Abstract
Mood disorders, especially depression, are a major cause of human disability. The loss of pleasure (anhedonia) is a common, severely debilitating symptom of clinical depression. Experimental animal models are widely used to better understand depression pathogenesis and to develop novel antidepressant therapies. In rodents, various experimental models of anhedonia have already been developed and extensively validated. Complementing rodent studies, the zebrafish (Danio rerio) is emerging as a powerful model organism to assess pathobiological mechanisms of affective disorders, including depression. Here, we critically discuss the potential of zebrafish for modeling anhedonia and studying its molecular mechanisms and translational implications.
Keywords: Anhedonia, animal models, antidepressant, behavior, zebrafish
Introduction: Anhedonia and Its Experimental Models
Affective disorders, especially depression, are a major cause of human disability (Steel et al., 2014). While the ability to experience pleasure and interest are essential for human well-being (Berridge and Kringelbach, 2015), the loss of pleasure (anhedonia) represents a common, severely debilitating clinical symptom of depression (Cooper et al., 2018; De Fruyt et al., 2020). Anhedonia is also highly comorbid with other prevalent psychiatric and neurological disorders, including schizophrenia (Gard et al., 2007), chronic pain (Garland et al., 2020), and Parkinson’s disease (Loas et al., 2012). In humans, anhedonia manifests as losing interest in reward and activities and spending less time experiencing and pursuing pleasure (American Psychiatric Association, 2013) (Table 1; Figure 1). Deficits in specific neuroanatomical areas (e.g., the prefrontal cortex, dorsal striatum, nucleus accumbens, and amygdala) (Rizvi et al., 2016; Auerbach et al., 2017) and neurotransmitter systems (e.g., dopamine, serotonin, opioids, glutamate, and gamma-aminobutyric acid [GABA]) have been consistently implicated in clinical anhedonia (Barbano and Cador, 2007; van Zessen et al., 2012).
Table 1.
Summary of Key Anhedonic-related/like Phenotypes in Humans, Rodents, and Zebrafish
| Humans | Rodents | Zebrafish |
|---|---|---|
| Reduced interest in activities and in time spent on activities and experiencing pleasure | Reduced reward behavior (e.g., in CPP tests) | Reduced reward behavior (e.g., in CPP, hypophagia) |
| Loss of appetite | Reduced consumption of palatable food | Loss of food preference in CPP, hypophagia |
| Social withdrawal | Social deficits (reduced social interaction or preference, low social hierarchy) | Social deficits (reduced social interaction, social preference and shoaling behavior, low social hierarchy), reduced motivation to fight |
| Lethargy, hypoactivity (motor retardation), loss of energy | Hypoactivity (motor retardation) | Hypoactivity (motor retardation) |
| Loss of libido | Reduced sexual behavior | Reduced sexual behavior |
| Reduced emotional abilities (e.g., having less verbal or nonverbal expressions) | Reduced ultrasonic vocalizations | |
| Reduced ability to learn from reward | Reduced reward learning | Reduced reward learning |
| Sensitivity of anhedonic phenotypes to antidepressants | Sensitivity of anhedonia-like phenotypes to antidepressants | Sensitivity of anhedonia-like phenotypes to antidepressants |
Abbreviation: CPP, conditioned place preference.
Figure 1.
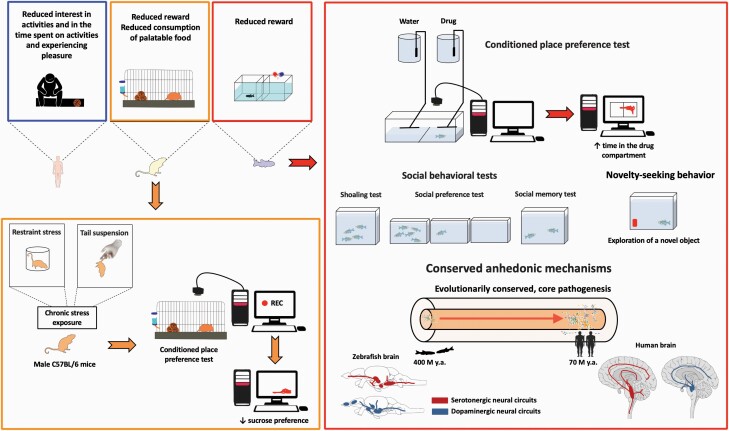
Summary of anhedonic phenotypes in humans, rodents, and zebrafish (see Tables 1 and 2 for details). Left panel shows that rodent anhedonia-like behavioral responses can be assessed by conditioned place preference (CPP) or sucrose preference (SP) tests (Cunningham et al., 2006; Scheggi et al., 2018a). For example, male C57BL/6 mice exposed to chronic stress display reduced SP, a behavioral sign of anhedonia (Strekalova et al., 2004). The right panel illustrates zebrafish CPP models developed to measure reward-like phenotypes, hence reflecting their potential to assess anhedonia (Mathur and Guo, 2010; Mathur et al., 2011; Hinz et al., 2013; Collier et al., 2014; Braida et al., 2020). For example, zebrafish clearly prefer reward-associated CPP compartments (e.g., paired with morphine, diazepam, fluoxetine, risperidone, and buspirone) (Lau et al., 2011; Abreu et al., 2016) and also offer several other behavioral tests assessing social phenotypes (relevant to social anhedonia) (Pham et al., 2012; Ogi et al., 2021) as well as novelty-seeking behavior (novel object or environment exploration), also seen in rodent anhedonia models (Strekalova et al., 2004), reflecting decreased exploration of novelty. Finally, like humans, zebrafish present generally similar, conserved brain structures and circuits (Parker et al., 2013b), including serotonergic and dopaminergic systems strongly involved in anhedonia pathogenesis.
Experimental animal models, especially based on rodents, are commonly used to study various aspects of anhedonia (Anisman and Matheson, 2005; Scheggi et al., 2018a). For example, rodent anhedonia-like states can be induced experimentally by unpredictable chronic stress (UCS), social defeat stress, and early-life stress (Anisman and Matheson, 2005; Scheggi et al., 2018a), often being comorbid with anxiety- and depression-like behavior, including behavioral “despair” (Brockhurst et al., 2015; Olejniczak et al., 2021), motor retardation (hypolocomotion), and social withdrawal (various social deficits) (Frenois et al., 2007; Wilson and Koenig, 2014).
As various experimental manipulations can cause anhedonia-like phenotypes in animals, there are also reliable paradigms to detect and quantify these phenotypes (Table 2). Specifically, rodent anhedonia-like states are traditionally assessed in the sucrose preference test (SP; behavioral test based on the animal’s natural preference for sweet vs neutral tastes), conditioned place preference test (CPP; a behavioral paradigm used to study rewarding or aversive properties), and social interaction test (a behavioral assay measuring time spent on social investigation, reflecting the animal’s sociability) (Cunningham et al., 2006; Scheggi et al., 2018a). For instance, mice exposed to UCS or repeated social defeat display reduced SP (Strekalova et al., 2004) and social interaction (García-Pardo et al., 2015), whereas rats in the CPP model prefer reward (e.g., amphetamine)-associated compartment, but not when exposed to UCS (Papp et al., 1991). Likewise, acute or chronic administration of phencyclidine, a non-competitive glutamate N-methyl-D-aspartate receptor antagonist, induces a robust social withdrawal phenotype (Snigdha and Neill, 2008) that can be quantified using rodent social preference and social interaction paradigms (Wilson and Koenig, 2014).
Table 2.
Selected Tests to Study Anhedonia in Humans, Rodents, and Zebrafish
| Anhedonia-like phenotypes | Humans | Rodents | Zebrafish |
|---|---|---|---|
| Impaired ability to learn about reward | Conditioned preference to a methamphetamine-associated contextual cue (Mayo et al., 2013; Mayo and de Wit 2015), Pavlovian-to-instrumental transfer task (Garofalo et al., 2020) |
CPP (Tzschentke 1998, 2007) and CPA (Zang et al., 2020) | CPP (Wong et al., 2014) and CPA (Wong et al., 2014) |
| Impaired ability to pursue reward (e.g., food, sex or social status) | Incentive key press/force grip (Aharon et al., 2001; Parsons et al., 2011), attentional blink (Field et al., 2009; Tibboel et al., 2010), EEfRT (Treadway et al., 2009) | Effort to obtain reward (Berridge and Valenstein 1991; Pecina et al., 2003), palatable food intake (Salamone et al., 1994, 2007), Pavlovian instrumental transfer (Wyvell and Berridge 2000, 2001), female urine sniffing test (Malkesman et al., 2010) | Reduced motivation to fight (Nakajo et al., 2020) |
| Impaired ability to experience pleasure | Self-reports (Jarratt-Barnham et al., 2020; El Sayed et al., 2021), facial expressions (Bylsma et al., 2008), rectal pressure variability (Georgiadis et al., 2006) | Facial “liking” reactions and “disliking” reactions (Grill and Norgren 1978a, 1978b) | — |
| General anhedonia | A wide range of anhedonia questionnaires | Social interaction test (File and Hyde, 1978) | Social preference (Ogi et al., 2020) |
| -— | SP test (Liu et al., 2018) | Food size preference (Onal and Langdon, 2016) | |
| Self-administration (Jones and Comer, 2013) | Intracranial self-stimulation (Redgrave and Dean, 1981), self-administration (Figlewicz et al., 2011; Huyts et al., 2019) | Self-administration (Bosse and Peterson, 2017) |
Abbreviations: CPA, conditioned place avoidance; CPP, conditioned place preference; EEfRT, effort expenditure for rewards task; SP, sucrose preference.
