Abstract
Melatonin and serotonin, products of tryptophan metabolism, are endogenous neurotransmitters and hormones. We have identified and quantified these metabolites in natural honey from Australia, USA, and Poland using a Xevo G2 XS qTof LC–MS. To help ensure correct product identification, some samples were prepurified by RP-HPLC based on the retention times of standards, prior to LC–MS. The concentrations of the metabolites of interest depended on the source of the honey. For Australian honey, levels for melatonin and 2-hydroxymelatonin were 0.91 and 0.68 ng/g, respectively. Melatonin was detected in one brand of US commercial honey at 0.48 ng/g, while a second brand contained serotonin at 88.2 ng/g. In Polish natural honey, 20.6 ng/g of serotonin and 40.8 ng/g of N-acetylserotonin (NAS) were detected, while in Polish commercial honey 25.9 ng/g of serotonin and 7.30 ng/g of NAS were present. We suggest that addictive and health-related properties of honey may be in part dependent on the presence of serotonin, melatonin, and their metabolites, and that these compounds may play a role in the colony activities of bees.
Keywords: honey, bees, melatonin, 2-hydroxymelatonin, serotonin, N-acetylserotonin
INTRODUCTION
Melatonin is an ancient molecule, present since the evolution of life as indicated by its detection in alphaproteobacteria and cyanobacteria.1,2 This pluripotent molecule with diverse actions is widely produced in nature by bacteria, eucarya, unicellular and multicellular fungi, plants, and animals including simple and complex vertebrates and invertebrates.3-8 In the animal kingdom it is produced from serotonin following its acetylation to produce N-acetylserotonin (NAS) with subsequent methylation generating the final product, melatonin.9-11 Melatonin is metabolized through hydroxylations at C2 and C6, through indolic and kynuric pathways as well as by nonenzymatic reactions initiated by UVR (predominantly UVB with possible action of UVA) or ROS.12,13
Serotonin, also an ancient molecule with pluripotent and diverse activities, is a product of sequential metabolic transformations of L-tryptophan which occur across different species such as vertebrates, invertebrates, including worms and insects, fungi, plants, and unicellular organisms.14-22 The rate-limiting step in serotonin synthesis in vertebrates is tryptophan hydroxylation catalyzed by tryptophan hydroxylase.23-25 Hydroxytryptophan can also be generated through nonenzymatic transformation of tryptophan by ultraviolet radiation (UVR) and reactive oxygen species (ROS).26 5-Hydroxytryptophan is decarboxylated by aromatic amino acid decarboxylase to produce serotonin.22 Similar enzymatic reactions of the serotogenic/melatogenic pathway are catalyzed by homologues or paralogs of the above enzymes in different organisms, including plants. In plants, tryptophan is synthesized via the shikimic acid pathway, which is then decarboxylated to tryptamine by tryptophan decarboxylase and further converted to serotonin by tryptamine 5-hydroxylase.3,6,7 Serotonin can also be metabolized to 5-mehoxytryptamine in plants with its further methylation to melatonin.3,8 In insects, serotonin and melatonin are synthesized by same routes as in vertebrates.27-30
Serotonin and melatonin function as pleiotropic signaling molecules regulating many physiological processes and counteracting pathology or environmental stresses across different species.4,6-8,10,11,18,22,31 In insects including honey bees, they play important roles as neurotransmitters, hormones, and bioregulators, regulating different endocrine, behavioral (including social behavior), immune, developmental, and protective functions, as well as biological rhythms.18,29,32-38 Serotonin regulates a wide range of physiological and pathological processes in humans by acting as a neurotransmitter, neurohormone, hormone, and biological modifier.15-17,22,39,40 Melatonin, in addition to regulating the circadian rhythm, regulates a variety of endocrine, immune, neural, metabolic, and protective functions.5,10,41 Many studies support that melatonin can be used as a health supplement due to its many biological functions such as its antioxidant,42-45 anticancer,46-49 and antiaging effects.50,51
Honey is a widely available and highly consumed natural product with nutritional value and may impart significant health benefits and be used as a therapeutic.52-59 While the health benefits of honey may not be fully grasped, many reports have indicated that honey contains numerous natural antioxidants such as polyphenols, flavonoids, phytochemicals, minerals, and vitamins.53,56,57,59-61 Previously, we reported the presence of vitamin D3 and its biologically active hydroxyderivatives in honey.62 In this study using highly sensitive analytical methods, we report the presence of serotonin, melatonin, and their derivatives in honey samples from Australia, the U.S.A, and Poland.
MATERIALS AND METHODS
Chemicals.
To extract honey, HPLC grade methylene chloride and ethyl acetate (Fisher Scientific, Hampton, NH) were used. LC–MS grade acetonitrile, water, and formic acid were purchased from Fisher Scientific (Hampton, NH) for HPLC or LC–MS. Melatonin, serotonin, and N-acetylserotonin were purchased from Sigma-Aldrich (St. Louis, MO) for standards. 2-Hydroxymelatonin was obtained from Santa Cruz Biotech.
Extraction of Honey and Prepurification.
