Abstract
Cyclooxygenase (COX-1 and COX-2)- and 5-lipoxygenase (5-LOX)-catalyzed biosynthesis of eicosanoids play important roles in inflammation and chronic diseases. The vitamin E family has four tocopherols and tocotrienols. We have shown that the metabolites of δ-tocopherol (δT) and δ-tocotrienol (δTE), i.e., δT-13’-carboxychromanol (COOH) and δTE-13’-COOH, respectively, inhibit COX-1/-2 and 5-LOX activity, but the nature of how they inhibit 5-LOX is not clear. Further, the impact of tocopherols and tocotrienols on COX-1/-2 or 5-LOX activity has not been fully delineated. In this study, we found that tocopherols and tocotrienols inhibited human recombinant COX-1 with IC50s of 1–12 μM, and suppressed COX-1-mediated formation of thromboxane in collagen-stimulated rat’s platelets with IC50s of 8–50 μM. None of the vitamin E forms directly inhibited COX-2 activity. 13’-COOHs inhibited COX-1 and COX-2 enzyme activity with IC50s of 3–4 and 4–10 μM, respectively, blocked thromboxane formation in collagen- and ionophore-stimulated rats’ platelets with IC50s of 1.5-2.5 μM, and also inhibited COX-2-mediated prostaglandins in stimulated cells. Using enzyme kinetics, we observed that δT-13’-COOH, δTE-13’-COOH and δTE competitively inhibited 5-LOX activity with Ki of 1.6, 0.8 and 2.2 μM, respectively. These compounds decreased leukotriene B4 from stimulated neutrophil-like cells without affecting translocation of 5-LOX from cytosol to the nucleus. Our study reveals inhibitory effects of vitamin E forms and 13’-COOHs on COX-1 activity and thromboxane formation in platelets, and elucidates mechanisms underlying their inhibition of 5-LOX. These observations are useful for understanding the role of these compounds in disease prevention and therapy.
Keywords: Tocopherol, Tocotrienol, 13′-carboxychromanol, Inflammation, Cancer, Lipoxygenase, Eicosanoids, Cyclooxygenase
1. Introduction
5-Lipoxygenase (5-LOX)- and cyclooxygenases (COX-1/-2)-catalyzed oxidation of arachidonic acid (AA) leads to formation of leukotrienes (LTs) and thromboxane/prostaglandins, respectively [1]. These eicosanoids are bioactive lipids regulating diverse biological functions and contributing to diseases. In particular, prostaglandin E (PGE2) and leukotriene B4 (LTB4) are potent mediators of inflammation and contribute to inflammation-associated diseases including asthma and cancer [1,2]. For instance, PGE2, LTB4, COX-2 and 5-LOX have been reported to be elevated in prostate and colon cancer tissues, and shown to stimulate cancer cell growth and promote chemotherapy drug resistance [1]. Further, thromboxane A2 (TxA2), which is synthesized by COX-1-mediated reaction in platelets, stimulates platelet aggregation, and overproduction of this eicosanoid increases the risk of cardiovascular diseases [3]. Given the role of these enzymes and related eicosanoids in inflammation and chronic diseases, targeting 5-LOX and COXs is considered effective for prevention and treatment of chronic diseases. For example, Zileuton, a 5-LOX inhibitor, significantly reduces allergen-induced nasal congestion, and blocks leukotriene formation in the nasal lavage fluids of patients with allergic rhinitis after challenge with specific allergens [4]. Further, COXs inhibitors are used for treating inflammation and prove effective for prevention of colorectal cancer [1]. Despite strong clinical implications, COXs inhibitors have well-documented adverse effects and zileuton is the only clinicallyused 5-LOX inhibitor.
Interestingly, we have shown that natural forms of vitamin E and metabolites are capable of inhibiting 5-LOX and COX-mediated eicosanoids in cell and animal studies [5,6]. The vitamin E family consists of α-, β-, γ- or δ-tocopherol (αT, βT, γT, and δT) and α-, β-, γ- or δ-tocotrienol (αTE, βTE, γTE, and δTE) (Fig. 1A). These vitamin E forms are readily metabolized by cytochrome P450-mediated ω-hydroxylation and oxidation of the 13’-carbon on the hydrophobic side chain to produce 13’-carboxychromanol (13’-COOH), which is further degraded by β-oxidation to form the terminal urinary-excreted metabolite 2-(β-carboxyethyl)-6-hydroxychroman (CEHC, or 3’-carboxychromanol) (Fig. 1B) [5]. We have shown that γT and δT are stronger than αT in inhibition of ionophore-stimulated formation of LTB4 and LTC4 in neutrophils via blocking Ca2+ influx and 5-LOX translocation from cytosol to the nucleus, a key step leading to 5-LOX activation, whereas these tocopherols do not directly inhibit human 5-LOX activity at physiologically relevant concentrations [7]. Unlike tocopherols, δT-13’-carboxychromanol (δT-13’-COOH) (Fig. 1B), a metabolite derived from δT, inhibits 5-LOX activity, and ionophore- or thapsigargin (THAP)-stimulated leukotriene (LT) formation in neutrophils [7]. Interestingly, δTE-13’-COOH, a metabolite of δTE with three double bonds in the side chain (Fig. 1B), appears to be slightly stronger than δT-13’-COOH in inhibition of 5-LOX [8]. Further, we have shown that 13’-COOHs are competitive inhibitors of COXs [9]. Therefore, δTE-13’-COOH, and δT-13’-COOH are dual inhibitors of COX/5-LOX. Consistently, Pein et al [10] confirmed that δTE-13’-COOH inhibits 5-LOX and showed that αT-13’-COOH, a metabolite from αT, is also a potent inhibitor of 5-LOX.
Fig. 1.
