Abstract
To investigate endogenous interference factors of the detection results of novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) IgM/IgG. Enzyme‐linked immunosorbent assay (ELISA) was used to detect SARS‐CoV‐2 IgM/IgG in sera of 200 patients without COVID‐19 infection, including rheumatoid factor (RF) positive group, antinuclear antibody (ANA) positive group, pregnant women group, and normal senior group, with 50 in each group and 100 normal controls. The level of SARS‐CoV‐2 IgG in pregnant women was significantly higher than that in the normal control group (p = 0.000), but there was no significant difference between other groups. The levels of SARS‐CoV‐2 IgM in the pregnant women group, normal senior group, ANA positive group, and RF positive group were significantly higher than that in the normal control group (p < 0.05), with significant higher false‐positive rates in these groups (p = 0.036, p = 0.004, p = 0.000, vs. normal control group). Serum RF caused SARS‐CoV‐2 IgM false‐positive in a concentration‐dependent manner, especially when its concentration was higher than 110.25 IU/L, and the urea dissociation test can turn the false positive to negative. ANA, normal seniors, pregnant women, and RF can lead to false‐positive reactivity of SARS‐CoV‐2 IgM and/or IgG detected using ELISA. These factors should be considered when SARS‐CoV‐2 IgM or IgG detection is positive, false positive samples caused by RF positive can be used for urea dissociation test.
Keywords: ELISA, false positive, interference factors, SARS‐CoV‐2 IgG, SARS‐CoV‐2 IgM
Key Points
Pregnancy may cause false‐positive results of SARS‐CoV‐2 IgG and IgM.
Serum RF caused a false‐positive result of SARS‐COV‐2 IgM when its concentration was higher than 110.25 IU/L and the urea dissociation test can turn the false positive to negative.
1. INTRODUCTION
SARS‐CoV‐2 is a serious threat to human health worldwide. Due to its high infectivity and virulence, it has become a global epidemic. 1 , 2 , 3 The disease caused by SARS‐CoV‐2 infection in humans has been named coronavirus disease 2019 (COVID‐19), which can cause multiple organ dysfunction. 4 , 5 The detection of IgM/IgG antibodies against SARS‐CoV‐2 has high sensitivity and specificity, which can be combined with RT‐PCR for the diagnosis of SARS‐CoV‐2 infection. 6 , 7 With the emergence of various virus variants (Alpha, Beta, Gamma, Delta, and Lambda variants), the false‐negative rate of SARS‐CoV‐2 RNA detection results also increased. In addition, as the number of COVID‐19 vaccination increases, rapid and accurate monitoring of serum levels of SARS‐CoV‐2 specific IgG and IgM can be used to timely understand the variation of the immune status of the vaccinated patients. The detection of SARS‐CoV‐2 specific IgG and IgM antibodies by enzyme‐linked immunosorbent assay (ELISA) is a quick and simple screening method. 7 Nowadays, the challenge of antibody analysis is to ensure its sensitivity and specificity. Although many newly developed tests are available, in many cases, ELISA serological tests could produce false‐positive results due to a variety of factors. 8 It was shown in multiple works that rheumatoid factor (RF) can interfere with the detection of antigens or antibodies in serum by ELISA, resulting in a false‐positive result. 9 Another study reported that there are certain false‐positive rates when pregnant women used ELISA to detect serum cytomegalovirus (CMV) IgG levels. 10 Some patients with autoimmune diseases can also cause false‐positive detection of serum SARS‐CoV antibodies by ELISA. 11 However, few systematic reports on the factors affecting the detection of SARS‐CoV‐2 IgG and IgM antibodies. In this study, to further understand the interference factors of ELISA in the detection of SARS‐CoV‐2 IgG and IgM antibodies in serum, four potential subjects which possibly show false positive detection results, including senior, pregnant women, patients with autoimmune diseases, and rheumatoid arthritis, were investigated to explore the endogenous influencing factors of ELISA in the detection of SARS‐CoV‐2 IgG and IgM antibodies, so as to provide a more reliable theoretical basis for clinical diagnosis, postvaccination monitoring, and epidemiological investigation.