Australian honey, collected in spring and summer and obtained directly from a local producer in Perth, was diluted with an equal volume of water and extracted with 2 volumes of CH2Cl2, three times. The combined extracts were dried under a stream of N2 gas at 30 °C. Honey from Nature Nate’s Corporate (McKinney, TX) was purchased in Walmart and named commercial honey sample 1. Commercial honey sample 2 was from Kirkland Signature, purchased at Costco. The natural Polish honey was collected during the summertime near Warsaw, while the commercial Polish one was purchased in Carrefour market. We extracted honey by two different methods using methylene chloride or ethyl acetate. For the methylene chloride extraction method, the honey was dissolved in a 3.5 volume of water and transferred to a 1 L extraction funnel. The aqueous layer was extracted with CH2Cl2 (3 × 1.5 volume of honey). The combined organic layers were washed with distilled water (3 × 1 volume of honey), brine (2 × 1 volume of honey), and dried over sodium sulfate. It was then filtered using a sintered glass Buchner funnel, and the solvent was evaporated using a rotary evaporator while maintaining the water bath temperature below 35 °C to give a semisolid (200 mg). For the ethyl acetate method, the honey was dissolved in distilled water (6× volume of honey) and transferred to a 1 L extraction funnel. The aqueous layer was extracted with ethyl acetate (3 × 6 volume of honey). The combined organic layers were dried over sodium sulfate, filtered, and evaporated using a rotary evaporator to give a semisolid (120 mg). For quantification, we selected the more efficient of the two extraction methods: methylene chloride for the extraction of melatonin and 2-hydroxymelatonin and ethyl acetate for serotonin and NAS, as shown in Table 1.
Table 1.
Quantification of Melatonin and Its Derivatives in Honeya
| compounds detected | natural honey from Australia |
commercial honey 1 from USA |
commercial honey 2 from USA |
natural honey from Poland |
commercial honey from Poland |
|---|---|---|---|---|---|
| melatonin (ng/g) | 0.91 ± 0.03b | 0.481 ± 0.004 | NQe | - | - |
| 2-hydroxymelatonin (ng/g) | 0.68 ± 0.03 | - | - | - | - |
| serotonin (ng/g) | NQd | NQd | 88.2 ± 1.0 | 20.6 ± 7.9 | 25.9 ± 0.2 |
| NAS (ng/g) | NQd | NQd | - | 40.8 ± 9.2 | 7.3 ± 0.3 |
Melatonin and 2-hydroxymelatonin were quantified using methylene chloride-extracted honey while serotonin NAS was quantified using ethyl acetate-extracted honey. NQ, Detected but not quantified. (−) Could not detect a clear separate peak by LC–MS.
After purification using C18 column.
Detected in honey extracted with methylene chloride.
Detected in honey extracted with ethyl acetate.
Liquid Chromatography–Mass Spectrometry (LC–MS).
Extracted samples were dissolved in acetonitrile and in some cases subjected to a HPLC prepurification step to isolate fractions of interest. This was carried out using a 1260 Infinity II HPLC system with a C18 column (250 mm × 4.6 mm, 5 μm particle size) (Waters, Milford, MA), using a gradient of acetonitrile in water (40–100%) at a flow rate of 0.5 mL/min for 15 min, then with 100% acetonitrile at a flow rate of 0.5 mL/min for 30 min followed by a flow rate of 1.5 mL/min for 20 min. All standards of samples of interest were well separated and displayed different retention times with this mobile phase (data not shown). Fractions with these retention times were then collected from the extracted honey and these samples, and in some cases the crude extracts as well, were analyzed using a Xevo G2 XS qTof LC–MS equipped with a Waters ACQUITY UPLC I-Class System (Waters, Milford, MA). LC–MS was performed using a Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm) (Agilent Technology, Santa Clara, CA). Elution was achieved with a gradient of acetonitrile in water (all containing 0.1% formic acid), 15% acetonitrile for 1.5 min, 15–30% for 0.1 min, 30% isocratically for 0.9 min, 30–100% for 0.5 min, 100% isocratically for 3 min, 100%–15% for 0.1 min, and 15% for 0.9 min at a flow rate of 0.3 mL/min. Positive mode masses were scanned from 100 to 1000 Da using the continuum mode with a scan time of 1 s. The capillary voltage was 1.7 kV with 40 V as cone voltage. The desolvation gas flow rate was set as 800 L/hour with source temperature of 120 °C. As the lockspray reference compound, Leucine enkephalin (200 ng/mL, m/z = 556.2771) was used at a flow rate of 10 μL/min with the lockspray interval being 10 s and a scan time of 1 s. Extracted ion chromatograms (EICs) were obtained using m/z = 233.1 [M + H]+, 255.1 [M + Na]+, and 174.1 [M + H – NH2CH3CO]+ for melatonin; 249.1 [M + H]+ for 2-hydroxymelatonin; 160.1 [M + H – NH3]+ for serotonin and 160.1 [M + H – NH2CH3CO]+ for NAS with Waters MassLynx 4.1 software.
RESULTS
Identification of Melatonin, 2-Hydroxymelatonin, Serotonin, and NAS in Honey.
Honey (from Australia) was extracted with methylene chloride, and fractions of interest were prepurified by HPLC using a C18 column (250 mm × 4.6 mm, 5 μm particle size). Both the crude and purified samples were then analyzed by LC–MS as described in the Materials and Methods. Analysis of purified samples resulted in melatonin, 2-hydroxymelatonin, and NAS being detected with m/z = 255.1 [M + Na]+, 249.1 [M + H]+, and 160.1 [M + H – NH2CH3CO]+ with RTs corresponding to the standards (Figure 1A). Serotonin, 2-hydroxymelatonin, and NAS were also detected in crude honey extract with m/z = 160.1 [M+H-NH3]+, 249.1 [M + H]+ and 160.1 [M + H – NH2CH3CO]+, respectively, with RTs corresponding to the standards (Figure 1B). NAS-d7 was used as internal standard, having the same RT as NAS (Figure 1A). Thus, the identification of melatonin, 2-hydroxymelatonin, and NAS in the sample is based on the characteristic masses being observed for each one and also from the retention times being identical to the standards in two different LC systems. Serotonin was identified from its expected mass and its identical retention time to the standard by LC–MS.
Figure 1.