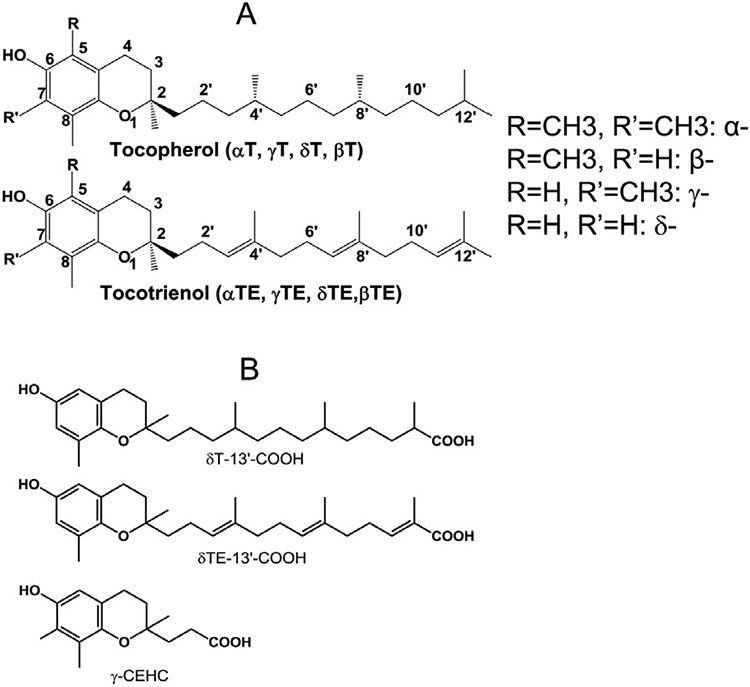
Structures of natural forms of vitamin E and metabolites. (A) Structures of α-, β-, γ-, δ-tocopherol (αT, βT, γT, δT) and α-, β-, γ-, δ-tocotrienol (αTE, βTE, γTE, δTE). (B) δT-, δTE-13’-carboxychromanol (δT-13’-COOH, δTE-13’-COOH); 2-(β-carboxyethyl)-6-hydroxchroman (γ-CEHC, or 3’-COOH).
Despite these findings, there are knowledge gaps concerning the effects of vitamin E forms and 13’-COOHs on 5-LOX and COX-1/-2 activity and their catalyzed eicosanoids. In particular, the mechanism underlying 13’-COOHs’ inhibition of 5-LOX is not known. Although Pein et al [10] suggested that δTE-13’-COOH and αT-13’-COOH are allosteric 5-LOX inhibitors, enzyme kinetics, which is considered vital for characterization of enzyme inhibition [11], has not been conducted and therefore the mechanism remains elusive. In addition, the impact of tocopherols and tocotrienols on COX-1 and its mediated eicosanoids has not been delineated. In this paper, we investigated the effect of vitamin E forms, δTE-13’-COOH and δT-13’-COOH on human recombinant COX-1 activity and COX-1 mediated thromboxane B2 (TXB2) formation in platelets. We also conducted enzyme kinetics and cell studies to elucidate the nature of how tocotrienols and 13’-COOHs inhibit 5-LOX and whether those compounds affect 5-LOX activation in neutrophil-like HL-60 cells.
2. Materials and methods
2.1. Materials
Human recombinant COX-1, COX-2, 5-LOX, and arachidonic acid (AA) were from Cayman Chemical (Ann Arbor, MI). Cell culture reagents were from American Type Culture Collection or Invitrogen. Collagen fibrils (type I) from equine tendons were obtained from Chrono-Log Corp (Havertown, PA). Zileuton (a 5-LOX inhibitor) was purchased from Tocris Cookson (Minneapolis, MN). The primary and secondary antibody for detecting 5-LOX were purchased from BD Biosciences Pharmingen and Santa Cruz Biotechnology, respectively. Human recombinant IL-1β, bacterial lipopolysaccharide (LPS), and all other chemicals were from Sigma (St. Louis, MO).
2.2. COX-1 and COX-2 activity assay using purified enzymes
The COX-1 and COX-2 activity assays were performed according to the procedure modified from Cayman Chemical. Briefly, test compounds were incubated with human recombinant COX-1 or COX-2 in the reaction buffer at room temperature for 10 min. Enzymatic reactions were initiated by addition of AA at a final concentration of 5 μM for 2 min and stopped by addition of 0.1 M HCl containing stannous chloride, which reduces prostaglandin (PG)G2, and PGH2 to PGF2α. PGF2α was quantified using ELISA assays.
2.3. Preparation of washed platelets and platelet activation
The protocol of animal use was approved by Purdue Animal Use and Care Committee. Blood was collected from the heart of rats and dispensed into centrifuge tubes containing 10% (v/v) anticoagulant solution (65 mM citric acid/85 mM sodium citrate/2% glucose, pH 7.4). Then, the blood was centrifuged at 210 X g for 10 min at room temperature. The supernatant of platelet-rich plasma was mixed with 50% Hanks’ balanced salt solution supplemented with 25 mM HEPES (HHBSS)/30% anticoagulant solution and further centrifuged (760 X g, 10 min). The platelet pellet was re-suspended in fresh HHBSS/10% anticoagulant and centrifuged at 760 X g for 10 min, and the pellet was resuspended in HHBSS/10% anticoagulant [12].
Isolated platelets (200μl at 108 cells/mL) were incubated with test compounds or DMSO (Ctrl) for 25 min at 37°C, and were then stimulated by 20 μ/mL collagen or 1–2 μM calcium ionophore (A23187) with addition of CaCl2 (final 1–2 mM) for 10 min. The reactions were terminated by adding 100 μL of methanol. After centrifugation, the supernatant was removed and TXB2 was analyzed using enzyme immunoassay (EIA) kit from Cayman Chemical Company.
2.4. COX-2 activity in the intact-cell assays
Murine RAW264.7 macrophages were routinely cultured in DMEM containing 10% fetal bovine serum (FBS). Human lung epithelial cancer cells (A549) were obtained from American Type Culture Collection and cultured in RPMI 1640 supplemented with 10% FBS. The effect on COX-2 activity was examined in intact-cell assays as previously described [13]. Briefly, COX-2 was induced in A549 cells or RAW264.7 macrophages by 0.5 ng/mL IL-1β for 6 h or 0.1 μg/mL LPS for 14–16 h, respectively. Cells were then incubated with fresh medium-1% FBS containing test compounds or DMSO for 10 min, and then added with 5 μM AA for 5 min. PGE2 or PGD2 accumulated in the media was measured as an index of COX-2 activity using ELISA (Cayman Chemical).
2.5. Assessment of 5-LOX activity in enzyme assays
Potential effects on the activity of 5-LOX were evaluated using the ferrous oxidation-xylenol orange assay (FOX assay) as previously described [14]. Briefly, human recombinant 5-LOX (4.5 U) was pre-incubated with tested compounds for 4 min at room temperature in 50 mM Tris-HCl buffer (pH 7.4) containing 0.4 mM CaCl2. Reactions were initiated by addition of AA (final 75 μM). Four min later, the reaction was terminated by FOX reagent containing 25 μM sulfuric acid, 100 μM xylenol orange, and 100 μM ferrous sulfate dissolved in methanol/water (9:1). After color development, the absorbance was measured at 560 and 575 nm using a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA).
