ABSTRACT
Neisseria gonorrhoeae infection is characterized by local and abundant recruitment of neutrophils. Despite neutrophils’ antimicrobial activities, viable N. gonorrhoeae is recovered from infected individuals, leading to the question of how N. gonorrhoeae survives neutrophil attack. One feature impacting N. gonorrhoeae-neutrophil interactions is the phase-variable opacity-associated (Opa) proteins. Most Opa proteins engage human carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) to facilitate bacterial binding and invasion. Neutrophils express two transmembrane CEACAMs, CEACAM1 and the granulocyte-specific CEACAM3. While N. gonorrhoeae isolated from infected individuals is frequently Opa+, expression of OpaD from strain FA1090, which interacts with CEACAMs 1 and 3, is associated with reduced N. gonorrhoeae survival after exposure to human neutrophils. In this study, we hypothesized that the receptor-binding capability of individual Opa proteins impacts bacterial survival in the presence of neutrophils. To test this hypothesis, we introduced opa genes that are constitutively expressed into a derivative of strain FA1090 with all 11 opa genes deleted. The engineered genes encode Opa proteins that bind CEACAM1 and -3, CEACAM1 but not CEACAM3, or neither CEACAM1 nor -3. N. gonorrhoeae expressing CEACAM3-binding Opa proteins survived significantly less well than bacteria expressing other Opa proteins when exposed to primary human neutrophils. The CEACAM3-binding N. gonorrhoeae had significantly greater association with and internalization by neutrophils. However, once internalized, bacteria were similarly killed inside neutrophils, regardless of Opa expression. Furthermore, Opa expression did not significantly impact neutrophil granule mobilization. Our findings indicate that the extent to which Opa proteins mediate nonopsonic binding is the predominant determinant of bacterial survival from neutrophils.
IMPORTANCE Neisseria gonorrhoeae, the cause of gonorrhea, is an urgent-threat pathogen due to increasing numbers of infections and increased antibiotic resistance. Many surface components of N. gonorrhoeae are phase variable, including the Opa protein family of adhesins and invasins. While Opa protein expression is selected for in vivo, bacteria expressing some Opa proteins are readily killed by neutrophils, which are recruited to sites of infection. The reason for this discrepancy has remained unresolved. Our work shows that Opa-dependent differences in bacterial survival after exposure to primary human neutrophils correlates with Opa-dependent bacterial binding and phagocytosis. These findings underscore how the ability of N. gonorrhoeae to change Opa expression through phase variation contributes to bacterial resistance to neutrophil clearance.
KEYWORDS: Neisseria, adhesin, binding, invasin, neutrophil, outer membrane protein, phagocytosis, phase variation
INTRODUCTION
Neisseria gonorrhoeae is the causative agent of the sexually transmitted infection gonorrhea. Gonorrhea is a major public health concern, as there is no protective immunity against future infections, due in part to the variable nature of immunodominant surface antigens (1, 2); for related reasons, there is currently no effective vaccine. Cases of gonorrhea are on the rise, as is resistance to antibiotics, with only ceftriaxone currently recommended for treatment by the U.S. Centers for Disease Control and Prevention (3). These issues emphasize the need to better understand the mechanisms by which N. gonorrhoeae infects and successfully colonizes its obligate human host to develop effective new therapeutics and vaccine targets.
N. gonorrhoeae infects mucosal surfaces, including the nasopharynx, rectum, male urethra, and female cervix. Women are more frequently asymptomatic than men and may not seek treatment (4). Despite a robust neutrophil-rich immune response, N. gonorrhoeae that is not cleared from infected tissues leads to tissue damage (5). Subsequently, N. gonorrhoeae infections that are untreated or escape treatment due to antibiotic resistance can lead to infertility in both sexes and pelvic inflammatory disease and ectopic pregnancy in women.
Neutrophils (the predominant type of polymorphonuclear leukocyte, PMN) are the first line of defense against many invading pathogens, including N. gonorrhoeae. Despite the rapid, robust response of neutrophils to many infectious agents, N. gonorrhoeae can evade many neutrophil effector functions, including neutrophil extracellular traps, reactive oxygen species (ROS) release, release of antimicrobial peptides and proteases, phagocytosis into degradative compartments, and nutritional immunity (6). Neutrophils recognize N. gonorrhoeae through both opsonic and nonopsonic mechanisms, dependent upon the receptor that is interacting with the bacteria (7). In particular, neutrophils phagocytose N. gonorrhoeae in a nonopsonic manner using the bacterial surface-expressed Opa proteins (8).
The opa family of genes, found in the pathogenic Neisseria, encode eight stranded beta barrel proteins that span the outer membrane. Each isolate of N. gonorrhoeae carries at least 10 opa genes, some of which are duplicates but in distinct chromosomal loci. Each is independently phase variable due to slipped-strand mispairing of a pentameric repeat in the signal sequence-coding portion of the gene. The protein has four extracellular loops, the second and third of which confer the ability of Opa proteins to bind cellular receptors (9). Relative to the rest of the Opa protein, these loops are hypervariable. The hypervariable loops of Opa proteins that bind the same receptors do not always share primary sequence similarity, leading to the assumption that it is the structure and resulting chemical environment of the binding surface, not sequence of the Opa hypervariable loops, that confer receptor binding specificity and selectivity (9). However, these molecular determinants remain to be defined.
While a subset of Opa proteins has been shown to be able to bind to heparan sulfate proteoglycans (HSPGs), the major family of Opa-binding receptors is human carcinoembryonic antigen-related cellular adhesion molecules (CEACAMs). CEACAMs are expressed on multiple cell types relevant to neisserial infection, including neutrophils, endothelial cells, and epithelial cells (10). Human neutrophils express CEACAMs 1, 3, 4, 6, and 8; of these, the neisserial Opa proteins analyzed to date have been shown to interact with CEACAMs 1, 3, and 6 as well as CEACAM5 on epithelial cells (8, 11–13). While CEACAMs 5 and 6 are glycophosphatidylinositol (GPI) anchored, CEACAMs 1 and 3 are transmembrane proteins with cytosolic tails that activate signaling within the cell. CEACAM1 is ubiquitously expressed. Its cytosolic tail contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) that recruits the SHP phosphatases to downregulate cellular activities such as cell proliferation and signaling through the T cell receptor (14, 15). In contrast, CEACAM3, which is restricted to granulocytes, including neutrophils, contains a C-terminal immunoreceptor tyrosine-based activation motif (ITAM). Recruitment of Src family and Syk tyrosine kinases to the CEACAM3 ITAM drives signaling pathways that result in actin-dependent phagocytosis, release of neutrophil granules, and production of reactive oxygen species (ROS) (16, 17). CEACAM3 is thought to have evolved on primate neutrophils as a way to combat the myriad microorganisms that exploit CEACAM1 as a colonization factor (18).
Opa protein expression is selected for in vivo, with specific Opa proteins appearing more often than others in a given strain background. Most N. gonorrhoeae bacteria that can be cultured from the secretions of infected individuals express at least one Opa protein (19). In male volunteers inoculated urethrally with predominantly phase-OFF Opa-negative N. gonorrhoeae, most bacteria that are recovered from symptomatic individuals express one or more Opa proteins (19). In male individuals urethrally challenged with Opa+ bacteria, Jerse et al. found that over time there was a selection for specific Opa proteins, and, once expressed, that protein stayed expressed from the first positive sample collected until infection was terminated by antibiotic treatment (20). What drives the selection for expression of particular Opa proteins in vivo is not yet clear. However, Sintsova et al. found that primary N. gonorrhoeae isolates from urethral and cervical infections more frequently expressed Opa proteins that do not have the ability to bind CEACAM3 (21).
Based on this observation and the understanding of the downstream activation of antimicrobial activity upon CEACAM3 binding, we hypothesized that avoidance of CEACAM3 binding confers a survival advantage to N. gonorrhoeae when exposed to neutrophils. To this end, we created an isogenic panel of N. gonorrhoeae strain FA1090 of different CEACAM-binding profiles, using Opa proteins that are constitutively expressed or are phase varied for expression. These Opa+ bacteria and their Opa-negative counterparts were examined for their survival after infection of primary human neutrophils. Our findings support a model in which the extent of association is the main determinant of N. gonorrhoeae resistance to neutrophil killing, where bacteria that do not bind CEACAM3 have a survival advantage.
RESULTS
N. gonorrhoeae strains expressing Opa proteins of different receptor-binding profiles differentially activate human neutrophils.
To investigate Opa-dependent interactions of N. gonorrhoeae with neutrophils, we established a panel of N. gonorrhoeae strains expressing single Opa proteins that are predicted to interact differently with human neutrophils (Fig. 1). Opa proteins were expressed in two genetic backgrounds, each with its own advantages. In one, constitutively expressed, nonvariable (nv) versions of opa genes were introduced into a piliated derivative of strain FA1090 in which its 11 opa genes were deleted, called Opaless (22). They were introduced into the opaD locus and driven by the opaD promoter (Opa50nv, OpaDnv) or into an intergenic site between aspC and lctP under ectopic control of the tac-lac promoter (Opa54nv). The other approach used phase-varied ON Opa expressors (OpaA+, OpaF+, and OpaI+) in the piliated FA1090 ΔopaBEGK background, in which the four phenotypically translucent opa genes of this strain were deleted. In this background, Opa expression can be followed by colony morphology, where each of the remaining Opa proteins confers a particular colony opacity phenotype, as well as Western blotting using monoclonal antibodies specific to each Opa protein of strain FA1090 (20). We confirmed that each of the used strains grew similarly in media (data not shown), as previously reported by our lab (22).
