Abstract
Object
The study aimed to utilize the peripheral blood immunological parameters and resulting individual and combined inflammatory indices [neutrophil/lymphocyte ratio (NLR), lymphocyte/monocyte ratio (LMR) and C-reactive protein/lymphocyte ratio (CLR)] in predicting the prognosis and mortality in COVID-19 patients.
Materials and methods
The measurements of individual and combined inflammatory indices (NLR, LMR and CLR) were performed at hospital admission and at last day of hospitalization for COVID-19 patients.
Results
Prominent elevation of NLR and CLR among patients with refractory disease admitted to Intensive Care Unit (ICU) and deceased patients was found when compared with moderate ill patients and healthy controls. Interestingly, NLR and CLR typically returned to near normal value as patients recover from severe infection. By contrast, deceased patients had persistent increased NLR and CLR until last day of hospitalization in ICU. ROC obtained for the above parameters showed that NLR and CLR were the most associated immunological parameters with the severity of COVID-19 disease. Using multivariate logistic regression analysis, CLR > 69.46 is an independent prognostic factors in identifying critically ill COVID-19 cases. Study of the combined markers NLR and CLR showed that most of patients admitted in ICU were characterized with high NLR combined with high CLR, while most of healthy subjects and non-ICU group have low NLR combined with low CLR.
Conclusion
The combination of NLR and CLR could improve the predictive efficacy compared to individual markers to segregate patients who will develop a severe disease from those with a mild pathology.
Keywords: COVID-19, SARS-CoV-2, NLR, CLR, Combined markers, Prognosis
1. Introduction
The new coronavirus, officially designated as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the cause of a disease outbreak that emerged in Wuhan, a city in Hubei’s province in China [1]. The outbreak of COVID-19 has caused a global crisis, as the disease continues to spread resulting in significant morbidity and mortality [2]. Although most of SARS-CoV-2 infected patients generally display mild-to-moderate symptoms and undergo spontaneous regression, only a few COVID-19 patients have developed into severe pneumonia, pulmonary edema, acute respiratory distress syndrome, or multiple organ failure leading to intensive care unit (ICU) admission and high mortality [3]. The immune response activated by SARS-CoV-2 infection was crucial for the clearance of invading pathogens. Uncontrolled inflammatory innate immune responses and impaired adaptive immune responses could cause harmful tissue damage. Acute COVID-19 is caused by tissue-directed immunopathology, especially in the lungs, rather than by the virus itself [4], [5]. A deregulated and overactive immune response resulting in excessive inflammation is a significant contributor to coronavirus-mediated lung damage and systemic pathology. It is likely that host immune profiling represents one of the main determinants of progression and deterioration in SARS-CoV-2 related pneumonia [6], [7]. It was of utmost importance to delineate changes of immune profiling in COVID-19 patients and look for useful immunological parameters to predict the outcome of infection as well as informing clinical risk-based stratification which can help for early intervention and to anticipate in which patients it can be more fatal. As a result, circulating biomarkers that show inflammation, as well as the immune cell subsets, can be good indicators of the prognosis of patients with COVID‐19. Of these, CRP levels, blood leukocytes cell subsets (neutrophils, eosinophils, basophils and monocytes) and inflammatory indices resulting from theses parameters (NLR, LMR and CLR) have been of great interest. These immunological parameters are quickly available from hemogram analysis which is a common routine and low‐cost techniques [8], [9]. The diagnostic and prognostic utility of these immunological parameters in COVID-19 disease have been the subject of hot debate [10]. Since these parameters are affected by many inflammatory conditions, there are many discrepancies in which parameter had superior predictive value performance as a prognosis tool to determine the mortality and severity risks in COVID-19 patients. Herein, we aimed to evaluate the prognostic value of a panel of immunological parameters including CRP levels, peripheral immune cell subsets, NLR, LMR and CLR in a Tunisian cohort of SARS-CoV-2 infected patients with distinct disease severity and fatal outcome. To date, no external validation and/or other literature evidence has been carried out to evaluate the feasibility of inflammatory indices combinations to assess COVID-19 disease severity. Therefore, this study aimed also to determine whether or not inflammatory indices combinations (NLR combined with LMR or NLR combined with CLR or LMR combined with CLR) could be more useful than individual indices in the discrimination of COVID 19 cases with different severity which might support the clinical condition’s risk assessment to aid the anticipation of severe complications and provide clues for therapeutic strategies.
2. Patients and methods
2.1. Study design and participants
A total of 33 healthy individuals without SARS-CoV-2 infection were recruited as healthy controls and 132 COVID-19 patients were enrolled in the study. We included all adult patients who were hospitalized for COVID-19 in Al-Amen Clinic and Habib Bougatfa Hospital in Bizerte, Tunisia from September 17, 2020 to May 12, 2021. All patients in our study had a laboratory-confirmed diagnosis of COVID-19 by RT-PCR on nasopharyngeal swab. We excluded patients, who had a non-confirmed diagnosis and those who received a medical history or treatment that altered their blood counts and, therefore, their circulating lymphocytes or CRP (e.g., chemotherapy, immunosuppressive therapy, active cancer, or hematological malignancies).
Patients were categorized into two groups: non-ICU group with moderate severity of COVID-19 (n = 52) and ICU group with severe to critical disease (n = 80). Disease severity classification of COVID-19 patients was performed according to the interim guidelines from the WHO and the National Health Commission of China [11], [12]: (i) The mild disease group was defined as patients displaying mild, clinical symptoms with no pneumonia on computerized tomography (CT) imaging. (ii) Patients with a moderate illness were characterized by fever, respiratory symptoms, and a CT imaging indicating the presence of pneumonia. (iii) Patients belonging to the severe disease group were those who met at least one of the following criteria: shortness of breath and respiratory rate ≥ 30 breaths/min; SpO2 ≤ 93% at a rest state; PaO2/FiO2 ≤ 300 mmHg; and/or lung infiltrates > 50% of the lung field within 24–48 h. (iv) Critical patients were defined as those meeting at least one of the following conditions: patients with respiratory failure who were in need of mechanical ventilation; patients displaying signs of cardiovascular shock; and patients with other organ failures, which required monitoring in the intensive care unit.
Blood samples were collected at the moment of the first in hospital consultation for COVID-19 symptoms, before any treatment. For critical patients admitted in ICU (n = 80), blood samples were also collected at the moment of the last day of hospitalization. The clinical outcomes (survival or death) of all patients were followed up to 30 days after admission in the hospital. The study was approved by the institutional ethics committee of Al-Amen Clinic and Habib Bougatpha Hospital. Informed consent was obtained from all subjects enrolled in the study.
2.2. Data collection
Data on demographic characteristics, comorbidities, severity assessment on admission, as well as clinical outcomes were retrieved from a medical record system. We collected data on age, sex, exposure history, chronic medical histories (hypertension, diabetes and asthma), symptoms from onset to hospital admission (fever, cough, dyspnea, headache, asthenia and flu symdrome), vital signs at hospital admission (heart rate and respiratory rate), treatment (vitaminotherapy, glucocorticoids, antibiotic treatment, anticoagulation treatment, oxygen therapy), as well as living status. Computed tomographic (CT) scans were also collected at admission to the hospital. All data were reviewed and validated by a team of trained physicians.
2.3. Laboratory measurements
2.3.1. Real-time reverse transcription PCR assay for SARS-CoV-2
Respiratory specimens were collected by trained technician and then shipped to the medical laboratory Biodhaouadi to detect SARS-CoV-2. The presence of SARS-CoV-2 in respiratory specimens was detected by real time reverse transcription (RT-PCR) methods. RNA was extracted automatically by AlphaPrep Viral DNA/RNA Extraction kit (ALPHAGENE Co., Ltd. Republic of Korea) in the nucleic extractor ARN viral NC 15 Plus (Hanwooltpc, Republic of Korea). Real TM Sacace Kit (Sacace Biotechnologies s.r.l, Como, Italy) was used for transcription to DNAc and RT-PCR. SARS-CoV-2 Real-TM is a multi-target Real-Time PCR test for the qualitative detection of SARS-CoV-2 (COVID-19 virus, 2019-nCoV) RNA in clinical samples. It detects 2 different specific genes of the SARS-Cov-2 virus genome, E-gene and N-gene. It includes a separate fluorescence channel for detection of a conserved region of SARS-like viruses which detects both SARS-Cov and SARS-Cov-2. Amplification was performed in Sa-Cycler-96 real time PCR System (Sacace Biotechnologies s.r.l, Como, Italy). Conditions for the amplifications were 35 °C for 20 min, 94 °C for 5 min, 5 cycles of 94 °C for 10 s, 5 cycles of 64 °C for 25 s followed by 45 cycles of 94 °C for 10 s and 64 °C for 25 s.
2.3.2. Clinical laboratory measurements
Initial clinical laboratory investigation included a complete blood count, serum biochemical test and infection-related biomarkers (CRP). Routine bacterial and fungal examinations were also performed.
