Abstract
Background and Aims
Alcohol consumption increased during the COVID‐19 pandemic in 2020 in the United States. We projected the effect of increased alcohol consumption on alcohol‐associated liver disease (ALD) and mortality.
Approach and Results
We extended a previously validated microsimulation model that estimated the short‐ and long‐term effect of increased drinking during the COVID‐19 pandemic in individuals in the United States born between 1920 and 2012. We modeled short‐ and long‐term outcomes of current drinking patterns during COVID‐19 (status quo) using survey data of changes in alcohol consumption in a nationally representative sample between February and November 2020. We compared these outcomes with a counterfactual scenario wherein no COVID‐19 occurs and drinking patterns do not change.
One‐year increase in alcohol consumption during the COVID‐19 pandemic is estimated to result in 8000 (95% uncertainty interval [UI], 7500–8600) additional ALD‐related deaths, 18,700 (95% UI, 17,600–19,900) cases of decompensated cirrhosis, and 1000 (95% UI, 1000–1100) cases of HCC, and 8.9 million disability‐adjusted life years between 2020 and 2040. Between 2020 and 2023, alcohol consumption changes due to COVID‐19 will lead to 100 (100–200) additional deaths and 2800 (2700–2900) additional decompensated cirrhosis cases. A sustained increase in alcohol consumption for more than 1 year could result in additional morbidity and mortality.
Conclusions
A short‐term increase in alcohol consumption during the COVID‐19 pandemic can substantially increase long‐term ALD‐related morbidity and mortality. Our findings highlight the need for individuals and policymakers to make informed decisions to mitigate the impact of high‐risk alcohol drinking in the United States.
Abbreviations
- ALD
alcohol‐associated liver disease
- CDC
Centers for Disease Control and Prevention
- DALY
disability‐adjusted life‐year
- F0
no fibrosis
- F1
mild fibrosis
- F2
moderate fibrosis
- F3
septal fibrosis
- NESARC
National Epidemiologic Survey on Alcohol and Related Conditions
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- UI
uncertainty interval
- YLL
life years lost due to disease and mortality
INTRODUCTION
Since the start of COVID‐19 pandemic in the United States, alcohol consumption has increased considerably—observed increases in consumption of up to 25% have been recorded in small samples.[ 1 , 2 , 3 ] Weekly sales of alcohol have also been observed to increase by as much as 400% and be sustained over many weeks.[ 4 , 5 ] A recent nationally representative survey showed considerable increases in alcohol consumption overall and by populations of interest including women, Black people, and households with children.[ 1 , 3 ] There is risk that these short‐term increases may manifest as long‐term trends in drinking at the societal level. There is an urgent need to understand the impact of these changes and inform the decisions of individuals, policymakers, and health care workers.
The burden of alcohol‐associated liver disease (ALD) has risen in the past decade, particularly among the young and women.[ 6 , 7 , 8 ] Short‐term increases in alcohol consumption can result in abrupt rises in alcohol use disorder and other long‐term changes in behavior and care,[ 9 ] which may cause complications such as injury and cirrhosis, leading to long‐term increase in ALD and mortality.
As the COVID‐19 pandemic continues to evolve, it is important to understand the immediate and long‐term effects of increased alcohol consumption on ALD to inform appropriate clinical and policy management. The objective of our study was to quantify projected ALD mortality in the context of increases in alcohol consumption during the COVID‐19 pandemic and compare outcomes with a counterfactual scenario without COVID‐19 and no changes in drinking patterns.
PARTICIPANTS AND METHODS
Model synopsis
In our model, individuals are born and their health characteristics and drinking levels are updated each year based on their age and sex. Individuals’ alcohol consumption is simulated consistent with rates found in national data sets, and their health status is updated each year based on their drinking levels. Based on their drinking levels, individuals have a likelihood of developing complications of the liver including decompensation and HCC, and they could die of ALD or other causes. The model simulated the long‐term population‐level outcomes under two scenarios: increased alcohol consumption during COVID‐19 and a counterfactual with no COVID‐19–related increase in alcohol consumption.
Model overview
We extended a previously developed microsimulation model of fibrosis progression in ALD for US adults[ 6 ] by accounting for current age cohort and sex‐based drinking rates as collected by the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC),[ 10 ] drinking trajectories in the US population,[ 11 ] and fibrosis progression rates for drinkers of varying levels that account for current age cohort and sex‐based drinking trajectories in the US population.[ 12 ] At any given time, a patient occupies one of the health states in the model, which is defined by a combination of their liver health stage and drinking level (Figure 1). Individuals transition from one health state to another based on progression and regression rates that are dependent on drinking level and sex. The model accounts for competing causes of mortality, both related and unrelated to alcohol use (not shown in Figure 1 for simplicity). Time advances in 1‐year increments. The model was developed in R, version 3.6.1 (R Foundation for Statistical Computing) using microsimulation code adapted from DARTH group.[ 13 ]
FIGURE 1.

