas for each metabolite, which is proportional to the relative concentration. We log-transformed and z-scored metabolites to approximate normality. Metabolites with ≤25% missingness were imputed with half of the minimum value (n=200); while metabolites with >25% missingness were removed from the analysis (n=8). A total of 293 metabolites were included in the current analysis.
Covariates
We obtained covariate information from biennial questionnaires (height, weight at age 18, age at menarche, parity, age at first birth, family history of breast cancer, personal history of any benign breast disease, alcohol consumption, physical activity, and alternative healthy eating index or AHEI), and at blood collection (weight and smoking status). The AHEI index is a score that measures adherence to a dietary pattern based on foods and nutrients most predictive of disease risk in clinical and epidemiological studies.(30) The AHEI index included 11 components (vegetables, servings/day; fruits, servings/day; whole grains, servings/day; sugar-sweetened beverages and fruit juice, servings/day; nuts and legumes, servings/day; red meat and processed meat, servings/day; trans-fat, % of energy; long-chain (n-3) fats (EPA+DHA), mg/day; poly-unsaturated fatty acids, % of energy; sodium, mg/day; alcohol, drinks/day) derived from a validated semi-quantitative food frequency questionnaire (FFQ).(30–32) A commonly used portion size was specified for each food item and participants were asked, on average, how frequent she had consumed that quantity over the past year.(31, 32) Each component had a minimum score of 0 and a maximum score of 10, and the total AHEI score ranged from 0 (non-adherence) to 110 (perfect adherence).(30) We modified the original AHEI index by excluding fruit, vegetables and alcohol consumptions. Age at first birth and parity were combined and classified as nulliparous, 1 or 2 children & <25 years old, 1 or 2 children & 25–29 years old, 1 or 2 children 30+ years old, 3+ children & <25 years old, 3+ children & 25+ years old. Weight change since age 18 was calculated as the difference in weight from age 18 to blood collection. Circulating total cholesterol was assessed using Roche Diagnostics reagents as previously described.(33) Estrogen receptor (ER) status was determined via central review of breast tissue microarrays or by pathology reports, if tissue was unavailable.(34)
Statistical analysis
Relative risks (RRs) and 95% confidence intervals (CIs) were calculated to examine associations between circulating carotenoids and breast cancer risk in this study subset using conditional logistic regression adjusting for breast cancer risk factors. Carotenoids differ in their activities (i.e., α-carotene, β-carotene, and β-cryptoxanthin have pro-vitamin A activities) and major food sources that contribute (i.e., lycopene more commonly found in tomatoes, while lutein/zeaxanthin from leafy greens). We therefore examined each carotenoid individually. We also estimated vitamin A potential based on retinol conversion efficiency to capture the overall effects of carotenoids with vitamin A activity using the following formula: α-carotene concentration + (2)* β-carotene concentration + (1/12)* β-cryptoxanthin concentration. We considered distant and proximate carotenoid measures separately. Specifically, we (i) regressed circulating levels of carotenoids on circulating total cholesterols; (ii) obtained residuals for each participant; (iii) added back the mean levels of carotenoids. We calculated quartiles of each individual carotenoid (cholesterol-standardized) based on distributions of the controls for distant blood measures. Tests for trend were calculated using the Wald test, with medians of quartiles modeled continuously. We identified individual metabolites associated with carotenoids among the controls using linear-mixed models with random intercept to incorporate two blood measures when available, taking into account within-person variation. A metabolite was considered to be metabolome-wide significant if it reached the false discovery rate (FDR) corrected p-value≤0.05 based on the Benjamini-Hochberg method.(35) Pathway analysis was performed using MetaboAnalyst, RRID:SCR_015539 (ULR: https://www.metaboanalyst.ca/).(36) We calculated a metabolomic signature for each carotenoid using LASSO regularized regression, and lambda was chosen to minimize mean cross-validation error (10-fold).(37) The metabolomic score for each carotenoid for any individual i in the NHS is defined as the sum of the relative level of each metabolite weighted by corresponding metabolite regression coefficient from LASSO regression: We examined associations between carotenoid-associated metabolomic signatures and breast cancer risk using multivariable conditional logistic regression adjusting for breast cancer risk factors. We applied the difference method to evaluate whether and to what extent were the carotenoid—breast cancer associations statistically accounted for by the metabolomic signatures. Specifically, we estimated the RR and 95% CI for (a) the association between carotenoids and breast cancer risk not adjusted for the metabolomic signatures (i.e., total effects) and (b) the association between carotenoids and breast cancer risk adjusted for the metabolomic signatures (i.e., indirect effects). (38, 39) Proportion mediated was calculated as the percentage of indirect effect over the sum of direct and indirect effect. All analyses were performed with SAS 9.4 and R 3.4.1.
RESULTS
Characteristics at blood draw for NHS participants who later developed breast cancer and their matched controls are presented in Table 1. We observed similar distributions between cases and controls for matching factors, including age and menopausal status. A higher proportion of cases were current smokers compared to controls (12% vs. 9% for distant and 6% vs. 4% for proximate blood collections). Family history of breast cancer was higher in cases (14%) compared with controls (10%). At the 2nd blood collection, women were approximately 11 years older and were generally more physically active. Cases had a lower AHEI score for distant blood collection (mean±SD, 42.7±9.6 for cases and 43.4±9.8 for controls); however, AHEI scores were similar between cases and controls for proximate blood collection. For both blood collections, cholesterol-adjusted plasma carotenoid levels were higher in controls.
Table 1.
Characteristics of breast cancer cases and matched controls by distant and proximate blood collection in the NHS.
| Distant blood collection (≥10 years) | Proximate blood collection (<10 years) | |||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
|
|
||||
| N=873 | N=873 | N=545 | N=545 | |
|
| ||||
| Age at blood draw (years) [mean (SD)] | 55.3 (6.9) | 55.5 (6.9) | 66.3 (6.9) | 66.5 (6.8) |
| BMI at blood draw (kg/m2) [mean (SD)] | 25.6 (4.3) | 25.1 (4.7) | 25.4 (4.2) | 25.2 (4.6) |
| Nulliparous [n (%)] | 55 (6%) | 44 (5%) | 39 (7%) | 27 (5%) |
| Parity (number of children) [mean (SD)] | 2.9 (1.5) | 3.1 (1.7) | 2.9 (1.5) | 3.1 (1.7) |
| Age at first birth (years) [mean (SD)] | 25.0 (3.0) | 24.9 (3.0) | 24.9 (2.9) | 24.7 (2.9) |
| Postmenopausal [n (%)] | 535 (61%) | 535 (61%) | 535 (98%) | 535 (98%) |
| Family history of breast cancer [n (%)] | 124 (14%) | 90 (10%) | 74 (14%) | 50 (9%) |
| Current smokers [n (%)] | 108 (12%) | 80 (9%) | 34 (6%) | 23 (4%) |
| Alcohol consumption (grams/day) [mean (SD)] | 7.4 (11.4) | 6.0 (8.8) | 6.4 (10.2) | 5.4 (8.6) |
| Physical activity (MET-hours/week) [median (IQR)] | 16.6 (21.5) | 16.3 (18.2) | 19.1 (20.1) | 20.2 (22.0) |
| AHEI † [mean (SD)] | 37.9 (9.0) | 38.5 (9.3) | 39.1 (8.4) | 39.4 (9.2) |
| Plasma carotenoids cholesterol-adjusted value (μg/dL) [median (IQR)] | ||||
| α-carotene | 6.3 (5.7) | 6.7 (5.6) | 6.2 (6.0) | 6.8 (7.1) |
| β-carotene | 20.3 (17.9) | 22.2 (19.2) | 19.6 (21.8) | 21.0 (22.3) |
| β-cryptoxanthin | 10.3 (7.3) | 10.6 (7.5) | 10.5 (8.5) | 10.3 (7.3) |
| Lycopene | 37.2 (18.5) | 39.8 (21.1) | 37.2 (22.6) | 40.2 (23.6) |
| Lutein & zeaxanthin | 20.4 (11.4) | 21.0 (11.9) | 20.8 (11.3) | 21.0 (11.4) |
| Estimated vitamin A potential‡ [median (IQR)] | 48.4 (40.8) | 52.6 (44.1) | 47.2 (49.8) | 50.4 (49.9) |
AHEI: Alternative healthy eating index, modified to exclude fruit, vegetable, and alcohol intake.
