Abstract
Stroke remains a leading cause of death and disability, with limited therapeutic options and suboptimal tools for diagnosis and prognosis. High throughput technologies such as proteomics generate large volumes of experimental data at once, thus providing an advanced opportunity to improve the status quo by facilitating identification of novel therapeutic targets and molecular biomarkers. While proteomics studies in animals are largely designed to decipher molecular pathways and targets altered in brain tissue after stroke, studies in human patients primarily focus on biomarker discovery in biofluids and, more recently, in thrombi and extracellular vesicles. Here, we offer a comprehensive review of stroke proteomics studies conducted in both animal and human specimen, and present our view on limitations, challenges, and future perspectives in the field. In addition, as a unique resource for the scientific community, we provide extensive lists of all proteins identified in proteomic studies as altered by stroke, and perform post-analysis of animal data to reveal stroke-related cellular processes and pathways.
Keywords: Cognitive Impairment, Proteomics, Cerebrovascular Disease/Stroke
1. Introduction
Stroke is a highly prevalent and often devastating disease that primarily affects the elderly1. It is a leading cause of death and long-term disability, presenting a major socioeconomic burden. In the past several decades, significant progress has been made in stroke prevention and acute stroke care, while only limited advances were achieved in identifying biomarkers to assist diagnosis/prognosis and developing therapies to counteract the harmful effects of stroke2.
There are two main stroke subtypes, ischemic and hemorrhagic, which arise from cerebral vessel blockage and bleeding into the brain parenchyma (intracerebral hemorrhage, ICH) or subarachnoid space (subarachnoid hemorrhage, SAH), respectively. Patients suffering from either stroke type often present with similar clinical features, making early accurate diagnosis challenging. Current stroke diagnosis almost exclusively relies on a patient’s neurologic presentation and neuroimaging, which is time-consuming, and requires highly experienced neurologists and advanced imaging facilities that are often not accessible. Identifying a rapid, reliable, and inexpensive stroke biomarker may greatly help to quickly diagnose stroke and stroke subtypes in patients, especially in resource-limited medical facilities, and ensure that they receive timely and appropriate medical care. In addition, therapeutic options following stroke are extremely limited. The treatment of ischemic stroke (IS) relies primarily on acute thrombolysis with tissue plasminogen activator (t-PA) and/or mechanical thrombectomy to restore blood flow, and there is no specific medication for hemorrhagic stroke (HS). Finding new strategies to increase post-stroke brain cell survival is essential, and will only succeed if we identify better targets through a deeper mechanistic understanding of stroke pathophysiology.
The dawn of the proteomics age has allowed researchers to obtain a comprehensive picture of the protein landscape altered in brain tissue, bodily fluids, and other biological materials following stroke. If utilized to its full potential, this information can greatly increase the chances of discovering stroke biomarkers and therapeutic targets. Proteomics is the large-scale analysis of the entire set of proteins (termed proteome) in a biologic system at a given time. Proteins carry out most essential cellular functions. Therefore, the proteome’s composition bears critical information related to an organism’s state. The proteome is highly dynamic and adapts in response to changes in the environment through transcriptional, translational, post-translational, and degradational processes. Proteomics is advantageous over other high throughput omics techniques as it assesses the end-product arising from a combination of adaptations on every level. This is particularly relevant to stroke, after which translation is stalled3 and altered mRNA levels, detected by transcriptomics, may not correlate with similar changes in protein levels. Additionally, post-translational protein modifications (PTMs), which cannot be investigated by any other omics method, may be important modulators of stroke outcome.
To date, stroke proteomics has used different sample sources by global or targeted mass spectrometry (MS) approaches as well as antibody- and aptamer-based arrays (Figure 1). Here, we summarize proteomics data from animal and human stroke studies, discuss their potential value for stroke diagnosis/prognosis and therapy, with focus on biomarker and target discovery, and provide thoughts about future research in this field.
Figure 1.

Schematic diagram summarizing sample sources, proteomics methods and applications of stroke proteomics studies executed in animal models and patients. AP, affinity purification; CSF, cerebrospinal fluid; ECF, extracellular fluid; EVs, extracellular vesicles; ICH, intracerebral hemorrhage; MS, mass spectrometry; SAH, subarachnoid hemorrhage. Image was partly created with BioRender.com.
2. Proteomics from brain tissue: in search of disease mechanisms and therapeutic targets
2.1. Animal studies
Global proteomic changes in the ischemic core and penumbra during acute, subacute, and chronic stroke phases were assessed in rodent, porcine, and nonhuman primate models of IS and HS to identify proteins involved in brain cell death, survival, and recovery. A list of all animal studies containing details about type of animal and stroke model used, sampling sources and timepoints, as well as lysis and proteomics methods used for protein identification can be found in Table S1. Over 20 studies identified >7,000 known and new differentially expressed proteins (DEPs) in different brain areas at various timepoints after stroke (DEPs listed in Table S2). Here, we provide a review of the major targets and associated molecular pathways identified across all studies. To determine pathways consistently altered throughout all post-stroke phases in each stroke type, we pooled the available data, and performed pathway and gene ontology (GO) enrichment analyses with DEPs that appeared in at least two proteomics studies (Table S3, sheet 1). Identified DEPs suggest changes in platelet regulation and complement/coagulation cascades in both stroke types, while protein phosphorylation and neuron-specific pathways were only altered by ischemia (Figure 2A). DEPs connected with platelets and coagulation, as well as phosphorylation, were upregulated, indicating heightened responses, while those associated with synaptic function were reduced (Figure 2B). Enriched GO terms among decreased DEPs after ischemia reiterated deficient neuronal function, while an increase in enzymatic activity, metabolism, and proteolysis was observed (Figure 2C). DEPs after HS were exclusively upregulated and mainly associated with extracellular material, regulation of enzymatic activity, and coagulation (Figure 2C). Below, we describe selected DEPs and protein classes that were consistently changed in different stroke phases across multiple proteomics studies. An omitted protein, however, should not be considered insignificant.
Figure 2.
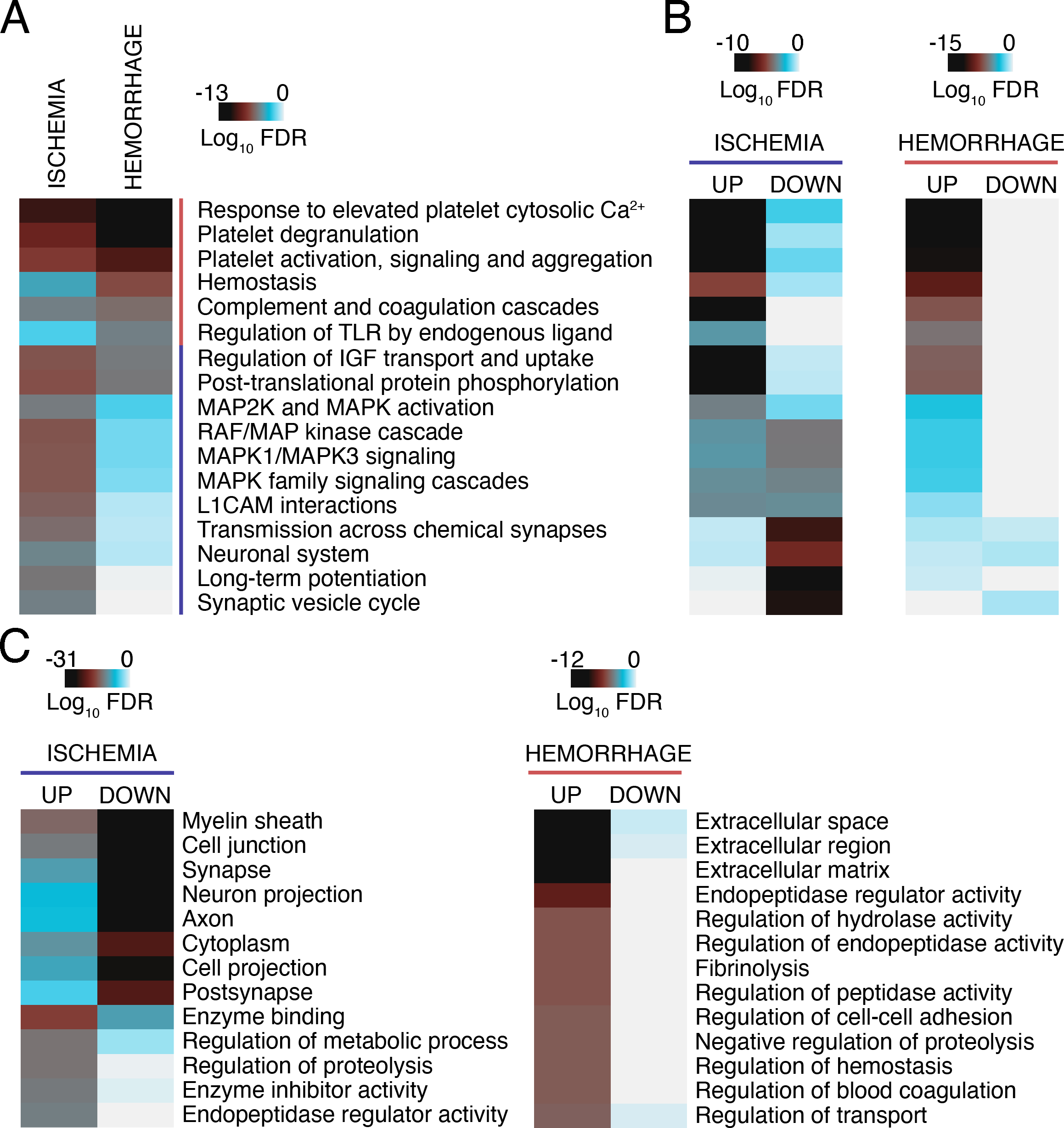
Heatmaps showing (A) (B) KEGG and Reactome pathways and (C) GO terms significantly enriched among DEPs in animal brain tissue after IS and HS. Datasets were derived from the following studies: Ischemia4–8,11–16,22–24,30,32,33,36–38,55,60,71, ICH6,39–41,50. Only DEPs that were identified in at least 2 proteomics studies were considered for analysis (Table S3, n=293 for ischemia and n=47 for ICH). Functional enrichment analysis was performed using g:Profiler166. Benjamini-Hochberg FDR-corrected P values were plotted on a Log10 scale. P values were considered significant for (A) (B) P<10−5 and (C) ischemia: GO-MF P<10−12, GO-BP P<10−13, GO-CC P<10−20; ICH: GO-MF P<10−7, GO-BP P<10−5, GO-CC P<10−9. FDR, false discovery rate.
2.1.1. IS - Acute phase
DEPs identified via proteomics using rodent tissue obtained after IS and 1–12h reperfusion confirm altered transcription, translation, protein phosphorylation, organelle function, and neurotransmission in this early phase after ischemia. For example, middle cerebral artery occlusion (MCAO; a commonly used IS model in animals) and 1 hour (h) reperfusion in mice significantly changed the abundance of histones, RNA-binding proteins (FXR2, FUS, SFPQ), ribosomal subunits, kinases (PKC, ITPKA, adenylate kinase-5), calcium-binding proteins (annexin-2, annexin-6), and ion channels (SLC6A11, VDAC1) in the ischemic penumbra4. Another proteomics study reported a similar trend toward upregulation of kinases (PKA, PKC), and RNA-binding and transcription factors (STAT6, PRP16, FUS), whereas neuronal proteins (PSD95, MAP2), and proteins associated with cellular adhesion and vesicular transport (clathrin heavy chain, Ras-related protein, paxillin) exhibited downregulation after MCAO and 0, 3, and 12h reperfusion in mice5. A third study performed in rats confirmed differential regulation of kinases (CAMK2A, phosphoglycerate kinase-1, PKC) and synaptic regulators (synaptophysin, synapsin-2, NSF, MAP6), as well as metabolic enzymes, such as adenosine kinase-1, adenosyl homocysteinase, alcohol dehydrogenase-1, and glutamate dehydrogenase-1, in the ischemic hemisphere after MCAO/3h reperfusion6. After MCAO/0.5h reperfusion, similar DEPs as in the penumbra were detected in the mouse ischemic core, with particular emphasis on mitochondrial respiration (NDUFA1, NDUFS5, UQCRQ) and vesicular transport (VAMP2, ATP6V1F)7. Histones, however, were not altered in the core7. Two DEPs from this study, GADD45G and catenin δ−2, were increased in circulating blood of human stroke patients, where they might prove promising as stroke prognostic and diagnostic biomarkers7.
Proteomics executed after 24–48h of permanent or transient ischemia in rodents identified DEPs that reflect post-stroke alterations in protein homeostasis, energy metabolism, and synaptic function/plasticity. 24h of MCAO significantly upregulated DRP2, HSC70-ps1, and SPNA-2 fragment in the ischemic penumbra in rats8. DRP2, a protein governing axonal growth, and synaptic plasticity and maturation9,10, was upregulated after 24h of MCAO in several other proteomics studies in rats11–15, presenting itself as a reliable marker for the ischemic brain. Interestingly, DRP2 upregulation appears to be restricted to MCAO without reperfusion, suggesting a normalizing effect of blood flow restoration. In contrast, proteomics found elevated SPNA-2 fragment levels in rodents after MCAO with and without reperfusion8,16. Fragmentation of SPNA-2, a protein required for maintaining neuronal membrane integrity, indicates ischemic stress and foreshadows neuronal cell death17. The products of SPNA-2 breakdown were also detected in the cerebrospinal fluid (CSF) of MCAO-treated rats18,19 and in patients with subarachnoid hemorrhage20, demonstrating the potential of SPNA-2 as a biomarker for IS and HS.
Heat shock proteins (HSPs) are well-known to be impacted by ischemia in the acute phase21, a finding that proteomics studies confirm. Both HSC70 and HSP60 were elevated after 24h of MCAO in the rat cortex8,11, while HSP90 and HSPA5 were decreased after MCAO and 24h reperfusion in the rat peri-infarct area22. Additionally, HSPs, specifically the inducible form of HSP7023, as well as HSP10 and HSP7016, were increased in rats after 48h MCAO with and without reperfusion. Proteomics also found that DRP2 and HSC70, among others, were increased in pituitary glands in rats after 24h of MCAO24, suggesting a common response to injury in cortical and neuroendocrine systems.
Thioredoxin, peroxiredoxin-2 and UCH-L1 are DEPs commonly detected by proteomics as downregulated in rodents in the acute stroke phase11,16. Lower levels of thioredoxin and peroxiredoxin-2, two key molecules in combating ischemic oxidative stress25,26, and UCH-L1, a deubiquitinase implicated in neuroprotection27, may actively exacerbate ischemic cell death. However, on the other hand, reduction in these and other identified proteins could also be only a consequence of already progressed tissue damage and death. While intracellular UCH-L1 decreased after IS and traumatic brain injury (TBI), elevated levels were found in the CSF of rats, warranting further investigation as a biomarker for certain types of brain injury19,28. Indeed, a serum biomarker test combining measurements of UCH-L1 and GFAP within 12h of head injury was recently approved by the FDA for diagnosing mild TBI29. Notably, levels of some DEPs, such as SPNA-2 fragment, HSP10, and HSP70, were altered regardless of occlusion time (0.5h vs 2h MCAO), while reduction in UCH-L1 and HSP90 was exclusively seen with longer occlusions16, potentially representing markers for ischemia severity. Other notable DEPs identified by proteomics in the acute phase in the ischemic penumbra are tropomodulin-2, calmodulin-1, superoxide dismutase-1, synapsin-1, MDH2, stathmin, and apolipoprotein-A18,11,22.
Proteomic analysis of the ventricular zone, which primarily contains migrating neurons, radial glial cells, and progenitor cells, revealed significantly increased levels of antiproteases and cytokine/growth factor-binding proteins, including α−2-macroglobulin, albumin, apolipoprotein-A1, SERPINA3K, and transferrin, in mice after 4h of MCAO30. Interestingly, in the penumbra these protein classes tend to be altered in the subacute and chronic phases of ischemia, which is described in the next section.
