Background:
Screening for polyomavirus infection after kidney transplantation is recommended by clinical practice guidelines, but cost-effectiveness of this strategy is uncertain. The aim of this study was to estimate the incremental costs and benefits of routine screening for polyomavirus infection compared with no screening in kidney transplant recipients.
Methods:
Probabilistic Markov models were constructed to compare the health and economic benefits of routine screening for polyomavirus infection using real-time polymerase chain reaction assay. A series of 1-way and probabilistic sensitivity analyses were conducted to define the most influential variables in the model.
Results:
Monthly screening for 6 mo followed by 3 monthly screenings until 12 mo after transplant was dominant (lower costs and improved outcomes). Compared with no screening, the incremental benefits of screening were 0.294 life-years saved and 0.232 quality-adjusted life-years saved. Total savings from screening were $6986 Australian dollars ($5057 US dollars). The cost-effectiveness ratios were most sensitive to the costs of transplantation and dialysis, age of transplantation, prevalence of viremia, and probability of death in patients with a history of polyomavirus-associated nephropathy. Probabilistic sensitivity analysis indicated that screening (compared with no screening) was the dominant strategy across all plausible ranges of transition probabilities.
Conclusions:
Screening for polyomavirus infections 1 year following transplantation appears to save money, improves survival, and improves quality of life in kidney transplant recipients.
Immunosuppression following kidney transplantation minimizes the risk of acute rejection and is needed to maintain long-term graft survival. However, prolonged suppression of the immune system increases the risk of opportunistic infections and reactivation of latent pathogenic viruses, such as polyomavirus infections.1 When unrecognized and untreated, polyomavirus BK (BKPyV) infection can result in nephropathy, ureteric strictures, premature graft loss, and return to dialysis.2 Viremia (BKPyV-DNAemia) is common during the first year after transplantation, affecting approximately 15% of transplant recipients, while 3% to 5% develop polyomavirus-associated nephropathy (PyVAN).3
The primary treatment strategy for identified polyomavirus infections is immunosuppression reduction. Conventional immunosuppression reduction approach may include judicious reduction or elimination of calcineurin inhibitors and antiproliferative agents or conversion to less potent immunosuppression therapy such as changing from tacrolimus to cyclosporine. These changes allow immune reconstitution during the period of viremia and facilitate viral clearance before it progresses to nephropathy and graft dysfunction.4–8 Once PyVAN is established, the risk of allograft loss is over 50% in 5 y.9 The slow evolution of viremia to PyVAN over a typical time frame of 12 to 18 mo allows early reduction of immunosuppression therapies and a window of opportunity to prevent the development of advanced stage PyVAN, provided the infection is promptly identified.10
Current recommendation by the American Society of Transplantation suggests routine screening for BKPyV-DNAemia monthly through month 9 and then every 3 mo until 2 y post-transplant and stepwise reduction in immunosuppression when the plasma BKPyV-DNAemia is greater than 1000 copies/mL for 3 wk or more.11 The Kidney Disease Improving Global Outcomes guideline recommends screening with quantitative nucleic acid tests monthly for the first 3 to 6 months, followed 3 monthly up until the end of the first posttransplant year.12 However, the evidence that underpins these recommendations is limited to observational data. No randomized controlled trials have been conducted to detect an improvement in graft function and survival or have assessed the potential harms associated with routine screening, including the development of de novo donor-specific antibodies (dnDSA) and rejection from immunosuppression reduction. Therefore, the best evidence to support or refute routine screening is reliant on the estimates derived from decision analytical modeling. A single published economic evaluation of screening for BKPyV-DNAemia indicates that routine screening is effective and cost-saving, but previous work did not account for retransplantation and the impact of immunosuppression reduction on the risk of dnDSA development.13 The aims of the study were to estimate the health care costs and benefits of screening for BKPyV, compared with no screening in contemporary kidney transplant practices, and to define the key variables that influenced the cost-effectiveness of routine screening.
MATERIALS AND METHODS
This study was reported according to the Consolidated Health Economic Evaluation Reporting Standards Statement.14 The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.15
Using a third-party payer’s perspective, 2 deterministic and probabilistic Markov models were developed to simulate the natural history of BKPyV infection in a hypothetical cohort of kidney transplant recipients (n = 10 000). We structured the models to include all the potential consequences of the infection, from viremia to the development of PyVAN, the downstream consequences of acute rejection, and the occurrence of dnDSA, graft loss, and death. The models were populated by collating and synthesizing all the relevant evidence (clinical, costs, and utilities) as input parameters. Uncertainties within the parameter estimates were assessed using 1-way and probabilistic sensitivity analyses. Costs and benefits were incorporated into each of these health states with the expected outcomes calculated by adding all the costs and effects across the states and weighting according to the time the patient is expected to be in each state. Although the models had no memory as to where and when the transplant recipients originated from and the timing of such transition, we had addressed this limitation by incorporated time dependency into the transition probabilities.16
Structure of the Model
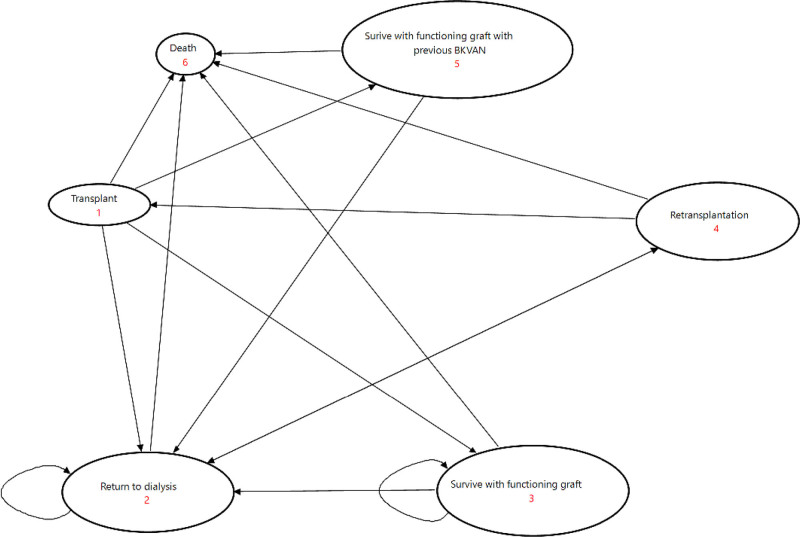
The state transition diagram of the model is shown in Figure 1. The starting age for the base model was 45 y (median age of transplantation in the United States and Australia). This model assumed monthly screening for BKPyV using real-time quantitative polymerase chain reaction (RT-PCR) assay until month 6 and then 3 monthly until 12 mo post-transplant. This strategy was chosen because the median time to the diagnosis of polyomavirus infection is 9.5 mo and consistent with the Kidney Disease Improving Global Outcomes recommendations.17 Of those who developed BKPyV-DNAemia, over 85% were diagnosed within the first 12 mo after transplantation.18 In the no-screening arm, we assumed no transplant recipients received screening. In the screening arm, BKPyV was identified through routine screening, and in some cases, PyVAN was confirmed with a graft biopsy. False-negative results were defined as patients with BKPyV infections (BKPyV-DNAemia and PyVAN) but were not detected on routine screening. False-positive results were defined as patients with positive BKPyV-DNAemia who never developed clinical nephropathy in the absence of immunosuppression reduction. In the no-screening arm, no transplant recipients received screening, and PyVAN was diagnosed when there was allograft dysfunction and confirmed histologically with biopsies.