Vitamin A potential calculated based on retinol conversion efficiency with the following formula: α-carotene concentration + (2)* β-carotene concentration + (1/12)* β-cryptoxanthin concentration.
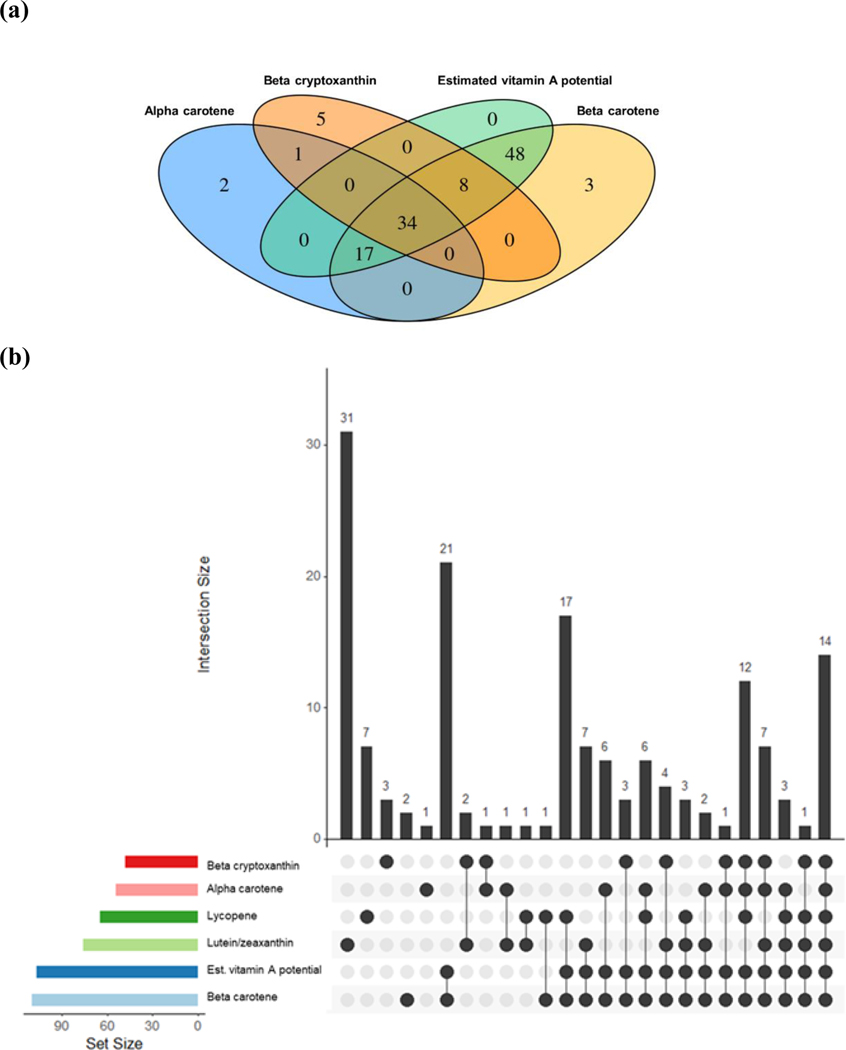
Our metabolome-wide association study (MWAS) identified individual metabolites associated with α-carotene (n=54), β-carotene (n=110), β-cryptoxanthin (n=48), estimated vitamin A potential (n=107), lutein/zeaxanthin (n=76), and lycopene (n=65) (FDR≤0.05) (Supplementary Figure 2). These include amino acids and derivatives, carboximidic acids, ceramides, diacylglycerols, fatty acyls, triacylglycerols, glycerophosphocholines, glycerophosphoethanolamines, indolyl carboxylic acids and derivatives, purines and derivatives, and cholesteryl esters. We observed comparable effect estimates in subsequent sensitivity analyses excluding women who took vitamin A supplements (4%) or current smokers (9%) (Supplementary Table 1). Fourteen metabolites were commonly selected across all carotenoids with comparable regression coefficients (Supplementary Table 2), including 1 amino acid (N-acetyl-tryptophan), 8 glycerolipids (C36:1 DAG, C36:2 DAG, C48:2 TAG, C50:2 TAG, C52:0 TAG, C52:2 TAG, C54:1 TAG, C54:2 TAG), 1 glycerophospholipid (C22:0 LPE), and 4 cholesteryl esters (C16:0 CE, C18:0 CE, C18:1 CE, C18:2 CE). There were many overlapping metabolites selected for carotenoids with provitamin A activity (which comprised 63%, 31%, 71% and 32% of FDR significant metabolites for α-carotene, β-carotene, β-cryptoxanthin, and estimated vitamin A potential) (Figure 1). For example, all metabolites identified for estimated vitamin A potential were also selected by β-carotene (n=107); α-carotene (total n=54) and β-carotene (total n=110) shared 51 metabolites in common, while β-carotene and β-cryptoxanthin (total n=48) had 42 metabolites in common. Carotenoids with no provitamin A activity (i.e., lutein/zeaxanthin and lycopene), on the other hand, were associated with more distinct metabolomic profiles, as 31 FDR significant metabolites were only associated with lutein/zeaxanthin (41% of total) and 7 only for lycopene (11% of total) (Figure 1). We observed modest to high Pearson correlations among circulating carotenoids (Supplementary Figure 3). Starting with the individual metabolites that were significantly associated (FDR≤0.05), we derived a metabolomic signature for each individual carotenoid through LASSO regularized regression (Figure 2). Twenty-nine (out of 54), 36 (out of 110), 26 (out of 48), 32 (out of 107), 46 (out of 76), 19 (out of 65) metabolites were selected by LASSO for α-carotene, β-carotene, β-cryptoxanthin, estimated vitamin A potential, lutein/zeaxanthin and lycopene, respectively. Network correlation plots for LASSO selected metabolites are presented in Supplementary Figure 4. Metabolites with absolute Pearson correlation >0.3 with others are connected. In general, plasmalogens and TAGs/DAGs were likely to be positively interconnected. Tryptophan was positive correlated with its acetylated derivative (N-acetyl tryptophan) when both were selected by LASSO (α-carotene, β-carotene and estimated vitamin A potential). Cholesteryl esters were inversely correlated with TAGs/DAGs, while positively correlated with phosphatidylethanolamine. Amino acids were generally less correlated with one another. Percent of variance explained (R2) by the metabolomic signature for the corresponding carotenoid ranged from 26% (lutein/zeaxanthin) to 6% (lycopene) in unadjusted regressions. R2 were attenuated when we further adjusted for breast cancer risk factors and lifestyle factors (i.e., R2 decreased from 18% in unadjusted models to 10% in multivariable models for β-carotene). Our secondary pathway analysis revealed that glycine, serine and threonine metabolism and arginine biosynthesis were associated with circulating β-carotene levels and glycerophospholipid metabolism were associated with lutein/zeaxanthin (FDR≤0.05) (Supplementary Table 4).
Figure 1.
Venn and upset diagrams of overlapping metabolites associated with different carotenoids that met FDR≤0.05: (a) for carotenoids with pro-vitamin A activity (α-carotene, β-carotene, β-cryptoxanthin, estimated vitamin A potential); (b) for all plasma carotenoids (α-carotene, β-carotene, β-cryptoxanthin, estimated vitamin A potential, lutein/zeaxanthin, lycopene).
Figure 2.
Metabolic representations of circulating levels of carotenoids based on 887 controls with repeated measures (n=1418). Metabolites that met FDR≤0.05 and are further selected by lasso regularized regression are presented. Effect estimates were expressed as per SD increase in carotenoids.
Mixed effects models with random intercept and adjusted for age at blood draw (continuous), fasting status (continuous), menopausal status and hormone therapy use (pre-menopausal, post-menopausal and use, post-menopausal and not use, not known), BMI at blood draw (continuous), alcohol consumption (continuous), physical activity (continuous), smoking status (never, ever), and individual components of AHEI excluding fruit, vegetable, and alcohol intake (whole grains, sugar-sweetened beverage and fruit juice, nuts and legumes, red meet and processed meat, trans-fat, long-chain (n-3) fats (EPA + DHA), poly-unsaturated fatty acids, and sodium).
