Abstract
There is accumulating evidence in the literature indicating that a number of patients with coronavirus disease 2019 (COVID-19) may experience a range of neuropsychiatric symptoms, persisting or even presenting following the resolution of acute COVID-19. Among the neuropsychiatric manifestations more frequently associated with ‘long COVID’ are depression, anxiety, post-traumatic stress disorder, sleep disturbances, fatigue and cognitive deficits, that can potentially be debilitating and negatively affect patients' wellbeing, albeit in the majority of cases symptoms tend to improve over time. Despite variations in results obtained from studies using different methodological approaches to define ‘long COVID’ syndrome, the most widely accepted factors associated with a higher risk of developing neuropsychiatric manifestations include the severity of foregoing COVID-19, the female sex, the presence of comorbidities, a history of mental health disease and an elevation in the levels of inflammatory markers, albeit further research is required to establish causal associations. To date, the pathophysiological mechanisms implicated in neuropsychiatric manifestations of ‘long COVID’ remain only partially elucidated, while the role of the indirect effects of the COVID-19 pandemic, such as social isolation and uncertainty concerning social, financial and health recovery post-COVID, have also been highlighted. Given the alarming effects of ‘long-COVID’, interdisciplinary cooperation for the early identification of patients who are at a high risk of persistent neuropsychiatric presentations, beyond COVID-19 recovery, is crucial to ensure that appropriate integrated physical and mental health support is provided, with the aim of mitigating the risks of long-term disability at a societal and individual level.
Keywords: COVID-19, severe acute respiratory syndrome coronavirus 2, long COVID, post-COVID syndrome, COVID-19 survivors, neuropsychiatric sequalae
1. Introduction
Over 2 years have passed since the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) as a global public health emergency (1). During this period, significant efforts have been made to describe, study, and understand the clinical manifestations of the disease and its repercussions on physical and mental health (2-7).
During the acute phase of COVID-19, apart from the typical systemic and pulmonary manifestations, such as fever, cough, sore throat and dyspnea, neuropsychiatric symptoms related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may occur (8-10). Neuropsychiatric manifestations of acute COVID-19 include hyposmia/anosmia, consciousness disorders, delirium, agitation, encephalopathy, encephalitis, acute ischemic stroke, hypoxic/ischemic brain injury, seizures, vertigo, numbness/paresthesia, anxiety, depression and insomnia, while new-onset psychosis has also been reported in the setting of acute COVID-19 infection (11-20).
Crucially, while the acute phase of COVID-19 has been well-characterized, the data concerning the long-term outcomes of the disease are comparatively rather limited (21). There is increasing evidence to indicate that a number of patients may experience new, recurring or ongoing symptoms, as well as clinical signs, that persist beyond the acute illness; a condition that is colloquially referred to as ‘long COVID’ (22-25). Over the course of the pandemic, several definitions have been proposed and various terms have been used to describe the long-term symptoms and consequences following SARS-CoV-2 infection, including ‘post-COVID conditions’, ‘chronic COVID’, ‘long-haul COVID’, ‘long-term COVID-19’, ‘post-acute COVID-19’, ‘post-acute COVID syndrome’, ‘post COVID syndrome’ and ‘post-acute sequelae of SARS-COV-2 infection (PASC)’ (21,22,26,27). However, it is important to note that i) the lack of a standardized definition for ‘long COVID’; ii) the use of different temporal criteria for ‘long COVID’ diagnosis (i.e., 3 weeks up to several months after SARS-CoV-2 infection); and iii) the inclusion and attribution of diverse symptoms, conditions or signs to ‘long COVID’, may hinder the accurate classification of patients and may thus limit the elucidation of ‘long COVID’ syndrome (22,23,26-28).
‘Long COVID’ can become debilitating for some patients, while it has also been associated with a higher risk of mortality (23,29). In particular, individuals with ‘long COVID’ may present a wide spectrum of clinical manifestations, both pulmonary and extrapulmonary ones (including nervous system and neurocognitive disorders, musculoskeletal pain, mental health disorders, metabolic disorders, cardiovascular disorders, gastrointestinal disorders, and anemia), as well as signs and symptoms related to poor physical wellbeing (including malaise and fatigue) (30).
Patients affected by ‘long COVID’ include also those who initially had mild or asymptomatic disease, pointing to residual effects that involve multiple organ systems, including the peripheral and central nervous system (CNS) (23,31,32). Research evidence suggests that the neuroinvasive and neurotrophic properties of SARS-CoV-2, as well as the rapid overproduction of cytokines and immune cell hyperactivation (i.e., cytokine storm) may influence the manifestation of neuropsychiatric symptoms during and after COVID-19 (33-35). Furthermore, the indirect effects of COVID-19, such as social isolation and feelings of loneliness, uncertainty of the prognosis or incomplete physical health recovery, changes in sleep and lifestyle behaviors and the economic burden may also affect the manifestation of neuropsychiatric symptoms (36,37).
The systematic research of neuropsychiatric manifestations in patients beyond the resolution of acute COVID-19 is thus crucial in order to broaden the current understanding regarding the sequelae experienced by COVID-19 survivors and may facilitate the development of targeted evidence-based approaches towards an integrated patient care.
2. Neuropsychiatric manifestations in COVID-19 survivors with ‘long COVID’
There is increasing scientific evidence to indicate that a significant proportion of COVID-19 survivors experience a range of neuropsychiatric symptoms persisting or even presenting months after the initial infection (29,32,38).
Of note, Mazza et al (39), in 2020, assessed 402 COVID-19 survivors at 1 month following treatment at the hospital emergency department, using self-rated psychometric instruments, and found that overall, 56% scored in the pathological range in at least one clinical dimension [i.e., including depression, anxiety, post-traumatic stress disorder (PTSD) and obsessive-compulsive symptomatology] (39). Moreover, females, patients with a pre-existing psychiatric diagnosis and patients who were managed at home exhibited increased levels in the majority of psychopathological measurements (39). In a later cohort study, reassessing a subsample of 226 COVID-19 survivors of the aforementioned study at 3 months, Mazza et al (40), reported that 35.8% of the patients still scored in the pathological range in at least one psychopathological dimension (40).
Furthermore, Huang et al (41) evaluated a sample of 1,733 patients who were hospitalized due to COVID-19, 6 months following symptom onset and demonstrated that a significant proportion (76%) still reported at least one neuropsychiatric symptom, with the most common being fatigue/muscle weakness (63%) and sleep disturbances (26%). In a later study, 1-year follow-up data were available from a subsample of 1,276 COVID-19 survivors (36). Of note, within 1 year after acute infection, the majority of individuals exhibited a good physical and functional recovery over time, and had returned to their original work and life, albeit their health status remained lower compared to non-COVID-19 participants (controls) matched for age, sex and comorbidities (36).
Notably, Taquet et al (42) reported that among 236,379 patients diagnosed with COVID-19, the estimated incidence of a neurological or psychiatric diagnosis in the succeeding 6 months was 33.62%, with a proportion of 12.84% receiving such a diagnosis for the first time, whereas the estimated incidence was even higher for patients who had been admitted to an intensive care unit (ICU).
Depression, anxiety, PTSD, sleep disturbances, fatigue and cognitive deficits are among the most commonly reported neuropsychiatric symptoms in published studies investigating ‘long COVID’ (20,43), and thus should be explicitly assessed when treating patients with symptoms beyond the phase of acute SARS-COV-2 infection.
Anxiety, depression and ‘long COVID’
According to previous systematic reviews, the most frequently reported psychiatric symptoms in the context of ‘long COVID’ are depression and anxiety (20,38). Considering that, particularly persisting, depression and anxiety symptoms are associated with substantial individual morbidity with severe repercussions on the quality of life of patients, the psychiatric manifestations of ‘long COVID’ may also pose a significant healthcare challenge with major societal implications (44,45).
Of note, a previous retrospective cohort study found that among 236,379 patients, 17.39% were diagnosed with anxiety disorder (7.11% received first such diagnosis) and 13.66% with mood disorder (4.22% received first such diagnosis) in the 6 months following COVID-19 diagnosis. Notably, as regards patients who were admitted in the ICU, estimated incidences were 22.43% for anxiety disorder (9.24% for first diagnosis) and 22.52% for mood disorder (8.07% for first diagnosis) (42).
Furthermore, another prospective cohort study, including 251 patients with COVID-19 reported that 29.6% of the survivors presented state anxiety one month after hospital discharge, that was persistent at the 3-month follow-up assessment (i.e., in 25.5% of patients), while no changes in anxiety/depression symptoms were noted at 3-month follow-up (evaluated with EuroQoL-5 Dimensions, EQ-5D) (46).
Notably, a previous cohort study including 134 patients with COVID-19 assessed at a median of ~3.8 months (46-167 days) post-discharge, reported that at follow-up, 47.8% of the survivors experienced anxiety and 39.6% a low mood, with female patients being at a higher risk in comparison to males (47).