2.1.2. IS - Subacute and chronic phases
Proteomics of the subacute and chronic phases of ischemic injury identified DEPs that potentially modify post-stroke synaptic function, lysosomal activity, and the inflammatory response, which plays a critical role in late-stage stroke pathophysiology31. After MCAO followed by 7 or 14d reperfusion, cathepsin and LAMP2 levels were increased in the ischemic penumbra of rodents, as were plasma, and complement and immune mediators such as complement C3, C-reactive protein, albumin, fibrinogens, annexin-2, transferrin and α−1-antitrypsin32. This suggests enhanced lysosomal activity and an activated inflammatory response. Proteomics and subsequent immunohistochemistry of the rat cortex after MCAO with 3 or 14d reperfusion showed increased expression of annexin-3, specifically in microglia33, revealing a new marker that has since been used to detect activated migrating microglia in the ischemic penumbra34. Large-scale analysis of the cortical proteome following permanent distal hypoxia-MCAO in mice identified many secreted DEPs such as apolipoprotein-E, α−2-macroglobulin, albumin, SERPINA3K and apolipoprotein-A1, at 1, 3, 7, 14, and 28d35, again indicating affected immune responses, coagulation, and plasmin signaling. A similar DEP profile was discovered in the mouse cortex after MCAO and 0, 6, 12, or 24h reperfusion36. Identified proteins included serpins, fibrinogens, albumin, apolipoprotein-A1, C3, S100A9, transthyretin, and transferrin, which were upregulated as early as 6h after onset of reperfusion. After global ischemia in mice, induced by bilateral common carotid artery occlusion (BCCAO), and 72h reperfusion, cortical, hippocampal, and striatal proteins involved in inflammation and oxidative stress (apolipoprotein-E, SERPINE2, clusterin, cystatin-C) were increased, while proteins regulating synaptic function (α-synuclein, synaptopodin, CAMK2A) were decreased37. Clusterin and cystatin-C were also elevated in plasma of IS patients within 24h of stroke onset37, warranting further investigation as blood biomarkers for acute IS. Substantiating rodent studies, proteomics revealed similar DEP classes in the penumbra of nonhuman primates after MCAO and 28d reperfusion38.
2.1.3. ICH – primary and secondary injury phases
ICH led to a significant upregulation of plasma proteins in the parenchyma of rodent brains. DEPs such as albumin, α−2-macroglobulin, apolipoprotein-A1, C3, fibrinogens, hemoglobin, haptoglobin, hemopexin, ITIH3, plasminogen, S100A9, and serpins were elevated in the primary injury phase after 3h6, and in the secondary injury phase at 24h39, 48h40, and 72h41 in rats and mice, attesting to an early and prolonged reaction to ICH. Parenchymal apolipoprotein-A1, albumin, α−2-macroglobulin, and serpins may contribute to hematoma clearance through heightened hemostasis and platelet degranulation, while elevated C3 and hemoglobin aggravate brain injury through excessive recruitment of immune cells42 and production of cytotoxic heme and iron43, respectively. Induction of hyperglycemia in rats led to augmented neuronal apoptosis 12h and 24h after ICH, which was accompanied by a reduction in albumin and other DEPs44. Low serum albumin levels were associated with poor functional outcome in patients with SAH, ICH, and IS45–47 by increasing the risk of for infections and directly aggravating brain injury48,49. The role of other identified plasma DEPs in ICH injury development remains unclear, and requires further assessment.
Contrary to plasma mediators, levels of intracellular proteins were reduced after ICH. Metabolic enzymes such as alcohol dehydrogenase-1, ATP-citrate synthase, ALDOC, glutamate dehydrogenase-1, lactoylglutathione lyase-1, L-lactate dehydrogenase-A, PFKM, and transketolase exhibited decreased levels at 3h after ICH, suggesting impaired metabolic performance early after the insult. The dysregulated metabolism was maintained after 48h and 72h40,41. Most enzymes were still decreased (e.g., ALDOC, L-lactate dehydrogenase-A, GOT1, ECHS1, NDUFA9, UQCRB, ATP5C1), while some were increased (e.g., ATP1A1, carbonic anhydrase-1, ITPKA, PFKM, copine-7). This demonstrates a bidirectional regulation of energy metabolism at this stage after ICH. Other intracellular protein classes with altered abundance in rodent and pig brain tissue after ICH included septins, neurofilaments, solute carriers, serine-threonine protein phosphatases, kinases, proteasomal subunits, HSPs, and synaptic proteins, among others6,39–41,50.
2.1.4. IS – cytoprotection
Proteomic analyses of tissue from animals protected from ischemic insult through genetic advantage, preconditioning or treatment with a cytoprotective agent aim to identify proteins that aid in protection from injury. We performed GO enrichment analysis of DEPs changed in vulnerable and protected tissue after focal IS in at least two studies (listed in Table S3, sheet 2). Protection led to a distinct upregulation of DEPs associated with preservation of neuronal and synaptic integrity, while largely normalizing changes observed in the vulnerable condition (Figure 3). The potential role of select DEPs in cytoprotection is described below.
Figure 3.
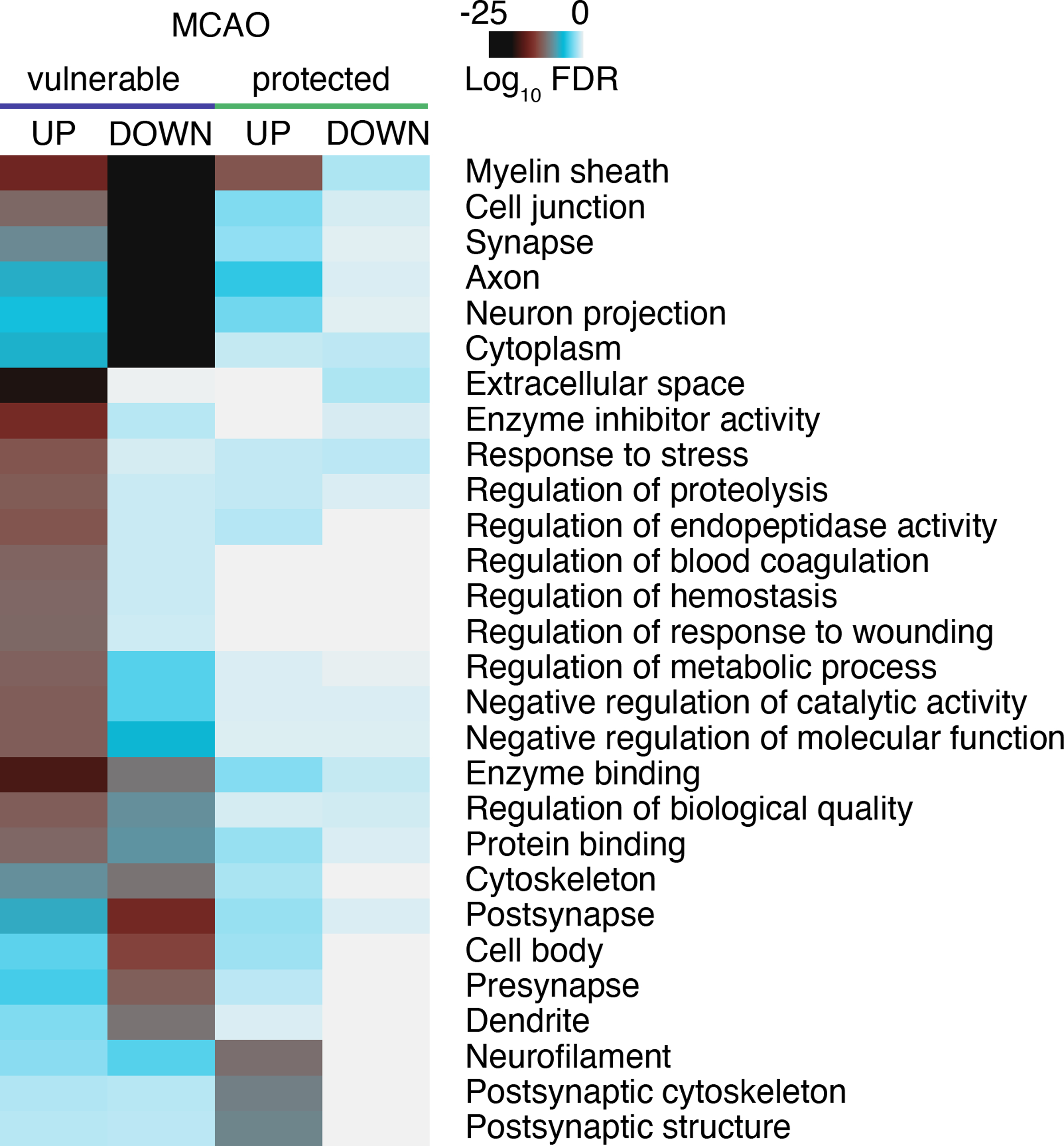
Heatmap comparing GO terms significantly enriched among post-stroke DEPs in animal brain tissue vulnerable to and protected from focal IS (MCAO). Datasets used for analysis were from the following studies: Vulnerable4–8,11–16,22–24,30,32,36,38,55,60,71, protected55,60,64,67,70,71,73. Enrichment analysis and data plotting were performed as in Figure 2. P values were considered significant for GO-MF P<10−8, GO-BP P<10−10, GO-CC P<10−8; n=98 for vulnerable UP, n=135 for vulnerable DOWN; n=8 for protected UP, n=7 for protected DOWN). FDR, false discovery rate; MCAO, middle cerebral artery occlusion.
2.1.4.1. Estrogen
Females are protected from IS during their reproductive years. Proteomics from ovariectomized female rats pretreated with vehicle or estradiol found that estradiol prevented the increase in HSP60 levels after 24h of MCAO observed in vehicle controls51. Meanwhile, PEA15 and PPP2A were elevated in estradiol-treated vs non-treated animals. In PEA15 knockout mice susceptibility of astrocytes to TNF-induced apoptosis was increased52; hence, reduced PEA15 could favor stroke-mediated apoptosis, while decreased PPP2A may contribute to ischemic injury by increasing pTau53. Interestingly, decreased HSP60 and increased PEA15 and PPP2A were already present in estradiol-treated animals before MCAO51, indicating that they could underlie the mechanism that protects females against stroke.
Additionally, estradiol protects against ischemia by increasing neuronal Stat3 activity54. To identify potential mediators of this effect, proteomics analyzed male and female neuronal Stat3 wild type and knockout mice after MCAO and 4d reperfusion. Stat3- and sex-specific DEPs consisted of metabolic (ACO2, superoxide dismutase-2, γ-enolase), synaptic (α-synuclein, synapsin-2), structural (14–3-3 protein β/α, profilin-2, DRP2), and transcriptional (Pur-α, BASP1) regulators55. Their involvement in the Stat3-dependent neuroprotection in females, however, has not yet been determined. One caveat of this study is that the proteome was assessed 4d after MCAO, thus differing extents of cell death in experimental groups could confound results.
2.1.4.2. Ischemic preconditioning
Exposure to a short episode of ischemia induces protection against a subsequent severe ischemic insult. Proteomic changes in response to ischemic preconditioning could explain the molecular mechanisms underlying the induced tolerance. At 24h after 10min sublethal MCAO, HSP70 and HSP27, whose overexpression results in less ischemic brain damage56,57, were upregulated in the rat cortex58. Other proteins that increased by preconditioning were guanylyl cyclase, PTAFR, β-actin, and muskulin-158. Guanylyl cyclase knockout mice exhibited increased infarct volume after MCAO59, hence upregulated guanylyl cyclase is expected to attenuate stroke injury. Noteworthy, the above study was executed in spontaneously hypertensive rats, and the extent to which hypertension influenced the proteome is unclear.
Another proteomics study found a distinctive increase in transcriptional repressors, particularly in polycomb group (PcG) proteins, in tolerant vs vulnerable mice after MCAO and 24h reperfusion60. This is one of the rare proteomics studies in which an effect of the identified proteins on outcome was confirmed. PcG protein knockdown precluded induction of ischemic tolerance, whereas exogenous overexpression conferred tolerance without the preconditioning stimulus. PcG proteins conferred protection by decreasing abundance of potassium channels, whose activity is detrimental to stroke outcome60. In addition to PcG proteins, ischemic preconditioning also impacted levels of certain ATPase subunits, histones, kinases, phosphatases, and Ras-related proteins, all with currently unknown importance in preconditioning.
2.1.4.3. Pre-injury treatment with cytoprotective agents
Pretreatment with the non-erythropoietic derivative asialoEPO 4h before insult led to robust protection against hypoxia-ischemia in neonatal rats61. Proteomics showed pre-insult induction of cortical p-SNAP2561, which may contribute to post-ischemic neuroprotection by priming SNARE complex formation and vesicle exocytosis62,63.
Fenofibrate and atorvastatin are hypolipidemic, neuroprotective drugs with pleotropic and partly overlapping functions involving PPARα induction. Administering these drugs 14d before MCAO in rats affected pre- and post-ischemic cortical levels of α-synuclein, 14–3-3 protein ζ/δ, and protein disulfide-isomerase64, which could confer protection by preserving synaptic and endoplasmic reticulum (ER) function.
2.1.4.4. Post-injury treatment with cytoprotective agents
Nicotinamide, a B3 vitamin and precursor to NAD+, protects against ischemic injury by acting as PARP inhibitor and scavenging of free radicals. Like in females51, proteomics found that, administration of nicotinamide 2h after occlusion prevented the increase in HSP60 and decrease in PEA15 and PPP2A observed in vehicle-treated rats after 24h of MCAO14. The same alterations of HSP60, PEA15 and PPP2A levels were also reported after ferulic acid treatment in rats after 24h of MCAO13. In addition to these proteins, nicotinamide increased cortical levels of NSE14; however, the meaning of this increase is unclear due controversial findings regarding post-stroke NSE tissue levels16,32. Ferulic acid on the other hand restored cortical levels of MAP2K1, GAPDH, IDH2 and adenosyl homocysteinase13, which could provide protection by maintaining energy metabolism and preserving adenosine levels.
Post-injury application of pituitary adenylate cyclase activating polypeptide (PACAP)38, a neuroprotective peptide resembling a hypothalamic hormone, promotes an increase in DRP2 levels at 6h rather than at 24h of MCAO in mice8,11–15,24,65, which could accelerate neurorepair9,10.
Post-treatment with the anti-inflammatory and antioxidant compound resveratrol restored cortical UCH-L1 levels in the rat after 24h of MCAO12, which could directly contribute to protection via promoting axonal repair66. Additionally, it restored peroxiredoxin-5 and IDH levels12, potentially relieving oxidative and mitochondrial stress.
Proteomics assessment after treatment with simvastatin 15min after occlusion revealed elevated GNAO1 and MAPRE, and lower HSP60, HSP75, and fumarate hydratase after 48h of MCAO67. HSP75 was also reduced in plasma of simvastatin-treated patients on days 3 and 5 after stroke67. While it may show promise as a biomarker, reduced HSP75 is not likely to have a beneficial effect on injury development, as HSP75 overexpression proved protective against ischemic injury68. Rather, decreased HSP75 may reflect reduced injury resulting from a mechanism involving another affected protein such as GNAO1. Since GNAO1 plays key roles in transducing GPCR signals to regulate neuronal excitability69, restoring GNAO1 function to improve stroke outcome should be further explored.
Proteomics revealed that post-treatment with rosiglitazone, a PPAR-γ-dependent neuroprotectant, in rats restored 14–3-3 protein ε levels after MCAO and 24h reperfusion70. This is one of the few studies with follow-up showing that the identified protein indeed underlies protection. While 14–3-3 protein ε siRNA knockdown abrogated rosiglitazone-dependent protection, its overexpression was sufficient to alleviate infarct volume without treatment70.
