Abstract
Background
Little is known about metabolic and nutrition characteristics of patients with coronavirus disease 2019 (COVID‐19) and persistent critical illness. We aimed to compare those characteristics in patients with PCI and COVID‐19 and patients without COVID‐19 infection (non‐CO)—primarily, their energy balance.
Methods
This is a prospective observational study including two consecutive cohorts, defined as needing intubation for >10 days. We collected demographic data, severity scores, nutrition variables, length of stay, and mortality.
Results
Altogether, 104 patients (52 per group) were included (59 ± 14 years old [mean ± SD], 75% men) between July 2019 and May 2020. SAPSII, Nutrition Risk Screening (NRS) score, proportion of obese patients, duration of intubation (18.2 ± 11.7 days), and mortality rates were similar. Patients with COVID‐19 (vs non‐CO) had lower SOFA scores (P = 0.013) and more frequently needed prone position (P < 0.0001) and neuromuscular blockade (P < 0.0001): lengths of ICU (P = 0.03) and hospital stays were shorter (P < 0.0001). Prescribed energy targets were below those of the ICU protocol. The energy balance of patients with COVID‐19 was significantly more negative after day 10. Enteral nutrition (EN) started earlier (P < 0.0001). During the first 10 days, COVID‐19 patients received more lipid (propofol sedation) and less protein. Higher admission C‐reactive protein (P = 0.002) decreased faster (P < 0.001). Whereas intestinal function was characterized by constipation in both groups during the first 10 days, diarrhea was less common in patients with COVID‐19 thereafter.
Conclusion
Compared with non‐CO patients, COVID‐19 patients were not more obese, had lower SOFA scores, and were fed more rapidly with EN, because of a more normal gastrointestinal function possibly due to fewer non–respiratory organ failures: their energy balances were more negative after the first 10 days. Propofol sedation reduced protein delivery.
Keywords: energy deficit, gastrointestinal function, hypophosphatemia, lipid, propofol, protein, refeeding syndrome risk, underfeeding
CLINICAL RELEVANCY STATEMENT
Data regarding metabolism and nutrition of critically ill patients with coronavirus disease 2019 (COVID‐19) are scarce, as clinical research has predominantly focused on drug treatments and respiratory management. The patients with COVID‐19 frequently require prolonged mechanical ventilation, and COVID‐19 evolves into persistent critical illness. However, the present data suggest that after the initial higher risk of refeeding syndrome, the principal risk is an underprescription of energy and protein in the presence of higher weight, leading to energy and protein deficit. Early enteral feeding was surprisingly easy with a near‐normal gastrointestinal function. The resulting low protein delivery requires close monitoring and adaptation of feeding solutions.
INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has resulted in an overwhelming number of intensive care unit (ICU) admissions, generally motivated by severe acute hypoxemic respiratory failure requiring mechanical ventilation. In the Lausanne ICU, the number of beds enabling mechanical ventilation was increased from 35 to 76 within 6 weeks. Research predominantly has focused on respiratory, renal, hematological, and infectious manifestations and organizational aspects, 1 and there are still limited data regarding the metabolic and nutrition status of the patients with COVID‐19 compared with other categories of patients without COVID‐19 (non‐CO).
Since the first systematic review by intensivists from Wuhan recommending enteral nutrition (EN) and energy goals of 25–30 kcal/day, 2 several societies, including the European Society for Clinical Nutrition and Metabolism (ESPEN), have attempted to provide guidance with the application of previous general critically ill patients’ recommendations to patients with COVID‐19. 2 , 3 , 4 However, objective data remain limited. Some authors reported unexpected and unprecedented challenges to achieve adequate nutrition with the gastrointestinal (GI) tract's limited tolerance of EN, possibly related to the need for heavy sedation and neuromuscular blockades. 5 , 6 The lack of prospective data was emphasized in the scoping review conducted by the American Society for Parenteral and Enteral Nutrition (ASPEN), which summarized the numerous unresolved questions regarding the specificities of the patients with COVID‐19. 7
From a metabolic point of view, the strongly inflammatory COVID‐19 disease and its treatment, with heavy sedation and frequent prone position, are indeed likely to modify energy needs, GI function, and response to treatment. Two recent studies finally provided guidance about energy targets by using indirect calorimetry (IC)–measured energy expenditure. 8 , 9 They show that energy expenditure neither was as elevated as previously hypothesized 3 nor was depressed. But other aspects of metabolism have not yet been described.
The risk of acute malnutrition, assessed by the Nutrition Risk Screening (NRS) score, seems elevated according to a cohort including 413 critically ill patients from Wuhan: there, the proportion of patients with high NRS scores (≥5) was surprisingly high, which was associated with significantly higher mortality. 10 This high score was attributable to strongly reduced feeding during the days preceding the admission, because of coughing and fever.
Observational studies show that about 5% of ICU patients will develop persistent critical illness (PCI), consuming up to 32.8% of total ICU bed‐days. 11 Also, these patients have a greater risk of death and disproportionately consume vast health resources compared with patients without PCI. 11 All these features are extremely important for bedside decision‐making and prognostication, especially during a pandemic, during which resources are limited. It has been reported that critically ill patients with COVID‐19 frequently have a prolonged ICU stay. 12 , 13 However, to our knowledge, no study has compared persitent critically ill patients with and without COVID‐19.
As the pandemic still rages worldwide, it is time to draw lessons from the first wave and develop practical orientation. Patients with COVID‐19 constitute a rather homogeneous population characterized by a predominant respiratory failure with comorbidities mainly linked to a metabolic syndrome. In the Lausanne ICU, many patients with COVID‐19 fulfilled the previously defined criteria for PCI, with prolonged ICU admissions. As our ICU had developed a program for general patients with PCI, 14 the present study aimed at prospectively collecting metabolism‐ and nutrition‐related variables of patients with COVID‐19 to compare them with the non‐CO patients in an attempt to identify specific problems related to their management, with an emphasis on energy balance.