2. MATERIALS AND METHODS
2.1. Patients
The study population consisted of 300 patients without COVID‐19 infection who were hospitalized in the First People's Hospital of Zigong City from February 1 to October 30, 2020. 50 normal seniors (25 males and 25 females) and 50 normal pregnant women were excluded from endocrine system, cardiovascular and cerebrovascular diseases, tumors, infections, autoimmune diseases, liver diseases, kidney diseases, and other system diseases; 50 antinuclear antibody (ANA) positive patients and 50 RF positive patients, all of them excluded endocrine system, cardiovascular and cerebrovascular diseases, tumors, infections, liver diseases, kidney diseases, and other system diseases. All patients (participants or individuals) were admitted to the hospital and tested for nucleic acid and all were negative, which ruled out COVID‐19 infection.
2.2. Mixed serum samples
Among the physical examination population, 15 normal people, aged 18–30, were selected to exclude systemic diseases such as endocrine system, cardiovascular and cerebrovascular diseases, tumors, infections, autoimmune diseases, liver diseases, and kidney diseases. The sera of 15 selected individuals were collected and divided into three groups on average. The sera of each group were mixed to form three mixed samples, which were shaken and mixed evenly for substitution.
2.3. Assay
SARS‐CoV‐2 nucleic acid was detected using real‐time PCR (RT‐PCR) (kit provided by Sichuan Maccura Biotechnology Co.; detection instrument provided by Shanghai Hongshi Biotechnology Co.). ELISA was used for SARS‐CoV‐2 IgM detection (kit provided by Zhuhai Lizhu Reagent Co.: lot no. 2020021408). The optical density in ELISA plates was measured using a microplate reader (Thermo Fisher Scientific).
2.4. Data collection
Epidemiological, clinical, laboratory, management, and outcome data were collected through a review of medical records. Real‐time RT‐PCR confirmed that all patients were negative for SARS‐CoV‐2.
2.5. ELISA
Serum (5 μl) was added to a 500 μl sample diluent and mixed. Then 100 μl volume of the diluted sample, negative control, and positive control were added to the wells of the plate coated with SARS‐CoV‐2 recombinant antigen (Mouse anti‐human IgM monoclonal antibody [μ chain]), and the plates were incubated at 37°C for 30 min. The plates were washed five times, and 100 μl anti‐human IgM/IgG horseradish peroxidase (HRP)‐labeled antibodies were added to the reaction system to form indirect immune complexes, which were incubated at 37°C for 30 min. After washing five times to remove the unbound substance, 100 μl substrate was added and incubated at 37°C for 30 min. After 50 μl of termination solution was added, the blank OD values were read at the wavelength of 450 nm. The results were interpreted according to the ratios of the sample optical density value and the cutoff optical density value (S/CO) as follows: positive, S/CO ≥ 1; negative, S/CO < 1.00 (The cutoff value of SARS‐CoV‐2 IgG = 0.093+OD average value of negative control; the cutoff value of SARS‐CoV‐2 IgM = 0.100+OD average value of negative control). ELISA method was used to detect SARS‐CoV‐2 IgG and IgM antibodies. Each serum sample was tested three times, and the results were expressed as the average value of S/CO.
2.6. Urea dissociation test of ELISA
Serum (5 μl) was added to a 500 μl sample diluent and mixed. Then 100 μl volume of the diluted sample, negative control, and positive control were added to the wells of the plate coated with SARS‐CoV‐2 recombinant antigen (Mouse anti‐human IgM monoclonal antibody [μ chain]), and the plates were incubated at 37°C for 30 min. The plates were washed five times, and 100 μl of PBS solution (containing 0, 1, 2, 4, 6, and 8 mol/L urea in different wells) was added and incubated at 37°C for 10 min. After three more washes, 100 μl anti‐human IgM/IgG (HRP)‐labeled antibodies were added to the reaction system to form indirect immune complexes, which were incubated at 37°C for 30 min. After washing five times to remove the unbound substance, 100 μl substrate was added and incubated at 37°C for 30 min. After 50 μl of termination solution was added, the blank OD values were read at the wavelength of 450 nm. The results were interpreted according to the ratios of the sample optical density value and the cutoff optical density value (S/CO) as follows: positive, S/CO ≥ 1; negative, S/CO < 1.00. The results of affinity index (AI) analyses were expressed as the ratios of the S/CO values measured at different dissociated urea concentrations to that of PBS with 0 mol/L urea. The AI threshold value was set as the middle value between the highest AI value determined for the false‐positive sample results with the outliers removed and the lowest AI value determined for all of the SARS‐CoV‐2 infection samples. The results were interpreted as follows: positive, AI value of sera greater than or equal to the AI threshold; negative, AI value of sera less than the AI threshold.
