Abstract
Adult gyrification provides a window into coordinated early neurodevelopment when disruptions predispose individuals to psychiatric illness. We hypothesized that the echoes of such disruptions should be observed within structural gyrification networks in early psychiatric illness that would demonstrate associations with developmentally relevant variables rather than specific psychiatric symptoms. We employed a new data-driven method (Orthogonal Projective Non-Negative Matrix Factorization) to delineate novel gyrification-based networks of structural covariance in 308 healthy controls. Gyrification within the networks was then compared to 713 patients with recent onset psychosis or depression, and at clinical high-risk. Associations with diagnosis, symptoms, cognition, and functioning were investigated using linear models. Results demonstrated 18 novel gyrification networks in controls as verified by internal and external validation. Gyrification was reduced in patients in temporal-insular, lateral occipital, and lateral fronto-parietal networks (pFDR < 0.01) and was not moderated by illness group. Higher gyrification was associated with better cognitive performance and lifetime role functioning, but not with symptoms. The findings demonstrated that gyrification can be parsed into novel brain networks that highlight generalized illness effects linked to developmental vulnerability. When combined, our study widens the window into the etiology of psychiatric risk and its expression in adulthood.
Keywords: clinical high risk, cortical folding, depression, psychosis, structural covariance
Introduction
Gyrification (i.e., the degree of cortical folding) is a fundamental property of the human brain, which primarily arises from a complex interplay of both genetic and biological (Borrell 2018), as well as biomechanical factors (Kroenke and Bayly 2018) acting during early cortical development that peaks at week 66/80 post-conception (Llinares-Benadero and Borrell 2019). Recent evidence also shows that glial cells formation and migration in the cortex and subcortical white matter influences to a great extent the formation of cortical folds (Rash et al. 2019). Associations with structural and functional connectivity reflect these interactions (White and Hilgetag 2011), while also highlighting the contribution of environmental factors that influence a protracted developmental course extending throughout adolescence (Cao et al. 2017). Beyond the consistently homogeneous primary gyri (i.e., convex ridges) and sulci (i.e., concave grooves) (Lohmann et al. 2008), whose morphology is only impacted by severe neurodevelopmental disorders (Barkovich 2010), this continued complex interplay is reflected in more fine-grained folding patterns showing high interindividual variability (Glasser et al. 2017) that is related to psychiatric illness (Guo et al. 2015).
Pertinent current questions relate to the morphology of gyrification abnormalities in mental illness, their psychiatric manifestation, and their origin. For example, both increased and decreased gyrification have been found in established psychoses (Palaniyappan et al. 2011; Nanda et al. 2014; Matsuda and Ohi 2018) and across depression and bipolar disorder (Depping et al. 2018). While there may be relationships between gyrification and illness-specific psychiatric symptoms (Matsuda and Ohi 2018), transdiagnostic cognitive disturbances with a putative neurodevelopmental basis could also mediate these relationships (Cao et al. 2017; Popovic et al. 2020). This hypothesis is supported by associations between gyrification and cognitive ability across species (Pillay and Manger 2007; Gautam et al. 2015; Gregory et al. 2016), the coexistence of cognitive and gyrification abnormalities in neurodevelopmental disorders (Kippenhan et al. 2005; Wallace et al. 2013) and adults born very preterm (Papini et al. 2020), and by relationships found between fronto-temporal folding and cognition in affective psychoses (Rodrigue et al. 2018). Neurodevelopmental contributions are also suggested by folding differences in individuals at clinical high-risk for psychosis (Sasabayashi et al. 2017) who develop a first episode (Das et al. 2018) and by the effects of perinatal stress on altered gyrification and mood disturbances (Mareckova et al. 2020). Notably, no study has yet investigated how gyrification relates to other clinical aspects usually impaired in mental illnesses, such as everyday functioning across the lifetime (i.e., social and occupational). Late adolescence and early adulthood might be the most informative timeframes to investigate such transdiagnostic cognitive and functioning phenomena because of the high comorbidity rate of symptoms, especially in the initial phases of psychiatric illness (Musliner et al. 2019; Thapar and Riglin 2020). However, existing results remain inconclusive, differing both quantitatively and qualitatively across studies (Matsuda and Ohi 2018).
A potential reason for inconsistent results could be the use of techniques that cannot harness the highly interconnected gyrification system of the brain, such as traditional brain atlases, which are based on coarse anatomical characteristics, or mass univariate vertex-wise approaches. Whole-brain structural covariance methods (Alexander-Bloch et al. 2013; Evans 2013) might be more powerful in this regard because they are known to produce cortical maps that are highly heritable, related to behavioral variation, and have their origin in coordinated developmental processes (Alexander-Bloch et al. 2013). Seed-based techniques have been the most popular in this domain where an existing brain atlas is used to discover relationships between nodes (Bassett et al. 2008; Yeh et al. 2010; Van Den Heuvel et al. 2013; Wang et al. 2016), yielding insights regarding gyrification in patients with established schizophrenia (Palaniyappan et al. 2015, 2016). However, these studies involve an assumption that traditional atlases (e.g., the Desikan-Killiany) are valid for gyrification networks and this may not be the case. Hypothesis-free, data-driven techniques may thus be best suited to obtain gyrification-specific maps. A preliminary study has been conducted using principal components analysis (PCA) (Das et al. 2018), but this technique is limited both in terms of replicability and by its fuzzy representation of variance, which cannot define clear boundaries between components (Sotiras et al. 2015).
In this study, we first aimed to delineate gyrification covariance patterns in healthy control individuals using a novel Non-Negative Matrix Factorization (NNMF) technique, following research investigating cortical thickness (Sotiras et al. 2017). NNMF produces a parsimonious “alphabet” of clearly separated and well-defined variance components (Yang and Oja 2010; Sotiras et al. 2015), thus overcoming limitations of other dimensionality reduction methods by enhancing results’ interpretability. We then aimed to assess developmental and sex effects on data-driven components, before investigating gyrification disruptions in early stages of psychosis and depression (i.e., recent-onset psychosis, ROP, and recent-onset depression, ROD) and in clinical high-risk (CHR) individuals. Merging these three unique patient populations allowed us to investigate whether potential gyrification abnormalities were shared across clinical manifestations and/or comorbidities. Further, we explored the specificity of the abnormalities in patients in relationship to diagnosis, symptom severity (i.e., psychosis, depression, and subjective cognitive disturbances), cognition, and occupational and social functioning. We expected to find novel gyrification covariance patterns in controls that demonstrated shared spatial abnormalities across clinical groups and were associated with cognition and functioning.
Materials and Methods
Participants
A total of 413 healthy controls and 901 patients with ROP, ROD, or CHR were recruited within the PRONIA study (www.pronia.eu), an international longitudinal project conducted across seven European sites (Supplementary Information, Koutsouleris et al. 2018). Previous work on the PRONIA sample investigated gray matter volume for diagnostic and prognostic purposes (Koutsouleris et al. 2018, 2020; Upthegrove et al. 2020) and its associations with childhood trauma (Popovic et al. 2020). A subsample of 329 controls and 754 patients was selected from five out of seven sites (Munich, Cologne, Basel, Turku, and Udine) based on the availability of surface-based neuroimaging data and the need to match sex and age during site correction (see Supplementary Information). The two excluded sites (Milan and Birmingham) were used as external validation (Supplementary Information). Following magnetic resonance imaging (MRI) processing and quality control (i.e., 45 surfaces visually inspected and 21 excluded, see Supplementary Information), 308 controls and 713 patients constituted the final sample (Supplementary Figure 1; Table 1). All participants provided written informed consent and the study protocol was approved by each ethical committee.
Table 1.
