Abstract
Context
Reporting temporal trends in adrenocortical carcinoma (ACC) helps guide management strategies.
Objective
This work aimed to report the trends in disease burden and clinical outcomes over time that cannot be adequately captured from individual clinical trials.
Methods
A retrospective study was held of ACC patients seen at a referral cancer center between February 1998 and August 2019. Clinical outcomes were compared between an early cohort (February 1998-June 2007) and a late cohort (July 2007-August 2019).
Results
A total of 621 patients included with a median age at diagnosis of 49.3 years (range, 0.5-86.6 years). There were 285 (45.9%) patients with hormonal overproduction. More patients in the late cohort had stage IV disease compared to the early cohort (36.8% vs 23.1%; P < .0001). Resection of the primary tumor was performed in 502 patients (80.8%). Complete resection (R0) was more common in the late cohort (165 [60.2%]) than in the early cohort (100 [44.6%]; P = .0005). Of 475 patients with metastatic disease (stage IV or recurrent metastatic disease), 352 (74.1%) received mitotane, 320 (67.4%) received chemotherapy, and 53 (11.2%) received immunotherapy. In the early cohort, 70 (33%) received 2 or more lines of therapy, whereas in the late cohort, 127 (48%) received 2 or more lines of therapy. The 5-year overall survival (OS) rates were 65%, 58%, 45%, and 10% for stage I, II, III, and IV disease, respectively, whereas the 2-year OS rates in patients with stage IV disease was 24% in the early cohort and 46% in the late cohort (P = .01).
Conclusion
ACC clinical outcomes improved over the past 2 decades as more patients had complete resection or received more lines of systemic therapy.
Keywords: adrenocortical carcinoma, recurrence, survival, surgery, mitotane, immunotherapy
Adrenocortical carcinoma (ACC) is a rare and aggressive malignancy with an annual incidence of 0.5 to 2.0 cases per million individuals (1, 2). The majority of ACC cases are sporadic, and less than 10% of ACC cases are associated with hereditary cancer syndromes such as Li-Fraumeni syndrome, Lynch syndrome, Beckwith-Wiedemann syndrome, Carney complex, and multiple endocrine neoplasia type I (3). The role of environmental factors in ACC development is still unclear, although smoking has been associated with increased rates of ACC in male patients (4). ACC has a bimodal distribution, with peaks in the first and fifth decades of life with a reported female predominance (5, 6). About 50% to 75% of patients present with features of adrenal steroid hormone excess, whereas 25% to 50% are hormonally silent. Of the ACCs in patients presenting with hormonal excess, 50% to 80% are cortisol-secreting, whereas 40% to 60% are androgen-secreting (2).
While prospective clinical trials are needed to ascertain the efficacy and safety of emerging therapies, these studies are not always feasible or available for patients with very rare diseases such as ACC. Furthermore, clinical trial participation may not be enough to capture the long-term outcomes of these patients beyond the time of trial participation. In such situations, cohort studies can provide clinically useful information to guide investigators and care providers despite the biases associated with this type of study design (7, 8).
Given the rarity of ACC, most of our existing guidelines and knowledge regarding management are based on retrospective analyses performed at major referral centers using retrospective registries (1, 9-13). Over the past 4 decades, multiple retrospective studies from our institution have detailed our clinical experience with ACC management and patient outcomes, including the roles of surgery, adjuvant therapy, and cytotoxic chemotherapy (14-19). Over the past few years we have witnessed drastic changes in therapies for many cancer types with the introduction of new classes of antineoplastic drugs including small kinase inhibitors and immune checkpoint inhibitors. Whereas the majority of phase 1 and 2 studies aim to determine the safety and efficacy of new drugs, these studies are not powered to ascertain the effect of novel medications and treatment strategies on long-term survival. This is particularly relevant to immunotherapy, where there may be a delayed effect on overall survival (OS) (20).
This report represents the largest published case series from our institution detailing ACC management and outcomes. Our aim was to report the trends in disease burden and clinical outcomes over time that cannot be adequately captured from individual clinical trials.
Materials and Methods
This study consisted of a retrospective review of patients with ACC who were evaluated at The University of Texas MD Anderson Cancer Center (MDACC) from February 1998 through August 2019. Institutional review board approval was obtained before conducting the data extraction and analysis. Patients were identified in the Tumor Registry database of the Department of Medical Informatics at MDACC. Patients with all histological subtypes of ACC were included. Patients were not restricted based on age. Data were collected from patients’ medical records to summarize demographics, laboratory test results, imaging findings, pathology studies, treatments, and clinical outcomes. Hormonal hypersecretion was identified from laboratory results or documentation in the medical records. The date of diagnosis was defined as the date of pathological confirmation (surgery or biopsy) when tissue confirmation was performed. In the absence of pathological diagnosis, we used the date of clinical presentation and first imaging compatible with ACC as the date of diagnosis. OS was calculated from the date of diagnosis to the date of death or last follow-up visit. Patients were censored at the last follow-up visit if recurrence or death had not occurred. For staging, we used the European Network for the Study of Adrenal Tumors staging system (21). Stage I ACC was defined as a tumor up to 5 cm in size confined to the adrenal gland without disease in nearby lymph nodes (N0) or at distant sites (M0). Stage II ACC was defined as an N0M0 tumor larger than 5 cm confined to the adrenal gland. Stage III ACC was defined as a tumor with disease in nearby nodes (N1), infiltration of surrounding tissue, or vascular extension without evidence of distant metastasis. Stage IV ACC was defined as a tumor with distant metastasis (M1).
