Abstract
Mercury toxicity from amalgam dental fillings and their potential for creating problems in the environment and for human health have prompted the development of new restorative materials. The leading alternatives among these are glass ionomer cements. According to current understanding, restorative materials that slowly release fluoride exert a local cariostatic effect. For this purpose, glass ionomer cements have desirable properties in that they help prevent recurrence of caries by releasing fluoride over a long period. Thus, they function in accord with the major cariostatic mechanism of fluoride, which is believed to be its action to promote remineralization and to influence the morphology of teeth by reducing enamel solubility and by suppressing oral cariogenic bacteria. Although the minimum local concentration of fluoride release required to inhibit demineralization has not been determined, it is reported that the cariostatic ability of fluoride releasing restorative materials is significant. Zirconomer defines a new class of restorative that promises the strength and durability of amalgam with the protective benefits of glass ionomer while completely eliminating the hazards of mercury. The inclusion of specially micronized zirconia fillers in the glass component of zirconomer reinforces the structural integrity of the restoration and imparts superior mechanical properties for the restoration of load-bearing permanent teeth. Combination of outstanding strength, durability, and sustained fluoride protection deems it ideal for multiple applications. The aim of the present study was to determine the fluoride release from glass ionomer cements and compare it with new material zirconomer.
Materials and methods
Sample preparation
Tablets of glass-ionomer cements and zirconomer were prepared. A dental floss was incorporated into the tablets during fabrication to allow suspension into the test medium. Each disk specimen was immersed in airtight polyethylene bottle containing 20 mL of deionized water and incubated at 37°C and stored for 24 hours.
Determination of fluoride ion release
Fluoride ion measurement was performed after 6 hours, 24 hours, 48 hours, 7 days, and 14 days under normal atmospheric conditions by fluoride ion selective electrode connected to an ion selective electrode meter.
Result and conclusion
Both the material tested in the study had the ability to release fluoride but higher fluoride release was observed by zirconomer as compared to GIC at all time intervals.
Clinical significance
From a clinical point of view, both the restorative materials release fluoride at all time intervals; however, addition of zirconia particles in zirconomer increases its strength and provides superior mechanical properties. Therefore, due to the combination of both good structural integrity and fluoride releasing properties, zirconomer can be used for restoration of load bearing teeth.
How to cite this article
Kukreja R, Singla S, Bhadoria N, et al. An In Vitro Study to Compare the Release of Fluoride from Glass Ionomer Cement (Fuji IX) and Zirconomer. Int J Clin Pediatr Dent 2022;15(1):35-37.
Keywords: Fluoride release, GIC, Zirconomer
Introduction
Zirconomer is a new class of restorative material that has been developed by addition of nano zirconia fillers in the glass component. The use of nano zirconia fillers in the glass component strengthens the restoration's structural stability. It greatly enhances mechanical qualities in posterior load-bearing regions, such as increased compressive strength and durability.1
The need to improve the mechanical properties of GICs is always a major concern.2,3 Zirconomer defines a new class of restorative material overcoming all the shortcoming of self hardening glass ionomer cement but the ability of GICs to remineralize and protect the tooth through ion release such as fluoride, calcium, and phosphate is desirable.4,5 Active fluoride release is essential factor for an ideal performance of the restorative material. Hence, this present in vitro study was aimed to compare the amount of fluoride produced from glass ionomer cement (Fuji IX) and zirconomer. It can be used in Class I and II cavities and also temporary replacement for fractured cusps, used in Pediatric and Geriatric restorations in root surfaces where overdentures rest and is also suitable for ART technique. Fluoride ions release is associated with all glass ionomer systems, which are useful for all young patients, are particularly advantageous for those susceptible to dental caries.6
Materials and Methods
An in vitro study was conducted to assess the amount of fluoride release from glass ionomer cement (Fuji IX) and zirconomer in the department of pediatric and preventive dentistry.