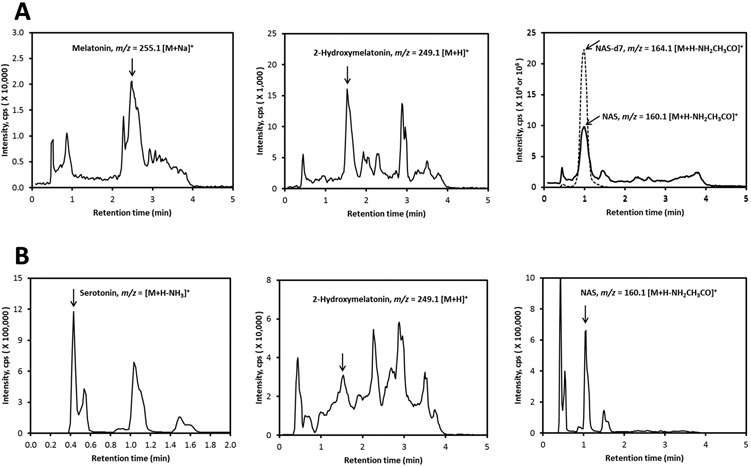
Detection of melatonin, 2-hydroxymelatonin, serotonin, and NAS in Australian honey. The honey was extracted with methylene chloride and analyzed after being prepurified on a C18 column (A) or directly (B) using a Zorbax Eclipse Plus C18 column connected to a Xevo G2 XS equipped with an ACQUITY UPLC I-Class System (Waters, Milford, MA). The extracted ion chromatograms (EICs) were obtained using m/z = 255.1 [M + Na]+, 249.1 [M + H]+, 160.1 [M + H – NH3]+ and 160.1 [M + H – NH2CH3CO]+ for melatonin, 2-hydroxymelatonin, serotonin, and NAS, respectively.
We also investigated two different commercial brands of honey from the United States for the presence of serotonin and melatonin and its derivatives. After extraction of the commercial honey sample 1 with methylene chloride followed by LC–MS, we detected peaks of melatonin and serotonin with m/z = 233.1 [M + H]+ and 160.1 [M + H – NH2CH3CO]+, respectively, with RTs corresponding to melatonin and serotonin standards (Figure 2B). In the prepurified samples in which HPLC was used to collect a fraction at the same RT as standard melatonin and NAS, we detected peaks having m/z = 255.1 [M + Na]+ and 160.1 [M + H – NH2CH3CO]+ (Figure 2A) by subsequent LC–MS, again with retention times matching standards. A second commercial honey sample (sample 2) was extracted with ethyl acetate and analyzed by LC–MS before and after prepurifying the sample with HPLC. As shown in Figure 3A, melatonin was detected with m/z = 174.1 [M + H – NH2CH3CO]+ having a RT corresponding to the standard. We also detected the peaks corresponding to melatonin and serotonin in a crude extract having the same RTs as standards, with m/z = 174.1 [M + H – NH2CH3CO]+ and 160.1 [M + H – NH3]+, respectively (Figure 3B).
Figure 2.
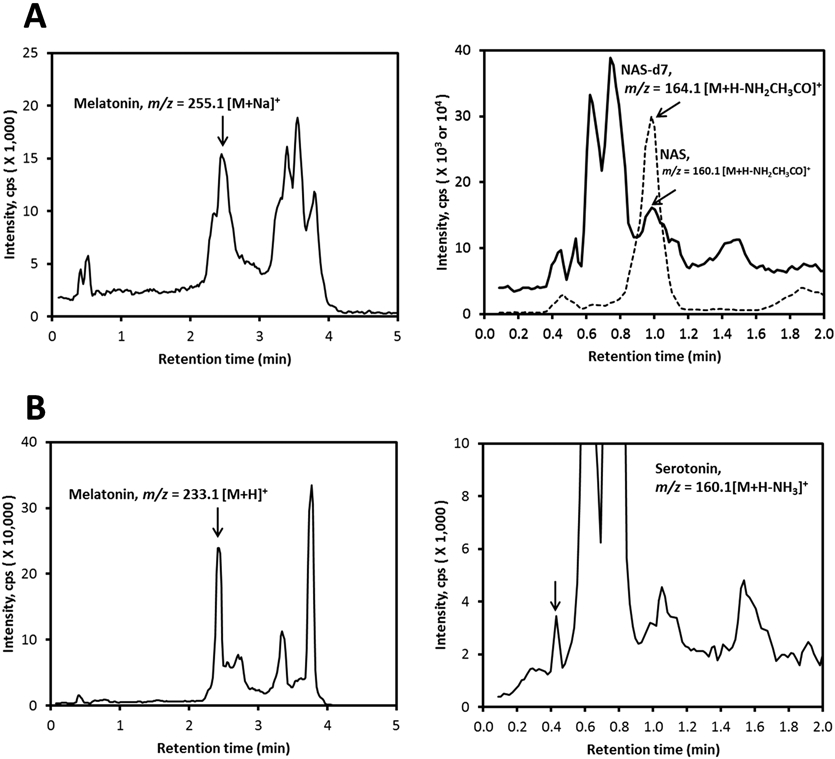
Detection of melatonin, serotonin, and NAS in commercial honey sample 1. The honey was extracted with methylene chloride and analyzed after prepurification of fractions of interest in a C18 column (A) or directly (B) using a Zorbax Eclipse Plus C18 column connected to a Xevo G2 XS equipped with an ACQUITY UPLC I-Class System (Waters, Milford, MA). The EICs were obtained using m/z = 255.1 [M + Na]+ or 233.1 [M + H]+, 160.1 [M + H – NH3]+ and 160.1 [M + H – NH2CH3CO]+ for melatonin, serotonin, and NAS, respectively.
Figure 3.