2.7. Statistical methods
Analysis of normality and homogeneity of variance is carried out on the measurement data. If the data conforms to the normal distribution, it is displayed by ±s, and t‐test was used for difference analysis; if the data does not conform to the normal distribution, it is expressed as the median (quartile)M(P25, P75), Mann–Whitney U‐test was used for variance analysis. Qualitative data were analyzed by the X 2 test. SPSS 21.0 statistical software was used for statistical analysis of the data, and Statistical significance was defined as p < 0.05. Other data were displayed as tables and line charts.
3. RESULTS
3.1. Precision of ELISA for detection of serum SARS‐CoV‐2 IgG and IgM
Three mixed sera were prepared and measured for SARS‐CoV‐2 IgG and IgM, respectively. Each mixed serum was measured 20 times in the same batch to obtain the intra‐batch precision (Table 1), and for 20 consecutive days to obtain the inter‐batch precision (Table 2). The coefficient variations (CVs) of intra‐batch and inter‐batch precision for all samples were ≤15%. It is concluded that this method has a certain accuracy in detecting serum SARS‐CoV‐2 IgG and IgM.
Table 1.
Detection of intra‐batch precision of serum SARS‐CoV‐2 IgG and IgM
| Sample | n | Mean (S/CO) | SD | CV (%) | |
|---|---|---|---|---|---|
| SARS‐CoV‐2 IgG | 1 | 20 | 15.745 | 0.961 | 6.1 |
| 2 | 20 | 8.581 | 0.776 | 9.0 | |
| 3 | 20 | 0.801 | 0.081 | 10.1 | |
| SARS‐CoV‐2 IgM | 1 | 20 | 15.534 | 1.569 | 0.812 |
| 2 | 20 | 8.984 | 1.119 | 0.111 | |
| 3 | 20 | 0.812 | 0.125 | 0.137 |
Table 2.
Detection of inter‐batch precision of serum SARS‐CoV‐2 IgG and IgM
| Sample | n | Mean (S/CO) | SD | CV (%) | |
|---|---|---|---|---|---|
| SARS‐CoV‐2 IgG | 1 | 20 | 15.080 | 1.204 | 8.0 |
| 2 | 20 | 8.905 | 1.012 | 11.4 | |
| 3 | 20 | 0.763 | 0.101 | 13.3 | |
| SARS‐CoV‐2 IgM | 1 | 20 | 15.047 | 1.870 | 12.4 |
| 2 | 20 | 8.667 | 1.182 | 13.6 | |
| 3 | 20 | 0.782 | 0.110 | 14.1 |
3.2. SARS‐CoV‐2 IgG and IgM levels in each group
The test results of negative controls were less than the cutoff value in the reagent specification, and the levels of all positive controls were far greater than the specified cutoff value. We also detected eight SARS‐CoV‐2 positive samples (confirmed by RT‐PCR), which were positive for SARS‐CoV‐2 IgG and IgM (Table 3). These indicated that the test was working properly.
Table 3.