FIGURE 1.
State transition diagram of the screen and no-screen arm. BK VAN, BK virus-associated nephropathy.
Screening allows early recognition of the disease (in this case, viremia) by using the reliable RT-PCR testing. Using published data and estimates from registries, we estimated the probability of viremia as the prevalence of detectable viremia during the first year of transplantation. We then estimated the true and false positive and negative rates based on the test performance estimates of the RT-PCT assay reported in the literature. Through early detection of viremia, this then allowed intervention (reduction in immunosuppression) to prevent the progression of viremia to PyVAN. We have also accounted for the detrimental consequences of immunosuppression reduction including acute rejection and the potential risk of graft loss associated with acute rejection.
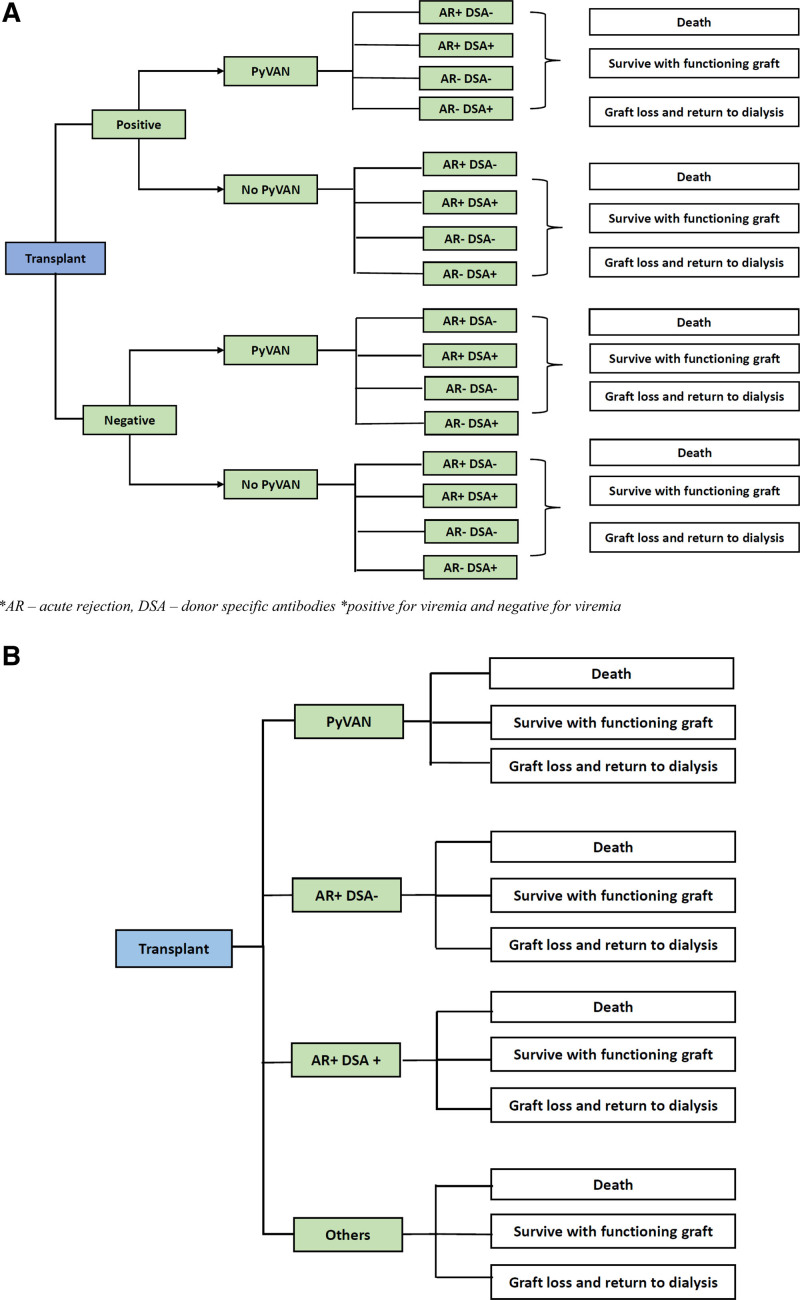
The trees (Figure 2A and B; Figure S1A and B, SDC, http://links.lww.com/TXD/A417), beginning with the decision node (blue boxes), are read from left to right. Screening and no screening are the two alternatives. Events stemming from the chance nodes (represented by green boxes) were assigned with single probabilities such that the total probability of all events originating from a chance node sums up to 1. Information of the probability of response to interventions, quality of life (QoL) implications, and costs were then used to populate the model. The expected values of health outcomes and costs of the different branches in the tree were then calculated. This process was repeated for all options to calculate the expected outcomes and costs of screening and no screening, which were then used to calculate the incremental cost-effectiveness ratio (ICER) of screening compared with no screening.
FIGURE 2.
Markov model comparing screening and no screening for BKPyV A, Decision tree for the screened arm. B, Decision tree for the no-screen arm. AR, acute rejection; DSA, donor-specific antibody; PyVAN, polyomavirus-associated nephropathy.
For patients diagnosed with BKPyV infections, stepwise reduction in immunosuppression was the first step. In general, immunosuppression reduction included a 25% to 50% reduction in antimetabolites and calcineurin inhibitors, followed by complete withdrawal of antimetabolites in patients who did not respond to immunosuppression reduction. Adjuvant therapies including intravenous immunoglobulin were considered in a proportion of patients (10%) with persistent infections.8 Cidofovir and quinolones were not included in the modeling, given the lack of clinical benefits.19 Transplant recipients with BKPyV infections could progress through one of these transition states: acute rejection with or without development of dnDSA, no acute rejection/dnDSA, or stable graft function without dnDSA during the first year post-transplant. Individuals could experience graft loss, death, or remain alive at the end of year one.
Transplant recipients who experienced graft loss could remain alive on dialysis, receive another transplant, or die on dialysis. Those who remained alive at the end of the year could either survive with a functioning graft, die, or experience allograft loss. Those who experienced graft loss could return to dialysis. The model also assumed a small proportion of patients with allograft loss chose not to proceed with any form of kidney replacement therapy. A proportion of patients on dialysis would withdraw from dialysis each year (and opted for palliative and conservative management) and die during the concurrent year. We also assumed that all transplant maintenance costs were similar across the screening and no-screening arms. At the end of each cycle, the model accrued the effectiveness and costs for each patient in the assigned health state. Cumulative benefits and costs were calculated after all patients were deceased.