METHODS
Study design
This prospective observational study includes two consecutive cohorts of patients with PCI who were on mechanical ventilation, as well as a retrospective analysis of the data. It was conducted with the approval of the Commission cantonale d’éthique de la recherche sur l’être humain (CER 2018‐02018 and CER‐2020‐01453) in the multidisciplinary adult ICU of the Lausanne University Hospital (registered at ClinicalTrials.gov; clinical trial identifiers: NCT03938961 and NCT05026151). The need to obtain individual consent was waived, and absence of refusal to use coded data was verified.
Study end point
The primary end point was the cumulative energy balance during the stay, defined as the difference between prescribed and delivered energy calculated daily for 30 days or the length of ICU stay. Analysis of energy balance addressed two different periods: the first 10 days of ICU stay and the period from day 10 to day 30 or ICU discharge, whichever came first. Secondary end points are time to nutrition initiation, number of fasting days, feeding route, energy and substrate delivery (protein, lipid, and glucose) during the ICU stay (considering also the first 10 days and subsequent days until day 30 or discharge), mean daily blood glucose values (arterial samples), insulin requirements, intestinal function, propofol dose, serum prealbumin values (admission and during stay), blood phosphate, triglycerides, C‐reactive protein (CRP), and mortality. Time on each feeding route (fasting; EN, parenteral nutrition [PN], or combined EN + PN; and oral) for each patient was recorded for a maximum of 30 days.
Patients
Inclusion criteria were age >18 years, enrollment in the ICU's PCI program for the pre–COVID‐19 period, and >10 days of invasive mechanical ventilation for both cohorts. 15 Exclusion criteria were admission for major burns >20% body surface, traumatic brain injury, and refusal to participate.
Study variables
Data of the first 30 days in the ICU were extracted from electronic medical records (EMRs) (version 5.46.44; Metavision iMDsoft). Recorded variables were age; severity of disease (Simplified Acute Physiology Score II [SAPS II] and admission Sequential Organ Failure Assessment [SOFA] scores); admission body weight (BW) (defined as the preadmission “dry” BW—ie, before fluid resuscitation); ideal BW (IBW); discharge BW; body mass index (BMI; [kg]/[m2]); NRS‐2002 score, 16 with emphasis on preadmission nutrition intake (NRS‐“A” for “alimentation”); time to feeding initiation; feeding route; bowel activity; energy, protein, and glucose intakes; energy balance; and frequency and timing of IC. Furthermore, requirement for continuous renal replacement therapy (CRRT), prone position, neuromuscular blockade (cisatracurium or rocuronium), duration of mechanical ventilation and ICU stay, and mortality (ICU and 90 days) were recorded. Laboratory variables were recorded during the first 30 days: blood creatinine, urea, triglycerides, and prealbumin (routine weekly value).
Nutrition protocol
The ICU's standard operating procedures (SOPs) are based on the ESPEN guidelines 17 and were not modified during the study period. Briefly, EN is recommended as the preferential route, to be initiated within the first 48–72 h, after hemodynamic stabilization. Gastric EN is started at a rate of 10–20 ml/h, depending on patient size and hemodynamic stability (never at full target, by ESPEN recommendation 17 ), and ramped up over 3–4 days to target according to GI tolerance. Gastric residual volumes (GRVs) are measured twice daily: metoclopramide and erythromycin are used in case of GRV >250 ml (no change for prone position). Postpyloric feeding is a rare option. PN may be used as supplemental nutrition (SPN) or as exclusive PN from day 4 and earlier in selected patients with malnutrition. An energy target of 20 kcal/kg/day is recommended during the first week, determined by using admission BW (IBW if BMI ≥ 30), to be increased thereafter to 25 kcal/kg (actual BW or IBW) and/or adapted by IC after fist week (ideally weekly in the intubated patients). For patients in whom IC is not feasible and after extubation, surrogate equations are the Harris‐Benedict equation in obese patients and 25 kcal/kg or Faisy‐Fagon 18 in patients with normal BW. The protein target is 1.2–1.3 g/kg BW for BMI ≤ 30 or IBW for BMI > 30. Lipid delivery should not exceed 1 g/kg/day. Substrate intakes include nutrition and nonnutrition sources (drug dilution and sedation). Insulin delivery is nurse driven, with a blood glucose target of 6–8 mmol/L in general ICU patients and 6–10 mmol/L in diabetic and obese patients.
For the IC protocol, the Q‐NRG (Cosmed, Italy) gas analyzer device is connected to the ventilator circuit next to the endotracheal tube, and the second analyzer is connected to the ventilator port after the filter. IC measurement is considered possible as soon as a steady‐state condition is reached, defined as a coefficient of variation of VO2 and VCO2 ≤5% for 5 min: the measure is continued for 10–15 min. 19 The Q‐NRG device is calibrated once monthly according to manufacturer instructions.
Two dietitians shared one supervisory position throughout the study period. Before the pandemic, they were physically attending the nurse morning report during weekdays. During the COVID‐19 phase, both dietitians were working remotely and did not realize IC studies: early morning meetings were replaced by daily phone calls and emails to nurses. The potential number of patients to supervise at a distance increased from 35 to 76.
Energy balance was calculated by computer daily as the difference between total energy delivery and prescribed value. Cumulative energy balances were the sum of daily balances. SOPs recommend an energy target of 20 kcal/kg in the first week, to be increased in the absence of IC to 25 kcal/kg thereafter. The increase was frequently not done in patients with COVID‐19, and targets remained at the initial level: the targets and balances were recalculated after day 10 and called “per protocol.”
Feeding solutions were the same in both periods (Table S1): pharmacy‐compounded PN was available for individual cases. Whatever the feeding route, an intravenous “stress micronutrient profile” was administered during the first 6 days (local ICU SOP): thiamin 100 mg, vitamin C 500 mg, zinc 5 mg, one vial multi–trace element (Addaven; Fresenius Kabi), and one vial multivitamin (Cernevit; Baxter).