Accordingly, another prospective uncontrolled cohort study that evaluated, via telephone interview, 478 patients who were hospitalized due to COVID-19, 4 months after discharge, indicated that 31.4% of these patients reported anxiety and 20.6% depression (48), while another 4-month follow-up study found that among 94 COVID-19 patients who presented COVID-19 related pneumonia with respiratory failure, 21% presented anxiety post-hospital discharge (49).
Of note, a retrospective, case series of 200 hospitalized patients with severe-to-critical COVID-19 infection reported that at 4-7 months from disease-onset, 20% of patients presented anxiety or a low mood, sometimes associated with intrusive thoughts or flashbacks, while patients with pre-existing mental health issues presented a deterioration of their symptoms both during hospitalization and after discharge (50).
Furthermore, another cohort study, including 402 COVID-19 survivors who were hospitalized, demonstrated that at a 1-month assessment, 31% presented with depression and 42% with anxiety. Crucially, a significant decrease was recorded from the 1- to the 3-month follow-up assessment with regards to anxiety, whereas depression rates were not altered. It is important to note that the severity of baseline systemic inflammation predicted the severity of depressive psychopathology at the 3-month follow-up (40). Accordingly, a recent multimodal magnetic resonance imaging study assessing 42 COVID-19 survivors without brain lesions, at ~3 months (90.59±54.66 days) after COVID-19 infection, revealed that the systemic immune-inflammation index measured in the emergency department predicted increased depression several weeks after the clearance of the virus, while the severity of depression symptoms was also associated with decreasing grey matter (GM) volumes in the anterior cingulate cortex (ACC) (51).
Of note, a multicenter observational study including 1,142 COVID-19 survivors reported that at ~7 months after hospital discharge 16.2% of the patients presented self-rated anxiety symptoms, while 19.7% depressive symptoms. The female sex, the number of days spent at the hospital, the number of pre-existing medical comorbidities, and the number of lingering symptoms at hospital admission were significantly associated with depressive symptoms, whereas only the number of symptoms at hospital admission was associated with anxiety (52).
Moreover, a longitudinal study that assessed 165 consecutive non-neurological patients 6 months after hospitalization due to COVID-19, reported that 26.7% of the patients presented depressive symptoms or anxiety (53). Similarly, a larger cohort study that assessed 1,733 hospitalized patients 6 months following symptom onset demonstrated that 23% of these patents experienced anxiety or depression (41). It is important to note that the 1-year follow-up findings suggested an exacerbation of psychiatric symptoms, as the proportion of individuals with anxiety or depression increased significantly from 23% at the 6-month assessment to 26% at the 12-month assessment (36). Another 1-year follow-up study including 171 discharged patients with COVID-19 without a mental health history revealed that 35.1% of these patients reported anxiety and 32.2% depression (54). Accordingly, a cohort study including 2,433 COVID-19 survivors, also identified anxiety symptoms (10.4%) 1 year after hospital discharge, albeit to a lesser extent (55).
Findings concerning the trajectory of depression and anxiety symptoms are contradictory, as there are reports of an increase over time [e.g., higher anxiety and depression from the 6- to the 12-month assessment (36)], or results, suggesting symptom amelioration [e.g., decrease in anxiety at 1 month after admission (56), and also from the 1- to the 3-month assessment (40), as well as a decrease in depression at 1-month after admission (56)]. However, there are also reports of non-significant changes noted at the follow-up assessment [e.g., persistent anxiety 1 month after hospital discharge (46), as well as depression 1 month after admission (57) that remained unaltered between the 1- and 3-month assessment (40)].
As regards factors that may be associated with depression and anxiety in patients with ‘long COVID’, a number of studies have identified an elevated risk among COVID-19 survivors with an increased disease severity, with differences arising among patients who were treated at home or in outpatient settings, those who were hospitalized or patients requiring treatment in the ICU (30,41,42,58), although this connection has been suggested to be rather weak for psychiatric compared to neurological outcomes (42). Nevertheless, de Graaf et al (59) indicated that depression and anxiety symptoms did not differentiate between hospitalized patients treated in the ICU and those in a general ward. Similar findings were obtained in another study on a sample of COVID-19 survivors, with no pre-existing neurological, psychiatric, or severe medical condition, referred to an outpatient rehabilitation program due to persistent symptoms and/or sequelae of COVID-19 >3 months following acute COVID-19 infection (60). Accordingly, other studies did not find either differences in anxiety or depression between COVID-19 survivors with varying degrees of severity (e.g., mild, moderate, severe) (61,62), while Yuan et al (63) reported that depression was not associated with the severity of initial infection or a history of hospital admittance. As regards the impact of comorbidities, while associations with either depression (28) or both anxiety and depression symptoms (64), have been suggested, other studies have not found any association between comorbidities and ‘long COVID’ symptoms (63,65,66).
There is evidence to indicate that patients with a history of mental health issues may be at a higher risk of presenting persisting depression symptoms (39,40); however, there are also findings revealing that among COVID-19 survivors with self-rated anxiety and depression, a high proportion does not have pre-existing mental health conditions (58). Furthermore, it should be noted that often, studies investigating mental health sequalae among COVID-19 survivors, either define psychiatric history as an exclusion criterion or do not report findings using standardized assessment with respect to this (43). Crucially, a number of studies suggest that females are, in general, more likely to display anxiety or depression at follow-up (40,41,47,67); however, the absence of such an association has also been reported (63).
Post-traumatic stress disorder and ‘long COVID’
Taking into consideration the findings of previous studies regarding previous coronavirus outbreaks, such as SARS and Middle East respiratory syndrome (MERS), that reported significantly elevated PTSD rates in survivors even after several months, it is evident that the investigation of PTSD with respect to ‘long COVID’ is of utmost importance (68,69).
Of note, in a previous study, among 402 patients assessed at 1 month after hospital treatment, 28% presented PTSD (39), while when a subsample of 226 survivors was reassessed at 3 months, a significant decrease from the 1- to the 3-month follow-up was identified with respect to PTSD symptoms (40). Nevertheless, another prospective cohort study, assessing 251 hospitalized patients both at 1 and 3 months post-discharge, reported that at 1 month, 24.5% of patients experienced PTSD, a proportion that did not change significantly at the 3-month post-discharge evaluation (46).
Furthermore, in a large cohort study including 760 hospitalized patients, assessed at a median of 65 days (i.e., ~9 weeks) post-discharge, 10.5% screened positive for PTSD, with patients having more physical symptoms at admission and at follow-up being more likely to present PTSD symptoms. Patients with PTSD were more likely to experience persistent symptoms of breathlessness, myalgia, anorexia and confusion, whereas they were less likely to have returned to work (70). Notably, in another large cohort study, among 767 COVID-19 survivors assessed at a median time of 81 days after discharge, 30.5% reported PTSD (71).
Crucially, in a cohort study, at 4 months after discharge, PTSD was identified in 14.2% of 478 patients hospitalized due to COVID-19(48). Similarly, in a prospective, longitudinal cohort study, among 1,077 patients assessed at a median of 5.9 months after hospital discharge, 12.2% reported symptoms compatible with PTSD (72). Nevertheless, another 4-month follow-up study including 238 hospitalized patients due to severe COVID-19 found that at 4 months after discharge, 42.9% of the patients presented PTSD symptoms, while 17.2% of the patients reported moderate-to-severe symptom severity (73).
Notably, a 12-month longitudinal study revealed that 24.6% of the 171 discharged patients with COVID-19, without a mental health history, reported PTSD at the follow-up assessment (54). Of note, another study using a multimodal brain imaging approach demonstrated that systemic immune-inflammation during the acute phase predicted increased the risk of PTSD symptoms at ~3 months (90.59±54.66 days) post-COVID, while such symptoms were also associated with decreasing GM volumes both, in the ACC and in the bilateral insular cortex (51).
As regards the risk factors, there is evidence to indicate that psychological distress at the onset of illness may be predictive of PTSD development (74), while higher levels of anxiety and depression symptoms during the first week of hospitalization have also been suggested to independently predict a higher risk of developing PTSD symptoms at 1 month after hospitalization (56).
The setting of care (e.g., ICU, general hospital ward, outpatient setting) has emerged as a risk factor for PTSD in some studies (58,74); however, this is not the case in others (40,59,73,75). Of note, in previous studies, no differences were found among COVID-19 survivors with different levels of disease severity (e.g., mild, moderate, severe, or critical disease) (61,62). In another study, hospitalization was identified as a protective factor against developing PTSD, when comparing to patients discharged from the emergency department (76).
With respect to physical comorbidities, the findings suggest that there is no association with PTSD development, particularly following adjustment for other confounding factors (73,76). Crucially, patients with pre-existing mental health problems are likely to be at a greater risk of presenting PTSD (39,40), even when controlling for other demographic or clinically relevant factors (75); nevertheless, non-significant associations have also been reported (77).
Furthermore, studies using either univariate or multivariate analyses, have demonstrated that PTSD symptoms more commonly present in females following COVID-19 (39,40,75,77), particularly when treated in an ICU (58). However, it is important to note that there is also evidence to indicate that when adjusting for other variables, the sex effect may lose statistical significance (46,76). On the other hand, Bellan et al (73) indicated that the male sex was independently associated with the presence of moderate-to-severe PTSD symptoms.
