Abstract
Purpose
To assess and compare the relative efficacy and safety of anti-SARS-CoV-2 antibody regimens for COVID-19.
Methods
This systematic review and random-effects network meta-analysis was conducted according to PRISMA-NMA. Literature searches were conducted across MEDLINE, EMBASE, PubMed, Web of Science, CENTRAL, and CNKI up to February 20th, 2022. Interventions were ranked using P scores.
Results
Fifty-five RCTs (N = 45,005) were included in the review. Bamlanivimab + etesevimab (OR 0.13, 95% CI 0.02–0.77) was associated with a significant reduction in mortality compared to standard of care/placebo. Casirivimab + imdevimab reduced mortality (OR 0.67, 95% CI 0.50–0.91) in baseline seronegative patients only. Four different regimens led to a significant decrease in the incidence of hospitalization compared to standard of care/placebo with sotrovimab ranking first in terms of efficacy (OR 0.20, 95% CI 0.08–0.48). No treatment improved incidence of mechanical ventilation, duration of hospital/ICU stay, and time to viral clearance. Convalescent plasma and anti-COVID IVIg both led to a significant increase in adverse events compared to standard of care/placebo, but no treatment increased the odds of serious adverse events.
Conclusion
Anti-SARS-CoV-2 mAbs are safe, and could be effective in improving mortality and incidence of hospitalization. Convalescent plasma and anti-COVID IVIg were not efficacious and could increase odds of adverse events. Future trials should further examine the effect of baseline seronegativity, disease severity, patient risk factors, and SARS-CoV-2 strain variation on the efficacy of these regimes.
Registration
PROSPERO-CRD42021289903.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-022-01825-8.
Keywords: SARS-CoV-2, Antibody, Convalescent plasma, Monoclonal antibody, Network meta-analysis
Introduction
In the midst of the ongoing SARS-CoV-2 pandemic, a variety of treatment regimens have been developed or repurposed for the management of COVID-19. Specifically, the efficacy and safety of several antiviral therapies, as well as immunomodulatory regimens, have been thoroughly investigated and described in the literature. However, many of the antiviral therapies, such as chloroquine, lopinavir–ritonavir, and ivermectin, have been consistently shown in randomized controlled trials (RCTs) and meta-analyses to confer little to no clinical efficacy against SARS-CoV-2 [1–3]. Meanwhile, immunomodulatory agents such as dexamethasone were found to only be effective among patients with severe disease receiving respiratory support, with little to no efficacy in patients with mild symptoms [4]. While vaccination remains one of the best strategies for the prevention of infections and disease progression, numerous obstacles—including vaccine hesitancy, logistical difficulties, and lack of access in low-to-middle income countries—continue to hamper its adoption [5, 6]. Furthermore, older adults and those with comorbidities such as diabetes, obesity, and immunosuppression can present with atypical symptoms, have varying immunity from vaccination, and continue to be at high risk for hospitalization and mortality [7, 8]. Therefore, a range of evidence-based, effective therapeutics that, either alone or in combination, can limit disease progression and improve outcomes are of great interest.
Anti-SARS-CoV-2 antibody therapies are a category of COVID-19 treatment regimens that are under active clinical investigation due to their promising mechanisms of action against SARS-CoV-2. As it is hypothesized that SARS-CoV-2 relies on the binding of human angiotensin-converting enzyme 2 (ACE2) receptors through receptor-binding domains on its spike proteins to gain cellular entry, current research and development efforts surrounding anti-SARS-CoV-2 antibody therapies have focused on blocking the spike protein binding sites using neutralizing antibodies [9]. Convalescent plasma from recovered COVID-19 patients was one of the first anti-SARS-CoV-2 antibody treatments to receive an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) in August 2020 [10]. The treatment relies on neutralizing antibodies produced by the donors during viral infection, although its efficacy against SARS-CoV-2 was contradictory during the early stages of the pandemic due to a paucity of convincing evidence from RCTs [11]. The availability of convalescent plasma and its derivative, intravenous immunoglobulin (IVIg) products, have also been constrained by increased demand and limited collection capacities during the pandemic.
Monoclonal antibodies (mAbs) represent an alternative type of anti-SARS-CoV-2 antibody therapy which can provide more specific, precise, and consistent protection against COVID-19 progression compared to convalescent plasma therapy. Anti-SARS-CoV-2 mAbs are generally developed from antibodies isolated from the blood of previously infected patients and re-engineered for mass production [12]. The first antiviral mAb therapy to receive EUA was bamlanivimab in November 2020 [13]. However, this EUA was later revoked in favor of mAb cocktails (such as bamlanivimab and etesevimab or casirivimab and imdevimab) due to concerns regarding the possibility of antigen escape [14]. Recent RCTs, such as the RECOVERY and BLAZE-1 trials, have associated the use of mAb combination therapies with reduced mortality, viral load, and COVID-related hospitalizations [15–17]; however, the efficacy and safety of mAb therapies has yet to be demonstrated in large, diverse patient populations. In addition to mAbs, multiple candidate animal-based polyclonal antibody therapies are also under active investigation for the treatment of COVID-19 [18, 19].
Due to the large number of different anti-SARS-CoV-2 antibody regimens currently under investigation, this systematic review and network meta-analysis (NMA) was conducted to assess and compare the relative efficacy and safety of different antibody regimens based on RCT data. We aimed to better understand the potential clinical benefit of anti-SARS-CoV-2 antibody therapies in reducing mortality, duration of hospitalization, length of ICU stay, time to viral clearance, and disease progression leading to hospitalization and/or invasive mechanical ventilation/ECMO, while also investigating the odds of adverse events and future research implications. The efficacy and safety of these therapies among patients with different severities of COVID-19 and baseline serology statuses were assessed as well.
Methods
We conducted a systematic review and NMA in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses incorporating NMA of healthcare interventions (PRISMA-NMA, see complete checklist in Table S1) [20]. This study was prospectively registered on PROSPERO (CRD42021289903) [21].
Study identification and eligibility criteria
We systematically searched the following databases for relevant studies from January 1st, 2020 until November 7th, 2021, with an updated search on December 27th, 2021 and February 20, 2022: (1) MEDLINE; (2) EMBASE; (3) PubMed Clinical Queries (COVID-19 General Filter); (4) Web of Science Core Collection; (5) Cochrane Central Register of Controlled Trials (CENTRAL); and (6) China National Knowledge Infrastructure (CNKI). The search strategies used are provided in Tables S2–S7. In addition to the database searches, we also hand-searched pre-print servers medRxiv and Research Square, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP), as well as the reference section of relevant systematic reviews.