2.6. LTB4 formation in stimulated neutrophil-like cells
Human promyeloblast HL-60 cells (from ATCC) were routinely maintained in RPMI 1640 medium supplemented with 10% FBS under 5% CO2. For induction of neutrophil differentiation, 2.2–2.4 × 105 cells/mL were incubated in RPMI 1640 containing 10% FBS plus 1.25% DMSO for 5 d [15-17]. Our data show that neutrophil morphology became obvious and 5-LOX expression peaked after 5-d differentiation. To study the effect on LTB4, differentiated HL-60 cells (1.6 × 106 ) were preincubated with vehicle control, vitamin E forms, δT-13’-COOH or δTE-13’-COOH in DMEM-1% FBS at 37°C for 10- or 30-min. Cells were then stimulated with THAP (1–2 μM) or A23187 (1–10 μM) for another 15 min. After brief centrifugation, the medium was collected, and LTB4 was measured by ELISA (Cayman Chemicals).
2.7. 5-LOX translocation by the western blotting
To study the effect on 5-LOX translocation, differentiated HL-60 cells were preincubated with tested compounds or DMSO for 30 min and then stimulated by THAP or A23187 for 15 min. The nuclear and cytosol fractions were isolated by NEPER nuclear and cytoplasmic extraction reagent (Thermo Scientific). The resulting solution was heated at 95°C for 8 min. Proteins (25–50 μg) were resolved on 10% SDS-PAGE gels, transferred onto a polyvinylidene floride membrane (Millippore), and probed by a 5-LOX antibody. Membranes were exposed to chemiluminescent reagent (PerkinElmer) and visualized on a Kodak film.
2.8. Enzyme kinetics to study the nature of how 13’-COOHs and δTE inhibits 5-LOX
The 5-LOX activity was measured by monitoring the initial rate of oxygen consumption (nanomoles per min) in a MT200 glass chamber using a model 1302 oxygen electrode (Warner Instruments, LCC). The standard assay solution contained 50 mM Tris (pH 7.4) and 0.4 mM CaCl2, in which human recombinant 5-LOX and test compounds or DMSO were added. After 10-min pre-incubation, the enzyme reaction was initiated by injection of AA (5–50 μM) into the reaction chamber. The initial rate of the oxygen consumption during the 5-LOX reaction was recorded using an oxygen microelectrode. The Km and Vmax of the reactions in the absence and presence of 13’-COOHs or δTE were calculated using nonlinear regression (Graph-Pad Prism 7.03). The nature of competitive inhibition is shown by Lineweaver-Burk plots.
2.9. Statistical analyses
For multiple statistical analyses, we performed one-way ANOVA with subsequent Dunnett’s test to compare a number of treatments to a control group. P<.05 or P<.01 were considered significant difference.
3. Results
3.1. The effect of 13’-COOHs and vitamin E forms on human COX-1 and COX-2 activity
Previously, we have shown that δT-13’-COOH, which is generated via metabolism of δT in A549 cells, competitively inhibits COX−1/−2 [9]. Here we investigated the effect of chemically-synthesized δT-13’-COOH and δTE-13’-COOH as well as natural forms of vitamin E on COX-1 and COX-2 in enzyme assays. Similar to naturally-formed metabolite from δT [9], the synthetic δT-13’-COOH inhibited human recombinant COX-1 and COX-2 with an apparent IC50 of 2.5 and 4 μM, respectively (Fig. 2A and 2B). δTE-13’-COOH appeared to be slightly less potent than δT-13’-COOH in inhibition of COX-1 and COX-2 with IC50 values of 4 and ~10 μM, respectively (Fig. 2A and 2B). Interestingly, vitamin E forms γT, δT, γTE, and δTE strongly inhibited COX-1 with IC50 values of 1–2.5 μM, and α-T inhibited COX-1 with IC50 of ~12 μM (Fig. 2C). Under the same condition, aspirin inhibited COX-1 activity with IC50 of 40 μM (data not shown). In contrast to the effect on COX-1, none of these vitamin E forms significantly inhibit COX-2 (Fig. 2D), consistent with our previous observation [9]. The IC50s of the test compounds on COX-1/COX-2 activity are summarized in the table (Fig. 2).
Fig. 2.
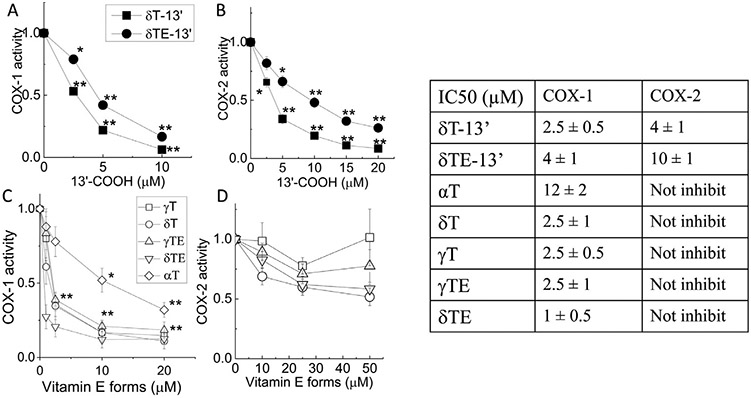
Effects of 13’-COOHs and natural forms of vitamin E on human recombinant COX-1 and COX-2 activity. Test compounds or solvent control (DMSO) were incubated with COX-1 or COX-2 at room temperature for 10 min, and AA at a final concentration of 5 μM was added for 2 min. The reaction was stopped by addition of 0.1 M HCl with Stannous chloride. PGF2α was quantified using ELISA assays. The effects of 13’-COOHs on COX-1 (A) or COX-2 (B) activity are shown in solid symbols. The effects of vitamin E forms on COX-1 (C) and COX-2 (D) activity are shown in open symbols. The COX-1 or COX-2 activity are calculated as the ratio of PGF2α formation in the presence of tested compounds to that in solvent control. Table: IC50s of 13’-COOHs and natural forms of vitamin E. Results were expressed as mean±SEM (n=3-13). *P<.05 and **P<.01 indicate significant differences between tested compounds and solvent controls. δT-13’: δT-13’-COOH; δTE-13’: δTE-13’-COOH; αT, γT, δT: α-, γ-, δ-tocopherol; γTE, δTE: γ-, δ-tocotrienol.