The findings are not consistent regarding the role of body mass index (BMI) with regards to PTSD. There is evidence to suggest that a higher BMI (i.e., obesity) is associated with PTSD, independently of other factors (75), while it has also been indicated that this association is more pronounced in patients treated in the ICU (58); nevertheless, other studies have not found any such associations (73,76). Further factors suggested to predict PTSD severity include previous traumatic events, prolonged COVID-19 symptoms, stigmatization and a negative view of the COVID-19 pandemic (77).
Sleep disturbances, fatigue, other neuropsychiatric symptoms and ‘long COVID’
Sleep difficulties of varying degrees, as well as fatigue, that can potentially be debilitating and negatively affect the quality of life of patients, are among the long-term effects that COVID-19 survivors may continue to experience or even present for the first time weeks to months following the initial infection (20,38). Furthermore, post-acute neuropsychiatric manifestations of ‘long COVID’ include memory impairment, concentration difficulties, headaches, disorientation or confusion and obsessive-compulsive symptoms, among others (31,78).
In a previous prospective cohort study that assessed 251 survivors, at 1 month after discharge, 41.8% of the patients experienced insomnia, 35.3% pain/discomfort, 26.8% problems in usual daily activities and 10.3% problems in self-care. Among the aforementioned symptoms, none was significantly altered at the 3-month post-discharge evaluation, apart from insomnia, which improved significantly (i.e., affecting 25.5% of patients) albeit a number of patients still experienced sleep disturbances (46).
Accordingly, in another study, 402 patients with COVID-19 who were assessed at 1 month after hospital treatment, 40% reported insomnia and 20% obsessive-compulsive symptoms (39). Crucially, a significant decrease from the 1- to the 3-month follow-up assessment (in a subsample of 226 survivors) was recorded for insomnia, whereas notably, obsessive-compulsive symptomatology worsened (40). Furthermore, a significant proportion of patients (78%) displayed poor performances in at least one cognitive domain, with executive functions (50%) and psychomotor coordination (57%) emerging as the most impaired, followed by attention and information processing (33%), verbal fluency (32%), working memory (24%) and verbal memory (10%). Notably, baseline systemic inflammation predicted neurocognitive performance at the 3-month follow-up (40).
Another study demonstrated that among 134 patients with COVID-19 assessed after a median of ~3.8 months post-discharge, 51.5% reported myalgia, 39.6% extreme fatigue, 37.3% memory impairment, 35.1% sleep disturbances, 25.4% attention deficits and 9.7% cognitive impairment. Females were more likely to suffer from sleep disturbances, fatigue, myalgia and memory impairment compared to males, while higher a BMI was significantly associated with myalgia and fatigue (47). Crucially, in a larger retrospective cohort study, including 932 hospitalized patients with COVID-19, a relatively low proportion with respect to fatigue was noted at 3 months after discharge (i.e., 1.8%) (79).
Of note, in another study with a 4-month follow-up, including 94 survivors who had COVID-19-related pneumonia with respiratory failure, at 4 months after their discharge, 52% of patients reported experiencing fatigue, 10% anorexia and 31% insomnia (49). Similarly, in a 4-month post-discharge assessment of 478 patients who were hospitalized due to COVID-19, the neuropsychiatric symptoms reported included insomnia (53.6%), fatigue (31.1%), memory difficulties (17.5%), persistent paresthesia (12.1%), mental slowness (10.1%), memory difficulties (10.0%) and headaches (5.5%), while cognitive impairment was found in 38.4% of patients. Notably, 51% of the COVID-19 survivors reported at least one symptom that did not exist prior to the disease (48). Accordingly, in another study among 200 hospitalized patients with severe-to-critical COVID-19 infection, it was found that at 4-7 months from disease-onset a significant proportion of patients experienced significant fatigue (53.5%), decreased mobility (37.5%) and pain (36.8%). Further neuropsychiatric symptoms included cognitive difficulties, mainly in concentration and short-term recall (12.5%), sleeping disturbances (14.6%), while 12.2% of patients became frail (50).
A prospective, longitudinal cohort study that assessed 1,077 patients at a median of 5.9 months following hospital discharge found that the most commonly reported persistent symptoms at follow-up were aching muscles/pain (57.2%), fatigue (worsening of symptoms reported by 56.2%), physical slowing down (49.9%), slowing down in thinking (42.4%), impaired sleep quality (worsening of symptoms reported by 41.8%), joint pain or swelling (47.8%), limb weakness (46.3%), difficulty with concentration (40.2%), short-term memory loss (42.0%) and headaches (33.4%). Moreover, less frequently reported symptoms included, among others, confusion/fuzzy head, dizziness or lightheadedness, altered personality/behavior and difficulty with communication. Of note, 29% of the patients reported that they felt fully recovered at follow-up, while non-recovery was associated with female sex, middle age (40-59 years), two or more comorbidities and more severe acute illness (72).
Of note, a previous cohort study, following a total of 952 patients with absent-to-mild COVID-19 symptoms for a median time period of 6.8 months (i.e., 442 and 353 patients observed over a period of 4 and 7 months after symptom onset, respectively), demonstrated that while the most common symptoms at disease onset included cough (64.4%), ageusia (59.1%), anosmia (54.3%), body aches (53.2%), headaches (53.1%) and fever (44.6%), the most frequently reported symptoms at 4-month follow-up were anosmia (12.4%), ageusia (11.1%), fatigue (9.7%) and shortness of breath (8.6%). Intriguingly, symptoms such as anosmia (14.7%), shortness of breath (13.6%), fatigue (14.7%) and ageusia (11.0%) were also present at the 7-month assessment, while headaches (3.7%) were also recorded (80).
Furthermore, a retrospective observational follow-up study including 797 COVID-19 survivors who were hospitalized, demonstrated that at 6 months post-discharge, 22.1% presented fatigue, 3.8% muscle weakness, 15.3% musculoskeletal pain, 5.3% headache, 3.4% paresthesia, 2.6% disorientation or confusion, and 4.9% sleep disturbances (67). Another 6-month follow-up cohort study that evaluated 796 patients with severe COVID-19 rehabilitation, found that the most common neuropsychiatric symptoms at follow-up comprised fatigue (25.3%) and sleep disorder (23.2%), while hypomnesia (8.7%), dizziness and headache (1.9%) were also recorded (81). Crucially, among 165 patients, 6 months after hospitalization due to COVID-19, fatigue (33.9%), memory/concentration complaints (31.5%), sleep disorders (31.5%), myalgias (30.3%) and the loss of dependency in instrumental activities of daily living (20.7%) emerged as the most common neuropsychiatric symptoms (53). In another cohort study, poor sleep quality was recorded 7 months after discharge in 34.5% of 1,142 patients who were hospitalized due to COVID-19, while females, patients with a higher number of days of hospital stay, a higher number of comorbidities and a higher number of symptoms at hospital admission were more likely to report poor sleep quality (52).
Notably, a retrospective cohort study reported that among 236,379 patients diagnosed with COVID-19, the estimated incidences at 6 months post-COVID were 1.40% for psychotic disorders (0.42% for first such diagnosis), 6.58% for substance use disorder (1.92% for first diagnosis), 5.42% for insomnia (2.53% for first diagnosis), 2.10, and 0.67% for dementia (42).
Furthermore, in another study, among 1,733 patients hospitalized due to COVID-19, within 6 months from symptom onset, 63% experienced symptoms of fatigue or muscle weakness, 27% pain or discomfort, 26% sleep difficulties, 6% dizziness, 2% myalgia and 2% headache (41). In a subsample of 1,276 COVID-19 survivors who were assessed both at 6 and 12 months following symptom onset, the majority of the aforementioned neuropsychiatric symptoms appeared to have improved. In particular, symptoms of fatigue or muscle weakness decreased from 52 to 20%, while sleep difficulties from 27 to 17%. However, a statistically significant increase was observed with respect to myalgia (from 3 to 4%) and headache (from 2 to 5%), whereas no statistically significant changes were noted for pain or discomfort (from 27 to 29%) and dizziness (from 6 to 5%) (36).
Notably, the neuropsychiatric symptoms reported in a retrospective cohort study that assessed 2,433 COVID-19 survivors at 1 year after their hospital discharge included fatigue (27.7%) and myalgia (7.9%), while patients with severe disease during hospitalization presented more frequently with post-COVID symptoms (55). Importantly, in the aforementioned study, an older age, female sex and severe disease during hospital stay were associated with a higher risk of fatigue, while an advanced age and severe disease were also associated with an increased risk of having more post-infection symptoms. Furthermore, another 1-year follow-up study on 171 discharged patients, reported neuropsychiatric symptoms including fatigue (48.5%), memory complaints (32.2%), headaches (15.8%) and paresthesia (7%). Notably, the most affected cognitive domain was semantic verbal fluency (32.7%) followed by immediate verbal memory/learning (20.5%), working memory/executive function (12.3%) and delayed verbal memory (7.6%) (54).
