Abstract
Background
Diabetes is one of the metabolic diseases characterized by hyperglycemia, with many complications. Diabetic foot ulcer (DFU) is a significant complication of diabetes. Various therapy procedures have been recently described for DFU improvement.
Methods
Using PubMed, Scopus, Science Direct, and Google Scholar to discover the therapeutic effects of bee products, this review study was conducted in 2018-2019 by searching PubMed, Scopus, Science Direct, and Google Scholar databases.
Results
Cell therapies with various cell candidates such as mesenchymal stem cells (MSCs) are increasingly introduced into routine medical care to manage skin wounds. The applying of these cells for tissue regeneration was initially based on the capability of MSCs to differentiate into specialized cells within the injured tissue. Paracrine signaling and differentiation mechanisms have both been contributed to improving tissue repair by MCSs. However, the role of MSCs differentiation is less due to the poor survival of these cells at the site of injury.
Conclusion
At the same time, paracrine signaling or their secretome is the primary mechanism of MSCs that stimulate neovascularization and re-epithelialization and mobilization of inhabitant stem cells. In this review study, we discuss the role of MSCs and their secretome that can improve the use of this new approach in treating ulcers and DFU.
Key Words: Mesenchymal stem cell, Diabetic foot ulcers, Cell therapy, Secretome
INTRODUCTION
Diabetes is a heterogeneous group of metabolic diseases resulting from defects in insulin secretion, insulin action, or complex of both1. This disease is described by hyperglycemia owing to the autoimmune destruction of β-cells in the pancreas (type 1) or insulin resistance, primarily due to obesity, with decreasing pancreatic insulin production and β-cell failure (type 2)2. Metabolic derangements associated with diabetes induce many complexities ranging from cardiovascular and cerebrovascular disorders to neuropathy, retinopathy, nephropathy, and insignificant wound healing. These complications can lead to death or abate the life quality 3. One of these complications is diabetic foot ulcer (DFU), estimated that 15% of patients with diabetes are suffered from DFU during their lifetime4, 5. DFUs are chronic, non-healing wounds that result in non-traumatic amputations in the world. DFU treatment, including management of wound debridement, preventing infection, revascularization procedures and other methods such as hyperbaric oxygen therapy, and negative-pressure wound therapy, has not provided adequate evidence of the efficacy and cost-effectiveness6, 7. The DFUs can dramatically lead to pain, diminish the quality of life, impair mobility, and finally, in some cases, result in amputation. Healing impairment of DFUs is caused due to several intrinsic factors such as neuropathy, vascular problems, and extrinsic factors like wound infection, callus formation, and excessive pressure to the site8.
Generally, wound healing is characterized by several steps interactive process that leads to the resuscitation of a functional dermis or epidermis layer and revascularization of the skin9. These phases of healing are defined by an inflammatory reaction, controlling bleeding, activating cells with coagulation proteins and complement mediators, then the recruitment and the infiltration of neutrophils and mast cells that clear the wound from dead cells, debris, foreign particles, and bacteria10. Regenerative medicine and stem cell-based therapies, particularly those using stem cells, are increasingly introduced into a promising therapeutic approach for managing wound healing. Because they can repair or replace injured tissue with their natural ability to produce cytokines, chemokines, and growth factors necessary for promoting angiogenesis and extracellular matrix remodeling to healing11, 12.
Numerous types of stem cells, such as mesenchymal stem cells (MSCs), have been reported to improve wound healing in DFUs13, 14. These pluripotent stem cells could differentiate into several types of fibroblasts, osteoblasts, chondrocytes, adipocytes, vascular endothelial cells, epithelial cells15, 16. Hitherto clinical trials have shown that autologous MSCs transplantation could promote wound healing in patients with DFUs17, 18. MSCs have therapeutic efficacy by migrating to the wound site, cellular differentiation, immune-modulation, secretion of growth factors, managing the anti-inflammatory activity, and epithelial cells proliferation and regeneration19. These cells can promote endogenous angiogenesis via microenvironmental modulation and expression of the Von Willebrand factor (vWF) and vascular endothelial growth factor (VEGF). Moreover, they stimulate epithelial stem cells recruitment through secrete of tumor necrosis factor-α (TNF-α) and reduce lymphocytes function in the inflammatory, lowering interferon-γ (IFN-γ) activity in the process20.
These approaches may be limited to the relatively invasive procedure required for sample collection and the marked reduction in cell number, proliferation, and differentiation capacity with age21. Therefore, multiple tissues (BM, placenta, adipose tissue, fetal lung, dental pulp, and umbilical cord blood) were used to achieve optimal MSC isolation22-24. Therefore, the choice of MSC source and purification protocol is significant for the therapeutic potential of these cells and the best conditions for DFUs healing25. For example, in Zeng et al. study26, the role of MSCs in wound healing was proved. They carried out the efficacy and safety PDMSCs hydrogel to improve the rate of wound healing. In this study, foot ulcers and function were improved without complications and recurrence by six months. Using the source placenta’s advantages include cell isolation by noninvasive methods, obtaining a more significant number of cells, and lower immunogenicity.
In this review study, we discuss the role of MSCs and their secretome that can improve the use of this new approach in treating skin ulcers such as DFUs.
