Abstract
Nicotine is the psychoactive component given tobacco has several main components and acts as an agonist for nicotinic acetylcholine receptors (nAChRs) in the nervous system. Although the ligand-gated cation channels known as nAChRs are found throughout the nervous system and body, this review focuses on neuronal nAChRs. Individuals with psychiatric diseases such as schizophrenia, comorbid substance use disorders, attention-deficit hyperactivity disorder, major depression, and bipolar disorder have increased rates of smoking. These psychiatric disorders are associated with various cognitive deficits, including working memory, deficits in attention, and response inhibition functions. The cognitive-enhancing effects of nicotine may be particularly relevant predictors of smoking initiation and continuation in this comorbid population. Individuals with schizophrenia make up a significant proportion of smokers. Literature suggests that patients smoke to alleviate cognitive deficiencies due to the stimulating effects of nicotine. This narrative review examines the role of nicotine on cognition in schizophrenia.
Keywords: nicotinic cholinergic receptors (nachrs), nicotine, tobacco, schizophrenia, smoking, cognition, varenicline
Introduction and background
Schizophrenia is a chronic mental condition with a lifetime prevalence of about 1% in the general population [1]. The disorder is characterized by positive, negative, and cognitive symptoms. Schizophrenia can commonly result in social impairments [2]. Although the actual etiology of schizophrenia remains unknown, environment, genetics, and altered brain neurobiology may play a role [3]. Attention, executive functioning, learning deficits, linguistic knowledge, and spatial working memory are all connected to cognitive impairment in schizophrenia [4,5]. Furthermore, despite their efficacy in the treatment of positive symptoms, antipsychotics may contribute to cognitive impairment in schizophrenia [6]. Several studies have shown that schizophrenia patients have an extremely high prevalence of smoking, increased rates, and intensity of tobacco smoking of almost 90% compared to only 33% in the general population and 45-70% in patients with other psychiatric diagnoses [7,8].
The increase in nicotine receptors caused by smoking has been linked to lower levels of social withdrawal, motivational responses, blunted emotions, and improved cognitive function [9-11]. Although nicotine has been shown to improve cognitive deficits in schizophrenia, the underlying neurobiological mechanism remains poorly understood [12]. A review by Levin and Rezvani (2006) reported that nicotinic co-therapy may be a useful adjunct in the treatment of schizophrenia, potentially lowering cognitive impairment [12]. Nicotinic acetylcholine receptors (nAchRs) have emerged as a possible therapeutic target for the treatment of schizophrenia-related neurocognitive dysfunctions [8-13]. Varenicline is a partial agonist and may be a useful therapy for treating not just nicotine dependency in schizophrenia but also the cognitive deficiencies that are one of the disorder’s primary symptom clusters [14]. This review examines the effect of nicotine on cognition in schizophrenia patients.
Review
Methodology
With the specific combination of keywords including “Nicotine/administration and dosage,” “Nicotine/adverse effects,” “Nicotine/agonists,” “Nicotine/chemical synthesis,” “Nicotine/chemistry,” “Nicotine/genetics,” “Nicotine/metabolism,” “Nicotine/physiology,” “Nicotine/therapeutic use,” “Nicotine/toxicity,” “Schizophrenia/anatomy and histology,” “Schizophrenia/chemically induced,” “Schizophrenia/chemistry,” “Schizophrenia/drug therapy,” “Schizophrenia/epidemiology,” “Schizophrenia/diagnosis,” “Neurobehavioral Manifestations/anatomy and histology,” “Neurobehavioral Manifestations/chemistry,” “Neurobehavioral Manifestations/diagnosis,” “Neurobehavioral Manifestations/etiology,” etc. an article search was conducted on Google Scholar, PubMed, MEDLINE, Scopus, and Cochrane. Search results were reviewed, and only articles describing the effect of nicotine on cognition in schizophrenia patients were selected. Articles published in the English language were included in this review. All study designs including cohort studies, case-control studies, and randomized controlled trials were analyzed in this review.
Results
A total of 1,202 articles were found in multiple databases, including Google Scholar, Cochrane, Scopus, PubMed, and MEDLINE, of which 888 were initially removed due to repetition and irrelevance. After analyzing the titles and abstracts at the first screening level, 635 articles were further removed. A total of 90 articles were included in this review.