Sensitivity Analyses
Assumptions were tested over a range of plausible values to assess the robustness of the uncertainties in the model’s parameter estimates using sensitivity analyses. Using 1-way sensitivity analyses, we identified the influential variables within the model. In addition to the baseline variables, we also tested the impact of discontinuing all antimetabolites or maintaining a reduced immunosuppression regimen until year 2 after the diagnosis of polyomavirus infections in the screening arm, on the overall cost-benefit ratios. Probabilistic sensitivity analysis was also undertaken. We assigned a distribution to each model parameter and sampled from that distribution using Monte Carlo simulation and estimated the expected value of the screening and no-screening arms. We used the log-normal distributions for relative risks and gamma distributions for costs and randomly sampled over 10 000 iterations for each variable of interest.
Input Parameters for the Model
Clinical Data
A comprehensive literature search was conducted to identify the best available data on the clinical events that occurred after transplantation in patients with and without BKPyV infections (Table 1; Table S1, SDC, http://links.lww.com/TXD/A417). Annual transition probabilities of the following variables in the patients with or without a history of PyVAN were sourced from transplant registries: probability of allograft loss and return to dialysis, death, and retransplantation. Other probabilities such as the annual incidence of acute rejection, development of dnDSA, utilities-based QoL, and test performance characteristics of the RT-PCR assays were sourced from published literature.20–27
TABLE 1.
Clinical, costs, and utilities data for the model
| Clinical data | Estimates | References | |
|---|---|---|---|
| Utility | 33,34 | ||
| Transplant | 0.74 | ||
| Graft loss and return to dialysis | 0.62 | ||
| Acute rejection | 0.59 | ||
| Diagnosis of BKVAN | 0.64 | ||
| Dialysis survival | |||
| Patient survival on dialysis | Age, y | 35,36 | |
| First year | 18–24 | 0.98 (0.96–0.99) | |
| 25–44 | 0.96 (0.96–0.97) | ||
| 45–64 | 0.94 (0.93–0.94) | ||
| 65–74 | 0.89 (0.88–0.90) | ||
| 75–84 | 0.84 (0.83–0.85) | ||
| ≥85 | 0.76 (0.72–0.79) | ||
| 2 y | 18–24 | 0.96 (0.94–0.97) | 35,36 |
| 25–44 | 0.93 (0.92–0.93) | ||
| 45–64 | 0.87 (0.87–0.88) | ||
| 65–74 | 0.79 (0.78–0.80) | ||
| 75–84 | 0.71 (0.69–0.72) | ||
| ≥85 | 0.57 (0.53–0.62) | ||
| 5 y | 18–24 | 0.94 (0.92–0.95) | 35,36 |
| 25–44 | 0.85 (0.83–0.86) | ||
| 45–64 | 0.94 (0.69–0.70) | ||
| 65–74 | 0.89 (0.88–0.90) | ||
| 75–84 | 0.34 (0.33–0.36) | ||
| ≥85 | 0.18 (0.15–0.23) | ||
| Transplant survival | |||
| Patient survival: deceased donor transplant | 35,36 | ||
| First year | 0.97 (0.97–0.98) | ||
| 5 y | 0.90 (0.88–0.91) | ||
| 10 y | 0.75 (0.73–0.77) | ||
| 15 y | 0.64 (0.61–0.66) | ||
| Graft survival: deceased donor transplant | 35,36 | ||
| First year | 0.97 (0.97–0.98) | ||
| 5 y | 0.90 (0.88–0.91) | ||
| 10 y | 0.75 (0.73–0.77) | ||
| 15 y | 0.48 (0.46–0.51) | ||
| Patient survival: living donor transplant | 35,36 | ||
| First year | 0.99 (0.98–1.00) | ||
| 5 y | 0.96 (0.94–0.97) | ||
| 10 y | 0.88 (0.86–0.89) | ||
| 15 y | 0.76 (0.74–0.79) | ||
| Graft survival: living donor transplant | 35,36 | ||
| First year | 0.98 (0.97–0.99) | ||
| 5 y | 0.89 (0.88–0.91) | ||
| 10 y | 0.75 (0.73–0.77) | ||
| 15 y | 0.55 (0.52–0.58) | ||
| Graft rejection | |||
| Probability of graft rejection: first 6 mo | 35,36 | ||
| Living donor | |||
| First graft | 0.191 | ||
| Subsequent grafts | 0.216 | ||
| Deceased donor | |||
| First graft | 0.185 | ||
| Subsequent grafts | 0.20 | ||
| Probability of acute rejection: first 12 mo | 0.214 | 37 | |
| Probability of acute rejection: subsequent years | 0.04 | 38 | |
| Acute rejection and DSA | |||
| Probability of acute rejection with BK infection | 0.215 | 21 | |
| Probability of acute rejection with high viremia | 0.34 | 22 | |
| Probability of acute rejection with low viremia | 0.17 | 22 | |
| Probability of acute rejection but no DSA in patients with PyVAN | 0.06 | 23 | |
| Probability of acute rejection with DSA in patients with PyVAN | 0.19 | 24,25 | |
| Probability of no acute rejection but has DSA in patients with PyVAN | 0.1 | 21,39 | |
| Probability of no acute rejection and no DSA in patients with PyVAN | 0.65 | 21,39 | |
| Graft dysfunction (no-screen arm) | |||
| Probability of graft dysfunction from all causes | 0.4 | 39 | |
| Probability of PyVAN in patients with graft dysfunction | 0.11 | 40,41 | |
| Probability of acute rejection in patients with graft dysfunction | 0.22 | 25 | |
| Graft loss in patients with PyVAN | |||
| Probability of graft loss in patients with PyVAN and acute rejection | 0.057 | 4,20 | |
| Probability of graft loss in patients with PyVAN but no rejection | 0.048 | 4,20 | |
| Graft loss in patients without PyVAN | |||
| Probability of graft loss from all causes | 0.147 | 27 | |
| Probability of graft loss after acute rejection | 0.038 | 20 | |
| Probability of graft loss from acute rejection | 0.03 | 42 | |
| Probability of graft loss without PyVAN | 0.046 | 20,30 | |
| BK infection within the first year | |||
| Probability of positive BKPCR within the first year | 0.10–0.30 | 20 | |
| Probability of positive BK viral load >10 000 if PCR is +ve | 0.25 | 20 | |
| Probability of positive BK viral load <10 000 if PCR is +ve | 0.75 | 20 | |
| Probability of PyVAN with BK viral load >10 000 | 0.87 | 20 | |
| Probability of PyVAN with BK viral load <10 000 | 0.31 | 20 | |
| Late diagnosis of BK: no-screening arm | |||
| Probability of graft loss from BK without monitoring | 0.46 | 26 | |
| Probability of retransplantation | 0.05 | 35 | |
| Recurrence of BK in retransplantation | |||
