Abstract
Background:
Hereditary alpha-tryptasemia (HαT) is characterized by elevated basal serum tryptase (BST) due to increased copies of the TPSAB1 gene. Individuals with HαT frequently present with multisystem complaints, including anaphylaxis and seemingly functional gastrointestinal (GI) symptoms.
Objective:
To determine the prevalence of HaT in an IBS cohort and associated immunological characteristics that may distinguish HaT from non-HaT patients.
Methods:
Tryptase genotyping by droplet digital PCR, flow cytometry, cytometry by time-of-flight (CyTOF), Immunohistochemistry, and other molecular biology techniques were employed.
Results:
HaT prevalence in an large IBS cohort was 5% (N=X/158). Immunophenotyping of HαT peripheral blood mononuclear cells (N≥27) revealed increased total and class-switched memory B cells. In the small bowel, expansion of tissue mast cells (MC) with expression of CD203c, HLA-DR and FcεRI, higher intestinal epithelial cell (IEC) pyroptosis, and increased class-switched memory B cells were observed. IgG profiles in sera from individuals with HαT (N=21) significantly differed from quiescent Crohn’s (N=20) and non-HαT controls (N=19), with increased antibodies directed against GI-associated proteins identified in individuals with HaT.
Conclusions:
Increased MC number and IEC pyroptosis in the small intestine, and class-switched memory B cells in both the gut and peripheral blood associated with IgG reactive to GI-related proteins distinguish HaT from functional GI disease. These innate and adaptive immunologic findings identified in association with HαT are suggestive of subclinical intestinal inflammation in symptomatic individuals.
Keywords: Mast cells, pyroptosis, mast cell activation, hereditary alpha-tryptasemia, small intestine, memory B cells, CyTOF, autoantibodies, DN T cells
Capsule Summary
Individuals with HαT have increased numbers of activated mast cells in the small intestine, accelerated intestinal epithelial cell pyroptosis, expanded class-switched memory B cells, and increased GI-associated antigen-directed antibody production.
INTRODUCTION
Hereditary alpha-tryptasemia (HαT) is an autosomal dominant genetic trait characterized by elevated basal serum tryptase (BST) resulting from increased copy number of the TPSAB1 gene encoding alpha-tryptase [1]. Several studies in the U.S. and Europe have demonstrated that this is common, present in 4-6% of the general Caucasian population, affecting an estimated 16 million people in the U.S. Increased TPSAB1 gene copy number and elevated BST are associated with multisystem complaints, including cutaneous flushing and pruritis [2], anaphylaxis [3, 4], and ostensibly functional gastrointestinal (GI) symptoms [1, 4-8]. A gene-dosage effect has been reported in otherwise healthy adults [1] as well as in individuals with systemic mastocytosis (SM) [4], whereby increased TPSAB1 copy number is associated with higher BST and more mast cell mediator-associated symptoms. HαT has also been shown to be 2-3 times more common among individuals with SM, where this trait not only augments MC mediator-associated symptoms but is also associated with increased anaphylaxis severity. However, in the absence of clonal mast cell disease, increased MC have also been observed in the bone marrow (BM) of individuals with HαT [5] suggesting that MC homeostasis may be affected by α-tryptase overexpression. Additionally, HαT modifies non-clonal mast cell disorders where it is also associated with more severe anaphylaxis, but the prevalence of HαT in GI disorders, such as IBS, where mast cells have been implicated is currently unknown. Likewise, whether mast cells are generally increased in tissues other than bone marrow, such as in the GI mucosa, and if increased how this may contribute to associated GI symptoms, has not been determined.
To date, the GI manifestations reported in association with HαT have largely been consistent with IBS-like presentations, based upon ROME III criteria [9, 10]. However, there are a number of observations that raise the possibility that HαT-associated GI symptoms may represent a distinct uncharacterized entity. Mast cells have been implicated in certain individuals with functional GI symptoms [1, 4-6, 8, 11], but this finding has been inconsistent, raising the possibility that increased mast cells in the gut of a subset of these individuals may be a finding associated with HαT, and potentially represent a specific endotype of IBS, or a distinct entity. Moreover, given that HαT is associated with increased systemic mast cell activation in the form of anaphylaxis [3, 4], it is possible that local activation of mast cells may contribute to GI symptoms in a similar manner, particularly if the number of mast cells in the gut is increased.
To better understand the basis for GI manifestations frequently reported by individuals with HαT, we first sought to determine the frequency of this trait among a large cohort of IBS patients. Together with subsequent immunophenotyping of peripheral blood, small intestinal tissue resident cells, and examination of markers indicative of innate and adaptive immunologic responses, we have identified unique features – including evidence of sub-clinical inflammation and breaches in the epithelial integrity resulting from inflammatory cell death (pyroptosis) – present among individuals with HαT. Collectively, these findings demonstrate that GI complaints associated with HαT are related to distinct immunologic findings that distinguish these individuals from those with functional GI disorders.
METHODS
Study participants
Informed consent was provided by all study participants on respective IRB-apGI sproved research protocols at NIAID (NCT01164241, NCT00852943), UF (IRB 201702274), UMMC (IRB 2019-0082), and Boston Children’s Hospital (BCH) Proband Study (IRB P00000529). From the approximately 500 or more individuals with HαT followed at these institutions, 21 were identified who had undergone scope with biopsy, had no other confounding diagnosis and no evidence of pathology on H&E that would have otherwise explained their clinical GI symptoms. As part of a validation effort, an additional thirty-five patients also had immunophenotyping of peripheral blood samples. As disease controls, patients with quiescent Crohn’s disease (CD) – defined as any subject with a previous CD diagnosis, who had no evidence of active inflammation on ileal biopsy at the time of the study [12] – who did not have HαT, provided informed consent and were enrolled on the same IRB-approved protocols.
Clinical and histopathological information were collected from the respective electronic medical record systems at UF, UMMC, or the NIH in accordance with approved human participant protocols. Patients’ symptoms were evaluated using the ROME III criteria (note: at time of study ROME IV had not been released) for IBS, [13] defined as those with at least 12 weeks of abdominal discomfort or pain, which need not be consecutive, in the preceding 12 months with at least 2 of following 3 features: 1. Relieved with defecation, 2. Onset associated with a change in stool frequency, or 3. Onset associated with a change in stool form (appearance) [9, 10]. Further, we evaluated the presence of HαT among Caucasians from a large well-characterized IBS cohort to determine the prevalence of this genetic trait in IBS patients. Supplementary Table 1 describes the GI clinical symptoms and targeted laboratory findings for the HαT participants whose intestinal tissues were used in this study.