METHODS
This review study was conducted in 2018-2019 by searching PubMed, Scopus, Science Direct, and Google Scholar databases using the keywords “Mesenchymal stem cell, Diabetic foot ulcers, Cell therapy, Secretome “ to investigate the therapeutic effects of bee products. Articles presented in conferences, theses, and abstracts of articles were excluded. In the initial search, 113 articles were found, and 22 articles were reviewed and criticized by removing duplicate articles and articles that did not have full access to them.
RESULTS
MSCs and stimulation of angiogenesis in DFU
Angiogenesis is a vital phase of the normal wound healing process, as newly formed vessels supply oxygen, a vital component for successful skin repair27. Therefore, MSCs can differentiate into various skin cell types, contributing to a repopulation of the wound bed with normal dermal construction and endothelial progenitor cells (EPCs)28. MSCs can play an essential role in angiogenesis as they are recruited to the wound bed following mobilization from endogenous sources, including stromal cell-derived factor-1 (SDF-1), VEGF, epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), angiopoietin (Ang)-1, keratinocyte growth factor (KGF), matrix metalloproteinase-9 (MMP-9), macrophage inflammatory protein (MIP)-1α and β and erythropoietin (EPO). These cytokines stimulate the recruitment, proliferation, and differentiation of EPCs that stimulate angiogenesis and tissue regeneration29-32.
It has been indicated that the pro-angiogenic growth factors such as IGF-1, Ang-2 had the highest concentration in the MSCs compared to VEGF. These pro-angiogenic factors enhance EPCs proliferation and neovascularization in tissue regeneration33, 34. MSCs mediated repair takes place through the release and actions of paracrine factors that are beneficial to EPCs. However, the increased levels of VEGF and primary fibroblast growth factor (bFGF) in MSCs conditioned medium could only partially account for the improved EPCs proliferation response in vitro35,36. Considerably, the survival of EPCs is increased by MSCs conditioned medium shown by the expansion of cell numbers through exerting cytokine effects37, 38 (Figure 1).
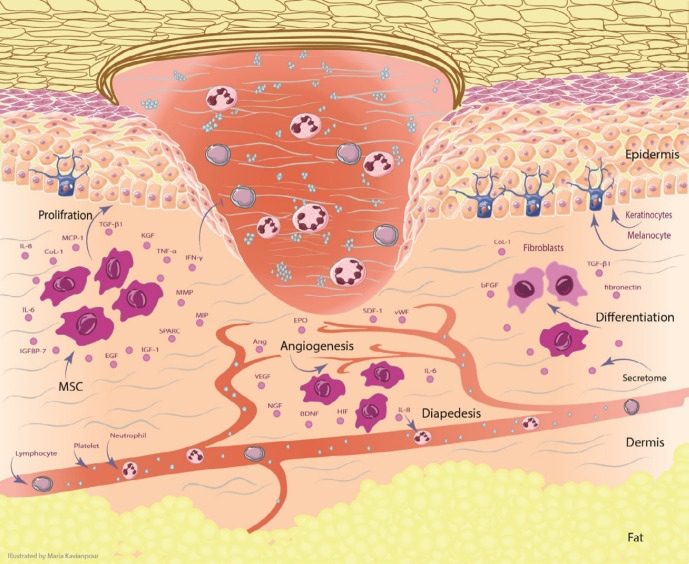
Figure 1.
The effect of MSCs and their secretome in the skin for DFU treatment. MSCs can migrate and increase angiogenesis through secreting VEGF, NGF, BDNF, Ang, SDF-1, vWF, EPO and HIF. the proliferation of keratinocytes plays the significant role in re-epithelialization. Keratinocyte function is improved by regulating IGF-1, EGF, MMP, TGF-β, KGF, MCP-1, and TIMP. MSCs can differentiate into fibroblasts by producing of Col-1, TGF-β, fibronectin, and bFGF. All of these processes can promote the wound healing in DFU. Abbreviation: Ang: angiopoietin, BDNF: brain-derived neurotrophic factor, bFGF: basic fibroblast growth factor, Col-1: collagen type 1, DFU: Diabetic foot ulcer, EGF: epidermal growth factor, EPO: erythropoietin, HIF: hypoxia-inducible factor, IGF-1: insulin-like growth factor-1, IL: interleukin, IGFBP-7: insulin-like growth factor binding protein-7, IFN-γ: interferon-γ, KGF: keratinocyte growth factor, MMP: matrix metalloproteinase, MIP: macrophage inflammatory protein, MCP-1: monocyte chemotactic protein-1, MSCs: mesenchymal stem cells, NGF: Nerve growth factor, PGE2: Prostaglandin E2, SDF-1: stromal cell-derived factor-1, SPARC: secreted protein acidic and rich in cysteine, TGF-β: transforming growth factor, TIMP: tissue inhibitors of metalloproteinase, TNF-α: tumor necrosis factor-α, VEGF: vascular endothelial growth factor, vWF: Von Willebrand factor
The increased levels of VEGF in the MSCs conditioned medium also could potentially mediate the complex interaction of MSCs and EPCs38, 39. Angiogenic factors significantly increased in the MSCs-conditioned medium compared with the conditioned medium from control constructs without cells. MMP-2, transforming growth factor (TGF)-β1, and bFGF have been up-regulated in conditioned medium of stimulated MSCs, but VEGF and hypoxia-inducible factor (HIF)-1α have been unchanged in response to mechanical stimulation of MSCs34, 40.