| Probability of recurrence in the second/subsequent transplants | 0.175 | 43 | |
| Probability of BKVAN in the second transplant with recurrence | 0.06 | 44 | |
| Survival of retransplants after previous graft loss | |||
| Patient survival | 43 | ||
| Years after transplant | 1 | 0.985 (0.93–1.00) | |
| 2 | 0.985 (0.93–1.00) | ||
| 3 | 0.985 (0.93–1.00) | ||
| Graft survival | 44 | ||
| Years after transplant | 1 | 0.96 (0.88–1.00) | |
| 2 | 0.94 (0.85–1.00) | ||
| 3 | 0.94 (0.85–1.00) | ||
| Costs and resource uses, $ (AUD) | 45–50 | ||
| Access surgery | 1043 | 800–1500 | |
| Biopsy | 607 | 500–750 | |
| Death | 6000 | 2000–10 000 | |
| Home hemodialysis | 50 045 | 45 000–100 000 | |
| Center hemodialysis | 85 987 | 60 000–120 000 | |
| Peritoneal dialysis | 70 304 | 50 000–100 000 | |
| Transplant: first year | 51 044 | 40 000–100 000 | |
| Transplant: subsequent years | 18 864 | 10 000–50 000 | |
| Immunosuppression reduction | 4380 | 2000–5000 | |
| Polyomavirus PCR test: initial (per test) | 29 | 20–50 | |
| Polyomavirus PCR test: monitoring | 762 | 500–1000 | |
| Luminex testing (per test) | 1600 | 500–2000 | |
| Treatment of acute rejection: ABMR | 18 308 | 10 000–30 000 | |
| Treatment of acute rejection: TCMR (steroid responsive) | 6030 | 5000–10 000 | |
| Treatment of acute rejection: TCMR (steroid resistant) | 43 330 | 30 000–50 000 | |
| Treatment using IVIG | 4032 | 2000–10 000 | |
| Discount costs | 0.05 | 0.03–0.08 | |
| Distributions | |||
| Prevalence of viremia | 0.18 (0.001) | Normal (mean, SD) | |
| Probability of graft loss in the no-screen arm | 0.46 (0.05) | Normal (mean, SD) | 26 |
| Probability of graft dysfunction in patients with PyVAN | 0.1 (0.05) | Normal (mean, SD) | 29,30,39 |
| Probability of retransplantation | 0.1 (0.05) | Normal (mean, SD) | 43 |
| Probability of death in patients with PyVAN | 0.0225 (0.005) | Normal (mean, SD) | 29 |
| Costs of transplant: subsequent years, $ (AUD) | 18 864 (0.85) | γ (α, λ) | |
| Costs of dialysis: return to dialysis after allograft loss, $ (AUD) | 113 932 (0.85) | γ (α, λ) |
AUD, Australian dollars; ABMR, antibody mediated rejection; BKVAN, BK virus-associated nephropathy; DSA, donor-specific antibody; PCR, polymerase chain reaction; PyVAN, polyomavirus-associated nephropathy; TCMR, T-cell mediated rejection.
Costs Data
Unit costs for screening, biopsy monitoring, treatment, and management strategies were estimated using a top-down approach and sourced from the published literature and country-specific costing agencies such as the Australian Refined Diagnosis Related Groups.28 All costs were reported in 2020 Australian dollars (AUD) but also presented in US dollars in the base-case and sensitivity analyses. The impact of variability in the cost schedule was also tested in the sensitivity analyses.
Model Outcomes
The model outcomes included the total costs and health outcomes (expressed in life-years [LYs] and quality-adjusted LYs [QALYs]) and the incremental costs and health benefits of screening for BKPyV infections compared with no screening. The ICER of screening compared with no screening was calculated using the following formula: ICER = (CostNew − CostComparator)(EffectivenessNew − EffectivenessComparator).
Future costs and benefits were discounted using a discount rate of 5% per annum, and half-cycle corrections were employed. We used TreeAge Pro Healthcare 2021 (TreeAge software; Williamstown, MA) and SAS 9.4 to develop and analyze the model. This study used only published data and existing collection of registry records that only contain nonidentifiable data and therefore was exempted from the Human Research Ethics Committee review.
RESULTS
Base Case
Assuming a starting age of 45 y, a cycle length of 1 y, with the model terminating after all recipients were deceased, the estimated total costs of posttransplant care were $350 947 AUD ($254 017 US dollars) in the screened arm, compared with $357 933 AUD ($259 090 US dollars) for the no-screening arm, resulting in 11.59 LYs and 8.416 QALYs in the screening arm and 11.296 LYs and 8.184 QALYs for no screening. The incremental benefits for screening were 0.294 LYs saved and 0.232 QALYs, with screening dominant and resulting in savings of $6986 AUD ($5057 US dollars). The Markov state cumulative probabilities of death and survival with a functioning graft for both the screen and no-screen arms after 50 cycles are shown in Figure S2 (SDC, http://links.lww.com/TXD/A417).
Sensitivity Analyses
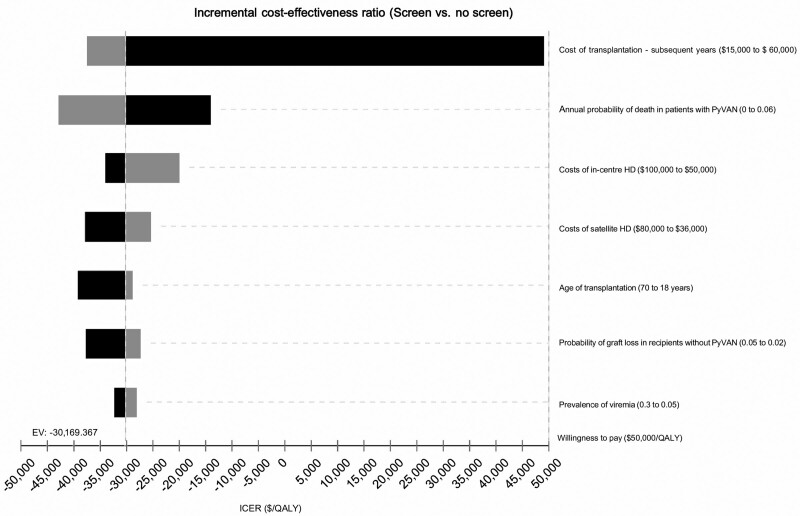
The most influential variables identified in the model were the costs of transplantation (maintenance immunosuppression and management after year one post-transplant), starting age of transplantation, costs of dialysis after allograft loss, probability of death in patients with a history of PyVAN, prevalence of BKPyV-DNAemia, and the probability of graft loss in patients without PyVAN and acute rejection. The extent of the variability associated with these variables on the incremental health outcomes and costs is shown in the tornado diagram (Figure 3) and Table 2. For example, if the age of transplantation is decreased from 70 y (higher values, represented by shades of black) to 18 y (lower values, represented by shades of gray), then the incremental benefits of screening would increase from 0.201 to 0.236 QALYs. However, the total savings from screening would reduce from $7884 to $6844, as younger recipients would incur greater resources used over their lifetime compared with the older counterparts. The overall ICER was reduced from −$39 294/QALY to −$28 933/QALY, suggesting screening in younger recipients would save less money but acquire slightly more health benefits over time (Figure S3, SDC, http://links.lww.com/TXD/A417).