Administration of the anticoagulant t-PA 4h after occlusion in rats altered cortical levels of many proteins involved in excitatory neurotransmission and signaling after 24h reperfusion71. Examples include glutamate decarboxylase-2, EAAT1, neurofilaments, MAPT, CAMK2D, Ras-related protein-10, and α-internexin. These changes may underlie some of the neurotoxic effects of t-PA seen after ischemia72.
Treatment with the reactive oxygen species (ROS) scavenger tetramethylpyrazine nitrone (TBN) 3h after MCAO in nonhuman primates conferred neuroprotection, which on the proteomic level, was associated with reduced mitochondrial and redox-sensitive proteins (e.g., COX5B, superoxide dismutase-1, and peroxiredoxin-2) and elevated cytoskeletal and tight junction proteins (claudin-11, MBP, and neurofilaments) at 28d reperfusion73.
2.1.4.5. Hypothermia
Hypothermia is one of the best-studied protective therapies for brain ischemia. Proteomic changes in response to hypothermia in rats included normalization of cortical DRP2 levels after 24h of MCAO15, with unknown consequences. Hypothermia also increased availability of BAIAP2L1 and profilin-2, perhaps to stabilize the cytoskeleton, and MAP2K1 and PPIA, to support metabolic and anti-apoptotic functions15. Proteomic changes in this study were observed only in the ischemic penumbra, not in the infarct core, signifying that protection of the salvageable area of the brain may involve one or more of the identified proteins.
2.1.5. IS and preconditioning - changes in protein interactions, modifications, and aggregation
Protein function is regulated not only by changes in its levels but, also by its interaction profile with other proteins, its subcellular localization, and its conjugation with posttranslational modifiers. Identifying such alterations in response to a preconditioning stimulus or stroke may increase our mechanistic understanding of stroke pathophysiology and aid in finding therapeutic targets. In this section, we describe studies that used affinity purification-coupled MS (AP-MS) to analyze preconditioning- and stroke-dependent changes in protein interaction complexes of PKC, PPP1 and 4E-BP2, PTMs such as SUMOylation and ubiquitination, and aggregation propensity of proteins.
2.1.5.1. Protein interactions
PKC and PPP1 activities are essential for hypoxic and ischemic preconditioning74–77. Several proteomics studies were executed to decipher the interactome around these important proteins in the presence and absence of preconditioning. PKCβ and ε bound to 49 and 39 proteins, respectively, in cortices of control and hypoxic preconditioned mice, revealing an extensive interaction network75,76. Notably, β and ε PKC showed a distinct, largely non-overlapping interaction profile, reflecting different roles. In response to preconditioning, ten proteins were differentially bound to PKCβ. Their identity predicted a role for PKCβ in preconditioning by altering energy metabolism, oxidative stress, protein folding and degradation, and axon guidance75. DRP2 was confirmed to be a novel PKC phosphorylation target75. Preconditioning led to increased DRP2 binding to PKCβ localized at the membrane, thereby preventing DRP2 dephosphorylation and cleavage after ischemia, which could enhance axonal resistance to neurotoxicity78. Eight proteins exhibited altered binding to PKCε after preconditioning (i.e., triosephosphate isomerase-1, HSC71, GAPDH, HSP60, GRP78, 14–3-3 protein γ, Pur-α, and ACO1)76. Potential effects of any of these changes on PKCε-dependent preconditioning have not yet been determined. According to current knowledge, PKCε-dependent preconditioning is achieved through positive regulation of mitochondrial respiration79.
AP-MS proteomics identified PPP1α- and γ-interacting proteins that significantly changed association in response to ischemia and ischemic tolerance produced by ischemic preconditioning80. In the ipsilateral cortex, ischemia altered interaction of PPP1α and γ with overlapping and distinct proteins. While both isoforms exhibited altered binding to HSC70 and DARPP32, changes in association with PDE6B, VCP, GRP78, neurofilament light polypeptide, DRP2, prelamin-A/C, γ-enolase, β-actin, and SIAH2 were specific to PPP1γ. Preconditioning restored some ischemic changes to control levels, while newly affecting others80. GRP78, neurofilament light polypeptide, and DARPP32 were known regulatory components of PPP181, whereas PDE6B, creatine kinase-β, and SIAH2 were novel interactors that remain unexplored. Only VCP binding to PPP1 has been independently confirmed82.
Cerebral ischemia causes translational arrest through binding of 4E-BP2 to eIF4E, inhibiting its function83. To reveal other potentially important proteins in this regulation, 4E-BP2-interacting proteins were identified by proteomics in the ischemia-resistant cortex and vulnerable hippocampus in sham and BCCAO animals84. Interestingly, the 4E-BP2 interaction profile in the ischemia-resistant cortex compared to the susceptible hippocampus was already vastly different at baseline, which could explain differing vulnerabilities of these areas. Of note, HSC70, DRP2, α-enolase, UCH-L1, adenosine kinase-1, GAPDH, and phosphoglycerate kinase-1, maintained their region-specific interaction with 4E-BP2 after ischemia84.
While proteomic association studies led to the discovery of many potential new regulators of PKC, PPP1, and 4E-BP2, follow-up studies on the functional implications of modified interactions in the context of ischemia and preconditioning are lacking.
2.1.5.2. PTMs and protein aggregation
In the acute phase, cerebral ischemia activates nuclear small-ubiquitin-like modifier (SUMO)2/3 conjugation, which is associated with neuroprotection85–87. To uncover molecular mechanisms that link nuclear SUMOylation to protection, targeted proteins were identified in the ischemia-resistant cortex after BCCAO and 1h reperfusion in SUMO-transgenic mice, using a SUMO-enrichment and proteomics approach88. Ischemia induced SUMOylation of 91 proteins, among them many RNA (heterogenous nuclear ribonucleoproteins, RBM14, DEAD-box helicase-5, and DExH-box helicase-9) and transcriptional (glucocorticoid receptor, TFII-I, TIF1B, and CTIP2) regulators, supporting a critical role for transcriptional and translational regulation in SUMO-mediated neuroprotection.
Like SUMOylation, ubiquitin conjugation is activated in the acute phase of ischemia89,90. However, in contrast to SUMO, the significance of ubiquitination in post-ischemic cell fate remains undetermined. To assess potential functions of ubiquitination, the ubiquitinated proteome was analyzed by ubiquitin-enrichment and subsequent proteomics in hippocampi of mice after BCCAO and 4h reperfusion91. Compared to sham, 272 proteins exhibited increased ubiquitination, including many synaptic regulators such as CAMK2A, PKC, creatine kinase-β, GRIN2B, DLGAP1, NSF, and SynGAP1, likely impacting synaptic function. Additionally, some translation factors (eIF4H and eEFA1) and chaperones (HSPA12A and DNAJA1) were highly ubiquitinated, potentially regulating the reported translational arrest after ischemia3.
Ubiquitin attaches to substrates as monomers or chains, and the conjugation type determines the impact of ubiquitin modification on protein function92,93. Proteomics found that ischemia induced ubiquitination via lysine 6-, 11-, 48-, and 63-conjugated chains91, indicating multiple involved mechanisms and regulation of a wide range of cellular processes. Notably, post-ischemic ubiquitinated proteins are predominantly detergent-insoluble89,91, suggesting sequestration into potentially toxic aggregates. This has not yet been verified.
Ischemia affects solubility of proteins in the acute phase, as found by proteomics of detergent-insoluble material obtained from cortices of mice after MCAO and 1hr reperfusion94. After ischemia, 196 proteins exhibited increased insolubility, the largest group being associated with RNA processing, including TDP43, FUS, HNRNPA1, NONO, and SFPQ. Accumulation of such proteins is commonly linked to neurodegenerative diseases (NDs), where it is terminal94. However, it is transient after ischemia if reperfusion occurs94. This indicates a potential functional role for RNA-binding protein sequestration following ischemia, instead of foreshadowing cell death. Other proteins with increased insolubility after ischemia were HSPs, ubiquitin, SUMO, eIF4, and kinases PKC and CAMK2. The mechanism by which these proteins become insoluble awaits to be established but could be related to protein aggregation or simple change in subcellular localization.
2.2. Human studies – IS
To date, several proteomics studies, published mostly by the Montaner group, have identified approximately 190 DEPs in postmortem human brains at different stroke stages (Table 1 and Table S4). For data interpretation, effects of high patient heterogeneity and potential changes in pathways after death must be considered. First, DEPs were assessed by global proteomics in the contralateral hemisphere, peri-infarct area, and infarct core of postmortem brains from three patients with MCAO and compared to three controls without any apparent neurologic condition95,96. In the infarcted tissue, 51 DEPs were identified. GFAP and albumin were increased in infarct and peri-infarct areas, likely reflecting astrogliosis and trapped blood or blood-brain barrier (BBB) damage, respectively. Other DEPs of interest include plasma proteins (C3, fibrinogens, and transferrin), vesicle transport proteins (NSF, GDI, and ARHGDIA), HSC70, gelsolin, and DRP2, all of which were also altered in infarcted animal brain tissue after MCAO7,8,11–16,23,24,30,32,36,38. Interestingly, gelsolin, cystatin-A, and DRP2 were changed not only in the brain parenchyma, but also in blood from stroke patients, where altered levels were associated with long-term outcome96.
Table 1.
Proposed biomarkers for stroke diagnosis and prognosis discovered by proteomics in patients. A comprehensive list of identified DEPs from each study can be found in Tables S4 (tissue) and S6 (biofluids).
| Samples | Analysis cohort (n) | Sampling time | Methods | Major findings | Samples | Blood biomarker utility | Parameters (Cutoff, sensitivity, specificity) | Ref. |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| PROTEOMICS DISCOVERY | BIOMARKER EVALUATION | |||||||
|
| ||||||||
| Blood | Blood | |||||||
|
| ||||||||
|
Plasma healthy, IS, HS |
healthy (n=21), IS (n=11), HS (n=10) | At admission | Protein chip, Gel-based MS | DEPs: ApoC-I, ApoC-III, SAA, AT-III (IS vs HS) | 6h after symptom onset, healthy (n=12), IS (n=16), HS (n=15) | Diagnosis: distinguish between IS and HS | ApoC-I (RFU, 94%, 73%), ApoC-III (RFU, 94%, 87%) | 140 |
|
Serum TIA, minor IS, mimics |
TIA (n=20), minor IS (n=15), mimics (n=12) | Within 48h of symptom onset | TMT, LC-MS/MS | DEPs: CP, C8γ, PBP (TIA/minor IS vs mimics) | Within 48h of symptom onset, TIA (n=22), minor IS (n=20), mimics (n=14) | Diagnosis: distinguish between TIA/minor IS and mimics | PBP (1.5μg/ml, 91%, 57%) | 137 |
|
Serum healthy, IS |
pooled samples each n=20 | Within 24h of symptom onset | iTRAQ, LC-MS/MS | 60 DEPs (IS vs healthy) | Within 24h of symptom onset; IS (n=50), healthy (n=35) | Diagnosis: distinguish between IS and healthy | ↑ vWF (N/A, 90%, 83%), ↓ ADAMTS13 (N/A, 98%, 90%), ↓ S100A7 (N/A, 97%, 91%) | 132 |
|
Serum TIA, minor IS, mimics |
TIA/minor IS suspected (n=545) | Within 24h of symptom onset | LC/MRM-MS | 16 DEPs (TIA/minor IS vs mimics) | Within 24h of symptom onset; TIA/minor IS (n=811), mimics (n=292) | Diagnosis: distinguish between TIA/minor IS and mimics | IGFBP-3, PON3 are promising TIA predictors but failed to achieve clinical relevance | 138,139 |
|
Whole blood IS, HS, mimics |
IS (n=19), HS (n=17), mimics (n=20) | At admission | Peptide array | Altered circulating antibody pool (IS/HS vs mimics) | Same cohort | Diagnosis: distinguish between IS/HS and mimics; between IS and HS | Model with 17 peptide probes: IS/HS vs mimics (N/A, 92%, 90%); IS vs HS (N/A, 93%, 90%) | 134 |
| Serum IS | IS (n=21) | 5 timepoints from imaging to 90d after IS | SELDI-TOF-MS/MS | 23 DEPs with significant change over time | Correlation analysis performed with proteomics data | Prognosis: vWF is associated with poor outcome (3mth) | N/D | 145 |
| Plasma healthy, IS with LVO | healthy (n=20), LVO IS (n=40) | Within 7d of symptom onset | iTRAQ, LC-MS/MS | DEPs: THBS1, LYVE1, IGF2, PPBP, SPP2, CDH1, APOC4-APOC2 | Within 7d of symptom onset, healthy, non-LVO IS, LVO IS (n=33/group) | Prognosis: ↑ IGF2, LYVE1, ↓ THBS1 are associated with favorable outcome (3mth) | N/D | 131 |
|
Plasma IS with LVO |
IS with LVO (n=40) | At the time of thrombectomy | Antibody-based | DEP: VCAM1 | Correlation analysis performed with proteomics data | Prognosis: ↑ VCAM1 is associated with poor outcome on discharge | N/D | 157 |
|
Serum IS w/o reperfusion therapy |
HT (n=5), non-HT (n=32) | At admission | iTRAQ, LC-MS/MS | 17 DEPs (e.g., A1M, FBLN1) (HT vs non-HT) | Correlation analysis performed with proteomics data | Prognosis: DEPs alone or in combination could predict HT | N/D | 143 |
|
HDL healthy, IS |
healthy (n=35), IS 24 and 96h post intervention (n=35, n=20) | 24 and 96h post intervention | PRM-MS/MS | 12 DEPs (e.g., APOE, CLU, SAA2, APOF) (IS vs healthy) | Correlation analysis performed with proteomics data | Prognosis: APOF, APOL1 levels correlate with recovery (3mth) | N/D | 147 |
| Other biomaterials | Blood | |||||||
|
CSF healthy, deceased |
healthy, deceased (each n=4) | Unspecified | 2-DE-MS | DEP: H-FABP (decreased vs healthy) | 40min to 3d after symptom onset, healthy (n=22), MI (n=20), IS (n=22) | Diagnosis: distinguish between stroke and healthy controls | H-FABP (OD405=0.531, 68.2%, 100%) | 123 |
|
ECF IS with malignant MCA infarction |
IS (n=6) | Within 24h after reperfusion | TMT, MALDI-TOF/TOF MS | 53 DEPs (e.g., UCH-L1, APOA4, UBIQ, AQP4) | Within 24h of symptom onset, healthy (n=14), IS (n=12), HS (n=2) | Diagnosis: ↑ GSTP1, S100B, PRDX1 distinguish between stroke and healthy controls | N/D | 125 |
| Thrombi IS with LVO | IS with LVO treated by mechanical thrombectomy (n=41) | 210–344min after IS | LC-MS/MS | DEPs: glycophorin-A, fibrinogen (cardioembolic vs atherothrombotic) | ESUS (n=12) | Diagnosis: distinguish between cardioembolic and atherothrombotic IS | Model with 40 parameters selected from 2456 proteomics and 5019 metabolomics features (N/A, 100%, 85.7%) | 149 |
|
Tissue (infarct, peri-infarct) healthy, IS |
healthy, IS (each n=3) | 6h postmortem | 2-DE-MS | 51 DEPs (e.g., FGG, CSTA, LDHA) (infarct core vs peri-infarct) | Within 4.5h of symptom onset, IS (n=60) | Prognosis: combination of ↑ GELS, CYTA, ↓ DRP2 predicts poor long-term outcome (3mth) | GELS (19.87ng/mL, 71.8%, 76.5%), CYTA (31.76ng/mL, 26.8%, 100%), DRP2 (2.09ng/mL, 97.1%, 28.6%) | 96 |
| Neurons + BBB (infarct, contralateral) IS | IS (n=7) | 4–9h postmortem | LC-ESI-MS/MS | 30 DEPs (e.g., SH3G2, EAA2, SRSF1, SAHH2) (infarct vs contralateral) | Within 6h of symptom onset, healthy (n=8), mimics (n=13), IS (n=45) | Prognosis: ↓ SAHH2 predicts better neurologic outcome at 24 and 48h after IS | SAHH2 (993.23pg/mL, 89%, 58%) | 97 |
Abbreviations: CSF, cerebrospinal fluid; DEP, differentially expressed protein; ECF, extracellular fluid; ESUS, embolic stroke of undetermined source; HS, hemorrhagic stroke; IS, ischemic stroke; LVO, large vessel occlusion; MCA, middle cerebral artery; MI, myocardial infarction; N/D, not determined; RFU, relative fluorescence unit; TIA, transient ischemic attack.