In several studies, researchers examined the role of MSCs and their secretome in increasing angiogenesis and wound healing. For example, the secretome of MSCs contained a higher concentration of growth factors and proteins relevant to wound healing such as IGF-1, collagen type 1 (CoL-1), KGF, hepatocyte growth factor (HGF), VEGF, Ang-2, MMP-1, and prostaglandin E2 than that of mouse bone marrow-derived allogeneic MSCs (allo-mBM-MSCs)30. On the other hand, insufficient wound healing subsequent administration of allo-mBM-MSCs in diabetic mice has been shown that trophic factors secreted by MSCs are critical for skin regeneration (Table 1).
Table 1.
Beneficial effect of MSCs on angiogenesis of DFU
| Study | Source | Dose of injection | Recipient | Site of injection | Outcome |
|---|---|---|---|---|---|
| Wu et al. 68 2017 |
P-MSCs | Each of four dose cohorts (3 × 106, 10× 106, 30× 106, and 100 × 106 cells) | 15 patients | Intramuscularly | These cells were generally safe and well-tolerated in DFUs and PAD. Outcomes from this study informed the doses, endpoints, biomarkers, and patient population for an ongoing phase 2 trial. |
| Seo et al. 65 2017 |
ADSCs combination with 50 μl of 100 nM Ex-4 | 2.5 × 105 cells | C57BL/6 mice | Intradermally around the wound | A combination of topical treatment of Ex-4 and injection of ADSCs has a better therapeutic effect. |
| Mayo et al. 30 2017 |
allo-BM-MSCs | 1×106 cells | NOD mice | Tropic on wound site | MSCs with secretomes are critical for skin regeneration. |
| Liang et al. 66 2017 |
P-MSCs | MSCs at 2 × 106 (high-dose group) 0.5 × 106 (low-dose group) Combination with insulin |
Nude rats | Intramuscularly | MSCs improved ischemia damage and functional recovery in diabetic rats. The combination therapy of cell treatment and insulin injection did not show increased improvement. |
| Edwards at al. 69 2014 |
WJ-MSC AD-MSC |
12-well plates at a density of 2.5 × 104 cells per well | Mouse | Tropic on wound site | Induced angiogenesis by VEGF-A, Ang-1, and aFGF. |
| O'Loughlin et al. 70 2013 |
Allogeneic nondiabetic BM-MSCs | 1 × 106 cells on a collagen scaffold | Rabbit | Topical application | This cell-based therapy provides a novel therapeutic strategy for increasing wound closure and augmenting angiogenesis, a central pathophysiological deficit in the non-healing DFU. |
| Kim et al.71 2012 |
AMMs | 1 × 106 cells | NOD/SCID Mice | Intra-dermally around the wounds | Secretion of angiogenic factors and enhanced engraftment/differentiation capabilities |
| Kirana et al. 72 2012 |
BMCs in comparison with TCRs CD90+ | 1 ml cell suspension each on an area of 3 · 5 cm, depth 4 cm | 30 patients | Intramuscular | Eighteen patients showed wound healing after 45 weeks. The total number of applicated cells was 3.8 times lower in the TRC group, but TRC patients received significantly higher CD90+ cells. |
| Amann et al. 73 2009 |
Autologous BMC | 3.0 +/- 1.7 x 109 | 51 patients | Intramuscular | BMCs transplantation is a safe procedure that can improve leg perfusion sufficiently to reduce significant amputations and permit durable limb salvage. |
| Falanga et al. 74 2007 |
BM aspirate | 1 × 106 cells per cm2 of wound area by fibrin polymer spray | Human and murine | Topically applied | Stimulation closure of full-thickness wounds in diabetic mice and blood vessels |
| Vojtaššák et al.14 2006 |
Iliac crest | 2 to 4 ml of the aspirate | 77-year-old patient | Into the edges of the wound | Increase in vascularity of the dermis and the dermal thickness of the wound. |
Abbreviation: ADSC: adipose-derived stem cell, Ang-1: angiopoietin-1, aFGF: acidic fibroblast growth factor, AMM: Amniotic mesenchymal stem cell, AD-MSC: adipose-derived mesenchymal stem cell, BM: Bone marrow, BMCs: Bone marrow mononuclear cells, DFU: Diabetic foot ulcer, Ex-4: Exendin-4, NOD/SCID: Non-obese diabetic/severe combined immunodeficiency, TRCs: tissue repair cells, P-MSCs: human placenta-derived mesenchymal stem cell, WJ-MSC: Wharton's jelly mesenchymal stem cell, VEGF-A: Vascular endothelial growth factor-A.
Kuo et al.41 investigated adipose-derived stem cells (ASCs) can accelerate diabetic wound healing and traffic in the engraftment of ASCs with significantly increased levels of EGF, VEGF, prolyl 4-hydroxylase (rPH), and Ki-67 expression compared to the controls. Immunofluorescence staining showed ASCs significantly accumulated in the subdermal layer of the wound margin and increased angiogenesis via vWF and VEGF expression after injection. ASC treatment caused neo angiogenesis and tissue regeneration with paracrine and autocrine mechanisms. Lastly, the rate of wound healing significantly boosted with MSCs in preclinical murine models42. For instance, injection of adipose MSCs (Ad-MSCs) in diabetic murine wound repairing models showed the capability of Ad-MSCs to notably raise the expression of VEGF and levels of angiogenesis in the wound bed. The results of preclinical animal model studies proposed that Ad-MSCs have tremendous power to relieve impaired angiogenesis mechanisms in chronic wounds41. Allogeneic transplantation of Ad-MSCs beside artificial skin into full-thickness wounds of diabetic mice caused mainly developed levels of vascularization and wound repairing43, 44.
