Abstract
Resistance to macrolides in pneumococci is generally mediated by methylation of 23S rRNA via erm(B) methylase which can confer a macrolide (M)-, lincosamide (L)-, and streptogramin B (SB)-resistant (MLSB) phenotype or by drug efflux via mef(A) which confers resistance to 14- and 15-membered macrolides only. We studied 20 strains with unusual ML or MSB phenotypes which did not harbor erm(B) or mef(A). The strains had been isolated from patients in Eastern Europe and North America from 1992 to 1998. These isolates were found to contain mutations in genes for either 23S rRNA or ribosomal proteins. Three strains from the United States with an ML phenotype, each representing a different clone, were characterized as having an A2059G (Escherichia coli numbering) change in three of the four 23S rRNA alleles. Susceptibility to macrolides and lincosamides decreased as the number of alleles in isogenic strains containing A2059G increased. Sixteen MSB strains from Eastern Europe were found to contain a 3-amino-acid substitution (69GTG71 to TPS) in a highly conserved region of the ribosomal protein L4 (63KPWRQKGTGRAR74). These strains formed several distinct clonal types. The single MSB strain from Canada contained a 6-amino-acid L4 insertion (69GTGREKGTGRAR), which impacted growth rate and also conferred a 500-fold increase in MIC on the ketolide telithromycin. These macrolide resistance mechanisms from clinical isolates are similar to those recently described for laboratory-derived mutants.
The most prevalent mechanisms of macrolide resistance in Streptococcus pneumoniae are mediated by mef(A), a gene encoding an efflux pump specific for 14- and 15-membered macrolides, and erm(B), a 23S rRNA methylase that dimethylates A2058, resulting in macrolide-lincosamide-streptogramin B resistance (MLSB) (10, 15, 16, 29, 38, 40, 51). The Mef(A) pump is found in other species of streptococci (12, 32, 41) and other bacterial genera (18, 28; H. Fraimow and C. Knob, Abstr. 97th Gen. Meet. Am. Soc. Microbiol., abstr. A-125, 1997). Erm(B) or another homologous methylase is found in almost every bacterial species, including macrolide-producing strains (10, 15, 29, 38, 40, 51). Both mechanisms are common, but geographically, North American pneumococcal isolates have a greater tendency to harbor mef(A) while European isolates more frequently contain erm(B) (5, 9, 13, 14, 33, 35).
During studies to determine the frequency as well as the nature of macrolide resistance in pneumococcal clinical isolates, we identified strains with unusual MSB or ML phenotypes. We have recently shown that mutations obtained in vitro in a highly conserved region of ribosomal protein L4 or in one of four different nucleotides in domain V of 23S rRNA are responsible for macrolide resistance in S. pneumoniae isolates (43; W. Fu, M. Anderson, S. Williams, A. Tait-Kamradt, J. Sutcliffe, and J. Retsema, Abstr. Fifth Int. Conf. Macrolides Azalides Streptogramins Ketolides Oxazolidinones, abstr. 7.10, 2000). This study documents our investigation of 20 clinical isolates with an MSB or ML phenotype. These isolates had been recovered from patients in Eastern Europe and North America from 1992 to 1998.
MATERIALS AND METHODS
Bacterial strains.
The clinical isolates of S. pneumoniae were collected as part of surveillance studies in Eastern Europe, Canada, and the United States (Table 1). Strain Bu41 was an isolate from the ear of a 1-year-old infant in Bulgaria, collected by A. Marton and provided by R. Hakenbeck. A series of strains (Sof289, Bul23, Bul29, Bul34, Bul40, Bul91, and Bul115) from separate individuals in Bulgaria collected in 1992 to 1995 was kindly provided by L. Setchanova. All but one of these strains were from patients under the age of four. These serotype 19F strains were previously characterized as a single clone called K1, and Sof289 is representative of this clone (33). Strains 395, 398, 404, 407, 381, 386, 387, and 442 are from Eastern Europe. Strain BM4418 is from a blood culture of a 64-year-old man with Crohn's disease who was hospitalized in Toronto, Ontario, Canada, for an episode of community-acquired bacteremic pneumococcal pneumonia. The strain was collected by the Toronto Invasive Bacterial Diseases Network (20) and provided by A. McGeer. 02J1200 is a 1996 blood isolate from a 5-year-old male in New Jersey, E40 is a 1996 serotype 23F isolate from Tennessee obtained from Linda McDougal (Centers for Disease Control and Prevention), and 117-891 was isolated in 1998 from the sputum of a 78-year-old male in Washington state.
TABLE 1.
Source and phenotype of clinical isolates
| Strain | Country | Year | Source | Phenotype |
|---|---|---|---|---|
| Bu41 | Bulgaria | Unknown | Middle ear exudate | MSB |
| Sof289 | Bulgaria | 1994 | Nasopharynx | MSB |
| Bul23 | Bulgaria | 1992 | Nose | MSB |
| Bul29 | Bulgaria | 1992 | Nose | MSB |
| Bul34 | Bulgaria | 1994 | Conjunctiva | MSB |
| Bul40 | Bulgaria | 1993 | Middle ear exudate | MSB |
| Bul91 | Bulgaria | 1995 | Middle ear exudate | MSB |
| Bul115 | Bulgaria | 1995 | Sputum | MSB |
| 395 | Bulgaria | 1994 | Sputum | MSB |
| 398 | Bulgaria | 1994 | Nose | MSB |
| 404 | Bulgaria | 1994 | Eye | MSB |
| 407 | Bulgaria | 1994 | Middle ear exudate | MSB |
| 381 | Slovakia | 1994 | Middle ear exudate | MSB |
| 386 | Slovakia | 1994 | Nasopharynx | MSB |
| 387 | Slovakia | 1994 | Nasopharynx | MSB |
| 442 | Poland | 1994 | Nasopharynx | MSB |
| BM4418 | Canada | 1994–1996 | Blood | MSB |
| E40 | Tennessee | 1993 | Blood | ML |
| 02J1200 | New Jersey | 1996 | Blood | ML |
| 117-891 | Washington | 1998 | Sputum | ML |
S. pneumoniae R6 and CP1000 were used as recipients in transformation experiments, and strain A9, a spontaneous streptomycin-resistant derivative of R6, served as a control in transformation experiments (1). Strains derived in this study are listed with their mutations in Tables 3 and 5.