FIGURE 3.
Tornado diagram showing the influential variables on the incremental cost-effectiveness ratios of the base model. EV, expected value; HD, hemodialysis; PyVAN, polyomavirus-associated nephropathy; QALY, quality-adjusted life-years.
TABLE 2.
One-way sensitivity analyses
| Variables | Costs (screen), $AUD | Costs (no screen), $AUD | Benefits (screen),QALYs | Benefits (no screen),QALYs | Incremental costs, $AUD | Incremental benefits,QALYs | ICER, ($/QALYs) |
|---|---|---|---|---|---|---|---|
| Costs of transplantation: subsequent years (assuming recipients returned to standard immunosuppression after year 1), $ (AUD) | |||||||
| 15 000 | 315 980 | 324 558 | 8.48 | 8.243 | −8578 | 0.236 | −36 319 |
| 37 500 | 534 706 | 533 127 | 8.48 | 8.243 | −1579 | 0.236 | 6689 |
| 48 750 | 644 069 | 637 411 | 8.48 | 8.243 | 6659 | 0.236 | 28 139 |
| 60 000 | 753 433 | 741 695 | 8.48 | 8.243 | 11 737 | 0.236 | 49 697 |
| Prevalence of viremia in the screened arm | |||||||
| 0.05 | 353 741 | 360 376 | 8.49 | 8.243 | −6635 | 0.247 | −26 869 |
| 0.15 | 353 550 | 360 376 | 8.48 | 8.243 | −6825 | 0.237 | −28 850 |
| 0.2 | 353 458 | 360 376 | 8.475 | 8.243 | −6921 | 0.231 | −29 908 |
| 0.3 | 353 359 | 360 376 | 8.47 | 8.243 | −7016 | 0.226 | −31 013 |
| Probability of death in recipients with PyVAN | |||||||
| 0.01 | 353 836 | 362 213 | 8.488 | 8.287 | 8376 | 0.201 | −41 708 |
| 0.035 | 353 248 | 358 883 | 8.472 | 8.208 | 5635 | 0.263 | −21 393 |
| 0.0475 | 352 954 | 357 647 | 8.463 | 8.179 | 4692 | 0.285 | −16 490 |
| 0.06 | 352 660 | 356 606 | 8.455 | 8.154 | 3945 | 0.301 | −13 104 |
| Costs of dialysis | |||||||
| 50 000 | 292 346 | 292 755 | 8.48 | 8.243 | 410 | 0.236 | −1734 |
| 85 000 | 325 848 | 329 775 | 8.48 | 8.243 | 3926 | 0.236 | −16 624 |
| 102 500 | 342 600 | 348 284 | 8.48 | 8.243 | 5685 | 0.236 | −24 070 |
| 120 000 | 359 351 | 366 794 | 8.48 | 8.243 | 7443 | 0.236 | −31 515 |
| Probability of graft loss in recipients without PyVAN and acute rejection | |||||||
| 0.02 | 342 572 | 349 721 | 8.98 | 8.704 | 7149 | 0.276 | −25 936 |
| 0.035 | 358 342 | 365 034 | 8.26 | 8.042 | 6691 | 0.218 | −30 640 |
| 0.043 | 364 837 | 371 333 | 7.962 | 7.768 | 6495 | 0.194 | −33 517 |
| 0.05 | 370 613 | 376 931 | 7.696 | 7.525 | 6317 | 0.171 | −36 853 |
| Age of transplantation, y | |||||||
| 18 | 353 543 | 360 376 | 8.48 | 8.243 | 6833 | 0.236 | −28 933 |
| 44 | 351 186 | 358 160 | 8.422 | 8.19 | 6973 | 0.232 | −30 063 |
| 57 | 345 657 | 352 898 | 8.285 | 8.062 | 7240 | 0.223 | −32 407 |
| 70 | 328 496 | 336 380 | 7.862 | 7.662 | 7884 | 0.201 | −39 294 |
| Costs of transplantation in recipients with prior PyVAN in the screen arm (assuming recipients remained on reduced immunosuppression in up to year 2 after the initial diagnosis), $ (AUD) | |||||||
| 8000 | 352 738 | 357 989 | 8.48 | 8.243 | 5251 | 0.236 | −22 233 |
| 13 432 | 353 140 | 357 989 | 8.48 | 8.243 | 4848 | 0.236 | −20 529 |
| 16 148 | 353 341 | 357 989 | 8.48 | 8.243 | 4647 | 0.236 | −19 678 |
| 18 864 | 353 542 | 357 989 | 8.48 | 8.243 | 4446 | 0.236 | −18 826 |
| Costs of transplantation in recipients with prior PyVAN in the no-screen arm (assuming recipients remained on reduced immunosuppression over the life course of the transplant), $ (AUD) | |||||||
| 9000 | 353 144 | 356 360 | 8.48 | 8.243 | 3216 | 0.236 | −13 618 |
| 14 500 | 353 144 | 358 599 | 8.48 | 8.243 | 5455 | 0.236 | −23 099 |
| 17 250 | 353 144 | 359 719 | 8.48 | 8.243 | 6575 | 0.236 | −27 839 |
| 20 000 | 353 144 | 360 838 | 8.48 | 8.243 | 7694 | 0.236 | −32 579 |
AUD, Australian dollars; ICER, incremental cost-effectiveness ratio; PyVAN, polyomavirus-associated nephropathy; QALY, quality-adjusted life-years.
If the costs of transplantation in subsequent years in both the screen and no-screen arms for patients with and without prior polyomavirus infections were increased from $15 000 AUD ($11 100 US dollars) to $60 000 AUD ($44 400 US dollars), then screening (compared with no screening) would vary from being cost-savings to incurring additional costs of $8578 AUD ($11 737 US dollars). However, the ICER remained below the willingness-to-pay threshold of $50 000 per LYs saved or QALYs. In this model, if the annual probability of death in patients with PyVAN was twice that of those without PyVAN, the incremental benefits of screening increased from 0.263 to 0.301 QALYs gained. If the probability of other causes of graft loss unrelated to PyVAN was reduced from 0.05 to 0.02 (ie, the probability of other competing causes of graft loss was reduced), then the incremental benefits of screening would increase from 0.171 to 0.276 QALYs. If the costs of return to dialysis (after allograft loss) were increased from a base rate of $50 000 AUD ($37 000 US dollars) to over $120 000 AUD ($87 000 US dollars) per annum, savings from screening would increase from around $410 AUD ($300 US dollars) to approximately $7500 AUD ($5500 US dollars). The model was also sensitive to the costs of a reduced immunosuppression regimen in patients with a history of PyVAN, with additional savings of around $5250 (compared with no screening) if the antimetabolites were discontinued or decreased up to 2 y after the initial diagnosis in the screening arm.