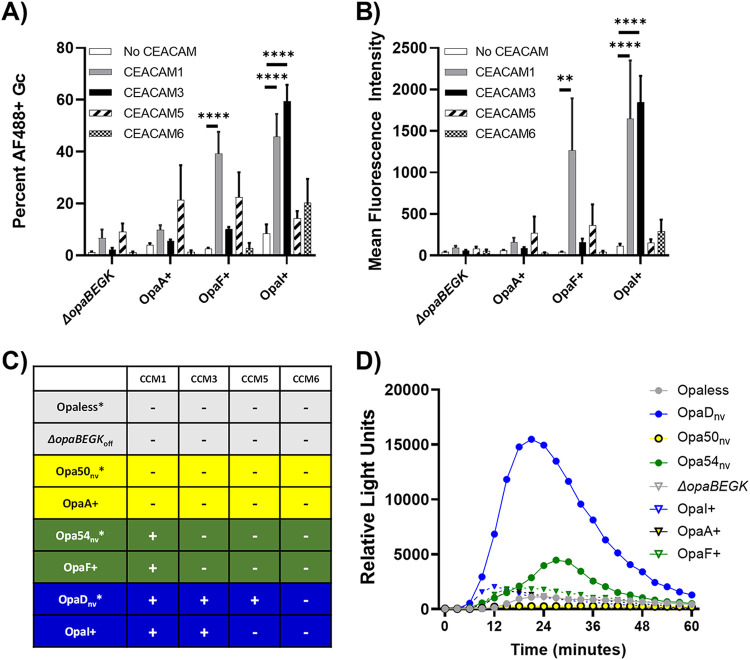
FIG 1.
Receptor-binding profile of selected Opa proteins and elicitation of the neutrophil oxidative burst. (A and B) N. gonorrhoeae predominantly expressing OpaA, OpaF, or OpaI in the ΔopaBEGK background, or the Opa− control, was incubated with GST-tagged recombinant N-CEACAM1 (gray), N-CEACAM3 (black), N-CEACAM5 (hatched), N-CEACAM6 (checked), or no protein as a control (white). Binding of CEACAM was recognized with an anti-GST antibody followed by Alexa Fluor 488-coupled goat anti-mouse IgG. The capacity of each N. gonorrhoeae strain used in this study to bind each CEACAM was determined by imaging flow cytometry. The percentage of the singlet bacterial population in the Alexa Fluor 488+ gate (A) and the mean fluorescence intensity (MFI) of Alexa Fluor 488 (B) were quantified. (C) Data are compiled from panel A and reference 23 (asterisks). Yellow, blue, and green colors are kept consistent throughout this study. Opa+ indicates phase-variable strains, and nv indicates non-phase-variable, locked-ON strains. (D) The indicated strains of N. gonorrhoeae at an MOI of 100 were exposed to primary human neutrophils in the presence of luminol. Production of reactive oxygen species was measured as relative light units of luminol-dependent chemiluminescence over 60 min. Circles denote non-phase-variable, locked strains of N. gonorrhoeae in Opaless; triangles indicate predominantly phase-ON or -OFF N. gonorrhoeae in the ΔopaBEGK background (Opa− bacteria are gray).
For this study, we investigated three categories of Opa proteins: those interacting with CEACAM1 and CEACAM3, those interacting with CEACAM1 but not CEACAM3, and those that do not interact with either CEACAM1 or -3. Additionally, we assessed the ability of these Opa proteins to interact with the neutrophil-expressed CEACAM6 and epithelium-restricted CEACAM5, the two other CEACAMs that Opa proteins are reported to bind. Receptor binding was determined by the ability of N. gonorrhoeae expressing defined Opa proteins to precipitate the soluble N-terminal domain of each of these CEACAMs (N-CEACAMs), analyzed by imaging flow cytometry (23). Results are reported as both the percentage of Alexa Fluor 488+ (CEACAM-binding) N. gonorrhoeae (Fig. 1A) and the mean Alexa Fluor 488 fluorescence intensity of the population (Fig. 1B).
The binding capabilities of the nonvariable strains used in this study have been previously reported by our group using the imaging flow cytometry binding assay (23). FA1090 OpaD binds to the N-domains of human CEACAM1, CEACAM3, and CEACAM5 but not CEACAM6, Opa54 of strain MS11 binds to CEACAM1 but not CEACAMs 3, 5, or 6, and Opa50 of strain MS11 does not interact with any CEACAMs (23). These data are consistent with prior reports about Opa54’s CEACAM-binding preferences (21, 23) and that Opa50 interacts with heparan sulfate proteoglycans and not CEACAMs (8). Applying the imaging flow cytometry binding assay to the predominantly phase-ON Opa+ N. gonorrhoeae, we found that FA1090 OpaI binds to both CEACAMs 1 and 3, OpaF of strain FA1090 interacts with CEACAM1 but not CEACAM3, and OpaA of strain FA1090 does not bind any CEACAMs (Fig. 1A and B). None of the newly tested Opa proteins bound to CEACAM5 or CEACAM6 (Fig. 1A and B). The nucleotide (see Fig. S1A in the supplemental material) and amino acid (Fig. S1B) sequences of each Opa used in this study are reported. The CEACAM binding profiles and genetic background for each strain in this study are presented in Fig. 1B. Opaless and ΔopaBEGKoff (ΔopaBEGK) are the non-Opa-expressing bacteria used throughout this study that did not interact with any CEACAMs (Fig. 1A and B) (23).
To begin to assess how expression of the different Opa proteins affects interaction with primary human neutrophils, we measured neutrophil production of reactive oxygen species (ROS) after exposure to equivalent numbers of CFU of N. gonorrhoeae, using luminol-dependent chemiluminescence. ROS production is a consequence of granule trafficking and cytoplasmic signaling events that result in assembly of NADPH oxidase, which produces superoxide and hydrogen peroxide, and myeloperoxidase, which uses hydrogen peroxide to generate hypochlorous acid (24). ROS does not directly contribute to neutrophil antimicrobial activity against N. gonorrhoeae (25, 26) but does reflect the activation state of neutrophils in response to infection. CEACAM1 and -3 binding OpaDnv and OpaI+ elicited a rapid (within 15 min) ROS response from neutrophils (Fig. 1D, blue lines). In contrast, the ROS response was slower (peak, ∼20 to 30 min) in response to the CEACAM1-only binder Opa54nv. OpaF+ N. gonorrhoeae, which also binds CEACAM1 and not CEACAM3, elicited a marginal ROS response from neutrophils, with a peak within 15 to 20 min of exposure (green lines with triangles). The non-CEACAM binding strains of N. gonorrhoeae elicited minimal release of ROS from neutrophils, similar to the Opaless and ΔopaBEGK backgrounds (yellow lines and gray lines). These findings indicate that Opa expression state affects gonococcal activation of neutrophils. In particular, bacteria that bind both CEACAM1 and CEACAM3 tended to stimulate a more rapid and/or potent oxidative response.
CEACAM3 engagement is associated with increased bacterial binding and phagocytosis by neutrophils.
We next tested how expression of different Opa proteins affected bacterial association with and phagocytosis by primary human neutrophils. To do so, we applied an imaging flow cytometry assay, which uses a spot count algorithm to quantify the association and phagocytosis of N. gonorrhoeae across tens of thousands of neutrophils per condition (27). These experiments used adherent, IL-8-treated primary human neutrophils as a surrogate for neutrophils that have migrated to sites of mucosal N. gonorrhoeae infection (28).
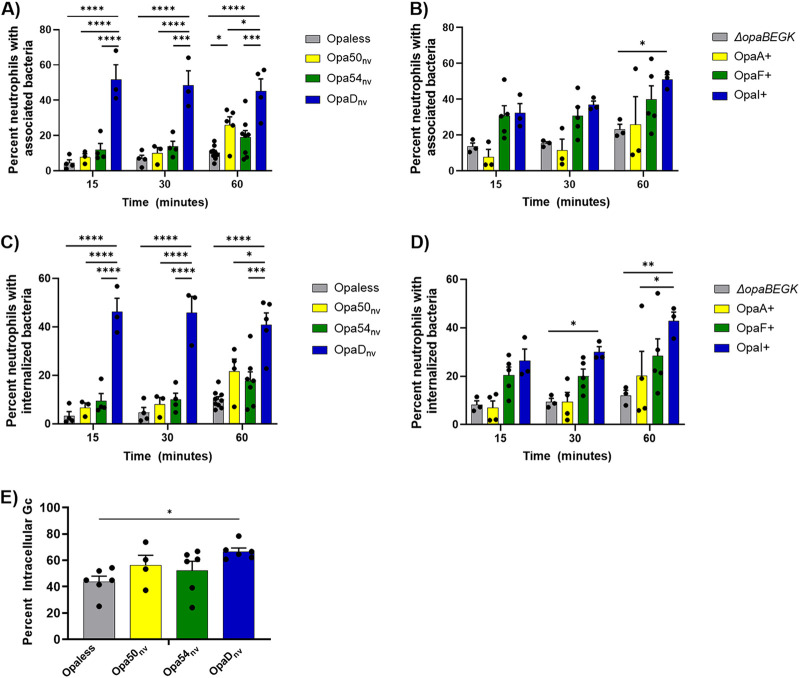
At 15 min postinfection at a multiplicity of infection (MOI) of 1, fewer than 20% of neutrophils were associated with the non-CEACAM or CEACAM1 only binding nonvariable N. gonorrhoeae (average for Opaless, 4.4%; Opa50nv, 7.7%; Opa54nv, 12%) (Fig. 2A). In contrast, at the same time point, CEACAM1 and CEACAM3 binding OpaDnv showed significantly more association with neutrophils (OpaDnv, 52%) (Fig. 2A). Similarly, at 30 min and 60 min, neutrophils were significantly more associated with OpaDnv N. gonorrhoeae than with Opaless, Opa50nv, or Opa54nv bacteria (Fig. 2A). At 60 min, significantly more neutrophils were also associated with Opa50nv than Opaless.