3.2. The effect of 13’-COOHs and vitamin E forms on COX-1-mediated TXB2 in rats’ platelets and COX-2-mediated PGE2 formation in macrophages or epithelial cells
To further evaluate the effects of 13’-COOHs and vitamin E forms on COX-1-catalyzed product formation in cells, we used freshly isolated rats’ platelets, which upon stimulation, produce TXA2 via a COX-1-catalyzed reaction and TXA2 is quickly converted to stable metabolite TXB2. In our study, when stimulated by collagen (20 μM) or A23187 (1–2 μM), rats’ platelets released 2.1–10 ng/mL or 18–75 ng/mL of TXB2, respectively, compared to the baseline of 0.5–1 ng/mL. As shown in Figure 3A and B, δT-13’-COOH and δTE-13’-COOH dose-dependently inhibited collagen- or A23187-induced TXB2 formation with IC50 values of 1.5–2.5 μM. Interestingly, γT, δT, γTE, and δTE inhibited TXB2 with estimated IC50 values of the order of 5–10 μM for δTE and γT, 10–20 μM for γTE and ~25 μM for δT in rats’ platelets stimulated by collagen, a physiologically relevant stimulator (Fig. 3C). However, when the platelets were triggered by A23187, the IC50s for γT, δT, γTE, and δTE increased to over 30 μM (Fig. 3D). αT decreased collagen- or A23187-stimulaed TXB2 with IC50s of ~50 μM (Fig. 3E). Overall, the relative inhibitory potency showed an order of δTE ≈ γT>γTE>αT, δT. These cellular data indicate that the ability of these vitamin E forms to inhibit COX-1 is not as strong as that shown in enzyme assays.
Fig. 3.
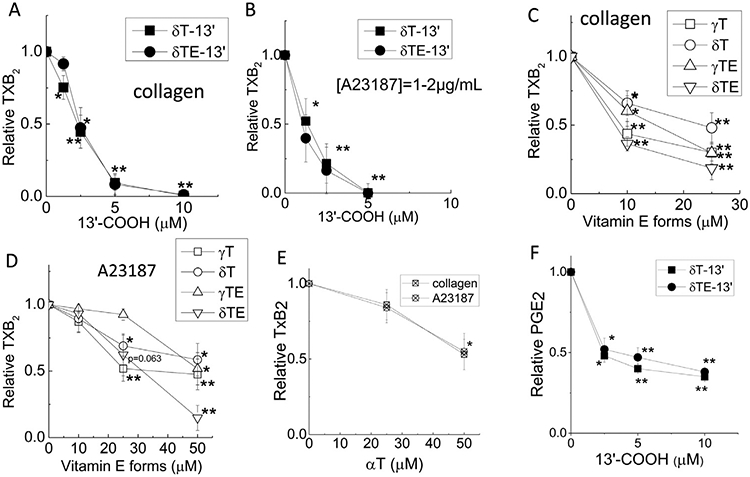
Effects of 13’-COOHs and natural forms of vitamin E on COX-1-catalyzed synthesis of thromboxane in rat platelets; The effect of 13’-COOHs on COX-2 mediated formation of PGE2 in A549 cells. Freshly isolated rat’s platelets were pretreated with vehicle or test compounds for 25 min, and then stimulated with 20 μg/mL collagen or 1–2 μM A23187 in the presence of 1–2 mM CaCl2 for 10 min. TXB2 was quantified using ELISA assays. The effects of 13’-COOHs on collagen (A)- and A23187 (B)-stimulated formation of TXB2 are shown in solid symbols. The effects of γT, δT, γTE and δTE on collagen (C)- and A23187 (D)-stimulated TXB2 are shown in open symbols. E – The effect of αT on TXB2 formation. Panel F – The effect of 13’-COOHs on PGE2 in IL-1β-stimulated A549 cells (see Materials and Methods). Relative TXB2 (PGE2) is the ratio of TXB2 (PGE2) concentrations in the presence of tested compounds to those in solvent controls under stimulated condition. Data are mean±SEM (n=7–8). *P<.05 and **P<.01 indicate significant differences between treatments and solvent controls. Abbreviations are listed under Figure 2.
In agreement with inhibition of COX-2 activity, δT-13’-COOH and δTE-13’-COOH inhibited COX-2-mediated PGE2 in IL-1β-stimulated A549 epithelial cells with IC50s of 2.5–5 μM (Fig. 3F). We also observed similar inhibitory effect of 13’-COOHs on PGE2 and PGD2 in LPS-stimulated macrophages (not shown).
3.3. 13’-COOHs are much stronger than CEHC in inhibiting 5-LOX activity; Tocotrienols, unlike tocopherols, inhibit 5-LOX activity
Like our previous observation with naturally-formed δT-13’-COOH [7], synthetic δT-13’-COOH inhibited the 5-LOX activity with IC50 of 1.6 μM. Under the same condition, δTE-13’-COOH inhibited 5-LOX with an IC50 of 0.5 μM (Fig. 4A) and zileuton, a known 5-LOX inhibitor, showed an IC50 of ~1 μM. γ-CEHC, a short-chain carboxychromanol, weakly inhibited 5-LOX with an IC50 of >50 μM (Table in Fig. 4). Further, tocotrienols differentially inhibited human 5-LOX, at the relative potency of γTE, δTE > αTE, with IC50s of 4.3, 4.3 and >10 μM, respectively (Fig. 4). Unlike these compounds, we previously show that tocopherols fail to inhibit 5-LOX under 50 μM [7].
Fig. 4.