Subsequently, laser microdissection was combined with label-free MS proteomics to analyze cell-specific proteomes of neurons and BBB structures isolated from the infarct area and contralateral regions of human IS brains97. This analysis identified 30 DEPs with little overlap to previous studies, including SAHH2, PACN1, and SRSF1 in neurons, and neuromodulin, PKCγ and SIRT2 in the BBB. The distinct DEPs identified in this study likely derive from different starting material, comparison group, and/or proteomics approach used in previous studies. In a cohort of patients, the authors found that lower circulating levels of SAHH2 at admission was an independent predictor of neurologic improvement after 24h97. However, there was no follow-up stroke biomarker study on SAHH2.
Recently, a combined proteomics and transcriptomics approach was implemented to identify DEPs in the infarct core vs contralateral hemisphere of six human postmortem stroke brains98. Only 60% of DEPs were equally regulated at both mRNA and protein levels, emphasizing the necessity of proteomics in the search for stroke therapeutic targets and biomarkers. Among the 66 DEPs, Ras-related protein-3C and sulfotransferase-4A1 were confirmed to be down-, and α−1-acid glycoprotein 1 and α−1-antitrypsin up-regulated in another cohort of brain samples. The increase in brain α−1-acid glycoprotein 1 and α−1-antitrypsin levels translated to a significant elevation of both proteins in blood from 11 acute IS patients treated with t-PA within the first 24h after stroke onset. Further exploration of these two proteins as biomarkers is needed.
Finally, a proteomics study not published by the Montaner group, used 8-plex iTRAQ labeling to quantitatively determine DEPs in the putamen, thalamus, and parietal lobe of postmortem human stroke brains99. Compared to location-matched controls, several proteins were increased in infarcted tissue, including vimentin, annexin-2, HSP70, MBP, and ferritin. All these DEPs were also altered in MCAO-treated animals4,5,23,32,71, indicating their involvement in stroke pathogenesis across both species. Additionally, all identified mitochondrial DEPs (e.g., SLC25A11, GOT2, and NDUFS1) were decreased in the infarcted tissue, signifying dysregulated mitochondrial function.
3. Proteomics assessment of biofluids: in search of stroke biomarkers
Stroke protein biomarkers would aid in stroke diagnosis, prognosis, and therapy efficacy evaluation (Figure 1). However, while protein biomarkers are routinely used in the clinic for some cardiovascular diseases (e.g., natriuretic peptides for heart failure), stroke biomarker discovery has not yet come to fruition. Most large clinical studies selected biomarker candidates based on current knowledge of stroke pathophysiology, which has had limited success. This was underscored by the Stroke-Chip study, which found that 21 commonly investigated biomarkers, including HSC70, NT-proBNP, matrix metalloproteinase-9, D-dimer, vWF, γ-enolase, S100B, interleukin-6, cellular fibronectin, and apolipoprotein-CIII, were unable to attain sufficient diagnostic accuracy in discriminating stroke from stroke mimics, even after combining with clinical variables100. Further, stroke subtype discrimination has been the subject of many clinical studies focusing on traditional biomarkers, of which GFAP currently is considered the most promising with excellent specificity to differentiate ICH (but not SAH) from IS101–103. However, its sensitivity to rule out ICH for thrombolytic therapy is a concern, and accompanying biomarkers are still needed. Some commonly studied biomarkers such as C-reactive protein104, neurofilament light polypeptide105,106, and C3107, have some predictive value for stroke outcome. None, however, acquired sufficient high-quality evidence with adequate performance to justify their clinical use. For recent general reviews on blood stroke biomarkers, see101,108.
Proteomics to assess post-stroke protein changes in body fluids, such as CSF, extracellular fluid (ECF), urine and blood, creates an advanced opportunity to identify novel biomarkers that are superior to the current candidates. While collecting CSF and ECF from patients is an invasive procedure, these fluids are attractive for biomarker discovery due to their proximity to the diseased brain. Blood is considered the best source for stroke biomarker discovery, as it is easily and non-invasively collected, and contains brain-specific, stroke-related proteins released from (dying) brain cells that enter the circulation once BBB integrity deteriorates. Urine is easy to obtain as well, contains a less complex proteome than CSF or blood, and has proven valuable for diagnosing heart disease and nephropathy109. Its utility in stroke diagnosis, however, is unclear.
More recently, stroke biomarker discovery has also been attempted in patient-derived blood clots and extracellular vesicles (EVs). With increased use of endovascular thrombectomy to treat stroke patients with large vessel occlusion (LVO), thrombi can be retrieved from living patients. This unique tissue sample could give essential information about stroke-related thrombogenesis and the origin of blood clot formation to diagnose IS subtypes (thrombotic vs embolic). EVs are present in various biofluids including blood, and carry proteins, nucleic acids, lipids, and metabolites from parent cells, thus providing information about diseased tissue as found in stroke110. Brain-derived EVs cross the BBB, and can be recovered from peripheral blood to analyze cargo.
In the following, we discuss proteomics studies for biomarker discovery in biofluids, blood clots, and EVs from stroked animals and human stroke patients. See Tables S1 and 1 for a complete list of animal and human studies, respectively, and Tables S5 and S6 for a listing of identified DEPs.
3.1. Animal studies
IS.
Only a few animal studies have examined post-stroke proteomic changes in biofluids from rodents and nonhuman primates (Table S1). While most of the ~900 identified DEPs require further assessment in humans to be considered potential biomarkers, some have been evaluated in stroke patients. One protein that was found altered in several rodent proteomics studies is transthyretin. Levels of transthyretin were increased in CSF111 and urine112, but decreased in plasma113 within 8–24h of MCAO or spontaneous IS. Low serum transthyretin levels were also observed in stroke patients within a 24h time window114. In addition to transthyretin, proteomics consistently identified DEPs connected to the inflammatory and coagulation response in blood112,113,115, urine112,115, and CSF112,115,116 after IS in rodents and nonhuman primates. Altered proteins in blood included C3, α−2-macroglobulin, haptoglobin, albumin, and transferrin, which were also modified in serum from stroke patients, where they may serve as prognostic tools for stroke outcome46,107,117–121. In the CSF, DEPs again included C3, α−2-macroglobulin, haptoglobin, and transferrin. Proteins related to immunosuppression and M1/M2 macrophage transition (adiponectin, coagulation factor XIII, α−1-acid glycoprotein 1, MBL2, and stabilin-1), angiogenesis (multimerin-2) and immunomodulation (immunoglobulin J-chain and IGLL1) were increased in CSF by pretreatment with the neuroprotective toll-like receptor (TLR)9 agonist D192935, potentially reflecting its therapeutic effects on the brain116.
Exclusively relying on inflammatory serum DEPs as stroke biomarkers in the clinic may be problematic due to their variability in response to many other conditions that affect the immune system. Therefore, it is important to include other proteins on a potential stroke biomarker panel. Using an aptamer-based proteomics approach, Simats et al identified 716 DEPs in rat CSF during the hyperacute stroke phase122. Among the top elevated proteins were many kinases such as creatine kinase-β, CAMK2A, CAMK2B, and CMPK. Creatine kinase-β and CMPK were also elevated in blood from human stroke patients within 6h of symptom onset, which could be useful for stroke diagnosis. While CAMK2B could not distinguish between stroke and control subjects, high plasma levels predicted poor outcomes in a cohort of stroke patients, as did CMPK122.
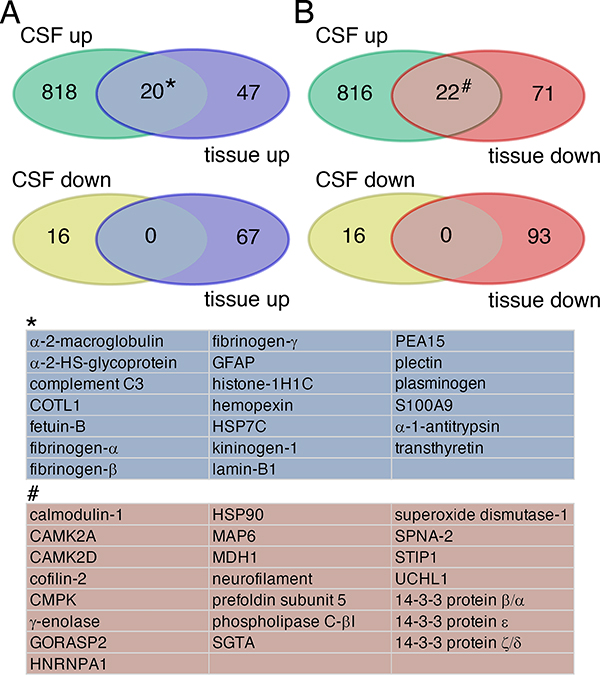
Although most identified inflammatory DEPs in CSF and plasma are extracellular by nature, some, such as kinases identified in this study, are normally localized intracellularly, and are likely derived from dying cells. By comparative post-analysis of animal tissue and fluid proteomics data (see Table S3, sheet 3, for a list), we found that increased presence of CAMK2 and CMPK in CSF and blood was accompanied by decreased tissue levels (Figure 4). The same was true for other common intracellular proteins found in fluids after stroke (γ-enolase, calmodulin-1, and UCH-L1). In contrast, elevation of “classic” plasma proteins, such as α−2-HS-glycoprotein, C3, and transthyretin, in post-stroke fluids correlated with an increase in tissue levels, likely indicating extravasation into the brain parenchyma (Figure 4).
Figure 4.
Venn Diagrams illustrating common DEPs after MCAO in animal brain tissue and CSF. (A) 20 DEPs in blue were upregulated in both tissue and CSF. They largely constitute “classic” plasma proteins. (B) 22 DEPs shaded in brown were decreased in tissue, while increased in CSF. They are mainly intracellular proteins. There is no overlap of proteins downregulated in CSF with any proteins detected in tissue. All DEPs used for this analysis are listed in Table S3. CSF, cerebrospinal fluid.
3.2. Human studies
3.2.1. CSF
IS and HS.
Proteomics comparing CSF between deceased and healthy subjects identified H-FABP as a marker of brain necrosis, which could be useful in diagnosing stroke with associated necrotic cell death123. Using quantitative measurement of H-FABP levels via ELISA in serum from healthy controls, as well as patients with hemorrhage, IS, and acute myocardial infarction, the authors were able to discriminate between the groups123. The specificity and sensitivity of serum H-FABP for stroke diagnosis were higher than for γ-enolase and S100B, two proteins that have been extensively assessed for this purpose101. In another study, targeted proteomics showed that γ-enolase and S100B, alongside five other proteins (GFAP, MBP, neurofilament medium polypeptide, α-internexin, and β-synuclein), were elevated in CSF from HS patients, where they were significantly higher than in controls and patients with IS124. Neurofilament medium polypeptide, α-internexin, and β-synuclein have not yet been evaluated as stroke biomarkers.
3.2.2. ECF
IS.
Proteomic analysis of ECF microdialysates from IS patients with malignant MCA infarction found 53 proteins that were increased in the ischemic penumbra and core compared to the unaffected contralateral side125. Several of these proteins, e.g., FABP, GFAP, S100B, and MBP, were also increased in CSF and blood after stroke123,124. Three biomarker candidate proteins, GSTP1, peroxiredoxin-1, and S100B, were confirmed elevated in blood in a cohort of stroke patients (n=14).
SAH and ICH.
Delayed cerebral vasospasm is a critical pathologic determinator of SAH outcome126, and identifying associated biomarkers would potentially aid in early clinical intervention. The ECF proteome from vasospastic and non-vasospastic SAH patients was compared 1.5d before the onset of symptomatic vasospasm127. Differential levels of GAPDH and HSP7C were detected, which, with validation, could be biomarkers for predicting development of symptomatic vasospasm after SAH.
Proteomics of ECF microdialysates collected from the peri-hemorrhagic zone and healthy cortex of ICH patients at time of surgery was performed to identify potential biomarkers that predict extent of secondary injury128. A few DEPs, including increased hemoglobin and transthyretin, and decreased haptoglobin and IGHA1, were identified, but no validation was performed. Consequently, the suitability of any of these proteins as ICH biomarkers has not yet been confirmed.
3.2.3. Urine
IS.
The proteome of urinary samples collected from stroke patients within 24h of symptom onset was compared to healthy controls129. Hemoglobin, ITIH4, β−2-microglobulin, ceruloplasmin, GGT2P, and collagen α−1(II) chain were among the DEPs identified; however, only the latter two passed rigorous adjustment for multiple testing as stand-alone biomarkers for discriminating between groups. To increase performance accuracy, the authors combined 35 DEPs to a biomarker panel, which enabled stroke diagnosis in a small patient cohort with good confidence. This panel has not yet been tested for capacity to distinguish between stroke and mimics.
3.2.4. Blood
3.2.4.1. Stroke diagnosis
Differentiating between IS patients and healthy controls.
An early proteomics study found significantly increased serum hemoglobin levels in acute IS patients within 3d of stroke onset compared to controls130, introducing it as a potential stroke biomarker. However, blood collection in this study occurred at a timepoint far beyond the current therapeutic window for IS (24h).
iTRAQ-based LC-MS/MS was used to assess proteomic changes in plasma from IS patients with LVO131. A panel of four elevated proteins, platelet basic protein, thrombospondin-1, LYVE1, and IGF2, after stroke was identified and, when used on a different patient cohort, was able to discern stroke LVO patients from controls. Further, IGF2, LYVE1, and thrombospondin-1 proved to be independent predictors of good outcome at a 3mth follow-up. Although this is a promising result, the blood collection timepoint of up to 7d after stroke onset, again makes the panel impractical from a therapeutic standpoint. The same approach with blood samples from IS patients within 24h after symptom onset led to discovery of 66 DEPs, many of them with known impact on stroke pathology132. Compared to healthy controls, C-reactive protein, β−2-microglobulin, lipopolysaccharide-binding protein, C3, ADAMTS13, vWF, and thrombospondin-4 were increased, while S100A7 and platelet basic protein, in contrast to the prior study, were decreased. After vWF, ADAMTS13, and S100A7 serum levels were further evaluated by ELISA, they showed excellent diagnostic value. However, validation analysis using a different cohort is needed.
Distinguishing stroke from stroke mimics.
One challenge of stroke diagnosis is distinguishing strokes from stroke mimics, other medical conditions with clinical symptoms similar to stroke, such as seizures, migraine, and delirium. Using targeted proteomics, 147 proteins were assessed for their potential to differentiate between stroke patients and patients with stroke-mimicking conditions133. Thirty DEPs were identified, and used to generate a model that exhibited high diagnostic value. Rapidly and simultaneously measuring 30 proteins, however, is not practical in the current clinical setting. Another study used a high-density peptide array to determine whether altering binding characteristics of circulating antibodies in plasma could be used for stroke diagnosis134. Combined antibody-binding intensities to 17 peptide probes demonstrated encouraging sensitivity and specificity to discriminate stroke from stroke mimics, indicating potential for antibody-based stroke diagnostics.
Discriminating between IS and TIA patients.
TIA is a risk factor for subsequent stroke that can be mitigated with appropriate prevention135. It remains difficult, however, to diagnose TIA patients, with once promising biomarkers failing to improve clinical practice136. One study used global quantitative proteomics to analyze serum samples from patients presenting with TIA, minor stroke, and mimicking conditions137. PBP was increased after stroke, which was further validated by ELISA in a second cohort of patients. While elevated PBP levels diagnosed stroke, they were unable to separate TIA from minor stroke patients. More comprehensive studies on biomarker-based distinction of minor stroke and TIA patients were recently performed by the SpecTRA study group138,139. In phase 1, targeted proteomics of plasma from patients within 24h of symptom onset led to the development of a 16-DEP panel, from which nine proteins were also significant univariate predictors of TIA vs mimics138. In phase 2, IGFBP3 and PON3 were validated as TIA biomarkers in two additional patient cohorts139, which if independently confirmed, would be of great clinical value.