FIG 2.
Expression of different Opa proteins differentially affects binding and phagocytosis of N. gonorrhoeae by primary human neutrophils. The indicated strains of N. gonorrhoeae (A and C, constitutively expressed, nonvariable; B and D, phase variable) were labeled with Tag-IT Violet (TIV) and incubated with adherent, IL-8-treated primary human neutrophils. At the indicated times, cells were fixed and stained with DyLight 650 (DL650)-labeled anti-N. gonorrhoeae antibody without permeabilization to recognize extracellular bacteria. Neutrophils were analyzed via imaging flow cytometry. Panels A and B report the percentage of single, intact neutrophils with ≥1 cell-associated bacterium (TIV+). Panels C and D indicate the percentage of neutrophils with ≥1 phagocytosed bacterium (TIV+ DL650−). Results are the average of n ≥ 3 biological replicates. Data were analyzed by two-way ANOVA with Tukey’s multiple comparisons, with the following indications of significance: *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001. Only statistical comparisons within a time point were made. (E) The indicated strains of N. gonorrhoeae were labeled with CFSE and then incubated with adherent, IL-8-treated neutrophils. After 60 min, cells were fixed and stained with AlexaFluor 647 (AF647)-labeled anti-bacteria antibody without permeabilization. Images were captured by fluorescence microscopy. The percentage of intracellular N. gonorrhoeae was determined by dividing the number of CFSE+ AF647− (intracellular) N. gonorrhoeae by the number of CFSE+ AF647+ (total) N. gonorrhoeae. Statistical comparisons were made for n ≥ 4 biological replicates using one-way ANOVA with Tukey’s multiple comparisons, with P < 0.05 (*) considered significant.
The phase-varied-ON strains followed similar patterns (Fig. 2B). At 15 and 30 min, OpaF+ and OpaI+ N. gonorrhoeae showed higher association with neutrophils than OpaA+ or ΔopaBEGK bacteria, but these differences were not statistically significant (ΔopaBEGK, 14%; OpaA+, 7.7%; OpaF+, 31%; OpaI+, 32%). By 60 min, OpaI+ N. gonorrhoeae was associated with more neutrophils than the other strains and was significantly increased over ΔopaBEGK bacteria (Fig. 2B). A histogram of the number of N. gonorrhoeae cells counted per neutrophil in each population is presented in Fig. S2.
The same data set was analyzed for bacterial phagocytosis, with the output being the percentage of neutrophils with intracellular N. gonorrhoeae (see Materials and Methods for details). Significantly more neutrophils contained intracellular OpaDnv N. gonorrhoeae than the other nonvariable bacteria at all measured time points postinfection (Fig. 2C). There were no significant differences between Opaless and Opa50nv or Opa54nv at any of the time points. Similar to the percent association data for the phase-varied-ON N. gonorrhoeae, neutrophils internalized more OpaI+ than OpaA+ or ΔopaBEGK strain at 30 and 60 min postinfection, with the difference between OpaI+ and OpaA+ being statistically significant at both time points and the difference between OpaI+ and ΔopaBEGK strain being statistically significant at 60 min (Fig. 2D). While there was a trend toward more phagocytosis of OpaI+ than OpaF+ N. gonorrhoeae as time progressed, this was not statistically significant.
The results with imaging flow cytometry were extended using an immunofluorescence assay that reports the percentage of cell-associated bacteria that are intracellular (28). Here, we focused on the nonvariable Opa strains so that the potential confounder of phase variation was removed. At 60 min postinfection, there were no significant differences among the Opa-expressing N. gonorrhoeae strains in the percentage of neutrophil-associated bacteria that were phagocytosed (Opa50nv, 56%; Opa54nv, 52%; OpaDnv, 67%) (Fig. 2E). However, the percentage of intracellular Opaless bacteria (44%) was significantly lower than that for OpaDnv, in keeping with prior reports (Fig. 2E) (29, 30).
Taken together, these results indicate that N. gonorrhoeae expressing different Opa proteins differentially interacts with human neutrophils. However, once bound to neutrophils, Opa+ N. gonorrhoeae is readily phagocytosed, regardless of which receptor(s) it engages.
Survival of N. gonorrhoeae from primary human neutrophils is modulated by bacterial Opa expression profile.
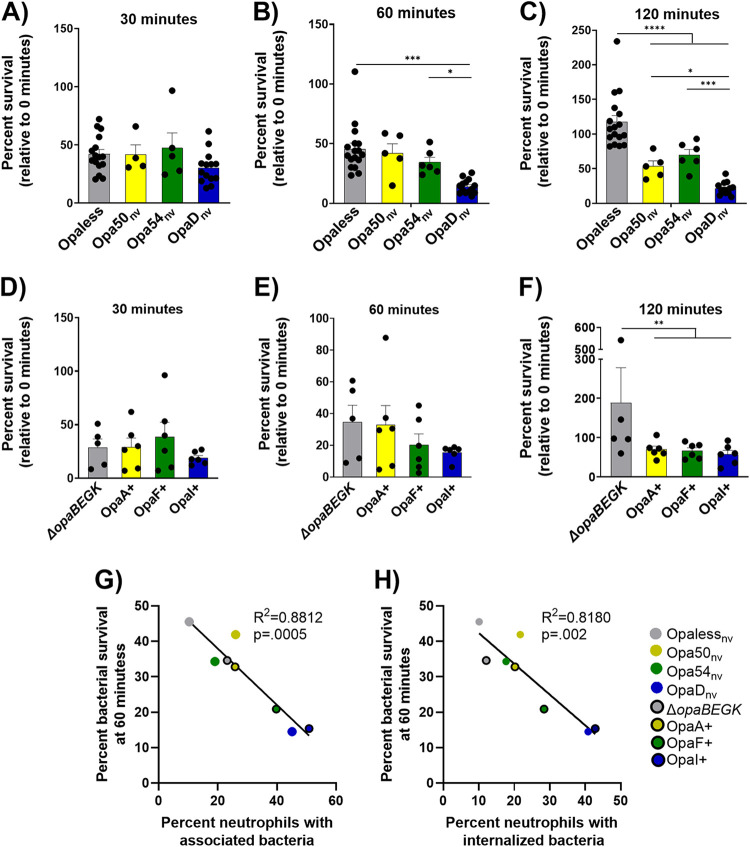
We examined how the association and phagocytosis of different Opa-expressing N. gonorrhoeae strains affected bacterial survival from neutrophils by enumerating CFU of bacteria from neutrophil lysates as a function of time (31). First examining the nonvariable strains, similar CFU numbers of Opaless, OpaDnv, Opa54nv, and Opa50nv were recovered after 30 min of neutrophil exposure (Fig. 3A). However, after 60 min, fewer CFU of OpaDnv N. gonorrhoeae were recovered than with any of the other strains, with the differences from Opaless and Opa54nv N. gonorrhoeae being statistically significant (Fig. 3B). By 120 min postinfection, the recovery of OpaDnv N. gonorrhoeae was significantly less than that of any of the other comparator strains (Fig. 3C). We also saw outgrowth of Opaless at 120 min postinfection, likely due to extracellular replication of the bacteria; the difference between Opaless and all of the Opa-expressing strains was statistically significant at this time point.
FIG 3.
Differential survival of N. gonorrhoeae of different Opa expression states after exposure to primary human neutrophils. Adherent, IL-8-treated neutrophils were synchronously exposed to constitutively expressed, nonvariable (A to C), or phase-variable (D to F) N. gonorrhoeae of the indicated Opa profile. Colors match the receptor-binding profile of each strain as in Fig. 1B. At 30 (A and D), 60 (B and E), and 120 (C and F) min postinfection, neutrophils were lysed and CFU of N. gonorrhoeae were enumerated from the lysates. Results are expressed as the average percentage of CFU at that time point divided by the CFU at the start of the experiment (0 min) ± standard errors for n ≥ 4 biological replicates. Statistical comparisons were by two-way ANOVA with post hoc Tukey multiple-comparison test, with the following pairwise significances: *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.0001. Separate ANOVAs were run to compare the variable strains and the nonvariable strains. Correlations between bacterial survival and association with neutrophils (G) and bacterial survival and phagocytosis by neutrophils (H) were calculated using a linear regression model. The R2 and P values are reported on the graphs.
The phase-varied-ON strains of N. gonorrhoeae followed the same trends as the Opa-nonvariable strains across all the time points tested, with no differences among ΔopaBEGK, OpaA+, OpaF+, and OpaI+ strains at 30 min (Fig. 3D). While more CFU of ΔopaBEGK and OpaA+ N. gonorrhoeae strains were recovered at 60 min than with OpaF+ or OpaI+ bacteria, these differences were not statistically significant (Fig. 3E). By 120 min, ΔopaBEGK bacteria were recovered in significantly greater numbers than any of the other Opa+ bacteria (Fig. 3F). Interestingly, the CEACAM-binding profile did not directly correlate with bacterial susceptibility to neutrophils: while the CEACAM1 and CEACAM3 binding OpaDnv strain survived significantly less well than any of its direct comparators, the OpaI+ strain, which also binds CEACAM1 and CEACAM3, survived similarly to the other Opa expressors. Overall, we saw an inverse correlation between association with neutrophils and survival of the bacteria as well as internalization into neutrophils and survival of bacteria (Fig. 3G and H).