2.4. Statistical analysis
All statistical analyses were performed by GraphPad Prism software 5 (GraphPad PRISMA 5.0 computer program) and MedCalc® software (version 20.022, Belgium). The statistical significance between groups was assessed by χ2 or Fisher’s exact test and the non-parametric Mann-Whitney U test. When more than two categories were analyzed, the one-way ANOVA test was used. The Spearman rank correlation coefficient was used for linear correlation analysis between groups. Continuous variables were expressed as mean and SEM. Receiver operating characteristic (ROC) curve and the effect size (Cohen’s d) analysis were conducted to evaluate the ability of the immunologic parameters in predicting severe disease. The optimal cut-off points were obtained by calculating Youden’s index. For all statistical analysis, P < 0.05 was considered statistically significant.
3. Results
3.1. Demographics and baseline characteristics of COVID-19 patients in non-ICU and ICU
A total of 132 patients with SARS-COV2 infection were included in this study. Among the patients, 52 cases were classified as moderate group in non-ICU and 80 categorized as severe group admitted in ICU. The baseline characteristics of patients were summarized in Table 1 . The mean age of the patients in ICU was 63 years (range; 30–87) and that of the non-ICU was 64 (range; 21–88) years old. The mean age of the control group was 53 (range; 24–86) years old (Table 1).
Table 1.
Demographic and laboratory findings of the COVID-19 Patients groups and the control group.
| Normal range | Control group (n = 33) | NO ICU (n = 52) | ICU (n = 80) | P | |
|---|---|---|---|---|---|
| Demographic | |||||
| Age. years | 53 (24–86) | 64 (21–88) | 63 (30–87) | 0.578 | |
| ≤50 years | 17 (51%) | 11 (21%) | 15 (18%) | 0.67 | |
| >50 years | 16 (49%) | 41 (79%) | 65 (82%) | ||
| Sex | |||||
| Female | 13 (39%) | 24 (46%) | 34 (42%) | 0.651 | |
| Male | 20 (61%) | 28 (54%) | 46 (58%) | ||
| Survivors | 33 (100%) | 48(93%) | 17 (22%) | <0.0001 | |
| Non-survivors | 0 (0%) | 4 (7%) | 63 (78%) | ||
| Lung Injury (%) | 20 (10–75) | 61 (15–90) | <0.0001 | ||
| Vital signs on admission | |||||
| SGI II Score | – | – | 32.79 (12–85) | ||
| APACHE II Score | – | – | 13.92 (3–32) | ||
| Blood routine | |||||
| Hemoglobin (g/dl) | 11.5–16 | 14.07 (10.3–19.6) | 13 (6.1–17.3) | 12.41 (5.5–17) | 0.117 |
| Red blood cell count (×106/µl) | 4.5–5.6 | 4.96 (3.91–6.5) | 4.52 (3.39–5.81) | 4.23 (2.05–6.46) | 0.0239 |
| Platelet (×103/µl) | 150–450 | 255.49 (151–407) | 232.8 (126.5–572) | 254.4 (36–622) | 0.202 |
| Infection-related biomarkers | |||||
| C-reactive protein (mg/l) | <10 | 19.46 (0.01–158.3) | 114.2 (1.93–274.1) | 171.1 (2–486.6) | 0.0011 |
Note: The P value is significant if < 0.05.
Legend: APACHE II = Acute Physiology And Chronic Health Evaluation II,SGI II = Simplified Gravity Index II.
Interstitial lung abnormalities were observed in chest computed tomography (CT) scans of all patients on admission. The mean lung injury was 61% (range; 15–90) in patients admitted in ICU, while in the non-ICU that was 20% (range; 10–75). The mean Simplified Gravity Index II (SGI II) of patients admitted in ICU was 32.79 (range; 12–85). In addition, the mean of Acute Physiology and Chronic Health Evaluation II (APACHE II) score of patients in ICU was 13.92 (range; 3–32) (Table1).
For the primary outcome, among 80 critically ill patients with SARS-COV2 infection, 63 (78%) patients had died and 17 (22%) patients had survived. Compared with the patients in the ICU, most of patients (93%) in the non-ICU were recovered and only 4 patients (7%) were deceased (Tables 1 and 2 ).
Table 2.
Demographic and clinical characteristics of the survival ICU group and deceased ICU group.
|
ICU (n = 80) |
P | ||
|---|---|---|---|
|
Survivors (n = 17) |
Non-Survivors (n = 63) | ||
| Demographic | |||
| Age. years | 50 (30–69) | 64 (36–87) | 0.003 |
| ≤50 years | 8 (47%) | 7 (11%) | 0.0008 |
| >50 years | 9 (53%) | 56 (89%) | |
| Sex | |||
| Female | 9 (53%) | 25 (40%) | 0.339 |
| Male | 8 (47%) | 38 (60%) | |
| Comorbidities | |||
| Hypertension | 7 (41%) | 33 (52%) | 0.423 |
| Diabetes | 7 (41%) | 31 (49%) | 0.56 |
| Obesity | 3 (17%) | 18 (28%) | 0.359 |
| Asthma | 4 (23%) | 2 (3%) | 0.005 |
| Smoking | 3 (17%) | 9 (14%) | 0.757 |
| Signs and symptoms at disease onset | |||
| Fever | 9 (53%) | 39 (61%) | 0.553 |
| Cough | 8 (47%) | 16 (25%) | 0.079 |
| Headache | 0 (0%) | 11 (17%) | 0.069 |
| Asthenia | 4 (23%) | 11 (17%) | 0.572 |
| Diarrhée | 0 (0%) | 5 (7%) | 0.264 |
| Dyspnea | 12 (70%) | 46 (73%) | 0.807 |
| Severe ARDS | 0 (0%) | 47 (74%) | <0.0001 |
| Flu Syndrome | 4 (23%) | 23 (36%) | 0.315 |
| Anosmia | 5 (29%) | 2 (3%) | 0.0007 |
| Vital signs on admission | |||
| Respiratory rate, per min | 34 (26–50) | 32 (8–60) | 0.43 |
| SpO2, % | 86 (60–98) | 83 (36–98) | 0.1712 |
| SGI II Score | 24 (13–36) | 34 (12–85) | 0.0072 |
| APACHE II Score | 9 (3–18) | 14 (4–32) | 0.0061 |
| Lung Injury (%) | 54 (30–80) | 63 (15–90) | 0.0537 |
| Oxygen support | |||
| Oxygenotheray | 17 (100%) | 33 (52%) | 0.0003 |
| Non-invasive ventilation | 8 (47%) | 44 (70%) | 0.079 |
| Invasive ventilation | 0 (0%) | 51 (81%) | <0.0001 |
| Treatment | |||
| Vitaminotherapy | 17 (100%) | 63 (100%) | |
| Glucocorticoids | 17 (100%) | 63 (100%) | |
| Antibiotic treatment | 17 (100%) | 63 (100%) | |
| Anticoagulation treatment | 17 (100%) | 63 (100%) | |
| Onset to admission,days | 10 (1–28) | 11 (2–75) | 0.5 |
| Hospital stay, days | |||
| ≤14 | 7 (41%) | 39 (62%) | 0.122 |
| >14 | 10 (59%) | 24 (38%) | |
| Complications | |||
| Bronchial superinfection | 0 (0%) | 15 (23%) | 0.029 |
| HCAP | 0 (0%) | 17 (26%) | 0.019 |
| Septic Choc | 0 (0%) | 27 (42%) | 0.0012 |
| Renal failure | 0 (0%) | 22 (34%) | 0.0052 |
| Multiviscerale failure | 0 (0%) | 8 (12%) | 0.135 |
Note: The P value is significant if <0.05.
Legend: APACHE II = Acute Physiology And Chronic Health Evaluation II, SGI II = Simplified Gravity Index II, ARDS = Acute Respiratory Distress Syndrome, SpO2 = oxygen saturation, HCAP = healthcare-associated pneumonia.
As shown in Table 2, the mean age of deceased patients in ICU was 64 (range; 36–87) years, which was significantly older than recovered patients 50 (range; 30–69) years (P = 0.003). Male sex was more predominant in deceased patients (38; 60%) than in recovered patients (8; 47%).
Compared with survivors, non-survivors in ICU were more likely to develop severe ARDS and to receive invasive mechanical ventilation (47 (74%) and 51 (81%)) (Table 2). Nosocomial infection was only noted in deceased patients in ICU. Among deceased patients in ICU, 23% developed bronchial superinfection, 26% patients who had ventilator-associated pneumonia (VAP) and 42% developed septic choc. Further comparison between recovered and deceased patients in ICU showed that multiviscerale failure especially renal failure was noted only in non-survivors patients (Table 2).