MSCs-Conditioned Medium Accelerate Keratino-cytes Proliferation
Keratinocytes are one of the most important cells of the epidermis. These cells have an essential role in the wound-healing mechanism because they are involved in all wound healing processes, including initiation, proliferation, and re-epithelialization45. In skin injuries, the migration of basal keratinocytes from the wound edge and cut epidermal appendages to the denuded wound surface is essential to carry over the newly reconstructed dermal. The stratified keratinocytes proliferate and differentiate to generate neo-epidermis, covering all wound surfaces and restoring the skin function45,46. For the effective plug of wounds, the proliferation of keratinocytes is needed to facilitate connection with other cell types involved in wound healing47. MSCs, improve tissue repair by mechanisms of differentiation and paracrine signaling that contributes by regenerating damaged tissue and regulates the local cellular responses to injury, respectively48.
Not only several MSC secretomes have been identified in wound healing, including TGF-β1, the chemokines IL-6, IL-8, monocyte chemotactic protein-1 (MCP-1), and CoL-1, fibronectin, secreted protein acidic and rich in cysteine (SPARC) and insulin-like growth factor binding protein-7 (IGFBP-7)49, but also promote human keratinocytes to produce cytokines such as IGF-1, EGF, MMP-2, MMP-9 and extracellular receptor kinase (Erk) signaling pathway tissue inhibitors of metalloproteinase (TIMP)-1 and -250. These data emphasize the importance of crosstalk between cells inhabitant in the injured tissue and the ectopically delivery MSCs. The administration of MSCs to either acute or diabetic wounds in rodents accelerates wound closure. Decreased wound size has also been observed when autologous MSCs were applied to human chronic wounds51.
Injection of allogeneic BM-MSCs around the wound increased re-epithelialization and angiogenesis and subsequently accelerated wound closure in diabetic mice compared to allogeneic neonatal dermal fibroblasts or vehicle control medium12. The auxiliary role of BM-MSCs in cutaneous reconstruction has been illustrated with the keratinocyte-specific protein keratin expression and completed glandular structures in the wound.
MSCs also secrete mitogens that stimulate the proliferation of keratinocytes, dermal fibroblasts, and epithelial cells in vitro52-54. Dermal fibroblasts secrete amounts of Col-1 and alter gene expression in response to either MSCs in co-culture or MSC-conditioned medium53, 55. In addition, the beneficial effects of MSCs-conditioned media on the keratinocytes, paracrine signaling activity, proliferation, and migration of keratinocytes have been demonstrated56. TGFβ1 shows MSCs paracrine signaling via decreasing suppression of differentiation, thus leading to increase proliferation of keratinocytes57, 58 (Table 2). Generally, MSCs transplantation stimulates proliferation and migration of the predominant cell types in the wound by release soluble factors59(Figure.1).
Table 2.
MSCs and Keratinocytes proliferation and wound healing
| Wound model | Dose of injection | Delivery method | Results | Mechanism | Ref. |
|---|---|---|---|---|---|
| Murine excisional wound treated with BM stromal progenitors | 7.5×105 cells | Topical | Enhanced epithelialization, granulation tissue formation, and angiogenesis | No evidence of MSC differentiation | 52 |
| allo-BM- MSCs | 1×106 cells | Topical | Accelerated wound closure and increased epithelialization, cellularity, and angiogenesis | MSC differentiation into epidermal keratinocytes | 12 |
| BM-MSC-CM | 0.7×106 in 60 µl PBS and 0.3×106 in 20 µl GFR Matrigel | Topical | Accelerated engraftment and wound closure with increased numbers of macrophages and endothelial progenitors | MSC paracrine signaling | 75 |
| BM- MSCs | 1×106 cells | Systemic delivery | Accelerated wound closure. | MSC differentiation into keratinocytes, endothelial cells, and pericytes | 16 |
| BM- stromal progenitors | ---------- | Topical | Ameliorating healing process in diabetic rats by the modification of keratinocyte functions. | Evaluated in human keratinocytes cultured in MSC-CM with high glucose levels. |
76 |
| human AD-MSC-CM | Topical (with collagen gel solution mixed with MSC- conditioned medium) | Accelerated wound closure by up-regulating the secretion of VEGF and bFGF. | MSC paracrine signaling | 54 | |
| BM- MSCs | 1×106 cells | Intravenous | MSCs localized to hair follicles, sebaceousglands, blood vessels, and dermis | MSC differentiation into keratinocytes | 77 |
| AD-MSC | ---------- | Topical | The activity of conditioned media on the keratinocytes with potential applications. VEGFA protein was increased in conditioned media. |
paracrine activity on keratinocyte proliferation and migration | 57 |
| UCB-MSCs | ---------- | ---------- | TGFβ1 from MSCs results in decreased suppression of differentiation with significantly increased proliferation of keratinocytes. | MSC paracrine signaling | 58 |
Abbreviations: AD: adipose-derived, BM: bone marrow, bFGF: basic fibroblast growth factor, CM: conditioned medium, GFR: Growth Factor Reduced, MSCs: mesenchymal stem cells, TGFβ1: Transforming growth factor-beta 1, UCB-MSCs: umbilical cord blood-derived MSCs, VEGF: Vascular endothelial growth factor
DISCUSSION
Chronic wounds such as DFU are challenging to heal, and insignificant improvement has been shown to prevent morbidity and disability in the past few decades60. The best present procedure for chronic wound treatment is often impermanent and achieves only a 50% healing rate because of the many aspects contributing to non-healing wounds, impairment in the production of cytokines by local inflammatory cells, and reduced fibroblasts angiogenesis are crucial12 (Figure 1).