State‐transition model of the natural history of ALD and drinking state. A patient is represented by the combination of one of the health states and one drinking state, shown here as rectangles and circles. Arrows between states represent annual transition probabilities, with the blue shaded states representing decompensated cirrhosis. Competing‐cause mortality, the probability of dying from other causes both related and unrelated to alcohol use, exists in every state but is not shown in our diagram for simplicity. F0, F1, and F2 represent no fibrosis (F0), mild fibrosis (F1), and moderate fibrosis (F2). F3 indicates septal fibrosis, F4 represents compensated fibrosis, and the darker blue stages represent various complications of decompensated fibrosis. The lighter shade of blue encompasses all the cirrhotic disease states. “HCC” indicates a patient has HCC, and mortality from stages F4 on contribute to the reported liver‐related mortality. The health states H1–H5 are tunnel states representing healthy liver predrinking states. These states are calibrated to reproduce the distribution of the age at first drink for the US population, as evidenced in the NESARC‐III
Data sources
Mortality data were collected from the Centers for Disease Control and Prevention (CDC) WONDER database for the years of interest.[ 14 ] The queries were placed for International Classification of Diseases, Tenth Revision codes K70.0 (alcohol‐associated fatty liver), K70.1 (alcohol‐associated hepatitis), K70.2 (alcohol‐associated fibrosis and sclerosis of liver), K70.3 (alcohol‐associated cirrhosis of liver), K70.4 (alcohol‐associated hepatic failure), and K70.9 (ALD) following procedures established for National Institute on Alcohol Abuse and Alcoholism (NIAAA) surveillance reports on liver cirrhosis mortality in the United States.[ 15 ] Other natural history transition probabilities are sourced from existing research as outlined in the Supporting Information (Tables S1 and S2).
We estimated individual drinking levels using the NESARC studies,[ 10 ] limited access data sets obtained from the NIAAA, which are nationally representative surveys of at least 36,309 US adults collected between 2001 and 2002 (NESARC‐I Wave 1) and April 2012 and June 2013 (NESARC‐III). The survey data allow for estimates of the 2001–2002 and 2012–2013 distribution of drinkers by age and sex. Compared against data collected 10 years prior during NESARC‐I, the NESARC‐III data also allowed for the consideration of changing rates of high‐risk drinking among age groups.[ 10 , 16 ]
Population
We model birth cohorts of US adults from 1920 to 2012. As an initial model input, we used male and female annual birth cohorts as reported by the US Census Bureau.
Drinking levels
Drinking levels consist of never yet consumed alcohol (denoted H1–H5 in the model), abstinence, low risk (<3 drinks per day), medium risk (3–4 drinks per day), high risk (4–7 drinks per day), and very high risk states (>7 drinks per day). Transitions are possible between every drinking level (abstinence, low risk, medium risk, high risk, and very high‐risk drinking), and transition rates are consistent with rates found in Barbosa et al.[ 11 ]
We calibrate transition probabilities in the tunnel states H1–H5 such that the distribution of simulated individuals’ initiation of drinking, the age at which they transition out of H5, matched initiation of drinking data in NESARC‐III data. Drinking rates by age and sex in 2012 from NESARC‐III are used as model input.
To capture an approximation of drinking before 2012, individuals are assigned a drinking level from the 2012 distribution collected in NESARC‐III for their age group once they initiate drinking and allowed to progress until 2012. In 2012, drinking rates returned to the initial level and progress again according to the drinking transition matrix.
Natural history of ALD
The following define the liver‐related health states: no fibrosis (F0), mild fibrosis (F1), moderate fibrosis (F2), and septal fibrosis (F3). Transition probabilities between states F1–F3 replicate fibrosis progression and regression in Lieber et al. and are responsive to changes in drinking rate.[ 12 ] Liver‐related health is defined by the following stages: no fibrosis (F0), mild fibrosis (F1), moderate fibrosis (F2), and septal fibrosis (F3). Transition probabilities between states F1–F3 replicate fibrosis progression and regression in Lieber et al. and are responsive to changes in drinking rate.[ 12 ]
Patients can further progress to compensated cirrhosis (F4) and decompensated cirrhosis (ascites, variceal bleeding, ascites with variceal bleeding, and encephalopathy) as well as HCC. From these advanced disease stages, patients can die of liver‐related mortality. Real‐world transition probabilities are used to estimate transition probabilities between compensated cirrhosis (F4), decompensated cirrhosis (ascites, variceal bleeding, ascites with variceal bleeding, and encephalopathy), and death consistent with the findings of Jepsen et al.[ 17 ] Mortality rates from advanced stages of liver disease are estimated from population studies that allowed for transplantation; although a transplantation state is not explicitly delineated in our model, we account for the competing event of transplantation before death.[ 17 ] Health state transitions were dependent on progression and regression rates associated with different levels of drinking.[ 12 ] All transition probabilities are summarized in Tables S1–S3.