TABLE 3.
The impact of mutational frequency at position 2059 in 23S rRNA on MICsa
| Strain | A2059:G2059b | MIC (μg/ml) of compound:
|
Disk diffusion zone diam (mm) for compound:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERY | CLR | AZM | JOS | TYL | SPI | TEL | LIN | CLX | STA | STB | CLX | ERY | STB | ||
| R6 | 4:0 | 0.02 | 0.05 | 0.10 | 0.10 | 0.39 | 0.20 | 0.006 | 0.39 | 0.05 | 25.00 | 3.12 | 30.0 | 30.5 | 22.1 |
| ML1 | 2:2 | 3.12 | 1.56 | 400 | 1.56 | 6.25 | 3.12 | 0.006 | 6.25 | 0.10 | 50.00 | 3.12 | 22.1 | 20.6 | 19.3 |
| Z10 | 1:3 | 12.5 | 6.25 | >400 | 200 | 200 | 400 | 0.006 | 12.5 | 0.39 | 50.00 | 3.12 | 18.2 | 14.0 | 19.4 |
| Z10-Spir | 0:4 | 25 | 6.25 | >400 | 200 | 400 | 800 | 0.006 | 12.5 | 0.78 | 50.00 | 3.12 | 13.8 | 0 | 18.4 |
Fourteen-membered macrolides: erythromycin (ERY) and clarithromycin (CLR); 15-membered macrolide: azithromycin (AZM); 16-membered macrolides: josamycin (JOS), tylosin (TYL), and spiramycin (SPI); ketolide: telithromycin (TEL); lincosamides: lincomycin (LIN) and clindamycin (CLX); streptogramins: streptogramin A (STA) and streptogramin B (STB).
The ratio of the number of 23S rRNA genes harboring adenine at position 2059 to the number of genes harboring guanine there.
TABLE 5.
MICs of macrolide-lincosamide-streptogramin B antibiotics against MSB strains and transformants
| Strain | MIC (μg/ml) of compounda:
|
Mutation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ERY | CLR | AZM | JOS | TEL | CLX | STB | PEN | ||
| BM4419 | 0.05 | 0.03 | 0.10 | 0.20 | 0.006 | 0.05 | 1.56 | 0.03 | None |
| BM4418 | 6.25 | 12.5 | 12.5 | 6.25 | 3.12 | 0.05 | 25 | 0.03 | 63KPWRQKGTGREKGTGRAR80 |
| CP1000 | 0.03 | 0.01 | 0.05 | 0.05 | 0.003 | 0.03 | 1.56 | 0.01 | None |
| BM4440 | 3.12 | 3.12 | 3.12 | 3.12 | 1.56 | 0.03 | 25 | 0.01 | 63KPWRQKGTGREKGTGRAR80 |
| R6 | 0.02 | 0.05 | 0.10 | 0.10 | 0.003 | 0.02 | 1.56 | 0.006 | None |
| R6 MSB | 50 | >100 | 6.25 | 50 | 0.024 | 0.02 | 12.5 | 0.01 | 63KPWRQKTPSRAR74 |
| Bu41 | 100 | >100 | 12.5 | 100 | 0.05 | 0.05 | 12.5 | 3.12 | 63KPWRQKTPSRAR74 |
Fourteen-membered macrolides: erythromycin (ERY) and clarithromycin (CLR); 15-membered macrolide: azithromycin (AZM); 16-membered macrolide: josamycin (JOS); ketolide: telithromycin (TEL); lincosamide: clindamycin (CLX); streptogramin: streptogramin B (STB). PEN, penicillin G.
Susceptibility testing.
Phenotypes were determined by disk diffusion using 2-μg-clindamycin, 15-μg-erythromycin, and 25-μg-streptogramin B disks as described previously (42). MICs were determined in microtiter trays using Mueller-Hinton broth supplemented with 2.5% lysed horse blood according to the recommendations of the NCCLS (23). The S. pneumoniae strain recommended by the NCCLS, ATCC 49619, was included in the MIC evaluations. All compounds were purchased from Sigma or made by published methods at Pfizer, Inc.
DNA amplification and sequencing.
Primers were purchased from Sigma/Genosys Biotechnologies (The Woodlands, Tex.). Primers for internal fragments of erm(A), erm(B), erm(C), erm(TR), msr(A), mef(A), mph(A), mph(B), mph(C), ere(A), and ere(B) have been described previously (39, 43). Primers for amplifying 23S rRNA, 3′ ends of individual 23S rRNA genes, and L4 and L22 ribosomal protein genes from S. pneumoniae have been described previously, as have methods for purification of PCR products and DNA sequencing (43).
PFGE.
Genomic DNA from each isolate was restricted with SmaI and analyzed by pulsed-field gel electrophoresis (PFGE) as previously described (43).
Transformation.
Synthetic competence-stimulating peptide I and the modified method of Havarstein et al. (7) were used to induce S. pneumoniae to move into a transformation-competent state. MSB transformants were selected on Todd-Hewitt agar containing 5% sheep blood and 0.5 μg of erythromycin per ml. When genomic DNA from BM4418 was transformed into CP1000, 2 μg of pristinamycin per ml was used as the selecting agent. ML transformants were selected with 0.05 or 0.1 μg of erythromycin per ml. Plates were incubated overnight at 37°C in 5% CO2. Cell suspensions were monitored for competence by determining the transformation frequency of streptomycin resistance from S. pneumoniae A9, which ranged from 0.1 to 0.5%.
In vitro translation and cellular viability.
Methods for isolation of S. pneumoniae ribosomes and in vitro translation have been described previously (42). To determine cellular viability, aliquots from strains growing in Todd-Hewitt broth containing 0.5% yeast extract were removed initially and hourly thereafter, diluted, and plated onto Trypticase soy agar containing 5% sheep blood. Following incubation at 37°C for 22 to 24 h, colonies were counted.