Treatment Use
The Department of Investigational Cancer Therapeutics at MDACC was established in July 2007, and this department has provided a growing number of ACC patients with protocol-based treatments. A subgroup analysis was performed to compare demographics, treatments, and clinical outcomes in patients from February 1998 to June 2007 (early cohort) and from July 2007 to August 2019 (late cohort) to identify and analyze any differences in outcomes of patients treated with or without investigational therapy.
Resection margins were determined based on pathology reports, operative reports, and perioperative documentation. Resection margins were defined as follows: R0, no evidence of tumor; R1, microscopic evidence of tumor at resection margins; R2, macroscopic evidence of tumor at resection margins; RX, unknown status of resection margins.
Systemic therapy was categorized as mitotane, systemic therapy (eg, traditional cytotoxic chemotherapy, tyrosine kinase inhibitors, insulin-like growth factor receptor 1 inhibitors), or immunotherapy. To analyze the use of systemic therapy, patients with metastatic disease were defined as those presenting with stage IV disease or experiencing metastatic recurrence after the resection of the primary ACC in patients with stage I, II, or III disease.
Statistical Analysis
Patients presenting at different time periods were compared using chi-square tests or the Fisher exact test for categorical variables and t tests or the Wilcoxon rank sum test for continuous variables. The Kaplan-Meier method was used to estimate OS, and log-rank tests were used to compare OS between strata. Multivariable Cox regression analysis was employed to evaluate associations of OS with clinical and treatment features. P values less than .05 were considered statistically significant, and all tests were 2-sided. Statistical analyses were performed using SAS software (version 9.4; SAS Institute) and R version 3.6.1.
Results
Demographics and Disease Burden
Table 1 describes the patient population in this study. A total of 621 patients were evaluated, consisting of 260 seen before July 2007 (early cohort) and 361 seen from July 2007 to August 2019 (late cohort). The median overall patient age at diagnosis of ACC was 49.3 years (range, 0.5-86.6 years). Nearly half the patients were aged 41 to 60 years at diagnosis, 30% were younger than 41 years, and 22% were older than 60 years. Pediatric-age patients (age < 18 years) accounted for only 3.2% (n = 20) of the population. Participants in the late cohort were somewhat older (median age, 50.2 y) than were those in the early cohort (median age, 47.4 years). The date of diagnosis was based on pathological confirmation in 595 patients (95.8%) while the date of diagnosis was made based on clinical presentation and highly suggestive imaging studies in 26 patients (4.2%). We found evidence of hormonal overproduction in 285 (45.9%) patients, with cortisol overproduction being the most common, followed by androgen overproduction. Ninety-six patients had a history of other malignancies, the most common of which was nonmelanomatous skin cancer (n = 24). Twelve (1.9%) patients had Li-Fraumeni syndrome, 7 (1.1%) had Lynch syndrome, and 6 (1.0%) had a family history of ACC but not as a part of Lynch or Li-Fraumeni syndrome. The proportions of patients who had stage I, II, III, and IV disease at diagnosis were 3.7%, 33.0%, 32.1%, and 31.2%, respectively. The proportion of patients with stage IV disease was statistically significantly higher in the late cohort than in the early cohort (36.8% vs 23.1%; P < .0001).
Table 1.
Demographic and clinical characteristics of the study patients (early cohort February 1998 to June 2007 and late cohort July 2007 to August 2019)
| No. (%) | ||||
|---|---|---|---|---|
| Characteristic | Overall (n = 621) | Early cohort (n = 260) | Late cohort (n = 361) | P |
| Median age at diagnosis, y (range) | 49.3 (0.5-86.6) | 47.4 (0.5-77.2) | 50.2 (1.1-86.6) | .009 |
| Sex | ||||
| Female | 383 (61.7) | 171 (65.8) | 212 (58.7) | .08 |
| Male | 238 (38.3) | 89 (34.2) | 149 (41.3) | |
| Race | ||||
| White | 516 (83.1) | 221 (85.0) | 295 (81.7) | .28 |
| Other | 105 (16.9) | 39 (15.0) | 66 (18.3) | |
| Hormonally functioning tumor | ||||
| No | 336 (54.1) | 146 (56.2) | 190 (52.6) | .38 |
| Yes | 285 (45.9) | 114 (43.8) | 171 (47.4) | |
| Laterality of ACC | ||||
| Right | 287 (46.2) | 117 (45.0) | 170 (47.1) | .61 |
| Left | 334 (53.8) | 143 (55.0) | 191 (52.9) | |
| History of other cancer | ||||
| No | 525 (84.5) | 224 (86.2) | 301 (83.4) | .35 |
| Yes | 96 (15.5) | 36 (13.8) | 60 (16.6) | |
| ENS@T stagea | ||||
| I | 23 (3.7) | 7 (2.7) | 16 (4.4) | < .0001 |
| II | 203 (33.0) | 110 (43.1) | 93 (25.8) | |
| III | 198 (32.1) | 79 (31.0) | 119 (33.0) | |
| IV | 192 (31.2) | 59 (23.1) | 133 (36.8) | |
Abbreviations: ACC, adrenocortical carcinoma; ENS@T, European Network for the Study of Adrenal Tumors.
a Staging information was missing for 5 patients (all in the early cohort).