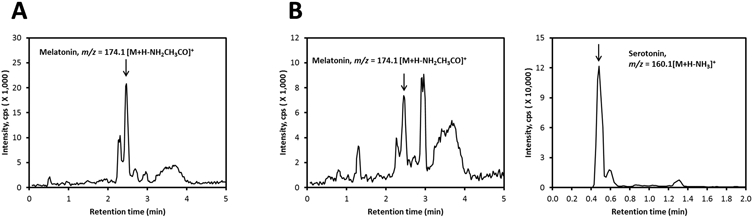
Detection of melatonin and serotonin in commercial honey sample 2. The honey was extracted with ethyl acetate and analyzed after prepurification on a C18 column (A) or directly (B) using a Zorbax Eclipse Plus C18 column connected to a Xevo G2 XS equipped with an ACQUITY UPLC I-Class System (Waters, Milford, MA). The EICs were obtained using m/z = 174.1 [M + H – NH2CH3CO]+ and 160.1 [M + H – NH3]+ for melatonin and serotonin, respectively.
Quantification of Melatonin, 2-Hydroxymelatonin, Serotonin, and NAS in Honey.
Selected peaks in the mass chromatograms were quantified using standard curves for the relevant compounds (Figure S1). The limits of quantification (LOQ) of each compound using their respective standard curve are shown in Figure S2. NAS quantification was done using the standard curve and calculating the ratio of the peak area of NAS/NAS-d7. All standard curves were linear over the range of measurement with coefficients of variation of r2 ≥ 0.996 (Figure S1). The limits of quantification (LOQ) were 0.33 to 20 pg, depending on the compound, and the S/N (signal-to-noise) ratio was over 20, meaning the detection and quantification were convincing (Figure S2). The quantifications were corrected by dilution factors and are described in Table 1. Melatonin and 2-hydroxymelatonin were measured in CH2Cl2-extracted honey while serotonin and NAS were measured in ethyl acetate-extracted honey because the recovery using methylene chloride was better for melatonin while the ethyl acetate method was better for serotonin and NAS (Tables S1, S2, and S3). The recovery rates for melatonin using methylene chloride and ethyl acetate were 75–87% and 58–66%, respectively. In contrast, the recovery rates for serotonin using methylene chloride and ethyl acetate were 15% and 134%, respectively. For NAS the recovery rates using methylene chloride and ethyl acetate were 18% and 82%, respectively.
Melatonin and 2-hydroxymelatonin in Australian honey extracted with methylene chloride were quantified using m/z = 255.1 [M + Na]+, and 249.1 [M + H]+, respectively. Melatonin in commercial honey sample 1 extracted with methylene chloride was quantified using m/z = 233.1 [M + H]+. Serotonin from commercial honey sample 2 extracted with ethyl acetate was quantified using m/z = 160.1 [M + H – NH3]+. For Polish honey extracted with ethyl acetate, m/z = 160.1 [M + H – NH3]+ was used for serotonin quantification while for NAS the ratio of NAS to NAS-d7 (160.1 [M + H – NH2CH3CO]+/NAS-d7 (m/z = 164.1 [M + H – NH2CH3CO]+) was used for quantification. Finally, l-tryptophan, the precursor to serotonin and melatonin, was clearly detected in honey (Figure S3).
DISCUSSION
Melatonin and serotonin are present in most if not all plants, vertebrates, and invertebrates including insects.1,3-8,14-22,30,31 2-Hydroxymelatonin is a predominant melatonin metabolite in plants.63,64 Therefore, it is logical to assume that bees produce NAS and can convert it to melatonin and 2-hydroxymelatonin, using serotonin as a substrate, with some of these products being transferred to their honey. There is already evidence that serotonin is produced by bees.32,35,65,66 However, despite some indications,37,38 analytical evidence for its transformation to NAS, melatonin, and other metabolites is lacking.
Using analytical chemistry methods we have detected intermediates of the serotonigenic/melatonigenic pathway in honey for the first time. Tryptophan, serotonin, NAS, melatonin and/or 2-hydroxymelatoinin were present in Australian, two commercial US, one natural Polish and one commercial Polish honey samples. Using LC–MS, melatonin was detected in natural Australian and two commercial US honeys at levels of 0.91 and 0.48 ng/g for Australian and commercial US honey 1, respectively. Serotonin was detected in all honey samples with levels ranging from 20.6 to 88.2 ng/g for commercial US honey 2 and two Polish honeys (Figure 4), illustrating the variation between the sources of the honey employed for its analysis. Our data provide analytical evidence that bees can transform serotonin to melatonin. Interestingly, 2-hydroxymelatonin was detected only in Australian honey which had a content of 0.7 ng/g, but the related metabolite, 6-hydroxymelatonin, was not detected. It should be noted that 2-hydroxymelatonin is an important product of melatonin metabolism in plants with important medicinal properties in humans.8,63,64 However, it has also been detected in skin samples and can be generated from melatonin by UVB irradiation,12,13 and can also be generated in mitochondria.67 In the case of NAS, it was detectable in all honey samples except commercial US honey 2 with levels of 40.8 and 7.3 ng/g for natural and commercial Polish honey, respectively. Although expected because it is an intermediate in the melatoninergic pathway, this is the first detection of NAS and of 2-hydroxymelatonin originating from insects in general.
Figure 4.
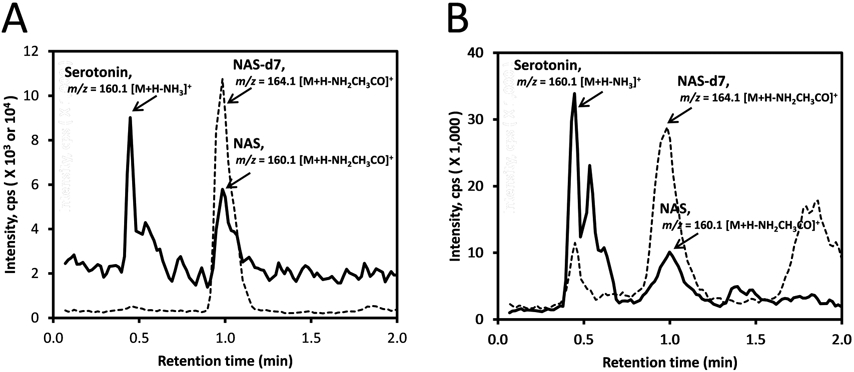
Measurement of serotonin and NAS in Polish honey. The honey was extracted with ethyl acetate and analyzed directly using a Zorbax Eclipse Plus C18 column connected to a Xevo G2 XS equipped with an ACQUITY UPLC I-Class System (Waters, Milford, MA). The extracted ion chromatograms (EICs) were obtained using m/z = 160.1 [M + H – NH3]+ for serotonin and 160.1 [M + H – NH2CH3CO]+ for NAS. (A) Polish natural honey; (B) Polish commercial honey.