See (Thomsen, 2015) for details.
Animal experimental models also emerge as a valuable tool to assess pharmacological rescue of anhedonia (Table 3), as chronic antidepressants relieve rodent anhedonia-like behaviors (Papp et al., 2003; Tsankova et al., 2006). For instance, following chronic stress exposure, vortioxetine, an antidepressant agonist of serotonin 5-HT1A receptors, reduces rat anhedonia (Martis et al., 2021), whereas agomelatine, an antidepressant MT1/MT2 melatonin receptor agonist and 5-HT2C serotonin receptor antagonist, rescues mouse anhedonia (Boulle et al., 2014). Likewise, a 24-hour treatment with ketamine, a non-selective N-methyl-D-aspartate receptor antagonist, causes an antidepressant-like effect in the UCS model and corrects rat anhedonia in the SP test (Jiang et al., 2017).
Table 3.
Selected Clinically Relevant Drugs to Treat Affective Anhedonia-Related Phenotypes in Humans, Rodents, and Zebrafish
| Substance | Human effects | Rodent effects | Zebrafish effects | References |
|---|---|---|---|---|
| Agomelatine | Reduces severity of anhedonia, depression and anxiety | Reduced anxiety- depression-like behaviors | (Gargoloff et al., 2016; Lapmanee et al., 2017) | |
| Amantadine | Antidepressant effect in bipolar depression | Antidepressant-like effects in the forced swim test, chronic mild stress paradigm, and reserpine test | (Raupp-Barcaro et al., 2018; Krzystanek and Pałasz, 2020) | |
| Bupropion | Antidepressant effect with robust improvement of self-reported anhedonia | Altered social anhedonia | (Tomarken et al., 2004; Lipina et al., 2013) | |
| Flibanserin | Improved libido in depressed women | Increased sucrose intake in stressed mice | (D’Aquila et al., 1997; Kennedy, 2010) | |
| Fluoxetine | Improved Montgomery-Åsberg Depression Rating Scale (including anhedonia) | Increased palatable sweet solution intake in stressed mice | Chronic administration promotes exploration and lowers whole-body cortisol levels | (Muscat et al., 1992; Corrigan et al., 2000; Egan et al., 2009; Cachat et al., 2010) |
| Ketamine | Reduced anhedonia in depressed patients | Increased sucrose preference in rats exposed to 21-day unpredictable chronic stress | (Li et al., 2011; Lally et al., 2014; Lally et al., 2015; Ballard et al., 2017) | |
| Maprotiline | Antidepressant effects | Antidepressant effects, reversed stress-induced anhedonia | (Li and Yan, 1989; Muscat et al., 1992) | |
| Moclobemide | Antidepressant effect, reduced social phobia | Reversed stress-induced anhedonia | (Moreau et al., 1993; Bonnet 2003) | |
| Pramipexole | Antidepressant effect | Reversed stress-induced anhedonia | (Willner et al., 1994; Corrigan et al., 2000) | |
| Sertraline | Antidepressant effect, reduced anhedonia in patients with major depression | Antidepressant effects | Reversed reserpine-induced depression and cognitive deficits | (Boyer et al., 2000; Ulloa et al., 2010; Zhang et al., 2018) |
Anhedonia-like phenotypes can also be induced by various genetic manipulations (Pucilowski et al., 1993; Cinque et al., 2012; Lipina et al., 2013). For example, chronic 4-week UCS decreases SP in the hypercholinergic Flinders Sensitive Line rats, a putative genetic animal model of depression, vs more “resilient” Flinders Resistant Line rats (Pucilowski et al., 1993). The Wistar Kyoto rats (originally bred as normotensive controls for the spontaneous hypertensive “SHR” rat line) are genetically prone to depression and also display reduced SP (Wright et al., 2020), whereas juvenile μ-opioid receptor knockout mice show anhedonia-like low interest in peers and socially rewarding environments (Cinque et al., 2012). The disrupted-in-schizophrenia-1 (Disc1-Q31L) mutant mice also exhibit depression-like behavior, reduced levels of monoamines in the nucleus accumbens, and overt social anhedonia (Lipina et al., 2013). Moreover, various environmental manipulations can also be used to induce anhedonia-like phenotypes in rodents (Ashkenazy et al., 2009). For instance, lengthening the light phase from a 12-:12-hour to a 22-:2-hour-light/-dark cycle induces a complex behavioral syndrome in C3H mice that includes anhedonia in the SP test (Becker et al., 2010). The loss of environmental enrichment (e.g., keeping animals in cages with crinkle paper, metal ladders, and plastic huts) can also elicit an anhedonia-like behavior in female rats (Morano et al., 2019).
Zebrafish Models Relevant to Anhedonia
Complementing rodent models, the zebrafish (Danio rerio) has become a valuable model organism to study central nervous system (CNS) pathogenesis (Meshalkina et al., 2017; Fontana et al., 2018), including affective disorders (de Abreu et al., 2018; Demin et al., 2020a). For example, various aquatic models of chronic stress (Marcon et al., 2016; Song et al., 2018; Demin et al., 2020b), social defeat (Nakajo et al., 2020), and early-life stress (Fontana et al., 2020; Fontana et al., 2021a; Hare et al., 2021) have recently been developed and successfully validated in zebrafish. Can these fish display a broader spectrum of evolutionarily conserved CNS traits and, for example, similarly to mammals, develop anhedonia-like phenotypes? Mounting evidence discussed further (Table 4) suggests potential relevance of zebrafish to modeling anhedonia. Here, we critically evaluate the developing utility of zebrafish models of anhedonia in terms of mechanisms and uses to investigate novel therapies for anhedonia alongside their translational implications.
Table 4.
Selected Open Questions Related to Zebrafish Anhedonia Models.
| Questions |
|---|
| Are there individual, strain, and sex differences in anhedonic responses in zebrafish? |
| What are reliable physiological (non-behavioral) biomarkers of anhedonia in mammals? Are these biomarkers shared between mammals and zebrafish? |
| Do anhedonia-like and sickness behavior-like phenotypes overlap in zebrafish models? |
| Do olfactory deficits (e.g., long-term anosmia) translate into zebrafish anhedonia? |
| Is there a clear hierarchy of motivations in animals and humans and how it relates to anhedonia in zebrafish? |
| Since depressive disorders are a heterogeneous group, where is the place for anhedonia in these clusters of endophenotypes? |
| Are there differences across age in zebrafish anhedonic responses? Can anhedonia-like phenotypes be measured in zebrafish larvae? |
| Can specific gene mutations influence anhedonia-like behaviors in zebrafish? |
| How does gene expression correlate with anhedonic responses in zebrafish models? |
| Can zebrafish anhedonia, if it exists, be epigenetically regulated? What are specific epigenetic mechanisms of such regulation? |
| What are specific neural circuits (e.g., involving habenula) implicated in zebrafish anhedonia-like states? |
| Can zebrafish models based on light or temperature be developed to assess zebrafish anhedonia? |
| Can zebrafish chronic pain models induce anhedonia-like phenotypes? |
| Can there be fully automated models and tests to assess zebrafish anhedonia? |
| Do zebrafish temperamental traits (e.g., boldness/shyness, pessimistic/optimistic bias) correlate with anhedonia-like states? |
| How can anhedonia be separated from fatigue in animal models, including zebrafish? |
| Can we model complex cognitive phenomena, such as motivation loss and avolition, in relation to zebrafish anhedonia? |
| Can different subtypes of clinical anhedonia be modeled in zebrafish? Are there state vs trait anhedonia models in zebrafish? |
| Can zebrafish models be developed for both specific and generalized anhedonia states? |
| Can zebrafish models of anhedonia overlap with (and be relevant to) some other related CNS states, such as cognitive inflexibility? |
| What is the complex dynamic relationship between depression and anhedonia? For example, is animals’ behavior in some models anhedonic-like because they are “depressed,” or, alternatively, can depression emerge first and then induce secondary anhedonia? |
Notably, while rodent models are presently widely used in translational neuroscience and neuropharmacology research, they are relatively time-consuming, expensive, and low-throughput (Nguyen et al., 2014b). Thus, developing complementary model systems is a critically important strategy to further advance the field. In rodents and humans, stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens, modulation of various neurotrophins, cell adhesion molecules, and synaptic proteins (Willner 2005; Bessa et al., 2009; Christoffel et al., 2011). Importantly, the nucleus accumbens is part of a complex network (receiving glutamatergic, monoaminergic, and cholinergic afferents) (Bessa et al., 2013) involved in motivation, reward, and reward-seeking behavior (Gold 2015). In teleost fishes, including zebrafish, a putative homolog of the mammalian nucleus accumbens is the ventral and dorsal telencephalic nuclei, which are rich in GABA (Kim et al., 2004) and dopamine receptors (O’Connell et al., 2011) and receive ascending dopaminergic inputs from the putative ventral tegmental area-like homolog (Rink and Wullimann, 2002; Panula et al., 2010). Zebrafish also share with mammals all major neurochemical (e.g., dopaminergic, serotonergic, and cholinergic) pathways (Rink and Wullimann, 2002; Filippi et al., 2010; Parker et al., 2013a) that are implicated in anhedonia pathogenesis.