Six reviewers (J.D., K.H., F.Z., D.R., H.B.R., S.H.) performed abstract/title and full-text screening independently and in duplicate to identify RCTs that satisfied the following eligibility criteria: (1) included patients diagnosed with COVID-19 through laboratory or radiographic methods; (2) compared the efficacy and/or safety of an anti-SARS-CoV-2 antibody product against standard of care or against another anti-SARS-CoV-2 antibody regimen; and (3) provided data for any of our outcomes of interest. While our initial protocol only included adult COVID-19 patients, this age restriction was removed prior to article screening to accommodate the updated inclusion criteria in major clinical trials such as BLAZE-1 [15, 16] and RECOVERY [17, 22] which enrolled a small number of adolescents in addition to adult patients. Disagreements during the screening process were resolved via consensus.
Definition of anti-SARS-CoV-2 antibody products
Anti-SARS-CoV-2 antibody products were defined as treatments involving exogenous antibodies that act upon epitopes on SARS-CoV-2. Keywords included in our search strategy relating to anti-SARS-CoV-2 antibody products were based upon treatments listed in the COVID-19 treatment guidelines from the National Institutes of Health (NIH) [23], which included: (1) anti-SARS-CoV-2 monoclonal antibodies (mAbs), e.g., sotrovimab, imdevimab, casirivimab, etesevimab, and bamlanivimab; (2) convalescent plasma from recovered COVID-19 patients; and (3) anti-SARS-CoV-2 IVIg manufactured from pooled convalescent plasma. Following our inclusion criteria, we specifically excluded antibody products that are not specific to SARS-CoV-2, such as immunomodulating mAbs and non-specific IVIgs.
Outcomes of interest
Our efficacy outcomes included: (1) time to viral clearance (negative SARS-CoV-2 RT-PCR test); (2) incidence of all-cause mortality; (3) duration of hospitalization; (4) duration of ICU hospitalization; (5) incidence of hospitalization; and (6) incidence of progression to invasive mechanical ventilation or ECMO. Our safety outcomes included incidence of all-cause adverse events and incidence of serious adverse events as defined by study investigators.
Data abstraction and risk of bias
Four reviewers (J.D., K.H., D.R., H.B.R.) independently performed data abstraction in duplicate using electronic extraction forms. The forms were constructed a priori as outlined on the PROSPERO registration. During the abstraction process, reviewers also assessed the risk of bias for each included study using the Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2) [24]. Disagreements during the data abstraction and risk assessment processes were resolved through consensus.
Statistical analysis
We conducted random-effects, frequentist NMAs using the netmeta library based on R. The treatment effect of dichotomous outcomes and continuous outcomes were expressed through odds ratio (OR) and mean difference (MD), respectively. Studies that reported zero incidence in any of the dichotomous outcomes were included in the network by adding a continuity correction factor of 0.5.
The relative efficacy and safety of each treatment arm was ranked using P scores. Similar to SUCRA scores reported in Bayesian NMAs, P scores represent the probability that a treatment is the most effective/safest treatment in the network. A P score of 1 indicates that the treatment is certain to be the most effective/safest treatment in the network, while a P score of 0 indicates that the treatment is certain to be the least effective/safe in the network [25]. Network structures were visualized using network plots, while the comparative efficacy and safety of treatment arms relative to standard of care/no intervention was shown using forest plots.
Exceptions to quantitative analyses
Five included studies [26–30] assessing the efficacy of convalescent plasma were not included in the NMAs as they used normal plasma as control. Because normal plasma may still have an immunomodulatory effect in COVID patients [31] and can result in transfusion reactions, combining these studies with other convalescent plasma trials (which used standard of care as controls) may diminish the treatment effects and adverse effects of convalescent plasma. Thus, results from these studies were only narratively described. Additionally, NMAs were not performed for the outcomes of time to viral clearance and length of ICU stay as only a small number of studies reported normally distributed duration data for these outcomes. Findings relating to these outcomes were described narratively.
Heterogeneity and inconsistency
We assessed the heterogeneity and inconsistency of our NMAs using Cochran’s Q statistics with a recommended threshold of P < 0.10 [32] and heterogeneity was further quantified using I2 statistics.
Subgroup analyses were conducted to explore potential sources of heterogeneity. We performed subgroup analyses based on disease severity, including only those who have non-severe or severe COVID-19 at baseline to compare the treatment rankings within these two groups of patients. Disease severity was defined post hoc according to the COVID-19 severity classification published by the FDA, which defined severe/critical disease as at least one of the following: (1) clinical signs indicative of severe systemic illness with COVID-19, such as respiratory rate ≥ 30 per minute, heart rate ≥ 125 beats per minute, SpO2 ≤ 93% on room air or PaO2/FiO2 ratio < 300; (2) respiratory failure, defined as requirement for endotracheal intubation and mechanical ventilation, oxygen delivered by high-flow nasal cannula, noninvasive positive pressure ventilation, or ECMO; (3) shock, defined as systolic blood pressure < 90 mmHg, diastolic blood pressure < 60 mmHg or requirement for vasopressors; and (4) multi-organ dysfunction/failure [33].
Additionally, we performed subgroup analyses based on baseline SARS-CoV-2 serology results. As shown in the final results from the ACTIV-3/TICO trial assessing bamlanivimab [34] and the RECOVERY trial [17] assessing casirivimab and imdevimab, anti-SARS-CoV-2 antibody products may be more efficacious in patients who were seronegative at baseline. While this subgroup analysis was originally planned for all reported outcomes, we only obtained sufficient subgroup data for analysis for the outcome of mortality incidence.
Lastly, we performed post hoc subgroup analyses based on hospitalization status (inpatients versus outpatients), as well as a post hoc sensitivity analysis restricting the follow-up duration of the mortality outcome to one month (defined as 30 ± 5 days) based on recommendations from peer-reviewers.
Missing data
For studies with missing outcome or outcome variance data, we attempted to contact the corresponding authors for unpublished information. If a study reported duration data as median with interquartile range (IQR) and the authors could not be reached, we imputed the mean and standard deviation (SD) using methods proposed by Luo et al. [35] and Wan et al. [36] provided that the data was normally distributed, as assessed using methods proposed by Shi et al. [37]. Data skewed from normal were described narratively.
Confidence of evidence and publication bias
We assessed the presence of publication bias using comparison-adjusted funnel plots [38]. As the treatment arms had to be sorted in a meaningful way to generate comparison-adjusted funnel plots, we ordered the treatment arms to define each comparison as an intervention versus a control treatment, with the assumption that publication bias is likely to exaggerate the effectiveness of the intervention treatment. Egger’s regression test was used to examine the presence of asymmetry in the comparison-adjusted funnel plots.
Confidence in our NMA findings was assessed using the Confidence in Network Meta-Analysis (CINeMA) web application [39]. Similar to the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework [40] used in pairwise meta-analyses, CINeMA assesses the confidence of evidence based on the following domains: (1) within-study bias; (2) reporting bias; (3) indirectness; (4) imprecision; (5) heterogeneity; and (6) incoherence.