Nicotine and Schizophrenia
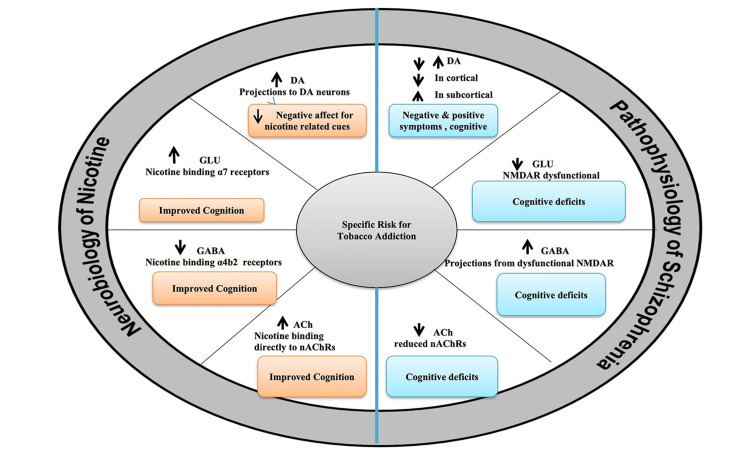
The nicotinic system in schizophrenia attracted initial interest after reports of greater smoking rates in schizophrenia patients compared to the general population [15-17]. The pathophysiology of schizophrenia and the neurobiology of nicotine is shown in Figure 1. Several characteristics of the smoking and schizophrenia relationship indicate a potential dysfunction in the nicotinic acetylcholine receptor system, suggesting that this dysfunction may play a key role in the disease progression. Unaffected relatives of patients with schizophrenia have higher rates of smoking, suggesting that physiological mechanisms driving smoking may also share a hereditary component similar to schizophrenia [18]. The majority of smokers (90%) start smoking before the onset of schizophrenia [17]. In adolescents who develop schizophrenia later in life, smoking rates are higher than in those who do not [19].
Figure 1. Pathophysiology of schizophrenia and neurobiology of nicotine.
DA: dopamine; GLU: glutamate; Ach: acetylcholine; nAchRs: nicotinic acetylcholine receptors; GABA: gamma aminobutyric acid; NMDAR: N-methyl-D-aspartate receptors
This evidence refutes the concept that rising smoking rates are due to a desire to relieve the consequences of schizophrenia symptoms. Higher smoking rates are linked to early disease onset, poorer quality of life, poorer prognosis, increased disease severity, and higher hospital admissions, signaling that the increased desire to smoke may be linked to important regulators of disease onset and progression [17,20-24]. Smoking has been linked to symptom relief in several early trials, suggesting that restoring a supposed nicotinic balance could improve illness prognosis significantly. Findings demonstrating a favorable impact of nicotine on different elements of cognitive performance have raised their interest in this regard [13,23]. A cohort study by Fang et al. evaluated the association between tobacco use and schizophrenia among 225 healthy controls and 244 schizophrenia patients [25]. Stratification analysis revealed that the rate of smoking was higher in male patients versus healthy controls, with male smokers having higher odds ratios for schizophrenia than non-smokers, despite the fact that there was no significant difference between schizophrenia patients and healthy controls in the entire sample.
Genetics of Smoking in Schizophrenia
Smoking is three times as prevalent in patients with schizophrenia compared to the general population [26]. While schizophrenia has several genetic risk factors, nicotine addiction is associated with numerous genes. The common genetic base (i.e., biological pleiotropy) of nicotine addiction and schizophrenia may explain why schizophrenia patients smoke so routinely and more severely compared to healthy controls [26,27]. Patients with schizophrenia who smoke do so to avoid either cognitive impairment and psychotic symptoms, or because smoking and schizophrenia share a genetic foundation, or perhaps smoking occurs before the onset of schizophrenia, making smoking a risk factor for schizophrenia [26]. A systematic analysis by Hu et al. detected 52 similar genes out of 331 genes were schizophrenia and 276 genes were nicotine addiction when they evaluated shared genes associated with both schizophrenia and smoking. The authors divided these shared genes for smoking and schizophrenia into multiple groups using network analysis, pathway analysis, and enrichment analysis. They discovered 12 significantly enriched pathways related to nicotine, cocaine, alcohol, and amphetamine addiction; serotonergic, dopaminergic, and glutamatergic synapse; neuroactive ligand-receptor interaction; cAMP signaling pathway; and estrogen signaling after performing pathway enrichment analysis [28]. Another study found pathways shared by schizophrenia and nicotine addiction associated with neural communication and neurotransmitter transduction, long-term potentiation, calcium signaling, and neuroactive ligand-receptor interaction pathway using large genome-wide association study datasets and Fagerström test for nicotine dependence in schizophrenia and concentration of plasma cotinine [26]. These findings suggested that nicotine addiction and schizophrenia may share a genetic relationship, that patients with schizophrenia smoke to relieve cognitive symptoms, or that nicotine addiction is a risk factor for schizophrenia [26]. Both studies suggested that assessing genes related to both polygenic disorders at a system level is necessary as nicotine addiction and schizophrenia could aid in the identification of shared genetic liability for both disorders, improve understanding of the relationship between the two disorders, and provide novel insights into the pathogenetic relationship between smoking and psychiatric disorders.