Daily protein, lipid, and glucose delivery was extracted from the EMR. Median protein delivery per kilogram of BW or IBW was calculated: a cumulative protein deficit of −300 g by day 20 was considered a critical cutoff. 20
Stools were defined as absent (>4 days: constipation), normal, diarrhea (more than three stools per day), fluid (response to enema), gas, or melena (black liquid stools).
SOPs recommend measuring GRVs every 12 h (more in case of high volumes): values ≥50 ml were recorded, and any value ≥500 ml/24 h was considered abnormal (number of days was recorded). Recommended prokinetics are metoclopramide (3 × 10–20 mg/day), followed by erythromycin (500 mg/day for 48 h).
Statistical analysis
There was no sample size calculation: consecutive non‐CO patient enrollment stopped with the first COVID‐19 wave, and patients were compared with the next consecutive patients with COVID‐19 who fulfilled the inclusion criterion of >10 days of invasive mechanical ventilation.
According to their distribution (the Shapiro‐Wilk test used for normality), data are reported as mean ± SD or median (IQR). Data analysis is limited to the first 30 days of the ICU stay. Two phases of the stay were considered: the first 10 days (days 1–10) and days 11–30, with the first corresponding to the minimal duration of mechanical ventilation and to the previously observed inflexion of high variability in nutrition delivery. 14 Comparisons between periods were conducted by using one‐way and two‐way analysis of variance (continuous variables), Wilcoxon rank tests, and chi‐squared tests (categorical variables). Kaplan‐Meier analysis was used to compare mortality. Data analyses were conducted by using JMP (version 14.2 for Windows; SAS Institute) and R version 4.0.3 (R Foundation for Statistical Computing, 2020). Significance level was set at P < 0.05.
RESULTS
Patients
Altogether, 104 patients (52 non‐CO and 52 with COVID‐19) were enrolled between July 2019 and May 2020 (CONSORT diagram in Figure 1): 52 (42.6%) of the 122 patients with COVID‐19 who were admitted during the first COVID‐19 wave fulfilled the PCI criteria. Table 1 summarizes their characteristics and clinical outcomes variables. Age, gender, and SAPS II and NRS scores were similar in both groups. Patients with COVID‐19 were heavier, but the proportion of obese patients was similar. Whereas only 15 of 52 (29%) non‐CO patients were admitted for acute respiratory distress syndrome (ARDS), this was the case for 50 of 52 (96%) patients with COVID‐19. McCabe (P = 0.002) and SOFA scores (P = 0.013) were lower in patients with COVID‐19, with a predominance of the respiratory component. Mechanical ventilation strategies differed significantly, with 42 (81%) of the patients with COVID‐19 (vs 1 non‐CO patient) being ventilated in a prone position for a median duration of 3 days (P < 0.0001). But the length of mechanical ventilation and of ICU and hospital stays did not differ, nor did ICU or hospital mortality (Figure S1). Mortality was similarly significantly higher in both groups in patients with an NRS score >5 (P = 0.042).
Figure 1.
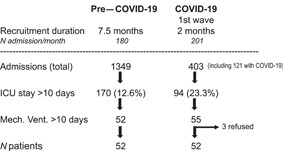
CONSORT diagram of the two cohorts: enrollment was between July 2019 and May 2020. COVID‐19, coronavirus disease 2019; ICU, intensive care unit; Mech. Vent., mechanical ventilation
Table 1.
Characteristics of the patients
| Non–COVID‐19 (n = 52) | COVID‐19 (n = 52) | P value | |
|---|---|---|---|
| Age, years | 60 (51–72) | 61 (56–71) | 0.197 |
| Sex, female/male | 13/39 | 13/39 | 1.00 |
| Adm BW, kg | 77 (63–90) | 85 (75–97) | 0.001 |
| Ideal BW, kg | 67 (61–72) | 70 (61–75) | 0.453 |
| Height, m | 1.72 (1.67–1.80) | 1.74 (1.68–1.80) | 0.345 |
| BMI, kg/m2 | 25.05 (21.07–31.48) | 27.72 (25.57–33.75) | 0.003 |
| Obesity (≥30), n (%) | 15 (28.9) | 20 (38.5) | 0.300 |
| Discharge BW, kg | 72.0 (57.0–84.4) | 86.0 (72.5–95.5) | 0.001 |
| Weight loss, kg | 4.0 (0.5–9.2) | 2.5 (0–6.7) | 0.513* |
| McCabe score | 0.002 | ||
| Nonfatal | 30 | 45 | |
| Fatal 5 years | 19 | 7 | |
| Fatal 6 months | 3 | 0 | |
| SAPS II | 41 (33–59) | 40 (33–46) | 0.138 |
| SOFA Adm score | 8 (6–11.2) | 7 (5–8) | 0.013 |
| Length of mech. vent., days | 15.5 (10.3–21.8) | 16.5 (11.6–22.8) | 0.422 |
| Patients on CRRT, n (%) | 14 (26.9) | 7 (13.5) | 0.059 |
| Length ICU stay, days | 26.6 (21.0–40.9) | 20.3 (15.8–29.0) | 0.030 |
| Length of hospital stay, days | 52.9 (27.4–79.7) | 29.04 (23.39–40.7) | <0.0001 |
| ICU mortality, n (%) | 10 (19.2) | 8 (15.4) | 0.604 |
| Hospital mortality, n (%) | 13 (25.0) | 9 (17.3) | 0.336 |
| Metabolic variables | |||
| Diabetes preadmission | 16/52 | 11/52 | 0.650 |
| NRS | 5 (4–6) | 5 (4–6) | 0.742 |
| NRS‐A‐food intake (median (IQR) (mean ± SD) | 1.5 (0–3) (1.4 ± 1.2) | 2 (1–3) (1.8 ± 1.0) | 0.089 |
| NRS ≥ 5, n (%) | 34 (65.4) | 35 (67.3) | 0.6338 |
| Harris‐Benedict, kcal | 1560 (1295–1158) | 1647 (1482–1863) | 0.007 |
| Energy goal of 20 kcal/kg/day | 1530 (1260–1800) | 1700 (1505–1930) | 0.001 |
| Time to feeding, days (median (IQR)) | 1.0 (0–2.0) | 0.5 (0–1.0) | <0.0001 |
| Mean ± SD | 1.33 ± 1.24 | 0.52 ± 0.54 | |
| CRP (Adm), mg/L | 108 (42–214) | 202 (130–283) | 0.003 |
| Prealbumin (Adm), g/L | 0.095 (0.07–0.14) | 0.070 (0.06–0.09) | 0.014 |
| Delta prealbumin, g/L | 0.025 (0.01–0.06) | 0.19 (0.09–0.32) | <0.0001 |
Note: Data are presented as median (IQR) unless described otherwise.