Results
Characteristics of included studies
After deduplication, we screened 3429 papers for eligibility (see Fig. 1). Fifty-five RCTs [15–19, 22, 26–31, 34, 41–82] (N = 45,005) were subsequently included in the systematic review with 10 unique intervention arms (not including the standard of care arm). Two included studies [50, 55] reported data on the same set of patients involved in the phase two portion of the BLAZE-1 trial but with different outcomes and subgroup data. Similarly, two included studies [34, 41] reported data on the same set of patients involved in the ACTIV-3/TICO trial assessing bamlanivimab, and two included studies [56, 77] reported interim and final data on patients involved in the COMET-ICE trial. The full list of patient characteristics are tabulated in Table S8, and the characteristics of studies included in the data synthesis is tabulated in Table S9.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for the identification and selection of randomized controlled trials. WoS web of science, CENTRAL cochrane central register of controlled trials, CNKI China national knowledge infrastructure
Based on RoB 2, 24 studies (43.6%) were rated as having a low risk of bias, 25 studies (45.5%) were rated as having some concerns in regards to risk of bias, and six studies (10.9%) were rated as having a high risk of bias (see Fig. 2 and Table S10). Studies given a some concerns rating were mostly due to unreported allocation concealment methods or the use of an open-label trial design. Four studies were given a high risk of bias rating in the missing data domain due to potentially unreported data for randomized patients lost from follow-up, and one study was given a high risk of bias rating in the randomization domain due to explicit reporting of a lack of allocation concealment during the randomization process.
Fig. 2.
Risk of bias bar charts showing the percentage of risk of bias ratings for each included study, as assessed using RoB 2
Comparative efficacy of anti-SARS-CoV-2 antibody regimens
Incidence of mortality
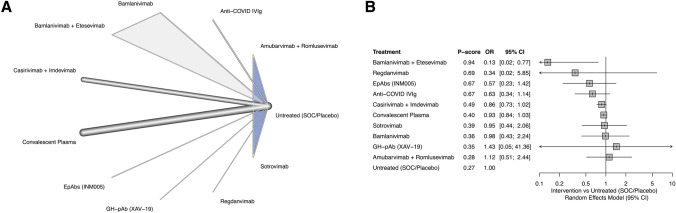
A total of 49 RCTs (N = 43,943) reported the incidence of mortality. Excluding five studies using plasma controls [26–30], 44 studies (N = 42,604) [15–19, 22, 31, 41–49, 52–55, 57–70, 72, 74–82] were included in the NMA. Follow-up durations for mortality ranged from 30 to 90 days. A combination of bamlanivimab and etesevimab (OR 0.13, 95% CI 0.02–0.77) was the only treatment arm associated with a significant reduction in the odds of mortality and it was ranked first in terms of efficacy (P score 0.94). There was very low heterogeneity (I2 = 6.7%, P = 0.41) and inconsistency (P = 0.21) within the network (see Fig. 3).
Fig. 3.
Results of the network meta-analysis for mortality incidence. A The network diagram for mortality incidence. Thickness of the edge connections represent the relative number of direct connections between the connected nodes. Shaded triangles represent multi-arm trials. B The forest diagram showing pooled ORs within each treatment arm compared to SOC for mortality incidence. Treatments were ranked from the most efficacious (i.e., the highest P-score) to the least efficacious (i.e., the lowest P score). ORs < 1 indicate beneficial treatment effects compared to SOC. SOC standard of care, IVIg intravenous immune globulin, OR odds ratio, CI confidence interval
The subgroup analyses by disease severity and hospitalization status did not reveal differences in the significance of the treatment effects when compared to the overall NMA (see Figure S1-S4). While bamlanivimab and etesevimab was still associated with significant reductions in mortality in these subgroup analyses, it was only assessed in the analysis including non-severe patients and in the analysis including outpatients. Similarly, the sensitivity analysis restricting the follow-up duration to one month did not reveal different findings either, with bamlanivimab + etesevimab still conferring a significant mortality reduction (see Figure S5).
Among COVID patients who were seropositive at baseline, neither convalescent plasma, casirivimab + imdevimab, nor bamlanivimab achieved a significant reduction in the odds of mortality in concordance with the overall NMA (see Figure S7). Contrarily, casirivimab + imdevimab was associated with a significant reduction in the odds of mortality among patients who were seronegative at baseline (OR 0.67, 95% CI 0.50–0.91; see Figure S6). Bamlanivimab + etesevimab was not assessed in any of the serology subgroups.
Across the five studies using normal plasma controls, only one [27] associated the use of convalescent plasma with significant mortality reductions compared to normal plasma (OR 0.44, 95% CI 0.22–0.91). The other four studies either did not find significant associations (Fisher P = 0.63 for Bennett-Guerrero et al. [28] or risk ratio [RR] 1.003, 95% CI 0.49–6.95 for Baldeón et al. [29]) or found numerically similar results between the intervention and control arms (6.3 versus 5.6% in NCT04421404 [26] or 0 versus 0.5% in Sullivan et al. [30]).
Incidence of hospitalization
A total of 9 RCTs [15, 16, 53, 55, 60, 72, 74, 77, 82] reported the incidence of hospitalization in 10,234 outpatients and were included in the NMA. At baseline, the trial by O’Brien et al. [82] only included asymptomatic outpatients, three studies [53, 60, 77] included outpatients who are not using supplemental oxygen, and four studies [15, 16, 55, 74] included outpatients with mild to moderate COVID-19 based on the FDA severity classification. All studies except publications reporting on the BLAZE-1 trial only included outpatients who had symptom onset less than a week before randomization.
Based on the NMA, sotrovimab, bamlanivimab + etesevimab, bamlanivimab monotherapy, and casirivimab + imdevimab were significantly associated with reduced odds of hospitalizations compared to standard of care. Sotrovimab ranked first in terms of efficacy (OR 0.20, 95% CI 0.08–0.48; P score 0.84), followed by bamlanivimab + etesevimab (OR 0.24, 95% CI 0.14–0.43; P score 0.75), bamlanivimab monotherapy (OR 0.28, 95% CI 0.10–0.84; P score 0.66) and casirivimab + imdevimab (OR 0.29, 95% CI 0.20–0.42; P score 0.65). Regdanvimab and convalescent plasma were the only analyzed treatment arms that did not achieve a significant treatment effect. Overall, there was no heterogeneity (I2 = 0%, P = 0.82) and no inconsistency (P = 0.63) within the network (see Fig. 4).
Fig. 4.
Results of the network meta-analysis for incidence of hospitalization. A The network diagram for incidence of hospitalization. Thickness of the edge connections represent the relative number of direct connections between the connected nodes. Shaded triangles represent multi-arm trials. B The forest diagram showing pooled ORs within each treatment arm compared to SOC for incidence of hospitalization. Treatments were ranked from the most efficacious (i.e., the highest P score) to the least efficacious (i.e., the lowest P score). ORs < 1 indicate beneficial treatment effects compared to SOC. SOC standard of care, OR odds ratio, CI confidence interval
Incidence of invasive mechanical ventilation/ECMO
A total of 19 studies [17, 19, 22, 41, 43, 44, 47–49, 51, 52, 57, 60, 63, 68, 70, 73, 77, 81] (N = 26,912) reported the incidence of progression to invasive mechanical ventilation and/or ECMO and were included in the NMA. No intervention was associated with significant reductions in the odds of invasive mechanical ventilation/ECMO (see Fig. 5). There was no heterogeneity (I2 = 0%, P = 0.49). Subgroup analyses by disease severity and hospitalization status did not yield any significant associations (see Figures S8–S11).