Nicotine and nAChR
Nicotine is a highly addictive chemical that aids in the start and continuation of tobacco usage [28]. The rewarding characteristics of nicotine are likely due to the rapid rate of transport to the brain after inhaling a cigarette puff. Nicotine acts primarily through nAChRs, which are ligand-gated ion channels composed of various pentameric combinations of three β subunits (β2-β4) and nine α subunits (α2-α10) organized around a central pore permeable to calcium, sodium, and potassium ions [29,30]. Most neuronal nAChRs in the central nervous system (CNS) are excitatory and fast-acting, control the release of other neurotransmitters such as norepinephrine, dopamine (DA), serotonin, glutamate, gamma-aminobutyric acid (GABA), and acetylcholine (ACh), and are found presynaptically [29,30]. However, the nAChRs on DA neurons in the ventral tegmental area (VTA), a key brain region for drug reinforcing, are situated postsynaptically. nAChRs are composed of homomeric receptors made up of a set of α subunits, or heteromeric receptors made up of a mixture of α and β subunits. The α4β2 and α7 subtypes of nAChRs are the most prominent in the brain [31].
The sensitivity of nAChRs to desensitization varies across subtypes. Glutamate-controlling nAChR subtypes, for example, desensitize more slowly than GABA-controlling nAChR subtypes [32]. Following extended nicotine exposure, this differential sensitivity to desensitization may result in higher glutamate release compared to GABA release. Increased DA release in the nucleus accumbens may result from a relative shortage of GABA over glutamate, which could be a key mechanism promoting tobacco use [32]. High nicotine doses or prolonged exposure result in antagonist-like nicotine activity as a result of nAChR desensitization [33]. Although the effects of nicotine on the upregulation and desensitization of nAChRs have been well documented, the roles these processes play in nicotine’s cognitive effects are complex and poorly understood. Nicotine appears to have an inverted J dose-response, with low doses or quick exposures benefiting cognitive function, and higher doses or protracted exposures either improving or impairing cognitive abilities [34]. The phasic and tonic activities of DA neurons are thought to mediate different aspects of goal-directed behavior; phasic activity facilitates cue-reward association and acquisition of incentive salience, whereas tonic activity is involved in response inhibition and behavioral flexibility [35]. The difficulty of clearly elucidating the mechanisms of nicotine’s effects on cognition is underscored by the varying brain expression patterns of nAChRs, their varying nicotine sensitivities, and the varying connections of nAChRs with other neurotransmitter systems that mediate cognitive function.
nAChR and Cognitive Function
Despite the fact that the neurobiological mechanisms behind the effects of nicotine on cognitive function remain unknown, new research at various levels continues to emerge. The hippocampal brain and prefrontal cortex regions have been linked to nicotine’s cognitive impacts. Moreover, it is possible that enhancing synaptic plasticity in particular brain circuits and improving signal-to-noise ratios improves cognition [35-37]. The nAChR subunits α2, α3, α4, α5, α7, β2, and β4 are likely to mediate the cognitive effects of nicotine, and, as mentioned below, the best evidence is for the implication of the α7 and β2 subunits [38,39].
α7 nAChR
α7 nAChRs generate ion channels that lack other nAChR subunits and have a high calcium permeability, allowing them to regulate the release of other neurotransmitters (e.g., glutamate) commonly found in the mammalian brain [33,40]. α7 nAChRs modulate synaptic plasticity similarly to NMDA-type glutamate receptors; however, they have a lower nicotine affinity than α4β2 nAChRs and do not desensitize at low nicotine doses [41,42]. These disparities in desensitization allow α7 nAChRs to remain active after α4β2 nAChRs have desensitized. Moreover, it has clinical therapeutic consequences, as explained below [43].
α7 nAChRs, which are abundant in the prefrontal cortex and hippocampus, have been demonstrated to influence numerous cognitive functions in both preclinical and human studies [44,45]. nAChR knock-out mice, for example, were shown to have more errors in sustained attention and working memory tasks than wild-type mice, according to multiple preclinical investigations [46,47]. However, the fact that these knock-out mice differ from wild-type mice in the density and distribution of other nAChR subtypes due to compensatory changes in the expression of other nAChR types during development makes interpretation of these data difficult [48].