Tryptase genotyping
Droplet digital PCR was performed as described [8] in order to determine tryptase genotypes of all study participants on a research basis at NIH (JJL) or using a clinical laboratory (Gene by Gene, Houston, TX).
Flow cytometry
Flow cytometry was performed in a CLIA-certified laboratory as described [14]. Briefly, EDTA anticoagulated PBMCs were isolated from whole blood using Ficoll (Ficoll-Paque PLUS; GE Healthcare, Pittsburgh, PA) gradient centrifugation. PBMCs were stained with fluorescent antibodies (anti-CD3, anti- CD4, anti-CD8, anti-CD45RA, anti-CD62L, anti-CD20, anti-CD19, anti-IgM, anti-CD27, anti-CD16 and anti-CD56). Events were captured using a FACSCanto II (Becton Dickinson, San Jose, CA), and analyzed using FCS Express software (De Novo, Glendale, CA).
Total basal serum tryptase quantification
Total basal serum tryptases (BST) were measured using a commercially available ImmunoCAP assay (Thermo Fisher Scientific, Phadia AB, Uppsala, Sweden) and performed in a CLIA-certified laboratory (Mayo Clinic, Rochester, NY).
Isolation of lamina propria mononuclear cells and CyTOF
Lamina propia mononuclear cells (LPMCs) were isolated from endoscopic biopsies from 3 patients with quiescent Crohn’s disease and 4 patients with HαT [12]. Samples were collected in 90% FBS with 10% DMSO and slow frozen as described [15]. Thawed ileal tissue samples were digested overnight on a shaker at 37° C in complete RPMI media with 2 μL of collagenase and 2 μL of DNase per 10 mL media. Undigested material was filtered out using a 100 μM filter. Single cells were resuspended in CyTOF staining buffer and counted. 1 x 106 cells/sample were prepared for CyTOF according to the Fluidigm protocol [16-18] with minor modifications. Cells were stained with Rh103 for viability, washed, blocked with Fc-Block and incubated with the cocktail of metal-coupled surface antibody for 30 min. Cells were then fixed in 1.6% formaldehyde and stained with Ir-DNA intercalator solution. Cells were resuspended in water containing 1:10 dilution of EQ beads and run on a Helios CyTOF machine (Fluidigm) at the HMS CyTOF Core. All antibodies were obtained from either Fluidigm directly conjugated or from the Harvard CyTOF core. Data were analyzed using premium CyTOBANK cloud-based software. Statistical analysis was performed using GraphPad Prism 7 software (GraphPad Software, San Diego, CA). See Supplemental Table 2 for antibody and metal list.
Immunohistochemistry
CD117 staining was performed in accordance as part of the standard of care by the hospital surgical pathology lab at the University of Florida. The mast cell numbers were counted by the staff GI pathologist in 10 high power fields per patient and each patient’s tissue was cut with at least 5 sections. The numbers were recorded in the patient’s medical record as part of their surgical pathology report.
The Molecular Pathology Core at the University of Florida carried out the heat-induced epitope retrieval method described below. Briefly, 4 μm sections were de-paraffinized, and were treated by Citra Steam (Biogenex #HK086-9K) for 30 minutes. Background Sniper (Biocare Medical #BS966M) were used for 15 minutes to reduce unspecific background staining. Sections were incubated with Mouse anti-human FcεRI (1:25, Abcam #ab54411) O/N 4°C. Stain was visualized using Mach 2 Mouse HRP polymer (Biocare Medical #MHRP520L) and the DAB chromagen (Vector Laboratories #SK-4105) and CAT hematoxylin counterstain (Biocare Medical #CATHE-M). The number of FcεRI positive cells was counted by 2 independent pathologists. They each counted 5 per High Power Field (HPF).
To detect HLA-DR expression level, the antibody from Abcam (#20181) was used. Sections were deparaffinized and were treated by Citra Steam (Biogenex #HK086-9K) for 30 minutes. Background Sniper (Biocare Medical #BS966M) was used for 15 minutes to reduce unspecific background staining. Sections were incubated with Mouse anti-human HLA-DR (1:3000) for 60 minutes followed by incubation with Mach 2 Mouse HRP polymer (Biocare Medical #MHRP520L) for 30 minutes, the DAB chromagen (Vector Laboratories #SK-4105) and CAT hematoxylin counterstain (Biocare Medical #CATHE-M). Images were obtained under 20X magnification with a Leica DM5500 B microscope system (Leica Microsystems Inc., Buffalo Grove, IL) running LAS X acquisition software (version 3.3.3). The optical density of HLA-DR was analyzed using Fiji Image J [19].
Quantification of IEC pyroptosis
Terminal ileal biopsies were collected from disease controls and individuals with HαT during colonoscopies and were sectioned at 5 μM. Disease controls were defined as subjects without history of atopy who underwent a lower endoscopy for other clinical indications. The slides were stained using Maximus Biological Assay staining kits (Maximus Diagnostics LLC, Little Rock, AR) for activated caspase and anti-CD3. Samples with a minimum of 10 intact villi per patient were analyzed for quantitation of ileal intestinal epithelial cell (IEC) pyroptosis by a gastrointestinal pathologist who was blinded to the patient’s disease status. Total number of IECs and IECs positive for activated caspase-1 were manually counted in 10 villi for the quantification. The stained slides were imaged with a Zeiss LSM 880 confocal microscope equipped with Airyscan (Zeiss USA, Dublin, CA).