States C1 and C2 are calibration states used to capture unidentified interactions, including genetic, age cohort, sex, diet, and other factors known to influence the progression of liver disease but not explicitly captured in the model. We calibrate the model overall by constructing a calibration function for the C1 and C2 transition probabilities to replicate observed mortality data from the CDC WONDER database for 2010–2014.[ 14 ] Individuals born after 1980 were calibrated as a group. We used a sequential algorithm that adjusted male and female calibration values independently while seeking to minimize our goodness of fit measure within error bounds for total cirrhosis mortality in our calibration period.
Simulated scenarios
To project model outcomes up to 2040, we consider two scenarios for drinking in the US population. Each scenario is implemented with a 1‐year duration of increase, consistent with current work showing drinking changes first recorded from February to March 2020 in the United States were durable through at least November 2020.[ 3 ] (1) “COVID‐19 base consumption scenario” describes an increase in alcohol consumption based on recorded increases in the Barbosa et al. study of individuals (n = 993) in the US population aged 21 and older.[ 1 , 3 ] The study found that average drinks per day and excessive drinking (drinking in excess of recommended drinking limits and binge drinking) increased significantly by 29% and 21% between February (before stay‐at‐home orders were issued) and November.[ 1 , 3 ] As a model input, we consider changes in drinking level by age and sex as recorded by the national survey. Individuals in the model either remain at their current drinking level or transition to a higher drinking level with rates that replicate the overall increase seen in the survey. Consumption increases are maintained for a 1‐year period. (2) The “counterfactual scenario” describes the absence of COVID‐19–related effects. This scenario assumed that age‐specific rates of alcohol consumption continue to evolve in the absence of COVID‐19.
Model outcomes
We project ALD‐related deaths inclusive of HCC mortality, the prevalence and incidence of decompensated cirrhosis and HCC, and disability‐adjusted life years (DALYs) lost to ALD from 2020 to 2040. DALYs—a combined measure of disability, prevalence of disease (in our case, decompensated cirrhosis and HCC), and quantity of life years lost due to disease and mortality (YLL)[ 18 , 19 , 20 ]—were calculated using disability weights from 2016 Global Burden of Disease Study values for decompensated cirrhosis and HCC.[ 21 ] YLLs were estimated by multiplying each death by the remaining life expectancy at the age of death and summing across the years. To calculate years lived with disability (YLD), we multiply the number of individuals in a health state by the disability weights defined by the Global Burden of Disease Study (0 for scores of F0–F4, 0.178 for decompensated cirrhosis, and 0.540 for HCC).[ 22 , 23 ] DALYs are then the sum of YLLs and YLDs.
Sensitivity analysis
We conducted a probabilistic sensitivity analysis to determine the uncertainty in our model projections in each scenario given the joint uncertainty of model parameters, in particular natural history of disease, transition rates, and drinking distribution. For this purpose, we first defined parameter uncertainty using recommended distributions for different types of parameters (see Table S2).[ 24 ] We then sampled parameter values from these distributions 1000 times and ran the model to determine model results. The populations used were 1/10th the size of the US birth cohort. We generated the 95% uncertainty intervals (UI) of model outcomes.
We also conducted one‐way sensitivity analysis on the drinking rate and duration of drinking increase. We created two hypothetical drinking trajectories, a low and high increase: (1) a “hypothetical low increase” in alcohol use corresponding to a 10% increase in overall drinking in the population and (2) a “hypothetical high increase” in alcohol use corresponding to a 40% increase in overall drinking in the population. These scenarios were created by increasing drinking by 10% and 40%, respectively in the NESARC‐III data and calculating what probability individuals had of transitioning from their current drinking state to a higher drinking state.
We conducted further sensitivity analysis to understand the impact of length of drinking increase: 3‐year and 5‐year sustained increase in alcohol consumption after 2020. We provide results for 3 and 5‐year increases in the Supporting Information.
RESULTS
We first validate our model‐predicted annual mortality due to ALD from 2014 to 2018 for men and women with those reported by the US national death registry data from the CDC WONDER database (Figure 2). We also compare alcohol‐related cirrhosis prevalence in the US population in our model against reported data in 2009 and 2015 in the peer‐reviewed literature (Table S5).[ 25 ]
FIGURE 2.