Treatment Use
Table 2 summarizes the surgical treatment and outcomes. A total of 502 patients (80.8%) underwent surgical resection of the primary tumor, including (87 [17.3%] at MDACC and 415 [82.7%] outside MDACC . The proportion of patients in the late cohort who underwent primary resection was statistically significantly smaller than that in the early cohort (274 [75.9%] vs 228 [87.7%]; P = .0002).
Table 2.
Surgical features and outcomes in the study patients
| No. (%) | ||||
|---|---|---|---|---|
| Feature | Overall (n = 621) | Early Cohort (n = 260) | Late cohort (n = 361) | P |
| Resection of primary tumor | .0002 | |||
| No | 119 (19.2) | 32 (12.3) | 87 (24.1) | |
| Yes | 502 (80.8) | 228 (87.7) | 274 (75.9) | |
| Site of surgery | < .0001 | |||
| Outside MD Anderson | 415 (82.7) | 206 (90.4) | 209 (76.3) | |
| At MD Anderson | 87 (17.3) | 22 (9.6) | 65 (23.7) | |
| Surgical methoda | .0004 | |||
| Open resection | 331 (78.4) | 150 (86.7) | 181 (72.7) | |
| Laparoscopic | 73 (17.3) | 22 (12.7) | 51 (20.5) | |
| Laparoscopic converted to open resection | 18 (4.3) | 1 (0.6) | 17 (6.8) | |
| Surgical marginsb | .0005 | |||
| R0 | 265 (53.2) | 100 (44.6) | 165 (60.2) | |
| R1/R2/RX | 233 (46.8) | 124 (55.4) | 109 (39.8) | |
a Method could not be determined for 80 patients.
b Margin status could not be determined for 4 patients.
The proportion of patients who had their primary surgeries performed at MDACC in the late cohort (65 [23.7%]) was statistically significantly higher than that in the early cohort (22 [9.6%]; P < .0001). None underwent laparoscopic resection at MDACC. In terms of surgical outcomes, the proportion of patients in the late cohort who had complete surgical resection (165 [60.2%]) was higher than in the early cohort (100 [44.6%]; P = .0004).
Adjuvant therapy was administered to 145 patients (93 females, 64.1%) with median age of 47.7 years. The median time from surgery to the initiation of systemic therapy was 49 days (interquartile range, 31-82 d). This group included 5 patients (3.4%) with stage I, 61 patients (42.1%) with stage II, 78 patients (53.8%) with stage III, and 1 patient with stage IV disease (0.7%). The reported resection margin was negative (R0) in 79 patients (54.5%). Adjuvant therapy regimens included 111 patients who received mitotane alone, 16 patients who received mitotane combined with platinum-based chemotherapy, 11 patients who received chemotherapy alone (platinum-based therapy), 4 patients who received mitotane combined with streptozocin, 2 patients with unknown adjuvant treatment, and 1 patient who received capecitabine combined with adjuvant radiation therapy.
Table 3 categorizes the use of systemic therapy in the whole cohort (N = 621) and compares the use of systemic options before and after July 2007. Of 475 patients with metastatic disease, 398 (83.8%) received systemic therapy (chemotherapy, mitotane, and/or immunotherapy), including 229 (86.7%) in the early cohort and 169 (80.1%) in the late cohort (P = .05). Specifically, 352 (74.1%) patients received mitotane, 320 (67.4%) received other chemotherapy, and 53 (11.2%) received immunotherapy. Combination cytotoxic therapy with mitotane was used more often in the late cohort (63.6% vs 50.2%, respectively; P = .003). Only 2 patients in the early cohort received immunotherapy for metastatic disease, compared with 51 in the late cohort.
Table 3.
Treatment use and types of systemic therapy (N = 621)
| No. (%) | Before July 2007 (N = 260) | ≥ July 2007 (N = 361) | P | |
|---|---|---|---|---|
| Mitotane | 421 (67.8) | 166 (63.9) | 255 (70.6) | .07 |
| Cytotoxic chemotherapy including targeted therapy | 347 (55.9) | 138 (53.1) | 209 (57.9) | .23 |
| Immunotherapy | 53 (8.5) | 2 (0.3) | 51 (8.2) | < .0001 |
Neoadjuvant chemotherapy was used in 29 patients (4.6%). Neoadjuvant therapy was used more often in the late cohort (n = 21) than in the early cohort (n = 8).
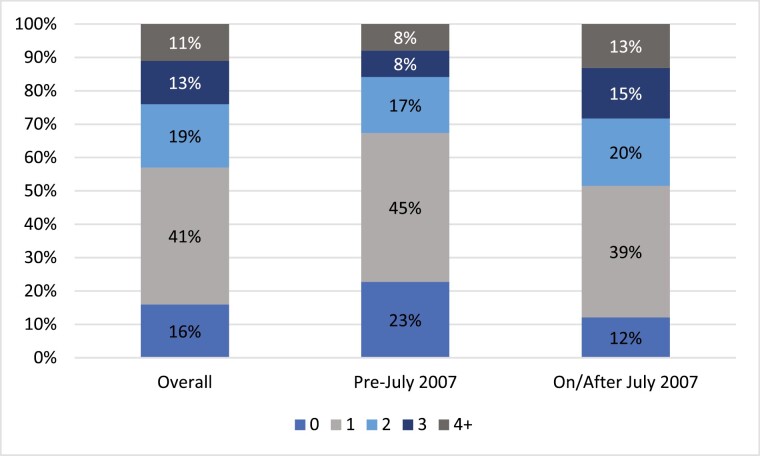
The median age of this group was 44.7 years and included 16 females (55.2%). The median time from systemic therapy initiation to surgery was 182 days (interquartile range, 159-241 d). These patients were staged as follows: 5 patients (17.2%) with stage II, 8 patients (27.6%) with stage III, and 16 patients (55.2%) with stage IV disease. Neoadjuvant therapy regimens included 23 patients who received mitotane in combination with platinum-based chemotherapy regimen (EDP [epirubicin, docetaxel, and cisplatin] in 17 patients, other regimens in 6 patients) and 6 patients received chemotherapy without mitotane (3 patients received platinum-based therapy, 1 patient received gemcitabine plus docetaxel, 1 patient received sorafenib, and 1 patient had an unknown regimen). Fig. 1 illustrates the numbers of lines of systemic therapy received by the 475 patients with metastatic disease (stage IV or recurrent metastatic disease). In the early cohort, 33% received 2 or more lines of therapy, whereas in the late cohort, 48% received 2 or more lines of therapy.