Fig. 5.
Results of the network meta-analysis for incidence of invasive mechanical ventilation/ECMO. A The network diagram for incidence of invasive mechanical ventilation/ECMO. Thickness of the edge connections represent the relative number of direct connections between the connected nodes. B The forest diagram showing pooled ORs within each treatment arm compared to SOC for incidence of invasive mechanical ventilation/ECMO. Treatments were ranked from the most efficacious (i.e., the highest P score) to the least efficacious (i.e., the lowest P score). ORs < 1 indicate beneficial treatment effects compared to SOC. SOC standard of care, IVIg intravenous immune globulin, OR odds ratio, CI confidence interval
Duration of hospitalization
Eleven studies [16, 19, 31, 44, 45, 51, 57, 62, 65, 72, 80] (N = 1931) reported hospitalization durations and were included in the NMA. No intervention was associated with significant reductions in the duration of hospitalization in the overall analysis (see Fig. 6) and the subgroup analyses (see Figures S12–S15). There was high heterogeneity in the overall NMA (I2 = 85.3%, P < 0.01), however heterogeneity was substantially reduced in the subgroup analysis including non-severe patients (I2 = 0%, P = 0.99) and outpatients (I2 = 0%, P = 0.99).
Fig. 6.
Results of the network meta-analysis for duration of hospitalization. A The network diagram for duration of hospitalization. Thickness of the edge connections represent the relative number of direct connections between the connected nodes. B The forest diagram showing pooled MDs within each treatment arm compared to SOC for duration of hospitalization. Treatments were ranked from the most efficacious (i.e., the highest P score) to the least efficacious (i.e., the lowest P score). MDs < 0 indicate beneficial treatment effects compared to SOC. SOC standard of care, IVIg intravenous immune globulin, MD mean difference, CI confidence interval
Twelve studies [17, 22, 27, 29, 43, 46, 47, 54, 61, 64, 69, 70] reported non-imputable data or used non-convalescent plasma as the control arm. Apart from one study which assessed the efficacy of casirivimab + imdevimab, all other studies assessed the efficacy of convalescent plasma against standard of care (or control plasma). Among studies with significance testing, none reported significant reductions in hospitalization duration associated with the use of anti-SARS-CoV-2 antibody products. The duration of hospitalization is numerically similar between the intervention and control arms in studies that did not perform significance testing. The results of the non-imputable studies are tabulated in Table S11.
Duration of ICU hospitalization
Five studies [19, 47, 49, 61, 65] reported data relating to duration of ICU hospitalization. Four studies assessed the efficacy of convalescent plasma versus standard of care, while one study assessed the efficacy of an equine hyperimmune serum (INM005). None of the studies reported a significant improvement in the duration of ICU hospitalization associated with an intervention; in fact, an unpublished study (NCT04385199) reported a substantially higher median duration in the convalescent plasma group compared to standard of care (19.5 days versus 13 days). The results of the included studies are tabulated in Table S12.
Time to viral clearance
Three studies [55, 59, 61] reported time to viral clearance. Two studies assessed the efficacy of convalescent plasma versus standard of care, while one study assessed the efficacy of bamlanivimab monotherapy and bamlanivimab + etesevimab combination therapy. None of the studies reported a significant reduction in the time to viral clearance associated with an intervention. The results of the included studies are tabulated in Table S13.
Comparative safety of anti-SARS-CoV-2 antibody regimens
Incidence of adverse events
A total of 30 studies (N = 11,403) reported the incidence of all-cause adverse events. Excluding two studies using plasma controls, 28 studies (N = 11,150) [15, 16, 19, 41, 42, 44, 45, 51, 53, 55, 57–61, 63–66, 69, 70, 74, 76–78, 80–82] were included in the NMA. All antibody products, except convalescent plasma and anti-COVID IVIg, demonstrated good safety profiles with no significant increase in the odds of adverse events. In particular, casirivimab + imdevimab was associated with a significant reduction in the odds of adverse events (OR 0.71, 95% CI 0.58–0.88) and ranked first in terms of safety (P score 0.94). Convalescent plasma was associated with a significant increase in adverse events (OR 1.40, 95% CI 1.13–1.72) and was ranked second-to-last in terms of safety (P score 0.12). Anti-COVID IVIg was also associated with a significant increase in adverse events (OR 1.56, 95% CI 1.06–2.30) and ranked last in terms of safety (P-score 0.07). There was no heterogeneity (I2 = 0%, P = 0.67) nor inconsistency (P = 0.18) within the network (see Fig. 7A, B).
Fig. 7.
Results of the network meta-analysis for safety outcomes. In the network diagrams, the thickness of the edge connections represent the relative number of direct connections between the connected nodes, and shaded triangles represent multi-arm trials. In the forest diagrams, treatments were ranked from the most efficacious (i.e., the highest P score) to the least efficacious (i.e., the lowest P score), with ORs < 1 indicating beneficial treatment effects compared to SOC. A The network diagram for incidence of adverse events. B The forest diagram showing pooled ORs within each treatment arm compared to SOC for incidence of adverse events. C The network diagram for incidence of serious adverse events. D The forest diagram showing pooled ORs within each treatment arm compared to SOC for incidence of serious adverse events. SOC standard of care, IVIg intravenous immune globulin, OR odds ratio, CI confidence interval
In the subgroup analyses by disease severity, we found that convalescent plasma was associated with a significant increase in the odds of adverse events in patients with non-severe disease (OR 1.92, 95% CI 1.18–3.14; see Figure S16) and in outpatients (OR 1.96, 95% CI 1.18–3.25; see Figure S19); however, we did not identify the same significant association in patients with severe disease (OR 1.25, 95% CI 0.90–1.73; see Figure S17) nor among inpatients (OR 1.26, 95% CI 0.99–1.61; see Figure S18). Anti-COVID IVIg was not assessed in the subgroup analyses by disease severity, but it was included in the analysis for inpatients and was associated with a significant increase in the odds of adverse events (OR 1.56, 95% CI 1.06–2.30).
In the two studies using plasma controls, both NCT04421404 [26] and the study by O’Donnell et al. [27] reported increased incidence of adverse events in patients receiving convalescent plasma compared to patients receiving normal plasma (12.5 versus 5.6% for NCT04421404 and 65.3 versus 55.6% for O’Donnell et al.).