The levels of serum SARS‐COV‐2 IgG and IgM in eight SARS ‐COV‐2 positive samples
| Sex | Age (y) | Day no.a | SARS‐CoV‐2 IgM S/CO (±s) | SARS‐CoV‐2 IgG S/CO (±s) | |
|---|---|---|---|---|---|
| Negative controls | 0.071 ± 0.006 | 0.068 ± 0.005 | |||
| Positive controls | 6.149 ± 0.856 | 5.905 ± 0.239 | |||
| Case 1 | Female | 52 | 18th | 1.059 ± 0.003 | 12.276 ± 0.126 |
| Case 2 | Female | 67 | 16th | 3.000 ± 0.012 | 7.082 ± 0.015 |
| Case 3 | Female | 48 | 9th | 5.175 ± 0.007 | 1.649 ± 0.055 |
| Case 4 | Male | 40 | 22th | 5.701 ± 0.024 | 6.757 ± 0.027 |
| Case 5 | Male | 34 | 24th | 1.272 ± 0.008 | 1.892 ± 0.060 |
| Case 6 | Female | 49 | 20th | 1.430 ± 0.002 | 2.677 ± 0.007 |
| Case 7 | Female | 42 | 20th | 1.835 ± 0.009 | 2.926 ± 0.010 |
| Case 8 | Female | 39 | 15th | 2.876 ± 0.070 | 7.323 ± 0.140 |
Serum SARS‐CoV‐2 IgG and IgM were detected on a day after illness onset.
In 100 normal controls, the medium levels of serum SARS‐CoV‐2 IgG and IgM were 0.078 (0.063, 0.158) and 0.284 (0.213, 0.345), respectively, and all were negative. The medium levels of SARS‐CoV‐2 IgG in sera of pregnant women group, senior group, ANA positive group and RF positive group were 0.218 (0.070, 0.395), 0.085 (0.060,0.223), 0.075 (0.060, 0.163) and 0.113 (0.048, 0.345) respectively. The level of SARS‐CoV‐2 IgG in normal pregnant women was significantly higher than that in the normal control group (p = 0.000), but no significant differences were detected between the other three groups and the normal control group (Figure 1A). Moreover, there were false positives in the normal pregnant women group and ANA positive group, and the false‐positive rates were 3/50 and 4/50, respectively, which was statistically significant compared with the normal control group (p = 0.036, p = 0.011; Figure 1B). For the serum SARS‐CoV‐2 IgM level, the detection results of normal pregnant women group, seniors group, ANA positive group and RF positive group were 0.395 (0.330, 0.498), 0.385 (0.308, 0.465), 0.368 (0.278, 0.508), and 0.856 (0.536, 1.547), respectively. The level of SARS‐CoV‐2 IgM in the normal pregnant women group, seniors group, ANA positive group, and RF positive group was significantly higher than that in the normal control group (p < 0.05; Figure 1C). In addition, there were false positives in the seniors' group, ANA positive group, and RF positive group, and the false‐positive rates were 3/50, 5/50, and 20/50, respectively. Compared with the normal control, it was statistically significant (p = 0.036, p = 0.004, p = 0.000; Figure 1D). Moreover, the concentration of RF leading to a high false‐positive rate in SARS‐COV‐2 IgM detection can cause false‐positive results, which need to be further studied.
Figure 1.