Sleep disturbances persisting in patients even months after acute COVID-19 infection have been highlighted in several studies, with some of these indicating that almost half of the survivors experience difficulties related to sleep (39,48), although there is evidence for improvement over time (40,46). The pivotal essential role of sleep in physical and mental health is well-established (82). Vice versa, there is evidence to indicate that insomnia and other mental health conditions not only share common pathophysiological causes, but also exhibit a bidirectional association, with disrupted sleep most likely being a contributory causal factor for the occurrence of major types of mental health disorders (83). Among patients with ‘long COVID’, the female sex (39,40,47,52,81), a history of mental health issues (39,40) and the number of comorbidities (52) are likely associated with an increased risk of experiencing sleep disturbances. Of note, there are studies that indicate no difference between patients admitted to hospital as compared to those discharged from the emergency department (76), or an association of sleep disorders with disease severity (41,62,81); however there are also studies that associate insomnia with the care setting/disease severity (e.g., patients without hospitalization, patients with hospitalization, patients with ICU admission or patients with encephalopathy) (30,42), or highlight the number of symptoms at hospital admission and the number of days spent at hospital, as risk factors for a poor sleep quality (52). Nevertheless, a previous study also found that patients that were not treated in the ICU presented a lower sleep quality and higher daytime dysfunction compared to those treated in the ICU, while no other differences were noted in any of the other sleep-related dimensions (i.e., latency, duration, efficacy, disturbance or medication use) (60).
Fatigue is among the most commonly reported symptoms that patients experience following the resolution of acute COVID-19, while according to a recent meta-analysis, ~32% of individuals experienced fatigue at ≥12 weeks following COVID-19 diagnosis (84). Females are, in general, at a greater risk of experiencing persisting fatigue post-COVID (41,47,67,81,85,86), either when treated in a hospital ward or in the ICU (58), with differences being observed even at 1 year of follow-up (36,55). Nevertheless, other studies have reported no such associations (87,88). An older age has been independently associated with fatigue or muscle weakness (41) and higher rates of fatigue (87) at 6 months post-discharge, as well as at the 1-year follow-up (55), whereas other findings suggesting an absence of significant association have also been reported at the 10-week (85) and 1-year follow-up following discharge (36). Furthermore, patients with a higher BMI are likely to experience increased fatigue (47,89), although findings not supporting this association have also been reported (58,85,87). Crucially, as regards the impact of COVID-19 severity on post-viral fatigue, the findings are not consistent. There are reports of severe disease linked to a higher risk of fatigue at 3 months (62) and 6 months after COVID-19 (30,53,90), as well as of fatigue or muscle weakness (41) at 6 months post-discharge and fatigue up to 1 year post-discharge (55); however, in the study sample of Huang et al this association has emerged only for the 6-month assessment (41), but not the 12 month assessment (36). Of note, another study assessing patients, with no neurological or psychiatric history, at >3 months after acute COVID-19 infection, indicated that there was no significant association between previous admission to ICU and fatigue (60), while another study assessing fatigue at a median of 10 weeks after the initial COVID-19 symptoms demonstrated that there was no association between COVID-19 severity (need for inpatient admission, supplemental oxygen or critical care) and fatigue following COVID-19(85). Accordingly, Shang et al (81) did not find any differences between severe and critical illness at 6-month follow-up assessment among patients with severe COVID-19. Other risk factors for fatigue may include high symptom load during COVID-19(89), the length of hospital stay (87), dyspnea during COVID-19(89), confusion during COVID-19(89), previous depression (89), a pre-existing diagnosis of depression/anxiety (85), sleep disturbances (62), as well as new neurological diagnoses (62). Of note, in the study by Huang et al (36), therapy using corticosteroids during the acute phase was associated with an increased risk of fatigue or muscle weakness at 1 year post-discharge, whereas intravenous immunoglobulin therapy in the acute phase decreased the risk of persistent fatigue or muscle weakness. Townsend et al (85) reported no association between routine laboratory markers of inflammation and cell turnover (leukocyte, neutrophil or lymphocyte counts, neutrophil-to-lymphocyte ratio, lactate dehydrogenase, C-reactive protein) or pro-inflammatory molecules [interleukin (IL)-6 or sCD25] and fatigue post COVID-19, while Liang et al (91) found that serum troponin-I levels during acute illness correlated positively with fatigue after hospital discharge.
Cognitive impairment persisting in patients with COVID-19, is particularly concerning, as apart from the individual physical/mental health and social repercussions, this may also translate into a major global healthcare and economic burden (84). Cognitive impairment/deficits reported following the resolution of acute COVID-19 symptoms, may include difficulties in concentration, memory, attention, language, executive function, encoding and verbal fluency, and visuospatial function, among others (20,78,92). A previous systematic review and meta-analysis regarding the psychiatric and neuropsychiatric presentations associated with severe coronavirus infections, such as SARS or MERS, demonstrated that during the acute illness, common cognitive problems among hospitalized patients included confusion [prevalence, 27.9%; 95% confidence interval (CI), 20.5-36.0], an impaired concentration or attention (prevalence, 38.2%; 95% CI, 29.0-47.9) and impaired memory (prevalence, 34.1%; 95% CI, 26.2-42.5), while an impaired concentration or attention (prevalence, 19.9%; 95% CI, 14.2-26.2) and memory impairment (prevalence, 18.9%; 95% CI, 14.1-24.2) were frequently reported during the post-illness stage as well (93). Notably, a recent meta-analysis revealed that ~22% of individuals experienced fatigue ≥12 weeks following the diagnosis of COVID-19(84). Disease severity has been associated with cognitive deficits, such as deficits related to memory [i.e., memory complaints (53), memory deficits (30), hypomnesia (81)], confusion (53) and visual disturbances (53). Nevertheless, there are studies that fail to support such a connection. In particular, de Graaf et al (2021) did not find any significant differences with regards to cognitive function between patients treated in a general hospital ward and those admitted to the ICU (59). Accordingly, in the study by De Lorenzo et al (76), patients admitted to hospital as compared to those discharged from the emergency department did not differ as regards self-reported cognitive impairment measures. Furthermore, a study assessing patients in an outpatient rehabilitation program due to persistent symptoms and/or sequelae of COVID-19, with no prior neurological or psychiatric history, indicated that there was no significant association between previous admission to the ICU and orientation, attention, verbal learning, long-term verbal memory, verbal recognition, working memory or executive control (60). Importantly, Méndez et al (94) did not find an association between sex and neurocognitive impairment at the 2-month post-discharge assessment, while Shang et al (81) did not find any differences in hypomnesia between male and female patients at the 6-month follow-up among patients with severe COVID-19. Nevertheless, according to other studies, females were more likely than males to report memory impairment (47), a poor performance in working memory (40), disorientation or confusion (67). Of note, delirium during hospitalization and stress-related symptoms have been found to be associated with an increased likelihood of neurocognitive impairment at 2-month assessment (94), while according to Mazza et al (40) neurocognitive impairments were associated with severity of depressive psychopathology, while processing speed, verbal memory and fluency, and psychomotor coordination were predicted by baseline systemic inflammation.
3. Pathophysiological mechanisms underlying neuropsychiatric symptoms of ‘long COVID’
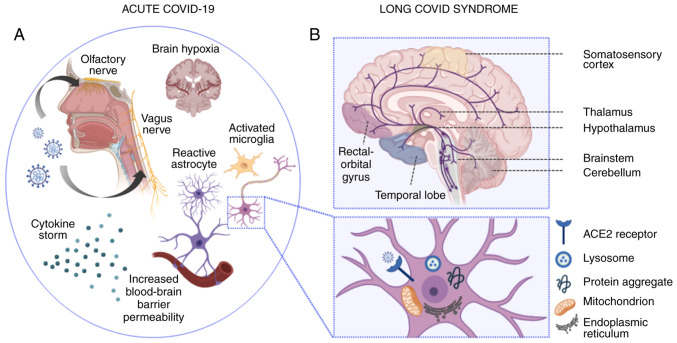
Significant strides have been made recently in the understanding of the underlying pathophysiology of neuropsychiatric complications following acute COVID-19 infection (Fig. 1). The neuroinvasive potential of coronaviruses has long been recognized; however, the precise route of entry of SARS-COV-2 in the nervous system remains only partially elucidated (95). The retrograde transport of viral antigens along the axons of both the olfactory neurons (96-99) and vagus nerve (100) has been suggested by research using animal models, while the Spike (S) viral protein has been shown to have the capacity of crossing the blood-brain barrier in mice (101). Additionally, SARS-COV-2 has the ability to enter host cells directly by binding its Spike protein to the angiotensin-I converting enzyme 2 (ACE2) (102), which is abundantly expressed in neural tissue-namely endothelial cells, neurons, glial cells, brain nuclei, etc. (103). The strong binding affinity of the Spike protein to ACE2(104) and the potential use of CD147 as a route of entry into cells (105), leads to a robust immune response, with infected macrophages releasing significant amounts of Th-1 (IL-1β, IL-6, interferon-γ, tumor necrosis factor-α, CXCL10 and CCL2) and Th-2 cytokines (IL-4, IL-10 and IL-1 receptor antagonist) (106,107). Notably, IL-6 is the main contributor to the dysregulated inflammatory response induced by the virus (108).