In general, several models and tests can be directly pertinent to studying anhedonia in zebrafish. One logical approach is to focus on reward-seeking behaviors, aiming to develop assays that characterize deficits in such responses in zebrafish. Indeed, as these fish display a rich behavioral repertoire with a wide range of reward-related behaviors (Kalueff et al., 2013), reduced reward-seeking behavior can be readily assessed in zebrafish by quantifying their impaired reward-like behavior in CPP and hypophagia models (Collier and Echevarria, 2013; Nguyen et al., 2014a) (Table 2). In both adult and larvae zebrafish, CPP models assess reward-like phenotypes (Mathur and Guo, 2010; Mathur et al., 2011; Hinz et al., 2013; Collier et al., 2014; Braida et al., 2020), hence reflecting their potential to characterize experimentally induced anhedonia-like states as well. Zebrafish do develop CPP towards a wide range of reward stimuli, including food, warm temperature (Rey et al., 2015), and various drugs, such as morphine, diazepam, fluoxetine, risperidone, and buspirone (Lau et al., 2011; Abreu et al., 2016). For example, in the CPP test, zebrafish prefer salvinorin A, a hallucinogenic drug, and morphine, over drug-free compartment (Braida et al., 2007; Mathur et al., 2011). However, preference for some rewards is abolished in anosmic zebrafish (Abreu et al., 2016, 2017), resembling smell loss–induced anhedonia in humans (Keller and Malaspina, 2013).
Social motivation is another powerful drive of human and animal behavior, whose loss can generally trigger anhedonia (Chevallier et al., 2012; Fontana et al., 2021b) (Figure 1). In fact, social anhedonia is one of the most common and debilitating types of anhedonia seen in clinical depression (Enneking et al., 2019). Importantly, like humans, zebrafish are highly social species (Suriyampola et al., 2016; Fontana et al., 2021b) and are therefore uniquely positioned to generate valuable translational insights into aberrant sociality linked to social anhedonia. Furthermore, multiple behavioral tests have been developed to assess zebrafish social phenotypes (Pham et al., 2012; Ogi et al., 2021) and are therefore highly relevant to measuring their social anhedonia as well. For example, the shoaling test examines group cohesion (e.g., shoal area and an average inter-fish distance) (Miller and Gerlai, 2007; Parker et al., 2013c; Gerlai 2014; Carreno Gutierrez et al., 2019), whereas the aquatic social preference test assesses the number of approaches and time spent near a conspecific (Gerlai et al., 2000; Norton et al., 2019). In contrast, exposure to acute and chronic stress decreases social interaction in adult zebrafish, manifested as shorter time near conspecifics in the social preference test and shorter average inter-fish distance in the shoaling test (Giacomini et al., 2016; Demin et al., 2020b).
Animal anhedonia in general is also commonly associated with decreased novelty-seeking behavior, such as novel object or novel environment exploration (Strekalova et al., 2004). In zebrafish, various protocols have been developed to assess their exploration of a novel object (May et al., 2016; Gaspary et al., 2018; Magyary 2019) or novel environments (Godwin et al., 2012; Stewart et al., 2012), thereby providing a potential tool to assess fish anhedonia by measuring reduced novelty-seeking. Interestingly, exploration of an unfamiliar conspecific fish (Madeira and Oliveira, 2017; Ribeiro et al., 2020) may combine both types of appetitive (social preference and novelty-seeking) behaviors, whose inhibition may potentially reflect both aspects of anhedonia in zebrafish and can conveniently be simultaneously assessed in one aquatic “combined” test. Reduction in other appetitive behaviors, such as sexual interaction (Spence and Smith, 2006), palatable food consumption (Lau et al., 2006), preference for warm temperature (Rey et al., 2015), and social status (Dahlbom et al., 2011), can all potentially reflect anhedonia-like states in zebrafish, therefore warranting further studies.
Various novel genetic models of zebrafish anhedonia can also be interesting to develop. For example, the “too few” zebrafish mutation reduces selective groups of dopaminergic and serotonergic neurons in the basal diencephalon and generates normal food preference but no preference for morphine (Lau et al., 2006). In contrast, pretreatment with dopamine receptor antagonists abolishes morphine preference in the wild-type fish, suggesting that preference for natural reward and addictive drug in zebrafish can be dissociable by a single-gene mutation that alters subregions of brain monoamine neurotransmitter systems (Lau et al., 2006). As such, genetic models with impaired reward in zebrafish (e.g., similar to the “too few” mutation) can be developed with potential relevance to mimicking anhedonia in this species.
Pharmacological Models of Anhedonia: From Rodents to Zebrafish?
Multiple pharmacological manipulations are used to treat depression pathogenesis (Table 3) and may therefore alleviate clinical anhedonia as part of their therapeutic profile (Cao et al., 2019). Paralleling clinical data, antidepressants fluoxetine and imipramine, as well as typical and atypical antipsychotic drugs, reverse stress-induced neurochemical alterations and reduce rodent anhedonia (Noda et al., 2000; Vardigan et al., 2010; Chatterjee et al., 2012; Bessa et al., 2013).
For instance, aripiprazole restores rodent motivation to receive reward impaired by UCS (Scheggi et al., 2018b); olanzapine not only prevents, but also reduces rodent stress-evoked anhedonia; whereas haloperidol prevents anhedonia when administered before (but not after) stress (Orsetti et al., 2006). In zebrafish, several anxiolytic, antipsychotic, and antidepressant drugs have also been tested following acute and chronic stress exposure (Demin et al., 2020a). Zebrafish chronically treated with fluoxetine, bromazepam, or nortriptyline display blunted behavioral and endocrine (e.g., whole-body cortisol levels) responses to UCS (Marcon et al., 2016; Song et al., 2018). However, putative, more specific roles of anxiolytic, antipsychotic, and antidepressant drugs in zebrafish anhedonia-like phenotypes merit further scrutiny.
Promises, Problems, and Limitations of Zebrafish Models of Anhedonia
As there is an urgent need to develop novel translational animal models of anhedonia, using animals to understand human diseases must also widen the spectrum of model organisms used. Indeed, the greater number of species studied increases the behavioral repertoire that can be evaluated and may more fully mimic the observed behaviors in the human syndromes or symptoms of these disorders. In line with this, as already mentioned, zebrafish possess a generally similar brain architectonics to that of mammals (Wullimann et al., 1996), including the reward circuits and shared neurotransmitters and hormones (Panula et al., 2010) traditionally associated with anhedonia states. Moreover, zebrafish models have also been developed for several common CNS disorders with frequent anhedonic phenotypes, including depression (Fonseka et al., 2016; de Abreu et al., 2018) and schizophrenia, as well as for chronic stress (Demin et al., 2019; Gawel et al., 2019; Campbell and Granato, 2020; Costa et al., 2021). In addition, zebrafish also offer several clear advantages to study anhedonia and its therapy. For example, these fish have a high degree (approximately 70%) of genetic homology to humans (Howe et al., 2013), including a large number of orthologous genes of the serotoninergic, dopaminergic, opioidergic, and GABA-ergic systems (Kim et al., 2004; Panula et al., 2006; Lillesaar 2011; Demin et al., 2018) all relevant to anhedonia. At the same time, the availability of modern gene-editing tools for zebrafish remarkably surpasses that of rodents (Liu et al., 2019; Sharma et al., 2021). As such, developing innovative genetic models of anhedonia in zebrafish may be an important, feasible, and promising strategy of research in this field.
Likewise, adult and especially larval zebrafish are particularly suitable for medium- and high-throughput CNS drug screening (Stewart et al., 2015; Khan et al., 2017). As such, the possibility of testing multiple antidepressants in zebrafish models enables not only targeting anhedonia-like phenotypes as part of their broader antidepressant action, but also may help identify anhedonia-specific CNS drugs as well, hence eventually discovering drugs that can differentially correct various subtypes of anhedonic behaviors. Zebrafish also have sophisticated behavioral responses that are easily assessed using automated video-tracking systems, increasing the efficiency and speed of time-intensive manual coding (Kalueff et al., 2013), which may be helpful for extracting anhedonia-related phenotypes and fostering an in-depth investigation of pharmacological correction of anhedonia-like phenotypes. Collectively, this supports zebrafish as a promising species to explore the pathobiology of anhedonia.
Notably, as depression is often triggered by stress, and given the high sensitivity of zebrafish to chronic stress, this aquatic species may be suitable not only for modeling depression-like states in general (Marcon et al., 2016; Song et al., 2018), but anhedonia in particular, as a core symptom of depression. For example, paralleling low dopamine levels [suggested to cause anhedonia in mammals (Gorwood, 2008)], UCS evokes depression-like motor retardation and reduced brain serotonin and dopamine levels in zebrafish (Nguyen et al., 2014b; Zakaria et al., 2021). Likewise, chronic treatment of adult zebrafish with the antidepressant fluoxetine normalizes anxiety- and motor retardation-like behavioral deficits, cortisol, and pro-inflammatory cytokines induced by UCS (Song et al., 2018). However, the effects of UCS on zebrafish anhedonic-like phenotypes are unclear and necessitate further studies. The role of other factors that induce or promote mental disorders like depression (e.g., psychological traumas associated with the loss of a family member) clinically can also be hypothetically used to develop more specific models that induce stress-related (including socially mediated) anhedonic-like phenotypes in zebrafish. If successful, such findings would further support zebrafish as a potentially promising candidate aquatic model organism to probe the link between chronic stress and depression- and anhedonia-like phenotypes.