Abbreviations: Adm, admission; BMI, body mass index; BW, body weight; COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; CRRT, continuous renal replacement therapy; ICU, intensive care unit; mech. vent., mechanical ventilation; NRS, Nutrition Risk Screening; SAPS II, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment.
Significant weight loss (P < 0.0001) but similar and parallel in both groups.
Sedation was mainly by propofol during the first 2 weeks in patients with COVID‐19, resulting in significantly higher doses exceeding 5000 mg/day vs 1800 mg/day in non‐CO patients (P < 0.0001) (Figure 2).
Figure 2.
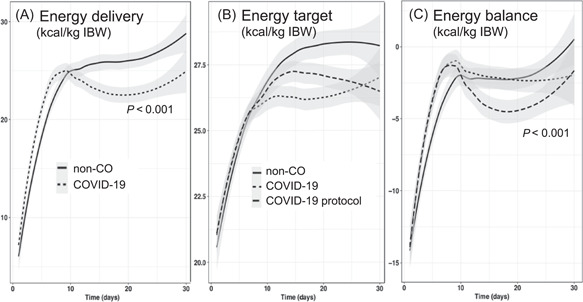
Evolution of energy delivery (A), energy target (B), and energy balance (C) of the non‐CO and COVID‐19 cohorts. In (A), the energy delivery per kilogram of IBW is significantly lower in the patients with COVID‐19 after day 10 (P < 0.001), as the non‐CO patients have a progressive adaptation of their targets and hence of deliveries. In (B) and (C), the prescribed (COVID‐19) and per‐protocol (COVID‐19 protocol) energy targets and balances are compared with those of the non‐CO patients. The prescribed values differ significantly from those on day 11 and from those of non‐CO patients. COVID‐19, coronavirus disease 2019; IBW, ideal body weight; non‐CO, non–COVID‐19
The number of patients requiring paralysis via neuromuscular blockade during mechanical ventilation was significantly higher in the COVID‐19 cohort (Table 2; P = 0.0002), as was the duration of paralysis (P < 0.0001).
Table 2.
Variables associated with gastrointestinal impact (Bowel activity: chi‐squared test)
| Non–COVID‐19 (n = 52) | COVID‐19 (n = 52) | P value | |
|---|---|---|---|
| Prone mech. vent., n | 1 | 42 | <0.0001 |
| Length of proning, median (IQR), days | 0 (0–0) | 3 (1–5.8) | <0.0001 |
| Myorelaxants, n | 39 | 51 | <0.0001 |
| Length, median (IQR), days | 4 (2–7) | 9 (6–12) | <0.0001 |
| High GRV (>500 ml/day), n (%) | 15 (29) | 23 (44) | 0.102 |
| Length, median (IQR), days | 2 (1–4) | 2 (2–4) | 0.934 |
| Bowel activity on days 1–10 (total 1035 days), n | 514 | 515 | 0.453 |
| Constipation, n (%) | 308 (59.2) | 321 (62.3) | NS |
| Enema fluid, n | 17 | 22 | |
| Diarrhea, n (%) | 106 (20.6) | 105 (20.4) | |
| Normal, n (%) | 83 (16.2) | 67 (13.0) | |
| Bowel activity on days 11–30 (total 1314 days), n | 732 | 593 | 0.0005 |
| Constipation, n (%) | 249 (34.0) | 214 (36.1) | |
| Enema fluid, n | 23 | 14 | |
| Diarrhea, n (%) | 244 (33.3) | 143 (23.9) | |
| Normal, n (%) | 216 (29.5) | 223 (37.6) |
Abbreviations: COVID‐19, coronavirus disease 2019; GRV, gastric residual volume; mech. vent., mechanical ventilation; NS, not significant.
Nutrition
Altogether, 2349 days were analyzed, evenly distributed among the periods (1035 days for the first 10‐day period and 1314 days for days 11–30). The time to feeding was significantly shorter in patients with COVID‐19, with a median time of 0.5 days (0.5 days [IQR, 0–1] vs 1.3 days [IQR, 0–2] in non‐CO patients; P < 0.0001) realized by early EN. Figure S2 shows that EN was predominant in both periods, being significantly more frequent and earlier in patients with COVID‐19, with less fasting and fewer PN and SPN days.
IC studies were conducted in 40 (72.3%) non‐CO patients with a time to first IC of 7 (IQR, 4–15) days, but studies were conducted in none of the patients with COVID‐19. Whereas the Faisy‐Fagon equation was used as a surrogate equation in 27 non‐CO patients, it was only used in four patients with COVID‐19.