Incidence of serious adverse events
A total of 30 studies (N = 19,887) patients reported the incidence of serious adverse events. Following the exclusion of two studies using plasma controls, 28 studies (N = 19,611) [15, 16, 19, 41, 42, 46, 48, 49, 51–53, 55, 58, 60, 61, 65, 68–74, 76–78, 80, 82] were included in the NMA. No antibody product was associated with a significant increase in the odds of serious adverse events. Casirivimab + imdevimab (OR 0.48, 95% CI 0.33–0.70) and sotrovimab (OR 0.46, 95% CI 0.29–0.73) were associated with significant reductions in the odds of serious adverse events, with sotrovimab ranking first (P score 0.82) and casirivimab + imdevimab ranking second (P score 0.79) in terms of safety. There was moderate heterogeneity (I2 = 32.7%, P = 0.15) and no inconsistency (P = 0.16) within the network (see Fig. 7C, D).
There was no change in significance in the subgroup analysis including non-severe patients and outpatients (see Figure S20 and Figure S23), however sotrovimab was not associated with a significant reduction in the incidence of serious adverse events in the subgroup analysis including inpatients (see Figure S22). Both casirivimab + imdevimab and sotrovimab were not assessed in the subgroup analysis including severe patients (see Figure S21).
Among the two studies using plasma controls, Bennett-Guerrero et al. [28] reported a numerically similar proportion of serious adverse events between the convalescent plasma and control plasma groups (27.1 versus 26.7%), while O’Donnell et al. [27] found a numerically decreased incidence of serious adverse events among the convalescent plasma group compared to control (26.5 versus 36.1%).
Publication bias and confidence of evidence
According to visual inspections of the comparison-adjusted funnel plots and Egger’s regression analysis, we only identified small study effects as an indication for publication bias for the outcome of mortality incidence (PEgger < 0.01). Missing small studies on the left side of the funnel indicates that small studies tend to exaggerate the effectiveness of intervention treatments compared to the control treatments in the outcome of mortality incidence (see Figures S24–S29).
Using CINeMA, we determined that the outcome of hospitalization incidence is mainly based on a moderate confidence of evidence, with concerns regarding imprecision in several indirect comparisons. Similarly, both of our safety outcomes were rated as having a mainly moderate confidence of evidence as well due to imprecision. The outcome of mortality incidence was rated as having a mainly low confidence of evidence due to the aforementioned publication/reporting bias in addition to imprecision. Lastly, the outcome of hospitalization duration and incidence of progression to invasive mechanical ventilation/ECMO were both rated as having a mainly very low confidence of evidence. This substantial downgrade in confidence was due to a combination of within-study bias (risk of bias of included studies), imprecision, and incoherence due to a lack of closed loops in the network (see Tables S14–S19).
Discussion
Main findings
Our systematic review and NMA included 55 RCTs (N = 45,005) to assess the efficacy and safety of anti-SARS-CoV-2 antibody products for the treatment of COVID-19. We found that all assessed antibody products did not significantly reduce the duration of hospitalization, nor incidence of progression to invasive mechanical ventilation/ECMO, based on mostly very low confidence of evidence. Moreover, none of the interventions were associated with a significant reduction in the time to viral clearance nor duration of ICU hospitalization.
Additionally, we found that bamlanivimab + etesevimab was associated with a 87% reduction in the odds of mortality in COVID-19 patients compared to standard of care, based on mostly low confidence of evidence; however this finding was based on data from outpatients and patients with non-severe COVID-19. Casirivimab + imdevimab demonstrated a 33% reduction in the odds of mortality in patients who were seronegative at baseline, but not among patients who were seropositive at baseline.
Among COVID-19 outpatients, we found that many anti-SARS-CoV-2 antibody products significantly reduced incidence of hospitalizations based on a moderate confidence of evidence. Sotrovimab was the most efficacious treatment arm, reducing the odds of hospitalization by 80% compared to standard of care. Bamlanivimab + etesevimab, bamlanivimab monotherapy, and casirivimab + imdevimab demonstrated a reduction of 76, 72, and 71% in the odds of hospitalization, respectively. Regdanvimab, a novel investigational anti-COVID mAb, was only assessed by a moderately sized RCT in our network and did not achieve a significant effect in reducing hospitalizations. Convalescent plasma was also not associated with a reduction in the odds of hospitalizations.
Most anti-SARS-CoV-2 antibody products demonstrated good safety profiles based on mostly moderate confidence of evidence. Only the use of convalescent plasma and anti-COVID IVIg was associated with a significant, 40 and 56% increase in the odds of adverse events, respectively. No antibody product was associated with a significant increase in the odds of serious adverse events.
Clinical implications and future directions
Similar to findings in other antiviral COVID-19 treatment strategies [83], we observed that anti-SARS-CoV-2 antibody products did not substantially improve the outcome of hospitalized patients, including duration of hospitalization and progression to mechanical ventilation. Previous studies have shown that viremia of SARS-CoV-2 peaks within a week of symptom onset [84], after which immunopathologies due to dysfunctional host immune responses are more likely to drive disease progression and result in mortality. Many COVID-19 patients were found to exhibit substantially elevated levels of proinflammatory cytokines which can lead to significant tissue damage [85]. Moreover, Schurink et al. found evidence of extensive systemic inflammatory responses in the lungs and other organs of diseased patients with COVID-19, with only sporadic presence of SARS-CoV-2-infected cells [86]. Given that patients who are most at-risk for COVID-related hospitalizations are generally hospitalized after a week of symptomatic infection [87]—at which point the disease progression is mainly driven by dysfunctional immune responses—these previous findings may explain why our NMA did not show any improvements in hospitalization-related outcomes. Despite the attention surrounding the promising efficacy of anti-SARS-CoV-2 antibody products, it is important for clinicians to recognize their limitations in hospitalized patients and patients with severe diseases.
Additionally, while previous meta-analyses have shown that convalescent plasma may yield significant benefits in the odds of mortality and clinical improvements [88], our review showed that convalescent plasma and its derivative IVIg was not associated with improvements in any outcomes of interest, including the incidence of hospitalization. Although it has been hypothesized that convalescent plasma may be more effective among patients who are seronegative at baseline [88], we found that convalescent plasma did not exhibit any efficacy in reducing mortality regardless of baseline serology status. Combined with increased odds of adverse events, including a considerable number of transfusion-related reactions, the use of convalescent plasma is not recommended based on the results of this review.
However, we found that most mAb products were effective at reducing the odds of hospitalization among outpatients with early infections. This suggests that anti-SARS-CoV-2 mAbs may be applicable in the setting of early infection among those with risk factors for disease progression, such as older adults, those with obesity, diabetes, and/or hypertension [89]. In addition, while vaccinations remain the preferred method of prophylaxis against SARS-CoV-2, mAb products may also provide short-term protection against viral infection in individuals who cannot produce productive immune responses to vaccinations such as those who may be older or immunocompromised. Antiviral mAb products can also play a role in populations that do not have timely access to vaccines, or patients with contraindications for vaccines.