Distinguishing IS from HS.
Having a reliable blood biomarker to facilitate early distinction between these two stroke types would greatly improve treatment selection. Plasma proteomics on samples obtained from healthy controls, and IS and HS patients at admission, showed higher levels of apolipoprotein-C-I and apolipoprotein-C-III in IS, which was further verified by ELISA140. Encouragingly, apolipoprotein-C-I and apolipoprotein-C-III were confirmed as suitable biomarkers in an independent study using targeted proteomics to measure apolipoprotein levels in plasma samples from stroke patients141. This study further indicated that the ischemic vs hemorrhagic groups were best differentiated by combining measurements of apolipoprotein-C-III and apolipoprotein-A1. Collectively, apolipoproteins make good biomarker candidates.
A recent proteomics study analyzed plasma samples from IS and ICH patients, and healthy controls, and identified nine biomarker candidates. Three of them, with higher levels in IS (CPN2, coagulation factor XII, and MASP1), were related to the blood coagulation system, and showed potential for differentiating between IS and ICH142.
3.2.4.2. Stroke prognosis
A few proteomics studies have aimed to identify prognostic blood biomarkers that predict functional recovery after stroke. The development of hemorrhagic transformation (HT) is a severe complication following IS that exacerbates brain damage. To optimize treatment of stroke patients at high risk for developing HT, a blood biomarker that predicts HT is of urgent clinical interest. The first systematic approach to identify such biomarkers was only recently performed using quantitative proteomics143. Analysis of serum samples taken from a small group of IS patients with no recanalization, some of which spontaneously developed HT (5 out of 37), identified 17 DEPs, including apolipoprotein C-II, HSP72, and CRHBP. Future studies are required to assess their predictive values.
Longitudinal quantitative proteomics of plasma collected from IS patients at three time points post stroke (days up to 12mth), was performed to track protein expression changes during recovery144. Several DEPs were identified, many associated with the complement pathway. However, none were independently evaluated as biomarkers. Another longitudinal proteomics study assessed serum samples from a cohort of IS patients treated with placebo or t-PA at five timepoints from baseline imaging to 90d after stroke145. The blood protein composition after stroke was highly dynamic, indicating the importance of timing blood sample collection for biomarker discovery. Twenty-six DEPs were altered with t-PA treatment, while 23 changed over the course of stroke progression. Baseline vWF levels correlated with functional recovery at 3mth after IS. Disappointingly, no other DEP was significantly associated with stroke subtype (atherosclerotic vs cardioembolic), stroke severity, HT, or cardiovascular risk factors.
High-density lipoprotein-cholesterol (HDL-C) levels are routinely used as an inverse risk biomarker for cardiovascular disease146, but little is known about changes in the HDL protein cargo after IS. A recent targeted proteomics study investigated the potential association between HDL proteome composition and functional recovery after IS147. Changes in nine proteins (apolipoproteins E, F, L1, M, and C-IV, as well as APMAP, LPA1, prenylcysteine oxidase-1, and PON1) significantly correlated with recovery scores at 3mth post stroke. Thus, these proteins may be useful as biomarkers to predict stroke recovery. With this encouraging data, further validation in a different cohort of stroke patients is strongly warranted.
3.2.5. Blood clots
To discover proteins suitable for use as biomarkers of stroke etiology, an exploratory proteomics study obtained thrombi from 20 patients with acute IS, and analyzed for DEPs that correlated with clinical features148. The levels of three DEPs (septin-2, phosphoglycerate kinase-1, and integrin α-M) correlated with serum low-density lipoprotein (LDL), while one protein, septin-7, corresponded to the erythrocyte sedimentation rate. However, due to the small sample size, only limited conclusions can be drawn from this study.
Analyzing thrombi from a larger cohort of patients (34 cardioembolic and 7 atherothrombotic), a recent omics study identified a distinct proteomic signature in the two etiologies, with higher glycophorin-A (indicative of more red blood cells) and fibrinogen in cardioembolic stroke patients149. Combining proteomic and metabolomic data offered an excellent predictive model for distinguishing the two stroke subtypes with a diagnostic performance of 100% sensitivity/85.7% specificity. However, the model uses 40 omics features, which is technically not feasible for current clinical use. Given the great diagnostic value of thrombi for stroke etiology, further research in this new field is needed.
3.2.6. EVs
Proteomic changes in circulating EVs following two different cardiovascular conditions were assessed by comparing serum EV content in 81 IS patients, 37 myocardial infarction patients, and 22 healthy controls150. Retrieval of EVs occurred within 24h and 72h of symptom onset after brain and heart infarction, respectively. EVs contained an average of 146 proteins, out of which only three, apolipoprotein-B, α−2-macroglobulin, and fibronectin, showed altered levels between ischemic and myocardial infarction. Disappointingly, no significant change in EV protein content was found between IS and control subjects. However, after separating the same stroke patient group into lesion subtypes (26 subcortical and 55 cortical-subcortical), LC-MS/MS analysis identified some proteins in EVs that were unique to one subtype, e.g., complement C1QA and caspase-14 in cortical-subcortical, and annexin-2 in subcortical lesions151. Compared to controls, EVs from cortical-subcortical patients had significantly elevated FGC, fibronectin, and vWF levels, indicating heightened atherosclerosis. In addition to investigating EV content by proteomics, post-stroke changes in EV cell populations based on cell origins (e.g., endothelial cells and platelets), are currently under investigation as prognostic biomarkers152,153.
4. Proteomics from biofluids: a reverse translational approach to identify mechanisms and targets
A few clinical proteomics studies followed a reverse translational approach to gain insight into pathologic mechanisms and therapeutic targets through DEP analysis of blood from stroke patients. Cervical artery dissection (CAD) is a leading cause of stroke in young adults. To understand the underlying pathophysiologic mechanisms, which are largely unknown, one study quantitatively compared the serum proteome between CAD stroke and non-CAD stroke patients from blood collected up to 10d from symptom onset154. Lipopolysaccharide-binding protein, VCAM1, ficolin-2, fibulin-1, apolipoprotein-C-I, and apolipoprotein-B were upregulated after CAD stroke, pointing to a difference in immune response, blood coagulation, and lipid metabolism.
To understand sex differences in stroke pathophysiology, comparative proteomics was used to analyze DEPs in plasma collected from male and female IS patients (mean age = ~82 years) prior to thrombolysis and within 24h of symptom onset155. Expression of 77 proteins differed between the two sexes, with higher levels of most DEPs in women. CBG, a negative regulator of cortisol bioactivity, was 16 times higher in females vs males, indicating involvement of cortisol signaling in the sexually dimorphic outcome. Interestingly, some of the identified DEPs with elevated levels in females are proteins involved in various stages of thrombosis, such as fibrinolysis, coagulation, and platelet activation, potentially underlying the higher risk for stroke in older females.
The increased use of thrombectomy enables collection of intracranial blood. Comparison of intracranial and systemic blood protein content can provide insights into the pathophysiologic changes in the stroke brain156. In a pilot study, plasma proteome analysis in patients showed that all DEPs, most significantly ficolin-2, fetuin-B, FAP, uromodulin, and PLTP, exhibit lower intracranial levels compared to systemic arterial blood. Whether these DEPs are stroke-specific or simply reflect basic differences between blood sources remains to be clarified. In a follow-up study, the authors showed that intracranial, but not systemic, levels of VCAM1 were positively associated with infarct and edema volume, suggesting a detrimental role of VCAM1 in stroke pathology157.
5. Challenges and future perspectives
Stroke proteomics, with its capability to analyze protein changes in a biological sample on a large scale, is a powerful approach that could advance stroke diagnosis and intervention by facilitating and expediting discovery of biomarkers and novel therapeutic targets. Since the birth of stroke proteomics, many intriguing DEPs have been identified in brain tissue as well as biofluids from stroke patients and animal models. None of these proteins, however, has led to a clinically approved stroke biomarker or therapy. The reasons for this translational gap are many.
Therapeutic target discovery by stroke proteomics is complicated by many factors. First, there is a relatively poor overlap of DEPs among current studies, which is likely due to the different experimental conditions used, particularly concerning stroke models, timing of sampling, and methods of protein preparation and proteomics. Second, there is a lack of follow-up studies confirming key findings that are truly related to stroke pathophysiology. While proteomics is highly effective in identifying proteins, it cannot inform the role of a DEP in stroke pathology or determine whether the altered protein level is a cause or consequence of injury. Third, inaccurate data interpretation may hinder discovery. As the name “DEP” implies, altered protein levels detected in tissue are commonly interpreted as a change in protein expression, which may not always be the case. For instance, many studies use little or no detergents during tissue extraction. This precludes analysis of insoluble cellular material such as the plasma membrane, the postsynapse, or aggregates, to which proteins could have translocated. Another reason for elusive therapeutic targets might be that they are not found by assessing protein level changes, but rather by evaluating changes in protein modifications after stroke, which are vastly understudied. Finally, all current experimental stroke studies were executed in young animals, which is an obvious mismatch to the clinic, where most stroke patients are elderly (>60 years), and functional outcome is inversely associated with age158. Thus, proteomics analysis of aged, stroked animals is urgently needed.
Biomarker discovery by stroke proteomics has been hampered by lack of candidates that consistently perform between different studies and lack of validation in large cohorts of heterogeneous stroke patients with sufficient ethnic and geographic diversity. Rather than relying on individual labs, it may be more productive to fund multi-lab or -center initiatives that ensure strict patient selection, standardized sample collection, and use of state-of-the-art proteomics technology and data analysis for biomarker discovery. Another strategy to improve biomarker performance is using a biomarker protein panel that covers different aspects of stroke pathophysiology, such as thrombosis, inflammation, and brain injury. Ideally, this panel should be feasible for clinical use and include stroke-specific brain-derived proteins, which are likely present in blood at low levels (pg-ng/mL). Identifying such proteins via proteomics presents a technical challenge, as the human blood has a highly complex and dynamic proteome159. Increasing sensitivity of mass spectrometers, and depleting high-abundance proteins or enriching stroke-specific proteins by affinity-purification with antibodies, for example, may help solve this problem. Alternatively, some proteomics studies have assessed protein changes in urine, which has a less complex proteome than blood.
Some newer approaches in stroke proteomics take advantage of enriched brain-specific proteins in EVs and thrombi. Several brain-cell types, including neurons and endothelial cells, release EVs into the blood during stroke. Analyzing their contents may provide new insights into stroke pathophysiology, and inform biomarker development. Current efforts are directed at optimizing EV isolation160. Additionally, while clot proteomics is still in its infancy, and needs further optimization, stroke centers have started to recognize the potential value of using thrombi for stroke diagnosis161, which will help move this field forward.
As omics technologies enter clinical research, combining analysis of complex biological data with multi-dimensional patient information requires a systems approach using the highest level statistical and bioinformatic methodologies162,163. Evolving methods of artificial intelligence and machine learning are well-suited for this purpose, and are beginning to be adopted in biomarker discovery164,165. It is with great hope that combining (prote)omics with these technologies will lead to a breakthrough in identifying biomarkers and therapeutic targets, and thereby revolutionize stroke diagnosis and care.
Supplementary Material
Acknowledgments.
The authors wish to thank Emma Hambright (BMRI, WCM) for editing and proofreading the manuscript.
Sources of Funding.
This work was supported by National Institutes of Health (NIH) grants R01NS109588 (KH), R01NS099590 (WY), and R01HL157354 (WY), and American Heart Association grant 18CSA34080277 (WY). In addition, support of KH by the Feil Family Foundation is gratefully acknowledged.