A growing body of evidence suggests that α7 nAChRs are involved in cognitive deficiencies in various neuropsychiatric disorders, including Alzheimer’s disease, schizophrenia, Parkinson’s disease, and autism spectrum disorders [40]. α7 nAChRs, for example, have been linked to the sensory gating failure seen in schizophrenia [49-51]. Sensory gating helps to distinguish between irrelevant and significant stimuli, and it may be at the root of both the sensory overload and cognitive deficiencies seen in schizophrenic patients. In the postmortem brains of schizophrenic patients, the density of α7 nAChRs in the hippocampus, a region controlling sensory gating, is reduced, and reductions in α7 nAChRs have been linked to sensory gating impairment in schizophrenia [52-55]. Neural α7 nAChR expression and functioning deficits have been extensively associated with cognitive and early sensory gating impairments in schizophrenia patients and their relatives. Nicotine and α7 nAChRs agonists have also been demonstrated to correct sensory gating defects in animal models and schizophrenia patients [56]. Overall, these results pointed to a biological mechanism that could be tested to explain why people with schizophrenia smoke at such high rates [57-61]. Consequently, targeting α7 nAChRs with pharmacological agonists for the treatment of cognitive abnormalities in schizophrenia may have an indirect advantage of facilitating smoking cessation [40,62]. Noda et al. recently published a study that revealed α7 nAChR is linked to disease progression and is involved in the therapeutic impact of nicotine in schizophrenia [63].
Because α7 nAChRs are the common subtype of homomeric and heteromeric receptors containing α7 subunits are uncommon a short-hand for receptor classification is whether the receptor is an α7 nAChR or a non-α7 nAChR [64,65]. This can be determined pharmacologically by measuring sensitivity to α-bungarotoxin (α-BTX), a powerful and selective antagonist of the neuronal seven homomeric receptors in the brain. The α-BTX-sensitive receptors have a low affinity for nicotine and have quick kinetics, whereas α-BTX-resistant receptors have a higher affinity for nicotine, slower kinetics, are heteromeric, and are desensitized to low agonist doses [64,65].
β2 nAChRs
The β2 subunit is abundant in the basal ganglia, thalamus, and hippocampus, and growing evidence suggests that the β2 subunit plays a central role in the cognitive effects of nicotine [66]. Behavioral flexibility, working memory, inhibitory control, and attention are all compromised in β2 knock-out mice [67-70]. Furthermore, in β2 knock-out mice, nicotine delivery has no effect on associative memory performance compared to wild-type mice [71]. Nicotine therapy only partially compensates for two knock-outs’ deficiencies in exploratory behavior [72]. Finally, drugs that stimulate α4β2 nAChRs have been shown to improve cognition. For example, varenicline improves learning deficiencies caused by either alcohol or nicotine deprivation in mice or drug use, or nicotine withdrawal in mice, and improves cognition in humans [73,74].
Cognitive Effects of Nicotine
Several laboratory investigations have examined the effects of cigarette smoking or pure nicotine delivery on cognitive ability. The fact that cigarette smoke contains numerous other chemicals, in addition to nicotine, that may have cognitive-enhancing benefits complicates the interpretation of findings after smoking cigarettes [75,76]. Furthermore, the amount of nicotine delivered by smoking varies greatly depending on the type of cigarette used and individual smoking patterns. Many investigations on the modulation of cognition by nicotine have employed pure nicotine delivered through intravenous infusion, oral inhaler subcutaneous injection, transdermal patch, or nasal spray to overcome these constraints [77-82]. Smoking after a period of abstinence enhances cognition. Moreover, cigarette smoking, or nicotine, had robust cognitive-enhancing effects, according to a review of studies conducted until the 1980s [83]. However, it is unclear whether these cognitive-enhancing benefits were secondary to withdrawal relief rather than direct cognitive enhancement [83,84]. Heishman et al. conducted a meta-analysis of 41 placebo-controlled studies that included nicotine delivery to either non-smokers or satiated smokers to separate the direct cognitive benefits of nicotine from withdrawal alleviation [85]. Nicotine had a significant favorable influence on short-term episodic memory, fine motor, and working memory function, according to the researchers. Furthermore, both “alerting attention” (maintenance of an alert state) and “orienting attention” (directing attention to sensory events) were favorably influenced [86]. The effect of nicotine on cognitive performance was not dose-dependent, either within or across areas of cognitive function, showing the diverse nature of nicotine pharmacodynamics.
One widely held belief is that schizophrenia people smoke more cigarettes than the general population to “self-medicate” the cognitive deficiencies indicated above [80,81]. The effects of nicotine on cognitive performance in schizophrenia have been studied in numerous clinical trials [87]. The degree of nicotine dependency, nicotine satiety, nicotine withdrawal, and mode of delivery, on the other hand, varied significantly between studies. Gum, transdermal patch, orally and nasally inhaled nicotine, and subcutaneous nicotine have all been used in research to improve cognition in schizophrenia patients [88]. Boggs et al. conducted a systematic evaluation of studies in which nicotine was given to people with schizophrenia and concluded that nicotine may improve attention/vigilance acutely [88]. Unfortunately, due to the short duration of these studies, no long-term improvements to attention have been established. Furthermore, while the studies looked at various cognitive tests with a variety of outcomes, the vast majority of studies failed to account for multiple comparisons. The research implies that nicotine does not improve overall cognitive performance, especially in the long term.