Figure 1.
Pathophysiological mechanisms implicated in acute and ‘long-COVID’ neuropsychiatric sequelae. (A) In acute COVID-19 infection, SARS-CoV-2 enters the CNS via hematogenous transmission or retrograde transport along the axons of the olfactory (94-97) and vagus nerve (100). Vagus nerve affection may lead to autonomic dysregulation, impaired cerebral autoregulation and subsequent brain hypoxia (100). Moreover, the SARS-CoV-2-induced cytokine storm results in impaired blood-brain barrier function and increased blood-brain barrier permeability. The latter induces the transmigration of virus-infected leukocytes into the CNS, the activation of microglial cells and astrocytes, which in turn trigger apoptotic cascades and demyelination, respectively. At the neuronal level (illustrated in the magnified inset), SARS-CoV-2 cell entry is mainly facilitated by the ACE2 receptor, located on the surface of neuronal cells. Intracellular inflammatory responses in the setting of acute SARS-CoV-2 infection induce lysosomal, mitochondrial and endoplasmic reticulum dysfunction, while protein misfolding and intracellular accumulation of protein aggregates may induce long-lasting neurodegenerative cascades (132). (B) In ‘long-COVID’ syndrome, neuropsychiatric deficits have been mainly associated with ongoing inflammatory, metabolic and degenerative processes, which have been linked to ‘ACE2-rich’ brain areas, extending from the somatosensory cortex to the rectal/orbital gyrus, the temporal lobe, the thalamus and hypothalamus, and further, to brainstem and cerebellar regions (133). Serotoninergic pathways are depicted in purple. Apart from serotoninergic pathways, further neurotransmitter imbalances (not illustrated), including acetylcholine, dopamine and histamine have been found to be linked to lingering neuropsychiatric symptoms noted in patients with ‘long-COVID’ (134). The image was created using BioRender (https://biorender.com). CNS, central nervous system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2.
Those direct effects of acute COVID-19 infection may subsequently contribute to the symptomatology noted in ‘long COVID’. It should, nevertheless, be noted that the exact mechanisms of ‘long COVID’ remain poorly delineated to date, while new evidence continues to emerge. Anxiety syndromes, depression and cognitive impairment are considered to have a multifactorial origin, with somatic, functional and psychosocial factors all contributing to the clinical picture. The psychological stressors of social isolation and infection with a novel potentially fatal virus, as well as the fear of infecting others or being stigmatized, all play a key role (109-111). The activation of the hypothalamus-pituitary-adrenal glands axis observed in patients with COVID-19 mediates glucocorticoid secretion (i.e., the main hormonal response to physical and mental stress stimuli) and is also one of the main neurobiological mechanisms in depression as it inhibits neurogenesis and decreases the proliferation and survival of nerve cells in the dentate gyrus of the hippocampus (112-114).
The cytokine storm as part of the systemic hyperinflammatory state observed in acute COVID-19 infection, also plays a key role in persistent maladies, precipitated by changes in cerebral perfusion, an increased permeability of the blood-brain barrier, changes in astrocytes involved in synaptogenesis and the imbalance of neurotransmitters (115). These molecules dysregulate neurogenesis, causing neurons, oligodendrocytes and glial cells to lose their physiological function (116). This occurring disruption of neuronal plasticity, synaptic function, myelination and blood-brain barrier maintenance can subsequently impair cognitive function and may lead to a number of the long-term neuropsychiatric symptoms of COVID-19(117). Of note, previous studies have demonstrated that the systemic immune-inflammation index (SII) was elevated at the 3-month follow-up in patients reporting depressive and cognitive impairment symptomatology (39,118). The SII is an objective marker of host systemic inflammation and immune response, implicating neutrophils, platelets and lymphocytes, cells involved in various inflammatory pathways. Individuals who presented with a marked decrease in the SII exhibited a decrease in the severity of depression, while by contrast, increased levels of SII had a negative effect on neurocognitive performance (memory, verbal fluency, speed of information processing, psychomotor coordination), since this elevation reflects prolonged systemic inflammation (40).
Another factor that also may account for the delayed sequelae of COVID-19 is the failure of reactive neuroglia to return to the physiological state. Neuroglia undergo complex remodeling in response to systemic pathology, a process known as gliosis, in order to remove pathogens, strengthen brain-organism barriers and contribute to the regenerative potential of the CNS (116). This reactive response results in the formation of a glial scar isolating the damaged area, protecting the adjacent healthy nervous tissue (119). The resolution of systemic pathology is what enables the restoration of homeostatic status of neuroglial cells. Post-mortem examinations of the brains of patients with COVID-19 have highlighted a perturbed glial homeostasis, with significant changes in both astrocytes and microglia (120). These alterations are consistent with morphological and functional astrocyte remodeling in chronic stress and major psychiatric diseases (121,122).
Autonomic dysfunction, either due to dysfunction related to the initial infection (123-126) or mediated by autoantibodies (127) (possibly to adrenoceptors and muscarinic receptors), has been hypothesized to cause orthostatic intolerance syndrome in patients with ‘long COVID’. The concomitant involvement of other organs, such as the heart or lungs, is prominent and associated with neurological sequelae, while perturbed viral infection-induced colon inflammation, gut microbial imbalance and α-synuclein upregulation play a role in the disruption of interplay between the gastrointestinal tract and the CNS (128).
The formation of thrombi due to endothelial dysfunction, hypercoagulability and the lingering cytokine storm in patients with ‘long COVID’ may be associated with a high incidence of thrombotic cerebral complications (129). Additionally, direct damage to the blood-brain barrier by the virus and hypertension due to elevation in ACE2 may cause hemorrhagic complications with persistent sequelae following the resolution of acute SARS-CoV-2 infection (130). The high susceptibility of white matter to ischemia renders it particularly vulnerable to ischemic changes. Furthermore, long-term alterations have recently been confirmed by the neuroradiological evidence of structural damage and impaired functional integrity of the brain, at a 3-month follow-up of COVID-19 survivors (131).
4. Conclusion
The present review article has provided insight into the long-term neuropsychiatric effects of COVID-19, while presenting a comprehensive overview of published epidemiological data to date, as well as research evidence on the pathophysiological mechanisms underlying the neuropsychiatric manifestations of ‘long COVID’. Considering the disconcerting effects of COVID-19 and the global dimensions of the pandemic, interdisciplinary cooperation is warranted for the early identification of patients who are at a high risk of developing persistent neuropsychiatric deficits following recovery from acute disease. To this end, it is of paramount importance to ensure that appropriate integrated physical and mental health support is provided, with the aim of mitigating the risks of long-term disability at an individual and societal level.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
VE, MIS, MD, NSi and MM wrote the original draft, and edited and critically revised the manuscript. GT, VZ, SPK, JNT, DAS, NSm and ER critically revised and edited the manuscript. All authors substantially contributed to the conception, writing and revision of the work, and have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but has no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1.Sohrabi C, Alsafi Z, O'Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifidis C, Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leaune E, Samuel M, Oh H, Poulet E, Brunelin J. Suicidal behaviors and ideation during emerging viral disease outbreaks before the COVID-19 pandemic: A systematic rapid review. Prev Med. 2020;141(106264) doi: 10.1016/j.ypmed.2020.106264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahil K, Cheaito MA, El Hayek R, Nofal M, El Halabi S, Kudva KG, Pereira-Sanchez V, El Hayek S. Suicide during COVID-19 and other major international respiratory outbreaks: A systematic review. Asian J Psychiatr. 2021;56(102509) doi: 10.1016/j.ajp.2020.102509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoumpourlis V, Goulielmaki M, Rizos E, Baliou S, Spandidos DA. [Comment] The COVID-19 pandemic as a scientific and social challenge in the 21st century. Mol Med Rep. 2020;22:3035–3048. doi: 10.3892/mmr.2020.