Furthermore, the characteristic sickness behavior, induced by infections and mediated by pro-inflammatory cytokines (Maier and Watkins 1998), is another pathological syndrome relevant to depression. Indeed, activated brain cytokines are associated with depression pathogenesis (Dantzer 2001), which, in turn, shares considerable phenomenological similarities with sickness behavior (Dantzer 2009; Maes et al., 2012). For example, they both share motor retardation, anorexia, weight loss, anhedonia, somatic symptoms (fatigue, hyperalgesia, and malaise), anxiety, and neurocognitive deficits (Maes et al., 2012). Similar to anhedonia, humans also exhibit decreased appetite during both illness and depression (Plata-Salamán, 1996; Simmons et al., 2016). Likewise, rodent infection and inflammation are commonly accompanied by reduced food intake (Bernstein et al., 1985; McHugh et al., 1993). For example, mimicking food anhedonia, the acute administration of endotoxin lipopolysaccharide reduces self-administration of sucrose pellets in rats (De La Garza et al., 2004). Similarly, rat sepsis model with polymicrobial abdominal infection evokes sickness behavior, fever and anhedonia-like low activity/energy (lethargy), SP, and body weight loss (Pereira de Souza Goldim et al., 2020).
Strikingly paralleling rodent findings, sickness behavior can be induced in adult zebrafish by formalin-inactivated bacteria, reducing fish locomotor activity (motor retardation), social preference (social anhedonia), and exploration of a novel object (reduced novelty-seeking behavior) in addition to upregulating brain expression of pro-inflammatory cytokines (e.g., interleukins [IL]-1β, IL-6, and tumor necrosis factor-α) (Kirsten et al., 2018). In line with this, pharmacological therapies can revert some of these deficits in various animal models (Yirmiya, 1996; Yirmiya et al., 2001; Sammut et al., 2002; Merali et al., 2003). For example, chronic treatment with a tricyclic antidepressant, imipramine, or fluoxetine abolishes reduced rat SP produced by acute lipopolysaccharide (Yirmiya, 1996; Yirmiya et al., 2001), interferon-α (Sammut et al., 2002), or IL-1β (Merali et al., 2003).
Anhedonia can be conceptually modeled in animals as reduced reward in multiple ways, from targeting simpler cognitions (e.g., reward valuation assessed by delay or effort) and reward responsiveness (e.g., anticipation, initial response to it, its satiation) to recapitulating more complex cognitive processes, such as learning from the reward (e.g., by assessing probabilistic and reinforcement learning or reward prediction errors) (Scheggi et al., 2018a). Thus, zebrafish models based on reduced reward anticipation or impaired reinforcement learning may also be a promising avenue of translational research modeling anhedonia across taxa.
At the same time, zebrafish models also have some clear limitations in terms of their translatability into human anhedonia, especially given certain differences from mammals in brain neuroanatomy (Parker et al., 2013b). For example, the cortex plays an important role in mammalian reward circuits (Haber and Knutson, 2010) in addition to the nucleus accumbens, ventral pallidum, and orbitofrontal cortex (OFC) (Der-Avakian and Markou, 2012). The OFC and ventral striatum receive inputs from sensory cortices and calculate the reward values, while the OFC projects reward value information to the rostral anterior cingulate cortex projecting to the prefrontal cortex. The latter has bidirectional connections with multiple areas, including the dorsal raphe, ventral tegmental area, and locus coeruleus, which play an important role in adaptive responses to reward and decision-making (Der-Avakian and Markou, 2012). However, since zebrafish lack a cortex (Parker et al., 2013b), they may not be appropriate models to study various aspects of cortically driven modulation of anhedonia.
Moreover, the small body size (Lakstygal et al., 2018) of zebrafish not only complicates their videorecording but also the development of operant and/or touchscreen-based assays for studying CPP deficits relevant to modeling anhedonia. The small size of zebrafish brain also complicates the development of intracranial self-stimulation protocols, commonly used to study anhedonia in rodents (Scheggi et al., 2018a), as well as real-time analyses of neurochemical markers in specific brain regions (Jones et al., 2015).
Inadequate maternal behavior is an important factor in triggering anhedonia in both humans (Widom et al., 2007) and rodents (Molet et al., 2016). For example, Flinders Sensitive Line rats display reduced motivation to lick, contact, and care for pups (Lavi-Avnon et al., 2005), which may underpin their anhedonia-like behavior as adults (Lavi-Avnon et al., 2005). However, maternal behavior is absent in some fishes, including zebrafish (Perrone Jr and Zaret, 1979), and therefore cannot be used to develop translationally relevant models of anhedonia based on maternal influences. Likewise, social defeat is a common model to induce depression-like anhedonia phenotypes in rodents (Hollis and Kabbaj, 2014; Riga et al., 2015) but is less straight-forward in zebrafish, whose repeated social defeat (e.g., by social subordination for 6 days) reduces the motivation to fight, likely representing a more specific and distinct social subtype of anhedonia (Nakajo et al., 2020).
Although anhedonia is commonly found in patients with chronic pain (Garland et al., 2020), and zebrafish present robust models to study pain (Costa et al., 2021), their potential to evoke anhedonia is unclear (Table 4). In addition, robust sex differences are also reported in anhedonia, as men score higher on physical anhedonia and social anhedonia scales (Miettunen and Jääskeläinen, 2010) and display greater anhedonic depression than women (Langvik et al., 2016). Likewise, female mice display longer latency to eat in the novelty-suppressed feeding test (Paden et al., 2020). Although robust sex differences are also noted for zebrafish behaviors [e.g., UCS increases aggression in males, but not in females (Rambo et al., 2017)], the role of sex in zebrafish anhedonia-like phenotypes remains unclear (Table 4) and warrants further studies.
Modeling zebrafish anhedonia also meets some translational challenges. For example, it is difficult to measure in fish several key clinical symptoms of anhedonia, such as negative feelings and reduced emotional verbal or nonverbal expressions. In addition, anhedonia is a highly heterogeneous phenotype, and in depressed patients it may differ from that in schizophrenic patients (Culig and Belzung, 2016). For example, the latter may be characterized by a disorganization, rather than a deficiency, in reward processing and cognitive function, including inappropriate energy expenditure and focus on irrelevant cues. In contrast, depressed patients display deficits in anticipatory pleasure, development of reward associations, and integration of information from past experience (Lambert et al., 2018). Thus, the possibility to distinguish between different types of experimental anhedonia in animals, including both rodents and zebrafish, is yet to be established. Likewise, animal models cannot assess some other anhedonia-related feelings (e.g., sadness, guilt, or suicidal thoughts), as such symptoms are limited to humans (Nestler and Hyman, 2010). Thus, modeling some subtypes of anhedonia (e.g., sadness-related anhedonia) in animals may be problematic.
Nevertheless, it is possible to model some other related anhedonia-like phenotypes (e.g., pessimistic bias), as telomerase-deficient zebrafish display more negative judgements in response to ambiguous stimuli than wild-type zebrafish (Espigares et al., 2021). Some other important conceptual questions remain in regard to animal anhedonia models in general, including zebrafish anhedonia (Table 4). For example, is animals’ behavior anhedonic-like because they are “depressed,” or, alternatively, does depression emerge first and then may induce anhedonia-like states? These possibilities must clearly be considered when planning experimental protocols to study complex dynamics of anhedonia pathogenesis in zebrafish and other species.
Despite these challenges, mounting evidence summarized here indicates several key characteristics—the presence of selected anhedonia-related behaviors in zebrafish and the availability of sensitive behavioral tests capable of assessing these anhedonic phenotypes—that, together with the growing number of experimental models evoking anhedonia-like states, support the zebrafish as a promising experimental model to probe the pathobiology of anhedonia. However, as many questions regarding anhedonia in zebrafish remain open (Table 4), further studies are needed to better understand the pathobiology of zebrafish anhedonia-like states as well as to develop novel therapies to correct these phenotypes in zebrafish-based screens. Overall, all advantages of zebrafish models discussed here make this animal a valuable and unique species to study anhedonia pathobiology.
Acknowledgments
The study is supported by the Southwest University Zebrafish Platform Construction Funds (Chongqing, China). A.V.K. is the Chair of the International Zebrafish Neuroscience Research Consortium (ZNRC) that coordinated this collaborative project. D.B.R.’s research is also supported by Programa de Excelencia Academica (PROEX)/Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES) (process no. 23038.005450/2020-19) and Program PQ-“Gaúcho” Fundacao de Amparo a Pesquisa do Rio Grande do Sul (FAPERGS) (process no. 19/2551-0001764-2) fellowship grants. A.C.V.V.G. is supported by the FAPERGS research fellowships 19/2551-0001-669-7. A.V.K. is supported by the Zebrafish Platform Construction Fund from the Southwest University (Chongqing, China). K.A.D. is supported by the Special Rector’s Fellowship for SPSU students, and state budgetary funds to Granov Russian Scientific Research Center of Radiology and Surgical Technologies (project 121040200141-4). The collaboration was supported by the Russian Science Foundation grant 20-65-46006. The work used the equipment of the Resource Fund of Applied Genetics MIPT (support grant 075-15-2021-684). K.N.Z., F.C. and G.O.M. are supported by Sirius University of Science and Technology. The funders had no role in the design, analyses and interpretation of the submitted study, or decision to publish.
Interest Statement
The authors declare no conflict of interest, financial or otherwise.