RESULTS
Of the 20 strains studied, 17 were found to have an MSB phenotype and 3 displayed an ML phenotype (Table 1; Fig. 1). MICs of representative macrolide-lincosamide-streptogramin B agents are shown in Table 2. Genomic DNA from these isolates was subjected to PCR analysis using primers specific for macrolide esterases [ere(A) and ere(B)], phosphotransferases [mph(A), mph(B), and mph(C)], an ATP binding cassette transporter [msr(A)], a proton motive force transporter [mef(A)], and rRNA methylases [erm(A), erm(TR) (belongs to class A) (29), erm(B), and erm(C)]. None of the isolates gave a PCR product specific to any of these known resistance determinants described for enteric bacteria, staphylococci, or streptococci.
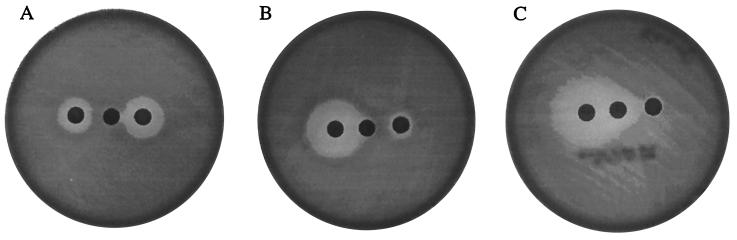
FIG. 1.
Phenotypes of pneumococcal clinical isolates. (A) 02J1200, ML pattern; (B) Bu41, MSB pattern; (C) BM4418, MSB pattern. Disks: left, 2 μg of clindamycin; center, 15 μg of erythromycin; right, 25 μg of streptogramin B.
TABLE 2.
MICs of macrolide-lincosamide-streptogramin B antibiotics against MSB and ML strains
| Strain | MIC (μg/ml) of compounda:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ERY | CLR | AZM | JOS | TEL | LIN | CLX | STA | STB | PEN | |
| E40 | 100 | 25 | >100 | >100 | 0.01 | 12.5 | 0.78 | 100 | 6.25 | 6.25 |
| 02J1200 | 50 | 12.5 | >100 | >100 | 0.01 | 12.5 | 0.78 | 100 | 3.12 | 0.01 |
| 117-891 | 50 | 12.5 | >100 | >100 | 0.01 | 12.5 | 0.78 | 50 | 3.12 | 0.03 |
| Bu41 | 100 | 12.5 | >100 | 100 | 0.03 | 0.78 | 0.05 | 100 | 12.5 | 6.25 |
| Sof289b | >100 | 25 | >100 | 100 | 0.1 | 0.78 | 0.1 | 100 | 12.5 | 3.12 |
| 395 | >100 | 25 | >100 | 100 | 0.1 | 1.56 | 0.1 | 100 | 12.5 | 1.56 |
| 398 | 100 | 25 | >100 | 100 | 0.03 | 0.78 | 0.05 | 100 | 12.5 | 3.12 |
| 404 | >100 | 25 | >100 | >100 | 0.2 | 1.56 | 0.1 | >100 | 25 | 6.25 |
| 407 | >100 | 25 | >100 | 100 | 0.05 | 1.56 | 0.1 | 100 | 25 | 6.25 |
| 381 | >100 | 25 | >100 | 100 | 0.05 | 0.39 | 0.05 | 100 | 25 | 6.25 |
| 386 | >100 | 50 | >100 | 100 | 0.1 | 0.39 | 0.1 | 100 | 25 | 3.12 |
| 387 | >100 | 50 | >100 | 100 | 0.1 | 0.39 | 0.1 | 100 | 25 | 1.56 |
| 442 | >100 | 25 | >100 | 100 | 0.1 | 0.78 | 0.1 | 100 | 12.5 | 6.25 |
| BM4418 | 6.25 | 12.5 | 12.5 | 6.25 | 3.12 | 0.39 | 0.05 | 50 | 25 | 0.01 |
| ATCC 49619 | 0.03 | 0.03 | 0.1 | 0.2 | 0.006 | 0.78 | 0.05 | 25 | 3.12 | 0.39 |
Fourteen-membered macrolides: erythromycin (ERY) and clarithromycin (CLR); 15-membered macrolide: azithromycin (AZM); 16-membered macrolide: josamycin (JOS); ketolide: telithromycin (TEL); lincosamides: lincomycin (LIN) and clindamycin (CLX); streptogramins: streptogramin A (STA) and streptogramin B (STB). PEN, penicillin G.
The MICs for Sof289 are representative of those for six other identical isolates (Bul23, Bul29, Bul34, Bul40, Bul91, and Bul115).
Analysis of strains with an ML phenotype.
Three strains, 02J1200, E40, and 117-891, had an ML phenotype (Fig. 1A). As these strains did not contain any of the known resistance mechanisms, we reasoned that mutations in the 23S rRNA alleles and/or in ribosomal proteins L4 and L22 might account for resistance. This was based on literature reports where mutations in L4, L22, or 23S rRNA genes were responsible for macrolide resistance in Escherichia coli and Bacillus spp. as well as our recent experience in characterizing resistance mechanisms in S. pneumoniae strains passaged with macrolides (2, 4, 25, 31, 34, 43, 46, 50, 52; Fu et al., Abstr. Fifth Int. Conf. Macrolides Azalides Streptogramins Ketolides Oxazolidinones). Using primers designed from the genomic database available for S. pneumoniae (43), we found that the sequences of the structural genes for L4 and L22 were identical to those of the wild-type strain, whereas the sequences for 23S rRNA revealed that all three strains had a mixture of adenine and guanine at position 2059 of domain V. The nature of the mutation was confirmed to be heterozygous, since the three isolates had an A2059G mutation in three of the four alleles when each allele was amplified separately and sequenced.