Aside from these findings in satisfied smokers, research has demonstrated disparities in nicotine’s impact on cognitive processes in abstinent smokers and non-smokers. While nicotine increased working memory performance among abstinent smokers, it had no effect on non-smokers [89]. A double-blind, placebo-controlled study by Ettinger et al. reported that nicotine improved basic attentional capabilities in non-smokers in a study, but not response inhibition or top-down executive attentional function [90]. Another experimental study of overnight abstinent smokers found that smoking nicotine-yielding cigarettes increased overall accuracy in an attentional processing task when compared to placebo cigarettes, indicating that nicotine overcomes cognitive deficiencies caused by nicotine deprivation [91].
Furthermore, a recent study examined the effects of stopping and restarting smoking on cognition in schizophrenia patients to see if the self-medication theory was correct [92]. Cognitive abilities were assessed while smoking as usual (baseline), one week later (extended abstinence), one day after quitting (early abstinence), and three weeks after restarting smoking (resumption). To analyze different cognitive domains impacted by schizophrenia, researchers used tests of processing speed, executive function, verbal fluency, working memory, verbal memory, conflict resolution, and attention. With smoking cessation, resumption or abstinence, there were no significant differences in general cognitive performance. Consequently, the findings of this study cast doubt on the prevalent and long-held “self-medication” concept of smoking and schizophrenia, call into question the magnitude of smoking and nicotine’s pro-cognitive effects, and advocate for smoking cessation in people with schizophrenia [92]. Strategies for assisting smoking cessation include behavioral counseling to enhance motivation and support attempts to quit and pharmacological intervention to reduce nicotine reinforcement and withdrawal. Three drugs are currently used as first-line pharmacotherapy for smoking cessation, nicotine replacement therapy, bupropion, and varenicline.
The current review has some limitations. The studies included in this review examined the effects of a single dose of nicotine in the short term. As a result, projecting the effects of chronic tobacco use from the findings of these researches, which focused on acute nicotine effects found in an experimental context, is difficult.
Conclusions
In schizophrenia, smoking is more common than in other populations. Nicotine has been shown to have cognitive-enhancing benefits in preclinical animals and human investigations, specifically in working memory, enhancement of fine motor control, attention, and episodic memory. The cognitive-enhancing properties of nicotine may play a role in cigarette smoking vulnerability, particularly in people with cognitive deficiencies, which include the majority of people with psychiatric disorders. Finally, future investigations are warranted to investigate the effectiveness of adjunctive nicotine agents on the preservation of cognitive and early sensory functions.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Activities of daily living, social functioning and their determinants in persons with psychotic disorder. Viertiö S, Tuulio-Henriksson A, Perälä J, et al. Eur Psychiatry. 2012;27:409–415. doi: 10.1016/j.eurpsy.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Schizophrenia and risk of dementia: a meta-analysis study. Cai L, Huang J. Neuropsychiatr Dis Treat. 2018;14:2047–2055. doi: 10.2147/NDT.S172933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The role of genetics in the etiology of schizophrenia. Gejman PV, Sanders AR, Duan J. Psychiatr Clin North Am. 2010;33:35–66. doi: 10.1016/j.psc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cognitive deficits and functional outcome in schizophrenia. Bowie CR, Harvey PD. Neuropsychiatr Dis Treat. 2006;2:531–536. doi: 10.2147/nedt.2006.2.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Insight in schizophrenia: relationship to positive, negative and neurocognitive dimensions. Joseph B, Narayanaswamy JC, Venkatasubramanian G. Indian J Psychol Med. 2015;37:5–11. doi: 10.4103/0253-7176.150797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antipsychotics, metabolic adverse effects, and cognitive function in schizophrenia. MacKenzie NE, Kowalchuk C, Agarwal SM, et al. Front Psychiatry. 2018;9:622. doi: 10.3389/fpsyt.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Kelly C, McCreadie RG. Am J Psychiatry. 1999;156:1751–1757. doi: 10.1176/ajp.156.11.1751. [DOI] [PubMed] [Google Scholar]