Melatonin and serotonin are beneficial to human health with properties which include enhancing sleep quality, regulating the circadian rhythm, regulating behavior, and modifying immune activity including anti-inflammatory actions. They also display endocrine, metabolic, anticancer, antiviral, antioxidative, photoprotective, and antiaging properties.4,5,11,15,16,21,22,41,50,68 Therefore, one may suggest that some of the beneficial health properties of Honey53,54,56-58,60,69-73 may be due, at least in part, to the presence of serotonin and melatonin. NAS and 2-hydroxymelatonin may also play a role from their antioxidative and other beneficial properties, although these have not been thoroughly investigated to date.8,21,63,64,74-76 Interestingly, the reported beneficial effects of honey on bones,55 may be secondary to not only serotonin action, but also to the presence of active forms of vitamin D in this product.62 The well recognized behavioral functions of serotonin22 may contribute to our inclusion of honey in our diet. In the case of melatonin, it is unclear whether the amount obtained from moderate honey consumption is sufficient to have beneficial effects on humans that are assigned to the honey.70,73,77-79 Importantly, the presence of serotonin and melatonin in honey may play an important developmental role in bees, affecting well-being of individual bees and their colony functions. The role of serotonin in these functions is well established,32-35,61,65,66,80-83 which includes learning and cognitive responses.34,84 The importance of melatonin in these functions remains to be investigated, but there are some indications for such a role.29,37,38
In summary, we report for the first time the detection of serotonin, NAS, melatonin, and 2-hydroxymelatonin in honey with considerable variation in levels of these bioactive compounds depending on the source of the honey. We suggest that the presence of these compounds may contribute to some health benefits assigned to the honey, and that they play a role in bee development, health, and colony functions.
Supplementary Material
Funding
The study was supported by NIH Grants 1R01AR073004-01A1, R01 AR071189-01A1, R21 AI149267-01A1, and VA merit grant (No. 1I01BX004293-01A1) to ATS. P.B. and A.F. were supported by Grants ETIUDA 7:2019/32/T/ST4/00505 and 2019/32/T/ST4/00575, respectively, from the National Science Centre, Poland.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsfoodscitech.1c00119.
Standard curves for melatonin and its derivatives used for quantification; limits of quantification (LOQ) for melatonin and its metabolites; detection of tryptophan in honey; recovery rates for melatonin and its metabolites in methylene chloride or ethyl acetate extraction (PDF)
The authors declare no competing financial interest.
Contributor Information
Tae-Kang Kim, Department of Dermatology, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States; VA Medical Center, Birmingham, Alabama 35294, United States.
Venkatram R. Atigadda, Department of Dermatology, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States
Pawel Brzeminski, Department of Dermatology, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States; Department of Chemistry, University of Warsaw, 02-093 Warsaw, Poland.
Adrian Fabisiak, Department of Dermatology, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States; Department of Chemistry, University of Warsaw, 02-093 Warsaw, Poland.
Edith K. Y. Tang, School of Molecular Sciences, The University of Western Australia, Perth, Western Australia 6009, Australia
Robert C. Tuckey, School of Molecular Sciences, The University of Western Australia, Perth, Western Australia 6009, Australia
Russel J. Reiter, Department of Cellular and Structural Biology, UT Health Science Center, San Antonio, Texas 77030, United States
Andrzej T. Slominski, Department of Dermatology, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States; VA Medical Center, Birmingham, Alabama 35294, United States.
REFERENCES
- (1).Tan DX; Hardeland R; Manchester LC; Korkmaz A; Ma S; Rosales-Corral S; Reiter RJ Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot 2012, 63 (2), 577–97. [DOI] [PubMed] [Google Scholar]
- (2).Tan DX; Manchester LC; Liu X; Rosales-Corral SA; Acuna-Castroviejo D; Reiter RJ Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res 2013, 54 (2), 127–38. [DOI] [PubMed] [Google Scholar]
- (3).Back K; Tan DX; Reiter RJ Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res 2016, 61 (4), 426–437. [DOI] [PubMed] [Google Scholar]
- (4).Cipolla-Neto J; Amaral F. G. d. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev 2018, 39 (6), 990–1028. [DOI] [PubMed] [Google Scholar]
- (5).Tan D; Reiter RJ Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2 (1), 44–66. [Google Scholar]
- (6).Hardeland R; Balzer I; Poeggeler B; Fuhrberg B; Uria H; Behrmann G; Wolf R; Meyer TJ; Reiter RJ On the primary functions of melatonin in evolution: mediation of photoperiodic signals in a unicell, photooxidation, and scavenging of free radicals. J. Pineal Res 1995, 18 (2), 104–11 [DOI] [PubMed] [Google Scholar]
- (7).Tan DX; Zheng X; Kong J; Manchester LC; Hardeland R; Kim SJ; Xu X; Reiter RJ Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int. J. Mol. Sci 2014, 15 (9), 15858–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zhao D; Wang H; Chen S; Yu D; Reiter RJ Phytomelatonin: An Emerging Regulator of Plant Biotic Stress Resistance. Trends Plant Sci. 2021, 26 (1), 70–82. [DOI] [PubMed] [Google Scholar]