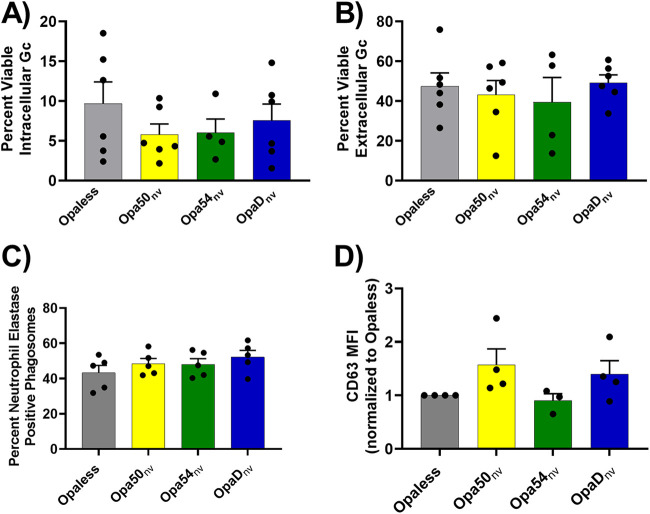
Opa-receptor interactions do not affect the survival or maturity of phagosomes containing Opa± N. gonorrhoeae inside human neutrophils.
Having observed a correlation between bacterial association with neutrophils and their resistance to neutrophil-mediated killing, we examined but ruled out other possibilities that could explain the difference in Opa+ N. gonorrhoeae survival from neutrophils. First, we found that Opa-expressing N. gonorrhoeae, once phagocytosed, exhibited similarly low intracellular survival in neutrophils, as determined based on their permeability to propidium iodide (32). Extracellular N. gonorrhoeae exhibited greater viability than intracellular bacteria, as previously reported (30), but there was no measurable difference in viability based on Opa expression in either the intracellular (Fig. 4A) or the extracellular (Fig. 4B) compartment. Next, we evaluated the maturity of the phagosome in which the phagocytosed N. gonorrhoeae was found based on acquisition of the primary granule protein neutrophil elastase (30). At 60 min, all Opa+ N. gonorrhoeae bacteria, regardless of receptor-binding profile, resided in phagosomes of similar maturity (Fig. 4C), with a trend toward reduced phagosome maturation for Opaless N. gonorrhoeae, as previously reported (P = 0.08) (30). There was also no significant difference in primary granule exocytosis in response to exposure to the different Opa+ N. gonorrhoeae variants, as reported by mean CD63 fluorescence intensity normalized to Opaless using flow cytometry (Fig. 4D).
FIG 4.
Opa expression state does not affect viability of N. gonorrhoeae in the intracellular or extracellular compartments of neutrophils or the release of neutrophil primary granules. (A and B) N. gonorrhoeae was incubated with adherent, IL-8-treated primary human neutrophils for 1 h. Infected neutrophils were exposed to AF647-coupled soybean lectin to recognize extracellular N. gonorrhoeae and then exposed to BacLight LIVE/DEAD viability dyes in the presence of saponin. The percentage of viable (SYTO9+) N. gonorrhoeae in the intracellular (AF647−) (A) and extracellular (AF647+) (B) compartments was quantified for n ≥ 4 biological replicates. There were no statistical differences among strains in either compartment using one-way ANOVA with Tukey’s multiple-comparison test. (C) Adherent, IL-8-treated neutrophils were exposed to CFSE-labeled N. gonorrhoeae for 60 min. Cells were fixed and stained without permeabilization with rabbit anti-N. gonorrhoeae antibody, followed by AF647-coupled goat anti-rabbit IgG, to label extracellular N. gonorrhoeae. Cells were refixed and exposed to mouse antineutrophil elastase IgG followed by Alexa Fluor 555-coupled goat anti-mouse IgG. The percentage of intracellular (CFSE+ AF647−) N. gonorrhoeae in neutrophil elastase-positive phagosomes was quantified. Results are from n ≥ 3 biological replicates. There were no statistically significant differences by one-way ANOVA with Tukey’s multiple-comparison test. (D) Adherent, IL-8-treated neutrophils were exposed to the indicated strains of N. gonorrhoeae for 60 min. Neutrophils were analyzed for the presence of the primary granule protein CD63 on the cell surface by flow cytometry. Data are presented as the mean fluorescence intensity (MFI) of CD63 and expressed relative to Opaless to account for human subject-intrinsic variability in CD63 expression. Results are from n ≥ 3 biological replicates. Shapes indicate individual matched data points from each experiment. There were no statistically significant differences by one-way ANOVA with Tukey’s multiple-comparison test.
Taken together, we conclude that avoiding association is the major route by which N. gonorrhoeae survives exposure to neutrophils. For a given bacterial strain, the N. gonorrhoeae bacteria that are most susceptible to killing by neutrophils express Opa proteins that most strongly increase neutrophil binding and phagocytosis.
Outgrowth of Opa-negative gonococci in a population of Opa-phase-ON bacteria that highly associate with neutrophils.
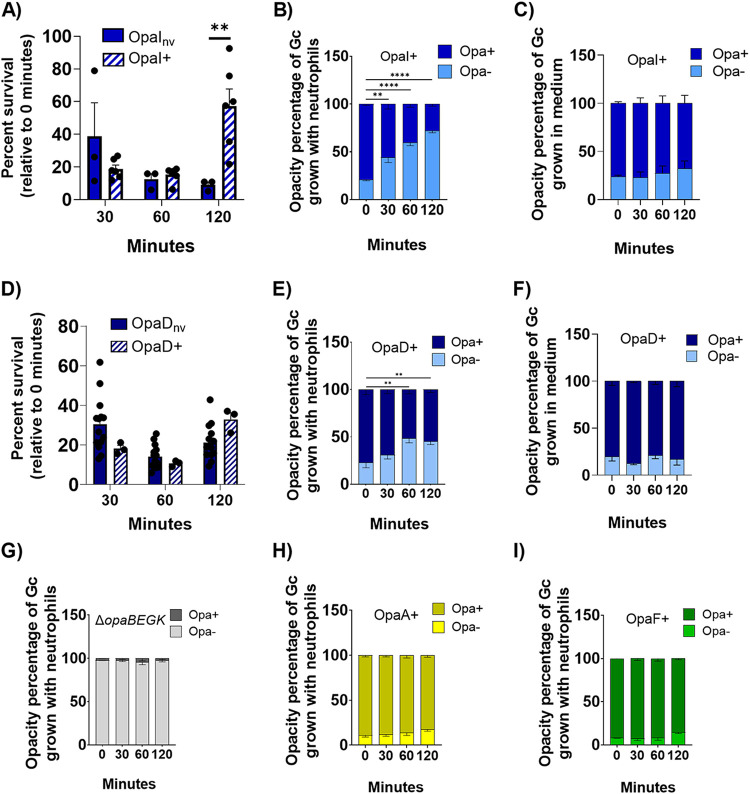
We were surprised to measure an increase in CFU numbers of OpaI+ bacteria recovered from neutrophils at 120 min, relative to 30 min and 60 min (Fig. 3F), given that OpaI binds to CEACAMs 1 and 3 and is readily bound and phagocytosed by neutrophils (Fig. 2C and D). To examine this Opa expressor further, we generated a strain of N. gonorrhoeae with a constitutively expressed, nonvariable opaI in the OpaD locus (OpaInv) (see Materials and Methods for details). While similar CFU counts of OpaInv and OpaI+ N. gonorrhoeae were enumerated at earlier times of infection, at 120 min significantly fewer OpaInv were recovered than OpaI+ bacteria (Fig. 5A). We hypothesized that this discrepancy was due to the phase variability of OpaI in this background. To test this, we quantified the opacity-related morphology of the colonies from the OpaI+ population after exposure to neutrophils and in the medium control (RPMI with 10% fetal bovine serum [FBS]). The percentage of Opa+ colonies in the OpaI+ inoculum (zero minutes) with neutrophils was similar to the percentage in the medium control, as expected (Fig. 5B and C). However, in the presence of neutrophils, the percentage of Opa+ colonies in the population significantly decreased over time, such that by 120 min, 72% of the colonies associated with neutrophils were Opa− (Fig. 5B). In the medium control, 76% of the starting OpaI+ population was Opa+, and this percentage did not significantly change over time (Fig. 5C). Within the OpaI+ population, greater numbers of Opa− (OpaI phase OFF) than Opa-expressing bacteria were recovered over time from neutrophils. This increase in the Opa− bacteria over time resulted in an increase in the overall survival of the OpaI+ population at 120 min of neutrophil exposure (Fig. S3A).
FIG 5.