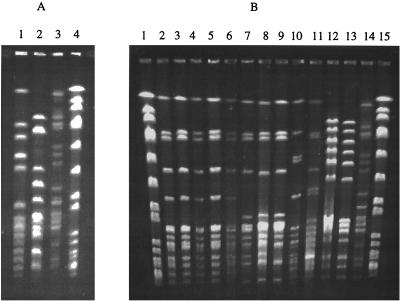
The ML strains were resistant to 14-, 15-, and 16-membered macrolides, with MICs ranging from 12.5 to >100 μg/ml (Table 2). As expected from their phenotype, the strains were also 16-fold-more resistant to the lincosamides clindamycin and lincomycin than was ATCC 49619. The isolates remained susceptible to streptogramin B and to the ketolide telithromycin. Only E40 was resistant to penicillin G. Genomic DNA from each isolate was restricted with SmaI and analyzed by PFGE (Fig. 2A). Each strain had a unique pattern, indicating that the strains did not share a recent common origin (45).
FIG. 2.
PFGE of SmaI digests of genomic DNA from ML and MSB strains. (A) Lane 1, strain 02J1200; lane 2, strain E40; lane 3, strain 117-891; lane 4, S. aureus SmaI markers. (B) Lanes 1 and 15, S. aureus SmaI markers; lane 2, Sof289 (Bulgaria); lane 3, Bu41 (Bulgaria); lane 4, 404 (Bulgaria); lane 5, 407 (Bulgaria); lane 6, 398 (Bulgaria); lane 7, 381 (Slovakia); lane 8, 386 (Slovakia); lane 9, 387 (Slovakia); lane 10, 395 (Bulgaria); lane 11, 442 (Poland); lane 12, BM4419 (susceptible revertant of BM4418); lane 13, BM4418 (Canada); lane 14, 117-891 (United States; ML isolate).
To confirm that the A2059G changes were sufficient to confer ML, the PCR product containing 23S rRNA from strain 117-891 was transformed into R6 and transformants were selected with low levels of erythromycin. The sequences of 23S rRNA, L4, and L22 genes of two transformants (ML1 and ML2) that were phenotypically ML were determined. Both had A2059G mutations in two of the four alleles of 23S rRNA, while the genes for the ribosomal proteins were wild type. When strain ML1 was phenotyped, colonies were noted in the zones of inhibition on erythromycin and clindamycin disks. Twelve “zone clones” were selected (Z1 to Z12) and streaked on Trypticase soy agar containing 5% sheep blood devoid of antibiotic. As shown by disk diffusion analysis, the majority of the zone clones had smaller zones of inhibition by erythromycin and clindamycin than did their parent, ML1, more analogous to the zone sizes seen with 117-891. When the zone clones were sequenced, all were found to have wild-type sequences for L4 and L22 ribosomal genes and 3 of the 12 had three of the four alleles of 23S rRNA as A2059G mutations. The remaining nine clones had two of the four alleles of 23S rRNA as A2059G. To see if there were any cells that had converted all four alleles to 2059G, samples from the wells containing inhibitory concentrations of spiramycin were streaked onto nonselective agar. The susceptibility of individual colonies growing from these wells to macrolide-lincosamide-streptogramin B antibiotics was determined by disk diffusion, and clones that had no zones of inhibition by erythromycin were identified (Z10-Spir, Table 3). Sequencing of the PCR products from 23S rRNA-specific primers revealed that all four alleles were present as A2059G with no changes in L4 or L22 sequences in Z10-Spir (Table 3).
The isogenic strains were checked for susceptibilities to macrolide-lincosamide-streptogramin B antibiotics (Table 3). MICs of azithromycin increased >100-fold with two or more A2059G changes. The MICs of erythromycin and clarithromycin increased about 100-fold with two A2059G changes and a further four- to eightfold with three or four A2059G changes. MICs of josamycin, tylosin, and spiramycin increased 10- to 20-fold with two A2059G changes and a further 30- to 100-fold with three or four A2059G changes. MICs of lincomycin but not clindamycin increased about 10-fold with two A2059G changes; MICs of lincomycin remained at similar values with three or four A2059G changes, while those of clindamycin increased a further four- to eightfold. MICs of telithromycin, streptogramin A, and streptogramin B were not affected by any of the A2059G changes. Disk diffusion analysis of these strains also confirmed that the effect of the mutation was to render the cells increasingly resistant to macrolides and lincosamides (Table 3).
Since mutations in the ribosome could alter the growth rate, viability was monitored for R6, ML1 (transformant with two A2059G changes), Z10 (zone clone with three A2059G changes), and Z10-Spir (four A2059G changes) in Todd-Hewitt broth–0.5% yeast extract at 35°C. The generation time increased as a function of the number of A2059G mutations. In two separate analyses, R6 had a generation time of 24 min while the generation times of ML1, Z10, and Z10-Spir were 29, 32, and 38 min, respectively.
Purification of ribosomes from this isogenic set and testing for translation inhibition by macrolide-lincosamide-streptogramin B antibiotics were done to ascertain ribosomal sensitivity. The 50% inhibitory concentrations (IC50) for macrolides increased 20- to over 50-fold as the number of alleles containing the A2059G mutation increased, while there was no change in the IC50 for telithromycin and a fourfold change for streptogramin B (Table 4).
TABLE 4.
In vitro poly(A)-directed translation using ribosomes from isogenic strains
| Strain | IC50 (μg/ml) of compounda:
|
|||||
|---|---|---|---|---|---|---|
| ERY | AZM | JOS | TEL | CLX | STB | |
| R6 | 0.02 | 0.02 | 0.2 | 0.02 | 0.03 | 0.15 |
| ML1 | 0.08 | 0.08 | >10 | 0.04 | 0.09 | 0.3 |
| Z10 | 0.9 | 1.2 | >10 | 0.02 | 0.3 | 0.3 |
| Z10-Spir | 0.5 | 0.8 | >10 | 0.04 | 0.6 | 0.6 |
| CP1000 | 0.07 | 0.01 | 0.003 | 0.005 | 0.03 | 0.1 |
| BM4440 | 10 | 7 | 8 | 0.4 | 0.05 | >10 |
| R6 | 0.01 | 0.01 | 0.2 | 0.08 | 0.01 | 0.3 |
| R6 MSB | >10 | >10 | >10 | 0.15 | 0.03 | >10 |
Fourteen-membered macrolide: erythromycin (ERY); 15-membered macrolide: azithromycin (AZM); 16-membered macrolide: josamycin (JOS); ketolide: telithromycin (TEL); lincosamide: clindamycin (CLX); streptogramin: streptogramin B (STB).