3.2. Immune cells subsets alterations in COVID-19 patients with different disease severity and outcome
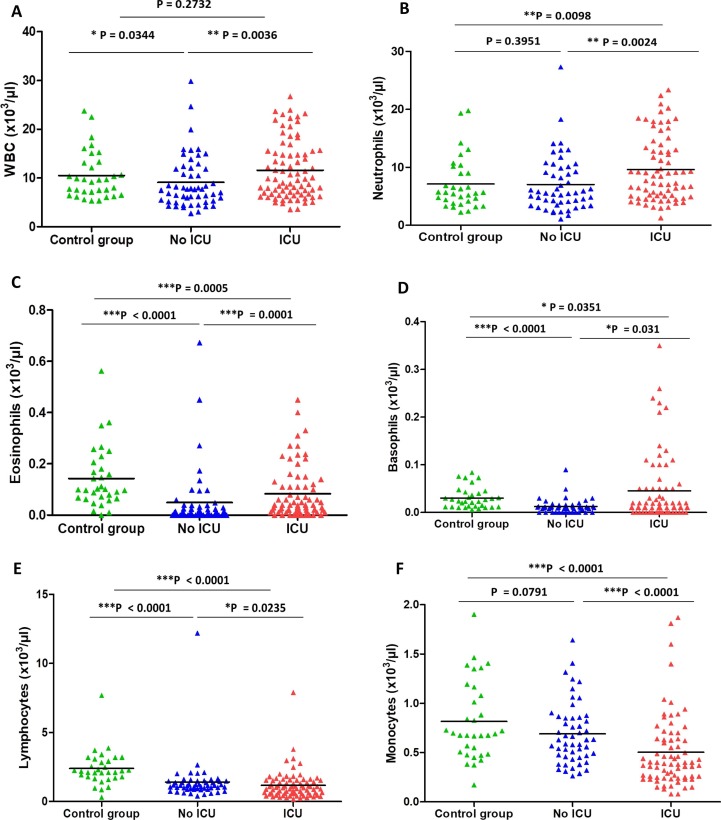
The quantification of peripheral blood immune subsets in patients admitted in ICU shown in Fig. 1 was compared with those in non-ICU and healthy controls. Our data showed that the WBC count was significantly increased in critical COVID-19 patients (11.61 (range; 3.57–26.7) × 103/µl) compared to the non-ICU group (9.1 (range; 2.76–29.84) × 103/µl), P = 0.0036) (Fig. 1). Furthermore, this increase was more pronounced in the fatal cases in ICU when compared to survived patients (12.45 vs. 8.32 × 103/µl; P = 0.0072) (Table 3 ). Similarly, neutrophils were significantly increased in critical COVID-19 patients (9.63 (range; 1.27–23.37) × 103/µl) compared to the non-ICU group (7.04 (range; 1.07–27.3) × 103/µl), P = 0.0024) (Fig. 1). Interestingly, this increase was more pronounced in the fatal cases in ICU when compared to survived patients (10.36 vs. 6.82 × 103/µl; P = 0.0275) (Table 3). A significant increase in eosinophils was found in the severe cases compared to the non-severe group (0.083 vs. 0.048 × 103/µl, P = 0.001). Also, there was a significant increase in the basophils in ICU group (0.045 (range; 0–0.35) × 103/µl) compared to the non-ICU group (0.012 (range; 0–0.09) × 103/µl), P = 0.031). Lymphopenia was also observed in the non-severe COVID-19 patients compared to control group, where lymphocyte count was 1.41 (range; 0.4–12.19) × 103/µl in the non-ICU group and it was 2.38 (range; 0.3–7.67) × 103/μl in healthy controls (P < 0.001). Moreover, this decrease was more pronounced in the critical cases when compared to non-severe COVID-19 patients (1.16 vs. 1.41 × 103/µl; P = 0.0235). Also, there was a significant decrease in the monocytes in ICU group (0.5 (range; 0.08–1.87) × 103/µl) compared to the non-ICU group (0.69 (range; 0.26–1.64) × 103/µl), P < 0.0001) (Fig. 1).
Fig. 1.
Number of immune cells subsets in healthy controls and COVID-19 patients. WBC (A) Neutrophils (B) Eosinophils (C) Basophils (D) Lymphocytes (E), and Monocytes (F) counts in different group.
Table 3.
Laboratory findings of the survival ICU group and deceased ICU group.
|
ICU (n = 80) |
P | ||
|---|---|---|---|
| Survivors (n = 17) | Non-Survivors (n = 63) | ||
| Blood routine | |||
| Hemoglobin (g/dl) | 12.42 (9.3–14.6) | 12.53 (5.5–17) | 0.5 |
| Red blood cell count (×106/µl) | 4.39 (3.87–5.3) | 4.22 (2.05–6.46) | 0.336 |
| Platelet (×103/µl) | 253.1 (127–382) | 254.7 (36–622) | 0.2718 |
| White blood cell count (×103/µl) | 8.32 (3.63–15.7) | 12.45 (3.57–26.7) | 0.0072 |
| Lymphocyte count (×103/µl) | 0.93 (0.18–1.98) | 1.22 (0.24–7.89) | 0.2778 |
| Monocyte count (×103/µl) | 0.49 (0.16–1.6) | 0.5 (0.08–1.87) | 0.3633 |
| Neutrophil count (×103/µl) | 6.82 (1.27–13.91) | 10.36 (3.09–23.37) | 0.0275 |
| Eosinophil count (×103/µl) | 0.055 (0–0.23) | 0.089 (0–0.45) | 0.2281 |
| Basophil count (×103/µl) | 0.008 (0–0.05) | 0.053 (0–0.35) | 0.0041 |
| Infection-related biomarkers | |||
| C-reactive protein (mg/l) | 116 (2–249.5) | 184.8 (19.52–486.6) | 0.0169 |
Note: The P value is significant if <0.05.
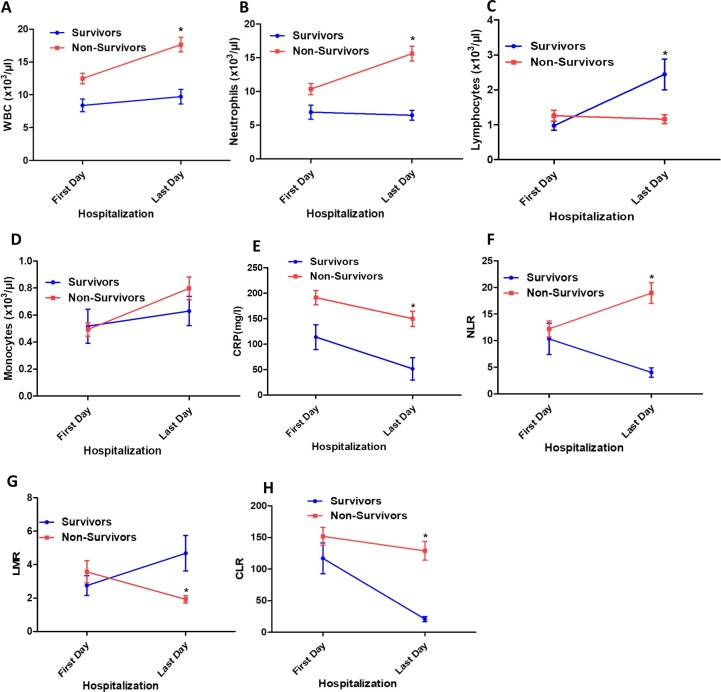
In addition to the changes occurring in immune cell populations, the study extended our analysis to the different immune subsets between first and last day of hospitalization in fatal cases versus recovered COVID-19 patients in ICU. Interestingly, our data revealed a more pronounced increase of the WBC count in the fatal cases at last day of hospitalization when compared to survived patients (12.49 vs. 17.65 × 103/µl; P < 0.0001). Among fatal cases, the neutrophils remain increased at last day of hospitalization, while it decreased in recovered patients (15.61 vs. 6.46 × 103/µl; P < 0.0001). Interestingly, lymphocytes count returned to the normal value at last day of hospitalization (2.44 ± 0.44 × 103/µl) in survived group, while it more decreased from first to last day of hospitalization in fatal cases (1.26 vs. 1.16; P = 0.039). From first to last day of hospitalization, the monocytes count increase in both fatal and survived COVID-19 cases (Fig. 2 ).
Fig. 2.
Comparison of immune cells subsets, CRP, LMR, NLR and CLR in survivors and non-survivors COVID-19 patients between first and last day of hospitalization in ICU. WBC (A), Neutrophils (B), Lymphocytes (C), Monocytes (D), CRP (E), NLR (F), LMR (G) and CLR (H) in patients COVID-19 group between first and last day of hospitalization in ICU.