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efstathiou V, Stefanou MI, Siafakas N, Makris M, Tsivgoulis G, Zoumpourlis V, Spandidos DA, Smyrnis N, Rizos E. Suicidality and COVID-19: Suicidal ideation, suicidal behaviors and completed suicides amidst the COVID-19 pandemic (Review) Exp Ther Med. 2022;23(107) doi: 10.3892/etm.2021.11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsamakis K, Tsiptsios D, Ouranidis A, Mueller C, Schizas D, Terniotis C, Nikolakakis N, Tyros G, Kympouropoulos S, Lazaris A, et al. COVID-19 and its consequences on mental health (Review) Exp Ther Med. 2021;21(244) doi: 10.3892/etm.2021.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsamakis K, Triantafyllis AS, Tsiptsios D, Spartalis E, Mueller C, Tsamakis C, Chaidou S, Spandidos DA, Fotis L, Economou M, Rizos E. COVID-19 related stress exacerbates common physical and mental pathologies and affects treatment (Review) Exp Ther Med. 2020;20:159–162. doi: 10.3892/etm.2020.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y, Yuan K, Wang Z, Liu WJ, Lu ZA, Liu L, Shi L, Yan W, Yuan JL, Li JL, et al. Neuropsychiatric manifestations of COVID-19, potential neurotropic mechanisms, and therapeutic interventions. Transl Psychiatry. 2021;11(499) doi: 10.1038/s41398-021-01629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, Huet K, Plzak J, Horoi M, Hans S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, Sultan M, Easton A, Breen G, Zandi M, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iltaf S Sr, Fatima M, Salman S Sr, Salam JU, Abbas S. Frequency of neurological presentations of coronavirus disease in patients presenting to a tertiary care hospital during the 2019 coronavirus disease pandemic. Cureus. 2020;12(e9846) doi: 10.7759/cureus.9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan S, Xiao M, Han F, Xia P, Bai X, Chen H, Zhang H, Ding X, Zhao H, Zhao J, et al. Neurological manifestations in critically ill patients with COVID-19: A retrospective study. Front Neurol. 2020;11(806) doi: 10.3389/fneur.2020.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong W, Mu J, Guo J, Lu L, Liu D, Luo J, Li N, Liu J, Yang D, Gao H. New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology. 2020;95:e1479–e1487. doi: 10.1212/WNL.0000000000010034. [DOI] [PubMed] [Google Scholar]
- 17.Frontera JA, Sabadia S, Lalchan R, Fang T, Flusty B, Millar-Vernetti P, Snyder T, Berger S, Yang D, Granger A, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology. 2021;96:e575–e586. doi: 10.1212/WNL.0000000000010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bo HX, Li W, Yang Y, Wang Y, Zhang Q, Cheung T, Wu X, Xiang YT. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol Med. 2021;51:1052–1053. doi: 10.1017/S0033291720000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, Chen Y, Zheng Y, You C, Tan J, Hu L, Zhang Z, Ding L. Factors related to mental health of inpatients with COVID-19 in Wuhan, China. Brain Behav Immun. 2020;89:587–593. doi: 10.1016/j.bbi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schou TM, Joca S, Wegener G, Bay-Richter C. Psychiatric and neuropsychiatric sequelae of COVID-19-A systematic review. Brain Behav Immun. 2021;97:328–348. doi: 10.1016/j.bbi.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, Hara M, Suett J, Dahmash D, Bugaeva P, et al. Characterising long COVID: A living systematic review. BMJ Global Health. 2021;6(e005427) doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention (CDC): Post-COVID Conditions: Information for Healthcare Providers. CDC, Atlanta, GA, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html. Updated July 9, 2021. [Google Scholar]
- 23.Deer RR, Rock MA, Vasilevsky N, Carmody L, Rando H, Anzalone AJ, Basson MD, Bennett TD, Bergquist T, Boudreau EA, et al. Characterizing long COVID: Deep phenotype of a complex condition. EBioMedicine. 2021;74(103722) doi: 10.1016/j.ebiom.2021.103722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 25.Stefanou MI, Palaiodimou L, Bakola E, Smyrnis N, Papadopoulou M, Paraskevas GP, Rizos E, Boutati E, Grigoriadis N, Krogias C, et al. Neurological manifestations of long-COVID syndrome: A narrative review. Ther Adv Chronic Dis. 2022;13(20406223221076890) doi: 10.1177/20406223221076890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajan S, Khunti K, Alwan N, Steves C, Greenhalgh T, MacDermott N, Sagan A, McKee M. In the wake of the pandemic. Preparing for Long COVID Policy Brief. 2021;(39) [PubMed] [Google Scholar]
- 28.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining Post-COVID symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An integrative classification. Int J Environ Res Public Health. 2021;18(2621) doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, Redfield S, Austin JP, Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38(101019) doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci Rep. 2021;11(16144) doi: 10.1101/2021.01.27.21250617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamontagne SJ, Winters MF, Pizzagalli DA, Olmstead MC. Post-acute sequelae of COVID-19: Evidence of mood & cognitive impairment. Brain Behav Immun Health. 2021;17(100347) doi: 10.1016/j.bbih.2021.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jesuthasan A, Massey F, Manji H, Zandi MS, Wiethoff S. Emerging potential mechanisms and predispositions to the neurological manifestations of COVID-19. J Neurol Sci. 2021;428(117608) doi: 10.1016/j.jns.2021.117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dąbrowska E, Galińska-Skok B, Waszkiewicz N. Depressive and neurocognitive disorders in the context of the inflammatory background of COVID-19. Life (Basel) 2021;11(1056) doi: 10.3390/life11101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flores G. SARS-COV-2 (COVID-19) has neurotropic and neuroinvasive properties. Int J Clin Pract. 2021;75(e13708) doi: 10.1111/ijcp.13708. [DOI] [PubMed] [Google Scholar]
- 36.Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, Hu P, Guo L, Liu M, Xu J, et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lagadinou M, Kostopoulou E, Karatza A, Marangos M, Gkentzi D. The prolonged effects of COVID-19. A New ‘Threat’? Eur Rev Med Pharmacol Sci. 2021;25:4611–4615. doi: 10.26355/eurrev_202107_26253. [DOI] [PubMed] [Google Scholar]
- 38.Shanbehzadeh S, Tavahomi M, Zanjari N, Ebrahimi-Takamjani I, Amiri-Arimi S. Physical and mental health complications post-COVID-19: Scoping review. J Psychosom Res. 2021;147(110525) doi: 10.1016/j.jpsychores.2021.110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazza MG, Palladini M, De Lorenzo R, Magnaghi C, Poletti S, Furlan R, Ciceri F, Rovere-Querini P, Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourmistrova NW, Solomon T, Braude P, Strawbridge R, Carter B. Long-term effects of COVID-19 on mental health: A systematic review. J Affect Disord. 2022;299:118–125. doi: 10.1016/j.jad.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young AS, Klap R, Shoai R, Wells KB. Persistent depression and anxiety in the United States: Prevalence and quality of care. Psychiatr Serv. 2008;59:1391–1398. doi: 10.1176/appi.ps.59.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renaud-Charest O, Lui LMW, Eskander S, Ceban F, Ho R, Di Vincenzo JD, Rosenblat JD, Lee Y, Subramaniapillai M, McIntyre RS. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J Psychiatr Res. 2021;144:129–137. doi: 10.1016/j.jpsychires.2021.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Lorenzo R, Cinel E, Cilla M, Compagnone N, Ferrante M, Falbo E, Patrizi A, Castellani J, Magnaghi C, Calvisi SL, et al. doi: 10.23736/S0031-0808.21.04399-8. Physical and psychological sequelae at three months after acute illness in COVID-19 survivors. Panminerva Med: Jun 1, 2021 (Epub ahead of print). doi: 10.23736/S0031-0808.21.04399-8. [DOI] [PubMed] [Google Scholar]
- 47.Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 Symptom Burden: What is Long-COVID and how should we manage it? Lung. 2021;199:113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, Gasnier M, Lecoq AL, Meyrignac O, Noel N. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boari GEM, Bonetti S, Braglia-Orlandini F, Chiarini G, Faustini C, Bianco G, Santagiuliana M, Guarinoni V, Saottini M, Viola S, et al. Short-term consequences of SARS-CoV-2-related pneumonia: A follow up study. High Blood Press Cardiovasc Prev. 2021;28:373–381. doi: 10.1007/s40292-021-00454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautam N, Madathil S, Tahani N, Bolton S, Parekh D, Stockley J, Goyal S, Qureshi H, Yasmin S, Cooper BG, et al. Medium-term outcome of severe to critically ill patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Clin Infect Dis. 2021;74:301–308. doi: 10.1093/cid/ciab341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benedetti F, Palladini M, Paolini M, Melloni E, Vai B, De Lorenzo R, Furlan R, Rovere-Querini P, Falini A, Mazza MG. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: A multimodal magnetic resonance imaging study. Brain Behav Immun Health. 2021;18(100387) doi: 10.1016/j.bbih.2021.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernández-de-Las-Peñas C, Gómez-Mayordomo V, de-la-Llave-Rincón AI, Palacios-Ceña M, Rodríguez-Jiménez J, Florencio LL, Velasco-Arribas M, Fuensalida-Novo S, Cigarán-Méndez M, Ambite-Quesada S, et al. Anxiety, depression and poor sleep quality as long-term post-COVID sequelae in previously hospitalized patients: A multicenter study. J Infect. 2021;83:496–522. doi: 10.1016/j.jinf.2021.