Analysis of strains with an MSB phenotype.
Strains that had an MSB phenotype displayed a large zone of inhibition around clindamycin and a reduced or no zone of inhibition for erythromycin or streptogramin B (Fig. 1B and C). These 17 strains did not harbor any known resistance determinants by PCR analysis, and sequencing of genes for 23S rRNA and L22 ribosomal protein did not reveal any mutation. By contrast, two types of mutations within the L4 sequence were found. The 16 Eastern European isolates contained a 3-amino-acid substitution (69GTG71 to TPS) resulting from mutations in a highly conserved region (63KPWRQKGTGRAR74) (Fig. 3). These strains were highly resistant to erythromycin, azithromycin, and josamycin (MIC, ≥100 μg/ml) and more moderately resistant to clarithromycin (MIC, 12.5 to 25 μg/ml) (Table 2). The strains remained susceptible to lincosamides and telithromycin, but all were resistant to streptogramin B and penicillin. We showed that the nucleotide changes (seven changes, two of which are silent) resulting in the 69GTG71 to TPS (nucleotide region 205 to 240) substitution are sufficient to confer MSB by analysis of R6 transformants transformed with the L4-specific PCR product from Bu41 (Table 5). Erythromycin-resistant transformants (i.e., R6 MSB in Table 5) contained the same seven changes in the nucleotide 205 to 240 region, and MICs for them were similar to MICs for the parent strain. Other changes outside of the nucleotide 205 to 240 region that were found in some of the wild-type strains and some of the transformants did not appear to impact the expression of MSB as measured by MICs of the transformants (data not shown).
FIG. 3.
Nucleotide and amino acid changes (boldface) in strains with MSB phenotypes.
The second type of L4 mutation was seen in the Canadian strain BM4418 and consisted of an 18-bp insertion resulting in the addition of six amino acids (69GTGREKGTGRAR) between 71G and 72R (Fig. 3). The MICs for this strain were different, most notably in that this strain was moderately resistant to all macrolides and more highly resistant to telithromycin (Table 2). Confirmation that this change in L4 sequence could confer an MSB phenotype was obtained from the study of transformants derived using genomic DNA to transform CP1000 (i.e., BM4440 in Table 5). BM4440 contained the same change as that in BM4418. Further, a susceptible revertant of BM4418, called BM4419, was obtained by growth of BM4418 for 80 generations in the absence of antibiotic. Sequence analysis of the genes for 23S rRNA and L4 and L22 ribosomal proteins in BM4419 revealed no mutations; the L4 DNA sequence revealed a clean deletion of the 18-bp insertion in BM4418 (Fig. 3).
The MICs of the macrolide-lincosamide-streptogramin B antibiotics against the isogenic pairs of MSB strains provided the best indicator of the impact of the L4 mutation (Table 5). A differential of ca. 300- to 500-fold for each of the macrolides, including the ketolide telithromycin, was seen in strains derived from BM4418 harboring the 6-amino-acid insert. Against the transformant R6 MSB containing the 69GTG71 to TPS mutation derived from Bu41, the increase in MICs of 14- and 16-membered macrolides was >2,000-fold while the differential observed for azithromycin (15-membered macrolide) was ca. 60-fold. The MIC of telithromycin against R6 MSB (0.02 μg/ml) was approximately eightfold higher than that against R6 (0.006 μg/ml), but still in the susceptible range. The large increase in resistance to telithromycin of BM4440 and BM4418 led to a MIC in the putative intermediate range of 2 to 3 μg/ml (M. M. Traczewski, S. D. Brown, and A. L. Barry, Abstr. Fifth Int. Conf. Macrolides Azalides Streptogramins Ketolides Oxazolidinones, abstr. 2.08, 2000; G. A. Pankuch, M. R. Jacobs, and P. C. Appelbaum, Abstr. Fifth Int. Conf. Macrolides Azalides Streptogramins Ketolides Oxazolidinones, abstr. 2.09, 2000). Comparison of IC50 of inhibition by different macrolide-lincosamide-streptogramin B antibiotics using the purified ribosomes from isogenic pairs R6 and R6 MSB and CP1000 and BM4440 indicated that resistance also occurred at the translational level (Table 4). However, the IC50 differential observed for telithromycin in CP1000 versus BM4440 was considerably less than that observed by MIC evaluation (Table 5).
When total DNA from the MSB isolates was digested with SmaI and examined by PFGE, four profiles were found (Fig. 2B). The first one (clone 1) consisted of Bulgarian strains Sof289, Bu41, 398, 404, and 407. The three isolates 381, 386, and 387 from Slovakia are clearly related to the first clone, differing by two bands and therefore assigned to clone 1 (45). The Bulgarian isolate 395 had a different restriction pattern by PFGE and was assigned to clone 2 (Fig. 2B, lane 10). Strains 442 from Poland (Fig. 2B, lane 11) and BM4418 from Canada also appeared to be separate clones.
Changes in ribosomal proteins have resulted in a temperature-sensitive phenotype in E. coli L4 mutants (3, 6). To determine if the L4 changes observed in S. pneumoniae strains resulted in growth defects, the generation times of R6 and its isogenic derivatives were measured in Todd-Hewitt–0.5% yeast extract broth at 35°C. R6 transformed with the L4 PCR product of BM4418 had a generation time of 38 min versus a generation time of 24 min for R6. However, R6 MSB (69GTG71 to TPS change) had the same generation time as did its isogenic parent, R6.