References
- Abreu MS, Giacomini AC, Gusso D, Rosa JG, Koakoski G, Kalichak F, Idalêncio R, Oliveira TA, Barcellos HH, Bonan CD, Barcellos LJ (2016) Acute exposure to waterborne psychoactive drugs attract zebrafish. Comp Biochem Physiol C Toxicol Pharmacol 179:37–43. [DOI] [PubMed] [Google Scholar]
- Abreu MS, Giacomini AC, Rodriguez R, Kalueff AV, Barcellos LJ (2017) Effects of ZnSO4-induced peripheral anosmia on zebrafish behavior and physiology. Behav Brain Res 320:275–281. [DOI] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC (2001) Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 32:537–551. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®). Washington DC: American Psychiatric Publication. [Google Scholar]
- Anisman H, Matheson K (2005) Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev 29:525–546. [DOI] [PubMed] [Google Scholar]
- Ashkenazy T, Einat H, Kronfeld-Schor N (2009) We are in the dark here: induction of depression- and anxiety-like behaviours in the diurnal fat sand rat, by short daylight or melatonin injections. Int J Neuropsychopharmacol 12:83–93. [DOI] [PubMed] [Google Scholar]
- Auerbach RP, Pisoni A, Bondy E, Kumar P, Stewart JG, Yendiki A, Pizzagalli DA (2017) Neuroanatomical prediction of anhedonia in adolescents. Neuropsychopharmacology 42:2087–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Wills K, Lally N, Richards EM, Luckenbaugh DA, Walls T, Ameli R, Niciu MJ, Brutsche NE, Park L, Zarate CA Jr (2017) Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. J Affect Disord 218:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano MF, Cador M (2007) Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 191:497–506. [DOI] [PubMed] [Google Scholar]
- Becker A, Bilkei-Gorzo A, Michel K, Zimmer A (2010) Exposure of mice to long-light: a new animal model to study depression. Eur Neuropsychopharmacol 20:802–812. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Treneer CM, Goehler LE, Murowchick E (1985) Tumor growth in rats: conditioned suppression of food intake and preference. Behav Neurosci 99:818–830. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML (2015) Pleasure systems in the brain. Neuron 86:646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Valenstein ES (1991) What psychological process mediates feeding evoked by electrical stimulation of the lateral hypothalamus? Behav Neurosci 105:3–14. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N (2009) The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14:764–73, 739. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Morais M, Marques F, Pinto L, Palha JA, Almeida OF, Sousa N (2013) Stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Transl Psychiatry 3:e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet U (2003) Moclobemide: therapeutic use and clinical studies. CNS Drug Rev 9:97–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossé GD, Peterson RT (2017) Development of an opioid self-administration assay to study drug seeking in zebrafish. Behav Brain Res 335:158–166. [DOI] [PubMed] [Google Scholar]
- Boulle F, Massart R, Stragier E, Païzanis E, Zaidan L, Marday S, Gabriel C, Mocaer E, Mongeau R, Lanfumey L (2014) Hippocampal and behavioral dysfunctions in a mouse model of environmental stress: normalization by agomelatine. Transl Psychiatry 4:e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P, Tassin JP, Falissart B, Troy S (2000) Sequential improvement of anxiety, depression and anhedonia with sertraline treatment in patients with major depression. J Clin Pharm Ther 25:363–371. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Pegorini S, Zani A, Guerini-Rocco C, Gori E, Sala M (2007) Hallucinatory and rewarding effect of salvinorin A in zebrafish: kappa-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology (Berl) 190:441–448. [DOI] [PubMed] [Google Scholar]
- Braida D, Ponzoni L, Moretti M, Viani P, Pallavicini M, Bolchi C, Appiani R, Bavo F, Gotti C, Sala M (2020) Behavioural and pharmacological profiles of zebrafish administrated pyrrolidinyl benzodioxanes and prolinol aryl ethers with high affinity for heteromeric nicotinic acetylcholine receptors. Psychopharmacology (Berl) 237:2317–2326. [DOI] [PubMed] [Google Scholar]
- Brockhurst J, Cheleuitte-Nieves C, Buckmaster CL, Schatzberg AF, Lyons DM (2015) Stress inoculation modeled in mice. Transl Psychiatry 5:e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J (2008) A meta-analysis of emotional reactivity in major depressive disorder. Clin Psychol Rev 28:676–691. [DOI] [PubMed] [Google Scholar]
- Cachat J, et al. (2010) Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc 5:1786–1799. [DOI] [PubMed] [Google Scholar]
- Campbell PD, Granato M (2020) Zebrafish as a tool to study schizophrenia-associated copy number variants. Dis Model Mech 13:dmm043877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Zhu J, Zuckerman H, Rosenblat JD, Brietzke E, Pan Z, Subramanieapillai M, Park C, Lee Y, McIntyre RS (2019) Pharmacological interventions targeting anhedonia in patients with major depressive disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry 92:109–117. [DOI] [PubMed] [Google Scholar]
- Carreño Gutiérrez H, Colanesi S, Cooper B, Reichmann F, Young AMJ, Kelsh RN, Norton WHJ (2019) Endothelin neurotransmitter signalling controls zebrafish social behaviour. Sci Rep 9:3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Jaiswal M, Palit G (2012) Comparative evaluation of forced swim test and tail suspension test as models of negative symptom of schizophrenia in rodents. ISRN Psychiatry 2012:595141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT (2012) The social motivation theory of autism. Trends Cogn Sci 16:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Russo SJ (2011) Structural and synaptic plasticity in stress-related disorders. Rev Neurosci 22:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque C, Pondiki S, Oddi D, Di Certo MG, Marinelli S, Troisi A, Moles A, D’Amato FR (2012) Modeling socially anhedonic syndromes: genetic and pharmacological manipulation of opioid neurotransmission in mice. Transl Psychiatry 2:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AD, Echevarria DJ (2013) The utility of the zebrafish model in conditioned place preference to assess the rewarding effects of drugs. Behav Pharmacol 24:375–383. [DOI] [PubMed] [Google Scholar]
- Collier AD, Khan KM, Caramillo EM, Mohn RS, Echevarria DJ (2014) Zebrafish and conditioned place preference: a translational model of drug reward. Prog Neuropsychopharmacol Biol Psychiatry 55:16–25. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Arulpragasam AR, Treadway MT (2018) Anhedonia in depression: biological mechanisms and computational models. Curr Opin Behav Sci 22:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL (2000) Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress Anxiety 11:58–65. [DOI] [PubMed] [Google Scholar]
- Costa FV, Rosa LV, Quadros VA, de Abreu MS, Santos ARS, Sneddon LU, Kalueff AV, Rosemberg DB(2021) The use of zebrafish as a non-traditional model organism in translational pain research: the knowns and the unknowns. Curr Neuropharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culig L, Belzung Ceds. (2016) Modeling affective symptoms of schizophrenia. In: Handbook of behavioral neuroscience, pp85–102. London: Elsevier. [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA (2006) Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1:1662–1670. [DOI] [PubMed] [Google Scholar]
- D’Aquila P, Monleon S, Borsini F, Brain P, Willner P (1997) Anti-anhedonic actions of the novel serotonergic agent flibanserin, a potential rapidly-acting antidepressant. Eur J Pharmacol 340:121–132. [DOI] [PubMed] [Google Scholar]
- Dahlbom SJ, Lagman D, Lundstedt-Enkel K, Sundström LF, Winberg S(2011) Boldness predicts social status in zebrafis (Danio rerio). Plos One 6:e23565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R (2001) Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun 15:7–24. [DOI] [PubMed] [Google Scholar]
- Dantzer R (2009) Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am 29:247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Abreu MS, Friend AJ, Demin KA, Amstislavskaya TG, Bao W, Kalueff AV (2018) Zebrafish models: do we have valid paradigms for depression? J Pharmacol Toxicol Methods 94:16–22. [DOI] [PubMed] [Google Scholar]
- De Fruyt J, Sabbe B, Demyttenaere K (2020) Anhedonia in depressive disorder: a narrative review. Psychopathology 53:274–281. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Fabrizio KR, Radoi GE, Vlad T, Asnis GM (2004) The non-steroidal anti-inflammatory drug diclofenac sodium attenuates lipopolysaccharide-induced alterations to reward behavior and corticosterone release. Behav Brain Res 149:77–85. [DOI] [PubMed] [Google Scholar]
- Demin KA, Meshalkina DA, Kysil EV, Antonova KA, Volgin AD, Yakovlev OA, Alekseeva PA, Firuleva MM, Lakstygal AM, de Abreu MS, Barcellos LJG, Bao W, Friend AJ, Amstislavskaya TG, Rosemberg DB, Musienko PE, Song C, Kalueff AV (2018) Zebrafish models relevant to studying central opioid and endocannabinoid systems. Prog Neuropsychopharmacol Biol Psychiatry 86:301–312. [DOI] [PubMed] [Google Scholar]
- Demin KA, Meshalkina DA, Volgin AD, Yakovlev OV, de Abreu MS, Alekseeva PA, Friend AJ, Lakstygal AM, Zabegalov K, Amstislavskaya TG, Strekalova T, Bao W, Kalueff AV (2019) Developing zebrafish experimental animal models relevant to schizophrenia. Neurosci Biobehav Rev 105:126–133. [DOI] [PubMed] [Google Scholar]
- Demin KA, Taranov AS, Ilyin NP, Lakstygal AM, Volgin AD, de Abreu MS, Strekalova T, Kalueff AV (2020a) Understanding neurobehavioral effects of acute and chronic stress in zebrafish. Stress 24:1–18. [DOI] [PubMed] [Google Scholar]