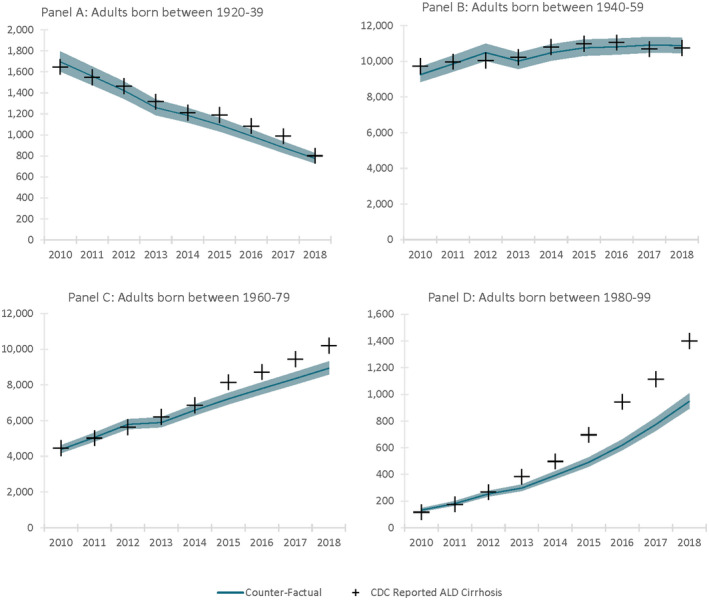
Birth cohort ALD disease mortality 2012–2018 with CDC mortality validation. Mortality is as reported in the US National Death Registry and predicted/projected mortality in our model from the start of the calibration period through the end of the validation period. Shaded regions represent the UIs generated by probabilistic sensitivity analysis. (A) Adults born between 1920 and 1939; (B) adults born between 1940 and 1959, (C) adults born between 1960 and 1979, and (D) adults born between 1980 and 1999
Under the counterfactual scenario, which assumed no COVID‐19–associated drinking changes, 948,700 (95% UI, 909,000–988,300) US adults, aged 18+, are projected to die of ALD‐related cirrhosis between 2020 and 2040, 1,046,100 (95% UI, 996,500–1,095,600) are projected to develop decompensated cirrhosis, and 142,800 (95% UI, 135,200–150,400) are projected to develop HCC during the same period (Table 1).
TABLE 1.
Cumulative projections for ALD disease morbidity and mortality for 3 and 20‐year horizons under COVID‐19 consumption scenario and counterfactual scenario
| Scenario | Results | 2020–2023 | 2020–2040 |
|---|---|---|---|
| COVID‐19 consumption | ALD cirrhosis mortality | 112,700 (107,800–117,700) | 956,700 (916,500–996,900) |
| Decompensated cirrhosis incidence | 116,000 (110,400–121,600) | 1,128,800 (1,075,200–1,182,400) | |
| HCC incidence | 16,900 (15,900–18,000) | 143,800 (136,200–151,500) | |
| Counterfactual | ALD cirrhosis mortality | 112,600 (107,700–117,500) | 948,700 (909,000–988,300) |
| Decompensated cirrhosis incidence | 112,900 (107,400–118,400) | 1,109,000 (1,056,600–1,161,400) | |
| HCC incidence | 17,000 (16,000–18,000) | 142,800 (135,200–150,400) | |
| Increase in outcomes because of COVID‐19 drinking | ALD cirrhosis mortality | 100 (100–200) | 8000 (7500–8600) |
| Decompensated cirrhosis incidence | 3100 (3000–3300) | 19,800 (18,600–21,000) | |
| HCC incidence | 00 (00–00) | 1000 (1000–1100) | |
| DALYs | 531,200 (526,600–535,700) | 8,902,000 (8,878,600–8,925,300) |
The table presents cumulative ALD morbidity and mortality for two time periods: 2020–2023 and 2020–2040. Data are presented as a mean of model results and the 95% UI generated by the probabilistic sensitivity analysis. Morbidity results include the incidence of decompensated cirrhosis and HCC, two diseases with significant health implications. In the COVID‐19 consumption scenario, some drinkers increase consumption during 2020, while in the counterfactual scenario, drinking progresses without any increases associated with COVID‐19.
Under the “COVID‐19 base consumption scenario,” 956,700 (95% UI, 916,500–996,900) US adults, aged 18+, are projected to die of ALD cirrhosis between 2020 and 2040, 1,064,800 (95% UI, 1,014,100–1,115,400) are projected to develop decompensated cirrhosis, and 143,800 (95% UI, 136,200–151,500) are projected to develop HCC (Table 1). Increase in alcohol consumption due to COVID‐19 in 2020 is associated with 8200 (95% UI, 7500–8600) additional deaths, 18,100 (95% UI, 16,100–18,200) additional decompensation cases, and 1000 (95% UI, 1000–1100) additional cases of HCC over the next 2 decades (Figure 3). We also estimated a pronounced spike in decompensation cases and mortality in the short term during 2020–2021. Between 2020 and 2023, alcohol consumption changes due to COVID‐19 will lead to 100 (100–200) additional deaths and 2800 (2700–2900) additional decompensated cirrhosis cases.
FIGURE 3.