DISCUSSION
Target modification of the adenine at position 2058 (E. coli numbering) in 23S rRNA by an Erm methylase is a common resistance mechanism seen in a variety of pathogenic bacteria (10, 29, 38, 40, 51). In S. pneumoniae, erm(B) is the predominant methylase and is generally part of Tn1545 or Tn3872 (19, 47). Alteration of A2058 to another residue has been described previously (8, 11, 17, 21, 22, 24, 26, 30, 36, 37, 43, 44, 48, 49; Fu et al., Abstr. Fifth Int. Conf. Macrolides Azalides Streptogramins Ketolides Oxazolidinones). Modification of adenine at position 2059 (A2059G transition) has been found in macrolide-resistant clinical strains of Mycobacterium spp. (21, 49), Helicobacter pylori (8, 24, 37, 48), and Propionibacterium spp. (30). In laboratory-derived mutants that contained A2059G, lincomycin resistance was conferred on Nicotiana plumbaginafoilia chloroplast (50), streptogramin A resistance was conferred on Halobacterium halobium (27), macrolide resistance was conferred on Mycoplasma pneumoniae (17), and macrolide resistance and increased lincosamide resistance were conferred on S. pneumoniae (43; Fu et al., Abstr. Fifth Int. Conf. Macrolides Azalides Streptogramins Ketolides Oxazolidinones). We explored the dose effect of this mutation by isolating isogenic strains carrying two, three, or four alleles of A2059G. As the number of alleles increased, so did the MICs of 14-, 15-, and 16-membered macrolides. The effect on clindamycin was not as pronounced in the isogenic strains, but for the three clinical strains that carry three 2059G alleles and one A2059 allele the MICs were ∼1 μg/ml, consistent with a resistant phenotype (≥1 μg/ml) according to NCCLS standards (Table 2).
The MSB phenotype was seen in laboratory studies when susceptible isolates of S. pneumoniae were passaged with macrolides (43). It was due to a C2611A or C2611G change in three or four alleles of 23S rRNA, respectively, or to L4 mutations in which there was either a change at amino acid 71 from glycine to cysteine or a 2-amino-acid insert (63KPWRQSQKGTGRAR) in a highly conserved region of L4. Interestingly, two types of mutations in L4 were observed in the clinical isolates, neither of which was seen in the laboratory-derived mutants. The majority of the clinical strains had mutations that resulted in the amino acid substitutions of TPS for 69GTG71. One isolate, BM4418, had a 6-amino-acid insert (69GTGREKGTGRAR) in the highly conserved region of L4 and differed from the other MSB isolates by its increased resistance to telithromycin. However, the latter mutant paid a price, as was noted by a 60% increase in generation time at 35°C. Thus, the L4 mutation 69GTG71 to TPS could be more easily maintained since it apparently does not alter growth.
As more macrolide-resistant isolates are characterized, it will be interesting to see how many contain 23S rRNA and/or ribosomal protein mutations in addition to or as the sole mechanism responsible for MLSB. Recently, there have been several reports of clinical strains of S. pneumoniae that do not contain either erm(B) or mef(A) (9; E. Di Modugno, A. Felici, M. Guerrini, H. Mottl, A. Zamperlin, and D. Sabatini, Abstr. Fifth Int. Conf. Macrolides Azalides Streptogramins Ketolides Oxazolidinones, abstr. 7.06, 2000; K. Waites, C. Johnson, B. Gray, K. Edwards, M. Crain, and W. Benjamin, Jr., Abstr. Fifth Int. Conf. Macrolides Azalides Streptogramins Ketolides Oxazolidinones, abstr. 7.08, 2000; E. Di Modugno, A. Felici, M. Guerrini, H. Mottl, P. Piccoli, and D. Sabatini, Abstr. Fifth Int. Conf. Macrolides Azalides Streptogramins Ketolides Oxazolidinones, abstr. 7.14, 2000). A surveillance study reported the number of erm(B)- and mef(A)-negative isolates to be as high as 2.2% of the total macrolide-resistant isolates (Di Modugno et al., Abstr. Fifth Int. Conf. Macrolides Azalides Streptogramins Ketolides Oxazolidinones, abstr. 7.06).
What are the clinical implications of these findings? Since the majority of clinical laboratories do not test streptogramin B, an ML phenotype will likely be interpreted as a strain carrying an Erm methylase, whereas a strain that is MSB may be interpreted as harboring mef(A). If the MSB phenotype results from a mutation in L4, the isolate is likely to be susceptible to clindamycin, whereas if it is the result of a C2611A/G change in 23S rRNA, as was seen previously for passaged isolates (43), there may be some uncertainty, as MICs of 0.2 to 0.4 μg of clindamycin per ml indicate intermediate resistance (23). The MICs of clindamycin for an ML strain also indicated intermediate to low-level resistance (0.39 to 0.78 μg/ml), and it remains to be determined if these isolates are susceptible to clindamycin therapy. Further, in MSB strain BM4418, we have seen a hint of one mechanism that may confer ketolide resistance. Use of disk diffusion analysis to infer the likely mechanism responsible for macrolide resistance needs to be modified in light of these new resistance mechanisms; however, using erythromycin, clindamycin, telithromycin, and streptogramin B disks will be helpful.
In summary, this work characterized two resistance mechanisms not previously recognized in clinical isolates of S. pneumoniae from Eastern Europe and North America. These isolates had changes in 23S rRNA genes (A→G at position 2059) or changes in a highly conserved region of ribosomal protein L4 (69GTG71 to TPS mutation or a 6-amino-acid L4 insertion, 69GTGREKGTGRAR). Introduction of new macrolide-lincosamide-streptogramin B agents will necessitate ongoing vigilance in testing the susceptibility of isolates to new and existing macrolide-lincosamide-streptogramin B agents and the determination of resistance mechanisms in strains with decreased susceptibility.
ACKNOWLEDGMENTS
We thank both Lori Brennan and Joan Duignan for their supporting technical roles. We also thank all members of the DNA sequencing lab from Pfizer Global Research and Development for their careful work. The strains received prior to their publication are gratefully acknowledged.