Energy delivery and balances
Figure 2 shows the evolution of energy target, delivery, and balance during the ICU stay. During the first 10 days, the median daily and cumulated energy balances were less negative in the patients with COVID‐19, with −2736 kcal (−4541 to −1423 kcal) vs −4038 kcal (−5522 to −2213 kcal) in the non‐CO patients (P = 0.04). After observing that the energy prescription was significantly below that of the ICU SOP after day 10 (P < 0.001), the energy targets per protocol were recalculated to 25 kcal/kg (BW or IBW). Figure 2B and C shows two different lines for the patients with COVID‐19: energy balances are significantly (P < 0.001) more negative when the SOP targets are applied (COVID‐19 protocol).
However, the weight loss between admission and discharge did not differ (−4 kg in non‐CO patients vs −2.5 kg in patients with COVID‐19; P = 0.513).
Protein
Mean protein intake during the first 10 days was higher in the patients with COVID‐19 (63.5 g/day vs 50.6 g/day; P = 0.0012) because of the earlier feeding start. However, protein delivery per kilogram (BW or IBW) over the entire stay was lower in patients with COVID‐19 (0.93 g/kg) than in non‐CO patients (1.03 g/kg) (P = 0.020) (Figure 3). The protein deficit by day 20 was accordingly higher, with a median value of −532 g [−820 to −330] vs −370 g [−545 to −153] in non‐CO patients (P = 0.0077). The number of patients exceeding the deficit cutoff of −300 g was modestly higher among patients with COVID‐19 than among non‐CO patients—24 (46.2%) vs 17 (32.7%), respectively (P = 0.160: nonsignificant).
Figure 3.
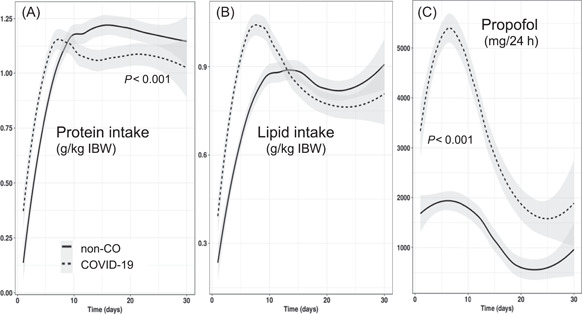
Evolution of the daily protein (A) and lipid (B) intakes and of the propofol (C) doses. Protein intake is significantly lower after day 10 in the COVID‐19 cohort (P < 0.001). Lipid intake is significantly higher because of the significantly higher propofol doses. COVID‐19, coronavirus disease 2019; IBW, ideal body weight
Lipid
Lipid intake was significantly higher during the first 10 days in the patients with COVID‐19, coming from the propofol lipids; the doses remained higher through the 30 days (Figure 3), resulting in the delivery of fat above the 1‐g/kg recommendation in 20 (38.5%) patients with COVID‐19 vs 6 non‐CO patients (P = 0.002).
Glucose
Glucose intakes (Figure 4) were similar during the first 10 days and became significantly lower in patients with COVID‐19 thereafter, in parallel with energy intake.
Figure 4.
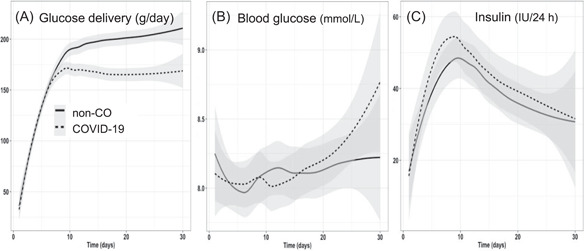
Evolution of glucose delivery (g/day), blood glucose, and 24‐h insulin doses. Despite significantly lower glucose intakes in the patients with COVID‐19 after day 10, this does not translate into significant changes in blood glucose or in 24‐h insulin requirements. COVID‐19, coronavirus disease 2019
Blood glucose and insulin
Prescribed blood glucose targets were frequently tighter (6–8 mmol/L) in the non‐CO patients (n = 29 vs 25) and wider (6–10 mmol/L) in the patients with COVID‐19 (n = 27 vs 23 in non‐CO patients) (Pearson, P = 0.005). But daily insulin doses did not differ, despite the lower glucose intake in patients with COVID‐19. Neither mean blood glucose values nor blood glucose variability differed.
Intestinal function
High GRVs occurred in both periods (Table 2) and persisted for a similar duration (0.8 vs 1.2 days in non‐CO patients and patients with COVID‐19, respectively; P = 0.082). Neuromuscular blockade was not associated with either high GRV (P = 0.207) or use of prokinetics (P = 0.759). During the first 10 days, absence of bowel activity (constipation) predominated in both groups: diarrhea was present for 20.4% of days in both groups (Figure S3). After day 10, the transit was significantly more frequently normal in patients with COVID‐19 (P = 0.0003), whereas diarrhea was more frequent in non‐CO patients.
Laboratory values
The inorganic phosphate (Pi) values observed during the first 48 h were lower in patients with COVID‐19 (P = 0.032), reverting to similar values thereafter (Figure 5) with phosphate repletion.
Figure 5.
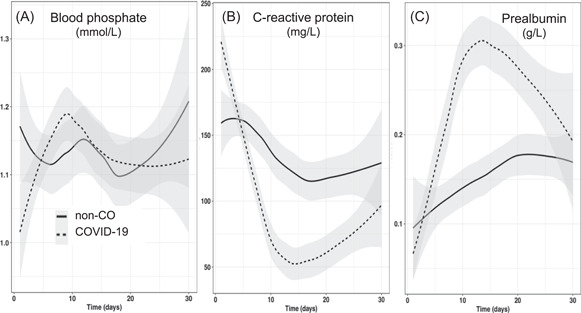
Evolution of blood phosphate, C‐reactive protein, and prealbumin levels over time. Admission blood phosphate level (first 48 h) was significantly lower in the COVID‐19 group (0.96 mmol/L [0.86–1.14] vs 1.11 mmol/L [0.86–134]; P = 0.033), whereas C‐reactive protein level was significantly higher (196 mg/L [130–281] vs 108 mg/L [42–214]; P = 0.003). COVID‐19, coronavirus disease 2019
The patients with COVID‐19 were admitted with significantly higher CRP values (Figure 5), which decreased significantly faster and remained lower than in non‐CO patients thereafter. This rapid resolution might be linked to the use of tocilizumab in 30 of 52 patients with COVID‐19.