MSCs, an ideal cell source for regenerative therapy with no ethical issues, play an essential role in DFU by promoting re-epithelialization, cell infiltration, and angiogenesis. Several issues have been introduced for the isolation of MSCs. However, there is a discrepancy about which source is the best for DFU treatment61,62 and the feasibility of autologous and allogeneic MSCs therapy of DFU. For example, adipose-derived MSCs (AD-MSCs) isolated from distal limbs of diabetic patients with DFU was not satisfactory as an autologous AD-MSC source because of its improper phenotype and function63.
Vojtaššák et al. injected autologous BM-derived directly to the wound and into the edges of the wound. BM-derived directly to the wound and into the edges of the wound. The wound was decreased in size and increased in vascularity and dermal thickness by day 29 of combination therapy. This study described a successful therapy of chronic diabetic ulcers by applying autologous graft and autologous somatic MSCs14. Based on the evidence, a significant complication of type 2 diabetes is the imperfection of stem cells so that the disease may alter endogenous MSCs. The efficiency of autologous MSCs therapies in diabetic patients was implicated64. However, preclinical and clinical data are pretty limited, and further studies need to be explored for this issue.
A combination of MSCs and some factors or drugs together can be more effective for the treatment of DFU. Seo et al. demonstrated that either topical Exendin-4 (Ex-4), a peptide agonist of the glucagon-like peptide (GLP) receptor that promotes insulin secretion, treatment or local injection of ADSCs are effective for the treatment of experimental skin wounds in diabetic mice. ADSCs injection increased migration and proliferation of keratinocytes. While Co-administration of Ex-4 and ADSC increased migration and proliferation of endothelial cells and resulted in more improvement of re-epithelization and wound closure65. On the other hand, in one study, diabetic nude rats with DFU were transplanted with MSCs (in different doses) or insulin. As a result of this study, P-MSCs differentiated and secreted angiogenic cytokines, so ischemia damage and functional recovery were improved; however, the combination therapy with insulin administration did not indicate increased recovery66.
Similar to other stem cells, MSCs release various secretomes, which can help in wound healing. For instance, Brini et al. have investigated the effect of human ADSCs and their secretome. Their results showed that MSC’s secretome contains brain-derived neurotrophic factor (BDNF), VEGF, IGF, which may be responsible for the advantage of stem cell therapy, which is now a prevalent theory. Their results demonstrate that human adipose stem cell (hASC) and hASC- modified media remedies may encourage paths to propose that their secretome67 likely moderates cell result. Mayo et al.30 have compared the beneficial effects of mouse BM-MSCs alone and co-administration of BM-MSCs, their secretome on the skin, wound healing in non-obese diabetic (NOD) mice. They have indicated the main variations in the wound repairing kinetics of injuries in the NOD treated with secretome analyzed to those received vehicle or BM-MSCs alone.
CONCLUSION
Inconsequently, this review indicated the beneficial effect of MSCs in skin regeneration and wound healing in DFU through paracrine signaling and differentiation mechanisms. However, the role of MSCs differentiation is less due to the poor survival of these cells at the site of injury. Whereas paracrine signaling is the primary mechanism of MSCs that stimulate neovascularization and re-epithelialization and mobilization of inhabitant stem cells. MSCs-based therapy needs further investigations to determine cells’ in vivo distribution and therapeutic mechanisms to optimize its use in personalized regenerative medicine.
Abbreviations
Ang: angiopoietin, BDNF: brain-derived neurotrophic factor, bFGF: basic fibroblast growth factor, Col-1: collagen type 1, DFU: Diabetic foot ulcer, EGF: epidermal growth factor, EPO: erythropoietin, Erk: extracellular receptor kinase, Ex-4: Exendin-4, GLP: glucagon-like peptide, HIF: hypoxia-inducible factor, hASC: human adipose stem cell, IGF-1: insulin-like growth factor-1, IL: interleukin, IGFBP-7: insulin-like growth factor binding protein-7, IFN-γ: interferon-γ, KGF: keratinocyte growth factor, MMP: matrix metalloproteinase, MIP: macrophage inflammatory protein, MCP-1: monocyte chemotactic protein-1, MSCs: mesenchymal stem cells, NOD: non-obese diabetic, NGF: Nerve growth factor, PGE2: Prostaglandin E2, SDF-1: stromal cell-derived factor-1, SPARC: secreted protein acidic and rich in cysteine, TGF-β: transforming growth factor, TIMP: tissue inhibitors of metalloproteinase, TNF-α: tumor necrosis factor-α, VEGF: vascular endothelial growth factor, vWF: Von Willebrand factor.