There are several limitations with the clinical usage and long-term applicability of mAbs that must be noted. Current mAb products generally target epitopes on the spike protein of SARS-CoV-2. As a key viral entry protein, the spike protein is uniquely exposed to selection pressure from host antibodies which can drive rapid antigenic drift and evolution [90]. In patients receiving mAb treatments, the selection pressure exerted by the mAbs can result in the genesis of resistant strains of SARS-CoV-2, which may substantially reduce the efficacy of mAb products. Specifically, the recent FDA withdrawal of EUA for bamlanivimab monotherapy was attributed to an increase in the number of bamlanivimab-resistant variants [91].
Current strategies for combating resistant COVID strains include the use of mAb cocktails (e.g., bamlanivimab + etesevimab, and casirivimab + imdevimab) or by targeting highly conserved regions of the spike protein that are vital to viral functions (e.g., sotrovimab, which targets an epitope shared between SARS-CoV and SARS-CoV-2). In light of the recent introduction of the omicron (B.1.1.529) variant, the latter approach of countering mAb resistance may also confer the benefit of maintaining the mAbs’ efficacy across different variants. Early in vitro studies have shown that the mAb cocktails assessed in this review (i.e., bamlanivimab + etesevimab and casirivimab + imdevimab) may not be effective against the omicron variant, while sotrovimab largely maintained its antiviral efficacy [92]. These preliminary findings, combined with the favorable hospitalization and safety results from this systematic review, may support the continued use and assessment of sotrovimab for treating patients infected with the omicron variant. Future mAb designs and usage should continue to take the possibility of antigen escape into consideration, and further in vivo investigations into the efficacy of mAb products in patients infected with novel variants, such as omicron and its sublineages (e.g., BA.2), should be conducted.
Lastly, pre-print data from the RECOVERY trial assessing casirivimab + imdevimab and final results from the ACTIV-3/TICO trial for bamlanivimab showed that mAb products may yield greater benefits among patients who are seronegative at baseline as opposed to patients who are seropositive at baseline. In our subgroup analyses for mortality incidence, we found that casirivimab + imdevimab only exhibited a reduction in the odds of mortality in patients who are seronegative at baseline based on results from multiple RCTs, which seem to support findings from the RECOVERY trial. While our serology subgroup findings are based on limited sample sizes, clinicians may need to consider the presence of host SARS-CoV-2 antibodies when making decisions about using anti-SARS-CoV-2 mAb products. Future trials assessing the efficacy of mAbs in different serological subgroups are needed to further clarify the role of serology status in the efficacy of mAb products.
Study limitations
The main limitation of this systematic review and NMA is the inability to assess the efficacy of antibody products across different COVID-19 variants as the distribution of viral variants was rarely reported in published RCTs. Because the efficacy of antibody products can change depending on mutations in the targeted epitope as shown in early in vitro studies involving the omicron variant and its sublineages, future RCTs and reviews should address differences in antibody efficacy between different COVID variants.
Furthermore, our review did not assess the efficacy and safety of antibody products in patients at high risks for progression to severe COVID-19. This was because the definition of high-risk populations was extremely heterogeneous during our preliminary research, in addition to a lack of subgroup data reporting involving these high-risk patient populations in published RCTs. It may be viable for trial investigators to use standardized screening tools and models, such as the COVID-19-AACC model [93], to systematically categorize patients into high- and low-risk subgroups and reduce the definition variability in subsequent investigations.
Lastly, our findings regarding the duration of hospitalization and incidence of progression to invasive mechanical ventilation/ECMO were based on a very low confidence of evidence due to risk of bias and incoherence in the network. Thus, results for these two outcome measures should be interpreted with caution. Additionally, we observed imprecision in results from several promising treatment arms, including anti-COVID IVIg and regdanvimab. More well-designed clinical trials, with larger sample sizes, are required to clarify the role of these treatments in COVID-19 management.
Conclusions
In this systematic review and NMA, we found that bamlanivimab + etesevimab significantly reduced mortality in COVID outpatients as well as patients with non-severe diseases, and most anti-SARS-CoV-2 mAbs were associated with reduced odds of hospitalization among COVID outpatients with sotrovimab being associated with the highest reduction in odds. Casirivimab + imdevimab only reduced mortality among patients who were seronegative at baseline. Both convalescent plasma and anti-COVID IVIg were not associated with efficacy in any outcomes and were associated with increased odds of adverse events. Clinicians should be aware of the limitations surrounding anti-SARS-CoV-2 antibody therapies for treating hospitalized patients and patients with severe diseases. While efficacious for outpatients in the current review, mAb treatments should be continuously evaluated against new COVID variants, such as omicron and the omicron sublineage BA.2.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Additional Figures (DOCX 4249 KB)
Supplementary file2 PRISMA-NMA Checklist (DOCX 75 KB)
Supplementary file3 Database Search Strategy (DOCX 36 KB)
Supplementary file4 Characteristics Table (DOCX 256 KB)
Supplementary file5 Characteristics of Included Studies and Patients by Outcome (Disease Severity and Hospitalization Status) (DOCX 18 KB)
Supplementary file6 Risk of Bias Ratings (DOCX 31 KB)
Supplementary file7 Summary of Findings for Unanalyzed Studies (DOCX 101 KB)
Supplementary file8 Confidence in Network Meta-Analysis (CINeMA) (DOCX 85 KB)
Supplementary file9 Pairwise Results of Treatment Comparisons (League Tables) (DOCX 32 KB)
Acknowledgements
None.
Author contributions
Conceptualization: JD, FZ; Methodology: JD; Investigation: JD, KH, FZ, DR, HBR, SH; Data Curation: JD; Formal Analysis: JD, KH, FZ; Project Administration: JD, KH; Resources: JD; Software: JD, FZ; Validation: KH, FZ; Visualization: JD; Writing-Original Draft: JD, KH, FZ; Writing-Review and Editing: DR, HBR, SH; Supervision: JD.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Deng J, Zhou F, Ali S, et al. Efficacy and safety of ivermectin for the treatment of COVID-19: a systematic review and meta-analysis. QJM: An Int J Med Published Online First. 2021 doi: 10.1093/qjmed/hcab247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng J, Zhou F, Heybati K, et al. Efficacy of chloroquine and hydroxychloroquine for the treatment of hospitalized COVID-19 patients: a meta-analysis. Future Virol Published Online First. 2021 doi: 10.2217/fvl-2021-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng J, Zhou F, Hou W, et al. Efficacy of lopinavir-ritonavir combination therapy for the treatment of hospitalized COVID-19 patients: a meta-analysis. Future Virol Published Online First. 2022 doi: 10.2217/fvl-2021-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordeiro LP, Linhares EONN, Nogueira FGO, et al. Perspectives on glucocorticoid treatment for COVID-19: a systematic review. Pharmacol Rep. 2021;73:728–735. doi: 10.1007/s43440-021-00225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khubchandani J, Sharma S, Price JH, et al. COVID-19 vaccination hesitancy in the united states: a rapid national assessment. J Community Health. 2021;46:270–277. doi: 10.1007/s10900-020-00958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tagoe ET, Sheikh N, Morton A, et al. COVID-19 vaccination in lower-middle income countries: national stakeholder views on challenges, barriers, and potential solutions. Front Public Health. 2021;9:709127. doi: 10.3389/fpubh.2021.709127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2021;72:e695–703. doi: 10.1093/cid/ciaa1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deepak P, Kim W, Paley MA, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreuzberger N, Hirsch C, Chai KL, et al. SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19. Cochrane Database Syst Rev. 2021;9:CD013825. doi: 10.1002/14651858.CD013825.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration (FDA). FDA EUA Letter of Authorization, Convalescent Plasma. 2021. https://www.fda.gov/media/141477/download. Accessed 8 Jan 2022.