REFERENCES
- 1.Avery O T, MacLeod C M, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from Pneumococcus type III. 1944. Mol Med. 1995;1:344–365. [PMC free article] [PubMed] [Google Scholar]
- 2.Burnette-Vick B, Champney W S, Musich P R. A temperature-sensitive mutant of Escherichia coli with an alteration in ribosomal protein L22. Genetica. 1994;94:17–25. doi: 10.1007/BF01429216. [DOI] [PubMed] [Google Scholar]
- 3.Chittum H S, Champney W S. Erythromycin inhibits the assembly of the large ribosomal subunit in growing Escherichia coli cells. Curr Microbiol. 1995;30:273–279. doi: 10.1007/BF00295501. [DOI] [PubMed] [Google Scholar]
- 4.Chittum H S, Champney W S. Ribosomal protein gene sequence changes in erythromycin-resistant mutants of Escherichia coli. J Bacteriol. 1994;176:6192–6198. doi: 10.1128/jb.176.20.6192-6198.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corso A, Severina E P, Petruk V F, Mauriz Y R, Tomasz A. Molecular characterization of penicillin-resistant Streptococcus pneumoniae isolates causing respiratory disease in the United States. Microb Drug Resist. 1998;4:325–327. doi: 10.1089/mdr.1998.4.325. [DOI] [PubMed] [Google Scholar]
- 6.Guthrie C, Nashimoto H, Nomura M. Structure and function of Escherichia coli ribosomes. 8. Cold-sensitive mutants defective in ribosome assembly. Proc Natl Acad Sci USA. 1969;63:384–391. doi: 10.1073/pnas.63.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havarstein L S, Coomaraswamy G, Morrison D A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulten K, Gibreel A, Skold O, Engstrand L. Macrolide resistance in Helicobacter pylori: mechanism and stability in strains from clarithromycin-treated patients. Antimicrob Agents Chemother. 1997;41:2550–2553. doi: 10.1128/aac.41.11.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston N J, De Azavedo J C, Kellner J D, Low D E. Prevalence and characterization of the mechanisms of macrolide, lincosamide, and streptogramin resistance in isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2425–2426. doi: 10.1128/aac.42.9.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneko T, McArthur H, Sutcliffe J. Recent developments in the area of macrolide antibiotics. Exp Opin Ther Patents. 2000;10:403–425. [Google Scholar]
- 11.Karlsson M, Fellstrom C, Heldtander M U, Johansson K E, Franklin A. Genetic basis of macrolide and lincosamide resistance in Brachyspira (Serpulina) hyodysenteriae. FEMS Microbiol Lett. 1999;172:255–260. doi: 10.1111/j.1574-6968.1999.tb13476.x. [DOI] [PubMed] [Google Scholar]
- 12.Kataja J, Seppala H, Skurnik M, Sarkkinen H, Huovinen P. Different erythromycin resistance mechanisms in group C and group G streptococci. Antimicrob Agents Chemother. 1998;42:1493–1494. doi: 10.1128/aac.42.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klugman K P, Capper T, Widdowson C A, Koornhof H J, Moser W. Increased activity of 16-membered lactone ring macrolides against erythromycin-resistant Streptococcus pyogenes and Streptococcus pneumoniae: characterization of South African isolates. J Antimicrob Chemother. 1998;42:729–734. doi: 10.1093/jac/42.6.729. [DOI] [PubMed] [Google Scholar]
- 14.Latini L, Ronchetti M P, Merolla R, Guglielmi F, Bajaksouzian S, Villa M P, Jacobs M R, Ronchetti R. Prevalence of mefE, erm and tet(M) genes in Streptococcus pneumoniae strains from Central Italy. Int J Antimicrob Agents. 1999;13:29–33. doi: 10.1016/s0924-8579(99)00097-7. [DOI] [PubMed] [Google Scholar]
- 15.Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1267–1272. doi: 10.1128/aac.35.7.1267. . (Erratum, 35:2165.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leclercq R, Courvalin P. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob Agents Chemother. 1991;35:1273–1276. doi: 10.1128/aac.35.7.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucier T S, Heitzman K, Liu S K, Hu P C. Transition mutations in the 23S rRNA of erythromycin-resistant isolates of Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1995;39:2770–2773. doi: 10.1128/aac.39.12.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luna V A, Coates P, Eady E A, Cove J H, Nguyen T T, Roberts M C. A variety of gram-positive bacteria carry mobile mef genes. J Antimicrob Chemother. 1999;44:19–25. doi: 10.1093/jac/44.1.19. [DOI] [PubMed] [Google Scholar]
- 19.McDougal L K, Tenover F C, Lee L N, Rasheed J K, Patterson J E, Jorgensen J H, LeBlanc D J. Detection of Tn917-like sequences within a Tn916-like conjugative transposon (Tn3872) in erythromycin-resistant isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2312–2318. doi: 10.1128/aac.42.9.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGeer A, Green K, Landry L, Talbot J, Goldenberg E the Toronto Invasive Bacterial Diseases Network. Assessing the potential impact of vaccination programs on invasive pneumococcal disease: data from population based surveillance. Can J Infect Dis. 1999;10:24A–26A. [Google Scholar]
- 21.Meier A, Kirschner P, Springer B, Steingrube V A, Brown B A, Wallace R J, Jr, Bottger E C. Identification of mutations in 23S rRNA gene of clarithromycin-resistant Mycobacterium intracellulare. Antimicrob Agents Chemother. 1994;38:381–384. doi: 10.1128/aac.38.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nash K A, Inderlied C B. Genetic basis of macrolide resistance in Mycobacterium avium isolated from patients with disseminated disease. Antimicrob Agents Chemother. 1995;39:2625–2630. doi: 10.1128/aac.39.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 24.Occhialini A, Urdaci M, Doucet-Populaire F, Bebear C M, Lamouliatte H, Megraud F. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother. 1997;41:2724–2728. doi: 10.1128/aac.41.12.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardo D, Rosset R. Genetic studies of erythromycin resistant mutants of Escherichia coli. Mol Gen Genet. 1974;135:257–268. doi: 10.1007/BF00268620. [DOI] [PubMed] [Google Scholar]