Using distant blood measures, the metabolomic signatures derived for α-carotene, β-carotene, β-cryptoxanthin, estimated vitamin A potential and lycopene were associated with lower breast cancer risk in unadjusted models (p≤0.05) (Table 2). Associations were in general attenuated in multivariable models additionally adjusting for breast cancer risk factors and lifestyle factors that may co-vary with circulating levels of carotenoids. Women in the highest quartile of β-carotene-associated metabolomic signature measured distant in time (≥10 years) had a 26% lower risk of breast cancer (RR=0.74, 95% CI [0.54, 1.01], p-trend=0.02) (Table 2). A similar magnitude of risk reduction was observed for women in the highest quartiles of metabolomic signature for α-carotene (RR=0.70, 95% CI [0.51, 0.97], p-trend=0.06), estimated vitamin A potential (RR=0.74, 95% CI [ 0.54, 1.01], p-trend=0.02) and lycopene (RR=0.80, 95% CI [0.60, 1.06], p-trend=0.08), compared to the lowest quartiles (Table 2). None of the carotenoid-associated metabolomic signatures derived for proximate blood measures were associated with lower breast cancer risk (Table 3).
Table 2.
Multivariable RRs (95% CI) of plasma metabolomic signatures measured distant in time to diagnosis (≥10 years) with breast cancer risk (N=873).
| Distant blood (≥ 10 years) | Quartiles | Continuous (SD increase) | |||||
|---|---|---|---|---|---|---|---|
| α-carotene (29 metabolites, R 2 † =16%) | Q1 (<−0.2) | Q2 (−0.2 to <0.03) | Q3 (0.03 to <0.3) | Q4 (≥0.3) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 0.82 (0.63, 1.08) | 0.82 (0.63, 1.08) | 0.62 (0.47, 0.82) | 0.001 | 0.86 (0.78; 0.95) | 0.003 |
| MV1 | 1.0 (ref) | 0.86 (0.65, 1.13) | 0.89 (0.67, 1.18) | 0.67 (0.49, 0.91) | 0.02 | 0.89 (0.80; 1.00) | 0.04 |
| MV2 | 1.0 (ref) | 0.87 (0.66, 1.16) | 0.90 (0.68, 1.21) | 0.67 (0.49, 0.92) | 0.02 | 0.90 (0.80; 1.00) | 0.05 |
| MV3 | 1.0 (ref) | 0.88 (0.66, 1.17) | 0.93 (0.69, 1.25) | 0.70 (0.51, 0.97) | 0.06 | 0.92 (0.82; 1.03) | 0.16 |
| β-carotene (36 metabolites, R 2 † =18%) | Q1 (<−0.2) | Q2 (−0.2 to <0.04) | Q3 (0.04 to <0.3) | Q4 (≥0.3) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 0.96 (0.74, 1.25) | 0.72 (0.55, 0.94) | 0.63 (0.48, 0.83) | 0.0002 | 0.85 (0.77; 0.94) | 0.001 |
| MV1 | 1.0 (ref) | 1.02 (0.78, 1.34) | 0.78 (0.58, 1.04) | 0.69 (0.52, 0.94) | 0.005 | 0.88 (0.79; 0.98) | 0.02 |
| MV2 | 1.0 (ref) | 1.06 (0.80, 1.40) | 0.79 (0.59, 1.06) | 0.71 (0.52, 0.96) | 0.007 | 0.88 (0.79; 0.98) | 0.03 |
| MV3 | 1.0 (ref) | 1.08 (0.82, 1.42) | 0.80 (0.59, 1.08) | 0.74 (0.54, 1.01) | 0.02 | 0.90 (0.80; 1.01) | 0.08 |
| β-cryptoxanthin (26 metabolites, R 2 † =23%) | Q1 (<−0.2) | Q2 (−0.2 to <−0.002) | Q3 (−0.002 to <0.2) | Q4 (≥0.2) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 0.90 (0.70, 1.16) | 0.62 (0.47, 0.82) | 0.80 (0.62, 1.04) | 0.02 | 0.90 (0.82; 0.99) | 0.04 |
| MV1 | 1.0 (ref) | 0.93 (0.72, 1.20) | 0.66 (0.49, 0.87) | 0.87 (0.66, 1.15) | 0.11 | 0.94 (0.84; 1.04) | 0.21 |
| MV2 | 1.0 (ref) | 0.96 (0.74, 1.25) | 0.66 (0.49, 0.88) | 0.88 (0.66, 1.18) | 0.12 | 0.94 (0.84; 1.05) | 0.25 |
| MV3 | 1.0 (ref) | 0.96 (0.74, 1.26) | 0.67 (0.50, 0.90) | 0.91 (0.68, 1.22) | 0.20 | 0.96 (0.85; 1.07) | 0.43 |
| Estimated vitamin A potential (32 metabolites, R 2 † =20%) | Q1 (<−0.2) | Q2 (−0.2 to <0.04) | Q3 (0.04 to <0.3) | Q4 (≥0.3) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 0.94 (0.73, 1.22) | 0.68 (0.52, 0.89) | 0.64 (0.49, 0.84) | 0.0002 | 0.85 (0.77; 0.94) | 0.0009 |
| MV1 | 1.0 (ref) | 1.00 (0.76, 1.30) | 0.73 (0.55, 0.98) | 0.70 (0.52, 0.94) | 0.004 | 0.88 (0.79; 0.98) | 0.02 |
| MV2 | 1.0 (ref) | 1.03 (0.78, 1.36) | 0.75 (0.56, 1.01) | 0.71 (0.52, 0.96) | 0.006 | 0.88 (0.78; 0.98) | 0.02 |
| MV3 | 1.0 (ref) | 1.05 (0.79, 1.38) | 0.76 (0.56, 1.03) | 0.74 (0.54, 1.01) | 0.02 | 0.90 (0.80; 1.01) | 0.06 |
| Lutein/zeaxanthin (46 metabolites, R 2 † =26%) | Q1 (<−0.2) | Q2 (−0.2 to <−0.004) | Q3 (−0.004 to <0.2) | Q4 (≥0.2) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 0.74 (0.57, 0.97) | 0.93 (0.71, 1.21) | 0.81 (0.61, 1.06) | 0.33 | 0.95 (0.86; 1.05) | 0.30 |
| MV1 | 1.0 (ref) | 0.75 (0.57, 0.99) | 0.97 (0.74, 1.28) | 0.88 (0.66, 1.16) | 0.73 | 0.98 (0.89; 1.09) | 0.74 |
| MV2 | 1.0 (ref) | 0.76 (0.57, 1.00) | 0.97 (0.73, 1.27) | 0.86 (0.65, 1.16) | 0.65 | 0.97 (0.87; 1.08) | 0.61 |
| MV3 | 1.0 (ref) | 0.75 (0.57, 0.99) | 1.00 (0.75, 1.33) | 0.93 (0.69, 1.25) | 0.98 | 1.01 (0.90; 1.12) | 0.93 |
| Lycopene (19 metabolites, R 2 † =6%) | Q1 (<−0.08) | Q2 (−0.08 to <0.02) | Q3 (0.02 to <0.1) | Q4 (≥0.1) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 0.86 (0.67, 1.11) | 0.77 (0.59, 1.00) | 0.72 (0.55, 0.94) | 0.01 | 0.89 (0.81; 0.97) | 0.01 |
| MV1 | 1.0 (ref) | 0.89 (0.69, 1.15) | 0.80 (0.62, 1.05) | 0.77 (0.58, 1.01) | 0.04 | 0.91 (0.82; 1.01) | 0.07 |
| MV2 | 1.0 (ref) | 0.91 (0.70, 1.18) | 0.81 (0.61, 1.06) | 0.77 (0.58, 1.02) | 0.04 | 0.91 (0.82; 1.01) | 0.07 |
| MV3 | 1.0 (ref) | 0.93 (0.71, 1.21) | 0.83 (0.63, 1.09) | 0.80 (0.60, 1.06) | 0.08 | 0.92 (0.83; 1.02) | 0.13 |
Variance explained by metabolomic score for the corresponding carotenoid from linear regression.
MV1 regression: conditional logistic regression models adjusted for BMI at age 18 (continuous), weight change since age 18 (continuous). MV2 regression: additionally adjusted for age at first birth / parity (nulliparous, 1 or 2 children & <25 years old, 1 or 2 children & 25–29 years old, 1 or 2 children 30+ years old, 3+ children & <25 years old, 3+ children & 25+ years old), age at menarche (continuous), alcohol consumption (continuous), physical activity (continuous), smoking status (never, ever), family history of breast cancer (yes, no), personal history of benign breast disease (yes, no). MV3 regression: additionally adjusted for individual components of AHEI excluding fruit, vegetable & alcohol intake (whole grains, sugar-sweetened beverage & fruit juice, nuts & legumes, red meet & processed meat, trans-fat, long-chain (n-3) fats (EPA + DHA), poly-unsaturated fatty acids, & sodium).