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pilotto A, Cristillo V, Cotti Piccinelli S, Zoppi N, Bonzi G, Sattin D, Schiavolin S, Raggi A, Canale A, Gipponi S, et al. Long-term neurological manifestations of COVID-19: prevalence and predictive factors. Neurol Sci. 2021;42:4903–4907. doi: 10.1007/s10072-021-05586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Méndez R, Balanzá-Martínez V, Luperdi SC, Estrada I, Latorre A, González-Jiménez P, Bouzas L, Yépez K, Ferrando A, Reyes S, et al. Long-term neuropsychiatric outcomes in COVID-19 survivors: A 1-year longitudinal study. J Intern Med. 2022;291:247–251. doi: 10.1111/joim.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Wang F, Shen Y, Zhang X, Cen Y, Wang B, Zhao S, Zhou Y, Hu B, Wang M, et al. Symptoms and Health Outcomes Among Survivors of COVID-19 Infection 1 Year After Discharge From Hospitals in Wuhan, China. JAMA Netw Open. 2021;4(e2127403) doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matalon N, Dorman-Ilan S, Hasson-Ohayon I, Hertz-Palmor N, Shani S, Basel D, Gross R, Chen W, Abramovich A, Afek A. Trajectories of post-traumatic stress symptoms, anxiety, and depression in hospitalized COVID-19 patients: A one-month follow-up. J Psychosom Res. 2021;143(110399) doi: 10.1016/j.jpsychores.2021.110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alemanno F, Houdayer E, Parma A, Spina A, Del Forno A, Scatolini A, Angelone S, Brugliera L, Tettamanti A, Beretta L. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: A COVID-rehabilitation unit experience. PLoS One. 2021;16(e0246590) doi: 10.1371/journal.pone.0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, Walshaw C, Kemp S, Corrado J, Singh R. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 59.de Graaf MA, Antoni ML, Ter Kuile MM, Arbous MS, Duinisveld AJF, Feltkamp MCW, Groeneveld GH, Hinnen SCH, Janssen VR, Lijfering WM, et al. Short-term outpatient follow-up of COVID-19 patients: A multidisciplinary approach. EClinicalMedicine. 2021;32(100731) doi: 10.1016/j.eclinm.2021.100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albu S, Zozaya NR, Murillo N, García-Molina A, Chacón CAF, Kumru H. What's going on following acute COVID-19? Clinical characteristics of patients in an out-patient rehabilitation program. NeuroRehabilitation. 2021;48:469–480. doi: 10.3233/NRE-210025. [DOI] [PubMed] [Google Scholar]
- 61.van den Borst B, Peters JB, Brink M, Schoon Y, Bleeker-Rovers CP, Schers H, van Hees HWH, van Helvoort H, van den Boogaard M, van der Hoeven H, et al. Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2021;73:e1089–e1098. doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rass V, Beer R, Schiefecker AJ, Kofler M, Lindner A, Mahlknecht P, Heim B, Limmert V, Sahanic S, Pizzini A, et al. Neurological outcome and quality of life 3 months after COVID-19: A prospective observational cohort study. Eur J Neurol. 2021;28:3348–3359. doi: 10.1111/ene.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan B, Li W, Liu H, Cai X, Song S, Zhao J, Hu X, Li Z, Chen Y, Zhang K, et al. Correlation between immune response and self-reported depression during convalescence from COVID-19. Brain Behav Immun. 2020;88:39–43. doi: 10.1016/j.bbi.2020.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong AW, Shah AS, Johnston JC, Carlsten C, Ryerson CJ. Patient-reported outcome measures after COVID-19: A prospective cohort study. Eur Respir J. 2020;56(200327) doi: 10.1183/13993003.03276-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomasoni D, Bai F, Castoldi R, Barbanotti D, Falcinella C, Mulè G, Mondatore D, Tavelli A, Vegni E, Marchetti G, d'Arminio Monforte A. Anxiety and depression symptoms after virological clearance of COVID-19: A cross-sectional study in Milan, Italy. J Med Virol. 2021;93:1175–1179. doi: 10.1002/jmv.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu C, Hu X, Song J, Yang D, Xu J, Cheng K, Chen D, Zhong M, Jiang J, Xiong W, et al. Mental health status and related influencing factors of COVID-19 survivors in Wuhan, China. Clin Transl Med. 2020;10(e52) doi: 10.1002/ctm2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romero-Duarte Á, Rivera-Izquierdo M, Guerrero-Fernández de Alba I, Pérez-Contreras M, Fernández-Martínez NF, Ruiz-Montero R, Serrano-Ortiz Á, González-Serna RO, Salcedo-Leal I, Jiménez-Mejías E, Cárdenas-Cruz A. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: The ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19(129) doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaseda ET, Levine AJ. Post-traumatic stress disorder: A differential diagnostic consideration for COVID-19 survivors. Clin Neuropsychol. 2020;34:1498–1514. doi: 10.1080/13854046.2020.1811894. [DOI] [PubMed] [Google Scholar]
- 69.Giannopoulou I, Galinaki S, Kollintza E, Adamaki M, Kympouropoulos S, Alevyzakis E, Tsamakis K, Tsangaris I, Spandidos DA, Siafakas N, et al. COVID-19 and post-traumatic stress disorder: The perfect ‘storm’ for mental health (Review) Exp Ther Med. 2021;22(1162) doi: 10.3892/etm.2021.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naidu SB, Shah AJ, Saigal A, Smith C, Brill SE, Goldring J, Hurst JR, Jarvis H, Lipman M, Mandal S. The high mental health burden of ʻLong COVIDʼ and its association with on-going physical and respiratory symptoms in all adults discharged from hospital. Eur Respir J. 2021;57(2004364) doi: 10.1183/13993003.04364-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Venturelli S, Benatti SV, Casati M, Binda F, Zuglian G, Imeri G, Conti C, Biffi AM, Spada MS, Bondi E, et al. Surviving COVID-19 in Bergamo province: A post-acute outpatient re-evaluation. Epidemiol Infect. 2021;149(e32) doi: 10.1017/S0950268821000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, Elneima O, Docherty AB, Lone NI, Leavy OC, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bellan M, Soddu D, Balbo PE, Baricich A, Zeppegno P, Avanzi GC, Baldon G, Bartolomei G, Battaglia M, Battistini S, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Network Open. 2021;4(e2036142) doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horn M, Wathelet M, Fovet T, Amad A, Vuotto F, Faure K, Astier T, Noël H, Henry M, Duhem S. Is COVID-19 associated with posttraumatic stress disorder? J Clin Psychiatry. 2020;82(9886) doi: 10.4088/JCP.20m13641. [DOI] [PubMed] [Google Scholar]
- 75.Tarsitani L, Vassalini P, Koukopoulos A, Borrazzo C, Alessi F, Di Nicolantonio C, Serra R, Alessandri F, Ceccarelli G, Mastroianni CM, et al. Post-traumatic stress disorder among COVID-19 survivors at 3-month follow-up after hospital discharge. J Gen Intern Med. 2021;36:1702–1707. doi: 10.1007/s11606-021-06731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Lorenzo R, Conte C, Lanzani C, Benedetti F, Roveri L, Mazza MG, Brioni E, Giacalone G, Canti V, Sofia V, et al. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PLoS One. 2020;15(e0239570) doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poyraz BÇ, Poyraz CA, Olgun Y, Gürel Ö, Alkan S, Özdemir YE, Balkan İİ, Karaali R. Psychiatric morbidity and protracted symptoms after COVID-19. Psychiatry Res. 2021;295(113604) doi: 10.1016/j.psychres.2020.113604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun LL, Wang J, Wang YS, Hu PF, Zhao ZQ, Chen W, Ning BF, Yin C, Hao YS, Wang Q, et al. Symptomatic features and prognosis of 932 hospitalized patients with coronavirus disease 2019 in Wuhan. J Dig Dis. 2021;22:271–281. doi: 10.1111/1751-2980.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, Horn C, Vanshylla K, Cristanziano VD, Osebold L, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6(100122) doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shang YF, Liu T, Yu JN, Xu XR, Zahid KR, Wei YC, Wang XH, Zhou FL. Half-year follow-up of patients recovering from severe COVID-19: Analysis of symptoms and their risk factors. J Intern Med. 2021;290:444–450. doi: 10.1111/joim.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grandner MA. Sleep, health, and society. Sleep Med Clin. 2017;12:1–22. doi: 10.1016/j.jsmc.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Freeman D, Sheaves B, Waite F, Harvey AG, Harrison PJ. Sleep disturbance and psychiatric disorders. Lancet Psychiatry. 2020;7:628–637. doi: 10.1016/S2215-0366(20)30136-X. [DOI] [PubMed] [Google Scholar]