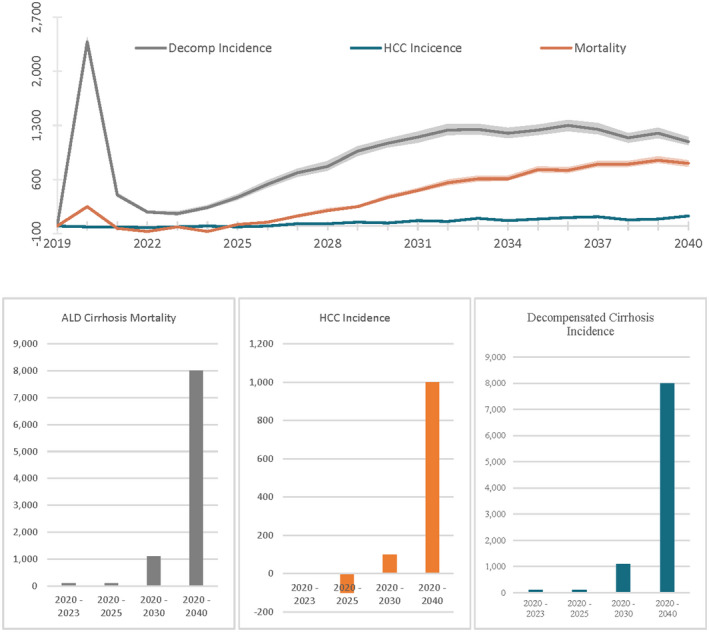
Difference in alcohol‐related liver mortality and morbidity between COVID‐19 consumption scenario and counterfactual for US adults aged 18+, 2019–2040. Annual and cumulative rates for morbidity and mortality differences between the COVID‐19 consumption scenario and counterfactual scenario associated with ALD. In the COVID‐19 consumption scenario, some drinkers increase consumption during 2020, while in the counterfactual scenario, drinking progresses without any increases associated with COVID‐19. Shaded regions represent the 95% UI associated with the probabilistic sensitivity analysis
Age cohort–related mortality
Alongside understanding increases in morbidity and mortality generally, we sought to explore the impact on specific age groups. For those born between 1920 and 1939, the model projects approximately 3170 deaths from 2020 to 2040 in the “COVID‐19 consumption scenario” versus 3190 deaths in the “counterfactual scenario,” and our projections indicate that for the birth cohorts born between 1940 and 1959, we expect 179,090 and 169,740 deaths, respectively. For those born between 1960 and 1979, we project 415,670 and 413,160 deaths, and finally, for those born between 1980 and 1999, we project 237,490 and 233,190 deaths in the “COVID‐19 consumption scenario” and the “counterfactual scenario,” respectively. Although the initial mortality increases are concentrated in the oldest birth cohorts, over the next 20 years, the additional ALD mortality burden shifts increasingly to the younger birth cohorts (Table 2).
TABLE 2.
Cumulative projections for ALD disease mortality by birth cohort for 3 and 20‐year horizons under COVID‐19 consumption scenario and counterfactual scenario
| Birth Cohort | Scenario | 2020–2023 | 2020–2040 |
|---|---|---|---|
| 1920–1939 | COVID‐19 consumption scenario | 1860 (1720–2000) | 3170 (2860–3470) |
| Counterfactual | 1860 (1720–2010) | 3190 (2880–3490) | |
| Difference | 00 (00–10) | ‐20 (‐20–20) | |
| 1940–1959 | COVID‐19 consumption scenario | 42,430 (40,810–44,050) | 170,090 (163,530–176,640) |
| Counterfactual | 42,340 (40,730–43,960) | 169,740 (163,160–176,320) | |
| Difference | 90 (80–90) | 350 (370–320) | |
| 1960–1979 | COVID‐19 consumption scenario | 45,560 (43,740–47,380) | 415,670 (399,700–431,640) |
| Counterfactual | 45,520 (43,710–47,330) | 413,160 (397,560–428,760) | |
| Difference | 40 (30–50) | 2510 (2140–2880) | |
| 1980–1999 | COVID‐19 consumption scenario | 7910 (7490–8330) | 237,490 (227,400–247,580) |
| Counterfactual | 7870 (7450–8290) | 233,190 (223,240–243,150) | |
| Difference | 40 (40–40) | 4300 (4160–4430) |
The table presents cumulative ALD mortality for two time periods: 2020–2023 and 2020–2040. Data are presented by 20‐year birth cohort as a mean of model results and the 95% UI generated by the probabilistic sensitivity analysis. In the base COVID‐19 consumption scenario, some drinkers increase consumption during 2020, while in the counterfactual scenario, drinking progresses without any increases associated with COVID‐19.
DALYs
COVID‐19–related drinking increases are also associated with substantial DALYs, a measure of both life years lost due to premature mortality and years of productive life lost due to disability. From 2020 to 2023, drinking increases during COVID‐19 are projected to lead to 531,200 DALYs relative to the status quo, a number that is projected to increase to 8,900,200 cumulative DALYs from 2020 through 2040 (Table 1).
Sensitivity analysis on alcohol consumption
We explored the impact of duration of drinking increase on disease burden and mortality. For that, we simulated an increase in drinking during COVID‐19 lasting for 3 and 5 years. Although a 1‐year increase in the base case scenario led to 8000 additional deaths, 3 and 5‐year sustained increases led to 9500 (95% UI, 9000–10,000) and 10,800 (95% UI, 10,400–11,300), respectively (Figure 4).
FIGURE 4.