Sociodemographic and clinical information of the analyzed sample
| PAT study-groups | HC vs. PAT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HC | Pooled PAT | CHR | ROD | ROP | F/X 2 | p | t/X 2 /z | p | |
| N (%) | 308 | 713 | 224 (31.4) | 226 (31.7) | 263 (36.9) | 21.331 | 0.006 | ||
| Age, mean (SD) | 25.7 (6.1) | 25.2 (5.9) | 24.2 (6.0) | 25.4 (5.6) | 25.8 (5.9) | 4.623 | 0.010 | 1.231 | 0.219 |
| Sex, females (%) | 183 (59.4) | 326 (45.7) | 113 (50.4) | 110 (48.7) | 103 (39.2) | 7.366 | 0.025 | 16.132 | <0.001 |
| Handedness, mean (SD)1 | 76.2 (44.5) | 68.8 (54.0) | 66 (49.7) | 73 (57.5) | 67.8 (54.0) | 0.848 | 0.429 | 2.011 | 0.045 |
| Ethnicity, N (%) | |||||||||
| Caucasian | 283 (92.8) | 584 (86.4) | 186 (83.0) | 195 (86.3) | 203 (77.2) | 19.814 | 0.003 | 9.566 | 0.023 |
| Asian | 4 (1.3) | 22 (3.3) | 3 (1.3) | 2 (0.9) | 17 (6.5) | ||||
| African | 1 (0.3) | 11 (1.6) | 3 (1.3) | 2 (0.9) | 6 (2.3) | ||||
| other | 17 (5.6) | 59 (8.7) | 18 (8.0) | 15 (6.6) | 26 (9.9) | ||||
| Urbanicity, N (%) | |||||||||
| > 500 000 | 108 (35.4) | 322 (47.9) | 74 (33.0) | 123 (54.4) | 125 (47.5) | 33.670 | <0.001 | 13.901 | 0.003 |
| 100 000–500 000 | 83 (27.2) | 158 (23.5) | 57 (25.4) | 31 (13.7) | 70 (26.6) | ||||
| 10 000–100 000 | 73 (23.9) | 126 (18.8) | 55 (24.6) | 39 (17.3) | 32 (12.2) | ||||
| <10 000 | 41 (13.4) | 66 (9.8) | 23 (10.3) | 21 (9.3) | 22 (8.4) | ||||
| Education, years (SD) | 15.5 (3.3) | 14.0 (5.4) | 13.5 (3.2) | 14.4 (3.0) | 14.2 (7.8) | 1.704 | 0.183 | 4.343 | <0.001 |
| Employment 2, N (%) | |||||||||
| Managers | 1 (0.2) | 9 (2.2) | 3 (1.3) | 1 (0.4) | 5 (1.9) | 19.501 | 0.244 | 39.910 | <0.001 |
| Professionals | 12 (2.0) | 67 (16.1) | 19 (8.5) | 25 (11.1) | 23 (8.7) | ||||
| Technicians | 133 (21.8) | 78 (18.7) | 14 (6.3) | 35 (15.5) | 29 (11.0) | ||||
| Clerical support workers | 111 (18.2) | 21 (5.03) | 7 (3.1) | 6 (2.7) | 8 (3.0) | ||||
| Service and sales workers | 36 (5.9) | 91 (21.8) | 30 (13.4) | 28 (12.4) | 33 (12.5) | ||||
| Agricoltural, forestry, fishery | 121 (19.8) | 3 (0.7) | 0 (0) | 2 (0.9) | 1 (0.4) | ||||
| Craft, trades workers | 5 (0.8) | 47 (11.3) | 13 (5.8) | 14 (6.2) | 20 (7.6) | ||||
| Plant/machine operators | 52 (8.5) | 10 (2.4) | 4 (1.8) | 3 (1.3) | 3 (1.4) | ||||
| Elementary occupations | 13 (2.1) | 91 (21.8) | 29 (12.9) | 19 (8.4) | 43 (16.3) | ||||
| Never employed, N (%) | 126 (20.7) | 237 (33.2) | 143 (63.8) | 149 (65.9) | 184 (70.0) | 2.146 | 0.342 | 0.587 | 0.444 |
| Relationship status, N(%) | |||||||||
| single | 136.0 (44.6) | 474 (70.2) | 147 (65.6) | 144 (63.7) | 183 (69.6) | 4.909 | 0.555 | 73.620 | <0.001 |
| married | 17.0 (5.6) | 40 (5.9) | 9 (4.0) | 15 (6.6) | 16 (6.1) | ||||
| partnership | 149.0 (48.9) | 148 (21.9) | 51 (22.8) | 51 (22.6) | 46 (17.5) | ||||
| separated/divorced | 3.0 (1.0) | 13 (1.9) | 3 (1.3) | 4 (1.8) | 6 (2.3) | ||||
| Functioning, mean (SD) | |||||||||
| GF:R current | 8.5 (0.7) | 5.5 (1.7) | 5.6 (1.7) | 5.9 (1.7) | 4.9 (1.7) | 45.390 | <0.001 | 23.467 | <0.001 |
| GF:R L past year | 8.2 (0.8) | 5.1 (1.7) | 5.4 (1.6) | 5.5 (1.6) | 4.5 (1.7) | 58.595 | <0.001 | 23.572 | <0.001 |
| GF:R H past year | 8.6 (0.7) | 7.0 (1.4) | 7.0 (1.1) | 7.4 (1.3) | 6.7 (1.6) | 25.429 | <0.001 | 17.809 | <0.001 |
| GF:R H lifetime | 8.6 (0.7) | 7.9 (0.9) | 7.9 (0.8) | 8.1 (0.9) | 7.8 (1.0) | 18.798 | <0.001 | 12.044 | <0.001 |
| GF:S current | 8.5 (0.7) | 6.0 (1.4) | 6.2 (1.3) | 6.2 (1.3) | 5.6 (1.5) | 31.338 | <0.001 | 23.073 | <0.001 |
| GF:S L past year | 8.2 (0.8) | 5.5 (1.4) | 5.8 (1.3) | 5.8 (1.3) | 5.0 (1.6) | 43.528 | <0.001 | 23.426 | <0.001 |
| GF:S H past year | 8.6 (0.7) | 7.0 (1.3) | 7.1 (1.2) | 7.2 (1.2) | 6.7 (1.3) | 16.401 | <0.001 | 19.371 | <0.001 |
| GF:S H lifetime | 8.7 (0.7) | 7.8 (0.9) | 7.8 (0.9) | 7.9 (0.9) | 7.8 (0.9) | 4.829 | 0.089 | 14.282 | <0.001 |
| Symptoms, mean (SD) | |||||||||
| SIPS-P | NA | 8.9 (7.2) | 8.1 (2.0) | 1.8 (4.3) | 15.7 (5.5) | 612.727 | <0.001 | NA | NA |
| SIPS-N | NA | 10.4 (6.6) | 10.1 (5.6) | 9.9 (6.6) | 11.1 (7.4) | 2.226 | 0.109 | NA | NA |
| SIPS-D | NA | 3.7 (3.4) | 3.4 (2.1) | 2.2 (2.8) | 5.2 (4.2) | 50.861 | <0.001 | NA | NA |
| SIPS-G | NA | 7.6 (4.1) | 7.8 (3.7) | 8.0 (4.0) | 7.1 (4.5) | 2.810 | 0.061 | NA | NA |
| COGDIS sum | NA | 7.6 (8.0) | 9.5 (4.8) | 3.0 (6.9) | 9.9 (9.3) | 60.923 | <0.001 | NA | NA |
| BDI | NA | 23.6 (12.1) | 25.4 (12.1) | 25.7 (11.2) | 20.2 (12.1) | 14.467 | <0.001 | NA | NA |
| Cognition, mean (SD) | |||||||||
| Social cognition3 | 19.4 (2.1) | 18.7 (2.5) | 18.9 (2.2) | 19.3 (2.1) | 18.1 (2.9) | 12.955 | <0.001 | 4.144 | <0.001 |
| Working memory4 | 17.9 (3.8) | 16.0 (4.0) | 16.6 (4.2) | 16.4 (4.0) | 15.0 (3.6) | 12.280 | <0.001 | 7.093 | <0.001 |
| Verbal fluency5 | 25.8 (5.7) | 22.1 (6.6) | 22.8 (6.7) | 23.4 (6.3) | 20.2 (6.3) | 16.129 | <0.001 | 8.381 | <0.001 |
| TMT-A | 27.5 (9.0) | 31.7 (12.1) | 31.5 (10.9) | 29.1 (11.4) | 34.1 (13.2) | 9.673 | <0.001 | −5.278 | <0.001 |
| DSST | 65.7 (10.4) | 57.0 (13.5) | 59.7 (11.9) | 61.5 (12.6) | 50.6 (13.2) | 49.093 | <0.001 | 9.932 | <0.001 |
| Verbal learning6 | 61.1 (6.9) | 56.8 (9.5) | 58.2 (8.5) | 58.6 (8.4) | 54.0 (10.5) | 17.602 | <0.001 | 7.076 | <0.001 |
| Reasoning7 | 21.2 (3.2) | 19.9 (4.1) | 20.4 (3.6) | 20.5 (3.7) | 18.8 (4.5) | 12.112 | <0.001 | 4.721 | <0.001 |
| CPT-IP (N correct) | 274.4 (13.0) | 265.8 (17.3) | 268.6 (18.3) | 269.7 (14.0) | 259.9 (17.3) | 23.632 | <0.001 | 7.716 | <0.001 |
| CPT-IP (N errors) | 10.3 (6.0) | 11.4 (7.0) | 12.1 (6.5) | 10.8 (7.6) | 11.4 (6.8) | 1.676 | 0.188 | −2.375 | 0.018 |
| Medication, N (%) | |||||||||
| Antipsychotics (AP) | NA | 104 (14.6) | 14 (6.3) | 5 (2.2) | 85 (32.3) | 299.510 | <0.001 | NA | NA |
| Antidepressants (AD) | NA | 146 (20.5) | 54 (24.1) | 83 (36.7) | 9 (3.4) | NA | NA | ||
| AP + AD | NA | 38 (5.3) | 11 (4.9) | 16 (7.1) | 11 (4.2) | NA | NA | ||
| Anxiolytics/Sedatives (Anx) | NA | 13 (1.8) | 6 (2.7) | 6 (2.7) | 1 (0.4) | NA | NA | ||
| AP + Anx | NA | 65 (9.1) | 6 (2.7) | 0 (0) | 59 (22.4) | NA | NA | ||
| AD+Anx | NA | 49 (6.9) | 10 (4.5) | 34 (15.0) | 5 (1.9) | NA | NA | ||
| AP + AD+Anx | NA | 28 (3.9) | 10 (4.5) | 4 (1.8) | 14 (5.3) | NA | NA | ||
Bold values represent significance after correction for multiple comparisons (False Discovery Rate, FDR < 0.01). Abbreviations: GF: Global Functioning Role (R) or Social (S), [0:10], higher scores indicate better functioning; L: lowest; H: highest; y: year; SIPS-P and N: Structured Interview for Prodromal Syndromes-Positive or Negative scale. Displayed are composite scores for all four subscales (i.e., sum of five Positive, six Negative, four Disorganized symptoms, and four items for General psychopathology) [0:6], higher scores indicate higher symptom severity; COGDIS: Schizophrenia Proneness Instrument, Cognitive Disturbances (sum of nine items); BDI: Beck’s Depression Inventory; TMT-A: Trail-Making Test-A; DSST: Digit-Symbol Substitution Test; CPT-IP: Continuous Performance Test-Identical Pairs; HC: Healthy Control; PAT: patients; CHR: Clinical High Risk; ROD: Recent Onset Depression; ROP: Recent Onset Psychosis. 1: Edinburgh Handedness score, [−100;100], higher scores indicate more pronounced right-handedness; 2: ISCO, International Standard Classification of occupation (www.ilo.org/public/english/bureau/stat/isco/isco08) [1–9]; 3: measured with Diagnostic Analysis of Nonverbal Accuracy (DANVA); 4: measured with forward + backward Digit Span; 5: correct words in Verbal Fluency (phonemic); 6: sum of 5-Rey Auditory Verbal Learning Test repetitions; 7: Matrix Reasoning subtest from the Wechsler Adult Intelligence Scale. Notes: cognition scores are those used to build the seven cognitive domains (refer to text).