Table 3.
Multivariable RRs (95% CI) of plasma metabolomic signatures measured proximate in time to diagnosis (<10 years) with breast cancer risk (N=545). Results expressed as per SD increase in metabolomic signature.
| Proximate blood (≤10 years) | Quartiles | Continuous (SD increase) | |||||
|---|---|---|---|---|---|---|---|
| α-carotene (29 metabolites, R 2 † =11%) | Q1(<−0.2) | Q2(−0.2 to <0.02) | Q4(0.02 to <0.2) | Q4(≥0.2) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 1.05 (0.76, 1.46) | 0.77 (0.55, 1.07) | 0.81 (0.57, 1.14) | 0.09 | 0.95 (0.84; 1.07) | 0.38 |
| MV1 | 1.0 (ref) | 1.09 (0.78, 1.51) | 0.80 (0.56, 1.13) | 0.86 (0.60, 1.24) | 0.23 | 0.98 (0.86; 1.12) | 0.78 |
| MV2 | 1.0 (ref) | 1.14 (0.81, 1.60) | 0.80 (0.56, 1.14) | 0.90 (0.62, 1.30) | 0.30 | 0.99 (0.87; 1.13) | 0.90 |
| MV3 | 1.0 (ref) | 1.17 (0.83, 1.66) | 0.83 (0.57, 1.19) | 0.97 (0.65, 1.46) | 0.53 | 1.03 (0.89; 1.19) | 0.71 |
| β-carotene (36 metabolites, R 2 † =18%) | Q1(<−0.3) | Q2(−0.3 to <−0.009) | Q3(−0.009 to <0.3) | Q4(≥0.3) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 1.04 (0.74, 1.45) | 0.78 (0.55, 1.12) | 0.87 (0.62, 1.23) | 0.23 | 0.96 (0.85; 1.09) | 0.52 |
| MV1 | 1.0 (ref) | 1.06 (0.76, 1.50) | 0.83 (0.58, 1.20) | 0.94 (0.66, 1.34) | 0.49 | 0.99 (0.87; 1.13) | 0.92 |
| MV2 | 1.0 (ref) | 1.04 (0.74, 1.47) | 0.83 (0.57, 1.21) | 0.93 (0.65, 1.34) | 0.50 | 1.00 (0.87; 1.14) | 0.99 |
| MV3 | 1.0 (ref) | 1.05 (0.74, 1.48) | 0.84 (0.57, 1.24) | 0.98 (0.67, 1.45) | 0.73 | 1.03 (0.89; 1.18) | 0.72 |
| β-cryptoxanthin (26 metabolites, R 2 † =23%) | Q1(<−0.2) | Q2(−0.2 to <−0.02) | Q3(−0.02 to <0.2) | Q4(≥0.2) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 0.75 (0.54, 1.03) | 0.78 (0.56, 1.07) | 0.82 (0.59, 1.14) | 0.23 | 0.96 (0.85; 1.08) | 0.46 |
| MV1 | 1.0 (ref) | 0.74 (0.54, 1.03) | 0.79 (0.57, 1.10) | 0.87 (0.61, 1.22) | 0.40 | 0.98 (0.87; 1.11) | 0.76 |
| MV2 | 1.0 (ref) | 0.69 (0.49, 0.97) | 0.74 (0.52, 1.04) | 0.83 (0.58, 1.18) | 0.30 | 0.97 (0.85; 1.10) | 0.65 |
| MV3 | 1.0 (ref) | 0.69 (0.48, 0.98) | 0.77 (0.54, 1.09) | 0.86 (0.59, 1.25) | 0.49 | 0.99 (0.87; 1.14) | 0.93 |
| Estimated vitamin A potential (32 metabolites, R 2 † =18%) | Q1(<−0.2) | Q2(−0.2 to <−0.02) | Q3(−0.02 to <0.2) | Q4(≥0.2) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 0.96 (0.69, 1.34) | 0.73 (0.51, 1.04) | 0.87 (0.62, 1.22) | 0.24 | 0.95 (0.84; 1.08) | 0.45 |
| MV1 | 1.0 (ref) | 0.99 (0.71, 1.38) | 0.77 (0.53, 1.12) | 0.94 (0.66, 1.33) | 0.50 | 0.99 (0.87; 1.12) | 0.84 |
| MV2 | 1.0 (ref) | 0.98 (0.70, 1.38) | 0.76 (0.52, 1.11) | 0.94 (0.65, 1.34) | 0.51 | 0.99 (0.87; 1.13) | 0.91 |
| MV3 | 1.0 (ref) | 0.99 (0.70, 1.40) | 0.78 (0.53, 1.15) | 1.00 (0.68, 1.47) | 0.78 | 1.02 (0.89; 1.18) | 0.77 |
| Lutein & zeaxanthin (46 metabolites, R 2 † =19%) | Q1(<−0.1) | Q2(−0.1 to <−0.002) | Q3(−0.002 to <0.1) | Q4(≥0.1) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 0.66 (0.47, 0.93) | 0.75 (0.53, 1.06) | 0.80 (0.57, 1.13) | 0.26 | 0.95 (0.84; 1.09) | 0.48 |
| MV1 | 1.0 (ref) | 0.68 (0.48, 0.96) | 0.79 (0.56, 1.12) | 0.84 (0.59, 1.20) | 0.43 | 0.98 (0.85; 1.12) | 0.73 |
| MV2 | 1.0 (ref) | 0.69 (0.49, 0.98) | 0.78 (0.55, 1.12) | 0.82 (0.57, 1.19) | 0.37 | 0.96 (0.84; 1.11) | 0.60 |
| MV3 | 1.0 (ref) | 0.70 (0.49, 1.00) | 0.82 (0.57, 1.18) | 0.90 (0.61, 1.32) | 0.67 | 0.99 (0.86; 1.15) | 0.93 |
| Lycopene (19 metabolites, R 2 † =2%) | Q1(<−0.09) | Q2(−0.09 to <0.001) | Q3(0.001 to <0.1) | Q4(≥0.1) | p-trend | RR (95% CI) | p |
| Unadjusted model | 1.0 (ref) | 1.09 (0.76, 1.54) | 1.14 (0.81, 1.62) | 0.93 (0.65, 1.34) | 0.82 | 1.03 (0.91; 1.16) | 0.68 |
| MV1 | 1.0 (ref) | 1.09 (0.77, 1.55) | 1.17 (0.82, 1.65) | 0.97 (0.67, 1.39) | 0.98 | 1.04 (0.92; 1.18) | 0.55 |
| MV2 | 1.0 (ref) | 1.11 (0.77, 1.60) | 1.23 (0.86, 1.77) | 1.01 (0.70, 1.47) | 0.79 | 1.06 (0.93; 1.21) | 0.38 |
| MV3 | 1.0 (ref) | 1.15 (0.79, 1.66) | 1.25 (0.86, 1.80) | 1.04 (0.71, 1.53) | 0.74 | 1.07 (0.94; 1.22) | 0.31 |
Variance explained by metabolomic score for the corresponding carotenoid from linear regression.
MV1 regression: conditional logistic regression models adjusted for BMI at age 18 (continuous), weight change since age 18 (continuous). MV2 regression: additionally adjusted for age at first birth / parity (nulliparous, 1 or 2 children & <25 years old, 1 or 2 children & 25–29 years old, 1 or 2 children 30+ years old, 3+ children & <25 years old, 3+ children & 25+ years old), age at menarche (continuous), alcohol consumption (continuous), physical activity (continuous), smoking status (never, ever), family history of breast cancer (yes, no), personal history of benign breast disease (yes, no). MV3 regression: additionally adjusted for individual components of AHEI excluding fruit, vegetable & alcohol intake (whole grains, sugar-sweetened beverage & fruit juice, nuts & legumes, red meet & processed meat, trans-fat, long-chain (n-3) fats (EPA + DHA), poly-unsaturated fatty acids, & sodium).