Sensitivity analysis projecting difference in alcohol‐related liver mortality and morbidity between a counterfactual scenario and COVID‐19 consumption scenario under varying duration of alcohol consumption in adults aged 18+. Annual and cumulative mortality differences between the counterfacture scenario and COVID‐19 scenario with sustained increase in alcohol consumption for 1, 3, and 5 years. In the base COVID‐19 consumption scenario some drinkers increase consumption during the 2020 based on a survey of changes in the US population. Consumption is increased for 1, 3, or 5 years and compared with a counterfactual drinking scenario that progresses without any increases associated with COVID‐19. Shaded regions represent the 95% UI associated with the probabilistic sensitivity analysis
We also include analysis on a “lower than measured” 10% increase in overall consumption and a “higher than measured” 40% increase in alcohol consumption during the COVID pandemic. In comparison with 8000 deaths over the next 2 decades in the base case scenario, a 40% increase in consumption would lead to 12,000 (95% UI, 12,900–11,000) deaths versus 3100 (95% UI, 2800–3300) deaths in the “10% increase scenario” (Figure S1).
DISCUSSION
ALD accounts for a significant health burden in the United States.[ 6 ] Alcohol consumption has increased substantially during the COVID pandemic.[ 1 , 2 ] Our results show that there may be a measurable effect on ALD‐related decompensation and mortality in the short term, and, without intervention, it can result in a long‐term disease burden of additional 8000 ALD‐related deaths, 18,700 cases of decompensated cirrhosis, and 1000 cases of HCC between 2020 and 2040. The observed increase in alcohol consumption has the potential to exasperate the condition of those already living with impaired liver function and lead to long‐term damage in those who increase consumption at an early age.
ALD is a major threat to public health
ALD already poses a significant disease burden in the United States. ALD now accounts for more than $5 billion in direct health care costs, and mortality due to ALD has increased substantially by 200% in people aged 25–34 over the last decade.[ 25 ] Even without COVID‐19−related increases in consumption, ALD will claim the lives of nearly 260,000 Americans over the next decade. Our models show that if the higher rates of risky alcohol consumption hold for 1 year, this burden will further rise. A key insight from these data, however, is that in the short term there will be a disproportionate rise in the excess morbidity and mortality attributed to ALD. Many with ALD, even those with compensated cirrhosis, are clinically stable, but marginal increases in consumption for these vulnerable patients are uniquely toxic.[ 7 , 26 ] The result, we find, is a spike in the short‐term incidence of decompensated cirrhosis that will stress a health care system that is still overwhelmed by the impact of the pandemic.[ 9 ]
The burden of ALD requires a public health approach
Our data show that COVID‐associated increases in alcohol consumption increase short and long‐term morbidity and mortality due to ALD. However, even in the counterfactual estimates, we expect a steady expansion of the public health impact of ALD. Our data show also that young persons will be disproportionately impacted. There is, accordingly, substantial value in interventions to reduce the burden of ALD. All ALD‐focused interventions aim to reducing alcohol consumption. At the individual level, one can use behavioral and pharmacological interventions, each proven to reduce the harms of alcohol use disorder.[ 27 ] However, the impact of these interventions remains limited owing to modest efficacy and inadequate linkage to care.[ 28 , 29 ] Our data underscore a population‐level problem for which a population‐level intervention may be indicated. Policies that reduce access to alcohol especially during the COVID‐19 pandemic or influence demand through minimum unit pricing may be the most effective tools.[ 30 , 31 , 32 , 33 ] In Scotland, a minimal unit price of 50 pence has resulted in a substantial decrease in alcohol consumption, primarily by the highest consumers.[ 34 ]
Contextual factors
Our results must be interpreted in the context of the study design. First, there is a lack of comprehensive information on the increase and distribution of increase of drinking in the US population. Accordingly, we use data from a representative sample of the US population taken in 2020 as a representation of the impact of drinking increases. To mitigate this limitation, we model the uncertainty in our estimates with extensive sensitivity analysis. Second, there are other (unaccounted) factors that could impact the risk of ALD‐related decompensation. The significant turmoil of this moment in the United States caused by COVID‐19 and the associated economic and political stressors may have had a disproportionate impact on individuals at increased risk of adverse health outcomes.[ 35 ] Individuals often increase alcohol intake to cope with emotional stress and chronic uncertainty; these coping mechanism have unclear long‐term consistency.[ 36 ] To address this concern, our increased consumption scenario only increases drinking for 1 year whereupon drinking transitions return to the pre‐2020 rates. Third, this modeling study does not account for the impact of COVID‐19 infection on those with preexisting cirrhosis or those at risk for future decompensation. Fourth, we do not model the impact of adverse trends on alcohol consumption on specific population subgroups. Given recent complementary results by Barbosa et al.,[ 3 ] a particular focus on at‐risk groups including women, Black people, and households with children, should be taken as increased morbidity, and mortality beyond the status quo should be expected to be concentrated in populations with the greatest increase in consumption and fibrosis susceptibility. Although the study does not specifically address the impacts in at‐risk communities, the general dynamics of consumption, liver damage, and morbidity and mortality described here should remain similar.