Funding
No Funding.
Availability of data and materials
The primary data for this study is available from the authors on direct request.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
Not applicable.
References
- 1.Khadem Hh, Farsad Na, Pourghassem Gb, Ali Aa, Nemati A. Effect Of Cinnamon supplementation on blood glucose and lipid levels in type2 diabetic patients. Archives of Advances In Biosciences. 2011;2(1):2–6. [Google Scholar]
- 2.Khamaisi M, Balanson SE. Stem Cells for Diabetes Complications: A Future Potential Cure. Rambam Maimonides Med J. 2017;8(1):e0008. doi: 10.5041/RMMJ.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choby B. Diabetes Update: Prevention and Management of Diabetes Complications. FP Essentials. 2017;456:36–40. [PubMed] [Google Scholar]
- 4.Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6(1):37–53. doi: 10.4239/wjd.v6.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jhamb S, Vangaveti VN, Malabu UH. Genetic and molecular basis of diabetic foot ulcers: clinical review. J Tissue Viability. 2016;25(4):229–236. doi: 10.1016/j.jtv.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Domínguez-Bendala J, Lanzoni G, Inverardi L, Ricordi C. Concise review: mesenchymal stem cells for diabetes Stem cells translational medicine. Stem Cells Transl Med. 2012 Jan;1(1):59–63. doi: 10.5966/sctm.2011-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3(1):4 . doi: 10.1007/s13300-012-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–43. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 9.Yeboah A, Maguire T, Schloss R, Berthiaume F, Yarmush ML. Stromal Cell-Derived Growth Factor-1 Alpha-Elastin Like Peptide Fusion Protein Promotes Cell Migration and Revascularization of Experimental Wounds in Diabetic Mice. Adv Wound Care. 2017;6(1):10–22. doi: 10.1089/wound.2016.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valero C, Javierre E, García-Aznar J, Menzel A, Gomez-Benito M. Challenges in the modeling of wound healing mechanisms in soft biological tissues. Ann Biomed Eng. 2015;43(7):1654–65. doi: 10.1007/s10439-014-1200-8. [DOI] [PubMed] [Google Scholar]
- 11.Marfia G, Navone SE, Di Vito C, et al. Mesenchymal stem cells: potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis. 2015;11(4):183–206. doi: 10.1080/15476278.2015.1126018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–59. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 13.Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting non-healing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12(5):359–66. doi: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]
- 14.Vojtaššák J, Danišovič L, Kubeš M, et al. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol. Lett. 2006;27(supplement 2):134–7. [PubMed] [Google Scholar]
- 15.Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106(6):984–91. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–7. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 17.Amann B, Luedemann C, Ratei R, Schmidt-Lucke JA. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009;18(3):371–80. doi: 10.3727/096368909788534942. [DOI] [PubMed] [Google Scholar]
- 18.Rigato M, Monami M, Fadini GP. Autologous Cell Therapy for Peripheral Arterial DiseaseNovelty and Significance: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ Res. 2017;120(8):1326–40. doi: 10.1161/CIRCRESAHA.116.309045. [DOI] [PubMed] [Google Scholar]
- 19.Rashtbar M, Hadjati J, Ai J, et al. Characterization of decellularized ovine small intestine submucosal layer as extracellular matrix‐based scaffold for tissue engineering. J Biomed Mater Res B Appl Biomater. 2017;106(3):933–944. doi: 10.1002/jbm.b.33899. [DOI] [PubMed] [Google Scholar]
- 20.Casado JG, Tarazona R, Sanchez-Margallo F. NK and MSCs crosstalk: the sense of immunomodulation and their sensitivity. Stem Cell Rev. 2013;9(2):184–9. doi: 10.1007/s12015-013-9430-y. [DOI] [PubMed] [Google Scholar]
- 21.Shetty P, Cooper K, Viswanathan C. Comparison of proliferative and multilineage differentiation potentials of cord matrix, cord blood, and bone marrow mesenchymal stem cells. Asian J Transfus Sci. 2010;4(1):14 . doi: 10.4103/0973-6247.59386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arno AI, Amini-Nik S, Blit PH, et al. Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res Ther. 2014;5(1):28. doi: 10.1186/scrt417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 24.Jeon YJ, Kim J, Cho JH, Chung HM, Chae JI. Comparative analysis of human mesenchymal stem cells derived from bone marrow, placenta, and adipose tissue as sources of cell therapy. J Cell Biochem. 2016;117(5):1112–25. doi: 10.1002/jcb.25395. [DOI] [PubMed] [Google Scholar]
- 25.Parikh PP, Liu Z-J, Velazquez OC. A Molecular and Clinical Review of Stem Cell Therapy in Critical Limb Ischemia. Stem Cells Int. 2017;2017:3750829. doi: 10.1155/2017/3750829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng X, Tang Y, Hu K, et al. Three-week topical treatment with placenta-derived mesenchymal stem cells hydrogel in a patient with diabetic foot ulcer: A case report. Medicine. 2017;96(51) doi: 10.1097/MD.0000000000009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flegg JA, Menon SN, Maini PK, McElwain DS. On the mathematical modeling of wound healing angiogenesis in skin as a reaction-transport process. Front Physiol. 2015;6: 262 . doi: 10.3389/fphys.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu C, Huang S, Gao D, et al. Angiogenic effect of mesenchymal stem cells as a therapeutic target for enhancing diabetic wound healing. Int J Low Extrem Wounds. 2014;13(2):88–93. doi: 10.1177/1534734614534977. [DOI] [PubMed] [Google Scholar]