Neutrophil challenge selects for phase-OFF expression of Opa proteins that drive association of N. gonorrhoeae with neutrophils, increasing overall survival of the population of N. gonorrhoeae. N. gonorrhoeae bacteria that were constitutively expressing (nv) or phase-ON (+) for OpaI (A) or OpaD (D) were exposed to adherent, IL-8-treated primary human neutrophils, and CFU were enumerated from bacterial lysates over time as for Fig. 3. Bacterial survival is expressed relative to the CFU enumerated at time zero. At each time point from panels A and D, opacity phenotype of the enumerated CFU was visually inspected and recorded. Results are reported as the percentage of colonies at the indicated time point that retained Opa expression (dark bars) or were Opa negative (light bars). No other opacity phenotypes other than the indicated OpaI+ (B) and OpaD+ (E) were observed. The same starting cultures of OpaI+ (B) and OpaD+ (E) as above were inoculated into media without neutrophils, and CFU of Opa− and Opa+ phenotypes were enumerated and plotted. No change was seen for with OpaI+ (C) or OpaD+ (F). ΔopaBEGK (G), OpaA+ (H), and OpaF+ (I) N. gonorrhoeae strains were exposed to neutrophils, and the opacity phenotypes of the CFU recovered at each time point were plotted as in panels B and E. Statistical comparisons were by two-way ANOVA with post hoc Tukey multiple-comparison test, with the following pairwise significances: *, P < 0.05, **, P < 0.01, ****, P < 0.0001.
We asked if the same observation would be made for OpaD-expressing bacteria, which also highly associated with neutrophils (Fig. 2A and B) and survived less well from neutrophils than the other Opa-nonvariable bacteria (Fig. 3A to C). To do so, we isolated OpaD phase-ON (OpaD+) bacteria from the ΔopaBEGK background and compared their survival to that of OpaDnv after exposure to neutrophils. OpaD+ trended toward surviving better than OpaDnv from neutrophils but was not statistically significantly different (Fig. 5D). Similar to OpaI+, there was a shift in the OpaD+ population after exposure to neutrophils, with significantly greater numbers of Opa phase-OFF bacteria by 120 min (77% Opa+ in the inoculum versus 55% at 120 min) (Fig. 5E). OpaD+ in the medium control maintained its Opa+ status over time (Fig. 5F). As with OpaI+, the Opa− bacteria outgrew the OpaD+ bacteria, skewing the survival of the OpaD+ population higher than what was seen for OpaDnv (Fig. S3B).
Similar analyses were performed for the parent of the variable strains, ΔopaBEGK strain, and the OpaA+ and OpaF+ expressors. After exposure to neutrophils, >95% of ΔopaBEGK colonies remained Opa− throughout the 120-min infection period (Fig. 5G). While there was a slight increase over time in the proportion of Opa− colonies in the OpaA+ (Fig. 5H) and OpaF+ (Fig. 5I) populations after exposure to neutrophils, this change was not statistically significant. Expression of other Opa proteins in the OpaA+ and OpaF+ backgrounds was not noted, as judged by colony opacity phenotype. There was no significant change in Opa expression state for these bacteria in the medium control, with ΔopaBEGK strain remaining predominantly Opa− and OpaA+ and OpaF+ and mostly Opa+ (Fig. S4A, B, and C). While there tended to be more Opa− than Opa+ colonies in these variable Opa populations 120 min postneutrophil infection, this change did not have a major impact on the overall survival of the ΔopaBEGK, OpaA+, and OpaF+ populations (Fig. S3C to E).
Taken together, these data indicate that the ability to phase vary Opa expression is advantageous for N. gonorrhoeae. A heterogeneous population allows the bacteria to avoid phagocytic killing by neutrophils, specifically in cases where the Opa+ bacteria are rapidly and efficiently phagocytosed.
DISCUSSION
Opa proteins are an important family of adhesins and invasins for N. gonorrhoeae, and most N. gonorrhoeae bacteria isolated from individuals with symptomatic uncomplicated gonorrhea are phenotypically Opa+. However, we and others have found that Opa+ bacteria are more susceptible to killing by neutrophils than those lacking Opa expression. In this work, we investigated how differential interaction with primary human neutrophils affects N. gonorrhoeae survival in an Opa-dependent manner. To do so, we used two panels of isogenic N. gonorrhoeae, one with or without constitutive expression of a single Opa and the other with single phase-varied-ON Opa+ bacteria and defined the receptor-binding profile of the Opa expressors. We found that Opa expression alone does not dictate the survival of N. gonorrhoeae after exposure to adherent, IL-8-treated primary human neutrophils. Instead, survival is impacted by the degree to which Opa expression affects N. gonorrhoeae association with and phagocytosis by neutrophils. In particular, bacteria expressing Opa proteins that do not bind to the granulocyte-specific CEACAM3 were more successful at avoiding phagocytosis and killing by neutrophils. While the phase-variable nature of Opa proteins makes them a less than ideal vaccine target, our results suggest therapeutics that promote the phagocytic killing activities of locally recruited neutrophils would be effective at combating N. gonorrhoeae, regardless of which Opa protein(s) the bacteria in the population expresses.
For this study, we examined six Opa proteins, two that do not interact with neutrophils via CEACAMs (OpaA of FA1090 and Opa50 of MS11), two that interact via CEACAM1 (OpaF of FA1090 and Opa54 of MS11), and two that interact via both CEACAM1 and CEACAM3 (OpaI and OpaD of FA1090), all in the FA1090 strain background. None of the Opa proteins in this study were found to interact with CEACAM6, and CEACAM5, which is bound by OpaD, is not expressed by neutrophils. The use of both phase-variable and nonvariable strains conferred advantages to the analyses in this study. N. gonorrhoeae with nonvariable Opa expression, in an Opa-deleted background (Opaless), allowed for exact control of which Opa protein was expressed on N. gonorrhoeae. Using predominantly expressing Opa+ N. gonorrhoeae in a background with limited Opa variation capacity (ΔopaBEGK) is more similar to bacterial phase variation dynamics in vivo, allowing us to assess the role of Opa phase variability and selection for Opa phenotypes in the context of infection. For N. gonorrhoeae expressing Opa proteins that bind both CEACAM1 and -3, the ability to phase-vary enhanced survival of the Opa phase-OFF bacteria in the population after exposure to neutrophils, increasing the recovery of N. gonorrhoeae in the infection mix over time. Thus, the ability of N. gonorrhoeae to avoid phagocytic killing by neutrophils is affected by both Opa expression status and the specific Opa protein being expressed.
The two Opa expressors in this study that were most readily phagocytosed and killed by neutrophils, OpaD and OpaI of FA1090, engage multiple CEACAMs, including CEACAM1 and CEACAM3 (23). Neutrophils constitutively express CEACAM3 at a relatively low level, regardless of their activation status, while CEACAM1 expression is upregulated with exposure to cytokines (33). The non-CEACAM binding strains could bind to heparan sulfate proteoglycan (HSPGs) to mediate interaction with neutrophils. Opa proteins are also highly positively charged at neutral pH (pI ∼11) and may interact with cell membranes in an ionic but non-receptor-mediated manner. To fully understand the mechanisms by which N. gonorrhoeae expressing OpaD and OpaI strongly associates with human neutrophils will require methods for manipulation of receptor expression in these primary, terminally differentiated cells, which are not currently available. It is also possible that in addition to characteristics of the Opa protein itself, the amount of Opa protein stably expressed on the bacterial surface influences bacterial interactions with neutrophils, although this is a less likely explanation for OpaInv and OpaDnv, where the nonvariable opa allele is under the control of the native opaD promoter. Future studies that investigate a broader array of Opa proteins for their receptor-binding and survival profiles after exposure to neutrophils will help to better understand how the N. gonorrhoeae Opa protein repertoire contributes to infectivity.
We were surprised to see that formation of mature phagosomes, release of granule content by neutrophils in response to N. gonorrhoeae, and, to some extent, release of ROS occurred regardless of which Opa protein was expressed. CEACAM1 is a canonically inhibitory receptor, containing an ITIM motif in its cytosolic domain. Other literature has shown a reduction in cellular activity and proliferation upon CEACAM1 binding (14, 15). In contrast, CEACAM3 contains an ITAM domain and has been shown to be activating (16, 17). Signaling downstream of CEACAMs involves recruitment of kinases leading to p47phox activation and consequent NADPH oxidase assembly (34, 35). In mouse promyelocytes transduced to express human CEACAMs, cross talk between receptors leads to activation of signals downstream of CEACAM3, when the cells are presented with N. gonorrhoeae expressing a CEACAM1-only binding Opa protein (36). Interaction of HSPGs, the secondary receptor of Opa proteins, with β-integrins has been shown to activate neutrophils in a similar manner (37). We anticipate that the similarities in phagosome maturation, degranulation, ROS, and death observed in the current study are due to Syk activation and Src recruitment downstream of CEACAM3 activation, precipitated by either CEACAM1 or CEACAM3 binding. Previous work by Sarantis and Gray-Owen showed that neutrophils were activated to a similar extent in response to either CEACAM1 or CEACAM3 being bound by N. gonorrhoeae (36). Our work is in agreement in that we demonstrate here that primary granules mobilize and are delivered to both the N. gonorrhoeae-containing phagosome as well as the neutrophil membrane. However, our work reports less pronounced differences between the Opa-expressing and Opaless strains than we reported previously (30). While we do not have a direct explanation for this difference, variation in human subjects’ neutrophils or other unidentified features of the infection milieu could contribute.