Consistent with our previous study,(2) we observed suggestive inverse associations between circulating levels of carotenoids, measured distant (≥10 years) in time to diagnosis, with breast cancer risk in this nested case-control study of 887 cases and 887 controls (Table 4), with comparable RR estimates. The associations were generally less evident for the proximate (<10 years) blood measures, possibly owing to the smaller sample size (545 cases and 545 controls). In subsequent analysis stratified by ER, RRs were similar for ER-positive and overall breast cancer comparing the top vs. bottom quartiles of carotenoids (Supplementary Table 3). However, the number of ER-negative diseases were limited (n ranges from 10 to 35 in each strata). We observed attenuations of effect estimates for the associations between β-carotene and estimated vitamin A potential with breast cancer risk in models additionally adjusting for the corresponding metabolomic signature for distant blood measurements (Table 4). For β-carotene, the quartile 4 (Q4) vs. quartile 1 (Q1) RR attenuated from 0.78 (p-trend=0.06) to 0.88 (p-trend=0.35) with adjustment for the metabolomic signature. For estimated vitamin A potential, the Q4 vs. Q1 RR attenuated from 0.80 (p=trend-0.08) to 0.91 (p-trend=0.41) adjusting for the metabolomic signature. In models controlling for lifestyle factors, although not statistically significant, 60% of β-carotene and 68% of estimated vitamin A potential associated breast cancer risk reduction were statistically accounted for by metabolomic scores for the distant blood measures (Table 4).
Table 4.
Multivariable RRs (95% CI) of circulating levels of carotenoid with breast cancer risk, and with additional adjustment for plasma metabolomics signature.
| α-carotene | Q1 | Q2 | Q3 | Q4 | p-trend |
|---|---|---|---|---|---|
| Cholesterol standardized value (μg/dL) | <4.42 | 4.42 to <6.74 | 6.74 to <10.06 | ≥10.06 | |
| Distant blood (≥ 10 years) | |||||
| Cases/controls, n | 265/219 | 203/217 | 200/218 | 205/219 | |
| RR (95% CI) MV | 1.0 (ref) | 0.77 (0.59, 1.02) | 0.80 (0.61, 1.05) | 0.85 (0.65, 1.12) | 0.30 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.80 (0.61, 1.05) | 0.83 (0.63, 1.10) | 0.92 (0.69, 1.24) | 0.66 |
| Proportion mediated (per SD increase) † | Not mediated | ||||
| Proximate blood (< 10 years) | |||||
| Cases/controls, n | 172/152 | 128/119 | 108/114 | 137/160 | |
| RR (95% CI) MV | 1.0 (ref) | 0.92 (0.64, 1.31) | 0.84 (0.59, 1.20) | 0.75 (0.54, 1.06) | 0.09 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.91 (0.64, 1.31) | 0.83 (0.57, 1.19) | 0.73 (0.51, 1.06) | 0.08 |
| Proportion mediated (per SD increase) †,‡ | 13.45% (p-value=0.89) | ||||
|
| |||||
| β-carotene | Q1 | Q2 | Q3 | Q4 | p-trend |
|
| |||||
| Cholesterol standardized value (μg/dL) | <14.06 | 14.06 to <22.16 | 22.16 to <33.3 | ≥33.3 | |
| Distant blood (≥ 10 years) | |||||
| Cases/controls, n | 256/218 | 235/219 | 195/218 | 187/218 | |
| RR (95% CI) MV | 1.0 (ref) | 0.94 (0.72, 1.24) | 0.83 (0.62, 1.09) | 0.78 (0.59, 1.04) | 0.06 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.99 (0.75, 1.32) | 0.89 (0.66, 1.20) | 0.88 (0.64, 1.22) | 0.35 |
| Proportion mediated (per SD increase) † | 60.18% (p-value=0.26) | ||||
| Proximate blood (< 10 years) | |||||
| Cases/controls, n | 166/147 | 143/142 | 95/103 | 141/153 | |
| RR (95% CI) MV | 1.0 (ref) | 0.89 (0.63, 1.26) | 0.82 (0.55, 1.21) | 0.82 (0.58, 1.16) | 0.25 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.87 (0.61, 1.24) | 0.79 (0.53, 1.19) | 0.78 (0.53, 1.15) | 0.21 |
| Proportion mediated (per SD increase) †,‡ | Not mediated | ||||
|
| |||||
| β-cryptoxanthin | Q1 | Q2 | Q3 | Q4 | p-trend |
|
| |||||
| Cholesterol standardized value (μg/dL) | <7.48 | 7.48 to <10.60 | 10.60 to <14.99 | ≥14.99 | |
| Distant blood (≥ 10 years) | |||||
| Cases/controls, n | 259/218 | 200/218 | 225/219 | 189/218 | |
| RR (95% CI) MV | 1.0 (ref) | 0.80 (0.61, 1.05) | 0.93 (0.71, 1.22) | 0.83 (0.63, 1.09) | 0.29 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.81 (0.62, 1.07) | 0.97 (0.73, 1.29) | 0.88 (0.64, 1.20) | 0.57 |
| Proportion mediated (per SD increase) † | 58.52% (p-value=0.51) | ||||
| Proximate blood (< 10 years) | |||||
| Cases/controls, n | 159/141 | 116/143 | 126/126 | 144/135 | |
| RR (95% CI) MV | 1.0 (ref) | 0.70 (0.50, 0.99) | 0.94 (0.67, 1.32) | 0.95 (0.67, 1.35) | 0.92 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.72 (0.51, 1.01) | 0.98 (0.68, 1.40) | 1.01 (0.68, 1.49) | 0.72 |
| Proportion mediated (per SD increase) †,‡ | Not mediated | ||||
|
| |||||
| Estimated vitamin A potential | Q1 | Q2 | Q3 | Q4 | p-trend |
|
| |||||
| Cholesterol standardized value (μg/dL) | <33.83 | 33.83 to <52.60 | 52.60 to <77.93 | ≥77.93 | |
| Distant blood (≥ 10 years) | |||||
| Cases/controls, n | 258/218 | 234/219 | 190/218 | 191/218 | |
| RR (95% CI) MV | 1.0 (ref) | 0.94 (0.71, 1.24) | 0.79 (0.60, 1.05) | 0.80 (0.60, 1.07) | 0.07 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.99 (0.75, 1.32) | 0.86 (0.64, 1.16) | 0.91 (0.66, 1.25) | 0.41 |
| Proportion mediated (per SD increase) † | 67.94% (p-value=0.26) | ||||
| Proximate blood (< 10 years) | |||||
| Cases/controls, n | 170/142 | 140/142 | 95/107 | 140/154 | |
| RR (95% CI) MV | 1.0 (ref) | 0.81 (0.57, 1.14) | 0.73 (0.50, 1.08) | 0.76 (0.54, 1.07) | 0.11 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.78 (0.55, 1.12) | 0.71 (0.47, 1.06) | 0.71 (0.48, 1.05) | 0.09 |
| Proportion mediated (per SD increase) †,‡ | Not mediated | ||||
|
| |||||
| Lutein / zeaxanthin | Q1 | Q2 | Q3 | Q4 | p-trend |
|
| |||||
| Cholesterol standardized value (μg/dL) | <16.05 | 16.05 to <21.01 | 21.01 to <27.98 | ≥27.98 | |
| Distant blood (≥ 10 years) | |||||
| Cases/controls, n | 237/219 | 229/218 | 217/217 | 190/219 | |
| RR (95% CI) MV | 1.0 (ref) | 0.96 (0.73, 1.26) | 0.95 (0.72, 1.26) | 0.81 (0.61, 1.09) | 0.18 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.96 (0.73, 1.26) | 0.95 (0.70, 1.28) | 0.80 (0.58, 1.12) | 0.22 |
| Proportion mediated (per SD increase) † | 3.47% (p-value=0.94) | ||||
| Proximate blood (< 10 years) | |||||
| Cases/controls, n | 157/140 | 123/134 | 148/148 | 117/123 | |
| RR (95% CI) MV | 1.0 (ref) | 0.84 (0.60, 1.19) | 0.85 (0.61, 1.20) | 0.89 (0.62, 1.28) | 0.53 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.85 (0.60, 1.20) | 0.87 (0.61, 1.23) | 0.91 (0.61, 1.36) | 0.65 |
| Proportion mediated (per SD increase) † | 18.74% (p-value=0.83) | ||||
|
| |||||
| Lycopene | Q1 | Q2 | Q3 | Q4 | p-trend |
|
| |||||
| Cholesterol standardized value (μg/dL) | <29.51 | 29.51 to <39.79 | 39.79 to <50.56 | ≥50.56 | |
| Distant blood (≥ 10 years) | |||||
| Cases/controls, n | 246/218 | 248/218 | 214/218 | 165/219 | |
| RR (95% CI) MV | 1.0 (ref) | 1.01 (0.77, 1.32) | 0.85 (0.64, 1.13) | 0.65 (0.48, 0.87) | 0.003 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 1.02 (0.78, 1.34) | 0.87 (0.66, 1.16) | 0.67 (0.50, 0.91) | 0.007 |
| Proportion mediated (per SD increase) † | 9.47% (p-value=0.22) | ||||
| Proximate blood (< 10 years) | |||||
| Cases/controls, n | 165/141 | 129/123 | 118/129 | 133/152 | |
| RR (95% CI) MV | 1.0 (ref) | 0.87 (0.61, 1.23) | 0.75 (0.52, 1.07) | 0.69 (0.548, 1.00) | 0.04 |
| RR (95% CI) MV with metabolomics signature | 1.0 (ref) | 0.86 (0.60, 1.21) | 0.73 (0.51, 1.05) | 0.66 (0.46, 0.96) | 0.02 |
| Proportion mediated (per SD increase)†,‡ | Not mediated | ||||
MV regression: conditional logistic regression models adjusted for BMI at age 18 (continuous), weight change since age 18 (continuous), age at first birth / parity (nulliparous, 1 or 2 children & <25 years old, 1 or 2 children & 25–29 years old, 1 or 2 children 30+ years old, 3+ children & <25 years old, 3+ children & 25+ years old), age at menarche (continuous), physical activity (continuous), alcohol consumption (continuous), smoking status (never, ever), family history of breast cancer (yes, no), & personal history of benign breast disease (yes, no).