3.3. Correlations between immune cells subsets and CRP in COVID-19 patients groups
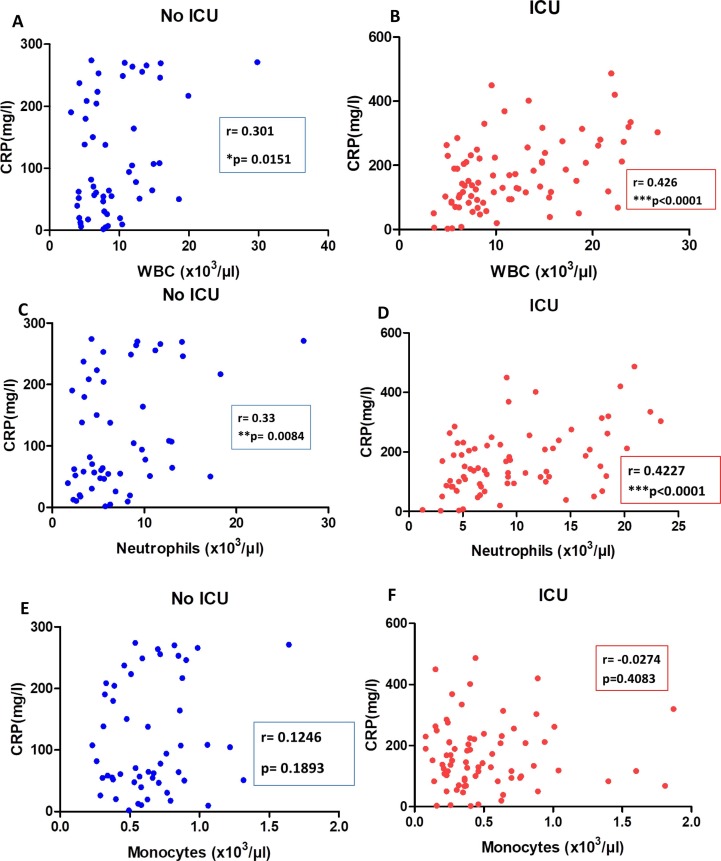
In order to gain a broader understanding of the immune response triggered by SARS-COV2 infection, the study examined the correlation between the different immune cells subsets and the CRP level (Fig. 3 ). The analysis revealed a positive correlation between WBC count and CRP level in non-ICU group and ICU group (r = 0.301, P = 0.0151 and r = 0.426, P < 0.0001; respectively). The neutrophils count was positively correlated with CRP level in non-severe and critical COVID-19 patients (r = 0.33, P = 0.0084 and r = 0.427, P < 0.0001; respectively). No correlation was found between monocytes and CRP concentration in both COVID-19 groups (Fig. 3). Similarly, there was no significant correlation between lymphocytes and CRP level in non-ICU and ICU groups (data not shown).
Fig. 3.
Correlations between CRP and immune cells subsets in COVID-19 patients groups. Correlations between CRP and WBC (A,B), and Neutrophils (C,D), and Monocytes (E,F) in COVID-19 patients admitted in No ICU and ICU.
There was a significant increase of C-reactive protein (CRP) in the COVID-19 patients admitted in ICU in comparison to non-ICU subjects (171.1 (range; 2–486.6) mg/l versus 114.2 (range; 1.93–274.1) mg/l; respectively, P = 0.0011) (Table 1). Furthermore, this increase was more pronounced in the fatal cases in ICU when compared to survived patients (184.8 (range; 19.52–486.6) mg/l vs. 116 (range; 2–249.5) mg/l; P = 0.0169) (Table 3).). As shown in Fig. 2, from first to last day of hospitalization, the CRP value decreased in both fatal and survived COVID-19 cases. However, this decrease was more pronounced in the survived patients compared to fatal cases at last day of hospitalization (51.52 vs. 150 mg/l, respectively). The other laboratory findings of the patients are illustrated in Tables 1 and 3.
3.4. The results of the assessed inflammatory indices of COVID-19 patients in non-ICU and ICU
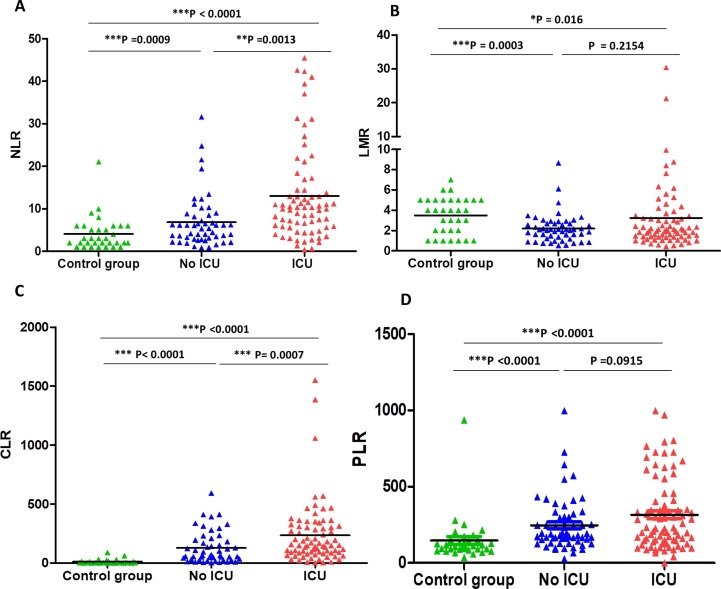
We investigated the individual diagnostic accuracy of NLR, LMR, CLR, and PLR in patients with COVID-19 compared to healthy controls. Hence, we compared NLR, LMR, CLR, and PLR values in healthy subjects, severe and moderate COVID-19 patients with COVID‐19. Fig. 4 shows these results. The value of NLR increased significantly in moderate group (6.84 (range; 0.88–31.57)) in comparison to the control group (4.06 (range; 1–21); P = 0.0009)). Furthermore, this increase was more pronounced in ICU cases (12.96 (range; 0.39–45.5)) when compared to non-ICU patients (6.84 (range; 0.88–31.57; P = 0.0013) (Fig. 4). In contrast, there was a significant decrease in the LMR in non-ICU group compared to control group (2.2 (0.53–8.67) and 3.48 (1–7); respectively, P = 0.0003)). There was no significant difference in the LMR between moderate and critical COVID-19 patients. Interestingly, there was a strong statistically significant difference between moderate and severe clinical outcomes in terms of the CLR variable, but there was no statistically significant difference in terms of PLR variable. The value of CLR increased significantly in moderate group (128.4 (range; 1.34–593.2)) in comparison to the control group (10.94 (range; 0.001–88.73); P < 0.0001)). Moreover, this increase was more pronounced in ICU cases (234.4 (range; 1.42–1551)) when compared to non-ICU patients (128.4 (range; 1.34–593.2); P = 0.0007) (Fig. 4). Interestingly, increased NLR and CLR were observed at last day of hospitalization in patients with a fatal outcome compared to those who survived in ICU (18.95 vs. 4.02; P < 0.0001 and 128.97 vs. 21.07; P < 0.0001, respectively). However, decreased LMR value was observed at last day of hospitalization in patients with a fatal outcome compared to those who survived in ICU (1.92 vs. 4.68; P < 0.0001) (Fig. 2).
Fig. 4.
NLR (A), LMR (B), CLR (C), and PLR (D) parameters of the control group and COVID-19 groups.
3.5. Potential immunologic markers to identify severe cases among COVID-19 patients
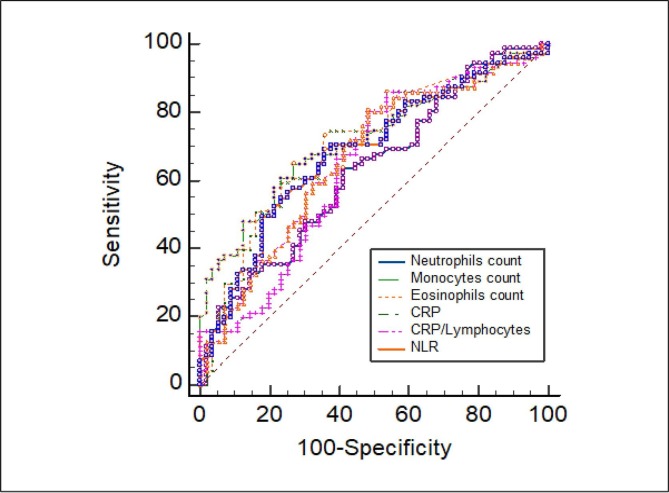
As previously described, low count of lymphocytes and monocytes cells and high count of WBC, neutrophils, eosinophils and basophils cells were associated with increased disease severity. Similarly, CRP, NLR and CLR values were associated with increased COVID-19 severity. ROC curve and area under ROC curve (AUC) were generated to evaluate the potential use of these parameters as diagnosis tool to identify severe cases. As shown in Fig. 5 and Table 4 , the number of monocytes, eosinophils cells and NLR, CLR, CRP had the highest diagnosis efficiency. The sensitivity, specificity and AUC of monocytes were 63.51%, 72.88% and 0.698, respectively at a cutoff (0.46 × 103/µl), P < 0.0001, and those of eosinophils were 73.97%, 60% and 0.685, respectively at a cutoff (0.019 × 103/µl), P = 0.0001. Also, the sensitivity, specificity and AUC of NLR were 69.86%, 62.71% and 0.685, respectively at a cutoff (6.69), P = 0.0001, and those of CLR were 81.25%, 51.79% and 0.668, respectively at a cutoff (69.46), P = 0.0004. The CRP achieved a sensitivity, specificity, and AUC for severe COVID-19 diagnosis of 86.25%, 46.67% and 0.659, respectively, at a cutoff (64.56 mg/l), P = 0.0008.
