Abstract
Background and Aim:
Gallstone disease has been related to a higher prevalence and incidence of chronic conditions, such as dyslipidemia, obesity, and cardiovascular disease (CVD). However, limited data are available regarding whether gallstone disease is related to mortality.
Methods:
We examined the relationship of a history of gallstone disease and risk of death, using Cox proportional hazards regression analysis, among 86,030 women from the Nurses’ Health Study and 43,949 men from the Health Professionals Follow-up Study.
Results:
During the up-to 32 years of follow-up, 34,011 all-cause deaths were confirmed, of which 8138 were CVD deaths and 12,173 were cancer deaths. For the participants with a history of gallstone disease compared to those without, the hazard ratio of total mortality was 1.16 (95% confidence interval [CI] 1.13−1.20), of CVD mortality 1.11 (1.05−1.17), of cancer mortality 1.15 (1.09−1.20), and of other mortality 1.19 (1.14−1.25) from a pooled-analysis of women and men (all P < 0.001). The multi-adjusted associations between gallstone disease and total mortality persisted among women and men, and among participants with various risk profiles including the different status of body mass index, hormone therapy use, diabetes, hypertension and hypercholesterolemia (all P for interaction≥0.09).
Conclusions:
These data suggest that gallstone disease is associated with a higher risk of total mortality and disease-specific mortality, including CVD and cancer mortality, independent of various traditional risk factors.
Keywords: mortality, gallstone disease, cohort studies
INTRODUCTION
Gallstone disease is a highly prevalent disorder 1, affecting 5–25% of adults in the Western world 2. Gallstone disease has been related to a variety of metabolic abnormalities such as dyslipidemia 3, 4, obesity 5, diabetes 6, 7, and metabolic syndrome 3, 4. Besides, gallstones distort secretion of bile acids 8 and subsequently affect gut microbiota 9, 10, which are recently related to cardiovascular and mortality risk 11–14.
We recently found that a history of gallstone disease was related to an increased risk of coronary heart disease in prospective cohorts 15. Among the participants of the third US National Health and Nutrition Examination Survey (NHANES III), gallstone disease was related to total and disease-specific mortality in a general American population 16. In this NHANES study, the sample size (n=14,228) and duration of follow-up time (mean follow-up, 14.3 years) were moderate, and the follow-up ended in 2006. Studies with even larger sample sizes and longer follow-up periods are however, needed, especially for less common causes of death where more statistical power may be needed to detect differences.
In the current study, we examined the association of gallstone disease and total and cause-specific mortality after ~30 years of follow-up in two large cohort studies with repeated measurements of gallstone diseases and various risk factors: the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS), which include more than nine times the number of participants and, about 15 times the total number of deaths as the NHNES III study 16.
METHODS
Study Populations
The Nurses’ Health Study (NHS) was started in 1976 with the enrollment of 121,700 female nurses aged 30 to 55 years who completed an initial questionnaire on medications, lifestyle and medical history. The Health Professionals Follow-up Study (HPFS) was started in 1986 with the enrollment of 51,529 male health professionals (including dentists, optometrists, osteopaths, pharmacists, podiatrists, and veterinarians) aged 40 to 75 years who filled out an initial similar questionnaire from 50 states. Through 2012, a response rate of approximately 90% has been achieved in both cohorts.
For the current study, the initial baseline year was the first year for which detailed information was available on gallstone disease, lifestyle, diet habits, and physical activity: 1980 for NHS, and 1986 for HPFS; such information has been updated every two years until the end of follow-up for this study: June 30, 2012, for the NHS and January 31, 2012, for the HPFS. Participants with a baseline history of cardiovascular disease (CVD; including myocardial infarction, coronary revascularization, angina pectoris and stroke), or cancer; those who had implausible total energy intake (<500 or >3500 kcal/d for the NHS, and <800 or >4200 kcal/d for the HPFS) were excluded from the analysis. After exclusions, a total of 84,124 women and 41,956 men remained in the analysis.
Assessment of gallstone disease
The exposure of interest was a history of gallstone disease, including unremoved gallstones and cholecystectomy. We reported the results on the overall history of gallstone disease as well as cholecystectomy in the current investigation. In NHS, biennial questionnaires were used to assess occurrence and date that gallstone symptoms started and/or cholecystectomy performed. Of 50 randomly selected nurses who self-reported cholecystectomy, all 43 who agreed to requests for additional information reiterated their earlier report. Cholecystectomy was confirmed in all 36 nurses for whom medical records were available 17. In HPFS, participants reported whether they had cholecystectomy or had received a diagnosis of gallstones from a physician at baseline. In the follow-up questionnaires, participants were asked whether their gallstone disease was symptomatic and whether the diagnosis had been confirmed by radiography or ultrasonography. To verify the self-report of diagnosed but unremoved gallstones and cholecystectomy, we reviewed a sample of 441 medical records of men who reported having gallstones or cholecystectomy; of these, 99% (all but 5) confirmed the diagnosis. Moreover, all of the self-reported symptoms and all but one of the self-reported diagnostic procedures were confirmed by medical record review 18.