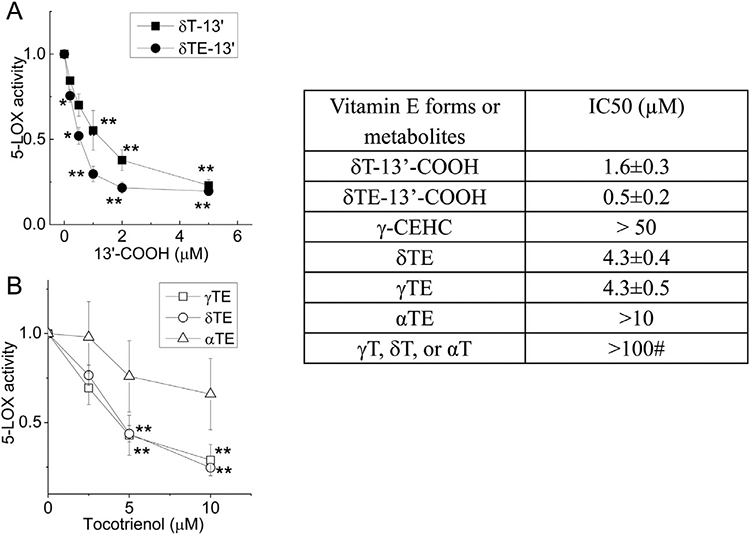
Effects of 13’-COOHs and tocotrienols on human recombinant 5-LOX activity. The effect of tested compounds on human recombinant 5-LOX was examined using FOX assay as described in Materials and Methods. A – The effect of δT- and δTE-13’-COOH on 5-LOX activity. B - The effect of αTE, γTE and δTE on 5-LOX activity. The 5-LOX activity is normalized to solvent controls. Table – IC50s of tested compounds. *P<.05 and **P<.01 indicate significant differences between the treatment and solvent controls. Data are mean±SEM (n=3–5). Abbreviations are listed under Figure 2. #(7) in the table.
3.4. γTE, δTE and 13’-COOH inhibited LTB4, but did not affect 5-LOX translocation from cytosol to the nucleus, in THAP- or A23187-stimulated neutrophil-like HL-60 cells
Ca2+-mobilizing agents such as THAP or calcium ionophores A23187 stimulate neutrophils via increasing cytosolic calcium, an essential step for 5-LOX activation and its mediated LTB4 formation [18]. A23187 increases membrane permeability while facilitating calcium influx, and THAP targets endoplasmic reticulum Ca2+− ATPase. In the present study, the differentiated HL-60 cells released LTB4 varying from 2.4–34.2 ng/mL when stimulated by 1–10 μM of THAP compared to 0.01–0.05 ng/mL by non-differentiated or unstimulated cells. We observed that δT-13’-COOH and δTE-13’-COOH, after pre-incubation with cells for 30 min, inhibited THAP-stimulated LTB4 formation with the IC50 of 7.5 μM (Fig. 5A). γTE and δTE, but not αTE, dose-dependently inhibited LTB4 with an IC50 of ~5 μM (Fig. 5B). Interestingly, when the pre-incubation time was reduced to 10 min, the inhibitory potency of δT-13’COOH, δTE-13’COOH, γTE, and δTE diminished as indicated by IC50 values of 11, 11, 9 and 8 μM, respectively (Data now shown). Additionally, 13’-COOHs and δTE inhibited A23187-stimulated LTB4 in neutrophil-like HL-60 cells (data not shown). Unlike γTE or δTE, γT and δT at up to at 50 μM had no significant effect on THAP-stimulated LTB4 (not shown), which is consistent with our previous observation [7].
Fig. 5.
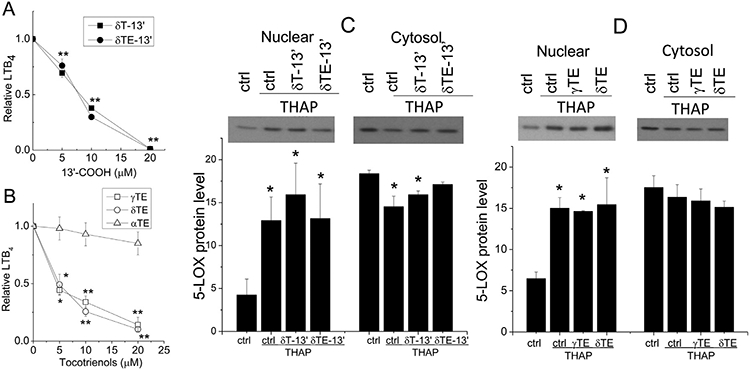
Effects of 13’-COOHs and tocotrienols on LTB4 formation and 5-LOX translocation in stimulated neutrophil-like HL-60 cells. Differentiated HL-60 cells were preincubated with 13’-COOHs or tocotrienols for 30 min and then activated by THAP (1–2 μM) for 15 min. LTB4 was measured by ELISA assays (Panel A, B). Relative LTB4 is the ratio of LTB4 in the presence of tested compounds to that in solvent controls under stimulated condition. The effect of 13’-COOHs (20 μM) or tocotrienols (20 μM) on 5-LOX in the cytosolic and nuclear fractions were probed by Western blotting (Panel C and D). The results are reported as mean±SEM (n=3–11). *P<.05 and **P<.01 indicate significant differences between the treatment and solvent control. Abbreviations are listed under Figure 2.
It is well established that leukotriene formation in granulocytes requires activation of 5-LOX through calcium signalling. Specifically, when leukocytes are stimulated, intracellular calcium is elevated, which leads to activation of PKC and MAPK signaling. Such activation and signaling events lead to translocation of 5-LOX from cytosol to the nuclear membrane where this enzyme interacts with 5-lipoxygenase-activating protein (FLAP) for 5-LOX-catalyzed oxygenation of AA [2]. We previously show that γT or δT, while do not inhibit 5-LOX activity, decrease LTB4 formation via blocking ionophore-stimulated Ca2+ influx and 5-LOX translocation [7]. In the present study, we observed that THAP triggered significant increase of 5-LOX in the nucleus and slight decrease in cytosol, consistent with 5-LOX translocation (Fig. 5). δT-13’-COOH, δTE-13’-COOH, γTE or δTE did not show any significant impact on THAP-induced 5-LOX translocation (Fig. 5C and 5D). Similar results were observed in A23187-stimulated cells (data not shown), although we previously observed that γTE modestly blocked A23187-stimulated 5-LOX translocation [7]. This discrepancy may be due to varied amounts of A23187, which may result in varied inhibition of LTB4 formation by tocopherols and γTE [7]. Nevertheless, the lack of affecting THAP-induced translocation suggests that δT-13’-COOH, δTE-13’-COOH, γTE, and δTE dampened LTB4 in cells by inhibition of 5-LOX without affecting 5-LOX translocation or the upstream signaling.