The first check of serum prealbumin levels occurred earlier in patients with COVID‐19 (first week in all patients with COVID‐19 [100%] vs in 17 [33%] non‐CO patients; P < 0.001): the first value was significantly lower in patients with COVID‐19 (P = 0.014). It increased faster than in non‐CO patients to rejoin the non‐CO values by the end of the stay.
Renal function
Plasma creatinine values did not differ significantly between periods (Figure S4). CRRT was used less frequently in patients with COVID‐19 (26.9% vs 13.5% of patients with COVID‐19; P = 0.06). Blood urea levels were significantly higher in the patients with COVID‐19 during the first 10 days vs non‐CO patients—9.6 mmol/L [7.0–14.6] vs 8.6 mmol/L, 4 , 5 , 6 , 12 respectively (P = 0.012).
During the first 10 days, median urea/creatinine ratios were similar and, therafter, tended to be higher in the non‐CO patients (days on CRRT excluded).
DISCUSSION
This prospective comparison of persistent critically ill non‐CO patients and patients with COVID‐19 shows some differences regarding the metabolic and nutrition risks of the population with COVID‐19. Whereas SAPS II scores were similar, the lower SOFA scores reflected the predominance of respiratory failure with limited involvement of other organs, which is confirmed by the lower McCabe values indicating admission for an acute respiratory disease.
The primary end point—energy delivery and balance—differed, being respectively lower and the energy deficit being larger compared with those of the non‐CO patients. Unexpectedly, we observed a systematic underprescription of energy after the first week, which resulted in proportional lower delivery of nutrients and a false perception of adequate energy balances (Figure 2). With the use of IC, energy targets get adapted and usually increase over time. 14 In the non‐CO patients, the increase of energy target prescription and delivery after day 10 is visible in the Figure 2, whereas both remain unchanged in the patients with COVID‐19, in whom the energy target should also have increased over time in accordance with the SOPs. The “non‐adaptation” results in the energy balance, which is calculated on the basis of the predictive equation target, looking reassuring and falsely “little negative.”
Although dietitians were able to follow up on patients with COVID‐19 by using a virtual private network (or “VPN”) connection to our EMRs and to interact with the nurses through phone/mail, they had lost IC information about energy expenditure, which is essential for individual adaptation. During the first wave, the risk of viral transmission through the calorimetry device was considered too high, despite the use of disposable equipment—in addition, the dietitians were not in hospital, and the nurses were overbooked and often untrained in ICU nutrition. Observing the experience and published recommendations, 8 , 9 this strategy might need reconsideration, at least in the very obese patients, in whom energy requirements are particularly difficult to predict.
Using an EMR that integrates energy from any source (nutrition and drugs) has the advantage of showing real energy intakes. Because of the heavy sedation level required by prone position and paralysis, the patients with COVID‐19 received a very high dose of propofol, which, despite having a 2% solution, resulted in a high lipid dose, delivering 300–400 kcal/day. 21 Because the message of overfeeding prevention has been strongly delivered in the ICU, the “place” occupied by lipids in the total energy count resulted in the reduction of feeding solutions and hence of protein delivery: in addition, the blood urea level was higher in the patients with COVID‐19, who presented a modestly higher number of acute kidney injuries but were less frequently treated with CRRT, because of restricted indications associated with a lack of team training.
Glucose delivery was similarly affected as protein, with significantly lower doses being delivered in patients with COVID‐19 after day 10: blood glucose control was easy. COVID‐19 has been associated with an increased risk of insulin resistance 22 ; blood glucose abnormalities are detected for prolonged periods after disease start. In a search for more pronounced insulin resistance, the analysis of arterial blood glucose values (mean and maximal) and the insulin doses revealed no difference. The lower amount of glucose delivered to the patients with COVID‐19 (−40 g/day) because of lower targets and large lipid doses may have masked the insulin resistance.
To evaluate the nutrition risk, this study used the NRS, which is recommended by the ESPEN guidelines 17 : scores >5 have been shown to be associated with mortality in general ICU patients, 14 which was confirmed in the present study (Figure S1B). Moreover, as observed by colleagues from Wuhan, 10 the feeding part of the NRS score (food intake before admission) was modestly higher in the patients with COVID‐19 (P = 0.088), resulting in a high risk of refeeding syndrome. Indeed, significantly lower phosphate values were observed in patients with COVID‐19 during the first 48 h, requiring active treatment. 23
EN was started significantly earlier in the patients with COVID‐19, who mostly suffered single organ failure: the rapid sequence “intubation → gastric tube insertion → control chest x‐ray → proning → starting EN” only takes 3–5 h. This rapidity in execution proved to be successful for the first 10 days’ feeding and possibly contributed to maintaining GI function. Very few patients needed prokinetics. It resulted in a significant reduction of days with fasting and a more rapid progression to the initially prescribed energy target, with PN and SPN being rarely required. The limited use of PN and SPN was further motivated by the lack of specific training of both nurses and doctors, as a substantial proportion came untrained to reinforce the ICU team. Because the use of PN and SPN is more complex, it will not be easily trusted under such crisis circumstances.