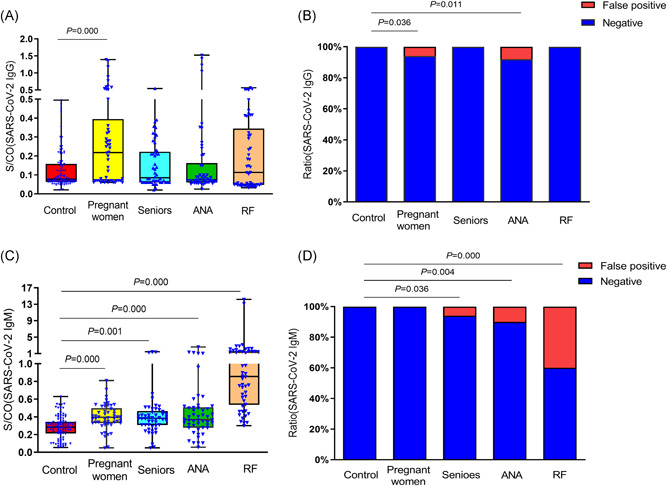
Detection results of SARS‐CoV‐2 IgG and IgM in serum of each group. (A) Detection results of SARS‐CoV‐2 IgG in serum of each group. (B) Detection results of SARS‐CoV‐2 IgM in serum of each group. (C) Comparison of false‐positive rate of SARS‐CoV‐2 IgG in serum of each group. (D) Comparison of the false‐positive rate of SARS‐CoV‐2 IgM in serum of each group
3.3. Comparison of SARS‐CoV‐2 IgM results and detection results before and after urea dissociation test of ELISA
The urea dissociation test of ELISA was carried out with PBS containing 0, 1, 2, 4, 6, and 8 mol/L urea in 20 RF‐IgM‐positive serum samples (SARS‐CoV‐2 IgM false‐positive serum specimens) and serum of 9 COVID‐19 patients with SARS‐CoV‐2 IgM positive detected by ELISA before urea dissociation. When urea dissociation concentration was 4 mol/L and AI calculation method was 0.432, 16 RF‐IGM positive serum samples were negative for SARS‐CoV‐2 IgM analysis, while 9 serum samples from COVID‐19 patients were still positive for SARS‐CoV‐2 IgM analysis (Figure 2). Through the urea dissociation test, the specificity of ELISA after dissociation was significantly higher than before dissociation.
Figure 2.
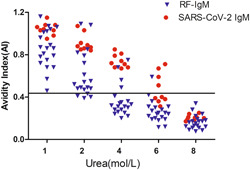
AI of SARS‐CoV‐2 IgM detected using different urea dissociation concentrations of ELISA. When the dissociation concentration of urea was 4 mol/L and the AI calculation method value was set to 0.432, the results determined for SARS‐CoV‐2 IgM in 16 sera with RF‐IgM positivity turned negative, whereas the results determined for SARS‐CoV‐2 IgM in the 9 sera from COVID‐19 patients remained positive. ELISA, enzyme‐linked immunosorbent assay; RF, rheumatoid factor
3.4. Effect of RF on serum SARS‐CoV‐2 IgG and IgM detection
To validate the effect of endogenous RF in serum on SARS‐CoV‐2 IgG and IgM, we collected 50 RF‐positive (≥20 IU/L) and non‐COVID‐19 infected patient serum samples and detected SARS‐CoV‐2 IgG and IgM by ELISA. The results showed that RF did not significantly interfere with the detection of SARS‐CoV‐2 IgG, but for SARS‐CoV‐2 IgM, 20 out of 50 samples showed false‐positive results, and the false‐positive rate was 40% (Figure 3A). And when the serum endogenous RF concentration was higher than 145 IU/L, it produced significant positive interference to IgM detection, leading to false‐positive results. The interference ability was gradually enhanced with the increase of serum RF concentration (Figure 3A).
Figure 3.
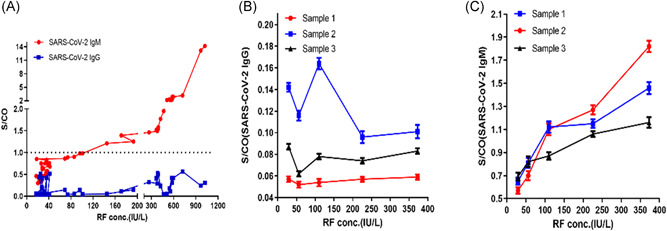
Effect of serum RF on detection of SARS‐CoV‐2 IgM and IgG. (A) S/CO values of SARS‐CoV‐2 IgG and IgM in serum of 50 non‐COVID‐19 infected patients (RF ≥ 20 IU/L). (B) S/CO values of SARS‐CoV‐2 IgG in three mixed serum samples with different RF concentrations. (C) S/CO values of SARS‐CoV‐2 IgM in three mixed serum samples with different RF concentrations. RF, rheumatoid factor
To further confirm the effect of RF on the detection of SARS‐CoV‐2 IgG and IgM by ELISA, we spiked RF standard solution with five different concentrations into three mixed serum samples to afford RF concentrations of measured 29.25, 56.25, 110.25, 225.75, and 372.75 IU/L, respectively. The results showed that there were no false‐positive results in the detection of SARS‐CoV‐2 IgG in the mixed serum samples of different RF concentrations, indicating that RF does not interfere with the qualitative detection of SARS‐CoV‐2 IgG in serum. However, for SARS‐CoV‐2 IgM detection, when the RF concentration exceeded 110.25 IU/L, it caused significant positive interference, resulting in false positives, and with the increase of serum RF concentration, the interference ability gradually increased. The results are shown in Figure 3B,C.