Ascertainment of Mortality
Deaths from all causes were reported by the next of kin or the postal authorities or identified by searching the National Death Index, between the return of the baseline questionnaire and the end of follow-up 19. More than 98% of deaths can be identified in each cohort 19, 20. For all deaths, we sought death certificates and, when appropriate, requested permission from the next of kin to review medical records. The underlying cause of death was assigned according to the International Classification of Diseases, 8th Revision (ICD-8). In this analysis we also specifically considered deaths due to CVD (ICD-8 codes 390.0–458.9 or 795.0–795.9) or cancer (ICD-8 codes 140.0–207.9).
Assessment of Covariates
In both cohorts, participants were asked at baseline about their date of birth. Information on body weight, height, family medical history of heart disease, cancer and diabetes, lifestyle characteristics (e.g., cigarette smoking and physical activity), disease diagnoses (diabetes, hypertension, and hypercholesterolemia), menopausal status and use of postmenopausal hormone therapy (HT, in women), and other characteristics was collected at baseline and in biennial validated follow-up questionnaires. Alcohol and other dietary information were first assessed at baseline and updated by validated food frequency questionnaires during follow-up. Detailed descriptions on the validity and reproducibility of self-reported body weight, physical activity, and alcohol consumption have been published elsewhere 21–23. A modified Alternate Healthy Eating Index (AHEI) score was calculated based on intakes of 10 foods and nutrients predictive of chronic disease risk, including fruits, vegetables, nuts and legumes, red or processed meat, sugar-sweetened beverages, alcohol, sodium, trans fat, long-chain ω−3 fats, and other polyunsaturated fats 24.
Statistical Analysis
We calculated person-years of follow-up from the return date of the first food frequency questionnaire to the date of death, loss to follow-up, or the end of follow-up, whichever came first. Cox proportional hazards models were used to calculate the hazard ratios and 95% confidence intervals of all cause and cause-specific mortality associated with a history of gallstone disease.
In multivariate analyses, we controlled for ethnicity (white, not white), a family history of myocardial infarction (yes, no), cancer (yes, no), or diabetes (yes, no), as well as time-varying covariates, including age (months), body mass index (BMI; kg/m2); smoking status (never, past, current); smoking amount and duration (0, 0–9, 10–24, 25–44, ≥45 pack-years); alcohol intake (women: 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, ≥15.0 g/d; men: 0, 0.1−4.9, 5.0−9.9, 10.0−14.9, 15.0−29.9, or ≥30.0 g/d); physical activity (in quintiles); marital status (married, not married), menopausal status and use of postmenopausal hormone therapy (HT) (premenopausal, postmenopausal and HT nonuser, postmenopausal and current HT user, postmenopausal and past HT user, missing; NHS only); regular aspirin use at least once per week (yes, no); a history of hypertension (yes, no), hypercholesterolemia (yes, no), or diabetes (yes, no); dietary cholesterol intake (in quintiles); daily total energy intake (in quintiles); and modified AHEI score (alcohol excluded; in quintiles). We used the data from subsequent questionnaires to update information on the time-varying covariates of age, BMI, smoking, alcohol intake, physical activity, marital status, HT use, and history of diseases. Indicator variables were used for any missing information on covariates.
To better represent long-term or habitual intake and to minimize within-person variation, we created and used the cumulative mean of energy-adjusted dietary intake, i.e. AHEI and dietary cholesterol intake, from all available dietary questionnaires from baseline through the end of follow-up 25. Because of the differences in sex, follow-up time, and the questionnaires in the two cohorts, we conducted analyses separately for each cohort to facilitate better control of confounding. An inverse-variance–weighted, fixed-effect meta-analysis was used to combine the results across the cohorts. The heterogeneity between cohorts was tested by Cochran’s Q test.
We repeated our analyses when stratifying by age (< 55 or ≥ 55 years), BMI (< 30 or ≥ 30 kg/m2), waist circumference (divided by cohort-specific median), current smoking and alcohol intake status (no or yes), AHEI (divided by cohort-specific median), physical activity (divided by cohort-specific median), disease status of type 2 diabetes, hypertension, and hyperlipidemia (no or yes), and HT use status (NHS only, no or yes). Effect modification by the above factors was assessed using the multiplicative interaction term between gallstone disease and the above factors, added to the multivariable model which included both main effect variables.