Mediation analysis was conducted treating circulating levels of carotenoids and plasma metabolomics as continuous variables.
Mediating effect could not be estimated, due to negative values.
DISCUSSION
In this large, nested case-control study of circulating carotenoids, blood metabolomics and breast cancer risk with repeated measures, we identified 48 to 110 metabolites associated with plasma levels of α-carotene, β-carotene, β-cryptoxanthin, estimated vitamin A potential, lutein/zeaxanthin, and lycopene, which included metabolites implicated in immune regulation (tryptophan and N-acetyl tryptophan), redox balance (plasmalogens, glutamine, orthinine and derivatives), membrane lipids and signal transduction (glycerolipid, glycerophospholipid, and sphingolipid), epigenetic regulations (acetylated and methylated metabolites), as well as β-oxidation (carnitines and glycerolipids). We observed a large number of overlapping metabolites selected by carotenoids with provitamin A activity (i.e., α-carotene, β-carotene, β-cryptoxanthin, estimated vitamin A potential), while more distinct metabolic profiles were observed for carotenoids without such activity (lutein/zeaxanthin, and lycopene). A metabolomic signature derived for each individual carotenoid explained modest amounts of variability in carotenoid levels. The metabolomic signatures derived for β-carotene and estimated vitamin A potential—measured ≥10 years before diagnosis—were associated with reduced breast cancer risk in multivariable models. We observed modest attenuations of effect estimates for the associations between measured β-carotene and breast cancer risk when we further adjusted for the metabolomic signatures in the distant blood samples, and a similar pattern was noted for estimated vitamin A potential. No carotenoid metabolomic signatures measured <10 years before diagnosis were associated with reduced breast cancer risk.
Compelling epidemiological evidence has linked circulating carotenoids with lower breast cancer risk. In our prior large-scale pooled analysis of 3,055 cases and 3,956 matched controls, which included the NHS, circulating levels of α-carotene, β-carotene, lutein/zeaxanthin and lycopene were significantly inversely associated with breast cancer risk.(1) In extended analyses in the NHS, we previously showed that women with higher circulating levels of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin and lycopene, measured both distant (≥10 years) and proximate (<10 years) in time to diagnosis, were associated at lowered breast cancer risk.(2) The associations were in general stronger for the distant blood measures.(2) Consistent with previous findings in the NHS, we found inverse associations between carotenoids, measured distant in time (≥10 years), with breast cancer risk. The associations between carotenoids at proximate blood collection and breast cancer risk were less apparent in this sub-population, possibly owning to the smaller sample size. The 10-year reproducibility of carotenoids in the NHS ranges from 0.30 to 0.54,(2) which was lower than the 2–3 year reproducibility measures (intraclass correlation ICCs: 0.73–0.88),(40) suggesting that carotenoids measured distant in time are not simply surrogate measures for recent exposures. The inverse associations between carotenoids—measured distant in time to diagnosis (≥10 years)—with breast cancer risk aligns with their anti-oxidative properties to scavenger free radicals and prevent macromolecular damage,(5) which is hypothesized to interrupt cancer-initiating processes and inhibit malignancy transformation.
Our study identified several metabolites significantly associated with circulating levels of carotenoids after correction for multiple testing. While these metabolites may reflect biologic activity of carotenoids, it is also possible that identified metabolites are correlated with carotenoids simply because they may share common food sources. For example, we observed higher proline betaine levels associated with increased β-cryptoxanthin concentrations, both of which are biomarkers for citrus fruit consumptions;(41) citrulline and β-carotene (commonly found in melons and pumpkins),(42) and betaine and lutein/zeaxanthin (commonly found in spinach and leafy vegetables),(43) were also positively associated with one another. In addition to external food sources, citrulline and betaine could also be generated internally from the urea cycle.(44) Higher circulating carotenoids were also associated with metabolites in the glycerolipid, glycerophospholipid, and sphingolipid super family. Carotenoids are lipophilic compounds that tend to reside in lipid membranes or lipophilic compartments,(5) which raises the possibility that some lipophilic metabolites identified may simply reflect compartmental co-localization with carotenoids. Nonetheless, we observed multiple carotenoid-associated lipophilic metabolites with plausible biological effects. Indeed, carotenoids are well-known experimentally for their roles in quenching free radicals and protecting nearby lipids from peroxidation.(5, 6, 45) Glycerolipid, glycerophospholipids and sphingolipids are key components of cell membranes that not only provide fundamental structural support, but also embed receptors, transporters, and enzymes that are responsible for numerous signal transductions.(46) The possible antioxidative role of carotenoids against membrane damage is pivotal for numerous cellular regulatory processes. Plasmalogens, which belong to the glycerophospholipid family and may play a role in protecting lipids from free radical damage, were positively associated with circulating carotenoids.(47) Other non-lipid free radical scavengers, such as arginine, glutamine, ornithine and derivatives, which play key roles in cellular redox balance (NADPH/NADP+),(48) were also associated with carotenoids. Future experimental research is warranted to verify whether, in addition to their short-distance direct free radical quenching activity, carotenoids could induce transcriptional activation of metabolites central to antioxidation.
We observed that multiple metabolites implicated in the urea cycle were positively associated with β-carotene and estimated vitamin A potential after multiple testing correction. Those included glutamine, citrulline, arginine, ornithine, glycine, guandinoacetic acid, serine, and derivatives. Compelling experimental studies show that down-regulation of the urea cycle reduces catabolism and nitrogen disposal, which enhances the ability of cancer cells to maximize nitrogen utilization for anabolic pyrimidine synthesis to facilitate tumor growth and proliferation.(49, 50) Although urea cycle dysregulation in cancer cells is key to tumor initiation and progression, additional experiments would be needed to examine whether carotenoid-associated changes in plasma metabolites involved in the urea cycle are causally related to tumorigenesis.
Few studies have examined metabolomic profiles of carotenoids. In the ATBC randomized trial among male smokers, pre-supplementation fasting serum retinol levels were associated with metabolites involved in the metabolism of tryptophan, arginine, proline, histidine, branched chain amino acid, and lipids, as well as carnitine transportation and Krebs cycle.(51) Top metabolites included N-acetyl-tryptophan, myo-inositol, 1-palmitoylglycerophosphoethanolamine and succinyl-carnitine.(51) Similar to the pre-supplementation analysis in the ATBC trial, we found positive associations between circulating carotenoids with tryptophan, arginine, proline, histidine and carnitine and derivatives, as well as several glycerophosphoethanolamines. Experimentally, activation of tryptophan metabolism through the kynurenine pathway regulates inflammation, oxidative stress and immune responses;(52) arginine and its downstream product ornithine also modulates redox balance intracellularly through oxidation-reduction of oxidative phosphorylation coenzymes (NAD+/NADPH and FAD+/FADH2).(48) Several acetylated and methylated amino acids were associated with circulating carotenoids in both the NHS and the pre-supplementary serum analysis in the ATBC trials (i.e., N-acetyl-tryptophan and N-acetyl-histidine); we also found higher N-acetyl-ornithine, N-acetyl-arginine, dimethylglycine, and N1-methyl-2-pyridone-5-carboxamide with higher carotenoids in the NHS, suggesting possible perturbations in acetylation and methylation that regulate gene expression through histone-chromatin remodeling. Interestingly, N-acetyl-tryptophan was among the strongest signal in the pre-supplementation analysis in the ATBC trial, and this amino acid derivative was also positively associated with all circulating levels of carotenoids in our analysis. N-terminal acetylation presents a pivotal post-translational modification step that alters the charge, hydrophobicity, and size of amino acids, which help prevent oxidative degradation of molecules and, facilitates protein-protein interactions and signal transduction.(53) The interconnectedness of tryptophan and N-acetylated-tryptophan (from our network correlation plots) provides further support that the tryptophan/kynurenine pathway—which regulates inflammation, oxidative stress and immune responses(52)—may play a role in carotenoid-associated breast cancer risk reduction.