- (9).Weissbach H; Redfield BG; Axelrod J Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin. Biochim. Biophys. Acta 1960, 43, 352–3. [DOI] [PubMed] [Google Scholar]
- (10).Acuna-Castroviejo D; Escames G; Venegas C; Diaz-Casado ME; Lima-Cabello E; Lopez LC; Rosales-Corral S; Tan DX; Reiter RJ Extrapineal melatonin: sources, regulation, and potential functions. Cell. Mol. Life Sci 2014, 71 (16), 2997–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Slominski A; Wortsman J; Tobin DJ The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005, 19 (2), 176–94. [DOI] [PubMed] [Google Scholar]
- (12).Fischer TW; Sweatman TW; Semak I; Sayre RM; Wortsman J; Slominski A.ƶe.; Fischer TW; Sweatman TW; Semak I; Sayre RM; Wortsman J; Slominski A Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006, 20 (9), 1564–6. [DOI] [PubMed] [Google Scholar]
- (13).Slominski AT; Semak I; Fischer TW; Kim TK; Kleszczynski K; Hardeland R; Reiter RJ Metabolism of melatonin in the skin: Why is it important? Exp Dermatol 2017, 26 (7), 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Reiter RJ; Rosales-Corral S; Tan DX; Jou MJ; Galano A; Xu B Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci 2017, 74 (21), 3863–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Martin AM; Young RL; Leong L; Rogers GB; Spencer NJ; Jessup CF; Keating DJ The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology 2017, 158 (5), 1049–1063. [DOI] [PubMed] [Google Scholar]
- (16).Spohn SN; Mawe GM Non-conventional features of peripheral serotonin signalling - the gut and beyond. Nat. Rev. Gastroenterol. Hepatol 2017, 14 (7), 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Lv J; Liu F The Role of Serotonin beyond the Central Nervous System during Embryogenesis. Front. Cell. Neurosci 2017, 11, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Buznikov GA; Lambert HW; Lauder JM Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001, 305 (2), 177–86. [DOI] [PubMed] [Google Scholar]
- (19).McGowan K; Guerina V; Wicks J; Donowitz M Secretory hormones of Entamoeba histolytica. Ciba Found Symp. 2008, 112, 139–54. [DOI] [PubMed] [Google Scholar]
- (20).Feldman JM; Lee EM Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid. Am. J. Clin. Nutr 1985, 42 (4), 639–43. [DOI] [PubMed] [Google Scholar]
- (21).Slominski AT; Kim TK; Kleszczynski K; Semak I; Janjetovic Z; Sweatman T; Skobowiat C; Steketee JD; Lin Z; Postlethwaite A; Li W; Reiter RJ; Tobin DJ Characterization of serotonin and N-acetylserotonin systems in the human epidermis and skin cells. J. Pineal Res 2020, 68 (2), e12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yabut JM; Crane JD; Green AE; Keating DJ; Khan WI; Steinberg GR Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev 2019, 40 (4), 1092–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kema IP; de Vries EG; Muskiet FA Clinical chemistry of serotonin and metabolites. J. Chromatogr., Biomed. Appl 2000, 747 (1–2), 33–48. [DOI] [PubMed] [Google Scholar]
- (24).Mockus SM; Vrana KE Advances in the molecular characterization of tryptophan hydroxylase. J. Mol. Neurosci 1998, 10 (3), 163–79. [DOI] [PubMed] [Google Scholar]
- (25).Tidemand KD; Peters GH; Harris P; Stensgaard E; Christensen HEM Isoform-Specific Substrate Inhibition Mechanism of Human Tryptophan Hydroxylase. Biochemistry 2017, 56 (46), 6155–6164. [DOI] [PubMed] [Google Scholar]
- (26).Ralf Paus L; Schallreuter KU; Bahadoran P; Picardo M; Slominski A; Elassiuty YE; Kemp EH; Giachino C; Liu JB; Luiten RM; Lambe T; Le Poole IC; Dammak I; Onay H; Zmijewski MA; Dell’Anna ML; Zeegers MP; Cornall RJ; Paus R; Ortonne JP; Westerhof W Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp. Dermatol 2008, 17 (2), 139–40. (discussion 141–160). [DOI] [PubMed] [Google Scholar]
- (27).Vieira R; Mancebo MJ; Perez-Maceira JJ; Aldegunde M Melatonin synthesis in the optic lobes and midbrain of the grasshopper Oedipoda caerulescens. Arch. Insect Biochem. Physiol 2019, 102 (4), e21605. [DOI] [PubMed] [Google Scholar]
- (28).Vivien-Roels B; Pevet P; Beck O; Fevre-Montange M Identification of melatonin in the compound eyes of an insect, the locust (Locusta migratoria), by radioimmunoassay and gas chromatography-mass spectrometry. Neurosci. Lett 1984, 49 (1–2), 153–7. [DOI] [PubMed] [Google Scholar]
- (29).Fan W; Li G; Zhang X; Wang Y; Wang C; Xu B; Guo X; Li H The role of melatonin and Tryptophan-5-hydroxylase-1 in different abiotic stressors in Apis cerana cerana. J. Insect Physiol 2021, 128, 104180. [DOI] [PubMed] [Google Scholar]
- (30).Vleugels R; Verlinden H; Broeck JV Serotonin, serotonin receptors and their actions in insects. Neurotransmitter 2015, 2, e314. [Google Scholar]
- (31).Arnao MB; Hernandez-Ruiz J Melatonin against environmental plant stressors: a review. Curr. Protein Pept. Sci 2021, DOI: 10.2174/1389203721999210101235422. [DOI] [PubMed] [Google Scholar]