Figure 1.
The numbers of lines of systemic therapy received by the patients with metastatic adrenocortical carcinoma (stage IV or recurrent disease) (n = 475).
A total of 134 (21.6%) patients (21.5% in the early cohort and 21.6% in the late cohort; P = .98) received radiation therapy, mostly for palliation of metastatic disease. Thermal ablation was used in 23 (4.8%) of 475 patients with metastatic disease and embolization in 37 (7.8%). Six (1.3%) patients underwent combined ablation and embolization.
Clinical Outcomes
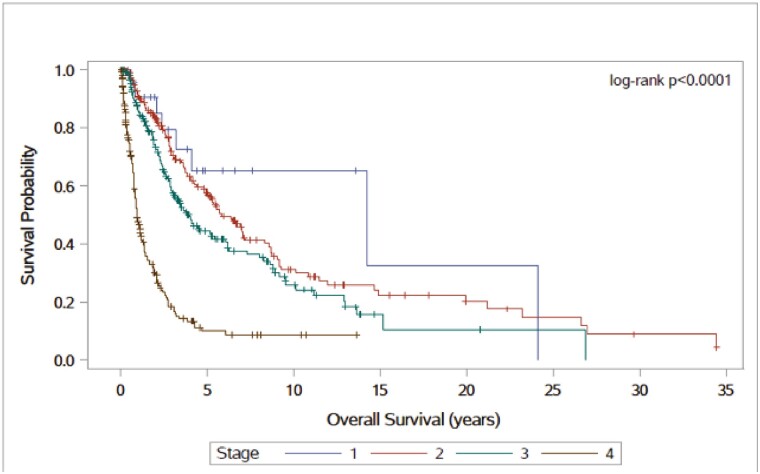
The median follow-up time was 2.10 years (range, 0.03-55.30 years) overall, 3.40 years (range, 0.04-55.30 years) in the early cohort, and 1.60 years (range, 0.03-11.30 years) in the late cohort (P ≤ .0001). When stratifying OS by disease stage (P < .0001 [log-rank test]), the median OS times were 14.2, 5.9, 4.0, and 1.0 years in patients with stage I, II, III, and IV ACC, respectively (Fig. 2). The 5-year survival rates were 65%, 58%, 45%, and 10% in patients with stage I, II, III, and IV disease, respectively.
Figure 2.
Overall survival time by adrenocortical carcinoma stage. A total of 621 patients: stage I (23, 3.7%), stage II (203, 33%), stage III (198, 32.1%), and stage IV disease (192, 31.2%).
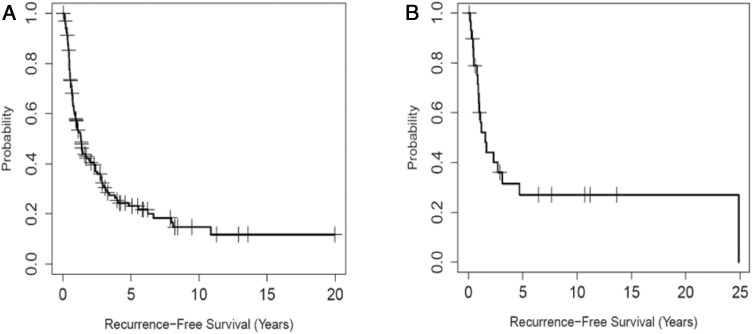
Fig. 3 illustrates the recurrence-free survival for patients who had adjuvant and neoadjuvant therapy. Over the study period, a relatively high proportion of patients received adjuvant therapy, and a high proportion of the surgeries performed had negative surgical margins (R0). The recurrence-free survival for the 145 patients who received adjuvant therapy was 57.1% at 1 year, 31.4% at 3 years, and 23.1% at 5 years. Fig. 3A illustrates the recurrence-free survival of this group. The 2-year OS rate increased from 75% to 83% during the study period (though not statistically significant, P = .83). In the 29 patients who received neoadjuvant therapy and had surgery, recurrence-free survival was 60.1% at 1 year, 36.1% at 3 years, and 27.1% at 5 years. Fig. 3B illustrates the recurrence-free survival in patients who received neoadjuvant therapy.
Figure 3.
A, Kaplan-Meier plot of recurrence-free survival (RFS) for patients who received adjuvant therapy after primary surgical resection (n = 145). B, Kaplan-Meier plot of RFS for patients who received neoadjuvant therapy (n = 29).