Antibody Determination
Plasma samples from 19 non-HαT controls (37% African American and 63% Caucasian), 21 HαT individuals (100% Caucasian), and 20 Crohn’s patients (40% African American, 55% Caucasian, and 5% Asian) were analyzed using a custom antigen microarray by GeneCopoeia (Rockville, MD). The 121 IgG and IgM specific self-antigens that were analyzed are listed in Supplemental Figure 3. The appropriate controls were performed. The methods described here were provided by GeneCopoeia. A 16-well slide gasket was applied to one side of a PA001 slide. Blocking buffer (100 μL) was added to each array well and incubated at room temperature for 30 minutes. Following incubation, the slide was washed 2X with PBST (100 μL for each well) for 5 minutes. PBST (90 μL) was added to each plasma sample or control mix and then added to each well of the slide (100 μL for each well). The samples were incubated at room temperature for 1 hour and washed with PBST (100 μL for each well) for 5 min. After washing with PBST, the samples were washed with the blocking buffer for 5 minutes and again with PBST for 5 minutes. The plasma samples were incubated with 100 μL of an anti-human IgG and anti-human IgM secondary antibody at 1:1000 in PBST. After incubation at room temp for 1 h the samples were washed 3X with PBST for 5 minutes and washed 2X with PBS for 5 minutes. The samples were then washed with nuclease-free water 2X for 5 minutes. The slide was scanned with GenePix 4000B microarray systems. A 532 nm channel was used to scan Cy3 fluorescence and a 635 nm channel to scan AlexaFluor-647 fluorescence. The fluorescent signals from the array were analyzed using GenePix Pro v7.0 software to obtain raw data, including foreground signals, background signals, SNR (signal-to-noise ratio), and Flags (bad data identified by the software). Next, the net fluorescence intensity (NFI) value was calculated NFI = foreground median - background median. The SNR and flags were filtered out. The net fluorescence value NFINoPBS was set after subtracting the value of PBS control and then normalized using Robust-Linear-Model (RLM) [20].
Principal Component Analysis (PCA) of the log transformed RLM [20] normalized NFI was performed using the prcomp command in R (version 3.6.3). The difference in the clustering of the groups based on antibody expression was tested using a linear model with generalized least squares (gls) (R nlme package version 3.1) of the form: PC ~ group + race + ε, where PC is the first or second principal component, group is HαT, non-HαT, or quiescent CD and race of the patient. The first two components were tested because they explain most of the variance between the samples [21].
Statistical analysis
Baseline demographics and clinical features were collected and included, respectively: age, sex, and ethnicity; clinical symptoms, autoimmune disorders, and laboratory findings. Continuous variables were described using means with standard deviations or medians with interquartile range; categorical variables were expressed as numbers and proportions.
The comparisons are based on student t test and nonparametric tests (i.e., Wilcoxon signed rank test, Mann-Whitney t test, and Pearson correlation coefficient), with p-values less than 0.05 considered to be significant. Statistical analysis was performed using GraphPad Prism 7 software.
RESULTS
Prevalence of HαT within an IBS cohort
In the initial cohort that defined HαT, 49 percent of individuals met criteria for IBS [1] by ROME III. According to ROME III, the patient must have at least 12 weeks of abdominal discomfort or pain, which need not be consecutive, in the preceding 12 months with at least 2 of following 3 features: 1) relieved with defecation; 2) onset associated with a change in stool frequency; or 3) onset associated with a change in stool form (appearance) [9]. These patients were referred for elevated serum tryptase or symptoms associated with mast cell activation. The prevalence of HαT among individuals with IBS had not been determined. To determine the validity of this association, a large well-characterized IBS cohort was genotyped. We found that only 5% of Caucasians (8/158) from this IBS cohort had HαT, a prevalence consistent with that of other general Caucasian populations [1, 3, 4, 6] . This observation strongly argues against HαT as a genetic driver of classical IBS and suggests that the abdominal symptoms associated with HαT may therefore be distinct from IBS.
Immunophenotypic and histopathological findings associated with HαT
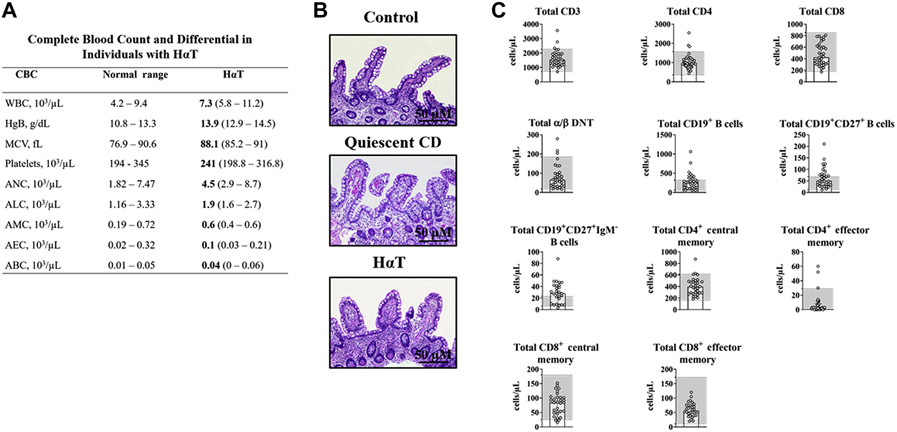
As shown in Figure 1A, individuals with HαT have a normal complete blood count and differential. Furthermore, standard histology obtained at the time of colonoscopy from the terminal ileum of an individual with HαT and stained with hematoxylin and eosin (H&E) was normal. For comparison, representative images of the terminal ileum from a non-HαT control or an individual with quiescent Crohn’s disease are shown (Figure 1B). There is no active inflammation seen on these three images. Evaluation of peripheral T and B cell subsets revealed normal T cell populations but expansion of both memory and class-switched memory B cells in 48% (N=13/27) of individuals with HαT (Figure 1C).
Figure 1. Complete blood count, peripheral T and B cell immunophenotype, and small bowel histology in HαT cohort.
Panel A. Complete blood count (CBC) with differential for individuals with HαT (n=20). Panel B. Absence of apoptosis or inflammatory infiltrates on representative hematoxylin and eosin (H&E)-stained terminal ileum section from control individuals (n=10) (top panel), quiescent Crohn’s disease (n=3) (center panel), and HαT (n=21) (bottom panel). Controls were individuals that had a right hemicolectomy due to cancer or diverticulitis and had a normal ileal margin. Images were obtained under 100x magnification. Scale bar = 50 μM. Panel C. Peripheral T and B cell subsets were evaluated in human peripheral blood from 27 to 35 individuals with HαT using flow cytometry. Gray boxes demonstrate the normal reference range among healthy individuals for this clinical laboratory test.