- 11.Katz LM. (A Little) Clarity on convalescent plasma for Covid-19. N Engl J Med. 2021;384:666–668. doi: 10.1056/NEJMe2035678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deb P, Molla MMA, Saif-Ur-Rahman KM. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf Health. 2021;3:87–91. doi: 10.1016/j.bsheal.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U. S Food and Drug Administration (FDA). FDA EUA Letter of Authorization (Revoked), Bamlanivimab. 2021. https://www.fda.gov/media/143602/download. Accessed 8 Jan 2022.
- 14.U.S. Food and Drug Administration (FDA). FDA EUA Revocation Letter, Bamlanivimab. 2021. https://www.fda.gov/media/147629/download. Accessed 8 Jan 2022.
- 15.Dougan M, Azizad M, Mocherla B, et al. A randomized, placebo-controlled clinical trial of bamlanivimab and etesevimab together in high-risk ambulatory patients with COVID-19 and validation of the prognostic value of persistently high viral load. Clin Infect Dis Published Online First. 2021 doi: 10.1093/cid/ciab912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RECOVERY Collaborative Group Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaborit B, Dailly E, Vanhove B, et al. Pharmacokinetics and safety of XAV-19, a swine glyco-humanized polyclonal Anti-SARS-CoV-2 antibody, for COVID-19-related moderate pneumonia: a randomized, double-blind, placebo-controlled. Phase IIa Study Antimicrob Agents Chemother. 2021;65:e0123721. doi: 10.1128/AAC.01237-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopardo G, Belloso WH, Nannini E, et al. RBD-specific polyclonal F(ab´) fragments of equine antibodies in patients with moderate to severe COVID-19 disease: a randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial. EClinicalMedicine. 2021;34:100843. doi: 10.1016/j.eclinm.2021.100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 21.Schiavo JH. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q. 2019;38:171–180. doi: 10.1080/02763869.2019.1588072. [DOI] [PubMed] [Google Scholar]
- 22.RECOVERY Collaborative Group Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021 doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 2020. https://www.covid19treatmentguidelines.nih.gov/. Accessed 22 Feb 2022. [PubMed]
- 24.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsue P. Effects of COVID-19 Convalescent Plasma (CCP) on Coronavirus-associated Complications in Hospitalized Patients (CAPRI). ClinicalTrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT04421404. Accessed 30 Dec 2021.
- 27.O’Donnell MR, Grinsztejn B, Cummings MJ, et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J Clin Invest. 2021 doi: 10.1172/JCI150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett-Guerrero E, Romeiser JL, Talbot LR, et al. Severe acute respiratory syndrome coronavirus 2 Convalescent plasma versus standard plasma in coronavirus disease 2019 infected hospitalized patients in new york: a double-blind randomized trial. Crit Care Med. 2021;49:1015–1025. doi: 10.1097/CCM.0000000000005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldeón ME, Maldonado A, Ochoa-Andrade M, et al. Effect of convalescent plasma as complementary treatment in patients with moderate COVID-19 infection. Transfus Med. 2022 doi: 10.1111/tme.12851. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan DJ, Gebo KA, Shoham S, et al. Randomized controlled trial of early outpatient COVID-19 treatment with high-titer convalescent plasma. medRxiv. 2021 doi: 10.1101/2021.12.10.21267485. [DOI] [Google Scholar]
- 31.Pouladzadeh M, Safdarian M, Eshghi P, et al. A randomized clinical trial evaluating the immunomodulatory effect of convalescent plasma on COVID-19-related cytokine storm. Intern Emerg Med. 2021;16:2181–2191. doi: 10.1007/s11739-021-02734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. New Jersey: Wiley; 2008. [Google Scholar]
- 33.U.S. Department of Health and Human Services, U.S. Food and Drug Administration Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration Center for Biologics Evaluation and Research (CBER). COVID-19: Developing Drugs and Biological Products for Treatment or Prevention (Guidance for Industry). 2021. https://www.fda.gov/media/137926/download. Accessed 22 Feb 2022.
- 34.ACTIV-3/TICO Bamlanivimab Study Group* Responses to a Neutralizing Monoclonal Antibody for Hospitalized Patients With COVID-19 According to Baseline Antibody and Antigen Levels: A Randomized Controlled Trial. Ann Intern Med Published Online First. 2021 doi: 10.7326/M21-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 36.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi J, Luo D, Weng H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11:641–654. doi: 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 38.Chaimani A, Higgins JPT, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082. doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundgren JD, Grund B, et al. ACTIV-3/TICO LY-CoV555 Study Group A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:905–14. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis Published Online First. 2021 doi: 10.1016/S1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal A, Mukherjee A, Kumar G, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali S, Uddin SM, Shalim E, et al. Hyperimmune anti-COVID-19 IVIG (C-IVIG) treatment in severe and critical COVID-19 patients: A phase I/II randomized control trial. EClinicalMedicine. 2021;36:100926. doi: 10.1016/j.eclinm.2021.100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.AlQahtani M, Abdulrahman A, Almadani A, et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Sci Rep. 2021;11:9927. doi: 10.1038/s41598-021-89444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avendaño-Solá C, Ramos-Martínez A, Muñez-Rubio E, et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. 2021 doi: 10.1172/JCI152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balcells ME, Rojas L, Le Corre N, et al. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: A randomized phase II clinical trial. PLoS Med. 2021;18:e1003415. doi: 10.1371/journal.pmed.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bar KJ, Shaw PA, Choi GH, et al. A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia. J Clin Invest. 2021 doi: 10.1172/JCI155114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bégin P, Callum J, Jamula E, et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med. 2021;27:2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen P, Datta G, Grace Li Y, et al. First-in-human study of bamlanivimab in a randomized trial of hospitalized patients with COVID-19. Clin Pharmacol Ther. 2021;110:1467–1477. doi: 10.1002/cpt.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devos T, Van Thillo Q, Compernolle V, et al. Early high antibody-titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma. Eur Respir J Published Online First. 2021 doi: 10.1183/13993003.01724-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eom JS, Ison M, Streinu-Cercel A, et al. Efficacy and safety of CT-P59 plus standard of care: a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate SARS-CoV-2 infection. Res Square Published Online First. 2021 doi: 10.21203/rs.3.rs-296518/v1. [DOI] [Google Scholar]
- 54.Gharbharan A, Jordans CCE, GeurtsvanKessel C, et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun. 2021;12:3189. doi: 10.1038/s41467-021-23469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 57.Holm K, Lundgren MN, Kjeldsen-Kragh J, et al. Convalescence plasma treatment of COVID-19: results from a prematurely terminated randomized controlled open-label study in Southern Sweden. BMC Res Notes. 2021;14:440. doi: 10.1186/s13104-021-05847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JY, Jang YR, Hong JH, et al. Safety, virologic efficacy, and pharmacokinetics of CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike receptor-binding protein: two randomized, placebo-controlled, phase i studies in healthy individuals and patients with mild SARS-CoV-2 infection. Clin Ther. 2021;43:1706–1727. doi: 10.1016/j.clinthera.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirenga B, Byakika-Kibwika P, Muttamba W, et al. Efficacy of convalescent plasma for treatment of COVID-19 in Uganda. BMJ Open Respir Res. 2021 doi: 10.1136/bmjresp-2021-001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korley FK, Durkalski-Mauldin V, Yeatts SD, et al. Early convalescent plasma for high-risk outpatients with Covid-19. N Engl J Med. 2021;385:1951–1960. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Körper S, Weiss M, Zickler D, et al. Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19. J Clin Invest. 2021 doi: 10.1172/JCI152264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menichetti F, Popoli P, Puopolo M, et al. Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial. JAMA Netw Open. 2021;4:e2136246. doi: 10.1001/jamanetworkopen.2021.36246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tatem G. Convalescent Plasma for Patients With COVID-19. ClinicalTrials.gov. 2021.https://clinicaltrials.gov/ct2/show/NCT04385199. Accessed 8 Jan 2022.