Our study raises the important specter of the near and long‐term impact of ALD. Even a short‐term increase in alcohol consumption during the pandemic can substantially increase morbidity and mortality associated with ALD in the long term due to behavioral changes. Our findings highlight the need for individuals and policy makers to make informed decisions to mitigate the impact of high‐risk alcohol drinking while staying at home during the COVID‐19 pandemic in the United States. Short‐term increases in consumption are associated with both an immediate bump in mortality and morbidity as well as long term increases many years later.
CONFLICT OF INTEREST
Elliot Tapper has served as a consultant to Norvartis, Axcella, Kaleido, and Allergan; served on advisory boards for Takeda, Mallinckrodt, Rebiotix, and Bausch Health; and received unrestricted research grants from Gilead and Valeant. Jagpreet Chhatwal has served as a consultant to Novo Nordisk and partner at Value Analytics Labs. Turgay Ayer has served as a consultant to Merck and partner at Value Analytics Labs. No other author has a conflict of interest.
ETHICS APPROVAL
The study (Protocol H18459) obtained ethics approval through the institutional review board of Georgia Institute of Technology.
AUTHOR CONTRIBUTIONS
Concept: Jovan Julien and Jagpreet Chhatwal. Analysis: Jovan Julien. Data acquisition: Jovan Julien, Carolina Barbosa, William Dowd. Analysis and interpretation of data: Jovan Julien, Turgay Ayer, Elliot Tapper, Jagpreet Chhatwal. Writing: Jovan Julien, Jagpreet Chhatwal. Revision: Turgay Ayer, Elliot Tapper, Carolina Barbosa, William Dowd, Jagpreet Chhatwal.
Supporting information
Supplementary Material
Julien J, Ayer T, Tapper EB, Barbosa C, Dowd WN, Chhatwal J. Effect of increased alcohol consumption during COVID‐19 pandemic on alcohol‐associated liver disease: A modeling study. Hepatology. 2022;75:1480–1490. doi: 10.1002/hep.32272
Funding information
This study was funded by the American Cancer Society Research Scholar (Grant RSG‐17‐022‐01‐CPPB to Jagpreet Chhatwal), the Robert Wood Johnson Health Policy Research Fellowship (Award Number 74817 to Jovan Julien), and the National Institutes of Health (Grant K23DK117055 to Elliot B. Tapper). Carolina Barbosa and William Dowd acknowledge financial support by the NIAAA under Award Number R01AA024423. The funding agreements ensured the authors’ independence in designing the study, interpreting the data, and writing and publishing the report. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This manuscript was prepared using a limited‐access data set obtained from the NIAAA and does not reflect the opinions or views of NIAAA or the US government
Contributor Information
Jovan Julien, Email: jovan@jovanjulien.com.
Jagpreet Chhatwal, Email: JagChhatwal@mgh.harvard.edu.
REFERENCES
- 1. Barbosa C, Cowell AJ, Dowd WN. Alcohol consumption in response to the COVID‐19 pandemic in the United States. J Addiction Med. 2021;15(4):341‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pollard MS, Tucker JS, Green HD. Changes in adult alcohol use and consequences during the COVID‐19 pandemic in the US. JAMA Network Open. 2020;3:e2022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barbosa C, Karriker‐Jaffe K, Dowd W. One year later: how have american drinking habits changed during the COVID‐19 pandemic. RTI International; 2021. https://www.rti.org/sites/default/files/fy21_covid_drinking_webinar_slides_final.pdf. Accessed 30 Aug 2021. [Google Scholar]
- 4. IZEA . Coronavirus impacts on alcohol & social media consumption. IZEA; 2020. [Google Scholar]
- 5. Drizly . How alcohol ecommerce sales are being impacted across North America. April 27, 2020; 2021. https://drizly.com/article/education/extras/how‐alcohol‐ecommerce‐sales‐are‐being‐impacted‐across‐north‐america/e‐1735e090. Accessed 27 Apr 2020.