Sample Preparation
The investigation employed glass ionomer cements and zirconomer tablets (10 numbers for each group).
Teflon-coated molds were used to make these tablets. A thick transparent sheet placed on a flat plane surface. This functioned as the mold's base. After that, the glass ionomer cements were mixed by hand according to the manufacturer's specifications. The mixture was poured into the mold and sealed with a mylar strip. The mold was then overlaid with a glass slide. Hand pressure was then used to extrude excess material and ensure that the specimen disks are uniform and void-free, and the cement was therefore allowed to set in the mold.
Set tablets were gently lifted out of the molds (Fig. 1). Each tablet was submerged in airtight polyethylene bottle filled with 10 mL of deionized water.
Fig. 1.
Specimen preparation
Determination of Fluoride Ion Release
Fluoride ion measurement was performed after 6 hours, 24 hours, 48 hours, 7 days, and 14 days under normal atmospheric conditions by fluoride ion selective electrode connected to an ion selective electrode meter. At the end, the tablet was removed from the bottle. 1 mL of total ionic strength adjustment buffer (TISAB) was added. The electrode was dipped into the solution and fluoride concentration was recorded.
Results
When LSD post hoc test was applied for pairwise comparison, it showed that at 6 hours, fluoride release was significantly higher than 24 hours; at 24 hours fluoride release was significantly higher than 14 day fluoride release. At 7 days, fluoride release was significantly higher than 48 hours and 14 day fluoride release (Fig. 2).
Fig. 2.
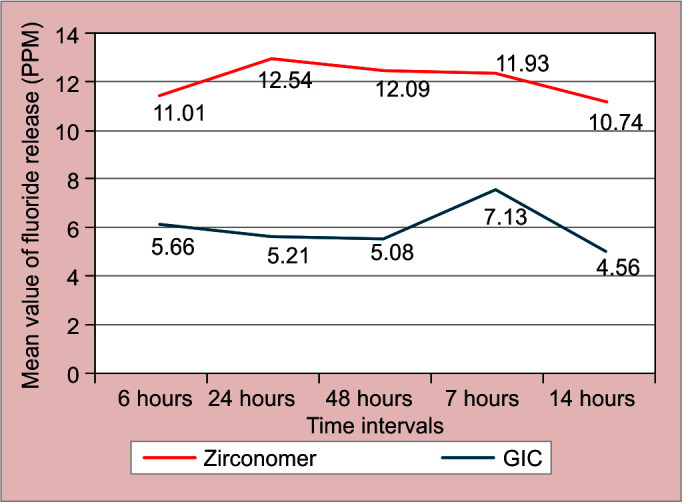
Mean and standard deviation (SD) of fluoride release (in PPM) from GIC and zirconomer at different time intervals
On comparing the fluoride release between GIC and zirconomer, significantly higher fluoride release by zirconomer was observed (Table 1).
Table 1.
Comparison of fluoride release between GIC and zirconomer at different time intervals (using Unpaired t-test)
| 6 hours | 24 hours | 48 hours | 7 days | 14 days | |
|---|---|---|---|---|---|
| t-test value | 40.500 | 140.513 | 85.54 | 36.923 | 31.577 |
| df | 2 | 2 | 2 | 2 | 2 |
| Mean difference | 5.35 | 7.33 | 7.01 | 4.80 | 6.18 |
| P value | < 0.01 | < 0.001 | < 0.001 | < 0.01 | < 0.01 |
Discussion
Various methods have been described to detect fluoride release previously. Fluoride ion selective electrode is the most frequently employed technique for measurement.7
In the present study, restorative material was placed into the Teflon mold and covered by mylar strip, supported by glass slide. This is to apply gentle and uniform pressure to extrude the excess material as shown in the previous studies conducted by Dhull et al.8 Several authors in previous studies have been changing the immersing solution regardless of the measurement intervals. McCabe (1998) suggested that daily changes of the immersing solution could minimize or even inhibit fluoride release by equilibration of the solution.8 Ion-specific electrode was used to measure fluoride-as it is more accurate, simple, and convenient.9 Zirconomer released considerably more fluoride in deionized water than glass-ionomer cements in this study. The highest values of cumulative fluoride release of zirconomer that can be related to incorporation of higher fluoride compounds compared to Fuji IX glass ionomer.