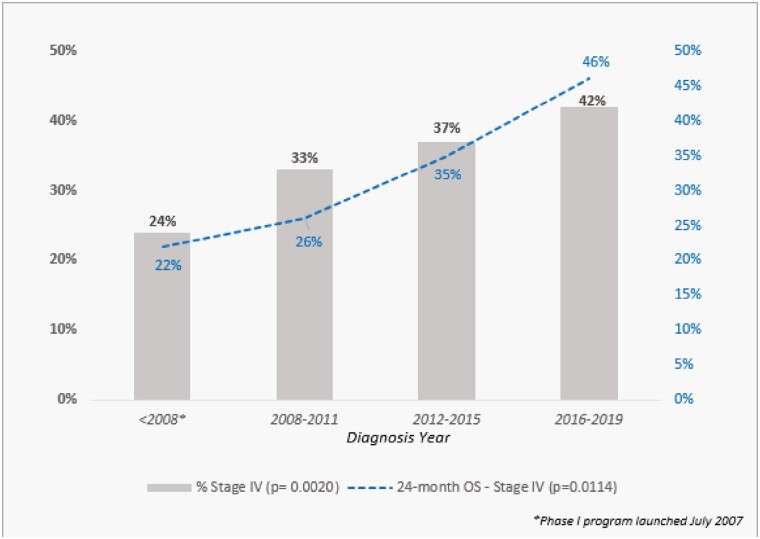
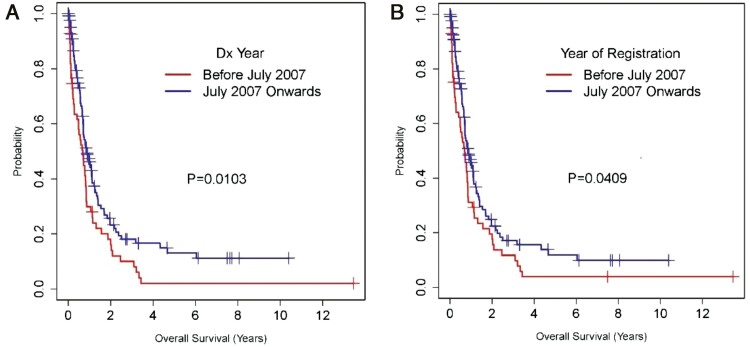
We also analyzed OS in patients with stage IV disease. Fig. 4 shows that the proportion of patients presenting with stage IV disease increased over time (P = .002). Also, the 2-year OS rate in this population improved nearly 2-fold, going from 24% in the early cohort to 46% in the late one (P = .011). Fig. 5A and 5B illustrate the OS of stage IV patients based on diagnosis year and based on analysis of survival from the time of registration for the first visit at our institution.
Figure 4.
The proportion of patients presenting with stage IV disease (n = 192) and 2-year overall survival (OS) rate in these patients across time.
Figure 5.
A and B illustrate the overall survival (OS) of stage IV patients based on diagnosis (Dx) year and based on the time of registration for the first visit at our institution, respectively.
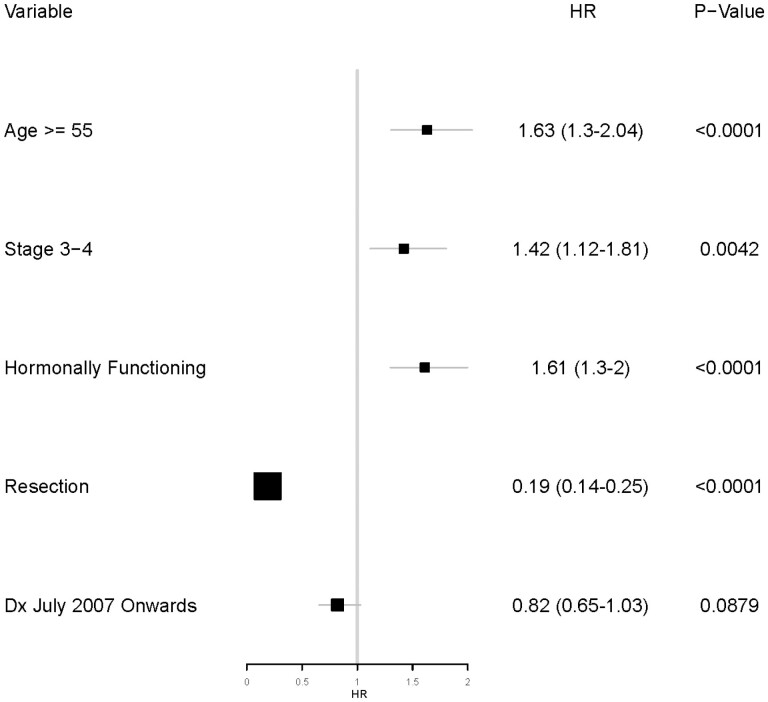
Multivariable analysis of the 621 patients demonstrated that age 55 years or older (hazard ratio [HR], 1.63; P < .0001), advanced disease stage (HR, 1.42; P = .004), and hormonally functional ACC (HR, 1.61; P < .0001) were associated with worse OS. However, surgical resection (HR, 0.19; P < .0001) was associated with better OS. There was a trend toward improved survival in patients presenting during or after July 2007 but did not reach statistical significance (HR, 0.82; P = .09). Fig. 6 shows the results of the multivariable analysis for the study cohort.
Figure 6.