- 8.Achieving smoking cessation in individuals with schizophrenia: special considerations. Cather C, Pachas GN, Cieslak KM, Evins AE. CNS Drugs. 2017;31:471–481. doi: 10.1007/s40263-017-0438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cognitive effects of nicotine: recent progress. Valentine G, Sofuoglu M. Curr Neuropharmacol. 2018;16:403–414. doi: 10.2174/1570159X15666171103152136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cognitive function as a transdiagnostic treatment target in stimulant use disorders. Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. J Dual Diagn. 2016;12:90–106. doi: 10.1080/15504263.2016.1146383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Millan MJ, Agid Y, Brüne M, et al. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 12.Elsevier. Smoking, schizophrenia linked by alterations in brain nicotine signals. [ Sep; 2014 ];https://www.sciencedaily.com/releases/2014/09/140916084827.htm 2014
- 13.Nicotinic-antipsychotic drug interactions and cognitive function. Levin ED, Rezvani AH. EXS. 2006;98:185–205. doi: 10.1007/978-3-7643-7772-4_10. [DOI] [PubMed] [Google Scholar]
- 14.The role of nicotine in schizophrenia. Featherstone RE, Siegel SJ. Int Rev Neurobiol. 2015;124:23–78. doi: 10.1016/bs.irn.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Effects of the nicotinic agonist varenicline on the performance of tasks of cognition in aged and middle-aged rhesus and pigtail monkeys. Terry AV Jr, Plagenhoef M, Callahan PM. Psychopharmacology (Berl) 2016;233:761–771. doi: 10.1007/s00213-015-4154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller SM. Impersonating Animals: Rhetoric, Ecofeminism, and Animal Rights Law. Michigan: Michigan State University Press; 2020. All animals are equal (but some are more equal than others): speciesist personhoods in the nonhuman rights project; pp. 33–58. [Google Scholar]
- 17.Prevalence of smoking among psychiatric outpatients. Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 18.Nicotine and familial vulnerability to schizophrenia: a discordant twin study. Lyons MJ, Bar JL, Kremen WS, et al. J Abnorm Psychol. 2002;111:687–693. doi: 10.1037//0021-843x.111.4.687. [DOI] [PubMed] [Google Scholar]
- 19.Smoking and vulnerability for schizophrenia. de Leon J. Schizophr Bull. 1996;22:405–409. doi: 10.1093/schbul/22.3.405. [DOI] [PubMed] [Google Scholar]
- 20.Nicotine dependence and symptoms in schizophrenia: naturalistic study of complex interactions. Aguilar MC, Gurpegui M, Diaz FJ, de Leon J. Br J Psychiatry. 2005;186:215–221. doi: 10.1192/bjp.186.3.215. [DOI] [PubMed] [Google Scholar]
- 21.Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Goff DC, Henderson DC, Amico E. Am J Psychiatry. 1992;149:1189–1194. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- 22.Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. Patkar AA, Gopalakrishnan R, Lundy A, Leone FT, Certa KM, Weinstein SP. J Nerv Ment Dis. 2002;190:604–610. doi: 10.1097/00005053-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Correlates of severity of smoking among persons with severe mental illness. Dixon L, Medoff DR, Wohlheiter K, et al. Am J Addict. 2007;16:101–110. doi: 10.1080/10550490601184415. [DOI] [PubMed] [Google Scholar]
- 24.Determinants of smoking behaviour in outpatients with schizophrenia. Herrán A, de Santiago A, Sandoya M, Fernández MJ, Diez-Manrique JF, Vázquez-Barquero JL. Schizophr Res. 2000;41:373–381. doi: 10.1016/s0920-9964(99)00082-1. [DOI] [PubMed] [Google Scholar]
- 25.Use of tobacco in schizophrenia: a double-edged sword. Fang Y, Wang W, Zhu C, Lin GN, Cheng Y, Zou J, Cui D. Brain Behav. 2019;9:0. doi: 10.1002/brb3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muscarinic receptor agonists and antagonists in the treatment of Alzheimer's disease. Clader JW, Wang Y. Curr Pharm Des. 2005;11:3353–3361. doi: 10.2174/138161205774370762. [DOI] [PubMed] [Google Scholar]
- 27.Genetic relationship between schizophrenia and nicotine dependence. Chen J, Bacanu SA, Yu H, et al. Sci Rep. 2016;6:25671. doi: 10.1038/srep25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Analyzing the genes related to nicotine addiction or schizophrenia via a pathway and network based approach. Hu Y, Fang Z, Yang Y, Rohlsen-Neal D, Cheng F, Wang J. Sci Rep. 2018;8:2894. doi: 10.1038/s41598-018-21297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Benowitz NL. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Mansvelder HD, Mertz M, Role LW. Semin Cell Dev Biol. 2009;20:432–440. doi: 10.1016/j.semcdb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Dani JA, Bertrand D. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 32.Diversity of native nicotinic receptor subtypes in mammalian brain. Zoli M, Pistillo F, Gotti C. Neuropharmacology. 2015;96:302–311. doi: 10.1016/j.neuropharm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. Buccafusco JJ, Beach JW, Terry AV Jr. J Pharmacol Exp Ther. 2009;328:364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Complex relationships of nicotinic receptor actions and cognitive functions. Levin ED. Biochem Pharmacol. 2013;86:1145–1152. doi: 10.1016/j.bcp.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Importance of the nicotinic acetylcholine receptor system in the prefrontal cortex. Wallace TL, Bertrand D. Biochem Pharmacol. 2013;85:1713–1720. doi: 10.1016/j.bcp.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Nicotinic receptors, memory, and hippocampus. Kutlu MG, Gould TJ. Curr Top Behav Neurosci. 2015;23:137–163. doi: 10.1007/978-3-319-13665-3_6. [DOI] [PubMed] [Google Scholar]