Abbreviations not described in the text (in alphabetical order)
- 4E-BP2
eukaryotic translation initiation factor 4E-binding protein-2
- ACO1
cytoplasmic aconitate hydratase
- ACO2
aconitate hydratase mitochondrial-2
- ADAMTS13
a disintegrin and metalloproteinase with thrombospondin motifs-13
- ALDOC
fructose-biphosphate aldolase-C
- APMAP
adipocyte plasma membrane-associated protein
- ARHGDIA
Rho GDP-dissociation inhibitor-1
- ATP1A1
sodium/potassium-transporting ATPase subunit α1
- ATP5C1
ATP synthase subunit γ, mitochondrial
- ATP6V1F
V-type proton ATPase subunit-1F
- BAIAP2L1
brain-specific angiogenesis inhibitor 1 associated protein-2L1
- BASP1
brain acid soluble protein-1
- CAMK2A
calcium-calmodulin-dependent protein kinase type II subunit α
- CAMK2B
calcium-calmodulin-dependent protein kinase type II subunit β
- CAMK2D
calcium/calmodulin-dependent protein kinase type II subunit δ
- CBG
corticosteroid-binding globulin
- CMPK
uridine monophosphate/cytidine monophosphate kinase
- COTL1
coactosin-like protein
- COX5B
cytochrome c oxidase subunit-5B
- CPN2
carboxypeptidase N subunit-2
- CRHBP
corticotropin-releasing factor-binding protein
- CTIP2
COUP-TF-interacting protein-2
- DARPP32
dopamine- and cAMP-regulated neuronal phosphoprotein
- DLGAP1
scaffolds disk-large associated protein-1
- DNAJA1
DnaJ homolog subfamily A member-1
- DRP2
dihydropyrimidinase-related protein-2
- EAAT1
excitatory amino acid transporter-1
- ECHS1
enoyl-CoA hydratase mitochondrial
- eEFA1
eukaryotic translation elongation factor-A1
- eIF4E
eukaryotic translation initiation factor-4E
- eIF4H
eukaryotic translation initiation factor-4H
- FAP
prolyl endopeptidase FAP
- FUS
fused in sarcoma
- FXR2
fragile X mental retardation syndrome-related proteins-2
- GADD45G
growth arrest and DNA damage-inducible protein 45G
- GAPDH
glyceraldehyde-3-phosphatase dehydrogenase
- GDI
Rab GDP dissociation inhibitor α
- GFAP
glial fibrillary acidic protein
- GGT2P
γ-glutamyltransferase-2, pseudogene
- GNAO1
guanine nucleotide-binding protein G(o) subunit α
- GORASP2
Golgi reassembly-stacking protein-2
- GOT1
aspartate aminotransferase cytoplasmic
- GOT2
aspartate aminotransferase, mitochondrial
- GPCR
G-protein coupled receptor
- GRIN2B
glutamate receptors ionotropic NMDA-2B
- GRP78
78kDa glucose-regulated protein
- GSTP1
glutathione S-transferase P
- H-FABP
fatty acid-binding protein, heart
- HNRNPA1
heterogenous nuclear ribonucleoprotein-A1
- HSC70-ps1
heat-shock cognate protein 70 pseudogene-1
- HSC70
heat-shock cognate protein-70
- HSC71
heat shock cognate 70kDa protein
- HSP7C
heat shock cognate 71kDa protein
- HSPA12A
heat shock 70 kDa protein-12A
- HSPA5
endoplasmic reticulum chaperone BiP
- IDH2
isocitrate dehydrogenase [NADP], mitochondrial
- IGF2
insulin-like growth factor-2
- IGFBP3
insulin-like growth factor-binding protein-3
- IGHA1
immunoglobulin heavy constant α1
- IGLL1
immunoglobulin λ like polypeptide-1
- ITIH3
inter-α-trypsin inhibitor heavy chain-H3
- ITIH4
inter-α-trypsin inhibitor heavy chain-H4
- ITPKA
inositol-triphosphate 3-kinase-A
- LAMP2
lysosome associated membrane glycoprotein-2
- LPA1
lysophosphatidic acid receptor-1
- LYVE1
lymphatic vessel endothelial hyaluronic acid receptor-1
- MAP2
microtubule-associated protein-2
- MAP2K1
dual specificity mitogen-activated protein kinase kinase-1
- MAP6
microtubule associated protein-6
- MAPRE1
microtubule-associated protein RP/EB family member-1
- MAPT
microtubule-associated protein Tau
- MASP1
mannan-binding lectin serine protease-1
- MBL2
mannose binding lectin
- MBP
myelin basic protein
- MDH1
malate dehydrogenase mitochondrial-1
- MDH2
malate dehydrogenase mitochondrial-2
- NDUFA1
NADH dehydrogenase [ubiquinone] 1 α subcomplex subunit-1
- NDUFA9
NADH dehydrogenase [ubiquinone] 1 α subcomplex subunit-9, mitochondrial
- NDUFS1
NADH-ubiquinone oxidoreductase 75 kDa subunit, mitochondrial
- NDUFS5
NADH dehydrogenase [ubiquinone] iron-sulfur protein-5
- NONO
non-POU domain-containing octamer-binding protein
- NSF
N-ethylmaleimide sensitive factor
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- p-SNAP25
phosphorylated synaptosomal-associated protein-25
- PACN1
protein kinase C and casein kinase substrate in neurons protein-1
- PARP
poly (ADP-ribose) polymerase
- PBP
phosphatidylethanolamine-binding protein-1
- PDE6B
rod cGMP-specific 3’,5’-cyclic phosphodiesterase subunit β
- PEA15
astrocytic phosphoprotein PEA15
- PFKM
ATP-dependent 6-phosphofructokinase, muscle type
- PKA
protein kinase-A
- PKC
protein kinase-C
- PLTP
phospholipid transfer protein
- PON1
serum paraoxonase/lactonase-1
- PON3
serum paraoxonase/lactonase-3
- PPARα
peroxisome proliferator-activated receptor α
- PPIA
peptidyl-prolyl cis-trans isomerase-A
- PPP1
serine-threonine protein phosphatase-1
- PPP2A
serine-threonine protein phosphatase-2A
- PRP16
pre-mRNA splicing factor ATP-dependent RNA helicase-16
- PSD95
postsynaptic density protein-95
- PTAFR
platelet-activating factor receptor
- RBM14
RNA-binding protein-14
- SAHH2
S-adenosylhomocysteine hydrolase-like protein-1
- SERPINA3K
serine protease inhibitor-3K
- SERPINE2
serpin peptidase inhibitor-E
- SFPQ
splicing factor proline and glutamine rich
- SGTA
Small glutamine-rich tetratricopeptide repeat-containing protein-α
- SIAH2
E3 ubiquitin-protein ligase SIAH2
- SIRT2
NAD-dependent protein deacetylase sirtuin-2
- SLC25A11
mitochondrial 2-oxoglutarate/malate carrier protein
- SLC6A11
solute carrier family 6A11
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptors
- SPNA-2
spectrin αII chain
- SRSF1
serine/arginine-rich splicing factor-1
- STAT6
signal transducer and activator of transcription-6
- STIP1
stress-induced phosphoprotein-1
- SynGAP1
Ras/Rap GTPase-activating protein-1
- TDP43
TAR DNA-binding protein-43
- TFII-I
transcription factor II-I
- TIF1B
transcription intermediary factor-1B
- UCH-L1
ubiquitin carboxyl-terminal hydrolase-1
- UQCRB
cytochrome b-c1 complex subunit-7
- UQCRQ
cytochrome B-C1 complex subunit-8
- VAMP2
vesicle-associated membrane protein-2
- VCAM1
vascular cell adhesion protein-1
- VCP
transitional endoplasmic reticulum ATPase
- VDAC1
voltage-dependent anion-selective channel protein-1
- vWF
von Willebrand factor
Footnotes
Disclosures. The authors declare no competing financial interests.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 2.Bosetti F, Koenig JI, Ayata C, Back SA, Becker K, Broderick JP, Carmichael ST, Cho S, Cipolla MJ, Corbett D, et al. Translational Stroke Research: Vision and Opportunities. Stroke. 2017;48:2632–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeGracia DJ. Regulation of mRNA following brain ischemia and reperfusion. Wiley Interdiscip Rev RNA. 2017;8. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Park S, Ha S, Kwon JS, Khan MR, Kang BG, Dawson TM, Dawson VL, Andrabi SA, Kang SU. Quantitative mass spectrometric analysis of the mouse cerebral cortex after ischemic stroke. PLoS One. 2020;15:e0231978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Focking M, Besselmann M, Trapp T. Proteomics of experimental stroke in mice. Acta Neurobiol Exp (Wars). 2006;66:273–278. [DOI] [PubMed] [Google Scholar]
- 6.Ren C, Guingab-Cagmat J, Kobeissy F, Zoltewicz S, Mondello S, Gao M, Hafeez A, Li N, Geng X, Larner SF, et al. A neuroproteomic and systems biology analysis of rat brain post intracerebral hemorrhagic stroke. Brain Res Bull. 2014;102:46–56. [DOI] [PubMed] [Google Scholar]
- 7.Simats A, Ramiro L, Garcia-Berrocoso T, Brianso F, Gonzalo R, Martin L, Sabe A, Gill N, Penalba A, Colome N, et al. A Mouse Brain-based Multi-omics Integrative Approach Reveals Potential Blood Biomarkers for Ischemic Stroke. Mol Cell Proteomics. 2020;19:1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen A, Liao WP, Lu Q, Wong WS, Wong PT. Upregulation of dihydropyrimidinase-related protein 2, spectrin alpha II chain, heat shock cognate protein 70 pseudogene 1 and tropomodulin 2 after focal cerebral ischemia in rats--a proteomics approach. Neurochem Int. 2007;50:1078–1086. [DOI] [PubMed] [Google Scholar]
- 9.Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–782. [DOI] [PubMed] [Google Scholar]
- 11.Koh PO. Proteomic analysis of focal cerebral ischemic injury in male rats. J Vet Med Sci. 2010;72:181–185. [DOI] [PubMed] [Google Scholar]
- 12.Shah FA, Gim SA, Kim MO, Koh PO. Proteomic identification of proteins differentially expressed in response to resveratrol treatment in middle cerebral artery occlusion stroke model. J Vet Med Sci. 2014;76:1367–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung JH, Cho EH, Cho JH, Won CK, Kim MO, Koh PO. Identification of proteins regulated by ferulic acid in a middle cerebral artery occlusion animal model-a proteomics approach. J Vet Med Sci. 2012;74:1401–1407. [DOI] [PubMed] [Google Scholar]
- 14.Sung JH, Kim MO, Koh PO. Proteomic identification of proteins differentially expressed by nicotinamide in focal cerebral ischemic injury. Neuroscience. 2011;174:171–177. [DOI] [PubMed] [Google Scholar]
- 15.Zgavc T, Hu TT, Van de Plas B, Vinken M, Ceulemans AG, Hachimi-Idrissi S, Sarre S, Michotte Y, Arckens L. Proteomic analysis of global protein expression changes in the endothelin-1 rat model for cerebral ischemia: rescue effect of mild hypothermia. Neurochem Int. 2013;63:379–388. [DOI] [PubMed] [Google Scholar]
- 16.Ottens AK, Bustamante L, Golden EC, Yao C, Hayes RL, Wang KK, Tortella FC, Dave JR. Neuroproteomics: a biochemical means to discriminate the extent and modality of brain injury. J Neurotrauma. 2010;27:1837–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutta S, Chiu YC, Probert AW, Wang KK. Selective release of calpain produced alphalI-spectrin (alpha-fodrin) breakdown products by acute neuronal cell death. Biol Chem. 2002;383:785–791. [DOI] [PubMed] [Google Scholar]
- 18.Pike BR, Flint J, Dave JR, Lu XC, Wang KK, Tortella FC, Hayes RL. Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alphaII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2004;24:98–106. [DOI] [PubMed] [Google Scholar]
- 19.Ren C, Zoltewicz S, Guingab-Cagmat J, Anagli J, Gao M, Hafeez A, Li N, Cao J, Geng X, Kobeissy F, et al. Different Expression of ubiquitin C-terminal hydrolase-L1 and alphaII-spectrin in Ischemic and Hemorrhagic Stroke: Potential Biomarkers in Diagnosis. Brain Res Mol Brain Res. 2013. [DOI] [PubMed] [Google Scholar]
- 20.Papa L, Rosenthal K, Silvestri F, Axley JC, Kelly JM, Lewis SB. Evaluation of alpha-II-spectrin breakdown products as potential biomarkers for early recognition and severity of aneurysmal subarachnoid hemorrhage. Sci Rep. 2018;8:13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JY, Kim JW, Yenari MA. Heat shock protein signaling in brain ischemia and injury. Neurosci Lett. 2020;715:134642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng F, Zhou YT, Zeng YF, Liu T, Yang ZY, Tang T, Luo JK, Wang Y. Proteomics Analysis of Brain Tissue in a Rat Model of Ischemic Stroke in the Acute Phase. Front Mol Neurosci. 2020;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brea D, Agulla J, Staes A, Gevaert K, Campos F, Sobrino T, Blanco M, Davalos A, Castillo J, Ramos-Cabrer P. Study of Protein Expression in Peri-Infarct Tissue after Cerebral Ischemia. Sci Rep. 2015;5:12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong X, Liang Q, Chen J, Fan R, Cheng T. Proteomics profiling of pituitary, adrenal gland, and splenic lymphocytes in rats with middle cerebral artery occlusion. Biosci Biotechnol Biochem. 2009;73:657–664. [DOI] [PubMed] [Google Scholar]
- 25.Boulos S, Meloni BP, Arthur PG, Bojarski C, Knuckey NW. Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J Neurosci Res. 2007;85:3089–3097. [DOI] [PubMed] [Google Scholar]
- 26.Takagi Y, Mitsui A, Nishiyama A, Nozaki K, Sono H, Gon Y, Hashimoto N, Yodoi J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci U S A. 1999;96:4131–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilguvar K, Tyagi NK, Ozkara C, Tuysuz B, Bakircioglu M, Choi M, Delil S, Caglayan AO, Baranoski JF, Erturk O, et al. Recessive loss of function of the neuronal ubiquitin hydrolase UCHL1 leads to early-onset progressive neurodegeneration. Proc Natl Acad Sci U S A. 2013;110:3489–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu MC, Akinyi L, Scharf D, Mo J, Larner SF, Muller U, Oli MW, Zheng W, Kobeissy F, Papa L, et al. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur J Neurosci. 2010;31:722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazarian JJ, Biberthaler P, Welch RD, Lewis LM, Barzo P, Bogner-Flatz V, Gunnar Brolinson P, Buki A, Chen JY, Christenson RH, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol. 2018;17:782–789. [DOI] [PubMed] [Google Scholar]
- 30.Gunton AN, Sanchez-Arias JC, Carmona-Wagner EO, Wicki-Stordeur LE, Swayne LA. Upregulation of inflammatory mediators in the ventricular zone after cortical stroke. Proteomics Clin Appl. 2017;11. [DOI] [PubMed] [Google Scholar]
- 31.Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Invest. 2020;130:2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen M, Jin Y, Zhang H, Sun X, Kuai Y, Tan W. Proteomic Analysis of Rat Cerebral Cortex in the Subacute to Long-Term Phases of Focal Cerebral Ischemia-Reperfusion Injury. J Proteome Res. 2019;18:3099–3118. [DOI] [PubMed] [Google Scholar]
- 33.Junker H, Suofu Y, Venz S, Sascau M, Herndon JG, Kessler C, Walther R, Popa-Wagner A. Proteomic identification of an upregulated isoform of annexin A3 in the rat brain following reversible cerebral ischemia. Glia. 2007;55:1630–1637. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Li Z, Ma Z, Deng M, Xing M, Wu J, Jiang S, Wang Q, Guo Q, Zou W. Annexin A3 as a Marker Protein for Microglia in the Central Nervous System of Rats. Neural Plast. 2021;2021:5575090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu RF, Fang T, Nelson A, Gyoneva S, Gao B, Hedde J, Henry K, Peterson E, Burkly LC, Wei R. Proteomic Characterization of the Dynamics of Ischemic Stroke in Mice. J Proteome Res. 2021;20:3689–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Dong L, Xiao Z, He W, Zhao J, Pan H, Chu B, Cheng J, Wang H. Integrated analysis of the proteome and transcriptome in a MCAO mouse model revealed the molecular landscape during stroke progression. J Adv Res. 2020;24:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song H, Zhou H, Qu Z, Hou J, Chen W, Cai W, Cheng Q, Chuang DY, Chen S, Li S, et al. From Analysis of Ischemic Mouse Brain Proteome to Identification of Human Serum Clusterin as a Potential Biomarker for Severity of Acute Ischemic Stroke. Transl Stroke Res. 2019;10:546–556. [DOI] [PubMed] [Google Scholar]
- 38.Law HC, Szeto SS, Quan Q, Zhao Y, Zhang Z, Krakovska O, Lui LT, Zheng C, Lee SM, Siu KW, et al. Characterization of the Molecular Mechanisms Underlying the Chronic Phase of Stroke in a Cynomolgus Monkey Model of Induced Cerebral Ischemia. J Proteome Res. 2017;16:1150–1166. [DOI] [PubMed] [Google Scholar]
- 39.Deng S, Feng S, Wang W, Zhao F, Gong Y. Biomarker and Drug Target Discovery Using Quantitative Proteomics Post-Intracerebral Hemorrhage Stroke in the Rat Brain. J Mol Neurosci. 2018;66:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu T, Zhou J, Cui H, Li P, Li H, Wang Y, Tang T. Quantitative proteomic analysis of intracerebral hemorrhage in rats with a focus on brain energy metabolism. Brain Behav. 2018;8:e01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dasari R, Zhi W, Bonsack F, Sukumari-Ramesh S. A Combined Proteomics and Bioinformatics Approach Reveals Novel Signaling Pathways and Molecular Targets After Intracerebral Hemorrhage. J Mol Neurosci. 2020;70:1186–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holste K, Xia F, Garton HJL, Wan S, Hua Y, Keep RF, Xi G. The role of complement in brain injury following intracerebral hemorrhage: A review. Exp Neurol. 2021;340:113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–652. [DOI] [PubMed] [Google Scholar]