Our derived metabolomic signatures were only modestly correlated with circulating carotenoid, and only the metabolic signatures for β-carotene and estimated vitamin A potential were associated with statistically significant lower breast cancer risk. This highlights the needs to investigate the vitamin A pathway in future work (since β-carotene and estimated vitamin A potential have pro-vitamin A activities).With the adjustment of the corresponding metabolomics signatures, we observed attenuation for breast cancer risk reduction for some (i.e., β-carotene and estimated vitamin A potential), but not other (α-carotene, lutein/zeaxanthin, and lycopene) carotenoids, suggesting that biological pathways not captured by our metabolomic analysis may also explain carotenoid-associated breast cancer risk reduction. The inverse associations between carotenoid-associated metabolomic signatures and breast cancer risk were stronger for the distant blood measures, possibility reflecting the more evident associations between circulating carotenoids and breast cancer risk in this nested case-control subset. It is estimated that the progression from normal mammary cells to atypical hyperplasia and invasive breast cancer may take as long as 10–30 years.(54) Because of the lengthy time between breast cancer initiation and disease manifestation, carotenoids and the metabolomic signatures measured ≥10 years prior to diagnosis may capture the potential anti-carcinogenic effects of carotenoids during cancer initiation and mild dysplasia formation, and suggests that carotenoid associated prevention strategies targeting the early phase of the carcinogenic continuum could potentially halt cancer progression. On the other hand, carotenoids and the metabolomic signatures measured <10 years before diagnosis, which is thought to reflect the later phase of cancer progression and manifestation, seem to be less relevant for breast cancer prevention in this nested case-control subset.
Our study has several strengths. We have measured carotenoids and metabolomics at 2 time points collected ~10 years apart with up to 20 years of follow-up, allowing us to evaluate the potential metabolomic effect of carotenoids along the breast cancer disease continuum (i.e., distant blood samples were more likely to capture cancer initiation processes, while proximate blood samples may reflect progression). Our study also has limitations. Most metabolites are more likely to reflect the impact of multiple dietary and lifestyle exposures on metabolic processes, and metabolites identified may not necessarily reflect biologically relevant carotenoid activity, making it difficult to identify functional relationships with causal interpretations. Our analysis included only known metabolites in the lipids and amino-acids family; future work is warranted to incorporate un-identified metabolite peaks as well as other metabolic pathways (i.e., specific fatty acids and carbohydrates) to better understand their effects. Our study included few ER-negative breast cancer cases (n=104 for distant and n=74 for proximate blood collection), and we have limited power to explore the metabolomic link of carotenoids and breast cancer risk among ER-negative cases. Future research is needed to include more hormone receptor negative cases to examine the relationship between carotenoid-associated metabolic signatures and breast cancer risk by molecular subtype. Although diet has a clear influence on plasma carotenoid levels,(55, 56) the absorption and metabolism of carotenoids may be influenced by age,(55, 57) gender,(55) genetic makeup,(58, 59) and BMI.(55, 60) We attempted to control for those non-dietary factors by (i) matching on age in the nested case-control study; (ii) adjusting for BMI in multivariable models; and (iii) using cholesterol standardized carotenoids values. However, we cannot rule out the possible influence of other non-dietary components on plasma carotenoid levels. The two blood collections were still considered sparse and more frequent measurements may be needed to reflect participants’ physiological status more accurately on a continuum. Participants in the NHS were predominately white registered nurses, and our findings may not be generalizable to other populations with different racial/ethnicity backgrounds, dietary and lifestyle patterns.
To conclude, our metabolomics analysis identified multiple metabolites associated with plasma levels of α-carotene, β-carotene, β-cryptoxanthin, estimated vitamin A potential, lutein/zeaxanthin, and lycopene. While some metabolites may simply share common food sources or reflect compartmental co-localization with carotenoids, other metabolites may have biological implications and may be involved in immune regulation, redox balance, anabolic synthesis of macromolecules, membrane lipids and signal transduction and β-oxidation. Metabolomic signatures derived for β-carotene and estimated vitamin A potential, measured ≥10 years before diagnosis, were associated with reduced breast cancer risk. These findings further support the role of higher carotenoid levels to reduce breast cancer risk.
Supplementary Material
Acknowledgements:
This work was supported by the Prevent Cancer Foundation (to C. Peng), the National Institutes of Health/National Cancer Institute [UM1 CA186107 (to M. Stampfer and A.H. Eliassen), P01 CA87969 (to A.H. Eliassen and R.M. Tamimi), U01 CA176726 (to W.C. Willett), T32 CA009001 (to M. Stampfer and A.H. Eliassen)]. The authors thank all participants and coordinators of the Nurses’ Health Studies for their valuable contribution, and the cancer registries in the following states for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA and WY. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
Conflicts of interest: No conflict of interest.
Data availability: The demographics and questionnaire data together with the plasma metabolomics profiles are not publicly available for the following reason: data contain information that could compromise research participant privacy. Requests to access these data should be made via http://www.nurseshealthstudy.org/researchers.