Probabilistic Sensitivity Analyses
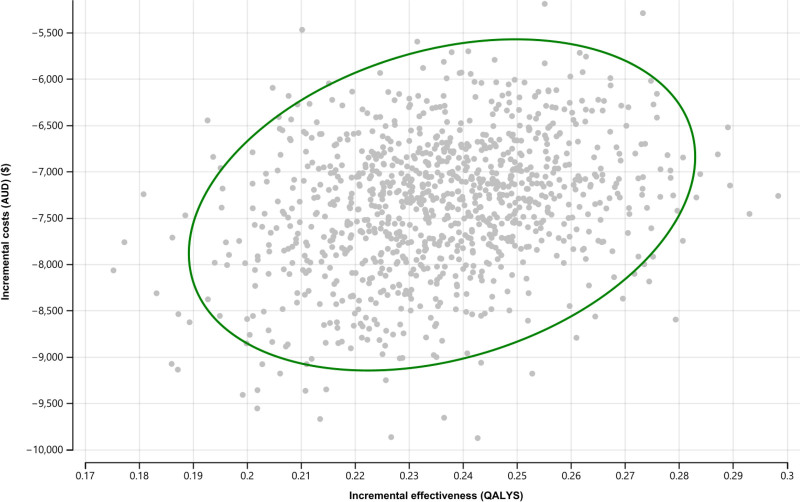
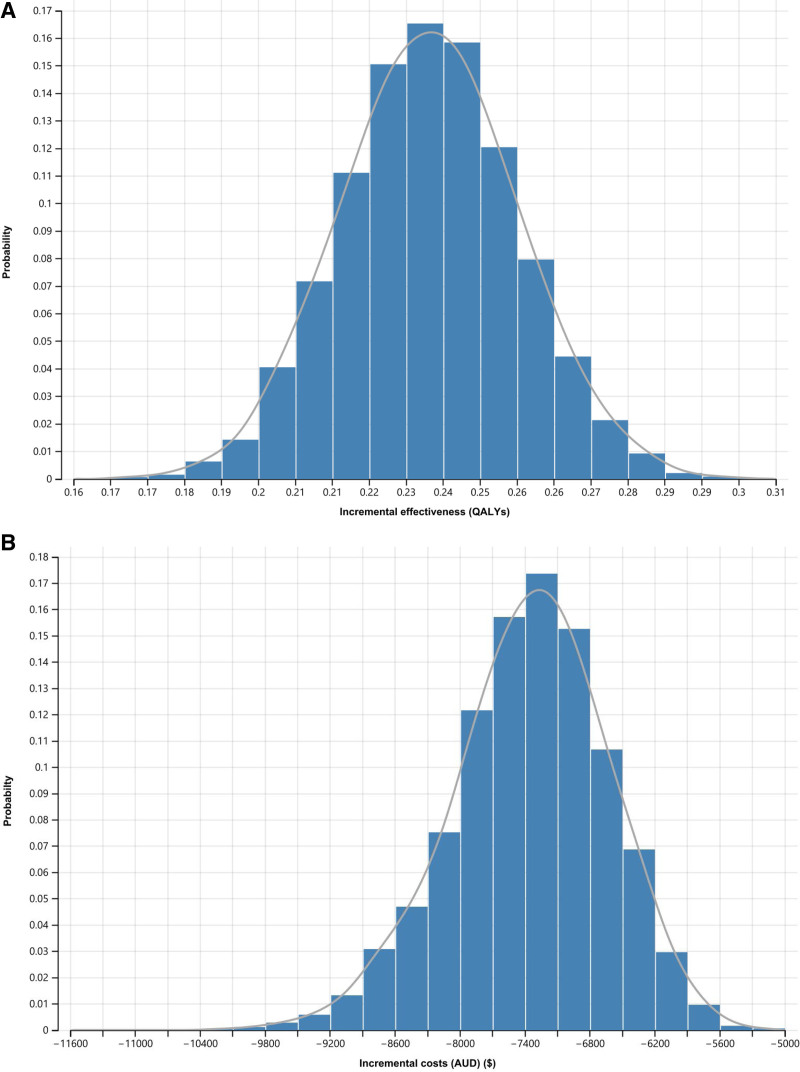
The scatter plot shown in Figure 4 shows the incremental costs and health outcomes and the uncertainties surrounding plausible range of mean parameter estimates in the screening and no-screening arms. The x-axis represents the incremental gains in QALYs, and the y-axis represents the incremental costs of screening compared with no screening. The scatter plot is located at the lower southeast quadrant of the cost-effectiveness plan, indicating screening is effective and cost-saving, compared with no screening (ie, dominant). Figure 5A and B shows the predicted probabilities that screening (compared with no screening) being cost-saving and effective is 100%, indicating that screening for BKPyV-DNAemia always dominated the no-screening strategy and across clinically relevant ranges and scenarios.
FIGURE 4.
Probabilistic sensitivity analyses showing the incremental cost-effectiveness ratios (ICERs) of screening vs no screening. AUD, Australian dollars; QALY, quality-adjusted life-years.
FIGURE 5.
A, Predicted probabilities that screening (compared with no screening) is effective. B, Predicted probabilities that screening (compared with no screening) is cost-saving. QALY, quality-adjusted life-years; AUD, Australian dollars.
DISCUSSION
This economic evaluation, derived from the best available evidence, demonstrates that universal screening for polyomavirus infections using RT-PCR to detect viremia within the first 12 mo post-transplant results in meaningful improvement in survival and QoL (0.2–0.3 LY/QALYs) and is cost-saving compared with no screening. In health economic terms, this means that screening is dominant (cost-savings and more cost-effective) compared with no screening. The extent of the survival benefits is dependent on the prevalence of viremia after transplant, age of transplantation, the survival probability of patients with PyVAN, and the annual incidence of allograft loss in recipients without a history of acute rejection and polyomavirus infections. The economic benefits of screening are influenced by the costs of transplantation after the first year. If viremia is cleared by reduced immunosuppression and the lowered costs of immunosuppression are maintained in subsequent years, screening could save up to $5200 AUD, compared with no screening.