Analyses were carried out with SAS software, version 9.3 (SAS Institute), at a two-tailed alpha level of 0.05.
RESULTS
Table 1 presents the baseline characteristics by the status of the history of gallstone disease (either reported presence of gallstones or history of cholecystectomy). In our study population of 86,030 women and 43,949 men, 7102 (8.3%) and 1416 (3.2%) had gallstone disease, respectively. Compared to those who did not have gallstone disease, participants who had were more likely to be older, hypertensive, have hypercholesterolemia, physically sedentary, be a current smoker and regular aspirin user or user of postmenopausal HT (in women), as well as have a higher BMI. In addition, a history of gallstone disease was associated with a worse diet quality indicated by a lower AHEI score.
Table 1.
Age-Adjusted Baseline Characteristics of Participates by Status of a History of Gallstone disease
| NHS | HPFS | |||
|---|---|---|---|---|
| History of gallstone disease | No | Yes | No | Yes |
| Participants No. | 78,928 | 7102 | 42,533 | 1416 |
| Age (years) * | 45.9(7.2) | 48.1(7.0) | 53.5(9.5) | 59.9(9.2) |
| White (%) | 97.5 | 98.4 | 90.9 | 91.2 |
| Married (%) | 91.4 | 90.5 | 90.2 | 90.7 |
| Body Mass Index (kg/m2) | 24.1(4.3) | 26.7(5.7) | 25.5(3.3) | 26.7(3.8) |
| Physical activity (metabolic equivalent/week) | 14.2(20.3) | 12.4(18.8) | 21.3(29.6) | 16.9(24.1) |
| Premenopausal (%) | 56.9 | 49.4 | - | - |
| Current use of postmenopausal hormone (%) | 8.2 | 11.2 | - | - |
| Current smoker (%) | 28.5 | 31.7 | 9.7 | 11.7 |
| Current alcohol drinker (%) | 69.1 | 62.6 | 76.8 | 70.8 |
| Alcohol (g/day) | 6.5(10.6) | 4.9(9.3) | 11.5(15.6) | 8.4(12.2) |
| Hypertension (%) | 15.0 | 24.3 | 20.0 | 25.0 |
| Hypercholesterolemia (%) | 5.0 | 7.6 | 10.4 | 13.2 |
| Family history of myocardial infarction ≤ 60 years (%) | 18.8 | 21.9 | 12.0 | 11.7 |
| Family history of diabetes (%) | 27.9 | 35.7 | 19.2 | 22.6 |
| Family history of cancer (%) | 16.0 | 15.4 | 15.4 | 16.2 |
| Regular Aspirin user (%) | 40.0 | 43.5 | 26.6 | 28.7 |
| Daily energy intake (kcal/day) | 1566.4(500.1) | 1562.9(515.8) | 1994.8(620.5) | 1991.6(645.8) |
| Daily cholesterol intake (mg/day) | 336.5(122.1) | 336.5(126.4) | 305.2(111.2) | 306.9(100.7) |
| Alternative Health Eating Index Score | 42.7(10.2) | 42.1(10.1) | 46.7(10.8) | 45.7(11.6) |
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
Value is not age adjusted.
In NHS, during up to 32 years of follow-up (2,556,075 person-years), we documented 21,547 deaths, of which 4602 were CVD deaths (1735 due to coronary heart disease and 1252 to stroke) and 8130 were cancer deaths (1887 due to lung cancer, 1341 to breast cancer, and 724 to colorectal cancer). In HPFS, with up to 26 years of follow-up (1,014,775 person-years), we documented 14,445 deaths, of which 4356 were CVD deaths (2480 due to coronary heart disease and 867 to stroke) and 4528 were cancer deaths (837 due to lung cancer, 642 to prostate cancer, and 466 to colorectal cancer). Table 2 presents the associations of a history of gallstone disease and cholecystectomy with the risk of total and major cause-specific mortality, including CVD mortality and cancer mortality. In age-adjusted analyses, a history of gallstone disease was significantly associated with a higher total and major cause-specific mortality in both cohorts. Further adjustment for other potential confounders attenuated such associations. In the fully adjusted model, a history of gallstone disease was associated with a 20% increased risk of all-cause mortality among women and a 9% increased risk among men. In addition, a history of gallstone disease was associated with a 15% increased risk of CVD death among women; and associated with a 15% increased risk of cancer death among both women and men. The association with CVD mortality was non-significant among men. The associations were similar for cholecystectomy (Table 2). The fully adjusted hazard ratios from pooled-analysis of women and men with a history of gallstone disease compared to those without were 1.16 (95% CI, 1.13, 1.20) for total mortality, 1.11 (1.05, 1.17) for CVD mortality, 1.15 (1.09, 1.20) for cancer mortality, and 1.19 (1.14, 1.25) for other mortality (Figure 1).