- 66.Ortigoza MB, Yoon H, Goldfeld KS, et al. Efficacy and safety of COVID-19 convalescent plasma in hospitalized patients: a randomized clinical trial. JAMA Intern Med Published Online First. 2021 doi: 10.1001/jamainternmed.2021.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rasheed AM, Fatak DF, Hashim HA, et al. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med. 2020;28:357–66. [PubMed] [Google Scholar]
- 68.Estcourt LJ, Turgeon AF, et al. Effect of convalescent plasma on organ support-free days in critically Ill patients with COVID-19: a randomized clinical trial. JAMA. 2021;326:1690–702. doi: 10.1001/jama.2021.18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sekine L, Arns B, Fabro BR, et al. Convalescent plasma for COVID-19 in hospitalised patients: an open-label, randomised clinical trial. Eur Respir J Published Online First. 2021 doi: 10.1183/13993003.01471-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385:e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV Antibody Cocktail in Outpatients with Covid-19. medRxiv Published Online First. 2021 doi: 10.1101/2021.06.09.21257915. [DOI] [Google Scholar]
- 74.Alemany A, Millat-Martinez P, Corbacho-Monné M, et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir Med Published Online First. 2022 doi: 10.1016/S2213-2600(21)00545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Santis GC, Oliveira LC, Garibaldi PMM, et al. High-Dose convalescent plasma for treatment of severe COVID-19. Emerg Infect Dis. 2022;28:548–555. doi: 10.3201/eid2803.212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.ITAC (INSIGHT 013) Study Group Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebo-controlled, phase 3, randomised trial. Lancet. 2022;399:530–40. doi: 10.1016/S0140-6736(22)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of the neutralizing SARS-CoV-2 antibody sotrovimab in preventing progression of COVID-19: a randomized clinical trial. medRxiv Published Online First. 2021 doi: 10.1101/2021.11.03.21265533. [DOI] [Google Scholar]
- 78.Portal-Celhay C, Forleo-Neto E, Eagan W, et al. Phase 2 dose-ranging study of the virologic efficacy and safety of the combination COVID-19 antibodies casirivimab and imdevimab in the outpatient setting. medRxiv Published Online First. 2021 doi: 10.1101/2021.11.09.21265912. [DOI] [Google Scholar]
- 79.Ray Y, Paul SR, Bandopadhyay P, et al. A phase 2 single center open label randomised control trial for convalescent plasma therapy in patients with severe COVID-19. Nat Commun. 2022;13:383. doi: 10.1038/s41467-022-28064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Somersan-Karakaya S, Mylonakis E, Menon VP, et al. Casirivimab and imdevimab for the treatment of hospitalized patients with COVID-19. medRxiv Published Online First. 2022 doi: 10.1101/2021.11.05.21265656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van den Berg K, Glatt TN, Vermeulen M, et al. Convalescent plasma in the treatment of moderate to severe COVID-19 pneumonia: a randomized controlled trial (PROTECT-Patient Trial) Sci Rep. 2022;12:2552. doi: 10.1038/s41598-022-06221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Brien MP, Forleo-Neto E, Sarkar N, et al. Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial. JAMA. 2022;327:432–441. doi: 10.1001/jama.2021.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao B, Hayden FG. Antiviral monotherapy for hospitalised patients with COVID-19 is not enough. Lancet. 2020;396:1310–1311. doi: 10.1016/S0140-6736(20)32078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–74. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Faes C, Abrams S, Van Beckhoven D, et al. Time between symptom onset, hospitalisation and recovery or death: statistical analysis of Belgian COVID-19 patients. Int J Environ Res Public Health. 2020 doi: 10.3390/ijerph17207560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vegivinti CTR, Pederson JM, Saravu K, et al. Efficacy of convalescent plasma therapy for COVID-19: a systematic review and meta-analysis. J Clin Apher. 2021;36:470–482. doi: 10.1002/jca.21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wolff D, Nee S, Hickey NS, et al. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021;49:15–28. doi: 10.1007/s15010-020-01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hurt AC, Wheatley AK. Neutralizing antibody therapeutics for COVID-19. Viruses. 2021 doi: 10.3390/v13040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab. Accessed 6 Jan 2022.
- 92.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 variant Omicron to antibody neutralization. bioRxiv Published Online First. 2021 doi: 10.1101/2021.12.14.472630. [DOI] [PubMed] [Google Scholar]
- 93.Dai Z, Zeng D, Cui D, et al. Prediction of COVID-19 patients at high risk of progression to severe disease. Front Public Health. 2020;8:574915. doi: 10.3389/fpubh.2020.574915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Additional Figures (DOCX 4249 KB)
Supplementary file2 PRISMA-NMA Checklist (DOCX 75 KB)
Supplementary file3 Database Search Strategy (DOCX 36 KB)
Supplementary file4 Characteristics Table (DOCX 256 KB)
Supplementary file5 Characteristics of Included Studies and Patients by Outcome (Disease Severity and Hospitalization Status) (DOCX 18 KB)
Supplementary file6 Risk of Bias Ratings (DOCX 31 KB)
Supplementary file7 Summary of Findings for Unanalyzed Studies (DOCX 101 KB)
Supplementary file8 Confidence in Network Meta-Analysis (CINeMA) (DOCX 85 KB)
Supplementary file9 Pairwise Results of Treatment Comparisons (League Tables) (DOCX 32 KB)