One of the major difficulties in the management of polyomavirus infection is the balance between over- and under-immunosuppression.29 Immunosuppression reduction remains the primary therapy for patients with polyomavirus infections. Defining the optimal immunosuppression therapy to avoid reactivation of the virus and at the same time preventing acute rejection and dnDSA development is the ultimate challenge, as these two events will eventually lead to kidney damage, allograft dysfunction, and subsequent graft loss.30 In our sensitivity analyses, we assumed a proportion of patients would remain on reduced immunosuppression even if they had cleared the viruses. The cost estimates of immunosuppression reduction greatly influenced cost-savings in both the screening and no-screening arms. However, uncertainties exist whether reduction in immunosuppression load will translate into longer term health benefits. Our study was built on previous research that has also considered the impact of reduced immunosuppression on the cost-benefit ratio of screening. Prior modeled analyses, using data from the United Network of Organ Sharing and the US Renal Data System databases also reported considerable savings of approximately $2000 US dollars with screening (compared with no screening), driven largely from immunosuppression reduction in the screened arm. In a scenario that the antimetabolites were ceased completely, the savings will further increase.13 In this current analysis using contemporary data, we have shown that the net benefits and savings from screening were considerably higher than the previous analysis.13
Our predictions show that screening incurred the greatest cost-savings if the underlying prevalence of PyVAN is high (>25%). This finding is expected because as the total number of patients with PyVAN increases, the overall costs of screening will be shared and offset by a greater number of individuals who may benefit from early intervention to prevent graft loss. However, even with a background prevalence viremia rate of only 5%, costs are reduced by approximately $6800 AUD. In contrast to the previous analyses,13 the absolute gains in the effectiveness of screening observed in the current model were reduced with higher prevalence of viremia. The prior model had assumed that immunosuppression reduction is effective in reducing the risk of developing advanced disease such as polyomavirus-associated nephropathy without the added risk of acute rejection, which improves allograft outcomes at a population level. However, in the current analyses, the model reflected the clinical scenario in which a proportion of patients managed with reduced immunosuppression developed acute rejection and allograft dysfunction from immunosuppression reduction. Therefore, the gains in health outcomes achieved through early detection and immunosuppression reduction were counterbalanced by the morbidity associated with a higher risk of acute rejection and subsequent graft loss. The model was also dependent on the inherent differences in the probability of death between patients with a history of PyVAN in the screening and no-screening groups. Apart from an increased risk of allograft loss in transplant recipients with PyVAN, progressive decline in allograft function over time may have contributed to the increased risk of death from other causes including other infections and cancer. Our study findings highlighted the critical importance of detecting the disease during the sojourn time, the time of the presymptomatic health status (early viremic state) before progression to graft dysfunction.
This study has several potential limitations. Our cost-effectiveness and cost-utility results are sensitive to some model inputs. However, many of these estimates, such as the prevalence of disease, the probability of death, and allograft loss attributed to PyVAN, are imprecise and may differ considerably between different sites and transplant units. Furthermore, many of these inputs such as costs and the impact of maintenance immunosuppression and medications after transplantation may change over time. It is also important to note that routine screening is not without harms. In this analysis, we had assumed a 1.5-fold increased risk of acute rejection and a 2-fold increase in the risk of dnDSA in patients with screened detected viremia, owing to the reduction in immunosuppression. However, if the risk of allograft loss associated with PyVAN was increased in the no-screening arm, then screening would incur greater benefits, and the relative harms associated with screening may be reduced, rendering screening more attractive and desirable than no screening. There may be reasons to suggest that the frequency of screening and screening intervals for polyomavirus infection should vary according to the risk factors for polyomavirus infections.31 Patients with risk factors such as use of T cell–depleting agents as induction therapy, prior acute rejection episodes, and human antigen leukocyte incompatibility may benefit from more frequent screening to increase the probability of detecting viremia early.32 In this model, we did not assess whether these additional risk factors combined with screening frequency influence cost-effectiveness. The present analysis also assumed that immunosuppression reduction strategies, cessation of antimetabolites, and the use of adjuvant therapies such as intravenous immunoglobulins are effective management strategies for BKPyV-DNAemia and PyVAN. However, none of these strategies have been assessed in randomized trials. We also have not considered patients’ preferences and perspectives in the analyses. Routine screening in the form of a regular blood test may pose added burden on our patients, as well as the fear and potential harms of false-positive or negative results, the implications of reduced immunosuppression, and the downstream consequences of acute rejection and allograft dysfunction.
In conclusion, using the best available existing data, routine screening for BKPyV-DNAemia using RT-PCR is cost-saving, improves survival, and improves overall QoL in kidney transplant recipients across all settings and assumptions, compared with no screening. Our findings support universal screening for all kidney transplant recipients for polyomavirus infections during the first 12 mo after transplantation, when the net immunosuppression load is at the highest level.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr Eric Tan for his assistance with the literature research and review.
Supplementary Material
Footnotes
Contributed equally as first authors.
Contributed equally as senior authors.
G.W., T.M.M., J.C.C., B.K., and D.A. contributed to research design and concepts. G.W., B.K., and T.M.M. contributed to data analysis. All authors contributed to writing and reviewing of the paper.
G.W. is supported by the NHMRC Career Development Fellowship (APP 1147657) and the NHMRC Investigator Grant (APP 1195414).
The authors declare no conflicts of interest.
Data sharing: The authors are willing to share the statistical codes and program upon requests.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277–1286. [DOI] [PubMed] [Google Scholar]
- 2.Bohl DL, Brennan DC. BK virus nephropathy and kidney transplantation. Clin J Am Soc Nephrol. 2007;2:S36–S46. [DOI] [PubMed] [Google Scholar]
- 3.Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5:2213–2221. [DOI] [PubMed] [Google Scholar]
- 4.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582–594. [DOI] [PubMed] [Google Scholar]
- 5.Josephson MA, Gillen D, Javaid B, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation. 2006;81:704–710. [DOI] [PubMed] [Google Scholar]
- 6.Moscarelli L, Caroti L, Antognoli G, et al. Everolimus leads to a lower risk of BKV viremia than mycophenolic acid in de novo renal transplantation patients: a single-center experience. Clin Transplant. 2013;27:546–554. [DOI] [PubMed] [Google Scholar]
- 7.Lipshutz GS, Flechner SM, Govani MV, et al. BK nephropathy in kidney transplant recipients treated with a calcineurin inhibitor-free immunosuppression regimen. Am J Transplant. 2004;4:2132–2134. [DOI] [PubMed] [Google Scholar]