Table 2.
Cohort-specific Hazard Ratios (95%) for Risk of All-cause and Major Cause-specific Death in Relation to A History of Gallstone Disease or Cholecystectomy
| Gallstone disease | Cholecystectomy | |||
|---|---|---|---|---|
|
| ||||
| No | Yes | No | Yes | |
| NHS (1980–2012) | ||||
| All-cause death | ||||
| Cases/person years Rate (/100k PY) |
16532/2183692 739 |
5015/372383 1347 |
16964/2221151 764 |
4583/334923 1368 |
| Model 1 HR (95% CI), Age-adjusted | 1 | 1.27 (1.23, 1.31) | 1 | 1.26 (1.22, 1.30) |
| Model 2 HR (95% CI), Multivariate | 1 | 1.20 (1.16, 1.24) | 1 | 1.18 (1.14, 1.22) |
| CVD-specific death | ||||
| Cases/person years Rate (/100k PY) |
3424/2196011 156 |
1178/376025 313 |
3532/2233777 158 |
1070/338258 316 |
| Model 1 HR (95% CI), Age-adjusted | 1 | 1.41 (1.32, 1.50) | 1 | 1.38 (1.29, 1.48) |
| Model 2 HR (95% CI), Multivariate | 1 | 1.15 (1.07, 1.23) | 1 | 1.12 (1.05, 1.21) |
| Cancer-specific death | ||||
| Cases/person years Rate (/100k PY) |
6475/2193105 295 |
1655/375546 441 |
6636/2230811 297 |
1494/337840 442 |
| Model 1 HR (95% CI), Age-adjusted | 1 | 1.14 (1.08, 1.21) | 1 | 1.12 (1.06, 1.19) |
| Model 2 HR (95% CI), Multivariate | 1 | 1.15 (1.09, 1.21) | 1 | 1.12 (1.06, 1.19) |
| Other death | ||||
| Cases/person years Rate (/100k PY) |
6633/2193161 302 |
2182/375113 582 |
6796/2230868 305 |
2019/337406 598 |
| Model 1 HR (95% CI), Age-adjusted | 1 | 1.31 (1.25, 1.38) | 1 | 1.30 (1.24, 1.37) |
| Model 2 HR (95% CI), Multivariate | 1 | 1.24 (1.18, 1.31) | 1 | 1.24 (1.17, 1.30) |
|
| ||||
| HPFS (1986–2012) | ||||
| All-cause death | ||||
| Cases/person years Rate (/100k PY) |
12449/946350 1315 |
1996/68425 2917 |
13004/963814 1349 |
1441/50961 2828 |
| Model 1 HR (95% CI), Age-adjusted | 1 | 1.18 (1.12, 1.23) | 1 | 1.15 (1.08, 1.21) |
| Model 2 HR (95% CI), Multivariate | 1 | 1.09 (1.04, 1.15) | 1 | 1.07 (1.01, 1.13) |
| CVD-specific death | ||||
| Cases/person years Rate (/100k PY) |
3715/954833 389 |
641/69754 919 |
3908/972655 402 |
448/51932 863 |
| Model 1 HR (95% CI), Age-adjusted | 1 | 1.18 (1.08, 1.29) | 1 | 1.12 (1.01, 1.24) |
| Model 2 HR (95% CI), Multivariate | 1 | 1.05 (0.96, 1.15) | 1 | 1.01 (0.91, 1.12) |
| Cancer-specific death | ||||
| Cases/person years Rate (/100k PY) |
3960/954596 415 |
568/69819 814 |
4088/972492 420 |
440/51924 847 |
| Model 1 HR (95% CI), Age-adjusted | 1 | 1.20 (1.10, 1.31) | 1 | 1.27 (1.15, 1.41) |
| Model 2 HR (95% CI), Multivariate | 1 | 1.15 (1.05, 1.25) | 1 | 1.21 (1.09, 1.34) |
| Other death | ||||
| Cases/person years Rate (/100k PY) |
4774/953836 501 |
787/69604 1131 |
5008/971616 515 |
553/51824 1067 |
| Model 1 HR (95% CI), Age-adjusted | 1 | 1.15 (1.06, 1.24) | 1 | 1.08 (0.98, 1.18) |
| Model 2 HR (95% CI), Multivariate | 1 | 1.08 (0.99, 1.17) | 1 | 1.01 (0.92, 1.11) |
Abbreviations: CI, Confidence Interval; HR, Hazard Ratios; PY, Person Years
Model1, adjusted for age;
Model2 adjusted for age (months), body mass index (kg/m2), white race (yes/no), marital status (yes/no), smoking status (never, past, current smoker), smoking amount and duration (0, 0–9, 10–24, 25–44, ≥45 pack years), alcohol drinking status (women: 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, ≥15.0 g/d; men: 0, 0.1−4.9, 5.0−9.9, 10.0−14.9, 15.0−29.9, or ≥30.0 g/d), physical activity (in quintile), family history of myocardial infarction (yes/no), diabetes (yes/no), and cancer (yes/no); post-menopausal hormone replacement (premenopausal, postmenopausal and HT nonuser, postmenopausal and current HT user, postmenopausal and past HT user, missing; NHS only), modified Alternative Health Eating Index Score (alcohol excluded; in quintiles), dietary cholesterol intake (in quintiles), daily energy intake (in quintiles); status of hypertension (yes/no), diabetes (yes/no), hypercholesterolemia (yes/no); regular aspirin use (yes/no).