4. DISCUSSION
SARS‐CoV‐2 continues to wreak havoc around the world and endanger human health. 12 Early detections, early diagnosis, and early isolation are effective measures to prevent the spread of COVID‐19. The diagnosis of COVID‐19 infection mainly relies on the detection of its RNA by RT‐PCR. 13 However, the detection process of SARS‐CoV‐2 RNA (including before, during, and after detection) is affected by various factors. In particular, the sample type, disease course, collection method, detection system, and other factors affect the positive detection rate of SARS‐CoV‐2 RNA, and significantly affect the early diagnosis of SARS‐CoV‐2 infection. 14 Several cases reported that SARS‐CoV‐2 RNA showed false‐negative results in multiple tests, especially with the emergence of various virus variants, the false‐negative rate increased as well. The serological analysis is based on the identification of specific antibodies to infectious sources in serum or other body fluids. It is used in epidemiological studies to determine the prevalence and transmission rate of a disease in the population. The method can also determine whether a particular person is infected, so as to assess their risk of illness and further transmission of infection. 8 Several reports confirmed that specific SARS‐CoV‐2 IgM and IgG were produced 3–5 days and 10–15 days after SARS‐CoV‐2 infection. 15 , 16
Serum SARS‐COV‐2 IgM and IgG tests are not affected by specimen collection. Together with SARS‐COV‐2 RNA test results, they can be used for the diagnosis of COVID‐19 infection and have been included in the Chinese Novel Coronavirus Guidelines (8th edition). The detection of SARS‐CoV‐2 IgG by ELISA has relatively high sensitivity and specificity. 12 , 17 To understand the accuracy of this method in detecting SARS‐CoV‐2 IgG and IgM in serum, we obtained that the ELISA method had a high precision in detecting SARS‐CoV‐2 IgG and IgM by detecting the intra‐batch precision and day‐time precision. However, due to the limitations of this method, it is also affected by a variety of factors, for example, RF can interfere with the colloidal gold method and ELISA method for the determination of SARS‐CoV‐2 IgM. 18 In addition, interference with autoantibodies, heterotropic antibodies, and alloantibodies (antibodies from allogeneic tissue) can lead to false positives in pregnant women, patients with autoimmune diseases, transplantation, and blood transfusions. 3 Specific antibodies in pregnant women have been reported to interfere with CMV IgG, Zika virus (ZIKV) IgG, and IgM detection. 10 , 19 However, the factors influencing the detection of SARS‐COV‐2 IgG and IgM antibodies are rarely comprehensively reported.
To further understand the endogenous interference factors of ELISA on the detection of serum SARS‐CoV‐2 IgM and IgG, we detected the serum SARS‐CoV‐2 IgM and IgG in the seniors, pregnant women, autoimmune diseases, rheumatoid arthritis, and other special groups. It was found that the IgG level was significantly higher than that in the normal control group, but there was no significant difference among other groups. In addition, there were false positives in the pregnant women group and ANA positive group, and the false‐positive rates were 3/50 and 4/50 respectively, which was statistically significant compared with the normal control group. The serum SARS‐CoV‐2 IgM levels in the pregnant women group, seniors group, ANA positive group, and RF positive group were significantly higher than that in the normal control group (p < 0.05). In addition, there were false positives in the seniors' group, ANA positive group, and RF positive group, and the false‐positive rates were 3/50, 5/50, and 20/50, respectively. Compared with the normal control group, it was statistically significant. When the serum endogenous RF concentration was higher than 145 IU/L, there was significant positive interference to IgM detection, resulting in false‐positive results. With the increase of serum RF concentration, the interference ability increased gradually. When the dissociation concentration of urea in ELISA was 4 mol/L and the dissociation time was 10 min, 16 of the 20 RF‐IgM positive sera produced false‐positive results for SARS‐CoV‐2 and IgM turned negative, while the 9 sera of COVID‐19 patients were unaffected. Therefore, the improved ELISA results not only ensure the detection sensitivity but also improve the corresponding specificity and reliability.