REFERENCE
- 1.Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao YT, Goodman MT, et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst 2012;104(24):1905–16. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eliassen AH, Liao X, Rosner B, Tamimi RM, Tworoger SS, Hankinson SE. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am J Clin Nutr 2015;101(6):1197–205. doi: 10.3945/ajcn.114.105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamimi RM, Colditz GA, Hankinson SE. Circulating carotenoids, mammographic density, and subsequent risk of breast cancer. Cancer Res 2009;69(24):9323–9. doi: 10.1158/0008-5472.CAN-09-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Gapstur SM, Giles GG, Giovannucci E, et al. Carotenoid intakes and risk of breast cancer defined by estrogen receptor and progesterone receptor status: a pooled analysis of 18 prospective cohort studies. Am J Clin Nutr 2012;95(3):713–25. doi: 10.3945/ajcn.111.014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britton G. Structure and properties of carotenoids in relation to function. FASEB J 1995;9(15):1551–8. [PubMed] [Google Scholar]
- 6.Burton GW, Ingold KU. beta-Carotene: an unusual type of lipid antioxidant. Science 1984;224(4649):569–73. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 7.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res 2013;54(7):1761–75. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palczewski G, Widjaja-Adhi MA, Amengual J, Golczak M, von Lintig J. Genetic dissection in a mouse model reveals interactions between carotenoids and lipid metabolism. J Lipid Res 2016;57(9):1684–95. doi: 10.1194/jlr.M069021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harari A, Coster ACF, Jenkins A, Xu A, Greenfield JR, Harats D, Shaish A, Samocha-Bonet D. Obesity and Insulin Resistance Are Inversely Associated with Serum and Adipose Tissue Carotenoid Concentrations in Adults. J Nutr 2020;150(1):38–46. doi: 10.1093/jn/nxz184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bie J, Langbeen A, Verlaet AAJ, Florizoone F, Immig I, Hermans N, Fransen E, Bols PEJ, Leroy J. The effect of a negative energy balance status on beta-carotene availability in serum and follicular fluid of nonlactating dairy cows. J Dairy Sci 2016;99(7):5808–19. doi: 10.3168/jds.2016-10870. [DOI] [PubMed] [Google Scholar]
- 11.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 2002;43(11):1773–808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 12.Carlberg C, Bendik I, Wyss A, Meier E, Sturzenbecker LJ, Grippo JF, Hunziker W. Two nuclear signalling pathways for vitamin D. Nature 1993;361(6413):657–60. doi: 10.1038/361657a0. [DOI] [PubMed] [Google Scholar]
- 13.Aktuna D, Buchinger W, Langsteger W, Meister E, Sternad H, Lorenz O, Eber O. [Beta-carotene, vitamin A and carrier proteins in thyroid diseases]. Acta Med Austriaca 1993;20(1–2):17–20. [PubMed] [Google Scholar]
- 14.Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res 2014;34(11):907–29. doi: 10.1016/j.nutres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Chew BP, Park JS. Carotenoid action on the immune response. J Nutr 2004;134(1):257S–61S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 16.Walrand S, Farges MC, Dehaese O, Cardinault N, Minet-Quinard R, Grolier P, Bouteloup-Demange C, Ribalta J, Winklhofer-Roob BM, Rock E, et al. In vivo and in vitro evidences that carotenoids could modulate the neutrophil respiratory burst during dietary manipulation. Eur J Nutr 2005;44(2):114–20. doi: 10.1007/s00394-004-0501-3. [DOI] [PubMed] [Google Scholar]
- 17.Bendich A, Shapiro SS. Effect of beta-carotene and canthaxanthin on the immune responses of the rat. J Nutr 1986;116(11):2254–62. doi: 10.1093/jn/116.11.2254. [DOI] [PubMed] [Google Scholar]
- 18.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334(18):1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 19.Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FL, Jr., Omenn GS, Valanis B, Williams JH, Jr. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst 2004;96(23):1743–50. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 20.Duffield-Lillico AJ, Begg CB. Reflections on the landmark studies of beta-carotene supplementation. J Natl Cancer Inst 2004;96(23):1729–31. doi: 10.1093/jnci/djh344. [DOI] [PubMed] [Google Scholar]
- 21.Blow N. Metabolomics: Biochemistry’s new look. Nature 2008;455(7213):697–700. doi: 10.1038/455697a. [DOI] [PubMed] [Google Scholar]
- 22.Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wagele B, Altmaier E, CardioGram, Deloukas P, Erdmann J, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011;477(7362):54–60. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, Arnold M, Erte I, Forgetta V, Yang TP, et al. An atlas of genetic influences on human blood metabolites. Nat Genet 2014;46(6):543–50. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lokody I. Translational genomics: A network of the human metabolome. Nat Rev Genet 2014;15(7):442. doi: 10.1038/nrg3758. [DOI] [PubMed] [Google Scholar]
- 25.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA 2006;296(2):193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, Longcope C, Speizer FE. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst 1995;87(17):1297–302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 27.Sisti JS, Lindstrom S, Kraft P, Tamimi RM, Rosner BA, Wu T, Willett WC, Eliassen AH. Premenopausal plasma carotenoids, fluorescent oxidation products, and subsequent breast cancer risk in the nurses’ health studies. Breast Cancer Res Treat 2015;151(2):415–25. doi: 10.1007/s10549-015-3391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, Deik AA, Bullock K, Pierce KA, Scott J, et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018;137(8):841–53. doi: 10.1161/CIRCULATIONAHA.117.029468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, Tworoger SS, Wolpin BM. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem 2013;59(11):1657–67. doi: 10.1373/clinchem.2012.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142(6):1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135(10):1114–26; discussion 27–36. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 32.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93(7):790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 33.Lucht SA, Eliassen AH, Bertrand KA, Ahern TP, Borgquist S, Rosner B, Hankinson SE, Tamimi RM. Circulating lipids, mammographic density, and risk of breast cancer in the Nurses’ Health Study and Nurses’ Health Study II. Cancer Causes Control 2019;30(9):943–53. doi: 10.1007/s10552-019-01201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, Deitz AC, Connolly JL, Schnitt SJ, Colditz GA, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res 2008;10(4):R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125(1–2):279–84. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 36.Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res 2009;37(Web Server issue):W652–60. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tibshirani R. Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society Series B (Methodological) 1996;58(1):267–88. [Google Scholar]
- 38.Rice MS, Tamimi RM, Bertrand KA, Scott CG, Jensen MR, Norman AD, Visscher DW, Chen YY, Brandt KR, Couch FJ, et al. Does mammographic density mediate risk factor associations with breast cancer? An analysis by tumor characteristics. Breast Cancer Res Treat 2018;170(1):129–41. doi: 10.1007/s10549-018-4735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nevo D, Liao X, Spiegelman D. Estimation and Inference for the Mediation Proportion. Int J Biostat 2017;13(2). doi: 10.1515/ijb-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV, Franke AA, Mantzoros CS, Ricchiuti V, Willett WC, Hankinson SE, et al. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev 2010;19(4):938–46. doi: 10.1158/1055-9965.EPI-09-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinzmann SS, Brown IJ, Chan Q, Bictash M, Dumas ME, Kochhar S, Stamler J, Holmes E, Elliott P, Nicholson JK. Metabolic profiling strategy for discovery of nutritional biomarkers: proline betaine as a marker of citrus consumption. Am J Clin Nutr 2010;92(2):436–43. doi: 10.3945/ajcn.2010.29672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allerton TD, Proctor DN, Stephens JM, Dugas TR, Spielmann G, Irving BA. l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018;10(7). doi: 10.3390/nu10070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho E, Zeisel SH, Jacques P, Selhub J, Dougherty L, Colditz GA, Willett WC. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr 2006;83(4):905–11. doi: 10.1093/ajcn/83.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ducker GS, Rabinowitz JD. One-Carbon Metabolism in Health and Disease. Cell Metab 2017;25(1):27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jomova K, Valko M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur J Med Chem 2013;70:102–10. doi: 10.1016/j.ejmech.2013.09.054. [DOI] [PubMed] [Google Scholar]
- 46.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008;9(2):112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 2012;1822(9):1442–52. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 2020;11(1):102. doi: 10.1038/s41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keshet R, Szlosarek P, Carracedo A, Erez A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat Rev Cancer 2018;18(10):634–45. doi: 10.1038/s41568-018-0054-z. [DOI] [PubMed] [Google Scholar]
- 50.Lee JS, Adler L, Karathia H, Carmel N, Rabinovich S, Auslander N, Keshet R, Stettner N, Silberman A, Agemy L, et al. Urea Cycle Dysregulation Generates Clinically Relevant Genomic and Biochemical Signatures. Cell 2018;174(6):1559–70 e22. doi: 10.1016/j.cell.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang J, Panagiotou OA, Anic GM, Mondul AM, Liao LM, Derkach A, Stolzenberg-Solomon R, Weinstein SJ, Albanes D. Metabolomic Profiling of Serum Retinol in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study. Sci Rep 2017;7(1):10601. doi: 10.1038/s41598-017-09698-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platten M, Nollen EAA, Rohrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov 2019;18(5):379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 53.Ree R, Varland S, Arnesen T. Spotlight on protein N-terminal acetylation. Exp Mol Med 2018;50(7):1–13. doi: 10.1038/s12276-018-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Umar A, Dunn BK, Greenwald P. Future directions in cancer prevention. Nat Rev Cancer 2012;12(12):835–48. doi: 10.1038/nrc3397. [DOI] [PubMed] [Google Scholar]
- 55.Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr 1996;126(1):129–37. doi: 10.1093/jn/126.1.129. [DOI] [PubMed] [Google Scholar]
- 56.El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr 2002;76(1):172–9. doi: 10.1093/ajcn/76.1.172. [DOI] [PubMed] [Google Scholar]
- 57.Weber D, Kochlik B, Demuth I, Steinhagen-Thiessen E, Grune T, Norman K. Plasma carotenoids, tocopherols and retinol - Association with age in the Berlin Aging Study II. Redox Biol 2020;32:101461. doi: 10.1016/j.redox.2020.101461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Da Costa LA, Garcia-Bailo B, Badawi A, El-Sohemy A. Genetic determinants of dietary antioxidant status. Prog Mol Biol Transl Sci 2012;108:179–200. doi: 10.1016/B978-0-12-398397-8.00008-3. [DOI] [PubMed] [Google Scholar]
- 59.Desmarchelier Charles BP. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends in Food Science & Technology 2017;69:270–80. [Google Scholar]
- 60.Nuss ET, Valentine AR, Zhang Z, Lai HJ, Tanumihardjo SA. Serum carotenoid interactions in premenopausal women reveal alpha-carotene is negatively impacted by body fat. Exp Biol Med (Maywood) 2017;242(12):1262–70. doi: 10.1177/1535370217706962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.