Figure 1. Pooled-analyzed hazard ratios of mortality comparing participants with a history of gallstone disease to those without, among 86,030 women from NHS and 43,949 men from HPFS.
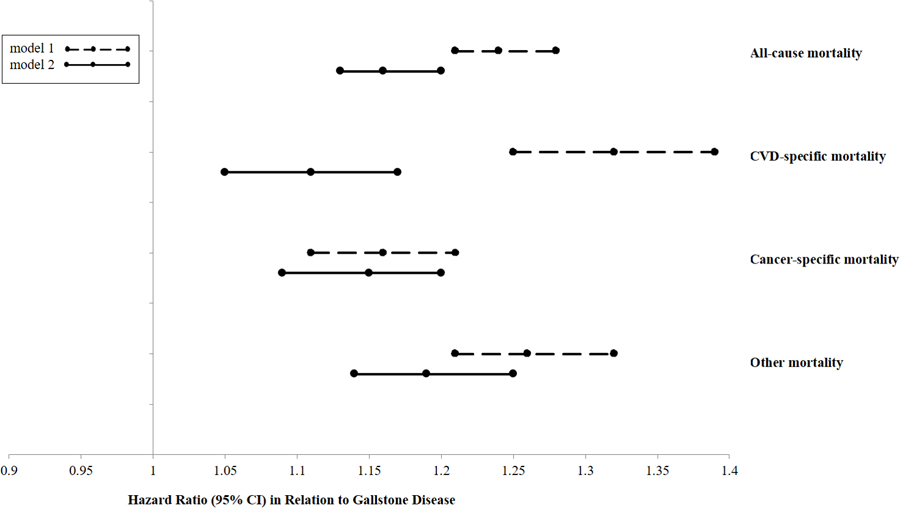
Model 1 adjusted for age; Model 2 adjusted for age (months), body mass index (kg/m2), white race (yes/no), marital status (yes/no), smoking status (never, past, current smoker), smoking amount and duration (0, 0–9, 10–24, 25–44, ≥45 pack years), alcohol drinking status (women: 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, ≥15.0 g/d; men: 0, 0.1−4.9, 5.0−9.9, 10.0−14.9, 15.0−29.9, or ≥30.0 g/d), physical activity (in quintile), family history of myocardial infarction (yes/no), diabetes (yes/no), and cancer (yes/no); post-menopausal hormone replacement (premenopausal, postmenopausal and HT nonuser, postmenopausal and current HT user, postmenopausal and past HT user, missing; NHS only), modified Alternative Health Eating Index Score (alcohol excluded; in quintiles), dietary cholesterol intake (in quintiles), daily energy intake (in quintiles); status of hypertension (yes/no), diabetes (yes/no), hypercholesterolemia (yes/no); regular aspirin use (yes/no).
P for heterogeneity in Model 2 was 0.003 for all-cause death, 0.12 for CVD-death, 0.96 for cancer-death, and 0.003 for other death.
Further subtype analyses revealed that a history of gallstone disease was associated with a higher risk of mortality from coronary heart disease (pooled hazard ratio [95% CI], 1.19 [1.10, 1.28]), but not from stroke (1.05 [0.94, 1.18]) (Supplemental Table 1). A history of gallstone disease was not significantly associated with mortality due to individual types of major cancer, including colorectal cancer, lung cancer, breast cancer, or prostate cancer (Supplemental Table 2). The associations for mortality from injury or poison were significant among women but not among men (Supplemental Table 3).