Intestinal MC from individuals with HαT are increased and correlate with elevated BST and expression of activation markers
We next examined biopsy specimens from a cohort of 21 symptomatic individuals with HαT who had GI symptoms and required endoscopy. The characteristics of these participants are described in Supplemental Table 1. Like those described in the original HαT cohorts [1, 6], these patients reported abdominal pain (90%), diarrhea (61%), constipation (42%), food sensitivity (23%), gastroesophageal reflux disease (GERD) (19%), and/or had objective evidence of GI dysmotility (14%). Moreover, more than half of this cohort had evidence of an overlapping autoimmune disorder (57%). Comparable to what was described in the NIH index cohort, the average basal serum tryptase (BST) was 14 ng/mL.
Similar to what has been reported in SM, BST levels in affected individuals with HαT positively correlated with the number of ileal mast cells per high power field (Pearson correlation coefficient = 0.1979, P = 0.0433) (Figure 2A). Individual tryptase levels, tryptase genotypes and mast cell numbers are listed in Supplemental Table 4. Further characterization of mast cells in the terminal ileum of HαT by CyTOF revealed that they were positive for CD203c, HLA-DR, and confirmed expression of FcεRI (Figure 2B).
Figure 2. Small intestinal mast cell number correlates with elevated basal serum tryptase and express markers for activation and antigen presentation in individuals with HαT.
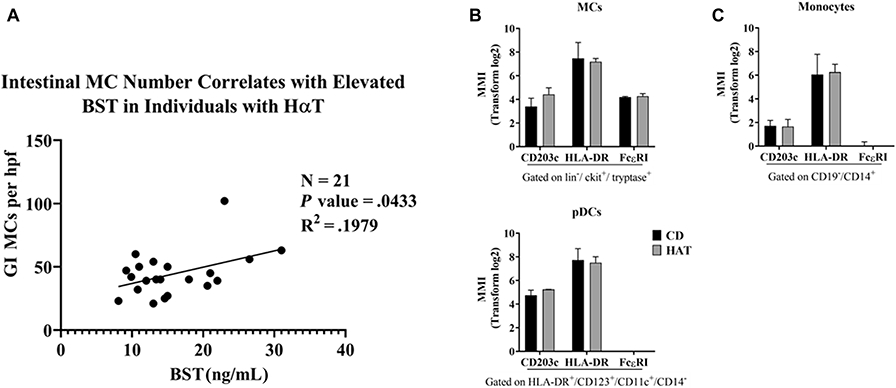
Panel A. Ileal mucosal mast cell (MC) counts by CD117 are significantly correlated (p ≤ 0.05) to the basal serum tryptase levels (BST) in individuals with HαT (n=21). Pearson correlation coefficient = 0.1979. Panel B. The mean metal intensity (MMI) of CD203c, HLA-DR, and FcεRI is shown in the terminal ileum of individuals with HαT compared to patients with quiescent Crohn’s disease (CD), n=3. Summary data shown as mean ± SD.
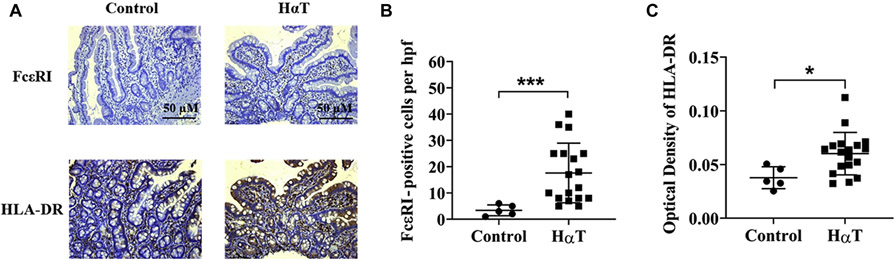
To corroborate our CyTOF findings in a larger HαT cohort (N=18), we evaluated the expression of FcεRI and HLA-DR in the duodenum by IHC. Mast cell number in the small intestine (duodenum or ileum) identified as CD117+ cells, averaged 40 per HPF, based upon five randomly examined fields [1, 5, 22]. In our small subset, we found that the ileum and duodenum had similar results. These results could be corroborated in a clinical pathology laboratory performing immunohistochemistry. There were less than 15 mast cells per HPF for the quiescent CD controls. FcεRI and HLA-DR expression was also increased in HαT samples compared with quiescent CD controls (Figure 3). Increased mast cell FcεRI expression has previously been reported in the foregut of atopic individuals [23], and increased HLA-DR expression – particularly in the gut epithelium – in these sections suggests innate and/or adaptive immune activation [24-26].
Figure 3. Increased FcεRI and HLA-DR in the duodenum from individuals with HαT.
Panel A. Immunohistochemical staining of FcεRI (top panel) and HLA-DR (bottom panel) from intestinal biopsies of the duodenum from a non-HαT control and HαT individual. Scale bar = 50 μM. Panel B. FcεRI positive cells per high power field. Non-HαT control, n=5 and HαT, n=18. Panel C. Optical density of HLA-DR based on IHC using Fiji ImageJ. Non-HαT control, n=5 and HαT, n=18. Summary data shown as mean ± SD. with p values determined by Mann-Whitney test. *p<0.05, ***p<0.001. Note: See mast cell enumeration in Figure 2B.
Increased IEC pyroptosis of the small intestine and distinct immunophenotypic findings in the terminal ileum of individuals with HαT
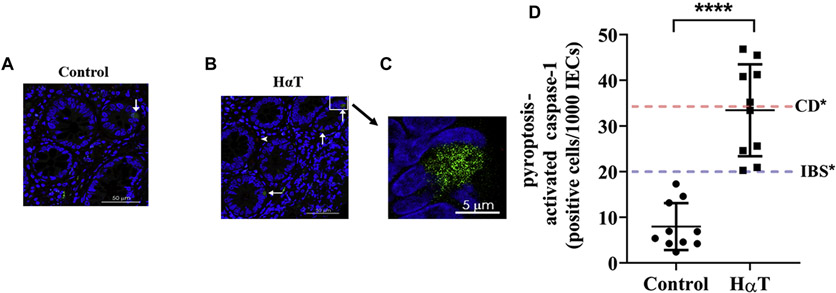
Given our findings of symptoms and signs (diarrhea, food sensitivities) suggestive of increase epithelial cell pyroptosis – a finding associated with gut barrier dysfunction [27-29], elevated mast cell numbers, and elevated HLA-DR in the small intestine, we set-out to determine whether innate and/or adaptive immune activation could be ongoing. We first examined innate immune activation by determining caspase-1 levels in ileal sections as a surrogate of pyroptosis (Figure 4A-C) [30]. There was a marked elevation of caspase-1 positive cells in HαT tissue sections (34 caspase-1 positive cells/1000 IECs) compared to sections from control individuals (6 positive cells/1000 IECs). This level of pyroptosis is moderately increased relative to published levels in IBS and comparable to those reported in Crohn’s disease (Figure 4D) [28].