Neuropsychological and Clinical Assessments
Patients underwent a comprehensive neuropsychological battery (Koutsouleris et al. 2018, Supplementary Information and Supplementary Table 8). To reduce multiple testing and build interpretable neurocognitive factors, we derived six cognitive domain scores and a global score of cognition following a similar approach to MATRICS (Nuechterlein et al. 2008). Data were checked for operator errors and outliers (i.e., 3 SD away from mean), and verbal learning scores were harmonized between the sites (Supplementary Information). Social cognition, working memory, speed of processing, verbal learning, reasoning, and attention summary scores were calculated. After standardization, a score of global cognition was computed by calculating the aggregate average across the six cognitive scores. In patients, social and role functioning were measured using the Global Functioning: Social Scale (GF:S) and Global Functioning: Role Scale (GF:R)(Cornblatt et al. 2007). Symptoms were evaluated using the Beck Depression Inventory (BDI)(Beck and Steer 1984), the Structured Interview for Psychosis-Risk Syndromes (SIPS)(Miller et al. 2003), from which the sum score was calculated separately for the positive (P), negative (N), disorganized (D), and general (G) symptoms items, and the “cognitive disturbances” items (COGDIS) from the Schizophrenia Proneness Instrument-Adult version (SPI-A(Schultze-Lutter et al. 2007); Supplementary Information).
MRI Data Acquisition and Processing
Participants were scanned using 3 T MRI scanners except for one site (Milan), which used a 1.5 T machine (Koutsouleris et al. 2018, Supplementary Table 2). Structural scans were visually inspected for motion artifacts and neuroanatomical abnormalities. The FreeSurfer software package (v6.0.0, https://surfer.nmr.mgh.harvard.edu/) was used to reconstruct the cortical surfaces from the T1-weighted structural MRI scans (Dale A.M., Fischl B. 1999; Fischl 2012, Supplementary Information). Quality control consisted in targeted inspection of the Euler number (Rosen et al. 2018) distribution in the sample followed by exclusion of scans (Supplementary Figure 2, Supplementary Information). Gyrification was calculated vertex-wise on each 3D cortical mesh using the local gyrification index (LGI, Schaer et al. 2012). This measure represents a ratio of the buried surface compared to a flat surface and its values range from 1, i.e., low gyrification, to 5, i.e., high gyrification. The LGI meshes were resampled to the fsaverage6 template (40.962 vertices per hemisphere) in order to reduce dimensionality, and then smoothed with a 5 mm Gaussian filter kernel (as usual for LGI, e.g., Sasabayashi et al. 2017).
Site Effects
The ComBat harmonization technique was used to mitigate site effects (Johnson et al. 2007). ComBat is an empirical Bayesian framework that removes site-effects variance while retaining age and sex effects to specifically investigate them in further analyses. To also maintain disease effects in the patient sample, ComBat was applied to the resampled and smoothed LGI-meshes in the controls before the estimates derived from the correction were applied, without modification, to the patients (Supplementary Information).
Non-Negative Matrix Factorization
We applied the orthonormal projective variant of NNMF (opNNMF, Sotiras et al. 2015), following Sotiras and colleagues (Sotiras et al. 2017), to the ComBat-corrected LGI maps of controls to detect Patterns of Structural Covariance (PSCs, see Supplementary Information). opNNMF is an unsupervised multivariate method that deconstructs a data matrix based on a nonnegative combination of its parts, or components. This method is particularly useful in the neuroimaging context for two main reasons: first, unlike PCA, it can aggregate variance in a parcellation-like way, in line with the hierarchical and modular organization of the human brain cortex. Second, it can generalize well to unseen data (Sotiras et al. 2015). To establish the optimal number of PSCs, we quantified the PSC goodness of fit using the reconstruction error (i.e., the amount of reconstructed variance in the data not explained by the components; Sotiras et al. 2017) and PSC generalizability using split-half analyses (i.e., applying NNMF independently on two randomly generated subsamples and calculating the components’ degree of overlap) (Supplementary Information, Supplementary Figure 6). The NNMF component solution was also externally validated in the two held-out sites by calculating the inner product between all components to identify maximal overlap and by visual inspection (Supplementary Information). The PSC maps were then applied to patients and the mean gyrification values for each component were extracted from both patients and controls (Supplementary Information, Supplementary Figure 1).
Investigation of Group and Clinical and Neuropsychological Associations
The associations between age, sex, and site and PSC gyrification values for controls and patients were first estimated using correlations, t-tests, and analyses of variance. Group effects (controls vs. patients, independent variable) were then investigated by fitting linear models for each PSC (dependent variable) with age and sex as covariates. We further investigated quadratic age effects and age-by-group interaction effects (Supplementary Information) and explored the contribution of each PRONIA study-group (i.e., ROP, ROD, CHR) (Supplementary Information). Supplemental analyses explored the potential confounding effect of scan quality (Supplementary Information).
PSCs showing a significant group effect (False Discovery Rate, FDR-correction P < 0.01, following Gregory et al. 2016) were then investigated using linear models to determine relationships with symptoms, cognition, and functioning in the patient sample. Supplementary analyses were conducted to test the potential confounding effects of MRI scan quality, education, and premorbid intelligence quotient (IQ) in order to investigate cognitive and functioning specificity (Supplementary Information). The same analyses were also performed in the control sample to investigate potential disease-independent associations between PSCs, cognition, and functioning. All analyses ran in Matlab (version R2020a) and results were FDR corrected (P < 0.01).
Data Availability
The code and models that support the findings of the current study are available on request from the corresponding author. The datasets generated and analyzed are not publicly available due to data restriction policies defined in the participants’ signed informed consent.
Results
Controls and patients differed in their sex and urbanicity distribution, educational and employment level, relationship status, and all functioning and cognitive domains, except for error numbers in the continuous performance test (CPT; P valuesFDR < 0.003, Table 1). No difference was found for age, handedness, and ethnicity (P valuesFDR > 0.02). The three clinical study groups differed from each other in terms of ethnicity, urbanicity, all functioning subscales (except GF:S highest lifetime), positive and depressive symptoms, COGDIS, cognitive abilities (except in CPT error numbers) and medication intake (P valuesFDR < 0.006). Between the three study-groups, age, sex, handedness, education, employment and relationship status, and negative and general symptoms were similar (P valuesFDR > 0.01). Site distribution of the pooled sample and sociodemographic information are reported in Supplementary Table 1.