- 84.Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, Rodrigues NB, Subramaniapillai M, Di Vincenzo JD, Cao B, et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 2021;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Townsend L, Dyer AH, Naughton A, Kiersey R, Holden D, Gardiner M, Dowds J, O'Brien K, Bannan C, Nadarajan P, et al. Longitudinal analysis of COVID-19 patients shows age-associated T cell changes independent of ongoing Ill-health. Front Immunol. 2021;12(676932) doi: 10.3389/fimmu.2021.676932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Rodríuez-Jiménez J, Palacios-Ceña M, Velasco-Arribas M, Guijarro C, de-la-Llave-Rincón AI, Fuensalida-Novo S, Elvira-Martínez CM, et al. Long-term post-COVID symptoms and associated risk factors in previously hospitalized patients: A multicenter study. J Infect. 2021;83:237–279. doi: 10.1016/j.jinf.2021.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.González-Hermosillo JA, Martínez-López JP, Carrillo-Lampón SA, Ruiz-Ojeda D, Herrera-Ramírez S, Amezcua-Guerra LM, Martínez-Alvarado MDR. Post-acute COVID-19 symptoms, a potential Link with Myalgic Encephalomyelitis/Chronic fatigue syndrome: A 6-month survey in a Mexican cohort. Brain Sci. 2021;11(760) doi: 10.3390/brainsci11060760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Menges D, Ballouz T, Anagnostopoulos A, Aschmann HE, Domenghino A, Fehr JS, Puhan MA. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS One. 2021;16(e0254523) doi: 10.1371/journal.pone.0254523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Prevalence and determinants of fatigue after COVID-19 in Non-hospitalized subjects: A Population-based study. Int J Environ Res Public Health. 2021;18(2030) doi: 10.3390/ijerph18042030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peghin M, Palese A, Venturini M, De Martino M, Gerussi V, Graziano E, Bontempo G, Marrella F, Tommasini A, Fabris M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27:1507–1513. doi: 10.1016/j.cmi.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang L, Yang B, Jiang N, Fu W, He X, Zhou Y, Ma WL, Wang X. Three-month Follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci. 2020;35:e418–e418. doi: 10.3346/jkms.2020.35.e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Daroische R, Hemminghyth MS, Eilertsen TH, Breitve MH, Chwiszczuk LJ. Cognitive impairment after COVID-19-A review on objective test data. Front Neurol. 2021;12(699582) doi: 10.3389/fneur.2021.699582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Méndez R, Balanzá-Martínez V, Luperdi SC, Estrada I, Latorre A, González-Jiménez P, Feced L, Bouzas L, Yépez K, Ferrando A, et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med. 2021;290:621–631. doi: 10.1111/joim.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, Talbot PJ. Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(14) doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li CW, Syue LS, Tsai YS, Li MC, Lo CL, Tsai CS, Chen PL, Ko WC, Lee NY. Anosmia and olfactory tract neuropathy in a case of COVID-19. J Microbiol Immunol Infect. 2021;54:93–96. doi: 10.1016/j.jmii.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin E, Lantos JE, Strauss SB, Phillips CD, Campion TR Jr, Navi BB, Parikh NS, Merkler AE, Mir S, Zhang C, et al. Brain imaging of patients with COVID-19: Findings at an academic institution during the height of the outbreak in New York City. AJNR Am J Neuroradiol. 2020;41:2001–2008. doi: 10.3174/ajnr.A6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu S, Wei N, Jiang J, Wu L, Sheng J, Zhou J, Fang Q, Chen Y, Zheng S, Chen F, et al. First report of manic-like symptoms in a COVID-19 patient with no previous history of a psychiatric disorder. J Affect Disord. 2020;277:337–340. doi: 10.1016/j.jad.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Najjar S, Najjar A, Chong DJ, Pramanik BK, Kirsch C, Kuzniecky RI, Pacia SV, Azhar S. Central nervous system complications associated with SARS-CoV-2 infection: Integrative concepts of pathophysiology and case reports. J Neuroinflammation. 2020;17(231) doi: 10.1186/s12974-020-01896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muus C, Luecken MD, Eraslan G, Waghray A, Heimberg G, Sikkema L, Kobayashi Y, Vaishnav ED, Subramanian A, Smilie C, et al. doi: 10.1101/2020.04.19.049254. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv: [DOI] [Google Scholar]
- 101.Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, Holden SJ, Raber J, Banks WA, Erickson MA. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2021;24:368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting Enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, Hattori T, Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P, Wei D, Zhang Y, Sun XX, Gong L, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(283) doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dantzer R. Neuroimmune Interactions: From the Brain to the immune system and vice versa. Physiol Rev. 2018;98:477–504. doi: 10.1152/physrev.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park MD. Macrophages: A Trojan horse in COVID-19? Nat Rev Immunol. 2020;20(351) doi: 10.1038/s41577-020-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cameron MJ, Bermejo-Martin JF, Danesh A, Muller MP, Kelvin DJ. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller AH, Raison CL. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ozbay F, Johnson DC, Dimoulas E, Morgan CA, Charney D, Southwick S. Social support and resilience to stress: From neurobiology to clinical practice. Psychiatry (Edgmont) 2007;4:35–40. [PMC free article] [PubMed] [Google Scholar]
- 112.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Pablos RM, Herrera AJ, Espinosa-Oliva AM, Sarmiento M, Muñoz MF, Machado A, Venero JL. Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J Neuroinflammation. 2014;11(34) doi: 10.1186/1742-2094-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Futtrup J, Margolinsky R, Benros ME, Moos T, Routhe LJ, Rungby J, Krogh J. Blood-brain barrier pathology in patients with severe mental disorders: A systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6(100102) doi: 10.1016/j.bbih.2020.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tremblay ME, Madore C, Bordeleau M, Tian L, Verkhratsky A. Neuropathobiology of COVID-19: The role for glia. Front Cell Neurosci. 2020;14(592214) doi: 10.3389/fncel.2020.592214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu H, Shi C, Hu S. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mazza MG, Lucchi S, Tringali AGM, Rossetti A, Botti ER, Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:229–236. doi: 10.1016/j.pnpbp.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 119.Escartin C, Galea E, Lakatos A, O'Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra A, Carmignoto G, Agarwal A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, Dodd SJ, Koretsky AP, Watts JA, Cheung V, et al. Microvascular Injury in the Brains of patients with Covid-19. N Engl J Med. 2021;384:481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: A unified hypothesis. Lancet Psychiatry. 2020;7:272–281. doi: 10.1016/S2215-0366(19)30302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verkhratsky A, Rodríguez JJ, Steardo L. Astrogliopathology: A central element of neuropsychiatric diseases? Neuroscientist. 2014;20:576–588. doi: 10.1177/1073858413510208. [DOI] [PubMed] [Google Scholar]
- 123.Barbic F, Heusser K, Minonzio M, Shiffer D, Cairo B, Tank J, Jordan J, Diedrich A, Gauger P, Zamuner RA, et al. Effects of prolonged head-down bed rest on cardiac and vascular baroreceptor modulation and orthostatic tolerance in healthy individuals. Front Physiol. 2019;10(1061) doi: 10.3389/fphys.2019.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beck L, Baisch F, Gaffney FA, Buckey JC, Arbeille P, Patat F, Ten Harkel AD, Hillebrecht A, Schulz H, Karemaker JM, et al. Cardiovascular response to lower body negative pressure before, during, and after ten days head-down tilt bedrest. Acta Physiol Scand Suppl. 1992;604:43–52. [PubMed] [Google Scholar]
- 125.Goldstein DS, Vernikos J, Holmes C, Convertino VA. Catecholaminergic effects of prolonged head-down bed rest. J Appl Physiol (1985) 1995;78:1023–1029. doi: 10.1152/jappl.1995.78.3.1023. [DOI] [PubMed] [Google Scholar]
- 126.Kamiya A, Michikami D, Fu Q, Iwase S, Hayano J, Kawada T, Mano T, Sunagawa K. Pathophysiology of orthostatic hypotension after bed rest: Paradoxical sympathetic withdrawal. Am J Physiol Heart Circ Physiol. 2003;285:H1158–H1167. doi: 10.1152/ajpheart.00965.2002. [DOI] [PubMed] [Google Scholar]
- 127.Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, Yombi JC, De Greef J, Pothen L, Yildiz H, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2021;268:751–757. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Follmer C. Viral infection-induced gut dysbiosis, neuroinflammation, and α-synuclein aggregation: Updates and perspectives on COVID-19 and neurodegenerative disorders. ACS Chem Neurosci. 2020;11:4012–4016. doi: 10.1021/acschemneuro.0c00671. [DOI] [PubMed] [Google Scholar]
- 129.McAlpine LS, Zubair AS, Maran I, Chojecka P, Lleva P, Jasne AS, Navaratnam D, Matouk C, Schindler J, Sheth KN, et al. Ischemic stroke, inflammation, and endotheliopathy in COVID-19 patients. Stroke. 2021;52:e233–e238. doi: 10.1161/STROKEAHA.120.031971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Z, Yang Y, Liang X, Gao B, Liu M, Li W, Chen Z, Wang Z. COVID-19 associated ischemic stroke and hemorrhagic stroke: Incidence, potential pathological mechanism, and management. Front Neurol. 2020;11(571996) doi: 10.3389/fneur.2020.571996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, Jia T, Zhao Y, Wang D, Xiao A, Yin B. Cerebral Micro-structural changes in COVID-19 patients-an MRI-based 3-month Follow-up study. EClinicalMedicine. 2020;25(100484) doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sulzer D, Antonini A, Leta V, Nordvig A, Smeyne RJ, Goldman JE, Al-Dalahmah O, Zecca L, Sette A, Bubacco L, et al. COVID-19 and possible links with Parkinson's disease and parkinsonism: From bench to bedside. NPJ Parkinsons Dis. 2020;6(18) doi: 10.1038/s41531-020-00123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, Guis S, Barthelemy F, Habert P, Ceccaldi M, et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48:2823–2833. doi: 10.1007/s00259-021-05215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Attademo L, Bernardini F. Are dopamine and serotonin involved in COVID-19 pathophysiology? Eur J Psychiatry. 2021;35:62–63. doi: 10.1016/j.ejpsy.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.