Forest plot showing the results of multivariable analysis for overall survival (N = 621).
Discussion
In this large cohort of ACC patients seen at our institution over the past 2 decades, we found improved clinical outcomes despite an increasing proportion of patients referred for advanced disease. The median age at diagnosis was 49.3 years, younger than what has been previously reported. In comparison, the median age at diagnosis was 55 years in a Surveillance, Epidemiology, and End Results Program database review (1973-2014) (11) and 56 years in a Dutch cancer registry (22). Hereditary syndromes contribute to small proportion of cases given the low rates of ACC cases in association with cancer predisposition syndromes (3.2%) or family history of ACC (1.0%) identified in our study population. Because genetic testing was not routinely offered to patients, the true prevalence of underlying hereditary mutations could be slightly higher. We previously offered genetic counseling and testing to ACC patients presenting before age 30 years or in older patients who have a strong family history of malignancy. With discovery of new syndromes associated with ACC (mainly Lynch syndrome), we recommend more frequent genetic testing in adults with ACC, as patients with underlying alterations in mismatch repair genes will require screening for other malignancies and are likely to be good candidates for immunotherapy when having advanced disease.
Whereas several studies have shown that the incidence of ACC has remained stable over time (11, 22), we believe that the increasing number of ACC patients seen at our institution over the past decade is partly the result of increased recognition of the importance of multidisciplinary ACC management, including having surgery performed at experienced centers. Thus, the effect of our institution’s referral pattern and the potential bias toward having more cases with advanced or recurrent disease may have limited our ability to capture changes in the incidence of small/early-stage ACC. Furthermore, we anticipate that some cases that were treated in low-volume centers and did not have expert pathology review may have been mischaracterized as adrenal adenomas or adrenal neoplasms of unknown malignant potential, as has been reported to occur in 13% of patients of ACC patients (23).
The increasing availability of clinical trials for ACC patients may also explain the increase in referrals of patients with stage IV disease from 59 (23.1%) in the early cohort to 133 (36.8%) in the late cohort (24-26). Reviews of German and Dutch ACC registries found that up to 40% of patients presented with stage I to II disease and 30% to 35% presented with stage IV disease (22, 23),. In the Dutch ACC registry, the median OS was 13.3 years in patients with stage I to II disease, 2.2 years in stage III disease, and 0.4 years in those with stage IV disease (22). In comparison, the median OS times were 14.2, 5.9, 4.0, and 1.0 years in patients with stage I, II, III, and IV ACC, respectively, in our cohort.
In this series, 502 patients (80.8%) had surgical resection of the primary tumor (87 [17.3%] at MDACC and 415 [82.7%] at another institution). Achieving negative resection margin (R0) is a very favorable prognostic factor in ACC (19, 27). A lower proportion of patients in the early cohort (44.6%) had R0 resection compared to the late cohort (60.2%). We also noted a higher proportion of patients having their primary surgery at MDACC in the late cohort (65 [23.7%]) than in the early one (22 [9.6%]; P < .0001). This is another predictor of improved ACC outcome, as surgical resection in high-volume centers has been associated with improved clinical outcomes (10, 28, 29). In addition, the use of cytotoxic therapy in the neoadjuvant setting as part of a considered multidisciplinary approach to patients presenting with advanced ACC is increasing in our group, and we previously found an association with improved outcome in patients with borderline resectable ACC treated with neoadjuvant systemic therapy (30). Furthermore, our group performs open resection in all cases of suspected ACC, as we and others have reported retrospective evidence of higher rates of peritoneal carcinomatosis in patients who had laparoscopic ACC resection compared with those who had open resection (31). The increasing use of adjuvant therapy in ACC patients may be associated with the slight improvement in the 2-year OS rate from 75% to 83% in these patients during the study period. Considering that adjuvant therapy is often offered to patients who are presumed to have high risk of recurrence, our finding of recurrence-free survival of 57.1% at 1 year and only 23.1% at 5-years illustrates the importance of identifying these patients by assessing prognostic factors such as Ki67%, margins, hormonal status, and stage and to consider offering mitotane plus chemotherapy in those deemed to have very high risk of recurrence (invasion of major vessels, Ki67 > 30%, or positive margins). Similar to findings in the Surveillance, Epidemiology, and End Results Program database (11), our study found that advanced stage, positive nodal disease, and hormonally functional ACC at diagnosis were associated with poorer OS and that young age at diagnosis and complete surgical resection of the primary tumor were associated with better OS. While we identified hormonal overproduction in 285 (45.9%) patients, we think that the true prevalence of hormonal activity in ACC is substantially higher. The retrospective nature of this review and the variation in hormonal testing methodology between providers and centers (including the possibility of partial testing) might have contributed to the lower prevalence of hormonal activity in our series (32).