Patterns of Structural Covariance
A total of 18 components best fit the gyrification data (Fig. 1, Supplementary Information). PSCs were spatially distinct and differed from structural and functional brain parcellations (Supplementary Information, Supplementary Figures 4 and 5). Figure 2 shows the cortical ribbon coverage when all PSCs are projected on the brain (see Supplementary Figure 8 for an individual representation of components). In the two completely held-out sites, 72% of components exhibited an inner product above 0.5 (Supplementary Figure 10) and were visually highly similar (Supplementary Figure 9). The mean inner product across PSC solutions was no different to the discovery sample split-half analysis result used to define the component solution (i.e., 0.622 and 0.623, respectively, P = 0.493; Supplementary Information). Post hoc analyses revealed significant associations of gyrification in controls across all PSCs with age (r ranging [−0.18;−0.55], pFDR < 0.01) and sex (t range [1.94;7.11], pFDR < 0.01) with no site differences (all pFDR > 0.01, Supplementary Table 5). In patients, similar demographic relationships were found (age: r range [−0.143;−0.475], pFDR < 0.001; sex: t range [2.10;10.70], pFDR < 0.01); three PSCs showed site effects (left PSC 6 and bilateral 17; Supplementary Table 6). Quadratic effects of age on gyrification were detected for five PSCs (i.e., PSC 3 and 9, and right PSC 18, Supplementary Information), however, the additional explained variance when adding the age quadratic term in linear models was minimal (Supplementary Information). No interactions between age and group were found in the pooled patient sample or within study-groups. No associations between PSCs in the patient sample and medication were found.
Figure 1 .

Split-half reproducibility (A) and reconstruction error (B) analyses. A: The median and mean inner product of the two independently estimated sets of components in the split-halves (y-axis) is represented as a function of the number of components estimated (x-axis). B: The gradient of the reconstruction error is reported as a function of the number of components estimated. Abbreviations: PSC: Pattern of Structural Covariance.
Figure 2 .
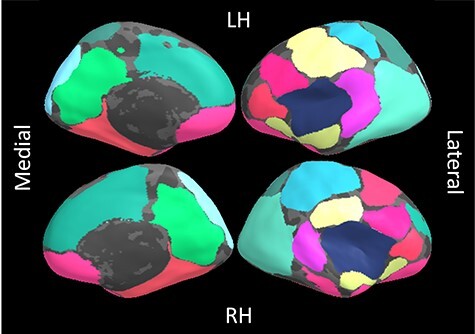
18 gyrification-based Patterns of Structural Covariance (PSC) overlapped on an inflated brain surface. RH: right hemisphere, LH: left hemisphere. The medial part of the brain is displayed on the left, the lateral on the right. Same colors correspond to the same PSC mapping bilaterally.
Effect of Group
A total of 14 gyrification components showed significant differences between controls and patients (models adjusted R2 [0.123;0.289]; maximum partial correlation coefficient for the specific effect of group −0.157, models’ pFDR [2.07E-29;1.57E-75]; coefficients’ t-values from −2.98 to −5.08; coefficients’ pFDR [0.003;4.60E-07], Supplementary Table 9). This pattern included, bilaterally, a temporal-insular area (PSC1 and 2, 5 left and 10 right and 12), a lateral occipital area (PSC 6 left and 5 right), and a lateral fronto-parietal area (PSC 7 left and 3 right). Within the right hemisphere, fronto-parietal (PSC 9, 13 and 18) and cingular gyrus areas (PSC 16) also differed between controls and patients (Fig. 3). These results were not influenced by MRI scan quality (Supplementary Information, Supplementary Table 10). The pattern of reduced gyrification in patients could almost completely be replicated in the external validation sample (Supplementary Information, Supplementary Table 11). Post hoc analyses of significant components demonstrated significance across all groups at a threshold of pFDR < 0.05, though marginal increases in effect size coupled with moderate significance increases were apparent for ROP and CHR for some PSCs (Supplementary Information, Supplementary Table 21). There were no differences between the ROD, ROP, and CHR groups in pair-wise comparisons (Supplementary Information).
Figure 3 .
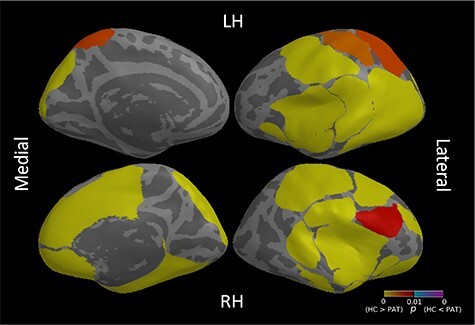
Effects of group (Healthy Controls, HC vs patients, PAT) resulting from the comparison of 29 gyrification Patterns of Structural Covariance (PSCs) between HC and PAT. The color table represents P values for each ROI. Only P values lower than 0.01 (uncorrected) are shown. ROIs are overlaid on an inflated common surface. RH: right hemisphere; LH: left hemisphere. The medial part of the brain is displayed on the left, the lateral on the right.
Associations with Cognitive and Clinical Measures
In patients, significant associations between 12/14 PSCs were found for cognition and functioning domains (Fig. 4, Supplementary Information, Supplementary Tables 12,16). For cognition, four temporal components (i.e., superior temporal gyrus, pars orbitalis and triangularis, and insula: PSC 1, 2 and 12) explained up to 3.8% of variance for working memory, speed of processing, reasoning, and global cognition (r range [0.116;0.196], pFDR < 0.002). Additionally, working memory was associated with the right angular gyrus and lateral occipital lobe (PSC 10 and 5, respectively r = 0.151, pFDR < 0.001 and r = 0.125, pFDR = 0.001), and global cognition with the left angular and medial temporal gyrus (PSC 5, r = 0.122, pFDR = 0.003). Results were not explained by either quality of MRI scans (Supplementary Information, Supplementary Table 13), or the patients’ educational level (Supplementary Information, Supplementary Table 14). Cognitive associations were found at a threshold of pFDR < 0.05 when controlling for premorbid IQ (r range [0.109;0.135], pFDR < 0.008, Supplementary Information, Supplementary Table 15, Supplementary Figure 11).
Figure 4 .
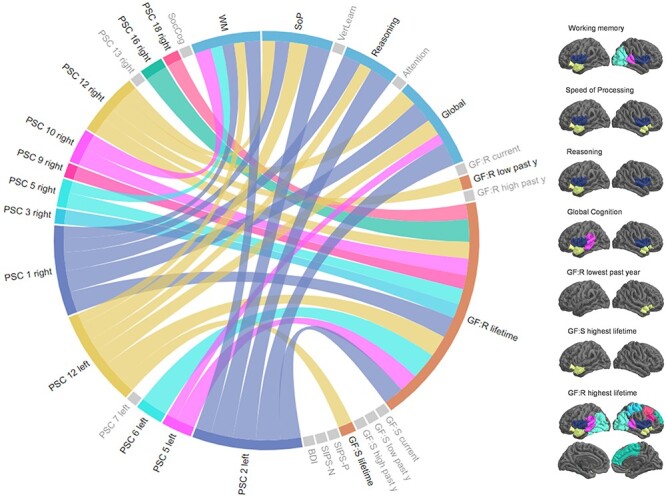
Associations between gyrification components and functioning and neurocognitive domains. The circular plot (left side) represents associations between components and neurocognitive (in blue) and functioning (in orange) domains, with thicker lines reflecting higher effect sizes/lower P values (FDR correction P < 0.01). Gray domains are those not significant in the analyses. The right panel displays the components associated with functioning and neurocognitive domains overlaid on a common cortical surface. Colors on the brains correspond to those in the plot. Abbreviations: PSC: Pattern of Structural Covariance; SocCog: social cognition; WM: working memory; SoP: speed of processing; VerLearn: verbal learning; Global: global cognition; GF:R/S: global functioning: Role/Social; lifetime: the highest functioning lifetime; low: lowest; high: highest; y: year; SIPS-P/N: Structured Interview for the Prodromal Syndrome, Positive/Negative symptoms; BDI: Beck Depression Inventory.