Fig. 5.
Area under the receiving operating curves of biomarkers as prognosticators of ICU admission. Legend: CRP = C-reactive protein; NLR = Neutrophils to Lymphocytes Ratio.
Table 4.
Predictive power of biomarkers as prognosticator of ICU admission.
| Variable | AUC | Cohen’s d | 95% CI | Criterion associated with best sensitivity and specificity at Youden index | Sensitivity | Specificity | Significance level P (Area = 0.5) |
|---|---|---|---|---|---|---|---|
| RBC (×106/µl) | 0.584 | 0.028 | 0.495–0.668 | ≤4.41 | 60% | 59.32% | 0.0926 |
| WBC (×103/µl) | 0.620 | 0.401 | 0.534–0.701 | >7.98 | 63.75% | 55.93% | 0.0127 |
| Lymphocyte count (×103/µl) | 0.577 | 1.07 | 0.490–0.660 | ≤0.72 | 37.5% | 83.05 | 0.1132 |
| Neutrophil count (×103/µl) | 0.629 | 0.452 | 0.540–0.711 | >6.288 | 64.38% | 57.63% | 0.0082 |
|
Monocyte count (×103/µl) |
0.698 | 0.506 | 0.613–0.775 | ≤0.46 | 63.51% | 72.88% | <0.0001 |
| Eosinophil count (×103/µl) | 0.685 | 0.137 | 0.598–0.762 | >0.019 | 73.97% | 60% | 0.0001 |
| Basophil count (×103/µl) | 0.583 | 0.615 | 0.493–0.668 | >0.049 | 30.14% | 98.28% | 0.0969 |
| C-reactive protein (mg/l) | 0.659 | 0.57 | 0.574–0.737 | >64.56 | 86.25% | 46.67% | 0.0008 |
| NLR | 0.685 | 0.584 | 0.598–0.763 | >6.69 | 69.86% | 62.71% | 0.0001 |
| CLR | 0.668 | 1.917 | 0.582–0.747 | >69.46 | 81.25% | 51.79% | 0.0004 |
| LMR | 0.554 | 0.345 | 0.465–0.640 | >3.05 | 28.38% | 88.14% | 0.2810 |
Note: The P value is significant if <0.05.
Legend: RBC = Red Blood Cells, WBC = White Blood Cells, NLR = Neutrophils to Lymphocytes Ratio, CLR = C-reactive protein to Lymphocytes Ratio, LMR = Lymphocytes to Monocytes Ratio.
To more evaluate the potential use of the above parameters as diagnosis tool to identify severe cases we next measure the effect size (Cohen's d) of each biomarker. For monocytes cells, CRP and NLR, the effect sizes were medium (0.506, 0.57 and 0.584; respectively). Interestingly, CLR had the largest effect size for the diagnosis of severe COVID-19 cases (1.917). (Table 4).
Multivariate regression analysis showed that neutrophils, eosinophils and NLR were positively correlated with the risk of COVID-19. Interestingly, CRP > 64.56 mg/l (OR: 6.272, P < 0.0001) and CLR > 69.46 (OR: 4.714, P < 0.0001) are considered independent diagnostic factors for sever COVID-19 infection. Nevertheless, the risks of WBC and monocytes were unclear (Table 5 ).
Table 5.
Logistic regression multivariate analysis for the prognosis of ICU admission.
| Variable | Odds Ratio | 95% confidence interval | P |
|---|---|---|---|
| WBC (>7.98 × 103/µl) | 1.963 | 1.124–3.427 | 0.0177 |
| Neutrophil count (>6.288 × 103/µl) | 2.478 | 1.394–4.403 | 0.0020 |
| Monocyte count (≤0.46 × 103/µl) |
1.963 | 1.124–3.427 | 0.0177 |
| Eosinophil count (>0.019 × 103/µl) | 3.210 | 1.760–5.855 | 0.0001 |
| C-reactive protein (>64.56 mg/l) | 6.272 | 3.090–12.730 | <0.0001 |
| NLR (>6.69) | 2.809 | 1.562–5.051 | 0.0006 |
| CLR (>69.46) | 4.714 | 2.449–9.07 | <0.0001 |
Note: The P value is significant if < 0.05.
Legend: WBC = White Blood Cells; NLR = Neutrophils to Lymphocytes Ratio, CLR = C-reactive protein to Lymphocytes Ratio.
3.6. Combined inflammatory indices and COVID-19 severity
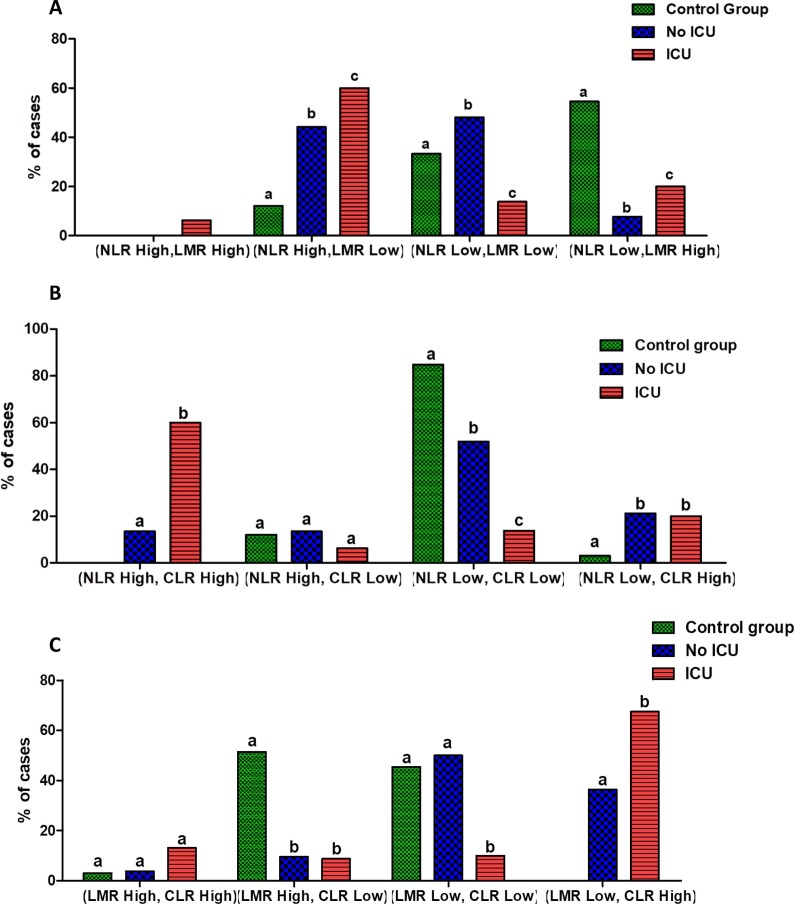
In the light of these data, we next investigated the combination of the three inflammatory markers NLR, LMR and CLR in the setting of COVID-19 disease. Fig. 6 shows these results. Based on Youden’s index, the optimal cutoff value was 6.69 for NLR, 3.05 for LMR and 69.46 for CLR (Table 4). Thus, we further classified patients depending to high and low levels of each inflammatory indices as fellow: NLR High: ≥6.69; NLR Low: <6.69; LMR High: ≥3.05; LMR Low: <3.05; CLR High: ≥69.46 and CLR Low: <69.46.
Fig. 6.
Combined NLR, LMR and CLR parameters in COVID-19 patients groups. Combined NLR and LMR parameters (A), Combined NLR and CLR parameters (B), and Combined LMR and CLR parameters (C) in COVID-19 patients groups. NLR High: ≥6.69; NLR Low: <6.69; LMR High: ≥3.05; LMR Low: <3.05; CLR High: ≥69.46 and CLR Low: <69.46.
The combination of NLR and LMR was significantly different in healthy subjects compared to COVID-19 patients groups. The (NLR High, LMR High) profile was absent in control group and non-severe COVID-19 patients. The (NLR Low, LMR High) profile was the most observed in the control group (n = 18; 54.54%), while the profiles (NLR High, LMR Low) and (NLR Low, LMR Low) were the most observed in moderate COVID-19 group (n = 22; 44.23% and n = 25; 48.07%, respectively). Among severe cases, the (NLR High, LMR Low) was the most predominant profile (n = 48; 60%) (Fig. 6). The study of the combination of NLR and CLR showed a significant predomination of the profile (NLR Low, CLR Low) in healthy subjects (n = 28; 84.84%) and in non-severe COVID-19 patients (n = 27;51.92%). Nevertheless, the profile (NLR High, LMR High) was the most observed in ICU groups (n = 48; 60%) (Fig. 6).