The associations between a history of gallstone disease and total mortality in general persisted among participants with various risk profiles defined by age, BMI, waist circumference, physical activity, AHEI score, alcohol and smoking status, HT use (in women), and disease status of diabetes, hypertension and hypercholesterolemia (Figure 2, all P for interaction > 0.05).
Figure 2. Stratified analysis of the association between gallstone history and total mortality.
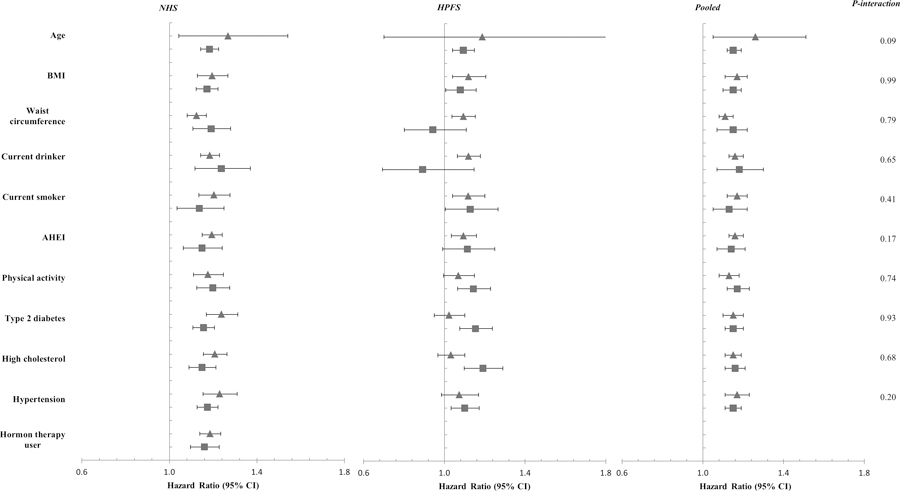
Covariates include age (months), body mass index (BMI, kg/m2), white race (yes/no), marital status (yes/no), smoking status (never, past, current smoker), smoking amount and duration (0, 0–9, 10–24, 25–44, ≥45 pack years), alcohol drinking status (women: 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, ≥15.0 g/d; men: 0, 0.1−4.9, 5.0−9.9, 10.0−14.9, 15.0−29.9, or ≥30.0 g/d), physical activity (in quintile), family history of myocardial infarction (yes/no), diabetes (yes/no), and cancer (yes/no); post-menopausal hormone replacement (premenopausal, postmenopausal and HT nonuser, postmenopausal and current HT user, postmenopausal and past HT user, missing; NHS only), modified Alternative Health Eating Index Score (AHEI, alcohol excluded; in quintiles), dietary cholesterol intake (in quintiles), daily energy intake (in quintiles); status of hypertension (yes/no), diabetes (yes/no), hypercholesterolemia (yes/no); regular aspirin use (yes/no).
 indicates a lower level of the stratifying variable, i.e., age < 55 years, BMI < 30 kg/m2, weight circumference, physical activity, and AHEI score was lower than the cohort-specific median, nondrinker, nonsmoker, free of type 2 diabetes, high cholesterol, or hypertension, and hormone therapy nonuser in women;
indicates a lower level of the stratifying variable, i.e., age < 55 years, BMI < 30 kg/m2, weight circumference, physical activity, and AHEI score was lower than the cohort-specific median, nondrinker, nonsmoker, free of type 2 diabetes, high cholesterol, or hypertension, and hormone therapy nonuser in women;
 indicates a higher level of the stratifying variable.
indicates a higher level of the stratifying variable.
DISCUSSION
In two large U.S. cohorts with long follow-up periods, we found that a history of gallstone disease was consistently associated with increased risk of total mortality and cancer mortality in men and women, and associated with increased risk of CVD mortality in women. These associations were independent of conventional demographic and lifestyle risk factors.
The associations of gallstone disease with overall, CVD- and cancer-specific mortality were suggested from previous investigations, while the most compelling analysis was from the NHANES III data 16. Ruhl et al. analyzed data from 14,228 participants in the US NHANES III (20–74 years old) who underwent gallbladder ultrasonography from 1988 to 1994, and reported that participants with gallstone disease had 30% higher total mortality, 40% higher CVD mortality but not significant higher cancer mortality, independent of multiple demographic and cardiovascular disease risk factors after an average follow-up period of 14 years 16. An early study of 383 Pima Indians found increased total mortality, especially the cancer mortality among participants with gallstone disease, independent of age and sex, during 20 years of follow-up 26. These previous studies measured gallstone disease and lifestyle factors only once at baseline, while in both the NHS and the HPFS, we collected detailed and repeated assessments of diet and risk factors, allowing for more careful control for potential confounding factors.