To further illustrate the effect of RF on SARS‐CoV‐2 IgM/IgG, we also found that there was no false‐positive result in the detection of SARS‐CoV‐2 IgG in the mixed serum samples with different RF concentrations, indicating that RF did not interfere with the qualitative detection of SARS‐CoV‐2 IgG in serum. However, for SARS‐CoV‐2 IgM detection, when the RF concentration exceeded 110.25 IU/L, it caused significant positive interference to IgM detection, resulting in false positives, and the interference ability gradually increased with the increase of serum RF concentration. This is consistent with the results of endogenous RF interference. It indicates that when the SARS‐CoV‐2 IgM test is positive, we should measure the RF value of this serum. If the RF concentration exceeds 110.25 IU/L, the result may be falsely positive, and other tests should be combined to determine whether the patient has recently been infected with SARS‐CoV‐2 or has been vaccinated. This study indicates that when SARS‐CoV‐2 IgM is detected by ELISA, serum RF IgM level should be evaluated and urea decomposition test should be carried out to avoid false positive, which is consistent with previous research results. 18 However, the urea dissociation test could not completely eliminate the interference of RF IgM, The methods to avoid these endogenous interference factors affecting the test results should be further studied.
In conclusion, although the ELISA method has high sensitivity and precision in detecting SARS‐CoV‐2 IgM/IgG in serum, it can be combined with RT‐PCR for the diagnosis of ‐CoV‐2 infection in SARS and can also continuously monitor SARS‐CoV‐2 IgM/IgG level in the human body inoculated with COVID‐19, so as to understand the variations of immune function in time and accurately, but in the analysis of endogenous factors affecting the detection of serum SARS‐CoV‐2 IgG and IgM antibodies by ELISA, it was found that the normal seniors and pregnant women could be false positive for SARS‐CoV‐2 IgG or IgM, and autoimmune diseases could also appear false positive for SARS‐CoV‐2 IgG or IgM. When the serum endogenous RF concentration exceeded 110.25 IU/L, there was a significant positive interference to the detection of SARS‐CoV‐2 IgM, leading to the occurrence of false positives, and the interference ability gradually increased with the increasing of the concentration. Therefore, when the SARS‐CoV‐2 IgM or IgG detection is positive, these endogenous factors should be considered, especially if RF concentrations are higher than 110.25 IU/L. Urea dissociation test can turn the false‐positive result of Sars‐Cov‐2 IgM into negative, so as to significantly improve the specificity of ELISA. At present, the mechanism of false‐positive results of serum SARS‐CoV‐2 IgM/IgG detected by ELISA in pregnant women, seniors, and ANA‐positive patients is still not clear, which may be related to the cross‐reaction of some antibodies in vivo. Further studies need to be carried out to find effective ways to reduce the false‐positive rate. In addition, studies with larger numbers of clinical samples are warranted and could strengthen the exciting preliminary results of this study.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
This study was reviewed and approved by the Ethics Committee of Zigong Academy of Medical Sciences & Zigong First People's Hospital. Individual‐level informed consent was not obtained given the use of pre‐existing, deidentified data.
AUTHOR CONTRIBUTIONS
Conceptualization: Weiping Liu and Yaohui Song. Formal analysis: Lin Li and Yaohui Song. Investigation: Weiping Liu and Yaohui Song. Resources: Xia Long, Kexing Wan, and Minggang Yin. Writing – original draft preparation: Weiping Liu. Writing – review and editing: Xia Long, Kexing Wan, Minggang Yin, Yi Yin, Bo Zhang, Lin Li, and Yaohui Song. Visualization: Yaohui Song. Project administration: Weiping Liu and Lin Li.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the intense individual effort and support from many sources to make this study possible. This study was supported by the Sichuan Medical Scientific Research Funding (Q18025).