Figure 4. Increased ileal IEC pyroptosis in individuals with HαT compared to controls.
Representative images of terminal ileum biopsies from control individuals (Panel A) and those with HαT (Panel B) demonstrating activated caspase-1 (green), a marker for pyroptosis. Panel C. The boxed area in B is shown at higher magnification. Cell nuclei are stained with DAPI (blue); white arrows indicate positive intestinal epithelial cells; white arrow head indicates an intra-epithelial T lymphocyte stained with anti-CD3 (red). Panel D. Number of cells positive for activated caspase-1 per 1000 intestinal epithelial cells (IECs) in control versus HαT individuals (n=10). *Blue and red lines indicate levels reported in IBS (20±7) and Crohn’s disease (35±6) [Liu et al., 2016]. Summary data shown as mean ± SD with p values determined by Mann-Whitney test. ****P < 0.0001.
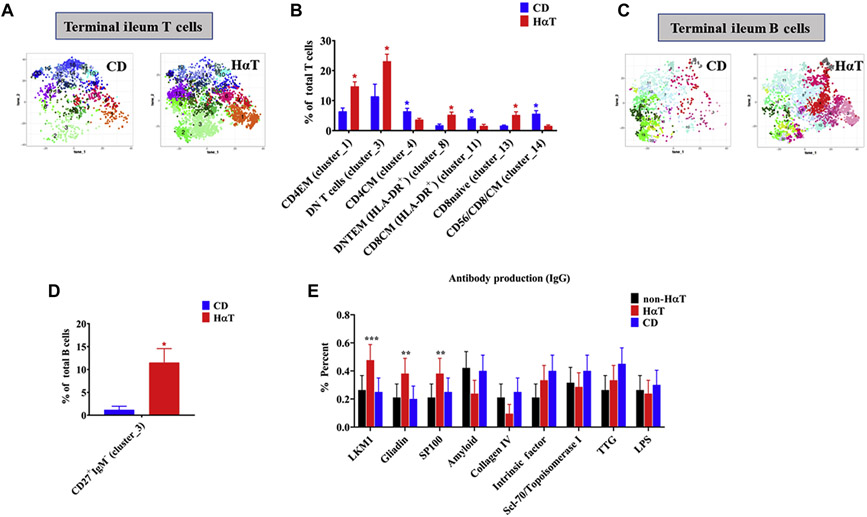
In order to examine immune cells in the gut in greater detail, samples from individuals with HαT and quiescent Crohn’s disease (CD) were extensively immunophenotyped using CyTOF (Figure 5A-D). Increased CD4 effector memory and double negative (DN) T cells were seen in HαT samples compared to quiescent CD samples (Figure 5A and B) (Supplemental Figure 1). Moreover, similar to the immunophenotyping result in peripheral blood, class-switched memory (CD27+IgM−) B cells were increased in the ileum of all individuals with HαT that were examined (Figure 5C and D) (Supplemental Figure 2). CyTOF results in quiescent CD samples was consistent with previously published data [31].
Figure 5. Terminal ileum T cells and B cells phenotype differs between HαT and quiescent CD.
Panel A. tSNE plots of automated clustering by FlowSOM for leukocytes in control (quiescent CD) and individuals with HαT. See Supplemental Figure 1A for enlarged picture of clusters. Panel B. Comparison of percent of total CD3 T cells between quiescent CD patients (n=3) and individuals with HαT (n=4). Summary data shown as mean ± SEM. with p values determined by student t test. *p<0.05. Panel C. tSNE plots of automated clustering by FlowSOM for leukocytes in control (quiescent CD) and individuals with HαT. Memory B cells (clusters: 2-6, 9 and 13), IgM− B cells (clusters: 14-19). See Supplemental Figure 2A for enlarged picture of clusters. Panel D. Comparison of percent of total CD27+IgM− memory B cells between quiescent CD patients (n=3) and individuals with HαT (n=4). Summary data shown as mean ± SEM. with p values determined by student t test. *p<0.05. Panel E. Antibody production (IgG) in individuals with HαT (n=21), CD (n=20), and non-HαT controls (n=19) and the GI significance. Summary data shown as mean ± SEM with p values determined by one sample t test. **p<0.01 and ***p<0.001.
A distinct antibody profile with increased GI tract-associated protein directed IgG in the peripheral blood of individuals with HαT
Because increased class-switched memory B cells were present in both peripheral blood and tissue of individuals with HαT, we sought to identify a potential functional consequence. Serum from 19 non-HαT controls who had undergone screening colonoscopy and were found to have no evidence of IBD, 21 individuals with HαT, and 20 individuals with quiescent Crohn’s disease (CD) were examined for the presence of antibodies to 121 self and disease associated antigens (See Supplemental Figure 3B). Whereas no differences in IgM against these antigens was observed between the 3 groups (Supplemental Figure 3A, Top Panel), the IgG profile among individuals with HαT were distinguished from both non-HαT controls and quiescent CD based upon PCA (HαT vs. CD, p=0.02; HαT vs. non HαT, p=0.03; CD vs. non HαT, p=0.86) (Supplemental Figure 3A, Bottom Panel). Race and ethnicity were accounted for in the analysis, and removing these variables had no effect on the result. Examination of differences in prevalence of IgG towards specific antigens revealed that GI tract-related antibody expression was increased in sera from individuals with HαT relative to the other groups, and included: LKM1, gliadin, and SP100 (Figure 5E). Of these, LKM1, an autoantibody associated with autoimmune hepatitis, was found to be most increased in HαT sera (48%) compared to CD (25%) or (26%) non-HαT controls (Figure 5E and Supplemental Table 3).