In the functioning domain, the left and right PSC 12 explained approximately 1.7% of variance of the patients’ highest social functioning lifetime and the lowest role functioning in the past year (pFDR < 0.001), respectively. Remaining significant associations were found specifically in the highest role functioning lifetime for 12 PSCs (r range [0.137;0.195], all pFDR < 0.001, Fig. 4, Supplementary Table 16). When controlling for premorbid IQ, three PSCs survived at a threshold of pFDR < 0.01, while results were overlapping for pFDR < 0.05 (Supplementary Information, Supplementary Table 18, Supplementary Figure 11). These relationships were not completely explained by educational level (Supplementary Information, Supplementary Table 17) or MRI scan quality (Supplementary Information, Supplementary Table 19). Associations with the cognitive and functioning domains could not be replicated in the validation cohort—potentially because of the smaller sample size. We found no significant associations with symptoms (Supplementary Table 20). In controls, no associations survived multiple comparisons correction, neither in the cognitive, nor in the functioning domain (all pFDR > 0.01, Supplementary Information, Supplementary Table 22).
Discussion
Novel and distinct gyrification covariance components were found, with a specific pattern of abnormality in early mental illness that was evident across all diagnostic groups and was not associated with symptoms. As hypothesized, the components showed rather small but significant associations with cognitive domains and functioning, which were not completely explained by premorbid intelligence, suggesting illness-related effects. While both age and sex effects were observed, we did not detect a specific interaction with diagnosis but did find increases of effect size when compared to controls in the psychosis and psychosis-prone study groups. These results point to transdiagnostic neurodevelopmental effects on gyrification occurring before late adolescence, which may precede the onset of illness.
Gyrification components were stable, replicable to some extent in new sites, demonstrated hemispheric symmetry, and were substantially novel. Quantitative comparisons indicated differences to traditional atlases (Supplementary Information, Supplementary Figure 4) and newer functional parcellations (Thomas Yeo et al. 2011) (Supplementary Figure 5), while qualitative comparisons indicated differences to thickness-based PSCs (Sotiras et al. 2017) and genetically derived structural brain networks (Chen et al. 2013). When compared to commonly used atlases in previous gyrification work (e.g., Desikan-Killiany), PSCs critically crossed gyral boundaries highlighting the importance of data-driven methods for gyrification research as opposed to traditional atlases. Hemispheric symmetry indicated shared structural and functional relationships, while some important exceptions highlighted known lateralized areas, e.g., Broca’s area (Knecht 2000; Toga and Thompson 2003). Expected linear negative relationships with age were found for the components based on hypotheses related to cortical restructuring processes during adolescence and in adult life (Klein et al. 2014; Cao et al. 2017), which occur against a background of early neurodevelopmental gyrogenesis (Llinares-Benadero and Borrell 2019). Given our restricted age window, the results reinforce research highlighting the importance of ongoing gyrification during adolescence and adulthood.
Decreased gyrification was found in patients, covering temporal, parieto-frontal, occipital, and insular areas bilaterally, and parieto-frontal and cingulate areas on the right hemisphere. The results are in line with previous research using traditional vertex-wise or atlas-based techniques, where hypogyrification has been detected in the temporal, insular, and frontal areas in schizophrenia (Palaniyappan and Liddle 2012), depression (Depping et al. 2018), and first-episode psychosis (Palaniyappan et al. 2013). Similar folding abnormalities were also found in a recent study investigating individuals at high-risk for psychosis (Sasabayashi et al. 2017), implicating bilateral supramarginal gyri, rostral middle frontal gyri, lateral occipital gyri, in addition to specific associations with the right pre- and postcentral gyri, superior frontal gyri, paracentral lobules, and cingulate. Our findings also add to this research field by providing a clearly defined gyrification covariance map that could be used in further studies, enabling a finer investigation of cortical geometry in both healthy and clinical populations. Furthermore, results also highlight a transdiagnostic signature of abnormal cortical folding, with slightly more evident disruptions in early psychosis and its risk, potentially suggesting a more distinctive neurodevelopmental component in this diagnostic group than in mood disorders (Craddock and Owen 2010). Altered gyrification in patients was not specific to current symptomatology, which has been reported in depression (Depping et al. 2018) and in cases of rare genetic disorders demonstrating disrupted gyrification with no symptoms (Caverzasi et al. 2019).
Of the several mechanisms, which are thought to be responsible for the gyrogenesis (e.g., axonal tension, differential growth, synaptogenesis; Llinares-Benadero and Borrell 2019), the involvement of radial glial cells during neurodevelopment (Rash et al. 2019) might play a particularly relevant role in psychopathology (Kato et al. 2017; Dietz et al. 2020). Disruptions of progenitor glial cells’ differentiation might in fact impact both patterns of cortical convolution and neurotransmission, which, in turn, might reflect pathophysiological manifestations of abnormal development. This cascade might start from gene expression levels involved in both pre- and post-natal cortex development, which have shown to be associated with cortical thickness profiles across disorders (Patel et al. 2021). Recent studies also point to a shared genetic liability across psychiatric disorders (Musliner et al. 2019), which supports our transdiagnostic findings of disrupted gyrification. Our findings thus reinforce the need to further investigate the neurobiological underpinnings of cortical folding networks throughout the lifespan within and across psychiatric disorders.
A bilateral cortical area covering the insula, pars orbitalis, triangularis, and opercularis (i.e., left PSC 2 and right 1) was also associated with multiple cognitive domains in patients—working memory, speed of processing, reasoning, and global cognition—with a mediation effect of premorbid IQ. These results are in line with limited previous research in established illness showing increased cortical curvature in schizophrenia related to lower premorbid and current intelligence (Jessen et al. 2019). Rather weak multivariate associations were also found between gyrification in frontotemporal areas (left pars orbitalis and triangularis, right pars opercularis and orbitalis, and insula) and general cognitive ability in bipolar, schizoaffective, and schizophrenic patients (Rodrigue et al. 2018). Interestingly, similar gyrification–cognition relationships detected in this study have been shown in healthy midlife individuals, where positive associations are found between gyrification and executive functions in lateral frontal cortex (Gautam et al. 2015), global cognition and superior temporal gyrus, as well as insular cortex and postcentral gyrus (Lamballais et al. 2020). Moreover, positive but weak associations with general intelligence have been demonstrated in children and adolescents (Gregory et al. 2016; Chung et al. 2017; Mathias et al. 2020). Of note, we found no cognitive associations in healthy young adults. On the one hand, it is possible that interindividual differences in gyrification–cognition relationships exist also for this different age range, but are too weak to be detectable, whereas they may be observable in psychiatric disorders due to being more pronounced as a result of illness and neurodevelopmental insults. On the other hand, associations found could point to unstable relationships between cortical convolution and cognitive abilities overall (Mathias et al. 2020). These might for instance be detectable only for subtypes of the population (Dwyer et al. 2020), or, although less plausible, only for specific cognitive domains. Further research is warranted to investigate whether and to what extent gyrification may mirror cognition in the healthy brain by comparing a broad spectrum of cognitive constructs in multicenter cohorts covering the full lifespan.
The consistent involvement of Broca’s area (and its contralateral equivalent) across cognitive domains might be due to its recognized double functional nature integrating both language-specific and multiple-demand networks participating in attention, working memory, planning and fluid intelligence (Fedorenko and Blank 2020). Bilateral insula associations may indicate both its involvement in feeling states and saliency, and its indirect connection with cognitive processes controlled prevalently in subregions of the prefrontal cortex (Namkung et al. 2017). The insular role both in emotional states and in motivational/cognitive processes might be one potential explanation for its disruptions found across a number of different psychiatric disorders (Goodkind et al. 2015; Namkung et al. 2017). Interestingly, the lack of insular relationships with symptoms in our study might point to gyrification abnormalities, which appear to be specific to the cognitive domain.
For the first time, we also detected a distributed pattern of gyrification-based PSCs (including fronto-temporal and lateral occipital areas bilaterally and pre-frontal, parietal and cingular areas on the right hemisphere) that was specifically correlated with the highest lifetime role functioning in the patient sample. Importantly, our results were only partly explained by premorbid intelligence, thus suggesting a specific cortical folding signature linked to the individual functioning and not driven by general cognition. When combined with the cognitive findings, these results may highlight the effects of abnormal gyrification on premorbid cognition and functioning over the lifetime that confer transdiagnostic risk to psychiatric illness during critical periods in adolescence and young adulthood. Such vulnerability is likely to be mediated by disruptions in coordinated, or synchronized, developmental processes, caused by pleiotropic genetic mechanisms, environmental factors, or insults before and during gyrogenesis (Alexander-Bloch et al. 2013). This hypothesis is supported by the lack of disrupted developmental pathways in our patient sample and suggests that future studies need to focus on younger at-risk samples (e.g., genetic risk samples) to identify candidate gyrification mechanisms that may be amenable to developmental interventions. Further research exploring which sulci are more evolutionary driven and therefore less prone to plastic reorganization (Schmitt et al. 2008; Rollins et al. 2020) would also be indicated in addition to multimodal covariance studies to assess the developmental interactions of different morphological measures (e.g., volume, density, and thickness).