The prevalence of GI problems reported in the literature is variable. In addition, mechanical ventilation while in the prone position with sedative and paralyzing drugs is also a risk factor of altered intestinal function. We were therefore expecting to observe more GI symptoms and diarrhea in these patients with severe COVID‐19. Short‐lived episodes of high GRV were found in a few cases in both groups, which is very reassuring regarding the safety of EN during prone sessions. Constipation predominated in non‐CO patients and patients with COVID‐19, being present in about 60% of patients during the first 10 days, whereas diarrhea occurred in 20.4% of patients in both groups. These positive findings regarding intestinal function contrast with worries that have been expressed in reviews. 24 Reported incidences of diarrhea vary between 2% and 50%. 25 , 26 A study from Wuhan did not signal major GI problems. 27 A metanalysis including 23 published and 6 preprint studies showed that 12% of patients with COVID‐19 were reported to manifest GI symptoms. 28 One study, using the paracetamol test, showed a 50% reduction of intestinal absorption. 29 The presence of GI symptoms has been associated with more frequent detection of RNA viruses in the stool 25 : the colonization of the GI mucous membrane is possibly causing the symptoms through alteration of the mucosal permeability. 30 In a cohort of 198 patients, Cereda et al signaled that difficult EN with large early energy deficits was associated with higher mortality. 31 A strict application of our SOPs might have favored a smoother clinical course.
Serum prealbumin levels upon admission have been suggested to be an indicator of prognosis in a cohort of 408 patients with COVID‐19: a cutoff of 0.15 g/L seems to indicate severity. 32 The majority of the patients with COVID‐19 started with very low values (Figure 5)—significantly lower than those of the non‐CO patients—that reverted to within‐normal values significantly faster vs non‐CO patients, with resolution of inflammation. The changes were so rapid that we could not rely on serum prealbumin levels to estimate feeding adequacy as we usually do. 33
Considering the greater severity of illness in the non‐CO patients, who suffered more multiorgan failure by SOFA, lower mortality rates might have been expected in the patients with COVID‐19. But because of the limited number of patients and the heterogeneity of diagnoses in the non‐CO group, comparison is hazardous. Moreover, the mortality of our selected COVID‐19 cohort is much lower than that reported by others (25%–42%). 13 Other factors such as lower protein and higher fat intakes, as well as an absence of individual adaptation of the energy target, might have contributed to a suboptimal outcome, but this remains to be verified.
Limitations
This is a prospective observational study comparing two consecutive groups: the time bias was small in terms of synchronicity and the protocols were identical, but there were differences as to the diagnosis, with heterogeneous principal diagnosis in non‐CO patients (15 of 52 with ARDS) and very homogeneous diagnosis in patients with COVID‐19 (50 of 52 with ARDS). Staffing changed massively, with a large proportion of ICU‐untrained nurses and physicians, whereas the dietitians were extramuros—all constraints being imposed by the pandemic. Also, we were unable to use IC to measure individual energy expenditure to adapt prescriptions. The calculated energy balances are therefore an inexact surrogate of IC‐based balances. This prevents us from drawing definitive conclusions. Despite these limitations, this study enabled the identification of certain nutrition characteristics of patients with COVID‐19 on the basis of the high quality of the data extracted from the EMRs in an ICU with strong SOPs.
CONCLUSION
Searching for specific COVID‐19 metabolic and nutrition characteristics, the study shows some significant differences. The patients had fewer comorbidities, had fewer organ failures, and were globally easier to feed enterally, with only rare cases of high GRV despite frequent prone position and heavy sedation and without other specific GI dysfunction. The practical difficulties were related to the higher prevalence of overweight patients while IC was unavailable. In an ICU accustomed to basing energy prescription on IC, the necessity of using equations that have been shown to favor overfeeding led to maintaining low targets, as the ICU had a strong incentive to avoid overfeeding: targets were not increased, even if this was part of the protocol. With the now available IC studies showing even higher values of energy expenditure in patients with COVID‐19 (25–35 kcal/kg), 8 this apprehension of overfeeding no longer needs to exist after day 10. The more restrictive use of renal replacement therapy, with rising urea values, was also an obstacle to respecting protein targets. To raise awareness about the energetic impact of propofol sedation, 21 we encourage searching for alternative sedation strategies that are neutral from a metabolic point of view.
CONFLICT OF INTEREST
None declared.
FUNDING INFORMATION
The study was funded exclusively by internal service resources.
AUTHOR CONTRIBUTIONS
Marina V. Viana, Olivier Pantet, and Mette M. Berger equally contributed to the conception and design of the research. Olivier Pantet, Mélanie Charrière, Doris Favre, Lise Piquilloud, Antoine G. Schneider, Claire‐Anne Hurni, and Mette M. Berger contributed to the acquisition and analysis of the data; Marina V. Viana, Olivier Pantet, and Mette M. Berger contributed to the interpretation of the data; Marina V. Viana and Mette M. Berger drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Supporting information
Supporting Information.Figure
Supporting Information.
Supporting Information.
ACKNOWLEDGMENTS
We thank Prof Philippe Eckert (past head of the ICU and actual General Director of CHUV, Lausanne) for internal resources to support of the study and Mrs Tatiana Kelevina (ICU nurse, CHUV, Lausanne) for assistance with data extraction. Open access funding provided by Universite de Lausanne.