Summary of Significant Immunological Findings between Individuals with HαT, IBS, Crohn’s Disease and Non-IBD in the Small Bowel Lamina Propria
Table 1 provides a comparison of the data presented above with published immunologic findings from healthy individuals compared to those observed in HαT, IBS, and both active and quiescent Crohn’s disease [22, 32-36]. In summary, mast cells in the small bowel of individuals with HαT are increased similar to that seen in Crohn’s disease. DN T cells and class switch memory B cells are expanded in HαT similar to Crohn’s disease.
Table 1.
Comparison of Significant Immunological Findings between Individuals with HαT, IBS, Crohn’s Disease and Non-IBD controls in the Small Bowel Lamina Propria
| Normal | IBS | Quiescent Crohn’s Disease |
Active Crohn’s Disease |
HαT | |
|---|---|---|---|---|---|
| Mast Cells | ≤ 15.0/HPF [22] | ≤25/HPF [32] | ≤12.0/HPF [33] | ≥55/HPF [34] | ≥40/HPF*≥30.0/HPF [22] |
| Double Negative (DN) T Cells | 1 – 5% of total T cells [35] | Data not available for this condition | 11.44% of total T cells * | 4-8% of total T cells [35] | 23.1% of total T cells * |
| CD27+ IgM− Memory B Cells | 0.4 – 2% of total B cells (In range with [36]) | Data not available for this condition | 1.18% of total B cells (In range with [36]) | No differences between Quiescent and Active CD [36] | 11.51% of total B cells * |
These data are original to this manuscript.
DISCUSSION
The current study describes several distinguishing immunologic characteristics present in the peripheral blood and gut of individuals with HαT. Increased numbers of mast cells – which has also been described in the bone marrow of individuals with HαT [5] – was observed in small intestine, and like systemic mastocytosis [3], positively correlated with total serum tryptase levels, as well as markers suggestive of mast cell activation. Increased pyroptosis of intestinal epithelial cells shown to result in reduced expression of epithelial cell-to-cell adhesion transcripts and in barrier dysfunction in vitro as demonstrated using transepithelial electrical resistance (TEER) measured in Ussing chambers [27, 29]. In HαT patients, pyroptosis was increased to a level comparable to quiescent inflammatory bowel disease (IBD) [27-29, 37], which may result from local innate immune activation. These observations were in turn associated with expansion of effector memory T cells as well as class-switched memory B cells, the latter of which were increased in both the gut and periphery. The class-switched memory B cell findings in HαT were distinctly different from those in clonal mast cell disease or systemic mastocytosis where they were previously noted to be decreased [14]. Moreover, an associated distinct profile of plasma IgG production directed towards GI-associated antigens was observed compared to healthy and disease controls and is suggestive of gut barrier impairment. Finally, despite reports that nearly half of individuals with HαT meet the ROME III diagnostic criteria for IBS, the prevalence of HαT in a large well-characterized IBS cohort was comparable to the general population. Taken together these clinical and epidemiologic data indicate that GI symptoms and their underlying immunopathology are distinct from both IBS and IBD, with evidence of sub-clinical inflammation placing HαT-associated symptoms somewhere between the two on the spectrum of GI disorders (Table 1 and Supplemental Table 1).
Whether HαT is the immediate cause for the GI symptoms and immunologic features observed in symptomatic individuals, or this genetic trait modifies some other environmental insult or genetic predisposition, remains unknown. However, findings from studies in multiple cohorts of individuals in the U.S. and Europe have demonstrated HαT as a major modifier of clonal and non-clonal mast cell disorders [3, 4]. One hypothesis to explain potential gut barrier abnormalities associated with HαT is based upon observations that PAR-2 is selectively activated by α-tryptase containing heterotetrameric mature tryptases that are increased in mast cells from individuals with HαT [2]. Activation of PAR-2 on gut epithelium has been demonstrated to increase gut permeability and may be a contributing mechanism for the gut barrier impairment suggested by our data [38].
The observed increase in class II MHC expression in gut epithelia and small intestinal MCs from individuals with HαT is intriguing, particularly in the context of increased class-switched memory B cells in the same tissue and peripheral blood, and increased GI-associated antigen directed IgG levels seen in individuals with HαT. Whether MCs may be acting as antigen presenting cells locally in tissue, as has been described [23], remains an area of ongoing investigation but would be one possible explanation for these findings.
The GI-associated antigen directed IgG may not necessarily be pathogenic. Indeed, none of the individuals in this study had canonical autoimmune hepatitis or primary biliary cholangitis – though some had previously abnormal liver studies with non-diagnostic work-ups – and celiac disease had been previously excluded by commercial clinical ELISA and duodenal biopsies. However, IgG to gliadin has been reported in association with connective tissue disorders and non-celiac gluten sensitivity [39, 40] and has been proposed as a biomarker for gut barrier failure [41]. Particularly when viewed in association with the identified IEC pyroptosis, these data are suggestive of barrier dysfunction and raise the question as to whether the increased number of mast cells bearing markers suggestive of ongoing activation – which are distinguished here from evidence of degranulation that was not observed – are the result, or cause, for barrier impairment. Certainly, mast cells are recruited to sites of inflammation and have been demonstrated as capable of contributing to gut permeability. Whether gut mast cell numbers in asymptomatic individuals with HαT are similarly increased remains unknown, but our data indicate that at least among symptomatic individuals there are increased mast cells in association with evidence of increased activation and impaired function of adjacent gut epithelia.
In conclusion, findings associated with what has previously been reported as functional GI symptoms associated with HαT, demonstrate evidence of immunologic activation and barrier dysfunction that are distinct from IBS and other functional GI disorders, and are instead on the spectrum of quiescent inflammatory GI disease. The precise role of mast cells and α-tryptase over-expression in these manifestations is an area of ongoing study, but increased GI-associated antigen-directed IgG production, selective PAR-2 activation, and local antigen presentation are putative contributing mechanisms. As additional functional insight is gained into the effects of α-tryptase over-expression in humans, the specific role of mast cells in these clinical and cellular phenotypes will be clarified. However, targeting mature tryptases has been shown to reduce mast cell activation and may be of particular utility in mast cell-associated mucosal inflammation [42]. Thus, neutralizing mature tryptases may be an effective future intervention among individuals with HαT and associated GI manifestations.