Furthermore, study of the combination of LMR and CLR shown predomination of both profiles (LMR High, CLR Low) and (LMR Low, CLR Low) in the control group (n = 17; 51.51% and n = 15; 45.45%, respectively). Among non-ICU, the (LMR Low, CLR Low) profile was the most observed (n = 26; 50%), while in ICU group the profile (LMR Low, CLR High) was the most predominate (n = 54; 67.5%) (Fig. 6).
4. Discussion
Since hyperinflammation in COVID-19 disease is known to be a major reason for poor prognosis of patients [13], biomarker combinations reflecting inflammation status may be a good alternative in this regard. Here, we study inflammatory biomarkers in moderate and critical COVID-19 patients with a particular emphasis on immune cell subsets in peripheral blood and subsequently on individual and combination inflammatory indices: NLR, LMR and CLR.
In line with previous reports, our data showed that the majority of patients, especially those who developed a severe disease with a fatal outcome, exhibited a significant increase of WBC and neutrophils counts (neutrophilia) but in contrast, drop of total lymphocyte counts (lymphopenia) [14], [15], [16]. Several studies have addressed the difference of baseline leukocyte counts between the clinical stages in COVID-19 patients. Chen G. et al. [17] reported that leukocytosis and lymphopenia were more common in severe cases than in moderate cases. Lymphopenia was reported as a common feature in patients with COVID-19, indicating abnormal immune function during SARS-CoV-2 infection [16]. Lymphocyte subsets played an important role in cellular immune regulation with each cell restricting and regulating each other. A number of studies had focused on characteristic of lymphocyte subsets in COVID-19 patients [18], [19]. Belaid B. et al. [19] suggested that the decrease of lymphocyte counts in COVID-19 patients is mainly due to the reduction of CD4+ T cells and B cells. Similarly, Wen X.S. et al. [20] found that CD4+ T and CD8+ T in the severe group had greater reductions than those in the mild group. Moreover, disease severity in lymphopenic patients with COVID-19 is more likely to be resulted in enhanced B and T lymphocyte apoptosis [21]. Recently, André and colleagues showed that CD4 and CD8 T cells from COVID-19 patients are more likely to die by apoptosis, and that blocking caspase activation using Q-VD prevents T cells from dying and enhances Th1 profiles [22].
Regarding neutrophil upregulation in patients with COVID-19, we can theorize a close association with lymphopenia. Qin C. et al. [14] reported that severe cases of COVID-19 were likely to have higher neutrophil count but lower lymphocyte count compared with non-severe patients. Mo P. et al. [23] investigated 155 patients with COVID-19 and found that refractory patients had higher level of neutrophils in comparison with general patients. It is known that infection with microbe can directly induce neutrophil recruitment to tissue sites [24]. Therefore, the impaired lymphocytes in patients with COVID-19 may easily lead to an infection with microbe, further promoting the activation and recruitment of neutrophils in the blood of patients [25]. In line with this idea, we found that some patients with critical disease have developed a bacterial superinfection that contributes to the exacerbation of the disease.
Interestingly, we noted that WBC, neutrophils and lymphocytes counts typically returned to near normal levels as patients recover from severe infection. Indeed, it has been shown that after a declining phase, all the lymphocytes tend to go back to their normal levels after the clearance of the virus [26]. By contrast, WBC and neutrophils counts remained high, while lymphocytes counts remained low throughout the course of infections with fatal outcomes. Deceased patients had persistent and more severe lymphopenia compared with recovered patients, suggesting that a cellular immune deficiency state was associated with poor prognosis [27]. It is widely accepted that lymphocytes play a central role in the defense mechanisms against viral respiratory infection. The study by Zhang H.J. et al. [28] showed that reduced absolute counts of lymphocyte blood levels that remain low can predict the death of patients with COVID-19. Belaid B. et al. [19] hypothesized that the lymphopenia is a direct consequence of the substantial cell migration to the site of infection where the immune response is initiated. Therefore, the proinflammatory environment might also contribute to the observed lymphopenia in COVID-19 patients. Consistent with this idea, Xu B. et al. [29] demonstrated increased inflammatory cytokines and suppressed T cell-mediated immunity in COVID-19. Moreover, they found lower counts of T lymphocyte subsets lymphocyte, CD3+ T cell, CD4+ T cell, CD8+ T cell, and B cell were associated with higher risks of in-hospital death of COVID-19 [29]. In line with these findings, previous reports have shown that the levels of IL-6 were negatively correlated with the lymphocyte counts in COVID-19 patients whereas convalescent patients were found to have restored their lymphocyte numbers paired with lower proinflammatory cytokine levels [18]. Interestingly, the IL-6 receptor antagonist, tocilizumab, was found to increase the number of circulating lymphocytes in COVID-19 patients [30].
Basophils and eosinophils, which play a greater role in other innate immune functions, such as allergic and anti-microbial responses, are nevertheless also impacted in COVID-19 [31]. In this study, we demonstrated that basophils and eosinophils levels were significantly higher in COVID-19 patients with critical disease, when compared to those with moderate diseases. Hence, ICU patients had a higher eosinophil count than non-ICU patients and also we identified eosinophil count higher than >0.019 × 103/µl as a risk factor for ICU admission. These findings are somehow different from those described earlier where the decline of basophils and eosinophils cells was more frequently observed in severe COVID-19 patients. The relatively limited data indicate depletion of basophils and eosinophils occurs in the blood in COVID-19, showing some associations with severe disease. Eosinopenia is frequent and has been linked to mortality in different settings during critical COVID-19 illness [31], [32]. By contrast, Chen R. et al. [33] found that eosinophil counts, while low at admission, ultimately rebounded in a cohort of patients who ultimately recovered from severe COVID-19. The significance of these changes in basophils and eosinophils numbers is not yet clear. It has been suggested, however, that decreased basophil and eosinophils counts in the blood could be attributed to migration to the lungs [31], [32]. A possible explanation for the discrepancy between our results and the earlier findings could be the differences in races and ethnicity. Of interest, Glickman J.W. et al. [34] found that the prognostic utility of peripheral eosinophil counts and percentages varied based on patient race and ethnicity.
Eosinophilia is not an isolated finding in severe COVID-19 patients and we showed that is typically accompanied by reduction in peripheral monocyte counts. In terms of absolute numbers, studies reported unchanged [35], increased [36] or decreased frequencies of the monocytic cell lineage in peripheral blood during COVID-19 [14]. The significance of these changes in monocyte numbers is not yet clear. IL-6 production by intermediate monocytes in COVID-19 has been described in association with cytokine storm and severe disease, and in general, increased IL-6 levels correlate with disease severity [13]. However, since an increase in intermediate monocytes in blood has been reported in both mild and severe disease, it is likely that other immune cells contribute to IL-6 production [36]. CRP is a non-specific acute-phase protein induced by IL-6 in the liver and a sensitive biomarker of inflammation, infection, and tissue damage. CRP expression level is usually low but increases rapidly and significantly during acute inflammatory responses [37]. The elevation of CRP in isolation or in combination with other markers may reveal bacterial or viral infections [38]. Our study explored the relationship between CRP and COVID-19 and found that patients with high CRP level were more likely to develop severe disease. Thus, a CRP level was significantly elevated in patients who are critically infected or deceased in comparison with patients in non-ICU or recovered. Furthermore, we found that CRP typically returned to near normal levels as patients recover from severe infection, while it remained high throughout the course of infections with fatal outcomes. We also found that an increased CRP level was correlated to WBC counts in non-ICU and ICU. Additionally, neutrophil, a major component of the leukocyte population, was positively correlated to CRP levels in both moderate and critical COVID-19 patients. Patients with severe virus infection are more likely to be co-infected with bacteria due to low immune functions, which would be another possible reason to explain the increased level of neutrophils and CRP shown in our study. The levels of CRP and other inflammatory markers, such as IL-6, IL-8, IL-2R, and IL-10, were noticed to be higher in severe cases than moderate COVID-19 cases [39]. Increased levels of CRP, cytokines, chemokines, neutrophils and decreased in lymphocytes in severe cases suggest a possible hyper-inflammatory response role in the pathogenesis of COVID-19 [13].