In 2012, the first randomized international trial in ACC patients (FIRM-ACT study) highlighted the suboptimal efficacy of the combined use of mitotane and cytotoxic chemotherapy (objective response rate, 23% when combining mitotane with cisplatin, etoposide, and doxorubicin vs 9% when combining mitotane with streptozocin) (33). Findings from this study spurred interest in exploring other novel treatment options. The introduction of small-molecule kinase inhibitors naturally resulted in multiple studies in ACC patients, but the majority of the drugs investigated failed to demonstrate statistically significant efficacy, potentially in part related to concomitant and/or recent mitotane use, increasing the clearance of these drugs through increased hepatic metabolism (34-36). Subsequent efforts to manage ACC based on the known increased expression of insulin-like growth factor receptor II in the vast majority of ACC cases resulted in another international randomized phase 3 clinical trial of the oral insulin-like growth factor receptor-1 (IGF-1R) inhibitor linsitinib, but the agent did not demonstrate statistically significant efficacy, and the investigators terminated the study early (37). In contrast, in our experience, treatment with drugs targeting IGF-1R signaling yielded durable stable disease (≥ 6 months) in 42% of ACC patients (24). Similarly, in other studies of IGF-1R-targeted agents in ACC patients, more prolonged disease stabilization was reported than for cytotoxic chemotherapy, suggesting that targeting the IGF-1R pathway in ACC patients remains a promising area for investigation and may be particularly relevant in combination with immune checkpoint inhibitors (25, 38). While our manuscript is not designed to ascertain the exact factors associated with the observed improved outcomes, we speculate that the recent introduction of immunotherapy could be in part a contributing factor in the improved OS in our cohort as those who respond to immunotherapy usually enjoy a prolonged period of disease control, with reported disease control rates with immunotherapy reportedly ranging from 52% to 56% (25, 39). Other potential factors could be the increasing use of tyrosine kinase inhibitors in combination with immune checkpoint inhibitors, yielding prolonged partial responses even after failing the same classes of drugs earlier when given as monotherapy, as recently reported (38). In the present study, we did not notice a variation in mitotane or cytotoxic chemotherapy use between the early and late cohorts. However, we did see the use of more lines of systemic therapy in the late cohort than in the early cohort, as shown in Fig. 1. In the multivariable analysis of the overall cohort (N = 621), the trend toward improved survival in patients seen during or after July 2007 did not reach statistical significance (HR 0.82; 95% CI, 0.65-1.03) but it remains clinically important. Potential explanations of this finding include the higher proportion of patients with advanced disease in the late cohort and the expected shorter duration of follow-up of patients seen near the end of the study. With the recent introduction of immune checkpoint inhibitors, the number of patients receiving treatment with immunotherapy has greatly increased, which may be a partial contributor to the improved OS in the late cohort (25, 38). Nevertheless, because we have seen an increasing number of referred patients with stage IV disease and other patients are often referred for recurrence or after failing other lines of therapy, we anticipate our reported experience is likely skewed toward reporting worse outcomes compared to other databases that could capture all ACC patients regardless of referral patterns.
Our findings must be interpreted while considering the inherent limitations of such a retrospective study design, including susceptibility to referral and selection biases. Also, our study is not designed or powered to ascertain the efficacy of specific individual lines or classes of systemic therapy, and our data do not capture other potentially important factors affecting outcomes of ACC patients, such as the use and efficacy of supportive measures, which were associated with improved survival in patients evaluated at our institution from 1980 to 1997 (17).
Conclusions
Despite the increasing number of patients with advanced disease seen in our institution over the past 2 decades, we observed an improvement in clinical outcomes. These improvements include a higher proportion of patients achieving complete resection as well as improved survival of patients with metastatic disease in the later part of the study associated with the use of more lines of therapy. A multidisciplinary approach to treatment is critical to optimizing outcomes of ACC patients.
Acknowledgments
Editorial assistance was provided by Don Norwood, MD Anderson Editing Services, Research Medical Library. MD Anderson Institutional Review Board approval (No. PA12-0933) was obtained to conduct this retrospective study. The MD Anderson Institutional Review Board approved a waiver of consent to publish deidentified clinical data.
Glossary
Abbreviations
- ACC
adrenocortical carcinoma
- IGF-1R
insulin-like growth factor receptor-1
- MDACC
The University of Texas MD Anderson Cancer Center
- OS
overall survival
Financial Support
This work was supported in part by the National Institutes of Health/National Cancer Institute (NIH/NCI award No. P30CA016672) and used the Biostatistics Resource Group.
Disclosures
The authors have nothing to disclose.