Compared to the more conserved CEACAM1, human CEACAM3 has evolved relatively recently and is specifically expressed on neutrophils and other granulocytes (38). It has been proposed that CEACAM3 expression is an evolutionary tactic by the human innate immune system to attempt to control infection by pathogens that target CEACAM1. In addition to Neisseria, multiple pathogens are known to express outer membrane proteins that bind to CEACAM1, including Haemophilus influenzae, Moraxella catarrhalis, and Helicobacter pylori, enabling bacterial colonization and survival (39–41). Some viruses also can utilize CEACAM1 as a cellular receptor (42). Similar to N. gonorrhoeae, none of the known species of CEACAM-binding bacteria have an adhesin that binds solely to CEACAM3. Our data and that of others show that N. gonorrhoeae with the ability to bind to CEACAM3 is more likely to be phagocytosed and killed by neutrophils (17, 30, 36, 43–48). Together these observations support a model where CEACAM3 plays an important role in controlling infections by human-targeting pathogens, but, in turn, these pathogens take advantage of recombination, mutation, and phase variation to generate an array of related adhesins, some of which evade binding to CEACAM3. This model is supported by the fact that there is a selection for Opa− bacteria after exposure to human neutrophils when the N. gonorrhoeae inoculum is predominantly expressing phase-variable CEACAM1 or CEACAM3 binding OpaI or OpaD proteins, which is not observed in bacterial populations expressing N. gonorrhoeae that do not engage CEACAM3.
Opa proteins are adhesins not only for human neutrophils, the focus of this work, but also human epithelial cells. In particular, Opa-CEACAM interactions enable successful infection by allowing binding to the epithelial cells at the site of infection as well as preventing shedding of those epithelial cells to drive longer-term colonization (49–51). In contrast, the enhanced phagocytosis of Opa+ N. gonorrhoeae by neutrophils leads to decreased bacterial survival, as we and others have shown. In addition, the predominance of Opa-expressing N. gonorrhoeae varies with the menstrual cycle, which has been attributed in part to sex hormone-based changes in expression of proteases and other innate immune effectors to which Opa+ N. gonorrhoeae is more sensitive (52). Along these lines, we reported that OpaD+ N. gonorrhoeae is more sensitive than Opaless to killing by bactericidal/permeability-increasing protein (30). Hormonal changes may also affect how well N. gonorrhoeae survives inside host cells (53).
Given the competing needs of N. gonorrhoeae to colonize epithelial surfaces yet avoid clearance by soluble and cellular immune effectors, phase variability of Opa proteins is advantageous to N. gonorrhoeae on a population level. In particular, as uncovered in this study, the phagocytic and antimicrobial activities of neutrophils drive selection in the population for N. gonorrhoeae that have phase-varied-OFF expression of CEACAM3-binding Opa proteins because of the enhanced phagocytic killing of the CEACAM3 expressors. However, N. gonorrhoeae expressing Opa proteins that are less rapidly phagocytosed and killed does not experience the same negative selection. These data, along with the understanding that Opa proteins are important for epithelial binding, suggest that the possession of numerous Opa genes, each independently phase variable and with their own receptor-binding properties, allows the N. gonorrhoeae population to constantly test its environment to maximize the ability to colonize while avoiding immune clearance. These results provide one explanation for why human gonorrheal exudates commonly contain Opa+ N. gonorrhoeae when some Opa+ bacteria are more susceptible to phagocytic killing by neutrophils. Since primary Opa sequence does not indicate receptor specificity or selectivity, the dynamics of opa gene recombination, mutation, and phase-variable expression are especially important to adapt to different conditions during infection and together enable the overall persistence of N. gonorrhoeae in its obligate human hosts.
MATERIALS AND METHODS
Bacteria used in this study.
All N. gonorrhoeae strains used in this study are in the FA1090 background, constitutively encoding the pilin variant 1-81-S2 due to a mutation in the G4 sequence upstream of pilE (22, 54). Opaless (ΔopaA-K) and OpaDnv (Opaless with a constitutively expressed, non-phase-variable opaD allele in the opaD locus) strains were described previously (22). OpaInv and Opa50nv were created in a manner similar to that of OpaDnv, with the non-phase-variable genes placed into the opaD locus. Opa50nv was previously described (55). OpaInv was created by transforming into Opaless a synthesized opaI with the OpaDnv nonvariable signal sequence, flanked by ∼500 bp upstream and downstream of the opaD locus (Genewiz). Transformants were selected by their colony opacity and confirmed by sequencing. Opa54nv was created by cloning a constitutively expressed, non-phase-variable version of opa54 from strain MS11 (gift of S. Gray-Owen, University of Toronto) (56) into the pKH35 complementation plasmid (57) and then incorporating the allele between lctP and aspC in Opaless by spot transformation and selection using chloramphenicol (0.5 μg/mL) (23). In Opa54nv, Opa54 expression is induced by growing N. gonorrhoeae in the presence of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). ΔopaBEGK strain, in which the four transparent Opa proteins that do not confer a strongly opaque phenotype on FA1090 were deleted in-frame (opaB, opaE, opaG, and opaK), was previously described (22). Predominantly OpaA+, OpaF+, and OpaI+ expressors in the ΔopaBEGK strain were selected by eye by their colony opacity. For Opa phase-variable bacteria, expression of the single Opa protein of interest was confirmed by Western blotting bacterial lysates with a panel of FA1090 Opa-specific antibodies (a gift from M. Hobbs, University of North Carolina) (58). Western blotting was similarly used to assess the Opa− predominance of the ΔopaBEGK population. The phase-variable opaA, opaF, and opaI sequences were extrapolated from the FA1090 genome sequence using the genomic locations previously reported (22). opaD (22), opa50 (55), and opa54 (23) sequences were published previously and confirmed after introduction into Opaless by DNA sequencing.
Bacterial growth conditions.
N. gonorrhoeae were grown overnight on gonococcal medium base (GCB; Difco) plus Kellogg’s supplements (59) at 37°C with 5% CO2. N. gonorrhoeae was grown in rich liquid medium (GCBL) with Kellogg’s supplements overnight with rotation at 30°C and then back diluted twice and grown with rotation at 37°C as previously described (28). Piliated N. gonorrhoeae was enriched at the final dilution by collecting naturally sedimented bacteria for transfer into fresh medium. Opa54nv was grown in the presence of 1 mM IPTG under all liquid conditions.
CEACAM binding of Opa± N. gonorrhoeae using imaging flow cytometry.
Glutathione S-transferase (GST)-tagged N-terminal domains of human CEACAM1 (N-CEACAM1) and CEACAM3 (N-CEACAM3) were purified as in reference 23. Opa+ N. gonorrhoeae or the ΔopaBEGK parent bacteria (1 × 108 CFU/mL) was incubated with GST-tagged N-CEACAM (N-CEACAM1 and N-CEACAM3) for 30 min at 37°C with end-over-end rotation. N. gonorrhoeae incubated without any N-CEACAM was used as a negative control. N. gonorrhoeae was then washed and stained to detect the presence of CEACAM with anti-GST antibody, as previously described (23). Bacteria were then resuspended in 2% paraformaldehyde with 5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI). Bacteria were processed using the ImageStream X Mk II imaging flow cytometer and analyzed with INSPIRE and IDEAS v. 6.2 software packages (Amnis Luminex Corporation). Cells were gated by singlets, focused cells, DAPI expression, and AF488 expression (23). The binding profiles of Opa50nv, Opa54nv, and OpaDnv were previously reported (23) as a percentage of the bacterial population positive for GST. For each sample, at least 40,000 cells were analyzed.
Neutrophil isolation.
Venous blood was collected from healthy human subjects in accordance with a protocol approved by the University of Virginia Institutional Review Board for Health Sciences Research (protocol number 13909). Neutrophils were isolated via dextran sedimentation followed by a Ficoll gradient as previously described (28). Neutrophils were resuspended in Dulbecco’s phosphate-buffered saline (DPBS; without calcium and magnesium; Thermo Scientific) containing 0.1% dextrose and used within 2 h of isolation.
Neutrophil ROS production.
Neutrophils (2 × 105) were resuspended in Morse’s defined medium (MDM [60]) in the presence of 20 μM luminol. N. gonorrhoeae was added at an MOI of 100. Luminol-dependent chemiluminescence was measured every 3 min for 1 h on a VICTOR3 Wallac luminometer (Perkin-Elmer) as previously described (34). One representative of ≥3 biological replicates is presented. Uninfected, untreated neutrophils were used as a negative control in each experiment (34).
Imaging flow cytometric analysis of bacterial association with and internalization by neutrophils.
N. gonorrhoeae was labeled with Tag-IT Violet proliferation and cell tracking dye (TIV) (BioLegend) in PBS with 5 mM MgSO4 for 15 min at 37°C. Bacteria were then added to neutrophils at an MOI of 1 that were adhered to plastic coverslips in 6-well plates. Neutrophils were suspended in RPMI with 10% fetal bovine serum (FBS) and pretreated with 10 nM human IL-8 (R&D Systems). At the indicated time points, cells were fixed with 4% paraformaldehyde in PBS and removed from the coverslips by gentle scraping as previously described (27). Extracellular bacteria were identified by staining with DyLight 650-conjugated (Thermo Scientific) goat anti-N. gonorrhoeae antibody (Biosource), diluted in PBS containing 10% normal goat serum at a final concentration of 1 μg/mL. Cells were then processed on the ImageStream X Mk II imaging flow cytometer and analyzed with INSPIRE and IDEAS v. 6.2 software packages (Luminex Corporation). Gating was completed as previously described (27). Briefly, focused, single cells were gated for low DL650 and then spot counted for TIV+ N. gonorrhoeae. Results are reported as the percentage of the neutrophil population with at least one bacterium that is associated (TIV+) (bound or internalized).