3.5. Enzyme kinetics reveal that 13’-COOHs and δTE are competitive inhibitors of 5-LOX
To elucidate the molecular mechanism underlying inhibition of 5-LOX, we used enzyme kinetics to investigate the nature of how δT-13’-COOH, δTE-13’-COOH, and δTE inhibit the 5-LOX activity. To this end, we evaluated the impact of these inhibitors on the initial rate of reaction (O2 consumption) with a series of substrate (AA) concentrations. We observed that these compounds decreased the initial rate of reaction in a concentration-dependent manner but did not appear to change the Vmax (Fig. 6A, 6C and 6E). The Lineweaver-Burk double reciprocal plot of our data consistently indicated that δT-13’-COOH, δTE-13’-COOH, and δTE acted as a competitive inhibitor of 5-LOX with Ki at 1.6 μM, 0.8 μM, and 2.2 μM, respectively, and the Km of AA was 2.1 μM (Fig. 6B, 6D, 6F). Similar Km values of AA were reported by others [19]. These data strongly suggest that these compounds can compete with AA for the binding to the substrate binding site of 5-LOX.
Fig. 6.
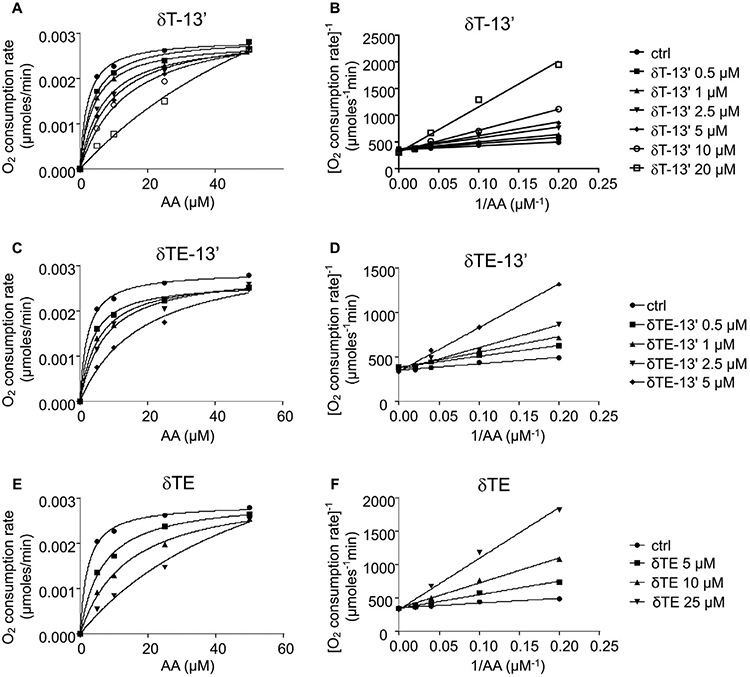
Enzyme kinetics for elucidating the nature of inhibition of 5-LOX. 13’-COOHs or δTE at indicated concentrations were pre-incubated with 5-LOX in 50 mM Tris (pH 7.4) containing 0.4 mM CaCl2 at room temperature for 10 min. AA (5–50 μM) was injected to initiate reactions. The initial rate of 5-LOX mediated reaction, i.e., the rate of oxygen consumption, was measured by an oxygen microelectrode. The nature of competitive inhibition is shown by Michaelis-Menten plots (A, C, and E) and Lineweaver-Burk plots (B, D, and F). δT-13’: δT-13’-COOH; δTE-13’: δTE-13’-COOH; δTE: δ-tocotrienol.
4. Discussion
A novel finding of our study is that δT-13’-COOH, δTE-13’-COOH and δTE are competitive inhibitors of 5-LOX, as indicated by enzyme kinetics that is considered essential for determining the mechanism of how an inhibitor interacts with an enzyme [11]. Previously, it was proposed that δTE-13’-COOH or αT-13’-COOH are allosteric inhibitors of 5-LOX, but enzyme kinetic experiments were not conducted to support such assumption [10]. Our enzyme kinetic data indicate that these compounds are capable of competing with AA for binding to the active site of 5-LOX. According to the estimated Ki and Km, δTE-13’-COOH appears to bind 5-LOX more strongly than AA, δTE or δT-13’-COOH. Since δTE-13’-COOH shows slightly stronger inhibition of 5-LOX than δT-13’-COOH, and short-chain carboxychromanols poorly inhibit 5-LOX [10], it is reasonable to assume that the long side chain with double bonds in δTE-13’-COOH can strengthen the interaction with the enzyme. This is in agreement with the observation that αT, γT or δT are poor inhibitors of 5-LOX compared to γTE and δTE. Furthermore, because δTE-13’-COOH is stronger than δTE in inhibiting 5-LOX, the carboxylate group at the 13’ position is important for the binding to 5-LOX. Additionally, the observation that δTE and γTE inhibits 5-LOX more strongly than αTE suggests that the methyl group at the 5-position on the chromanol ring may be too bulky for αTE to fit the enzyme binding site, although this speculation requires further investigation.
Consistent with inhibition of 5-LOX enzyme activity, 13’-COOHs, δTE and γTE, but not αTE, inhibited THAP- or ionophore-stimulated formation of LTB4 in neutrophils. Meanwhile, tocotrienols and 13’-COOHs do not affect 5-LOX translocation, a critical step for 5-LOX activation. In contrast, δT and γT, while do not inhibit 5-LOX, decreased ionophore-stimulated LTB4 via blocking the increase of intracellular calcium and 5-LOX translocation [7]. Despite agreement in inhibition of 5-LOX and decreasing LTB4 formation in cells, there are noticeable differences in the results between enzyme assays and cell-based studies. For instance, while 13’-COOHs (IC50s of 0.5–1.6 μM) showed stronger inhibition of 5-LOX than γTE or δTE (IC50s of 4.3 μM), tocotrienols (IC50 of 4–5 μM) inhibit LTB4 more effectively than 13’-COOHs (IC50s: 7–8 μM) in cell studies. This discrepancy may be due to the difference in the cellular uptake of these compounds. Since 5-LOX activation involves translocation from cytosol to the nucleus, it is important for inhibitors to be inside cells for effective inhibition of 5-LOX. However, 13’-COOHs may not readily enter cells because of partially-charged carboxylate group under physiological pH, unlike tocotrienols that are known to be well up-taken by cells [20]. Consistent with this idea, 30-min pre-incubation with 13’-COOHs enhanced inhibitory potency of LTB4 compared to 10-min pretreatment. In addition, the IC50s of δTE-13’-COOH in our study for inhibiting 5-LOX or LTB4 in cells are higher than those observed by Pein et al (35 nM and ~0.26 μM for inhibition of 5-LOX and LTB4 in cells, respectively) [10]. Since IC50s are known to vary with experimental conditions, this discrepancy is likely caused by potential difference in the amount of the enzyme and the ratio of tested compounds to the number of cells.