- (32).French AS; Simcock KL; Rolke D; Gartside SE; Blenau W; Wright GA The role of serotonin in feeding and gut contractions in the honeybee. J. Insect Physiol 2014, 61, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Dolen G; Darvishzadeh A; Huang KW; Malenka RC Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013, 501 (7466), 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wright GA The role of dopamine and serotonin in conditioned food aversion learning in the honeybee. Commun. Integr. Biol 2011, 4 (3), 318–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Thamm M; Balfanz S; Scheiner R; Baumann A; Blenau W Characterization of the 5-HT1A receptor of the honeybee (Apis mellifera) and involvement of serotonin in phototactic behavior. Cell. Mol. Life Sci 2010, 67 (14), 2467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bloch G; Hazan E; Rafaeli A Circadian rhythms and endocrine functions in adult insects. J. Insect Physiol 2013, 59 (1), 56–69. [DOI] [PubMed] [Google Scholar]
- (37).Li G; Zhang Y; Ni Y; Wang Y; Xu B; Guo X Identification of a melatonin receptor type 1A gene (AccMTNR1A) in Apis cerana cerana and its possible involvement in the response to low temperature stress. Naturwissenschaften 2018, 105 (3–4), 24. [DOI] [PubMed] [Google Scholar]
- (38).Meyer-Rochow VB; Vakkuri O Head and abdominal melatonin of summer and winter bees. J. Pineal Res 2002, 32 (4), 275–6. [DOI] [PubMed] [Google Scholar]
- (39).Millan MJ; Marin P; Bockaert J; Mannoury la Cour C Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol. Sci 2008, 29 (9), 454–64. [DOI] [PubMed] [Google Scholar]
- (40).Alexander SP; Christopoulos A; Davenport AP; Kelly E; Marrion NV; Peters JA; Faccenda E; Harding SD; Pawson AJ; Sharman JL; Southan C; Davies JA; Collaborators C THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors. Br. J. Pharmacol 2017, 174, S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Reiter RJ; Rosales-Corral SA; Tan DX; Acuna-Castroviejo D; Qin L; Yang SF; Xu K Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int. J. Mol. Sci 2017, 18 (4), 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Tan DX; Manchester LC; Esteban-Zubero E; Zhou Z; Reiter RJ Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20 (10), 18886–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Kleszczynski K; Bilska B; Stegemann A; Flis DJ; Ziolkowski W; Pyza E; Luger TA; Reiter RJ; Bohm M; Slominski AT Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int. J. Mol. Sci 2018, 19 (12), 3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Milan AS; Calpena Campmany AC; Naveros BC Antioxidant Nanoplatforms for Dermal Delivery: Melatonin. Curr. Drug Metab 2017, 18, 437. [DOI] [PubMed] [Google Scholar]
- (45).Reiter RJ; Mayo JC; Tan DX; Sainz RM; Alatorre-Jimenez M; Qin L Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res 2016, 61 (3), 253–78. [DOI] [PubMed] [Google Scholar]
- (46).Slominski AT; Zmijewski MA; Semak I; Zbytek B; Pisarchik A; Li W; Zjawiony J; Tuckey RC Cytochromes p450 and skin cancer: role of local endocrine pathways. Anti-Cancer Agents Med. Chem 2014, 14 (1), 77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Bizzarri M; Proietti S; Cucina A; Reiter RJ Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opin. Ther. Targets 2013, 17 (12), 1483–96. [DOI] [PubMed] [Google Scholar]
- (48).Chuffa LGA; Reiter RJ; Lupi LA Melatonin as a Promising Agent to Treat Ovarian Cancer: Molecular Mechanisms. Carcinogenesis 2017, 38, 945. [DOI] [PubMed] [Google Scholar]
- (49).Ma Z; Yang Y; Fan C; Han J; Wang D; Di S; Hu W; Liu D; Li X; Reiter RJ; Yan X Melatonin as a potential anticarcinogen for non-small-cell lung cancer. Oncotarget 2016, 7 (29), 46768–46784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Bocheva G; Slominski RM; Slominski AT Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci 2019, 20 (11), 2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Kleszczynski K; Fischer TW Melatonin and human skin aging. Derm.-Endocrinol 2012, 4 (3), 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Terzo S; Mule F; Amato A Honey and obesity-related dysfunctions: a summary on health benefits. J. Nutr. Biochem 2020, 82, 108401. [DOI] [PubMed] [Google Scholar]
- (53).Seraglio SKT; Silva B; Bergamo G; Brugnerotto P; Gonzaga LV; Fett R; Costa ACO An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int 2019, 119, 44–66. [DOI] [PubMed] [Google Scholar]
- (54).Ramsay EI; Rao S; Madathil L; Hegde SK; Baliga-Rao MP; George T; Baliga MS Honey in oral health and care: A mini review. J. Oral Biosci 2019, 61 (1), 32–36. [DOI] [PubMed] [Google Scholar]
- (55).Kamaruzzaman MA; Chin KY; Mohd Ramli ES A Review of Potential Beneficial Effects of Honey on Bone Health. Evid Based Complement Alternat Med. 2019, 2019, 8543618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Cianciosi D; Forbes-Hernandez TY; Afrin S; Gasparrini M; Reboredo-Rodriguez P; Manna PP; Zhang J; Bravo Lamas L; Martinez Florez S; Agudo Toyos P; Quiles JL; Giampieri F; Battino M Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23 (9), 2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Samarghandian S; Farkhondeh T; Samini F Honey and Health: A Review of Recent Clinical Research. Pharmacognosy Res. 2017, 9 (2), 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Ajibola A Novel Insights into the Health Importance of Natural Honey. Malays J. Med. Sci 2015, 22 (5), 7–22. [PMC free article] [PubMed] [Google Scholar]
- (59).Bogdanov S; Jurendic T; Sieber R; Gallmann P Honey for nutrition and health: a review. J. Am. Coll. Nutr 2008, 27 (6), 677–89. [DOI] [PubMed] [Google Scholar]
- (60).Ciucure CT; Geana EI Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal 2019, 30 (4), 481–492. [DOI] [PubMed] [Google Scholar]