Supplementary Material
Key Messages.
Individuals with HαT have increased small intestinal MC numbers.
Intestinal epithelial cell pyroptosis is increased in individuals with HαT at levels similar to quiescent Crohn’s disease.
HαT is associated with increased peripheral and tissue class-switched memory B cells and GI-associated antibody production.
ACKNOWLEDGEMENTS
This work was supported by the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine, a generous donation from the Mary Parker foundation to the University of Florida, extramural funding to Drs. Konnikova and Glover via 1R21TR002639-01A1, CCFA funding to Dr. Konnikova CDA 422348, and intramural NIH funding to Dr. Lyons via the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, NIH. RZG is supported by UF Health Cancer Center funds. The authors would also like to thank the staff of the Molecular Pathology Core at the College of Medicine, University of Florida for the histology and immunohistochemistry work.
Abbreviations
- HαT
Hereditary alpha-tryptasemia
- BST
basal serum tryptase
- GI
gastrointestinal
- IBS
irritable bowel syndrome
- IEC
intestinal epithelial cell
- ddPCR
droplet digital PCR
- CyTOF
Cytometry by Time of Flight
- IHC
Immunohistochemistry
- PBMCs
peripheral mononuclear cells
- SM
systemic mastocytosis
- BM
bone marrow
- MCs
mast cells
- SCF
stem cell factor
- IL-3
Interleukin 3
- DCs
dendritic cells
- PAR-2
proteinase-activated receptor 2
- MAPK
mitogen-activated kinase
- IBS
irritable bowel syndrome
- LKM1
Liver/Kidney Microsomal Type 1
- LPMC
lamina propria mononuclear cells
- IBD
inflammatory bowel disease
- DTT
dithiothreitol
- NFI
net fluorescence intensity
- RLM
Robust-Linear-Model
- PCA
Principal Component Analysis
- gls
generalized least squares
- GERD
gastroesophageal reflux disease
- TI
terminal ileum
- HPF
high power field
- CD
Crohn’s disease
- DN T
double negative T cells
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- IL-1
Interleukin-1
Footnotes
Disclosure of conflicts of interest
EMD and JL are patent holders of Maximus Diagnostic Technologies LLC. None of the other authors have any conflicts of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet, 2016. 48(12): p. 1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le QT, Lyons JJ, Naranjo AN, Olivera A, Lazarus RA, Metcalfe DD, et al. Impact of naturally forming human alpha beta-tryptase heterotetramers in the pathogenesis of hereditary alpha-tryptasemia. J Exp Med, 2019. 216(10): p. 2348–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyons JJ, Chovanec BS, O’Connell MP, Bonadonna P, Metcalfe DD, Korosec P. Heritable risk for severe anaphylaxis associated with increased alpha-tryptase-encoding germline copy number at TPSAB1. J Allergy Clin Immunol, 2020. [DOI] [PubMed] [Google Scholar]
- 4.Greiner G, Sprinzl B, Gorska A, Ratzinger F, Gurbisz M, Witzeneder N, et al. Hereditary alpha tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons JJ, Sun G, Stone KD, Nelson C, Wisch L, O’Brien M, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol, 2014. 133(5): p. 1471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robey RC, Wilcock A, Bobin H, Beaman G, Myers B, Grattan C, et al. Hereditary Alpha-Tryptasemia: UK Prevalence and Variability in Disease Expression. J Allergy Clin Immunol Pract, 2020. [DOI] [PubMed] [Google Scholar]
- 7.Valent P, Oude Elberinkm JNG, Gorska A, Lange M, Zanotti R, van Anrooij B, et al. The Data Registry of the European Competence Network on Mastocytosis (ECNM): Set Up, Projects, and Perspectives. J Allergy Clin Immunol Pract, 2019. 7(1): p. 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons JJ. Hereditary Alpha Tryptasemia: Genotyping and Associated Clinical Features. Immunol Allergy Clin North Am, 2018. 38(3): p. 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology, 2006. 130(5): p. 1377–90. [DOI] [PubMed] [Google Scholar]
- 10.Lyons JJ, Stotz SC, Chovanec J, Liu Y, Lewis KL, Nelson C, et al. A common haplotype containing functional CACNA1H variants is frequently coinherited with increased TPSAB1 copy number. Genet Med, 2018. 20(5): p. 503–512. [DOI] [PubMed] [Google Scholar]
- 11.Valent P, Cerny-Reiterer S, Herrmann H, Mirkina I, George TI, Sotlar K, et al. Phenotypic heterogeneity, novel diagnostic markers, and target expression profiles in normal and neoplastic human mast cells. Best Pract Res Clin Haematol, 2010. 23(3): p. 369–78. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Doty AL, Iqbal A, Glover SC. The differential frequency of Lineage(−)CRTH2(−)CD45(+)NKp44(−)CD117(−)CD127(+)ILC subset in the inflamed terminal ileum of patients with Crohn's disease. Cell Immunol, 2016. 304-305: p. 63–8. [DOI] [PubMed] [Google Scholar]
- 13.Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med, 2017. 376(26): p. 2566–2578. [DOI] [PubMed] [Google Scholar]
- 14.Kulinski JM, Eisch R, Young ML, Rampertaap S, Stoddard J, Monsale J, et al. Skewed Lymphocyte Subpopulations and Associated Phenotypes in Patients with Mastocytosis. J Allergy Clin Immunol Pract, 2020. 8(1): p. 292–301 e2. [DOI] [PubMed] [Google Scholar]
- 15.Konnikova L, Boschetti G, Rahman A, Mitsialis V, Lord J, Richmond C, et al. High-dimensional immune phenotyping and transcriptional analyses reveal robust recovery of viable human immune and epithelial cells from frozen gastrointestinal tissue. Mucosal Immunol, 2018. 11(6): p. 1684–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang SG, Guidos CJ Method for Tagging Antibodies with Metals for Mass Cytometry Experiments. Methods Mol Biol, 2019. 1989: p. 47–54. [DOI] [PubMed] [Google Scholar]