Another interesting finding is that vitamin E forms inhibit human recombinant COX-1 in enzyme assays, and moderately blocked COX-1-mediated formation of TxB2 in rats’ platelets stimulated by collagen, a physiologically relevant stimulus of platelets. However, inhibition of TxB2 formation by all vitamin E forms was weak in A23187-stimulated platelets. The reason for different potency in inhibition of COX-1 between enzyme assays and platelet studies is not clear, but this discrepancy underscores the importance of evaluation of inhibitors in both enzyme and cell-based assays. Further, similar to our previous observation [21], vitamin E forms do not inhibit COX-2 in enzyme assays. Unlike the vitamin E forms, 13’-COOHs inhibited COX-1 activity and strongly blocked collagen- and A23187-induced formation of TXB2 in platelets. Compared with inhibition of COX-1, 13’-COOHs showed relatively weak inhibition of COX-2, especially δTE-13’-COOH. We previously show that δT-13’-COOH is a competitive inhibitor for COX-1 and COX-2 [9]. These observations, together with the fact that 13’-COOHs inhibit 5-LOX, indicate that δT-13’-COOH and δTE-13’-COOH are dual inhibitors for COXs and 5-LOX, although δTE-13’-COOH mainly blocks COX-1 and 5-LOX.
Our findings of vitamin E forms’ impact on 5-LOX- and COX-1-mediated reactions have physiological implications. LTB4 produced by 5-LOX-catalyzed reaction is a potent chemotactic agent and plays significant roles in regulation of inflammatory response and cancer development [22,23]. Leukotriene antagonists and 5-LOX inhibitors have been used to treat asthma and inflammatory diseases, and show cancer preventive effects [2,24]. Our present study, together with previous research, provides mechanistic insights into anti-inflammatory effects of vitamin E forms. In particular, our current study shows that δTE and γTE competitively inhibits 5-LOX activity at physiologically-achievable concentrations. We previously reported that γT and δT, while do not inhibit 5-LOX, decrease ionophore-stimulated LTB4 or LTC4 in neutrophils via blocking calcium influx and 5-LOX activation [7]. Consistent with these mechanistic findings, γTE attenuates house dust mite-induced asthma in mice [25]. γT mitigates airway inflammation and colitis, decreases leukotrienes, and suppresses colitis-associated colon cancer in animal models [26-29]. In addition to the effect on 5-LOX, the observation that γT and αT inhibit COX-1 and thromboxane formation in platelets may partially explain their impact on platelet aggregation. Specifically, supplementation of γT-rich tocopherols has been reported to inhibit platelet aggregation [30]. Supplementation of high dose of αT (over 400IU) inhibits platelet aggregation and is associated with an increased risk of hemorrhagic stroke [31,32]. These anti-aggregation effects may be, in part, rooted in inhibition of COX-1-mediated thromboxane biosynthesis.
Our current mechanistic data support anti-inflammatory and anticancer effects of 13’-COOHs observed in vivo and suggest that these vitamin E metabolites may contribute to the beneficial effects of specific forms of vitamin E. COX- and 5-LOX-mediated reactions play significant roles in inflammation and colon cancer development [1]. Consistent with inhibition of these enzymes, δTE-13’-COOH inhibits colitis-associated colon tumorigenesis in mice [8,33]. Further, αT-13’-COOH have been shown to suppress immune cell infiltration and leukotrienes in zymosan-induced murine peritonitis, and block ovalbumin-induced bronchial hyper-reactivity and elevation of LTC4 in mice [10]. In addition, via metabolism of tocopherols and tocotrienols, 13’-COOHs may contribute to in vivo anticancer and anti-inflammatory effects of vitamin E forms. We and others have shown that after oral intake of αT, γT-rich tocopherols or δTE/γTE mixture, 13’-COOHs are elevated in the plasma of rodents or humans to 20–50 nM [10,34], which is, however, lower than the IC50s for inhibiting LTB4 in neutrophils (ranging from 0.26–7.5 μM). Despite low in the blood, 13’-COOHs are found at high levels in the feces of animals supplemented with vitamin E forms [27,29,33,34]. Future studies on 13’-COOHs’ availability in tissue are needed to further evaluate the role of metabolites in vitamin Es protective effects.
In summary, we have shown that different forms of vitamin E and metabolite 13’-COOHs inhibit COX-1 and its mediated thromboxane in stimulated platelets. δTE, δTE-13’-COOH and δT-13’-COOH are competitive inhibitors of 5-LOX and block LTB4 formation in neutrophils, while do not have impact on 5-LOX translocation. These observations provide mechanistic insights into anti-inflammatory activities of vitamin E forms and metabolites.
Abbreviations:
- AA
arachidonic acid
- αT, βT, γT, δT
α-, β-, β-, δ-tocopherol
- αTE, βTE, γTE, δTE
α-, β-, γ-, δ-tocotrienol
- CEHC (3’-COOH)
2-(β-carboxyethyl)-6-hydroxchroman or 3’-carboxychromanol
- 13’-COOH
13’-carboxychromanol
- COX−1/−2
cyclooxygenase-1/−2
- LT
leukotriene
- LTB4 or LTC4
leukotriene B4 or C4
- 5-LOX
5-lipoxygenase
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2α
- THAP
thapsigargin
- TXA(B)2
thromboxane A(B)2
Footnotes
Author disclosures: no conflicts of interest. The work of this manuscript was in part supported by USDA Hatch 1022869 and Purdue Research Foundation.
Dear Dr. Richardson, (author’s question: is this letter necessary? I have never seen this kind of letter to be included in a publication)
Thank you for sending us the reviewers’ comments on our manuscript entitled, “Different forms of vitamin E and metabolite 13’-carboxychromanols inhibit cyclooxygenase-1-catalyzed thromboxane in platelets, and tocotrienols and 13’-carboxychromanols are competitive inhibitors of 5-lipoxygenase.” We appreciate you and the reviewers who have thoroughly reviewed the manuscript.