- (61).Negri P; Villalobos E; Szawarski N; Damiani N; Gende L; Garrido M; Maggi M; Quintana S; Lamattina L; Eguaras M Towards Precision Nutrition: A Novel Concept Linking Phytochemicals, Immune Response and Honey Bee Health. Insects 2019, 10 (11), 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Kim TK; Atigadda V; Brzeminski P; Fabisiak A; Tang EKY; Tuckey RC; Slominski AT Detection of 7-Dehydrocholesterol and Vitamin D3 Derivatives in Honey. Molecules 2020, 25 (11), 2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Byeon Y; Tan DX; Reiter RJ; Back K Predominance of 2-hydroxymelatonin over melatonin in plants. J. Pineal Res 2015, 59 (4), 448–54. [DOI] [PubMed] [Google Scholar]
- (64).Yang Y; Zhou R; Park SY; Back K; Bae WK; Kim KK; Kim H 2-Hydroxymelatonin, a Predominant Hydroxylated Melatonin Metabolite in Plants, Shows Antitumor Activity against Human Colorectal Cancer Cells. Molecules 2017, 22 (3), 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Nouvian M; Deisig N; Reinhard J; Giurfa M Seasonality, alarm pheromone and serotonin: insights on the neurobiology of honeybee defence from winter bees. Biol. Lett 2018, 14 (8), 20180337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Blenau W; Thamm M Distribution of serotonin (5-HT) and its receptors in the insect brain with focus on the mushroom bodies: lessons from Drosophila melanogaster and Apis mellifera. Arthropod Struct. Dev 2011, 40 (5), 381–94. [DOI] [PubMed] [Google Scholar]
- (67).Semak I; Naumova M; Korik E; Terekhovich V; Wortsman J; Slominski A A novel metabolic pathway of melatonin: oxidation by cytochrome C. Biochemistry 2005, 44 (26), 9300–7. [DOI] [PubMed] [Google Scholar]
- (68).Slominski AT; Hardeland R; Zmijewski MA; Slominski RM; Reiter RJ; Paus R Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J. Invest. Dermatol 2018, 138 (3), 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Chan BK; Haron H Insights into Putative Health Implications of Gelam (Melaleuca cajuputi) Honey: Evidence from In-Vivo and In-Vitro Studies. Med. Sci 2016, 4 (1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Ayazi P; Mahyar A; Yousef-Zanjani M; Allami A; Esmailzadehha N; Beyhaghi T Comparison of the Effect of Two Kinds of Iranian Honey and Diphenhydramine on Nocturnal Cough and the Sleep Quality in Coughing Children and Their Parents. PLoS One 2017, 12 (1), e0170277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Niaz K; Maqbool F; Bahadar H; Abdollahi M Health Benefits of Manuka Honey as an Essential Constituent for Tissue Regeneration. Curr. Drug Metab 2018, 18 (10), 881–892. [DOI] [PubMed] [Google Scholar]
- (72).Pasupuleti VR; Sammugam L; Ramesh N; Gan SH Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longevity 2017, 2017, 1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Fakhr-Movahedi A; Mirmohammadkhani M; Ramezani H Effect of milk-honey mixture on the sleep quality of coronary patients: A clinical trial study. Clin Nutr ESPEN 2018, 28, 132–135. [DOI] [PubMed] [Google Scholar]
- (74).Janjetovic Z; Jarrett SG; Lee EF; Duprey C; Reiter RJ; Slominski AT Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci. Rep 2017, 7 (1), 1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Skobowiat C; Brozyna AA; Janjetovic Z; Jeayeng S; Oak ASW; Kim TK; Panich U; Reiter RJ; Slominski AT Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. J. Pineal Res 2018, 65 (2), e12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Janjetovic Z; Nahmias ZP; Hanna S; Jarrett SG; Kim TK; Reiter RJ; Slominski AT Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J. Pineal Res 2014, 57 (1), 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Cohen HA; Rozen J; Kristal H; Laks Y; Berkovitch M; Uziel Y; Kozer E; Pomeranz A; Efrat H Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics 2012, 130 (3), 465–71. [DOI] [PubMed] [Google Scholar]
- (78).Paul IM; Beiler J; McMonagle A; Shaffer ML; Duda L; Berlin CM Jr. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007, 161 (12), 1140–6. [DOI] [PubMed] [Google Scholar]
- (79).Warren MD; Pont SJ; Barkin SL; Callahan ST; Caples TL; Carroll KN; Plemmons GS; Swan RR; Cooper WO The effect of honey on nocturnal cough and sleep quality for children and their parents. Arch Pediatr Adolesc Med. 2007, 161 (12), 1149–53. [DOI] [PubMed] [Google Scholar]
- (80).Schlenstedt J; Balfanz S; Baumann A; Blenau W Am5-HT7: molecular and pharmacological characterization of the first serotonin receptor of the honeybee (Apis mellifera). J. Neurochem 2006, 98 (6), 1985–98. [DOI] [PubMed] [Google Scholar]
- (81).Pribbenow B; Erber J Modulation of antennal scanning in the honeybee by sucrose stimuli, serotonin, and octopamine: behavior and electrophysiology. Neurobiol. Learn. Mem 1996, 66 (2), 109–20. [DOI] [PubMed] [Google Scholar]
- (82).Blenau W; Erber J Behavioural pharmacology of dopamine, serotonin and putative aminergic ligands in the mushroom bodies of the honeybee (Apis mellifera). Behav. Brain Res 1998, 96 (1–2), 115–24. [DOI] [PubMed] [Google Scholar]
- (83).Schurmann FW; Klemm N Serotonin-immunoreactive neurons in the brain of the honeybee. J. Comp. Neurol 1984, 225 (4), 570–80. [DOI] [PubMed] [Google Scholar]
- (84).Bateson M; Desire S; Gartside SE; Wright GA Agitated honeybees exhibit pessimistic cognitive biases. Curr. Biol 2011, 21 (12), 1070–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