- 26.Pernodet J L, Boccard F, Alegre M T, Blondelet-Rouault M H, Guerineau M. Resistance to macrolides, lincosamides and streptogramin type B antibiotics due to a mutation in an rRNA operon of Streptomyces ambofaciens. EMBO J. 1988;7:277–282. doi: 10.1002/j.1460-2075.1988.tb02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porse B T, Garrett R A. Sites of interaction of streptogramin A and B antibiotics in the peptidyl transferase loop of 23 S rRNA and the synergism of their inhibitory mechanisms. J Mol Biol. 1999;286:375–387. doi: 10.1006/jmbi.1998.2509. [DOI] [PubMed] [Google Scholar]
- 28.Portillo A, Ruiz-Larrea F, Zarazaga M, Alonso A, Martinez J L, Torres C. Macrolide resistance genes in Enterococcus spp. Antimicrob Agents Chemother. 2000;44:967–971. doi: 10.1128/aac.44.4.967-971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts M C, Sutcliffe J, Courvalin P, Jensen L B, Rood J, Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43:2823–2830. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross J I, Eady E A, Cove J H, Jones C E, Ratyal A H, Miller Y W, Vyakrnam S, Cunliffe W J. Clinical resistance to erythromycin and clindamycin in cutaneous propionibacteria isolated from acne patients is associated with mutations in 23S rRNA. Antimicrob Agents Chemother. 1997;41:1162–1165. doi: 10.1128/aac.41.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnier J, Gewitz H S, Behrens S E, Lee A, Ginther C, Leighton T. Isolation and characterization of Bacillus stearothermophilus 30S and 50S ribosomal protein mutations. J Bacteriol. 1990;172:7306–7309. doi: 10.1128/jb.172.12.7306-7309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seppala H, Nissinen A, Yu Q, Huovinen P. Three different phenotypes of erythromycin-resistant Streptococcus pyogenes in Finland. J Antimicrob Chemother. 1993;32:885–891. doi: 10.1093/jac/32.6.885. [DOI] [PubMed] [Google Scholar]
- 33.Setchanova L, Tomasz A. Molecular characterization of penicillin-resistant Streptococcus pneumoniae isolates from Bulgaria. J Clin Microbiol. 1999;37:638–648. doi: 10.1128/jcm.37.3.638-648.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharrock R A, Leighton T, Wittmann H G. Macrolide and aminoglycoside antibiotic resistance mutations in the Bacillus subtilis ribosome resulting in temperature-sensitive sporulation. Mol Gen Genet. 1981;183:538–543. doi: 10.1007/BF00268778. [DOI] [PubMed] [Google Scholar]
- 35.Shortridge V D, Doern G V, Brueggemann A B, Beyer J M, Flamm R K. Prevalence of macrolide resistance mechanisms in Streptococcus pneumoniae isolates from a multicenter antibiotic resistance surveillance study conducted in the United States in 1994–1995. Clin Infect Dis. 1999;29:1186–1188. doi: 10.1086/313452. [DOI] [PubMed] [Google Scholar]
- 36.Stamm L V, Bergen H L. A point mutation associated with bacterial macrolide resistance is present in both 23S rRNA genes of an erythromycin-resistant Treponema pallidum clinical isolate. Antimicrob Agents Chemother. 2000;44:806–807. doi: 10.1128/aac.44.3.806-807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone G G, Shortridge D, Versalovic J, Beyer J, Flamm R K, Graham D Y, Ghoneim A T, Tanaka S K. A PCR-oligonucleotide ligation assay to determine the prevalence of 23S rRNA gene mutations in clarithromycin-resistant Helicobacter pylori. Antimicrob Agents Chemother. 1997;41:712–714. doi: 10.1128/aac.41.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutcliffe J. Resistance to macrolides mediated by efflux mechanisms. Curr Opin Investig Drugs. 1999;1:403–412. [Google Scholar]
- 39.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutcliffe J, Mueller J, Utt E. Antibiotic resistance mechanisms of bacterial pathogens. In: Demain A L, Davies J E, editors. Manual of industrial microbiology and biotechnology. 2nd ed. Washington, D.C.: ASM Press; 1999. pp. 759–788. [Google Scholar]
- 41.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tait-Kamradt A, Clancy J, Cronan M, Dib-Hajj F, Wondrack L, Yuan W, Sutcliffe J. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2251–2255. doi: 10.1128/aac.41.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tait-Kamradt A, Davies T, Cronan M, Jacobs M R, Appelbaum P C, Sutcliffe J. Mutations in 23S rRNA and L4 ribosomal protein account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob Agents Chemother. 2000;44:2118–2125. doi: 10.1128/aac.44.8.2118-2125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor D E, Ge Z, Purych D, Lo T, Hiratsuka K. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother. 1997;41:2621–2628. doi: 10.1128/aac.41.12.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tipper D J, Johnson C W, Ginther C L, Leighton T, Wittmann H G. Erythromycin resistant mutations in Bacillus subtilis cause temperature sensitive sporulation. Mol Gen Genet. 1977;150:147–159. doi: 10.1007/BF00695395. [DOI] [PubMed] [Google Scholar]
- 47.Trieu-Cuot P, Poyart-Salmeron C, Carlier C, Courvalin P. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 1990;18:3660. doi: 10.1093/nar/18.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Versalovic J, Shortridge D, Kibler K, Griffy M V, Beyer J, Flamm R K, Tanaka S K, Graham D Y, Go M F. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996;40:477–480. doi: 10.1128/aac.40.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallace R J, Jr, Meier A, Brown B A, Zhang Y, Sander P, Onyi G O, Bottger E C. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob Agents Chemother. 1996;40:1676–1681. doi: 10.1128/aac.40.7.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weisblum B. Resistance to macrolide-lincosamide-streptogramin antibiotics. In: Fischetti V, editor. Gram-positive pathogens. Washington, D.C.: ASM Press; 2000. pp. 682–698. [Google Scholar]
- 52.Wittmann H G, Stoffler G, Apirion D, Rosen L, Tanaka K, Tamaki M, Takata R, Dekio S, Otaka E. Biochemical and genetic studies on two different types of erythromycin resistant mutants of Escherichia coli with altered ribosomal proteins. Mol Gen Genet. 1973;127:175–189. doi: 10.1007/BF00333665. [DOI] [PubMed] [Google Scholar]