Liu W, Long X, Wan K, et al. The endogenous factors affecting the detection of serum SARS‐CoV‐2 IgG/IgM antibodies by ELISA. J Med Virol. 2022;94:1976‐1982. 10.1002/jmv.27557
Weiping Liu and Xia Long contributed equally to this study.
Contributor Information
Lin Li, Email: spphll@163.com.
Yaohui Song, Email: syh760701293@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tung C, Sangi‐Haghpeykar H, Levison J. Rapid versus standard testing for prenatal HIV screening in a predominantly Hispanic population. J Perinatol. 2010;30(1):30‐32. [DOI] [PubMed] [Google Scholar]
- 4. Irsara C, Egger A, Prokop W, et al. Clinical validation of the Siemens quantitative SARS‐CoV‐2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clin Chem Lab Med. 2021;59(8):1453‐1462. [DOI] [PubMed] [Google Scholar]
- 5. Song Y, Zhong H, Li L, et al. Dynamic monitoring of immune function indexes in COVID‐19 patients. Aging. 2020;12(24):24596‐24603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iddir M, Brito A, Dingeo G, et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID‐19 crisis. Nutrients. 2020;12(6):1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meng QB, Peng JJ, Wei X, et al. Clinical application of combined detection of SARS‐CoV‐2‐specific antibody and nucleic acid. World J Clin Cases. 2020;8(19):4360‐4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Komarov A, Kaznadzey A, Li Y, Kireeva M, Mazo I. Dual‐antigen system allows elimination of false positive results in COVID‐19 serological testing. Diagnostics. 2021;11(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trier NH, Houen G. Determination of rheumatoid factors by ELISA. Methods Mol Biol. 2019;1901:263‐270. [DOI] [PubMed] [Google Scholar]
- 10. Hitz D, Exler S, Daiminger A, Bartelt U, Enders M. Low‐level positive results in the Liaison CMV IgG II assay may misclassify pregnant woman as immune. Diagn Microbiol Infect Dis. 2020;97(2):115029. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Sun S, Shen H, et al. Cross‐reaction of SARS‐CoV antigen with autoantibodies in autoimmune diseases. Cell Mol Immunol. 2004;1(4):304‐307. [PubMed] [Google Scholar]
- 12. Gutiérrez‐Cobos A, Gómez de Frutos S, Domingo García D, et al. Evaluation of diagnostic accuracy of 10 serological assays for detection of SARS‐CoV‐2 antibodies. Eur J Clin Microbiol Infect Dis. 2021;40(5):955‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giacobbo A, Rodrigues M, Zoppas Ferreira J, Bernardes A, de Pinho M. A critical review on SARS‐CoV‐2 infectivity in water and wastewater. What do we know? Sci Total Environ. 2021;774:145721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lascarrou JB, Colin G, Le Thuaut A, et al. Predictors of negative first SARS‐CoV‐2 RT‐PCR despite final diagnosis of COVID‐19 and association with outcome. Sci Rep. 2021;11(1):2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou P, Yang XL, Wang XG, et al. Addendum: a pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588(7836):E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu X, Zhang R, An T, et al. Impact of heat‐inactivation on the detection of SARS‐CoV‐2 IgM and IgG antibody by ELISA. Clin Chim Acta. 2020;509:288‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Q, Du Q, Guo B, et al. A method to prevent SARS‐CoV‐2 IgM false positives in gold immunochromatography and enzyme‐linked immunosorbent assays. J Clin Microbiol. 2020;58(6):e00375‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lustig Y, Koren R, Biber A, Zuckerman N, Mendelson E, Schwartz E. Screening and exclusion of Zika virus infection in travellers by an NS1‐based ELISA and qRT‐PCR. Clin Microbiol Infect. 2020;26(12):1687.e7‐1687.e11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