- 29.Zou J-P, Huang S, Peng Y, et al. Mesenchymal Stem Cells/Multipotent Mesenchymal Stromal Cells (MSCs) Potential Role in Healing Cutaneous Chronic Wounds. Int J Low Extrem Wounds. 2012;11(4):244–53. doi: 10.1177/1534734612463935. [DOI] [PubMed] [Google Scholar]
- 30.de Mayo T, Conget P, Becerra-Bayona S, Sossa CL, Galvis V, Arango-Rodríguez ML. The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. Plos One. 2017;12(6):e0177533. doi: 10.1371/journal.pone.0177533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Zhu F, Zhang M, et al. Stromal cell-derived factor-1 enhances wound healing through recruiting bone marrow-derived mesenchymal stem cells to the wound area and promoting neovascularization. Cells Tissues Organs. 2013;197(2):103–13. doi: 10.1159/000342921. [DOI] [PubMed] [Google Scholar]
- 32.Yildirimer L, Thanh NTK, Seifalian AM. Skin regeneration scaffolds: a multimodal bottom-up approach. Trends Biotechnol. 2012;30(12):638–48. doi: 10.1016/j.tibtech.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Aguilera V, Briceño L, Contreras H, et al. Endothelium trans differentiated from Wharton’s jelly mesenchymal cells promote tissue regeneration: potential role of soluble pro-angiogenic factors. PLoS One. 2014;9(11):e111025. doi: 10.1371/journal.pone.0111025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Zhao Y, Hao H, et al. Mesenchymal Stem Cell–Conditioned Medium Improves the Proliferation and Migration of Keratinocytes in a Diabetes-Like Microenvironment. Int J Low Extrem Wounds. 2015;14(1):73–86. doi: 10.1177/1534734615569053. [DOI] [PubMed] [Google Scholar]
- 35.Liang X, Ding Y, Zhang Y, Tse H-F, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–59. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 36.Fan C-G, Zhang Q-j, Zhou J-r. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2011;7(1):195–207. doi: 10.1007/s12015-010-9168-8. [DOI] [PubMed] [Google Scholar]
- 37.Pereira T, Ivanova G, Caseiro AR, et al. MSCs conditioned media and umbilical cord blood plasma metabolomics and composition. PLoS One. 2014;9(11):e113769. doi: 10.1371/journal.pone.0113769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10(3):244–58. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joensuu K, Uusitalo‐Kylmälä L, Hentunen TA, Heino TJ. Angiogenic potential of human mesenchymal stromal cell and circulating mononuclear cell co‐cultures is reflected in the expression profiles of pro-angiogenic factors leading to endothelial cell and pericyte differentiation. J Tissue Eng Regen Med. 2018;12(3):775–83. doi: 10.1002/term.2496. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Xu Y, Zhao J, et al. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9(4):e96161. doi: 10.1371/journal.pone.0096161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo Y-R, Wang C-T, Cheng J-T, Kao G-S, Chiang Y-C, Wang C-J. Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant. 2016;25(1):71–81. doi: 10.3727/096368915X687921. [DOI] [PubMed] [Google Scholar]
- 42.Kaibuchi N, Iwata T, Yamato M, Okano T, Ando T. Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater. 2016;42:400–10. doi: 10.1016/j.actbio.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Kato Y, Iwata T, Morikawa S, Yamato M, Okano T, Uchigata Y. Allogeneic transplantation of an adipose-derived stem cell sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes. 2015;64(8):2723–34. doi: 10.2337/db14-1133. [DOI] [PubMed] [Google Scholar]
- 44.Malhotra S, Hu MS, Marshall CD, et al. Mesenchymal stromal cells as cell-based therapeutics for wound healing. Stem Cells Int. 2016;2016:4157934. doi: 10.1155/2016/4157934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomathysankar S, Halim AS, Yaacob NS. Proliferation of keratinocytes induced by adipose-derived stem cells on a chitosan scaffold and its role in wound healing, a review. Arch. Plast. Surg. 2014;41(5):452–7. doi: 10.5999/aps.2014.41.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370–8. doi: 10.1111/bjd.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al‐Shaibani MB, Wang Xn, Lovat PE, Dickinson AM. Cellular Therapy for Wounds: Applications of Mesenchymal Stem Cells in Wound Healing. Wound healing–new insights into ancient challenges. London: InTech. 2016:99–131. [Google Scholar]
- 48.Wang L-T, Ting C-H, Yen M-L, et al. Human mesenchymal stem cells (MSCs) for treatment towards immune-and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23(1):76. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walter MNM, Wright KT, Fuller HR, MacNeil S, Johnson WEB. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: An in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010;316(7):1271–81. doi: 10.1016/j.yexcr.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Cao Y, Gang X, Sun C, Wang G. Mesenchymal Stem Cells Improve Healing of Diabetic Foot Ulcer. J. Diabetes Res. 2017;2017:9328347. doi: 10.1155/2017/9328347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta GJ, Karki K, Jain P, Saxena AK. Autologous Bone Marrow Aspirate Therapy for Skin Tissue Engineering and Tissue Regeneration. Adv Wound Care. 2017;6(4):135–142. doi: 10.1089/wound.2016.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Javazon EH, Keswani SG, Badillo AT, et al. Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen. 2007;15(3):350–9. doi: 10.1111/j.1524-475X.2007.00237.x. [DOI] [PubMed] [Google Scholar]