- 8.Kable K, Davies CD, O’connell PJ, et al. Clearance of BK virus nephropathy by combination antiviral therapy with intravenous immunoglobulin. Transplant Direct. 2017;3:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch HH, Babel N, Comoli P, et al. ; ESCMID Study Group of Infection in Compromised Hosts. European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clin Microbiol Infect. 2014;20(Suppl 7):74–88. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. n Engl j Med. 2002;347:488–496. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch HH, Randhawa PS; AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13528. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes Transplant Work G. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. [DOI] [PubMed] [Google Scholar]
- 13.Kiberd BA. Screening to prevent polyoma virus nephropathy: a medical decision analysis. Am J Transplant. 2005;5:2410–2416. [DOI] [PubMed] [Google Scholar]
- 14.Husereau D, Drummond M, Petrou S, et al. ; ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–250. [DOI] [PubMed] [Google Scholar]
- 15.Tourism PitISoT, Society OTCbTT, International Society of Nephrology in Istanbul T, April. The declaration of istanbul on organ trafficking and transplant tourism. Clin J Am Soc Nephrol. 2008;3:1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong G, Howard K, Webster AC, et al. How is health economics relevant to transplant clinicians? Transplantation. 2014;98:124–130. [DOI] [PubMed] [Google Scholar]
- 17.Ahuja M, Cohen EP, Dayer AM, et al. Polyoma virus infection after renal transplantation. Use of immunostaining as a guide to diagnosis. Transplantation. 2001;71:896–899. [DOI] [PubMed] [Google Scholar]
- 18.Radtke J, Dietze N, Fischer L, et al. Incidence of BK polyomavirus infection after kidney transplantation is independent of type of immunosuppressive therapy. Transpl Infect Dis. 2016;18:850–855. [DOI] [PubMed] [Google Scholar]
- 19.Knoll GA, Humar A, Fergusson D, et al. Levofloxacin for BK virus prophylaxis following kidney transplantation: a randomized clinical trial. JAMA. 2014;312:2106–2114. [DOI] [PubMed] [Google Scholar]
- 20.Myint TM, Turner RM, Craig JC, et al. Test performance characteristics of quantitative nucleic acid testing for polyomaviruses in kidney and kidney-pancreas transplant recipients. Clin Transplant. 2013;27:E571–E579. [DOI] [PubMed] [Google Scholar]
- 21.Baek CH, Kim H, Yu H, et al. Risk factors of acute rejection in patients with BK nephropathy after reduction of immunosuppression. Ann Transplant. 2018;23:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elfadawy N, Flechner SM, Schold JD, et al. Transient versus persistent BK viremia and long-term outcomes after kidney and kidney-pancreas transplantation. Clin j Am Soc Nephrol. 2014;9:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlenstiel-Grunow T, Sester M, Sester U, et al. BK polyomavirus-specific T cells as a diagnostic and prognostic Mmrker for BK polyomavirus infections after pediatric kidney transplantation. Transplantation. 2020;104:2393–2402. [DOI] [PubMed] [Google Scholar]
- 24.Burek Kamenaric M, Ivkovic V, Kovacevic Vojtusek I, et al. The role of HLA and KIR immunogenetics in BK virus infection after kidney transplantation. Viruses. 2020;12:E1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Höcker B, Schneble L, Murer L, et al. Epidemiology of and risk factors for BK polyomavirus replication and nephropathy in pediatric renal transplant recipients: an international CERTAIN registry study. Transplantation. 2019;103:1224–1233. [DOI] [PubMed] [Google Scholar]
- 26.Weiss AS, Gralla J, Chan L, et al. Aggressive immunosuppression minimization reduces graft loss following diagnosis of BK virus-associated nephropathy: a comparison of two reduction strategies. Clin j Am Soc Nephrol. 2008;3:1812–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan BD, Wong G, Jiang Q, et al. Longitudinal study of BK polyomavirus outcomes, risk factors, and kinetics in renal transplantation patients. Microb Pathog. 2020;142:104036. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25–30. [DOI] [PubMed] [Google Scholar]
- 29.Shen CL, Wu BS, Lien TJ, et al. BK polyomavirus nephropathy in kidney transplantation: balancing rejection and infection. Viruses. 2021;13:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gard L, van Doesum W, Niesters HGM, et al. A delicate balance between rejection and BK polyomavirus associated nephropathy; a retrospective cohort study in renal transplant recipients. PLoS One. 2017;12:e0178801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Favi E, Puliatti C, Sivaprakasam R, et al. Incidence, risk factors, and outcome of BK polyomavirus infection after kidney transplantation. World J Clin Cases. 2019;7:270–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maung Myint T, Chong CHY, Wyld M, et al. Polyoma BK virus in kidney transplant recipients: screening, monitoring and management. Transplantation. 2022;106:e76–e89. [DOI] [PubMed] [Google Scholar]
- 33.Wong G, Howard K, Chapman J, et al. How do people with chronic kidney disease value cancer-related quality of life? Nephrology (Carlton). 2012;17:32–41. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan A, Teixeira-Pinto A, Lim WH, et al. Health-related quality of life in people across the spectrum of CKD. Kidney Int Rep. 2020;5:2264–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan G, Elasma M, Nikky I, et al. Probability of graft and patients outcomes in patients with polyomavirus infections. 2021. [Google Scholar]
- 36.United Organ For Organ Sharing Annual Report. 2018. Available at https://unos.org/.
- 37.Clayton PA, McDonald SP, Russ GR, et al. Long-term outcomes after acute rejection in kidney transplant recipients: an ANZDATA analysis. J Am Soc Nephrol. 2019;30:1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ANZDATA Registry 42nd Report, Chapter 7: graft losses, Australia and New Zealand dialysis and transplant registry, Adelaide, Australia. 2019. Available at https://wwwanzdataorgau.
- 39.Zhang Y, Ahmed H, Haririan A, et al. Granulomatous inflammation in BK polyomavirus-associated nephropathy. Transpl Infect Dis. 2018;20:e12939. [DOI] [PubMed] [Google Scholar]
- 40.Kharel A, Djamali A, Jorgenson MR, et al. Risk factors for progression from low level BK dnaemia to unfavorable outcomes after BK management via immunosuppressive reduction. Transpl Infect Dis. 2021;23:e13561. [DOI] [PubMed] [Google Scholar]
- 41.Sawinski D, Trofe-Clark J. BKV viremia and development of De Novo DSA in renal transplant recipients. Clin Transpl. 2015;31:249–256. [PubMed] [Google Scholar]
- 42.ANZDATA registry, 41st report, Chapter 7, transplantation, Australia and New Zealand dialysis and transplant registry, adelaide, Australia. 2018. Available at https://www.anzdata.org.au. 2018.
- 43.Dharnidharka VR, Abdulnour HA, Araya CE. The BK virus in renal transplant recipients-review of pathogenesis, diagnosis, and treatment. Pediatr Nephrol. 2011;26:1763–1774. [DOI] [PubMed] [Google Scholar]
- 44.Geetha D, Sozio SM, Ghanta M, et al. Results of repeat renal transplantation after graft loss from BK virus nephropathy. Transplantation. 2011;92:781–786. [DOI] [PubMed] [Google Scholar]
- 45.Hua DK, Howard K, Craig JC, et al. Cost-effectiveness of cidofovir treatment of polyomavirus nephropathy in kidney transplant recipients. Transplantation. 2012;93:188–194. [DOI] [PubMed] [Google Scholar]
- 46.Australian Government Department of Health and Aging. Medicare benefits schedule book. Canberra, ACT: AIHW. 2019. [Google Scholar]
- 47.Australian Government Department of Health and Aging. Pharmaceutical benefits scheme: AIHW. Available at https://www.aihw.gov.au. 2021.
- 48.Australian Government Australia Institute of Health and Welfare. AR-DRG. Available at https://www.aihw.gov.au/reports/hospitals/ar-drg-data-cubes/contents/data-cubes
- 49.Wong G, Howard K, Chapman JR, et al. Comparative survival and economic benefits of deceased donor kidney transplantation and dialysis in people with varying ages and co-morbidities. PLoS One. 2012;7:e29591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong G, Li MW, Howard K, et al. Health benefits and costs of screening for colorectal cancer in people on dialysis or who have received a kidney transplant. Nephrol Dial Transplant. 2013;28:917–926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.