- Demin KA, et al. (2020b) Understanding complex dynamics of behavioral, neurochemical and transcriptomic changes induced by prolonged chronic unpredictable stress in zebrafish. Sci Rep 10:19981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A (2012) The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sayed S, Shokry D, Gomaa SM (2021) Post-COVID-19 fatigue and anhedonia: A cross-sectional study and their correlation to post-recovery period. Neuropsychopharmacol Rep 41:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enneking V, Krüssel P, Zaremba D, Dohm K, Grotegerd D, Förster K, Meinert S, Bürger C, Dzvonyar F, Leehr EJ, Böhnlein J, Repple J, Opel N, Winter NR, Hahn T, Redlich R, Dannlowski U (2019) Social anhedonia in major depressive disorder: a symptom-specific neuroimaging approach. Neuropsychopharmacology 44:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espigares F, Abad-Tortosa D, Varela SAM, Ferreira MG, Oliveira RF(2021) Short telomeres drive pessimistic judgement bias in zebrafish. Biol Lett 17(3):20200745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Munafò MR, Franken IH (2009) A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull 135:589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett-Jay JL, Kittleson S, Sipols AJ, Zavosh A(2011) Sucrose self-administration and CNS activation in the rat. Am J Physiol Regul Integr Comp Physiol 300(4):R876–R884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Hyde JR (1978) Can social interaction be used to measure anxiety? Br J Pharmacol 62:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi A, Mahler J, Schweitzer J, Driever W (2010) Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J Comp Neurol 518:423–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseka TM, Wen XY, Foster JA, Kennedy SH (2016) Zebrafish models of major depressive disorders. J Neurosci Res 94:3–14. [DOI] [PubMed] [Google Scholar]
- Fontana BD, Mezzomo NJ, Kalueff AV, Rosemberg DB (2018) The developing utility of zebrafish models of neurological and neuropsychiatric disorders: a critical review. Exp Neurol 299:157–171. [DOI] [PubMed] [Google Scholar]
- Fontana BD, Gibbon AJ, Cleal M, Sudwarts A, Pritchett D, Miletto Petrazzini ME, Brennan CH, Parker MO(2020) Moderate early life stress improves adult zebrafish (Danio rerio) working memory but does not affect social and anxiety-like responses. Dev Psychobiol 63:54–64. [DOI] [PubMed] [Google Scholar]
- Fontana BD, Gibbon AJ, Cleal M, Norton WHJ, Parker MO (2021a) Chronic unpredictable early-life stress (CUELS) protocol: early-life stress changes anxiety levels of adult zebrafish. Prog Neuropsychopharmacol Biol Psychiatry 108:110087. [DOI] [PubMed] [Google Scholar]
- Fontana BD, Müller TE, Cleal M, de Abreu MS, Norton WHJ, Demin KA, Amstislavskaya TG, Petersen EV, Kalueff AV, Parker MO, Rosemberg DB(2021b) Using zebrafish (Danio rerio) models to understand the critical role of social inter actions in mental health and wellbeing. Progress in Neurobiology 208:101993. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N (2007) Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 32:516–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pardo MP, Blanco-Gandía MC, Valiente-Lluch M, Rodríguez-Arias M, Miñarro J, Aguilar MA (2015) Long-term effects of repeated social stress on the conditioned place preference induced by MDMA in mice. Prog Neuropsychopharmacol Biol Psychiatry 63:98–109. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF (2007) Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res 93:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargoloff PD, Corral R, Herbst L, Marquez M, Martinotti G, Gargoloff PR (2016) Effectiveness of agomelatine on anhedonia in depressed patients: an outpatient, open-label, real-world study. Hum Psychopharmacol 31:412–418. [DOI] [PubMed] [Google Scholar]
- Garland EL, Trøstheim M, Eikemo M, Ernst G, Leknes S (2020) Anhedonia in chronic pain and prescription opioid misuse. Psychol Med 50:1977–1988. [DOI] [PubMed] [Google Scholar]
- Garofalo S, Sagliano L, Starita F, Trojano L, di Pellegrino G (2020) Subliminal determinants of cue-guided choice. Sci Rep 10:11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspary KV, Reolon GK, Gusso D, Bonan CD (2018) Novel object recognition and object location tasks in zebrafish: influence of habituation and NMDA receptor antagonism. Neurobiol Learn Mem 155:249–260. [DOI] [PubMed] [Google Scholar]
- Gawel K, Banono NS, Michalak A, Esguerra CV (2019) A critical review of zebrafish schizophrenia models: time for validation? Neurosci Biobehav Rev 107:6–22. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Kortekaas R, Kuipers R, Nieuwenburg A, Pruim J, Reinders AA, Holstege G (2006) Regional cerebral blood flow changes associated with clitorally induced orgasm in healthy women. Eur J Neurosci 24:3305–3316. [DOI] [PubMed] [Google Scholar]
- Gerlai R (2014) Social behavior of zebrafish: from synthetic images to biological mechanisms of shoaling. J Neurosci Methods 234:59–65. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Lahav M, Guo S, Rosenthal A(2000) Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav 67:773–782. [DOI] [PubMed] [Google Scholar]
- Giacomini ACVV, Abreu MS, Giacomini LV, Siebel AM, Zimerman FF, Rambo CL, Mocelin R, Bonan CD, Piato AL, Barcellos LJG (2016) Fluoxetine and diazepam acutely modulate stress induced-behavior. Behav Brain Res 296:301–310. [DOI] [PubMed] [Google Scholar]
- Godwin J, Sawyer S, Perrin F, Oxendine SE, Kezios ZD(2012) Adapting the open field test to assess anxiety-related behavior in zebrafish. In: Zebrafish protocols for neurobehavioral research, pp181–189. Totowa, NJ: Springer. [Google Scholar]
- Gold PW (2015) The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry 20:32–47. [DOI] [PubMed] [Google Scholar]
- Gorwood P (2008) Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci 10:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Norgren R (1978a) The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res 143:281–297. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R (1978b) The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143:263–279. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare AJ, Zimmer AM, LePabic R, Morgan AL, Gilmour KM(2021) Early-life stress influences ion balance in developing zebrafish (Danio rerio). J Comp Physiol B 191:69–84. [DOI] [PubMed] [Google Scholar]
- Hinz FI, Aizenberg M, Tushev G, Schuman EM (2013) Protein synthesis-dependent associative long-term memory in larval zebrafish. J Neurosci 33:15382–15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis F, Kabbaj M (2014) Social defeat as an animal model for depression. Ilar J 55:221–232. [DOI] [PubMed] [Google Scholar]
- Howe K, et al. (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyts B, Brabant C, Tirelli E (2019) Pitolisant and intravenous cocaine self-administration in mice. Eur J Pharmacol 851:63–68. [DOI] [PubMed] [Google Scholar]
- Jarratt-Barnham I, Saleh Y, Husain M, Kirkpatrick B, Fernandez-Egea E (2020) The influence of negative and affective symptoms on anhedonia self-report in schizophrenia. Compr Psychiatry 98:152165. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wang Y, Sun X, Lian B, Sun H, Wang G, Du Z, Li Q, Sun L (2017) Short- and long-term antidepressant effects of ketamine in a rat chronic unpredictable stress model. Brain Behav 7:e00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Comer SD (2013) A review of human drug self-administration procedures. Behav Pharmacol 24:384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LJ, McCutcheon JE, Young AM, Norton WH (2015) Neurochemical measurements in the zebrafish brain. Front Behav Neurosci 9:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, et al. (2013) Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10:70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Malaspina D (2013) Hidden consequences of olfactory dysfunction: a patient report series. BMC Ear Nose Throat Disord 13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S (2010) Flibanserin: initial evidence of efficacy on sexual dysfunction, in patients with major depressive disorder. J Sex Med 7:3449–3459. [DOI] [PubMed] [Google Scholar]
- Khan KM, Collier AD, Meshalkina DA, Kysil EV, Khatsko SL, Kolesnikova T, Morzherin YY, Warnick JE, Kalueff AV, Echevarria DJ (2017) Zebrafish models in neuropsychopharmacology and CNS drug discovery. Br J Pharmacol 174:1925–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Nam RH, Yoo YM, Lee CJ(2004) Identification and functional evidence of GABAergic neurons in parts of the brain of adult zebrafish (Danio rerio). Neurosci Lett 355:29–32. [DOI] [PubMed] [Google Scholar]
- Kirsten K, Soares SM, Koakoski G, Carlos Kreutz L, Barcellos LJG (2018) Characterization of sickness behavior in zebrafish. Brain Behav Immun 73:596–602. [DOI] [PubMed] [Google Scholar]
- Krzystanek M, Pałasz A (2020) Possibility of a new indication for amantadine in the treatment of bipolar depression—case series study. Pharmaceuticals 13:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakstygal AM, de Abreu MS, Lifanov DA, Wappler-Guzzetta EA, Serikuly N, Alpsyshov ET, Wang D, Wang M, Tang Z, Yan D, Demin KA, Volgin AD, Amstislavskaya TG, Wang J, Song C, Alekseeva P, Kalueff AV (2018) Zebrafish models of diabetes-related CNS pathogenesis. Prog Neuropsychopharmacol Biol Psychiatry 92:48–58. [DOI] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA (2014) Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry 4:e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA Jr (2015) Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol 29:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Da Silva S, Ceniti AK, Rizvi SJ, Foussias G, Kennedy SH (2018) Anhedonia in depression and schizophrenia: a transdiagnostic challenge. CNS Neurosci Ther 24:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langvik E, Hjemdal O, Nordahl HM (2016) Personality traits, gender differences and symptoms of anhedonia: what does the Hospital Anxiety and Depression Scale (HADS) measure in nonclinical settings? Scand J Psychol 57:144–151. [DOI] [PubMed] [Google Scholar]