- 17.Han G, Spitzer MH, Bendall SC, Fantl WJ, Nolan GP. Metal-isotope-tagged monoclonal antibodies for high-dimensional mass cytometry. Nat Protoc, 2018. 13(10): p. 2121–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann FJ, Simonds EF, Vivanco N, Bruce T, Borges L, Nolan GP, et al. Scalable Conjugation and Characterization of Immunoglobulins with Stable Mass Isotope Reporters for Single-Cell Mass Cytometry Analysis. Methods Mol Biol, 2019. 1989: p. 55–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods, 2012. 9(7): p. 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sboner A, Karpikov A, Chen G, Smith M, Mattoon D, Freeman-Cook L, et al. Robust-linear-model normalization to reduce technical variability in functional protein microarrays. J Proteome Res, 2009. 8(12): p. 5451–64. [DOI] [PubMed] [Google Scholar]
- 21.Saitta S, Raphael B, Smith IFC. Combining Two Data Mining Methods for System Identification. 2006. Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- 22.Hamilton MJ, Zhao M, Giannetti MP, Weller E, Hufdhi R, Novak P, et al. Distinct Small Intestine Mast Cell Histologic Changes in Patients With Hereditary Alpha-tryptasemia and Mast Cell Activation Syndrome. Am J Surg Pathol, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bannert C, Bidmon-Fliegenschnee B, Stary G, Hotzy F, Stift J, Nurko S, et al. Fc-epsilon-RI, the high affinity IgE-receptor, is robustly expressed in the upper gastrointestinal tract and modulated by mucosal inflammation. PLoS One, 2012. 7(7): p. e42066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagnoff MF, Immunology of the intestinal tract. Gastroenterology, 1993. 105(5): p. 1275–80. [DOI] [PubMed] [Google Scholar]
- 25.Wosen JE, Mukhopadhyay D, Macaubas C, Mellins ED. Epithelial MHC Class II Expression and Its Role in Antigen Presentation in the Gastrointestinal and Respiratory Tracts. Front Immunol, 2018. 9: p. 2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabe H, Malmquist M, Barkman C, Ostman S, Gjertsson I, Saalman R et al. Distinct patterns of naive, activated and memory T and B cells in blood of patients with ulcerative colitis or Crohn's disease. Clin Exp Immunol, 2019. 197(1): p. 111–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu JJ, Davis EM, Wine E, Lou Y, Rudzinski JK, Alipour M, et al. Epithelial cell extrusion leads to breaches in the intestinal epithelium. Inflamm Bowel Dis, 2013. 19(5): p. 912–21. [DOI] [PubMed] [Google Scholar]
- 28.Liu JJ, Kay TM, Davis EM, Lou Y, Kao D, Claggerr B, et al. Epithelial Cell Extrusion Zones Observed on Confocal Laser Endomicroscopy Correlates with Immunohistochemical Staining of Mucosal Biopsy Samples. Dig Dis Sci, 2016. 61(7): p. 1895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nojkov B, Zhou SY, Dolan RD, Davis EM, Appelman HD, Guo X, et al. Evidence of Duodenal Epithelial Barrier Impairment and Increased Pyroptosis in Patients With Functional Dyspepsia on Confocal Laser Endomicroscopy and "Ex Vivo" Mucosa Analysis. Am J Gastroenterol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuchiya K, Nakajima S, Hosokima S, Nguyen DT, Hattori T, Le TM, et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat Commun, 2019. 10(1): p. 2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin SJS, Bai L, Haileselassia Y, Garay G, Yun C, Becker L, et al. Mass cytometry reveals systemic and local immune signatures that distinguish inflammatory bowel diseases. Nat Commun, 2019. 10(1): p. 2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakate S, Demeo M, John R, Tobin M, Keshavarzian A. Mastocytic enterocolitis: increased mucosal mast cells in chronic intractable diarrhea. Arch Pathol Lab Med, 2006. 130(3): p. 362–7. [DOI] [PubMed] [Google Scholar]
- 33.Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, Dainese R, Bernard G, Anty R, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut, 2014. 63(5): p. 744–52. [DOI] [PubMed] [Google Scholar]
- 34.Gelbmann CM, Mestermann S, Gross V, Kollinger M, Scholmerich J, Falk W. Strictures in Crohn's disease are characterised by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut, 1999. 45(2): p. 210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrasco A, Fernandez-Banares F, Pedrosa E, Salas A, Loras C, Rosinach M, et al. Regional Specialisation of T Cell Subsets and Apoptosis in the Human Gut Mucosa: Differences Between Ileum and Colon in Healthy Intestine and Inflammatory Bowel Diseases. J Crohns Colitis, 2016. 10(9): p. 1042–54. [DOI] [PubMed] [Google Scholar]
- 36.Mitsialis V, Wall S, Liu P, Ordovas-Montanes J, Parmet T, Vukovic M, et al. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn's Disease. Gastroenterology, 2020. 159(2): p. 591–608.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osterman MT, VanDussen KL, Gordon IO, Davis EM, Li K, Simpson K, et al. Epithelial Cell Biomarkers Are Predictive of Response to Biologic Agents in Crohn's Disease. Inflamm Bowel Dis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem, 2005. 280(36): p. 31936–48. [DOI] [PubMed] [Google Scholar]
- 39.Volta U, Tovoli F, Cicola R, Parisi C, Fabbri A, Piscaglia M, et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J Clin Gastroenterol, 2012. 46(8): p. 680–5. [DOI] [PubMed] [Google Scholar]
- 40.Infantino M, Meacci F, Grossi V, Macchia D, Manfredi M. Anti-gliadin antibodies in non-celiac gluten sensitivity. Minerva Gastroenterol Dietol, 2017. 63(1): p. 1–4. [DOI] [PubMed] [Google Scholar]
- 41.Tornai T, Palyu E, Vitalis Z, Tornai I, Tornai D, Antal-Szalmas P, et al. Gut barrier failure biomarkers are associated with poor disease outcome in patients with primary sclerosing cholangitis. World J Gastroenterol, 2017. 23(29): p. 5412–5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maun HR, Jackman JK, Choy DF, Loyet KM, Staton TL, Jia G, et al. An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma. Cell, 2019. 179(2): p. 417–431.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.