Our study has some limitations. First, as in most factor analyses, the number of components chosen to best represent the variance decomposition, although normally supported by stability metrics, was ultimately selected by the investigators. Many other solutions might as well be explanatory of gyrification patterns. However, in our sample, a smaller number of PSCs showed a lower grade of resolution (Supplementary Information), with similar PSC being fused in spreader components. Second, the variance explained in our clinical and neuropsychological models was low, in line with previous findings (Mathias et al. 2020) and, in general, research in psychology (Schäfer and Schwarz 2019). Third, our replication might have been hindered either by residual site effects (potentially caused by coil differences), or the sample size of the controls. Although the PSCs were promisingly partly generalizable to an independent subsample, further replication of our gyrification components in larger samples is required to validate their future use. Future investigation of the role of other psychopathologies in the transdiagnostic signature observed in our study is also highly warranted. Finally, even though our study lacks a deep biological investigation of cortical folding from a mechanistic perspective, it nevertheless represents an important step forward in the field that could lead to mechanistic studies (e.g., in combination with diffusion tensor imaging).
Our work adds knowledge to previous gyrification research by establishing a novel covariance map, whose components revealed cortical folding abnormalities in early psychosis, risk-states and, to a lesser extent, depression, irrespective of the psychopathological features. Relationships between cortical folding and cognition suggested a neurodevelopmental origin that was supported by a further association with role functioning. When combined, our results highlight the importance of studying gyrification before late adolescence to delineate genetic, developmental, and environmental mechanisms that potentially influence this morphological measure and put adolescents and young adults at risk of mental illness.
Supplementary Material
Contributor Information
Rachele Sanfelici, Department of Psychiatry and Psychotherapy, Ludwig-Maximilian University, Munich, 80336, Germany; Max Planck School of Cognition, Leipzig, 04103, Germany.
Anne Ruef, Department of Psychiatry and Psychotherapy, Ludwig-Maximilian University, Munich, 80336, Germany.
Linda A Antonucci, Department of Psychiatry and Psychotherapy, Ludwig-Maximilian University, Munich, 80336, Germany; Department of Basic Medical Science, Neuroscience and Sense Organs, University of Bari Aldo Moro, Bari, 70124, Italy.
Nora Penzel, Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital, University of Cologne, Cologne, 50937, Germany.
Aristeidis Sotiras, Department of Radiology and Institute of Informatics, Washington University in St. Luis, st. Luis, MO63110, USA.
Mark Sen Dong, Department of Psychiatry and Psychotherapy, Ludwig-Maximilian University, Munich, 80336, Germany.
Maria Urquijo-Castro, Department of Psychiatry and Psychotherapy, Ludwig-Maximilian University, Munich, 80336, Germany.
Julian Wenzel, Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital, University of Cologne, Cologne, 50937, Germany.
Lana Kambeitz-Ilankovic, Department of Psychiatry and Psychotherapy, Ludwig-Maximilian University, Munich, 80336, Germany; Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital, University of Cologne, Cologne, 50937, Germany.
Meike D Hettwer, Max Planck School of Cognition, Leipzig, 04103, Germany.
Stephan Ruhrmann, Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital, University of Cologne, Cologne, 50937, Germany.
Katharine Chisholm, Institute for Mental Health, University of Birmingham, Birmingham, B15 2TT, UK; Department of Psychology, Aston University, Birmingham, B4 7ET, UK.
Anita Riecher-Rössler, Medical Faculty, University of Basel, Basel, 4051, Switzerland.
Peter Falkai, Department of Psychiatry and Psychotherapy, Ludwig-Maximilian University, Munich, 80336, Germany; Max-Planck Institute of Psychiatry, Munich, 80804, Germany.
Christos Pantelis, Melbourne Neuropsychiatry Centrem University of Melbourne & Melbourne Health, Melbourne, 3053, Australia.
Raimo K R Salokangas, Department of Psychiatry, University of Turku, Turku, 20700, Finland.
Rebekka Lencer, Department of Psychiatry and Psychotherapy, University of Münster, Münster, 48149, Germany; Department of Psychiatry and Psychotherapy, University of Lübeck, Lübeck, 23538, Germany.
Alessandro Bertolino, Department of Basic Medical Science, Neuroscience and Sense Organs, University of Bari Aldo Moro, Bari, 70124, Italy.
Joseph Kambeitz, Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital, University of Cologne, Cologne, 50937, Germany.
Eva Meisenzahl, Department of Psychiatry and Psychotherapy, Medical Faculty, Heinrich-Heine University, Düsseldorf, 40629, Germany.
Stefan Borgwardt, Department of Psychiatry and Psychotherapy, University of Lübeck, Lübeck, 23538, Germany; Department of Psychiatry (Psychiatric University Hospital, UPK), University of Basel, Basel, 4002, Switzerland.
Paolo Brambilla, Department of Neurosciences and Mental Health, Fondazione IRCCS Ca' Grande Ospedale Maggiore Policlinico, Milano, 20122, Italy; Department of Pathophysiology and Transplantation, University of Milan, Milan, 20122, Italy.
Stephen J Wood, Centre for Youth Mental Health, University of Melbourne, Melbourne, 3052, Australia; Orygen, Melbourne, 3052, Australia; School of Psychology, University of Birmingham, Birmingham, B15 2TT, UK.
Rachel Upthegrove, Institute for Mental Health, University of Birmingham, Birmingham, B15 2TT, UK; Early Intervention Service, Birmingham Women’s and Children’s NHS foundation Trust, Birmingham, B4 6NH, UK.
Frauke Schultze-Lutter, Department of Psychiatry and Psychotherapy, Medical Faculty, Heinrich-Heine University, Düsseldorf, 40629, Germany; Department of Psychology and Mental Health, Faculty of Psychology, Airlangga University, Surubaya, 60286, Indonesia; University Hospital of Child and Adolescent Psychiatry and Psychotherapy, University of Bern, Bern, 3000, Switzerland.
Nikolaos Koutsouleris, Department of Psychiatry and Psychotherapy, Ludwig-Maximilian University, Munich, 80336, Germany; Max-Planck Institute of Psychiatry, Munich, 80804, Germany; Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, SE5 8AF, UK.
Dominic B Dwyer, Department of Psychiatry and Psychotherapy, Ludwig-Maximilian University, Munich, 80336, Germany.
Funding
This work was supported by PRONIA: a Collaboration Project funded by the European Union under the 7th Framework Program under grant agreement number 602152. BMBF and Max Planck Society (grant agreement number M526300) funded R.S. Structural European Funding of the Italian Minister of Education (Attraction and International Mobility—AIM—action, grant agreement No 1859959) funded L.A.A. NIH/NIA supported A.S. (grant R01AG06710). National Health and Medical Research Council Senior Principal Research Fellowship (grants 628386 and 1105825) and European Union–National Health and Medical Research Council (grant 1075379) supported C.P., S.R., A.R-R., and N.K. reported receiving grants from the European Union (EU) during the conduct of the study.
Notes
The PRONIA consortium: Principal investigator and primary contact is Prof. Nikolaos Koutsouleris (nikolaos.koutsouleris@med.uni-muenchen.de). PRONIA consortium members listed here performed the screening, recruitment, rating, examination, and follow-up of the study participants and were involved in implementing the examination protocols of the study, setting up its information technological infrastructure, and organizing the flow and quality control of the data analyzed in this article between the local study sites and the central study database.
Department of Psychiatry and Psychotherapy, Ludwig-Maximilian-University, Munich, Germany: Shalaila Haas, Alkomiet Hasan, Claudius Hoff, Ifrah Khanyaree, Aylin Melo, Susanna Muckenhuber-Sternbauer, Yanis Köhler, Ömer Öztürk, Nora Penzel, David Popovic, Adrian Rangnick, Sebastian von Saldern, Rachele Sanfelici, Moritz Spangemacher, Ana Tupac, Maria Fernanda Urquijo, Johanna Weiske, Antonia Wosgien, Camilla Krämer.
Department of Psychiatry and Psychotherapy, University of Cologne, Cologne, Germany: Karsten Blume, Dominika Julkowski, Nathalie Kaden, Ruth Milz, Alexandra Nikolaides, Mauro Seves, Silke Vent, Martina Wassen.
Department of Psychiatry (Psychiatric University Hospital, UPK), University of Basel, Switzerland: Christina Andreou, Laura Egloff, Fabienne Harrisberger, Ulrike Heitz, Claudia Lenz, Letizia Leanza, Amatya Mackintosh, Renata Smieskova, Erich Studerus, Anna Walter, Sonja Widmayer.