- 37.Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Desensitization of neuronal nicotinic receptors. Quick MW, Lester RA. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- 39.Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Changeux JP. Nat Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- 40.Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Kenney JW, Gould TJ. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Therapeutic potential of α7 nicotinic acetylcholine receptors. Bertrand D, Lee CH, Flood D, Marger F, Donnelly-Roberts D. Pharmacol Rev. 2015;67:1025–1073. doi: 10.1124/pr.113.008581. [DOI] [PubMed] [Google Scholar]
- 42.Heterogeneity and complexity of native brain nicotinic receptors. Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Biochem Pharmacol. 2007;74:1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Giniatullin R, Nistri A, Yakel JL. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 45.A cog in cognition: how the alpha 7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Leiser SC, Bowlby MR, Comery TA, Dunlop J. Pharmacol Ther. 2009;122:302–311. doi: 10.1016/j.pharmthera.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Hoyle E, Genn RF, Fernandes C, Stolerman IP. Psychopharmacology (Berl) 2006;189:211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Performance deficit of alpha7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Fernandes C, Hoyle E, Dempster E, Schalkwyk LC, Collier DA. Genes Brain Behav. 2006;5:433–440. doi: 10.1111/j.1601-183X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 48.Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, Sharkey J. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- 49.Smoking and cognitive deficits in schizophrenia: a pilot study. Taiminen TJ, Salokangas RK, Saarijärvi S, Niemi H, Lehto H, Ahola V, Syvälahti E. Addict Behav. 1998;23:263–266. doi: 10.1016/s0306-4603(97)00028-2. [DOI] [PubMed] [Google Scholar]
- 50.Role of alpha7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Nomikos GG, Schilström B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH. Behav Brain Res. 2000;113:97–103. doi: 10.1016/s0166-4328(00)00204-7. [DOI] [PubMed] [Google Scholar]
- 51.Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Adler LE, Hoffer LD, Wiser A, Freedman R. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 52.Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Martin-Ruiz CM, Haroutunian VH, Long P, Young AH, Davis KL, Perry EK, Court JA. Biol Psychiatry. 2003;54:1222–1233. doi: 10.1016/s0006-3223(03)00348-2. [DOI] [PubMed] [Google Scholar]
- 53.Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Freedman R, Hall M, Adler LE, Leonard S. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 54.Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Breese CR, Lee MJ, Adams CE, et al. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 55.Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Potter D, Summerfelt A, Gold J, Buchanan RW. Schizophr Bull. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Martin LF, Freedman R. Int Rev Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- 57.Is it possible to be schizophrenic yet neuropsychologically normal? Palmer BW, Heaton RK, Paulsen JS, et al. Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 58.Premorbid intellectual functioning and risk of schizophrenia and spectrum disorders. Reichenberg A, Weiser M, Caspi A, et al. J Clin Exp Neuropsychol. 2006;28:193–207. doi: 10.1080/13803390500360372. [DOI] [PubMed] [Google Scholar]
- 59.Do people with schizophrenia who have objective cognitive impairment identify cognitive deficits on a self report measure? Medalia A, Thysen J, Freilich B. Schizophr Res. 2008;105:156–164. doi: 10.1016/j.schres.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 60.Smoking and mental illness. Leonard S, Adler LE, Benhammou K, et al. Pharmacol Biochem Behav. 2001;70:561–570. doi: 10.1016/s0091-3057(01)00677-3. [DOI] [PubMed] [Google Scholar]
- 61.Prevalence of smoking in psychiatric patients. Poirier MF, Canceil O, Baylé F, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:529–537. doi: 10.1016/s0278-5846(01)00304-9. [DOI] [PubMed] [Google Scholar]
- 62.Nicotinic acetylcholine receptors: from basic science to therapeutics. Hurst R, Rollema H, Bertrand D. Pharmacol Ther. 2013;137:22–54. doi: 10.1016/j.pharmthera.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 63.Involvement of nicotinic acetylcholine receptors in behavioral abnormalities and psychological dependence in schizophrenia-like model mice. Noda Y, Uchida M, Mouri A, et al. Eur Neuropsychopharmacol. 2020;41:92–105. doi: 10.1016/j.euroneuro.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Gotti C, Zoli M, Clementi F. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Neuronal and extraneuronal nicotinic acetylcholine receptors. Zoli M, Pucci S, Vilella A, Gotti C. Curr Neuropharmacol. 2018;16:338–349. doi: 10.2174/1570159X15666170912110450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Dani JA. Int Rev Neurobiol. 2015;124:3–19. doi: 10.1016/bs.irn.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions. Feduccia AA, Chatterjee S, Bartlett SE. Front Mol Neurosci. 2012;5:83. doi: 10.3389/fnmol.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Guillem K, Bloem B, Poorthuis RB, et al. Science. 2011;333:888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- 69.Attention-deficit/hyperactivity disorder: a plausible mouse model? Granon S, Changeux JP. Acta Paediatr. 2006;95:645–649. doi: 10.1080/08035250600719747. [DOI] [PubMed] [Google Scholar]