- 6. Julien J, Ayer T, Bethea ED, Tapper EB, Chhatwal J. Projected prevalence and mortality associated with alcohol‐related liver disease in the USA, 2019–40: a modelling study. The Lancet Public Health. 2020;5:e316–23. [DOI] [PubMed] [Google Scholar]
- 7. Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White AM, Castle IJP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol‐related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44:178–87. [DOI] [PubMed] [Google Scholar]
- 9. Tapper EB, Asrani SK. COVID‐19 pandemic will have a long‐lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73(2):441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grant B, Chu A, Sigman R, Amsbary M, Kali J, Sugawara Y, et al. Source and accuracy statement: national epidemiologic survey on alcohol and related conditions‐III (NESARC‐III). Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 2014:1–125. https://www.niaaa.nih.gov/sites/default/files/NESARC_Final_Report_FINAL_1_8_15.pdf. Accessed 20 Apr 2020. [Google Scholar]
- 11. Barbosa C, Dowd WN, Aldridge AP, Timko C, Zarkin GA. Estimating long‐term drinking patterns for people with lifetime alcohol use disorder. Med Decis Making. 2019;39:765–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lieber CS, Weiss DG, Groszmann R, Paronetto F, Schenker S, Group VACS . I. Veterans Affairs Cooperative Study of polyenylphosphatidylcholine in alcoholic liver disease: effects on drinking behavior by nurse/physician teams. Alcohol Clin Exp Res. 2003;27:1757–64. [DOI] [PubMed] [Google Scholar]
- 13. Krijkamp EM, Alarid‐Escudero F, Enns EA, Jalal HJ, Hunink MGM, Pechlivanoglou P. Microsimulation modeling for health decision sciences using R: a tutorial. Med Decis Making. 2018;38:400–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. CDC WONDER . Multiple Cause of Death Files, 1999–2019. In: Underlying cause of death 1999–2019. Updated 2020. https://wonder.cdc.gov/ucd‐icd10.html. Accessed 7 May 2020. [Google Scholar]
- 15. Yoon Y‐H, Chen CM. Surveillance Report# 105: liver cirrhosis mortality in the United States: national, state, and regional trends, 2000–2013. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism (NIAAA); 2016. [Google Scholar]
- 16. Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12‐month alcohol use, high‐risk drinking, and DSM‐IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry. 2017;74:911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population‐based cohort study. Hepatology. 2010;51:1675–82. [DOI] [PubMed] [Google Scholar]
- 18. Anand S, Hanson K. Disability‐adjusted life years: a critical review. J Health Economics. 1997;16:685–702. [DOI] [PubMed] [Google Scholar]
- 19. Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- 20. Murray CJ. Quantifying the burden of disease: the technical basis for disability‐adjusted life years. Bull World Health Organ. 1994;72:429. [PMC free article] [PubMed] [Google Scholar]
- 21. Hay SI, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd‐Allah F, et al. Global, regional, and national disability‐adjusted life‐years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1260–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Global Health. 2015;3:e712–23. [DOI] [PubMed] [Google Scholar]
- 23. Devleesschauwer B, Havelaar AH, Maertens de Noordhout C, Haagsma JA, Praet N, Dorny P, et al. Calculating disability‐adjusted life years to quantify burden of disease. Int J Public Health. 2014;59:565–9. [DOI] [PubMed] [Google Scholar]
- 24. Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR‐SMDM modeling good research practices task force working group–6. Med Decis Making. 2012;32:722–32. [DOI] [PubMed] [Google Scholar]
- 25. Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68:872–82. [DOI] [PubMed] [Google Scholar]
- 26. Lucey MR, Connor JT, Boyer TD, Henderson JM, Rikkers LF, Group DS . Alcohol consumption by cirrhotic subjects: patterns of use and effects on liver function. Am J Gastroenterol. 2008;103:1698–706. [DOI] [PubMed] [Google Scholar]
- 27. Peng JL, Patel MP, McGee B, Liang T, Chandler K, Tayarachakul S, et al. Management of alcohol misuse in patients with liver diseases. J Investig Med. 2017;65:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Addolorato G, Mirijello A, Barrio P, Gual A. Treatment of alcohol use disorders in patients with alcoholic liver disease. J Hepatol. 2016;65:618–30. [DOI] [PubMed] [Google Scholar]
- 29. Rogal S, Youk A, Zhang H, Gellad WF, Fine MJ, Good CB, et al. Impact of alcohol use disorder treatment on clinical outcomes among patients with cirrhosis. Hepatology. 2020;71:2080–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xuan Z, Blanchette JG, Nelson TF, Nguyen TH, Hadland SE, Oussayef NL, et al. Youth drinking in the United States: Relationships with alcohol policies and adult drinking. Pediatrics. 2015;136:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Naimi TS, Blanchette J, Nelson TF, Nguyen T, Oussayef N, Heeren TC, et al. A new scale of the U.S. alcohol policy environment and its relationship to binge drinking. Am J Prev Med. 2014;46:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Black H, Gill J, Chick J. The price of a drink: levels of consumption and price paid per unit of alcohol by Edinburgh's ill drinkers with a comparison to wider alcohol sales in Scotland. Addiction. 2011;106:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holmes J, Meng Y, Meier PS, Brennan A, Angus C, Campbell‐Burton A, et al. Effects of minimum unit pricing for alcohol on different income and socioeconomic groups: a modelling study. Lancet. 2014;383:1655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Donnell A, Anderson P, Jané‐Llopis E, Manthey J, Kaner E, Rehm J. Immediate impact of minimum unit pricing on alcohol purchases in Scotland: controlled interrupted time series analysis for 2015‐18. BMJ. 2019;366:l5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pfefferbaum B, North CS. Mental health and the Covid‐19 pandemic. N Engl J Med. 2020;383:510–2. [DOI] [PubMed] [Google Scholar]
- 36. Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we Deal with the present and prepare for the future. Psychiatr Q. 2020;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