- 44.Chiu CD, Chen TY, Chin LT, Shen CC, Huo J, Ma SY, Chen HM, Chu CH. Investigation of the effect of hyperglycemia on intracerebral hemorrhage by proteomic approaches. Proteomics. 2012;12:113–123. [DOI] [PubMed] [Google Scholar]
- 45.Limaye K, Yang JD, Hinduja A. Role of admission serum albumin levels in patients with intracerebral hemorrhage. Acta Neurol Belg. 2016;116:27–30. [DOI] [PubMed] [Google Scholar]
- 46.Dziedzic T, Slowik A, Szczudlik A. Serum albumin level as a predictor of ischemic stroke outcome. Stroke. 2004;35:e156–158. [DOI] [PubMed] [Google Scholar]
- 47.Shang F, Zhao H, Cheng W, Qi M, Wang N, Qu X. Predictive Value of the Serum Albumin Level on Admission in Patients With Spontaneous Subarachnoid Hemorrhage. Front Surg. 2021;8:719226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morotti A, Marini S, Lena UK, Crawford K, Schwab K, Kourkoulis C, Ayres AM, Edip Gurol M, Viswanathan A, Greenberg SM, et al. Significance of admission hypoalbuminemia in acute intracerebral hemorrhage. J Neurol. 2017;264:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. [DOI] [PubMed] [Google Scholar]
- 50.Sidyakin AA, Kaysheva AL, Kopylov AT, Lobanov AV, Morozov SG. Proteomic Analysis of Cerebral Cortex Extracts from Sus scrofa with Induced Hemorrhagic Stroke. J Mol Neurosci. 2018;65:28–34. [DOI] [PubMed] [Google Scholar]
- 51.Sung JH, Cho EH, Min W, Kim MJ, Kim MO, Jung EJ, Koh PO. Identification of proteins regulated by estradiol in focal cerebral ischemic injury--a proteomics approach. Neurosci Lett. 2010;477:66–71. [DOI] [PubMed] [Google Scholar]
- 52.Kitsberg D, Formstecher E, Fauquet M, Kubes M, Cordier J, Canton B, Pan G, Rolli M, Glowinski J, Chneiweiss H. Knock-out of the neural death effector domain protein PEA15 demonstrates that its expression protects astrocytes from TNFalpha-induced apoptosis. J Neurosci. 1999;19:8244–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song B, Ao Q, Wang Z, Liu W, Niu Y, Shen Q, Zuo H, Zhang X, Gong Y. Phosphorylation of tau protein over time in rats subjected to transient brain ischemia. Neural Regen Res. 2013;8:3173–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dziennis S, Jia T, Ronnekleiv OK, Hurn PD, Alkayed NJ. Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection. J Neurosci. 2007;27:7268–7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Domenico F, Casalena G, Jia J, Sultana R, Barone E, Cai J, Pierce WM, Cini C, Mancuso C, Perluigi M, et al. Sex differences in brain proteomes of neuron-specific STAT3-null mice after cerebral ischemia/reperfusion. J Neurochem. 2012;121:680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- 57.Yenari MA, Fink SL, Sun GH, Chang LK, Patel MK, Kunis DM, Onley D, Ho DY, Sapolsky RM, Steinberg GK. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann Neurol. 1998;44:584–591. [DOI] [PubMed] [Google Scholar]
- 58.Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neurochem. 2004;89:73–89. [DOI] [PubMed] [Google Scholar]
- 59.Atochin DN, Yuzawa I, Li Q, Rauwerdink KM, Malhotra R, Chang J, Brouckaert P, Ayata C, Moskowitz MA, Bloch KD, et al. Soluble guanylate cyclase alpha1beta1 limits stroke size and attenuates neurological injury. Stroke. 2010;41:1815–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stapels M, Piper C, Yang T, Li M, Stowell C, Xiong ZG, Saugstad J, Simon RP, Geromanos S, Langridge J, et al. Polycomb group proteins as epigenetic mediators of neuroprotection in ischemic tolerance. Sci Signal. 2010;3:ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Zhu C, Wang X, Gerwien JG, Schrattenholz A, Sandberg M, Leist M, Blomgren K. The nonerythropoietic asialoerythropoietin protects against neonatal hypoxia-ischemia as potently as erythropoietin. J Neurochem. 2004;91:900–910. [DOI] [PubMed] [Google Scholar]
- 62.Katayama N, Yamamori S, Fukaya M, Kobayashi S, Watanabe M, Takahashi M, Manabe T. SNAP-25 phosphorylation at Ser187 regulates synaptic facilitation and short-term plasticity in an age-dependent manner. Sci Rep. 2017;7:7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagy G, Matti U, Nehring RB, Binz T, Rettig J, Neher E, Sorensen JB. Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment. J Neurosci. 2002;22:9278–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gele P, Vingtdeux V, Potey C, Drobecq H, Ghestem A, Melnyk P, Buee L, Sergeant N, Bordet R. Recovery of brain biomarkers following peroxisome proliferator-activated receptor agonist neuroprotective treatment before ischemic stroke. Proteome Sci. 2014;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hori M, Nakamachi T, Rakwal R, Shibato J, Ogawa T, Aiuchi T, Tsuruyama T, Tamaki K, Shioda S. Transcriptomics and proteomics analyses of the PACAP38 influenced ischemic brain in permanent middle cerebral artery occlusion model mice. J Neuroinflammation. 2012;9:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu H, Povysheva N, Rose ME, Mi Z, Banton JS, Li W, Chen F, Reay DP, Barrionuevo G, Zhang F, et al. Role of UCHL1 in axonal injury and functional recovery after cerebral ischemia. Proc Natl Acad Sci U S A. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campos-Martorell M, Salvador N, Monge M, Canals F, Garcia-Bonilla L, Hernandez-Guillamon M, Ayuso MI, Chacon P, Rosell A, Alcazar A, et al. Brain proteomics identifies potential simvastatin targets in acute phase of stroke in a rat embolic model. J Neurochem. 2014;130:301–312. [DOI] [PubMed] [Google Scholar]
- 68.Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab. 2009;29:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hescheler J, Rosenthal W, Trautwein W, Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. Nature. 1987;325:445–447. [DOI] [PubMed] [Google Scholar]
- 70.Wu JS, Cheung WM, Tsai YS, Chen YT, Fong WH, Tsai HD, Chen YC, Liou JY, Shyue SK, Chen JJ, et al. Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14–3-3 epsilon upregulation. Circulation. 2009;119:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merali Z, Gao MM, Bowes T, Chen J, Evans K, Kassner A. Neuroproteome changes after ischemia/reperfusion injury and tissue plasminogen activator administration in rats: a quantitative iTRAQ proteomics study. PLoS One. 2014;9:e98706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Z, Zhang G, Sun Y, Szeto SS, Law HC, Quan Q, Li G, Yu P, Sho E, Siu MK, et al. Tetramethylpyrazine nitrone, a multifunctional neuroprotective agent for ischemic stroke therapy. Sci Rep. 2016;6:37148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brooks G, Hearse DJ. Role of protein kinase C in ischemic preconditioning: player or spectator? Circ Res. 1996;79:627–630. [DOI] [PubMed] [Google Scholar]
- 75.Bu X, Zhang N, Yang X, Liu Y, Du J, Liang J, Xu Q, Li J. Proteomic analysis of cPKCbetaII-interacting proteins involved in HPC-induced neuroprotection against cerebral ischemia of mice. J Neurochem. 2011;117:346–356. [DOI] [PubMed] [Google Scholar]
- 76.Feng S, Li D, Li Y, Yang X, Han S, Li J. Insight into hypoxic preconditioning and ischemic injury through determination of nPKCepsilon-interacting proteins in mouse brain. Neurochem Int. 2013;63:69–79. [DOI] [PubMed] [Google Scholar]
- 77.Ladilov Y, Maxeiner H, Wolf C, Schafer C, Meuter K, Piper HM. Role of protein phosphatases in hypoxic preconditioning. Am J Physiol Heart Circ Physiol. 2002;283:H1092–1098. [DOI] [PubMed] [Google Scholar]
- 78.Yang X, Zhang X, Li Y, Han S, Howells DW, Li S, Li J. Conventional protein kinase Cbeta-mediated phosphorylation inhibits collapsin response-mediated protein 2 proteolysis and alleviates ischemic injury in cultured cortical neurons and ischemic stroke-induced mice. J Neurochem. 2016;137:446–459. [DOI] [PubMed] [Google Scholar]
- 79.Morris-Blanco KC, Dave KR, Saul I, Koronowski KB, Stradecki HM, Perez-Pinzon MA. Protein Kinase C Epsilon Promotes Cerebral Ischemic Tolerance Via Modulation of Mitochondrial Sirt5. Sci Rep. 2016;6:29790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cid C, Garcia-Bonilla L, Camafeita E, Burda J, Salinas M, Alcazar A. Proteomic characterization of protein phosphatase 1 complexes in ischemia-reperfusion and ischemic tolerance. Proteomics. 2007;7:3207–3218. [DOI] [PubMed] [Google Scholar]
- 81.Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–256. [DOI] [PubMed] [Google Scholar]
- 82.Weith M, Seiler J, van den Boom J, Kracht M, Hulsmann J, Primorac I, Del Pino Garcia J, Kaschani F, Kaiser M, Musacchio A, et al. Ubiquitin-Independent Disassembly by a p97 AAA-ATPase Complex Drives PP1 Holoenzyme Formation. Mol Cell. 2018;72:766–777 e766. [DOI] [PubMed] [Google Scholar]
- 83.Ayuso MI, Martinez-Alonso E, Cid C, Alonso de Lecinana M, Alcazar A. The translational repressor eIF4E-binding protein 2 (4E-BP2) correlates with selective delayed neuronal death after ischemia. J Cereb Blood Flow Metab. 2013;33:1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinez-Alonso E, Guerra-Perez N, Escobar-Peso A, Regidor I, Masjuan J, Alcazar A. Differential Association of 4E-BP2-Interacting Proteins Is Related to Selective Delayed Neuronal Death after Ischemia. Int J Mol Sci. 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang W, Sheng H, Warner DS, Paschen W. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab. 2008;28:269–279. [DOI] [PubMed] [Google Scholar]
- 86.Datwyler AL, Lattig-Tunnemann G, Yang W, Paschen W, Lee SL, Dirnagl U, Endres M, Harms C. SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2011;31:2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee YJ, Mou Y, Maric D, Klimanis D, Auh S, Hallenbeck JM. Elevated global SUMOylation in Ubc9 transgenic mice protects their brains against focal cerebral ischemic damage. PLoS One. 2011;6:e25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang W, Sheng H, Thompson JW, Zhao S, Wang L, Miao P, Liu X, Moseley MA, Paschen W. Small ubiquitin-like modifier 3-modified proteome regulated by brain ischemia in novel small ubiquitin-like modifier transgenic mice: putative protective proteins/pathways. Stroke. 2014;45:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hochrainer K, Jackman K, Anrather J, Iadecola C. Reperfusion rather than ischemia drives the formation of ubiquitin aggregates after middle cerebral artery occlusion. Stroke. 2012;43:2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hochrainer K, Jackman K, Benakis C, Anrather J, Iadecola C. SUMO2/3 is associated with ubiquitinated protein aggregates in the mouse neocortex after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2015;35:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iwabuchi M, Sheng H, Thompson JW, Wang L, Dubois LG, Gooden D, Moseley M, Paschen W, Yang W. Characterization of the ubiquitin-modified proteome regulated by transient forebrain ischemia. J Cereb Blood Flow Metab. 2014;34:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hochrainer K Protein Modifications with Ubiquitin as Response to Cerebral Ischemia-Reperfusion Injury. Transl Stroke Res. 2018;9:157–173. [DOI] [PubMed] [Google Scholar]
- 93.Kahles T, Poon C, Qian L, Palfini V, Srinivasan SP, Swaminathan S, Blanco I, Rodney-Sandy R, Iadecola C, Zhou P, et al. Elevated post-ischemic ubiquitination results from suppression of deubiquitinase activity and not proteasome inhibition. Cell Mol Life Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kahl A, Blanco I, Jackman K, Baskar J, Milaganur Mohan H, Rodney-Sandy R, Zhang S, Iadecola C, Hochrainer K. Cerebral ischemia induces the aggregation of proteins linked to neurodegenerative diseases. Sci Rep. 2018;8:2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cuadrado E, Rosell A, Colome N, Hernandez-Guillamon M, Garcia-Berrocoso T, Ribo M, Alcazar A, Ortega-Aznar A, Salinas M, Canals F, et al. The proteome of human brain after ischemic stroke. J Neuropathol Exp Neurol. 2010;69:1105–1115. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Berrocoso T, Penalba A, Boada C, Giralt D, Cuadrado E, Colome N, Dayon L, Canals F, Sanchez JC, Rosell A, et al. From brain to blood: New biomarkers for ischemic stroke prognosis. J Proteomics. 2013;94:138–148. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Berrocoso T, Llombart V, Colas-Campas L, Hainard A, Licker V, Penalba A, Ramiro L, Simats A, Bustamante A, Martinez-Saez E, et al. Single Cell Immuno-Laser Microdissection Coupled to Label-Free Proteomics to Reveal the Proteotypes of Human Brain Cells After Ischemia. Mol Cell Proteomics. 2018;17:175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramiro L, Garcia-Berrocoso T, Brianso F, Goicoechea L, Simats A, Llombart V, Gonzalo R, Hainard A, Martinez-Saez E, Canals F, et al. Integrative Multi-omics Analysis to Characterize Human Brain Ischemia. Mol Neurobiol. 2021;58:4107–4121. [DOI] [PubMed] [Google Scholar]
- 99.Datta A, Akatsu H, Heese K, Sze SK. Quantitative clinical proteomic study of autopsied human infarcted brain specimens to elucidate the deregulated pathways in ischemic stroke pathology. J Proteomics. 2013;91:556–568. [DOI] [PubMed] [Google Scholar]
- 100.Bustamante A, Lopez-Cancio E, Pich S, Penalba A, Giralt D, Garcia-Berrocoso T, Ferrer-Costa C, Gasull T, Hernandez-Perez M, Millan M, et al. Blood Biomarkers for the Early Diagnosis of Stroke: The Stroke-Chip Study. Stroke. 2017;48:2419–2425. [DOI] [PubMed] [Google Scholar]
- 101.Dagonnier M, Donnan GA, Davis SM, Dewey HM, Howells DW. Acute Stroke Biomarkers: Are We There Yet? Front Neurol. 2021;12:619721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katsanos AH, Makris K, Stefani D, Koniari K, Gialouri E, Lelekis M, Chondrogianni M, Zompola C, Dardiotis E, Rizos I, et al. Plasma Glial Fibrillary Acidic Protein in the Differential Diagnosis of Intracerebral Hemorrhage. Stroke. 2017;48:2586–2588. [DOI] [PubMed] [Google Scholar]
- 103.Luger S, Jaeger HS, Dixon J, Bohmann FO, Schaefer J, Richieri SP, Larsen K, Hov MR, Bache KG, Foerch C, et al. Diagnostic Accuracy of Glial Fibrillary Acidic Protein and Ubiquitin Carboxy-Terminal Hydrolase-L1 Serum Concentrations for Differentiating Acute Intracerebral Hemorrhage from Ischemic Stroke. Neurocrit Care. 2020;33:39–48. [DOI] [PubMed] [Google Scholar]
- 104.VanGilder RL, Davidov DM, Stinehart KR, Huber JD, Turner RC, Wilson KS, Haney E, Davis SM, Chantler PD, Theeke L, et al. C-reactive protein and long-term ischemic stroke prognosis. J Clin Neurosci. 2014;21:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uphaus T, Bittner S, Groschel S, Steffen F, Muthuraman M, Wasser K, Weber-Kruger M, Zipp F, Wachter R, Groschel K. NfL (Neurofilament Light Chain) Levels as a Predictive Marker for Long-Term Outcome After Ischemic Stroke. Stroke. 2019;50:3077–3084. [DOI] [PubMed] [Google Scholar]
- 106.Chen CH, Chu HJ, Hwang YT, Lin YH, Lee CW, Tang SC, Jeng JS. Plasma neurofilament light chain level predicts outcomes in stroke patients receiving endovascular thrombectomy. Journal of Neuroinflammation. 2021;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang P, Zhu Z, Zang Y, Bu X, Xu T, Zhong C, Wang A, Peng H, Guo D, Zheng X, et al. Increased Serum Complement C3 Levels Are Associated With Adverse Clinical Outcomes After Ischemic Stroke. Stroke. 2021;52:868–877. [DOI] [PubMed] [Google Scholar]