Data Availability
Some data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30(5):872-878. [DOI] [PubMed] [Google Scholar]
- 2. Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lerario AM, Moraitis A, Hammer GD. Genetics and epigenetics of adrenocortical tumors. Mol Cell Endocrinol. 2014;386(1-2):67-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Habra MA, Sukkari MA, Hasan A, et al. Epidemiological risk factors for adrenocortical carcinoma: a hospital-based case-control study. Int J Cancer. 2020;146(7):1836-1840. [DOI] [PubMed] [Google Scholar]
- 5. Wajchenberg BL, Albergaria Pereira MA, Medonca BB, et al. Adrenocortical carcinoma: clinical and laboratory observations. Cancer. 2000;88(4):711-736. [PubMed] [Google Scholar]
- 6. Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23(2):273-289. [DOI] [PubMed] [Google Scholar]
- 7. Euser AM, Zoccali C, Jager KJ, Dekker FW. Cohort studies: prospective versus retrospective. Nephron Clin Pract. 2009;113(3):c214-c217. [DOI] [PubMed] [Google Scholar]
- 8. Sun P, Garrison LP. Retrospective outcomes studies for orphan diseases: challenges and opportunities. Curr Med Res Opin. 2012;28(4):665-667. [DOI] [PubMed] [Google Scholar]
- 9. Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons Study Group. World J Surg. 2001;25(7):891-897. [DOI] [PubMed] [Google Scholar]
- 10. Kerkhofs TMA, Verhoeven RH, Bonjer HJ, et al. Dutch Adrenal Network . Surgery for adrenocortical carcinoma in the Netherlands: analysis of the national cancer registry data. Eur J Endocrinol. 2013;169(1):83-89. [DOI] [PubMed] [Google Scholar]
- 11. Sharma E, Dahal S, Sharma P, et al. The characteristics and trends in adrenocortical carcinoma: a United States population based study. J Clin Med Res. 2018;10(8):636-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tran TB, Postlewait LM, Maithel SK, et al. Actual 10-year survivors following resection of adrenocortical carcinoma. J Surg Oncol. 2016;114(8):971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fassnacht M, Dekkers OM, Else T, et al. European Society of Endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1-G46. [DOI] [PubMed] [Google Scholar]
- 14. Nader S, Hickey RC, Sellin RV, Samaan NA. Adrenal cortical carcinoma. A study of 77 cases. Cancer. 1983;52(4):707-711. [DOI] [PubMed] [Google Scholar]
- 15. Venkatesh S, Hickey RC, Sellin RV, Fernandez JF, Samaan NA. Adrenal cortical carcinoma. Cancer. 1989;64(3):765-769. [DOI] [PubMed] [Google Scholar]
- 16. Lee JE, Berger DH, el-Naggar AK, et al. Surgical management, DNA content, and patient survival in adrenal cortical carcinoma. Surgery. 1995;118(6):1090-1098. [DOI] [PubMed] [Google Scholar]
- 17. Vassilopoulou-Sellin R, Schultz PN. Adrenocortical carcinoma. Clinical outcome at the end of the 20th century. Cancer. 2001;92(5):1113-1121. [DOI] [PubMed] [Google Scholar]
- 18. Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol. 2010;17(1):263-270. [DOI] [PubMed] [Google Scholar]
- 19. Ayala-Ramirez M, Jasim S, Feng L, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol. 2013;169(6):891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoos A, Eggermont AMM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fassnacht M, Johanssen S, Quinkler M, et al. ; German Adrenocortical Carcinoma Registry Group; European Network for the Study of Adrenal Tumors. . Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer. 2009;115(2):243-250. [DOI] [PubMed] [Google Scholar]
- 22. Kerkhofs TMA, Verhoeven RHA, Van der Zwan JM, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013;49(11):2579-2586. [DOI] [PubMed] [Google Scholar]
- 23. Johanssen S, Hahner S, Saeger W, et al. Deficits in the management of patients with adrenocortical carcinoma in Germany. Dtsch Arztebl Int. 2010;107(50):885-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naing A, Lorusso P, Fu S, et al. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer. 2013;108(4):826-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Habra MA, Stephen B, Campbell M, et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J Immunother Cancer. 2019;7(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith DC, Kroiss M, Kebebew E, et al. A phase 1 study of nevanimibe HCl, a novel adrenal-specific sterol O-acyltransferase 1 (SOAT1) inhibitor, in adrenocortical carcinoma. Invest New Drugs. 2020;38(5):1421-1429. [DOI] [PubMed] [Google Scholar]
- 27. Margonis GA, Kim Y, Prescott JD, et al. Adrenocortical carcinoma: impact of surgical margin status on long-term outcomes. Ann Surg Oncol. 2016;23(1):134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lombardi CP, Raffaelli M, Boniardi M, et al. Adrenocortical carcinoma: effect of hospital volume on patient outcome. Langenbecks Arch Surg. 2012;397(2):201-207. [DOI] [PubMed] [Google Scholar]
- 29. Langenhuijsen J, Birtle A, Klatte T, Porpiglia F, Timsit MO. Surgical management of adrenocortical carcinoma: impact of laparoscopic approach, lymphadenectomy, and surgical volume on outcomes—a systematic review and meta-analysis of the current literature. Eur Urol Focus. 2016;1(3):241-250. [DOI] [PubMed] [Google Scholar]
- 30. Bednarski BK, Habra MA, Phan A, et al. Borderline resectable adrenal cortical carcinoma: a potential role for preoperative chemotherapy. World J Surg. 2014;38(6):1318-1327. [DOI] [PubMed] [Google Scholar]
- 31. Cooper AB, Habra MA, Grubbs EG, et al. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surg Endosc. 2013;27(11):4026-4032. [DOI] [PubMed] [Google Scholar]
- 32. Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91(7):2650-2655. [DOI] [PubMed] [Google Scholar]
- 33. Fassnacht M, Terzolo M, Allolio B, et al. FIRM-ACT Study Group . Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189-2197. [DOI] [PubMed] [Google Scholar]
- 34. Berruti A, Sperone P, Ferrero A, et al. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur J Endocrinol. 2012;166(3):451-458. [DOI] [PubMed] [Google Scholar]
- 35. Kroiss M, Quinkler M, Johanssen S, et al. Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab. 2012;97(10):3495-3503. [DOI] [PubMed] [Google Scholar]
- 36. O’Sullivan C, Edgerly M, Velarde M, et al. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J Clin Endocrinol Metab. 2014;99(4):1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fassnacht M, Berruti A, Baudin E, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426-435. [DOI] [PubMed] [Google Scholar]
- 38. Bedrose S, Miller KC, Altameemi L, et al. Combined lenvatinib and pembrolizumab as salvage therapy in advanced adrenal cortical carcinoma. J Immunother Cancer. 2020;8(2):e001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raj N, Zheng Y, Kelly V, et al. PD-1 blockade in advanced adrenocortical carcinoma. J Clin Oncol. 2020;38(1):71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.