- Lapmanee S, Charoenphandhu J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N(2017) Agomelatine, venlafaxine, and running exercise effectively prevent anxiety- and depression-like behaviors and memory impairment in restraint stressed rats. Plos One 12(11):e0187671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Bretaud S, Huang Y, Lin E, Guo S (2006) Dissociation of food and opiate preference by a genetic mutation in zebrafish. Genes Brain Behav 5:497–505. [DOI] [PubMed] [Google Scholar]
- Lau BY, Mathur P, Gould GG, Guo S (2011) Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc Natl Acad Sci U S A 108:2581–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi-Avnon Y, Yadid G, Overstreet DH, Weller A (2005) Abnormal patterns of maternal behavior in a genetic animal model of depression. Physiol Behav 84:607–615. [DOI] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Yan HQ (1989) Maprotiline (ludiomil) treatment of mental depression–a clinical report of 65 cases. Proc Chin Acad Med Sci Peking Union Med Coll 4:139–141. [PubMed] [Google Scholar]
- Lillesaar C (2011) The serotonergic system in fish. J Chem Neuroanat 41:294–308. [DOI] [PubMed] [Google Scholar]
- Lipina TV, Fletcher PJ, Lee FH, Wong AH, Roder JC (2013) Disrupted-in-schizophrenia-1 Gln31Leu polymorphism results in social anhedonia associated with monoaminergic imbalance and reduction of CREB and β-arrestin-1,2 in the nucleus accumbens in a mouse model of depression. Neuropsychopharmacology 38:423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Petree C, Requena T, Varshney P, Varshney GK (2019) Expanding the CRISPR toolbox in zebrafish for studying development and disease. Front Cell Dev Biol 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG (2018) Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 13:1686–1698. [DOI] [PubMed] [Google Scholar]
- Loas G, Krystkowiak P, Godefroy O (2012) Anhedonia in Parkinson’s disease: an overview. J Neuropsychiatry Clin Neurosci 24:444–451. [DOI] [PubMed] [Google Scholar]
- Madeira N, Oliveira RF (2017) Long-term social recognition memory in zebrafish. Zebrafish 14:305–310. [DOI] [PubMed] [Google Scholar]
- Maes M, Berk M, Goehler L, Song C, Anderson G, Gałecki P, Leonard B (2012) Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyary I (2019) Floating novel object recognition in adult zebrafish: a pilot study. Cogn Process 20:359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR (1998) Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev 105:83–107. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, Chen G, Crawley JN, Manji HK (2010) The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry 67:864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcon M, Herrmann AP, Mocelin R, Rambo CL, Koakoski G, Abreu MS, Conterato GM, Kist LW, Bogo MR, Zanatta L, Barcellos LJ, Piato AL (2016) Prevention of unpredictable chronic stress-related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology (Berl) 233:3815–3824. [DOI] [PubMed] [Google Scholar]
- Martis LS, Højgaard K, Holmes MC, Elfving B, Wiborg O (2021) Vortioxetine ameliorates anhedonic-like behaviour and promotes strategic cognitive performance in a rodent touchscreen task. Sci Rep 11:9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Guo S (2010) Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol Dis 40:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Lau B, Guo S (2011) Conditioned place preference behavior in zebrafish. Nat Protoc 6:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May Z, Morrill A, Holcombe A, Johnston T, Gallup J, Fouad K, Schalomon M, Hamilton TJ (2016) Object recognition memory in zebrafish. Behav Brain Res 296:199–210. [DOI] [PubMed] [Google Scholar]
- Mayo LM, de Wit H (2015) Acquisition of responses to a methamphetamine-associated cue in healthy humans: self-report, behavioral, and psychophysiological measures. Neuropsychopharmacology 40:1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo LM, Fraser D, Childs E, Momenan R, Hommer DW, de Wit H, Heilig M (2013) Conditioned preference to a methamphetamine-associated contextual cue in humans. Neuropsychopharmacology 38:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh KJ, Weingarten HP, Keenan C, Wallace JL, Collins SM (1993) On the suppression of food intake in experimental models of colitis in the rat. Am J Physiol 264:R871–R876. [DOI] [PubMed] [Google Scholar]
- Merali Z, Brennan K, Brau P, Anisman H (2003) Dissociating anorexia and anhedonia elicited by interleukin-1beta: antidepressant and gender effects on responding for “free chow” and “earned” sucrose intake. Psychopharmacology (Berl) 165:413–418. [DOI] [PubMed] [Google Scholar]
- Meshalkina DA, Kysil EV, Warnick JE, Demin KA, Kalueff AV (2017) Adult zebrafish in CNS disease modeling: a tank that’s half-full, not half-empty, and still filling. Lab Anim (NY) 46:378–387. [DOI] [PubMed] [Google Scholar]
- Miettunen J, Jääskeläinen E (2010) Sex differences in Wisconsin Schizotypy Scales–a meta-analysis. Schizophr Bull 36:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Gerlai R(2007) Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav Brain Res 184:157–166. [DOI] [PubMed] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, Stern H (2016) Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry 6:e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano R, Hoskins O, Smith BL, Herman JP (2019) Loss of environmental enrichment elicits behavioral and physiological dysregulation in female rats. Front Behav Neurosci 12:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Jenck F, Martin JR, Mortas P, Haefely W (1993) Effects of moclobemide, a new generation reversible Mao-A inhibitor, in a novel animal model of depression. Pharmacopsychiatry 26:30–33. [DOI] [PubMed] [Google Scholar]
- Muscat R, Papp M, Willner P (1992) Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology (Berl) 109:433–438. [DOI] [PubMed] [Google Scholar]
- Nakajo H, Tsuboi T, Okamoto H (2020) The behavioral paradigm to induce repeated social defeats in zebrafish. Neurosci Res 161:24–32. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE (2010) Animal models of neuropsychiatric disorders. Nat Neurosci 13:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Stewart AM, Kalueff AV (2014) Aquatic blues: modeling depression and antidepressant action in zebrafish. Prog Neuropsychopharmacol Biol Psychiatry 55:26–39. [DOI] [PubMed] [Google Scholar]
- Noda Y, Kamei H, Mamiya T, Furukawa H, Nabeshima T (2000) Repeated phencyclidine treatment induces negative symptom-like behavior in forced swimming test in mice: imbalance of prefrontal serotonergic and dopaminergic functions. Neuropsychopharmacology 23:375–387. [DOI] [PubMed] [Google Scholar]
- Norton WHJ, Manceau L, Reichmann F (2019) The visually mediated social preference test: a novel technique to measure social behavior and behavioral disturbances in zebrafish. Methods Mol Biol 2011:121–132. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Fontenot MR, Hofmann HA (2011) Characterization of the dopaminergic system in the brain of an African cichlid fish, Astatotilapia burtoni. J Comp Neurol 519:75–92. [DOI] [PubMed] [Google Scholar]
- Ogi A, Licitra R, Naef V, Marchese M, Fronte B, Gazzano A, Santorelli FM (2020) Social preference tests in zebrafish: a systematic review. Front Vet Sci 7:590057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogi A, Licitra R, Naef V, Marchese M, Fronte B, Gazzano A, Santorelli FM (2021) Social preference tests in zebrafish: a systematic review. Front Vet Sci 7:590057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejniczak I, Ripperger JA, Sandrelli F, Schnell A, Mansencal-Strittmatter L, Wendrich K, Hui KY, Brenna A, Ben Fredj N, Albrecht U (2021) Light affects behavioral despair involving the clock gene Period 1. Plos Genet 17:e1009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önal U, Langdon C(2016) Diet size preference of zebrafish (Danio rerio) larvae fed on cross-linked protein-walled capsules. Zebrafish 13:556–562. [DOI] [PubMed] [Google Scholar]
- Orsetti M, Colella L, Dellarole A, Canonico PL, Ferri S, Ghi P (2006) Effects of chronic administration of olanzapine, amitriptyline, haloperidol or sodium valproate in naive and anhedonic rats. Int J Neuropsychopharmacol 9:427–436. [DOI] [PubMed] [Google Scholar]
- Paden W, Barko K, Puralewski R, Cahill KM, Huo Z, Shelton MA, Tseng GC, Logan RW, Seney ML (2020) Sex differences in adult mood and in stress-induced transcriptional coherence across mesocorticolimbic circuitry. Transl Psychiatry 10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Sallinen V, Sundvik M, Kolehmainen J, Torkko V, Tiittula A, Moshnyakov M, Podlasz P (2006) Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish 3:235–247. [DOI] [PubMed] [Google Scholar]
- Panula P, Chen YC, Priyadarshini M, Kudo H, Semenova S, Sundvik M, Sallinen V (2010) The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol Dis 40:46–57. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R (1991) An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 104:255–259. [DOI] [PubMed] [Google Scholar]
- Papp M, Gruca P, Boyer PA, Mocaër E (2003) Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacology 28:694–703. [DOI] [PubMed] [Google Scholar]
- Parker MO, Brock AJ, Walton RT, Brennan CH(2013a) The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front Neural Circuits 7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MO, Brock AJ, Walton RT, Brennan CH (2013b) The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front Neural Circuits 7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MO, Brock AJ, Millington ME, Brennan CH (2013c) Behavioural phenotyping of casper mutant and 1-pheny-2-thiourea treated adult zebrafish. Zebrafish 10:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CE, Young KS, Kumari N, Stein A, Kringelbach ML (2011) The motivational salience of infant faces is similar for men and women. Plos One 6:e20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X (2003) Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci 23:9395–9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira de Souza Goldim M, et al. (2020) Sickness behavior score is associated with neuroinflammation and late behavioral changes in polymicrobial sepsis animal model. Inflammation 43:1019–1034. [DOI] [PubMed] [Google Scholar]