- 108.Misra S, Montaner J, Ramiro L, Arora R, Talwar P, Nath M, Kumar A, Kumar P, Pandit AK, Mohania D, et al. Blood biomarkers for the diagnosis and differentiation of stroke: A systematic review and meta-analysis. Int J Stroke. 2020;15:704–721. [DOI] [PubMed] [Google Scholar]
- 109.Latosinska A, Siwy J, Faguer S, Beige J, Mischak H, Schanstra JP. Value of Urine Peptides in Assessing Kidney and Cardiovascular Disease. Proteomics Clin Appl. 2021;15:e2000027. [DOI] [PubMed] [Google Scholar]
- 110.Shah R, Patel T, Freedman JE. Circulating Extracellular Vesicles in Human Disease. N Engl J Med. 2018;379:2180–2181. [DOI] [PubMed] [Google Scholar]
- 111.Suzuyama K, Shiraishi T, Oishi T, Ueda S, Okamoto H, Furuta M, Mineta T, Tabuchi K. Combined proteomic approach with SELDI-TOF-MS and peptide mass fingerprinting identified the rapid increase of monomeric transthyretin in rat cerebrospinal fluid after transient focal cerebral ischemia. Brain Res Mol Brain Res. 2004;129:44–53. [DOI] [PubMed] [Google Scholar]
- 112.Sironi L, Tremoli E, Miller I, Guerrini U, Calvio AM, Eberini I, Gemeiner M, Asdente M, Paoletti R, Gianazza E. Acute-phase proteins before cerebral ischemia in stroke-prone rats: identification by proteomics. Stroke. 2001;32:753–760. [DOI] [PubMed] [Google Scholar]
- 113.Chen R, Vendrell I, Chen CP, Cash D, O’Toole KG, Williams SA, Jones C, Preston JE, Wheeler JX. Proteomic analysis of rat plasma following transient focal cerebral ischemia. Biomark Med. 2011;5:837–846. [DOI] [PubMed] [Google Scholar]
- 114.Ambrosius W, Michalak S, Kazmierski R, Andrzejewska N, Kozubski W. Predictive value of serum transthyretin for outcome in acute ischemic stroke. PLoS One. 2017;12:e0179806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sironi L, Guerrini U, Tremoli E, Miller I, Gelosa P, Lascialfari A, Zucca I, Eberini I, Gemeiner M, Paoletti R, et al. Analysis of pathological events at the onset of brain damage in stroke-prone rats: a proteomics and magnetic resonance imaging approach. J Neurosci Res. 2004;78:115–122. [DOI] [PubMed] [Google Scholar]
- 116.Stevens SL, Liu T, Bahjat FR, Petyuk VA, Schepmoes AA, Sontag RL, Gritsenko MA, Wu C, Wang S, Shukla AK, et al. Preconditioning in the Rhesus Macaque Induces a Proteomic Signature Following Cerebral Ischemia that Is Associated with Neuroprotection. Transl Stroke Res. 2019;10:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang T, Xiang L. Elevated Plasma Haptoglobin Level as a Potential Marker for Poor Prognosis in Acute Cerebral Infarction. Eur Neurol. 2018;79:154–160. [DOI] [PubMed] [Google Scholar]
- 118.Zhou H, Wang A, Meng X, Lin J, Jiang Y, Jing J, Zuo Y, Wang Y, Zhao X, Li H, et al. Low serum albumin levels predict poor outcome in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc Neurol. 2021;6:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Altamura C, Squitti R, Pasqualetti P, Gaudino C, Palazzo P, Tibuzzi F, Lupoi D, Cortesi M, Rossini PM, Vernieri F. Ceruloplasmin/Transferrin system is related to clinical status in acute stroke. Stroke. 2009;40:1282–1288. [DOI] [PubMed] [Google Scholar]
- 120.Hu X, Li Y, Cheng P, Wu A, Li G. Serum Level of Transferrin Unique Peptide Is Decreased in Patients With Acute Ischemic Stroke. Front Neurol. 2021;12:619310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gori AM, Giusti B, Piccardi B, Nencini P, Palumbo V, Nesi M, Nucera A, Pracucci G, Tonelli P, Innocenti E, et al. Inflammatory and metalloproteinases profiles predict three-month poor outcomes in ischemic stroke treated with thrombolysis. J Cereb Blood Flow Metab. 2017;37:3253–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simats A, Garcia-Berrocoso T, Ramiro L, Giralt D, Gill N, Penalba A, Bustamante A, Rosell A, Montaner J. Characterization of the rat cerebrospinal fluid proteome following acute cerebral ischemia using an aptamer-based proteomic technology. Sci Rep. 2018;8:7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zimmermann-Ivol CG, Burkhard PR, Le Floch-Rohr J, Allard L, Hochstrasser DF, Sanchez JC. Fatty acid binding protein as a serum marker for the early diagnosis of stroke: a pilot study. Mol Cell Proteomics. 2004;3:66–72. [DOI] [PubMed] [Google Scholar]
- 124.Martinez-Morillo E, Garcia Hernandez P, Begcevic I, Kosanam H, Prieto Garcia B, Alvarez Menendez FV, Diamandis EP. Identification of novel biomarkers of brain damage in patients with hemorrhagic stroke by integrating bioinformatics and mass spectrometry-based proteomics. J Proteome Res. 2014;13:969–981. [DOI] [PubMed] [Google Scholar]
- 125.Dayon L, Turck N, Garci-Berrocoso T, Walter N, Burkhard PR, Vilalta A, Sahuquillo J, Montaner J, Sanchez JC. Brain extracellular fluid protein changes in acute stroke patients. J Proteome Res. 2011;10:1043–1051. [DOI] [PubMed] [Google Scholar]
- 126.Dodd WS, Laurent D, Dumont AS, Hasan DM, Jabbour PM, Starke RM, Hosaka K, Polifka AJ, Hoh BL, Chalouhi N. Pathophysiology of Delayed Cerebral Ischemia After Subarachnoid Hemorrhage: A Review. J Am Heart Assoc. 2021;10:e021845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maurer MH, Haux D, Sakowitz OW, Unterberg AW, Kuschinsky W. Identification of early markers for symptomatic vasospasm in human cerebral microdialysate after subarachnoid hemorrhage: preliminary results of a proteome-wide screening. J Cereb Blood Flow Metab. 2007;27:1675–1683. [DOI] [PubMed] [Google Scholar]
- 128.Tobieson L, Ghafouri B, Zsigmond P, Rossitti S, Hillman J, Marklund N. Dynamic protein changes in the perihaemorrhagic zone of Surgically Treated Intracerebral Haemorrhage Patients. Sci Rep. 2019;9:3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dawson J, Walters M, Delles C, Mischak H, Mullen W. Urinary proteomics to support diagnosis of stroke. PLoS One. 2012;7:e35879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang P, Lo LH, Chen YC, Lin RT, Shiea J, Liu CK. Serum free hemoglobin as a novel potential biomarker for acute ischemic stroke. J Neurol. 2009;256:625–631. [DOI] [PubMed] [Google Scholar]
- 131.Qin C, Zhao XL, Ma XT, Zhou LQ, Wu LJ, Shang K, Wang W, Tian DS. Proteomic profiling of plasma biomarkers in acute ischemic stroke due to large vessel occlusion. J Transl Med. 2019;17:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sharma R, Gowda H, Chavan S, Advani J, Kelkar D, Kumar GS, Bhattacharjee M, Chaerkady R, Prasad TS, Pandey A, et al. Proteomic Signature of Endothelial Dysfunction Identified in the Serum of Acute Ischemic Stroke Patients by the iTRAQ-Based LC-MS Approach. J Proteome Res. 2015;14:2466–2479. [DOI] [PubMed] [Google Scholar]
- 133.Penn AM, Saly V, Trivedi A, Lesperance ML, Votova K, Jackson AM, Croteau NS, Balshaw RF, Bibok MB, Smith DS, et al. Differential Proteomics for Distinguishing Ischemic Stroke from Controls: a Pilot Study of the SpecTRA Project. Transl Stroke Res. 2018;9:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Connell GC, Stafford P, Walsh KB, Adeoye O, Barr TL. High-Throughput Profiling of Circulating Antibody Signatures for Stroke Diagnosis Using Small Volumes of Whole Blood. Neurotherapeutics. 2019;16:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. [DOI] [PubMed] [Google Scholar]
- 136.Dolmans LS, Rutten F, Bartelink MEL, van Dijk EJ, Nederkoorn PJ, Kappelle J, Hoes AW, group M-Ts. Serum biomarkers in patients suspected of transient ischaemic attack in primary care: a diagnostic accuracy study. BMJ Open. 2019;9:e031774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.George PM, Mlynash M, Adams CM, Kuo CJ, Albers GW, Olivot JM. Novel TIA biomarkers identified by mass spectrometry-based proteomics. Int J Stroke. 2015;10:1204–1211. [DOI] [PubMed] [Google Scholar]
- 138.Penn AM, Bibok MB, Saly VK, Coutts SB, Lesperance ML, Balshaw RF, Votova K, Croteau NS, Trivedi A, Jackson AM, et al. Verification of a proteomic biomarker panel to diagnose minor stroke and transient ischaemic attack: phase 1 of SpecTRA, a large scale translational study. Biomarkers. 2018;23:392–405. [DOI] [PubMed] [Google Scholar]
- 139.Penn AM, Bibok MB, Saly VK, Coutts SB, Lesperance ML, Balshaw RF, Votova K, Croteau NS, Trivedi A, Jackson AM, et al. Validation of a proteomic biomarker panel to diagnose minor-stroke and transient ischaemic attack: phase 2 of SpecTRA, a large scale translational study. Biomarkers. 2018;23:793–803. [DOI] [PubMed] [Google Scholar]
- 140.Allard L, Lescuyer P, Burgess J, Leung KY, Ward M, Walter N, Burkhard PR, Corthals G, Hochstrasser DF, Sanchez JC. ApoC-I and ApoC-III as potential plasmatic markers to distinguish between ischemic and hemorrhagic stroke. Proteomics. 2004;4:2242–2251. [DOI] [PubMed] [Google Scholar]
- 141.Lopez MF, Sarracino DA, Prakash A, Athanas M, Krastins B, Rezai T, Sutton JN, Peterman S, Gvozdyak O, Chou S, et al. Discrimination of ischemic and hemorrhagic strokes using a multiplexed, mass spectrometry-based assay for serum apolipoproteins coupled to multi-marker ROC algorithm. Proteomics Clin Appl. 2012;6:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malicek D, Wittig I, Luger S, Foerch C. Proteomics-Based Approach to Identify Novel Blood Biomarker Candidates for Differentiating Intracerebral Hemorrhage From Ischemic Stroke-A Pilot Study. Front Neurol. 2021;12:713124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qi Z, Yuan S, Zhou X, Ji X, Liu KJ. Isobaric Tags for Relative and Absolute Quantitation-Based Quantitative Serum Proteomics Analysis in Ischemic Stroke Patients With Hemorrhagic Transformation. Front Cell Neurosci. 2021;15:710129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nguyen VA, Riddell N, Crewther SG, Faou P, Rajapaksha H, Howells DW, Hankey GJ, Wijeratne T, Ma H, Davis S, et al. Longitudinal Stroke Recovery Associated With Dysregulation of Complement System-A Proteomics Pathway Analysis. Front Neurol. 2020;11:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dagonnier M, Cooke IR, Faou P, Sidon TK, Dewey HM, Donnan GA, Howells DW. Discovery and Longitudinal Evaluation of Candidate Biomarkers for Ischaemic Stroke by Mass Spectrometry-Based Proteomics. Biomark Insights. 2017;12:1177271917749216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hewing B, Moore KJ, Fisher EA. HDL and cardiovascular risk: time to call the plumber? Circ Res. 2012;111:1117–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Plubell DL, Fenton AM, Rosario S, Bergstrom P, Wilmarth PA, Clark WM, Zakai NA, Quinn JF, Minnier J, Alkayed NJ, et al. High-Density Lipoprotein Carries Markers That Track With Recovery From Stroke. Circ Res. 2020;127:1274–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rao NM, Capri J, Cohn W, Abdaljaleel M, Restrepo L, Gornbein JA, Yong WH, Liebeskind DS, Whitelegge JP. Peptide Composition of Stroke Causing Emboli Correlate with Serum Markers of Atherosclerosis and Inflammation. Front Neurol. 2017;8:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Suissa L, Guigonis JM, Graslin F, Robinet-Borgomano E, Chau Y, Sedat J, Lindenthal S, Pourcher T. Combined Omic Analyzes of Cerebral Thrombi: A New Molecular Approach to Identify Cardioembolic Stroke Origin. Stroke. 2021;52:2892–2901. [DOI] [PubMed] [Google Scholar]
- 150.Otero-Ortega L, Alonso-Lopez E, Perez-Mato M, Laso-Garcia F, Gomez-de Frutos MC, Diekhorst L, Garcia-Bermejo ML, Conde-Moreno E, Fuentes B, Alonso de Lecinana M, et al. Similarities and Differences in Extracellular Vesicle Profiles between Ischaemic Stroke and Myocardial Infarction. Biomedicines. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Otero-Ortega L, Alonso-Lopez E, Perez-Mato M, Laso-Garcia F, Gomez-de Frutos MC, Diekhorst L, Garcia-Bermejo ML, Conde-Moreno E, Fuentes B, de Lecinana MA, et al. Circulating Extracellular Vesicle Proteins and MicroRNA Profiles in Subcortical and Cortical-Subcortical Ischaemic Stroke. Biomedicines. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jodicke RA, Huo S, Krankel N, Piper SK, Ebinger M, Landmesser U, Floel A, Endres M, Nave AH. The Dynamic of Extracellular Vesicles in Patients With Subacute Stroke: Results of the “Biomarkers and Perfusion-Training-Induced Changes After Stroke” (BAPTISe) Study. Front Neurol 2021;12:731013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huo S, Krankel N, Nave AH, Sperber PS, Rohmann JL, Piper SK, Heuschmann PU, Landmesser U, Endres M, Siegerink B, et al. Endothelial and Leukocyte-Derived Microvesicles and Cardiovascular Risk After Stroke: PROSCIS-B. Neurology. 2021;96:e937–e946. [DOI] [PubMed] [Google Scholar]
- 154.Yang Y, Peng J, Wang S, Huang J, Ran H, Chen K, Zhou Z. Serum-Based Proteomics Reveals Lipid Metabolic and Immunoregulatory Dysregulation in Cervical Artery Dissection With Stroke. Front Neurol. 2020;11:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.O’Connell GC, Walsh KB, Burrage E, Adeoye O, Chantler PD, Barr TL. High-throughput profiling of the circulating proteome suggests sexually dimorphic corticosteroid signaling following ischemic stroke. Physiol Genomics. 2018;50:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Maglinger B, Frank JA, McLouth CJ, Trout AL, Roberts JM, Grupke S, Turchan-Cholewo J, Stowe AM, Fraser JF, Pennypacker KR. Proteomic changes in intracranial blood during human ischemic stroke. J Neurointerv Surg. 2021;13:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maglinger B, Sands M, Frank JA, McLouth CJ, Trout AL, Roberts JM, Grupke S, Turchan-Cholewo J, Stowe AM, Fraser JF, et al. Intracranial VCAM1 at time of mechanical thrombectomy predicts ischemic stroke severity. J Neuroinflammation. 2021;18:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, Zangerle A, Werner P, Ferrari J, Schmidauer C, et al. Functional recovery after ischemic stroke--a matter of age: data from the Austrian Stroke Unit Registry. Neurology. 2012;78:279–285. [DOI] [PubMed] [Google Scholar]
- 159.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. [DOI] [PubMed] [Google Scholar]
- 160.Stenz KT, Just J, Blauenfeldt RA, Drasbek KR. Extracellular Vesicles in Acute Stroke Diagnostics. Biomedicines. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fraser JF, Collier LA, Gorman AA, Martha SR, Salmeron KE, Trout AL, Edwards DN, Davis SM, Lukins DE, Alhajeri A, et al. The Blood And Clot Thrombectomy Registry And Collaboration (BACTRAC) protocol: novel method for evaluating human stroke. J Neurointerv Surg. 2019;11:265–270. [DOI] [PubMed] [Google Scholar]
- 162.Montaner J, Ramiro L, Simats A, Tiedt S, Makris K, Jickling GC, Debette S, Sanchez JC, Bustamante A. Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nat Rev Neurol. 2020;16:247–264. [DOI] [PubMed] [Google Scholar]
- 163.Joshi A, Rienks M, Theofilatos K, Mayr M. Systems biology in cardiovascular disease: a multiomics approach. Nat Rev Cardiol. 2021;18:313–330. [DOI] [PubMed] [Google Scholar]
- 164.Mi X, Zou B, Zou F, Hu J. Permutation-based identification of important biomarkers for complex diseases via machine learning models. Nat Commun. 2021;12:3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mann M, Kumar C, Zeng WF, Strauss MT. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 2021;12:759–770. [DOI] [PubMed] [Google Scholar]
- 166.Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47:W191–W198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.