Institute for Mental Health & School of Psychology, University of Birmingham, United Kingdom: Chris Day, Sian Lowri Griffiths, Mariam Iqbal, Mirabel Pelton, Pavan Mallikarjun, Alexandra Stainton, Ashleigh Lin, Paris Lalousis.
Department of Psychiatry, University of Turku, Finland : Alexander Denissoff, Anu Ellilä, Tiina From, Markus Heinimaa, Tuula Ilonen, Päivi Jalo, Heikki Laurikainen, Antti Luutonen, Akseli Mäkela, Janina Paju, Henri Pesonen, Reetta-Liina Säilä, Anna Toivonen, Otto Turtonen.
Department of Psychiatry (Psychiatric University Hospital LVR/HHU Düsseldorf), University of Düsseldorf, Germany: Sonja Botterweck, Norman Kluthausen, Gerald Antoch, Julian Caspers, Hans-Jörg Wittsack.
Department of Psychiatry and Psychotherapy, and Department of Child Adolescence Psychiatry, Psychotherapy and Psychosomatics, University of Muenster, Germany:
Marian Surmann, Udo Dannlowski, Olga Bienek, Georg Romer.
General Electric Global Research Inc., USA.
Ana Beatriz Solana, Manuela Abraham, Timo Schirmer.
Workgroup of Paolo Brambilla, University of Milan, Italy:
Department of Neuroscience and Mental Health, Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, University of Milan, Milan, Italy: Carlo Altamura, Marika Belleri, Francesca Bottinelli, Adele Ferro, Marta Re
Programma2000, Niguarda Hospital, Milan: Emiliano Monzani, Maurizio Sberna
San Paolo Hospital, Milan: Armando D’Agostino, Lorenzo Del Fabro
Villa San Benedetto Menni, Albese con Cassano (CO): Giampaolo Perna, Maria Nobile, Alessandra Alciati
Workgroup of Paolo Brambilla at the University of Udine, Italy
Department of Medical Area, University of Udine, Udine, Italy: Matteo Balestrieri, Carolina Bonivento, Giuseppe Cabras, Franco Fabbro
IRCCS Scientific Institute “E. Medea”, Polo FVG, Udine: Marco Garzitto, Sara Piccin
Conflict of interest: R.S., R.U., and N.K. report educational fees from Lundbeck/Otsuka, outside the submitted work. R.U. reports receiving personal fees from Sunovion Pharmaceuticals, Inc, outside the submitted work. A.B. has received lecture fees from Otsuka, Janssen, Lundbeck, and consultant fees from Biogen. C.P. participated in advisory boards for Janssen-Cilag, AstraZeneca, Lundbeck, and Servier and received honoraria for talks presented at educational meetings organized by AstraZeneca, Janssen-Cilag, Eli Lilly, Pfizer, Lundbeck, and Shire, outside the submitted work. N.K. and E.M. reported having a patent to US20160192889A1 licensed. No other conflicts of interest were reported.
References
- Alexander-Bloch A, Giedd JN, Bullmore E. 2013. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 14:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ. 2010. Current concepts of polymicrogyria. Neuroradiology. 52:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. 2008. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 28:9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. 1984. Internal consistencies of the original and revised beck depression inventory. J Clin Psychol. 40:1365–1367. [DOI] [PubMed] [Google Scholar]
- Borrell V. 2018. How cells fold the cerebral cortex. J Neurosci. 38:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Mwangi B, Passos IC, Wu MJ, Keser Z, Zunta-Soares GB, Xu D, Hasan KM, Soares JC. 2017. Lifespan Gyrification trajectories of human brain in healthy individuals and patients with major psychiatric disorders. Sci Rep. 7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverzasi E, Battistella G, Chu SA, Rosen H, Zanto TP, Karydas A, Shwe W, Coppola G, Geschwind DH, Rademakers R, et al. 2019. Gyrification abnormalities in presymptomatic c9orf72 expansion carriers. J Neurol Neurosurg Psychiatry. 90:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fiecas M, Gutiérrez ED, Panizzon MS, Eyler LT, Vuoksimaa E, Thompson WK, Fennema-Notestine C, Hagler DJ, Jernigan TL, et al. 2013. Genetic topography of brain morphology. Proc Natl Acad Sci U S A. 110:17089–17094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YS, Hyatt CJ, Stevens MC. 2017. Adolescent maturation of the relationship between cortical gyrification and cognitive ability. Neuroimage. 158:319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD. 2007. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 33:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Owen MJ. 2010. The Kraepelinian dichotomy - going, going... but still not gone. Br J Psychiatry. 196:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, SML . 1999. Cortical surface-based analysis. Neuroimage. 194:179–194. [DOI] [PubMed] [Google Scholar]
- Das T, Borgwardt S, Hauke DJ, Harrisberger F, Lang UE, Riecher-Rössler A, Palaniyappan L, Schmidt A. 2018. Disorganized gyrification network properties during the transition to psychosis. JAMA Psychiatry. 75:613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depping MS, Thomann PA, Wolf ND, Vasic N, Sosic-Vasic Z, Schmitgen MM, Sambataro F, Wolf RC. 2018. Common and distinct patterns of abnormal cortical gyrification in major depression and borderline personality disorder. Eur Neuropsychopharmacol. 28:1115–1125. [DOI] [PubMed] [Google Scholar]
- Dietz AG, Goldman SA, Nedergaard M. 2020. Glial cells in schizophrenia: a unified hypothesis. The Lancet Psychiatry. 7:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DB, Kalman JL, Budde M, Kambeitz J, Ruef A, Antonucci LA, Kambeitz-Ilankovic L, Hasan A, Kondofersky I, Anderson-Schmidt H, et al. 2020. An investigation of psychosis subgroups with prognostic validation and exploration of genetic underpinnings: the PsyCourse study. JAMA Psychiatry. 77:523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC. 2013. Networks of anatomical covariance. Neuroimage. 80:489–504. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Blank IA. 2020. Broca’s area is not a natural kind. Trends Cogn Sci. 24:270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. 2012. FreeSurfer. Neuroimage. 62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P, Anstey KJ, Wen W, Sachdev PS, Cherbuin N. 2015. Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults. Behav Brain Res. 287:331–339. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, et al. 2017. Europe PMC funders group Europe PMC funders author manuscripts a multi-modal parcellation of human cerebral cortex. Nature. 536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, et al. 2015. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MD, Kippenhan JS, Dickinson D, Carrasco J, Mattay VS, Weinberger DR, Berman KF. 2016. Regional variations in brain gyrification are associated with general cognitive ability in humans. Curr Biol. 26:1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Iwabuchi S, Balain V, Feng J, Liddle P, Palaniyappan L. 2015. Cortical folding and the potential for prognostic neuroimaging in schizophrenia. Br J Psychiatry. 207:458–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen K, Rostrup E, Mandl RCW, Nielsen MO, Bak N, Fagerlund B, Glenthoj BY, Ebdrup BH. 2019. Cortical structures and their clinical correlates in antipsychotic-naïve schizophrenia patients before and after 6 weeks of dopamine D 2/3 receptor antagonist treatment. Psychol Med. 49:754–763. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A. 2007. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 8:118–127. [DOI] [PubMed] [Google Scholar]
- Kato TA, Myint AM, Steiner J. 2017. Editorial: minding glial cells in the novel understandings of mental illness. Front Cell Neurosci. 11:10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippenhan JS, Olsen RK, Mervis CB, Morris CA, Kohn P, Meyer-Lindenberg A, Berman KF. 2005. Genetic contributions to human Gyrification: sulcal morphometry in Williams syndrome. J Neurosci. 25:7840–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Rotarska-Jagiela A, Genc E, Sritharan S, Mohr H, Roux F, Han CE, Kaiser M, Singer W, Peter JU. 2014. Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PLoS One. 9:e84914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S. 2000. Handedness and hemispheric language dominance in healthy humans. Brain. 12:2512–2518. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Dwyer DB, Degenhardt F, Maj C, Urquijo-Castro MF, Sanfelici R, Popovic D, Oeztuerk O, Haas SS, Weiske J, et al. 2020. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 78:195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Kambeitz-Ilankovic L, Ruhrmann S, Rosen M, Ruef A, Dwyer DB, Paolini M, Chisholm K, Kambeitz J, Haidl T, et al. 2018. Prediction models of functional outcomes for individuals in the clinical high-risk state for psychosis or with recent-onset depression: a multimodal, multisite machine learning analysis. JAMA Psychiatry. 75:1156–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CD, Bayly PV. 2018. How forces fold the cerebral cortex. J Neurosci. 38:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamballais S, Vinke EJ, Vernooij MW, Ikram MA, Muetzel RL. 2020. Cortical gyrification in relation to age and cognition in older adults. Neuroimage. 212:116637. [DOI] [PubMed] [Google Scholar]