N. gonorrhoeae survival in the presence of primary human neutrophils.
Neutrophils were treated with IL-8 in RPMI 1640 medium with 10% FBS at 37°C with 5% CO2 and were allowed to adhere to 13-mm-diameter plastic coverslips (Sarstedt) for at least 30 min prior to infection. Mid-logarithmic-phase N. gonorrhoeae was exposed to neutrophils at an MOI of 1 and centrifuged together at 12°C to synchronize infection. At the indicated time points, neutrophils were lysed in 1% saponin, lysates were serially diluted and plated, and CFU were enumerated from lysates after overnight growth (28). Results are reported as the CFU enumerated at the indicated time point, divided by the number of CFU associated with neutrophils at time zero min × 100%.
Determination of intracellular and extracellular bacterial viability.
Adherent, IL-8-treated neutrophils were exposed to N. gonorrhoeae as for the bacterial survival assays, except N. gonorrhoeae was added at MOI of 10. After 60 min, N. gonorrhoeae was incubated for 10 min at room temperature in 0.1 M morpholinepropanesulfonic acid, pH 7.2, plus 1 mM MgCl2 containing 5 μg/mL Alexa Fluor 647-coupled soybean agglutinin (Thermo Fisher) to recognize extracellular bacteria. Cells were then permeabilized with 0.1% saponin, and viable and nonviable N. gonorrhoeae bacteria were detected using the BacLight LIVE/DEAD viability kit (Invitrogen) as previously described (32).
Neutrophil phagosome maturity.
Adherent, IL-8-treated neutrophils were exposed to N. gonorrhoeae at an MOI of 1 as described above, except bacteria were first labeled with carboxyfluorescein succinimidyl ester (CFSE) at 1:1,000 in PBS with 5 mM MgSO4 for 25 min at 37°C. After 60 min, cells were fixed and processed for immunofluorescence microscopy as in reference 61. Cells were blocked in PBS with 10% normal goat serum (NGS) for 10 min at room temperature. Extracellular N. gonorrhoeae was stained using an anti-Neisseria gonorrhoeae antibody, followed by Alexa Fluor 647-coupled goat anti-rabbit IgG (Life Technologies). Cells were then permeabilized in PBS with 10% NGS and 0.2% saponin and stained with an antibody against neutrophil elastase (AHN-10) (Millipore), followed by Alexa Fluor 555-coupled goat anti-mouse IgG (Life Technologies).
Degranulation.
Surface expression of primary granule markers was determined as previously described (62). N. gonorrhoeae was incubated with primary neutrophils adhered to glass slides for 1 h at 37°C. Cells were then lifted for 10 min on ice using 5 mM EDTA and washed twice in DPBS with 0.1% dextrose. They were then stained with phycoerythrin (PE)-CD63 (BioLegend) for 30 min on ice as an indicator of primary granule exocytosis. Isotype controls (PE-IgG1; BioLegend) were stained using the same protocol. Data were acquired using a Cytek Aurora Borealis spectral flow cytometer and analyzed using FCS Express (De Novo Software). The mean fluorescence intensity of each of the samples was normalized to the Opaless sample as a biological negative control.
Fluorescence microscopy.
Images were acquired on a Nikon Eclipse E800 UV-visible fluorescence microscope with Hamamatsu Orca-ER digital camera and analyzed using Nis-Elements (Nikon). At least 5 images were taken for each individual experiment, and ≥50 individual bacteria/phagosomes were counted.
Statistics.
For all experiments except for the chemiluminescence assay, results are depicted as the means ± standard errors for ≥3 independent experiments (different subjects’ neutrophils and different bacterial cultures). Statistics were calculated using GraphPad Prism (version 9.3.1) analysis software. For all experiments, a P value of <0.05 was considered significant. Specific statistical tests are reported for each figure with analysis of variance (ANOVA) used for multiple comparisons for parametric data.
ACKNOWLEDGMENTS
This work was supported by R01 AI097312 and R21 AI157539 (A.K.C.) and NIH R35 GM131829 (L.C.). A.M.A. and L.M.W. were supported in part by T32 AI007046. L.M.W. was supported in part by the Robert R. Wagner Fellowship at the University of Virginia. M.B.D. was supported by F32 GM136076.
We thank Scott Gray-Owen (University of Toronto) for Opa54-expressing MS11, Marcia Hobbs (University of North Carolina, Chapel Hill) for the gift of anti-FA1090 Opa monoclonal antibodies, Asya Smirnov for assistance with imaging flow cytometry, the UVA Flow Cytometry Core Facility for assistance with spectral flow cytometry, former Criss lab member Louise Ball for creation of the OpaInv strain, and members of the Criss lab for feedback and discussion.
Footnotes
Supplemental material is available online only.
Contributor Information
Alison K. Criss, Email: akc2r@virginia.edu.
Conrad W. Mullineaux, Queen Mary University of London
REFERENCES
- 1.Stern A, Brown M, Nickel P, Meyer TF. 1986. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell 47:61–71. 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- 2.Jordan PW, Snyder LAS, Saunders NJ. 2005. Strain-specific differences in Neisseria gonorrhoeae associated with the phase variable gene repertoire. BMC Microbiol 5:21. 10.1186/1471-2180-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. 2021. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 70:1–187. 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Judson FN. 1990. Gonorrhea. Med Clin North Am 74:1353–1366. 10.1016/s0025-7125(16)30485-0. [DOI] [PubMed] [Google Scholar]
- 5.Criss AK, Genco CA, Gray-Owen SD, Jerse AE, Seifert HS. 2021. Challenges and controversies concerning Neisseria gonorrhoeae-neutrophil interactions in pathogenesis. mBio 12:e00721-21. 10.1128/mBio.00721-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer A, Criss AK. 2018. Gonococcal defenses against antimicrobial activities of neutrophils. Trends Microbiol 26:1022–1034. 10.1016/j.tim.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson J, Sparks E, Zeligs B, Siam MA, Parrott C. 1974. Studies on gonococcus infection. V. Observations on in vitro interactions of gonococci and human neutrophils. Infect Immun 10:633–644. 10.1128/iai.10.3.633-644.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. 1997. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J 16:3435–3445. 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bos MP, Kao D, Hogan DM, Grant CCR, Belland RJ. 2002. Carcinoembryonic antigen family receptor recognition by gonococcal Opa proteins requires distinct combinations of hypervariable Opa protein domains. Infect Immun 70:1715–1723. 10.1128/IAI.70.4.1715-1723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuespert K, Pils S, Hauck CR. 2006. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol 18:565–571. 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T, Gotschlich EC. 1996. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA 93:14851–14856. 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virji M, Makepeace K, Ferguson DJ, Watt SM. 1996. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol 22:941–950. 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 13.Popp A, Dehio C, Grunert F, Meyer TF, Gray-Owen SD. 1999. Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell Microbiol 1:169–181. 10.1046/j.1462-5822.1999.00017.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee HSW, Ostrowski MA, Gray-Owen SD. 2008. CEACAM1 dynamics during neisseria gonorrhoeae suppression of CD4+ T lymphocyte activation. J Immunol 180:6827–6835. 10.4049/jimmunol.180.10.6827. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Chen L, Qiao S-W, Nagaishi T, Blumberg RS. 2008. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J Immunol 180:6085–6093. 10.4049/jimmunol.180.9.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitter T, Agerer F, Peterson L, Münzner P, Hauck CR. 2004. Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J Exp Med 199:35–46. 10.1084/jem.20030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCaw SE, Schneider J, Liao EH, Zimmermann W, Gray-Owen SD. 2003. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol Microbiol 49:623–637. 10.1046/j.1365-2958.2003.03591.x. [DOI] [PubMed] [Google Scholar]
- 18.Adrian J, Bonsignore P, Hammer S, Frickey T, Hauck CR. 2019. Adaptation to host-specific bacterial pathogens drives rapid evolution of a human innate immune receptor. Curr Biol 29:616–630. 10.1016/j.cub.2019.01.058. [DOI] [PubMed] [Google Scholar]