- 70.Contributions of β2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Cole RD, Poole RL, Guzman DM, Gould TJ, Parikh V. Psychopharmacology (Berl) 2015;232:1207–1217. doi: 10.1007/s00213-014-3754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chronic nicotine exposure has dissociable behavioural effects on control and beta2-/- mice. Besson M, Suarez S, Cormier A, Changeux JP, Granon S. Behav Genet. 2008;38:503–514. doi: 10.1007/s10519-008-9216-1. [DOI] [PubMed] [Google Scholar]
- 72.Chemical constituents and bioactivity of tobacco smoke. Hoffmann D, Wynder EL. http://pubmed.ncbi.nlm.nih.gov/3623665/ IARC Sci Publ. 1986:145–165. [PubMed] [Google Scholar]
- 73.Executive and social behaviors under nicotinic receptor regulation. Granon S, Faure P, Changeux JP. Proc Natl Acad Sci U S A. 2003;100:9596–9601. doi: 10.1073/pnas.1533498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Picciotto MR, Zoli M, Léna C, et al. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- 75.Varenicline ameliorates ethanol-induced deficits in learning in C57BL/6 mice. Gulick D, Gould TJ. Neurobiol Learn Mem. 2008;90:230–236. doi: 10.1016/j.nlm.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Raybuck JD, Portugal GS, Lerman C, Gould TJ. Behav Neurosci. 2008;122:1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biomarkers to assess the utility of potential reduced exposure tobacco products. Hatsukami DK, Benowitz NL, Rennard SI, Oncken C, Hecht SS. Nicotine Tob Res. 2006;8:600–622. doi: 10.1080/14622200600858166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M. Neuropsychopharmacology. 2014;39:1431–1440. doi: 10.1038/npp.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.The effects of nicotine on attention and working memory in never-smokers. Kleykamp BA, Jennings JM, Blank MD, Eissenberg T. Psychol Addict Behav. 2005;19:433–438. doi: 10.1037/0893-164X.19.4.433. [DOI] [PubMed] [Google Scholar]
- 80.Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Psychopharmacology (Berl) 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- 81.Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Poltavski DV, Petros T. Physiol Behav. 2006;87:614–624. doi: 10.1016/j.physbeh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 82.What aspects of human performance are truly enhanced by nicotine? Heishman SJ. Addiction. 1998;93:317–320. doi: 10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- 83.Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- 84.Nicotine and smoking: a review of effects on human performance. Heishma SJ, Taylor RC, Henningfield JE. Exp Clin Psychopharmacol. 1994;2:345–395. [Google Scholar]
- 85.Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. D'Souza MS, Markou A. Neuropharmacology. 2012;62:1564–1573. doi: 10.1016/j.neuropharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meta-analysis of the acute effects of nicotine and smoking on human performance. Heishman SJ, Kleykamp BA, Singleton EG. Psychopharmacology (Berl) 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Research on attention networks as a model for the integration of psychological science. Posner MI, Rothbart MK. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- 88.Going up in smoke? A review of nAChRs-based treatment strategies for improving cognition in schizophrenia. Boggs DL, Carlson J, Cortes-Briones J, Krystal JH, D’Souza DC. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4442779/ Curr Pharm Des. 2014;20:5077–5092. doi: 10.2174/1381612819666131216121019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Double dissociation of working memory and attentional processes in smokers and non-smokers with and without nicotine. Grundey J, Amu R, Ambrus GG, Batsikadze G, Paulus W, Nitsche MA. Psychopharmacology (Berl) 2015;232:2491–2501. doi: 10.1007/s00213-015-3880-7. [DOI] [PubMed] [Google Scholar]
- 90.Effects of nicotine on response inhibition and interference control. Ettinger U, Faiola E, Kasparbauer AM, Petrovsky N, Chan RC, Liepelt R, Kumari V. Psychopharmacology (Berl) 2017;234:1093–1111. doi: 10.1007/s00213-017-4542-8. [DOI] [PubMed] [Google Scholar]
- 91.Nicotine deprivation influences P300 markers of cognitive control. Evans DE, Maxfield ND, Van Rensburg KJ, Oliver JA, Jentink KG, Drobes DJ. Neuropsychopharmacology. 2013;38:2525–2531. doi: 10.1038/npp.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Minimal effects of prolonged smoking abstinence or resumption on cognitive performance challenge the "self-medication" hypothesis in schizophrenia. Boggs DL, Surti TS, Esterlis I, et al. Schizophr Res. 2018;194:62–69. doi: 10.1016/j.schres.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]