Viana MV, Pantet O, Charrière M, et al. Specific nutrition and metabolic characteristics of critically ill patients with persistent COVID‐19. J Parenter Enteral Nutr. 2022;46:1149‐1159. 10.1002/jpen.2334
REFERENCES
- 1. Mateen BA, Wilde H, Dennis JM, et al. Hospital bed capacity and usage across secondary healthcare providers in England during the first wave of the COVID‐19 pandemic: a descriptive analysis. BMJ Open. 2021;11(1):e042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shang Y, Pan C, Yang X, et al. Management of critically ill patients with COVID‐19 in ICU: statement from front‐line intensive care experts in Wuhan, China. Ann Intensive Care. 2020;10(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barazzoni R, Bischoff SC, Breda J, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS‐CoV‐2 infection. Clin Nutr. 2020;39(6):1631‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weber TK, Leandro‐Merhi VA, Bernasconi I, de Oliveira MRM. Nutritional therapy in hospital care of in‐patients with Covid‐19: evidence, consensus and practice guidelines. Rev Nutr. 2020;33:e200212. [Google Scholar]
- 5. Arkin N, Krishnan K, Chang MG, Bittner EA. Nutrition in critically ill patients with COVID‐19: challenges and special considerations. Clin Nutr. 2020;39(7):2327‐2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaafarani HMA, El Moheb M, Hwabejire JO, et al. Gastrointestinal complications in critically ill patients with COVID‐19. Ann Surg. 2020;272(2):e61‐e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mechanick JI. Clinical nutrition research and the COVID‐19 pandemic: a scoping review of the ASPEN COVID‐19 task force on nutrition research. JPEN J Parenter Enteral Nutr. 2021;45(1):13‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whittle J, Molinger J, MacLeod D, Haines K, Wischmeyer PE, Group Leep‐Covid Study . Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID‐19. Crit Care. 2020;24(1):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singer P, Pichard C, De Waele E. Practical guidance for the use of indirect calorimetry during COVID 19 pandemic. Clin Nutr Exp. 2020;33:18‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao X. Evaluation of nutrition risk and its association with mortality risk in severely and critically ill COVID‐19 patients. JPEN J Parenter Enteral Nutr. 2021;45(1):32‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bagshaw SM, Stelfox HT, Iwashyna TJ, Bellomo R, Zuege D, Wang X. Timing of onset of persistent critical illness: a multi‐centre retrospective cohort study. Intensive Care Med. 2018;44(12):2134‐2144. [DOI] [PubMed] [Google Scholar]
- 12. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Network Covid‐ICU Group on behalf of the REVA Network and the Covid‐ICU Investigators . Clinical characteristics and day‐90 outcomes of 4244 critically ill adults with COVID‐19: a prospective cohort study. Intensive Care Med. 2021;47(1):60‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viana MV, Pantet O, Bagnoud G, et al. Metabolic and nutritional characteristics of long‐stay critically ill patients. J Clin Med. 2019;8(7):985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaw M, Viglianti EM, McPeake J, et al. Timing of onset, burden, and postdischarge mortality of persistent critical illness in Scotland, 2005‐2014: a retrospective, population‐based, observational study. Crit Care Explor. 2020;2(4):e0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(4):321‐336. [DOI] [PubMed] [Google Scholar]
- 17. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48‐79. [DOI] [PubMed] [Google Scholar]
- 18. Faisy C, Guerot E, Diehl JL, Labrousse J, Fagon JY. Assessment of resting energy expenditure in mechanically ventilated patients. Am J Clin Nutr. 2003;78(2):241‐249. [DOI] [PubMed] [Google Scholar]
- 19. Oshima T, Delsoglio M, Dupertuis YM, et al. The clinical evaluation of the new indirect calorimeter developed by the ICALIC project. Clin Nutr. 2020;39(10):3103‐3111. [DOI] [PubMed] [Google Scholar]
- 20. Yeh DD, Fuentes E, Quraishi SA, et al. Adequate nutrition may get you home: effect of caloric/protein deficits on the discharge destination of critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2016;40:37‐44. [DOI] [PubMed] [Google Scholar]
- 21. Charrière M, Ridley E, Hastings J, Bianchet O, Scheinkestel C, Berger MM. Propofol sedation substantially increases the caloric and lipid intake in critically ill patients. Nutrition. 2017;42:64‐68. [DOI] [PubMed] [Google Scholar]
- 22. Montefusco L, Ben Nasr M, D'Addio F, et al. Acute and long‐term disruption of glycometabolic control after SARS‐CoV‐2 infection. Nat Metab. 2021;3(6):774‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doig GS, Simpson F, Heighes PT, et al. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel‐group, multicentre, single‐blind controlled trial. Lancet Respir Med. 2015;3(12):943‐952. [DOI] [PubMed] [Google Scholar]
- 24. Reintam Blaser A, Gunst J, Arabi YM. The gut in COVID‐19. Intensive Care Med. 2021;47(9):1024‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D'Amico F, Baumgart DC, Danese S, Peyrin‐Biroulet L. Diarrhea during COVID‐19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol. 2020;18(8):1663‐1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang W, Feng Z, Rao S, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69(6):1141‐1143. [DOI] [PubMed] [Google Scholar]
- 27. Zheng Y, Gao Y, Wu B, Huang L, Chen Y, Cai X. Characteristics and outcomes of patients with COVID‐19 in Hainan, South China. Medicine (Baltimore). 2021;100(11):e24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parasa S, Desai M, Thoguluva Chandrasekar V, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta‐analysis. JAMA Netw Open. 2020;3(6):e2011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Southren DL, Nardone AD, Haastrup AA, Roberts RJ, Chang MG, Bittner EA. An examination of gastrointestinal absorption using the acetaminophen absorption test in critically ill patients with COVID‐19: a retrospective cohort study. Nutr Clin Pract. 2021;36(4):853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cardinale V, Capurso G, Ianiro G, Gasbarrini A, Arcidiacono PG, Alvaro D. Intestinal permeability changes with bacterial translocation as key events modulating systemic host immune response to SARS‐CoV‐2: a working hypothesis. Dig Liver Dis. 2020;52(12):1383‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cereda E, Guzzardella A, Klersy C, et al. Early caloric deficit is associated with a higher risk of death in invasive ventilated COVID‐19 patients. Clin Nutr. Published online March 2, 2021;180:1345. 10.1016/j.clnu.2021.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cui N, Tong H, Li Y, et al. Role of prealbumin in predicting the prognosis of severely and critically ill Covid‐19 patients. Am J Trop Med Hyg. 2021;105(3):718‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shenkin A. Serum prealbumin: is it a marker of nutritional status or of risk of malnutrition? Clin Chem. 2006;52(12):2177‐2179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.Figure
Supporting Information.
Supporting Information.