- 53.Kim W-S, Park B-S, Sung J-H, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 54.Lee EY, Xia Y, Kim WS, et al. Hypoxia‐enhanced wound‐healing function of adipose‐derived stem cells: Increase in stem cell proliferation and up‐regulation of VEGF and bFGF. Wound Repair Regen. 2009;17(4):540–7. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 55.Smith AN, Willis E, Chan VT, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010;316(1):48–54. doi: 10.1016/j.yexcr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park S-R, Kim J-W, Jun H-S, Roh JY, Lee H-Y, Hong I-S. Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing. Mol Ther. 2018;26(2):606–17. doi: 10.1016/j.ymthe.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ong HT, Redmond SL, Marano RJ, et al. Paracrine Activity from Adipose-Derived Stem Cells on In Vitro Wound Healing in Human Tympanic Membrane Keratinocytes. Stem Cells Dev. 2017;26(6):405–18. doi: 10.1089/scd.2016.0204. [DOI] [PubMed] [Google Scholar]
- 58.Sah SK, Kim HY, Lee JH, et al. Effects of Human Mesenchymal Stem Cells Coculture on Calcium-Induced Differentiation of Normal Human Keratinocytes. Stem Cells. 2017;35(6):1592–602. doi: 10.1002/stem.2593. [DOI] [PubMed] [Google Scholar]
- 59.Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316(14):2213–9. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. The Lancet. 2005;366(9498):1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 61.Vija L, Farge D, Gautier J-F, et al. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009;35(2):85–93. doi: 10.1016/j.diabet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Anzalone R, Iacono ML, Loria T, et al. Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev. 2011;7(2):342–63. doi: 10.1007/s12015-010-9196-4. [DOI] [PubMed] [Google Scholar]
- 63.Kočí Z, Turnovcová K, Dubský M, et al. Characterization of human adipose tissue‐derived stromal cells isolated from diabetic patient’s distal limbs with critical ischemia. Cell Biochem Funct. 2014;32(7):597–604. doi: 10.1002/cbf.3056. [DOI] [PubMed] [Google Scholar]
- 64.Shin L, Peterson DA. Impaired therapeutic capacity of autologous stem cells in a model of type 2 diabetes. Stem Cells Transl. Med. 2012;1(2):125–35. doi: 10.5966/sctm.2012-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seo E, Lim JS, Jun J-B, Choi W, Hong I-S, Jun H-S. Exendin-4 in combination with adipose-derived stem cells promotes angiogenesis and improves diabetic wound healing. J Transl Med. 2017;15(1):35 . doi: 10.1186/s12967-017-1145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang L, Li Z, Ma T, et al. Transplantation of human placenta-derived mesenchymal stem cells alleviates critical limb ischemia in diabetic nude rats. Cell Transplant. 2017;26(1):45–61. doi: 10.3727/096368916X692726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brini AT, Amodeo G, Ferreira LM, et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci Rep. 2017;7(1):9904. doi: 10.1038/s41598-017-09487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu SC, Pollak R, Frykberg RG, et al. Safety and efficacy of intramuscular human placenta‐derived mesenchymal stromal‐like cells (cenplacel [PDA‐002]) in patients who have a diabetic foot ulcer with peripheral arterial disease. Int. Wound J. 2017;14(5):823–9. doi: 10.1111/iwj.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edwards SS, Zavala G, Prieto CP, et al. Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton’s jelly in dermal regeneration. Angiogenesis. 2014;17(4):851–66. doi: 10.1007/s10456-014-9432-7. [DOI] [PubMed] [Google Scholar]
- 70.O’Loughlin A, Kulkarni M, Creane M, et al. Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer. Diabetes. 2013;62(7):2588–94. doi: 10.2337/db12-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim S-W, Zhang H-Z, Guo L, Kim J-M, Kim MH. Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS One. 2012;7(7):e41105. doi: 10.1371/journal.pone.0041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirana S, Stratmann B, Prante C, et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int J Clin Pract. 2012;66(4):384–93. doi: 10.1111/j.1742-1241.2011.02886.x. [DOI] [PubMed] [Google Scholar]
- 73.Amann B, Lüdemann C, Ratei R, Schmidt-Lucke J. Autologous bone-marrow stem-cell transplantation for induction of arteriogenesis for limb salvage in critical limb ischaemia. Zentralbl Chir. 2009;134(4):298–304. doi: 10.1055/s-0029-1224532. [DOI] [PubMed] [Google Scholar]
- 74.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow–derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13(6):1299–312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 75.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kato J, Kamiya H, Himeno T, et al. Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. J Diabetes Complications. 2014;28(5):588–95. doi: 10.1016/j.jdiacomp.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006;326(3):725–36. doi: 10.1007/s00441-006-0270-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data for this study is available from the authors on direct request.