Due to the fact that blood leukocytes cells may are not accurate enough as earlier prognostic factors of poor outcome and effective in determining the severity of the disease, we have tried to demonstrate that other parameters resulting from the leukocyte formula may be more useful. Neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-C-reactive protein ratio (LCR), Glasgow prognostic score (GPS), systemic inflammation score (SIS) and prognostic nutritional index (PNI) are novel biomarkers of systemic inflammation, which are closely related to esophageal cancer and colorectal cancer. Previous studies reported that theses serum systemic inflammatory markers can predictive of response to chemotherapy and survival in patients with malignancy [40], [41]. Hence, we investigated NLR, LMR and C-reactive protein-to-lymphocyte ratio (LCR) as useful predictors for the prognosis of patients with SARS-COV2 pneumonia. The reference range of NLR and LMR was previously established only for Chinese healthy adults, which may make the results unrepresentative and impossible to be directly applied to subjects of our Tunisian cohort [42]. In the present study, our results first proved our hypothesis and indicated a prominent elevation of NLR among patients with refractory disease admitted to ICU and deceased patients when compared with moderate ill patients and healthy controls. An elevated NLR reflect the severity of COVID-19 and the immune status of the patients was a consequence of neutrophilia and lymphopenia throughout the course of infections with fatal outcomes [43]. Although the exact cutoff for the NLR until now is lacking, Forget et al., have identified that normal NLR values in an adult, non-geriatric, population in good health are between 0.78 and 3.53 [44]. Interestingly, our analysis revealed that NLR typically returned to near normal value as patients recover from severe infection. By contrast, deceased patients had persistent increased NLR until last day of hospitalization in ICU. Our data argue with previous studies that also revealed significantly elevated of NLR value in patients who are critically infected or deceased in comparison with patients in isolation wards or recovered [43]. Previous studies suggested that the magnitude of the increase in NLR indicated the extent of the impairment of immune system by the viral infection. Therefore, NLR may serve as a useful factor to reflect the intensity of imbalance of inflammation and immune responses in COVID-19 patients [43], [45].
Previous reports have revealed decreased LMR value among patients of ICU thus rendering declined ratio of LMR as an indicator of poor prognosis [46], [47]. However, our findings argue against the involvement of LMR as an indicator of severe COVID-19 outcome. No significant difference in LMR value has been found between moderate and severe COVID-19 patients; while, the moderate COVID-19 patients showed reduced LMR value compared to healthy controls. Our study also evaluated the LMR value from first and last day of hospitalization in ICU between survivors and deceased patients. Unlike NLR, declined LMR was maintained at last day of hospitalization in patients with a fatal outcome compared to those who survived in ICU. In line with our finding, previous study revealed that decreased LMR value as an indicator of increased chances of mortality among patients suffering from COVID-19 disease in ICU [46], [47].
Since the SARS-CoV2 viral load has been highly correlated with CRP value and lymphocyte count [38], [15], we hypothesized that CLR could help predict of disease severity. Few previous studies have been carried out to evaluate the feasibility of (lymphocyte-to-C-reactive protein ratio) LCR to assess COVID-19 disease severity [47], [48]. By contrast, this study aimed to determine CLR and not LCR whether or not could be useful in the discrimination of COVID 19 cases with different levels of severity. In the current study, elevated CLR value was detected among patients under treatment in non-ICU as compared with healthy controls. Furthermore, prominently elevated CLR was observed in critically ill as compared to outcomes of patients in non-ICU. Importantly, it was observed in the current study that CLR in the deceased group remained high, while these values returned to normal more quickly in survivor’s patients. Similar results were documented in another study, which showed that the LCR is able to distinguish COVID-19 infected patients of different severity (mild/moderate, severe and critically ill) [47], [48]. Erdogan A. et al. [47], [48] demonstrated that the LCR was significantly decreased in severe cases, suggesting that this marker could reflect the severity of COVID-19 disease. Supporting this hypothesis, data from several studies have revealed that the balance between host immune response and hyperinflammatory response plays a key role in prognosis in COVID-19 disease [13]. Therefore, lower LCR levels in severe patients could be the result of fewer lymphocytes leading to immune dysfunction and higher CRP levels reflecting the severe systemic inflammatory response of the patients [47], [48].
Effective prediction criteria can allow physicians to provide an appropriate medical care for the patients with severe COVID-19. Currently, Omicron is the dominant variant in several countries. Although the risk factors for the mortality are much more important than predicting the ICU submission in consideration the epidemic of the strain Omicron, prediction of severe COVID-19 cases remains a challenge since the emergence of new variants is an event that will continue to be repeated as time progresses [49]. In this study, ROC analysis was performed for the assessed blood immune cell subsets and inflammatory indices to identify severe COVID-19 patients. ROC curve analyses showed that eosinophils and monocytes count were the most associated immune cells subsets with the severity of COVID-19 disease. In addition, CRP, NLR and CLR were also associated with the severity of COVID-19 disease. Potential progonostic factors for severe COVID-19 were analyzed by a multivariable logistic regression. The results indicated that CRP level > 64.56 mg/l and CLR > 69.46 are two independent prognostic factors in identifying critically ill COVID-19 cases. Interestingly, CLR had the largest effect size for the diagnosis of severe COVID-19 cases. Therefore, our data indicated that CLR could be superior to NLR for the detection of COVID-19 disease severity. These findings are in accordance with previous studies that revealed that only LCR showed a reasonable ability to distinguish mild/moderate patients from severe patients, while the NLR was not able to discriminate these two groups from each other. These results also indicated that perhaps LCR could be used to triage patients with COVID-19 disease more effectively [47]. One reason for this difference between LCR and NLR trends may be that the relatively higher level of viral load in severe patients could contribute to the lower levels of lymphocyte counts and higher levels of CRP, compared to mild/moderate patients [47], [48]. A recent study by Zheng S. et al. [50] demonstrated that patients with severe disease have a later viral load peak as compared to those with mild disease. Authors have also suggested a correlation between virus persistence and poor disease outcomes.
To the best of our knowledge, here the study is the first to analyze the three combined markers NLR, LMR and CLR together with disease severity of COVID-19. Our study clearly showed that the risk of critical ill was associated with high NLR combined with low LMR among patients with refractory disease admitted to ICU. However, healthy controls were mostly characterized with low NLR combined with high LMR. Study of the combined markers NLR and CLR showed that most of patients admitted in ICU were characterized with high NLR combined with high CLR, while most of healthy subjects and non-ICU group have low NLR combined with low CLR. Furthermore, study of the combined markers LMR and CLR showed that the majority of patients admitted in ICU have low LMR combined with high CLR. The differentiation of severe patients from mild/ moderate patients is essential for adequate management of the disease which may prevent unnecessary hospitalization and decrease delayed treatment, which is associated with mortality risk because of silent hypoxia in severe cases [51]. In the current study, it was observed that among combined markers only NLR and CLR combination showed a reasonable ability to distinguish mild/moderate patients from severe patients. Therefore, we suggested that combined NLR and CLR may be more valuable and useful in identifying critically ill COVID-19 cases. Our findings also indicated that combined NLR and CLR could be used to triage patients with COVID-19 disease more effectively than individually inflammatory indices.
The study presents here the first description of immunologic characteristics of a cohort of Tunisian patients with COVID-19 that confirms some data described in previous reports. The study also showed that the immune cell subsets and CRP levels as well as inflammatory indices (NLR, LMR and CLR) resulting from the leukocyte formula was correlated with the severity of the disease and fatal outcome. To the best of our knowledge, this is the first work to describe the combination inflammatory indices: NLR, LMR and CLR as powerful prognostic factors for the early identification of severe COVID-19 cases. Importantly, we identified combined markers NLR and CLR as a valuable prognostic marker in discriminating severe COVID-19 patients.
5. Conclusion
In conclusion, in our study NLR combined to CLR seems to predict better than NLR and CLR alone critically cases. The results of this study have several clinical implications and strengths. Since NLR and CLR could be quickly calculated based on a blood routine test on admission, clinicians may identify high risk COVID19 patients at an early stage. Thus, treatments can be modified accordingly to reduce the in-hospital death. Nevertheless, further prospective investigations are necessary to define precisely the immunologic profile during the course of the disease in order to improve the clinical and therapeutic management of COVID-19 patients.
6. Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Awatef Ben Jemaa: Investigation, Conceptualization, Methodology, Data curation, Writing – review & editing. Noura Salhi: Data curation, Investigation. Meriam Ben Othmen: Conceptualization, Methodology. Hana Ben Ali: Data curation, Investigation. Jihene Guissouma: Data curation, Investigation. Hatem Ghadhoune: Data curation, Investigation. Ridha Oueslati: . Hamdi Dhaouadi: Investigation, Conceptualization, Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The Authors thank medical and nursing staffs of Al-Amen Clinic and Habib Bougatfa Hospital in Bizerte, Tunisia for their precious contribute in management of patients suffering for SARS-CoV2. They also thank technical staff of Biodhaouadi Laboratory for the technical support.
References
- 1.Ludwig S., Zarbock A. Coronaviruses and SARS-CoV-2: a brief overview. Anesth. Analg. 2020;131:93–96. doi: 10.1213/ane.0000000000004845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, WHO Coronavirus (COVID-19) dashboard, Available at: https://covid19.who.int/. Accessed on 29 Dec 2021.
- 3.B. Aylward, W. Liang, Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19), HO-China Jt Mission Coronavirus. Dis. 2019 (2020) 16-24.