The results of this study about Fuji IX is in accordance with another study results. Low fluoride release in F IX is attributed to glass filler content with fewer monovalent ions cross-linking the polymer chains holding them close together, leading to less water transport and, consequently less fluoride release.
Restorative materials with a high fluoride release generally have lower mechanical properties.10 Therefore, they may not be as durable clinically as lower fluoride releasing materials, particularly in load-bearing areas. Mechanically stronger materials usually release only a small amount of fluoride.
But zirconomer is developed to exhibit strength that is consistent with amalgam. The homogeneous incorporation of zirconia particles in the glass component further reinforces the material for lasting durability and high tolerance to occlusal load.11
Conclusion
In caries active patients, the preferable choice of material needs to have good fluoride releasing ability. Within the limitations of present study, it can be concluded that both the materials tested in the study had the ability to release fluoride but higher fluoride release was observed by zirconomer as compared to GIC at all time intervals.
Clinical Significance
From a clinical point of view, both the restorative materials release fluoride at all time intervals; however, addition of zirconia particles in zirconomer increases its strength and provides superior mechanical properties. Therefore, due to the combination of both good structural integrity and fluoride releasing properties, zirconomer can be used for restoration of load-bearing teeth.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Zirconomer [Package Insert]. Japan: Shofu Inc.; 2012. [Google Scholar]
- 2.Peutzfeldt A, García-Godoy F, Asmussen E. Surface hardness and wear of glass ionomers and compomers. Am J Dent. 1997;10(01):15–17. [PubMed] [Google Scholar]
- 3.Zafar MS, Ahmed N. Effects of wear on hardness and stiffness of restorative dental materials. Life Sci J. 2014;11:11–18. [Google Scholar]
- 4.Craig RG. St Louis: Mosby; 1997. Restorative dental materials. [Google Scholar]
- 5.Croll TP, Nicholson JW. Glass ionomer cements in pediatric dentistry: Review of the literature. Pediatr Dent. 2002;24:423–429. doi: 10.1080/26415275.2022.2033623. [DOI] [PubMed] [Google Scholar]
- 6.Forsten L. Short- and long-term fluoride release from glass ionomers. Scand J Dent Res. 1991;99:241–245. doi: 10.1111/j.1600-0722.1991.tb01891.x. [DOI] [PubMed] [Google Scholar]
- 7.Yap AU, Tham SY, Zhu LY, et al. Short-term fluoride release from various aesthetic restorative materials. Oper Dent. 2002;27:259–265. [PubMed] [Google Scholar]
- 8.Dhull KS, Nandlal B. Comparative evaluation of fluoride release from PRG-composites and compomer on application of topical fluoride: An in-vitro study. J Indian Soc Pedod Prev Dent. 2009;27:27–32. doi: 10.4103/0970-4388.50813. [DOI] [PubMed] [Google Scholar]
- 9.Dhull KS, Nandlal B. Effect of low‐concentration daily topical fluoride application on fluoride release of giomer and compomer: An in vitro study. J Indian Soc Pedod Prev Dent. 2011;29:39–45. doi: 10.4103/0970-4388.79930. [DOI] [PubMed] [Google Scholar]
- 10.McCabe JF. Resin-modified glass-ionomers. Biomaterials. 1998;19:521–527. doi: 10.1016/s0142-9612(98)00132-x. [DOI] [PubMed] [Google Scholar]
- 11.Moreau JL, Hockin HK. Fluoride releasing restorative materials: Effects of pH on mechanical properties and ion release. Dent Mater. 2010;26(11):e227–e235. doi: 10.1016/j.dental.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]