The mechanisms by which gallstone disease may affect various causes of death differ. Gallstone patients have a higher prevalence of cardiovascular risk factors, such as obesity 5, diabetes 6, 7, and metabolic syndrome 3, 4. Previous observational studies have related gallstone disease with CVD development 15, 27–29, and abnormalities in lipids may partly explain the effects of gallstone disease as a risk factor of CVD 27. Obesity may also increase the risk of some cancers, including breast cancer, colorectum cancer, and possibly prostate cancer 30. A novel hypothesis that gallstones result in gut microbiota dysbiosis leading to generation of atherosclerosis-promoted metabolites, had been raised as a possible underlying mechanism 15, though solid evidence is still sparse. In addition, accumulating data have suggested that the intestinal microbiota might play a role in the etiology of cancer by influencing inflammation, DNA damage and apoptosis, partially via microbial metabolites 31.
To our knowledge, our study is the largest population with the longest follow-up of its kind to examine association between gallstone disease and mortality. The statistical power of our study enabled us to examine various cause-specific deaths such as CVD- and cancer-mortality. We were able to control for numerous demographic, dietary and lifestyle risk factors, including the general dietary pattern and various disease status. We had repeated measurements of the status of gallstone disease and various covariates throughout the follow-up period, and minimized the possibility of misclassification of exposure and maximized the accuracy of time-varying covariates. Follow-up rates in both our cohorts were high, with over 90% of participants being either actively followed or having a recorded death. Mortality ascertainment was over 98%, therefore loss to follow-up or underestimating deaths was less likely to bias our results.
There were several limitations to our study that merit consideration. We could not identify participants with asymptomatic gallstone disease, and our prevalence of baseline gallstone disease by self-reports was lower than that determined by ultrasonography from US NHANES III 32. Because of our prospective study design, any misclassification of gallstone exposure was unlikely to be related to the study outcome mortality and more likely to attenuate the true association toward the null. As typically in large population studies, we acknowledge the potential for residual confounding, which was partially related to the unavailability of blood biochemical indices (such as the lipid profiles and insulin resistance). However, the sensitivity analyses in a previous study 16 did not reveal these as important confounders influencing the association of gallstone disease with total and specific mortality, suggesting that our findings are unlikely to be biased by these unmeasured factors. The positive association with mortality was consistently observed in the current and previous investigations, suggesting that the associations are robust to various degrees of measurement errors and residual confounding. The epidemiology and characteristic of gallstone disease are divergent among different races. For example, the prevalence of gallstone disease is higher in Hispanic populations and Native populations in America, intermediate in Asian populations, and lower in African populations.33 In Western countries, most gallstones are consisted of cholesterol crystals, while in East Asia, the predominant type is brown pigment stones.34 However, our study populations were relatively homogenous for race (>90% were Whites), and the generalizability of our findings might be limited. Additionally, we did not measure gut microbiota and related biomarkers, and therefore were unable to explore the contribution of these metabolites to the associations observed.
In summary, these data from two large prospective cohort studies indicated significant associations of gallstone diseases with a higher risk of total mortality and disease-specific mortality, including CVD and cancer mortality, independent of various traditional risk factors. Further studies are needed to confirm our findings and investigate the mechanisms.
Supplementary Material
Acknowledgements:
We would like to thank the participants and staff of the NHS and the HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
Financial support:
This study was supported by the National Institutes of Health had no role in the design, conduct, analysis, or reporting of this study. The cohorts were supported by grants of UM1 CA186107, R01 HL034594, UM1 CA176726, UM1 CA167552, and R01 HL35464 from the National Institutes of Health. The current study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, and HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, and DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States – Israel Binational Science Foundation Grant 2011036. Dr. Qi was a recipient of the American Heart Association Scientist Development Award (0730094N), and Shanghai Thousand Talents Program for Distinguished Scholars. Dr. Zheng was supported by a fellowship from the American Diabetes Association (7–12-MN-34). The National Heart, Lung, and Blood Institute had no role in the design, conduct, analysis, or reporting of this study.
Abbreviations used in this paper:
- AHEI
Alternate Healthy Eating Index
- BMI
body mass index
- CIs
confidence intervals
- CVD
cardiovascular disease
- HPFS
the Health Professionals Follow-up Study
- HT
hormone therapy
- ICD-8
International Classification of Diseases, 8th Revision
- NHANES III
National Health and Nutrition Examination Survey
- NHS
the Nurses’ Health Study
Footnotes
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Ethics, consent and permissions: The study protocol was approved by the Human Research Committee of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health. The completion of the self-administered questionnaire was considered to imply written informed consent.