- Llinares-Benadero C, Borrell V. 2019. Deconstructing cortical folding: genetic, cellular and mechanical determinants. Nat Rev Neurosci. 20:161–176. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Von Cramon DY, Colchester ACF. 2008. Deep sulcal landmarks provide an organizing framework for human cortical folding. Cereb Cortex. 18:1415–1420. [DOI] [PubMed] [Google Scholar]
- Mareckova K, Miles A, Andryskova L, Brazdil M, Nikolova YS. 2020. Temporally and sex-specific effects of maternal perinatal stress on offspring cortical gyrification and mood in young adulthood. Hum Brain Mapp. 41:4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias SR, Knowles EEM, Mollon J, Rodrigue A, Koenis MMC, Alexander-Bloch AF, Winkler AM, Olvera RL, Duggirala R, Göring HHH, et al. 2020. Minimal relationship between local Gyrification and general cognitive ability in humans. Cereb Cortex. 30:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Ohi K. 2018. Cortical gyrification in schizophrenia: current perspectives. Neuropsychiatr Dis Treat. 14:1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, Mcqlashan TH, Rosen JL, Cadenhead K, Ventura J, Mcfarlane W, Perkins DO, Pearlson QD, Woods SW. 2003. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 29:703–715. [DOI] [PubMed] [Google Scholar]
- Musliner KL, Mortensen PB, McGrath JJ, Suppli NP, Hougaard DM, Bybjerg-Grauholm J, Bækvad-Hansen M, Andreassen O, Pedersen CB, Pedersen MG, et al. 2019. Association of Polygenic Liabilities for major depression, bipolar disorder, and schizophrenia with risk for depression in the Danish population. JAMA Psychiatry. 76:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung H, Kim SH, Sawa A. 2017. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci. 40:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda P, Tandon N, Mathew IT, Giakoumatos CI, Abhishekh HA, Clementz BA, Pearlson GD, Sweeney J, Tamminga CA, Keshavan MS. 2014. Local gyrification index in Probands with psychotic disorders and their first-degree relatives. Biol Psychiatry. 76:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, et al. 2008. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 165:203–213. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. 2012. Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. Neuroimage. 60:693–699. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. 2011. Folding of the prefrontal cortex in schizophrenia: regional differences in gyrification. Biol Psychiatry. 69:974–979. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Marques TR, Taylor H, Handley R, Mondelli V, Bonaccorso S, Giordano A, McQueen G, DiForti M, Simmons A, et al. 2013. Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA psychiatry. 70:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Marques TR, Taylor H, Mondelli V, Reinders AATS, Bonaccorso S, Giordano A, DiForti M, Simmons A, David AS, et al. 2016. Globally efficient brain organization and treatment response in psychosis: a connectomic study of Gyrification. Schizophr Bull. 42:1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Park B, Balain V, Dangi R, Liddle P. 2015. Abnormalities in structural covariance of cortical gyrification in schizophrenia. Brain Struct Funct. 220:2059–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini C, Palaniyappan L, Kroll J, Froudist-Walsh S, Murray RM, Nosarti C. 2020. Altered cortical Gyrification in adults who were born very preterm and its associations with cognition and mental health. Biol Psychiatry Cogn Neurosci Neuroimaging. 5:640–650. [DOI] [PubMed] [Google Scholar]
- Patel Y, Parker N, Shin J, Howard D, French L, Thomopoulos SI, Pozzi E, Abe Y, Abé C, Anticevic A, et al. 2021. Virtual histology of cortical thickness and shared neurobiology in 6 psychiatric disorders. JAMA Psychiatry. 78:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay P, Manger PR. 2007. Order-specific quantitative patterns of cortical gyrification. Eur J Neurosci. 25:2705–2712. [DOI] [PubMed] [Google Scholar]
- Popovic D, Ruef A, Dwyer DB, Antonucci LA, Eder J, Sanfelici R, Kambeitz-Ilankovic L, Oztuerk OF, Dong MS, Paul R, et al. 2020. Traces of trauma: a multivariate pattern analysis of childhood trauma, brain structure, and clinical phenotypes. Biol Psychiatry. 88:829–842. [DOI] [PubMed] [Google Scholar]
- Rash BG, Duque A, Morozov YM, Arellano JI, Micali N, Rakic P. 2019. Gliogenesis in the outer subventricular zone promotes enlargement and gyrification of the primate cerebrum. Proc Natl Acad Sci U S A. 116:7089–7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue AL, McDowell JE, Tandon N, Keshavan MS, Tamminga CA, Pearlson GD, Sweeney JA, Gibbons RD, Clementz BA. 2018. Multivariate relationships between cognition and brain anatomy across the psychosis Spectrum. Biol Psychiatry Cogn Neurosci Neuroimaging. 3:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins CPE, Garrison JR, Arribas M, Seyedsalehi A, Li Z, Chan RCK, Yang J, Wang D, Liò P, Yan C, et al. 2020. Evidence in cortical folding patterns for prenatal predispositions to hallucinations in schizophrenia. Transl Psychiatry. 10:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AFG, Roalf DR, Ruparel K, Blake J, Seelaus K, Villa LP, Ciric R, Cook PA, Davatzikos C, Elliott MA, et al. 2018. Quantitative assessment of structural image quality. Neuroimage. 169:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabayashi D, Takayanagi Y, Takahashi T, Koike S, Yamasue H, Katagiri N, Sakuma A, Obara C, Nakamura M, Furuichi A, et al. 2017. Increased occipital Gyrification and development of psychotic disorders in individuals with an at-risk mental state: a Multicenter study. Biol Psychiatry. 82:737–745. [DOI] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Schmansky N, Fischl B, Thiran J-P, Eliez S. 2012. How to measure cortical folding from MR images: a step-by-step tutorial to compute local Gyrification index. J Vis Exp. 59:e3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer T, Schwarz MA. 2019. The meaningfulness of effect sizes in psychological research: differences between sub-disciplines and the impact of potential biases. Front Psychol. 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot RK, Wallace GL, Ordaz S, Taylor KN, Kabani N, Greenstein D, Lerch JP, Kendler KS, Neale MC, et al. 2008. Identification of genetically mediated cortical networks: a multivariate study of pediatric twins and siblings. Cereb Cortex. 18:1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. 2007. Schizophrenia Proneness Instrument, Adult Version (SPI-A). Roma: Giovanni Fioriti Ed. [Google Scholar]
- Sotiras A, Resnick SM, Davatzikos C. 2015. Finding imaging patterns of structural covariance via non-negative matrix factorization. Neuroimage. 108:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiras A, Toledo JB, Gur RE, Gur RC, Satterthwaite TD, Davatzikos C. 2017. Patterns of coordinated cortical remodeling during adolescence and their associations with functional specialization and evolutionary expansion. Proc Natl Acad Sci. 114:3527–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Riglin L. 2020. The importance of a developmental perspective in psychiatry: what do recent genetic-epidemiological findings show. Mol Psychiatry. 25:1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, et al. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. 2003. Mapping brain asymmetry. Nat Rev Neurosci. 4:37–48. [DOI] [PubMed] [Google Scholar]
- Upthegrove R, Lalousis P, Mallikarjun P, Chisholm K, Griffiths SL, Iqbal M, Pelton M, Reniers R, Stainton A, Rosen M, et al. 2020. The psychopathology and neuroanatomical markers of depression in early psychosis. Schizophr Bull. 47:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RCW, Cahn W, Goni J, Pol HEH, Kahn RS. 2013. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 70:783–792. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Robustelli B, Dankner N, Kenworthy L, Giedd JN, Martin A. 2013. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 136:1956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wang K, Qu H, Zhou J, Li Q, Deng Z, Du X, Lv F, Ren G, Guo J, et al. 2016. Disorganized cortical thickness covariance network in major depressive disorder implicated by aberrant hubs in large-scale networks. Sci Rep. 6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Hilgetag CC. 2011. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 23:339–352. [DOI] [PubMed] [Google Scholar]
- Yang Z, Oja E. 2010. Linear and nonlinear projective nonnegative matrix factorization. IEEE Trans Neural Networks. 21:734–749. [DOI] [PubMed] [Google Scholar]
- Yeh PH, Zhu H, Nicoletti MA, Hatch JP, Brambilla P, Soares JC. 2010. Structural equation modeling and principal component analysis of gray matter volumes in major depressive and bipolar disorders: differences in latent volumetric structure. Psychiatry Res – Neuroimaging. 184:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code and models that support the findings of the current study are available on request from the corresponding author. The datasets generated and analyzed are not publicly available due to data restriction policies defined in the participants’ signed informed consent.