- 4.Jesenak M., Brndiarova M., Urbancikova I., Rennerova Z., Vojtkova J., Bobcakova A., Ostro R., Banovcin P. Immune parameters and COVID-19 infection—associations with clinical severity and disease prognosis. Front. Cell. Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vabret N., Britton G.J., Gruber C., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Li S., Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold. Spring. Harb. Perspect. Biol. 2014;6:1–16. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng J., Qi D., Yuan G., et al. Diagnostic value of peripheral hematologic markers for coronavirus disease 2019 (COVID‐19): a multicenter, cross‐sectional study. J. Clin. Lab. Anal. 2019;34(2020) doi: 10.1002/jcla.23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagunas-Rangel F.A. Neutrophil‐to‐lymphocyte ratio and lymphocyte‐to‐C‐reactive protein ratio in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. J. Med. Virol. 2019;92(2020):1733–1734. doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bivona G., Agnello L., Ciaccio M. Biomarkers for prognosis and treatment response in COVID-19 patients. Ann. Lab. Med. 2021;41:540–548. doi: 10.3343/alm.2021.41.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance 28 January 2020, WHO. 10 (2020).
- 12.N.H.C. Chinese, state A of T, Diagnosis and treatment protocol for novel coronavirus pneumonia, Chin. Med. J. (Engl). 133 (2020) 1087-1095, 10.1097/cm9.0000000000000819. [DOI] [PMC free article] [PubMed]
- 13.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan L.i., Wang Q.i., Zhang D., Ding J., Huang Q., Tang Y.-Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal. Transduct. Target. Ther. 2020;5(1) doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. Respir. Med. 2020;8:475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/jci137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Li S., Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belaid B., Lamara Mahammad L., Mihi B., Rahali S.Y., Djidjeli A., Larab Z., Berkani L., Berkane I., Sayah W., Merah F., Lazli N.Z., Kheddouci L., Kadi A., Ouali M., Khellafi R., Mekideche D., Kheliouen A., Ayoub S., Hamidi R.M., Derrar F., Gharnaout M., Allam I., Djidjik R. T cell counts and IL-6 concentration in blood of North African COVID-19 patients are two independent prognostic factors for severe disease and death. J. Leukoc. Biol. 2022;111(1):269–281. doi: 10.1002/JLB.4COVA1020-703R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen X.-S., Jiang D., Gao L., Zhou J.-Z., Xiao J., Cheng X.-C., He B., Chen Y., Lei P., Tan X.-W., Qin S., Zhang D.-Y. Clinical characteristics and predictive value of lower CD4(+)T cell level in patients with moderate and severe COVID-19: a multicenter retrospective study. BMC. Infect. Dis. 2021;21(1) doi: 10.1186/s12879-020-05741-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cizmecioglu A., Akay Cizmecioglu H., Goktepe M.H., Emsen A., Korkmaz C., Esenkaya Tasbent F., Colkesen F., Artac H. Apoptosis-induced T-cell lymphopenia is related to COVID-19 severity. J. Med. Virol. 2021;93(5):2867–2874. doi: 10.1002/jmv.26742. [DOI] [PubMed] [Google Scholar]
- 22.André S., Picard M., Cezar R., et al. T cell apoptosis characterizes severe Covid-19 disease. Cell. Death. Differ. 2022:1–14. doi: 10.1038/s41418-022-00936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo P., Xing Y., Xiao Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2021;73:e4208–e4213. doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reusch N., De Domenico E., Bonaguro L., Schulte-Schrepping J., Baßler K., Schultze J.L., Aschenbrenner A.C. Neutrophils in COVID-19. Front. Immunol. 2021;12:652470. doi: 10.3389/fimmu.2021.652470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L.i., Liu S., Liu J., Zhang Z., Wan X., Huang B.o., Chen Y., Zhang Y.i. COVID-19: immunopathogenesis and Immunotherapeutics. Signal. Transduct. Target. Ther. 2020;5(1) doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He R., Lu Z., Zhang L., et al. The clinical course and its correlated immune status in COVID-19 pneumonia. Clin. Virol. 2020;127:104361. doi: 10.1016/j.jcv.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantenys-Molina S., Fernández-Cruz E., Francos P., et al. Lymphocyte subsets early predict mortality in a large series of hospitalized COVID-19 patients in Spain. Clin. Exp. Immunol. 2021;203:424–432. doi: 10.1111/cei.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H.J., Qi G.Q., Gu X., et al. Lymphocyte blood levels that remain low can predict the death of patients with COVID-19. Medicine (Baltimore) 2021;100 doi: 10.1097/md.0000000000026503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu B., Fan C.Y., Wang A.L., et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J. Infect. 2020;81:e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cacciapuoti S., De Rosa A., Gelzo M., et al. Immunocytometric analysis of COVID patients: A contribution to personalized therapy? Life. Sci. 2020;261:118355. doi: 10.1016/j.lfs.2020.118355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong X., Cheng A., Yuan X., et al. Characteristics of peripheral white blood cells in COVID-19 patients revealed by a retrospective cohort study. BMC. Infect. Dis. 2021;21:1236. doi: 10.1186/s12879-021-06899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L., Zhang Y.-P., Yang X., Liu X. Eosinopenia is associated with greater severity in patients with coronavirus disease 2019. Allergy. 2021;76(2):562–564. doi: 10.1111/all.14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen R., Sang L., Jiang M., Yang Z., Jia N., Fu W., Xie J., Guan W., Liang W., Ni Z., Hu Y.u., Liu L., Shan H., Lei C., Peng Y., Wei L.i., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zheng J., Zhang N., Li Y., He J., Li J., Li S., Zhong N. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J. Allergy. Clin. Immunol. 2020;146(1):89–100. doi: 10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glickman J.W., Pavel A.B., Guttman-Yassky E., et al. The role of circulating eosinophils on COVID-19 mortality varies by race/ethnicity. Allergy. 2020;76:925–927. doi: 10.1111/all.14708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombardi A., Trombetta E., Cattaneo A., et al. Early phases of COVID-19 are characterized by a reduction in lymphocyte populations and the presence of atypical monocytes. Front. Immunol. 2020;11:560330. doi: 10.3389/fimmu.2020.560330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L.Y.C., Biggs C.M., Jamal S., et al. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell. Rep. Med. 2021;2:100269. doi: 10.1016/j.xcrm.2021.100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy. Clin. Immunol. 2020;146(1):128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu F., Li L., Xu M., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhar S.K., Vishnupriyan K., Damodar S., et al. L-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon. 2021;7:e06155. doi: 10.1016/j.heliyon.2021.e06155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li K.J., Xia X.F., Su M., et al. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC. Cancer. 2019;19:1004. doi: 10.1186/s12885-019-6157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inamoto S., Kawada K., Okamura R., Hida K., Sakai Y. Prognostic impact of the combination of neutrophil-to-lymphocyte ratio and Glasgow prognostic score in colorectal cancer: a retrospective cohort study. Int. J. Colorectal. Dis. 2019;34(7):1303–1315. doi: 10.1007/s00384-019-03316-z. [DOI] [PubMed] [Google Scholar]
- 42.Wang J., Zhang F., Jiang F., et al. Distribution and reference interval establishment of neutral-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) in Chinese healthy adults. J. Clin. Lab. Anal. 2021;35:e23935. doi: 10.1002/jcla.23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang A.P., Liu J.P., Tao W.Q., et al. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forget P., Khalifa C., Defour J.P., et al. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC. Res. Notes. 2017;10:12. doi: 10.1186/s13104-016-2335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vafadar Moradi E., Teimouri A., Rezaee R., Morovatdar N., Foroughian M., Layegh P., Rezvani Kakhki B., Ahmadi Koupaei S.R., Ghorani V. Increased age, neutrophil-to-lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID-19 mortality. Am. J. Emerg. Med. 2021;40:11–14. doi: 10.1016/j.ajem.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asghar M.S., Khan N.A., Haider Kazmi S.J., Ahmed A., Hassan M., Jawed R., Akram M., Rasheed U., Memon G.M., Ahmed M.U., Tahniyat U., Tirmizi S.B. Hematological parameters predicting severity and mortality in COVID-19 patients of Pakistan: a retrospective comparative analysis. J. Community. Hosp. Intern. Med. Perspect. 2020;10(6):514–520. doi: 10.1080/20009666.2020.1816276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erdogan A., Can F.E., Gönüllü H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR ratio in COVID-19 patients. J. Med. Virol. 2021;93:5555–5559. doi: 10.1002/jmv.27097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ullah W., Basyal B., Tariq S., et al. Lymphocyte-to-C-Reactive Protein Ratio: A Novel Predictor of Adverse Outcomes in COVID-19. J. Clin. Med. Res. 2020;12:415–422. doi: 10.14740/jocmr4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.I.R. Mendiola-Pastrana E. López-Ortiz J.G. Río de la Loza-Zamora et al. SARS-CoV-2 Variants and Clinical Outcomes: A Systematic Review, Life. (Basel). 12 (2022) 170, 10.3390/life12020170. [DOI] [PMC free article] [PubMed]
- 50.Zheng S., Fan J., Yu F., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. B.M.J. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo L., Jin Z., Gan T.J., et al. Silent hypoxemia in patients with COVID-19 pneumonia: a review. Med. Sci. Monit. 2021;27:e930776. doi: 10.12659/msm.930776. [DOI] [PMC free article] [PubMed] [Google Scholar]