- Perrone M Jr, Zaret TM (1979) Parental care patterns of fishes. Am Naturalist 113:351–361. [Google Scholar]
- Pham M, Raymond J, Hester J, Kyzar E, Gaikwad S, Bruce I, Fryar C, Chanin S, Enriquez J, Bagawandoss S(2012) Assessing social behavior phenotypes in adult zebrafish: shoaling, social preference, and mirror biting tests. In: Zebrafish protocols for neurobehavioral research, pp231–246. Totowa, NJ: Springer. [Google Scholar]
- Plata-Salamán CR (1996) Anorexia during acute and chronic disease. Nutrition 12:69–78. [DOI] [PubMed] [Google Scholar]
- Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS (1993) Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiol Behav 54:1215–1220. [DOI] [PubMed] [Google Scholar]
- Rambo CL, Mocelin R, Marcon M, Villanova D, Koakoski G, de Abreu MS, Oliveira TA, Barcellos LJG, Piato AL, Bonan CD (2017) Gender differences in aggression and cortisol levels in zebrafish subjected to unpredictable chronic stress. Physiol Behav 171:50–54. [DOI] [PubMed] [Google Scholar]
- Raupp-Barcaro IF, Vital MA, Galduróz JC, Andreatini R (2018) Potential antidepressant effect of amantadine: a review of preclinical studies and clinical trials. Braz J Psychiatry 40:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Dean P (1981) Intracranial self-stimulation. Br Med Bull 37:141–146. [DOI] [PubMed] [Google Scholar]
- Rey S, Digka N, MacKenzie S(2015) Animal personality relates to thermal preference in wild-type zebrafish, Danio rerio. Zebrafish 12:243–249. [DOI] [PubMed] [Google Scholar]
- Ribeiro D, Nunes AR, Gliksberg M, Anbalagan S, Levkowitz G, Oliveira RF (2020) Oxytocin receptor signalling modulates novelty recognition but not social preference in zebrafish. J Neuroendocrinol 32:e12834. [DOI] [PubMed] [Google Scholar]
- Riga D, Theijs JT, De Vries TJ, Smit AB, Spijker S (2015) Social defeat-induced anhedonia: effects on operant sucrose-seeking behavior. Front Behav Neurosci 9:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink E, Wullimann MF (2002) Development of the catecholaminergic system in the early zebrafish brain: an immunohistochemical study. Brain Res Dev Brain Res 137:89–100. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH (2016) Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev 65:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, McCullough LD, Carriero DL, Berkowitz RJ (1994) Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol Biochem Behav 49:25–31. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM (2007) Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 191:461–482. [DOI] [PubMed] [Google Scholar]
- Sammut S, Bethus I, Goodall G, Muscat R (2002) Antidepressant reversal of interferon-alpha-induced anhedonia. Physiol Behav 75:765–772. [DOI] [PubMed] [Google Scholar]
- Scheggi S, De Montis MG, Gambarana C (2018a) Making sense of rodent models of anhedonia. Int J Neuropsychopharmacol 21:1049–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggi S, Pelliccia T, Gambarana C, De Montis MG (2018b) Aripiprazole relieves motivational anhedonia in rats. J Affect Disord 227:192–197. [DOI] [PubMed] [Google Scholar]
- Sharma P, Sharma BS, Verma RJ (2021) CRISPR-based genome editing of zebrafish. In: Progress in molecular biology and translational science (Singh V, ed), pp 69–84. New York: Academic Press. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Burrows K, Avery JA, Kerr KL, Bodurka J, Savage CR, Drevets WC (2016) Depression-related increases and decreases in appetite: dissociable patterns of aberrant activity in reward and interoceptive neurocircuitry. Am J Psychiatry 173:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha S, Neill JC (2008) Efficacy of antipsychotics to reverse phencyclidine-induced social interaction deficits in female rats–a preliminary investigation. Behav Brain Res 187:489–494. [DOI] [PubMed] [Google Scholar]
- Song C, Liu BP, Zhang YP, Peng Z, Wang J, Collier AD, Echevarria DJ, Savelieva KV, Lawrence RF, Rex CS, Meshalkina DA, Kalueff AV (2018) Modeling consequences of prolonged strong unpredictable stress in zebrafish: complex effects on behavior and physiology. Prog Neuropsychopharmacol Biol Psychiatry 81:384–394. [DOI] [PubMed] [Google Scholar]
- Spence R, Smith C(2006) Mating preference of female zebrafish, Danio rerio, in relation to male dominance. Behavioral Ecology 17:779–783. [Google Scholar]
- Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, Silove D (2014) The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol 43:476–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM, Gerlai R, Kalueff AV (2015) Developing highER-throughput zebrafish screens for in-vivo CNS drug discovery. Front Behav Neurosci 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM, Gaikwad S, Kyzar E, Kalueff AV (2012) Understanding spatio-temporal strategies of adult zebrafish exploration in the open field test. Brain Res 1451:44–52. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P (2004) Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 29:2007–2017. [DOI] [PubMed] [Google Scholar]
- Suriyampola PS, Shelton DS, Shukla R, Roy T, Bhat A, Martins EP (2016) Zebrafish social behavior in the wild. Zebrafish 13:1–8. [DOI] [PubMed] [Google Scholar]
- Thomsen KR (2015) Measuring anhedonia: impaired ability to pursue, experience, and learn about reward. Front Psychol 6:1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibboel H, De Houwer J, Field M (2010) Reduced attentional blink for alcohol-related stimuli in heavy social drinkers. J Psychopharmacol 24:1349–1356. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Freid C, Addington S, Shelton RC (2004) Assessing the effects of bupropion SR on mood dimensions of depression. J Affect Disord 78:235–241. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH (2009) Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. Plos One 4:e6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006) Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–525. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462. [DOI] [PubMed] [Google Scholar]
- Ulloa JL, Castañeda P, Berríos C, Díaz-Veliz G, Mora S, Bravo JA, Araneda K, Menares C, Morales P, Fiedler JL (2010) Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav 97:213–221. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD (2012) Activation of VTA GABA neurons disrupts reward consumption. Neuron 73:1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM (2010) MK-801 produces a deficit in sucrose preference that is reversed by clozapine, D-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: relevance to negative symptoms associated with schizophrenia? Pharmacol Biochem Behav 95:223–229. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ (2007) A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry 64:49–56. [DOI] [PubMed] [Google Scholar]
- Willner P (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110. [DOI] [PubMed] [Google Scholar]
- Willner P, Lappas S, Cheeta S, Muscat R (1994) Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology (Berl) 115:454–462. [DOI] [PubMed] [Google Scholar]
- Wilson CA, Koenig JI (2014) Social interaction and social withdrawal in rodents as readouts for investigating the negative symptoms of schizophrenia. Eur Neuropsychopharmacol 24:759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, von Keyserlingk MA, Richards JG, Weary DM(2014) Con ditioned place avoidance of zebrafish (Danio rerio) to three chemicals used for euthanasia and anaesthesia. Plos One 9:e88030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Gilmour G, Dwyer DM (2020) Wistar Kyoto rats display anhedonia in consumption but retain some sensitivity to the anticipation of palatable solutions. Front Behav Neurosci 14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullimann MF, Rupp B, Reichert H (1996) Introduction: neuroanatomy for a neurogenetic model system. In: Neuroanatomy of the zebrafish brain: a topological atlas (Wullimann MF, Rupp B, Reichert H, eds), pp 1–5. Basel, Switzerland: Birkhäuser Basel. [Google Scholar]
- Wyvell CL, Berridge KC (2000) Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 20:8122–8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC (2001) Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci 21:7831–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R (1996) Endotoxin produces a depressive-like episode in rats. Brain Res 711:163–174. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, Weihe E, Weidenfeld J (2001) Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology 24:531–544. [DOI] [PubMed] [Google Scholar]
- Zakaria F, Akhtar MT, Wan Ibrahim WN, Abu Bakar N, Muhamad A, Shohaimi S, Maulidiani M, Ahmad H, Ismail IS, Shaari K (2021) Perturbations in amino acid metabolism in reserpine-treated zebrafish brain detected by 1H nuclear magnetic resonance-based metabolomics. Zebrafish 18:42–54. [DOI] [PubMed] [Google Scholar]
- Zang KK, Xiao X, Chen LQ, Yang Y, Cao QL, Tang YL, Lv SS, Cao H, Zhang L, Zhang YQ (2020) Distinct function of estrogen receptors in the rodent anterior cingulate cortex in pain-related aversion. Anesthesiology 133:165–184. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu X, Sun M, Zhang Q, Li T, Li X, Xu J, Zhao X, Chen D, Feng X (2018) Reversal of reserpine-induced depression and cognitive disorder in zebrafish by sertraline and Traditional Chinese Medicine (TCM). Behav Brain Funct 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]