References:
- [1].Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet 2006; 368: 230–9. [DOI] [PubMed] [Google Scholar]
- [2].Kratzer W, Mason RA, Kachele V. Prevalence of gallstones in sonographic surveys worldwide. J Clin Ultrasound 1999; 27: 1–7. [DOI] [PubMed] [Google Scholar]
- [3].Chen LY, Qiao QH, Zhang SC, Chen YH, Chao GQ, Fang LZ. Metabolic syndrome and gallstone disease. World J Gastroenterol 2012; 18: 4215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ata N, Kucukazman M, Yavuz B, et al. The metabolic syndrome is associated with complicated gallstone disease. Can J Gastroenterol 2011; 25: 274–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stender S, Nordestgaard BG, Tybjaerg-Hansen A. Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a Mendelian randomization study. Hepatology 2013; 58: 2133–41. [DOI] [PubMed] [Google Scholar]
- [6].Weikert C, Weikert S, Schulze MB, et al. Presence of gallstones or kidney stones and risk of type 2 diabetes. Am J Epidemiol 2010; 171: 447–54. [DOI] [PubMed] [Google Scholar]
- [7].Xu M, Li Y, Zheng Y, Qi L. Gallstones and risk of type 2 diabetes In process.
- [8].Berr F, Pratschke E, Fischer S, Paumgartner G. Disorders of bile acid metabolism in cholesterol gallstone disease. J Clin Invest 1992; 90: 859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yokota A, Fukiya S, Islam KB, et al. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes 2012; 3: 455–9. [DOI] [PubMed] [Google Scholar]
- [10].Wu T, Zhang Z, Liu B, et al. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics 2013; 14: 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Charach G, Grosskopf I, Rabinovich A, Shochat M, Weintraub M, Rabinovich P. The association of bile acid excretion and atherosclerotic coronary artery disease. Therap Adv Gastroenterol 2011; 4: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013; 368: 1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19: 576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zheng Y, Xu M, Li Y, et al. Gallstones and Risk of Coronary Heart Disease: Prospective Analysis of 270 000 Men and Women From 3 US Cohorts and Meta-Analysis. Arterioscler Thromb Vasc Biol 2016; 36: 1997–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ruhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the United States. Gastroenterology 2011; 140: 508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. The New England journal of medicine 1999; 341: 777–84. [DOI] [PubMed] [Google Scholar]
- [18].Leitzmann MFWW, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E. A prospective study of coffee consumption and the risk of symptomatic gallstone disease in men. JAMA 1999; 281: 2106–12. [DOI] [PubMed] [Google Scholar]
- [19].Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 1994; 140: 1016–9. [DOI] [PubMed] [Google Scholar]
- [20].Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol 1984; 119: 837–9. [DOI] [PubMed] [Google Scholar]
- [21].Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990; 1: 466–73. [DOI] [PubMed] [Google Scholar]
- [22].Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol 1991; 133: 810–7. [DOI] [PubMed] [Google Scholar]
- [23].Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996; 7: 81–6. [DOI] [PubMed] [Google Scholar]
- [24].Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012; 142: 1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999; 149: 531–40. [DOI] [PubMed] [Google Scholar]
- [26].Grimaldi CH, Nelson RG, Pettitt DJ, Sampliner RE, Bennett PH, Knowler WC. Increased mortality with gallstone disease: results of a 20-year population-based survey in Pima Indians. Annals of internal medicine 1993; 118: 185–90. [DOI] [PubMed] [Google Scholar]
- [27].Bortnichak EA, Freeman DH Jr., Ostfeld AM, et al. The association between cholesterol cholelithiasis and coronary heart disease in Framingham, Massachusetts. Am J Epidemiol 1985; 121: 19–30. [DOI] [PubMed] [Google Scholar]
- [28].Wirth J, Giuseppe RD, Wientzek A, et al. Presence of gallstones and the risk of cardiovascular diseases: The EPIC-Germany cohort study. Eur J Prev Cardiol 2013. [DOI] [PubMed]
- [29].Olaiya MT, Chiou HY, Jeng JS, Lien LM, Hsieh FI. Significantly Increased Risk of Cardiovascular Disease among Patients with Gallstone Disease: A Population-Based Cohort Study. PLoS One 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Research. AIfC. Updated Estimate on Obesity-Related Cancers
- [31].Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nature reviews Microbiology 2014; 12: 661–72. [DOI] [PubMed] [Google Scholar]
- [32].Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999; 117: 632–9. [DOI] [PubMed] [Google Scholar]
- [33].Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nature reviews Disease primers 2016; 2: 16024. [DOI] [PubMed] [Google Scholar]
- [34].Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best practice & research Clinical gastroenterology 2006; 20: 981–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