We have carefully studied the comments and have revised the manuscript accordingly. Point-to-point clarifications have been made in response to the reviewers’ comments. The changes in the revised manuscript are highlighted in the revision.
We appreciate the insightful comments and suggestions, which helped improve the manuscript. We are submitting the revised manuscript, and hope that the revision can now be accepted for publication.
Sincerely yours,
Qing Jiang
Declaration of competing interest
The authors declare that there are no conflicts of interest related to this article.
References
- [1].Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer 2010;10:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochimica et bio-physica acta 2015;1851:331–9. [DOI] [PubMed] [Google Scholar]
- [3].Lebas H, Yahiaoui K, Martos R, Boulaftali Y. Platelets are at the nexus of vascular diseases. Front Cardiovasc Med 2019;6:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Knapp HR. Reduced allergen-induced nasal congestion and leukotriene synthesis with an orally active 5-lipoxygenase inhibitor. N Engl J Med 1990;323:1745–8. [DOI] [PubMed] [Google Scholar]
- [5].Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med 2014;72:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jiang Q. Natural forms of vitamin e as effective agents for cancer prevention and therapy. Adv Nutr 2017;8:850–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jiang Z, Yin X, Jiang Q. Natural forms of vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J Immunology 2011;186:1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jang Y, Park NY Rostgaard-Hansen AL Huang J, Jiang Q. Vitamin E metabolite 13′-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice. Free Radic Biol Med 2016;95:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc Natl Acad Sci U S A 2008;105:20464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pein H, Ville A, Pace S, Temml V, Garscha U, Raasch M, et al. Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase. Nat Commun 2018;9:3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Holdgate GA, Meek TD, Grimley RL. Mechanistic enzymology in drug discovery: a fresh perspective. Nat Rev Drug Discov 2018;17:115–32. [DOI] [PubMed] [Google Scholar]
- [12].Ouellet M, Riendeau D, Percival MD. A high level of cyclooxygenase-2 inhibitor selectivity is associated with a reduced interference of platelet cyclooxygenase-1 inactivation by aspirin. Proc Natl Acad Sci U S A 2001;98:14583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mitchell JA, Saunders M, Barnes PJ, Newton R, Belvisi MG. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor kappaB) activation: role of arachidonic acid. Mol Pharmacol 1997;51:907–12. [DOI] [PubMed] [Google Scholar]
- [14].Cho YS, Kim HS, Kim CH, Cheon HG. Application of the ferrous oxidation-xylenol orange assay for the screening of 5-lipoxygenase inhibitors. Anal Biochem 2006;351:62–8. [DOI] [PubMed] [Google Scholar]
- [15].Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A 1978;75:2458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kargman S, Rouzer CA. Studies on the regulation, biosynthesis, and activation of 5-lipoxygenase in differentiated HL60 cells. J Biol Chem 1989;264:13313–20. [PubMed] [Google Scholar]
- [17].Scoggan KA, Nicholson DW, Ford-Hutchinson AW. Regulation of leukotriene-biosynthetic enzymes during differentiation of myelocytic HL-60 cells to eosinophilic or neutrophilic cells. Eur J Biochem 1996;239:572–8. [DOI] [PubMed] [Google Scholar]
- [18].Werz O. 5-lipoxygenase: cellular biology and molecular pharmacology. Curr Drug Targets Inflamm Allergy 2002;1:23–44. [DOI] [PubMed] [Google Scholar]
- [19].Smyrniotis CJ, Barbour SR, Xia Z, Hixon MS, Holman TR. ATP allosterically activates the human 5-lipoxygenase molecular mechanism of arachidonic acid and 5(S)-hydroperoxy-6(E),8(Z),11(Z),14(Z)-eicosatetraenoic acid. Biochemistry 2014;53:4407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jiang Q, Rao X, Kim CY, Freiser H, Zhang Q, Jiang Z, et al. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int J Cancer 2012;130:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha- tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A 2000;97:11494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 2001;294:1871–5. [DOI] [PubMed] [Google Scholar]
- [23].Murphy RC, Gijon MA. Biosynthesis and metabolism of leukotrienes. Biochem J 2007;405:379–95. [DOI] [PubMed] [Google Scholar]
- [24].Peters-Golden M, Henderson WR Jr. Leukotrienes. N Engl J Med 2007;357:1841–54. [DOI] [PubMed] [Google Scholar]
- [25].Peh HY, Ho WE, Cheng C, Chan TK, Seow AC, Lim AY, et al. Vitamin E isoform gamma-tocotrienol downregulates house dust mite-induced asthma. J Immunol 2015;195:437–44. [DOI] [PubMed] [Google Scholar]
- [26].Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev Res (Phila) 2009;2:143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jiang Q, Jiang Z, Hall YJ, Jang Y, Snyder PW, Bain C, et al. Gamma-to-copherol attenuates moderate but not severe colitis and suppresses moderate colitis-promoted colon tumorigenesis in mice. Free Radic Biol Med 2013;65:1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, et al. Ozone enhancement of lower airway allergic inflammation is prevented by gamma-to-copherol. Free Radic Biol Med 2007;43:1176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu KY, Nakatsu CH, Jones-Hall Y, Kozik A, Jiang Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic Biol Med 2021;163:180–9. [DOI] [PubMed] [Google Scholar]
- [30].Liu M, Wallmon A, Olsson-Mortlock C, Wallin R, Saldeen T. Mixed tocopherols inhibit platelet aggregation in humans: potential mechanisms. Am J Clin Nutr 2003;77:700–6. [DOI] [PubMed] [Google Scholar]
- [31].Freedman JE, Farhat JH, Loscalzo J, Keaney JF Jr. alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation 1996;94:2434–40. [DOI] [PubMed] [Google Scholar]
- [32].Sesso HD. Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. Jama 2008;300:2123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang C, Zhao Y, Im S, Nakatsu C, Jones-Hall Y, Jiang Q. Vitamin E delta-tocotrienol and metabolite 13′-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice: Tocotrienol and 13′-carboxychromanol modulate gut microbiota. J Nutr Biochem 2020;89:108567. [DOI] [PubMed] [Google Scholar]
- [34].Liu KY, Jiang Q. Tocopherols and tocotrienols are bioavailable in rats and primarily excreted in feces as the intact forms and 13′-carboxychromanol metabolites. J Nutr 2020;150:222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