- 19.James JF, Swanson J. 1978. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun 19:332–340. 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerse AE, Cohen MS, Drown PM, Whicker LG, Isbey SF, Seifert HS, Cannon JG. 1994. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med 179:911–920. 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sintsova A, Wong H, MacDonald KS, Kaul R, Virji M, Gray-Owen SD. 2015. Selection for a CEACAM receptor-specific binding phenotype during Neisseria gonorrhoeae infection of the human genital tract. Infect Immun 83:1372–1383. 10.1128/IAI.03123-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ball LM, Criss AK. 2013. Constitutively Opa-expressing and Opa-deficient neisseria gonorrhoeae strains differentially stimulate and survive exposure to human neutrophils. J Bacteriol 195:2982–2990. 10.1128/JB.00171-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner LM, Palmer A, Smirnov A, Belcher Dufrisne M, Columbus L, Criss AK. 2020. Imaging flow cytometry analysis of CEACAM binding to Opa-expressing Neisseria gonorrhoeae. Cytometry A 97:1081–1089. 10.1002/cyto.a.24037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen GT, Green ER, Mecsas J. 2017. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol 7:373. 10.3389/fcimb.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey SG, Shafer WM, Spitznagel JK. 1986. Neisseria gonorrhoeae survive intraleukocytic oxygen-independent antimicrobial capacities of anaerobic and aerobic granulocytes in the presence of pyocin lethal for extracellular gonococci. Infect Immun 52:384–389. 10.1128/iai.52.2.384-389.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frangipane JV, Rest RF. 1992. Anaerobic growth of gonococci does not alter their Opa-mediated interactions with human neutrophils. Infect Immun 60:1793–1799. 10.1128/iai.60.5.1793-1799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smirnov A, Solga MD, Lannigan J, Criss AK. 2020. Using imaging flow cytometry to quantify neutrophil phagocytosis. Methods Mol Biol Clifton NJ 2087:127–140. 10.1007/978-1-0716-0154-9_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragland SA, Criss AK. 2019. Protocols to interrogate the interactions between Neisseria gonorrhoeae and primary human neutrophils. Methods Mol Biol Clifton NJ 1997:319–345. 10.1007/978-1-4939-9496-0_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smirnov A, Solga MD, Lannigan J, Criss AK. 2015. An improved method for differentiating cell-bound from internalized particles by imaging flow cytometry. J Immunol Methods 423:60–69. 10.1016/j.jim.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson MB, Ball LM, Daily KP, Martin JN, Columbus L, Criss AK. 2015. Opa+ Neisseria gonorrhoeae exhibits reduced survival in human neutrophils via Src family kinase-mediated bacterial trafficking into mature phagolysosomes. Cell Microbiol 17:648–665. 10.1111/cmi.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Criss AK, Katz BZ, Seifert HS. 2009. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol 11:1074–1087. 10.1111/j.1462-5822.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson MB, Criss AK. 2013. Fluorescence microscopy methods for determining the viability of bacteria in association with mammalian cells. J Vis Exp 79:e50729. 10.3791/50729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stocks SC, Kerr MA. 1993. Neutrophil NCA-160 (CD66) is the major protein carrier of selectin binding carbohydrate groups Lewisx and Sialyl Lewisx. Biochem Biophys Res Commun 195:478–483. 10.1006/bbrc.1993.2068. [DOI] [PubMed] [Google Scholar]
- 34.Smirnov A, Daily KP, Criss AK. 2014. Assembly of NADPH oxidase in human neutrophils is modulated by the opacity-associated protein expression state of Neisseria gonorrhoeae. Infect Immun 82:1036–1044. 10.1128/IAI.00881-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Criss AK, Seifert HS. 2008. Neisseria gonorrhoeae suppresses the oxidative burst of human polymorphonuclear leukoctes. Cell Microbiol 10:2257–2270. 10.1111/j.1462-5822.2008.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarantis H, Gray-Owen SD. 2012. Defining the roles of human carcinoembryonic antigen-related cellular adhesion molecules during neutrophil responses to Neisseria gonorrhoeae. Infect Immun 80:345–358. 10.1128/IAI.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller LA, Hong JJ, Kinch MS, Harrison ML, Geahlen RL. 1999. The engagement of beta1 integrins on promonocytic cells promotes phosphorylation of Syk and formation of a protein complex containing Lyn and beta1 integrin. Eur J Immunol 29:1426–1434. . [DOI] [PubMed] [Google Scholar]
- 38.Bonsignore P, Kuiper JWP, Adrian J, Goob G, Hauck CR. 2020. CEACAM3—a prim(at)e invention for opsonin-independent phagocytosis of bacteria. Front Immunol 10:3160. 10.3389/fimmu.2019.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tchoupa AK, Lichtenegger S, Reidl J, Hauck CR. 2015. Outer membrane protein P1 is the CEACAM-binding adhesin of Haemophilus influenzae. Mol Microbiol 98:440–455. 10.1111/mmi.13134. [DOI] [PubMed] [Google Scholar]
- 40.Conners R, Hill DJ, Borodina E, Agnew C, Daniell SJ, Burton NM, Sessions RB, Clarke AR, Catto LE, Lammie D, Wess T, Brady RL, Virji M. 2008. The Moraxella adhesin UspA1 binds to its human CEACAM1 receptor by a deformable trimeric coiled-coil. EMBO J 27:1779–1789. 10.1038/emboj.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Königer V, Holsten L, Harrison U, Busch B, Loell E, Zhao Q, Bonsor DA, Roth A, Kengmo-Tchoupa A, Smith SI, Mueller S, Sundberg EJ, Zimmermann W, Fischer W, Hauck CR, Haas R. 2017. Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat Microbiol 2:1–12. 10.1038/nmicrobiol.2016.233. [DOI] [PubMed] [Google Scholar]
- 42.Matsuyama S, Taguchi F. 2002. Communication between S1N330 and a region in S2 of murine coronavirus spike protein is important for virus entry into cells expressing CEACAM1b receptor. Virology 295:160–171. 10.1006/viro.2002.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen T, Bolland S, Chen I, Parker J, Pantelic M, Grunert F, Zimmermann W. 2001. The CGM1a (CEACAM3/CD66d)-mediated phagocytic pathway of Neisseria gonorrhoeae expressing opacity proteins is also the pathway to cell death. J Biol Chem 276:17413–17419. 10.1074/jbc.M010609200. [DOI] [PubMed] [Google Scholar]
- 44.Sarantis H, Gray-Owen SD. 2007. The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase-dependent pathway. Cell Microbiol 9:2167–2180. 10.1111/j.1462-5822.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 45.Sintsova A, Sarantis H, Islam EA, Sun CX, Amin M, Chan CHF, Stanners CP, Glogauer M, Gray-Owen SD. 2014. Global analysis of neutrophil responses to Neisseria gonorrhoeae reveals a self-propagating inflammatory program. PLoS Pathog 10:e1004341. 10.1371/journal.ppat.1004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth A, Mattheis C, Muenzner P, Unemo M, Hauck CR. 2013. Innate recognition by neutrophil granulocytes differs between Neisseria gonorrhoeae strains causing local or disseminating infections. Infect Immun 81:2358–2370. 10.1128/IAI.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitter T, Pils S, Weibel S, Agerer F, Peterson L, Buntru A, Kopp K, Hauck CR. 2007. Opa proteins of pathogenic neisseriae initiate Src kinase-dependent or lipid raft-mediated uptake via distinct human carcinoembryonic antigen-related cell adhesion molecule isoforms. Infect Immun 75:4116–4126. 10.1128/IAI.01835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Booth JW, Telio D, Liao EH, McCaw SE, Matsuo T, Grinstein S, Gray-Owen SD. 2003. Phosphatidylinositol 3-kinases in carcinoembryonic antigen-related cellular adhesion molecule-mediated internalization of Neisseria gonorrhoeae. J Biol Chem 278:14037–14045. 10.1074/jbc.M211879200. [DOI] [PubMed] [Google Scholar]
- 49.Muenzner P, Hauck CR. 2020. Neisseria gonorrhoeae blocks epithelial exfoliation by nitric-oxide-mediated metabolic cross talk to promote colonization in mice. Cell Host Microbe 27:793–808. 10.1016/j.chom.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Yu Q, Wang L-C, Di Benigno S, Gray-Owen SD, Stein DC, Song W. 2019. Neisseria gonorrhoeae infects the heterogeneous epithelia of the human cervix using distinct mechanisms. PLoS Pathog 15:e1008136. 10.1371/journal.ppat.1008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muenzner P, Rohde M, Kneitz S, Hauck CR. 2005. CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J Cell Biol 170:825–836. 10.1083/jcb.200412151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cole JG, Fulcher NB, Jerse AE. 2010. Opacity proteins increase Neisseria gonorrhoeae fitness in the female genital tract due to a factor under ovarian control. Infect Immun 78:1629–1641. 10.1128/IAI.00996-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards JL. 2010. Neisseria gonorrhoeae survival during primary human cervical epithelial cell infection requires nitric oxide and is augmented by progesterone. Infect Immun 78:1202–1213. 10.1128/IAI.01085-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cahoon LA, Seifert HS. 2009. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science 325:764–767. 10.1126/science.1175653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin JN, Ball LM, Solomon TL, Dewald AH, Criss AK, Columbus L. 2016. Neisserial Opa protein-CEACAM interactions: competition for receptors as a means of bacterial invasion and pathogenesis. Biochemistry 55:4286–4294. 10.1021/acs.biochem.6b00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kupsch EM, Knepper B, Kuroki T, Heuer I, Meyer TF. 1993. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J 12:641–650. 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramsey ME, Hackett KT, Kotha C, Dillard JP. 2012. New complementation constructs for inducible and constitutive gene expression in Neisseria gonorrhoeae and Neisseria meningitidis. Appl Environ Microbiol 78:3068–3078. 10.1128/AEM.07871-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hobbs MM, Malorny B, Prasad P, Morelli G, Kusecek B, Heckels JE, Cannon JG, Achtman M. 1998. Recombinational reassortment among opa genes from ET-37 complex Neisseria meningitidis isolates of diverse geographical origins. Microbiology 144:157–166. 10.1099/00221287-144-1-157. [DOI] [PubMed] [Google Scholar]
- 59.Kellogg DS, Peacock WL, Deacon WE, Brown L, Pirkle DI. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol 85:1274–1279. 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morse SA, Bartenstein L. 1980. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol 26:13–20. 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- 61.Johnson MB, Criss AK. 2013. Neisseria gonorrhoeae phagosomes delay fusion with primary granules to enhance bacterial survival inside human neutrophils. Cell Microbiol 15:1323–1340. 10.1111/cmi.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ragland SA, Schaub RE, Hackett KT, Dillard JP, Criss AK. 2017. Two lytic transglycosylases in Neisseria gonorrhoeae impart resistance to killing by lysozyme and human neutrophils. Cell Microbiol 19:10.1111/cmi.12662. 10.1111/cmi.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S4. Download jb.00035-22-s0001.pdf, PDF file, 0.7 MB (712KB, pdf)